Abstract
Schizophrenia (SCZ) has been associated with serotonergic and endocannabinoid systems dysregulation, but difficulty in obtaining in vivo neurological tissue has limited its exploration. We investigated CB1R-5-HT2AR heteromer expression and functionality via intracellular pERK and cAMP quantification in olfactory neuroepithelium (ON) cells of SCZ patients non-cannabis users (SCZ/nc), and evaluated whether cannabis modulated these parameters in patients using cannabis (SCZ/c). Results were compared vs healthy controls non-cannabis users (HC/nc) and healthy controls cannabis users (HC/c). Further, antipsychotic effects on heteromer signaling were tested in vitro in HC/nc and HC/c. Results indicated that heteromer expression was enhanced in both SCZ groups vs HC/nc. Additionally, pooling all 4 groups together, heteromer expression correlated with worse attentional performance and more neurological soft signs (NSS), indicating that these changes may be useful markers for neurocognitive impairment. Remarkably, the previously reported signaling properties of CB1R-5-HT2AR heteromers in ON cells were absent, specifically in SCZ/nc treated with clozapine. These findings were mimicked in cells from HC/nc exposed to clozapine, suggesting a major role of this antipsychotic in altering the quaternary structure of the CB1R-5-HT2AR heteromer in SCZ/nc patients. In contrast, cells from SCZ/c showed enhanced heteromer functionality similar to HC/c. Our data highlight a molecular marker of the interaction between antipsychotic medication and cannabis use in SCZ with relevance for future studies evaluating its association with specific neuropsychiatric alterations.
Keywords: schizophrenia, antipsychotics, cognition, cannabis, human olfactory neuroepithelium
Introduction
Schizophrenia (SCZ) is a complex mental disorder that causes substantial clinical, familial, and economic burden.1,2 Despite advances in preclinical, neuroimaging and genetic research, clear links between etiology, pathophysiology and biological processes, and their relationship to specific behavioral or cognitive symptoms remain unidentified.3–6 This is due to remarkable heterogeneity in the clinical presentation, potentially involving diverse behavioral and cognitive phenotypes, and to the challenge of obtaining viable neurological tissue for the study of underlying neurophysiological processes, among other factors. Recently, the study of the olfactory neuroepithelium (ON) has emerged as a promising tool to help unveil molecular processes involved in some neuropsychiatric disorders, including SCZ.7–12 The human ON is a specialized epithelial tissue that lies on the roof of the nasal cavity,13 and is relatively easy to collect, can proliferate in vitro and differentiate into multiple cell types, including neurons and glia.14
Dysregulations in serotoninergic and endocannabinoid systems have been related to SCZ. Hence, polymorphisms in genes coding for serotonin type 2A receptors (5-HT2AR) have been associated with a risk for SCZ.15 Importantly, these receptors also mediate the action of atypical antipsychotics,16 and antipsychotic-related differential dysregulations in 5-HT2AR expression have been found in postmortem brains of SCZ.17 Additionally, cannabinoid type 1 receptors (CB1R) binding alterations have also been described in postmortem studies of SCZ patients.18,19 Further pointing to the potential relevance of these 2 receptors for SCZ, 2,5-dimethoxy-4-iodoamphetamine (DOI) induces a characteristic hallucinogen response in rodents, attributed to activation of 5-HT2AR, which is increased by endocannabinoids.20,21 Moreover, studies have shown relevant associations between cannabis use and several behavioral, functional and neurocognitive alterations in SCZ,22–26 but the underlying molecular mechanisms of these interactions are unknown. Recently, our group demonstrated that 5-HT2AR and CB1R form heteromers in the brain of mice, where they specifically mediate the memory impairment induced by delta9-tetrahydrocannabinol (THC), the main psychoactive constituent of cannabis.27 Additionally, we revealed the presence of functional CB1R-5-HT2AR heteromers (CB1R-5-HT2AR-HET) in ON cells of human cannabis users and control subjects, showing a significant negative correlation between its expression levels and attention and working memory performance.9 Therefore, a potential functional interaction between CB1R and 5-HT2AR could mediate some behavioral and/or cognitive symptoms in SCZ patients. However, the presence and functionality of this CB1R-5-HT2AR-HET in patients with SCZ remains to be examined.
Antipsychotics are the cornerstone of successful acute treatment and relapse prevention in SCZ.28–31 They are classified as typical, such as haloperidol (HAL), and atypical, such as clozapine (CLZ), olanzapine (OLZ), aripiprazole (ARP), or risperidone (RIS), among others. Although cognitive impairment is a core feature of SCZ,32 and is present across the course of the illness,33,34 specific antipsychotic effects on molecular markers of neurocognition have not been fully characterized.35,36 The 5-HT2AR plays an important role in both the therapeutic and side-effect profile of antipsychotics.16 Interestingly, recent preclinical studies have demonstrated that chronic treatment with atypical, but not with typical antipsychotics induces a 5-HT2AR-dependent increase of HDAC2 epigenetic function via NF-κB, which leads to cognitive dysfunction.37 Moreover, in HEK 293 cells expressing the D2R-5-HT1AR heterodimer, exposure to CLZ or ARP potentiated the functionality of this heteromer via an increase in ERK activation.38 Therefore, it would be relevant to understand whether different types of antipsychotics could differentially modulate the functionality of the CB1R-5-HT2AR-HET in ON cells.
Based on the above literature, we tested the following hypotheses: 1) SCZ patients would show alterations in the formation/functionality of CB1R-5-HT2AR-HET in ON cells, which could be associated to alterations in clinical, functional and cognitive variables; 2) Cannabis use in SCZ patients would modulate changes in heteromer functionality, and 3) Exposure to different types of antipsychotics could have differential effects on CB1R-5-HT2AR-HET signaling.
Methods and Materials
Study Design and Subjects
A cross-sectional study was conducted in SCZ patients non-cannabis users (SCZ/nc) and cannabis users (SCZ/c), and in healthy controls non-cannabis users (HC/nc) and cannabis users (HC/c). Before nasal exfoliation, subjects underwent a complete physical exam, and medical, psychiatric, sociodemographic, family history data were collected, and neuropsychological assessments were performed, as previously described.9 The complete medical records and type/dose of the antipsychotic treatment received in the last 6 months, and other psychopharmacological treatments were collected. The study was approved by the local institutional ethics committee (CEIC-PSMAR). Details about recruitment, inclusion/exclusion criteria, and clinical and functional assessments are detailed in supplementary information.
Neuropsychological Assessment
Attention and working memory performance were evaluated using the spatial span direct recall and the spatial span inverse recall tests, respectively, using the Cambridge Neuropsychological Test Automated Battery (CANTAB 2017), as was social and emotional cognition. The digit span direct recall and the digit span inverse recall were appraised with WAIS-III39 tests. Measures of span length were used for the analysis. Executive functions were evaluated with the semantic verbal fluency test,40 and premorbid intelligence estimation with the vocabulary test (WAIS-III).39
Quantification of Cannabis Consumption in Plasma
To estimate the quantity of cannabis consumed in cannabis users, we measured the plasma concentrations of THC, its initial psychoactive metabolite (11-hydroxy-THC; 11-OHTHC), and its main non-psychoactive metabolite (11-nor-9-carboxy-Δ-9-THC; THC-COOH). An extraction protocol from Waters Corporation was followed with some modifications, as previously reported.9
ON Cell Culture and Biochemical and Molecular Assays
Primary cultures were grown for 3–4 weeks in Dulbecco’s Modified Eagle Medium/Ham F-12 (DMEM/F12) containing 10% FBS, 2% glutamine and 1% streptomycin-penicillin with 10% FBS at 37°C and 5% CO2, as previously described,9 before passaging into flasks. Biochemical and functional assays were carried at passage 4 to ensure that no major epigenetic changes took place in our cell cultures.41 ON cells in monolayer culture show various differentiation stages according to their morphological characteristics.9,42–44 Thus, they can be considered as immature olfactory neurons. The expression of CB1R-5-HT2AR-HET was determined using the proximity ligation assay (PLA), and heteromer signaling was determined through adenylate cyclase (cAMP) and the ERK1/2 phosphorylation pathway (pERK) to evaluate cross-talk and cross-antagonism following treatment with CB1 and 5-HT2A receptor agonists and antagonists, as previously described.9
In vitro Pharmacological Study
To model the effects of chronic administration of antipsychotics and their modulation by cannabis use, we exposed ON cells from HC/nc and HC/c in vitro to 3 antipsychotics with different molecular conformations acting at the 5-HT2AR: ARP, HAL, and CLZ (Sigma Aldrich, Madrid, Spain) for 12 days, and then determined CB1R-5-HT2AR-HET signaling via cAMP production and pERK activation, as previously described.9 Details of the experimental procedure are explained in supplementary information.
Computational Models of Ligand-5-HT2AR Complexes
CLZ, HAL, and ARP were docked into the structures of 5-HT2AR in complex with the homologous RIS (PDB id 6A93) or zotepine (6A94) ligands,45 and THC was docked into CB1R in complex with the homologous AM11542 ligand (5XRA).46 These structures were used to build CB1R-5-HT2AR-HET via transmembrane (TM) helices 5&6 using the structure of the µ-opioid receptor (4DKL).47
Statistical Analyses
Normality of continuous variables was tested with a Shaphiro-Wilk W test. Continuous variables that were not normally distributed were analyzed with the Kruskal–Wallis test. Categorical variables were analyzed using Chi-Square tests, followed by pair-wise post-hoc and/or the false discovery rate (FDR) correction, as appropriate. For neuropsychological data, an adjusted regression model considering tobacco use (present/absent) as a fixed factor was performed. Correlation analyses were performed using Spearman test. The cAMP and pERK data were analyzed with 2-way repeated-measures ANOVAs (group as between-subjects factor and cell treatment as within-subjects factor), followed by the LSD post-hoc test. Data were analyzed with the PASW Statistics v.18, and JMP software v.14. All statistical tests were 2-sides with α = .05.
Results
Demographic and clinical data are shown in table 1 and supplementary table 1. Groups did not differ in age, sex, history of nasal trauma, surgery or rhinitis. Body mass index was significantly higher in SCZ/nc than HC/nc, and the percentage of tobacco users was significantly lower in HC/nc than in HC/c and SCZ/c. Socioeconomic status was significantly lower in SCZ/nc than HC/nc, but not after correction for multiple testing. Both SCZ groups had significantly lower functionality scores vs HC/nc and HC/c, but SCZ/c exhibited significantly lower scores than SCZ/nc. Conversely, while neurological soft signs (NSS) were higher in both SCZ groups vs HC/nc and HC/c, SCZ/c showed significantly lower scores vs SCZ/nc. Depression and psychosis severity scores did not differ among groups. Antipsychotic treatments for SCZ patients are shown in supplementary table 2.
Table 1.
Demographic Characteristics of the Different Groups Included in the Study
| Healthy Controls Non-Cannabis Users (HC/nc) (n = 18) | Healthy Controls Cannabis Users (HC/c) (n = 20) | Schizophrenia Non-Cannabis Users (SCZ/nc) (n = 16) | Schizophrenia Cannabis Users (SCZ/c) (n = 10) | |
|---|---|---|---|---|
| Age (y)† | 28 (26.8–33) | 28.5 (22.5–33.3) | 32 (30–43) | 30.5 (26.5–42.3) |
| Gender; male (%) | 11 (61.1) | 15 (75.0) | 11 (68.8) | 10 (100.0) |
| BMI (kg/m2)† | 22.7 (20.4–25.3) | 23.1 (18.6–25.6) | 29.4 (25.4–35.0)** /## | 25.7 (24.1–28.2) |
| History of nasal trauma/surgery; n (%) | 4 (22.2) | 6 (30.0) | 3 (18.8) | 1 (10.0) |
| History of rhinitis; n (%) | 2 (11.1) | 4 (20.0) | 0 (0) | 2 (20.0) |
| Tobacco use | ||||
| Users – n, (%) | 6 (35.3) | 16 (80.0) | 10 (62.5) | 10 (100.0) |
| Units per week† | 42 (28.8–140) | 28 (14–70) | 175 (79–280)## | 105 (70–210)# |
| Length of use (y)† | 9 (5–15.8) | 6.5 (2.8–17.8) | 15 (7.5–17) | 15 (4–25.3) |
| Cannabis use | ||||
| Age first use (y)‡ | n/a | 15.7 (1.9) | n/a | 16.3 (2.3) |
| Units per week† | n/a | 8 (4.3–21) | n/a | 18 (4.8–21) |
| Length of use (y)† | n/a | 8 (3.3–13.8) | n/a | 13.5 (9.3–23.5)# |
Note: For continuous, non-normally distributed variables (†) results are reported in median (first quartile-third quartile) and provided statistics refer to the Kruskal–Wallis test. For continuous, normally distributed variables (‡) results are reported in mean (SD) and provided statistics refer to ANOVA followed by post-hoc test. Body mass index (BMI) [χ 2 (3) = 20.13; P < .001], and the percentage of tobacco users [χ 2 (3) =14.25, P < .01] were significantly different between groups.
*P < .05; **P < .01; ***P < .001 vs HC/nc; #P < .05, ##P < .01, ##P < .001 vs HC/c.
Neuropsychological Performance
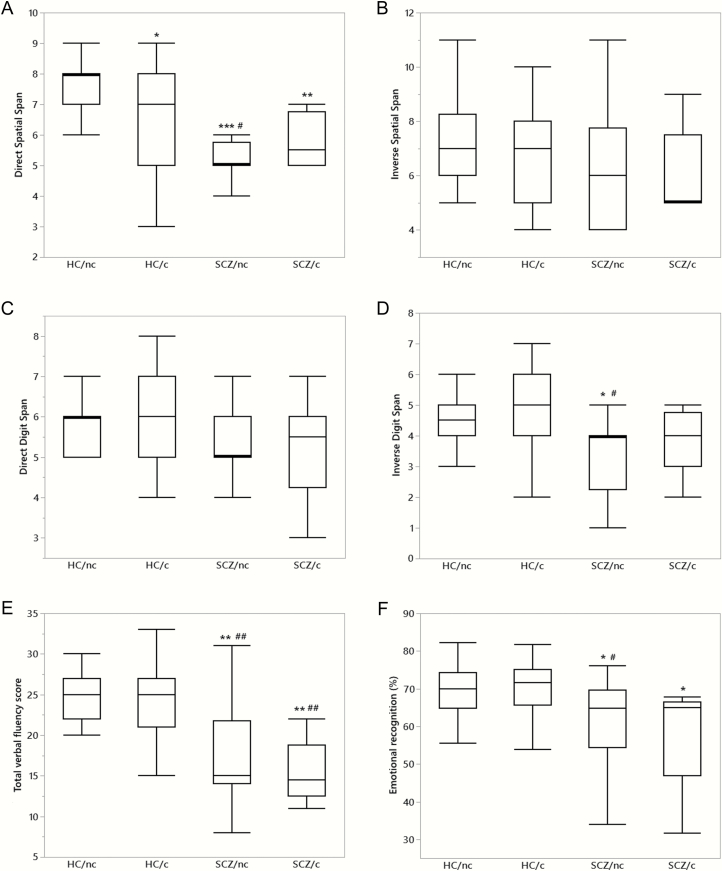
Neuropsychological data are shown in figure 1. Attention (figure 1A) was significantly worse in HC/c (n = 20), SCZ/nc (n = 16), and SCZ/c (n = 10) vs HC/nc (n = 18). Spatial span inverse recall (figure 1B), and digit span direct recall (figure 1C) did not significantly differ between groups. SCZ/nc also showed significantly worse performance than HC/c. Working memory (figure 1D) was significantly worse in SCZ/nc than HC/c and HC/c, while SCZ/c showed similar scores vs HC/nc and HC/c. Semantic verbal fluency performance (figure 1E) was significantly worse in SCZ/nc and in SCZ/c vs both healthy control groups. A significantly worse performance in emotional recognition (figure 1F) was also detected in both SCZ groups vs HC/nc, but SCZ/nc also showed worse performance than HC/c. These comparisons remained significant after adjusting for tobacco use. No significant group differences were found in the remaining neuropsychological tests.
Fig. 1.
Neuropsychological assessments in healthy subjects who do not use cannabis (HC/nc), healthy subjects who use cannabis (HC/c), schizophrenia patients who do not use cannabis (SCZ/nc), and schizophrenia patients who use cannabis (SCZ/c). Significant differences between groups were observed [χ 2(3) = 22.54, P < 0.001] in the direct spatial span test (A), but not in the inverse spatial span or the direct digit span tests (B and C). The inverse digit span (D) [χ 2(3) = 11.23, P < .01], semantic verbal fluency [χ 2(3) = 24.45, P < 0.001] (E), and emotional recognition [χ 2(3) = 11.30, P < 0.01] (F) performance were also different between groups. The data are box plots displaying the minimum, first quartile, median, third quartile, and maximum. *P < .05, **P < .01, ***P < .001 vs HC/nc; #P < .05, ##P < .01, ###P < .001 vs HC/c.
Increased Expression of CB1R-5-HT2AR-HET in ON Cells of SCZ Patients
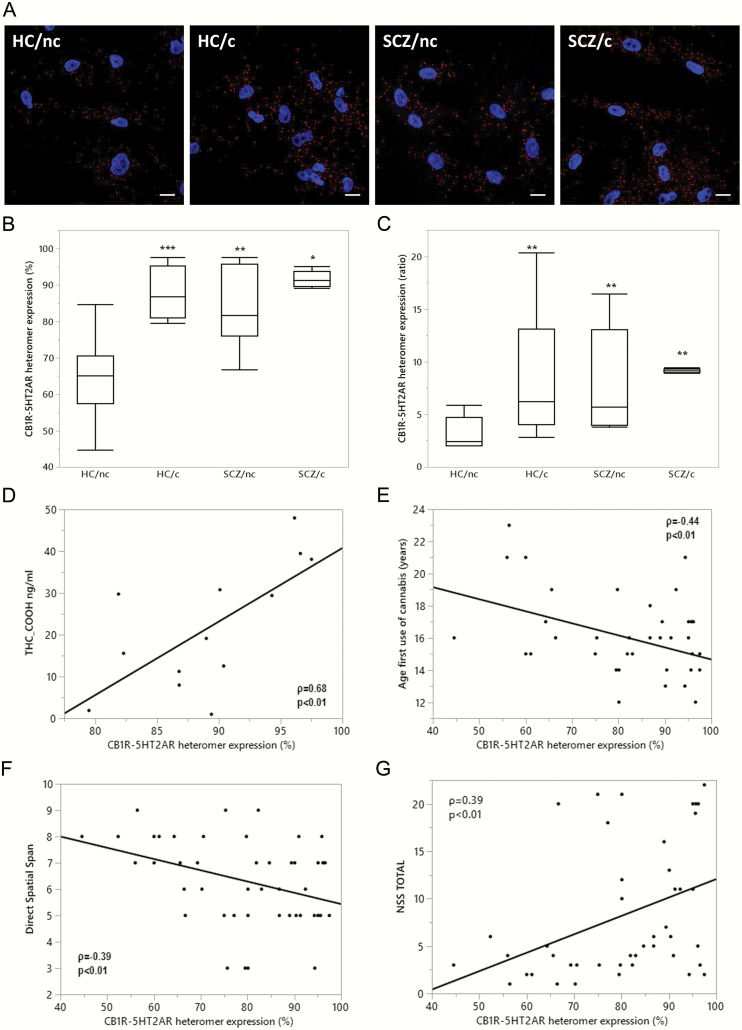
Representative photomicrographs of CB1R-5-HT2AR-HET expression, quantified with PLA in ON cells of HC/nc, HC/c, SCZ/nc, and SCZ/c, are shown in figure 2A. The percentage of cells containing heteromers (figure 2B) was significantly higher in SCZ/nc, SCZ/c, and HC/c vs HC/nc. Similarly, the number of heteromers per positive cells was significantly higher in SCZ/nc, SCZ/c, and HC/c vs HC/nc (figure 2C).
Fig. 2.
CB1R-5-HT2AR-HET expression in healthy subjects who do not use cannabis (HC/nc), healthy subjects who use cannabis (HC/c), schizophrenia patients who do not smoke cannabis (SCZ/nc), and schizophrenia patients who use cannabis (SCZ/c). (A) Representative confocal microscopy images of ON cells processed in proximity ligation assays showing heteromers appearing as red spots, and cell nuclei stained with DAPI (blue). Scale bars = 20 μm. (B) The percentage of cells containing heteromers [χ 2(3) = 23.13, P < .001], and (C) the number of heteromers per positive cells (ratio) [χ 2(3) = 19.69, P < .001] differed significantly between groups. The data are represented in box plots displaying the minimum, first quartile, median, third quartile, and maximum. *P < .05,**P < .01,***P < .001 vs HC/nc. Significant spearman correlations between the percentage of cells expressing 5-HT2AR-CB1R-HET and: (D) plasma concentrations of THC-COOH in subjects who use cannabis (n = 13) (ρ = 0.68; P < .01); (E) age of onset of cannabis use in all subjects who have tried cannabis at least once in their lifetime (N = 35) (ρ = −0.44; P < .01); (F) attention in the direct spatial span test (ρ = −0.39, P < .01), and (G) neurological soft-signs (NSS)(G) neurological soft-signs (NSS) (ρ = 0.39, P < .01) in the entire population.
Associations Between CB1R-5-HT2AR-HET Expression, Cannabis Use, and Neurocognitive Performance
The percentage of heteromer expression in ON cells of HC/c and SCZ/c positively correlated with plasma levels of THC-COOH (figure 2D), and negatively with age of onset of cannabis use in the population who tried cannabis at least once in their lifetime (n = 35; figure 2E). In the entire population, heteromer expression correlated negatively with attention performance (figure 2F) and positively with the number of NSS (figure 2G). Similar results were obtained for the mean number of heteromers per positive cells (Data not shown). These comparisons remained significant after adjusting for tobacco use. No significant correlations were found for neurocognitive performance or other clinical or functional variables and heteromer expression within groups.
Functional Characteristics of CB1R-5-HT2AR-HET in ON Cells of SCZ Patients
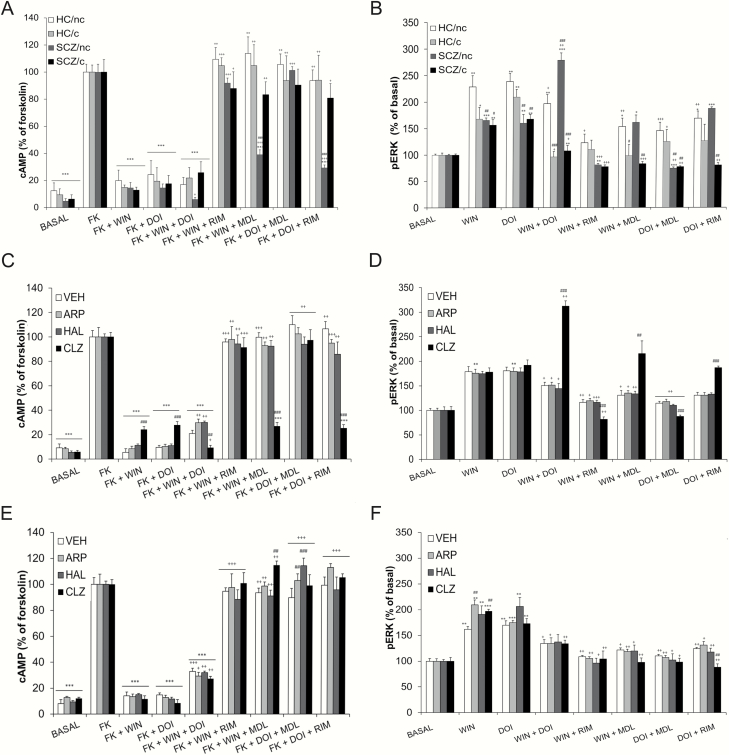
We determined heteromer signaling by measuring cAMP levels (decrease of forskolin-induced cAMP), and through activation of the ERK1/2 phosphorylation pathway in ON cells of HC/nc, HC/c, SCZ/nc, and SCZ/c (figure 3A). Importantly, we have previously found that the formation of the CB1R-5-HT2AR heteromer leads to a switch in G-protein coupling for 5-HT2AR from Gq to Gi proteins.27 Thus, cells stimulated with forskolin and treated with the CB1R agonist WIN 55,212–2 (WIN) or the 5-HT2AR agonist DOI showed reduced cAMP production in all groups, as expected for Gi-coupled receptors. Also, when both receptors were stimulated together, an additive decrease of cAMP was not observed (presence of negative cross-talk) in HC/nc, HC/c, and SCZ/c. In contrast, in SCZ/nc the co-administration of WIN+DOI leads to a further significant decrease of cAMP (figure 3A, absence of negative cross-talk). Similar results were obtained for pERK, where a further increased activation was observed, revealing no negative cross-talk only in SCZ/nc (figure 3B). Furthermore, the CB1R antagonist rimonabant (RIM) and the 5-HT2AR antagonist MDL 100,907 (MDL) blocked the decrease of forskolin-induced cAMP triggered by WIN and DOI in HC/c, HC/nc, and SCZ/c (cross-antagonism). However, in SCZ/nc, RIM did not block the decrease of cAMP triggered by DOI, and MDL did not block the decrease of cAMP triggered by WIN, indicating no cross-antagonism (figure 3A). Similar results were obtained for pERK, where no cross-antagonism was observed only in SCZ/nc (figure 3B).
Fig. 3.
CB1R-5-HT2AR-HET functionality in ON cells. (A and B) cells were obtained from healthy subjects who do not use cannabis (HC/nc), healthy subjects who use cannabis (HC/c), schizophrenia patients who do not smoke cannabis (SCZ/nc), and schizophrenia patients who use cannabis (SCZ/c). (A) cAMP production (B) pERK activation *P < .05,**P < .01,***P < .001 vs basal values; +P < .05, ++P < .01, +++P < .001 vs WIN or DOI treatment; #P < .05, ##P < .01, ###P < .001 vs HC/nc. (C–F) ON cells obtained from healthy subjects non-cannabis users (HC/nc) (C and D) and from healthy subjects cannabis users (HC/c) (E and F) treated in vitro with vehicle (VEH), aripiprazole (ARP), haloperidol (HAL), or clozapine (CLZ) during 12 days. (C) cAMP production in HC/nc, (D) pERK activation in HC/nc. (E) cAMP production in HC/c. (F) pERK activation in HC/c. *P < .05, **P < .01, ***P < .001 vs basal values; + P < .05, ++P < .01, +++P < .001 vs WIN or DOI treatment and ##P < .01, ###P < .001 vs the VEH group. Data are mean+SEM. ANOVA values for each panel are shown in supplementary table 3.
To understand whether the effects observed were due to a specific type of antipsychotic treatment, the data from SCZ/nc receiving CLZ/OLZ and ARP/RIS were analyzed separately. Results showed the known signature of the heteromer (negative cross-talk and cross-antagonism) for both cAMP and pERK in cells from SCZ/nc treated with ARP/RIS, while cells from SCZ/nc treated with CLZ/OLZ did not show cross-talk or cross-antagonism (supplementary figure 1).
CB1R-5-HT2AR-HET Functionality in ON Cells Following Repeated Antipsychotic Treatment in vitro
To investigate whether the changes in heteromer functionality observed with CLZ in SCZ/nc were specifically related to its interaction with the disease, we exposed ON cells from HC/nc and HC/c users to different types of antipsychotics and evaluated heteromer signaling (Fig. 3). ON cells from HC/nc stimulated with forskolin and treated with WIN or DOI showed a decrease of cAMP in all groups exposed repeatedly to VEH, CLZ, ARP, and HAL compared to forskolin alone. However, in the CLZ group, this decrease was significantly lower vs the other groups. Remarkably, the co-administration of WIN+DOI lead to a greater decrease of cAMP only in cells treated with CLZ (no negative cross-talk), in contrast to cells exposed to VEH, ARP, or HAL (negative cross-talk) (Fig. 3A). Similarly, WIN+DOI lead to significantly higher pERK levels only in cells exposed to CLZ (no negative cross-talk) (Fig. 3B). Moreover, cross-antagonism was observed in both cAMP and pERK measurements in cells exposed to VEH, ARP, and HAL, but not in cells treated with CLZ. These data are consistent with the results obtained in SCZ/nc treated with CLZ, suggesting that heteromer signaling alterations in SCZ may be mostly due to CLZ treatment.
Cannabis use prevented the signaling changes induced by CLZ since opposing results were obtained in cells from HC/c, where negative cross-talk and bidirectional cross-antagonism appeared in both cAMP (Fig. 3C) and pERK (Fig. 3D) measurements in all groups (VEH, CLZ, ARP, and HAL).
Discussion
ON cells are pro-neuronal tissue that can be easily obtained from a living human and have quickly become a promising tool to characterize underlying cellular and biochemical processes related to brain disorders, including SCZ.7,48 We previously reported increased CB1R-5-HT2AR-HET expression in ON cells of cannabis users vs control subjects.9 Here, we extend these findings by showing that the expression of this heteromer is also enhanced in SCZ patients vs control subjects, irrespective of cannabis use. Conversely, we discovered that the previously reported signaling properties (presence of negative cross-talk and cross-antagonism) of CB1R-5-HT2AR-HET in ON cells were absent in SCZ/nc, but not in SCZ/c.
In this study, the positive correlation between heteromer expression levels in ON cells with plasma concentrations of THC-COOH found in our previous study with HC/c users,9 remained significant when including SCZ/c. Furthermore, in all subjects that had tried cannabis at least once in their lifetime, age of first onset of cannabis use was positively correlated with heteromer expression. In agreement, studies in animal models have reported that acute THC administration increased the number of CB1R-5-HT2AR-HET in the brain of mice,27 and chronic THC increased the number of dopamine D1-D2 receptor heteromers in striatal neurons of monkeys.49 Our findings suggest that possible epigenetic changes taking place in SCZ,50–52 and following chronic cannabis use,49 may favor CB1R-5-HT2AR-HET formation.
We found that HC/c and both groups of SCZ patients showed deficits in the direct recall spatial span test, but not in the inverse recall spatial span test. These processes differ in that both require attention, but the inverse recall also engages working memory’s central executive functions.53,54 Thus, our results suggest that both cannabis use and SCZ alter the attentional component of cognitive processing. However, SCZ/nc also showed deficits in the inverse recall digit span test, but not in the direct recall digit span test. Again, the inverse recall digit span test is more cognitively demanding (working memory) than the direct digit recall. Thus, SCZ/nc may be more cognitively compromised than HC/c and SCZ/c since they present both attention and working memory deficits. While HC/c and both groups of SCZ patients exhibited a significant increase in heteromer expression, in the entire population, enhanced heteromer formation correlated with worse attention performance, and with more NSS (abnormalities in sensorimotor integration increased in SCZ vs HC55). Thus, while increased formation of heteromers in ON cells may biologically link to neurocognitive impairment and sensorimotor integration, more data are needed to corroborate these results. Future studies in postmortem brains of patients may shed light into the relevant corticostriatal circuits involved, where highly expressed CB1R and 5-HT2AR modulate these processes.56,57
We have previously provided evidence that the negative cross-talk and bidirectional cross-antagonism properties of CB1R-5-HT2AR-HET are due to an interaction of CB1R with 5-HT2AR via TMs 5&6.27 The experimental data for HC/nc, HC/c, and SCZ/c agree with this proposal (supplementary figure 2, top panels). On the other hand, the data from SCZ/nc points to a different quaternary structure of the heteromer (supplementary figure 2, lower panels), where the outward movement of TM6 for G-protein binding in both protomers is feasible, triggering further decrease of cAMP or further increase of pERK (no negative cross-talk), and antagonist binding to either protomer also stabilizes the inactive conformations of TMs 5&6, but does not stabilize the subsequent formation of the very stable 4-helix association, facilitating receptor activation (no cross-antagonism). Thus, our findings suggest that in ON cells of SCZ/nc, the biochemical signature of the CB1R-5-HT2AR-HET is modified due to a slightly different heteromeric interface. Furthermore, a separate analysis of the data in SCZ/nc indicated that only cells from patients treated with CLZ/OLZ, but not those treated with ARP/RIS, showed altered heteromer signaling. These findings are consistent with those obtained in vitro following repeated exposure to CLZ in cells from HC/nc and suggest a major role of CLZ in heteromer conformational changes in SCZ patients, although confirmatory studies should be carried out in antipsychotic-naïve patients.
Moreover, we found that cannabis use prevents these alterations in heteromer signaling in cells from SCZ/c, where enhanced negative cross-talk and cross-antagonism was observed, similar to HC/c. These opposite changes in pERK and cAMP signaling in ON cells from SCZ/nc and SCZ/c were associated with neurocognitive impairment in both groups. pERK1/2 and cAMP have been related to neuronal plasticity and memory processes,58 and alterations in these intracellular markers have been observed in postmortem brain samples of SCZ patients,59 and pluripotent stem cells (iPSCs) in a DISC1 model of SCZ.60 Our findings in ON cells are consistent with these data, and suggest that changes in the expression and functionality of CB1R-5-HT2AR-HET may be useful markers for neurocognitive impairment in SCZ.
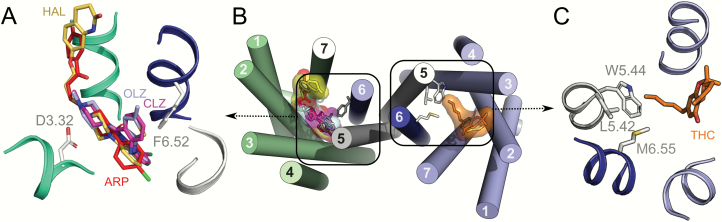
Aiming to understand this molecular signature of CLZ and OLZ, relative to HAL and ARP, we show in figure 4A and 4B the docking models of these molecules on the 5-HT2AR. These ligands bind to the same orthosteric pocket, but CLZ/OLZ occupy an additional volume near TMs 5&6, whereas HAL/ARP expand toward the extracellular environment. We propose that the specific binding properties of CLZ/OLZ may trigger transcriptional mechanisms that ultimately lead to an alternative heteromeric interface in the CB1R-5-HT2AR-HET. Consistent with this notion, previous studies have shown that chronic treatment with CLZ, but not HAL induces epigenetic alterations in the histone deacetylase, HDAC2 in a 5-HT2AR-dependent manner in mice, and in postmortem brains of SCZ patients.37 These changes in gene expression may modulate the quaternary structure of the CB1R-5-HT2AR-HET by altering the expression of additional proteins that either associate with the heteromer or modify the heteromer post-translationally. Although not related with drug effects, we have recently reported that post-translational modifications, involving phosphorylated amino acids in the C-tail modulated the quaternary structure of another dimer, the A2AR-CB1R heteromer.61 This CLZ/OLZ-induced alternative interface tolerates simultaneous activation of both receptors (absence of negative cross-talk) and does not show the cross-antagonism signature. Since these changes in the quaternary structure of the heteromer due to CLZ/OLZ are prevented by cannabis use, we docked THC, the most prevalent of the active compounds found in cannabis, to CB1R (figure 4B and 4C). The model shows that the alkyl chain of THC expands towards TMs 5&6 to occupy a hydrophobic pocket formed by L5.42, W5.45, and M6.55. We propose that exposure to cannabis (THC) stabilizes the known conformation of TMs 5&6 of CB1R, such that binding of CLZ to 5-HT2AR cannot trigger the alternative interface (supplementary figure 3).
Fig. 4.
Molecular model of CB1R-5-HT2AR-HET. (A) Detailed view of 5-HT2AR bound to CLZ (fuscia), OLZ (light blue), HAL (yellow), or ARP (red), showing that the aromatic ring of CLZ/OLZ, in contrast to the others, expands towards TM6 and interacts with F6.52. (B) Quaternary structure of the CB1R-5-HT2AR-HET to illustrate how CLZ/OLZ and THC interact with amino acids near the heteromerization interface (TMs 5&6) that could trigger changes in the interface. (C) Binding of THC (orange) to CB1R shows that the alkyl chain interacts with L5.42, W5.45, and M6.55 in TMs 5&6.
In conclusion, we report that both SCZ/nc and SCZ/c show an increase in CB1R-5-HT2AR-HET expression in ON cells, as well as altered heteromer functionality. In SCZ/nc treated with CLZ, stimulation of the heterodimer leads to a further increase in pERK1/2 and a further decrease in cAMP signaling, suggesting alterations in the heterodimer interface possibly due to changes in its quaternary structure. Conversely, in SCZ/c stimulation of the heterodimer leads to further reduction in pERK1/2 and further increase of cAMP (enhanced cross-talk). Both of these effects were associated with neurocognitive impairment. These findings indicate that CLZ treatment alters the functionality of CB1R-5-HT2AR-HET, and that cannabis use prevents these changes, possibly due to its specific interaction with hydrophobic residues at TMs 5&6 of CB1R. Our results also emphasize the utility of using the ON cell model in neuropsychiatric research.
Funding
This work was supported by grants from DIUE de la Generalitat de Catalunya, Secretaria General D’Economía i Coneixement (2014-SGR-680 and 2017-SGR-1497), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación de España y fondos FEDER (PI14/00210 and PI18/00053 to P.R.), FIS-FEDER Funds, Ministerio de Economía y Competitividad de España (SAF-2017-87629-R to E.I.C. and V.C., SAF-2015-69762-R to J.M.F-F., SAF2015-74627-JIN to A.C., PID2019-109240RB-I00 to L.P.), Programa de Excelencia María de Maeztu, Ministerio de Ciencia e Innovación de España (MDM-2014-0370), Red de Trastornos Adictivos, Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación de España, Centro de Investigación Biomédica en Red de Salud Mental, Instituto de Salud Carlos III, Centro de Investigación Biomédica en Red de Fisiopatología de la Obesidad y Nutrición, Instituto de Salud Carlos III, Centro de Investigación Biomédica en Red de Enfermedades Neurodegenerativas, Instituto de Salud Carlos.
Supplementary Material
Acknowledgments
We would like to thank Jordi García and Mitona Pujadas for their excellent technical assistance. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Fusar-Poli P, McGorry PD, Kane JM. Improving outcomes of first-episode psychosis: an overview. World Psychiatry. 2017;16(3):251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chong HY, Teoh SL, Wu DB, Kotirum S, Chiou CF, Chaiyakunapruk N. Global economic burden of schizophrenia: a systematic review. Neuropsychiatr Dis Treat. 2016;12:357–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flores G, Morales-Medina JC, Diaz A. Neuronal and brain morphological changes in animal models of schizophrenia. Behav Brain Res. 2016;301:190–203. [DOI] [PubMed] [Google Scholar]
- 4. Potkin SG, Macciardi F, Guffanti G, et al. Identifying gene regulatory networks in schizophrenia. Neuroimage. 2010;53(3):839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Howes OD, McCutcheon R, Owen MJ, Murray RM. The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry. 2017;81(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van der Merwe C, Passchier R, Mufford M, Ramesar R, Dalvie S, Stein DJ. Polygenic risk for schizophrenia and associated brain structural changes: a systematic review. Compr Psychiatry. 2019;88:77–82. [DOI] [PubMed] [Google Scholar]
- 7. Lavoie J, Sawa A, Ishizuka K. Application of olfactory tissue and its neural progenitors to schizophrenia and psychiatric research. Curr Opin Psychiatry. 2017;30(3):176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horiuchi Y, Kano S, Ishizuka K, et al. Olfactory cells via nasal biopsy reflect the developing brain in gene expression profiles: utility and limitation of the surrogate tissues in research for brain disorders. Neurosci Res. 2013;77(4):247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galindo L, Moreno E, López-Armenta F, et al. Cannabis users show enhanced expression of CB1-5HT2A receptor heteromers in olfactory neuroepithelium cells. Mol Neurobiol. 2018;55(8):6347–6361. [DOI] [PubMed] [Google Scholar]
- 10. Borgmann-Winter K, Willard SL, Sinclair D, et al. Translational potential of olfactory mucosa for the study of neuropsychiatric illness. Transl Psychiatry. 2015;5:e527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mackay-Sim A. Concise review: patient-derived olfactory stem cells: new models for brain diseases. Stem Cells. 2012;30(11):2361–2365. [DOI] [PubMed] [Google Scholar]
- 12. Benítez-King G, Valdés-Tovar M, Trueta C, et al. The microtubular cytoskeleton of olfactory neurons derived from patients with schizophrenia or with bipolar disorder: implications for biomarker characterization, neuronal physiology and pharmacological screening. Mol Cell Neurosci. 2016;73:84–95. [DOI] [PubMed] [Google Scholar]
- 13. Moran DT, Rowley JC III, Jafek BW, Lovell MA. The fine structure of the olfactory mucosa in man. J Neurocytol. 1982;11(5):721–746. [DOI] [PubMed] [Google Scholar]
- 14. Matigian N, Abrahamsen G, Sutharsan R, et al. Disease-specific, neurosphere-derived cells as models for brain disorders. Dis Model Mech. 2010;3(11–12):785–798. [DOI] [PubMed] [Google Scholar]
- 15. Miyamoto S, LaMantia AS, Duncan GE, Sullivan P, Gilmore JH, Lieberman JA. Recent advances in the neurobiology of schizophrenia. Mol Interv. 2003;3(1):27–39. [DOI] [PubMed] [Google Scholar]
- 16. Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10(1):79–104. [DOI] [PubMed] [Google Scholar]
- 17. Muguruza C, Moreno JL, Umali A, Callado LF, Meana JJ, González-Maeso J. Dysregulated 5-HT(2A) receptor binding in postmortem frontal cortex of schizophrenic subjects. Eur Neuropsychopharmacol. 2013;23(8):852–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jenko KJ, Hirvonen J, Henter ID, et al. Binding of a tritiated inverse agonist to cannabinoid CB1 receptors is increased in patients with schizophrenia. Schizophr Res. 2012;141(2–3):185–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zavitsanou K, Garrick T, Huang XF. Selective antagonist [3H]SR141716A binding to cannabinoid CB1 receptors is increased in the anterior cingulate cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(2):355–360. [DOI] [PubMed] [Google Scholar]
- 20. Darmani NA. Cannabinoids of diverse structure inhibit two DOI-induced 5-HT(2A) receptor-mediated behaviors in mice. Pharmacol Biochem Behav. 2001;68(2):311–317. [DOI] [PubMed] [Google Scholar]
- 21. Ibarra-Lecue I, Mollinedo-Gajate I, Meana JJ, Callado LF, Diez-Alarcia R, Urigüen L. Chronic cannabis promotes pro-hallucinogenic signaling of 5-HT2A receptors through Akt/mTOR pathway. Neuropsychopharmacology. 2018;43(10):2028–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Di Forti M, Morgan C, Selten JP, Lynskey M, Murray RM. High-potency cannabis and incident psychosis: correcting the causal assumption - Authors’ reply. Lancet Psychiatry. 2019;6(6):466–467. [DOI] [PubMed] [Google Scholar]
- 23. van der Meer FJ, Velthorst E; Genetic Risk and Outcome of Psychosis (GROUP) Investigators Course of cannabis use and clinical outcome in patients with non-affective psychosis: a 3-year follow-up study. Psychol Med. 2015;45(9):1977–1988. [DOI] [PubMed] [Google Scholar]
- 24. Bergé D, Mané A, Salgado P, et al. Predictors of relapse and functioning in first-episode psychosis: a two-year follow-up study. Psychiatr Serv. 2016;67(2):227–233. [DOI] [PubMed] [Google Scholar]
- 25. Mané A, Bergé D, Penzol MJ, et al. ; PEPs Group Cannabis use, COMT, BDNF and age at first-episode psychosis. Psychiatry Res. 2017;250:38–43. [DOI] [PubMed] [Google Scholar]
- 26. Ruiz-Veguilla M, Callado LF, Ferrin M. Neurological soft signs in patients with psychosis and cannabis abuse: a systematic review and meta-analysis of paradox. Curr Pharm Des. 2012;18(32):5156–5164. [DOI] [PubMed] [Google Scholar]
- 27. Viñals X, Moreno E, Lanfumey L, et al. Cognitive impairment induced by delta9-tetrahydrocannabinol occurs through heteromers between cannabinoid CB1 and serotonin 5-HT2A receptors. PLoS Biol. 2015;13(7):e1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buchanan RW, Kreyenbuhl J, Kelly DL, et al. ; Schizophrenia Patient Outcomes Research Team (PORT) The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Keating D, McWilliams S, Schneider I, et al. Pharmacological guidelines for schizophrenia: a systematic review and comparison of recommendations for the first episode. BMJ Open. 2017;7(1):e013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063–2071. [DOI] [PubMed] [Google Scholar]
- 31. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019; 394(10202): P939– P951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keefe RSE. The longitudinal course of cognitive impairment in schizophrenia: an examination of data from premorbid through posttreatment phases of illness. J Clin Psychiatry. 2014;75(Suppl 2):8–13. [DOI] [PubMed] [Google Scholar]
- 34. Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23(3):315–336. [DOI] [PubMed] [Google Scholar]
- 35. Husa AP, Moilanen J, Murray GK, et al. Lifetime antipsychotic medication and cognitive performance in schizophrenia at age 43 years in a general population birth cohort. Psychiatry Res. 2017;247:130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Désaméricq G, Schurhoff F, Meary A, et al. Long-term neurocognitive effects of antipsychotics in schizophrenia: a network meta-analysis. Eur J Clin Pharmacol. 2014;70(2):127–134. [DOI] [PubMed] [Google Scholar]
- 37. Ibi D, de la Fuente Revenga M, Kezunovic N, et al. Antipsychotic-induced Hdac2 transcription via NF-κB leads to synaptic and cognitive side effects. Nat Neurosci. 2017;20(9):1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Łukasiewicz S, Błasiak E, Szafran-Pilch K, Dziedzicka-Wasylewska M. Dopamine D2 and serotonin 5-HT1A receptor interaction in the context of the effects of antipsychotics - in vitro studies. J Neurochem. 2016;137(4):549–560. [DOI] [PubMed] [Google Scholar]
- 39. Wechsler D. Escala de Inteligencia de Wechsler III. Madrid, Spain: Pearson. Educación; 1997. [Google Scholar]
- 40. Benton A, Hamsher K. Multilingual Aphasia Exam. 3rd ed Iowa City, IA: The University of Iowa; 1983. [Google Scholar]
- 41. Pastor DM, Poritz LS, Olson TL, et al. Primary cell lines: false representation or model system? a comparison of four human colorectal tumors and their coordinately established cell lines. Int J Clin Exp Med. 2010;3(1):69–83. [PMC free article] [PubMed] [Google Scholar]
- 42. Benítez-King G, Riquelme A, Ortíz-López L, et al. A non-invasive method to isolate the neuronal linage from the nasal epithelium from schizophrenic and bipolar diseases. J Neurosci Methods. 2011;201(1):35–45. [DOI] [PubMed] [Google Scholar]
- 43. Galvan-Arrieta T, Trueta C, Cercos MG, et al. The role of melatonin in the neurodevelopmental etiology of schizophrenia: a study in human olfactory neuronal precursors. J Pineal Res. 2017;63(3) e12421. [DOI] [PubMed] [Google Scholar]
- 44. Ortiz-López L, González-Olvera JJ, Vega-Rivera NM, et al. Human neural stem/progenitor cells derived from the olfactory epithelium express the TrkB receptor and migrate in response to BDNF. Neuroscience. 2017;355:84–100. [DOI] [PubMed] [Google Scholar]
- 45. Kimura KT, Asada H, Inoue A, et al. Structures of the 5-HT2A receptor in complex with the antipsychotics risperidone and zotepine. Nat Struct Mol Biol. 2019;26(2):121–128. [DOI] [PubMed] [Google Scholar]
- 46. Hua T, Vemuri K, Nikas SP, et al. Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature. 2017;547(7664):468–471. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47. Manglik A, Kruse AC, Kobilka TS, et al. Crystal structure of the µ-opioid receptor bound to a Morphinan antagonist. Nature. 2012;485(7398):321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jiménez-Vaca AL, Benitez-King G, Ruiz V, et al. Exfoliated human olfactory neuroepithelium: a source of neural progenitor cells. Mol Neurobiol. 2018;55(3):2516–2523. [DOI] [PubMed] [Google Scholar]
- 49. Hasbi A, Madras BK, Bergman J, et al. Δ-Tetrahydrocannabinol increases dopamine D1-D2 receptor heteromer and elicits phenotypic reprogramming in adult primate striatal neurons. iScience. 2020;23(1):100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Roth TL, Lubin FD, Sodhi M, Kleinman JE. Epigenetic mechanisms in schizophrenia. Biochim Biophys Acta. 2009;1790(9):869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wockner LF, Noble EP, Lawford BR, et al. Genome-wide DNA methylation analysis of human brain tissue from schizophrenia patients. Transl Psychiatry. 2014;4:e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bioque M, Mas S, Costanzo MC, et al. ; PEPs GROUP Gene-environment interaction between an endocannabinoid system genetic polymorphism and cannabis use in first episode of psychosis. Eur Neuropsychopharmacol. 2019;29(6):786–794. [DOI] [PubMed] [Google Scholar]
- 53. Baddeley A, Logie R, Bressi S, Della Sala S, Spinnler H. Dementia and working memory. Q J Exp Psychol A. 1986;38(4):603–618. [DOI] [PubMed] [Google Scholar]
- 54. Hester RL, Kinsella GJ, Ong B. Effect of age on forward and backward span tasks. J Int Neuropsychol Soc. 2004;10(4):475–481. [DOI] [PubMed] [Google Scholar]
- 55. Dazzan P, Murray RM. Neurological soft signs in first-episode psychosis: a systematic review. Br J Psychiatry Suppl. 2002;43:s50–s57. [DOI] [PubMed] [Google Scholar]
- 56. Fernández-Ruiz J. The endocannabinoid system as a target for the treatment of motor dysfunction. Br J Pharmacol. 2009;156(7):1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Goodman J, Packard MG. The influence of cannabinoids on learning and memory processes of the dorsal striatum. Neurobiol Learn Mem. 2015;125:1–14. [DOI] [PubMed] [Google Scholar]
- 58. Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14(3):311–317. [DOI] [PubMed] [Google Scholar]
- 59. McGuire JL, Depasquale EA, Funk AJ, et al. Abnormalities of signal transduction networks in chronic schizophrenia. npj Schizophr. 2017;3(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bentea E, Depasquale EAK, O’Donovan SM, et al. Kinase network dysregulation in a human induced pluripotent stem cell model of DISC1 schizophrenia. Mol Omics. 2019;15(3):173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Köfalvi A, Moreno E, Cordomí A, et al. Control of glutamate release by complexes of adenosine and cannabinoid receptors. BMC Biol. 2020;18(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.