Abstract
Treatment for critical illness typically focuses on a patient’s short-term physical recovery; however, recent work has broadened our understanding of the long-term implications of illness and treatment strategies. In particular, survivors of critical illness have significantly elevated risk of developing lasting cognitive impairment and psychiatric disorders. In this review, we examine the role of endogenous and exogenous glucocorticoids in neuropsychiatric outcomes following critical illness. Illness is marked by acute elevation of free cortisol and adrenocorticotropic hormone suppression, which typically normalize after recovery; however, prolonged dysregulation can sometimes occur. High glucocorticoid levels can cause lasting alterations to the plasticity and structural integrity of the hippocampus and prefrontal cortex, and this mechanism may plausibly contribute to impaired memory and cognition in critical illness survivors, though specific evidence is lacking. Glucocorticoids may also exacerbate inflammation-associated neural damage. Conversely, current evidence indicates that glucocorticoids during illness may protect against the development of post-traumatic stress disorder. We propose future directions for research in this field, including determining the role of persistent glucocorticoid elevations after illness in neuropsychiatric outcomes, the role of systemic vs neuroinflammation, and probing unexplored lines of investigation on the role of mineralocorticoid receptors and the gut–brain axis. Progress toward personalized medicine in this area has the potential to produce tangible improvements to the lives patients after a critical illness, including Coronavirus Disease 2019.
Keywords: glucocorticoid, critical illness, sepsis, ARDS, cortisol, brain
Each year more than 9 million Americans are admitted to an intensive care unit (ICU) (1, 2) and roughly 7 out of every 8 patients survive (3). However, the impact of critical illness on an individual’s life does not end with their “recovery” in the traditional sense; their experiences in the ICU can have lasting effects on their health and quality of life. Critical illness survivors often suffer from neuropsychiatric impairment, including post-traumatic stress disorder (PTSD), depression, anxiety, and cognitive impairment (4-9).
Critical illness is accompanied by a sustained stress response. Our understanding of the biology of this stress response has its roots in the early twentieth century, when Hans Selye pioneered the notion that various forms of acute illness led to the same physiological syndrome mediated by hormones from the anterior pituitary and the adrenals (10). By 1950, adrenal steroid hormones had been isolated and used clinically for their anti-inflammatory properties, and the Nobel Prize in Physiology or Medicine was awarded to Kendall, Reichstein, and Hench for these discoveries. Scientists began to outline a function for endogenous glucocorticoids in maintaining homeostasis by constraining all aspects of the body’s response to stress (11, 12). In the late twentieth century and early twenty-first, Bruce McEwen reshaped the field through his demonstration that glucocorticoids bind to receptors in the central nervous system, where they directly influence brain architecture and behavior. He introduced the term allostatic overload to describe the overwhelming accumulation of multiple stress responses, particularly the repeated influx of glucocorticoids, and its contribution to the etiology of mental and physical illness (13).
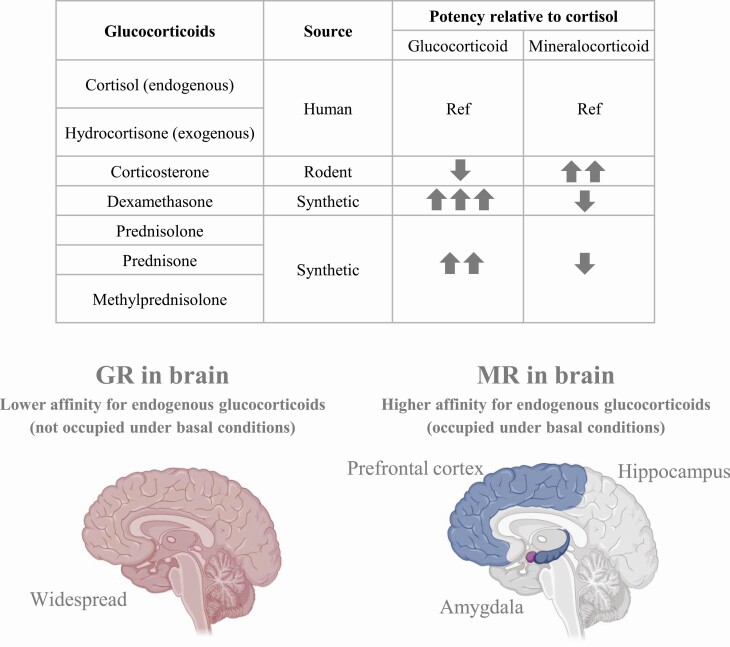
Glucocorticoids are widely used in intensive care for their anti-inflammatory and cardiovascular effects (11, 14-16). Of the exogenous glucocorticoids, hydrocortisone is identical to endogenous cortisol, while prednisone, prednisolone, dexamethasone, and others are synthetic and vary in potency and receptor affinity (Fig. 1). One study estimated that over a quarter of ICU patients receive glucocorticoids (17). The choice of glucocorticoid has important implications. Glucocorticoids bind to 2 different types of intracellular receptors, glucocorticoid receptors (GRs) and mineralocorticoid receptors (MRs). These complexes alter gene expression via classic genomic pathways following nuclear translocation or engage rapid nongenomic signaling pathways (18-21). In the brain, GR expression is widespread, while MR is expressed more selectively, mostly in limbic regions ((22) and Fig. 1).
Figure 1.
Top: Chart showing the relative potency of several endogenous and synthetic glucocorticoids for the glucocorticoid receptor (GR) and mineralocorticoid receptor (MR). Bottom: schematic illustrating the widespread localization of the lower affinity (for endogenous glucocorticoids) GR in the brain with more focused localization of the higher affinity MR to limbic regions. Created with Biorender.com.
The general study of glucocorticoids during stress has demonstrated a role for both receptors in modulating cognition and affective behavior (13, 23, 24). The relative contribution of MR and GR to the glucocorticoid effect in different brain areas will depend on multiple factors including receptor distribution, the specific glucocorticoid and its concentration, and the local activity of steroidogenic enzymes (25). For example, the binding affinity of MR for the endogenous glucocorticoid cortisol is 10-fold greater than that of GR, meaning that under basal conditions MRs are mostly ligand bound whereas GRs are mostly unoccupied ((26) and Fig. 1). Blood–brain barrier permeability is another important consideration; for example, dexamethasone is typically actively excluded from the brain unless given at sufficiently high doses, but illness may affect barrier permeability (27-29). Thus, changes in the level of circulating endogenous glucocorticoids and the specific exogenous glucocorticoid and its dose could have implications for brain function in critically ill patients and ICU survivors. The findings will be particularly important for informing treatment strategies in the current Coronavirus Disease 2019 (COVID-19) pandemic, as dexamethasone is one of the few treatments to actually reduce mortality in COVID-19 patients (30). In this review, we examine the role of endogenous and exogenous glucocorticoids in neuropsychiatric outcomes following critical illness.
Materials and Methods
In order to review an unbiased representation of the available literature, we performed a structured PubMed search and supplemented the results with articles known to us and their references.
While much of the relevant literature is clinical, rodent studies attempt to mimic aspects of human critical illness by administering acute infectious or acute inflammatory stimuli. Widely used rodent models to induce acute inflammatory illness include cecal ligation and puncture (CLP) and injection of endotoxin (lipopolysaccharide [LPS]). These models are not equivalent, with CLP producing a sustained illness due to abdominal infection with endogenous gut flora requiring supportive treatment (eg, antibiotics, fluids) and LPS producing a shorter response that lacks the hemodynamic alterations seen in human populations (31). We included these terms in our search to capture relevant animal research.
Our search strategy identified articles containing at least 1 medical subject headings (MeSH terms) from each of 3 categories:
1. Glucocorticoid OR corticosterone OR cortisol OR corticosteroids OR adrenal insufficiency
AND
2. Critical illness OR critical care OR sepsis OR shock OR acute lung injury OR adult respiratory distress syndrome OR infections OR cecal ligation OR endotoxin
AND
3. Anxiety OR depression OR PTSD OR memory OR cognition OR delirium.
From the results, we then excluded articles that focused on chronic illnesses, such as HIV or chronic fatigue syndrome, or neonatal immune challenge. The original search returned 227 articles, of which 165 were excluded and 62 were reviewed (32).
Hypothalamic–Pituitary–Adrenal Axis Dynamics During Critical Illness and Recovery
The endogenous glucocorticoids cortisol (humans) and corticosterone (rodents) represent the main final product of the hypothalamic–pituitary–adrenal (HPA) axis. At the time of presentation, critically ill patients demonstrate elevated blood cortisol, the extent of which correlates with the severity of the illness and can be interpreted as an adaptive response. Despite the high cortisol levels, adrenocorticotropic hormone (ACTH) levels are not high (33). The heightened cortisol levels can partially be attributed to elevated synthesis, but increases are mostly due to reduced cortisol metabolism and low levels of binding globulins (34). Diminished levels of binding proteins, such as albumin and corticosteroid binding globulin, increase the proportion of free cortisol available to tissue and can explain why critically ill patients often only show modest increases in total cortisol levels (33). Relatively low ACTH levels are likely due to negative feedback from elevated free cortisol levels (35). Regardless of length of ICU stay, both ACTH and cortisol levels are increased above normal 1 week after discharge, suggesting recovery from central ACTH suppression (36). However, a few pieces of evidence suggest that in some cases, primary adrenal dysfunction may be seen during the illness or recovery period. In patients with prolonged critical illness, the adrenal glands showed loss of the typical zonation, decreased lipid content, and reduced steroidogenic enzyme expression, suggesting decreased adrenal reserve (37). Furthermore, in mice, we have seen normal corticosterone with high ACTH levels 3 weeks after sepsis induced by cecal ligation and puncture compared with a sham condition (38), suggesting appropriate central compensation for decreased adrenal corticosterone production. The clinical significance of these findings remains to be seen.
Long-term perturbations of HPA axis functioning after acute inflammatory illness have been described in a few animal studies. For example, in rats, a single 1 mg/kg LPS injection caused long-term desensitization of the HPA axis to subsequent LPS challenge 4 weeks later (39). On the other hand, mice showed increased adrenal weight 5 weeks after CLP, suggesting chronic activation of the HPA axis (24). The clinical relevance of these findings is not clear, as these parameters have not been well studied in human critical illness survivors.
We will now consider how alterations in glucocorticoid levels, glucocorticoid sensitivity, and HPA axis dynamics during or after critical illness could influence brain function and neuropsychiatric outcomes in critical illness survivors.
Cognitive Outcomes
Impact of illness and glucocorticoids on brain architecture and function
In clinical studies, critical illness increased long-term cognitive morbidity, particularly by impairing memory, verbal fluency, and executive function (4, 5, 40). Findings in murine studies have been mixed: LPS caused no long-term difference in performance in the Morris Water Maze, 8-arm radial maze, or novel object recognition tests (41), whereas CLP caused impairment in learning and memory dysfunction in a clock maze task which lasted several months (42). These disparate outcomes could be due to differences in the duration of illness, character of the inflammatory response, HPA axis outcomes, or other differences between these stimuli. CLP in particular increased HPA axis activity long after recovery in mice (24). Sustained, high levels of glucocorticoids could directly or indirectly influence cognition by impacting hippocampal or prefrontal cortical function.
Outside the realm of critical illness, glucocorticoids are known to directly impact cognitive functioning and memory. They have a biphasic effect on synaptic plasticity: at low doses, the MR activity of glucocorticoids enhances long-term potentiation, whereas at higher doses, the GR activity inhibits long-term potentiation, facilitates long-term depression, and decreases excitability in the hippocampus (43), which is dependent on GR and MR (44-47). High levels of glucocorticoids alter the function and morphology of the hippocampus, where GRs are expressed abundantly, and impair learning and memory (48). The deleterious effect of chronic high levels of glucocorticoids on brain architecture and function is most evident in Cushing’s syndrome, which is associated with atrophy in the hippocampus and prefrontal cortex alongside deteriorating cognitive function and mood (49-51). The combination of high glucocorticoids and inflammation may be especially deleterious. While traditionally considered to have anti-inflammatory effects, glucocorticoids were found to suppress anti-inflammatory signaling molecules in the hippocampus, thereby increasing the inflammatory response to excitotoxic injury and worsening hippocampal neuron death (52). This suggests that high levels of endogenous or exogenous glucocorticoids could exacerbate inflammation-associated hippocampal damage.
This inference seems to be supported by research demonstrating the damaging effects of acute inflammation on the hippocampus. Some, but not all (53), studies in rodent models have found that illness-induced inflammation causes structural hippocampal damage. One study found that CLP in mice resulted in decreased spine density on dendritic processes of CA1 neurons, which was correlated with memory impairment in a clock maze task and was visible 4 months but not 2 weeks after the onset of illness, a sign of progressive degeneration (42). Another mouse study saw the same delayed deterioration 2 months after CLP, but only in the apical dendritic tree of the dorsal hippocampus, and found it was specifically associated with worse spatial memory in a navigational test (54). A third study of CLP-exposed mice also saw decreased spine density in hippocampal neurons 2 months and 6 months postsurgery, and in addition, found the same effect in the basolateral amygdala (BLA) associated with impaired contextual fear memory (55). Thus, there is evidence that sepsis in particular can cause lasting hippocampal structural damage with associated impairment in cognitive function in rodents. While glucocorticoids may plausibly mediate this effect or exacerbate inflammation-associated damage, their specific contributions have not been studied.
The prefrontal cortex may also be involved in the poor cognitive outcomes after critical illness, since it is involved in executive functioning and highly sensitive to glucocorticoids. Elevation of glucocorticoids due to chronic stress significantly decreases apical spine density and length in the medial Prefrontal cortex (PFC) in rats, decreasing synaptic input to the region by one-third (56). In humans, chronic psychological stress shows similar disruption to PFC plasticity through impaired PFC functional connectivity and performance on attention-shifting tasks (57). In rats, 4 weeks of corticosterone and dexamethasone treatment induced structural deterioration in the PFC and impaired PFC-dependent functions, such as spatial working memory and behavioral flexibility (58). There is also evidence that the PFC is sensitive to inflammatory damage, which can be amplified by glucocorticoids (59). These findings suggest that prolonged elevations in endogenous or exogenous glucocorticoids associated with critical illness could lead to poor cognitive outcomes through actions in the PFC.
In summary, inflammation directly affects the integrity of the hippocampus and prefrontal cortex, and high levels of glucocorticoids may exacerbate these effects and worsen the damage at least in part by exacerbating neuroinflammation. Thus, sustained high levels of glucocorticoids during and potentially after illness could contribute to structural hippocampal and PFC damage, leading to cognitive impairment. This hypothesis is supported by 1 animal study showing that a low dose of dexamethasone after CLP in rats normalized corticosterone and ACTH levels and prevented memory impairment in an inhibitory avoidance task (60). Dexamethasone, which is relatively excluded from the blood–brain barrier at low doses, would shut off endogenous glucocorticoid production via feedback to the pituitary and deplete the brain of glucocorticoids (61). Further studies are needed to test this hypothesis on the role of elevated glucocorticoids in structural hippocampal and prefrontal cortical damage and resulting cognitive impairment in critical illness survivors.
Glucocorticoids and delirium
In the ICU, incidence and duration of delirium is an independent risk factor for disability in activities of daily living (62, 63). Duration of delirium is also an independent risk factor for worse motor-sensory function (63) and impaired global cognition and executive function (5) in the following year. In a study of mechanically ventilated ICU patients, over 70% had cognitive impairment at 3- and 12-month follow-up, and duration of delirium but not duration of ventilation was an independent predictor of this outcome (64). Thus, ICU delirium is a risk factor for cognitive impairment and physical disability after hospitalization (65).
The neural mechanisms of delirium remain ambiguous, and it is difficult to study in animals (66-70). There is significant overlap between the defining features of postoperative delirium and long-term cognitive impairment, including disturbances of attention, memory, and thought processes, implying that these short- and long-term consequences of critical illness may share underlying neurobiology (71). It has been hypothesized that endogenous glucocorticoids contribute to the development of delirium. Indeed, endogenous glucocorticoid levels predicted the occurrence of delirium in several studies. For instance, higher perioperative cortisol levels are associated with an increased risk of postoperative delirium (72-77). In a small group of geriatric patients with acute lower respiratory infection, less cortisol suppression in response to dexamethasone predicted the occurrence of delirium (78).
However, not all studies have shown this relationship between endogenous glucocorticoid levels and delirium. One recent study found no association between cortisol levels and delirium after cardiac surgery (79). Additionally, a multivariate analysis of delirium biomarkers in ICU patients found that cortisol levels were not independently associated with occurrence of delirium (75). In addition, examination of healthy volunteers with experimental endotoxemia revealed that cortisol was not sufficient to cause either delirium or cognitive impairment (80, 81). The association between cortisol nonsuppression to dexamethasone (78) and delirium could be explained by impaired hippocampal function at baseline, with resulting impairment in negative feedback to the HPA axis. We therefore find it much more likely that high cortisol and delirium are both associated with higher severity of illness and underlying hippocampal damage, without any clear evidence for causality between them.
The literature regarding the impact of exogenous glucocorticoids on delirium is conflicting. One retrospective study found that early methylprednisolone treatment was associated with lower risk of delirium in lung cancer patients with postoperative acute lung injury (82), though the treatment also improved lung injury and lessened the duration of mechanical ventilation, confounding the interpretation. Of 2 prospective cohort studies, 1 found that systemic corticosteroid administration was associated with an increased risk for delirium in ICU patients (83), while the other did not find an association (84). Of 2 randomized controlled trials of hydrocortisone, 1 small study in postoperative cardiac surgery patients found no difference in the risk of delirium with hydrocortisone (85). In contrast, in the HYPRESS trial, a large, randomized controlled trial of hydrocortisone treatment for severe sepsis, hydrocortisone was associated with lower rates of delirium (86). Considering its large and randomized nature, these findings are interesting, and hydrocortisone was not associated with other measured clinical benefits that could confound the interpretation (though there were several undesirable side effects, such as hyperglycemia and neuromuscular weakness). Previous randomized trials of hydrocortisone in sepsis (87-89) did not include delirium as an endpoint. Nonetheless, based on the totality of the literature reviewed above, we conclude there is no consistent evidence that glucocorticoids directly impact delirium in critically ill patients. Future randomized controlled trials of exogenous glucocorticoids in critically ill patients should include delirium as an endpoint in order to improve our understanding of their effect on delirium.
Psychiatric Outcomes
Glucocorticoids and fear memory
Pertaining to critical illness, fear memories could be described as emotionally arousing, traumatic memories of the ICU experience. Glucocorticoids have a complex impact on fear-related memory (23, 90-93). Similar to the effect on cognition, their actions are dose-dependent, forming an inverted U-shape. In both animals (94-96) and humans (97, 98), administration of a moderate dose of glucocorticoids shortly after training enhances long-term consolidation of various forms of memory, including contextual fear memory, while administration of a high dose impairs it. Glucocorticoids are also timing dependent; elevated levels immediately before retention testing hampers the retrieval of contextual fear-conditioned memory and inhibitory avoidance memory (99). These effects on consolidation and retrieval are reliant on sex (97, 100) and on interaction with the noradrenergic system (101). Glucocorticoids may also enhance fear generalization (102).
The amygdala, especially the BLA, is particularly important for the consolidation of emotionally arousing experiences, including fear memory (103). Evidence indicates that glucocorticoid enhancement of fear memory may occur via direct effects on the amygdala. For example, activation of GRs in the BLA increases activity, transmission, and dendritic growth, and enhances fear memory consolidation (93, 103, 104), and the administration of a GR antagonist to either ventral hippocampus or BLA decreased fear memory retention (105).
The effect of circulating glucocorticoids on fear memory formation and retention in critically ill patients will likely be influenced by their level, timing, and potentially and changes in GR or MR expression or function. Severe inflammatory illness decreases functional GR expression in multiple tissues. potentially decreasing sensitivity to glucocorticoids (106, 107). In rats, the LPS-induced release of cytokines temporarily decreased the affinity of MR for corticosterone (108). Also in rats, 7 days of daily LPS injections decreased hippocampal GR in male rats, actually increasing it in females (109). Any effect of glucocorticoids on the formation or persistence of traumatic memories, in particular, could influence the development of PTSD or negative affect in critical illness survivors.
Glucocorticoids and PTSD
It is common for critical illness survivors to develop PTSD, although the reported prevalence varies widely from 16% to 44% (4, 6, 110-114). In nonillness populations, some but not all studies have shown an association of PTSD with low cortisol levels, including basal cortisol and/or lower cortisol response at the time of trauma (111, 115-118). It is possible that low basal cortisol levels are simply a marker for increased vulnerability to PTSD. Alternatively, low cortisol levels could be involved in the pathogenesis of PTSD, facilitating both traumatic memory formation and retrieval, as discussed above.
In critical illness, there is also some evidence for an association between HPA axis dynamics and PTSD. In a small study of adult ARDS patients, individuals with lower cortisol levels in the ICU showed a trend toward higher PTSD symptoms 6 months later (112). In long-term survivors of ARDS, low basal cortisol levels correlated with more traumatic memories of the illness (119). On the other hand, this association was not true in children, as 1 study actually found that higher evening salivary cortisol levels were associated with more PTSD symptoms 3 to 6 months after discharge from the pediatric ICU (120). Genetic variants in GR and CRH receptors predict risk of PTSD after critical illness in adults and children (121-123) and the modulatory role of dexamethasone (124), further suggesting the involvement of glucocorticoid signaling in PTSD risk after critical illness.
Because of the association between low cortisol levels and PTSD, exogenous glucocorticoid treatment has been explored and, indeed, has shown promise for the prevention of PTSD after a traumatic experience, including critical illness (125, 126). For example, pediatric sepsis patients who received glucocorticoids had lower PTSD intrusion symptom scores 3 to 6 months later (127). In an observational study of adult acute lung injury patients, higher proportion of ICU days on glucocorticoids was associated with decreased PTSD symptoms after controlling for other factors including ICU days, sepsis days, and midazolam and opioid usage (114). In 2 small studies, administration of hydrocortisone reduced the incidence of PTSD in patients with septic shock, with no difference in illness severity scores or ICU days (128, 129). Moreover, in a randomized controlled trial of hydrocortisone for the prevention of PTSD after cardiac surgery, patients treated with hydrocortisone had lower PTSD symptoms at 6 months despite no effect of the treatment on ICU days, duration of mechanical ventilation, or pressor needs (130). Finally, in a randomized trial of high intraoperative doses of the potent synthetic glucocorticoid dexamethasone (1 mg/kg up to 100 mg) during cardiac surgery (131), dexamethasone treatment was associated with lower rates of PTSD in follow-up in female patients and in patients with specific variants of the GR gene (124, 132). This high dose of dexamethasone almost certainly reached the brain parenchyma. While dexamethasone also conferred clinical benefit in this trial, the clinical benefit was seen in all patients, while the PTSD benefit was only noted in specific subsets of patients. Of note, since dexamethasone is a pure GR agonist and the effect was dependent on GR polymorphisms, the effect of glucocorticoids during illness on the development of PTSD may be GR mediated. In conclusion, the available evidence suggests that exogenous glucocorticoid treatment during critical illness may prevent PTSD in survivors independent of any additional physiological benefit.
How might glucocorticoid treatment prevent PTSD in critical illness survivors? The goal of much PTSD prophylaxis is to interfere with the most common symptom: intrusive fearful memories, since high incidence of traumatic memories is strongly associated with PTSD after critical illness (119, 130). As discussed above, high doses of glucocorticoids administered with appropriate timing may block fear memory formation or retrieval, although moderate doses would be expected to enhance fear memory, depending on their timing. However, several studies showed that the effect of glucocorticoids on PTSD in critical illness survivors was not associated with reduced numbers or types of traumatic memories (129, 130, 133). More work is needed, particularly in the controlled setting of rodent models, to understand how continuous elevation of glucocorticoids in the context of the ongoing stress of critical illness influences the formation of memories, especially emotionally salient memories, and subsequent fear generalization.
If not via traumatic memories, how could glucocorticoids prevent development of PTSD? Animal studies do provide some evidence that glucocorticoid treatment at the time of acute stress may prevent changes in hippocampal and amygdala architecture and associated changes in affective behavior (134-136). In 1 study, a more stress-susceptible rat strain was found to have lower corticosterone levels, and administration of corticosterone prior to acute predator odor stress diminished the effect of the stressor on elevated plus maze exploration and acoustic startle 7 days later (137). Whether these changes in rodent affective behavior and limbic system architecture after stress and glucocorticoid treatment truly represent a neurobiological substrate of PTSD is not clear. Moreover, they would need to be reconciled with the earlier hypothesis that illness-induced elevations in glucocorticoids cause hippocampal and prefrontal cortical damage, resulting in cognitive impairment in critical illness survivors. A clearer understanding of the neural circuits perturbed in survivors of acute illness, their relationship to behavior, and the impact of glucocorticoids on these circuits is needed for true understanding.
Glucocorticoids and mood disorders
In critical illness survivors, there is significant comorbidity between PTSD and mood disorders, such as anxiety and depression (110, 112, 113). Most studies show substantially higher incidence of mood disorders than PTSD in critical illness survivors; in 1 study of a mixed medical and surgical ICU survivor population, depression was about 5 times more likely than PTSD (8). As discussed previously, high levels of glucocorticoids can cause dendritic atrophy in the hippocampus and PFC, 2 key brain areas implicated in depression and anxiety. Central nervous system exposure to prolonged and severe elevations in free cortisol levels, such as is seen during critical illness, could therefore contribute to the increased risk of developing mood disorders.
The clinical literature mostly suggests that exogenous glucocorticoid treatment during illness has little role in the development of mood disorders after recovery. Most studies have found that neither administration of hydrocortisone or dexamethasone around the time of illness, nor preadmission use of glucocorticoids, had any effect on the subsequent occurrence of depression or anxiety (85, 132, 138). One study found that the number of days of corticosteroid treatment in the ICU was associated with less severe anxiety after acute lung injury (112). If glucocorticoid treatment does prevent the development of PTSD, then by doing so it could also decrease the likelihood of developing a comorbid mood disorder.
After illness, overactivity of the HPA axis could contribute to the development of mood disorders through similar mechanisms as seen, for example, in Cushing’s syndrome (139). For example, mice who survived CLP showed higher blood corticosterone after swimming and heavier adrenal weights up to 50 days after the illness, accompanied by avoidance of bright open areas and increased immobility in the forced swim test classically interpreted as “anxiety”- and “depressive”-like behavior (24). In that study, GR expression was also increased in the ventral hippocampus of sepsis survivor mice, where increased GR signaling has been associated with anxiety-like behavior. In another study, rats who survived pneumococcal meningitis showed elevated ACTH and corticosterone levels accompanied by anhedonia and evidence of neuroinflammation; all of these findings were reversed with imipramine treatment (140). This suggests that in mice and rats HPA axis overactivity is associated with negative affect in illness survivors, suggesting it could contribute to the development of mood disorders after critical illness. MRs may also play a role as studies have shown that MRs modulate stress-induced anxiety-like behavior (141), that MR expression in certain limbic regions is reduced individuals with depression (142, 143), and that certain MR genetic variants increase vulnerability to depression (144, 145). Research in this area should focus on understanding whether human critical illness survivors show long-term HPA axis overactivity, whether HPA axis overactivity after illness causes negative affect, and the receptor mechanisms.
Role of Systemic Inflammation
Peripheral and central elevations in proinflammatory cytokines have been implicated in PTSD and depression (146, 147). In addition to the role of acute inflammation-associated damage describe above, ongoing neuroinflammation could plausibly mediate some of the brain-based sequelae of critical illness. Indeed, mice who survive abdominal or pneumosepsis exhibit evidence of persistent neuroinflammation (53, 148-150). There is some evidence that cytokine elevations contribute to the acute changes in memory and affective behavior after LPS injection in mice (146). Furthermore, treatment of mice with a nuclear factor kappa B (NFKB) inhibitor to block the transcription of immune-regulated genes after LPS administration improved signs of neuroinflammation and also improved several measures of affective behavior (41).
Since glucocorticoids are often exploited for their anti-inflammatory properties, they could protect the brain by suppressing its exposure to peripherally and centrally derived cytokines. Adrenalectomized mice showed higher circulating proinflammatory cytokines in response to a viral challenge (151), adding further evidence to the extensive literature detailing the profound immune modulatory effects of glucocorticoids (152, 153). Moreover, exogenous administration of stress doses of hydrocortisone to patients with septic shock significantly reduced levels of some, but not all, circulating proinflammatory cytokines (154). However, as noted above, in some parts of the brain glucocorticoids could have a local proinflammatory effect. It is also interesting that chronic stress primes neuroinflammatory responses in a glucocorticoid-dependent manner (155). Thus, the glucocorticoid state of the patient preceding illness may be important for the eventual outcome. Whether endogenous or exogenous glucocorticoids may alter brain outcomes through their immune and inflammatory inhibiting or promoting properties has not yet been explored.
Conclusion and Directions for Future Research
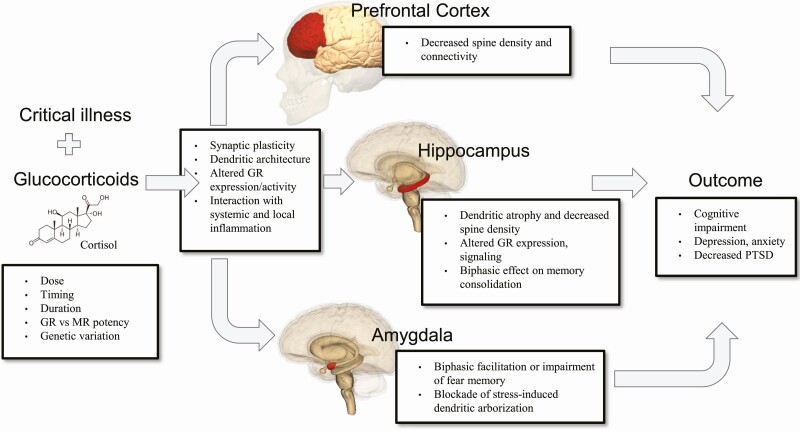
Critical illness is marked by massive increases in endogenous free glucocorticoid and is often accompanied by exogenous glucocorticoid treatment. In this mini-review, we have discussed evidence that glucocorticoids may be both detrimental and beneficial to neuropsychiatric outcomes after intensive care (Fig. 2). The strongest evidence suggests that high levels of circulating glucocorticoids during critical illness may decrease the risk of PTSD, though perhaps not via their effect on traumatic memories. Acute, extreme elevations in circulating glucocorticoid during illness may cause or exacerbate inflammation-induced hippocampal and prefrontal cortical damage, while persistent HPA axis overactivity in survivors (seen in several rodent models) may also contribute to the development of cognitive impairment and mood disorders. The relative contributions of systemic and local central nervous system inflammation in brain function after illness is important to disentangle, as glucocorticoids suppress systemic inflammation, but this is not always true locally in the brain. One additional completely underexplored area is the role of gut-brain communication in HPA axis functioning and neuropsychiatric outcomes after critical illness (156-160).
Figure 2.
Endogenous and exogenous glucocorticoids directly and indirectly impact brain function in critical illness survivors. Patient factors and characteristics of the specific glucocorticoid will influence this outcome. Prolonged elevations in glucocorticoids may contribute to depression and cognitive impairment via effects on hippocampal and prefrontal cortical synaptic plasticity, dendritic architecture, and function. Glucocorticoids may prevent the development of PTSD perhaps via blockade of stress-induced dendritic growth in the amygdala. Brain images are licensed under the Creative Commons Attribution-Share Alike 2.1 Japan and reproduced without alteration.
The effective exploitation of glucocorticoid biology to improve overall patient survival while enhancing brain recovery and minimizing brain harms is a prodigious challenge. Because of the importance of dose, timing, potency, inflammation, and genetic variation, it is an opportunity for personalized medicine. Critically ill patients are subjected to a number of factors that will also influence brain outcomes, including hypoxia, loss of diurnal rhythm, and treatments with numerous other centrally acting medications including analgesic and anesthetic agents. As critical care moves into the era of big data, many of these patient- and illness-specific factors will become available for measurement, interpretation, and action in real time.
Consideration of the effect of glucocorticoids on the brain during critical illness is especially timely in light of the current COVID-19 pandemic. COVID-19 is accompanied by a severe inflammatory response as well as high cortisol levels during the acute illness period: Admission cortisol levels were higher in COVID-19 patients than in those initially suspected of having the infection but ultimately not diagnosed with COVID-19 (161). Evidence is emerging for acute neuropsychiatric complications of COVID-19, including patients presenting with new-onset neuropsychiatric disorders (162, 163). Moreover, COVID-19 patients will be at high risk for delirium due to the severity of the disease and treatment-related factors (164). The combination of severe inflammation, high cortisol levels, specific neuropathologic effects of the disease, and high incidence of delirium in COVID-19 patients could result in especially high long-term neuropsychiatric morbidity in these patients. Interestingly, recently dexamethasone, 6 mg daily for up to 10 days, has emerged as the only treatment shown to reduce mortality in critically ill patients with COVID-19 (30). The mechanism is highly likely to include constraint of deleterious aspects of the inflammatory and immune response in sick patients with COVID-19; dexamethasone is not indicated for the prevention of infection. It will be important to follow up on the neuropsychiatric outcomes of COVID-19 patients from the RECOVERY trial to determine the effect of dexamethasone.
In conclusion, glucocorticoids may have both beneficial and detrimental effects on the brain of critically ill patients and survivors due to direct actions on brain regions important for cognition, affect, and fear memory, and direct effects on systemic and neuroinflammation. Harnessing the “good” while minimizing the “bad” in these compounds will rely on a better understanding of their mechanisms as well as how to exploit them in each individual patient. Cooperation between preclinical and clinical investigators on this topic is crucial and timely, as the findings have direct relevance to brain outcomes for survivors of all severe illnesses, including COVID-19.
Acknowledgments
Financial Support: University of Michigan College of Literature, Science and the Arts Honors Summer Fellowship (A.H.); National Institutes of Health grant MH116267 (J.S.S.).
Glossary
Abbreviations
- ACTH
adrenocorticotropic hormone
- BLA
basolateral amygdala
- CLP
cecal ligation and puncture
- COVID-19
Coronavirus Disease 2019
- GR
glucocorticoid receptor
- HPA
hypothalamic–pituitary–adrenal
- ICU
intensive care unit
- LPS
lipopolysaccharide
- MR
mineralocorticoid receptor
- NFKB
nuclear factor kappa B
- PFC
prefrontal cortex
- PTSD
post-traumatic stress disorder
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Witt WP, Weiss AJ, Elixhauser A. Overview of Hospital Stays for Children in the United States, 2012: Statistical Brief #187. 2014 Dec. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. [PubMed] [Google Scholar]
- 2. Barrett ML, Smith MW, Elixhauser A, Honigman LS, Pines JM. Utilization of Intensive Care Services, 2011: Statistical Brief #185. 2014 Dec. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. [PubMed] [Google Scholar]
- 3. Zimmerman JE, Kramer AA, Knaus WA. Changes in hospital mortality for United States intensive care unit admissions from 1988 to 2012. Crit Care. 2013;17(2):R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mikkelsen ME, Christie JD, Lanken PN, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185(12):1307-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pandharipande PP, Girard TD, Jackson JC, et al. ; BRAIN-ICU Study Investigators . Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hong X, Currier GW, Zhao X, Jiang Y, Zhou W, Wei J. Posttraumatic stress disorder in convalescent severe acute respiratory syndrome patients: a 4-year follow-up study. Gen Hosp Psychiatry. 2009;31(6):546-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davydow DS, Zatzick D, Hough CL, Katon WJ. A longitudinal investigation of posttraumatic stress and depressive symptoms over the course of the year following medical-surgical intensive care unit admission. Gen Hosp Psychiatry. 2013;35(3):226-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jackson JC, Pandharipande PP, Girard TD, et al. ; Bringing to light the Risk Factors And Incidence of Neuropsychological dysfunction in ICU survivors (BRAIN-ICU) study investigators . Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008;70(4):512-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Selye H. A syndrome produced by diverse nocuous agents. Nature 1936;138:32. [DOI] [PubMed] [Google Scholar]
- 11. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55-89. [DOI] [PubMed] [Google Scholar]
- 12. Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5(1):25-44. [DOI] [PubMed] [Google Scholar]
- 13. McEwen BS, Bowles NP, Gray JD, et al. Mechanisms of stress in the brain. Nat Neurosci. 2015;18(10):1353-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martino EA, Baiardo Redaelli M, Sardo S, et al. Steroids and survival in critically ill adult patients: a meta-analysis of 135 randomized trials. J Cardiothorac Vasc Anesth. 2018;32(5):2252-2260. [DOI] [PubMed] [Google Scholar]
- 15. Spencer-Segal JL. Future directions for corticosteroids in treatment of sepsis. JAMA Intern Med. 2019;179(6):845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dieleman JM, de Wit GA, Nierich AP, et al. ; DExamethasone for Cardiac Surgery (DECS) Study Group . Long-term outcomes and cost effectiveness of high-dose dexamethasone for cardiac surgery: a randomised trial. Anaesthesia. 2017;72(6):704-713. [DOI] [PubMed] [Google Scholar]
- 17. Rady MY, Johnson DJ, Patel B, Larson J, Helmers R. Corticosteroids influence the mortality and morbidity of acute critical illness. Crit Care. 2006;10(4):R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karst H, Berger S, Turiault M, Tronche F, Schütz G, Joëls M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci U S A. 2005;102(52):19204-19207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karst H, Berger S, Erdmann G, Schütz G, Joëls M. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc Natl Acad Sci U S A. 2010;107(32):14449-14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Di S, Itoga CA, Fisher MO, et al. Acute stress suppresses synaptic inhibition and increases anxiety via endocannabinoid release in the basolateral amygdala. J Neurosci. 2016;36(32):8461-8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Joëls M, Sarabdjitsingh RA, Karst H. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacol Rev. 2012;64(4):901-938. [DOI] [PubMed] [Google Scholar]
- 22. Meijer OC, Buurstede JC, Schaaf MJM. Corticosteroid receptors in the brain: transcriptional mechanisms for specificity and context-dependent effects. Cell Mol Neurobiol. 2019;39(4):539-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Quervain D, Schwabe L, Roozendaal B. Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat Rev Neurosci. 2017;18(1):7-19. [DOI] [PubMed] [Google Scholar]
- 24. Spencer-Segal JL, Singer BH, Laborc K, et al. Sepsis survivor mice exhibit a behavioral endocrine syndrome with ventral hippocampal dysfunction. Psychoneuroendocrinology. 2020;117:104679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Kloet ER, Meijer OC, de Nicola AF, de Rijk RH, Joëls M. Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front Neuroendocrinol. 2018;49:124-145. [DOI] [PubMed] [Google Scholar]
- 26. Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117(6):2505-2511. [DOI] [PubMed] [Google Scholar]
- 27. De Kloet R, Wallach G, McEwen BS. Differences in corticosterone and dexamethasone binding to rat brain and pituitary. Endocrinology. 1975;96(3):598-609. [DOI] [PubMed] [Google Scholar]
- 28. Meijer OC, de Lange EC, Breimer DD, de Boer AG, Workel JO, de Kloet ER. Penetration of dexamethasone into brain glucocorticoid targets is enhanced in mdr1A P-glycoprotein knockout mice. Endocrinology. 1998;139(4):1789-1793. [DOI] [PubMed] [Google Scholar]
- 29. Honig G, Mader S, Chen H, et al. Blood-brain barrier deterioration and hippocampal gene expression in polymicrobial sepsis: an evaluation of endothelial MyD88 and the vagus nerve. PLoS One. 2016;11(1):e0144215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. Published online July 17, 2020. doi: 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- 31. Buras JA, Holzmann B, Sitkovsky M. Model organisms: animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4(10):854-865. [DOI] [PubMed] [Google Scholar]
- 32. Hill A, Spencer-Segal JL. Search Results. Mendeley Public Gr. 2020. https://www.mendeley.com/community/glucocorticoids-and-the-brain-after-critical-illness-search-results/documents/. Accessed December 15, 2020.
- 33. Peeters B, Langouche L, Van den Berghe G. Adrenocortical stress response during the course of critical illness. Compr Physiol. 2017;8(1):283-298. [DOI] [PubMed] [Google Scholar]
- 34. Boonen E, Vervenne H, Meersseman P, et al. Reduced cortisol metabolism during critical illness. N Engl J Med. 2013;368(16):1477-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peeters B, Meersseman P, Vander Perre S, et al. ACTH and cortisol responses to CRH in acute, subacute, and prolonged critical illness: a randomized, double-blind, placebo-controlled, crossover cohort study. Intensive Care Med. 2018;44(12):2048-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peeters B, Meersseman P, Vander Perre S, et al. Adrenocortical function during prolonged critical illness and beyond: a prospective observational study. Intensive Care Med. 2018;44(10):1720-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boonen E, Langouche L, Janssens T, et al. Impact of duration of critical illness on the adrenal glands of human intensive care patients. J Clin Endocrinol Metab. 2014;99(11):4214-4222. [DOI] [PubMed] [Google Scholar]
- 38. Spencer-Segal JL, Xing Y, Kounelis S, Laborc K, Akil H, Hammer G. SAT-358 corticosterone treatment during sepsis results in a primary adrenal defect in male survivors. J Endocr Soc. 2019;3(1): SAT-358. [Google Scholar]
- 39. Vallès A, Martí O, Harbuz MS, Armario A. A single lipopolysaccharide administration is sufficient to induce a long-term desensitization of the hypothalamic-pituitary-adrenal axis. Neuroscience. 2002;112(2):383-389. [DOI] [PubMed] [Google Scholar]
- 40. Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF Jr. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171(4):340-347. [DOI] [PubMed] [Google Scholar]
- 41. Anderson ST, Commins S, Moynagh PN, Coogan AN. Lipopolysaccharide-induced sepsis induces long-lasting affective changes in the mouse. Brain Behav Immun. 2015;43:98-109. [DOI] [PubMed] [Google Scholar]
- 42. Chavan SS, Huerta PT, Robbiati S, et al. HMGB1 mediates cognitive impairment in sepsis survivors. Mol Med. 2012;18(1):930-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5(2):205-216. [DOI] [PubMed] [Google Scholar]
- 44. Baulieu EE. Contragestion and other clinical applications of RU 486, an antiprogesterone at the receptor. Obstet Gynecol Surv. 1990;45(3):197-198. [DOI] [PubMed] [Google Scholar]
- 45. Joëls M, de Kloet ER. Mineralocorticoid receptor-mediated changes in membrane properties of rat CA1 pyramidal neurons in vitro. Proc Natl Acad Sci U S A. 1990;87(12): 4495-4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Joëls M, de Kloet ER. Control of neuronal excitability by corticosteroid hormones. Trends Neurosci. 1992;15(1):25-30. [DOI] [PubMed] [Google Scholar]
- 47. Joëls M, Karst H, DeRijk R, de Kloet ER. The coming out of the brain mineralocorticoid receptor. Trends Neurosci. 2008;31(1):1-7. [DOI] [PubMed] [Google Scholar]
- 48. Herbert J, Goodyer IM, Grossman AB, et al. Do corticosteroids damage the brain? J Neuroendocrinol. 2006;18(6):393-411. [DOI] [PubMed] [Google Scholar]
- 49. Brzozowska MM, Kepreotis S, Tsang F, Fuentes-Patarroyo SX. Improvement in cognitive impairment following the successful treatment of endogenous Cushing’s syndrome-a case report and literature review. BMC Endocr Disord. 2019;19(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bauduin SEEC, van der Wee NJA, van der Werff SJA. Structural brain abnormalities in Cushing’s syndrome. Curr Opin Endocrinol Diabetes Obes. 2018;25(4):285-289. [DOI] [PubMed] [Google Scholar]
- 51. Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biol Psychiatry. 1992;32(9):756-765. [DOI] [PubMed] [Google Scholar]
- 52. Sorrells SF, Munhoz CD, Manley NC, Yen S, Sapolsky RM. Glucocorticoids increase excitotoxic injury and inflammation in the hippocampus of adult male rats. Neuroendocrinology. 2014;100(2-3):129-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Singer BH, Newstead MW, Zeng X, et al. Cecal ligation and puncture results in long-term central nervous system myeloid inflammation. PLoS One. 2016;11(2):e0149136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Volpe BT, Berlin RA, Frankfurt M. The brain at risk: the sepsis syndrome and lessons from preclinical experiments. Immunol Res. 2015;63(1-3):70-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huerta PT, Robbiati S, Huerta TS, et al. Preclinical models of overwhelming sepsis implicate the neural system that encodes contextual fear memory. Mol Med. 2016;22:789-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Radley JJ, Rocher AB, Miller M, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16(3):313-320. [DOI] [PubMed] [Google Scholar]
- 57. Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci U S A. 2009;106(3):912-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cerqueira JJ, Pêgo JM, Taipa R, Bessa JM, Almeida OF, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005;25(34):7792-7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. de Pablos RM, Villarán RF, Argüelles S, et al. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci. 2006;26(21):5709-5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cassol OJ Jr, Comim CM, Petronilho F, et al. Low dose dexamethasone reverses depressive-like parameters and memory impairment in rats submitted to sepsis. Neurosci Lett. 2010;473(2):126-130. [DOI] [PubMed] [Google Scholar]
- 61. Karssen AM, Meijer OC, Berry A, Sanjuan Piñol R, de Kloet ER. Low doses of dexamethasone can produce a hypocorticosteroid state in the brain. Endocrinology. 2005;146(12):5587-5595. [DOI] [PubMed] [Google Scholar]
- 62. Abelha FJ, Luís C, Veiga D, et al. Outcome and quality of life in patients with postoperative delirium during an ICU stay following major surgery. Crit Care. 2013;17(5):R257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brummel NE, Jackson JC, Pandharipande PP, et al. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42(2):369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Goldberg TE, Chen C, Wang Y, et al. Association of delirium with long-term cognitive decline: a meta-analysis. JAMA Neurol. 2020;77(11):1373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190-1222. [DOI] [PubMed] [Google Scholar]
- 67. Maclullich AM, Ferguson KJ, Miller T, de Rooij SE, Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res. 2008;65(3):229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. Br J Anaesth. 2009;103(Suppl 1):i41-i46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14(2): 87-98. [DOI] [PubMed] [Google Scholar]
- 70. Peng M, Zhang C, Dong Y, et al. Battery of behavioral tests in mice to study postoperative delirium. Sci Rep. 2016;6(June):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Meagher DJ, Moran M, Raju B, et al. Phenomenology of delirium: assessment of 100 adult cases using standardised measures. Br J Psychiatry. 2007;190(FEB.):135-141. [DOI] [PubMed] [Google Scholar]
- 72. Kazmierski J, Banys A, Latek J, Bourke J, Jaszewski R. Cortisol levels and neuropsychiatric diagnosis as markers of postoperative delirium: a prospective cohort study. Crit Care. 2013;17(2):R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kazmierski J, Banys A, Latek J, et al. Mild cognitive impairment with associated inflammatory and cortisol alterations as independent risk factor for postoperative delirium. Dement Geriatr Cogn Disord. 2014;38(1-2):65-78. [DOI] [PubMed] [Google Scholar]
- 74. Mu DL, Wang DX, Li LH, et al. High serum cortisol level is associated with increased risk of delirium after coronary artery bypass graft surgery: a prospective cohort study. Crit Care. 2010;14(6):R238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. van den Boogaard M, Kox M, Quinn KL, et al. Biomarkers associated with delirium in critically ill patients and their relation with long-term subjective cognitive dysfunction; indications for different pathways governing delirium in inflamed and noninflamed patients. Crit Care. 2011;15(6):R297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pfister D, Siegemund M, Dell-Kuster S, et al. Cerebral perfusion in sepsis-associated delirium. Crit Care. 2008;12(3):R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nguyen DN, Huyghens L, Zhang H, Schiettecatte J, Smitz J, Vincent JL. Cortisol is an associated-risk factor of brain dysfunction in patients with severe sepsis and septic shock. Biomed Res Int. 2014;2014:712742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. O’Keeffe ST, Devlin JG. Delirium and the dexamethasone suppression test in the elderly. Neuropsychobiology. 1994;30(4):153-156. [DOI] [PubMed] [Google Scholar]
- 79. Eshmawey M, Arlt S, Ledschbor-Frahnert C, Guenther U, Popp J. Preoperative depression and plasma cortisol levels as predictors of delirium after cardiac surgery. Dement Geriatr Cogn Disord. 2019;48(3-4):207-214. [DOI] [PubMed] [Google Scholar]
- 80. van den Boogaard M, Ramakers BP, van Alfen N, et al. Endotoxemia-induced inflammation and the effect on the human brain. Crit Care. 2010;14(3):R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Grigoleit JS, Oberbeck JR, Lichte P, et al. Lipopolysaccharide-induced experimental immune activation does not impair memory functions in humans. Neurobiol Learn Mem. 2010;94(4):561-567. [DOI] [PubMed] [Google Scholar]
- 82. Choi H, Shin B, Yoo H, et al. Early corticosteroid treatment for postoperative acute lung injury after lung cancer surgery. Ther Adv Respir Dis. 2019;13:1753466619840256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schreiber MP, Colantuoni E, Bienvenu OJ, et al. Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med. 2014;42(6):1480-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wolters AE, Veldhuijzen DS, Zaal IJ, et al. Systemic corticosteroids and transition to delirium in critically Ill patients. Crit Care Med. 2015;43(12):e585-e588. [DOI] [PubMed] [Google Scholar]
- 85. Hauer D, Weis F, Campolongo P, et al. Glucocorticoid-endocannabinoid interaction in cardiac surgical patients: relationship to early cognitive dysfunction and late depression. Rev Neurosci. 2012;23(5-6):681-690. [DOI] [PubMed] [Google Scholar]
- 86. Keh D, Trips E, Marx G, et al. ; SepNet–Critical Care Trials Group . Effect of hydrocortisone on development of shock among patients with severe sepsis: the HYPRESS randomized clinical trial. JAMA. 2016;316(17):1775-1785. [DOI] [PubMed] [Google Scholar]
- 87. Sprung CL, Annane D, Keh D, et al. ; CORTICUS Study Group . Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111-124. [DOI] [PubMed] [Google Scholar]
- 88. Annane D, Renault A, Brun-Buisson C, et al. ; CRICS-TRIGGERSEP Network . Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018;378(9):809-818. [DOI] [PubMed] [Google Scholar]
- 89. Venkatesh B, Finfer S, Cohen J, et al. ; ADRENAL Trial Investigators and the Australian–New Zealand Intensive Care Society Clinical Trials Group . Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018;378(9):797-808. [DOI] [PubMed] [Google Scholar]
- 90. Sandi C, Pinelo-Nava MT. Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plast. 2007;2007:78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain James L McGaugh and Benno Roozendaal. Curr Opin Neurobiol. 2002;12(2):205-210. [DOI] [PubMed] [Google Scholar]
- 92. Hauer D, Kaufmann I, Strewe C, Briegel I, Campolongo P, Schelling G. The role of glucocorticoids, catecholamines and endocannabinoids in the development of traumatic memories and posttraumatic stress symptoms in survivors of critical illness. Neurobiol Learn Mem. 2014;112:68-74. [DOI] [PubMed] [Google Scholar]
- 93. Finsterwald C, Alberini CM. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: from adaptive responses to psychopathologies. Neurobiol Learn Mem. 2014;112:17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur J Neurosci. 1997;9(4):637-642. [DOI] [PubMed] [Google Scholar]
- 95. Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behav Neurosci. 1997;111(3):503-511. [PubMed] [Google Scholar]
- 96. Cordero MI, Sandi C. A role for brain glucocorticoid receptors in contextual fear conditioning: dependence upon training intensity. Brain Res. 1998;786(1-2):11-17. [DOI] [PubMed] [Google Scholar]
- 97. Andreano JM, Cahill L. Glucocorticoid release and memory consolidation in men and women. Psychol Sci. 2006;17(6):466-470. [DOI] [PubMed] [Google Scholar]
- 98. Lupien SJ, Wilkinson CW, Brière S, Ménard C, Ng Ying Kin NM, Nair NP. The modulatory effects of corticosteroids on cognition: studies in young human populations. Psychoneuroendocrinology. 2002;27(3):401-416. [DOI] [PubMed] [Google Scholar]
- 99. de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394(6695):787-790. [DOI] [PubMed] [Google Scholar]
- 100. Bentz D, Michael T, Wilhelm FH, et al. Influence of stress on fear memory processes in an aversive differential conditioning paradigm in humans. Psychoneuroendocrinology. 2013;38(7):1186-1197. [DOI] [PubMed] [Google Scholar]
- 101. Roozendaal B, De Quervain DJF, Schelling G, McGaugh JL. A systemically administered β-adrenoceptor antagonist blocks corticosterone-induced impairment of contextual memory retrieval in rats. Neurobiol Learn Mem. 2004;81(2):150-154. [DOI] [PubMed] [Google Scholar]
- 102. Roozendaal B, Mirone G. Opposite effects of noradrenergic and glucocorticoid activation on accuracy of an episodic-like memory. Psychoneuroendocrinology. 2020;114:104588. [DOI] [PubMed] [Google Scholar]
- 103. Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10(6):423-433. [DOI] [PubMed] [Google Scholar]
- 104. Roozendaal B, McGaugh JL. Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobiol Learn Mem. 1997;67(2):176-179. [DOI] [PubMed] [Google Scholar]
- 105. Donley MP, Schulkin J, Rosen JB. Glucocorticoid receptor antagonism in the basolateral amygdala and ventral hippocampus interferes with long-term memory of contextual fear. Behav Brain Res. 2005;164(2):197-205. [DOI] [PubMed] [Google Scholar]
- 106. Meduri GU, Muthiah MP, Carratu P, Eltorky M, Chrousos GP. Nuclear factor-kappaB- and glucocorticoid receptor alpha- mediated mechanisms in the regulation of systemic and pulmonary inflammation during sepsis and acute respiratory distress syndrome. Evidence for inflammation-induced target tissue resistance to glucocorticoids. Neuroimmunomodulation. 2005;12(6):321-338. [DOI] [PubMed] [Google Scholar]
- 107. Vassiliou AG, Floros G, Jahaj E, et al. Decreased glucocorticoid receptor expression during critical illness. Eur J Clin Invest. 2019;49(4):e13073. [DOI] [PubMed] [Google Scholar]
- 108. Schöbitz B, Sutanto W, Carey MP, Holsboer F, de Kloet ER. Endotoxin and interleukin 1 decrease the affinity of hippocampal mineralocorticoid (type I) receptor in parallel to activation of the hypothalamic-pituitary-adrenal axis. Neuroendocrinology. 1994;60(2):124-133. [DOI] [PubMed] [Google Scholar]
- 109. Brkic Z, Francija E, Petrovic Z, et al. Distinct modifications of hippocampal glucocorticoid receptor phosphorylation and FKBPs by lipopolysaccharide in depressive female and male rats. J Psychopharmacol. 2017;31(9):1234-1249. [DOI] [PubMed] [Google Scholar]
- 110. Ginzburg K. Comorbidity of PTSD and depression following myocardial infarction. J Affect Disord. 2006;94(1-3):135-143. [DOI] [PubMed] [Google Scholar]
- 111. Delahanty DL, Raimonde AJ, Spoonster E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biol Psychiatry. 2000;48(9):940-947. [DOI] [PubMed] [Google Scholar]
- 112. Spencer-Segal JL, Hyzy RC, Iwashyna TJ, Standiford TJ. Psychiatric symptoms in survivors of acute respiratory distress syndrome effects of age, sex, and immune modulation. Ann Am Thorac Soc. 2017;14(6):960-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hatch R, Young D, Barber V, Griffiths J, Harrison DA, Watkinson P. Anxiety, Depression and Post Traumatic Stress Disorder after critical illness: a UK-wide prospective cohort study. Crit Care. 2018;22(1):310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bienvenu OJ, Gellar J, Althouse BM, et al. Post-traumatic stress disorder symptoms after acute lung injury: a 2-year prospective longitudinal study. Psychol Med. 2013;43(12):2657-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32(4):301-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387-392. [DOI] [PubMed] [Google Scholar]
- 117. Fink G. Stress controversies: post-traumatic stress disorder, hippocampal volume, gastroduodenal ulceration. J Neuroendocrinol. 2011;23(2):107-117. [DOI] [PubMed] [Google Scholar]
- 118. Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Ann N Y Acad Sci. 2009;1179:56-69. [DOI] [PubMed] [Google Scholar]
- 119. Hauer D, Weis F, Krauseneck T, Vogeser M, Schelling G, Roozendaal B. Traumatic memories, post-traumatic stress disorder and serum cortisol levels in long-term survivors of the acute respiratory distress syndrome. Brain Res. 2009;1293:114-120. [DOI] [PubMed] [Google Scholar]
- 120. Als LC, Picouto MD, O’Donnell KJ, et al. Stress hormones and posttraumatic stress symptoms following paediatric critical illness: an exploratory study. Eur Child Adolesc Psychiatry. 2017;26(5):511-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hauer D, Weis F, Papassotiropoulos A, et al. Relationship of a common polymorphism of the glucocorticoid receptor gene to traumatic memories and posttraumatic stress disorder in patients after intensive care therapy. Crit Care Med. 2011;39(4):643-650. [DOI] [PubMed] [Google Scholar]
- 122. Davydow DS, Kohen R, Hough CL, Tracy JH, Zatzick D, Katon WJ. A pilot investigation of the association of genetic polymorphisms regulating corticotrophin-releasing hormone with posttraumatic stress and depressive symptoms in medical-surgical intensive care unit survivors. J Crit Care. 2014;29(1):101-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Amstadter AB, Nugent NR, Yang BZ, et al. Corticotrophin-releasing hormone type 1 receptor gene (CRHR1) variants predict posttraumatic stress disorder onset and course in pediatric injury patients. Dis Markers. 2011;30(2-3): 89-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kok L, Hillegers MH, Veldhuijzen DS, et al. Genetic variation in the glucocorticoid receptor and psychopathology after dexamethasone administration in cardiac surgery patients. J Psychiatr Res. 2018;103:167-172. [DOI] [PubMed] [Google Scholar]
- 125. Sijbrandij M, Kleiboer A, Bisson JI, Barbui C, Cuijpers P. Pharmacological prevention of post-traumatic stress disorder and acute stress disorder: a systematic review and meta-analysis. Lancet Psychiatry. 2015;2(5):413-421. [DOI] [PubMed] [Google Scholar]
- 126. Amos T, Stein DJ, Ipser JC. Pharmacological interventions for preventing post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2014;(7):CD006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Corbet Burcher G, Picouto MD, Als LC, et al. Post-traumatic stress after PICU and corticosteroid use. Arch Dis Child. 2018;103(9):887-889. [DOI] [PubMed] [Google Scholar]
- 128. Schelling G, Stoll C, Kapfhammer HP, et al. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder and health-related quality of life in survivors. Crit Care Med. 1999;27(12):2678-2683. [DOI] [PubMed] [Google Scholar]
- 129. Schelling G, Briegel J, Roozendaal B, Stoll C, Rothenhäusler HB, Kapfhammer HP. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biol Psychiatry. 2001;50(12):978-985. [DOI] [PubMed] [Google Scholar]
- 130. Schelling G, Kilger E, Roozendaal B, et al. Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: a randomized study. Biol Psychiatry. 2004;55(6):627-633. [DOI] [PubMed] [Google Scholar]
- 131. Dieleman JM, Nierich AP, Rosseel PM, et al. ; Dexamethasone for Cardiac Surgery (DECS) Study Group . Intraoperative high-dose dexamethasone for cardiac surgery: a randomized controlled trial. JAMA. 2012;308(17):1761-1767. [DOI] [PubMed] [Google Scholar]
- 132. Kok L, Hillegers MH, Veldhuijzen DS, et al. ; Dexamethasone for Cardiac Surgery Study Group . The effect of dexamethasone on symptoms of posttraumatic stress disorder and depression after cardiac surgery and intensive care admission: longitudinal follow-up of a randomized controlled trial. Crit Care Med. 2016;44(3):512-520. [DOI] [PubMed] [Google Scholar]
- 133. Weis F, Kilger E, Roozendaal B, et al. Stress doses of hydrocortisone reduce chronic stress symptoms and improve health-related quality of life in high-risk patients after cardiac surgery: a randomized study. J Thorac Cardiovasc Surg. 2006;131(2):277-282. [DOI] [PubMed] [Google Scholar]
- 134. Rao RP, Anilkumar S, McEwen BS, Chattarji S. Glucocorticoids protect against the delayed behavioral and cellular effects of acute stress on the amygdala. Biol Psychiatry. 2012;72(6):466-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Zohar J, Yahalom H, Kozlovsky N, et al. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. Eur Neuropsychopharmacol. 2011;21(11):796-809. [DOI] [PubMed] [Google Scholar]
- 136. Cohen H, Matar MA, Buskila D, Kaplan Z, Zohar J. Early post-stressor intervention with high-dose corticosterone attenuates posttraumatic stress response in an animal model of posttraumatic stress disorder. Biol Psychiatry. 2008;64(8):708-717. [DOI] [PubMed] [Google Scholar]
- 137. Cohen H, Zohar J, Gidron Y, et al. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry. 2006;59(12): 1208-1218. [DOI] [PubMed] [Google Scholar]
- 138. Medici CR, Gradus JL, Pedersen L, Sørensen HT, Østergaard SD, Christiansen CF. No impact of preadmission anti-inflammatory drug use on risk of depression and anxiety after critical illness. Crit Care Med. 2017;45(10):1635-1641. [DOI] [PubMed] [Google Scholar]
- 139. Pivonello R, Simeoli C, De Martino MC, et al. Neuropsychiatric disorders in Cushing’s syndrome. Front Neurosci. 2015;9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Barichello T, Milioli G, Generoso JS, et al. Imipramine reverses depressive-like parameters in pneumococcal meningitis survivor rats. J Neural Transm (Vienna). 2012;119(6):653-660. [DOI] [PubMed] [Google Scholar]
- 141. Nasca C, Bigio B, Zelli D, Nicoletti F, McEwen BS. Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol Psychiatry. 2015;20(6):755-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Medina A, Seasholtz AF, Sharma V, et al. Glucocorticoid and mineralocorticoid receptor expression in the human hippocampus in major depressive disorder. J Psychiatr Res. 2013;47(3):307-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Klok MD, Alt SR, Irurzun Lafitte AJ, et al. Decreased expression of mineralocorticoid receptor mRNA and its splice variants in postmortem brain regions of patients with major depressive disorder. J Psychiatr Res. 2011;45(7):871-878. [DOI] [PubMed] [Google Scholar]
- 144. Klok MD, Giltay EJ, Van der Does AJ, et al. A common and functional mineralocorticoid receptor haplotype enhances optimism and protects against depression in females. Transl Psychiatry. 2011;1(12):e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. DeRijk RH, de Kloet ER, Zitman FG, van Leeuwen N. Mineralocorticoid receptor gene variants as determinants of HPA axis regulation and behavior. Endocr Dev. 2011;20:137-148. [DOI] [PubMed] [Google Scholar]
- 146. Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Daskalakis NP, Cohen H, Nievergelt CM, et al. New translational perspectives for blood-based biomarkers of PTSD: From glucocorticoid to immune mediators of stress susceptibility. Exp Neurol. 2016;284(Pt B):133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Denstaedt SJ, Spencer-Segal JL, Newstead MW, et al. S100A8/A9 drives neuroinflammatory priming and protects against anxiety-like behavior after sepsis. J Immunol. 2018;200(9):3188-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Denstaedt SJ, Spencer-Segal JL, Newstead M, et al. Persistent neuroinflammation and brain-specific immune priming in a novel survival model of murine pneumosepsis. Shock. 2020;54(1):78-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Anderson ST, Commins S, Moynagh P, Coogan AN. Chronic fluoxetine treatment attenuates post-septic affective changes in the mouse. Behav Brain Res. 2016;297:112-115. [DOI] [PubMed] [Google Scholar]
- 151. Silverman MN, Macdougall MG, Hu F, Pace TW, Raison CL, Miller AH. Endogenous glucocorticoids protect against TNF-alpha-induced increases in anxiety-like behavior in virally infected mice. Mol Psychiatry. 2007;12(4):408-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Yeager MP, Guyre PM, Munck AU. Glucocorticoid regulation of the inflammatory response to injury. Acta Anaesthesiol Scand. 2004;48(7):799-813. [DOI] [PubMed] [Google Scholar]
- 153. Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17(4):233-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Briegel J, Jochum M, Gippner-Steppert C, Thiel M. Immunomodulation in septic shock: hydrocortisone differentially regulates cytokine responses. J Am Soc Nephrol. 2001;12(Suppl 17):70-74. [PubMed] [Google Scholar]
- 155. Fonken LK, Frank MG, Gaudet AD, et al. Neuroinflammatory priming to stress is differentially regulated in male and female rats. Brain Behav Immun. 2018;70:257-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Santos J, Yang PC, Söderholm JD, Benjamin M, Perdue MH. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut. 2001;48(5):630-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Maes M, Kubera M, Leunis JC, Berk M. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J Affect Disord. 2012;141(1):55-62. [DOI] [PubMed] [Google Scholar]
- 158. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712. [DOI] [PubMed] [Google Scholar]
- 159. Han SK, Kim DH. Lactobacillus mucosae and bifidobacterium longum synergistically alleviate immobilization stress-induced anxiety/depression in mice by suppressing gut dysbiosis. J Microbiol Biotechnol. 2019;29(9):1369-1374. [DOI] [PubMed] [Google Scholar]
- 160. Jang HM, Lee HJ, Jang SE, Han MJ, Kim DH. Evidence for interplay among antibacterial-induced gut microbiota disturbance, neuro-inflammation, and anxiety in mice. Mucosal Immunol. 2018;11(5):1386-1397. [DOI] [PubMed] [Google Scholar]
- 161. Tan T, Khoo B, Mills EG, et al. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020;8(8):659-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry 2020;2(20):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Kotfis K, Williams Roberson S, Wilson JE, Dabrowski W, Pun BT, Ely EW. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care. 2020;24(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.