Abstract
Indicator traits associated with disease resiliency would be useful to improve the health and welfare of feedlot cattle. A post hoc analysis of data collected previously (Kayser et al., 2019a) was conducted to investigate differences in immunologic, physiologic, and behavioral responses of steers (N = 36, initial BW = 386 ± 24 kg) that had differential haptoglobin (HPT) responses to an experimentally induced challenge with Mannheimia haemolytica (MH). Rumen temperature, DMI, and feeding behavior data were collected continuously, and serial blood samples were collected following the MH challenge. Retrospectively, it was determined that 9 of the 18 MH-challenged steers mounted a minimal HPT response, despite having similar leukocyte and temperature responses to other MH-challenged steers with a greater HPT response. Our objective was to examine differences in behavioral and physiological responses between MH-challenged HPT responsive (RES; n = 9), MH-challenged HPT nonresponsive (NON; n = 9), and phosphate-buffered saline-inoculated controls (CON; n = 18). Additionally, 1H NMR analysis was conducted to determine whether the HPT-responsive phenotype affected serum metabolite profiles. The RES steers had lesser (P < 0.05) cortisol concentrations than NON and CON steers. The magnitude of the increases in neutrophil concentrations and rumen temperature, and the reduction in DMI following the MH challenge were greatest (P < 0.05) in RES steers. Univariate analysis of serum metabolites indicated differences between RES, NON, and CON steers following the MH challenge; however, multivariate analysis revealed no difference between HPT-responsive phenotypes. Prior to the MH challenge, RES steers had longer (P < 0.05) head down and bunk visit durations, slower eating rates (P < 0.01) and greater (P < 0.05) daily variances in bunk visit frequency and head down duration compared with NON steers, suggesting that feeding behavior patterns were associated with the HPT-responsive phenotype. During the 28-d postchallenge period, RES steers had decreased (P < 0.05) final BW, tended (P = 0.06) to have lesser DMI, and had greater (P < 0.05) daily variances in head down and bunk visit durations compared with NON steers, which may have been attributed to their greater acute-phase protein response to the MH challenge. These results indicate that the HPT-responsive phenotype affected feeding behavior patterns and may be associated with disease resiliency in beef cattle.
Keywords: bovine respiratory disease, disease resilience, feeding behavior, Mannheimia haemolytica, metabolite profiling, nuclear magnetic resonance
Introduction
As bovine respiratory disease (BRD) remains the greatest threat to the health of beef cattle, research investigating mitigation strategies is of paramount importance. With ever-increasing public scrutiny on the use of antimicrobial technology in agriculture, identification of animals that are naturally more disease resilient would be valuable (König and May, 2019). Disease resilience is the ability to maintain performance even with infection pressure present, and to return rapidly to pre-infection state following immune activation (Colditz and Hine, 2016; Putz et al., 2018). Resilient animals could be managed differently, requiring fewer health interventions. Certain physiological responses following immune challenges, such as haptoglobin (HPT) concentration, may represent degree of immunocompetence and disease resilience in cattle. These divergent responses may be related to other biomarkers (such as feeding behavior), which could be used to identify disease-resilient animals. A post hoc analysis of data previously collected (Kayser et al., 2019a) revealed that HPT response was substantially different in steers challenged with Mannheimia haemolytica (MH), despite similar temperature and leukocyte responses. Therefore, the objectives of this study were to determine whether differential HPT response to a MH challenge was an indicator for other immunologic, physiologic, and behavior response differences, and to determine whether HPT-responsive phenotype altered serum metabolite profiles. We hypothesized that HPT response phenotype would be related to disease resiliency as measured by these physiologic responses.
Materials and Methods
All animal care and use procedures were in accordance with the guidelines for use of Animals in Agricultural Teaching and Research as approved by the Texas A&M University Institutional Animal Care and Use Committee and Institutional Biosafety Committee (IACUC # 2015-0379; IBC # 2015-068).
Experimental animals and design
This study utilized 36 Angus cross steers (initial BW = 386 ± 24 kg) ~11 mo of age, originating from the Texas A&M University McGregor Research Center (McGregor, TX) and Beef Cattle Systems (College Station, TX) herds. Vaccines for viral respiratory pathogens were subcutaneously administered at 5 mo of age and 3 wk before weaning (Triangle 5; Boehringer Ingelheim, St. Joseph, MO). At 5 mo of age, steers were also vaccinated for clostridial disease (Covexin 8; Merck, Madison, NJ). Steers were required to be seronegative for MH (whole-cell agglutination test; Texas Veterinary Medical Diagnostic Laboratory) to qualify for study enrollment, and the steers were confirmed not persistently infected with bovine viral diarrhea virus (BVDV). Steers were stratified by source, exit velocity, initial BW, and pre-trial ADG, then randomly assigned into treatments in a 2 × 2 factorial arrangement with dietary live yeast (Saccharomyces cerevisiae boulardii strain CNCM I-1079; Proternative Advantage; Lallemand Animal Nutrition) and MH inoculation (1.2 to 1.4 × 109 CFU/10-mL dose) being the 2 factors. Steers remained in the same 4 pens equipped with GrowSafe feed bunks (GrowSafe Systems Ltd., Calgary, Canada) for the duration of the study. Steers in 2 pens were fed diets containing live yeast, with steers in the other 2 pens fed a control diet. Steers were adapted to the diet, and thereafter DMI and feeding behavior were collected for 28 d prior to MH or phosphate-buffered saline (PBS) inoculation. Each pen housed equal numbers from each inoculation group. Live yeast supplementation did not affect any of the response variables, thus the effects of that treatment will not be addressed in this study.
Complete animal management and MH inoculation procedures are described at length by Kayser et al. (2019a). Inoculum was prepared as described by Mosier et al. (1995). The MH serotype A1 was grown for 18 hr at 37 °C in 7% CO2 on trypticase soy agar with 5% sheep blood. Next, colonies were inoculated into brain-heart infusion broth and incubated for an additional 18 hr at 37 °C. Bacteria were centrifuged at 3,000 × g for 15 min at 4 °C, washed with PBS twice, and finally resuspended in PBS. Inoculum was transported on ice 17 km to the site of inoculation. Plate counts were later used to confirm bacterial concentration of 1.2 to 1.4 × 108 CFU/mL. On day 0, all animals were challenged with PBS or MH. Control steers were processed first to avoid risk of cross-contamination. Briefly, an endoscope was passed through ventral meatus of the left nostril through to the right apical lung bronchus, where a 10-mL dose containing 1.2 × 109 CFU MH was administered followed by a 60-mL PBS flush. The CON steers were likewise inoculated with 70 mL of PBS. Steers were observed twice daily by 2 experienced evaluators for clinical signs of BRD, and a clinical illness score was assigned that included signs of depression, inappetence, and respiratory distress (scores of 1 to 4 [1 = mild, 2 = moderate, 3 = severe, and 4 = moribund] for each criterion). Steers with a clinical illness score of 3 or greater were removed from the pen, rectal temperature measured, and antimicrobial therapy administered if temperature exceeded 40.5 °C. Steers were returned to home pen following the health evaluations.
Data collection
Steers were fitted with rumen biothermal boluses (ThermoBolus, Medria, Châteauborg, France) that recorded rumen temperature at 5-min intervals, with a proprietary algorithm used to remove the effects of drinking events. During the study, steers were housed in pens equipped with electronic feed bunks (GrowSafe Systems Ltd.) that continuously recorded individual-animal feed intake and feeding behavior traits that included frequency and duration of bunk visit events, nonfeeding interval, head down duration, and time to bunk. Meal traits were extrapolated from bunk visit traits using the Meal Criterion Calculation software v.1.8.7154.27227 (http://nutritionmodels.tamu.edu/mcc.html). Day-to-day variation in DMI, bunk visit and meal traits was calculated as the root mean square error (RMSE) within animal by regressing each trait on trial day.
Blood samples (7-mL EDTA and 10-mL Vacutainers with no additive; Becton, Dickson and Company, Franklin Lakes, NJ) and BW were collected on days −4, 0 to 3, 5, 7, 10, and 14, relative to MH or PBS inoculation. The EDTA blood samples were analyzed for complete blood count analysis using an automated hemocytometer (ADVIA 120, Siemens Healthcare Diagnostics, Tarrytown, NY; Texas Veterinary Medical Diagnostic Laboratory). HPT analysis of serum samples was conducted using a commercial ELISA kit (Bovine Haptoglobin ELISA kit, Immunology Consultants Laboratory, Inc., Portland, OR) at the West Texas A&M University Animal Health Laboratory (Canyon, TX). The inter- and intraassay CVs of the haptoglobin assay were 16.1% and 11.4%, respectively. Serum cortisol was measured using a solid-phase radioimmunoassay (DSL-2100; Diagnostic Systems Labs, Webster, TX). The interassay CV of the cortisol assay was 8.8%. Plasma samples were stored at −80 °C for subsequent metabolite analysis at the NMR Center at Montana State University (Bozeman, MT).
Preparation of serum samples for NMR metabolomics analysis
Prior to NMR analysis, plasma samples were thawed at 4 °C and centrifuged at 12,000 × g for 5 min at 4 °C to remove cells and other precipitated material. Samples were diluted 1:1 with MeOH, incubated at −20 °C for 30 min, and then centrifuged at 14,000 × g for 10 min. Thereafter, the supernatant was diluted 1:1 with chloroform, and then centrifuged at 10,000 × g for 8 min. The top polar layer was removed, placed in a 1.5-mL polypropylene tube in a vacuum concentrator overnight with no heat, and stored at −80 °C until subsequent analysis. Dried extracts were resuspended in 600 µL of NMR buffer consisting of 25 mM NaH2PO4/Na2HPO4, 0.4 mM imidazole, 0.25 mM 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS) in 90% H2O/10% D2O, pH 7.0. Following resuspension, samples were centrifuged at 21,000 rpm for 1 min to pellet insoluble debris, and then transferred to 5-mm NMR tubes for NMR metabolomics analysis.
1H NMR experiments
NMR spectra of serum metabolite extracts were collected at 300 K using a Bruker 600 MHz AVANCE III solution NMR spectrometer equipped with a 5-mm triple resonance (1H, 13C, and 15N) sensitivity-enhanced CryoProbe, automatic sample loading system (SampleJet), and Topspin software (Bruker version 3.2). The 1D 1H NMR spectra were recorded using the Bruker gradient-based water suppression “zgesgp” pulse sequence (Ramm Sander et al., 2013; Fuchs et al., 2019), with 256 scans, a 1H spectral window of 7211.539Hz, 64K data points, and a dwell time interval of 69 µs, resulting in a data acquisition time of 4.5 s. Recovery delay times between acquisitions were set to 2.0 s with a prescan delay of 10.0 µs resulting in a total relaxation recovery delay of 5 s between scans. The 1D 1H NMR spectra were phase- and baseline-corrected manually, and pH and chemical shift calibrated using the Chenomx NMR Suite program (version 8.4; Edmonton, Canada). The Chenomx spectral reference library for 600 MHz (1H Larmor Frequency) magnetic field strength NMR was used to identify and quantify metabolites in resulting 1D 1H NMR spectra of metabolite mixtures (Weljie et al., 2006). Sodium trimethylsilylpropanesulfonate (DSS) was added to each NMR sample at a fixed concentration of 0.25 mM, and served as an internal chemical shift reference and internal calibration of relative 1H signal intensities, enabling metabolite quantitation.
Statistical analysis
The original study was a randomized complete block design with treatments arranged as a 2 × 2 factorial (live yeast and MH inoculation serving as the 2 factors), with individual animal as experimental unit. As the effect of live yeast supplementation was not significant for any of the response variables, the fixed effect of live yeast supplementation was not included in models for this study. Retrospectively, it was discovered 9 of the 18 steers that were inoculated with MH produced a minimal HPT response, whereas the other 9 MH-challenged steers produced a substantial HPT response. The classification of MH-challenged steers as HPT responsive or HPT nonresponsive was based on a threshold of 20 mg/dL/d area under the curve values using Proc Expand (SAS 9.4, SAS Institute, Cary, NC). Thus, the steers were classified as HPT responsive (RES) or nonresponsive (NON) following the MH challenge, and PBS-inoculated controls (CON). Preliminary analysis revealed that initial exit velocity was a significant covariate in the analysis of the feeding behavior data collected for 28 d prior to the MH challenge, thus initial exit velocity was included as a fixed effect. For the analyses of DMI and feeding behavior data collected during the 28-d post MH-challenge period, initial exit velocity was no longer a significant covariate and thus was not included in the model.
Feeding behavior, rumen temperature, and blood data were analyzed in a repeated measures analysis using the mixed procedure of JMP (JMP, Version 14.0; SAS Institute, Cary, NC) with autoregressive covariance structure. The model included fixed effects of day, classification, and the interaction thereof. If that interaction was significant at P < 0.05, group means were compared using Student’s t-test. Repeated measures analysis of rumen temperature was completed on an hourly basis for 36 hr prior to and following MH inoculation due to the transient nature of the febrile response. To remove diurnal effect, summary statistics of rumen temperature for the duration of the trial were computed for the third quarter of the day (1200 to 1800 hours). Body weights of individual steers were regressed on day of trial using general regression platform (JMP, 14.0) to model growth rates, and the regression coefficients used to calculate initial and final BW, and ADG for each period. Statistical significance was declared at P ≤ 0.05 and tendencies at P ≤ 0.10.
Metabolite concentrations data obtained from the NMR studies were analyzed using univariate and multivariate statistical analysis to assess whether distinct serum metabolite profiles could be used to discriminate between BRD infected and healthy animals. Metabolite concentrations normalized by sum were further log transformed to ensure a Gaussian distribution of the data and auto-scaled (i.e., mean centered and divided by the standard deviation) prior to principal component analysis (PCA) which was accomplished using the MetaboAnalyst software 4.0 (Chong et al., 2019). Univariate analysis was conducted using least square mean metabolomic data from days 2 to 5, relative to MH inoculation, to elucidate maximal metabolic effects of experimental BRD. Statistical significance of metabolite level difference was assessed by unpaired parametric t-test with Mann–Whitney and Bonferroni correction.
Results and Discussion
The MH-challenge model used in this study did not affect clinical illness scores between MH- versus PBS-inoculated steers. It is likely that the lack of clinical signs of BRD observed in the current study was due to the relatively low-potency MH inoculum (1.4 × 109 CFU/dose) administered and the subjects being of heavier BW (386 kg). Corrigan et al. (2007) administered a similar dose (1.3 × 109 CFU/dose) in calves 150 kg lighter than calves in this study, and reported mild clinical symptoms. Other studies that administered less-potent inoculums (6 × 107 to 5.4 × 108 CFU/dose) in calves of substantially lighter BW than the present study reported mild-to-severe clinical symptoms (Forbes et al., 2011; Baruch et al., 2019; Kayser et al., 2019b). Hanzlicek et al. (2010) delivered a substantially more concentrated MH challenge (4 × 1010 CFU/dose) to 200 kg calves and observed moderate clinical symptoms and lung lesions upon necropsy. Theurer et al. (2013) and Amrine et al. (2014) observed slight-to-moderate signs of clinical illness in calves weighing ~200 kg that received a nearly 10-fold more concentrated MH dose (1 × 1010 CFU/dose). Therefore, it can be deduced that subject BW and inoculum dosage affect clinical response to an MH challenge.
Hematology
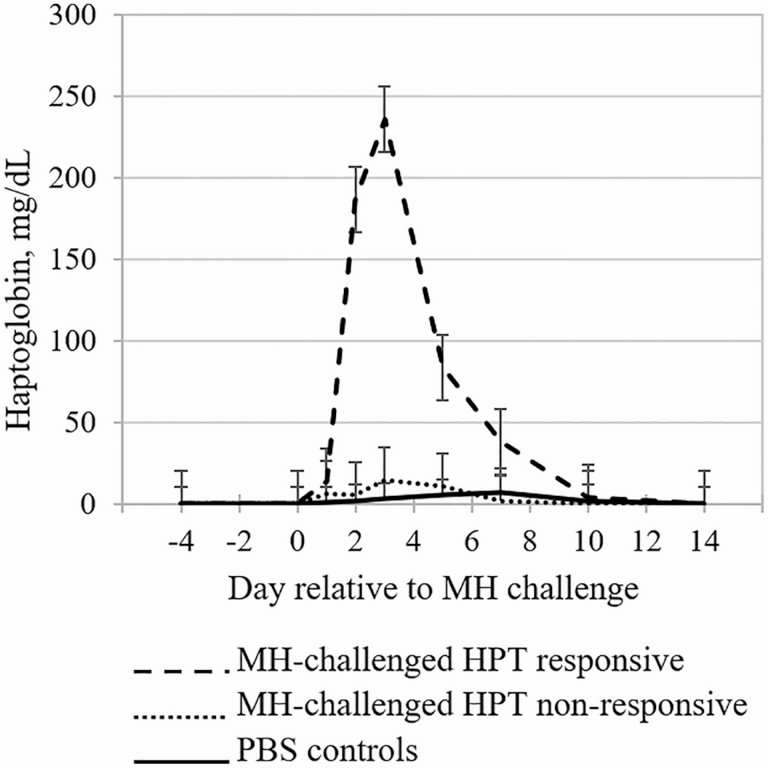
Effects of HPT-responsive phenotype on physiological responses, and DMI and feeding behavior responses are presented in Table 1. The MH-challenged steers classified as RES exhibited greater (P < 0.01) HPT concentration on days 2 through 5 post-MH challenge compared with NON and CON steers (Figure 1). The HPT concentrations of lighter-weight MH-challenged calves reported by Corrigan et al. (2007) are substantially lesser compared with observations in the present study, though timing of the HPT response was similar. El-Deeb et al. (2020) evaluated acute-phase proteins and proinflammatory cytokines in BRD-diagnosed feedlot calves, and concluded that HPT and other acute phase proteins exhibited utility as diagnostic and prognostic biomarkers for BRD, as HPT was rapidly elevated in morbid calves, and quickly returned to baseline levels following antimicrobial therapy. Humblet et al. (2004) observed that serum HPT concentrations were greater in BRD-diagnosed calves that required anti-inflammatory and antimicrobial therapy for recovery from disease compared with BRD-diagnosed calves that recovered with antimicrobial therapy alone. Burciaga-Robles et al. (2009) found that HPT concentration on feedlot arrival was least in healthy calves, intermediate for calves that subsequently were treated once for BRD, and greatest in calves that were treated multiple times for BRD. Holland et al. (2011) sorted calves into group pens based on low, medium, or high serum HPT on feedlot arrival. Calves with the increased HPT concentrations had reduced ADG and DMI, greater morbidity rates and a greater proportion of calves requiring 3 or more treatments to recovery from BRD during the 2-wk postarrival period compared with calves categorized as having decreased HPT concentrations. Results from these studies indicate that serum HPT concentration may be associated with immunocompetence and disease resilience. In contrast, Young et al. (1996) reported limited predictability of HPT; they measured serum HPT in 366 calves on days 0, 40, and 65 relative to feedlot arrival and recorded incidence of BRD 10 d following each blood collection. Predictive value of HPT was calculated for each collection day and determined to be low, due to the inconsistency with which calves that had elevated HPT actually developed clinical BRD. Further, <60% of the calves with elevated HPT from at least one of the collection days had lung lesions at slaughter, which indicates the non-specific nature of the HPT response. Therefore, HPT response may indicate immunocompetence or disease resilience, but results are inconsistent regarding its predictability of disease.
Table 1.
The effects of haptoglobin (HPT) responsive phenotype on physiological responses and feeding behavior from days −4 to 14 relative to inoculation with MH or PBS (control) in cattle
| MH challenged | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Item1 | HPT responsive | HPT nonresponsive | PBS control | SE | Group | Day | Group × day |
| Feeding behavior traits | |||||||
| DMI, kg/d | 8.76 | 9.80 | 10.2 | 0.49 | 0.08 | 0.01 | 0.01 |
| BV eating rate, g/min | 95.8b | 118a | 120a | 4.6 | 0.01 | 0.01 | 0.03 |
| BV duration, min/d | 104 | 95.3 | 97.1 | 6.5 | 0.57 | 0.01 | 0.45 |
| BV frequency, events | 26.7 | 31.2 | 29.3 | 2.6 | 0.23 | 0.01 | 0.47 |
| Rumen temperature traits | |||||||
| Temperature, °C | 39.6 | 39.5 | 39.5 | 0.1 | 0.27 | 0.01 | 0.01 |
| Temperature SD, °C | 0.574a | 0.541ab | 0.482b | 0.033 | 0.03 | 0.01 | 0.01 |
| Hematologic variables | |||||||
| Haptoglobin, mg/dL | 62.9 | 4.37 | 2.21 | 6.17 | 0.01 | 0.01 | 0.01 |
| Cortisol, ng/mL | 25.4b | 31.0a | 32.6a | 2.3 | 0.05 | 0.07 | 0.11 |
| Neutrophils, K/µL | 4.68a | 4.24a | 2.76b | 0.31 | 0.01 | 0.01 | 0.01 |
| Monocytes, K/µL | 0.41 | 0.56 | 0.43 | 0.06 | 0.17 | 0.31 | 0.70 |
| Eosinophils, K/µL | 0.29 | 0.33 | 0.35 | 0.06 | 0.71 | 0.47 | 0.85 |
| Lymphocytes, K/µL | 6.30 | 6.62 | 6.82 | 0.51 | 0.70 | 0.01 | 0.01 |
| Erythrocytes, K/µL | 7.95 | 8.09 | 8.10 | 0.22 | 0.84 | 0.01 | 0.24 |
| Hematocrit, % | 33.1 | 34.5 | 34.2 | 0.9 | 0.47 | 0.01 | 0.66 |
| Hemoglobin, g/dL | 12.3 | 12.7 | 12.8 | 0.4 | 0.51 | 0.01 | 0.12 |
| MCH, pg | 15.5 | 15.9 | 15.8 | 0.3 | 0.62 | 0.07 | 0.88 |
| MCV, fL | 41.8 | 42.7 | 42.3 | 0.8 | 0.74 | 0.01 | 0.57 |
| Platelets, K/µL | 579 | 492 | 526 | 35 | 0.22 | 0.01 | 0.01 |
1BV, bunk visit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin.
a–cDifferent letters indicate significant (P < 0.05) differences among means within a row.
Figure 1.
Least squares means of serum HPT concentrations by day relative to an experimentally induced challenge with MH or PBS (control). Differential HPT responsiveness to MH challenge was used to classify cattle into HPT-responsive and nonresponsive phenotypes. Means within day with separated error bars differ at P ≤ 0.05.
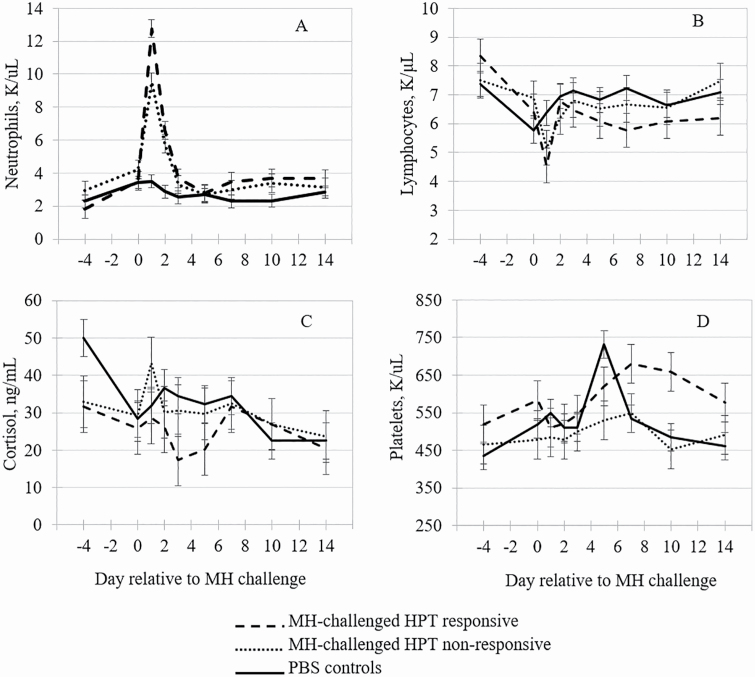
The MH-challenged steers exhibited greater (P < 0.01) neutrophil concentrations than CON steers on days 1 and 2 post-MH challenge, but the magnitude of increase was 35% greater (P < 0.01) in RES than NON steers (Figure 2A). Neutrophils are phagocytic leukocytes and are the first leukocyte to respond in immune insult or injury. The greater neutrophil concentrations observed in RES steers are logical, as neutrophils release chemokines that then recruit acute-phase proteins such as HPT (Malech et al., 2014). Collectively, the MH-challenged steers exhibited numerically decreased lymphocytes for 7 d following MH inoculation compared with the CON steers. However, the RES steers exhibited decreased (P < 0.05) lymphocytes compared with the CON steers on days 1 and 7, while NON was intermediate (Figure 2B). Although the lymphocyte concentrations were depressed in the RES steers, the values were within standard reference limits (Jones and Allison, 2007) and were within the range reported by Hanzlicek et al. (2010) following an experimentally induced MH challenge. Burciaga-Robles et al. (2010) similarly found that neutrophil concentrations were greater and lymphocyte concentrations were lesser in calves exposed to BVDV prior to an MH challenge compared with calves that were MH-challenged only, indicating that the severity of the disease insult altered the leukogram profile.
Figure 2.
The effects of HPT-responsive phenotype on (A) neutrophil, (B) lymphocyte, (C) cortisol, and (D) platelet concentrations by day relative to inoculation with MH or PBS (control) in cattle. Means within day with separated error bars differ at P ≤ 0.05.
Although the HPT classification × day interaction was not significant (P = 0.11) for cortisol, there was a classification effect with the RES steers expressing reduced (P = 0.05) cortisol concentrations compared with NON and CON steers (Figure 2C). Decreased cortisol response in RES steers may have enabled the increased immunologic response following the MH challenge, as glucocorticoids are known to be anti-inflammatory (Dhabhar, 2008). Indeed, Dong et al. (2018) reported that cortisol inhibited production of proinflammatory cytokines in lipopolysaccharide-stimulated macrophages. In some cases, the inflammation associated with an immune response is more damaging to the host than the pathogen the immune response is targeting, indicating modest concentrations of cortisol may help to moderate the immune response following a health challenge (Lawrence and Gilroy, 2007; Jose and Madan, 2016).
There was an HPT classification × day interaction (P < 0.01) for platelets, with CON steers having the greatest concentration on day 5 compared with RES and NON steers. We cannot provide plausible explanation for the thrombocytosis that occurred on day 5 in CON steers as no outliers were observed. However, the RES steers had greater concentrations of platelets on days 7 to 14 than CON or NON steers (Fig. 2D); the thrombocytosis response in RES steers is expected, as RES steers also had greater neutrophil concentrations, which are known to release platelet activating factor (Whiteley et al., 1992). Although the role of platelets in the inflammatory cascade has not been fully elucidated, Hanedan et al. (2015) reported numerically greater platelet concentrations in BRD-diagnosed calves, and elevated platelet concentrations have been associated with poor prognosis in human pneumonia patients (Prina et al., 2013).
Feeding behavior
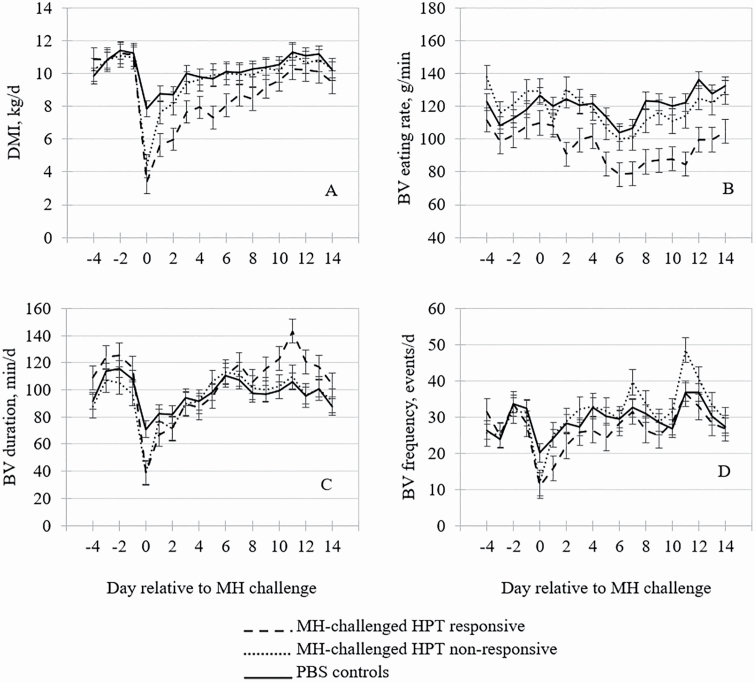
The effects of HPT-responsive phenotype on feeding behavior are presented in Table 1. Feed intake was depressed (P < 0.01) in the MH-challenged steers compared with CON steers following inoculation. However, the magnitude of the depression in DMI was greater in RES vs. NON steers, with DMI remaining reduced (P < 0.05) in RES steers compared with NON and CON steers until day 8 post-MH challenge (Figure 3A). The depression in feed intake prior to onset of clinical BRD has been well documented (Quimby et al., 2001; Wolfger et al., 2015; Kayser et al., 2019c). Though cytokines were not directly measured in this study, it is likely RES steers had greater circulating concentrations of cytokines than NON steers, which have been found to be elevated in BRD-diagnosed calves (Ozkanlar et al., 2012). Proinflammatory cytokines are known to induce the anorexia as well as promote synthesis of HPT and other acute-phase proteins (McCarthy, 2000; Dantzer, 2004). Greater cytokine concentrations in the RES steers would have contributed to the observed prolonged DMI depression compared with the NON steers. Similarly, Holland et al. (2011) reported that calves with greater concentrations of HPT on feedlot arrival had depressed DMI compared with calves that had lesser HPT concentrations. Additionally, bunk visit eating rate in RES steers was decreased (P < 0.05) from day 2 through 14 post-MH inoculation compared to CON and NON steers (Figure 3B). The inappetence brought on by proinflammatory cytokines could have also contributed to the decreased bunk visit eating rate in the RES steers in this trial; however, bunk visit frequency and duration were not affected by HPT classification for during this period (Figure 3C and D).
Figure 3.
The effects of HPT-responsive phenotype on (A) DMI, (B) bunk visit eating rate, (C) bunk visit duration, and (D) bunk visit frequency by day relative to challenge with MH or PBS (control) in cattle. Means within day with separated error bars differ at P ≤ 0.05.
Febrile response
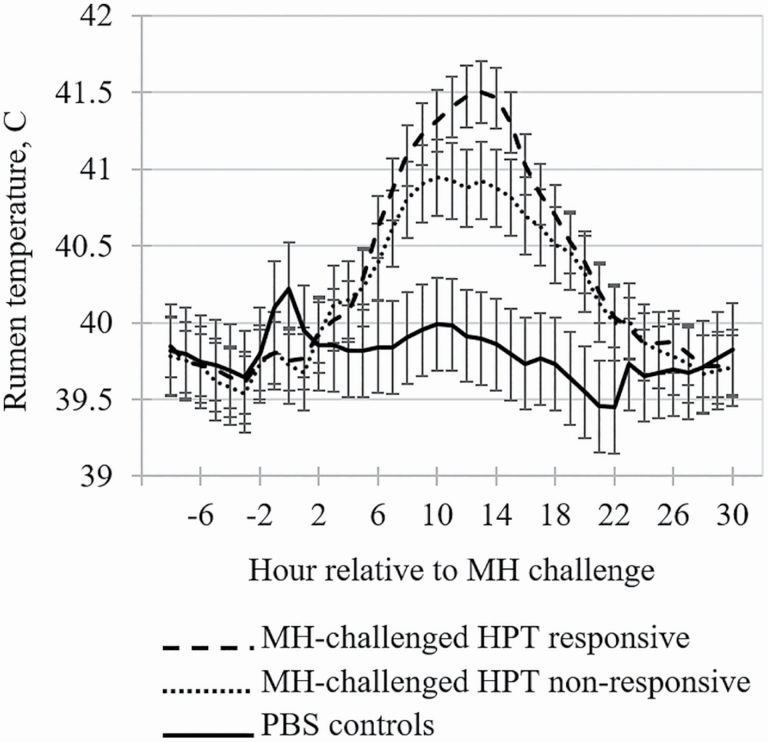
Main effects of HPT-responsive phenotype on temperature are presented in Table 1. The rumen temperature data from hours −8 through 28 relative to MH inoculation are presented in Figure 4. Collectively, the MH-challenged steers exhibited a mild, transient fever starting at hour 5 post-MH challenge similar to that reported by Corrigan et al. (2007); however, the RES steers in the present study had greater (P < 0.01) rumen temperature from 9 to 16 h post-MH challenge than the NON steers. Holland et al. (2011) reported that rectal temperature of calves with greater HPT concentrations at feedlot arrival tended to be greater during first treatment of BRD compared to calves with lesser HPT concentrations on arrival. Additionally, Burdick et al. (2011) demonstrated that bulls with more excitable temperaments had greater basal cortisol concentrations and lesser peak rectal temperature responses to a lipopolysaccharide challenge than bulls with calm temperaments. These findings would suggest that the lower-magnitude febrile response to the MH challenge in NON steers may have been associated with their increased basal cortisol concentrations compared with RES steers. Temperature is a non-specific response to infection mounted by the innate immune system in order to make the host less hospitable to pathogens, and temperature responses remain the most common diagnostic method for defining BRD cases in commercial operations, as well as in experimental-challenge models (Hanzlicek et al., 2010; Theurer et al., 2013; Timsit et al., 2016). It appears that in MH-inoculated calves, disease severity affects the zenith of the febrile response but not the duration. Eberhart et al. (2017) administered a more concentrated MH inoculum (3 to 5 × 109 CFU/dose) to 5-mo-old dairy calves and reported the challenged calves experienced a severe fever that returned to baseline within 24 hr. Inoculating cattle with a virus such as bovine herpes virus-1 prior to MH inoculation results in a more prolonged fever, though peak temperature is similar to MH-only models used in the present study (Baruch et al., 2019; Kayser et al., 2019b).
Figure 4.
The effects of HPT-responsive phenotype on rumen temperature by hour relative to challenge with MH or PBS (control) in cattle. Means within day with separated error bars differ at P ≤ 0.05.
Metabolomics
The 1H NMR spectra of 364 plasma metabolites were evaluated for this study, resulting in the unambiguous identification and quantification of 32 polar metabolites. Univariate analysis was conducted to examine the effect of HPT classification on relative metabolite concentrations between days 2 and 5 relative to MH challenge; these results are presented in Table 2. The RES steers had decreased (P < 0.05) concentrations of allantoin, glucose, glutamine, and l-lactic acid compared with NON and CON steers. Additionally, RES steers had greater (P < 0.05) concentrations of d-lactic acid and phenylalanine, and tended (P = 0.06) to have greater 3-hydroxybutyrate concentrations compared with NON and CON steers.
Table 2.
The effects of HPT-responsive phenotype on metabolite concentrations from days 2 to 5 relative to inoculation with MH or PBS (control) in cattle.
| MH challenged | |||||
|---|---|---|---|---|---|
| Metabolite | HPT responsive | HPT nonresponsive | PBS control | SEM | P-value |
| 3-Hydroxybutyrate | 0.261 | 0.208 | 0.211 | 0.017 | 0.06 |
| 3-Hydroxyisobutyrate | 0.032 | 0.024 | 0.025 | 0.003 | 0.13 |
| 3-Hydroxyisovalerate | 0.027 | 0.026 | 0.027 | 0.003 | 0.98 |
| Acetate | 0.366 | 0.401 | 0.361 | 0.026 | 0.52 |
| Alanine | 0.190 | 0.193 | 0.184 | 0.007 | 0.66 |
| Allantoin | 0.242b | 0.310a | 0.283ab | 0.017 | 0.03 |
| Betaine | 0.179 | 0.172 | 0.165 | 0.009 | 0.47 |
| Creatine | 0.110 | 0.102 | 0.111 | 0.007 | 0.67 |
| Creatinine | 0.071 | 0.071 | 0.071 | 0.003 | 0.99 |
| Dimethyl sulfone | 0.867 | 0.878 | 0.830 | 0.035 | 0.53 |
| Formate | 0.083 | 0.089 | 0.083 | 0.006 | 0.74 |
| Fructose | 0.372 | 0.317 | 0.328 | 0.032 | 0.48 |
| Glucose | 4.78ab | 5.10a | 4.43b | 0.20 | 0.05 |
| Glutamine | 0.206b | 0.249a | 0.230ab | 0.010 | 0.02 |
| Glycine | 0.169 | 0.174 | 0.161 | 0.008 | 0.44 |
| Hippurate | 0.021 | 0.024 | 0.025 | 0.002 | 0.26 |
| Histidine | 0.029 | 0.035 | 0.031 | 0.002 | 0.15 |
| Isoleucine | 0.136 | 0.143 | 0.136 | 0.006 | 0.66 |
| Leucine | 0.211 | 0.225 | 0.212 | 0.008 | 0.48 |
| d-Lactic acid | 0.021a | 0.019ab | 0.017b | 0.001 | 0.03 |
| l-Lactic acid | 2.05b | 2.82a | 2.05b | 0.22 | 0.03 |
| Malonate | 3.37 | 3.38 | 3.21 | 0.13 | 0.52 |
| Methanol | 0.477 | 0.470 | 0.532 | 0.165 | 0.95 |
| Methionine | 0.026 | 0.026 | 0.028 | 0.001 | 0.48 |
| Phenylalanine | 0.088a | 0.080ab | 0.074b | 0.003 | 0.01 |
| Pyruvate | 0.069 | 0.071 | 0.059 | 0.005 | 0.17 |
| Succinate | 0.005 | 0.006 | 0.005 | 0.001 | 0.69 |
| Threonine | 0.155 | 0.125 | 0.143 | 0.010 | 0.13 |
| Tryptophan | 0.043 | 0.043 | 0.044 | 0.002 | 0.92 |
| Tyrosine | 0.078 | 0.090 | 0.085 | 0.004 | 0.12 |
| Urea | 13.5 | 12.6 | 13.8 | 1.0 | 0.72 |
| Valine | 0.292 | 0.317 | 0.290 | 0.012 | 0.24 |
a–cDifferent letters indicate significant (P < 0.05) differences among means within a row.
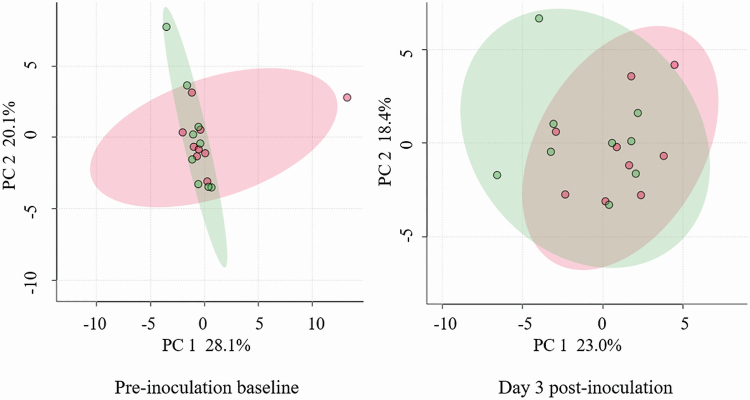
In contrast to the univariate analysis, multivariate analysis using unsupervised 2-dimensional principal component analysis (2D-PCA) was unable to differentiate RES from NON steers, nor combined MH-challenged steers from CON steers, based on distinct serum metabolite profiles. The 2D-PCA score plots of the metabolite analyses between RES and NON are presented for day of MH inoculation (day 0) and the day of peak HPT response (day 3) in Figure 5. These results demonstrate a lack of separation of metabolite profiles due to HPT phenotype or due to the MH-challenge treatment. The lack of difference in metabolite profiles is in all likelihood associated with this being a subclinical MH challenge or potentially indicative of partial sample degradation.
Figure 5.
2D principal component (PC) analysis scores plots (2D-PCA) scores plots resulting from the analysis of plasma metabolic profiles of haptoglobin-responsive (green circles) versus nonresponsive (red circles) cattle, following inoculation with MH, with shaded regions illustrating respective 95% confidence intervals.
Blakebrough-Hall et al. (2020) recently conducted a 1H NMR metabolomic analysis of serum samples that were collected on day of diagnosis with BRD (n = 149) and randomly selected healthy cohorts (n = 148). They identified 28 individual metabolites with different concentrations between BRD-diagnosed and healthy cattle. Five of the metabolites (3-hydroxybutyrate, glucose, glutamine, lactic acid, and phenylalanine) identified by Blakebrough-Hall et al. (2020) were similar those that were found to be different between RES vs. NON and CON steers in this study. In contrast to our findings, Blakebrough-Hall et al. (2020) reported distinctive metabolite profiles differences based on PCA of metabolite profiles between BRD-diagnosed and healthy cohorts. Blakebrough-Hall et al. (2020) concluded that hydroxybutyrate, phenylalanine, lactate, tyrosine, citrate, and leucine were metabolites of importance in identifying cattle with BRD. Cattle in the study by Blakebrough-Hall (2020) were visually diagnosed with natural BRD based on clinical symptoms, whereas the MH-challenge induced in the present study induced only mild subclinical responses. This could indicate that metabolic profiles of cattle do not measurably deviate with subclinical BRD. However, metabolomics analysis has successfully distinguished healthy dairy cows from those that would develop postpartum diseases such as mastitis and metritis up to 4 wk prior to parturition with sensitivity of 85% (Hailemariam et al., 2014). Additionally, 1H NMR metabolomic analysis of sera distinguished healthy calves from calves experimentally infected with Mycobacterium avium prior to onset of clinical symptoms (De Buck et al., 2014). Buhler et al. (2019) found that auction-sourced calves had differential metabolite profiles compared with calves that were sourced from a single ranch, suggesting that metabolomics could be of use to identify calves at greater risk for BRD. Further research is warranted to expand metabolite identification coverage and to further identify metabolites that are responsive to a more severe experimentally induced challenge to aid in the discovery of biomarkers of predictive value of the onset of BRD.
Performance and feeding behavior of pre- and postchallenge periods
The effects of HPT-responsive phenotype on performance, DMI and feeding behavior patterns are presented in Tables 3 and 4 for the 28-d prior to and 28-d following the MH challenge, respectively. During the 28-d prechallenge period, there were no differences in BW, ADG, DMI, or G: F between RES, NON and CON steers. However, during the 28-d postchallenge period, RES steers tended (P = 0.06) to have diminished DMI and decreased (7%) (P < 0.05) final BW compared with NON and CON steers. The difference in performance was likely associated with RES steers’ energy allocation to mount greater immunological responses following the MH-challenge, further indicating that NON steers exhibited greater disease resilience. Holland et al. (2011) also observed decreased BW and DMI in calves that had detectable HPT concentrations on feedlot arrival compared with cattle that arrived with undetectable concentrations of HPT.
Table 3.
The effects of HPT-responsive phenotype on performance and feeding behavior patterns for 28 d prior to inoculation with MH or PBS (control) in cattle.
| MH challenged | |||||
|---|---|---|---|---|---|
| Item1 | HPT responsive | HPT nonresponsive | PBS control | SE | P-value |
| No. of animals | 9 | 9 | 18 | ||
| Performance and growth traits | |||||
| Initial BW, kg | 344 | 358 | 355 | 7 | 0.37 |
| Final BW, kg | 392 | 402 | 401 | 8 | 0.63 |
| ADG, kg | 1.71 | 1.56 | 1.62 | 0.08 | 0.46 |
| DMI, kg | 11.3 | 10.6 | 11.3 | 0.3 | 0.20 |
| G: F | 0.152 | 0.146 | 0.143 | 0.012 | 0.55 |
| Bunk visit (BV) traits | |||||
| BV frequency, events | 57.9 | 48.5 | 51.8 | 3.9 | 0.23 |
| BV duration, min | 140a | 112b | 121ab | 7 | 0.03 |
| BV eating rate, g/min | 84.5c | 103a | 97.9b | 4.7 | 0.03 |
| Meal traits | |||||
| Meal criterion, min | 5.38 | 5.76 | 5.68 | 1.08 | 0.97 |
| Meal frequency, events | 14.3 | 14.0 | 15.2 | 1.5 | 0.77 |
| Meal duration, min | 193 | 160 | 167 | 10 | 0.07 |
| Meal eating rate, g/min | 62.7 | 71.3 | 71.8 | 3.5 | 0.11 |
| Intensity traits | |||||
| Head down duration, min/d | 78.2a | 46.4c | 65.0b | 7.8 | 0.02 |
| Time to bunk, min | 25.1 | 30.6 | 30.6 | 8.0 | 0.84 |
| Day-to-day variation, RMSE1 | |||||
| DMI, kg/d | 1.42 | 1.34 | 1.50 | 0.09 | 0.37 |
| BV frequency, events | 17.1a | 11.6b | 15.0ab | 1.2 | 0.01 |
| BV duration, min | 26.9 | 23.7 | 23.7 | 1.5 | 0.19 |
| Meal frequency, events | 3.13 | 3.25 | 3.24 | 0.32 | 0.96 |
| Meal duration, min | 32.5 | 27.9 | 30.2 | 2.7 | 0.48 |
| Head down duration, min | 17.9a | 12.2b | 15.3ab | 1.4 | 0.03 |
| Time to bunk, min | 30.5 | 34.6 | 35.0 | 8.2 | 0.90 |
1Day-to-day variance was calculated as the RMSE.
a–cDifferent letters indicate significant (P < 0.05) differences among means within a row.
Table 4.
The effects of HPT-responsive phenotype on performance and feeding behavior patterns for 28 d postinoculation with MH or PBS (control) in cattle
| MH challenged | |||||
|---|---|---|---|---|---|
| Item | HPT responsive | HPT nonresponsive | PBS control | SE | P-value |
| No. of animals | 9 | 9 | 18 | ||
| Performance and growth traits | |||||
| Initial BW, kg | 378 | 393 | 394 | 8 | 0.27 |
| Final BW, kg | 405b | 434a | 434a | 9 | 0.04 |
| ADG, kg/d | 1.29 | 1.48 | 1.47 | 0.07 | 0.20 |
| DMI, kg/d | 9.31 | 10.3 | 10.6 | 0.44 | 0.06 |
| G:F | 0.136 | 0.144 | 0.139 | 0.011 | 0.60 |
| Bunk visit (BV) traits | |||||
| BV frequency, events | 30.0 | 34.5 | 32.1 | 2.5 | 0.46 |
| BV duration, min | 108 | 95.7 | 98.1 | 6.2 | 0.31 |
| BV eating rate, g/min | 86.8b | 109a | 111a | 4.5 | 0.01 |
| Meal traits | |||||
| Meal criterion, min | 6.22 | 6.69 | 5.58 | 0.71 | 0.43 |
| Meal frequency, events | 11.4 | 10.8 | 12.3 | 0.8 | 0.34 |
| Meal duration, min | 135 | 132 | 126 | 7 | 0.59 |
| Meal eating rate, g/min | 70.1b | 79.3ab | 85.7a | 3.5 | 0.01 |
| Intensity traits | |||||
| Head down duration, min | 64.8 | 45.7 | 56.6 | 5.9 | 0.09 |
| Time to bunk, min | 56.5 | 41.4 | 38.1 | 8.1 | 0.19 |
| Day-to-day variation, RMSE1 | |||||
| DMI, kg | 1.77a | 1.47ab | 1.21b | 0.12 | 0.01 |
| BV frequency, events | 8.89 | 9.76 | 7.44 | 0.82 | 0.07 |
| BV duration, min | 27.7a | 19.3b | 15.3c | 1.6 | 0.01 |
| Meal frequency, events | 2.62 | 2.22 | 2.11 | 0.21 | 0.15 |
| Meal duration, min | 34.5a | 28.6a | 19.3b | 2.2 | 0.01 |
| Head down duration, min | 17.5a | 10.1b | 10.5b | 1.2 | 0.01 |
| Time to bunk, min | 71.6 | 63.2 | 51.1 | 7.8 | 0.10 |
1Day-to-day variance was calculated as the RMSE.
a–cDifferent letters indicate significant (P < 0.05) differences among means within a row.
During the 28-d prechallenge period, RES steers had 25% longer (P < 0.05) bunk visit duration and 41% longer (P ≤ 0.01) head down duration compared with NON steers, with CON steers being intermediate. Further, RES steers tended (P = 0.07) to have 21% longer meal duration than NON steers. As a consequence, RES steers had 22% slower (P < 0.01) bunk visit eating rate and tended to have slower meal eating rate than NON or CON steers during this 28-d prechallenge period. During the 28-d postchallenge period, RES steers still exhibited 26% slower (P < 0.01) bunk visit eating rate and a tendency for slower meal eating rate compared with NON and CON steers. As bunk visit and meal durations were not different between HPT classifications postchallenge, the slower eating rates in RES steers during this period were due to the tendency for reduced DMI in association with the pronounced febrile and leukocyte responses discussed above. In BRD-diagnosed bulls, Jackson et al. (2016) reported faster bunk visit eating rates 1 to 3 d prior to onset of clinical symptoms. As the RES steers had 22% lower (P = 0.05) serum cortisol concentrations than NON steers both prior to and following inoculation, we posit that the relatively slower eating rates exhibited both prior to and following the MH challenge are due to the RES steers having more calm temperaments than NON steers. Cattle with calm temperaments have been reported to have lesser basal cortisol concentrations than cattle with excitable temperaments (Curley et al., 2006; Burdick et al., 2011), and have greater DMI, longer bunk visit duration, and slower eating rate (Smith et al., 2017; Olson et al., 2019). Although RES steers had reduced serum cortisol and bunk visit eating rate prior to inoculation which suggests a calmer temperament, a single-point measure of prestudy exit velocity was not affected by HPT classification (data not shown).
In the 28-d prechallenge period, RES steers exhibited 32% greater (P < 0.04) day-to-day variation in both bunk visit frequency and head down duration compared to NON steers, with CON steers being intermediate. Likewise, during the 28-d postchallenge period, RES steers expressed 30% and 42% greater (P < 0.01) day-to-day variations in bunk visit duration and head down duration, respectively, and tended (P < 0.10) to express greater day-to-day variations in DMI and meal duration compared with NON and CON steers. In aggregate, RES steers exhibited greater daily variance in feeding behavior patterns than NON steers both prior to and following the MH challenge. Recent research has investigated the utility of day-to-day variation in feed intake and feeding duration as potential indicators of disease resiliency in pigs (Putz et al., 2018). In their natural-disease challenge model, infection pressure was maintained at the feeding facility by continually introducing diseased pigs from other facilities, resulting in 26% overall mortality rate. Daily variances in both DMI and feeding duration were found to be moderately heritable (0.21 to 0.26), and had a positive genetic correlation (0.37 to 0.62) to mortality and morbidity rates in pigs. Putz et al. (2018) concluded that decreased day-to-day variance may be indicative of resilience and presents a phenotype for selection of animals with improved disease resilience. Further, less day-to-day variance in daily milk production has been associated with improved health and longevity in dairy cows (Elgersma et al., 2018). Dikmen and Mateescu (2019) determined decreased day-to-day variance in vaginal temperature was positively associated with heat tolerance; therefore, more temperature-consistent heifers were deemed to be more heat resilient. Gijzel et al. (2017) concluded greater day-to-day variations in self-evaluated physical, mental, and social health metrics were associated with decreased disease resilience in geriatric human patients. Collectively, these results suggest the greater day-to-day variance in feeding behavior patterns of RES steers may be associated with decreased disease resiliency and could in part explain the hyperimmune reaction to a subclinical health challenge with no apparent benefit in reduction of disease severity or recovery when compared with NON seers.
Furthermore, basal cortisol concentration has been shown to be associated with disease resilience. Piglet survivability was shown to be positively correlated with size of adrenals and serum cortisol concentration (Leenhouwers et al., 2002) and rats expressing greater corticosterone concentrations were shown to be more tolerant of a heat stress challenge (Michel et al., 2007). Richeson et al. (2016) reported decreased HPT response in animals administered the glucocorticoid dexamethasone, frequently used to experimentally mimic natural cortisol. Mormède et al. (2011) proposed that improved disease resilience observed with greater basal cortisol concentrations is because animals that have increased basal HPA axis activity adapt better to stressors and thus recover more quickly following a perturbation. This would suggest that the greater basal cortisol seen in NON steers could be a marker of resilience. More research is needed to more fully explore traits for utility in identifying resilient animals, and to determine whether our preliminary results indicating that animals exhibiting high levels of variance in behavior or production, and low basal cortisol, are in fact less resilient to disease in the commercial setting. Future projects will include use of auction-source animals to help elucidate whether results observed in this experimentally induced BRD model are reflected in natural BRD cases.
Conclusion
Identification of disease-resilient animals could result in decreased morbidity due to BRD in feedlots. Results of the current study indicate that although the MH-challenged steers exhibited immunological, physiological, and behavioral responses to the experimentally induced challenge, these responses were more pronounced in the MH-challenged steers that mounted a greater HPT response. Steers that were HPT-responsive displayed increased neutrophils, pyrexia, and day-to-day variation in feeding behavior, and depressed cortisol, DMI, and eating rate. However, the disease challenge may not have been severe enough to illicit changes in metabolite profiles, as there were no differences in observed clinical illness scores. We propose that these HPT-responsive steers were less resilient to BRD caused by MH, as literature has shown association in low basal cortisol concentration and high day-to-day variation in feeding behavior as indicators of diminished disease resilience. Therefore, HPT response and feeding behavior may be related and may be indicator traits of disease resilience. Further research is needed to investigate how temperament, basal cortisol, and acute-phase protein response relate to disease resilience.
Glossary
Abbreviations
- BRD
bovine respiratory disease
- BVDV
bovine viral diarrhea virus
- CBC
complete blood count
- G: F
gain: feed
- HPT
haptoglobin
- MH
Mannheimia haemolytica
- PBS
phosphate-buffered saline
- RMSE
root mean square error
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Amrine, D. E., B. J. White, R. L. Larson, and D. A. Mosier. . 2014. Pulmonary lesions and clinical disease response to Mannheimia haemolytica challenge 10 days following administration of tildipirosin or tulathromycin. J. Anim. Sci. 92:311–319. doi: 10.2527/jas.2013-6577 [DOI] [PubMed] [Google Scholar]
- Baruch, J., N. Cernicchiaro, C. A. Cull, K. F. Lechtenberg, J. S. Nickell, and D. G. Renter. . 2019. Performance of multiple diagnostic methods in assessing the progression of bovine respiratory disease in calves challenged with infectious bovine rhinotracheitis virus and Mannheimia haemolytica. J. Anim. Sci. 97:2357–2367. doi: 10.1093/jas/skz107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakebrough-Hall, C., A. Dona, M. J. D’occhio, J. McMeniman, and L. A. González. . 2020. Diagnosis of bovine respiratory disease in feedlot cattle using blood 1H NMR metabolomics. Sci. Rep. 10:115. doi: 10.1038/s41598-019-56809-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhler, V. M., K. R. Cash, D. J. Hurley, and B. C. Credille. . 2019. Characterization and comparison of cell-mediated immune responses following ex vivo stimulation with viral and bacterial respiratory pathogens in stressed and unstressed beef calves1. J. Anim. Sci. 97:2739–2749. doi: 10.1093/jas/skz155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burciaga-Robles, L. O., B. P. Holland, D. L. Step, C. R. Krehbiel, G. L. McMillen, C. J. Richards, L. E. Sims, J. D. Jeffers, K. Namjou, and P. J. McCann. . 2009. Evaluation of breath biomarkers and serum haptoglobin concentration for diagnosis of bovine respiratory disease in heifers newly arrived at a feedlot. Am. J. Vet. Res. 70:1291–1298. doi: 10.2460/ajvr.70.10.1291 [DOI] [PubMed] [Google Scholar]
- Burciaga-Robles, L. O., D. L. Step, C. R. Krehbiel, B. P. Holland, C. J. Richards, M. A. Montelongo, A. W. Confer, and R. W. Fulton. . 2010. Effects of exposure to calves persistently infected with bovine viral diarrhea virus type 1b and subsequent infection with Mannheimia haemolytica on clinical signs and immune variables: model for bovine respiratory disease via viral and bacterial interaction. J. Anim. Sci. 88:2166–2178. doi: 10.2527/jas.2009-2005 [DOI] [PubMed] [Google Scholar]
- Burdick, N. C., J. A. Carroll, L. E. Hulbert, J. W. Dailey, M. A. Ballou, R. D. Randel, S. T. Willard, R. C. Vann, and T. H. Welsh, Jr. 2011. Temperament influences endotoxin-induced changes in rectal temperature, sickness behavior, and plasma epinephrine concentrations in bulls. Innate Immun. 17:355–364. doi: 10.1177/1753425910379144 [DOI] [PubMed] [Google Scholar]
- Chong, J., D. S. Wishart, and J. Xia. . 2019. Using metaboanalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinformatics 68:e86. doi: 10.1002/cpbi.86. [DOI] [PubMed] [Google Scholar]
- Colditz, I., and B. Hine. . 2016. Resilience in farm animals: biology, management, breeding and implications for animal welfare. Anim. Prod. Sci. 56:1961–1983. doi: 10.1071/AN15297 [DOI] [Google Scholar]
- Corrigan, M. E., J. S. Drouillard, M. F. Spire, D. A. Mosier, J. E. Minton, J. J. Higgins, E. R. Loe, B. E. Depenbusch, and J. T. Fox. . 2007. Effects of melengestrol acetate on the inflammatory response in heifers challenged with Mannheimia haemolytica. J. Anim. Sci. 85:1770–1779. doi: 10.2527/jas.2006-396 [DOI] [PubMed] [Google Scholar]
- Curley, K. O., Jr, J. C. Paschal, T. H. Welsh, Jr, and R. D. Randel. . 2006. Technical note: exit velocity as a measure of cattle temperament is repeatable and associated with serum concentration of cortisol in Brahman bulls. J. Anim. Sci. 84:3100–3103. doi: 10.2527/jas.2006-055 [DOI] [PubMed] [Google Scholar]
- Dantzer, R 2004. Cytokine-induced sickness behavior: a neuroimmune response to activation of innate immunity. Eur. J. Pharmacol. 500:399–411. doi: 10.1016/j.ejphar.2004.07.040 [DOI] [PubMed] [Google Scholar]
- De Buck, J., R. Shaykhutdinov, H. W. Barkema, and H. J. Vogel. . 2014. Metabolomic profiling in cattle experimentally infected with Mycobacterium avium subsp. paratuberculosis. PLoS One 9:e111872. doi: 10.1371/journal.pone.0111872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar Firdaus, S 2008. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection versus immunopathology. Allergy Asthma Clin. Immunol. 4:2–11. doi: 10.1186/1710-1492-4-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikmen, S., and R. G. Mateescu. . 2019. PSXI-37 Differences in thermoregulation ability and genetic parameters of skin traits in Angus, Brahman and their crossbreds. J. Anim. Sci. 97 Suppl. 3:386. doi: 10.1093/jas/skz258.768 [DOI] [Google Scholar]
- Dong, J., J. Li, L. Cui, Y. Wang, J. Lin, Y. Qu, and H. Wang. . 2018. Cortisol modulates inflammatory responses in LPS-stimulated RAW264.7 cells via the NF-κB and MAPK pathways. BMC Vet. Res. 14:30. doi: 10.1186/s12917-018-1360-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart, N. L., J. M. Storer, M. Caldwell, A. M. Saxton, and P. D. Krawczel. . 2017. Behavioral and physiologic changes in Holstein steers experimentally infected with Mannheimia haemolytica. Am. J. Vet. Res. 78:1056–1064. doi: 10.2460/ajvr.78.9.1056 [DOI] [PubMed] [Google Scholar]
- El-Deeb, W., I. Elsohaby, M. Fayez, H. V. Mkrtchyan, D. El-Etriby, and M. ElGioushy. . 2020. Use of procalcitonin, neopterin, haptoglobin, serum amyloid A and proinflammatory cytokines in diagnosis and prognosis of bovine respiratory disease in feedlot calves under field conditions. Acta Trop. 204:105336. doi: 10.1016/j.actatropica.2020.105336 [DOI] [PubMed] [Google Scholar]
- Elgersma, G. G., G. de Jong, R. van der Linde, and H. A. Mulder. . 2018. Fluctuations in milk yield are heritable and can be used as a resilience indicator to breed healthy cows. J. Dairy Sci. 101:1240–1250. doi: 10.3168/jds.2017-13270 [DOI] [PubMed] [Google Scholar]
- Forbes, A. B., C. Ramage, J. Sales, D. Baggott, and W. Donachie. . 2011. Determination of the duration of antibacterial efficacy following administration of gamithromycin using a bovine Mannheimia haemolytica challenge model. Antimicrob. Agents Chemother. 55:831–835. doi: 10.1128/AAC.00552-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs, A. L., S. M. Schiller, W. J. Keegan, M. C. Ammons, B. Eilers, B. Tripet, and V. Copie. . 2019. Quantitative 1H NMR metabolomics reveal distinct metabolic adaptations in human macrophages following differential activation. Metabolites. 9:248. doi: 10.3390/metabo9110248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijzel, S. M. W., I. A. van de Leemput, M. Scheffer, M. Roppolo, M. G. M. Olde Rikkert, and R. J. F. Melis. . 2017. Dynamical resilience indicators in time series of self-rated health correspond to frailty levels in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 72:991–996. doi: 10.1093/gerona/glx065 [DOI] [PubMed] [Google Scholar]
- Hailemariam, D., R. Mandal, F. Saleem, S. M. Dunn, D. S. Wishart, and B. N. Ametaj. . 2014. Identification of predictive biomarkers of disease state in transition dairy cows. J. Dairy Sci. 97:2680–2693. doi: 10.3168/jds.2013-6803 [DOI] [PubMed] [Google Scholar]
- Hanedan, B., A. Kirbas, E. Dorman, O. Timurkan Mehmet, M. Kandemir, and O. Alkan. . 2015. Cardiac troponin-i concentration in weaned calves with bovine respiratory disease. Acta Vet. 65:454–462. doi: 10.1515/acve-2015-0038 [DOI] [Google Scholar]
- Hanzlicek, G. A., B. J. White, D. Mosier, D. G. Renter, and D. E. Anderson. . 2010. Serial evaluation of physiologic, pathologic, and behavioral changes related to disease progression of experimentally induced Mannheimia haemolytica pneumonia in post weaned calves. Am. J. Vet. Res. 71:359–369. doi: 10.2460/ajvr.71.3.359 [DOI] [PubMed] [Google Scholar]
- Holland, B. P., D. L. Step, L. O. Burciaga-Robles, R. W. Fulton, A. W. Confer, T. K. Rose, L. E. Laidig, C. J. Richards, and C. R. Krehbiel. . 2011. Effectiveness of sorting calves with high risk of developing bovine respiratory disease on the basis of serum haptoglobin concentration at the time of arrival at a feedlot. Am. J. Vet. Res. 72:1349–1360. doi: 10.2460/ajvr.72.10.1349 [DOI] [PubMed] [Google Scholar]
- Humblet, M. F., J. Coghe, P. Lekeux, and J. M. Godeau. . 2004. Acute phase proteins assessment for an early selection of treatments in growing calves suffering from bronchopneumonia under field conditions. Res. Vet. Sci. 77:41–47. doi: 10.1016/j.rvsc.2004.02.009 [DOI] [PubMed] [Google Scholar]
- Jackson, K. S., G. E. Carstens, L. O. Tedeschi, and W. E. Pinchak. . 2016. Changes in feeding behavior patterns and dry matter intake before clinical symptoms associated with bovine respiratory disease in growing bulls. J. Anim. Sci. 94:1644–1652. doi: 10.2527/jas.2015-9993 [DOI] [PubMed] [Google Scholar]
- Jones, M. L., and R. W. Allison. . 2007. Evaluation of the ruminant complete blood cell count. Vet. Clin. North Am. Food Anim. Pract. 23:377–402, v. doi: 10.1016/j.cvfa.2007.07.002 [DOI] [PubMed] [Google Scholar]
- Jose, S., and R. Madan. . 2016. Neutrophil-mediated inflammation in the pathogenesis of Clostridium difficile infections. Anaerobe 41:85–90. doi: 10.1016/j.anaerobe.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser, W. C., G. E. Carstens, K. S. Jackson, W. E. Pinchak, A. Banerjee, and Y. Fu. . 2019. Evaluation of statistical process control procedures to monitor feeding behavior patterns and detect onset of bovine respiratory disease in growing bulls. J. Anim. Sci. 97:1158–1170. doi: 10.1093/jas/sky486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser, W. C., G. E. Carstens, I. L. Parsons, T. H. Welsh, K. E. Washburn, S. D. Lawhon, W. E. Pinchak, J. T. Richeson, E. Chevaux, and A. L. Skidmore. . 2019. Effects of Mannheimia haemolytica challenge with or without supplementation of Saccharomyces cerevisiae boulardii strain CNCM I-1079 on immune upregulation and behavior in beef steers. J. Anim. Sci. 97:596–609. doi: 10.1093/jas/sky447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser, W. C., G. E. Carstens, K. E. Washburn, T. H. Welsh, S. D. Lawhon, S. M. Reddy, W. E. Pinchak, E. Chevaux, and A. L. Skidmore. . 2019. Effects of combined viral-bacterial challenge with or without supplementation of Saccharomyces cerevisiae boulardii strain CNCM I-1079 on immune upregulation and DMI in beef heifers. J. Anim. Sci. 97:1171–1184. doi: 10.1093/jas/sky483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König, S., and K. May. . 2019. Invited review: phenotyping strategies and quantitative-genetic background of resistance, tolerance and resilience associated traits in dairy cattle. Animal. 13:897–908. doi: 10.1017/S1751731118003208 [DOI] [PubMed] [Google Scholar]
- Lawrence, T., and D. W. Gilroy. . 2007. Chronic inflammation: a failure of resolution? Int. J. Exp. Pathol. 88:85–94. doi: 10.1111/j.1365-2613.2006.00507.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenhouwers, J. I., E. F. Knol, P. N. de Groot, H. Vos, and T. van der Lende. . 2002. Fetal development in the pig in relation to genetic merit for piglet survival. J. Anim. Sci. 80:1759–1770. doi: 10.2527/2002.8071759x [DOI] [PubMed] [Google Scholar]
- Malech, H. L., F. R. Deleo, and M. T. Quinn. . 2014. The role of neutrophils in the immune system: an overview. Methods Mol. Biol. 1124:3–10. doi: 10.1007/978-1-62703-845-4_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy, D. O 2000. Cytokines and the anorexia of infection: potential mechanisms and treatments. Biol. Res. Nurs. 1:287–298. doi: 10.1177/109980040000100405 [DOI] [PubMed] [Google Scholar]
- Michel, V., A. Peinnequin, A. Alonso, A. Buguet, R. Cespuglio, and F. Canini. . 2007. Decreased heat tolerance is associated with hypothalamo-pituitary-adrenocortical axis impairment. Neuroscience 147:522–531. doi: 10.1016/j.neuroscience.2007.04.035 [DOI] [PubMed] [Google Scholar]
- Mormède, P., A. Foury, E. Terenina, and P. W. Knap. . 2011. Breeding for robustness: the role of cortisol. Animal 5:651–657. doi: 10.1017/S1751731110002168 [DOI] [PubMed] [Google Scholar]
- Mosier, D. A., K. R. Simons, and J. G. Vestweber. . 1995. Passive protection of calves with pasteurella haemolytica antiserum. Am. J. Vet. Res. 56:1317–1321. [PubMed] [Google Scholar]
- Olson, C. A., G. E. Carstens, A. D. Herring, D. S. Hale, W. C. Kayser, and R. K. Miller. . 2019. Effects of temperament at feedlot arrival and breed type on growth efficiency, feeding behavior, and carcass value in finishing heifers. J. Anim. Sci. 97:1828–1839. doi: 10.1093/jas/skz029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkanlar, Y., M. S. Aktas, O. Kaynar, S. Ozkanlar, E. Kirecci, and L. Yildiz. . 2012. Bovine respiratory disease in naturally infected calves: clinical signs, blood gases and cytokine response. Revue Méd. Vét. 163:123–130. [Google Scholar]
- Prina, E., M. Ferrer, O. T. Ranzani, E. Polverino, C. Cillóniz, E. Moreno, J. Mensa, B. Montull, R. Menéndez, R. Cosentini, . et al. 2013. Thrombocytosis is a marker of poor outcome in community-acquired pneumonia. Chest 143:767–775. doi: 10.1378/chest.12-1235 [DOI] [PubMed] [Google Scholar]
- Putz, A. M., J. C. S. Harding, M. K. Dyck, F. Fortin, G. S. Plastow, and J. C. M. Dekkers; PigGen Canada 2018. Novel resilience phenotypes using feed intake data from a natural disease challenge model in wean-to-finish pigs. Front. Genet. 9:660. doi: 10.3389/fgene.2018.00660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quimby, W.F., B. F. Sowell, J. G. P. Bowman, M. E. Branine, M. E. Hubbert, and H. W. Sherwood. . 2001. Application of feeding behavior to predict morbidity of newly received calves in a commercial feedlot. Can. J. Anim. Sci. 81:315–320. doi: 10.4141/A00-098. [DOI] [Google Scholar]
- Ramm Sander, P., M. Peer, M. Grandl, U. Bogdahn, G. Schmitz, and H. R. Kalbitzer. . 2013. NMR spectroscopy of macrophages loaded with native, oxidized or enzymatically degraded lipoproteins. PLoS One 8:e56360. doi: 10.1371/journal.pone.0056360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeson, J. T., J. A. Carroll, N. C. Burdick Sanchez, N. D. May, H. D. Hughes, S. L. Roberts, P. R. Broadway, K. P. Sharon, and M. A. Ballou. . 2016. Dexamethasone treatment differentially alters viral shedding and the antibody and acute phase protein response after multivalent respiratory vaccination in beef steers. J. Anim. Sci. 94:3501–3509. doi: 10.2527/jas.2016-0572 [DOI] [PubMed] [Google Scholar]
- Smith, P. S., G. E. Carstens, C. A. Runyan, J. F. Ridpath, J. E. Sawyer, and A. D. Herring. . 2017. Effects of vaccine treatment and temperament classification on intake and feeding behavior responses to bovine viral diarrhea virus challenge in beef steers. J. Anim. Sci. 95:42. doi: 10.2527/asasann.2017.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurer, M. E., D. E. Anderson, B. J. White, M. D. Miesner, D. A. Mosier, J. F. Coetzee, J. Lakritz, and D. E. Amrine. . 2013. Effect of Mannheimia haemolytica pneumonia on behavior and physiologic responses of calves during high ambient environmental temperatures. J. Anim. Sci. 91:3917–3929. doi: 10.2527/jas.2012-5823 [DOI] [PubMed] [Google Scholar]
- Timsit, E., N. Dendukuri, I. Schiller, and S. Buczinski. . 2016. Diagnostic accuracy of clinical illness for bovine respiratory disease (BRD) diagnosis in beef cattle placed in feedlots: a systematic literature review and hierarchical Bayesian latent-class meta-analysis. Prev. Vet. Med. 135:67–73. doi: 10.1016/j.prevetmed.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Weljie, A. M., J. Newton, P. Mercier, E. Carlson, and C. M. Slupsky. . 2006. Targeted profiling: quantitative analysis of 1 H NMR metabolomics data. Anal. Chem. 78:4430–4442. doi: 10.1021/ac060209g [DOI] [PubMed] [Google Scholar]
- Whiteley, L. O., S. K. Maheswaran, D. J. Weiss, T. R. Ames, and M. S. Kannan. . 1992. Pasteurella haemolytica A1 and bovine respiratory disease: pathogenesis. J. Vet. Intern. Med. 6:11–22. doi: 10.1111/j.1939-1676.1992.tb00980.x [DOI] [PubMed] [Google Scholar]
- Wolfger, B., K. S. Schwartzkopf-Genswein, H. W. Barkema, E. A. Pajor, M. Levy, and K. Orsel. . 2015. Feeding behavior as an early predictor of bovine respiratory disease in North American feedlot systems. J. Anim. Sci. 93:377–385. doi: 10.2527/jas.2013-8030 [DOI] [PubMed] [Google Scholar]
- Young, C. R., T. E. Wittum, L. H. Stanker, L. J. Perino, D. D. Griffin, and E. T. Littledike. . 1996. Serum haptoglobin concentrations in a population of feedlot cattle. Am. J. Vet. Res. 57:138–141. [PubMed] [Google Scholar]