Abstract
Objectives
Uncertainty has surrounded the duration of immunity against SARS CoV-2. This concerns both the duration of vaccine immunity and the duration of natural immunity. We aim to critically review the information available today, and draw practical conclusions.
Methods
This is a narrative review of the recently published information on the topic, compared with the knowledge we already have of the behavior of various viral infectious agents.
Results
It is too early to have any meaningful information on the duration of vaccine immunity against SARS CoV-2. For those who already had the infeciton, the rate of reinfection is very low. Most reinfections are due to laboratory errors, to incomplete cure of the primary infection, to the supervening immunodeficiency of the host, or to pre-existing immunodeficiency made evident by the SARS CoV-2 infection. The available studies on the immunology of the infection converge in indicating that it generates a robust and persistent immunity. This behavior does not differ from that of respiratory viruses known to date: in naturally occurring viral respiratory infections, reinfections are exceptional.
Conclusions and implications
The civil community awaits suggestions from scientists not only to protect susceptible people, but to be able to safely resume activities made uncertain by the pandemic. From the information we have to-date, we suggest that, in principle, patients who have already overcome the infection should not be prioritized to the SARS CoV-2 vaccine. Instead, they could be provided with an immunological passport that allows them to resume a normal social life.
Keywords: SARS-COV-2, Pediatric, Immune passport, Immunization
Introduction
A recent public statement post by the US Centers for Disease Control and Prevention (CDC) lists several facts about SARS-COV-2 vaccines.1 Among the reported facts, it is stated that "People who have gotten sick with COVID-19 may still benefit from getting vaccinated". This suggestion stems from the consideration that, in this moment, experts do not know the actual duration of immunity in individuals who have had SARS-COV-2 disease. It is stated that according to some evidence, naturally developed immunity does not last long. Now that vaccine efficacy is demonstrated, such attitudes can have an important impact on vaccine policies at a time when priority choices lie ahead.2
As clinicians dealing with dozens of patients every day, allergists and pediatricians are used to scanning the scientific literature, drawing synthetic conclusions from multiple and heterogeneous information. Perhaps for this reason, we are perplexed about the hypothesis that SARS-CoV-2, a virus that almost 1 year ago we did not even know existed, may have an immunological behavior different from all other known viruses. We still lack reliable information on several aspects of the SARS-CoV-2 vaccines. How long does the protection last? Are they safe in the long term? Is it possible that the evanescence of the immunological memory induced by the vaccine could lead to susceptibility for the disease again after re-exposure? Is there an association between the degree of protection, the age of the vaccinated, and any other coexisting conditions?3 Amid these uncertainties, what we do lack is the reasonable certainty about the duration of SARS-CoV-2 immunity, both natural and induced.
Reinfections are exceptional
To the best of our knowledge, there are no cases of successful experimental reinfection with the same strain of SARS-CoV-2. In a rhesus macaque model, re-challenge with the virus has only a limited effect and does not produce infections.4 Thus, in primates immunologic control is effective against re-exposure.
SARS-CoV-2 displays some genetic variability (see infra). In humans, it has been shows that significant cross-reactivity among strains confers mutual protection.5 How to explain, then, the Korean reported cases of patients who, after becoming negative to viral RNA, subsequently demonstrated active infections in the short term?6 The explanation provided by the same scientists was a possible false negative PCR during the course of a single infection, rather than an early reinfection.7 In September, a meta-analysis of published data did not support the possibility of reinfections.8 In January 2021, when 78,810,611 cases of confirmed SARS CoV-2 infections have been reported,9 only 4 cases of confirmed reinfections are published.10
Still, such clinical reports aroused an important media echo with significant consequences. On their basis, World Health Organization (WHO) officially issued the skeptical claim that there is no evidence that those who are cured of COVID-19, despite having antibodies, are protected from a second infection.11 This is even more remarkable, as the Korean Centers for Disease Control & Prevention, in accordance with the observations, adapted their nomenclature from “re-positive cases” to “PCR re-detected after discharge from isolation”.12
It is well known that any virus can achieve some initial replication in an immune competent person, which is subdued in a state of acquired immunity. In the swabs of these patients, fragments of RNA can be found rather than intact genomes of the virus, therefore without the possibility of any transmission. In accordance, none of the patients with presumed reinfection was able to transmit any infection to the contacts.49
SARS-CoV-2 displays low genetic variability
For more than 30 years, we have known that reinfections with human coronaviruses are possible in the immunocompetent host, not due to inability to exert an effective immunity, but to antigen drift by genetic mutations of the virus.13 This could also be the case for SARS-CoV-2. On December 24, 2020, 290,562 genetic sequences of SARS-CoV-2 isolates from all over the world were deposited in Gisaid's databases.14 Of these, 128,879, over 45%, came from the United Kingdom, where a major sequencing effort has taken place since April 2020. The so-called "English variant" B.1.1.7, which has dominated the public debate for some days now, reflects the increased attention that the country devoted to sequencing; it is associated with increased transmission rate, but not with increased virulence.15 The P.1 Brazilian mutation seems potentially dangerous as it includes several mutations of known biological importance (E484K, K417T, and N501Y).16 To date, there is no evidence that it is associated with reinfections even in Manaus, but surveillance has just started and could hold surprises. Immune evasion from genomic variants is in our opinion the most serious danger in this pandemic, but in the vast prairie of infectious subjects that the virus encountered in 2020, the numerous viral mutations that have been reported from the majority of the world countries (Fig. 1) have not been associated with clinical behavior significantly different from the original strain.
Fig. 1.
Genomic epidemiology of SARS CoV-2.14
With a mutation rate of 1.12 × 10−3 mutations per site-year, the variability of SARS-CoV-2 is similar to the 0.80/2.38 × 10−3 mutations per site-year attributed to SARS-CoV-A.17 This rate is far lower that the majority of other RNA viruses, in particular influenza A, influenza B, and Vesicular stomatitis virus.18 A relative resistance to mutation is typical of coronaviruses, which contain an RNA exoribonuclease with proofreading activity, able to stabilize the genome across generations.19
Genetic mutations are in the nature of the evolutional dynamics of any virus. The new genomes that appear, take over by direct competition with others, and then eventually disappear, are not necessarily more pathogenic. The reported reinfections, all oligo-symptomatic, are often associated with mutated viral strains20,21,22 However, the low genetic variability of the virus seems not able to produce antigenic variations as to substantially escape the host's immunological reaction. For example, the different SARS-CoV-2 genetic variants D614D and D614G are responsible for the early Chinese outbreak and the American cases, respectively.50 Although the behavior of these strains is different, the latter being associated with higher replication and transmissibility,51 naturally formed neutralizing antibodies against D614D are able to exert their activity on the D614G variants at least 6 months after the infection. In line, serum assay performance for IgG, IgM, and IgA was reliable in terms of the new spike protein variant with increased infectivity.23 Thus, also due to their low genetic variability,24 reinfection by divergent SARS-CoV-2-strains seems not likely. It would be surprising that a Chinese patient who got immunity in Wuhan would be susceptible to SARS-CoV-2 in Washington State.
Antibodies are short-lived
Within 19 days after symptom onset, all patients produce antiviral immunoglobulin-G,25 and 93% of the patients recovered from COVID-19 display high specific neutralizing IgG titer.26 Early case reports showing that IgG and IgM against the viral spike and nucleocapsid antigens disappear 80 days after the infection27 have been confirmed by large studies in health care personnel,28 asymptomatic infections,29 mild infections,30 and patients requiring hospitalization.31 The short life span of antibodies against SARS-CoV-2, together with the report of cases of SARS-CoV-2 reinfection,14 generated at the public level the impression of non-durability of SARS-CoV-2 immunity.32 The specificity of serological tests might be improved by using independent SARS-CoV-2 antigens in serological assays,33 as it is known that nucleocapsid antibodies can be detected only until 5–7 months, while neutralizing and spike-specific antibodies persist longer. Notably, anti-spike protein IgG, IgM, and IgA antibodies have been associated with the highest neutralizing capacity.34 It was shown recently that with the decline in IgG, IgM, and IgA after 3 months the neutralizing capacity of the antibodies also decreases.35 However, this information from case reports or small case series has been counterbalanced by the emergence of an increasing literature that suggests that antibodies can last longer than reported. A study in 5882 members of a low-seroprevalence community in Arizona in the United States, including multiple antibody assays, in the limited observation time span, was able to detect persistent neutralizing antibodies for at least 5–7 months after SARS-CoV-2 infection.26 Thus, it is possible that not all antibodies vanish, but only those that have been searched for.
Robust and persistent
Infectious studies and daily practice teach us that antibodies are only part of the anamnestic immune response, and a fundamental role is played by the adaptive cellular immunity in the form of memory cells. Immunity against the flu 1918 pandemics have been found 90 years after the infection.36 Many studies indicate that even for SARS-CoV-2 the immunity can be robust and persistent.
In convalescent patients, antibodies to SARS-CoV-2 have been shown to persist up to 7 months, albeit at lower levels compared to the response that can be detected in the first few months.5 These antibodies proved capable of blocking the engraftment of different genetic variants of the virus. In addition, the authors noted the presence of CD4+ and CD8+ cells producing γ-interferon with no tendency to depression in the observation period so far. Another study found that, despite limited levels of neutralizing antibodies, receptor-binding domain (RBD)-specific antibodies with potent antiviral activity are present in all individuals tested.37
Other findings indicate a persistent T-cellular immunity. Rhesus macaques have been found immune to successive viral challenge4, with polyfunctional SARS-CoV-2-specific T cells, characterized by a stem-like memory phenotype, being present in the convalescent-phase. These cells are present in exposed family members even if they are seronegative, as well as in patients with a history of asymptomatic and mild infection.38
The whole concert of T- and B-cell immunity seems to be fully in action in the response to the virus. A recent paper evaluated the persistence of SARS-COV-2 antibodies together with specific memory cells, in particular, Spike-specific memory B cells, SARS-CoV-2-specific CD4+ T cells, and SARS-CoV-2-specific CD8+ T cells. This allowed assessing the relationships between different aspects of immunological memory.52 The majority of patients demonstrated a complete response to each of the immune memory compartments within 1–2 months of infection, while at 5 months 40% of them remained positive in 3 of the 5 compartments, with a heterogeneity of responses that fanned out over time. Remarkably, no patient was negative to all the immunologic markers. As Fig. 2 demonstrates, the memory for SARS CoV-2 during the early (1–2 months), intermediate (3–4 months), or late phase (5+ months) remained traceable in each case.
Fig. 2.
Immune memory to SARS-CoV-2 during the early phase (1–2 mo, black line), medium phase (3–4 mo, red line), or late phase (5+ mo, blue line).52 (Re-used with permission.) (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of the article.)
Similar data have been replicated in another group of patients 6–8 months after the infection.53 In this caseload, while the serum levels of anti-SARS-CoV-2 IgG antibodies showed decline, virus-specific T and/or memory B cell responses were found increased with time and were maintained throughout the study period. Thus, at a time interval from the SARS-CoV-2 infection the organism tends to keep track of its passage in the compartments most exquisitely dedicated to the reorganization of a defense in the event of a new attack. These data point in the direction that SARS CoV-2 infection happens once, and raise the hope that a vaccine could be sufficient once in a lifetime.
Our comment
It is part of our daily clinical practice to follow paths of deductive reasoning. The classic figure of the syllogism is applicable in the case of SARS CoV-2. We knew that respiratory viruses give permanent immunity: this simple assumption forms the basis of the practice of attenuated live virus vaccines that have defeated the most dangerous of them, such as smallpox and measles.39 It was, therefore, logical to believe right from the start that this virus may determine permanent immunity.40 It is true that we know little about the kinetics of the immune response to SARS CoV-2; but which viral disease due to a stable agent does not end in a long-term immunity after viral clearance? Even if the precise duration of memory is not yet determined, it is unlikely that it lasts only for a few weeks. We fully share the opinion of Nicole Baumgarth, that “the expressions of doubt with which scientific papers necessarily open their introductions are intended to pose the scientific question, and must not be mistaken for statements of lack of knowledge”.36 In the analytical reasoning of those called to investigate the immunological aspects of SARS-CoV-2, it is mandatory to evaluate all the multifaceted aspects of immunity against this specific virus. In some of the articles in which antibodies to major antigens, cellular immunity, and natural immunity were studied, the premise sounds: "we know nothing about this specific aspect of the immune response to SARS-COV2". This tribute to the absence of evidence should not be interpreted as evidence of absence of permanent immunity. Each of these studies offers an in-depth contribution for some immunological aspects, but must not make us lose sight of the complexity of the interaction between different immunological lines, aimed at permanent immunity.41 We were therefore not surprised by the recent findings on the persistence of antiviral immunity.52 They confirm what we always have known, but often not stated.
Practical consequences
These observations carry important practical implications.
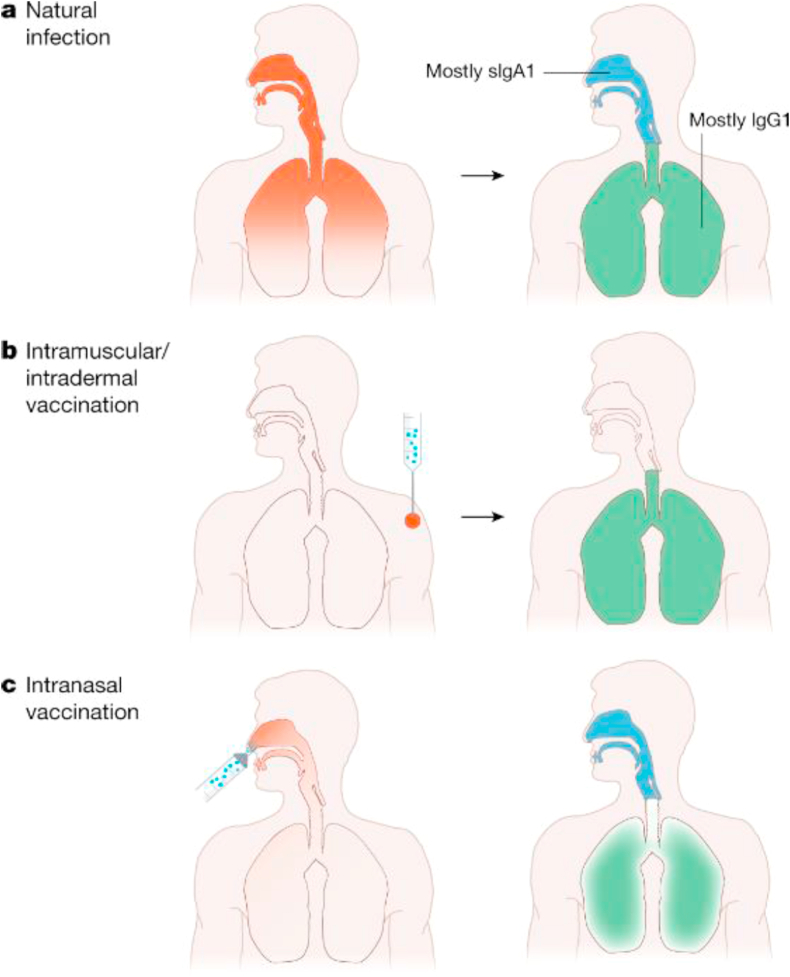
First, those who have already contracted the infection should not be prioritized to SARS-CoV-2 immunization. Although previous infection does not contradict boosting the natural immune response by a vaccine, immunizing already immune subjects is generally not needed in viral infections. The long-desired immunization will hopefully induce disease-attenuating immunity for all those who have not had the infection, but as they are mainly parenteral, they are expected to induce IgG, but no secretory IgA42 (Fig. 3). Therefore, there are doubts that they can confer sterilizing immunity,43 even though it is logical that formed antibodies in an immune individual inhibit the cellular entry and amplification of the virus, making the viral load and correlating risk of transmission to still permissive persons neglectable. We expect that natural immunity lasts over time, while the duration of the vaccine immunity could be limited in time.
Fig. 3.
Natural infection elicits IgA and IgG antibodies to protect the mucous surfaces and internal organs. It elicits sterilizing immunity. It is foreseeable that the antibodies produced by vaccinations administered intramuscularly or intradermally cover only the internal organs. The antibodies produced by intranasal vaccination are expected to cover the internal organs with possible less efficacy, but with good efficacy the upper airways.43 (re-used with permission).
Second, appropriate programs for the identification of immune subjects could allow them to be exempt from the restrictive measures imposed by the authorities in epidemic situations. We realize that the concession of the "Immune passport" is complex, with not only scientific but ethical, organizational, and economic aspects.44 However, with the increase in the slice of the population that has already contracted the infection, it will be inevitable that somehow these people will be recognized and completely freed. Lockdown measures heavily curtail human activities,45,46 and we believe that it is ethically not acceptable to restrict the mobility of people who do not pose a risk to others. With all the necessary technological devices, we believe that identifying the individuals who can move freely, attend hotels, and carry out their work activities, is imperative. Among the relevant aspects of the SARS-CoV-2 immunity that are being elucidated, the following are important:
-
a.
Will a medical asseveration of infection be enough for a certification of immunity? Along the current CDC Advisory Committee on Immunization Practices indications, for infectious diseases such as chickenpox, a diagnosis witnessed by a health-care provider constitutes evidence of immunity; in these cases, there is no need of chickenpox immunization.47
-
b.
As diagnosis in this case cannot disregard the evidence of a passage of the virus at the oropharyngeal level and/or the presence of circulating specific antibodies, which is the reliability of current diagnostic tests? Which are the most appropriate to indicate a former infection?
-
c.
Does SARS-CoV-2 infection confer sterilizing immunity at least to the specific viral strain? This is of great importance in view of the future epidemic scenarios.48
With all these limitations, in our opinion the Pros outweigh the Cons (Table 1). We propose that allergists and pediatricians actively participate in the research on the current epidemic. As specialists in clinical immunology, we are the best-positioned clinicians to become promoters of these instances in our respective communities. We propose that our profession become not only a bulwark against disease, but also a promoter of safe well-being. This will be another help to our patients: to favor the rebirth of human relations, trade, and cultural exchanges heavily penalized by the pandemic.
Table 1.
Pros and Cons of the establishment of the immunological passport for patients recovered from SARS CoV-2.
Cons:
|
Pros:
|
Author contribution
AF initiated the concept and made the first draft.
EJJ participated in the the development of the document.
Both authors reviewed and approved the final manuscript.
Ethics approval and consent to participate
Not applicable; the manuscript does not report on or involve the use of any animal or human data or tissue.
Consent for publication
Both authors agree to the publication of the work.
Availability of data and materials
This is a review article.
Funding
This is a review article that did not need or receive any funding.
Declaration of competing interest
The authors have no competing interests to declare in relevance to the article.
References
- 1.https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-benefits/facts.html, accessed December 4, 2020.
- 2.Bloom B.R., Nowak G.J., Orenstein W. When will we have a vaccine?" - understanding questions and answers about covid-19 vaccination. N Engl J Med. 2020 Dec 3;383:2202–2204. doi: 10.1056/NEJMp2025331. [DOI] [PubMed] [Google Scholar]
- 3.Krause P.R., Fleming T.R., Longini I.M., WHO Ad Hoc Expert Group on the Next Steps for Covid-19 Vaccine Evaluation Placebo-controlled trials of covid-19 vaccines - why we still need them. N Engl J Med. 2021 Jan 14;384:e2. doi: 10.1056/NEJMp2033538. Epub ahead of print. PMID: 33264543. [DOI] [PubMed] [Google Scholar]
- 4.Chandrashekar A., Liu J., Martinot A.J. SARSCoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020;369:812–817. doi: 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan Y., Liu F., Xu X. Durability of neutralizing antibodies and T-cell response post SARS-CoV-2 infection. Front Med. 2020;14(6):746–751. doi: 10.1007/s11684-020-0822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korean Centers for Disease Control . 2020. Findings from Investigation and Analysis of Re-positive Cases.http://www.cdc.go.kr Available at: [Google Scholar]
- 7.Xiao A.T., Tong Y.X., Zhang S. False negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: rather than recurrence. J Med Virol. 2020;92(10):1755–1756. doi: 10.1002/jmv.25855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arafkas M., Khosrawipour T., Kocbach P. Current meta-analysis does not support the possibility of COVID-19 reinfections. J Med Virol. 2020 Sep 8 doi: 10.1002/jmv.26496. Epub ahead of print. PMID: 32897549. [DOI] [PubMed] [Google Scholar]
- 9.https://coronavirus.jhu.edu/map.html, accessed December 24, 2020.
- 10.Iwasaki A. What reinfections mean for COVID-19. Lancet Infect Dis. 2021;21:3–5. doi: 10.1016/S1473-3099(20)30783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.https://www.who.int/news-room/commentaries/detail/immunity-passports-in-the-context-of-covid-19, accessed December 2, 2020.
- 12.https://is.cdc.go.kr/upload_comm/syview/doc.html?fn=158993708884700.pdf&rs=/upload_comm/docu/0030/, accessed December 7, 2020.
- 13.Reed S.E. The behaviour of recent isolates of human respiratory coronavirus in vitro and in volunteers: evidence of heterogeneity among 229E-related strains. J Med Virol. 1984;13(2):179–192. doi: 10.1002/jmv.1890130208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genomic epidemiology of hCoV-19. https://www.gisaid.org/. (as updated by Nextstrain, https://nextstrain.org/, Hatfield et al Bioinformatics), accessed December 24, 2020.
- 15.https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563, accessed January 16, 2020.
- 16.https://virological.org/t/genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-manaus-preliminary-findings/586, accessed January 16, 2020.
- 17.Zhao Z., Li H., Wu X. Moderate mutation rate in the SARS coronavirus genome and its implications. BMC Evol Biol. 2004 06 28;4(1):21. doi: 10.1186/1471-2148-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanjuán R., Nebot M.R., Chirico N., Mansky L.M., Belshaw R. Viral mutation rates. J Virol. 2010;84:9733–9748. doi: 10.1128/JVI.00694-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minskaia E., Hertzig T., Gorbalenya A.E. Discovery of an RNA virus 3 35 exoribonuclease that is critically involved in coronavirus RNA synthesis. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5108–5113. doi: 10.1073/pnas.0508200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.To K.K., Hung I.F., Ip J.D. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1275. ciaa1275. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J.S., Kim S.Y., Kim T.S. Evidence of severe acute respiratory syndrome coronavirus 2 reinfection after recovery from mild coronavirus disease 2019. Clin Infect Dis. 2020 Nov 21 doi: 10.1093/cid/ciaa1421. ciaa1421. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zehender G., Lai A., Bergna A. Genomic characterization and phylogenetic analysis of SARS-COV-2 in Italy. J Med Virol. 2020 Mar 29 doi: 10.1002/jmv.25794. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klumpp-Thomas C., Kalish H., Hicks J. D614G spike variant does not alter IgG, IgM, or IgA spike seroassay performance. J Infect Dis. 2020 Dec 1 doi: 10.1093/infdis/jiaa743. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turakhia Y., De Maio N., Thornlow B. Stability of SARS-CoV-2 phylogenies. PLoS Genet. 2020 Nov 18;16(11) doi: 10.1371/journal.pgen.1009175. Epub ahead of print. PMID: 33206635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long Q.X., Liu B.Z., Deng H.J. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 26.Del Fante C., Franchini M., Baldanti F. A retrospective study assessing the characteristics of COVID-19 convalescent plasma donors and donations. Transfusion. 2020 Nov 24 doi: 10.1111/trf.16208. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu A., Wang W., Zhao X. Disappearance of antibodies to SARS-CoV-2 in a -COVID-19 patient after recovery. Clin Microbiol Infect. 2020;26:1703–1705. doi: 10.1016/j.cmi.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Self W.H., Tenforde M.W., Stubblefield W.B., CDC COVID-19 Response Team. IVY Network Decline in SARS-CoV-2 antibodies after mild infection among frontline health care personnel in a multistate hospital network - 12 states, april-august 2020. MMWR Morb Mortal Wkly Rep. 2020 Nov 27;69(47):1762–1766. doi: 10.15585/mmwr.mm6947a2.PMID:33237893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lei Q., Li Y., Hou H.Y. Antibody dynamics to SARS-CoV-2 in asymptomatic COVID-19 infections. Allergy. 2020 Oct 10 doi: 10.1111/all.14622. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibarrondo F.J., Fulcher J.A., Goodman-Meza D. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild covid-19. N Engl J Med. 2020 Sep 10;383(11):1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isho B., Abe K.T., Zuo M. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol. 2020 Oct 8;5(52) doi: 10.1126/sciimmunol.abe5511. PMID: 33033173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.https://uk.reuters.com/article/us-china-health-reinfection-explainer/explainer-coronavirus-reappears-in-discharged-patients-raising-questions-in-containment-fight-idUKKCN20M124, accessed November 26 2020.
- 33.Ripperger T.J., Uhrlaub J.L., Watanabe M. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity. 2020 Nov 17;53:925–933. doi: 10.1016/j.immuni.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazzini L., Martinuzzi D., Hyseni I. Comparative analyses of SARS-CoV-2 binding (IgG, IgM, IgA) and neutralizing antibodies from human serum samples. J Immunol Methods. 2020 Nov 27:112937. doi: 10.1016/j.jim.2020.112937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seow J., Graham C., Merrick B. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020 Dec;5:1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu X., Tsibane T., McGraw P.A. Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors. Nature. 2008;455:532–536. doi: 10.1038/nature07231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robbiani D.F., Gaebler C., Muecksch F. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sekine T., Perez-Potti A., Rivera-Ballesteros O. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.https://www.nationalgeographic.com/history/magazine/2015/06-07/vaccines/, accessed December 2, 2020.
- 40.Baumgarth N., Nikolich-Žugich J., Lee F.E., Bhattacharya D. Antibody responses to SARS-CoV-2: let's stick to known knowns. J Immunol. 2020;205:2342–2350. doi: 10.4049/jimmunol.2000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azkur A.K., Akdis M., Azkur D. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75:1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su F., Patel G.B., Hu S., Chen W. Induction of mucosal immunity through systemic immunization: phantom or reality? Hum Vaccines Immunother. 2016;12:1070–1079. doi: 10.1080/21645515.2015.1114195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020 Oct;586(7830):516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 44.Kofler N., Baylis F. Ten reasons why immunity passports are a bad idea. Nature. 2020;581:379–381. doi: 10.1038/d41586-020-01451-0. [DOI] [PubMed] [Google Scholar]
- 45.Klimek L., Huppertz T., Alali A. A new form of irritant rhinitis to filtering facepiece particle (FFP) masks (FFP2/N95/KN95 respirators) during COVID-19 pandemic. World Allergy Organ J. 2020 Oct;13(10):100474. doi: 10.1016/j.waojou.2020.100474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dell'Edera A., Borghesan F., Favero E. Venom immunotherapy during COVID-19 pandemic: experience from a university allergy center in northern Italy. World Allergy Organ J. 2020 Dec;13(12):100489. doi: 10.1016/j.waojou.2020.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marin M., Güris D., Chaves S.S., Schmid S., Seward J.F. Advisory committee on immunization practices, Centers for disease control and prevention (CDC). Prevention of varicella: recommendations of the advisory committee on immunization practices (ACIP) MMWR Recomm Rep (Morb Mortal Wkly Rep) 2007;56(RR-4):1–40. [PubMed] [Google Scholar]
- 48.Shaman J., Galanti M. Will SARS-CoV-2 become endemic? Science. 2020 Oct 30;370(6516):527–529. doi: 10.1126/science.abe5960. [DOI] [PubMed] [Google Scholar]
- 49.Yang C., Jiang M., Wang X. Viral RNA level, serum antibody responses, and transmission risk in recovered COVID-19 patients with recurrent positive SARS-CoV-2 RNA test results: a population-based observational cohort study. Emerg Microbes Inf. 2020 Dec;9(1):2368–2378. doi: 10.1080/22221751.2020.1837018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mueller N.F., Wagner C., Frazar C.D. Viral genomes reveal patterns of the SARS-CoV-2 outbreak in Washington State. medRxiv. 2020 doi: 10.1101/2020.09.30.20204230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou B., Thao T.T.N., Hoffmann D. SARS-CoV-2 spike D614G variant confers enhanced replication and transmissibility. bioRxiv. 2020 doi: 10.1101/2020.10.27.357558. [DOI] [Google Scholar]
- 52.Dan J.M., Mateus J., Kato Y. Immunological memory to SARS-CoV-2 assessed for greater than six months after infection. Science. 2021 Feb 5;371(6529):eabf4063. doi: 10.1126/science.abf4063. Epub 2021 Jan 6. PMID: 33408181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sherina N., Piralla A., Du L. Persistence of SARS-CoV-2 specific B- and T-cell responses in convalescent COVID-19 patients 6-8 months after the infection. bioRxiv. 2020 doi: 10.1101/2020.11.06.371617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review article.