Abstract
The effect of hay type on the microbiome of the equine gastrointestinal tract is relatively unexplored. Our objective was to characterize the cecal and fecal microbiome of mature horses consuming alfalfa or Smooth Bromegrass (brome) hay. Six cecally cannulated horses were used in a split-plot design run as a crossover in two periods. The whole plot treatment was ad libitum access to brome or alfalfa hay fed over two 21-d acclimation periods with subplots of sampling location (cecum and rectum) and sampling hour. Each acclimation period was followed by a 24-h collection period where cecal and fecal samples were collected every 3 h for analysis of pH and volatile fatty acids (VFA). Fecal and cecal samples were pooled and sent to a commercial lab (MR DNA, Shallowater, TX) for the amplification of the V4 region of the 16S rRNA gene and sequenced using Illumina HiSeq. The main effects of hay on VFA, pH, and taxonomic abundances were analyzed using the MIXED procedure of SAS 9.4 with fixed effects of hay, hour, location, period, and all possible interactions and random effect of horse. Alpha and beta diversities were analyzed using the R Dame package. Horses fed alfalfa had greater fecal than cecal pH (P ≤ 0.05), whereas horses fed brome had greater cecal than fecal pH (P ≤ 0.05). Regardless of hay type, total VFA concentrations were greater (P ≤ 0.05) in the cecum than in feces, and alfalfa resulted in greater (P ≤ 0.05) VFA concentrations than brome in both sampling locations. Alpha diversity was greater (P ≤ 0.05) in fecal compared with cecal samples. Microbial community structure within each sampling location and hay type differed from one another (P ≤ 0.05). Bacteroidetes were greater (P ≤ 0.05) in the cecum compared with the rectum, regardless of hay type. Firmicutes and Firmicutes:Bacteroidetes were greater (P ≤ 0.05) in the feces compared with cecal samples of alfalfa-fed horses. In all, fermentation parameters and bacterial abundances were impacted by hay type and sampling location in the hindgut.
Keywords: bacteria, fermentation, hay, hindgut, horse, microbiome
Introduction
As hindgut fermenters, horses rely on a highly functional cecal microbiome capable of fermenting structural carbohydrates into volatile fatty acids (VFA). Next-generation sequencing (NGS) of the 16S rRNA gene (Brunstein, 2016) allows the identification of these microbial populations. Utilizing these technologies, microbial shifts due to diet and other exogenous factors can be identified.
Studies designed to evaluate the gastrointestinal (GI) microbiome and fermentation parameters of horses on forage-only diets have relied largely on fecal sampling (Costa et al., 2012; Shepherd et al., 2012; Fernandes et al., 2014; Julliand et al., 2018; Stewart et al., 2018), euthanasia (Dougal et al., 2013; Costa et al., 2015), or nasogastric sampling (Julliand et al., 2018). Costa et al. (2015) reported that microbiota varied greatly between GI compartments of horses. Similarly, Dougal et al. (2012) found that the bacterial community within the cecum of horses euthanized for non-research purposes was more closely related to one another than those of the right dorsal colon or feces. The authors contributed these differences to GI anatomy and the varying substrates within each section (Dougal et al., 2012).
Though not as common as euthanasia or fecal microbiome studies, cecally cannulated horses have been used to evaluate the impact of concentrates (i.e., cereal grains and byproducts) on microbial shifts (Coverdale et al., 2004; Venable et al., 2017; Warzecha et al., 2017). Firmicutes have been reported to be the most abundant phyla in fecal samples of live horses (Costa et al., 2012; Shepherd et al., 2012; Fernandes et al., 2014) and all digestive compartments of euthanized horses fed various diets (Dougal et al., 2013; Costa et al., 2015). Yet, Daly et al. (2012) reported Bacteroidetes to be in greater abundance than Firmicutes in colonic samples from concentrate-fed compared with grass-fed horses. This may be attributed to an increase in some genera within Bacteroidetes that favor nonstructural carbohydrates for fermentation and are more resilient to acidic conditions (Daly et al., 2012). Others (Daly et al., 2012; Fernandes et al., 2014) have reported that Fibrobacter and Ruminococcaceae were greater in grass-fed horses compared with horses fed concentrates as they degrade fiber and may be suppressed by more acidic environments. Julliand et al. (2018) reported increased amylolytic, pectinolytic, and lactate-utilizing bacteria in fecal samples of horses fed dehydrated alfalfa pellets compared with sunflower meal pellets. However, fecal pH was similar between the two diets. Warzecha et al. (2017) provided supporting data whereby horses consuming a high-starch diet had decreased Ruminococcus and increased Prevotella populations when compared with a high-fiber diet.
Indeed, previous studies give a solid base of information regarding the effect of diet on the cecal microbiome. Researchers relied on culture-based techniques before NGS became available, but only a small percentage of cecal bacterial species can be grown in culture (Julliand et al., 1999; Creevey et al., 2014). The ability of cereal grains to alter the cecal microbiome is well defined. However, there are no reports that describe the differences in the cecal microbiome of cannulated horses fed a legume vs. those fed a cool-season grass hay, arguably the two most common forage types for horses. Therefore, our objective was to quantify the fecal and cecal microbiome via NGS of mature horses consuming alfalfa or Smooth Bromegrass (brome) hay and to evaluate subsequent fermentation parameters, including pH and VFA concentration.
Materials and Methods
Animals
All animal protocols were approved by the Kansas State University Institutional Animal Care and Use Committee. Experimental units consisted of six mature Quarter Horses (12 ± 0.83 yr; 537 ± 16.3 kg; three mares and three geldings) previously fitted with cecal cannulae (Beard et al., 2011). All horses except one had undergone cannulation surgery no less than 3 yr prior to the study. One mare was fitted with a cannula 6 mo prior to the study. No horses had received antibiotic treatment for at least 4 mo prior to the study. Before the initiation of the study, all horses were housed together in a dry lot and fed ad libitum brome (Bromus inermis). On day 1, horses were housed within their respective treatment groups in adjacent dry lots (21.6 × 22.6 m). Each lot was equipped with an automatic waterer, hay feeder, and salt block.
Experimental design and dietary treatments
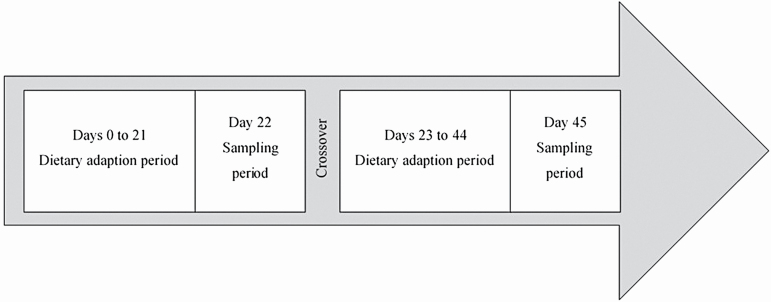
The experiment consisted of a split-plot crossover design (Figure 1) where the whole plot consisted of hay type (alfalfa or brome) with subplots of sampling time (hour) and sampling location within the GI tract (cecum and rectum). On day 0, horses were randomly assigned to one of two dietary treatments: ad libitum brome (n = 3) or ad libitum alfalfa (Medicago sativa; n = 3), and they remained on their respective diet for an adaptive period of 22 d. Horses were group fed and hay was pitched into feeders at 0700 and 1900 hours as needed to allow horses ad libitum access to hay. Brome hay was pitched from a round bale, while alfalfa was pitched from a large square bale. On day 22, cecal and fecal samples were collected. On day 23, horses were moved into the opposite pen to consume the alternate hay type and the protocol repeated. Refusals of hay per pen were recorded at 0700 and 1900 hours on the final 4 d of each treatment period to determine dry matter intake (DMI).
Figure 1.
Experimental design. On day 0, horses were randomly assigned to one of two dietary treatments: ad libitum brome (Bromus inermis; n = 3) or ad libitum alfalfa (Medicago sativa; n = 3), and they remained on their respective diet for 22 d. On day 22, two cecal and fecal samples were collected from each horse every 3 h for 24 h. On day 23, horses were moved into the opposite pen to consume the alternate hay type and the protocol repeated.
Sample collection and laboratory analyses
Hay samples were collected prior to the study with a hay core sampler (#07190, AgraTronix, Streetsboro, OH) and sent to a commercial laboratory (Dairy One Forage Lab, Ithaca, NY) for proximate analysis (Table 1).
Table 1.
Proximate analysis (DM basis) of hay1
| Item | Brome | Alfalfa |
|---|---|---|
| DM, % | 93.90 | 90.30 |
| Crude protein, % | 7.10 | 19.30 |
| Crude fat, % | 3.60 | 2.30 |
| Neutral detergent fiber, % | 62.30 | 47.20 |
| Acid detergent fiber, % | 38.90 | 38.70 |
| Digestible energy, Mcal/kg | 2.18 | 2.07 |
| Calcium, % | 0.40 | 1.63 |
| Phosphorus, % | 0.10 | 0.29 |
| Magnesium, % | 0.12 | 0.22 |
| Potassium, % | 1.43 | 2.39 |
| Sodium, % | 0.01 | 0.02 |
| Iron, mg/kg | 147.00 | 730.00 |
| Zinc, mg/kg | 11.00 | 24.00 |
| Copper, mg/kg | 5.00 | 10.00 |
| Manganese, mg/kg | 55.00 | 41.00 |
1Fed ad libitum to horses.
Cecal and fecal samples were collected every 3 h for 24 h on day 22 of each period. Horses were placed into stocks, plugs were removed from cannulae, and cecal contents were collected via gravity flow. Fecal samples were collected via rectal grab. All samples for microbial analysis were collected in sterile 15-mL conical centrifuge tubes (Nunc Conical Sterile Polypropylene Centrifuge Tubes, Thermo-Fisher Scientific, Waltham, MA). Cecal samples were collected directly into conical tubes, and approximately 10 g of the inner portion of fecal balls was placed into conical tubes. Samples were immediately placed in a −20 °C freezer for 24 h before being transported and stored in a −80 °C freezer until deoxyribonucleic acid (DNA) extraction and microbial sequencing were performed.
An additional sample of cecal fluid and fecal matter was collected at each time point from each horse and immediately strained through four layers of cheesecloth into a 180-mL container (Specimen Storage Containers, #4A0180, Thermo-Fisher Scientific, Waltham, MA). Strained cecal and fecal fluid were immediately measured for pH via a portable pH meter (Thermo Scientific Orion 3 Star Portable pH Meter, Waltham, MA). From each sample, three 1-mL aliquots of strained fluid were transferred by pipette into microcentrifuge tubes containing 0.25 mL of 25% metaphosphoric acid (wt/vol) for deproteination. Samples were then stored at −20 °C until VFA analyses.
Deproteinated cecal and fecal fluid were thawed and centrifuged at 17,000 × g for 30 min. Aqueous supernatant was transferred to gas chromatography (GC) vials and analyzed for VFA concentrations on an Agilent 7890 GC (Agilent Technologies, Santa Clara, CA) fitted with a 15 m × 0.53 mm × 0.5 µm film thickness Nukol capillary column (Supelco columns; Sigma-Aldrich, St. Louis, MO) and flame ionization detector. Hydrogen was used as the carrier gas at a flow rate of 35 mL/min. The initial oven temperature was 80 °C for 1 min and increased 20 °C/min for 6 min to reach a final temperature of 200 °C for 6 min. Inlet and detector temperatures were 250 °C. Quantification of VFAs was completed by comparison against known standards (Supelco Volatile Fatty Acid Standard Mix, Sigma-Aldrich, St. Louis, MO) containing acetate, propionate, isobutyrate, butyrate, isovalerate, valerate, isocaproate, caproate, and heptanoate.
Two cecal and fecal samples per treatment and per horse were used for DNA extraction. Cecal and fecal microbiome samples were collected every 3 h over the 24 h sampling period from each horse in each sampling location. Samples were pooled relative to when hay was pitched (0700 and 1900 hours) within the sampling location for each horse. In brief, cecal samples from time points 0900, 1200, 1500, and 1800 hours were pooled in equal proportions as were samples from 2100, 2400, 0300, and 0600 (final) hours per horse to represent the microbiome relative to 0700 and 1900 hours when hay was pitched, respectively. To pool samples, individual aliquots were vortexed (Scientific Industries Vortex-Genie 2, Houston, TX) until thawed and kept on ice to minimize shifts in microbial populations. One gram of each original sample was added into a sterile 15-mL conical centrifuge tube using sterilized lab scoops (Stainless Steel Lab Scoops, Thermo-Fisher Scientific, Waltham, MA). Pooled samples were vortexed and frozen at −80 °C. In total, 24 pooled cecal samples and 24 pooled fecal samples were shipped on dry ice to MR DNA (MR DNA, Shallowater, TX) for DNA extraction, amplification of the V4 region of the 16S gene, and sequencing using Illumina HiSeq protocols.
Raw sequence data were processed through Qiime version 1.9.1 (Caporaso et al., 2012). Raw sequences were joined and depleted of barcodes and primers. USEARCH (Edgar, 2010) was used as the main filter for noisy sequences, chimera checking, and operational taxonomic units (OTUs) picking. OTUs were defined by clustering at 3% divergence (97% similarity) against an open reference and final OTUs were taxonomically classified against the 16S rRNA Greengenes 13.8 database (McDonald et al., 2012).
Statistical analyses
Data were analyzed utilizing the MIXED procedure of SAS 9.4 (SAS Institute Inc., Cary, NC). Main and linear effects of hay type on VFA concentrations, pH, and microbial abundance using fixed effects of hay, period, location, hour (VFA and pH only), and all possible interactions, random effect of horse, and repeated measures of hour and location were analyzed. Degrees of freedom were determined using the Kenward–Rogers approximation. Differences were defined at P ≤ 0.05; a tendency was declared at 0.05 < P ≤ 0.10. The PDiff option of SAS was used to determine differences between least-squares means.
Alpha and beta diversities were analyzed using the R Dame package (Ihaka and Gentleman, 1996; Piccolo et al., 2018). Diversity indices used to evaluate alpha diversity included the observed OTU, Shannon index, and Fisher’s alpha index with data at the OTU level. Alpha diversity comparisons were made via the Mann–Whitney U test. Beta diversity was analyzed using permutational multivariate analysis of variance to assess group differences, and data were plotted on a 3D principal coordinate analysis (PCoA) plot based on a weighted Unifrac distance matrix.
Results
Dry matter intake
Whole pen DMI did not differ between hay types (P = 0.64). Horses consumed an average of 43.93 kg dry matter (DM) brome/d and an average of 45.52 kg DM alfalfa/d. This was 2.73% and 2.82% of the horses’ body weight in brome and alfalfa, respectively.
Cecal and fecal pH
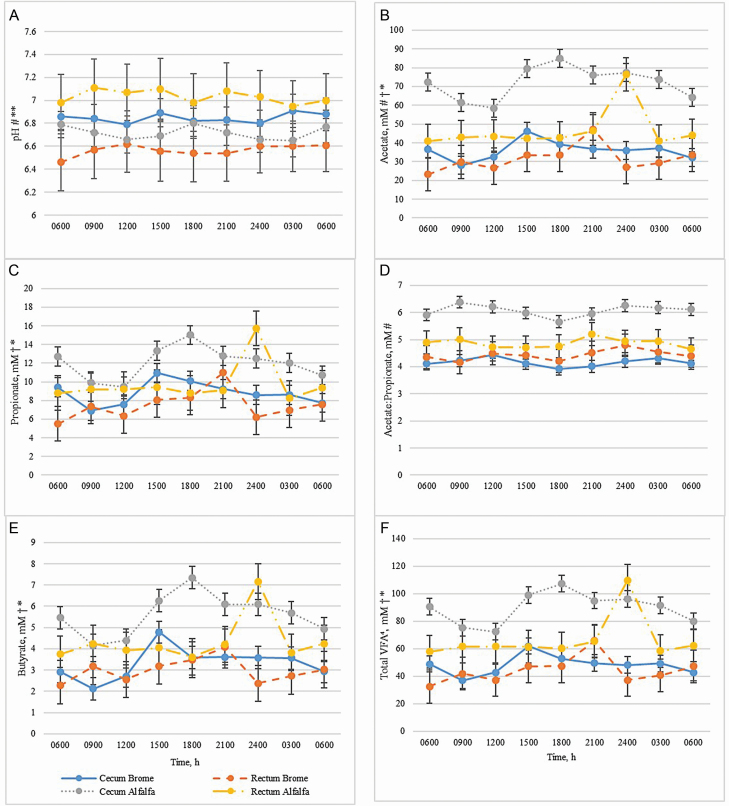
All time points (h) presented are in relation to the morning feeding with hour 0 at 0700 hours. There were no main effects of location or hour on pH (P > 0.46; Figure 2) nor were there interactions between hay and hour (P = 0.46) or between hay, hour, and location on pH (P = 0.62). An interaction between hay and location was detected (P < 0.01) as horses consuming brome had a greater (P = 0.01) pH in cecal fluid compared with fecal material. The opposite was observed in horses consuming alfalfa, as they had greater fecal pH compared with cecal fluid pH (P < 0.01). Within the cecum, horses fed brome had greater pH (P = 0.05) than those fed alfalfa. Conversely, fecal pH was greater (P < 0.01) in alfalfa-fed horses compared with their brome-fed counterparts.
Figure 2.
Effect of hay type on pH and fermentation parameters in the cecal and fecal matter of horses. Hay type (brome or alfalfa) was fed ad libitum to horses; sampling locations included cecum and rectum. (A) pH, (B) acetate, (C) propionate, (D) A:P, (E) butyrate, and (F) total VFA (includes acetate, propionate, butyrate, isobutyrate, isovalerate, valerate, isocaproate, caproate, and heptanoate). Symbols on the y-axis in (A) to (F) denote the following interactions: Hay type × Location (#P ≤ 0.05; ##0.05 < P ≤ 0.10); Hay type × Hour (†P ≤ 0.05; ††0.05 < P ≤ 0.10); Hay type × Hour × Location (*P ≤ 0.05; **0.05 < P ≤ 0.10).
Cecal and fecal VFA
Interactions between hay, hour, and location were observed with acetate, propionate, butyrate, and total VFA concentrations (P ≤ 0.05; Figure 2). Cecal concentrations of acetate, butyrate, and total VFA were elevated (P ≤ 0.05) in alfalfa-fed horses at all sampling times compared with cecal samples from those consuming brome. In alfalfa-fed horses, acetate concentrations were greater (P < 0.01) in cecal samples at hours 0, 9, 12, 15, 21, and 24 compared with fecal samples from the same horses. While concentrations of acetate were greater (P < 0.01) in cecal material from alfalfa-fed horses at all time points than in the cecum of brome-fed horses, acetate in fecal samples between the two groups only differed at hour 18 (P = 0.0002). Propionate concentrations were greater (P ≤ 0.04) in cecal samples of alfalfa-fed horses at hours 0, 3, 12, 15, 18, 21, and 24 compared with cecal samples of brome-fed horses and were greater (P < 0.01) in fecal samples of alfalfa-fed horses than in fecal samples of brome-fed horses at hour 18. Fecal concentrations of butyrate and total VFA in alfalfa-fed horses also were greater (P < 0.01) than in brome-fed horses at hour 18. Total VFA concentrations in alfalfa-fed horses were elevated (P ≤ 0.05) in cecal samples compared with fecal samples at hours 0, 9, 12, 15, and 21. No differences (P > 0.05) were detected in acetate, propionate, acetate:propionate (A:P), butyrate, or total VFA concentrations in brome-fed horses between cecal and fecal samples. A hay by hour interaction was detected (P ≤ 0.05) for acetate, propionate, butyrate, and total VFA concentrations. Furthermore, a hay by location interaction (P ≤ 0.05) was observed for acetate and A:P, as both were elevated in cecal samples of alfalfa-fed horses compared with cecal samples of brome-fed horses (P < 0.01) and fecal samples of alfalfa-fed horses (P ≤ 0.01).
Microbial composition
A total of 3,201,298 reads were sequenced from 48 samples. Read length was 600 bp, and the average number of reads per sample was 66,639 ± 17,767, with a minimum of 33,356 and a maximum of 107,390 reads per sample observed. Taxa that did not appear consistently (<0.1% of sample) were removed for statistical analyses. Taxa were considered “unassigned” if they were unable to be matched against our reference database with at least 97% similarity.
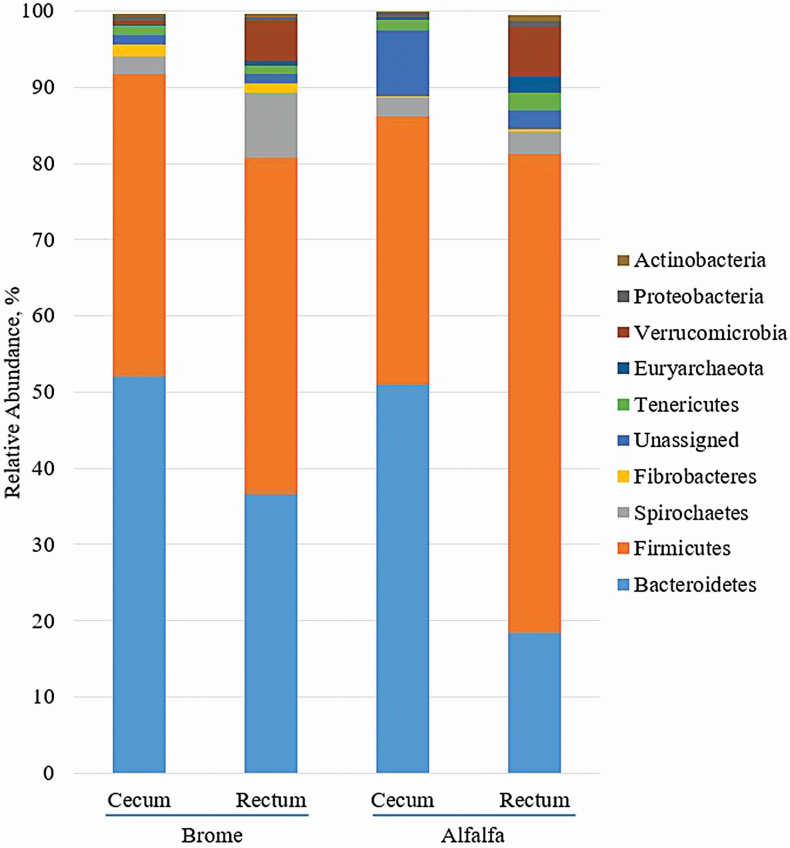
Approximately, nine phyla were identified through taxonomic classification (Figure 3). Bacteroidetes was the most abundant phylum detected in the cecum of brome-fed and alfalfa-fed horses (52% and 51.03%, respectively), whereas Firmicutes was the most abundant phylum detected in the rectum of brome-fed and alfalfa-fed horses (44.17% and 62.83%, respectively).
Figure 3.
Effect of hay type and sampling location on the relative abundance of phyla detected in horses. Hay type (brome or alfalfa) was fed ad libitum to horses; sampling locations included the cecum and rectum.
A hay by location interaction (P ≤ 0.05) was noted for the phyla Bacteroidetes, Firmicutes, Spirochaetes, unassigned, Tenericutes, and Actinobacteria, as well as Firmicutes:Bacteroidetes (F:B), and there was a tendency (P = 0.06) for an interaction with Proteobacteria. Bacteroidetes was greater (P < 0.01) in cecal samples of brome and alfalfa-fed horses compared with fecal samples and was more (P < 0.01) abundant in fecal samples of brome-fed horses compared with fecal samples from those consuming alfalfa. Fecal abundance of Firmicutes were greater (P < 0.01) in cecal samples within alfalfa-fed horses, which ultimately led to an increased F:B (P < 0.01). Spirochaetes was observed in greater (P < 0.01) abundance in fecal samples of brome-fed horses compared with cecal samples of either group and fecal samples of alfalfa-fed horses. Tenericutes was greater (P < 0.01) in fecal samples of alfalfa-fed horses compared with cecal samples of either group and fecal samples of brome-fed horses. Bacteria within Actinobacteria were more abundant (P ≤ 0.02) in fecal samples of brome- and alfalfa-fed horses compared with cecal samples, with a greater (P = 0.01) percentage in fecal samples of alfalfa-fed horses compared with brome. Verrucomicrobia was greater (P < 0.01) in fecal samples compared with cecal samples regardless of hay type. Fibrobacteres was greater (P < 0.01) in brome-fed horses than alfalfa-fed horses at both locations sampled.
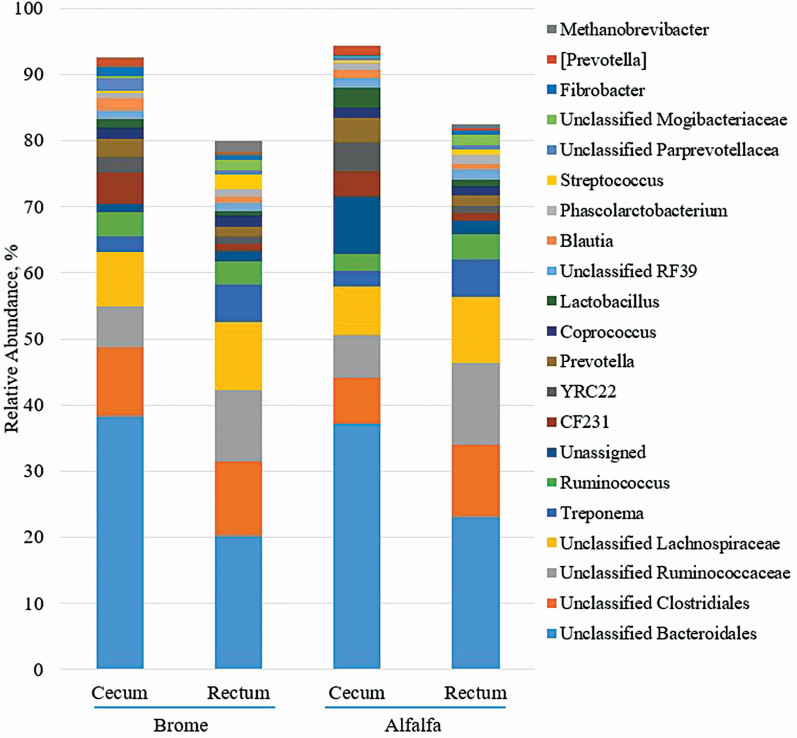
Approximately, 86 genera were observed following taxonomic classification. Of those, 21 comprised ≥1% relative abundance (Figure 4) A hay by location interaction (P ≤ 0.05) was observed for unclassified Clostridiales, unassigned, YRC22, Lactobacillus, Unclassified Paraprevotellacea, and Fibrobacter. Cecal abundance of Fibrobacter, unclassified Clostridiales, and CF231 was greater (P = 0.02, P < 0.01, and P = 0.21, respectively) in horses consuming brome compared with those fed alfalfa. Meanwhile, Unassigned and Lactobacillus had greater (P < 0.01) cecal abundance in horses fed alfalfa compared with brome. Cecal abundance of unclassified Bacteroidales, YRC22, and Prevotella was greater (P < 0.01) and Prevotella tended to be greater (P = 0.06) compared with fecal abundance, regardless of hay type. Ruminococcus was unaffected (P > 0.17) by hay type, location, and any possible interactions.
Figure 4.
Effect of hay type and sampling location on the relative abundance of genera detected in horses. Hay type (brome or alfalfa) was fed ad libitum to horses; sampling locations included the cecum and rectum. Genera that were <1% in relative abundance were omitted.
Alpha and beta diversities
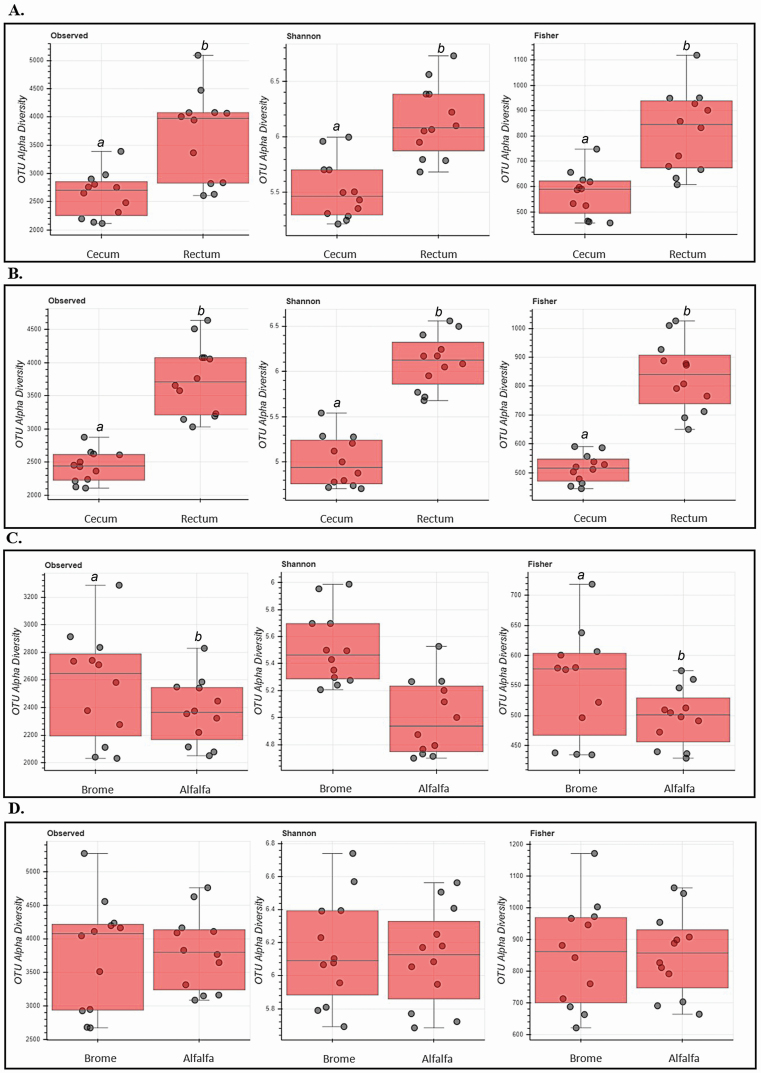
Alpha diversity differed between locations within treatment at the OTU level (Figure 5). Fecal samples had greater diversity based on the Fisher alpha (P < 0.01), observed OTU (P < 0.01), and Shannon (P < 0.01) indices compared with cecal samples regardless of hay type. In cecal samples, OTU index did not differ (P > 0.10) between horses fed brome or alfalfa; however, the Shannon index was greater (P < 0.01) and the Fisher alpha index tended to be greater (P = 0.09) in horses fed brome. No differences were detected (P > 0.10) in alpha diversity measures between hay types in fecal samples.
Figure 5.
Effect of hay type and sampling location on alpha diversity measures. Alpha diversity was measured using observed OTU, Shannon, and Fisher alpha indices. Hay type (brome or alfalfa) was fed ad libitum to horses; sampling locations included the cecum and rectum. (A) Effect of brome on alpha diversity measures in sampling locations. (B) Effect of alfalfa on alpha diversity measures in sampling locations. (C) Effect of the cecum on alpha diversity measures based on hay type. (D) Effect of the rectum on alpha diversity measures based on hay type. abMeans within the same box plot with a different letter are different (P ≤ 0.05).
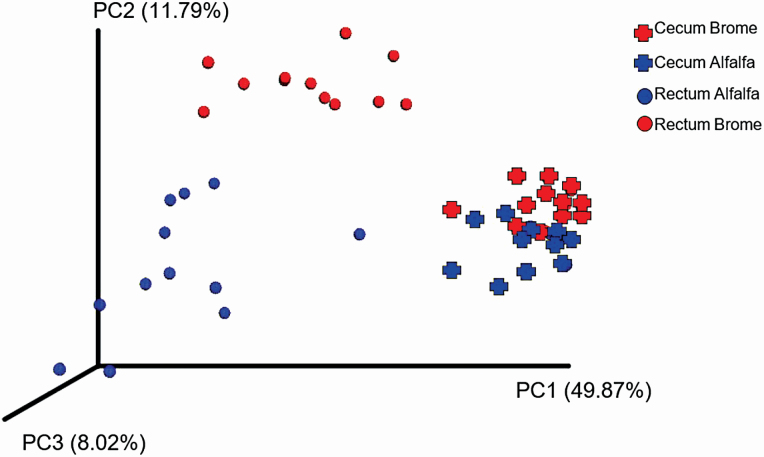
Based on dissimilarity and distance measures to assess beta diversity, a treatment by location interaction was detected (P ≤ 0.05). Microbiome samples were different based on location (F = 16.48; P = 0.02) and hay type (F = 13.91; P = 0.02). The differing bacterial environments can be visualized based on clustering in the PCoA plot in Figure 6.
Figure 6.
Effect of hay type and sampling location on beta diversity. Beta diversity was analyzed using the weighted Unifrac distance matrix, visualized on a PCoA plot, and comparisons were made using permutational multivariate analysis of variance. Hay type (brome or alfalfa) was fed ad libitum to horses; sampling locations included the cecum and rectum; An interaction effect of hay type by location (P < 0.05) was found.
Discussion
DMI did not differ between hays, which was unexpected. While daily digestible energy intake was similar between horses fed brome or alfalfa (31.91 and 31.41 Mcal/d/horse, respectively), it appears that alfalfa was digested more readily in the proximal components of the hindgut given the greater concentration of VFA in the cecum of alfalfa-fed horses compared with their brome-fed counterparts. This would be expected given the lower neutral detergent fiber of alfalfa compared with brome. The greater crude protein content of alfalfa may have also contributed to increased VFA concentration; however, this would have only occurred if nitrogen was limited in the cecum in brome-fed horses. Although VFA absorption was not evaluated, it can be theorized that more proximal fermentation would allow more time and access to colonocytes for VFA absorption, thus increasing the energy available to the animal despite similar calculated energy consumption.
The greater VFA concentration in alfalfa-fed horses can also be attributed to changes in the microbiome as relative abundances of Streptococcus, Lactobacillus, and YRC22 were greater compared with brome-fed horses. Similar data by Warzecha et al. (2017) showed increased abundances of Streptococcus, Lactobacillus, and YRC22 with greater VFA concentrations in horses consuming a more readily fermentable diet (1.8 g non-structural carbohydrates [NSC]/kg body weight [BW]) compared with a low-quality warm-season grass hay. Julliand et al. (2018) also reported an increase in the same bacteria in alfalfa-fed horses along with Prevotella and Fibrobacter succinogenes, which have potential pectinolytic activity, possibly contributing to the increased VFAs in alfalfa-fed horses. Because Prevotella degrade hemicellulose, pectin, and peptides (Wallace et al., 1997; Nagaraja, 2016), Prevotella were likely elevated in the cecum of alfalfa-fed horses due to the increased availability of structural carbohydrates and protein.
Cecal and fecal VFA concentrations did not differ in brome-fed horses while those consuming alfalfa had increased VFA concentrations in the cecum compared with feces, with the exception of hour 18. Dougal et al. (2012) and de Fombelle et al. (2003) also reported greater VFA concentrations in the cecum than the small colon of euthanized horses fed varied forage:concentrate diets. Total cecal VFA concentration in alfalfa-fed horses averaged 89 mM, which was slightly greater than that reported by Warzecha et al. (2017). Warzecha et al. (2017) stated horses fed up to 1.8 g NSC/kg BW with ad libitum coastal bermudagrass hay had total VFA concentrations of approximately 80 mM after 7 d of adapting to their diet.
Differences in pH values reflected the differences in VFA concentrations, as those compartments with increased VFA generally had reduced pH. This relationship was especially notable in the cecum of alfalfa-fed horses. While not measured in the current study, lactate concentrations may have been greater in alfalfa-fed horses, thereby further reducing pH. This theory is supported by Julliand et al. (2018) who reported increased lactate concentration in fecal samples from horses fed alfalfa pellets compared with sunflower meal. Regardless of hay type, cecal and fecal pH values were similar to those reported previously (Coverdale et al., 2004; Hussein et al., 2004; Jordan et al., 2018). While cecal and fecal pH in brome-fed horses was expected to be similar, a reduced pH was observed in feces. This was largely attributed to one horse who was an outlier with low fecal pH (pH < 6) while consuming brome.
Daly et al. (2001) observed a majority of bacterial sequences from luminal contents of the hindgut of euthanized grass-fed horses to be within the Clostridiaceae family, particularly cellulolytic Clostridium spp., along with Butyrivibrio spp., Ruminococcus spp., and Eubacterium spp. Daly et al.’s (2012) report of increased Fibrobacter, Ruminococcus, and unclassified Clostridiales in grass-fed horses is similar to the results for brome-fed horses in the current study, presumably due to the need for more fibrolytic fermentation within the cecum.
Firmicutes have been reported as the most abundant phyla in the feces of horses fed ryegrass-clover pasture (Fernandes et al., 2014) and orchardgrass hay (Shepherd et al., 2012). The current data support these observations as Firmicutes was the dominant phylum in feces regardless of forage type. This was largely driven by greater abundances of Lachnospiraceae, Ruminococcaceae, and Clostridiales, which is similar to data reported by Fernandes et al. (2014). It has been reported that a gut microbiome with Firmicutes as the most abundant phylum puts humans at increased risk for cardiovascular disease and obesity (Singh et al., 2017). However, Lachnospiraceae and Ruminococcaceae produce butyrate, which promotes healthy gut mucosa, and have been reported in the hindgut of healthy horses consuming forage (Willing et al., 2009; Costa et al., 2012, 2015). Conversely, Stewart et al. (2018) reported Bacteroidetes as the dominant phylum, followed by Firmicutes, in fecal samples from horses consuming timothy hay. Similar to the current findings, unclassified Bacteroidales was predominant, which has been reported as a member of the core microbiome in the equine hindgut (Dougal et al., 2013; Julliand and Grimm, 2016).
In ruminants, Ruminococcus flavefaciens, Ruminococcus albus, and Fibrobacter succinogenes are considered the most abundant cellulose-degrading ruminal microbes (Nagaraja, 2016). Julliand et al. (1999) identified R. flavefaciens to be the most abundant cellulolytic bacteria followed by F. succinogenes in the cecum of horses fed a 70% legume-orchardgrass hay:30% concentrate diet using culture-based sequencing. Ruminococcus albus was not detected in horses by Julliand et al. (1999) with oligonucleotide probes. Although most of the bacteria were not identified at the species level in the current study, F. succinogenes and R. flavefaciens were and likely play a role in cellulolytic degradation in the hindgut of hay-fed horses.
Treponema was more abundant in fecal samples regardless of hay type. This may be due to the fact that Treponema utilizes products of cellulose fermentation; therefore, as cellulose continues to be degraded throughout the hindgut, Treponema increases (Stanton and Canale-Parola, 1980; Paster and Canale-Parola, 1982).
Alpha diversity is used to evaluate microbial diversity within samples. In accordance with our findings, Dougal et al. (2012) noted increased Shannon diversity in fecal samples compared with cecal samples. Diversity typically decreases as soluble carbohydrates increase. This is likely a result of increased VFA production, which leads to decreased pH, which ultimately inhibits some bacteria. Accordingly, Warzecha et al. (2017) reported decreased Shannon index values after horses were fed a high starch concentrate (up to 1.8 g NSC/kg BW, as-fed).
In this study, beta diversity was used to assess differences in diversity between hay type and location. Fernandes et al. (2014) reported no detectable difference in beta diversity at the genus level from fecal samples of horses adapted to a commercial ensiled conserved forage-grain diet or ad libitum ryegrass-clover pasture. Costa et al. (2015) reported similar microbial diversity in varying compartments of the hindgut (cecum, pelvic flexure, small colon, and rectum) in euthanized horses of various ages and breeds fed grass hay and concentrate. Because they found similarity in diversity and bacterial communities at the phylum level between fecal, cecal, and large colonic samples, Costa et al. (2015) concluded that fecal samples can be used to adequately represent the main fermentation chambers in horses. In the current study, however, dissimilarity in microbial community structure at the OTU level was observed, with hay × location interactions. In these hay-fed horses, fecal samples were not representative of the cecal microbial environment. With differences noted in beta diversity, microbial populations, VFA concentrations, and pH between cecal and fecal samples, it appears that relying on information collected from feces to represent the entirety of the equine hindgut may result in inaccurate assumptions and conclusions. Equine feces are commonly used as an inoculum for in vitro procedures given their availability and ease of collection. However, authors must be careful when drawing conclusions from these studies. Certainly, utilizing feces to estimate microbial communities and their fermentative process within the cecum would result in erroneous assumptions.
Iron (Fe) concentration differed drastically between the two forages and may help explain diversity differences between treatments. Although the effect of Fe on the equine gut microbiome is unknown, recent data in mice demonstrate that excessive unabsorbed Fe may have deleterious effects on the gut microbiome. Mahalhal et al. (2018) found that mice consuming a diet with 400 ppm Fe had reduced species richness in fecal samples compared with those consuming a 100-ppm Fe diet. A recent review suggests that both ferric and ferrous forms may enhance the virulence of enteric pathogens, decrease Bifidobacteriaceae and Lactobacillaceae, increase coliforms, and induce reactive oxygen species, thereby causing stress to local bacteria (Kortman et al., 2014). Given that consumption of Fe-rich forage is commonplace in the equine industry, research is warranted in this area.
Conclusions
This is the first published report to document differences in pH, VFA concentrations, and the microbiome of the cecum and feces in horses fed cool-season grass hay and alfalfa. Because VFA concentrations were elevated in the cecum of alfalfa-fed horses, it appears that alfalfa was fermented more rapidly and more proximally in the hindgut. This led to decreased pH and altered microbial populations, which were reflected in decreased alpha diversity measures in the cecum. Despite reduced alpha diversity, the more rapid production of VFA likely yielded greater VFA absorption and thus energy availability to the animal in those consuming alfalfa, despite similar calculated dietary energy. Fecal samples differed in essentially all parameters evaluated when compared with cecal samples; therefore, fecal samples are not representative of microbial populations or fermentation parameters of the cecum.
Acknowledgments
This project was partially funded by Kansas State University and the Kansas Agricultural Experiment Station. This is contribution number 20-129-J of the Kansas Agricultural Experiment Station.
Glossary
Abbreviations
- A:P
acetate:propionate
- DM
dry matter
- DMI
dry matter intake
- DNA
deoxyribonucleic acid
- F:B
Firmicutes:Bacteroidetes
- GC
gas chromatography
- GI
gastrointestinal
- NGS
next-generation sequencing
- OTU
operational taxonomic unit
- PCoA
principal coordinates analysis
- VFA
volatile fatty acids
Authors’ contribution
All authors approve the submitted version and agree to personally account for their own contributions. R.J.S. contributed to the study design, study execution, data analysis and interpretation, and preparation of the manuscript. J.S.D., T.L.D., and J.M.L. contributed to the study design, data analysis and interpretation, and preparation of the manuscript. Q.R. and D.G.M. contributed toward the 16s analysis. C.I.V. and Q.K. contributed to statistical analyses of the data.
Conflict of interest statement
The authors do not have any competing financial or non-financial interests in relation to this study.
Literature Cited
- Beard, W. L., T. L. Slough, and C. D. Gunkel. . 2011. Technical Note: A 2-stage cecal cannulation technique in standing horses. J. Anim. Sci. 89:2425–2429. doi: 10.2527/jas.2010-3718 [DOI] [PubMed] [Google Scholar]
- Brunstein, J 2016. rRNA sequencing for bacterial identification. MLO. Med. Lab. Obs. 48:28–29. [PubMed] [Google Scholar]
- Caporaso, J. G., C. L. Lauber, W. A. Walters, D. Berg-Lyons, J. Huntley, N. Fierer, S. M. Owens, J. Betley, L. Fraser, M. Bauer, . et al. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6:1621–1624. doi: 10.1038/ismej.2012.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, M. C., L. G. Arroyo, E. Allen-Vercoe, H. R. Stämpfli, P. T. Kim, A. Sturgeon, and J. S. Weese. . 2012. Comparison of the fecal microbiota of healthy horses and horses with colitis by high throughput sequencing of the V3-V5 region of the 16S rRNA gene. PLoS One. 7:e41484. doi: 10.1371/journal.pone.0041484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, M. C., G. Silva, R. V. Ramos, H. R. Staempfli, L. G. Arroyo, P. Kim, and J. S. Weese. . 2015. Characterization and comparison of the bacterial microbiota in different gastrointestinal tract compartments in horses. Vet. J. 205:74–80. doi: 10.1016/j.tvjl.2015.03.018 [DOI] [PubMed] [Google Scholar]
- Coverdale, J. A., J. A. Moore, H. D. Tyler, and P. A. Miller-Auwerda. . 2004. Soybean hulls as an alternative feed for horses. J. Anim. Sci. 82:1663–1668. doi: 10.2527/2004.8261663x [DOI] [PubMed] [Google Scholar]
- Creevey, C. J., W. J. Kelly, G. Henderson, and S. C. Leahy. . 2014. Determining the culturability of the rumen bacterial microbiome. Microb. Biotechnol. 7:467–479. doi: 10.1111/1751-7915.12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly, K., C. J. Proudman, S. H. Duncan, H. J. Flint, J. Dyer, and S. P. Shirazi-Beechey. . 2012. Alterations in microbiota and fermentation products in equine large intestine in response to dietary variation and intestinal disease. Br. J. Nutr. 107:989–995. doi: 10.1017/S0007114511003825 [DOI] [PubMed] [Google Scholar]
- Daly, K., C. S. Stewart, H. J. Flint, and S. P. Shirazi-Beechey. . 2001. Bacterial diversity within the equine large intestine as revealed by molecular analysis of cloned 16S rRNA genes. FEMS Microbiol. Ecol. 38:141–152. doi: 10.1111/j.1574-6941.2001.tb00892.x [DOI] [Google Scholar]
- Dougal, K., G. de la Fuente, P. A. Harris, S. E. Girdwood, E. Pinloche, and C. J. Newbold. . 2013. Identification of a core bacterial community within the large intestine of the horse. PLoS One. 8:e77660. doi: 10.1371/journal.pone.0077660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougal, K., P. A. Harris, A. Edwards, J. A. Pachebat, T. M. Blackmore, H. J. Worgan, and C. J. Newbold. . 2012. A comparison of the microbiome and the metabolome of different regions of the equine hindgut. FEMS Microbiol. Ecol. 82:642–652. doi: 10.1111/j.1574-6941.2012.01441.x [DOI] [PubMed] [Google Scholar]
- Edgar, R. C 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. doi: 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- Fernandes, K. A., S. Kittelmann, C. W. Rogers, E. K. Gee, C. F. Bolwell, E. N. Bermingham, and D. G. Thomas. . 2014. Faecal microbiota of forage-fed horses in New Zealand and the population dynamics of microbial communities following dietary change. PLoS One. 9:e112846. doi: 10.1371/journal.pone.0112846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fombelle, A., M. Varloud, A.-G. Goachet, E. Jacotot, C. Philippeau, C. Drogoul, and V. Julliand. . 2003. Characterization of the microbial and biochemical profile of the different segments of the digestive tract in horses given two distinct diets. Anim. Sci. 77:293–304. doi: 10.1017/S1357729800059038 [DOI] [Google Scholar]
- Hussein, H. S., L. A. Vogedes, G. C. Fernandez, and R. L. Frankeny. . 2004. Effects of cereal grain supplementation on apparent digestibility of nutrients and concentrations of fermentation end-products in the feces and serum of horses consuming alfalfa cubes. J. Anim. Sci. 82:1986–1996. doi: 10.2527/2004.8271986x [DOI] [PubMed] [Google Scholar]
- Ihaka, R., and R. Gentleman. . 1996. R: a language for data analysis and graphics. J. Comput. Graph. Stat. 5:299–314. doi: 10.1080/10618600.1996.10474713 [DOI] [Google Scholar]
- Jordan, K. V., J. S. Drouillard, T. L. Douthit, and J. M. Lattimer. . 2018. Effects of sodium caseinate on hindgut fermentation and fiber digestion in horses. J. Anim. Sci. 97:813–819. doi: 10.1093/jas/sky436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julliand, V., and P. Grimm. . 2016. Horse Species Symposium: The microbiome of the horse hindgut: history and current knowledge1. J. Anim. Sci. 94:2262–2274. doi: 10.2527/jas.2015-0198 [DOI] [PubMed] [Google Scholar]
- Julliand, S., A. Martin, and V. Julliand. . 2018. Effect of dehydrated alfalfa on equine gastric and faecal microbial ecosystems. Livest. Sci. 215:16–20. doi: 10.1016/j.livsci.2017.05.005 [DOI] [Google Scholar]
- Julliand, V., A. de Vaux, L. Millet, and G. Fonty. . 1999. Identification of Ruminococcus flavefaciens as the predominant cellulolytic bacterial species of the equine cecum. Appl. Environ. Microbiol. 65:3738–3741. doi: 10.1128/AEM.65.8.3738-3741.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortman, G. A., M. Raffatellu, D. W. Swinkels, and H. Tjalsma. . 2014. Nutritional iron turned inside out: intestinal stress from a gut microbial perspective. FEMS Microbiol. Rev. 38:1202–1234. doi: 10.1111/1574-6976.12086 [DOI] [PubMed] [Google Scholar]
- Mahalhal, A., J. M. Williams, S. Johnson, N. Ellaby, C. A. Duckworth, M. D. Burkitt, X. Liu, G. L. Hold, B. J. Campbell, D. M. Pritchard, . et al. 2018. Oral iron exacerbates colitis and influences the intestinal microbiome. PLoS One 13:e0202460. doi: 10.1371/journal.pone.0202460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, D., M. N. Price, J. Goodrich, E. P. Nawrocki, T. Z. DeSantis, A. Probst, G. L. Andersen, R. Knight, and P. Hugenholtz. . 2012. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 6:610–618. doi: 10.1038/ismej.2011.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraja, T. G 2016. Microbiology of the rumen. In: Millen, D., M. De Beni Arrigoni, and R. Lauritano Pacheco, editors. Rumenology. Cham: Springer; p. 39–61. [Google Scholar]
- Paster, B. J., and E. Canale-Parola. . 1982. Physiological diversity of rumen spirochetes. Appl. Environ. Microbiol. 43:686–693. doi: 10.1128/AEM.43.3.686-693.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo, B. D., U. D. Wankhade, S. V. Chintapalli, S. Bhattacharyya, L. Chunqiao, and K. Shankar. . 2018. Dynamic assessment of microbial ecology (DAME): a web app for interactive analysis and visualization of microbial sequencing data. Bioinformatics 34:1050–1052. doi: 10.1093/bioinformatics/btx686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd, M. L., W. S. Swecker, R. V. Jensen, and M. A. Ponder. . 2012. Characterization of the fecal bacteria communities of forage-fed horses by pyrosequencing of 16S rRNA V4 gene amplicons. FEMS Microbiol. Lett. 326:62–69. doi: 10.1111/j.1574-6968.2011.02434.x [DOI] [PubMed] [Google Scholar]
- Singh, R. K., H. W. Chang, D. Yan, K. M. Lee, D. Ucmak, K. Wong, M. Abrouk, B. Farahnik, M. Nakamura, T. H. Zhu, . et al. 2017. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 15:73. doi: 10.1186/s12967-017-1175-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton, T. B., and E. Canale-Parola. . 1980. Treponema bryantii Sp. nov., a rumen spirochete that interacts with cellulolytic bacteria. Arch. Microbiol. 127:145–156. doi: 10.1007/BF00428018 [DOI] [PubMed] [Google Scholar]
- Stewart, H. L., D. Pitta, N. Indugu, B. Vecchiarelli, J. B. Engiles, and L. L. Southwood. . 2018. Characterization of the fecal microbiota of healthy horses. Am. J. Vet. Res. 79:811–819. doi: 10.2460/ajvr.79.8.811 [DOI] [PubMed] [Google Scholar]
- Venable, E. B., K. A. Fenton, V. M. Braner, C. E. Reddington, M. J. Halpin, S. A. Heitz, J. M. Francis, N. A. Gulson, C. L. Goyer, S. D. Bland, . et al. 2017. Effects of feeding management on the equine cecal microbiota. J. Equine Vet. Sci. 49:113–121. doi: 10.1016/j.jevs.2016.09.010 [DOI] [Google Scholar]
- Wallace, R. J., N. McKain, G. A. Broderick, L. M. Rode, N. D. Walker, C. J. Newbold, and J. Kopecny. . 1997. Peptidases of the rumen bacterium, Prevotella ruminicola. Anaerobe. 3:35–42. doi: 10.1006/anae.1996.0065 [DOI] [PubMed] [Google Scholar]
- Warzecha, C. M., J. A. Coverdale, J. E. Janecka, J. L. Leatherwood, W. E. Pinchak, T. A. Wickersham, and J. C. McCann. . 2017. Influence of short-term dietary starch inclusion on the equine cecal microbiome. J. Anim. Sci. 95:5077–5090. doi: 10.2527/jas2017.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing, B., A. Vörös, S. Roos, C. Jones, A. Jansson, and J. E. Lindberg. . 2009. Changes in faecal bacteria associated with concentrate and forage-only diets fed to horses in training. Equine Vet. J. 41:908–914. doi: 10.2746/042516409x447806 [DOI] [PubMed] [Google Scholar]