Abstract
Electronic cigarettes are the most commonly used nicotine containing product among teenagers. The oral epithelium is the first site of exposure and our recent work revealed considerable diversity among e-liquids for composition and level of chemical constituents that impact nicotine deposition in a human oral-trachea cast and affect the formation of reactive carbonyls. Here, we evaluate the dose response for cytotoxicity and genotoxicity of e-cigarette-generated aerosols from 10 diverse flavored e-liquid products with and without nicotine compared with unflavored in 3 immortalized oral epithelial cell lines. Three e-liquids, Blue Pucker, Love Potion, and Jamestown caused ≥20% cell toxicity assessed by the neutral red uptake assay. Nine products induced significant levels of oxidative stress up to 2.4-fold quantified by the ROS-Glo assay in at least 1 cell line, with dose response seen for Love Potion with and without nicotine across all cell lines. Lipid peroxidation detected by the thiobarbituric acid reactive substances assay was less common among products; however, dose response increases up to 12-fold were seen for individual cell lines. Micronuclei formation indicative of genotoxicity was increased up to 5-fold for some products. Blue Pucker was the most genotoxic e-liquid, inducing micronuclei across all cell lines irrespective of nicotine status. A potency score derived from all assays identified Blue Pucker and Love Potion as the most hazardous e-liquids. These in vitro acute exposure studies provide new insight about the potential for some flavored vaping products to induce significant levels of oxidative stress and genotoxicity.
Keywords: electronic cigarette, oral epithelial cells, cytotoxicity, genotoxicity
Electronic cigarettes (e-cigarettes) are the most commonly used nicotine containing product among youth with 10% and 27% of U.S. middle-school and high school students, respectively reporting use in the past 30 days (Cullen et al., 2019). In addition, in 2019, 3.2% of U.S. adults (non-Hispanic White, African American, and Hispanics) were current e-cigarette users (Dai and Leventhal, 2019). Although generally thought to be a safer alternative to cigarettes, e-cigarettes contain nicotine and users have access to over 7000 unique flavorings that along with nicotine liquid can be added to the humectants propylene glycol (PG) and vegetable glycerin (VG) to comprise the complex e-liquid that is then vaporized and inhaled into the lungs (Tierney et al., 2016; Zhu et al., 2014). The nicotine vapor is readily absorbed through the mucosal membranes of the oral pharynx and respiratory tract to exert its highly addictive affects and to modulate blood pressure and adrenaline levels (Goniewicz et al, 2014; Haass and Kübler, 1997). Heating the e-liquid also causes thermal decomposition of the PG and VG to generate carbonyls that include the carcinogens formaldehyde and acetaldehyde and the toxicant acrolein (Farsalinos and Gillman, 2018; Khlystov and Samburova, 2016).
We recently completed a study evaluating the constituents of 19 e-liquids and their effect on carbonyl formation (Zhou et al., 2020). Total chemical burden ranged from 0.35 to 14.6 mg/ml with multiple alcohols and aldehydes detected across the e-liquids. A quantitative assessment of carbonyls in the vapor formed during heating of the e-liquids clearly showed a profound effect of voltage whereby aerosols generated at 4 V contained no detectable formaldehyde, whereas 5 V led to levels up 2100 ng/ml. Several of the e-liquids showed significant increases in acetaldehyde and acrolein compared with unflavored liquid that was independent of voltage and presence of nicotine. These results, replicated on a smaller scale in the literature, indicate the potential hazard of some e-liquids with respect to exposing the respiratory tract to higher levels of reactive carbonyls to elevate risk for head and neck cancer in the chronic e-cigarette user (Kosmider et al., 2014; Ogunwale et al., 2017). In fact, 2 cases of oral carcinoma associated with reported use of 20–30 e-cigarettes per day over 10–13 years have been described (Nguyen et al., 2017). Epidemiological studies will be required to provide better estimates of risk for head and neck cancer from long-term use of e-cigarettes.
In vitro studies have compared total particulate matter (TPM) or complete aerosol generated from cigarettes and e-cigarettes for a multitude of effects on normal cell functions (reviewed in Merecz-Sadowska et al., 2020). Scheffler et al. (2015) exposed 2 primary undifferentiated bronchial epithelial cell lines at the air-liquid interface to aerosol from a cigarette (60 puffs) or an e-cigarette (200 puffs) and observed 24 h after exposure significant levels of cytotoxicity and oxidative stress for both products, albeit much greater effects with the cigarette. E-cigarette effects were similar with and without nicotine. Similar studies using H292 cells, a human pulmonary mucoepidermoid carcinoma cell line exposed to 55 puffs of e-cigarette aerosols showed reduced cell viability and release of inflammatory cytokines that varied in magnitude across e-liquids with the same level of nicotine (Leigh et al., 2016). The Calu3 lung adenocarcinoma cell line also showed dose-dependent reductions in proliferation and viability in response to 13 different flavored e-liquids following direct administration or aerosol delivery of 0–35 puffs 24 h after exposure (Rowell et al., 2017). The magnitude of response again varied by e-liquid likely reflecting differences (type and/or amount) in chemicals across products. The oral-pharynx is the region of highest exposure to particles and gases from tobacco products and smoking cigarettes is strongly associated with increasing periodontal disease and risk for head and neck cancer (Sundar et al., 2016). Sundar et al. (2016) also showed that aerosol exposure (300 puffs) to e-cigarettes, particular those with flavoring, induce inflammation and prosenescence responses in oral epithelial cells and periodontal fibroblasts. All these findings support the potential for e-cigarettes to cause cellular stress in vivo, but there have been few systematic studies in respiratory epithelial cells that have compared aerosols from different e-liquids with defined dose rates and patterns of exposure for cytotoxicity, oxidative stress, and genotoxicity. Exposures in these studies were lower than daily consumption by many e-cigarette users, 23% and 8% who report consuming 4–5 or > 8 ml of e-liquid per day that equates to 1200 and 2000 puffs (McLaren, 2015).
The purpose of this study was to extend our work on examining health effects for the oral-pharynx by evaluating the cytotoxicity and genotoxicity of e-cigarette-generated aerosols containing diverse flavoring products with and without nicotine in oral epithelial cell lines. Dose response studies were conducted using an aerosol delivery system that exposed 3 immortalized oral epithelial cell lines to unflavored e-cigarette aerosol and 10 flavored products with and without nicotine (1.2% [12 mg/ml]). Cell lines were evaluated for cytotoxicity (neutral red uptake [NRU] assay), oxidative stress (ROS-Glo and thiobarbituric acid reactive substances [TBARs] assay), and genotoxicity (comet and micronuclei assays).
MATERIAL AND METHODS
Cell lines
Three immortalized cell lines were selected for study rather than primary cells that senesce after 7–10 population doublings given the magnitude of the studies and the need for rigor with respect to comparing findings across products and endpoints. Two lines, designated MOE1A and MOE1B oral epithelial cells obtained from 28- and 32-year-old never smoker males, were immortalized by introducing hTERT, CDK4R2C, and cyclin D1 (Kibe et al., 2011). P53C234 was also introduced into MOE1B. The third line, MSK-LEUK1 (MSK1 [spontaneously immortalized]) was established from a dysplastic leukoplakia lesion adjacent to a squamous cell carcinoma of the tongue in a 46-year old nonsmoking female (Michaluart et al., 1999). The MSK1 cells have undergone cytogenetic characterization and their karyotype shows a few changes that include loss of 1 copy of chromosome 4, gains for 22q11.2q12, 7p and 8p (Singh et al., 2001). These 3 immortalized cell lines do not grow in soft agar or form tumors as xenografts on nude mice (Kibe et al., 2011; Michaluart et al., 1999). MOE1A and MOE1B were grown in KSFM media and MSK1 was grown in defined KSFM media. MOE1A cells were at passage 8, MOE1B at passage 6, and MSK1 at passage 37 at the start of the studies. Upon study completion, MOE1A was at passage 23, MOE1B at passage 22, and MSK1 at passage 54.
Flavor chemicals
Ten e-liquids were obtained from AVAIL VAPOR LLC who provides research grade products that come with certificate of analysis confirming nicotine content and lack of diacetyl (< 38 ppm) and its alternative acetyl propionyl (< 100 ppm; Kreiss et al, 2002). The ratio of PG and VG selected was 70%:30% as the vehicle along with a freebase nicotine content of 1.2% (12 mg/ml). This PG/VG ratio achieves a good vapor as characterized by a visible cloud and the nicotine content is common with never smoker first-time users (Zhou et al., 2020). This vehicle with and without nicotine also served as the unflavored product for all studies. The selection of the e-liquids was based on the diversity of chemical constituents in the products determined previously and the inclusion of menthol, full flavored tobacco, and vanillin flavoring (Zhou et al., 2020). The flavored e-liquids in 70%PG/30%VG with and without nicotine selected were Arctic Blast, Blue Pucker, Jamestown, Love Potion, Mardi Gras, Midnight Splash, Port Royale, Tobacco Row, Tortuga, and Uptown. In addition, because users are also known to use 30%PG/70%VG, limited studies were conducted with this ratio only in presence of nicotine. Those studies evaluated unflavored and the e-liquids Love Potion, Mardi Gras, Port Royale, Tortuga, and Uptown.
Aerosol exposure
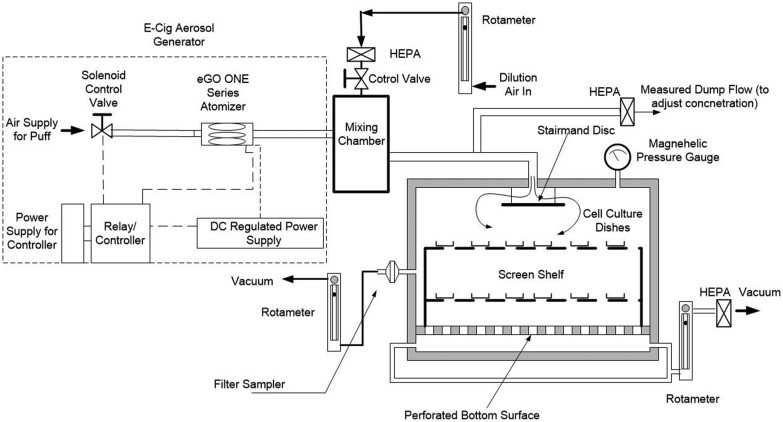
The aerosol generation system consists of an aerosol exposure chamber, a Joyetech eGo ONE 1100 mAh e-cigarette with a 2.5 ml tank capacity and a 0.5-ohm stainless-steel atomizer (the battery was replaced by a DC-regulated power supply), a mixing chamber, and a solenoid valve controlled by a relay (Figure 1). The concentration in the exposure chamber is controlled by the flow rate of dilution air, whereas the puffing protocol is controlled by the solenoid valve and its relay. The aerosols were generated at 5 V using a puffing protocol based on the topography results of e-cigarette users consisting of ∼52 ml puffs of 2.6 s duration at intervals of 18 s (Behar et al., 2015). The amount and concentration of aerosol delivered was quantified by collection of TPM onto a filter. Cells were exposed for 20 min, reflecting an average smoking period for an e-cigarette user, to aerosol generated over a dose range containing 150, 250, 350, and 450 mg TPM/m3. This exposure system accommodates culture dishes up to 100 mm and cells are simply growing attached to the surface of the plate covered with a small volume of serum free RPMI media during the exposure for the various assays and endpoints. The tops of the culture dishes are removed prior to placement in the exposure box. An orbital shaker facilitates exchange between the exposure atmosphere and cell media. The use of undifferentiated cells allows the assessment of genotoxic endpoints such as micronuclei formation that require at least one cell replication following exposure. The Kentucky reference 3R4F cigarette was used as a positive control for all assays. Target doses for exposures were reduced slightly to 100, 200, 300, and 400 mg TPM/m3 to avoid overt toxicity at the higher doses.
Figure 1.
Schematic of the electronic cigarette aerosol generation system and exposure chamber.
NRU assay
The NRU assay is commonly used to assess cytotoxicity (Repetto et al., 2008). Four replicates of each cell line in 96-well plates were exposed in 100 µl of RPMI serum free media at approximately 60% confluence to vehicle (air) or the e-cigarette aerosol. Plates were visualized under an inverted microscope prior to the wash step and incubation with neutral red growth media. There was no increase in floating cells or lifting of cells off the plate that differed from control cells. Following 24 h postexposure, the cells were incubated with neutral red growth media for 2 h, washed and the dye was solubilized by addition of neutral red solubilization solution (1% acetic acid in 50% ethanol) and the absorbance is read at 540 nm.
Oxidative stress assays
Oxygen species are highly reactive (eg, superoxide, singlet oxygen) and have a short half-life in solution with most being converted to H2O2 (Lushchak, 2014). The ROS-Glo assay (Promega) uses an H2O2 substrate that directly reacts with H2O2 to produce a luciferin precursor that is then reacted with a detection solution to convert the precursor to luciferin. This provides luciferase to generate a light signal proportional to the level of H2O2 present. Cells at approximately 60% confluence in 48-well dishes containing 100 µl of RPMI serum free media were exposed in quadruplicate over the dose range for 20 min. The exposure media was removed (no wash) and replaced with fresh media and incubated for 90 min for detection and H2O2 was then quantified as relative light units (RLUs). The number of viable cells quantified by Cell-Titer assay (Promega) was used to normalize the RLUs detected.
Lipid peroxidation is a well-defined mechanism of cellular damage in animals and plants. Lipid peroxides are unstable indicators of oxidative stress in cells that decompose to form more complex and reactive compounds such as malondialdehyde (MDA) and 4-hydroxynonenal, natural bi-products of lipid peroxidation (Ghani et al., 2017). TBARs is a well-established assay for screening and monitoring lipid peroxidation. MDA forms an adduct with thiobarbituric acid that can be measured colorimetrically. Cells at approximately 60% confluence in 6-well dishes containing 1 ml of RPMI serum free media were exposed in quadruplicate over the dose range for 20 min and placed in the incubator for an additional 100 min. Following incubation, the cells were washed and cell lysates were collected. TBARs levels were determined from a MDA equivalence standard curve that is linear over a range of 0.98–125 µM. Assay results from 4 replicates are normalized by measuring total protein by bicinchoninic acid in the cell lysate.
Genotoxicity assays
The alkaline comet assay is used for the detection of DNA strand breaks in cells or nuclei following exposure to potentially genotoxic materials (McKelvey-Martin et al., 1993). Under alkaline conditions (>pH 13), the comet assay can detect single and double stranded breaks, resulting, eg, from direct interactions with DNA, alkali labile sites or as a consequence of transient DNA strand breaks resulting from DNA excision repair (McKelvey-Martin et al., 1993). Cells at approximately 60% confluence in 6-well dishes containing 1 ml of RPMI serum free media were exposed in triplicate, removed from the plate by scraping, and the Trevigen Alkaline Comet Assay kit was used to conduct the comet assay. Comet images were captured at 4× magnification on a microscope equipped with epifluorescence and a CCD camera. All slides for analysis were randomly coded and scored “blinded” to prevent scoring bias. At least 150 scoreable comets without overlapping tails and not located at the edge of the slides per treatment per cell line were analyzed using Trevigen Comet Analysis Software. The % tail DNA (also known as % tail intensity) was used for the evaluation and interpretation of results, and was determined by the DNA fragment intensity in the tail expressed as a percentage of the cell’s total intensity.
The micronucleus assay was used to assess genotoxic potency of the e-liquid aerosols (Fenech, 2005). Micronuclei are formed during the metaphase/anaphase transition of mitosis. They may arise from a whole lagging chromosome (aneugenic event leading to chromosome breakage) or an acentric chromosome fragment that detached from a chromosome after breakage (clastogenic event). Cells at approximately 60% confluence in 48-well plates containing 100 µl of RPMI serum free medium were exposed to air or aerosols (4 replicates) over the 4-dose range for 20 min followed by a 4-h incubation at 37°C. Media was removed, cells were washed, and normal growth media added to support growth for 48 h with confluence at harvest ≤ 90%. S9, a mixture of liver microsomes and cytosol that is often included in the exposures to support metabolic activation of compounds within the aerosols was not used because at the lowest dose recommended the S9 caused considerable death with these cell lines. Cells were processed for micronuclei scoring as described in the Litron protocol (https://litronlabs.com/ Products/In-Vitro-Micronucleus) and a Becton Dickinson FACSCaliber flow cytometer was used to collect the data. The Litron flow cytometry-based assay is high throughput and can count living cells based on specific dyes that allow mono-, bi, and polynucleated cells with and without micronuclei to be rapidly identified and quantified. Based on manufacturer’s recommendation, 10 000 cells were enumerated as positive or negative for micronuclei. Apoptotic cells are also counted separate from living cells.
Data analysis
The extent of cytotoxicity determined by the NRU assay was assessed by dividing absorbance readings of the different doses and replicates by the absorbance seen with the vehicle control. The IC50 and/or maximum cytotoxicity for each e-liquid per cell line was calculated with probit analysis, a type of regression used to analyze binomial response variables in which the sigmoid dose-response curves are transformed to a straight line to allow for assessment by least squares or maximum likelihood.
Exposures varied ±20% of the target dose. Therefore, data were dose-normalized for all exposures using model selection to fit the intercept using linear or quadratic models relative to the exposure TPM for the ROS-Glo, TBARs, comet, and micronuclei assays. The difference between the target TPM and exposure TPM was used with the selected model to adjust the data from the actual exposure to target TPM. In addition, outliers were identified using Grubb’s analysis and excluded from the dataset (Grubbs and Beck, 1972). There were no outliers for ROS-Glo or comet assay. There were 14 outliers out of 690 (2%) and 105 outliers out of 1965 (5.6%) assays for TBARs and micronuclei. Spearman coefficient was used to assess the relationship between total chemical constituents or carbonyls determined in Zhou et al. (2020) and potency score.
ROS data were analyzed with a 1-sided paired t test to evaluate the hypothesis that the exposures would increase oxidative stress for each e-liquid versus the air control, with and without nicotine. Then, ROS values were normalized to cell number at each exposure and converted to a ROS fold change by comparing to each sample’s respective air control ROS value divided by air control cell number. The dose response for increase in ROS fold change for each test article across exposure levels, with and without nicotine, was tested with analysis of variance. Doses and test articles were included in the model and adjusted as covariates. Evaluating the statistical significance of the test article times the dose interaction assessed the differential test article dose-response effect.
The TBARs’ concentrations were determined from the standard curve and then normalized by respective protein levels. The adjusted TBARs concentrations were log-transformed for analysis. The comparison for significance was tested for each product and exposure, with and without nicotine, by analysis of variance and results were expressed as fold change compared with the air control. The fold difference was expressed as a mean and 95% C). Comet assay was log-transformed and analyzed as described for TBARs’ concentrations.
Statistical significance for micronuclei was based on testing the difference between any of the treatment groups versus air control using Poisson regression to address the fact that counts (number of cells with micronuclei or apoptotic cells) per 10 000 interrogated cells was used as the readout for the assay. Our goal was to assess whether a product could induce micronucleus formation, thus 1-sided p-values were calculated. A trend test across doses was conducted to evaluate significance.
RESULTS
Cytotoxic Effects of E-Cigarette Liquids
The Kentucky reference cigarette induced dose-dependent reduction in cell number detected by the NRU assay with IC50s of 271–312 mg TPM/m3 and maximum cytotoxicity of 65–76% across the 3 oral cell lines (Table 1). In contrast, IC50s or dose response was generally not seen for any e-liquid in 70%PG/30%VG with maximum cytotoxicity of 0–36% (Table 1). The presence or absence of nicotine did not appear to be a factor in the level of cytotoxicity; however, interestingly in the unflavored e-liquid with nicotine, no cytotoxicity was seen in any cell line. Similar results were seen for e-liquids in 30%PG/70%VG, the exception being for Uptown where MOE1A cells showed a dose response for cytotoxicity that reached 44% (Supplementary Table 1). The NRU assay reflects a continuous outcome and when converted to a binary outcome can readily detect a 20% change that is highly unlikely (< 5%) to be a false positive. Three e-liquids (Blue Pucker, Jamestown, and Love Potion) met this criterium in more than 1 cell line, largely in the presence of nicotine (Table 1).
Table 1.
Effect of E-Cigarette Liquids and Nicotine on Cytotoxicity of Oral Epithelial Cell Lines
| E-Liquid a | Cell Lines |
|||||
|---|---|---|---|---|---|---|
| MOE1A |
MOE1B |
MSK1 |
||||
| (–) | (+) | (–) | (+) | (–) | (+) | |
| (Maximum cytotoxicity, %) | ||||||
| Unflavored | 17.5 | 0.0 | 27.3 | 0.0 | 13.5 | 0.0 |
| Arctic Blast | 15.2 | 11.7 | 15.5 | 6.0 | 13.7 | 16.8 |
| Blue Pucker | 0.0 | 15.6 | 3.6 | 28.0 | 0.3 | 26.0 |
| Jamestown | 14.5 | 28.1 | 2.6 | 15.0 | 15.5 | 28.3 |
| Love Potion | 21.4 | 0.0 | 5.3 | 33.0 | 4.0 | 0.0 |
| Mardi Gras | 9.3 | 20.1 | 13.2 | 18.9 | 8.6 | 6.5 |
| Midnight Splash | 8.6 | 8.6 | 2.1 | 2.1 | 5.1 | 5.1 |
| Port Royale | 5.5 | 4.1 | 10.0 | 13.3 | 4.4 | 18.3 |
| Tobacco Row | 3.6 | 10.1 | 36.0 | 27.4 | 0.0 | 14.7 |
| Tortuga | 8.5 | 5.7 | 15.2 | 0.0 | 10.4 | 6.5 |
| Uptown | 2.6 | 10.4 | 9.7 | 22.7 | 16.0 | 8.9 |
| Kentucky reference cigarette 3R4F | 72.6 | 65.1 | 76.0 | |||
Abbreviations: (–), no nicotine; (+), nicotine.
All E-liquids contain 70%PG/30%VG.
Induction of Oxidative Stress by E-Cigarette Liquids
The Kentucky reference cigarette caused a significant dose-dependent induction in oxidative stress detected by the ROS-Glo assay with fold changes of 1.5–2.2 seen at the 400 mg TPM/m3 dose (Table 2 andSupplementary Table 2). Positivity for an e-liquid for each assay (eg, ROS-Glo, TBARs) required significance for at least 2 of the doses. The effect of e-liquids on oxidative stress are summarized in Table 2 that provides the highest significant fold change irrespective of dose and whether a dose response was seen for a product by nicotine status and cell line (see Supplementary Table 2 for complete dataset). There was no significant induction of oxidative stress seen for unflavored e-liquid (Table 2). The induction of oxidative stress at levels up to 2.3-fold greater than air control with and without nicotine were seen for Blue Pucker in MOE1A and Love Potion in MOE1B and MSK1 and Tortuga in MOE1A and MOE1B. Significant dose response was also seen for Love Potion in the presence of nicotine across all cell lines (Table 2). Port Royale also caused significant increases in oxidative stress in all cell lines in the absence of nicotine with dose response seen for MOE1B and MSK1 lines. Vapors generated from e-liquids in 30%PG/70%VG showed very little induction of oxidative stress with no evidence of dose response (Supplementary Table 3).
Table 2.
Effect of E-Cigarette Liquids and Nicotine on Oxidative Stress of Oral Epithelial Cell Lines Detected by the ROS-Glo Assay
| E-Liquid a | Cell Lines |
|||||
|---|---|---|---|---|---|---|
| MOE1A |
MOE1B |
MSK1 |
||||
| (–) | (+) | (–) | (+) | (–) | (+) | |
| (Maximum Significant Fold Change) |
||||||
| Unflavored | NS | NS | NS | NS | NS | NS |
| Arctic Blast | NS | NS | NS | 1.3 | NS | NS |
| Blue Pucker | 1.2 | 2.3 | NS | 1.6 | NS | 16 |
| Jamestown | NS | NS | NS | 1.3 | NS | NS |
| Love Potion | NS | 1.9* | 1.8 | 1.9* | 1.5 | 1.8* |
| Mardi Gras | 1.2 | NS | NS | 1.5 | 1.3 | 1.3 |
| Midnight Splash | 1.2 | NS | 1.5* | NS | NS | NS |
| Port Royale | 1.9 | NS | 2.4* | NS | 1.5* | NS |
| Tobacco Row | 1.2 | NS | NS | 1.3 | 1.2 | 1.5 |
| Tortuga | 1.3 | 1.2 | 1.4 | 1.1 | 1.2 | NS |
| Uptown | NS | NS | NS | NS | NS | 1.3 |
| Kentucky reference cigarette 3R4F | 2.2* | 1.5* | 2.0* | |||
Abbreviations: (–), no nicotine; (+), nicotine; NS, not significant.
All e-liquids contain 70%PG/30% VG.
p < .05 for dose response trend test.
Lipid peroxidation detected by the TBARs assay was less common and more variable across replicates for detecting effects of the Kentucky reference cigarette and the e-liquids. Dose response was not seen with the cigarette; however, maximum fold increases of 2.2, 3.7, and 3.5 were observed for MOE1A, MOE1B, and MSK1 cell lines, respectively (Table 3 andSupplementary Table 4). Love Potion, Midnight Splash, and Port Royale induced the largest fold changes (10.6–12.3) in lipid peroxidation, albeit in only a single cell line in the presence or absence of nicotine (Table 3 and Supplementary Table 4). Dose response was also seen for Blue Pucker for MOE1B, Love Potion for MOE1B, and MSK1, and Port Royale for MOE1A. Vapors generated from e-liquids in 30%PG/70%VG showed no significant dose response for lipid peroxidation with unflavored for MSK1, Love Potion for MOE1A, Mardi Gras for MOE1B, and Uptown for MOE1A and MOE1B (Supplementary Table 5).
Table 3.
Effect of E-Cigarette Liquids and Nicotine on Oxidative Stress of Oral Epithelial Cell Lines Detected by TBARs Assay
| E-Liquid a | Cell Lines |
|||||
|---|---|---|---|---|---|---|
| MOE1A |
MOE1B |
MSK1 |
||||
| (–) | (+) | (–) | (+) | (–) | (+) | |
| (Maximum Significant Fold Change) |
||||||
| Unflavored | NS | NS | NS | NS | NS | NS |
| Arctic Blast | NS | NS | 5.5 | 2.5 | NS | NS |
| Blue Pucker | NS | NS | 3.2* | NS | NS | NS |
| Jamestown | NS | NS | NS | NS | 1.2 | NS |
| Love Potion | 3.1 | NS | NS | 3.7* | 6.8 | 12.3* |
| Mardi Gras | NS | NS | NS | NS | NS | NS |
| Midnight Splash | 1.1 | 1.2 | NS | 1.3 | 10.6 | NS |
| Port Royale | 2.8 | 11.8* | NS | NS | NS | NS |
| Tobacco Row | NS | NS | NS | 1.1 | NS | 1.5 |
| Tortuga | NS | NS | NS | NS | NS | NS |
| Uptown | NS | NS | NS | NS | NS | NS |
| Kentucky Reference Cigarette 3R4F | 2.2 | 3.7 | 3.5 | |||
Abbreviations: (–) No nicotine; (+) nicotine; NS, not significant.
All e-liquids contain 70%PG/30% VG.
p < .05 for dose response trend test.
Induction of DNA Damage by E-Cigarette Liquids
The Kentucky reference cigarette caused dose-dependent increase in DNA damage detected by the comet assay. Maximum fold-changes seen were 3.8, 5.1, and 18.7 for MOE1A, MOE1B, and MSK1 cell lines. In contrast, none of the e-liquids delivered in 70%PG/30%VG or 30%PG/70%VG with or without nicotine caused significance increase in DNA damage assessed by the comet assay (not shown).
Micronuclei were also formed in response to cigarette exposure with increases up to 1.8-, 2.8-, and 4.0-fold seen for MOE1A, MOE1B, and MSK1 cell lines, respectively (Table 4 andSupplementary Table 6). Unflavored e-liquid-induced micronuclei formation in MOE1A without nicotine and MSK1 with and without nicotine (Table 4). Blue Pucker was the most potent product, inducing micronuclei across all cell lines irrespective of nicotine status (Table 4 andSupplementary Table 5). Jamestown and Uptown also induced micronuclei in all 3 cell lines, but not always with and without nicotine present. Dose response increases were also present with Unflavored, Jamestown, Port Royale, Tobacco Road, Tortuga, and Uptown for one or more of the cell lines. A similar profile across products for micronuclei induction was also seen with vapors generated from 30%PG/70%VG (Supplementary Table 7). There were no significant differences in number of apoptotic cells across e-liquids.
Table 4.
Effect of E-Cigarette Liquids and Nicotine on Micronuclei Formation in Oral Epithelial Cell Lines
| E-Liquid a | Cell Lines |
|||||
|---|---|---|---|---|---|---|
| MOE1A |
MOE1B |
MSK1 |
||||
| (–) | (+) | (–) | (+) | (–) | (+) | |
| (Maximum Significant Fold Change) |
||||||
| Unflavored | 1.3* | NS | NS | NS | 1.3* | 1.8 |
| Arctic Blast | NS | NS | NS | NS | NS | NS |
| Blue Pucker | 1.7 | 1.5 | 1.4 | 1.5 | 2.0 | 1.6 |
| Jamestown | NS | 1.4* | 2.8* | 2.1* | NS | 1.5* |
| Love Potion | 1.4 | NS | NS | NS | NS | NS |
| Mardi Gras | 2.5 | 1.4 | NS | 1.7 | NS | NS |
| Midnight Splash | NS | 2.0 | NS | NS | NS | 5.4 |
| Port Royale | NS | NS | NS | 1.3* | NS | 1.5 |
| Tobacco Row | NS | NS | 1.7 | NS | 2.5 | 4.7* |
| Tortuga | 1.6* | NS | NS | NS | NS | NS |
| Uptown | NS | 2.0 | 2.4* | NS | 2.2 | NS |
| Kentucky Reference Cigarette 3R4F | 1.8 | 2.8 | 4.0* | |||
Abbreviations: (–), no nicotine; (+), nicotine; NS, not significant.
All e-liquids contain 70%PG/30%VG.
p < .05 for dose response trend test.
Potency Score, Chemical and Carbonyl Burdens for E-Liquids
A potency score for each e-liquid was determined by awarding one point for each exposure that resulted in ≥ 20% cytotoxicity assessed by the NRU assay. One point was given for each exposure when significant for ROS-Glo, TBARs, and micronuclei and one point when dose response was also seen in these assays. The top 2 e-liquids with 14 and 17 points were Blue Pucker and Love Potion (Table 5). The lowest score of 3 was seen for Arctic Blast, whereas the remaining e-liquids had potency scores of 6–12 (Table 5).
Table 5.
Potency Score, Chemical, and Carbonyl Burdens for E-Liquids
| E-Liquid a |
Assay
b
|
||||||
|---|---|---|---|---|---|---|---|
| NRU | ROS | TBARs | MN | Total Score c | Chemicals (mg/ml) d | Carbonyls (ng/Puff) e | |
| Unflavored | 1 | 0 | 0 | 5 | 6 | 0.46 | 101 |
| Arctic Blast | 0 | 1 | 2 | 0 | 3 | 8.20 | 2576 |
| Blue Pucker | 2 | 3 | 2 | 5 | 14 | 1.94 | 1232 |
| Jamestown | 2 | 1 | 1 | 8 | 12 | 0.35 | 186 |
| Love Potion | 2 | 8 | 6 | 1 | 17 | 3.12 | 1319 |
| Mardi Gras | 1 | 4 | 0 | 3 | 8 | 1.90 | 2171 |
| Midnight Splash | 0 | 3 | 4 | 2 | 9 | 0.88 | 4090 |
| Port Royale | 0 | 5 | 3 | 3 | 11 | 14.60f | 2156 |
| Tobacco Row | 2 | 4 | 2 | 4 | 10 | 4.35 | 690 |
| Tortuga | 0 | 5 | 0 | 2 | 7 | 9.69 | 424 |
| Uptown | 1 | 1 | 0 | 4 | 6 | 1.59 | 1930 |
All e-liquids contain 70%PG/30%VG.
One point for each exposure causing ≥20% cytotoxicity assessed by NRU, 1 point for each exposure when significant for ROS-Glo, TBARs, and MN (micronuclei) and 1 point when dose response was seen.
There was no correlation between chemicals (p = .96) or carbonyls (p = .62) and total score.
Total level of 30 chemical constituents previously assessed (Zhou et al., 2020).
Combined level of carbonyls: formaldehyde, acetaldehyde, and acrolein previously quantified from e-liquid vapor containing nicotine at 5 V (Zhou et al., 2020).
Menthol comprised 12.26 mg/ml of the chemical burden.
Potency for cytotoxicity and genotoxicity may be associated with chemical constituents and the production of toxic carbonyls formaldehyde, acetaldehyde, and acrolein. These endpoints were assessed previously and are also summarized in Table 5 (Zhou et al., 2020). The total level of 30 chemical constituents listed in Supplementary Table 8 was < 1 mg/ml in 3 of the e-liquids (Unflavored, Jamestown, and Midnight Splash), 1–< 5 mg/ml in 5 e-liquids (Blue Pucker, Love Potion, Mardi Gras, Tobacco Row, and Uptown), and > 5 mg/ml in 3 e-liquids (Arctic Blast, Port Royale, and Tortuga). Carbonyl burden defined by summing the levels of formaldehyde, acetaldehyde, and acrolein present in a single puff was determined previously (Zhou et al, 2020). Four e-liquids (Unflavored, Jamestown, Tobacco Row, and Tortuga) contained carbonyl levels of 101–690 ng/puff, whereas levels in the remaining products exceeded 1200 ng/puff with Midnight Splash showing the highest value of 4090 ng/puff (Table 5). Neither total chemical constituents nor carbonyls were significantly associated with the potency score.
DISCUSSION
These studies demonstrate heterogeneity across e-liquid products in producing cytotoxicity and genotoxicity in oral epithelial cell lines exposed to the generated aerosols. Oxidative stress assessed by the ROS-Glo assay and genotoxicity by the micronuclei assay were the most sensitive readouts for detecting effects by the e-liquid aerosols. Because cell lines were sensitive to the toxic effects of the S9/microsome activation system precluding it’s use, the bioactive potential toxicants that could contribute to the positivity of the micronuclei assay were not assessed. Although there was variation in response by nicotine status and across cell lines, there were products that were consistent across the assays for inducing cytotoxicity, oxidative stress, and genotoxicity. Blue Pucker and Love Potion had the highest potency score derived from all the assays. These 2 products were previously shown to significantly increase the deposition of nicotine compared with unflavored PG/VG in a human oral-trachea cast model (Zhou et al., 2020).
The total amount of chemicals for these 2 products did not differ from other studied e-liquids; however, there were some distinction with regard to type and amount of chemicals (Zhou et al., 2020). Blue Pucker contained the highest levels of ethyl acetate, ethyl butyrate, and limonene compared with the other e-liquids and an intermediate level of maltol. Ethyl acetate, ethyl butyrate, and maltol were also present at levels of 70%, 95%, and 166% in Love Potion compared with Blue Pucker. In addition, levels of ethyl maltol, vanillin, and ethyl vanillin were higher in Love Potion relative to most of the other e-liquids (Zhou et al., 2020). Other studies have evaluated the cytotoxicity of common individual flavor chemicals in the e-liquids in human lung cell lines using the methylthiazol tetrazolium assay (Behar et al., 2018; Hua et al., 2019). Ethyl maltol, ethyl vanillin, and vanillin ranked in the top 6 chemicals for most toxic to the lung BEAS-2B cells (Hua et al., 2019). A second study using lung A549 cells also corroborated toxicity for vanillin, ethyl maltol, and maltol while importantly demonstrating that aerosolization at 5 V had a transfer efficiency of ≥ 70% for most chemicals (Behar et al., 2018). Inhalation of ethyl acetate, but not limonene caused irritation to the respiratory tract (Burkhart et al., 1996; Falk-Filipsson et al., 1993). Together, these studies provide some biological plausibility for the higher potency of Blue Pucker and Love Potion. However, one can also not rule out that the constituent profile of an e-liquid can influence the physiochemical properties of the aerosol to allow increased exposure of cells to the aerosol. Aerosols of both e-liquids generated reactive carbonyls formaldehyde, acetaldehyde, and acrolein at levels that also likely contribute to the observed cytotoxic and genotoxic stress (Zhou et al., 2020).
There were differences in potency across e-liquids for induction of oxidative stress, lipid peroxidation, and genotoxicity in the oral epithelial cell lines. Response by cell line also differed for some products, potentially stemming from the genetic differences of the people from which the lines were derived, that in turn would affect many cellular processes that include metabolism, DNA damage and repair. This finding agrees with prior comparative studies of e-liquids in different lung epithelial-derived cell lines evaluating many toxicological endpoints (Leigh et al., 2016; Merecz-Sadowska et al., 2020; Rowell et al., 2017; Scheffler et al., 2015; Sundar et al., 2016). Because e-cigarette products are not potent inducers of cytotoxicity or mutagenicity, our criteria for positivity was relaxed to having at least 2 of 4 doses show a significant effect. This likely contributed to the incongruent significant findings between e-liquids in the presence and absence of nicotine. However, Love Potion demonstrated strong evidence of oxidative stress detected by the ROS-Glo assay with positivity and dose response in the presence of nicotine for all 3 cell lines that was recapitulated in the TBARs assay in the MOE1B and MSK1 cell lines. Blue Pucker was also significant in the ROS-Glo for all 3 cell lines. Unflavored e-liquid showed no significant effect for the ROS-Glo or TBARs assays, while significance was present for micronuclei formation for MOE1A and MSK1. The literature and our own studies confirm that heating the e-liquid causes thermal decomposition of the PG and VG to produce formaldehyde that produces DNA crosslinks and single-strand breaks, whereas acetaldehyde produces single- and double-strand breaks, all of which if not repaired can lead to micronuclei formation (Farsalinos and Gillman, 2018; Khlystov and Samburova, 2016; Zhou et al., 2020). Thus, positivity for micronuclei with unflavored e-liquid is not surprising; however, Blue Pucker caused significant increases in micronuclei at 2 or more doses across all 3 cell lines with and without nicotine and Jamestown was positive with dose response in all 3 cell lines in the presence of nicotine. With respect to cytotoxicity assessed by the NRU assay, effects were modest for all e-liquids with no distinction compared with unflavored. Our findings of differential toxicological effects by e-liquids was corroborated by Muthumalage et al. (2019) who showed differential effects on levels of cell-free ROS generated by JUUL pod flavors and in vitro exposures of immortalized lung epithelial cells to flavor aerosols showed varied effects for endpoints being measured (eg, PGE2 production) that included no difference from air control, differed significantly at 1 dose, or showed significant dose response.
These in vitro acute exposure studies provide new insight into the potential effects of some flavored vaping products with respect to low levels of oxidative stress and genotoxicity that may be augmented by chronic exposure. The increased deposition in the oral-trachea cast from our prior work combined with this genotoxic stress to oral epithelial cells by the Blue Pucker and Love Potion flavors further compounds the effects of some e-liquids. This heightened exposure is likely to also impact the large airways as supported by in vitro studies showing sensitivity of lung epithelial cells to toxicological effects of some e-liquids (Leigh et al., 2016; Rowell et al., 2017; Scheffler et al., 2015).
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Author Contributions
S.A.B. designed the studies with input from C.S.T., S.K., and T.K. C.S.T., L.M.P., C.M.Y., C.L.T., D.E.J., and K.D. conducted the studies. G.W. and W.W.D. conducted the analyses. S.A.B. wrote the manuscript and all authors reviewed the article and provided feedback.
FUNDING
This work was supported largely by National Institute of Health grant (R01 DE026013 to S.A.B.) and in part by (P30CA11800 to C. Willman) from the National Institutes of Health, National Cancer Institute, (grant/award number: CA11800), and National Institute of Dental and Craniofacial Research (grant/award number: DE026013).
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary Material
REFERENCES
- Behar R. Z., Hua M., Talbot P. (2015). Puffing topography and nicotine intake of electronic cigarette users. PLoS One 10, e0117222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar R. Z., Luo W., McWhirter K. J., Pankow J. F., Talbot P. (2018). Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci. Rep. 8, 8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhart K. K., Britt A., Petrini G., O’Donnell S., Donovan J. W. (1996). Pulmonary toxicity following exposure to an aerosolized leather protector. J. Toxicol. Clin. Toxicol. 34, 21–24. [DOI] [PubMed] [Google Scholar]
- Cullen K. A., Gentzke A. S., Sawdey M. D., Chang J. T., Anic G. M., Wang T. W., Creamer M. R., Jamal A., Ambrose B. K., King B. A. (2019). e-Cigarette use among youth in the United States, 2019. JAMA 322, 2095–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H., Leventhal A. M. (2019). Prevalence of e-cigarette use among adults in the United States, 2014-2018. JAMA 322, 1824–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk-Filipsson A., Lof A., Hagberg M., Hjelm E. W., Wang Z. (1993). d-limonene exposure to humans by inhalation: Uptake, distribution, elimination, and effects on the pulmonary function. J. Toxicol. Health 38, 77–88. [DOI] [PubMed] [Google Scholar]
- Farsalinos K. E., Gillman G. (2018). Carbonyl emissions in E-cigarette aerosol: a systematic review and methodological considerations. Front. Physiol. 8, 1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenech M. (2005). In vitro micronucleus technique to predict chemosensitivity. Methods Mol. Med. 111, 3–32. [DOI] [PubMed] [Google Scholar]
- Ghani A., Barril C., Bedgood D. R., Prenzler P. D. (2017). Measurement of antioxidant activity with thiobarbituric acid reactive substances assay. Food Chem. 230, 195–207. [DOI] [PubMed] [Google Scholar]
- Goniewicz M. L., Knysak J., Gawron M., Kosmider L., Sobczak A., Kurek J., Prokopowicz A., Jablonska-Czapla M., Rosik-Dulewska C., Havel C., et al. (2014). Levels of selected carcinogens and toxicants in vapor from electronic cigarettes. Tob. Control 23, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs F. E., Beck G. (1972). Extension of sample sizes and percentage points for significance tests of outlying observations. Technometrics 14, 847–854. [Google Scholar]
- Haass M., Kübler W. (1997). Nicotine and sympathetic neurotransmission. Cardiovasc. Drugs Ther. 10, 657–665. [DOI] [PubMed] [Google Scholar]
- Hua M., Omaiye E. E., Luo W., McWhirter K. J., Pankow J. F., Talbot P. (2019). Identification of cytotoxic flavor chemicals in top-selling electronic cigarette refill fluids. Sci. Rep. 9, 2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khlystov A., Samburova V. (2016). Flavoring compounds dominate toxic aldehyde production during E-cigarette vaping. Environ. Sci. Technol. 50, 13080–13085. [DOI] [PubMed] [Google Scholar]
- Kibe T., Kishida M., Kamino M., Iijima M., Chen L., Habu M., Miyawaki A., Hijioka H., Nakamura N., Kiyono T., et al. (2011). Immortalization and characterization of normal oral epithelial cells without using HPV and SV40 genes. Oral Sci. Int. 8, 20–28. [Google Scholar]
- Kosmider L., Sobczak A., Fik M., Knysak J., Zaciera M., Kurek J., Goniewicz M. L. (2014). Carbonyl compounds in electronic cigarette vapors: Effects of nicotine solvent and battery output voltage. Nicotine Tob. Res. 16, 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiss K., Gomaa A., Kullman G., Fedan K., Simoes E. J., Enright P. L. (2002). Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. New Engl. J. Med. 347, 330–338. [DOI] [PubMed] [Google Scholar]
- Leigh N. J., Lawton R. I., Hershberger P. A., Goniewicz M. L. (2016). Flavourings significantly affect toxicity of aerosol generated from electronic nicotine delivery systems (ENDS). Tob. Control 25, ii81–ii87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushchak V. I. (2014). Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Bio. Interact. 224, 164–175. [DOI] [PubMed] [Google Scholar]
- McKelvey-Martin V. J., Green M. H. L., Schmezer P., Pool-Zobel B. L., De Meo M. P., Collins A. (1993). The single cell gel electrophoresis assay (comet assay): A European review. Mut. Res. 288, 47–63. [DOI] [PubMed] [Google Scholar]
- McLaren N. (2015). Big Survey 2014–Initial Findings Eliquid Available at: http://vaping.com/data/big-survey-2014-initial-findings-eliquid. Assessed February 27, 2015.
- Merecz-Sadowska A., Sitarek P., Zielinska-Blizniewska H., Malinowska K., Zajdel K., Zakonnik L., Zajdel R. (2020). A summary of in vitro and in vivo studies evaluating the impact of e-cigarette exposure on living organisms and the environment. Int. J. Mol. Sci. 21, 652–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaluart P., Masferrer J. L., Carothers A. M., Subbaramaiah K., Zweifel B. S., Koboldt C., Mestre J. R., Grunberger D., Sacks P. G., Tanabe T., et al. (1999). Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res. 59, 2347–2352. [PubMed] [Google Scholar]
- Muthumalage T., Lamb T., Friedman M. R., Rahman I. (2019). E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci. Rep. 9, 19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H., Kitzmiller J. P., Nguyen K. T., Nguyen C. D., Bui T. C. (2017). Oral carcinoma associated with chronic use of electronic cigarettes. Otolaryngology 7, 1000304. [Google Scholar]
- Ogunwale M. A., Li M., Ramakrishnam Raju M. V., Chen Y., Nantz M. H., Conklin D. J., Fu X.-A. (2017). Aldehyde detection in electronic cigarette aerosols. ACS Omega 2, 1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetto G., del Peso A., Zurita J. L. (2008). Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 3, 1125–1131. [DOI] [PubMed] [Google Scholar]
- Rowell T. R., Reeber S. L., Lee S. L., Harris R. A., Nethery R. C., Herring A. H., Glish G. L., Tarran R. (2017). Flavored e-cigarette liquids reduce proliferation and viability in the Calu3 airway epithelial cell line. Am. J. Physiol. Lung Cell Mol. Physiol. 313, L52–L66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler S., Dieken H., Krischenowski O., Forster C., Branscheid D., Aufderheide M. (2015). Evaluation of e-cigarette liquid vapor and mainstream cigarette smoke after direct exposure of primary human bronchial epithelial cells. Int. J. Environ. Res. Public Health 12, 3915–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Gogineni S. K., Sacks P. G., Shaha A. R., Shah J. P., Stoffel A., Rao P. H. (2001). Molecular and cytogenetic characterization of head and neck squamous cell carcinoma and refinement of 3q amplification. Cancer Res. 61, 4506–4513. [PubMed] [Google Scholar]
- Sundar I. K., Javed F., Romanos G. E., Rahman I. (2016). E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget 7, 77196–77204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney P. A., Karpinski C. D., Brown J. E., Luo W., Pankow J. F. (2016). Flavour chemicals in electronic cigarette fluids. Tob. Control 25, e10–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S. H., Sun J. Y., Bonnevie E., Cummins S. E., Gamst A., Yin L., Lee M. (2014). Four hundred brands and sixty brands of e-cigarettes and counting: Implications for product regulation. Tob. Control 23, iii3–iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Irshad H., Dye W. W., Wu G., Tellez C. S., Belinsky S. A. (2020). Voltage and e-liquid composition affect nicotine deposition with the oral cavity and carbonyl formation. Tob. Control. Epub. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.