Abstract
Environmental occurrence and biomonitoring data for per- and polyfluoroalkyl substances (PFAS) demonstrate that humans are exposed to mixtures of PFAS. This article presents a new and systematic analysis of available PFAS toxicity study data using a tiered mixtures risk assessment framework consistent with United States and international mixtures guidance. The lines of evidence presented herein include a critique of whole mixture toxicity studies and analysis of dose-response models based on data from subchronic oral toxicity studies in rats. Based on available data to-date, concentration addition and relative potency factor methods are found to be inappropriate due to differences among sensitive effects and target organ potencies and noncongruent dose-response curves for the same effect endpoints from studies using the same species and protocols. Perfluorooctanoic acid and perfluorooctane sulfonic acid lack a single mode of action or molecular initiating event and our evaluation herein shows they also have noncongruent dose-response curves. Dose-response curves for long-chain perfluoroalkyl sulfonic acids (PFSAs) also significantly differ in shapes of the curves from short-chain PFSAs and perfluoroalkyl carboxylic acids evaluated, and additional differences are apparent when curves are evaluated based on internal or administered dose. Following well-established guidance, the hazard index method applied to perfluoroalkyl carboxylic acids and PFSAs grouped separately is the most appropriate approach for conducting a screening level risk assessment for nonpolymeric PFAS mixtures, given the current state-of-the science. A clear presentation of assumptions, uncertainties, and data gaps is needed before dose-additivity methods, including hazard index , are used to support risk management decisions. Adverse outcome pathway(s) and mode(s) of action information for perfluorooctanoic acid and perfluorooctane sulfonic acid and for other nonpolymer PFAS are key data gaps precluding more robust mixtures methods. These findings can guide the prioritization of future studies on single chemical and whole mixture toxicity studies.
Keywords: PFAS, per- and polyfluoroalkyl substances, mixtures, human health, risk assessment
The conventional approach to human health risk assessments of chemicals in the environment involves one-at-a-time evaluations of chemicals. For cumulative risk assessments involving coexposure to chemical mixtures, often simplifying assumptions are made regarding dose additivity, response additivity, and interactions (eg, synergism or antagonism). In rare cases, comprehensive evaluations of multiple lines of evidence support quantitative estimates of cumulative risks for broad chemical classes such as total petroleum hydrocarbons, polychlorinated biphenyls, organophosphates, and dioxin-like compounds. A variety of mixtures risk assessment methods and decision frameworks have been developed (reviewed in European Food Safety Authority Scientific Committee et al., 2019; Rotter et al., 2018). As discussed by Teuschler (2007), several key questions should be addressed prior to using mixtures risk assessment methods, including: (1) When is it appropriate to generalize and assume dose or response additivity?; (2) What information is needed to determine that 2 or more chemical components of the mixture share a common mode of action (MoA) or have similarly shaped dose-response curves?; (3) What evidence is needed to estimate the toxicity of the mixture if whole mixture toxicity study data are lacking?; and (4) How should the fraction of unidentified chemicals that may be present in a mixture be addressed? Many of the common chemical mixtures risk assessment methods involve inferences about responses at relatively low doses, using dose-response information from studies with single components often administered at doses higher than environmentally relevant levels. More complete information on low dose responses is needed to refine quantitative approaches and more fully utilize data from studies with component chemicals. Indeed, data either on the exact mixture of concern, or on a “sufficiently similar” whole mixture are frequently critical data gaps (USEPA, 2000). With improved analytical methods and increasing number of chemicals used in commercial application, methods are needed to address the fraction of a mixture that is composed of chemicals lacking toxicity data, or the fraction of the mixture composed of yet unidentified chemicals that may partly contribute to an observed toxicity. Such is the case for nonpolymeric per- and polyfluoroalkyl substances (PFAS).
PFAS are a large and diverse group of chemicals whose exact definition is not agreed upon by experts worldwide. Generally speaking, PFAS can be identified by the presence of at least one fully fluorinated carbon-carbon bond (Buck et al., 2011; Wang et al., 2017). PFAS can be subdivided into 2 broad classes: polymers and nonpolymers. Nonpolymeric PFAS are either fully fluorinated (perfluorinated) or partially fluorinated (polyfluorinated). Releases of PFAS from specific manufacturing locations or from the use of aqueous film-forming foam (AFFF) has led to the presence of a large array of nonpolymeric PFAS congeners in the environment (Anderson et al., 2016; Backe et al., 2013; Barzen-Hanson et al., 2017; McCord and Strynar, 2019). Drinking water systems in the United States that are impacted by PFAS usually have various nonpolymeric PFAS present (Guelfo and Adamson, 2018) and serum analysis of the general population consistently detects several of the persistent perfluoroalkyl acids (PFAAs; CDC, 2019; Jain, 2018). Thus, there is the potential for humans and ecological receptors to be exposed to an uncertain and complex mixture of nonpolymeric PFAS.
Exposure to such mixtures poses technical challenges for assessing the potential for health effects, and regulatory and public health agencies worldwide have disparate strategies for addressing this risk. To date, some regulatory environmental guidance values apply to individual PFAAs, whereas others are based on the sum of concentrations (ie, concentration-addition) of multiple PFAAs in drinking water or groundwater (see https://pfas-1.itrcweb.org/fact-sheets/ (last accessed August 06, 2020) for an up-to-date list of regulatory values). The U.S. Environmental Protection Agency’s (USEPA) current lifetime drinking water health advisory for perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) of 70 parts per trillion (ppt) for the sum of their concentrations is perhaps the most relevant example of concentration-addition. The USEPA based this concentration-additivity approach on their determination that the 2 chemicals not only share similar toxic endpoints (developmental effects), but also have equal oral reference doses, when rounded to one significant figure (USEPA, 2016a,b). State agencies in Connecticut, Massachusetts, and Vermont followed suit by applying a similar assumption of concentration additivity but for a broader suite of compounds, including PFOA, PFOS, perfluorononanoic acid (PFNA), perfluorohexanesulfonic acid (PFHxS), and perfluoroheptanoic acid (PFHpA); however, the data supporting the assumption of additivity for this range of compounds were not provided by the State agencies.
In 2017, the Australian Environmental Health Standing Committee and the Food Standards Australia and New Zealand (FSANZ) took the position that although there was insufficient information to establish a guidance level for PFHxS, it was reasonable to use the same value for PFHxS as PFOS because the structures of the 2 compounds are similar, and there was some evidence of similar potency of PFHxS and PFOS in activating peroxisome proliferator-activated receptor alpha (PPARα; FSANZ, 2017). However, they did not find sufficient similarity between perfluoroalkyl sulfonic acids (PFSAs) and perfluoroalkyl carboxylic acids (PFCAs) to support an assumption of concentration additivity across these 2 classes of PFAAs. Therefore, in Australia and New Zealand, PFOS and PFHxS concentrations are summed, whereas PFOA and PFOS concentrations are not.
The U.S. Agency for Toxic Substances and Disease Registry (ATSDR) applies yet a different approach to address human health risks associated with exposure to mixtures of PFAS. As a matter of policy, ATSDR health guideline values (eg, Environmental Media Evaluation Guides) are applicable to a single substance (ATSDR, 2005). ATSDR’s revised draft toxicological profile for PFAAs concluded that “…although there is some evidence of similar health outcomes for some compounds, there is evidence of qualitative and mechanistic differences” that preclude extrapolating findings across PFAS chemicals (ATSDR, 2018b). ATSDR found the available data on interactions among PFAS chemicals, and between PFAS and other chemicals, to be insufficient to quantitatively evaluate mixtures within a toxicity evaluation. Recent site-specific Health Consultations by the Agency show that they address potential risk associated with exposure to a mixture of PFAAs by using the dose-additivity hazard index (HI) approach (described below) of summing the ratio of each chemical’s exposure concentration compared with its health-based criteria (ATSDR, 2020).
To date, Health Canada and the National Institute for Public Health and the Environment in the Netherlands (RIVM) appear to be the only regulatory agencies to have explicitly applied some aspect of a mixtures risk assessment framework to PFAS. Health Canada modeled its framework on mixtures guidance from the World Health Organization/International Programme on Chemical Safety (WHO/IPCS; Meek, 2013; Meek et al., 2011; WHO, 2017) and determined that a dose-additive HI approach for PFOA and PFOS in drinking water is appropriate for the protection of human health. This conclusion was based on the likelihood of coexposure and a determination of toxicological similarity (eg, similar MoAs and toxic effects) for PFOA and PFOS. RIVM, however, derived relative potency factors (RPFs) for 19 PFAAs, including PFOA and PFOS, and selected PFOA as the index chemical to extrapolate to other PFAAs (Zeilmaker et al., 2018). RIVM acknowledges numerous simplifying assumptions and limitations, including: (1) focusing on liver hypertrophy as the basis for comparing each PFAA, even though this is not the most sensitive effect across all of the chemicals studied; (2) extrapolating RPFs from chemicals with a similar carbon chain length for 7 PFAAs with data gaps; (3) assuming that the shapes of the dose-response curves are congruent, such that a constant ratio (calculated from benchmark doses [BMDs]) applies across the entire dose-response curve for each chemical; and (4) additivity cannot be fully verified until additional whole mixture toxicity studies are conducted.
In summary, regulatory approaches to addressing risk associated with exposure to a mixture of PFAS are inconsistent. Scientific-based approaches are necessary, including use of established mixtures risk assessment methods and comprehensive evaluations of available data on individual PFAS and PFAS whole mixtures studies.
MATERIALS AND METHODS
Following current USEPA mixtures guidance (USEPA, 2000), we examine the existing nonpolymeric PFAS database, including dose-response information, and apply established mixtures risk assessment methods to these data to determine what, if any, mixtures effects may occur, and what mixtures risk assessment approach is appropriate given the available data. Dose-response analysis is incorporated as an additional line of evidence to support grouping of component chemicals as well as to assess if relative potency varies (in terms of proportionality in the response mean and variance) across an environmentally relevant range of exposures.
Mixtures Risk Assessment Framework
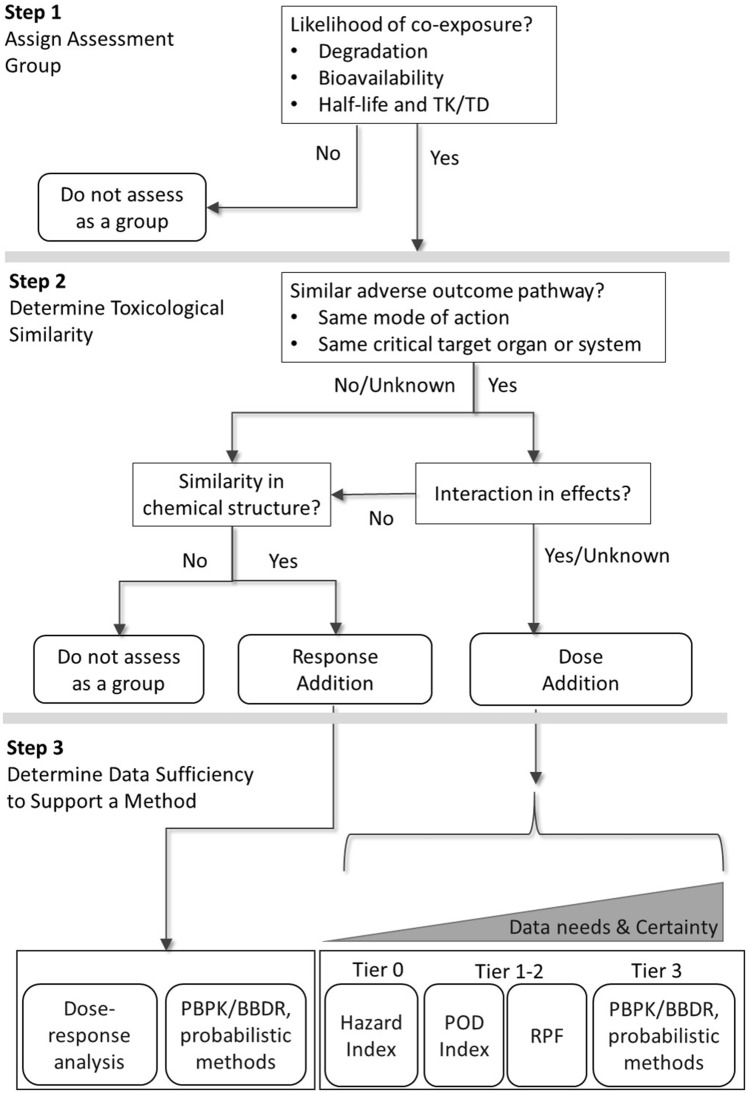
Figure 1 illustrates a 3-step decision framework that we applied to evaluate mixtures of nonpolymeric PFAS. This framework was adapted from similar component-based mixtures frameworks proposed by USEPA (Teuschler, 2007; USEPA, 2000, 2007), WHO/IPCS (Meek, 2013; Meek et al., 2011), EFSA (European Food Safety Authority Scientific Committee et al., 2019), and ATSDR (ATSDR, 2018a). Rotter et al. (2018) provides a comprehensive review and comparison of these and many other frameworks applied and adapted by regulatory authorities for use in human health risk assessment.
Figure 1.
Decision flow chart illustrating a component-based mixtures risk assessment framework for per- and polyfluoroalkyl substances. Refer to Table 1 for a summary of key elements of the tiered approach. BBDR, biologically based dose-response model; PBPK, physiologically-based pharmacokinetic model; POD, point of departure; RPF, relative potency factor; TK/TD, toxicokinetic/toxicodynamic.
Key elements of the framework are illustrated in Figure 1 and briefly summarized below.
Step 1 informs the initial list of chemicals that are assigned to an assessment group based on the likelihood that coexposures may occur. The chemicals can be directly measured in potential exposure media and/or estimated based on models that account for environmental degradation, potential for bioavailability, and frequency and duration of exposure relative to pharmacokinetic (PK) properties (eg, serum elimination half-life). Subsequent steps serve to refine the groupings based on additional lines of evidence.
Step 2 involves an assessment of the toxicological similarity based on MoA, most sensitive effect endpoints, likelihood of interactions, and chemical structure. In general, dose addition should apply when component chemicals share a similar adverse outcome pathway (AOP), meaning there is specific evidence of a common MoA, or more broad evidence of impairment of the same target organ or biological systems. See the discussion in Adams et al. (2017) for a proposed standardized target organ and biological systems framework. Response addition applies when components act on different systems or produce effects that do not influence each other (ie, “no-interaction” condition such that the response to the first component is the same whether or not a second component chemical is present; USEPA, 2000). Dose addition and response addition then represent default approaches for toxicologically similar and toxicologically independent chemicals, respectively (USEPA, 2000).
In addition to grouping chemicals based on toxicological similarity, other considerations proposed by mixtures frameworks include physicochemical similarities (European Food Safety Authority Scientific Committee et al., 2019). This evaluation may result in different subgroups of components such that each subgroup is evaluated as a separate mixture. Each evaluation of the framework requires professional judgment when available information is inconsistent or does not clearly point to a single decision path. Under these conditions, USEPA (2000) recommends that if either a dose- or response-addition method is applied (as outlined in Step 3), caveats regarding assumptions and uncertainties should be clearly communicated. Therefore, for mixtures of diverse compounds, such as can be found with nonpolymeric PFAS compounds, it is important that Step 2 includes an evaluation of the similarity of chemical structures when considering the use of response addition. This evaluation may result in different subgroups of components such that each subgroup is evaluated as a separate mixture. It should be noted that when there is a common apical endpoint, multiple mixtures may be included into one integrated assessment using probabilistic risk estimates or in a qualitative evaluation.
Step 3 involves the selection of an appropriate mixtures method for chemicals that are grouped together. The concepts that distinguish between dose and response additivity help to guide the computational approaches that are applicable. The original USEPA guidelines for mixtures risk assessment released in 1986 referred to dose addition for nongenotoxic toxicants acting by a similar MoA or affecting common organs, whereas response addition was applied to carcinogenic risk, a risk metric that conveys a probability or likelihood of increased incidence of cancer in a population (USEPA, 1986). Dose addition can be thought of as a condition when components of a mixture act as dilutions of one another (European Food Safety Authority Scientific Committee et al., 2019; Hertzberg et al., 2013; USEPA, 2000). Therefore, a distinguishing factor among methods is how the relative potencies inform the weights applied to each component dose. The term response addition should be interpreted with care because the component responses themselves are not summed, but rather the probabilities of no response are multiplied and subtracted from 1 in order to represent the concept of independent joint action (European Food Safety Authority Scientific Committee et al., 2019; Meek et al., 2011; USEPA, 2000). Concentration-additivity approach is a special case of mixtures additivity methods that requires an assumption of a common MoA or toxic effect endpoint and requires that the component chemicals be equipotent across a broad range of environmentally relevant doses. This approach is different from dose-addition methods that apply component-specific weights to the concentrations and is generally not well-supported by available data.
Response addition
Two mixtures methods are possible when response addition is supported and dose-response data on components are available. Because response addition involves the product of the probabilities of no response, the most basic approach is to select representative dose-response models for each component. For each dose-response model, the concentration of the ith component can be converted to a probability of no-response, 1−pi. If a physiologically based PK (PBPK) model or biologically based dose response (BBDR) is available, then measures of internal dose can be converted to estimates of human equivalent administered dose, from which 1−pi can then be estimated.
Dose addition
USEPA (Moody and Field, 2000; USEPA, 2000) and WHO/IPCS (Meek, 2013; Meek et al., 2011) present a tiered approach to selecting an appropriate method given the available toxicity data on individual components (or suitable proxy chemicals) and the level of certainty in key assumptions, summarized in Table 1. The intent of the framework is to promote a sequential and transparent evaluation of multiple lines of evidence, such that each consecutive tier applies a refinement, supported by the data, and a progression from a conservative (health protective) to a more realistic (predictive) quantitative analysis of risk. The candidate methods associated with dose addition are organized in a tiered manner, with increasing tiers generally requiring additional data, but affording greater certainty in the toxicity assessment (Table 1).
Table 1.
Key Elements of Various Tiered Methods for Mixtures Risk Assessment
| Definition | Exposure Assessment | Hazard Assessment | Risk Characterization | Example Mixture Methods | |
|---|---|---|---|---|---|
| Tier 0 |
|
|
|
|
|
| Tier 1-2 |
|
|
|
|
|
| Tier 3 |
|
|
|
|
|
Abbreviations: BBDR, biologically based dose-response model; BMD, benchmark dose; MoA, mode of action; NOAEL, no-observed-adverse-effect level; PBPK, physiologically based pharmacokinetic model; POD, point of departure; RPF, relative potency factor.
Perhaps the most common, and often default, method involves the summation of ratios of doses to chemical-specific reference values—the HI method. The HI approach requires chemical component-specific toxicity values, which limits its application. The HI method scales the potency to each chemical’s toxicity value, and usually has been applied to noncancer endpoints:
where, and, HI, hazard index (unitless); HQi, hazard quotient for the ith component chemical (unitless); Dosei, average daily dose for the ith component chemical (mg/kg/day); RfDi, oral reference dose for the ith component chemical (mg/kg/day); can be any relevant toxicity reference value, such as USEPA reference concentration or ATSDR minimal risk level
The HI method for mixtures is most commonly applied under an assumption of dose additivity among chemicals using measured or estimated concentrations. Mixtures frameworks differ on how to address components with different or multiple effect endpoints and target organ systems. Early guidance from the USEPA suggests that grouping component chemicals based on similar target organs is required for dose addition approaches (USEPA, 2000) and the Agency’s guidance for conducting risk assessments at national Superfund sites calls for only considering the possible additivity for chemicals with the same critical target organ (USEPA, 1989). However, more recent guidance from the USEPA is more consistent with other U.S. agencies and international authorities. According to USEPA (USEPA, 2007), EFSA (European Food Safety Authority Scientific Committee et al., 2019), and ATSDR (ATSDR, 2018a), for example, component chemicals may be grouped together in a “Tier 0” (see Table 1) mixtures assessment even if the most sensitive effect target organs are dissimilar, as a preliminary and initial screen. If there is a potential for risk based on the preliminary screening assessment (ie, if HI > 1), refinement should then be made using the “Tier 1 or 2” approaches, including evaluating target organ-specific HIs or using the Target Organ Toxicity Dose (TTD) HI approach. The TTD accommodates the assessment of mixtures whose components may produce toxic effects in common target organs of the same species dependent on exposure level (ATSDR, 2018a). Target organ-specific toxicity values (TTDs) are used in dose addition methods, if available, in place of the most sensitive effect toxicity value (eg, RfD or minimal risk level) if the critical target organs or biological systems differ.
Target organ-specific TTD-based HIs are calculated as follows:
where, HIrenal, hazard index for endpoints associated with adverse effects on kidney function; HIhepatic, hazard index for endpoints associated with adverse effects on liver function; Dosei, average daily dose for the ith component chemical (mg/kg/day); TTDi, renal, target organ-specific toxicity value for renal effects for the ith component chemical (mg/kg/day); TTDi, hepatic, target organ-specific toxicity value for hepatic effects for the ith component chemical (mg/kg/day). Note: the doses and TTDs should all be for the same species.
USEPA and others acknowledge that the application of HI as a default method without consideration of similarity in target organ likely overestimates the risk (USEPA, 2007). The U.S. National Academy of Sciences supports combining chemicals with different initiating events, MoAs, or target organs when there is a common adverse outcome (eg, phthalate exposure may lead to androgen insensitivity syndrome via different MoAs and target organs; National Research Council, 2008). Consideration of the AOP is an alternative means for grouping chemicals and recent studies have suggested a joint impact of chemicals with different MoAs acting on the same AOP (Conley et al., 2018; Kortenkamp, 2020; Lichtenstein et al., 2020). The calculation of a HI based on HQs derived from toxicity values for different target organs or systems is currently a matter of professional judgment. Importantly, HI is considered a “Tier 0” screening method because it generally does not involve a closer examination of toxic similarity or dose-response relationships among component chemicals. Tier 1 and tier 2 evaluations incorporate additional component-specific information.
The summation of ratios of doses to chemical-specific points of departure (PODi) is referred to as the POD index (PODI) method (European Food Safety Authority Scientific Committee et al., 2019). The PODI is given by:
where, PODI, point of departure index (unitless); Dosei, average daily dose for the ith component chemical (mg/kg/day); PODi, point of departure dose for the ith component chemical (mg/kg/day).
The PODI is included as a Tier 1 method given that additional dose-response analysis may be required to generate comparable POD metrics (eg, BMDs that correspond to the same benchmark response [BMR] level). As a metric of risk, the PODI differs from HI in that chemical-specific uncertainty factors, which are built into the RfD or TTDs, may not be accounted for.
The relative potency scaled to an index chemical “A” is referred to as a RPF and is given by:
where, RPFi, relative potency factor for ith component chemical; PODi, point of departure dose for ith component chemical; PODA, point of departure dose for the index chemical “A”.
The index chemical is typically the chemical in the group for which the most toxicity information is known, and for PFAAs would likely be PFOA or PFOS. The RPF approach typically requires a significant level of effort regarding evaluation of toxicological similarity (Hertzberg et al., 2013; USEPA, 2000). Furthermore, multiple RPF applications may be used to address different exposures (eg, routes) and different endpoints, resulting in possibly multiple potential chemical groupings depending on the risk assessment scenario and goals (USEPA, 2000). Once established, the RPF is then used to scale the concentrations of component chemicals to estimate an equivalent concentration of the index chemical, as if each component chemical is essentially a dilution of the index chemical (Finney, 1942). From here, the sum of the concentrations is compared with the toxicity reference value of the index chemical, either as a HQ or a margin of exposure (the inverse of the HQ; reviewed in more detail in Benford et al., 2010):
where, CA*, mixture’s equivalent concentration, ie, the concentration of index chemical after accounting for all components of the mixture, including the concentration of the index chemical (CA), which has RPF = 1; Ci, concentration of ith component chemical; RPFi, relative potency factor for ith component chemical; CA* may then applied in a standard dose-response assessment and compared with the RfD for the index chemical in order to derive a final HQ for the mixture:
In a Tier 1 evaluation, dose-response relationships are closely evaluated to examine and verify assumptions regarding relative potency and interactions across a relevant dose range for each component, which can guide the selection of an appropriate index chemical for a group. The supplementary Material illustrates how graphical tools recommended by USEPA (USEPA, 2000), such as isoboles, can be applied using dose-response data on PFAAs (Supplementary Figure 1). A Tier 2 assessment might involve calculating multiple sets of RPFs for a mixture if there are sufficient data to evaluate multiple sensitive effect endpoints. Multiple RPFs can also be used if there are different exposure route-specific potencies (USEPA, 2000). Given that the rank order of component chemicals in terms of potency can vary across endpoints as well as selected BMR levels (due to noncongruent dose-response curves), such an analysis can provide a more comprehensive risk characterization.
PBPK or BBDR models and probabilistic methods may also be applicable under the assumption that the mixture exhibits dose additivity. Instead of using these methods to refine estimates of response for each component (as discussed above for use in response additivity), these methods can be used to refine estimates of component-specific PODs, RfDs, and HQs discussed above as part of a “Tier 3” evaluation (Haddad, 2001; Sarigiannis and Gotti, 2008).
Finally, it should be noted that there are also mixtures risk assessment methods that combine dose addition and response addition into a hybrid “integrated addition” approach for multiple component mixtures (Altenburger et al., 2005; Flippin et al., 2009; Rider and LeBlanc, 2005; Rider et al., 2010; Teuschler et al., 2004; USEPA, 2007). This approach applies concepts of both similar and independent MoA in that when there is a common apical endpoint, the multiple similar groups of component chemicals can have separate dose-additive assessments that are then combined via response additional into overall probabilistic risk estimates.
PFAS Toxicology Literature Review and Data Sources
Relevant PFAS toxicological studies were located via searches of public databases including published peer-reviewed literature and online toxicity data curated by regulatory agencies. Key sources of relevant information included the U.S. National Library of Medicine (NLM) and National Institutes of Health (NIH) PubMed, the Registry of Toxic Effects of Chemical Substances, and the NLM Toxicology Data Network. Primary studies were reviewed, and secondary sources (review papers) on mixtures assessment frameworks were also considered. USEPA and NIH have also run selected PFAS through their Tox21 high-throughput assays; data are available via the USEPA Chemistry Dashboard (https://comptox.epa.gov/dashboard, last accessed August 06, 2020).
The U.S. National Toxicology Program (NTP) conducted rodent bioassays and kinetic studies of several PFAAs and, in 2018, released data tables for a suite of 28-day oral gavage studies in which male and female Harlan Sprague Dawley (SD) rats were dosed with PFOS, PFHxS, PFBS, perfluorodecanoic acid (PFDA), PFNA, PFOA, and PFHxA (NTP, 2018a,b). These studies were conducted under standardized conditions and, therefore, provide a useful foundation for comparing dose-response relationships attributable to different chemistries, with minimal confounding due to variability in study designs and testing laboratories.
The USEPA ToxCast Chemical Inventory List (EPAPFASINV) was reviewed to identify all PFAS that have been tested for bioactivity in ToxCast/Tox21 high-throughput assays (https://comptox.epa.gov/dashboard/chemical_lists/EPAPFASINVIVO, last accessed August 06, 2020). As of September 2019, ToxCast data were available for 21 unique CASRNs, including several PFSAs (eg, perfluorobutanesulfonic acid (PFBS), PFHxS, and PFOS), PFCAs (eg, PFHxA, PFHpA, PFOA, PFNA, PFDA, and PFUnDA), and fluorotelomers (eg, 8:2 and 6:2 fluorotelomer alcohol (FTOH)). We identified chemicals by CASRN, and sorted the findings by bioactivity outcome (ie, “Active” vs. “Inactive”) and intended target family.
Dose-Response Evaluation
BMD modeling was conducted using USEPA’s BMD software (BMDS version 2.7) in accordance with USEPA guidance (USEPA, 2012a). For dichotomous datasets (eg, liver hypertrophy), the BMR was set to 10% extra risk. For continuous datasets, the BMR was set to either 10% (eg, decreased body weight) or one control standard deviation (eg, decreased cholesterol and decreased relative kidney weight) when no sufficient biological basis for setting a BMR was available. Dichotomous datasets were modeled via the gamma, logistic, log-logistic, log-probit, probit, Weibull, and quantal-linear models, while continuous datasets were modeled using the exponential (models 2–5), Hill, linear, polynomial (models 2 and 3), and power models. Model fit was assessed based on an evaluation of multiple criteria, including the p-value for goodness-of-fit, the Akaike information criterion, scaled residuals at doses near the BMD, and visual inspection of the dose-response curves, consistent with USEPA guidance (USEPA, 2012a). Examples of model output are given in the Supplementary Material and referenced herein.
Data tables from the 2018 NTP 28-day oral gavage studies conducted with Harlan SD rats exposed to PFHxA, PFOA, PFNA, PFDA, PFBS, PFHxS, and PFOS reporting incidence of hepatocellular hypertrophy, serum cholesterol, relative kidney weight, and body weight for male rats were downloaded from the NTP website and were used for BMD modeling (NTP, 2018a,b). Data for PFBA were obtained from a 28-day oral gavage study conducted with male SD rats (Butenhoff et al., 2012). Data for perfluoroundecanoic acid (PFUnA) and perfluorododecanoic acid (PFDoA) were obtained from 42-day oral gavage studies conducted with SD rats (Kato et al., 2015; Takahashi et al., 2014). Data for 8:2 and 6:2 FTOH were obtained from 90-day oral gavage studies conducted with male SD rats (Ladics et al., 2008; Serex et al., 2014).
BMD modeling based on internal serum levels would be preferable for PFAAs because interpretations of dose-response are less likely to be confounded by differences in chemical- and species-specific kinetics (Vogs et al., 2019). However, serum levels have not been consistently reported, and remain a significant data gap in the available literature when comparing relative potencies of PFAAs. Therefore, most of the BMD modeling reported here was conducted with administered dose. The NTP studies demonstrate an approximately linear relationship between administered dose and internal serum level (24 h after the final dose) for PFNA, PFDA, and PFOS, slight supralinearity (increasing slope with increasing dose) for PFBS and PFHxA, and sublinearity (plateauing for serum levels) for PFHxS and PFOA at the higher administered doses (Supplementary Figure 2; NTP, 2018a,b). Nonlinearities may contribute uncertainty in inferences regarding the assessment of groupings of mixtures of PFAAs based on these NTP studies, as illustrated in Dose-Response for Hepatocellular Hypertrophy Section below using data on hepatocellular hypertrophy as an example.
RESULTS
Specific lines of evidence identified in the mixtures framework are summarized below, including studies on PKs, nuclear receptor binding activity, and target organ toxicity.
Review of Whole Mixtures Studies
There are currently less than a dozen published whole mixture toxicity studies with PFAS, which involve dosing mostly binary combinations (pairs) of PFAAs, largely PFOA and PFOS. The available studies used a variety of methods to evaluate potential interactions. Critique of each study’s methods is beyond the scope herein; we report only the author’s conclusions. Based on stated conclusions from the limited data available to date, it appears that PFOA and PFOS mixtures have complicated toxicological interactions and there is no consistent finding that supports a single assumption regarding mixture effects (Carr et al., 2013; Hoover et al., 2019; Hu and Hu, 2009; Hu et al., 2014; Ojo et al., 2020; Wei et al., 2009; Wolf et al., 2014). In vitro whole mixture studies, while more common, are inconclusive and demonstrate that the differences in study design (eg, choice of in vitro model, chemical mixture, and dose) can affect outcomes from exposure to mixtures of PFAAs. The few studies that have evaluated mixture effects in vivo demonstrate that findings for similar combinations of PFAAs vary depending on dose, test organism, and endpoint evaluated. Health Canada (2018a,b) cites results from a conference abstract in which CD-1 mice were administered binary mixtures of PFOA and PFOS. Health Canada determined this study supports dose additivity for some reproductive and developmental parameters, including maternal weight gain, pup body weight, and maternal and neonatal liver weight. However, for the neonatal mortality endpoint, an antagonistic interaction was observed—the mixture of PFOS and PFOA caused less mortality than exposure to component PFAAs alone. This is consistent with a recent study of nine nonpolymeric PFAS (5 PFCAs, 3 PFSAs, and 6:2 FTOH) on the behavioral effects of zebrafish larvae across multiple concentration ranges that shows that the mixture was less potent than certain PFAAs alone (Menger et al., 2020). Ding et al. (2013) evaluated binary mixtures of PFOA and PFOS on zebrafish embryonic development and demonstrated that the interactions changed from additive to synergistic to antagonistic depending on the molar ratios. Yang et al. (2019) assessed binary mixtures of PFOA and PFOS in aquatic invertebrates (Daphnia magna) and report synergistic effects on acute mortality and on some, but not all developmental endpoints. Finally, Flynn et al. (2019) dosed larval American bullfrogs with binary mixtures of PFOA and PFOS and report additive, synergistic, or no mixture effects, depending upon the endpoint evaluated and mixture dose. The whole mixture toxicity studies available to date remain inconsistent (ie, “Yes/Unknown” in Step 2 of Figure 1). Dose addition assumptions for PFAAs are not yet fully characterized by the available whole mixture toxicity data.
A current critical data gap is mixtures studies with nonpolymeric PFAS (not just PFOA and PFOS), using environmentally relevant (ie, part per trillion) doses and a focus on human relevant endpoints. Research currently funded by the Department of Defense Strategic Environmental Research and Development Program (SERDP) and Environmental Security Technology Certification Program (ESTCP) is investigating effects in amphibians and avian species from exposure to whole mixtures of nonpolymeric PFAS in AFFF formulations (see: https://www.serdp-estcp.org/Featured-Initiatives/Per-and-Polyfluoroalkyl-Substances-PFASs, last accessed August 06, 2020).
Elimination Kinetics by PFAA Carbon Chain Length
Biomonitoring studies report a wide range of elimination half-lives for PFAAs in humans, however, the pattern of differences between short- and long-chain PFAAs is consistent (Table 2). Human serum elimination rates for short-chain PFAAs (defined as ≤6 fully fluorinated carbons for PFCAs and 5 fully fluorinated carbons for PFSAs) are relatively rapid, ranging from a few days to several months for PFBA, PFHxA, PFHpA, and PFBS. This is in contrast with long-chain PFAAs such as PFHxS, PFOS, and PFOA that have reported serum elimination half-lives ranging 2.3–8.5 years. Similar estimates are not available for several long-chain PFCAs (ie, PFNA, PFDA, or PFUnA) in human serum, but estimates based on measurements of urine (which reflects renal clearance) also indicate a greater potential for biopersistence of long-chain PFCAs, with half-lives ranging from 1.7 to 12 years. PK studies for 8:2 and 6:2 FTOHs are typically unable to report a half-life given the concentration of test material quickly drops below detection limits due to rapid metabolism to terminal carboxylic acids or other compounds.
Table 2.
Estimates of Human Serum and Urine Elimination Half-Lives of PFAAs
| Half-Life Type | PFAA Group | PFAS | Chain Length | Elimination Half-Life | References |
|---|---|---|---|---|---|
| Serum | PFCA | PFBA | C4 | 2.9 daysa | Chang et al. (2008) |
| PFHxA | C6 | 32 daysb | Russell et al. (2015) | ||
| PFHpA | C7 | 70 daysb | Russell et al. (2015) | ||
| PFOA | C8 | 3.5 yearsb to 3.8 yearsa | Olsen et al. (2007) | ||
| 2.3 yearsc | Bartell et al. (2010) | ||||
| 2.7 yearsd | Li et al. (2018) | ||||
| PFSA | PFBS | C4 | 25.8 daysb | Olsen et al. (2009) | |
| PFHxS | C6 | 7.3 yearsb to 8.5 yearsa | Olsen et al. (2007) | ||
| 5.3 yearsd | Li et al. (2018) | ||||
| PFOS | C8 | 3.4 yearsd | Olsen et al. (2007) | ||
| 4.8 yearsb to 5.4 yearsa | Li et al. (2018) | ||||
| Urinary | PFCA | PFHpA | C7 | 1.2—1.5 yearsa; 0.82—1.0 yearsb | Zhang et al. (2013) |
| PFOA | C8 | 2.1—2.6 yearsa; 1.2—1.5 yearsb | |||
| PFNA | C9 | 2.5—4.3 yearsa; 1.7—3.2 yearsb | |||
| PFDA | C10 | 4.5—12 yearsa; 4.0—7.1 yearsb | |||
| PFUnA | C11 | 4.5—12 yearsa; 4.0—7.4 yearsb | |||
| PFSA | PFHxS | C6 | 7.7—35 yearsa; 7.1—25 yearsb | ||
| PFOS | C8 | 6.2—27 yearsa; 5.8—18 yearsb |
Arithmetic mean.
Geometric mean.
Median.
Assumed to be arithmetic mean, but not stated.
Although PK parameter estimates from animal and human data may provide one line of evidence to support broad grouping strategies based on chain-length (eg, group short- and long-chain PFAS separately in Step 1 of Figure 1), kinetics information alone may be of limited utility. Given that human biomonitoring data provide a snapshot in time, or preferably multiple measurements over a time period in the same cohort, a critical simplifying assumption is that exposures to co-occurring chemicals have not changed during the interval between measurements. However, if a primary source has been mitigated, or conversely, if a baseline source continues but is unaccounted for, estimates of kinetic parameters from human data can be highly uncertain. This uncertainty is particularly relevant for short-chain PFAAs that are likely to exhibit more rapid fluctuations in serum and urine following a change in exposure. Moreover, if exposures to short-chain PFAAs are on-going or of sufficient duration compared with long-chain PFAAs, the internal dose metrics that may lead to a toxic effect are most relevant and critical for risk assessment.
Relevance of Complexity in MoA to Mixtures Assessment
An important question in mixtures risk assessment is the extent to which knowledge regarding MoA or AOP is needed to support one or more mixtures methods. Meek (2013) states that in the context of mixtures assessment, chemicals can reasonably be assigned to the same assessment group if there is a biologically plausible (emphasis added) sequence of key events leading to an observed effect supported by robust experimental observations and mechanistic data, a more tractable decision criteria than requiring a full understanding of MoA at the molecular level. For example, the current target lipid model for dose additivity of mixtures of polyaromatic hydrocarbons (PAHs) is based on the idea that PAHs can cause narcosis (disruption of cellular function) through a shared site of action (target lipids) in aquatic organisms (Di Toro et al., 2000; French-McCay, 2002). Similar to the HI approach discussed previously, an assessment of a mixture of PAHs is evaluated by summing the toxic units—chemical-specific ratios of the molar concentration in water divided by the molar concentration that yields 50% mortality (LC50). For PFAAs, Peters and Gonzalez (2011) previously argued that there is compelling evidence that the mechanism for toxicity induced by PFAA exposure is complex, likely mediated by more than one nuclear receptor, and variable for different PFAA compounds.
Although, numerous in vivo gene expression and in vitro reporter assay studies have demonstrated that activation of PPARα may be involved in many of the toxicities associated with PFAAs (Rosen et al., 2008a,b; Wolf et al., 2008, 2012), PPARα does not appear to mediate all of the effects associated with PFAA exposure (see Supplementary Tables 1 and 2). Studies suggest that multiple nuclear receptors likely play a role in mediating the toxicities observed in a single target organ (see also Elcombe et al., 2010 and reviewed in Health Canada, 2018a,b). Rosen and colleagues (Rosen et al., 2008a,b) exposed wild-type and PPARα knockout mice to PFOA and the PPARα agonist WY-14,643 (WY) and measured transcriptional changes in the liver. Although gene expression changes were found to be primarily mediated by PPARα, PFOA also induced a subset of genes involved in xenobiotic metabolism through the nuclear receptor CAR (constitutive activated/androstane receptor). Similarly, NTP (2019) also found that a broad suite of PFAAs (ie, PFHxA, PFOA, PFNA, PFDA, PFBS, PFHxS, and PFOS) could induce the expression of PPARα- and CAR-related genes in the liver, indicating that hepatotoxic effects of PFAAs may be mediated through multiple nuclear receptors. Further demonstrating the complexity of the MoA, experiments conducted with wild-type and PPARα-knockout mice indicate that PFOA, PFNA, and PFOS induce developmental toxicity through different MoAs. For example, PFNA-induced developmental toxicity in wild-type mice is not observed in PPARα-knockout mice, indicating that PFNA may primarily induce developmental toxicity through PPARα (Wolf et al., 2010). Alternatively, PPARα appears to only mediate some of the developmental and reproductive effects associated with PFOA, as gestational exposure to PFOA induces full litter resorptions in wild-type and knockout mice, while other developmental effects are only observed in wild-type mice (Abbott et al., 2007). Finally, gestational exposure to PFOS induced neonatal lethality and delayed eye opening in both wild-type and PPARα-knockout mice, indicating that many developmental effects associated with PFOS are likely mediated through a MoA independent of PPARα (Abbott et al., 2009).
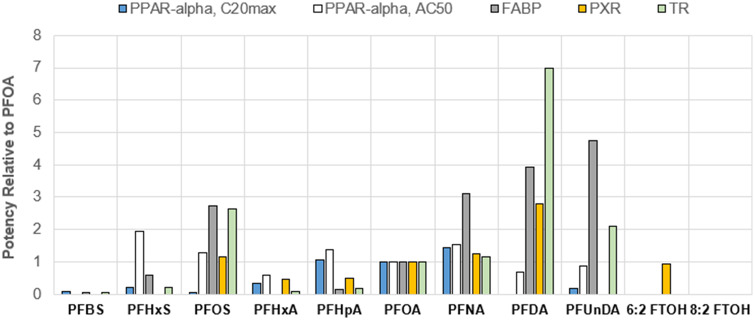
Although the aforementioned studies highlight the roles for PPARα and CAR, they do not capture the full suite of nuclear receptors that have been identified as potentially contributing to toxicity associated with PFAAs. In addition to PPARα and CAR, in vitro reporter gene studies have demonstrated that PFAAs can bind to and activate the thyroid receptor (Ren et al., 2015), the human pregnane X receptor (Zhang et al., 2017), and PPAR gamma (Zhang et al., 2014). USEPA’s high-throughput Tox21 in vitro dataset indicates short- and long-chain PFCAs, PFSAs, and FTOHs can interact with around 2 dozen different nuclear receptors (Supplementary Table 1). Intriguingly, there are clear chain-length dependent effects. Short-chain PFBS and PFHxA demonstrate relatively low activity, interacting with just 0–2 nuclear receptors, whereas long-chain PFAS can interact with as many as 6–16 different nuclear receptors. In addition to interacting with fewer nuclear receptors, short-chain PFCAs and PFSAs also tend to have weaker binding affinity toward many nuclear receptors and proteins (Figure 2 and Supplementary Table 2). For example, PFBS and PFHxA exhibit relatively weak potency to induce thyroid receptor activity with IC50 values of >1000 and >500 µM, respectively, whereas PFOA and PFOS exhibit order-of-magnitude higher potencies with IC50 values of 42 and 16 µM, respectively (Ren et al., 2015). This difference in relative potency suggests that the MoA for toxicity of long-chain PFAAs may be different and more complicated. However, generalizations regarding MoA may not apply to chemicals grouped by chain-length alone. For example, short-chain PFHpA (C7) interacts with a similar number of nuclear receptors as PFOA, indicating that PFHpA may have a MoA more like that of PFOA than PFHxA.
Figure 2.
Relative potency of perfluoroalkyl acids and FTOHs based on reactivity with various human nuclear receptors, using PFOA as the index chemical. See Supplementary Table 2 for corresponding tabular summary of binding activity metrics and values. PPAR-α, C20max, human peroxisome proliferator-activated receptor alpha, concentration that produces 20% of the maximal response; PPAR-alpha, AC50, human peroxisome proliferator-activated receptor alpha, half-maximal activity concentration; FABP, human liver fatty acid binding protein; PXR, human pregnane X receptor; TR, human thyroid receptor.
Collectively, these results provide a compelling line of evidence to guide a mixtures approach for PFAAs away from the use of the RPF approach (Figure 1, Step 3). No single nuclear receptor or molecular initiating event is likely to be responsible for all of the observed toxicities associated with short- and long-chain PFCAs, PFSAs, and FTOHs. Therefore, it is unlikely that grouping strategies and mixtures methods that focus on a specific nuclear receptor (eg, PPARα) will be predictive of human risk. Consistent with the mixtures framework (Figure 1, Step 2), given uncertainty in grouping chemistries based on a common MoA, we explored dose-response information for chemicals that share the same effect endpoint (eg, target organ toxicity).
Dose-Response for Hepatocellular Hypertrophy
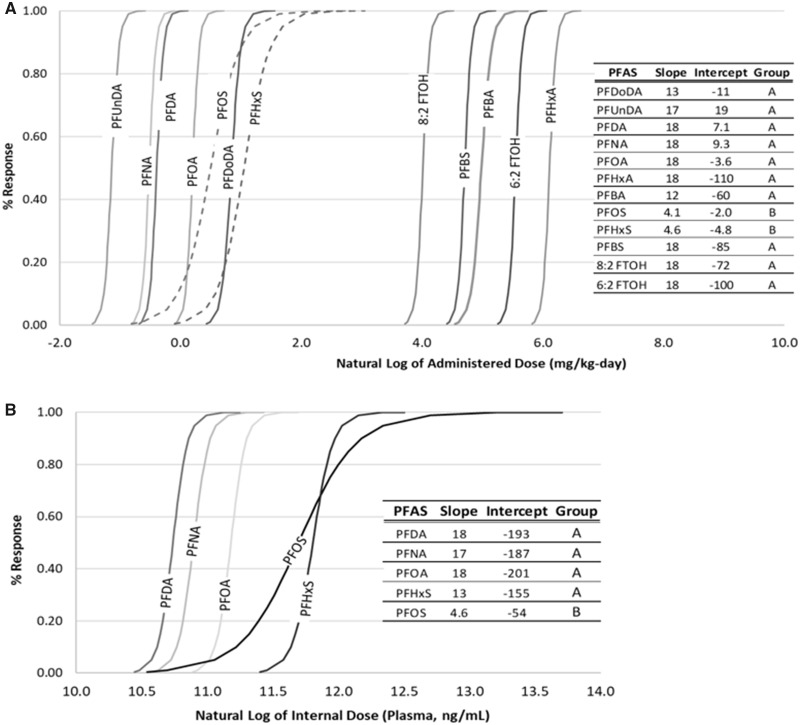
The liver is a well-established target organ for many PFCAs and PFSAs. According to the diagnostic criteria outlined in Hall et al. (Hall et al., 2012), hepatocellular hypertrophy and hepatomegaly is a common nonadverse, adaptive response following activation of nuclear receptors such as PPARα or CAR. This response should only be considered adverse if it coincides with histopathology (ie, necrosis or inflammation) or clinical chemistry (eg, biologically relevant changes in aspartate transaminase, alanine aminotransferase, alkaline phosphatase) that indicates organ damage, both of which sometimes, but not always have been observed following PFAA administration to rodents. Regardless, RIVM recently proposed an RPF approach for assessing PFAA mixture toxicity based upon hepatocellular hypertrophy. To build upon RIVM’s work and to investigate the appropriateness of this mixtures approach using a different dataset, we performed BMD modeling on the incidence of hepatocellular hypertrophy observed in male rats orally exposed for 28 days to PFBA, PFHxA, PFOA, PFNA, PFDA, PFBS, PFHxS, and PFOS (Butenhoff et al., 2012; NTP, 2018a,b), PFUnDA and PFDoDA for 42 days (Kato et al., 2015; Takahashi et al., 2014) or 6:2 FTOH and 8:2 FTOH for 90 days (Ladics et al., 2008; Serex et al., 2014). The log-logistic model provides an adequate fit for all modeled datasets and results are summarized in Supplementary Table 3. BMDs are lowest for long-chain PFAAs (ranging 0.281 mg/kg/day for PFUnDA to 1.96 mg/kg/day for PFDoDA), compared with short-chain PFAAs (ie, 121, 97.9, and 392 mg/kg/day for PFBA, PFBS, and PFHxA, respectively) and FTOHs (ie, 48.9 and 228 mg/kg/day for 8:2 and 6:2 FTOH, respectively1).
These results clearly demonstrate short-chain PFAAs (eg, PFBA, PFHxA, and PFBS) are less potent inducers of hepatocellular hypertrophy than long-chain PFAAs (eg, PFOA and PFOS), and are approximately equipotent as FTOHs. In partial agreement with RIVM’s analysis, shapes of the dose-response curves were similar for short- and long-chain PFCAs, and PFBS (slopes ranged from 12.0 to 18.0; Figure 3A). Geometrically congruent curves may indicate that it is appropriate to group these specific PFAAs for purposes of hepatotoxic risk assessment or if assuming the dose-response curves for liver toxicity are representative for other endpoints (Figure 1, Step 2—leading to dose addition for selected component chemicals and endpoints). However, a contradictory finding to RIVM is evident from the dose-response curves for PFOS (slope = 4.1) and PFHxS (slope = 4.6), which are approximately congruent with each other, but not with the dose-response curves for PFCAs and PFBS (Figure 3A). This finding indicates that long-chain PFSAs (ie, PFOS and PFHxS) should not be grouped with short-chain PFSAs (ie, PFBS) or any PFCAs. This finding contradicts an assumption of dose additivity of PFOA and PFOS currently applied by USEPA and other agencies, since proportional BMDs do not occur across the dose ranges associated with adverse effects for either chemical in NTP’s 28-day study.
Figure 3.
Log-logistic dose response curves for incidence of hepatocellular hypertrophy based upon (A) administered dose and (B) internal dose (plasma levels).
Interestingly, dose-response curves for long-chain PFCAs and PFSAs based upon internal dosimetry (ie, plasma PFAA level 24 h after the final administered dose) support a slightly different grouping strategy (see Supplementary Table 3). As can be seen from Figure 3B, the slopes of the internal serum liver-toxicity dose-response curves for PFDA, PFNA, PFOA, and PFHxS are similar and range from 13 to 18, whereas the slope of the dose-response curve for PFOS is 4.6. Again, this result indicates that PFOS should not be grouped with long-chain PFCAs, while it may be appropriate to group PFHxS with long-chain PFCAs based upon internal dosimetry. This also further highlights the need to correlate internal serum levels with a broader range of nonpolymeric PFAS and toxicity outcomes.
Dose-Response for Effects on Serum Cholesterol
To date, a biologically plausible MoA has not yet been established to explain how increased exposure to PFAAs could cause an elevation in serum cholesterol levels in humans. The role of PPARα in lipid metabolism is well established and suggests that an inverse relationship with serum cholesterol is more likely. Prolonged activation of PPARα leads to increased lipid metabolism, thereby reducing serum cholesterol levels. This hypothesis is supported by a recent phase I clinical trial with PFOA, which demonstrated that when human serum levels of PFOA are comparable with the relatively high levels achieved in rodent studies, cholesterol levels decline rather than increase (Convertino et al., 2018). In rodent models, exposure to PFAAs tends to reduce total serum cholesterol levels (Supplementary Figure 3; Kennedy et al., 2004). However, some epidemiology studies suggest individuals with higher serum levels of PFOA, PFOS, PFNA, and PFDA also tend to have higher serum total cholesterol and low-density lipoprotein (LDL)-cholesterol (reviewed in ATSDR, 2018b). An explanation for the inconsistency in human and animal data is uncertain.
Regardless of the limitations and uncertainties surrounding serum cholesterol specifically, there is evidence that PFAAs can alter lipid metabolism in humans and animal models. Therefore, we conducted BMD modeling on administered dose and serum total cholesterol levels to understand the potency and dose-response relationship for short- and long-chain PFCAs, PFSAs, and FTOHs. Oral exposure to both short- and long-chain PFCAs for 28 days had a weak effect on total serum cholesterol levels in male rats, and the majority of the data was not amenable to BMD modeling (Supplementary Figure 3A and 3B). Alternatively, strong dose-response relationships were observed for all PFSAs, with BMDs of 54.4 mg/kg/day PFBS (exponential model 2/3 with modeled variance), 1.71 mg/kg/day PFHxS (Hill model with constant variance), and 0.0972 mg/kg/day PFOS (exponential model 4 with constant variance; Supplementary Figure 3C). Similar to the PFCAs, oral exposure to 8:2 and 6:2 FTOH for 90 days had minimal impact on total serum cholesterol levels in male rats and the datasets were not amenable to BMD modeling (Supplementary Figure 3D).
Disparate responses in serum cholesterol following exposure to PFCAs and PFSAs may support separate groupings for PFCAs and PFSAs (Figure 1, Step 3—chemical groups informed by dose-response analysis). However, in humans, both PFCAs (PFOA, PFNA, PFDA) and PFSAs (PFOS) have been associated with similar impacts on serum cholesterol levels, which conflicts with the modeled rodent dataset. Clearly, additional data are required to better understand the MoA underlying a potential increase in total cholesterol in humans before any conclusions regarding grouping for this endpoint can be made.
Analysis of Chemical Structure Similarity
Nonpolymeric PFAS comprise a large set of chemicals and chemical structures (Buck et al., 2011; Chelcea et al., 2020; Wang et al., 2017). These PFAS encompass a broad range of Markush structures, with a wide array of chemical and physical properties. Moreover, nonpolymeric PFAS comprise cationic, anionic, and zwitterionic forms, among others. USEPA has attempted to speciate nonpolymeric PFAS based, primarily, on overarching chemical identifiers; eg, USEPA has reported their speciation efforts for perfluoroalkyl sulfonamides in the ToxPrint chemotype database (Patlewicz, 2019). Even within a specific group like perfluoroalkyl sulfonamides, there can be a large number of compounds with diverse additional Markush groups, which furthers adds a level of complexity when assessing toxicity and conducting a mixtures risk assessment.
Nonpolymeric PFAS can adopt a wide array of different 3D structures—depending upon chain length—but can also adopt different conformations in vivo. This is largely an overlooked area of research for nonpolymeric PFAS but could be important when attempting any quantitative structure activity relationship analysis and molecular modeling analysis. Furthermore, the binding affinity, hydrogen bonding, structure orientation, binding kinetics (or lack thereof), can be important when assessing the toxicokinetic/toxicodynamic properties of nonpolymeric PFAS. Moreover, even with USEPA’s attempt at speciation of nonpolymeric PFAS, and grouping PFAS based on a chemical identifier, these specific groups can also encompass a wide array of physical and chemical properties. The speciation into Markush groups, varying chemical and physical properties with each group, and the number of unknowns, further supports the notion that there is currently no support for a simplifying assumption that all nonpolymeric PFAS can grouped for purposes of mixtures assessment (Figure 1, Steps 1 and 2).
Summary
In summary, whole mixtures or binary component mixture studies suggest that dose-additivity assumptions for PFAAs are not yet supported by the available whole mixture toxicity data. Although some nonpolymeric PFAS may share similar target organs dependent upon exposure level, the most sensitive effects, as defined by regulatory agencies in the United States, including developmental endpoints and immune endpoints, are not amenable to in-depth mixtures assessment (Tier 1 or higher in Figure 1, Step 3) and AOPs have not been clearly elucidated for multiple PFAAs and the same apical endpoint. Only liver data from animal bioassays are amenable to comparing the shape of dose-response curves across a range of nonpolymeric PFAS. For most of the PFAAs, the available data for increased relative kidney weight are not amenable to dose-response modeling (see Supplementary Table 4 for examples of Hill dose-response model parameters for PFNA, PFDoDA, PFBs, PFHxS, and PFOS). Similarly, the NTP datasets for body weight are also not amenable to dose-response analysis for PFBA, PFUnDA, PFDoDA, and PFHxS (see Supplementary Table 5 for examples of power dose-response model parameters for remaining PFAAs). For total cholesterol, with the exception of PFBA, data on PFCAs were not amenable to dose-response modeling; however, differences in the shapes of the dose-response data presented graphically is illustrative (see Supplementary Figure 3). Developmental and reproductive endpoints have either not been tested across a large enough range of nonpolymeric PFAS in similar study designs, have inconsistent endpoints (eg, reduced body weight vs delayed eye opening) or are actually not appropriate endpoints of concern for some PFAAs such as PFHxA (Iwai et al., 2019). Based on the liver data alone, however, different approaches for grouping for mixtures risk assessment are apparent whether the evaluation is based on internal serum dose (the preferred approach) or based on administered dose. The available data currently suggest that PFOS should not be grouped with long-chain PFCAs for mixtures risk assessment, and some PFCAs (PFOA, PFNA, and PFDA) may be grouped together based on similar toxicities and similar dose-response slopes. It is not clear, however, that those same conclusions would hold for different toxicity endpoints, such as effects on development or the immune system.
DISCUSSION
Regulatory and public health agencies around the globe are developing and implementing guidance and regulations to address the environmental risks associated with nonpolymeric PFAS. Just as the chemical-specific action levels vary greatly, agencies have also addressed the issue of mixtures quite differently. A fundamental data gap is that toxicity values (eg, oral RfDs) have only been derived for a handful of nonpolymeric PFAS (eg, PFHxA, PFHxS, PFOA, PFOS, PFBS) and clear MoAs or AOPs have not been defined. Furthermore, the best information to support assumptions about mixture toxicity of chemicals with toxicity values would be data on the whole mixture or sufficiently similar mixtures. However, to date, there are currently less than a dozen published whole mixture toxicity studies with PFAS, most of which involve dosing binary combinations of only a few PFAAs, and these data reveal no consistent finding that supports a single interpretation of these data. Therefore, it is yet unclear if mixture effects (dose or response addition) are of concern for exposure to nonpolymeric PFAS. Herein, we applied well-established frameworks for assessing risk to a mixture of PFAS when only individual chemical data are available.
The initial step in any mixtures assessment framework involves identifying the subset of chemicals for which coexposure may be occurring and for which the mixtures risk assessment may be appropriate. For PFAS, this is challenged by the currently available analytical limitations. Nonetheless, empirical environmental sampling data and/or information on nonpolymeric PFAS of concern in commercial products can be used to estimate exposure groups. It should be noted that exposure to polymeric PFAS is unlikely to present a significant human health risk due to their high-molecular weight (MW), low absorbance, and low reactivity, which contributes to a general lack of bioavailability (Henry et al., 2018; USEPA, 2012b). Although the clearance or elimination rate of PFAAs with different chain lengths (ie, “long” vs “short chain”) has shown to vary dramatically, use of half-life alone is not likely a sufficient discriminator to determine the mixture of concern without additional information about the magnitude and frequency of exposures relative to the half-lives. Together, the relative half-lives and the exposure scenario will determine the internal dose profile of a mixture. If on-going exposures have been mitigated and the purpose of the risk assessment is forward-looking, then grouping nonpolymeric PFAS based on their elimination kinetics may be appropriate. However, if exposures are on-going and occur potentially on a daily basis, or if the risk assessment’s purpose is to evaluate past risk during on-going coexposure, kinetic half-life differences are of little relevance given that the various nonpolymeric PFAS will likely coexist in vivo.
Second, one should assess the toxicological similarity based on MoA and most sensitive effect endpoints (and related AOPs) of the identified components in the mixture. Available data continue to demonstrate that toxicity induced by PFAA exposure may occur across several biological systems and is not mediated by a single nuclear receptor. Our evaluation of USEPA’s in vitro dataset shows that short- and long-chain PFCAs, PFSAs, and FTOHs can interact with around 2 dozen different nuclear receptors (Supplementary Table 1). Therefore, it is unlikely that grouping strategies and mixtures methods that focus on a specific nuclear receptor (eg, PPARα) will be predictive of human risk to a mixture of nonpolymeric PFAS. However, there are clear toxicological similarities based on chain-length, because short-chain PFAAs demonstrate relatively low activity, interacting with 0–2 nuclear receptors with weaker binding affinity, while long-chain PFAAs can interact with as many as 6–16 different nuclear receptors (Supplementary Table 1). Thus, if the relevant mixture of concern includes both long- and short-chain PFAS, subdividing the components based on chain-length may make sense given that there are differences in sensitive target organs and PFAS do not appear to act via a similar MoA. Another consideration when assessing candidates for grouping based on similarities in dose-response relationships is to evaluate concentrations expressed on a molar basis (eg, mol/l), essentially normalizing mass-per-volume (eg, g/l) by MW (g/mole). For example, Vogs et al. (2019) examined relative potencies of PFOS, PFHxS, PFOA, and PFBS using the zebrafish embryo model and compared POD ratios expressed in terms of molar concentrations of internal and external dose. Normalizing by MW may reduce a source of variability when evaluating the support for dose-additivity assumptions and deriving toxicity weighting factors used to generate a weighted summation of dose or concentration, as has been effectively demonstrated for PAHs with the toxic unit approach (Di Toro et al., 2000; French-McCay, 2002).
With an unknown MoA and lack of appropriate single molecular target, it is clear that RPF (and toxic equivalency factors) approaches that would group short- and long-chain PFCAS, PFSAs, and FOTHs are not supported by the data. We next explored the dose-response relationships of various nonpolymeric PFAS across similar endpoints to assess the applicability of dose or concentration additivity or HI methods. In general, dose addition most directly applies when component chemicals act on similar biological systems (eg, target organs, such as the liver or systems such as the reproductive system) and elicit a common response (USEPA, 2000). To date, hepatocellular hypertrophy and kidney effects remain the only endpoints for which there are similar toxicity data from similar study designs, for multiple nonpolymeric PFAS. We conducted BMD modeling on the incidence of hepatocellular hypertrophy observed in male rats orally exposed for 28 days to PFBA, PFHxA, PFOA, PFNA, PFDA, PFBS, PFHxS, and PFOS (Butenhoff et al., 2012; NTP, 2018a,b), PFUnDA and PFDoDA for 42 days (Kato et al., 2015; Takahashi et al., 2014) or 6:2 FTOH and 8:2 FTOH for 90 days (Ladics et al., 2008; Serex et al., 2014) to evaluate the potency and dose-response relationships across these nonpolymeric PFAS. Our analyses demonstrate that for the PFAS for which we have applicable data, it is evident that these nonpolymeric PFAS are not equipotent across a range of doses. Short-chain PFAAs and the FTOHs evaluated are less potent inducers of hepatocellular hypertrophy than long-chain PFAAs. The slopes of the dose-response curves were approximately the same for PFCAs (short and long chain) and PFBS. PFOS and PFHxS also exhibited congruent shapes with each other, but not with PFCAs (Figure 3). This finding indicates that long-chain PFSAs (ie, PFOS and PFHxS) should not be grouped with short-chain PFSAs (ie, PFBS) or any PFCAs, suggesting that the concentration-addition method used by the USEPA and several state agencies, is not supported by the currently available data. It is unknown how well these conclusions, based on analysis of hepatoxicity in the rat, are applicable across different target organs or in humans. However, EPA and NTP have developed a structurally diverse library of 150 PFAS, which they are testing for hepatotoxicity, immunotoxicity, developmental toxicity, mitochondrial toxicity, developmental neurotoxicity, hepatic clearance, and toxicokinetics in a suite of high-throughput in vitro assays (Patlewicz, 2019; Thomas 2019). By maximizing structural diversity, this research may inform read-across efforts and PFAS grouping strategies to support human health risk assessment.
Until additional data become available, the use of a default screening-level HI method applied to noncancer endpoints may be the only option for a preliminary mixtures assessment for nonpolymeric PFAS for chemicals in the same assessment group, consistent with USEPA, ATSDR, EFSA, and WHO guidance and the Health Canada and ATSDR approaches. For demonstration purposes, we developed a hypothetical site mixtures risk assessment for a dataset consisting of a variety of short- and long-chain PFCAs and PFSAs (Tables 3 and 4). Under this hypothetical scenario, most of the individual PFAS concentrations would exceed most of the drinking water screening levels reported by U.S. federal and state agencies. We can compare this outcome with alternative approaches by applying the default risk equation for residential exposure to noncarcinogens in groundwater and the exposure factors for drinking water ingestion rate and body weight to reflect values recommended by USEPA for infant receptors, as the most sensitive receptor for noncarcinogens. In this example, the HQ is ≤1 for each chemical; however, the HI for all components combined is 1.6 (=2 when rounded to one significant figure), which exceeds a target risk threshold of HI ≤ 1, indicating a need to conduct a refined assessment and the potential for risk (Table 3). If PFCAs and PFSAs are summed separately, neither group would yield a HI > 1, suggesting no unacceptable risk. Therefore, the choice of how to combine chemical-specific risk estimates may change the interpretation of risk in this example.
Table 3.
Hypothetical Example Illustrating Application of the HI Approach for Infants Consuming Drinking Water
| Chemical | C (ng/l) | DW (l/day) | BW (kg) | EF (days/year) | Dose a (mg/kg/day) | Oral RfD (mg/kg/day) | Critical Effect Target Organ | HQ b | Source for RfD |
|---|---|---|---|---|---|---|---|---|---|
| PFNA | 11 | 0.78 | 15 | 350 | 5.5E-07 | 2E-06 | liver | 0.3 | Health Canada (2019) |
| PFOA | 43 | 0.78 | 15 | 350 | 2.1E-06 | 2E-05 | development | 0.1 | USEPA (2016b) |
| PFHxA | 87 | 0.78 | 15 | 350 | 4.3E-06 | 0.25 | kidney | 0.00002 | Luz et al. (2019) |
| PFOS | 446 | 0.78 | 15 | 350 | 2.2E-05 | 2E-05 | development | 1 | USEPA (2016a) |
| PFHxS | 92 | 0.78 | 15 | 350 | 4.6E-06 | 6E-05 | liver | 0.1 | Health Canada (2019) |
| PFBS | 21 | 0.78 | 15 | 350 | 1.0E-06 | 2E-03 | kidney | 0.0007 | USEPA (2014) |
| Sum: | 700 | Sum (HI): | 1.6 | ||||||
Abbreviations: BW, infant body weight; C, concentration; DW, infant drinking water ingestion rate; EF, exposure frequency; HQ, hazard quotient.
Dose = (C/1 × 106) × DW × (EF/365)/BW.
HQ = dose/RfD.
Table 4.
Hypothetical Example Illustrating Application of the RPF Approach
| Chemical | POD Value (mg/kg/day) a | POD ratio b | RPF (Unitless) | C (ng/l) | Equiv. Conc. c (ng/l) | % of Mixture |
|---|---|---|---|---|---|---|
| PFNA | 0.528 | PFOA/PFNA | 2 | 11 | 23 | 34.23% |
| PFOA | 1.08 | PFOA/PFOA | 1 | 43 | 43 | 65.42% |
| PFHxA | 392 | PFOA/PFHxA | 0.003 | 87 | 0.23 | 0.35% |
| Sum: | 141 | 66 | 100% | |||
| PFOS | 0.957 | PFOS/PFOS | 1 | 446 | 446 | 90.12% |
| PFHxS | 1.77 | PFOS/PFHxS | 0.5 | 92 | 49 | 9.84% |
| PFBS | 97.9 | PFOS/PFBS | 0.01 | 21 | 0.21 | 0.04% |
| Sum: | 559 | 495 | 100% |
| Chemical | C (ng/l) | DW (l/day) | BW (kg) | EF (days/year) | Dosed (mg/kg/day) | Oral RfD (mg/kg/day) | HQe |
|---|---|---|---|---|---|---|---|
| PFOAequiv | 66 | 0.78 | 15 | 350 | 3.3E-06 | 2E-05 | 0.2 |
| PFOSequiv | 495 | 0.78 | 15 | 350 | 2.5E-05 | 2E-05 | 1 |
|
| |||||||
Abbreviations: BMD, benchmark dose; BW, infant body weight; C, concentration; DW, infant drinking water ingestion rate; EF, exposure frequency; Equiv. Conc., concentration equivalent to the index chemical; HQ, hazard quotient; POD, point of departure; RPF, relative potency factor.
The POD is the BMD calculated for a BMR of 10% change using the best-fit dose-response model calculated with BMDS. NTP (2018a,b) 28-day oral gavage study with rats; liver hypertrophy.
The POD ratio is the BMD of the index chemical (either PFOA or PFOS) divided by the BMD of the chemical of interest.
cEquivalent concentration of the index chemical, after adjusting for relative potency. Equivalent concentration = RPF × C.
Dose = (C/1 × 106) × DW × (EF/365)/BW.
HQ = dose/RfD.
Additionally, a rudimentary example of an RPF calculation is shown (Table 4), using PODs calculated from the 28-day rat study results reported by NTP for liver hypertrophy (NTP, 2018a,b). In this example, the PODs are taken directly from the animal studies (based on a 10% response level), rather than converted to a human-equivalent dose (HED). The RPF method is not fully demonstrated in this example because the predicted mixture response is not estimated from the dose-response curves of the index chemicals (PFOA and PFOS; USEPA, 2000). Such an approach would require a different method of derivation of the HED than used to calculate the current oral RfDs (ie, multiplying clearance rate by the average serum level corresponding to a POD; USEPA, 2016a,b). Note how the RPFs for the short-chain PFAAs (ie, PFHxA and PFBS) are orders of magnitude lower than their respective index chemicals—PFOA for the PFCAs, and PFOS for the PFSAs. In this example, the final HQs, after summing equivalent concentrations of the index chemicals, are ≤1 separately and when added together. Each approach has significant limitations, and moreover, the example shows how the decision outcome varies depending on the method selected. Critical data gaps remain, including whole mixture toxicity tests, evaluations of toxicity across an expanded suite of nonpolymeric PFAS and endpoints (including developmental outcomes), and better defined MoAs or AOPs. Different decisions regarding aggregation of component chemicals of a mixture can lead to different risk assessment conclusions; therefore, transparent discussion of key assumptions, supporting lines of evidence, and their quantitative impacts are necessary if a mixture approach is utilized for PFAS risk assessment.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This work was funded, in part, by the FluoroCouncil of the American Chemistry Council. The researchers’ scientific conclusions and professional judgments were not subject to the funders’ control and the contents of this article reflect solely the view of the authors.
Supplementary Material
ACKNOWLEDGMENTS
The authors would also like to thank Sarah E. Hansen for her support in preparing the article, and our colleagues including Richard H. Anderson and 2 independent peer-reviewers for their constructive comments on early drafts.
Footnotes
For 8:2 FTOH, the incidence of hepatocellular hypertrophy increased from 0% at 25 mg/kg/day (no observed effect level (NOEL)) to 100% at 125 mg/kg/day (lowest observed effect level (LOEL)). Therefore, due to dose spacing there is uncertainty around the shape of the dose-response curve and the BMD estimate.
REFERENCES
- Abbott B. D., Wolf C. J., Das K. P., Zehr R. D., Schmid J. E., Lindstrom A. B., Strynar M. J., Lau C. (2009). Developmental toxicity of perfluorooctane sulfonate (PFOS) is not dependent on expression of peroxisome proliferator activated receptor-alpha (PPARα) in the mouse. Reprod. Toxicol. 27, 258–265. [DOI] [PubMed] [Google Scholar]
- Abbott B. D., Wolf C. J., Schmid J. E., Das K. P., Zehr R. D., Helfant L., Nakayama S., Lindstrom A. B., Strynar M. J., Lau C., et al. (2007). Perfluorooctanoic acid induced developmental toxicity in the mouse is dependent on expression of peroxisome proliferator activated receptor-alpha. Toxicol. Sci. 98, 571–581. [DOI] [PubMed] [Google Scholar]
- Adams V. H., McAtee M. J., Johnson M. S. (2017). Implementation of the basic hazard index screening for health risks associated with simultaneous exposure to multiple chemicals using a standardized target organ and systems framework: Hazard Index Screening Using Standardized TTOS Framework. Integr. Environ. Assess. Manag. 13, 852–860. [DOI] [PubMed] [Google Scholar]
- Altenburger R., Schmitt H., Schüürmann G. (2005). Algal toxicity of nitrobenzenes: Combined effect analysis as a pharmacological probe for similar modes of interaction. Environ. Toxicol. Chem. 24, 324. [DOI] [PubMed] [Google Scholar]
- Anderson R. H., Long G. C., Porter R. C., Anderson J. K. (2016). Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere 150, 678–685. [DOI] [PubMed] [Google Scholar]
- ATSDR. (2005) Public Health Assessment Guidance Manual (Update) Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA. [Google Scholar]
- ATSDR. (2018. a) Framework for Assessing Health Impacts of Multiple Chemicals and Other Stressors (Update). Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA. [Google Scholar]
- ATSDR. (2018. b) Toxicological Profile for Perfluoroalkyls, Draft for Public Comment. Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA. [Google Scholar]
- ATSDR. (2020) Health Consultation: Per- and Polyfluoroalkyl Substances (PFAS) in the Pease Tradeport Public Water System. EPA PWS ID: 1951020; Pease Air Force Base; Portsmouth, Newington, and Greenland, New Hampshire. EPA Facility ID: NH7570024847 Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services, Public Health Service, Atlanta, Georgia.
- Backe W. J., Day T. C., Field J. A. (2013). Zwitterionic, cationic, and anionic fluorinated chemicals in aqueous film forming foam formulations and groundwater from U.S. Military bases by nonaqueous large-volume injection HPLC-MS/MS. Environ. Sci. Technol. 47, 5226–5234. [DOI] [PubMed] [Google Scholar]
- Bartell S. M., Calafat A. M., Lyu C., Kato K., Ryan P. B., Steenland K. (2010). Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ. Health Perspect. 118, 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzen-Hanson K. A., Roberts S. C., Choyke S., Oetjen K., McAlees A., Riddell N., McCrindle R., Ferguson P. L., Higgins C. P., Field J. A., et al. (2017). Discovery of 40 classes of per- and polyfluoroalkyl substances in historical aqueous film-forming foams (AFFFs) and AFFF-impacted groundwater. Environ. Sci. Technol. 51, 2047–2057. [DOI] [PubMed] [Google Scholar]
- Benford D., Bolger P. M., Carthew P., Coulet M., DiNovi M., Leblanc J.-C., Renwick A. G., Setzer W., Schlatter J., Smith B., et al. (2010). Application of the margin of exposure (MOE) approach to substances in food that are genotoxic and carcinogenic. Food Chem. Toxicol. 48, S2–S24. [DOI] [PubMed] [Google Scholar]
- Buck R. C., Franklin J., Berger U., Conder J. M., Cousins I. T., de Voogt P., Jensen A. A., Kannan K., Mabury S. A., van Leeuwen S. P., et al. (2011). Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 7, 513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenhoff J. L., Bjork J. A., Chang S.-C., Ehresman D. J., Parker G. A., Das K., Lau C., Lieder P. H., van Otterdijk F. M., Wallace K. B., et al. (2012). Toxicological evaluation of ammonium perfluorobutyrate in rats: Twenty-eight-day and ninety-day oral gavage studies. Reprod. Toxicol. 33, 513–530. [DOI] [PubMed] [Google Scholar]
- Carr C. K., Watkins A. M., Wolf C. J., Abbott B. D., Lau C., Gennings C. (2013). Testing for departures from additivity in mixtures of perfluoroalkyl acids (PFAAs). Toxicology 306, 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2019) Fourth National Report on Human Exposure to Environmental Chemicals Update. U.S Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA.
- Chang S.-C., Das K., Ehresman D. J., Ellefson M. E., Gorman G. S., Hart J. A., Noker P. E., Tan Y.-M., Lieder P. H., Lau C., et al. (2008). Comparative pharmacokinetics of perfluorobutyrate in rats, mice, monkeys, and humans and relevance to human exposure via drinking water. Toxicol. Sci. 104, 40–53. [DOI] [PubMed] [Google Scholar]
- Chelcea I. C., Ahrens L., Örn S., Mucs D., Andersson P. L. (2020). Investigating the OECD database of per- and polyfluoroalkyl substances – Chemical variation and applicability of current fate models. Environ. Chem. [Google Scholar]
- Conley J. M., Lambright C. S., Evans N., Cardon M., Furr J., Wilson V. S., Gray L. E. (2018). Mixed “Antiandrogenic” chemicals at low individual doses produce reproductive tract malformations in the male rat. Toxicol. Sci. 164, 166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convertino M., Church T. R., Olsen G. W., Liu Y., Doyle E., Elcombe C. R., Barnett A. L., Samuel L. M., MacPherson I. R., Evans T. R. J., et al. (2018). Stochastic pharmacokinetic-pharmacodynamic modeling for assessing the systemic health risk of perfluorooctanoate (PFOA). Toxicol. Sci. 163, 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Toro D. M., McGrath J. A., Hansen D. J. (2000). Technical basis for narcotic chemicals and polycyclic aromatic hydrocarbon criteria. I. Water and tissue. Environ. Toxicol. Chem. 19, 1951–1970. [DOI] [PubMed] [Google Scholar]
- Ding G., Zhang J., Chen Y., Wang L., Wang M., Xiong D., Sun Y. (2013). Combined effects of PFOS and PFOA on zebrafish (Danio rerio) embryos. Arch Environ. Contam. Toxicol. 64, 668–675. [DOI] [PubMed] [Google Scholar]
- Elcombe C. R., Elcombe B. M., Foster J. R., Farrar D. G., Jung R., Chang S.-C., Kennedy G. L., Butenhoff J. L. (2010). Hepatocellular hypertrophy and cell proliferation in Sprague–Dawley rats following dietary exposure to ammonium perfluorooctanoate occurs through increased activation of the xenosensor nuclear receptors PPARα and CAR/PXR. Arch. Toxicol. 84, 787–798. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority Scientific Committee, More, S. J., Bampidis, V., Benford, D., Bennekou, S. H., Bragard, C., Halldorsson, T. I., Hernández‐Jerez, A. F., Koutsoumanis, K., Naegeli, H., et al (2019). Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA J. 17, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney D. J. (1942). The analysis of toxicity tests on mixtures of poisons. Ann. Appl. Biol. 29, 82–94. [Google Scholar]
- Flippin J. L., Hedge J. M., DeVito M. J., LeBlanc G. A., Crofton K. M. (2009). Predictive modeling of a mixture of thyroid hormone disrupting chemicals that affect production and clearance of thyroxine. Int. J. Toxicol. 28, 368–381. [DOI] [PubMed] [Google Scholar]
- Flynn R. W., Chislock M. F., Gannon M. E., Bauer S. J., Tornabene B. J., Hoverman J. T., Sepúlveda M. S. (2019). Acute and chronic effects of perfluoroalkyl substance mixtures on larval American bullfrogs (Rana catesbeiana). Chemosphere 236, 124350. [DOI] [PubMed] [Google Scholar]
- French-McCay D. P. (2002). Development and application of an oil toxicity and exposure model, OilToxEx. Environ. Toxicol. Chem. 21, 2080–2094. [PubMed] [Google Scholar]
- FSANZ. (2017) Hazard Assessment Report – Perfluorooctane Sulfonate (PFOS), Perfluorooctanoic Acid (PFOA), Perfluorohexane Sulfonate (PFHxS). Food Standards Australia New Zealand.
- Guelfo J. L., Adamson D. T. (2018). Evaluation of a national data set for insights into sources, composition, and concentrations of per- and polyfluoroalkyl substances (PFASs) in U.S. drinking water. Environ. Pollut. 236, 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad S. (2001). A PBPK modeling-based approach to account for interactions in the health risk assessment of chemical mixtures. Toxicol. Sci. 63, 125–131. [DOI] [PubMed] [Google Scholar]
- Hall A. P., Elcombe C. R., Foster J. R., Harada T., Kaufmann W., Knippel A., Küttler K., Malarkey D. E., Maronpot R. R., Nishikawa A., et al. (2012). Liver hypertrophy: A review of adaptive (adverse and non-adverse) changes—Conclusions from the 3rd International ESTP Expert Workshop. Toxicol. Pathol. 40, 971–994. [DOI] [PubMed] [Google Scholar]
- Health Canada. (2018. a) Guidelines for Canadian Drinking Water Quality: Guideline Technical Document: Perfluorooctane Sulfonate (PFOS). Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. [Google Scholar]
- Health Canada. (2018. b) Guidelines for Canadian Drinking Water Quality: Guideline Technical Document: Perfluorooctanoic Acid (PFOA). Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. [Google Scholar]
- Health Canada. (2019) Summary Table: Health Canada Draft Guidelines, Screening Values and Toxicological Reference Values (TRVs) for Perfluoroalkyl Substances (PFAS). Health Canada, Ottawa, Ontario. [Google Scholar]
- Henry B. J., Carlin J. P., Hammerschmidt J. A., Buck R. C., Buxton L. W., Fiedler H., Seed J., Hernandez O. (2018). A critical review of the application of polymer of low concern and regulatory criteria to fluoropolymers: Fluoropolymers PLC. Integr. Environ. Assess. Manag. 14, 316–334. [DOI] [PubMed] [Google Scholar]
- Hertzberg R. C., Pan Y., Li R., Haber L. T., Lyles R. H., Herr D. W., Moser V. C., Simmons J. E. (2013). A four-step approach to evaluate mixtures for consistency with dose addition. Toxicology 313, 134–144. [DOI] [PubMed] [Google Scholar]
- Hoover G., Kar S., Guffey S., Leszczynski J., Sepúlveda M. S. (2019). In vitro and in silico modeling of perfluoroalkyl substances mixture toxicity in an amphibian fibroblast cell line. Chemosphere 233, 25–33. [DOI] [PubMed] [Google Scholar]
- Hu X.-Z., Hu D.-C. (2009). Effects of perfluorooctanoate and perfluorooctane sulfonate exposure on hepatoma Hep G2 cells. Arch. Toxicol. 83, 851–861. [DOI] [PubMed] [Google Scholar]
- Hu J., Li J., Wang J., Zhang A., Dai J. (2014). Synergistic effects of perfluoroalkyl acids mixtures with J-shaped concentration–responses on viability of a human liver cell line. Chemosphere 96, 81–88. [DOI] [PubMed] [Google Scholar]
- Iwai H., Hoberman A. M., Goodrum P. E., Mendelsohn E., Anderson J. K. (2019). Addendum to Iwai and Hoberman (2014)—Reassessment of developmental toxicity of PFHxA in mice. Int. J. Toxicol. 38, 183–191. [DOI] [PubMed] [Google Scholar]
- Jain R. B. (2018). Time trends over 2003–2014 in the concentrations of selected perfluoroalkyl substances among US adults aged ≥20 years: Interpretational issues. Sci. Total Environ. 645, 946–957. [DOI] [PubMed] [Google Scholar]
- Kato H., Fujii S., Takahashi M., Matsumoto M., Hirata-Koizumi M., Ono A., Hirose A. (2015). Repeated dose and reproductive/developmental toxicity of perfluorododecanoic acid in rats. Environ. Toxicol. 30, 1244–1263. [DOI] [PubMed] [Google Scholar]
- Kennedy G. L., Butenhoff J. L., Olsen G. W., O’Connor J. C., Seacat A. M., Perkins R. G., Biegel L. B., Murphy S. R., Farrar D. G. (2004). The toxicology of perfluorooctanoate. Crit. Rev. Toxicol. 34, 351–384. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A. (2020). Which chemicals should be grouped together for mixture risk assessments of male reproductive disorders?. Mol. Cell. Endocrinol. 499, 110581. [DOI] [PubMed] [Google Scholar]
- Ladics G. S., Kennedy G. L., O'Connor J., Everds N., Malley L. A., Frame S. R., Gannon S., Jung R., Roth T., Iwai H., et al. (2008). 90-day oral gavage toxicity study of 8-2 fluorotelomer alcohol in rats. Drug Chem. Toxicol. 31, 189–216. [DOI] [PubMed] [Google Scholar]
- Li Y., Fletcher T., Mucs D., Scott K., Lindh C. H., Tallving P., Jakobsson K. (2018). Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup. Environ. Med. 75, 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein D., Luckert C., Alarcan J., de Sousa G., Gioutlakis M., Katsanou E. S., Konstantinidou P., Machera K., Milani E. S., Peijnenburg A., et al. (2020). An adverse outcome pathway-based approach to assess steatotic mixture effects of hepatotoxic pesticides in vitro. Food Chem. Toxicol. 139, 111283. [DOI] [PubMed] [Google Scholar]
- Luz A. L., Anderson J. K., Goodrum P., Durda J. (2019). Perfluorohexanoic acid toxicity, part I: Development of a chronic human health toxicity value for use in risk assessment. Regul. Toxicol. Pharmacol. 103, 41–55. [DOI] [PubMed] [Google Scholar]
- McCord J., Strynar M. (2019). Identification of per- and polyfluoroalkyl substances in the cape fear river by high resolution mass spectrometry and nontargeted screening. Environ. Sci. Technol. 53, 4717–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek M. E. (2013). International experience in addressing combined exposures: Increasing the efficiency of assessment. Toxicology 313, 185–189. [DOI] [PubMed] [Google Scholar]
- Meek M. E., Boobis A. R., Crofton K. M., Heinemeyer G., Van Raaji M, Vickers C. (2011). Risk assessment of combined exposure to multiple chemicals: A WHO/IPCS framework. Regulatory Toxicology and Pharmacology 60, S1–S14. [DOI] [PubMed] [Google Scholar]
- Menger F., Pohl J., Ahrens L., Carlsson G., Örn S. (2020). Behavioural effects and bioconcentration of per- and polyfluoroalkyl substances (PFASs) in zebrafish (Danio rerio) embryos. Chemosphere 245, 125573. [DOI] [PubMed] [Google Scholar]
- Moody C. A., Field J. A. (2000). Perfluorinated surfactants and the environmental implications of their use in fire-fighting foams. Environ. Sci. Technol. 34, 3864–3870. [Google Scholar]
- National Research Council. (2008) Phthalates and Cumulative Risk Assessment. The Task Ahead National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- NTP. (2018. a) TOX-96: Toxicity Report Tables & Curves. Pathology Tables, Survival and Growth Curves from NTP Short-Term Studies. U.S Department of Health and Human Services, National Institute of Environmental Health, National Toxicology Program, Research Triangle Park, North Carolina. [Google Scholar]
- NTP. (2018. b) TOX-97: Toxicity Report Tables & Curves. Pathology Tables, Survival and Growth Curves from NTP Short-Term Studies. U.S Department of Health and Human Services, National Institute of Environmental Health, National Toxicology Program, Research Triangle Park, North Carolina. [Google Scholar]
- NTP. (2019) NTP Technical Report on the Toxicity Studies of Perfluoroalkyl Carboxylates (Perfluorohexanoic Acid, Perfluorooctanoic Acid, Perfluorononanoic Acid, and Perfluorodecanoic Acid) Administered by Gavage to Sprague Dawley (Hsd: Sprague Dawley SD) Rats. National Toxicology Program, U.S. Department of Health and Human Services, Public Health Service, Research Triangle Park, North Carolina. [Google Scholar]
- Ojo A. F., Peng C., Ng J. C. (2020). Combined effects and toxicological interactions of perfluoroalkyl and polyfluoroalkyl substances mixtures in human liver cells (HepG2). Environ. Pollut. 263, 114182. [DOI] [PubMed] [Google Scholar]
- Olsen G. W., Burris J. M., Ehresman D. J., Froehlich J. W., Seacat A. M., Butenhoff J. L., Zobel L. R. (2007). Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 115, 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen G. W., Chang S.-C., Noker P. E., Gorman G. S., Ehresman D. J., Lieder P. H., Butenhoff J. L. (2009). A comparison of the pharmacokinetics of perfluorobutanesulfonate (PFBS) in rats, monkeys, and humans. Toxicology 256, 65–74. [DOI] [PubMed] [Google Scholar]
- Patlewicz G. (2019) Perspectives on the development, evaluation and application of in silico approaches for predicting toxicity. The United Stated Environmental Protection Agency's Center for Computational Toxicology and Exposure. Presentation. 10.23645/epacomptox.10299371.v1. [DOI]
- Peters J. M., Gonzalez F. J. (2011). Why toxic equivalency factors are not suitable for perfluoroalkyl chemicals. Chem. Res. Toxicol. 24, 1601–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X.-M., Zhang Y.-F., Guo L.-H., Qin Z.-F., Lv Q.-Y., Zhang L.-Y. (2015). Structure–activity relations in binding of perfluoroalkyl compounds to human thyroid hormone T3 receptor. Arch. Toxicol. 89, 233–242. [DOI] [PubMed] [Google Scholar]
- Rider C. V., LeBlanc G. A. (2005). An integrated addition and interaction model for assessing toxicity of chemical mixtures. Toxicol. Sci. 87, 520–528. [DOI] [PubMed] [Google Scholar]
- Rider C. V., Furr J. R., Wilson V. S., Gray L. E. Jr. (2010). Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity. Int. J. Androl. 33, 443–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen M. B., Abbott B. D., Wolf D. C., Corton J. C., Wood C. R., Schmid J. E., Das K. P., Zehr R. D., Blair E. T., Lau C., et al. (2008). Gene profiling in the livers of wild-type and PPARα-null mice exposed to perfluorooctanoic acid. Toxicol. Pathol. 36, 592–607. [DOI] [PubMed] [Google Scholar]
- Rosen M. B., Lee J. S., Ren H., Vallanat B., Liu J., Waalkes M. P., Abbott B. D., Lau C., Corton J. C. (2008). Toxicogenomic dissection of the perfluorooctanoic acid transcript profile in mouse liver: Evidence for the involvement of nuclear receptors PPARα and CAR. Toxicol. Sci. 103, 46–56. [DOI] [PubMed] [Google Scholar]
- Rotter S., Beronius A., Boobis A. R., Hanberg A., van Klaveren J., Luijten M., Machera K., Nikolopoulou D., van der Voet H., Zilliacus J., et al. (2018). Overview on legislation and scientific approaches for risk assessment of combined exposure to multiple chemicals: The potential EuroMix contribution. Crit. Rev. Toxicol. 48, 796–814. [DOI] [PubMed] [Google Scholar]
- Russell M. H., Himmelstein M. W., Buck R. C. (2015). Inhalation and oral toxicokinetics of 6:2 FTOH and its metabolites in mammals. Chemosphere 120, 328–335. [DOI] [PubMed] [Google Scholar]
- Sarigiannis D., Gotti A. (2008). Biology-based dose-response models for health risk assessment of chemical mixtures. Fresen. Environ. Bull. 17, 1439–1451. [Google Scholar]
- Serex T., Anand S., Munley S., Donner E. M., Frame S. R., Buck R. C., Loveless S. E. (2014). Toxicological evaluation of 6:2 fluorotelomer alcohol. Toxicology 319, 1–9. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Ishida S., Hirata-Koizumi M., Ono A., Hirose A. (2014). Repeated dose and reproductive/developmental toxicity of perfluoroundecanoic acid in rats. J. Toxicol. Sci. 39, 97–108. [DOI] [PubMed] [Google Scholar]
- Teuschler L. (2007). Deciding which chemical mixtures risk assessment methods work best for what mixtures⋆. Toxicol. Appl. Pharmacol. 223, 139–147. [DOI] [PubMed] [Google Scholar]
- Teuschler L. K., Rice G. E., Wilkes C. R., Lipscomb J. C., Power F. W. (2004). A feasibility study of cumulative risk assessment methods for drinking water disinfection by-product mixtures. J. Toxicol. Environ Health A 67, 755–777. [DOI] [PubMed] [Google Scholar]
- Thomas R. (2019). A Chemical Category-Based Approach for Selecting and Screening PFAS for Toxicity and Toxicokinetic Testing. The United State Environmental Protection Agency's Center for Computational Toxicology and Exposure. Presentation. https://doi.org/10.23645/epacomptox.9742535.v1 [Google Scholar]
- USEPA. (1986) Guidelines for the Health Risk Assessment of Chemical Mixtures Risk Assessment Forum. U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- USEPA. (1989) Risk Assessment Guidance for Superfund. Volume I. Human Health Evaluation Manual (Part A). Office of Emergency and Remedial Response, U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- USEPA. (2000) Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures Risk Assessment Forum. U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- USEPA. (2007) Concepts, Methods and Data Sources for Cumulative Health Risk Assessment of Multiple Chemicals, Exposures and Effects: A Resource Document. National Center for Environmental Assessment, United States Environmental Protection Agency, Cincinnati, OH. [Google Scholar]
- USEPA. (2012. a) Benchmark Dose Technical Guidance Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- USEPA. (2012. b) TSCA Work Plan Chemicals: Methods Document Office of Pollution Prevention and Toxics, U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- USEPA. (2014) Provisional Peer-Reviewed Toxicty Values for Perfluorobutane Sulfonate (CASRN 375-73-5) and Related Compound Potassium Perfluorobutane Sulfonate (CASRN 29420-49-3). Superfund Health Risk Technical Support Center, National Center for Environmental Assessment, Office of Research and Development, U.S. Environmental Protection Agency, Cincinnati, OH. [Google Scholar]
- USEPA. (2016. a) Health Effects Support Document for Perfluorooctane Sulfonate (PFOS). Health and Ecological Criteria Division, Office of Water. U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- USEPA. (2016. b) Health Effects Support Document for Perfluorooctanoic Acid (PFOA). Health and Ecological Criteria Division, Office of Water, U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- Vogs C., Johanson G., Näslund M., Wulff S., Sjödin M., Hellstrandh M., Lindberg J., Wincent E. (2019). Toxicokinetics of perfluorinated alkyl acids influences their toxic potency in the zebrafish embryo (Danio rerio). Environ. Sci. Technol. 53, 3898–3907. [DOI] [PubMed] [Google Scholar]
- Wang Z., DeWitt J. C., Higgins C. P., Cousins I. T. (2017). A never-ending story of per- and polyfluoroalkyl substances (PFASs)? Environ. Sci. Technol. 51, 2508–2518. [DOI] [PubMed] [Google Scholar]
- Wei Y., Shi X., Zhang H., Wang J., Zhou B., Dai J. (2009). Combined effects of polyfluorinated and perfluorinated compounds on primary cultured hepatocytes from rare minnow (Gobiocypris rarus) using toxicogenomic analysis. Aquat. Toxicol. 95, 27–36. [DOI] [PubMed] [Google Scholar]
- WHO. (2017) Chemical Mixtures in Source Water and Drinking-Water World Health Organization, Geneva. [Google Scholar]
- Wolf C. J., Rider C. V., Lau C., Abbott B. D. (2014). Evaluating the additivity of perfluoroalkyl acids in binary combinations on peroxisome proliferator-activated receptor-α activation. Toxicology 316, 43–54. [DOI] [PubMed] [Google Scholar]
- Wolf C. J., Schmid J. E., Lau C., Abbott B. D. (2012). Activation of mouse and human peroxisome proliferator-activated receptor-alpha (PPARα) by perfluoroalkyl acids (PFAAs): Further investigation of C4–C12 compounds. Reprod. Toxicol. 33, 546–551. [DOI] [PubMed] [Google Scholar]
- Wolf C. J., Takacs M. L., Schmid J. E., Lau C., Abbott B. D. (2008). Activation of mouse and human peroxisome proliferator-activated receptor alpha by perfluoroalkyl acids of different functional groups and chain lengths. Toxicol. Sci. 106, 162–171. [DOI] [PubMed] [Google Scholar]
- Wolf C. J., Zehr R. D., Schmid J. E., Lau C., Abbott B. D. (2010). Developmental effects of perfluorononanoic acid in the mouse are dependent on peroxisome proliferator-activated receptor-alpha. PPAR Res. 2010, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.-B., Zhao Y.-Z., Tang Y., Gong H.-Q., Guo F., Sun W.-H., Liu S.-S., Tan H., Chen F. (2019). Antioxidant defence system is responsible for the toxicological interactions of mixtures: A case study on PFOS and PFOA in Daphnia magna. Sci. Total Environ. 667, 435–443. [DOI] [PubMed] [Google Scholar]
- Zeilmaker M. J., Fragki, S., Verbruggen, E. M. J., Bokkers, B. G. H., and Lijzen, J. P. A. (2018) Mixture exposure to PFAS: A Relative Potency Factor approach. Gecombineerde blootstelling aan PFAS: een benadering met factoren voor relatieve potentie.
- Zhang Y., Beesoon S., Zhu L., Martin J. W. (2013). Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ. Sci. Technol. 47, 10619–10627. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-M., Dong X.-Y., Fan L.-J., Zhang Z.-L., Wang Q., Jiang N., Yang X.-S. (2017). Poly- and perfluorinated compounds activate human pregnane X receptor. Toxicology 380, 23–29. [DOI] [PubMed] [Google Scholar]
- Zhang L., Ren X.-M., Wan B., Guo L.-H. (2014). Structure-dependent binding and activation of perfluorinated compounds on human peroxisome proliferator-activated receptor γ. Toxicol. Appl. Pharmacol. 279, 275–283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.