Abstract
The coronavirus disease 2019 (COVID‐19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has rapidly spread across the globe, causing innumerable deaths and a massive economic catastrophe. Exposure to household members with confirmed COVID‐19 is the most common source of infection among children. Children are just as likely as adults to get infected with SARS‐CoV‐2. Most children are asymptomatic and when symptoms occur, they are usually mild. Infants <12 months old are at a higher risk for severe or critical disease. COVID‐19 is diagnosed the same way in pediatric population as adults by testing specimen obtained from upper respiratory tract for nucleic acid amplification test (NAAT) using reverse transcriptase viral polymerase chain reaction (RT‐PCR). The common laboratory findings in hospitalized patient include leukopenia, lymphopenia, and increased levels of inflammatory markers. Chest X‐ray findings are variable and computed tomography scans of the chest may show ground glass opacities similar to adults or non‐specific findings. Prevention is the primary intervention strategy. Recently the U.S. Food and Drug Administration (FDA) has provided emergency authorization of the Pfizer‐BioNTech COVID‐19 vaccine and many other vaccine candidates are in the investigational stage. There is limited data in children on the use of antivirals, hydroxychloroquine, azithromycin, monoclonal antibody, and convalescent plasma. Oxygen therapy is required in hypoxic children (saturation <92%). Similar to adults, other measures to maintain oxygenation such as high flow nasal cannula, CPAP, or ventilatory support may be needed. Ventilatory management strategies should include use of low tidal volumes (5–6 cc/kg), high positive expiratory pressure, adequate sedation, paralysis, and prone positioning. Recently, a new entity associated with COVID‐19 called multisystem inflammatory syndrome in children (MIS‐C) has emerged. Clinical, laboratory, and epidemiological criteria are the basis for this diagnosis. Management options include ICU admission, steroids, intravenous gamma globulin, aspirin, anakinra, and anticoagulants. Vasoactive‐inotropic score (VIS) is used to guide vasopressor support.
Keywords: Angiotensin‐Converting Enzyme 2 (ACE2), COVID‐19, Pediatric Multi‐System Inflammatory Syndrome (MISC), SARS‐COV‐2, steroid, Vasoactive‐Inotropic Score (VIS)
1. INTRODUCTION AND BACKGROUND
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), now referred to as coronavirus disease 2019 (COVID‐19), has gained worldwide attention since its first detection in Wuhan, Hubei province, China, in December 2019. The World Health Organization (WHO) declared this condition as a global pandemic and has since emerged as a significant public health emergency with devastating effects on the global economy. 1
For the third time this century, the world faces a new, highly contagious virus strain spread across the globe, causing numerous deaths and a catastrophic economic impact. The first pandemic of 2002 was caused by severe acute respiratory syndrome coronavirus (SARS‐CoV). This outbreak originated in Guangdong, China. The mode of transmission was mostly by droplets. Health care professionals were at an increased risk of contracting this disease. 2 The second outbreak occurred in 2012 with the Middle Eastern respiratory syndrome virus (MERS‐CoV), which first appeared in the Middle East around the Arabian Peninsula and spread around the globe. This virus also placed health care professionals at a higher risk of contracting the disease. WHO reported that this MERS‐CoV virus is still in circulation, having a case fatality rate of 35% compared to 9.5% for SARS‐CoV‐2.
The world is currently experiencing the third wave of coronavirus infection, this time with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). This crisis started in December 2019, when Chinese health officials in Wuhan noticed a cluster of patients with severe respiratory distress secondary to pneumonia. The infections were reported to the Chinese Bureau of the World Health Organization (WHO). On January 7, 2020, a new strain of coronavirus was isolated from these clusters of patients and named novel coronavirus 2019 (2019‐nCoV). The virus was tagged with the official name COVID‐19 by the WHO on February 11, 2020. The case fatality rate of SARS‐CoV‐2 is reported to be ∼2%–3%. 3
Children account for 9%–12% of patients diagnosed with COVID‐19 infection. 4 In general, 90% of children positive for SARS‐CoV‐2 are “asymptomatic” or have mild‐to‐moderate symptoms. In a review of 2572 pediatric cases, only 15 children needed intensive care with 3 reported deaths. 5 Another study of children admitted to PICUs in the United States and Canada showed that 18 (38%) of the 48 children hospitalized required invasive ventilation with 16 survivals and 2 deaths. 6 Severe illness may occur in children younger than 1 year of age and those with other comorbidities or underlying conditions. 7 The first confirmed pediatric case of severe acute respiratory syndrome (SARS‐CoV‐2) infection was in Shenzhen in January 2020. 8 By the end of January 2020, China reported 16 cases originating from an infected family member.
The clinical presentation and epidemiological characteristics of SARS‐CoV‐2 are still relatively unclear, and new data are emerging every day. In April 2020, there was a high alert about a disease entity in children related to COVID‐19, based on a study in the United Kingdom (UK). The clinical features of this new entity included symptoms of toxic shock syndrome and incomplete Kawasaki disease. This emerging syndrome was called pediatric multi‐system inflammatory syndrome (PMIS). 9 On May 14, 2020, the Center for Disease Control and Prevention (CDC) published guidelines and an advisory on the same condition coining a new name, “multisystem inflammatory syndrome in children” (MIS‐C). The objective of this review is to offer clinicians a comprehensive overview of the currently available information about this condition. We hope to provide the readers, especially emergency physicians, with a comprehensive and concise review of the literature that can aid in early recognition and appropriate initial stabilization and management of children affected by COVID‐19.
2. METHODS
We conducted a search in PubMed, Medline, and Cochrane database for research articles published on COVID‐19 from December 2019 to December 2020 using the search term COVID‐19, Pediatrics, SARS‐CoV‐2, Epidemiology, Guidelines. We focused on original research, case reports, case series, and treatment guidelines by authoritative health institutions. All records were retrieved, including original articles, letters to editor, editorials, and case reports, in English and records with English translation were downloaded and reviewed. Preprints, in‐press articles, and accepted‐for‐publication studies were also evaluated, given the current scarcity of evidence.
2.1. Virology
Coronaviruses are zoonotic viruses that can cause disease in both mammals and birds. The structure of COVID‐19 is a single‐stranded positive‐sense RNA (+ssRNA) (∼30 kb) with a 5′‐cap structure and 3′poly‐A tail with a crown‐like shape because of the presence of glycoproteins on the surface. These belong to the subfamily of Coronaviridae and order Nidovirales. The 4 genera described are: alpha coronavirus (alphaCoV), α, beta coronavirus (betaCoV), β, gamma coronavirus (deltaCoV), γ, and delta coronavirus (gamma CoV), δ.
Alpha coronavirus includes species that can cause human illness (human coronavirus 229E and human coronavirus NL63). Beta coronavirus has 4 lineages, subgroups A, B, C, and D. The subgroup A includes beta coronavirus 1 (human coronavirus OC43 and human coronavirus HKU1). Subgroup B comprises severe acute respiratory syndrome‐related coronavirus (SARS‐CoV, SARS‐CoV‐2). Subgroup C includes the Middle East respiratory syndrome‐related coronavirus (MERS)‐CoV.
The most common human coronaviruses are HCoV‐OC43, HCoV‐HKU1 HCoV‐229E, and HCoV‐NL63. They are responsible for causing upper respiratory infections. The SARS‐CoV‐2 is shaped round or elliptic, often pleomorphic, with a diameter of ∼60–140 nm. SARS‐CoV‐2 receptor binding gene region is similar to that found in SAR CoV that uses the angiotensin‐converting enzyme 2 (ACE2) receptor to penetrate and enter the cell. 10
SARS‐CoV‐2 is sensitive to the action of ultraviolet rays and heat. It can also be rendered inactive by ethanol, lipid solvents, including ether (75%), peroxyacetic acid chlorine‐containing disinfectant, and chloroform (except for chlorhexidine).
2.2. Pathophysiology
SARS‐CoV‐2 infection consists of 2 phases: infection and host cell response.
2.2.1. Infection of host cell
The life cycle of the virus begins with the infection of the host cell, divided into 5 phases: attachment, penetration, biosynthesis, maturation, and release.
The SARS‐CoV‐2 has four structural proteins: spike (S), membrane (M), envelope (E), and nucleocapsid (N). This is depicted in Figures 1 and 2. 11 , 12
The virus binds to a receptor on the surface of the host cell (attachment) and enters the cell via endocytosis or fusion to the membrane (penetration). Once inside the cell, the virus enters the host cell nucleus via viral mRNA. The viral mRNA is used to make viral protein (biosynthesis). The newly produced viral particles are then released (maturation and release). 12 Transmembrane trimetric glycoprotein make up the spike projecting from the viral surface, thus determining the diversity of coronaviruses and host tropism. In the spike, there are 2 functional subunits: the S1 subunit controls the binding to the host cell receptor, and the S2 subunit is for the fusion with cellular membranes. 13
Angiotensin‐converting enzyme 2 (ACE2) is the functional receptor for SARS‐CoV. Based on structural and functional analysis, SARS‐CoV‐2 binds to ACE2 receptors.
Once the SARS‐CoV‐2 binds to the ACE2 receptors, the spike protein undergoes protease cleavage. The cleavage occurs in a 2‐step process. After the initial cleavage at the S1/S2 cleavage site, S1 and S2 subunits remain non‐covalently bound, and the distal S1 subunit plays a part in the stabilization of the membrane‐anchored S2 subunit at the prefusion state.
The expression of angiotensin‐converting enzyme 2 (ACE2) is very high in the following organs like lungs, heart, ileum, kidney, and bladder. 14
SARS‐CoV‐2 has a furin cleavage site (“RPPA” sequence) at the S1/S2 site. Even though the S1/S2 site is exposed to cleavage by other proteases such as transmembrane protease serine 2 (TMPRSS2) and cathepsin L, the ubiquitous expression of furin may explain the pathogenicity of SARS‐CoV‐2. 15
2.2.2. Host response
Viral particles typically penetrate the epithelial cells of the alveolar space via the apical side and destroy them. The macrophages, epithelial, alveolar, and dendritic cells mediate the innate immune response. T cell responses to SARS‐CoV‐2 begin by antigen presentation via dendritic cells and macrophages. The virus‐infected apoptotic epithelial cells can be phagocytized by dendritic cells and macrophages, followed by antigen presentation to the T cells. 16 Typically, patients with severe diseases demonstrate lymphopenia.
There is an increase in plasma concentrations of proinflammatory cytokines, including interleukin 6 and 10 (IL‐6, IL‐10), granulocyte‐colony stimulating factor (G‐CSF), monocyte chemoattractant protein 1 (MCP1), macrophage inflammatory protein (MIP)1α, and tumor necrosis factor α (TNFα) 33. The infected lung epithelial cells generate IL‐8 in addition to IL‐6. IL‐8 is a chemoattractant for neutrophils and T cells. The attracted neutrophils then induce the lung injury associated with the infection.
3. DIFFERENCES BETWEEN ADULT AND PEDIATRIC INFECTION
Children have milder symptoms of COVID‐19 infection and are often asymptomatic compared to adults. Several proposed hypotheses explain this difference. The first hypothesis suggests that the expression level of ACE2 may differ between adults and children. One study showed that ACE2 receptors abundantly expressed on well‐differentiated ciliated epithelial cells. 17 ACE2 expression on the epithelium of the alveolar cells may be lower in the pediatric population. The second hypothesis proposes that pediatric patients have a qualitatively different response to the SARS‐CoV‐2 virus relative to adults. By the processes of aging, continuous antigen stimulation and thymic involution will eventually lead to shifts in T cell subset distribution from naïve T cells to central memory T cells, effector T cells, and effector memory T cells. 18 The third hypothesis rests on the premise that the simultaneous presence of other viruses in the epithelial lining of the lungs and airways in young children causes the SARS‐CoV‐2 virus to compete with them limiting its growth and proliferation. 18
One or a combination of these mechanisms may explain why SARS‐CoV‐2 has less severity in the pediatric population. A better understanding of these mechanisms may aid in developing appropriate therapy.
4. EPIDEMIOLOGY
The case definition for pediatric COVID‐19 has been variable in the literature. The Chinese Centers for Disease Control and Prevention reported that only 2% of the 72,314 reported cases occurred in children that were less than 19 years of age. 19 More recent data in the United States shows that children account for 9%–12% of patients diagnosed with COVID‐19 infection (in general, 90% of children positive for SARS‐CoV‐2 are asymptomatic or have mild to moderate symptoms). 20 By mid‐November 2020, the number of pediatric COVID‐19 cases surpassed the 1 million mark. 21 It appears that COVID‐19 occurs more often among the 12‐ to 17‐year age group compared to the 5‐ to 11‐year age group in the United States. 22
In a multicenter study of 48 children admitted with COVID‐19 to PICUs in Canada and the United States, 40 patients (83%) had preexisting comorbidities and most (35; 73%) presented with respiratory symptoms. Eleven patients (38%) had a failure of 2 or more organs, and 1 patient (2%) required ECMO. 6 Garg et al described their experience using data derived from the COVID‐NET registry. Between March 1 and July 25, 2020, 576 pediatric COVID‐19 cases were reported. 23 The COVID‐19‐associated hospitalization rate among children (<18 years) was 8.0 per 100,000 populations; most were in the <2 years age group (24.8 per 100,000) and belonged to the Hispanic/Latino or black ethnic groups (16.4 and 10.5 per 100,000, respectively). The hospitalization rate for white children was 2.1/100,000. Among the hospitalized children, 33.2% (69/207) were admitted to an ICU, with 5.8% (12/207) requiring mechanical ventilation. There was 1 reported death in this study. 4
As of July 29, 2020, 570 cases were reported to the CDC that met the case definition of MIS‐C. Clinical course consistent with previous MIS‐C reports occurred in 203 patients, characterized predominantly by shock, cardiac dysfunction, abdominal pain, markedly elevated inflammatory markers, and positive SARS‐CoV‐2 test. The remaining 367 patients had manifestations that appeared to overlap with acute COVID‐19 infection or had features of Kawasaki disease. Most patients required (n = 364 patients, 63.9%) care in an ICU, and 10 patients (1.8%) died. 24 A review of a small case series of 9 mothers infected with SARS‐CoV‐2 showed no evidence of mother‐to‐infant vertical transmission of SARS‐CoV‐2 and no evidence of transmission through breast milk. Pregnant women and newborns infected with SARS‐CoV‐2 are at increased risk of developing severe pneumonia. 25 Of the countries with the highest disease burden, the percentage of pediatric cases is reported here: United States, 2% of confirmed cases of COVID‐19 were among persons <18 years of age 5 ; China, 2.2% of confirmed cases of COVID‐19 were among persons <19 years of age 26 ; Italy, 1.2% of COVID‐19 cases were among children <18 years of age; 27 and Spain, 0.8% of confirmed cases of COVID‐19 were among persons <18 years of age. 28
5. MODE OF TRANSMISSION
SARS‐CoV‐2 mode of transmission in children is similar to those of adults. Transmission between humans is mostly via respiratory droplets through aerosolized viral particles. Close contact or proximity can also be a source of transmission of SARS‐CoV‐2 via direct or indirect contact with mucous membranes in the eyes, mouth, or nose. Most children are thought to be exposed to the virus from an adult at home. 29 Currently, no evidence exists of vertical transmission from mother to child. 30 Although children can transmit the virus to others, the transmission rate is unclear with younger children less likely to transmit compared to children >10 years old. 31
6. CLINICAL MANIFESTATIONS
The incubation period is 2–14 days with a median of 6 days after exposure, similar to that in adults. 32 A large proportion of children with COVID‐19 are asymptomatic. When symptomatic, manifestations include fever, nasal congestion/rhinorrhea, dyspnea, loss of sense of smell and taste, cough, sore throat, diarrhea, nausea/vomiting, fatigue, headache, myalgia, and poor feeding/poor appetite. 33 Unlike adults, children with COVID‐19 may not always present with fever and cough. 34 In one pediatric case report, the only presenting symptom was diarrhea. 35 In another case series of 9 hospitalized infants in China with confirmed COVID‐19, only 50% of them presented with fever. 36
7. CLINICAL COURSE AND COMPLICATIONS
The clinical course of SARS‐CoV‐2 based on >2000 cases in children described in China is shown in Table 1.
TABLE 1.
Clinical course of COVID 19 in children
| Signs and symptoms | Percentage | |
|---|---|---|
| Asymptomatic | Absence of signs or symptoms; normal chest imaging | 4 |
| Mild | Mild symptoms; fever, cough, myalgia, fatigue | 51 |
| Moderate | Pneumonia with symptoms or subclinical disease with abnormal chest imaging | 39 |
| Severe | Dyspnea; hypoxia; central cyanosis | 5 |
| Critical | Acute respiratory distress syndrome; respiratory failure; shock, multi‐organ dysfunction | 0.6 |
Infants account for 1.2% of the US population, but make up ∼15% of pediatric COVID‐19 cases and 0.3% of all COVID‐19 patients. 37 In comparison to adults, fewer children with COVID‐19 require hospitalization (6%–20%), and few (0.6%–2%) require ICU admission. Hospitalization is common among children <1 year of age and those with underlying conditions such as cardiovascular disease, chronic lung disease, asthma, and immunosuppression. 5 Of the 149,760 laboratory‐confirmed COVID‐19 cases in the United States occurring between February 12–April 2, 2020, 3 deaths occurred among children with laboratory‐confirmed SARS‐CoV‐2 infection. However, the role of SARS‐CoV‐2 infection in the cause of these deaths remains unclear. 5 Children have fewer complications (shock and acute respiratory distress syndrome) compared to adults. Current data on suspected and confirmed SARS‐CoV‐2 infection suggests that infants <12 months of age may be at greater risk of severe or critical disease relative to older children. 38
8. DIAGNOSIS
The criteria for testing are still evolving. Initially testing was done for high‐risk patients (chronic heart or lung disease, immunocompromised, severe obesity) with a clinical picture suggestive of COVID‐19, for those presenting with severe illness and ones who had close contact to a COVID‐positive patient. Close contact is defined as a distance of <6 feet for at least 15 minutes from a person with confirmed or probable SARS‐CoV‐2 infection. With increasing availability of testing, the threshold to testing has been significantly lowered. Additionally, most children undergoing procedures or needing hospitalization for any reason are recommended to get tested for COVID‐19. Diagnosis of COVID‐19 can be established by using RT‐PCR of a nasal swab, throat swab, saliva, tracheal aspirate, or bronchoalveolar lavage, in adults. COVID 19 is diagnosed the same way in pediatric population as adults by testing specimen obtained from upper respiratory tract, for nucleic acid amplification test (NAAT) using RT‐ PCR. 39 The routine use of bronchoscopy is discouraged because the aerosolized particles generated during the procedure can affect health care workers. If the diagnosis is uncertain and safety precautions have been observed, then this can be used as an option. For intubated pediatric patients, tracheal aspirate and non‐bronchoscopic bronchoalveolar lavage facilitates the specimen collection. 40 One negative test does not exclude SARS‐CoV‐2 infection, especially if the individual is highly exposed or if the test is performed using a nasopharyngeal swab specimen just at the beginning of the infection. In such instances, it is advisable to repeat the test or collect a deeper respiratory tract sample. 41 The RT‐PCR presently is the reference standard. There is no published literature outlining the sensitivity or specificity among children compared to adults. It is important to remember that a positive test may remain positive for several weeks. 42 According to the CDC, the testing protocol for specimen and clinical criteria are similar for children and adult patients. However, there are specific considerations for neonates. All neonates born of mothers who have tested positive for COVID‐19 should undergo testing for SARS‐CoV02 by PCR within 24 hours of age regardless of symptomatology. A repeat test is suggested within 48 hours if the first test is negative. In the case that results are unavailable or the neonate is to be discharged within 48 hours, a single test suffices to direct further management. It is not advisable to test neonates too soon after birth because of the high probability of false negatives in neonates. The CDC does not recommend the use of serum antigen tests for the diagnosis or management of acute infection in neonates. 43
9. LABORATORY ABNORMALITIES
In many asymptomatic or mildly symptomatic children, the labs may be normal. The most common laboratory abnormalities among hospitalized patients include leukopenia or leukocytosis, lymphopenia, elevated levels of alanine aminotransferase, aspartate aminotransferase, and lactate dehydrogenase. Inflammatory markers like CRP, ESR, procalcitonin, D‐dimer, and ferritin levels may also be increased, particularly in MIS‐C. 44 , 45 Elevated troponin in COVID 19 patients is associated with myocarditis and may be associated with poor prognosis. 46
10. RADIOLOGY
Chest x‐ray to detect lung involvement has low sensitivity, and CT chest should be considered when there is a respiratory compromise. Radiological findings in pediatric patients with COVID‐19 are similar to those of adults, comprising of unilateral or bilateral infiltrates and some show ground‐glass opacities, especially in the lower lobes. 47 Sonogram and CT scan of the chest may show nonspecific findings or ground glass appearance similar to adults. 48
Some patients have bilateral multi‐lobular and subsegmental areas of atelectasis, and the number of involved lung segments correlates with disease severity. The opacifications tend to coalesce as the disease progresses. CT scan alone should not be used for the diagnosis of COVID‐19 as it may not be a valuable screening tool for COVID‐19. 49 According to Buda et al, the use of ultrasound is somewhat attractive because it is non‐invasive, portable, and may be performed several times during the course of illness. The transthoracic lung ultrasound is generally used to detect pulmonary edema in patients with acute respiratory distress syndrome (ARDS) with a sensitivity and specificity of 0.78 to 0.9. 50 , 51 However, in the COVID‐19 pandemic, the use of ultrasound for the detection of viral pneumonia has gained significant popularity and may detect changes earlier than a routine chest X‐ray. 52
11. MEDICAL MANAGEMENT OF COVID‐19 INFECTION
Initial assessment should make the determination if the patient has a mild (not ill, minimal symptoms, no hypoxia), moderate (fever, significant myalgia, mild hypoxia >93% without shortness of breath) or severe illness (dyspnea, ill appearing, hypoxia <92%). Patients with mild symptoms can be managed at home with supportive care, such as antipyretics for fever maintenance of adequate hydration, appropriate isolation and education of caregivers regarding emergency warning signs. 53 Patients with moderate to severe symptoms should be hospitalized for specialized care. Most affected children are managed similar to adults.
Therapies are still evolving, and there is no silver bullet at the present time. The best strategy at the current time is prevention through social distancing (6 feet apart), wearing of masks, and strict hand hygiene. 20 The American Academy of Pediatrics recommends that children older than 2 years should wear cloth face coverings, especially when social distancing is not possible. 54 In the hospital setting, strict infection control should be practiced including frequent hand washing, appropriate donning and doffing of personal protective equipment (PPE) and special precautions during high risk (eg, aerosolizing) procedures. Eye protection with goggles or face shields is recommended in the health care settings. 55 Early testing and isolation of those with positive tests combined with contact tracing can further help reduce the transmission of this virus.
12. PHARMACOLOGICAL THERAPY
There is currently no evidence to support the use of prophylactic antibiotics to prevent COVID‐19 infection, although this may be considered for the initial management of patients presenting with shock for presumed bacterial sepsis.
The RECOVERY trial in United Kingdom showed reduced mortality at 28 days for COVID‐19‐affected hospitalized adult patients who were on supplemental oxygen or on mechanical ventilation. In patients hospitalized with COVID‐19, the administration of dexamethasone resulted in lower 28‐day mortality among those who were receiving either invasive mechanical ventilation or oxygen alone at randomization but not among those receiving no respiratory support. 56 At present, no conclusive evidence exists about the use of steroids in children, although there may be benefit in patients that require mechanical ventilation. 57 Previous experience with the use of steroids to treat SARS‐CoV and MERS‐CoV showed increased mortality, higher secondary infections, and occurrence of complications such as psychosis, hyperglycemia, delayed viral clearance, and enhanced risk of mutation of the pathogen. 58 If steroids are used, it is prudent to use at minimal effective doses for the shortest time. 58 The use of anticoagulation therapy is recommended in patients with early‐stage COVID‐19, especially when the D‐dimer value is 4 times higher. The presence of inflammation and other disease‐related factors can cause overactivation of coagulation, thereby increasing the risk of ischemic events and disseminated intravascular coagulation (DIC). 59
13. ANTIVIRAL DRUGS
The use of antiviral drugs in the treatment of COVID‐19 follows the experience with SARS‐CoV and MERS‐CoV. There is limited data on the use of antiviral drugs in children. Remdesivir has recently received emergency FDA authorization for use in hospitalized adults and pediatric patients <12 years. It also received emergency use authorization for hospitalized pediatric patients weighing 3.5 to <40 kg or <12 years of age and weighing ≥3.5 kg for compassionate use. 60 The COVID‐19 Treatment Guidelines Panel recommends the use of remdesivir to treat hospitalized patients with SpO2 ≤94% on ambient air or those who require supplemental oxygen. The panel recommends a 5‐day course for non‐intubated patients. Data are insufficient, but for patients on mechanical ventilation or ECMO, experts recommend a 10‐day course. 61 Another investigational drug, lopinavir/ritonavir, an antiretroviral drug combination that inhibits protease, has shown some efficacy in its ability to reduce the viral load in COVID‐19 patients. 62 The NIH treatment guideline panel has recommended against the use of this drug. Other drugs not recommended by the panel include chloroquine and hydrochloroquine with or without azithromycin, ivermectin, and other HIV protease inhibitors.
14. MONOCLONAL ANTIBODIES
On November 21, 2020, the FDA issued emergency use authorization for monoclonal antibodies, casirivimab and imdevimab, to be administered together, for the treatment of mild to moderate COVID‐19 in adults and children <12 years and weighing at least 40 kg, who are at high risk for progressing to severe COVID‐19 disease. 63 , 64
14.1. Passive immunotherapy
The use of passive immunotherapy is another option.
Convalescent plasma has shown potential benefits in adults with SARS‐CoV‐2 but has theoretical risks. The mechanism of action of convalescent plasma is via neutralizing antibodies binding to the virus, rendering it inert. 65 The initial series of convalescent plasma administration in adults with COVID‐19 showed potential benefits without apparent side effects. 66
In a recent case report, 4 pediatric patients with SARS‐CoV‐2 and acute respiratory distress syndrome were given convalescent plasma. The administration of convalescent plasma was not associated with antibody‐dependent enhancement (ADE) and did not dampen the endogenous antibody response. 67
15. RESPIRATORY SUPPORT
Oxygen therapy is beneficial for patients with hypoxia to keep oxygen saturation above 94%. It is recommended to avoid the use of a nasal mask or face mask as the risk of dissemination of aerosolized particles is very high. Oxygen therapy should be administered using a close circuit system. High‐flow by nasal cannula, a face mask, or non‐invasive ventilation (preferably using a continuous positive airway pressure helmet or full‐face interface) or bilevel positive airway pressure machine is recommended. Endotracheal intubation should not be delayed if patients develop acute lung injury or ARDS. The recommendation is to use low tidal volume to prevent volutrauma and barotrauma and high PEEP for alveolar recruitment. Intubated patients should be sedated and paralyzed. Prone positioning is an option for patients with minimal improvement in oxygenation. 68 Extracorporeal membrane oxygenation could be an option if mechanical ventilation fails to resolve hypoxemia. 69
The WHO published guidelines on the management of pediatric and adult patients with COVID‐19. 70 The Surviving Sepsis Campaign has also published guidelines on the management of septic shock and sepsis‐associated organ dysfunction in children with COVID‐19 infection. The publication is titled “Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis‐Associated Organ Dysfunction in Children.” 71 Vaccine against COVID‐19 has been developed by several pharmaceutical companies and implementation of vaccination in adults will start shortly. 72
16. MIS‐C ASSOCIATED WITH COVID‐19
The CDC case definition for MIS‐C includes the following: an individual under 21 years presenting with fever, laboratory evidence of inflammation and evidence of clinically severe illness requiring hospitalization with multisystem (>2) organ involvement (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic, or neurologic) and no plausible alternative diagnoses; and positive testing for current or recent SARS‐CoV‐2 infection by a RT‐PCR, serology, or antigen test, or COVID‐19 exposure within the 4 weeks before the onset of symptoms.
The fever should be at least 38°C, or it can be subjective, lasting for at least 24 hours. The evidence of inflammation includes elevated C‐reactive protein (CRP), erythrocyte sedimentation rate (ESR), fibrinogen, procalcitonin, d‐dimer, ferritin, lactic acid dehydrogenase (LDH), or interleukin 6 (IL‐6), elevated neutrophils, reduced lymphocytes, and low albumin. 73
17. DIAGNOSIS OF MIS‐C
To establish the diagnosis of MIS‐C, the following tests are recommended: SARS‐CoV‐2 RT‐PCR nasopharyngeal swab, COVID ELISA serology testing, blood culture if sepsis suspected, and respiratory pathogen PCR panel if available.
Other tests in the workup include a CBC with differential, comprehensive metabolic panel, liver function test, blood gas analysis with lactate, C‐reactive protein, erythrocyte sedimentation rate, serum ferritin, procalcitonin, LDH levels, PT/PTT, fibrinogen, D‐dimers, proBNP, troponin, CPK, and triglycerides. Other tests to consider include IL‐6, ANA, C3, C4, von Willebrand antigen, cytokine panel, and CXCL9. Urinalysis is also included with microscopy, urine creatinine, and protein. Rheumatology workup is indicated If ANA is elevated >1:640. Other investigations include anti‐dsDNA, anti‐extractable nuclear antigen (smith, rnp, ssa, ssb), ANCA, lupus anticoagulant, anti‐β2‐glycoprotein, anti‐cardiolipin, ASO, and anti‐DNAse. If there is unprovoked thrombosis, lupus anticoagulant, anti‐β2‐glycoprotein, and anti‐cardiolipin level should be obtained for evaluation. A chest X‐ray with EKG and transthoracic echocardiogram focused on cardiac function and coronary arteries should also be performed.
18. MIS‐C CLASSIFICATION OF DISEASE SEVERITY
Pediatric patients are broadly classified into 3 groups based on disease severity. Mild disease presents with no requirement for vasoactive support, minimal to no respiratory support, and minimal organ injury. The moderate category counts a vasoactive‐inotropic score (VIS) ≤10, significant supplemental oxygen requirement, mild or isolated organ injury. Severely ill patients have a VIS >10, non‐invasive or invasive ventilatory support, moderate or severe organ injury, including moderate to severe ventricular dysfunction. The VIS was derived from the previously described inotropic score which quantifies the amount of cardiovascular support required by pediatric patients postoperatively and includes dopamine, dobutamine, epinephrine, milrinone, vasopressin, and norepinephrine. The VIS is calculated using the formula: dopamine dose (μg/kg/min) + dobutamine (μg/kg/min) + 100 × epinephrine dose (μg/kg/min + 100 × norepinephrine dose + 10 × milrinone (μg/kg/min). 74
19. MEDICAL MANAGEMENT OF MIS‐C
For MIS‐C, there is currently no standardized treatment regimen and supportive treatment is the main strategy. Several treatment strategies have been used by various centers that include intravenous immunoglobulins, various immune modulators, steroids, aspirin, and anticoagulant therapies, but clinical evidence of benefit is yet to be established. The role for antiviral therapy also remains unclear. The American Academy of Pediatrics recommends a multidisciplinary approach involving available pediatric subsists such as intensivists, hospitalists, cardiologists, rheumatologists, and hematologists and immunologists. 75 Patients with MIS‐C and abnormal BNP or troponin levels should have a thorough evaluation of cardiac function with frequent EKGs, echocardiograms, and in some cases, cardiac MRI to closely monitor cardiac function until normalization of function. For patients with cardiac involvement, immunomodulatory therapy should be initiated with intravenous gamma globulin (2 g/kg) and steroids. 76 For all patients with MIS‐C, broad‐spectrum antibiotics, anticoagulation therapy, and low dose aspirin should be initiated. Glucocorticoids at the dose of 1–2 mg/kg are initiated and for non‐responders, high dose pulse steroid at 10–30 mg/kg should be considered, especially for those requiring inotropic support. For patients refractory to steroid and IVIG therapy, anakinra (IL‐1 receptor antagonist) at 2–10 mg/kg should be considered.
Presently, tocilizumab is not recommended for most pediatric patients with no response to the steroid. It is recommended to consider low dose aspirin (3–5 mg/kg) until the normalization of platelet count. For patients with coagulation abnormalities with elevated d‐dimer levels, anticoagulation should be considered with the use of enoxaparin.
20. OTHER PEDIATRIC CONSIDERATIONS
20.1. Return to sports
Asymptomatic and mild COVID‐19‐positive children should only be cleared after passing a screening test by their primary care provider and allowed to return 10 days after the positive test. Patients with moderate to severe disease should not exercise until cleared by a cardiologist. The American Academy of Pediatrics recommends wearing of cloth face coverings at all times during practice and sporting activities except for swimming, wrestling, cheerleading, or gymnastics. 77
20.2. Return to school
The American Academy of Pediatrics strongly advocates that the goal should be to have students physically present in school and administrators take all measures to mitigate the risk of COVID‐19 spread through physical distancing, wearing masks, and thorough disinfection. 78
21. CONCLUSIONS
SAR‐CoV2 or COVID‐19 is an infectious disease that has quickly emerged as a global pandemic making an unprecedented and devastating social and economic impact. Affected children do not appear to have the same degree of severity of illness as seen in adults. MIS‐C is a new entity that is uniquely seen in pediatric patients infected with COVID‐19 and usually requires hospitalization to an intensive care unit and managed by a multidisciplinary team of specialists. There is a need for more research to understand the disease in children and develop optimal therapies and effective vaccines. Vaccines against COVID‐19 have been developed by several pharmaceutical companies and implementation of vaccination in adults will start shortly.
As of December 11, 2020, the U.S. Food and Drug Administration has issued the first emergency use authorization for a vaccine for the prevention of COVID‐19 caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in individuals 16 years of age and older.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The authors wish to acknowledge the contribution of Clarisse Pierre and Dorisse Pierre for the diagram in Figure 1 (COVID‐19 viral structure) and Figure 2 (COVID‐19 transcription and replication).
FIGURE 1.
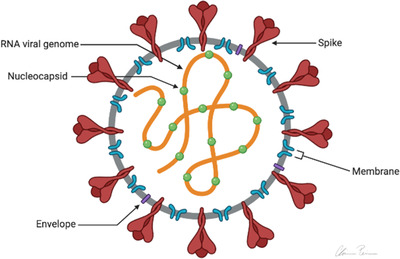
COVID‐19 viral structure. Diagram of the structure of the COVID‐19 virus. 11 Adapted from structure and genome of SARS‐CoV‐2 (COVID‐19) with diagram
FIGURE 2.
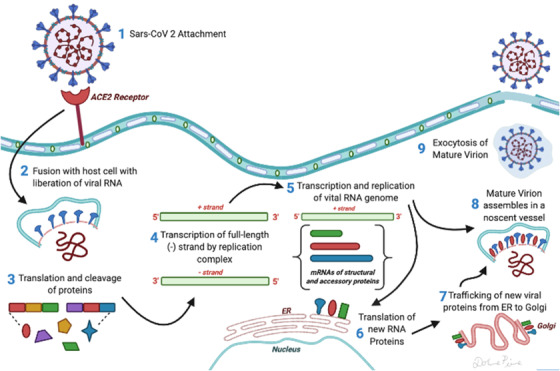
Transcription and replication of COVID‐19 virus in a host's cell. 12 Adapted from spotlight on COVID‐19: infection
Adeyinka A, Bailey K, Pierre L, Kondamudi N. COVID 19 infection: Pediatric perspectives. JACEP Open. 2021;2:e12375 10.1002/emp2.12375
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
Supervising Editor: Sing‐Yi Feng, MD.
REFERENCES
- 1. Guo Y‐R, Cao Q‐D, Hong Z‐S, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak ‐ an update on the status. Mil Med Res. 2020; 7(1): 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guarner J. Three emerging coronaviruses in two decades. Am J Clin Pathol. 2020; 153(4): 420‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hui DS, Azhar EI, Kim Y‐J, Memish ZA, Oh M‐D, Zumla A. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018; 18(8): e217‐e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. CDC . COVID‐19 Cases, Deaths, and Trends in the US | CDC COVID Data Tracker. Centers for Disease Control and Prevention. Published March 28, 2020. Accessed December 11, 2020. https://covid.cdc.gov/covid-data-tracker
- 5. CDCMMWR . Coronavirus Disease 2019 in Children — United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69 10.15585/mmwr.mm6914e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID‐19) infection admitted to US and canadian pediatric intensive care units. JAMA Pediatr. 10.1001/jamapediatrics.2020.1948. Published online May 11, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang S, Tuo J, Huang X, et al. Epidemiology characteristics of human coronaviruses in patients with respiratory infection symptoms and phylogenetic analysis of HCoV‐OC43 during 2010–2015 in Guangzhou. PLOS ONE. 2018; 13(1): e0191789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. TEZER H, BEDİR DEMİRDAĞ T. Novel coronavirus disease (COVID‐19) in children. Turk J Med Sci. 2020; 50(3): 592‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levin M. Childhood multisystem inflammatory syndrome — a new challenge in the pandemic. N Engl J Med. 10.1056/NEJMe2023158. Published online June 29, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID‐19. J Pharm Anal. 2020; 10(2): 102‐108.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sapkota A. Structure and Genome of SARS‐CoV‐2 (COVID‐19) with diagram. Microbe Notes. Published April 5, 2020. Accessed July 18, 2020. https://microbenotes.com/structure-and-genome-of-sars-cov-2/
- 12. Spotlight on COVID‐19: Infection. InvivoGen. Published March 27, 2020. Accessed July 18, 2020. https://www.invivogen.com/spotlight-covid-19-infection
- 13. Yuki K, Fujiogi M, Koutsogiannaki S. COVID‐19 pathophysiology: a review. Clin Immunol Orlando Fla. 2020; 215: 108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bosch BJ, van der Zee R, de Haan CAM, Rottier PJM, The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol. 2003;77(16):8801‐8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS‐CoV‐2 on virus entry and its immune cross‐reactivity with SARS‐CoV. Nat Commun. 2020;11(1):1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fujimoto I, Pan J, Takizawa T, Nakanishi Y. Virus clearance through apoptosis‐dependent phagocytosis of influenza A virus‐infected cells by macrophages. J Virol. 2000;74(7):3399‐3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. ‐ PubMed ‐ NCBI. Accessed May 17, 2020. https://www.ncbi.nlm.nih.gov/pubmed/16282461 [DOI] [PMC free article] [PubMed]
- 18. Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint J‐P, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev. 2006;127(3):274‐281. [DOI] [PubMed] [Google Scholar]
- 19. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in china: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 20. CDC. Coronavirus Disease 2019 (COVID‐19). Centers for Disease Control and Prevention. Published February 11, 2020. Accessed December 11, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/pediatric-hcp.html
- 21. Jenco M. Pediatric COVID‐19 cases surpass ‘tragic’ 1 million mark. AAP News. Published online December 9, 2020. Accessed December 11, 2020. https://www.aappublications.org/news/2020/11/16/covid19children111620 [Google Scholar]
- 22. Leeb RT. COVID‐19 trends among school‐aged children — United States, March 1–September 19, 2020. MMWR Morb Mortal Wkly Rep. 2020;69 10.15585/mmwr.mm6939e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garg S. Hospitalization rates and characteristics of patients hospitalized with laboratory‐confirmed coronavirus disease 2019 — COVID‐NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69 10.15585/mmwr.mm6915e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Godfred‐Cato S. COVID‐19–Associated multisystem inflammatory syndrome in children — United States, March–July 2020. MMWR Morb Mortal Wkly Rep. 2020;69 10.15585/mmwr.mm6932e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. The Lancet. 2020;395(10226):809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. WHO Coronavirus Disease (COVID‐19) Dashboard. Accessed December 12, 2020. https://covid19.who.int
- 27. Livingston E, Bucher K. Coronavirus disease 2019 (COVID‐19) in Italy. JAMA. 2020;323(14):1335‐1335. [DOI] [PubMed] [Google Scholar]
- 28. Tagarro A, Epalza C, Santos M, et al. Screening and severity of coronavirus disease 2019 (COVID‐19) in children in Madrid, Spain. JAMA Pediatr. Published online April 8, 2020. 10.1001/jamapediatrics.2020.1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Posfay‐Barbe KM, Wagner N, Gauthey M, et al. COVID‐19 in children and the dynamics of infection in families. Pediatrics. 2020;146(2). 10.1542/peds.2020-1576 [DOI] [PubMed] [Google Scholar]
- 30. Lu C‐W, Liu X‐F, Jia Z‐F. 2019‐nCoV transmission through the ocular surface must not be ignored. Lancet Lond Engl. 2020;395(10224):e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goldstein E, Lipsitch M, Cevik M. On the effect of age on the transmission of SARS‐CoV‐2 in households, schools, and the community. J Infect Dis. 2020;(jiaa691). 10.1093/infdis/jiaa691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. The Incubation period of coronavirus disease 2019 (COVID‐19) from publicly reported confirmed cases: estimation and application | annals of internal medicine. Accessed May 17, 2020. https://www.acpjournals.org/doi/10.7326/M20-0504 [DOI] [PMC free article] [PubMed]
- 33. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382(17):1663‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID‐19 among children in China. Pediatrics. Published online March 16, 2020:e20200702. 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 35. Ji L‐N, Chao S, Wang Y‐J, et al. Clinical features of pediatric patients with COVID‐19: a report of two family cluster cases. World J Pediatr WJP. Published online March 16, 2020. 10.1007/s12519-020-00356-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Novel Coronavirus Infection in Hospitalized Infants Under 1 Year of Age in China | Global Health | JAMA | JAMA Network. Accessed May 18, 2020. https://jamanetwork.com/journals/jama/fullarticle/2761659 [DOI] [PMC free article] [PubMed]
- 37. Pascarella G, Strumia A, Piliego C, et al. COVID‐19 diagnosis and management: a comprehensive review. J Intern Med. n/a(n/a). 10.1111/joim.13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. ‐ PubMed ‐ NCBI. Accessed May 17, 2020. https://www.ncbi.nlm.nih.gov/pubmed/32193831 [DOI] [PMC free article] [PubMed]
- 39. Patel A. Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak — United States, December 31, 2019–February 4, 2020. MMWR Morb Mortal Wkly Rep. 2020;69 10.15585/mmwr.mm6905e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA. Published online March 11, 2020. 10.1001/jama.2020.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Laboratory testing for 2019 novel coronavirus (2019‐nCoV) in suspected human cases. Accessed June 24, 2020. https://www.who.int/publications-detail-redirect/10665-331501
- 42. Liguoro I, Pilotto C, Bonanni M, et al. SARS‐COV‐2 infection in children and newborns: a systematic review. Eur J Pediatr. Published online May 18, 2020:1‐18. 10.1007/s00431-020-03684-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. CDC. Coronavirus Disease 2019 (COVID‐19) . Centers for Disease Control and Prevention. Published February 11, 2020. Accessed May 17, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/pediatric-hcp.html
- 44. Zimmermann P, Curtis N. COVID‐19 in children, pregnancy and neonates: a review of epidemiologic and clinical features. Pediatr Infect Dis J. 2020;39(6):469‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Laboratory testing for 2019 novel coronavirus (2019‐nCoV) in suspected human cases. Accessed May 18, 2020. https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117
- 46. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID‐19 infection: different points from adults. Pediatr Pulmonol. 2020;55(5):1169‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shelmerdine SC, Lovrenski J, Caro‐Domínguez P, Toso S. Coronavirus disease 2019 (COVID‐19) in children: a systematic review of imaging findings. Pediatr Radiol. Published online June 18, 2020:1‐14. 10.1007/s00247-020-04726-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Merkus PJFM, Klein WM. The value of chest CT as a COVID‐19 screening tool in children. Eur Respir J. 2020;55(6). 10.1183/13993003.01241-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Buda N, Segura‐Grau E, Cylwik J, Wełnicki M. Lung ultrasound in the diagnosis of COVID‐19 infection—a case series and review of the literature. Adv Med Sci. 2020;65(2):378‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Soldati G, Demi M, Demi L. Ultrasound patterns of pulmonary edema. Ann Transl Med. 2019;7(Suppl 1):S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kharasch S, Duggan NM, Cohen AR, Shokoohi H. Lung ultrasound in children with respiratory tract infections: viral, bacterial or COVID‐19? A Narrative Review. Open Access Emerg Med. 2020;12:275–285. 10.2147/OAEM.S238702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. CDC. Coronavirus Disease 2019 (COVID‐19). Centers for Disease Control and Prevention. Published February 11, 2020. Accessed December 11, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-home-care.html
- 54. News Articles | American Academy of Pediatrics. Accessed December 12, 2020. https://www.aappublications.org/news/2020/08/13/covid19facecoverings081320%C2%A0…
- 55. Marra AR, Edmond MB, Popescu SV, Perencevich EN. Examining the need for eye protection for coronavirus disease 2019 (COVID‐19) prevention in the community. Infect Control Hosp Epidemiol.:1‐2. 10.1017/ice.2020.314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. RECOVERY Collaborative Group , Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid‐19 ‐ preliminary report. N Engl J Med. Published online July 17, 2020. 10.1056/NEJMoa2021436 [DOI] [Google Scholar]
- 57. Corticosteroids . COVID‐19 Treatment Guidelines. Accessed October 19, 2020. https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/
- 58. Zhou W, Liu Y, Tian D, et al. Potential benefits of precise corticosteroids therapy for severe 2019‐nCoV pneumonia. Signal Transduct Target Ther. 2020;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hypothesis for potential pathogenesis of SARS‐CoV‐2 infection‐a review of immune changes in patients with viral pneumonia. ‐ PubMed ‐ NCBI. Accessed May 18, 2020. https://www.ncbi.nlm.nih.gov/pubmed/32196410 [DOI] [PMC free article] [PubMed]
- 60. Remdesivir. COVID‐19 Treatment Guidelines. Accessed December 11, 2020. https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/remdesivir/
- 61. Al‐Tawfiq JA, Al‐Homoud AH, Memish ZA. Remdesivir as a possible therapeutic option for the COVID‐19. Travel Med Infect Dis. 2020;34:101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lim J, Jeon S, Shin HY, et al. Case of the index patient who caused tertiary transmission of COVID‐19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID‐19 infected pneumonia monitored by quantitative RT‐PCR. J Korean Med Sci. 2020;35(6):e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Casirivimab. In: Drugs and Lactation Database (LactMed). National Library of Medicine (US); 2006. Accessed December 14, 2020. http://www.ncbi.nlm.nih.gov/books/NBK564279/
- 64. Statement on Casirivimab Plus Imdevimab EUA. COVID‐19 Treatment Guidelines. Accessed December 14, 2020. https://www.covid19treatmentguidelines.nih.gov/statement-on-casirivimab-plus-imdevimab-eua/
- 65. Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID‐19. J Clin Invest. 2020;130(6):2757‐2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shen C, Wang Z, Zhao F, et al. Treatment of 5 Critically Ill Patients With COVID‐19 With Convalescent Plasma. JAMA. 2020;323(16):1582‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Diorio C, Anderson EM, McNerney KO, et al. Convalescent plasma for pediatric patients with SARS‐CoV‐2‐associated acute respiratory distress syndrome. Pediatr Blood Cancer. 2020;67(11):e28693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome. a systematic review and meta‐analysis. Ann Am Thorac Soc. 2017;14(Supplement_4):S280–S288. [DOI] [PubMed] [Google Scholar]
- 69. Preparing for the Most Critically Ill Patients With COVID‐19: The Potential Role of Extracorporeal Membrane Oxygenation | Critical Care Medicine | JAMA | JAMA Network. Accessed June 24, 2020. https://jamanetwork.com/journals/jama/fullarticle/2761778 [DOI] [PubMed]
- 70. Clinical management of COVID‐19 . Accessed June 24, 2020. https://www.who.int/publications-detail-redirect/clinical-management-of-covid-19
- 71. Surviving Sepsis Campaign International Guidelines for the M… : Pediatric Critical Care Medicine. Accessed June 24, 2020. https://journals.lww.com/pccmjournal/pages/articleviewer.aspx?year=2020&issue=02000&article=00020&type=Fulltext
- 72. Peiris M, Leung GM. What can we expect from first‐generation COVID‐19 vaccines? Lancet Lond Engl. 2020;396(10261):1467‐1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. CDC . Multisystem Inflammatory Syndrome in Children (MIS‐C). Centers for Disease Control and Prevention. Published February 11, 2020. Accessed December 11, 2020. https://www.cdc.gov/mis-c/hcp/
- 74. McIntosh AM, Tong S, Deakyne SJ, Davidson JA, Scott HF. Validation of the vasoactive‐inotropic score in pediatric sepsis. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18(8):750‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Multisystem Inflammatory Syndrome in Children (MIS‐C) Interim Guidance. Accessed December 11, 2020. http://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/multisystem-inflammatory-syndrome-in-children-mis-c-interim-guidance/
- 76. Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, et al. American college of rheumatology. clinical guidance for pediatric patients with multisystem inflammatory syndrome in children (MIS‐C) associated with SARS‐CoV‐2 and hyperinflammation in COVID‐19. Arthritis Rheumatol 2020;72:1791‐1805.‐ Google Search. Accessed December 12, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. COVID‐19 Interim Guidance: Return to Sports. Accessed December 11, 2020. http://services.aap.org/en/pages/2019‐novel‐coronavirus‐covid‐19‐infections/clinical‐guidance/covid‐19‐interim‐guidance‐return‐to‐sports/
- 78. COVID‐19 Planning Considerations: Guidance for School Re‐entry. Accessed December 11, 2020. http://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/covid-19-planning-considerations-return-to-in-person-education-in-schools/


