Abstract
Background
Previous pilot studies have shown the feasibility of preoperative chemotherapy in patients with medulloblastoma, but benefits and risks compared with initial surgery have not been assessed.
Methods
Two therapeutic strategies were retrospectively compared in 92 patients with metastatic medulloblastoma treated at Gustave Roussy between 2002 and 2015: surgery at diagnosis (n = 54, group A) and surgery delayed after carboplatin and etoposide-based neoadjuvant therapy (n = 38, group B). Treatment strategies were similar in both groups.
Results
The rate of complete tumor excision was significantly higher in group B than in group A (93.3% vs 57.4%, P = 0.0013). Postoperative complications, chemotherapy-associated side effects, and local progressions were not increased in group B. Neoadjuvant chemotherapy led to a decrease in the primary tumor size in all patients; meanwhile 4/38 patients experienced a distant progression. The histological review of 19 matched tumor pairs (before and after chemotherapy) showed that proliferation was reduced and histological diagnosis feasible and accurate even after neoadjuvant chemotherapy. The 5-year progression-free and overall survival rates were comparable between groups. Comparison of the longitudinal neuropsychological data showed that intellectual outcome tended to be better in group B (the mean predicted intellectual quotient value was 6 points higher throughout the follow-up).
Conclusion
Preoperative chemotherapy is a safe and efficient strategy for metastatic medulloblastoma. It increases the rate of complete tumor excision and may improve the neuropsychological outcome without jeopardizing survival.
Key Points
1. Preoperative chemotherapy increases the rate of complete tumor removal.
2. No additional risk (toxic or disease progression) is linked to the delayed surgery.
3. Preoperative chemotherapy could have a positive impact on the neuropsychological outcome of patients.
Keywords: childhood brain tumor, medulloblastoma, neuropsychological outcome , preoperative chemotherapy, surgery
Importance of the Study.
In various cancers, neoadjuvant chemotherapy is often used to treat metastatic disease and facilitate surgery of the primary tumor, but it is rarely proposed for brain tumors. Preoperative chemotherapy is feasible in metastatic medulloblastomas but its benefits and risks have not been assessed. This study compared retrospectively the 2 therapeutic strategies (surgery at diagnosis and surgery after neoadjuvant chemotherapy) in 92 patients with metastatic medulloblastoma. The results confirmed the efficacy of neoadjuvant chemotherapy radiologically and histologically, and showed that it increases the complete tumor excision rate with no additional risk for patients. Histological diagnosis of medulloblastoma was still feasible after neoadjuvant chemotherapy. Our study also suggests that this strategy could have a positive impact on patients’ neuropsychological outcome. Moreover, the response rate to neoadjuvant chemotherapy may help to better tailor post-surgery therapy in patients with very high risk and not chemosensitive medulloblastoma.
Medulloblastoma is one of the most common malignant brain tumors in childhood. Young age at diagnosis, metastatic disease, incomplete surgical excision (residual disease >1.5 cm2),1,2 large-cell anaplastic histology,3,4 and several biological markers (especially MYC amplification)5,6 have been associated with poor outcome. Advances in molecular profiling have allowed classifying medulloblastoma into 4 subgroups: wingless (WNT), sonic hedgehog (SHH), Group 3, and Group 4.7–9 The 2016 update of the World Health Organization (WHO) classification subsequently defined 5 entities: medulloblastoma WNT activated, medulloblastoma SHH activated with or without TP53 mutation, and Group 3 and 4 medulloblastomas.10 The role of residual tumor (>1.5 cm2), confirmed as a high risk factor in the recently published HIT-SIOP-PNET4 phase III trial,11 justifies the development of therapeutic strategies that could improve complete excision rate at minimal cost in terms of neuropsychological outcome.
Modern management strategies with multimodal and more intensive treatments, including high-dose chemotherapy (HDC), have improved the 5-year overall survival (OS) to about 70–80% even for high-risk patients.9,12,13 As more children with medulloblastoma are now being cured, the quality of survival becomes a major concern. The cerebellum has a critical role in motor skills and coordination, as well as in cognitive and executive functions.14,15 Children treated for a malignant tumor in the posterior fossa within and/or adjacent to the cerebellum are therefore at risk of intellectual impairment due to the tumor and its treatment, especially medulloblastoma survivors.16–21 Age at radiotherapy, radiation doses, and boost volume have a tremendous effect on cognition that worsens with time.22–27 Moreover, tumor location and brainstem infiltration, hydrocephalus, splitting of the vermis, damage of the dentate nuclei during surgery, and posterior fossa syndrome are associated with poorer neurocognitive outcome.28–32 We hypothesized that preoperative chemotherapy could decrease the surgical morbidity and improve its efficacy. We have shown the feasibility of this approach previously,33 and its role has been suggested by others in infants.34,35 Here, we compared tumor control and morbidity after preoperative chemotherapy or standard upfront surgery in a population-based single center study.
Patients and Methods
Patients
This study retrospectively analyzed data from 92 children with metastatic medulloblastoma followed at our center between 2002 and 2015. Most patients (n = 65) were operated at the Neurosurgical Department of the Necker Sick Children’s Hospital, Paris, France. Inclusion criteria were:
-
•
Newly diagnosed metastatic medulloblastoma treated by chemotherapy and/or radiotherapy between 2002 and 2015
-
•
Patients younger than 18 years at diagnosis
-
•
Histologically proven diagnosis of medulloblastoma after surgical excision or biopsy (if clinical condition allowed surgery)
-
•
Metastatic stage evaluated by the positivity of MRI and/or lumbar cerebrospinal fluid (CSF) sampling at diagnosis and subsequent evaluations
-
•
No contraindication to chemotherapy
-
•
Treatment of hydrocephalus when present
Data were retrospectively extracted from the medical files.
Medulloblastoma Management
This study compared 2 strategies according to the intention-to-treat principle: (i) standard upfront surgery at diagnosis (group A) and (ii) delayed surgery after an initial biopsy (for histology-proven confirmation of medulloblastoma) and neoadjuvant chemotherapy (group B). Patients were not randomized but were assigned pragmatically to one of the 2 groups on the basis of the neurosurgeon’s choice. Both groups were treated during the same period of time with similar treatment regimens, according to the ongoing protocols at the time of diagnosis. Inclusions in both cohorts were distributed similarly during the recruiting period. In group B, all children received neoadjuvant chemotherapy before surgery: a combination of carboplatin (160 mg/m2/d, days 1–5) and etoposide (100 mg/m2/d, days 1–5) at conventional doses without hyperhydration.36 Post-surgery treatments were chosen according to the patients’ age and risk factors. The irradiation modalities changed slightly over time for both groups (technical improvement, dose modification according to the age and type of associated chemotherapy, reduction of the boost volume). Children older than 5 years received conventional-dose chemotherapy followed by radiotherapy (protocol SFOPTC9437) or tandem HDC (2 courses of thiotepa13 or 2 courses of melphalan38) with autologous stem cell transplantation (ASCT), followed by standard-dose (36 Gy) craniospinal irradiation (CSI). In children younger than 5 years, post-surgery management was adjusted to avoid radiotherapy or to decrease the irradiation dose. They received sequential HDC with ASCT followed by local radiation therapy to the posterior fossa (50–55 Gy) or reduced-dose CSI (18 or 24 Gy) according to the PNET HR protocol for children younger than 5 years (NCT00180791), or one course of tandem HDC with busulfan and thiotepa,39,40 or sequential HDC without radiotherapy (HRMB-5 protocol, NCT02025881). Informed consent of the parents or guardians was obtained for each patient, according to the institutional review board guidelines and the specific guidelines of each trial.
Radiological Investigations
All children underwent neuraxis MRI at diagnosis for evaluation of tumor size and staging. The tumor size was defined as the sum of the products of the largest perpendicular diameters evaluated on brain MRIs. The responses to neoadjuvant chemotherapy were assessed by MRI at the end of treatment. Criteria used for response evaluation were those recommended by the Response Assessment in Pediatric Neuro-Oncology committee41: complete response (CR) was defined as the disappearance of the whole lesion; partial response (PR) as a decrease of at least 50% of the sum of the products of the largest perpendicular diameters; and progressive disease (PD) as a ≥25% increase in tumor size. Stable disease was defined as MRI features that do not meet the criteria for PR or PD. Early postoperative cerebral MRI, within 72 hours after surgery, was performed to assess the tumor resection extent.
Surgical Procedure and Complications
Histological confirmation of medulloblastoma before chemotherapy was requested if possible. It was obtained through either a biopsy of the primary tumor or a metastasis, or tumor excision. Surgery was considered complete (R0), when there was no evidence of residual tumor during the surgery and on the postoperative MRI. The excision was considered partial (R1) in the presence of a macroscopic residue and/or residual tumor >1.5 cm2 on postoperative MRI. The neurological immediate postoperative complications (cerebellar syndrome kinetic and/or static, cerebellar mutism, nystagmus, dysmetria, tremor and/or ataxia, cranial nerve palsies) were evaluated in each child. The severity of neurological postoperative complications was rated from 0 = absent to 3 = severe (grade ≥3 on the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) scale). The postoperative course was considered complicated if prolonged postoperative obtundation or life-threatening complications led to new surgery or prolonged stay in the neurosurgical intensive care unit. Severe postoperative complications were recorded—such as neurological postoperative status grade ≥3 of the NCI-CTC, infection (meningitis or ventriculitis), hydrocephalus, air embolism, or hemorrhage after tumor resection.
Histological Tumor Analysis
Formalin-fixed paraffin-embedded specimens from all patients with available tumor tissue samples at diagnosis and/or after neoadjuvant chemotherapy were reviewed by 3 experienced neuropathologists. Standard histological analysis, including immunostaining with anti–beta-catenin, -YAP1, -GAB1, -Ki67, and -p53 antibodies, was used to identify the medulloblastoma type, according to the criteria defined by the 2007 and 2016 WHO classifications.10,42 N-myc and Myc amplification were analyzed by fluorescent in situ hybridization. Subgrouping with methylation profiling or NanoString was not available for most patients who underwent surgery before 2010. Matched tumor pairs (at diagnosis before chemotherapy and after surgery) were analyzed when available in group B.
Neuropsychological Evaluation
Neuropsychological evaluations were done according to a French national protocol and described previously.24 Specifically, aged-adapted Wechsler scales were used to assess the intellectual quotient (IQ), except in children younger than 3 years who were evaluated with the Brunet-Lezine scale. The scores of the subtests of the used scales were used to determine the Full-Scale Intellectual Quotient (FSIQ) and the Perceptual Reasoning/Organization Index (PRI), which evaluates the ability to interpret and organize visually presented nonverbal information. Complete neuropsychological testing was scheduled at diagnosis and usually 1, 3, 5 years or more after.
Statistical Analysis
Continuous variables were expressed as medians and interquartile ranges, and categorical variables as numbers and percentages. Baseline was defined as the date of diagnosis. Patients’ characteristics were compared between groups using the chi-square or Fisher’s exact test (as appropriate) for categorical variables, and the Mann–Whitney U-tests for continuous variables. Progression-free survival (PFS), event-free survival (EFS), and OS were calculated using the Kaplan–Meier method. PFS and EFS were defined as the time from the diagnosis date until the date of disease progression or first relapse/event, or last contact (death due to treatment-related toxicity was excluded). OS was defined as the time from the diagnosis date until death from any cause, or last contact. Survival rates are provided with the 95% confidence interval (CI) estimated using the Rothman method.
Longitudinal neuropsychological measures were analyzed using linear mixed models with random intercepts and slopes. Separate models were built for each neuropsychological outcome. Each explicative covariate was introduced in the model with an interaction term with time as continuous measure (using the diagnosis date as origin). A first-order autoregressive covariance matrix was used because it showed the lowest Akaike information criteria in linear mixed models including the treatment group. This choice was confirmed in the final models. The neuropsychological score changes in groups A and B were adjusted to age (older or younger than 5 y), radiotherapy total dose to the brain (0, 18, 24, or 36 Gy), hydrocephalus (yes or no), and medulloblastoma histological subtype (nodular/desmoplastic compared with the other subtypes). Interactions between explanatory variables were explored. Explanatory variables for fixed effects were selected in a stepwise procedure to retain the best model. Each model fit was checked using Cholesky’s scaled residuals plots. All tests were two-sided and P < 0.05 was considered statistically significant. Statistical analyses were done using SAS version 9.4.
Results
Patient Characteristics
The 92 patients (32 girls and 60 boys) who met the inclusion criteria were distributed into group A (n = 54; 59%) and group B (n = 38; 41%). The median age at diagnosis was 5.0 years (range, 0.1–18.0). Sex, age at diagnosis, and medulloblastoma histological subtypes and initial tumor size at the primary site were comparable between groups A and B (Table 1). Global treatment strategies and the patients’ outcome also were similar between groups (Table 1). Conversely, hydrocephalus and place of surgery (Necker Sick Children’s Hospital in Paris vs other centers) were significantly more frequent in group B (P = 0.0025 and P = 0.0005, respectively).
Table 1.
Patients’ clinical characteristics, treatments, and outcome
| Group A | Group B | P-value | |
|---|---|---|---|
| n = 54 (%) | n = 38 (%) | ||
| Boys/girls | 36 /18 (66.7 / 33.3) | 24/14 (63.2 / 36.8) | 0.7279 |
| Median age, y (range) | 4.7 (0.8–18.0) | 5.4 (0.1–13.9) | |
| Age class at diagnosis | 0.3970 | ||
| ≤5 y | 29 (53.7) | 17 (44.7) | |
| >5 y | 25 (46.3) | 21 (55.3) | |
| Histological diagnosis of medulloblastoma | 0.0542 | ||
| Classic | 35 (64.8) | 33 (86.8) | |
| Nodular/desmoplastic | 10 (18.5) | 2 (5.3) | |
| Anaplastic/large cell | 7 (13.0) | 1 (2.6) | |
| Other or NOS | 2 (3.7) | 2 (5.3) | |
| Hydrocephalus (yes/no) | 31/23 (57.4 / 42.6) | 33/5 (86.8 / 13.2) | 0.0025 |
| Place of the surgery (Necker/ other centers) | 30/24 (56 / 44) | 34 / 4 (89 / 11) | 0.0005 |
| Median initial tumor size at the primary site, cm2 (range) | 12.66 (3.20–22.14) | 12.56 (2.42–31.30) | 0.8726 |
| Treatment regimen combined with surgery* | 0.2990 | ||
| CC + RT | 2 (3.7) | 1 (2.6) | |
| CC + HDC + standard RT | 22 (40.7) | 23 (60.5) | |
| CC + HDC + decreased RT CC + HDC without RT | 28 (51.9) 2 (3.7) | 13 (34.2) 1 (2.6) | |
| Outcome (dead/alive) | 25/29 (46.3 / 53.7) | 16/22 (42.1 / 57.9) | 0.6905 |
Abbreviations: NOS not otherwise specified, CC conventional chemotherapy, RT radiotherapy, HDC high-dose chemotherapy.
* Intent to treat.
Surgical Management at Diagnosis
At diagnosis, 64 children (69.6%) had clinical signs of hydrocephalus. CSF shunting was performed, when needed, during the first surgery or afterward. Endoscopic third ventriculostomy was the preferred modality for obstructive hydrocephalus treatment. Among the 64 patients with clinical signs of hydrocephalus, no difference in terms of persistent CSF drainage with a ventriculoperitoneal shunt (VPS) was observed between the 2 groups: 18 patients had a VPS (n = 10 in group A and n = 8 in group B). In group A, tumor was removed 2.5 days (range, 0–14) after diagnostic MRI. In group B, a minimal tumor biopsy was carried out at diagnosis for the histological proof of medulloblastoma, except in 3 children because of their initial clinical instability. In these 3 patients, the histological diagnosis of medulloblastoma was obtained after tumor removal following neoadjuvant chemotherapy.
Neoadjuvant Chemotherapy and Radiological Evaluation
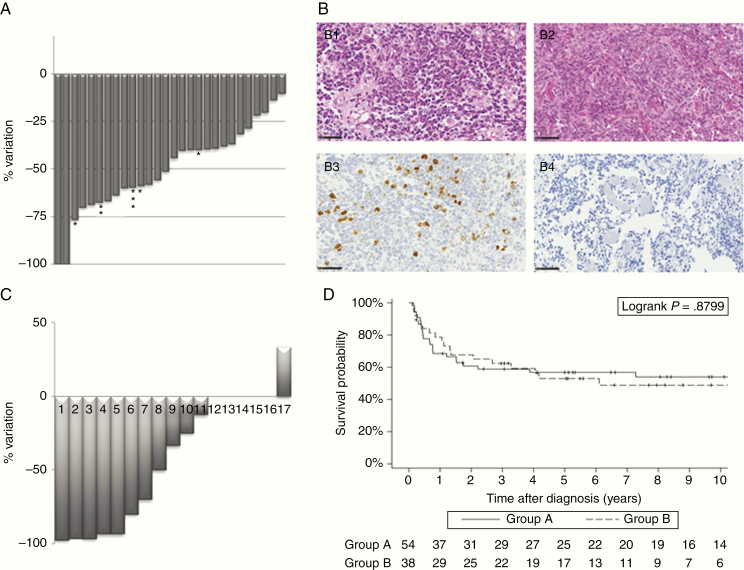
The median time between tumor biopsy at diagnosis and neoadjuvant chemotherapy start was 8 days in group B, compared with 15 days between tumor excision and beginning of chemotherapy in group A, P < 0.0001. In group B, all children (n = 38) received etoposide and carboplatin before tumor removal: 1 course (n = 1), 2 courses (n = 31), 3 courses (n = 4), 4 courses (n = 1), or 8 courses (n = 1), according to the physician’s choice. This led to a decrease in the primary tumor size in all patients (see Fig. 1A for an example), 4/38 patients experiencing meanwhile a distant progression. No isolated local failure or progression of the primary tumor was observed. In 28/38 children in group B, tumor size on the brain MRI at diagnosis and at the end of neoadjuvant chemotherapy was remeasured centrally. Response was evaluated after 2 courses (n = 23 patients), 3 courses (n = 3 patients), 4 courses (n = 1 patient), or 8 courses (n = 1 patient). This radiological review showed that CR was observed in 2/28 patients (7%), PR in 12/28 patients (43%), and minor response or stable disease in 14/28 patients (50%) (Fig. 1D). One patient, with a PR after 2 courses of chemotherapy, subsequently presented a metastatic progression during HDC and quickly died from it. In group A, a disease progression was observed in 6 children during conventional chemotherapy. Progression on conventional chemotherapy was therefore similar in both groups (6/54 patients, ie, 11% vs 4/38 patients, ie, 10%). As CSF sampling was not systematically performed in patients with overt metastases on MRI, CSF response could not be assessed accurately in all patients with M stage above 1. Only 2 patients in group B had a positive CSF without metastases to the brain or spine at diagnosis (M1 disease). CSF sampling was repeated in these 2 patients to assess the response of metastatic disease. CSF was negative at the end of neoadjuvant chemotherapy.
Fig. 1.
Response to neoadjuvant chemotherapy. (A) Radiological response to chemotherapy on brain MRI (sagittal sections T1 gadolinium) performed before and after neoadjuvant chemotherapy (scale bar 5 cm). Changes in the size of the primary tumor after neoadjuvant chemotherapy measured radiologically in 28 patients in group B with MRI at diagnosis and at the end of neoadjuvant chemotherapy available for comparison. Response was evaluated after 2 courses (23 patients), * 3 courses (3 patients), ** 4 courses (1 patient) or *** 8 courses (1 patient). (B) Medulloblastoma histopathological analysis before and after neoadjuvant chemotherapy. Sections showing: (B1 and B2) small blue round cell tumors consisting of densely packed undifferentiated embryonal cells (HPS), still existing after the adjuvant treatment (HPS) and (B3 and B4) significant decrease of the proliferation index (evaluated by MIB1 labeling index) between first surgery and the surgery after treatment (magnification 400x, scale bar 50µm). (C) Proliferation index changes after neoadjuvant chemotherapy in 17 patients from group B with available matched tumor pairs. (D) Progression-free survival in group A (surgery at diagnosis) and group B (surgery after neoadjuvant therapy) in function of time (years).
Results of Primary Tumor Surgery and Histological Features
All patients in group A underwent surgery with the aim of maximal primary tumor removal (n = 54). In group B, the posterior fossa tumor excision was performed after conventional neoadjuvant chemotherapy ± subsequent HDC in 30/38 patients (79%). The median time between biopsy and tumor removal was 64 days (range, 34–210). Surgery could not be performed in 8 patients: 5 children with progressive disease during conventional neoadjuvant chemotherapy (n = 4) or HDC (n = 1); 1 child died before surgery because of hemorrhage from a metastasis while the local disease was controlled; and 2 children had mostly metastatic disease with a very small primary tumor that completely disappeared after adjuvant chemotherapy. The rate of complete excision of the primary tumor was significantly higher in group B than in group A: 28/30 patients (93.3%) versus 31/54 (57.4%), respectively (P = 0.0013) (Table 2). Four patients in each group developed transient postoperative akinetic mutism after tumor removal. Postoperative neurological status and incidence of postoperative complications were similar in both groups (non-neurological postoperative complications: 14/54 = 25.9% vs 8/30 = 26.7%, P = n.s.; severe postoperative complications: 19/54 = 35.2% vs 7/30 = 23.3%, P = n.s.) (Table 2). In group A, severe postoperative complications were observed in 19 patients: severe neurological complications (grade ≥3 of the NCI-CTC scale) including akinetic mutism (n = 14), sometimes associated with hydrocephalus that required transient external shunt or VPS (n = 4), hemorrhage or subdural air collection that sometimes required a shunt (n = 4), and meningitis (n = 1). In group B, severe postoperative complications were observed in 7 patients: akinetic mutism (n = 4), hydrocephalus that required transient external shunt or VPS (n = 2), and cerebellar ischemic stroke (n = 1).
Table 2.
Extension of resection of the primary medulloblastoma at diagnosis (group A) or after neoadjuvant chemotherapy (group B) and postoperative complications
| Group A N = 54 (%) | Group B N = 30 (%) | P-value | |
|---|---|---|---|
| Tumor resection | 0.0013 | ||
| Complete tumor resection (R0) Partial tumor resection (R1) | 31 (57.4) 23 (42.6) | 28 (93.3) 2 (6.7) | |
| Nonneurological postoperative complications (hydrocephalus, infection, hemorrhage or subdural air collection) No Yes | 40 (74.1) 14 (25.9) | 22 (73.3) 8 (26.7) | 0.9410 |
| Neurological postoperative status None = 0 Mild (NCI-CTC grade 1) = 1 Moderate (NCI-CTC grade 2) = 2 Severe (NCI-CTC grade 3, 4, or 5), including akinetic mutism = 3 | 15 (27.8) 17 (31.5) 8 (14.8) 14 (25.9) | 10 (33.3) 10 (33.3) 6 (20) 4 (13.3) | 0.5810 |
| Severe postoperative complications: severe neurological status (3), or other severe postoperative complications Not severe Severe | 35 (64.8) 19 (35.2) | 23 (76.7) 7 (23.3) | 0.2602 |
In group B, of the 30 patients who had secondary surgery, 3 did not have an initial biopsy at the time of diagnosis. Of the 27 remaining patients who underwent 2 surgeries (biopsy at diagnosis and surgery after chemotherapy), only 19 matched tumor pair samples were available for comparison. Histological examination showed that live medulloblastoma cells could still be found after the second surgery despite the preoperative chemotherapy. In 16 of these patients, the medulloblastoma histological and immunophenotypic profiles (classic, non-WNT, and non-SHH in all samples) did not change (Fig. 1B1 and 1 B2). In the other 3 patients, tumors were considered as not otherwise specified at the time of the biopsy, and were characterized as classic, non-WNT/non-SHH medulloblastoma after tumor removal. Comparison of the proliferation index between paired samples was possible in 17 cases and it was significantly decreased by more than 50% in 8/17 patients after neoadjuvant treatment (Fig. 1B3 and 1 B4 and Fig. 1C).
Outcome
The median follow-up was 10 years in group A and 8 years in group B. The 5-year OS rates were 60% (95% CI: 47–72) in group A and 68% (95% CI: 52–81) in group B (log rank P = n.s., available in Supplementary Material). In all patients but 2, death was due to progressive disease or relapse. Only one child in each group died from treatment-related toxicity without progressive disease (veno-occlusive disease in one patient and metastatic hemorrhage in the other). The 5-year PFS rates were 57% (95% CI: 43–69) in group A and 53% (95% CI: 37–68) in group B (log rank P = n.s.) (Fig. 1E), and the EFS rate was 51% in both groups (log rank P = n.s., available in Supplementary Material). Disease progression or recurrence is summarized in Table 3. Local control (Table 3) was similar in both groups (22% in group A and 13% in group B, P = n.s.). Seven second malignancies occurred: high-grade glioma in 4 patients (4.9, 5.9, 6.5, and 8 y after the medulloblastoma diagnosis) and thyroid carcinoma in 3 patients (6.1, 9.3, and 12.9 y after the first diagnosis).
Table 3.
Disease progression or recurrence in groups A and B
| Disease Progression and Recurrence | Group A n = 54 | Group B n = 38 |
|---|---|---|
| Local and metastatic disease progression | 4 | 2 |
| Metastatic disease progression | 6 | 3 |
| Local recurrence | 6 | 1 |
| Local and metastatic recurrence | 2 | 2 |
| Metastatic recurrence | 6 | 10 |
| Median time to disease progression or recurrence, y (range) | 0.56 (0.2–7.3) | 1.1 (0.15–6.1) |
| Median follow-up, 50% (95% CI), y | 10 (8–13) | 8 (5.5–11) |
Neurocognitive Behavior
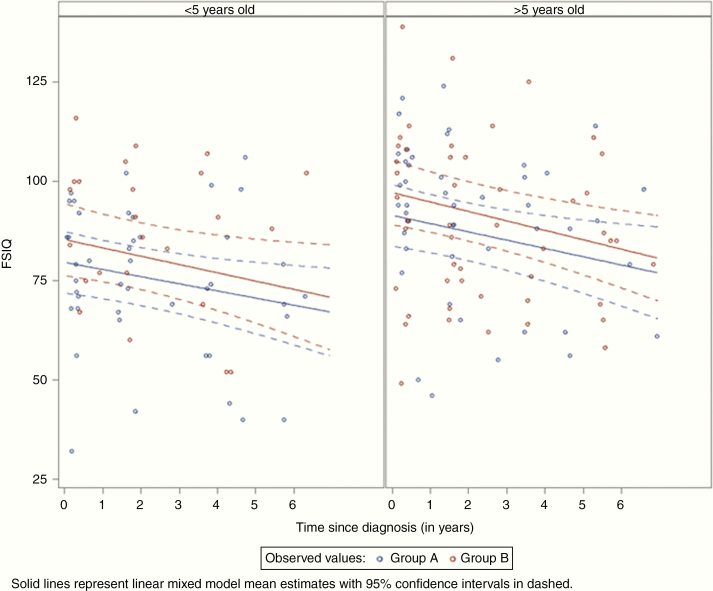
Neuropsychological evaluations were available for 69 patients (75% of the whole cohort; n = 39 in group A and n = 30 in group B) (median number of evaluations per patient = 2; range, 1–6): 183 FSIQ and 166 PRI scores. In linear mixed models with continuous time measurement, the FSIQ and PRI scores significantly decreased with time (P < 0.0001 and 0.0044) and were significantly correlated with the age class at diagnosis (P = 0.0182 and 0.0392) and the radiotherapy total doses delivered to the brain (P = 0.0417 and <.0001) in both groups. Hydrocephalus and medulloblastoma histological subtype (nodular/desmoplastic compared with the other subtypes) were not associated with the FSIQ and PRI scores. The predicted mean FSIQ and PRI estimates from the mixed model at 1, 3, 5, and 7 years after diagnosis (Table 4) highlighted that they tended to be higher in group B than in group A, although not significantly (P = 0.1764 and 0.1789, respectively). In linear mixed models, the FSIQ score progressively worsened over time, with no significant difference between groups (Fig. 2).
Table 4.
Predicted mean FSIQ and PRI estimates (mixed model) for groups A and B at 1, 3, 5, and 7 years after diagnosis
| Time, y | T0 | T1 | T3 | T5 | T7 | |
|---|---|---|---|---|---|---|
| Predicted mean FSIQ | Group A | 85.5 (79–92) | 83 (77–90) | 79 (73–86) | 75 (68–82) | 71 (63–79) |
| Group B | 91 (84–99) | 89 (82–96) | 85 (78–92) | 81 (73–88) | 77 (68–85) | |
| Predicted mean PRI | Group A | 84 (77–91) | 82 (76–89) | 79 (73–85) | 76 (69–83) | 73 (64–81) |
| Group B | 90 (82–98) | 88.5 (81–96) | 85 (79–92) | 82 (75–89) | 79 (70–87) |
Fig. 2.
Changes of the predicted FSIQ in patients in function of the age class at diagnosis (≤5 and >5 y).
Discussion
This study showed a significantly higher complete tumor excision rate in patients operated after preoperative chemotherapy in group B versus in group A (57.4%, similar rate to what is usually reported40,43). Carboplatin-etoposide regimen was very effective in the neoadjuvant setting, as shown in recurrent medulloblastoma,33,36 and led to a decrease of the primitive tumor size and an earlier efficient treatment of metastases before tumor removal. In group B, the complete tumor resection rate (93.3%) was similar to that described in our previous pilot study33 and for various brain tumors in infants and young children (85% and 89%, respectively), thanks to the effectiveness of preoperative chemotherapy on the size of the primary tumor, especially in embryonal tumors.34,35 Neoadjuvant chemotherapy enabled the surgery to be performed in the absence of raised intracranial pressure, on a smaller tumor and in a patient in better clinical condition. The higher rate of complete tumor excision may represent a prognostic advantage, as past studies have shown that residual tumor had an adverse prognostic impact.11 Maximum safe surgical resection should remain the standard of care in medulloblastoma. These goals were achieved with the strategy of tumor excision performed after neoadjuvant chemotherapy. Despite the significantly higher complete excision rate for patients in group B, we did not observe a higher rate of acute postoperative complications, especially neurological impairment. Late severe neurological complications were rare. Their low number of cases would not allow expectation of a statistically significant difference between both groups. Neuropsychological evaluation is more subtle. Analysis of the extensive longitudinal neuropsychological data showed that in both groups, IQ decline was correlated with young age at diagnosis, radiation doses to the brain, and time, as previously reported.25,44 Hydrocephalus did not have any effect, although its frequency was not balanced between groups. As hydrocephalus was more frequent in group B, this may have limited the neuropsychological benefit of delayed surgical therapy in this group because hydrocephalus is known to worsen the neuropsychological outcome.29 Although no significant difference was found between groups, children in group B tended to have a better neuropsychological outcome, based on the FSIQ and PRI scores after surgery and after treatment completion. This trend might be explained by the reduced surgery impact on normal brain due to the smaller tumor size and, possibly, hydrocephalus treatment before primary tumor surgery. A recent study showed that the medulloblastoma molecular subgroups influence the intellectual outcome, especially the SHH subgroup.9,45 Unfortunately, appropriate subgrouping was not available for part of our patients. Nevertheless, as desmoplastic/nodular medulloblastomas are almost exclusively SHH-activated tumors, analysis of this variable showed no significant correlation between the desmoplastic/nodular histological profile and neuropsychological impairment.
No negative impact of neoadjuvant chemotherapy was reported: no toxic complication due to chemotherapy or additional disease progression, especially local progression, linked to the delayed surgery of the primary tumor. Disease progression rate during etoposide-carboplatin courses was similar and no isolated local failure was observed in group B before surgery. No detrimental effect of delayed surgery after neoadjuvant chemotherapy was observed on survival, as indicated by the similar 5-year PFS and OS rates in the 2 groups. These results are comparable with those already published for patients with high-risk medulloblastoma,9,12,13 but better than reported in our pilot study.33 The safety of this approach in terms of tumor control suggests that a very aggressive surgery approach at diagnosis might not be needed and that second look surgery could be scheduled after chemotherapy.
The histological study of 19 matched tumor pairs led to the conclusion that diagnosis is still possible on residual medulloblastoma tumors despite the preoperative chemotherapy with no change in the histological subtype. This finding is of paramount importance because it allowed the safe and consistent histological proof to be obtained in every patient. Molecular grouping should be conserved, although not shown in this study, since it is conserved at relapse as demonstrated previously.46 This should not question the need for a biopsy at diagnosis, since alternative diagnoses may need different treatment approaches. Neoadjuvant chemotherapy significantly decreased the proliferation index in almost half of them. The sample size was too small to measure the influence of the histological response on survival.
One of the interesting perspectives raised by our study showing the safety of preoperative chemotherapy is the possibility to use this therapeutic window to test new drugs upfront. Response rate here with the standard combination of etoposide and carboplatin36 is high and setup a landmark. Previous studies have also shown that the response to preoperative chemotherapy could predict outcome in metastatic medulloblastomas47 and could therefore help to identify high-risk patients who do not respond to conventional chemotherapy.
Our study has some limitations. Patients were not randomized between surgery upfront and neoadjuvant chemotherapy before surgery, and the choice was mostly based on the interpretation of tumor operability by the neurosurgeon. Therefore, higher-risk patients (presence of hydrocephalus, worse clinical or neurological status) were mostly in group B. Nevertheless, our results show that the complete tumor excision rate was higher and neuropsychological behavior tended to be better in this group. This suggests that this strategy, initially proposed to patients with metastatic disease to improve disease control and start chemotherapy earlier, could be discussed also for patients with localized medulloblastoma where surgical difficulties are anticipated due to the higher rate of complete tumor resection.
Conclusion
Preoperative chemotherapy in patients with metastatic medulloblastoma is advantageous. It allows an early efficient treatment of the whole disease, increases the rate of complete tumor removal, and could have a positive impact on the neuropsychological outcome of patients. Our study did not show any additional risk concerning postoperative complications, disease control, and patient outcome in the group with chemotherapy before the surgery. Histological diagnosis was still feasible and reliable after preoperative chemotherapy. These findings are important because this strategy may be integrated and tested in future protocols for the treatment of children with metastatic medulloblastoma.
Supplementary Material
Funding
None.
Conflict of interest statement. No conflicts.
Authorship statement. Léa Guerrini-Rousseau, Sophie Huybrechts, Virginie Kieffer-Renaux, Stéphanie Bolle, Frédéric Dhermain, Audrey Longaud, Dominique Valteau-Couanet, Christelle Dufour, and Jacques Grill provided clinical data. Christian Sainte-Rose, Stéphanie Puget, Michel Zerah, Thomas Roujeau, Thomas Blauwblomme, and Kévin Beccaria performed surgery and provided surgical data. Pascale Varlet, Felipe Andreiuolo, and Arnault Tauziede-Espariat provided histological data and performed the histological review. Rachid Abbas provided the statistical analysis. Chantal Kalifa, Christelle Dufour, Jacques Grill, and Léa Guerrini-Rousseau contributed to the study design. All authors contributed to writing, reviewing and editing.
References
- 1. Albright AL, Wisoff JH, Zeltzer PM, Boyett JM, Rorke LB, Stanley P. Effects of medulloblastoma resections on outcome in children: a report from the Children’s Cancer Group. Neurosurgery. 1996;38(2):265–271. [DOI] [PubMed] [Google Scholar]
- 2. Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17(3):832–845. [DOI] [PubMed] [Google Scholar]
- 3. Ellison DW, Kocak M, Dalton J, et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. 2011;29(11):1400–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Massimino M, Antonelli M, Gandola L, et al. Histological variants of medulloblastoma are the most powerful clinical prognostic indicators. Pediatr Blood Cancer. 2013;60(2):210–216. [DOI] [PubMed] [Google Scholar]
- 5. Pfister S, Remke M, Benner A, et al. Outcome prediction in pediatric medulloblastoma based on DNA copy-number aberrations of chromosomes 6q and 17q and the MYC and MYCN loci. J Clin Oncol. 2009;27(10):1627–1636. [DOI] [PubMed] [Google Scholar]
- 6. Ryan SL, Schwalbe EC, Cole M, et al. MYC family amplification and clinical risk-factors interact to predict an extremely poor prognosis in childhood medulloblastoma. Acta Neuropathol. 2012;123(4):501–513. [DOI] [PubMed] [Google Scholar]
- 7. Northcott PA, Korshunov A, Witt H, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29(11):1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kool M, Korshunov A, Remke M, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123(4):473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramaswamy V, Remke M, Bouffet E, et al. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016;131(6):821–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 11. Lannering B, Rutkowski S, Doz F, et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol. 2012;30(26):3187–3193. [DOI] [PubMed] [Google Scholar]
- 12. Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. [DOI] [PubMed] [Google Scholar]
- 13. Dufour C, Kieffer V, Varlet P, et al. Tandem high-dose chemotherapy and autologous stem cell rescue in children with newly diagnosed high-risk medulloblastoma or supratentorial primitive neuro-ectodermic tumors. Pediatr Blood Cancer. 2014;61(8):1398–1402. [DOI] [PubMed] [Google Scholar]
- 14. Riva D, Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000;123(Pt 5):1051–1061. [DOI] [PubMed] [Google Scholar]
- 15. Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010;20(3):236–260. [DOI] [PubMed] [Google Scholar]
- 16. Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5(7):399–408. [DOI] [PubMed] [Google Scholar]
- 17. Di Rocco F, Jucá CE, Zerah M, Sainte-Rose C. Endoscopic third ventriculostomy and posterior fossa tumors. World Neurosurg. 2013;79(2 Suppl):S18.e15–S18.e19. [DOI] [PubMed] [Google Scholar]
- 18. Hanzlik E, Woodrome SE, Abdel-Baki M, Geller TJ, Elbabaa SK. A systematic review of neuropsychological outcomes following posterior fossa tumor surgery in children. Childs Nerv Syst. 2015;31(10):1869–1875. [DOI] [PubMed] [Google Scholar]
- 19. Palmer SL, Armstrong C, Onar-Thomas A, et al. Processing speed, attention, and working memory after treatment for medulloblastoma: an international, prospective, and longitudinal study. J Clin Oncol. 2013;31(28):3494–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kieffer V, Chevignard MP, Dellatolas G, et al. Intellectual, educational, and situation-based social outcome in adult survivors of childhood medulloblastoma. Dev Neurorehabil. 2019;22(1):19–26. [DOI] [PubMed] [Google Scholar]
- 21. Schreiber JE, Gurney JG, Palmer SL, et al. Examination of risk factors for intellectual and academic outcomes following treatment for pediatric medulloblastoma. Neuro Oncol. 2014;16(8):1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Ruiter MA, van Mourik R, Schouten-van Meeteren AY, Grootenhuis MA, Oosterlaan J. Neurocognitive consequences of a paediatric brain tumour and its treatment: a meta-analysis. Dev Med Child Neurol. 2013;55(5):408–417. [DOI] [PubMed] [Google Scholar]
- 23. Grill J, Renaux VK, Bulteau C, et al. Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int J Radiat Oncol Biol Phys. 1999;45(1):137–145. [DOI] [PubMed] [Google Scholar]
- 24. Kieffer-Renaux V, Bulteau C, Grill J, Kalifa C, Viguier D, Jambaque I. Patterns of neuropsychological deficits in children with medulloblastoma according to craniospatial irradiation doses. Dev Med Child Neurol. 2000;42(11):741–745. [DOI] [PubMed] [Google Scholar]
- 25. Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22(4):706–713. [DOI] [PubMed] [Google Scholar]
- 26. Lafay-Cousin L, Bouffet E, Hawkins C, Amid A, Huang A, Mabbott DJ. Impact of radiation avoidance on survival and neurocognitive outcome in infant medulloblastoma. Curr Oncol. 2009;16(6):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moxon-Emre I, Bouffet E, Taylor MD, et al. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol. 2014;32(17):1760–1768. [DOI] [PubMed] [Google Scholar]
- 28. Di Rocco C, Chieffo D, Pettorini BL, Massimi L, Caldarelli M, Tamburrini G. Preoperative and postoperative neurological, neuropsychological and behavioral impairment in children with posterior cranial fossa astrocytomas and medulloblastomas: the role of the tumor and the impact of the surgical treatment. Childs Nerv Syst. 2010;26(9):1173–1188. [DOI] [PubMed] [Google Scholar]
- 29. Grill J, Viguier D, Kieffer V, et al. Critical risk factors for intellectual impairment in children with posterior fossa tumors: the role of cerebellar damage. J Neurosurg. 2004;101(2 Suppl):152–158. [DOI] [PubMed] [Google Scholar]
- 30. Puget S, Boddaert N, Viguier D, et al. Injuries to inferior vermis and dentate nuclei predict poor neurological and neuropsychological outcome in children with malignant posterior fossa tumors. Cancer. 2009;115(6):1338–1347. [DOI] [PubMed] [Google Scholar]
- 31. Hardy KK, Bonner MJ, Willard VW, Watral MA, Gururangan S. Hydrocephalus as a possible additional contributor to cognitive outcome in survivors of pediatric medulloblastoma. Psychooncology. 2008;17(11):1157–1161. [DOI] [PubMed] [Google Scholar]
- 32. Schreiber JE, Palmer SL, Conklin HM, et al. Posterior fossa syndrome and long-term neuropsychological outcomes among children treated for medulloblastoma on a multi-institutional, prospective study. Neuro Oncol. 2017;19(12):1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grill J, Lellouch-Tubiana A, Elouahdani S, et al. Preoperative chemotherapy in children with high-risk medulloblastomas: a feasibility study. J Neurosurg. 2005;103(4 Suppl):312–318. [DOI] [PubMed] [Google Scholar]
- 34. Iwama J, Ogiwara H, Kiyotani C, et al. Neoadjuvant chemotherapy for brain tumors in infants and young children. J Neurosurg Pediatr. 2015;15(5):488–492. [DOI] [PubMed] [Google Scholar]
- 35. Van Poppel M, Klimo P, Dewire M, et al. Resection of infantile brain tumors after neoadjuvant chemotherapy: the St. Jude experience. J Neurosurg Pediatr. 2011;8(3):251–256. [DOI] [PubMed] [Google Scholar]
- 36. Gentet JC, Doz F, Bouffet E, et al. Carboplatin and VP 16 in medulloblastoma: a phase II study of the French Society of Pediatric Oncology (SFOP). Med Pediatr Oncol. 1994;23(5):422–427. [DOI] [PubMed] [Google Scholar]
- 37. Verlooy J, Mosseri V, Bracard S, et al. Treatment of high risk medulloblastomas in children above the age of 3 years: a SFOP study. Eur J Cancer. 2006;42(17):3004–3014. [DOI] [PubMed] [Google Scholar]
- 38. Vassal G, Tranchand B, Valteau-Couanet D, et al. Pharmacodynamics of tandem high-dose melphalan with peripheral blood stem cell transplantation in children with neuroblastoma and medulloblastoma. Bone Marrow Transplant. 2001;27(5):471–477. [DOI] [PubMed] [Google Scholar]
- 39. Kalifa C, Hartmann O, Demeocq F, et al. High-dose busulfan and thiotepa with autologous bone marrow transplantation in childhood malignant brain tumors: a phase II study. Bone Marrow Transplant. 1992;9(4):227–233. [PubMed] [Google Scholar]
- 40. Grill J, Sainte-Rose C, Jouvet A, et al. ; French Society of Paediatric Oncology Treatment of medulloblastoma with postoperative chemotherapy alone: an SFOP prospective trial in young children. Lancet Oncol. 2005;6(8):573–580. [DOI] [PubMed] [Google Scholar]
- 41. Warren KE, Vezina G, Poussaint TY, et al. Response assessment in medulloblastoma and leptomeningeal seeding tumors: recommendations from the Response Assessment in Pediatric Neuro-Oncology committee. Neuro Oncol. 2018;20(1):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thompson EM, Hielscher T, Bouffet E, et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol. 2016;17(4):484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Edelstein K, Spiegler BJ, Fung S, et al. Early aging in adult survivors of childhood medulloblastoma: long-term neurocognitive, functional, and physical outcomes. Neuro Oncol. 2011;13(5):536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moxon-Emre I, Taylor MD, Bouffet E, et al. Intellectual outcome in molecular subgroups of medulloblastoma. J Clin Oncol. 2016;34(34):4161–4170. [DOI] [PubMed] [Google Scholar]
- 46. Ramaswamy V, Remke M, Bouffet E, et al. Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol. 2013;14(12):1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dufour C, Beaugrand A, Pizer B, et al. Metastatic medulloblastoma in childhood: Chang’s classification revisited. Int J Surg Oncol. 2012;2012:245385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.