Abstract
Clinical studies have identified a cytokine storm in the third stage of disease progression in critical ill patients with coronavirus disease 2019 (COVID-19). Hence, effectively suppressing the uncontrolled immune response of the host towards the invaded viruses in a cytokine storm is a critical step to prevent the deterioration of patient conditions and decrease the rate of mortality. Therapeutic monoclonal antibodies (mAbs) are found to be effective for the management of acute respiratory distress syndrome in patients with COVID-19. In this review, we compiled all therapeutic mAbs targeting cytokine storm, which are in clinical trials for its repurposing in the management of COVID-19. Compilation of clinical trial data indicated that therapeutic monoclonal antibodies targeting interleukins (IL-6, IL-1ra, IL-8, IL-1β, IL-17A, IL-33), interferon-gamma, tumor necrosis factor-alpha, P-selectin, connective tissue growth factor, plasma kallikrein, tumor necrosis factor superfamily 14, granulocyte macrophage colony stimulating factor, colony stimulating factor 1 receptor, C–C chemokine receptor type 5, cluster of differentiation 14 and 147, vascular endothelial growth factor, programmed cell death protein-1, Angiopoietin - 2, human factor XIIa, complementary protein 5, natural killer cell receptor G2A, human epidermal growth factor receptor 2, immunoglobulin-like transcript 7 receptor, complement component fragment 5a receptor and viral attachment to the human cell were under investigation for management of severely ill patients with COVID-19. Among these, about 65 clinical trials are targeting IL-6 inhibition as the most promising one and Tocilizumab, an IL-6 inhibitor is considered to be the potential candidate to treat cytokine storm associated with the COVID-19.
Keywords: COVID-19, SARS-CoV-2, ARDS, Cytokine storm, Therapeutic monoclonal antibody
COVID-19; SARS-CoV-2; ARDS; Cytokine storm; Therapeutic monoclonal antibody
1. Overview
Coronavirus disease 2019 (COVID-19) is the infectious disease, which affects primarily the lungs, caused by newly identified coronavirus termed as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Patient infected with SARS-CoV-2 was initially discovered at Wuhan, China in December 2019 and is still spreading across the globe (Singhal, 2020). The COVID-19 outbreak was declared as pandemic on March 11, 2020 by the world health organization (WHO) (Coperchini et al., 2020). By September 9, 2020 the number of deaths raised to 896K amongst 27.5 million confirmed cases of COVID-19. The majority of the patients infected with SARS-CoV-2 in this COVID-19 pandemic are clinically asymptomatic or develop mild clinical manifestations like cough, mild fever, or muscle soreness. However, nearly 10–20% of patients (especially geriatric and patients with other co-morbidity) will develop pneumonia and acute respiratory distress syndrome (ARDS) (Soy et al., 2020). This COVID-19 pandemic created global crises of both public health as well as economic and thus emerged the need for rapid development of vaccines and therapeutic countermeasures (Sempowski et al., 2020). One approach for finding appropriate therapeutics for treating COVID-19 is rapid repurposing of existing drugs (Guy et al., 2020; Harrison, 2020; Lima et al., 2020; Rameshrad et al., 2020; Serafin et al., 2020). Several treatments are under investigation and are tested through clinical trials. The main focus of such clinical trials is the repurposing of existing drugs to effectively treat COVID-19.
Clinical studies have identified markers of cytokine storm in the critically ill patient infected with SARS-CoV-2 and thus correlated the severe deterioration of COVID-19 patients with excessive and uncontrolled production of cytokines. Clinicians are focusing on targeting the suppression of cytokine storm to save the lives of patients (Wan et al., 2020; Ye et al., 2020). Therapeutic monoclonal antibodies (mAbs) are effectively used earlier in respiratory infection in Influenza, SARS, middle east respiratory syndrome (MERS), and Ebola (AminJafari and Ghasemi, 2020; Jahanshahlu and Rezaei, 2020; Khan et al., 2017). Therefore, clinicians prefer mAbs targeting cytokine storm for the management of ARDS associated with COVID-19. In this article, we compiled and reviewed all therapeutic mAbs targeting cytokine storm, which are in clinical trials for its repurposing in management of COVID-19 along with a basic discussion about the SARS-CoV-2 virus, different stages of progression of the disease, pathogenesis of cytokine storm emphasizing the potential targets and the impact of cytokine storm with its mitigation.
2. SARS-CoV-2
The novel SARS-CoV-2 is recently identified and added as a new member along previously known members like MERS-CoV and severe acute respiratory syndrome coronavirus (SARS-CoV) into the family of β-coronavirus, which causes severe pulmonary pneumonia and potentially fatal ARDS (Guo et al., 2020). It contains mainly four structural proteins including small envelope glycoprotein, spike glycoprotein, membrane glycoprotein, and nucleocapsid protein, and several accessory proteins. Glycoprotein protrudes from the surface of the virus, binds with angiotensin converting enzyme-2 (ACE-2), and facilitates the binding and entry of viruses into the host cells (Astuti and Ysrafil, 2020). Endocytosis of the ACE-2 enzyme along with viral particles of SARS-CoV-2 triggered the loss of ACE-2 mediated cardiovascular protection (Wu et al., 2020a).
3. Phases of disease progression in COVID-19
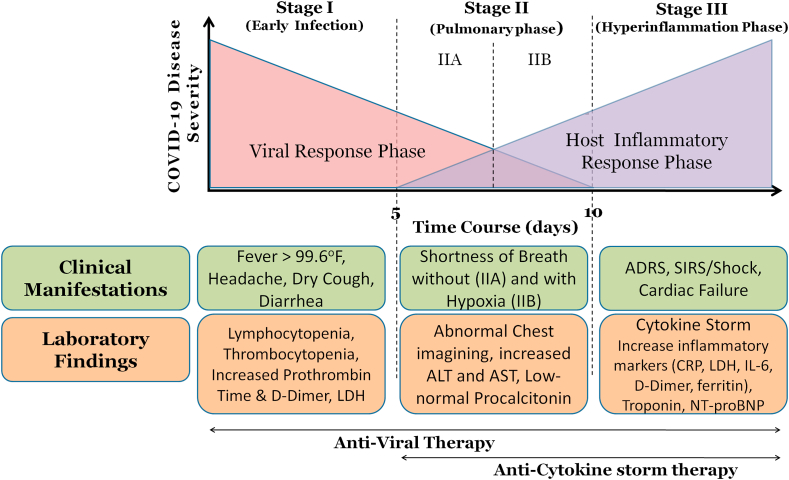
The disease advancement of COVID-19 is categorized into three different phases: the early infection phase, the pulmonary phase, and the hyper inflammation phase (Figure 1).
Figure 1.
Disease advancement Phases of COVID-19.
3.1. Phase I: early infection: viral invasion and replication
The early infection stage occurs at the instance of inoculation and early establishment of disease. After an incubation period, upper respiratory infection is caused due to invasion of SARS-CoV-2 into the mucosal membranes, especially oral-pharyngeal and nasal membranes. Mild symptoms such as dry cough, malaise, and fever are observed. The virus binds to target cells through ACE-2 and induces an activation of the transmembrane serine protease 2 (TMPRSS2) for S protein priming (Hoffmann et al., 2020). ACE-2 is found on the epithelial cells of the intestine, kidney, lung, and blood vessels (Fang et al., 2020). Symptomatic relief is considered a primary treatment target at this stage. Excellent recovery is witnessed in patients who can successfully limit the virus at this initial stage of disease.
3.2. Phase II: pulmonary involvement without (IIa) and with (IIb) hypoxia
The Phase-II of established pulmonary disease is remarkably characterized by multiplication of SARS CoV-2, and localized pulmonary inflammation. During this stage, clinical manifestations observed in the patients include pneumonia, with fever, cough, and hypoxemia defined as the ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2 < 300 mm Hg) (Siddiqi and Mehra, 2020). Chest imaging shows diffuse infiltrates or ground-glass opacities in the bilateral lung fields (Bernheim et al., 2020). Blood tests reveal increased lymphocytopenia. Inflammation markers may be found normal or unremarkably elevated (Hassan et al., 2020). Most hospitalization occurs for close observation and management during this stage. Various supportive measures and the previously mentioned antiviral therapies are suggested as a treatment to this stage.
A strong immune system plays a vital role to eliminate the virus even in the elderly and immunocompromised patient with severe clinical manifestations (Lin et al., 2020; Shi et al., 2020; Siddiqi and Mehra, 2020). The SARS CoV-2 continues to infect the lower respiratory tract and leads to pneumonia with progression of disease. The symptoms like dyspnea and hypoxemia are also getting worse (Hassan et al., 2020; Lin et al., 2020; Russell et al., 2020).
3.3. Phase III: systemic hyperinflammation
Fortunately, the COVID-19 is progressive to its third and the most severe stage in a very few patients, who are accompanied with ARDS and hypercytokinemia syndrome (cytokine storm) as the distinct characteristic of pathogenesis (Mehta et al., 2020; Siddiqi and Mehra, 2020). During this stage, the virus continues to replicate and damage the tissues in the ACE-2 expressing organs especially in the lungs. Helper, regulatory and suppressor T cell counts decrease significantly (Qin et al., 2020; Russell et al., 2020). In the patients with most severe disease, the prognosis can be significantly worsened by the hyper production of Interleukins (ILs) (IL-1, IL-2, IL-4, IL-6, IL-7, IL-10, IL-12, IL-13, IL-17); macrophage colony-stimulating factor (M-CSF), granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony stimulating factor (GM-CSF); interferon-gamma (IFN-γ), fibroblast growth factor; IFN-γ induced protein 10 (IP-10); monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1 alpha (MIP 1-α); tumor necrosis factor (TNF-α), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), C-reactive protein, D-dimer and ferritin (Costela-Ruiz et al., 2020; Huang et al., 2020). High levels of N-terminal pro B-type natriuretic peptide and troponin can also be observed. The remarkable raise in the cytokines level in these severe cases specifies cytokine storm (also termed as cytokine-release syndrome) with subsequent ARDS, which is the primary cause of mortality in critically ill patients with COVID-19 (Fu et al., 2020; Mehta et al., 2020; Shi et al., 2020; Zhang et al., 2020a, Zhang et al., 2020b, Zhang et al., 2020c, Zhang et al., 2020d, Zhang et al., 2020e).
4. Cytokine storm
Cytokine storm is an uncontrolled immune response of the host towards viral infection characterized by sharp increase in proinflammatory cytokines (Ragab et al., 2020). Cytokine storm is seen more commonly in chimeric antigen receptor T-cell (CAR-T) therapy (Norelli et al., 2018) and in immune diseases such as viral infections (Teijaro, 2017) and organ transplantation sepsis (Chousterman et al., 2017). Previous clinical findings postulate that cytokine storm is considered to participate as a crucial player in the deterioration of condition of patients with COVID-19 (Chen et al., 2020a, Chen et al., 2020b; Gao et al., 2020; Sun et al., 2020; Wan et al., 2020; Ye et al., 2020). Condition deteriorated from pneumonia through ARDS, culminating in systemic inflammation and ultimately multi-system organ failure (Ragab et al., 2020). Laboratory and clinical findings seen in SARS-CoV-2 infected patients are similar to that of previously occurring infections with influenza virus, MERS-CoV and SARS-CoV. This suggests that the pathogenesis of cytokine storm in all these infections falls under the same umbrella (Soy et al., 2020).
Cytokine storm is characterized by the abnormal release of circulating cytokines. There are five types of cytokines, which include interferons (IFNs), ILs, tumor necrosis factor, chemokines, and colony stimulating factor. IFNs are involved in the regulation of innate immunity and antiproliferative effects. IFNs are having an immunomodulatory effect and primarily express genes that encode proteins having antiviral properties (Friedman, 2008). ILs are proinflammatory and are involved in the growth and differentiation of leukocytes. Chemokines are proinflammatory and control chemotaxis, leukocyte recruitment. Colony-stimulating factors simulate differentiation and proliferation of hematopoietic progenitor cells and participate in amplification cascade of inflammatory response. Lastly, tumor necrosis factor is considered as the central cytokine in viral infection, which stimulates cytotoxic T cells (Coperchini et al., 2020; Tisoncik et al., 2012).
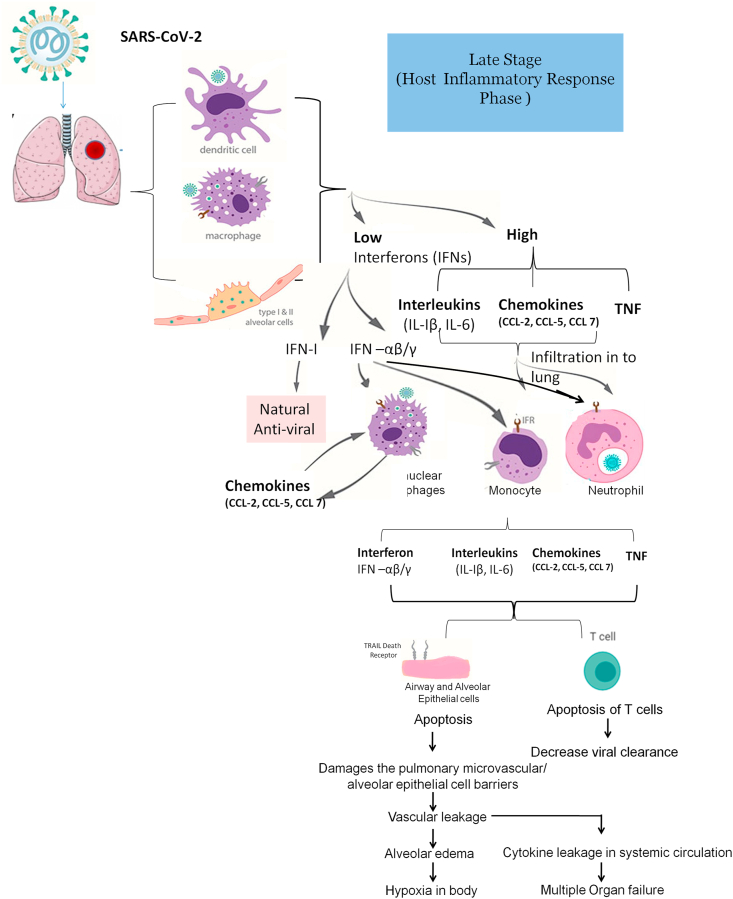
Figure 2 depicts the pathogenesis of cystokine storm. In the initial stage of viral infection, there is the delayed release of IFNs, chemokines, and cytokines occurring in dendritic cells, macrophages, and pulmonary epithelial cells. In the later stage of viral infection, the cells secrete lower amount of the IFNs and higher amount of interleukin (IL), tumor necrosis factor (TNF) and C–C motif chemokine ligand (CCL) (Cheung et al., 2005; Lau et al., 2013; Law et al., 2005). IFNs are antiviral while IL (IL-1 β, IL-6), TNF and CCL (CCL-2, CCL-3, and CCL-5) are proinflammatory cytokines. IFNs (IFN–I, IFN-α/β) are the natural host immune defence response produced by the body against viral infections in the initial stages of viral infection (Channappanavar et al., 2019; García-Sastre and Biron, 2006). However, due to delayed and release of IFNs in lower amounts impedes the body's antiviral response against invading pathogens (Channappanavar et al., 2019). Later the increased release of chemokines attracts and subsequently an excessive infiltration of many inflammatory cells, such as monocytes and neutrophils into the lung tissue and thus results in the injury of the lung (Ye et al., 2020). The delayed release of IFN- α/β activates mononuclear macrophages through their receptors present on their surfaces (Channappanavar et al., 2016). Activated mononuclear macrophages results in release of chemokines (such as CCL2, CCL7, and CCL12), which are monocyte chemoattractant and causes additional accretion of mononuclear macrophages which in turn generate augmented levels of proinflammatory cytokines (IL1- β, IL-6 and TNF). Elevated levels of these proinflammatory cytokines results in the apoptosis of cytotoxic T lymphocytes, which additionally hampers the clearance of viral particles. IFN- α/β and IFN- γ causes the apoptosis of alveolar and airway epithelial cells via acting on TRAIL–death receptor 5 (Herold et al., 2008; Högner et al., 2013) and results in subsequent damage of the pulmonary microvasculature and disruption of alveolar epithelial cell barriers and results in vascular leakage and subsequently alveolar edema, which eventually leading to hypoxemia in the body. Leakage in the systemic circulation further leads to multiple organ failure (Ye et al., 2020).
Figure 2.
Pathogenesis of cytokine Storm with Potential Target.
Data regarding the correlation between COVID-19 and cytokine storm are still limited. However, reports of clinical findings in a patient affected with SARS CoV-2 revealed that pro-inflammatory cytokines such as IL-1β, IL-2, IL-4, IL-6, IL-7, IL-8, IL-9, IL-10, IFN-γ, GM-CSF, TNF-α are significantly elevated (Wu et al., 2020b). Clinical studies show that the levels of IL-6 are significantly elevated in the complicated cases of COVID-19 and highly correlated with clinical outcomes (Coomes and Haghbayan, 2020).
5. Role of therapeutic monoclonal antibodies to reduce cytochrome strom
Several biological agents like convalescent plasma, neutralize antibodies, mAbs, IFNs aiming towards inflammatory cytokines and cytokines receptors have been preferred for the management of ARDS in patients as potential candidates. They alleviate the effects of cytokines released in response to the COVID-19 and reduce lung damage in severely ill patients. Several studies have trailed strategies to dampen inflammatory responses. We would like to focus on the significance of therapeutic mAbs targeting cytokine storm in the management of COVID-19 based on ongoing clinical trials.
6. Methodology
Data were collected from the website of Clinical trials.gov up to 9 September 2020 and analysed for the number of mAbs under clinical trials. The mAbs are classified as per their biological targets and further scrutinized as per the registered phase of clinical trials to identify the most druggable biological target and potential candidate to treat COVID-19 related cytokine storm.
7. Interpretation
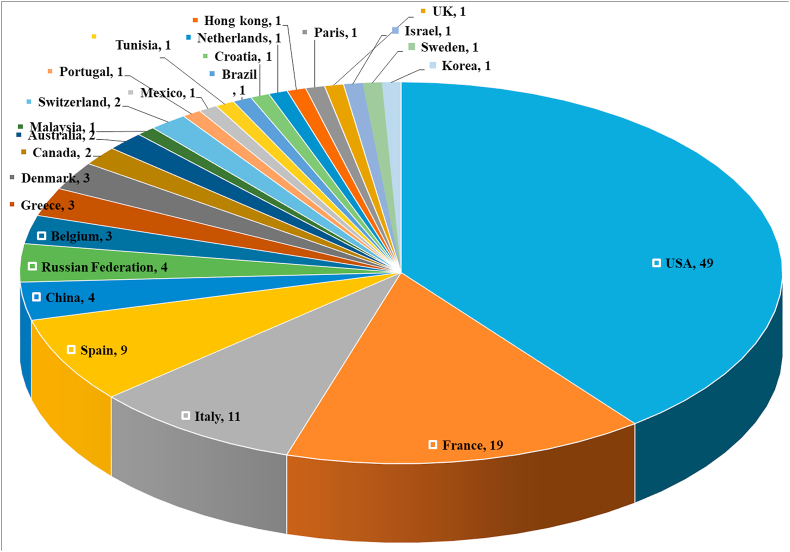
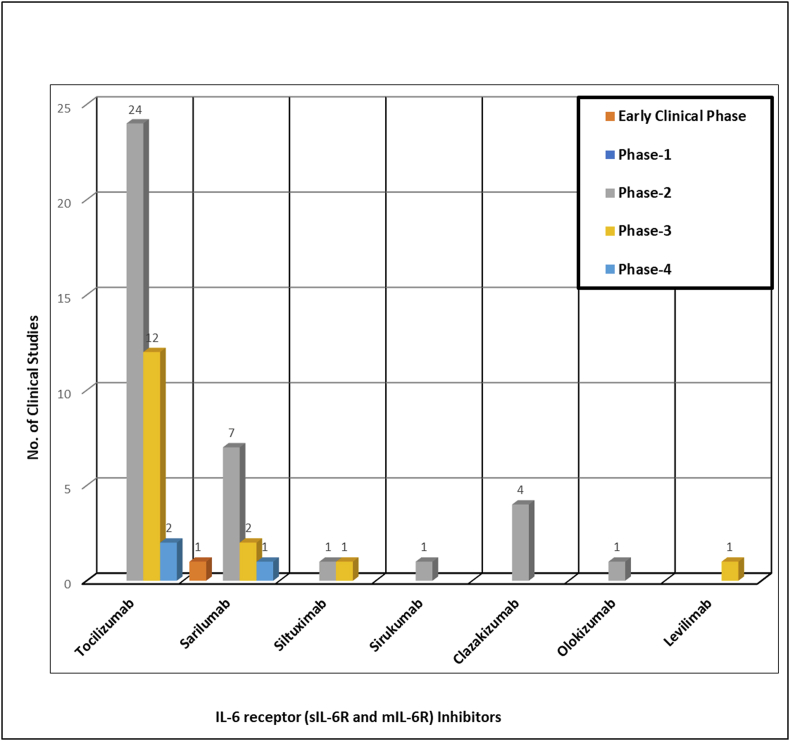
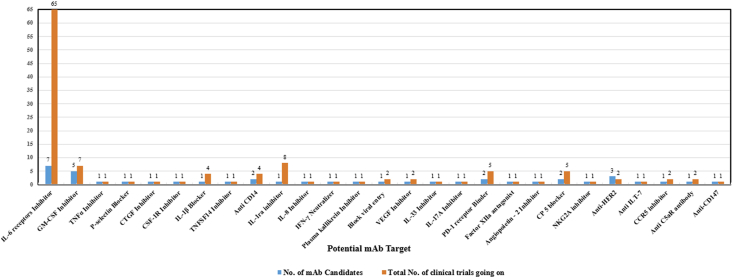
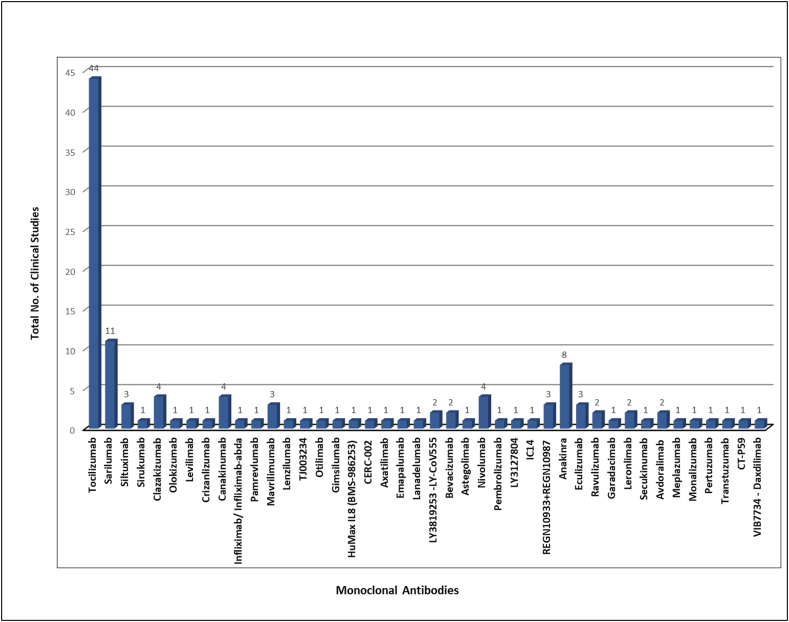
A total of 125 clinical trials are registered for the repurposing of mAbs in the management of ARDS in COVID-19 infected patients spread throughout the world but the majority of trials registered in the USA, France, and Italy (Figure 3). Detailed information regarding the name of mAb, its target, and the status of registered or ongoing trial is summarized in Table 1. It is very evident that though several mAbs are under investigation in clinical trials targeting various cytokines IL-6, IL-1ra, IL-8, IL-1β, IL-17A, IL-33, IFN-γ, TNF-α, P- selectin, connective tissue growth factor (CTGF), plasma kallikrein, tumor necrosis factor superfamily (TNFSF)14, GM-CSF, colony stimulating factor 1 receptor (CSF-1R), C–C chemokine receptor type 5 (CCR5), cluster of differentiation (CD) 14 and 147, VEGF, programmed cell death protein-1 (PD-1), angiopoietin - 2, human factor XIIa, complementary protein 5, natural killer cell receptor G2A (NKG2A), human epidermal growth factor receptor 2 (HER2), immunoglobulin-like transcript 7 (ILT7) receptor, complement component fragment 5a receptor (C5aR) and viral attachment to the human cell (Figure 4 and Table 2). However, the majority of mAb that are under clinical trials are IL-6 inhibitors for healing patients with moderate and severe COVID-19 (Figure 4). There are seven candidates that target IL-6 (tocilizumab, sarilumab, siltuximab, sirukumab, clazakizumab, olokizumab, levilimab). The maximum clinical studies are undergoing on tocilizumab among all the mAbs candidates as well as mAbs targeting IL-6 (Figure 5 and Figure 6). Therefore, tocilizumab is considered to be the most promising candidate for management of cytochrome storm in COVID-19.
Figure 3.
Comparison of ongoing clinical studies of therapeutic monoclonal antibodies for management of Covid-19 in different countries.
Table 1.
Status of ongoing clinical trials for repurposing of Therapeutic Monoclonal Antibodies in management of Covid-19.
| Sr. No | Drug Name | Title of Study | Country | Current Status | Phase of Clinical Study | Link | Registration No | Entered | Last update posted date |
|---|---|---|---|---|---|---|---|---|---|
| IL-6 receptors (sIL-6R and mIL-6R) Inhibitor | |||||||||
| 1 | Toclizumab | A RCT - Safety & Efficacy of Tocilizumab - Tx of Severe COVID-19: ARCHITECTS | USA | Recruiting | Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04412772 | NCT04412772 | 02-06-2020 | 02-06-2020 |
| Tocilizumab for Prevention of Respiratory Failure in Patients With Severe COVID-19 Infection | USA | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04377659 | NCT04377659 | 06-05-2020 | 21-08-2020 | ||
| Efficacy of Tocilizumab on Patients With COVID-19 | USA | Active, not recruiting | Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04356937 | NCT04356937 | 22-04-2020 | 24-08-2020 | ||
| Tocilizumab for Patients With Cancer and COVID-19 Disease | USA | Suspended | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04370834 | NCT04370834 | 01-05-2020 | 13-08-2020 | ||
| The Use of Tocilizumab in the Management of Patients Who Have Severe COVID-19 With Suspected Pulmonary Hyper inflammation | Israel | Recruiting | Phase-4 | https://clinicaltrials.gov/ct2/show/NCT04377750 | NCT04377750 | 06-05-2020 | 06-05-2020 | ||
| A Study in Patients With COVID-19 and Respiratory Distress Not Requiring Mechanical Ventilation, to Compare Standard-of-care With Anakinra and Tocilizumab Treatment The Immunomodulation-CoV Assessment (ImmCoVA) Study | Sweden | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04412291 | NCT04412291 | 02-06-2020 | 26-06-2020 | ||
| Efficacy of Tocilizumab in Modifying the Inflammatory Parameters of Patients With COVID-19 (COVITOZ-01) (COVITOZ-01) | Spain | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04435717 | NCT04435717 | 17-06-2020 | 17-06-2020 | ||
| Serum IL-6 and Soluble IL-6 Receptor in Severe COVID-19 Pneumonia Treated With Tocilizumab (UHID-COVID19) | Croatia | Not yet recruiting | Not mentioned | https://clinicaltrials.gov/ct2/show/NCT04359667 | NCT04359667 | 24-04-2020 | 09-06-2020 | ||
| A Study to Evaluate the Efficacy and Safety of Remdesivir Plus Tocilizumab Compared With Remdesivir Plus Placebo in Hospitalized Participants With Severe COVID-19 Pneumonia (REMDACTA) | USA | Recruiting | Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04409262 | NCT04409262 | 01-06-2020 | 13-08-2020 | ||
| A Study to Investigate Intravenous Tocilizumab in Participants With Moderate to Severe COVID-19 Pneumonia (MARIPOSA) | USA | Active, not recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04363736 | NCT04363736 | 27-04-2020 | 30-06-2020 | ||
| A Study to Evaluate the Efficacy and Safety of Tocilizumab in Hospitalized Participants With COVID-19 Pneumonia | USA | Active, not recruiting | Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04372186 | NCT04372186 | 01-05-2020 | 21-08-2020 | ||
| A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia | USA | Completed | Phase-3 | https://Clinicaltrials.gov/ct2/show/NCT04320615 | NCT04320615 | 25-03-2020 | 31-07-2020 | ||
| Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community- Acquired Pneumonia | Australia | Recruiting | Phase-4 | https://Clinicaltrials.gov/show/NCT02735707 | NCT02735707 | 13-04-2020 | 21-07-2020 | ||
| Tocilizumab vs CRRT in Management of Cytokine Release Syndrome (CRS) in COVID-19 | China | Recruiting | Not mentioned | https://Clinicaltrials.gov/show/NCT04306705 | NCT04306705 | 13-03-2020 | 17-03-2020 | ||
| Favipiravir Combined With Tocilizumab in the Treatment of Corona Virus Disease 2019 | China | Recruiting | Not applicable | https://Clinicaltrials.gov/show/NCT04310228 | NCT04310228 | 17-03-2020 | 10-04-2020 | ||
| Tocilizumab for SARS-CoV2 (COVID-19) Severe Pneumonitis | Italy | Active, not recruiting | Phase-2 | https://Clinicaltrials.gov/show/NCT04315480 | NCT04315480 | 19-03-2020 | 13-04-2020 | ||
| Tocilizumab in COVID-19 Pneumonia (TOCIVID-19) | Italy | Recruiting | Phase-2 | https://Clinicaltrials.gov/show/NCT04317092 | NCT04317092 | 20-03-2020 | 13-07-2020 | ||
| Treatment of COVID-19 Patients With Anti-interleukin Drugs | Belgium | Recruiting | Phase-3 | https://Clinicaltrials.gov/show/NCT04330638 | NCT04330638 | 01-04-2020 | 09-07-2020 | ||
| Tocilizumab to Prevent Clinical Decompensation in Hospitalized, Non-critically Ill Patients With COVID-19 Pneumonitis | USA | Completed | Phase-2 | https://Clinicaltrials.gov/show/NCT04331795 | NCT04331795 | 02-04-2020 | 03-08-2020 | ||
| CORIMUNO-19 - Tocilizumab Trial - TOCI (CORIMUNO-TOCI) | France | Active, not recruiting | Phase-2 | https://Clinicaltrials.gov/show/NCT04331808 | NCT04331808 | 02-04-2020 | 28-04-2020 | ||
| Clinical Trial of Combined Use of Hydroxychloroquine, Azithromycin, and Tocilizumab for the Treatment of COVID-19 | Spain | Recruiting | Phase-2 | https://Clinicaltrials.gov/show/NCT04332094 | NCT04332094 | 02-04-2020 | 07-04-2020 | ||
| Efficacy and Safety of Tocilizumab in the Treatment of SARS-CoV-2 Related Pneumonia | Italy | Recruiting | Not mentioned | https://Clinicaltrials.gov/show/NCT04332913 | NCT04332913 | 03-04-2020 | 13-04-2020 | ||
| Prospective Study in Patients With Advanced or Metastatic Cancer and SARS-CoV-2 Infection | France | Suspended | Phase-2 | https://Clinicaltrials.gov/show/NCT04333914 | NCT04333914 | 03-04-2020 | 16-06-2020 | ||
| Checkpoint Blockade in COVID-19 Pandemic | Spain | Recruiting | Phase-2 | https://Clinicaltrials.gov/show/NCT04335305 | NCT04335305 | 06-04-2020 | 09-06-2020 | ||
| Personalised Immunotherapy for SARS-CoV-2 (COVID-19) Associated With Organ Dysfunction | Greece | Recruiting | Phase-2 | https://Clinicaltrials.gov/show/NCT04339712 | NCT04339712 | 09-04-2020 | 22-04-2020 | ||
| Study to Evaluate the Efficacy and Safety of Tocilizumab Versus Corticosteroids in Hospitalised COVID-19 Patients With High Risk of Progression | Malaysia | Not yet recruiting | Phase-3 | https://Clinicaltrials.gov/show/NCT04345445 | NCT04345445 | 14-04-2020 | 14-04-2020 | ||
| Efficacy of Early Administration of Tocilizumab in COVID-19 Patients | Italy | Terminated | Phase-2 | https://Clinicaltrials.gov/show/NCT04346355 | NCT04346355 | 15-04-2020 | 22-06-2020 | ||
| Anti-il6 Treatment of Serious COVID-19 Disease With Threatening Respiratory Failure | Denmark | Recruiting | Phase-2 | https://ClinicalTrials.gov/show/NCT04322773 | NCT04322773 | 26-03-2020 | 07-04-2020 | ||
| Clinical Trial to Evaluate the Effectiveness and Safety of Tocilizumab for Treating Patients With COVID-19 Pneumonia | Spain | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04445272 | NCT04445272 | 24-06-2020 | 24-06-2020 | ||
| Tocilizumab to Prevent Clinical Decompensation in Hospitalized, Non-critically Ill Patients With COVID-19 Pneumonitis (COVIDOSE) | USA | Completed | Phase-2 | https://Clinicaltrials.gov/show/NCT04331795 | NCT04331795 | 02-04-2020 | 03-08-2020 | ||
| The Fleming [FMTVDM] Directed CoVid-19 Treatment Protocol | USA | Enrolling by invitation | Phase-2, Phase-3 | https://Clinicaltrials.gov/show/NCT04349410 | NCT04349410 | 16-04-2020 | 09-07-2020 | ||
| Tocilizumab in the Treatment of Coronavirus Induced Disease (COVID-19) (CORON-ACT) | Switzerland | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04335071 | NCT04335071 | 06-04-2020 | 28-04-2020 | ||
| Tocilizumab Versus Methylprednisolone in the Cytokine Release Syndrome of Patients With COVID-19 | Portugal | Not yet recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04377503 | NCT04377503 | 06-05-2020 | 06-05-2020 | ||
| Tocilizumab Treatment in Patients With COVID-19 | Mexico | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04363853 | NCT04363853 | 27-04-2020 | 17-06-2020 | ||
| Assessment of Efficacy and Safety of Tocilizumab Compared to DefeROxamine, Associated With Standards Treatments in COVID-19 (+) Patients Hospitalized In Intensive Care in Tunisia (TRONCHER) | Tunisia | Not yet recruiting | Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04361032 | NCT04361032 | 24-04-2020 | 27-08-2020 | ||
| Prospective Study in Patients With Advanced or Metastatic Cancer and SARS-CoV-2 Infection (IMMUNONCOVID) | France | suspended | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04333914 | NCT04333914 | 03-04-2020 | 16-06-2020 | ||
| Ultra Low Doses of Therapy With Radiation Applicated to COVID-19 (ULTRA-COVID) | Spain | Recruiting | Not applicable | https://clinicaltrials.gov/ct2/show/NCT04394182 | NCT04394182 | 19-05-2020 | 19-05-2020 | ||
| Safety and Efficacy of Tocilizumab in Moderate to Severe COVID-19 With Inflammatory Markers (TOCIBRAS) | Brazil | Terminated | Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04403685 | NCT04403685 | 27-05-2020 | 26-08-2020 | ||
| Tocilizumab for SARS-CoV-2 (COVID-19) Severe Pneumonitis | Italy | Active, not recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04315480 | NCT04315480 | 19-03-2020 | 13-04-2020 | ||
| Tocilizumab for the Treatment of Cytokine Release Syndrome in Patients With COVID-19 (SARS-CoV-2 Infection) | USA | Withdrawn | Phase - 3 | https://clinicaltrials.gov/ct2/show/NCT04361552 | NCT04361552 | 24-04-2020 | 18-06-2020 | ||
| Tocilizumab in Coronavirus-19 Positive Patients | Canada | Not yet recruiting | Phase - 3 | https://clinicaltrials.gov/ct2/show/NCT04423042 | NCT04423042 | 09-06-2020 | 22-07-2020 | ||
| Comparison of Tocilizumab Plus Dexamethasone vs. Dexamethasone for Patients With Covid-19 (TOCIDEX) | France | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04476979 | NCT04476979 | 20-07-2020 | 18-08-2020 | ||
| A Trial Using ANAKINRA, TOCILIZUMAB Alone or in Association With RUXOLITINIB in Severe Stage 2b and 3 of COVID19-associated Disease (INFLAMMACOV) | France | Not yet recruiting | Phase - 3 | https://clinicaltrials.gov/ct2/show/NCT04424056 | NCT04424056 | 09-06-2020 | 23-06-2020 | ||
| Toclizumam Versus Dexamethasone in Severe Covid-19 Cases | Eygpt | Completed | Not applicable | https://clinicaltrials.gov/ct2/show/NCT04519385 | NCT04519385 | 19-08-2020 | 25-08-2020 | ||
| 2 | Sarilumab | Sarilumab for Patients With Moderate COVID-19 Disease | USA | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04359901 | NCT04359901 | 24-04-2020 | 30-07-2020 |
| Evaluation of the Efficacy and Safety of Sarilumab in Hospitalized Patients With COVID-19 | USA | Active, not recruiting | Phase-2, Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04315298 | NCT04315298 | 19-03-2020 | 01-09-2020 | ||
| Cohort Multiple Randomized Controlled Trials Open-label of Immune Modulatory Drugs and Other Treatments in COVID-19 Patients -Sarilumab Trial - CORIMUNO-19 - SARI (CORIMUNO-SARI) | France | Active, not recruiting | Phase-2, Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04324073 | NCT04324073 | 27-03-2020 | 15-04-2020 | ||
| Sarilumab COVID-19 | Canada | Active, not recruiting | Phase - 3 | https://clinicaltrials.gov/ct2/show/NCT04327388 | NCT04327388 | 31-03-2020 | 17-08-2020 | ||
| Efficacy of Subcutaneous Sarilumab in Hospitalised Patients With Moderate-severe COVID-19 Infection (SARCOVID) (SARCOVID) | Spain | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04357808 | NCT04357808 | 22-04-2020 | 04-08-2020 | ||
| Study of Immune Modulatory Drugs and Other Treatments in COVID-19 Patients: Sarilumab, Azithromycin, Hydroxychloroquine Trial - CORIMUNO-19 - VIRO (CORIMUNO-VIRO) | France | suspended | Phase-2, Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04341870 | NCT04341870 | 10-04-2020 | 06-05-2020 | ||
| Anti-il6 Treatment of Serious COVID-19 Disease With Threatening Respiratory Failure | Denmark | Recruiting | Phase-2 | https://Clinicaltrials.gov/show/NCT04322773 | NCT04322773 | 26-03-2020 | 07-04-2020 | ||
| Study on the Use of Sarilumab in Patients With COVID-19 Infection | Italy | Not yet recruiting | Early Phase-1 | https://clinicaltrials.gov/ct2/show/NCT04386239 | NCT04386239 | 13-05-2020 | 13-05-2020 | ||
| Clinical Trial of Sarilumab in Adults With COVID-19 (SARICOR) | Spain | Not yet recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04357860 | NCT04357860 | 22-04-2020 | 27-04-2020 | ||
| Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community- Acquired Pneumonia (REMAP-CAP) | Australia | Recruiting | Phase-4 | https://clinicaltrials.gov/ct2/show/NCT02735707 | NCT02735707 | 13-04-2020 | 21-07-2020 | ||
| Efficacy and Safety of Novel Treatment Options for Adults With COVID-19 Pneumonia (CCAP) | Denmark | Recruiting | Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04345289 | NCT04345289 | 14-04-2020 | 31-07-2020 | ||
| 3 | Sirkumab | A Study to Evaluate the Efficacy and Safety of Sirukumab in Confirmed Severe or Critical Confirmed Coronavirus Disease (COVID)-19 | Belgium | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04380961 | NCT04380961 | 08-05-2020 | 02-09-2020 |
| 4 | Clazakizumab | Study for the Use of the IL-6 Inhibitor Clazakizumab in Patients With Life-threatening COVID-19 Infection | USA | Not yet recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04381052 | NCT04381052 | 08-05-2020 | 08-05-2020 |
| A Randomized Placebo-controlled Safety and Dose-finding Study for the Use of the IL-6 Inhibitor Clazakizumab in Patients With Life-threatening COVID-19 Infection | USA | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04343989 | NCT04343989 | 14-04-2020 | 06-05-2020 | ||
| Clazakizumab (Anti-IL- 6 Monoclonal) Compared to Placebo for COVID19 Disease | USA | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04348500 | NCT04348500 | 16-04-2020 | 05-06-2020 | ||
| Use of the Interleukin-6 Inhibitor Clazakizumab in Patients With Life-threatening COVID-19 Infection | USA | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04363502 | NCT04363502 | 27-04-2020 | 19-08-2020 | ||
| 5 | Olokizumab | Study of the Efficacy and Safety of a Single Administration of Olokizumab and RPH-104 With Standard Therapy in Patients With Severe Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection (COVID-19) | Russian Federation | Recruiting | Phase-2, Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04380519 | NCT04380519 | 08-05-2020 | 08-05-2020 |
| 6 | Siltuximab | Efficacy and Safety of Siltuximab vs. Corticosteroids in Hospitalized Patients With COVID-19 Pneumonia | Spain | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04329650 | NCT04329650 | 01-04-2020 | 17-04-2020 |
| An Observational Case-control Study of the Use of Siltuximab in ARDS Patients Diagnosed With COVID-19 Infection (SISCO) | Italy | Completed | Not mentioned | https://clinicaltrials.gov/ct2/show/NCT04322188 | NCT04322188 | 26-03-2020 | 01-06-2020 | ||
| Treatment of COVID-19 Patients With Anti-interleukin Drugs | Belgium | Recruiting | Phase-3 | https://Clinicaltrials.gov/show/NCT04330638 | NCT04330638 | 01-04-2020 | 09-07-2020 | ||
| 7 | Levilimab | A Clinical Trial of the Efficacy and Safety of Levilimab (BCD-089) in Patients With Severe COVID-19 (CORONA) | Russian Federation | Active, not recruiting | Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04397562 | NCT04397562 | 21-05-2020 | 11-06-2020 |
|
GM-CSF Inhibitor | |||||||||
| 8 | Lenzilumab | Phase 3 Study to Evaluate Efficacy and Safety of Lenzilumab in Hospitalized Patients With COVID-19 Pneumonia | USA | Recruiting | Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04351152 | NCT04351152 | 17-04-2020 | 05-08-2020 |
| 9 | Mavrilimumab | Mavrilimumab to Reduce Progression of Acute Respiratory Failure in COVID-19 Pneumonia and Systemic Hyper-inflammation | USA | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04399980 | NCT04399980 | 22-05-2020 | 14-07-2020 |
| Mavrilimumab in Severe COVID-19 Pneumonia and Hyper-inflammation (COMBAT-19) (COMBAT-19) | Italy | Not yet recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04397497 | NCT04397497 | 21-05-2020 | 26-05-2020 | ||
| Study of Mavrilimumab (KPL-301) in Participants Hospitalized With Severe Corona Virus Disease 2019 (COVID-19) Pneumonia and Hyper-inflammation | USA | Recruiting | Phase-2, Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04447469 | NCT04447469 | 25-06-2020 | 17-08-2020 | ||
| 10 | TJ003234 | Study of TJ003234 (Anti-GM-CSF Monoclonal Antibody) in Subjects With Severe Coronavirus Disease 2019 (COVID-19) | USA | Recruiting | Phase-1, Phase-2 |
https://clinicaltrials.gov/ct2/show/NCT04341116 | NCT04341116 | 10-04-2020 | 14-07-2020 |
| 11 | Gimsilumab | A Study to Assess the Efficacy and Safety of Gimsilumab in Subjects With Lung Injury or Acute Respiratory Distress Syndrome Secondary to COVID-19 (BREATHE). | USA | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04351243 | NCT04351243 | 17-04-2020 | 04-09-2020 |
| 12 | Otilimab | Investigating Otilimab in Patients With Severe Pulmonary COVID-19 Related Disease (OSCAR) | USA | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04376684 | NCT04376684 | 06-05-2020 | 02-09-2020 |
|
TNFα Inhibitor | |||||||||
| 13 | Infliximab/Infliximab-abda | A Phase 2 Trial of Infliximab in Coronavirus Disease 2019 (COVID-19). | USA | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04425538 | NCT04425538 | 11-06-2020 | 11-06-2020 |
|
P-selectin Blocker | |||||||||
| 14 | Crizanlizumab | Crizanlizumab for Treating COVID-19 Vasculopathy | USA | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04435184 | NCT04435184 | 17-06-2020 | 13-07-2020 |
|
CTGF Inhibitor | |||||||||
| 15 | Pamrevlumab | Study of the Efficacy and Safety of Intravenous Pamrevlumab, in Hospitalized Patients With Acute COVID-19 Disease | USA | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04432298 | NCT04432298 | 16-06-2020 | 24-06-2020 |
|
CSF-1R Inhibitor | |||||||||
| 16 | Axatilimab | A Phase 2 Study to Evaluate Axatilimab for Hospitalized Patients With Respiratory Involvement Secondary to COVID-19 | USA | Suspended | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04415073 | NCT04415073 | 04-06-2020 | 11-08-2020 |
|
IL-1β Blocker | |||||||||
| 17 | Canakinumab | Observational Study, Use of Canakinumab Administered Subcutaneously in the Treatment COVID-19 Pneumonia | Italy | Not yet Recruiting | Not mentioned | https://clinicaltrials.gov/ct2/show/NCT04348448 | NCT04348448 | 16-04-2020 | 16-04-2020 |
| Study of Efficacy and Safety of Canakinumab Treatment for CRS in Participants With COVID-19-induced Pneumonia (CAN-COVID) | USA | Recruiting | Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04362813 | NCT04362813 | 27-04-2020 | 24-08-2020 | ||
| Canakinumab in Covid-19 Cardiac Injury (The Three C Study) | USA | Active, not recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04365153 | NCT04365153 | 28-04-2020 | 03-09-2020 | ||
| Canakinumab in Patients With COVID-19 and Type 2 Diabetes (CanCovDia) | Switzerland | Not yet Recruiting | Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04510493 | NCT04510493 | 12-08-2020 | 19-08-2020 | ||
|
TNFSF14 Inhibitor | |||||||||
| 18 | CERC-002 - An anti-LIGHT fully human monoclonal antibody | Clinical Trial to Evaluate CERC-002 in Adults With COVID-19 Pneumonia and Acute Lung Injury | USA | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04412057 | NCT04412057 | 02-06-2020 | 01-09-2020 |
|
Anti CD14 | |||||||||
| 19 | REGN10933 + REGN10987 combination therapy | Safety, Tolerability, and Efficacy of Anti-Spike (S) SARS-CoV-2 Monoclonal Antibodies for Hospitalized Adult Patients With COVID-19 | USA | Recruiting | Phase-1, Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04426695 | NCT04426695 | 11-06-2020 | 04-09-2020 |
| Study Assessing the Efficacy and Safety of Anti-Spike SARS CoV-2 Monoclonal Antibodies for Prevention of SARS CoV-2 Infection Asymptomatic in Healthy Adults Who Are Household Contacts to an Individual With a Positive SARS-CoV-2 RT-PCR Assay | USA | Recruiting | Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04452318 | NCT04452318 | 30-06-2020 | 01-09-2020 | ||
| Study Assessing the Safety, Tolerability, Pharmacokinetics, and Immunogenicity of Repeated Subcutaneous Doses of Anti-Spike (S) SARS-CoV-2 Monoclonal Antibodies (REGN10933 + REGN10987) in Adult Volunteers as Related to COVID-19 | USA | Recruiting | Phase-1 | https://clinicaltrials.gov/ct2/show/NCT04519437 | NCT04519437 | 19-08-2020 | 19-08-2020 | ||
| 20 | IC14 - An antibody to the CD14 pattern-recognition receptor | IC14 (Anti-CD14) Treatment in Patients With SARS-CoV-2 (COVID-19) | USA | Not yet recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04391309 | NCT04391309 | 18-05-2020 | 24-06-2020 |
|
IL-1ra inhibitor | |||||||||
| 21 | Anakinra | Efficacy of Intravenous Anakinra and Ruxolitinib During COVID-19 Inflammation (JAKINCOV) | France | Not yet recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04366232 | NCT04366232 | 28-04-2020 | 09-06-2020 |
| Early Identification and Treatment of Cytokine Storm Syndrome in Covid-19 | USA | Not yet recruiting | Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04362111 | NCT04362111 | 24-04-2020 | 24-04-2020 | ||
| A Trial Using ANAKINRA, TOCILIZUMAB Alone or in Association With RUXOLITINIB in Severe Stage 2b and 3 of COVID19-associated Disease (INFLAMMACOV) | France | Not yet recruiting | Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04424056 | NCT04424056 | 09-06-2020 | 23-06-2020 | ||
| Anakinra for COVID-19 Respiratory Symptoms (ANACONDA) | France | Recruiting | Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04364009 | NCT04364009 | 27-04-2020 | 14-05-2020 | ||
| Personalised Immunotherapy for SARS-CoV-2 (COVID-19) Associated With Organ Dysfunction (ESCAPE) | Greece | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04339712 | NCT04339712 | 09-04-2020 | 22-04-2020 | ||
| suPAR-guided Anakinra Treatment for Validation of the Risk and Management of Respiratory Failure by COVID-19 (SAVE) (SAVE) | Greece | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04357366 | NCT04357366 | 22-04-2020 | 27-04-2020 | ||
| CORIMUNO-ANA: Trial Evaluating Efficacy Of Anakinra In Patients With Covid-19 Infection (CORIMUNO-ANA) | Paris | Not yet recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04341584 | NCT04341584 | 10-04-2020 | 14-04-2020 | ||
| Efficacy and Safety of Emapalumab and Anakinra in Reducing Hyperinflammation and Respiratory Distress in Patients With COVID-19 Infection. | Italy | Recruiting | Phase-2, Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04324021 | NCT04324021 | 27-03-2020 | 12-08-2020 | ||
|
IL-8 Inhibitor | |||||||||
| 22 | HuMax IL8 (BMS-986253) | Anti-Interleukin-8 (Anti-IL-8) for Patients With COVID-19 | USA | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04347226 | NCT04347226 | 15-04-2020 | 01-05-2020 |
|
IFN-γ Neutralizer | |||||||||
| 23 | Emapalumab - | Efficacy and Safety of Emapalumab and Anakinra in Reducing Hyperinflammation and Respiratory Distress in Patients With COVID-19 Infection. | Italy | Recruiting | Phase-2, Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04324021 | NCT04324021 | 27-03-2020 | 12-08-2020 |
|
Plasma kallikrein Inhibitor | |||||||||
| 24 | Lanadelumab | Lanadelumab for Treatment of COVID-19 Disease | Netherlands | Not yet recruiting | Phase-1, Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04422509 |
NCT04422509 |
09-06-2020 |
24-07-2020 |
|
Block viral attachment and entry into human cells | |||||||||
| 25 | LY3819253 -LY-CoV555 is a potent, neutralizing IgG1 monoclonal antibody (mAb) | A Study of LY3819253 (LY-CoV555) in Participants With Early Mild to Moderate COVID-19 Illness (BLAZE-1) | USA | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04427501 | NCT04427501 | 11-06-2020 | 25-08-2020 |
| A Study of LY3819253 (LY-CoV555) in Participants Hospitalized for COVID-19 |
USA |
Active, not recruiting |
Phase-1 |
https://clinicaltrials.gov/ct2/show/NCT04411628 |
NCT04411628 |
02-06-2020 |
05-08-2020 |
||
|
Angiopoietin - 2 Inhibitor | |||||||||
| 26 | LY3127804 | A Study of LY3127804 in Participants With COVID-19 | USA | Active, not recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04342897 | NCT04342897 | 13-04-2020 | 20-08-2020 |
|
VEGF Inhibitor | |||||||||
| 27 | Bevacizumab | Bevacizumab in Severe or Critically Severe Patients With COVID-19 Pneumonia-RCT (BEST-RCT) | China | Recruiting | Not applicable | https://clinicaltrials.gov/ct2/show/NCT04305106 | NCT04305106 | 12-03-2020 | 26-03-2020 |
| Trial Evaluating Efficacy and Safety of Bevacizumab (Avastin®/Zeribev®) in Patients With COVID-19 Infection, Nested in the Corimmuno-19 Cohort (CORIMMUNO-BEVA) | France | Not yet recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04344782 | NCT04344782 | 14-04-2017 | 17-04-2017 | ||
|
IL-33 Inhibitor | |||||||||
| 28 | Astegolimab | A Study to Evaluate the Safety and Efficacy of MSTT1041A (Astegolimab) or UTTR1147A in Patients With Severe COVID-19 Pneumonia (COVASTIL) | USA | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04386616 | NCT04386616 | 13-05-2020 | 02-09-2020 |
|
IL-17A Inhibitor | |||||||||
| 29 | Secukinumab | COLchicine Versus Ruxolitinib and Secukinumab In Open Prospective Randomized Trial (COLORIT) | Russia | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04403243 | NCT04403243 | 27-05-2020 | 27-05-2020 |
|
PD-1 receptor Binder | |||||||||
| 30 | Nivolumab | Efficiency and Security of NIVOLUMAB Therapy in Obese Individuals With COVID-19 (COrona VIrus Disease) Infection (NIVISCO) | France | Not yet recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04413838 | NCT04413838 | 04-06-2020 | 04-06-2020 |
| Trial Evaluating Efficacy and Safety of Nivolumab (Optivo®) in Patients With COVID-19 Infection, Nested in the Corimmuno-19 Cohort.(CORIMUNO-NIVO) | France | Not yet recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04343144 | NCT04343144 | 13-04-2020 | 14-04-2020 | ||
| COVID-19: A Pilot Study of Adaptive Immunity and Anti-PD1 | Hong Kong | Not yet recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04356508 | NCT04356508 | 22-04-2020 | 22-04-2020 | ||
| Prospective Study in Patients With Advanced or Metastatic Cancer and SARS-CoV-2 Infection (IMMUNONCOVID) | France | suspended | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04333914 | NCT04333914 | 03-04-2020 | 16-06-2020 | ||
| 31 | Pembrolizumab | Checkpoint Blockade in COVID-19 Pandemic | Spain | Recruiting | Phase-2 | https://ClinicalTrials.gov/show/NCT04335305 | NCT04335305 | 06-04-2020 | 09-06-2020 |
|
Human Factor XIIa antagonist | |||||||||
| 32 | Garadacimab | Treatment With CSL312 in Adults With Coronavirus Disease 2019 (COVID-19) | USA | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04409509 | NCT04409509 | 01-06-2020 | 21-08-2020 |
|
CCR5 inhibitor | |||||||||
| 33 | Leronlimab | Study to Evaluate the Efficacy and Safety of Leronlimab for Mild to Moderate COVID-19 | USA | Active, not recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04343651 | NCT04343651 | 13-04-2020 | 20-08-2020 |
| Study to Evaluate the Efficacy and Safety of Leronlimab for Patients With Severe or Critical Coronavirus Disease 2019 (COVID-19) | USA | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04347239 | NCT04347239 | 15-04-2020 | 31-08-2020 | ||
|
Anti C5aR antibody | |||||||||
| 34 | Avdoralimab | Avdoralimab an Anti-C5aR Antibody, in Patients With COVID-19 Severe Pneumonia (FORCE) | France | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04371367 | NCT04371367 | 01-05-2020 | 01-05-2020 |
| Prospective Study in Patients With Advanced or Metastatic Cancer and SARS-CoV-2 Infection | France | Suspended | Phase-2 | https://ClinicalTrials.gov/show/NCT04333914 | NCT04333914 | 03-04-2020 | 16-06-2020 | ||
|
Anti-CD147 | |||||||||
| 35 | Meplazumab | Clinical Study of Anti-CD147 Humanized Meplazumab for Injection to Treat With 2019-nCoV Pneumonia | China | Recruiting | Phase-1, Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04275245 | NCT04275245 | 19-02-2020 | 19-02-2020 |
|
Complementary protein 5 blocker | |||||||||
| 36 | Ravulizumab | Efficacy and Safety Study of IV Ravulizumab in Patients With COVID-19 Severe Pneumonia | USA | Recruiting | Phase-3 | https://clinicaltrials.gov/ct2/show/NCT04369469 | NCT04369469 | 30-04-2020 | 11-08-2020 |
| mulTi-Arm Therapeutic Study in Pre-ICu Patients Admitted With Covid-19 - Repurposed Drugs (TACTIC-R) (TACTIC-R) | UK | Recruiting | Phase-4 | https://clinicaltrials.gov/ct2/show/NCT04390464 | NCT04390464 | 15-05-2020 | 18-05-2020 | ||
| 37 | Eculizumab | CORIMUNO19-ECU: Trial Evaluating Efficacy and Safety of Eculizumab (Soliris) in Patients With COVID-19 Infection, Nested in the CORIMUNO-19 Cohort (CORIMUNO19-ECU) | France | Recruiting | Phase-2 | https://clinicaltrials.gov/ct2/show/NCT04346797 | NCT04346797 | 15-04-2020 | 27-04-2020 |
| SOLIRIS® (Eculizumab) Treatment of Participants With COVID-19 | France | Available | Not mentioned | https://clinicaltrials.gov/ct2/show/NCT04355494 | NCT04355494 | 21-04-2020 | 18-08-2020 | ||
| Eculizumab (Soliris) in Covid-19 Infected Patients (SOLID-C19) | USA | Available | Not mentioned | https://clinicaltrials.gov/ct2/show/NCT04288713 | NCT04288713 | 28-02-2020 | 30-03-2020 | ||
|
NKG2A inhibitor | |||||||||
| 38 | Monalizumab | Prospective Study in Patients With Advanced or Metastatic Cancer and SARS-CoV-2 Infection | France | Suspended | Phase-2 | https://ClinicalTrials.gov/ct2/show/NCT04333914 | NCT04333914 | 03-04-2020 | 16-06-2020 |
|
Anti-HER2 | |||||||||
| 39 | Pertuzumab | An Expanded Access Study to Provide at Home Subcutaneous Administration of Pertuzumab and Trastuzumab Fixed-Dose Combination (PH FDC SC) for Patients With HER2-Positive Breast Cancer During the COVID-19 Pandemic | USA | Available | Not mentioned | https://clinicaltrials.gov/ct2/show/NCT04395508 | NCT04395508 | 20-05-2020 | 02-09-2020 |
| 40 |
Trastuzumab |
An Expanded Access Study to Provide at Home Subcutaneous Administration of Pertuzumab and Trastuzumab Fixed-Dose Combination (PH FDC SC) for Patients With HER2-Positive Breast Cancer During the COVID-19 Pandemic |
USA |
Available |
Not mentioned |
https://clinicaltrials.gov/ct2/show/NCT04395508 |
NCT04395508 |
20-05-2020 |
07-07-2020 |
|
Anti ILT-7 | |||||||||
| 41 | VIB7734 - Daxdilimab | Treatment and Prevention of Acute Lung Injury (ALI) in Patients With COVID-19 Infection (ALI) | USA | Recruiting | Phase-1 | https://clinicaltrials.gov/ct2/show/NCT04526912 | NCT04526912 | 26-08-2020 | 02-09-2020 |
|
Other | |||||||||
| 42 | CT-P59 | To Evaluate the Safety, Tolerability and Pharmacokinetics of CT-P59 in Healthy Subjects | Korea | Recruiting | Phase-1 | https://clinicaltrials.gov/ct2/show/NCT04525079 | NCT04525079 | 25-08-2020 | 25-08-2020 |
IL, Interleukin. ILT, Immunoglobulin-like transcript. TNFα, Tumor Necrosis Factor alpha. CTGF, Connective Tissue Growth Factor. GM-CSF, Granulocyte Macrophage Colony Stimulating Factor. TNFSF, Tumor Necrosis Factor Superfamily. CSF-1R, Colony Stimulating Factor 1 Receptor. IFN- Interferon, VEGF, Vascular Endothelial growth Factor. PD, Programmed Cell Death Protein. CD, Cluster of Differentiation. CCR, Chemokine Receptor. NKG2A, Natural Killer Group 2 Member ACell Receptor. HER, Human Epidermal Growth Factor Receptor. C5aR, Complementary component fragment 5a receptor.
Figure 6.
Phase wise representation of ongoing clinical trials of various mAbs candidates targeting IL-6 inhibition.
Table 2.
Registered phase wise clinical trials for different biological targets of Therapeutic Monoclonal Antibodies in management of COVID-19 associated Cytokine Storm.
| Biological Target | Name of mAbs | Total no. of Clinical Trials going on | Early Phase-1 | Phase-1 | Phase-2 | Phase-3 | Phase-4 | Phase not mentioned |
|---|---|---|---|---|---|---|---|---|
| IL-6 receptors Inhibitor | 65 | 1 | - | 38 | 16 | 3 | 7 | |
| Tocilizumab | 44 | - | 24 | 12 | 2 | _ | 6 | |
| Sarilumab | 11 | - | 7 | 2 | 1 | 1 | _ | |
| Sirukumab | 3 | - | 1 | 1 | - | - | 1 | |
| Clazakizumab | 1 | - | 1 | - | - | - | - | |
| Olokizumab | 4 | - | 4 | - | - | - | - | |
| Siltuximab | 1 | - | 1 | - | - | - | - | |
| Levilimab | 1 | - | - | 1 | - | - | - | |
| GM-CSF Inhibitor | 7 | - | 1 | 5 | 1 | - | - | |
| Lenzilumab | 1 | - | - | - | 1 | - | - | |
| Mavrilimumab | 3 | - | - | 3 | - | - | - | |
| TJ003234 | 1 | - | 1 | - | - | - | - | |
| Gimsilumab | 1 | - | - | 1 | - | - | - | |
| Otilimab | 1 | - | - | 1 | - | - | - | |
| TNFα Inhibitor | Infliximab/Infliximab-abda | 1 | - | - | 1 | - | - | - |
| P-selectin Blocker | Crizanlizumab | 1 | - | - | 1 | - | - | - |
| CTGF Inhibitor | Pamrevlumab | 1 | - | - | 1 | - | - | - |
| CSF-1R Inhibitor | Axatilimab | 1 | - | - | 1 | - | - | - |
| IL-1β Blocker | Canakinumab | 4 | - | - | 1 | 2 | - | 1 |
| TNFSF14 Inhibitor | CERC-002 - An anti-LIGHT fully human monoclonal antibody | 1 | - | - | 1 | - | - | - |
| Anti CD14 | 4 | - | 2 | 1 | 1 | - | - | |
| REGN10933 + REGN10987 combination therapy | 3 | - | 2 | - | 1 | - | - | |
| Atibuclimab (IC-14) | 1 | - | - | 1 | - | - | - | |
| IL-1ra Inhibitor | Anakinra | 8 | - | - | 5 | 3 | - | - |
| IL-8 Inhibitor | HuMax IL8 (BMS-986253) | 1 | - | - | 1 | - | - | - |
| IFN-γ Neutralizer | Emapalumab | 1 | - | - | 1 | - | - | - |
| Plasma kallikrein Inhibitor | Lanadelumab | 1 | - | 1 | - | - | - | - |
| Block viral entry | LY3819253 | 2 | - | 1 | 1 | - | - | - |
| VEGF Inhibitor | Bevacizumab | 2 | - | 1 | - | - | - | 1 |
| IL-33 Inhibitor | Astegolimab | 1 | - | - | 1 | - | - | - |
| IL-17A Inhibitor | Secukinumab | 1 | - | - | 1 | - | - | - |
| PD-1 receptor Binder | 5 | - | - | 5 | - | - | - | |
| Nivolumab | 4 | - | - | 4 | - | - | - | |
| Pembrolizumab | 1 | - | - | 1 | - | - | - | |
| Human Factor XIIa antagonist | Garadacimab | 1 | - | - | 1 | - | - | - |
| Angiopoietin - 2 Inhibitor | LY3127804 | 1 | - | - | 1 | - | - | - |
| Complementary Protein 5 blocker | 5 | - | - | 1 | 1 | 1 | 2 | |
| Ravulizumab | 2 | - | - | - | 1 | 1 | - | |
| Eculizumab | 3 | - | - | 1 | - | - | 2 | |
| NKG2A Inhibitor | Monalizumab | 1 | - | - | 1 | - | - | - |
| Anti-HER2 | 1 | - | - | - | - | - | 1 | |
| Pertuzumab | 1 | - | - | - | - | - | 1 | |
| Transtuzumab | 1 | - | - | - | - | - | 1 | |
| Anti ILT-7 | VIB7734 -Daxdilimab | 1 | - | 1 | - | - | - | - |
| CCR5 inhibitor | Leronlimab | 2 | - | - | 2 | - | - | |
| Anti C5aR antibody | Avdoralimab | 2 | - | - | 2 | - | - | - |
| Anti-CD147 | Meplazumab | 1 | - | 1 | - | - | - | - |
| Other | CT-P59 | 1 | - | 1 | - | - | - | - |
IL, Interleukin. ILT, Immunoglobulin-like transcript. TNFα, Tumor Necrosis Factor alpha. CTGF, Connective Tissue Growth Factor. GM-CSF, Granulocyte Macrophage Colony Stimulating Factor. TNFSF, Tumor Necrosis Factor Superfamily. CSF-1R, Colony Stimulating Factor 1 Receptor. IFN- Interferon, VEGF, Vascular Endothelial growth Factor. PD, Programmed Cell Death Protein. CD, Cluster of Differentiation. CCR, Chemokine Receptor. NKG2A, Natural Killer Group 2 Member ACell Receptor. HER, Human Epidermal Growth Factor Receptor. C5aR, Complementary component fragment 5a receptor.
Figure 4.
Depiction of total number of ongoing clinical trials and mAbs Candidate for different biological targets of COVID-19.
Figure 5.
Representation of number of clinical trials undergoing for each mAbs for repurposing in Covid-19 management.
8. Discussion
It is evident that augmented levels of IL-6 were observed in patients infected with SARS-CoV-2 and correlated with disease severity (Zhang et al., 2020a, Zhang et al., 2020b, Zhang et al., 2020c, Zhang et al., 2020d, Zhang et al., 2020e; Aziz et al., 2020; Mojtabavi et al., 2020; Ji et al., 2020; Udomsinprasert et al., 2020). Levels of IL-6 seem to be associated with inflammatory response, respiratory failure, needing for mechanical ventilation and/or intubation and mortality in COVID-19 patients (Herold et al., 2020). Therefore study shows that it is considered to be a prognosticator in patients affected by SARS-CoV-2 (Grifoni et al., 2020). IL-6 is crucial for the production of T helper 17 (Th17) cells in the dendritic cell-T cell interaction [Tanaka et al., 2016]. Thus augmented IL-6 may explicate the exaggeratedly activated Th17 cells seen in patients infected with SARS-CoV-2 [Xu et al., 2020].
IL-6 is a small glycoprotein that has significant anti-inflammatory and pro-inflammatory properties. The anti-inflammatory function of IL-6 is mediated by classic signalling whereas trans-signalling is a key regulator for the proinflammatory properties (Scheller et al., 2011). In trans-signalling, IL-6 binds to the soluble form of the IL-6 receptor (sIL-6R) and forms a complex with gp130 dimer. This complex can activate IL-6-sIL-6R- Janus Kinase (JAK) -Signal transducer and activator of transcription 3 (STAT3) signalling (Zhang et al., 2020a, Zhang et al., 2020b, Zhang et al., 2020c, Zhang et al., 2020d, Zhang et al., 2020e). This leads to the secretion of VEGF, MCP-1, IL-8 and additional IL-6 along with reduction of E-cadherin expression on endothelial cells. This series of events ultimately aggravates the cytokine storm through increased vascular permeability and leakage (Tanaka et al., 2016). Selective blockage of this trans-signaling pathway is likely to have the beneficial effect of blocking inflammation without the undesirable off-target effects of broad immune suppression.
Among all IL-6 inhibitors, Tocilizumab (trade name RoActemra) is highly evaluated in clinical trials for management of COVID-19 patients with alarming signs of cytokine storm (Figure 6) and therefore preferred and recommended as a potential treatment option for critically ill patients with COVID-19 (Zhang et al., 2020a, Zhang et al., 2020e). Tocilizumab a recombinant humanized monoclonal antibody directed against IL-6 receptor and inhibiting its signal transduction pathway is approved by FDA for the treatment of cytokine storm associated with several inflammatory and autoimmune diseases, such as giant cell arteritis, rheumatoid arthritis, systemic juvenile idiopathic arthritis and polyarticular juvenile idiopathic arthritis. It is the first and only IL-inhibitor approved by FDA for the treatment of CAR-T cell-induced cytokine release syndrome (CRS) (Atal and Fatima, 2020; Tocilizumab: Drug information - UpToDate, 2020). It is effective and safe for treating both paediatric (with two or more years of age) as well as adults. Tocilizumab is being studied for managing ARDS in severe ill patients with COVID-19 alone or in combination with other antiviral therapies like hydroxychloroquine, remdesivir and favipiravir and broad spectrum antibiotics in several clinical trials (Antwi-Amoabeng et al., 2020; Borku Uysal et al., 2020; Campochiaro et al., 2020; Christou et al., 2020; Farooqi et al., 2020; Jordan et al., 2020; Mazzitelli et al., 2020; Moreno-Pérez et al., 2020; Quartuccio et al., 2020; Toniati et al., 2020; Trujillo et al., 2020; Wang et al., 2020). These clinical studies concluded that intravenous administration of Tocilizumab at a dose of 8 mg/kg results in reduced serum IL-6 level and rapid clinical improvement of COVID-19 pneumonia with ARDS (Toniati et al., 2020) in majority of participants. Other IL-6 inhibitors, siltuximab and sarilumab also studied clinically for the management of COVID-19 (Maes et al., 2020). FDA approved siltuximab for multicentric Castleman's disease while FDA approves sarilumab for rheumatoid arthritis similar to that of Tocilizumab (Atal and Fatima, 2020).
Clazakizumab is an investigational humanised monoclonal antibody against IL-6, which is also considered a potential candidate for the management of cytochrome strom associated with COVID-19. Although not yet FDA approved, It is primarily registered for phase II clinical trials for the treatment of kidney transplant recipients with late antibody-mediated rejection. Clinical trials of clazakizumab for treating critical cases of COVID-19 are under investigation (Vaidya et al., 2020). Olocizumab is also an investigational monoclonal antibody against IL-6. Tocilizumab and sarilumab are under evaluation in phase IV clinical trials, and siltuximab is in phase III for COVID-19 patients.
Apart from that canakinumab (monoclonal antibody against human IL-1β), anakinra (recombinant human IL-1R antagonist), and emapalumab (monoclonal antibody against human IFN-γ) are promising mAbs to treat the cytokine storm associated with severe forms of COVID-19.
A novel monoclonal antibody cocktail REGN10933 and REGN10987 intervening under clinical Phase-III is futurized to be considered as the most promising therapeutic strategy amongst all as it is able to thwart SARS-CoV-2 at the initial phase only by targeting the spike protein on its surface (Renn et al., 2020).
Therapeutic monoclonal antibodies are also exploring targets other than cytokines for the prevention and treatment of COVID-19.
9. Conclusion
Several mAbs are under investigation in clinical trials targeting various cytokines IL-6, IL-1ra, IL-8, IL-1β, IL-17A, IL-33, IFN-γ, TNF-α, P- selectin, CTGF, plasma kallikrein, TNFSF14, GM-CSF, CSF-1R, CCR5, CD14, CD147, VEGF, PD-1, Angiopoietin - 2, human factor XIIa, complementary protein 5, NKG2A, HER2, ILT7 receptor, C5aR and viral attachment to the human cell. However, elevated levels of pro-inflammatory cytokine IL-6 were observed to be a constant indicator of poor outcome in critically ill COVID-19 patients with pneumonia and ARDS. Cytokine IL-6 is the best-documented cytokine that correlates well with viral load, severity, criticality, and prognosis of patients with COVID-19. Most of the clinical studies conducted so far are targeting IL-6 for the management of severe stage of COVID-19. There are seven candidates that target IL-6 (tocilizumab, sarilumab, siltuximab, sirukumab, clazakizumab, olokizumab, levilimab). Among these the maximum clinical studies are reported on tocilizumab. Therefore, tocilizumab is considered to be the most promising candidate for management of cytochrome storm in COVID-19.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- AminJafari A., Ghasemi S. The possible of immunotherapy for COVID-19: a systematic review. Int. Immunopharm. 2020;83:106455. doi: 10.1016/j.intimp.2020.106455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antwi-Amoabeng D., Kanji Z., Ford B., Beutler B.D., Riddle M.S., Siddiqui F. Clinical outcomes in COVID-19 patients treated with tocilizumab: an individual patient data systematic review. J. Med. Virol. 2020 doi: 10.1002/jmv.26038. 26038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astuti I., Ysrafil Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:407–412. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atal S., Fatima Z. IL-6 inhibitors in the treatment of serious COVID-19: a promising therapy? Pharmaceut. Med. 2020;34:223–231. doi: 10.1007/s40290-020-00342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz M., Fatima R., Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.25948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N., Diao K., Lin B., Zhu X., Li K., Li S., Shan H., Jacobi A., Chung M. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borku Uysal B., Ikitimur H., Yavuzer S., Ikitimur B., Uysal H., Islamoglu M.S., Ozcan E., Aktepe E., Yavuzer H., Cengiz M. Tocilizumab challenge: a series of cytokine storm therapy experiences in hospitalized COVID-19 pneumonia patients. J. Med. Virol. 2020 doi: 10.1002/jmv.26111. 26111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campochiaro C., Della-Torre E., Cavalli G., De Luca G., Ripa M., Boffini N., Tomelleri A., Baldissera E., Rovere-Querini P., Ruggeri A., Monti G., De Cobelli F., Zangrillo A., Tresoldi M., Castagna A., Dagna L. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur. J. Intern. Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause Lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Zheng J., Wohlford-Lenane C., Abrahante J.E., Mack M., Sompallae R., McCray P.B., Meyerholz D.K., Perlman S. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J. Clin. Invest. 2019;129:3625–3639. doi: 10.1172/JCI126363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang Xiaoyun, Chen H., Yu H., Zhang Xiaoping, Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Liu H.G., Liu W., Liu J., Liu K., Shang J., Deng Y., Wei S. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:203–208. doi: 10.3760/cma.j.issn.1001-0939.2020.03.013. [DOI] [PubMed] [Google Scholar]
- Cheung C.Y., Poon L.L.M., Ng I.H.Y., Luk W., Sia S.-F., Wu M.H.S., Chan K.-H., Yuen K.-Y., Gordon S., Guan Y., Peiris J.S.M. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 2005;79:7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017;39:517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- Christou M.S., Mohamed M.S., Tsigarides J. Tocilizumab – a beacon of hope in the management of severe COVID-19? J. Med. Virol. 2020 doi: 10.1002/jmv.26466. 26466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomes E.A., Haghbayan H. Interleukin-6 in Covid-19: a systematic review and <scp>meta-analysis</scp>. Rev. Med. Virol. 2020 doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020 doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi F., Dhawan N., Morgan R., Dinh J., Nedd K., Yatzkan G. Treatment of severe COVID-19 with tocilizumab mitigates cytokine storm and averts mechanical ventilation during acute respiratory distress: a case report and Literature review. Trop. Med. Infect. Dis. 2020;5:112. doi: 10.3390/tropicalmed5030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R.M. Clinical uses of interferons. Br. J. Clin. Pharmacol. 2008 doi: 10.1111/j.1365-2125.2007.03055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol. Sin. 2020;35:266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Li T., Han M., Li X., Wu D., Xu Y., Zhu Y., Liu Y., Wang X., Wang L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020;92:791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sastre A., Biron C.A. Type 1 interferons and the virus-host relationship: a lesson in détente. Science (80- ) 2006 doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- Grifoni E., Valoriani A., Cei F., Lamanna R., Gelli A.M.G., Ciambotti B., Vannucchi V., Moroni F., Pelagatti L., Tarquini R., Landini G., Vanni S., Masotti L. Interleukin-6 as prognosticator in patients with COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J., Tan K.-S., Wang D.-Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil. Med. Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy R.K., DiPaola R.S., Romanelli F., Dutch R.E. Rapid repurposing of drugs for COVID-19. Science (80- ) 2020;368:829–830. doi: 10.1126/science.abb9332. [DOI] [PubMed] [Google Scholar]
- Harrison C. Coronavirus puts drug repurposing on the fast track. Nat. Biotechnol. 2020;38:379–381. doi: 10.1038/d41587-020-00003-1. [DOI] [PubMed] [Google Scholar]
- Hassan S.A., Sheikh F.N., Jamal S., Ezeh J.K., Akhtar A. Coronavirus (COVID-19): a review of clinical features, diagnosis, and treatment. Cureus. 2020 doi: 10.7759/cureus.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold S., Steinmueller M., Von Wulffen W., Cakarova L., Pinto R., Pleschka S., Mack M., Kuziel W.A., Corazza N., Brunner T., Seeger W., Lohmeyer J. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J. Exp. Med. 2008;205:3065–3077. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold T., Jurinovic V., Arnreich C., Lipworth B.J., Hellmuth J.C., von Bergwelt-Baildon M., Klein M., Weinberger T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020;146:128–136. doi: 10.1016/j.jaci.2020.05.008. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Högner K., Wolff T., Pleschka S., Plog S., Gruber A.D., Kalinke U., Walmrath H.D., Bodner J., Gattenlöhner S., Lewe-Schlosser P., Matrosovich M., Seeger W., Lohmeyer J., Herold S. Macrophage-expressed IFN-β contributes to apoptotic alveolar epithelial cell injury in severe influenza virus pneumonia. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahlu L., Rezaei N. Monoclonal antibody as a potential anti-COVID-19. Biomed. Pharmacother. 2020 doi: 10.1016/j.biopha.2020.110337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji P., Zhu J., Zhong Z., Li H., Pang J., Li B., Zhang J. Association of elevated inflammatory markers and severe COVID-19: a meta-analysis. Medicine (Baltim.) 2020;99 doi: 10.1097/MD.0000000000023315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan S.C., Zakowski P., Tran H.P., Smith E.A., Gaultier C., Marks G., Zabner R., Lowenstein H., Oft J., Bluen B., Le C., Shane R., Ammerman N., Vo A., Chen P., Kumar S., Toyoda M., Ge S., Huang E. Compassionate use of tocilizumab for treatment of SARS-CoV-2 pneumonia. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F.N., Qazi S., Tanveer K., Raza K. A review on the antagonist Ebola: a prophylactic approach. Biomed. Pharmacother. 2017;96:1513–1526. doi: 10.1016/j.biopha.2017.11.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K.P., Lau C.C.Y., Chan K.-H., Li C.P.Y., Chen H., Jin D.-Y., Chan J.F.W., Woo P.C.Y., Yuen K.-Y. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J. Gen. Virol. 2013;94:2679–2690. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- Law H.K.W., Cheung C.Y., Ng H.Y., Sia S.F., Chan Y.O., Luk W., Nicholls J.M., Peiris J.S.M., Lau Y.L. Chemokine up-regulation in SARS-coronavirus–infected, monocyte-derived human dendritic cells. Blood. 2005;106:2366–2374. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima W.G., Brito J.C.M., Overhage J., Nizer W.S. da C. The potential of drug repositioning as a short-term strategy for the control and treatment of COVID-19 (SARS-CoV-2): a systematic review. Arch. Virol. 2020 doi: 10.1007/s00705-020-04693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg. Microbes Infect. 2020;9:727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes B., Bosteels C., De Leeuw E., Declercq J., Van Damme K., Delporte A., Demeyere B., Vermeersch S., Vuylsteke M., Willaert J., Bollé L., Vanbiervliet Y., Decuypere J., Libeer F., Vandecasteele S., Peene I., Lambrecht B. Treatment of severely ill COVID-19 patients with anti-interleukin drugs (COV-AID): a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:468. doi: 10.1186/s13063-020-04453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzitelli M., Arrighi E., Serapide F., Pelle M.C., Tassone B., Lionello R., Marrazzo G., Laganà D., Costanzo F.S., Matera G., Trecarichi E.M., Torti C. Use of subcutaneous tocilizumab in patients with COVID-19 pneumonia. J. Med. Virol. 2020 doi: 10.1002/jmv.26016. 26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojtabavi H., Saghazadeh A., Rezaei N. Interleukin-6 and severe COVID-19: a systematic review and meta-analysis. Eur. Cytokine Netw. 2020 doi: 10.1684/ecn.2020.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Pérez O., Andres M., Leon-Ramirez J.-M., Sánchez-Payá J., Rodríguez J.C., Sánchez R., García-Sevila R., Boix V., Gil J., Merino E. Experience with tocilizumab in severe COVID-19 pneumonia after 80 days of follow-up: a retrospective cohort study. J. Autoimmun. 2020;102523 doi: 10.1016/j.jaut.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norelli M., Camisa B., Barbiera G., Falcone L., Purevdorj A., Genua M., Sanvito F., Ponzoni M., Doglioni C., Cristofori P., Traversari C., Bordignon C., Ciceri F., Ostuni R., Bonini C., Casucci M., Bondanza A. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 2018;24:739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.-S. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartuccio L., Sonaglia A., Pecori D., Peghin M., Fabris M., Tascini C., De Vita S. Higher levels of IL-6 early after tocilizumab distinguish survivors from nonsurvivors in COVID-19 pneumonia: a possible indication for deeper targeting of IL-6. J. Med. Virol. 2020 doi: 10.1002/jmv.26149. 26149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020 doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameshrad M., Ghafoori M., Mohammadpour A.H., Nayeri M.J.D., Hosseinzadeh H. A comprehensive review on drug repositioning against coronavirus disease 2019 (COVID19) Naunyn. Schmiedebergs. Arch. Pharmacol. 2020 doi: 10.1007/s00210-020-01901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn A., Fu Y., Hu X., Hall M.D., Simeonov A. Fruitful neutralizing antibody pipeline brings hope to defeat SARS-cov-2. Trends Pharmacol. Sci. 2020 doi: 10.1016/j.tips.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell B., Moss C., George G., Santaolalla A., Cope A., Papa S., Van Hemelrijck M. Associations between immune-suppressive and stimulating drugs and novel COVID-19—a systematic review of current evidence. Ecancermedicalscience. 2020;14 doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta Mol. Cell Res. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Sempowski G.D., Saunders K.O., Acharya P., Wiehe K.J., Haynes B.F. 2020. Pandemic Preparedness: Developing Vaccines and Therapeutic Antibodies for COVID-19. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafin M.B., Bottega A., Foletto V.S., da Rosa T.F., Hörner A., Hörner R. Drug repositioning is an alternative for the treatment of coronavirus COVID-19. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical–therapeutic staging proposal. J. Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J. Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soy M., Keser G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020;39:2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Li H., Lu X.X., Xiao H., Ren J., Zhang F.R., Liu Z.S. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J. Pediatr. 2020;16:251–259. doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Narazaki M., Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8:959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- Teijaro J.R. Cytokine storms in infectious diseases. Semin. Immunopathol. 2017 doi: 10.1007/s00281-017-0640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocilizumab: Drug information - UpToDate 2020. https://www.uptodate.com/contents/tocilizumab-drug-information [WWW Document], URL. (accessed 9.7.20)
- Toniati P., Piva S., Cattalini M., Garrafa E., Regola F., Castelli F., Franceschini F., Airò P., Bazzani C., Beindorf E.-A., Berlendis M., Bezzi M., Bossini N., Castellano M., Cattaneo S., Cavazzana I., Contessi G.-B., Crippa M., Delbarba A., De Peri E., Faletti A., Filippini M., Filippini M., Frassi M., Gaggiotti M., Gorla R., Lanspa M., Lorenzotti S., Marino R., Maroldi R., Metra M., Matteelli A., Modina D., Moioli G., Montani G., Muiesan M.-L., Odolini S., Peli E., Pesenti S., Pezzoli M.-C., Pirola I., Pozzi A., Proto A., Rasulo F.-A., Renisi G., Ricci C., Rizzoni D., Romanelli G., Rossi M., Salvetti M., Scolari F., Signorini L., Taglietti M., Tomasoni G., Tomasoni L.-R., Turla F., Valsecchi A., Zani D., Zuccalà F., Zunica F., Focà E., Andreoli L., Latronico N. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia. Italy. Autoimmun. Rev. 2020;19:102568. doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo H., Caravaca-Fontán F., Sevillano Á., Gutiérrez E., Fernández-Ruiz M., López-Medrano F., Hernández A., Aguado J.M., Praga M., Andrés A. Tocilizumab use in kidney transplant patients with covid-19. Clin. Transplant. 2020 doi: 10.1111/ctr.14072. [DOI] [PubMed] [Google Scholar]
- Udomsinprasert W., Jittikoon J., Sangroongruangsri S., Chaikledkaew U. Circulating levels of interleukin-6 and interleukin-10, but not tumor necrosis factor-alpha, as potential biomarkers of severity and mortality for COVID-19: systematic review with meta-analysis. J. Clin. Immunol. 2020 doi: 10.1007/s10875-020-00899-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya G., Czer L.S.C., Kobashigawa J., Kittleson M., Patel J., Chang D., Kransdorf E., Shikhare A., Tran H., Vo A., Ammerman N., Huang E., Zabner R., Jordan S. Successful treatment of severe COVID-19 pneumonia with clazakizumab in a heart transplant recipient: a case report. Transplant. Proc. 2020 doi: 10.1016/j.transproceed.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., Xiao Q., Xiao K., Yi Z., Qiang M., Xiang J., Zhang B., Chen Y. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) medRxiv. 2020 2020.02.10.20021832. [Google Scholar]
- Wang L., Peng X., Wang Z.H., Cai J., Zhou F.C. Tocilizumab in the treatment of a critical COVID-19 patient: a case report. Eur. Rev. Med. Pharmacol. Sci. 2020;24:5783–5787. doi: 10.26355/eurrev_202005_21372. [DOI] [PubMed] [Google Scholar]
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J., Sheng J., Quan L., Xia Z., Tan W., Cheng G., Jiang T. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J., Zhou Xing, Xu S., Huang H., Zhang L., Zhou Xia, Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou Xin, Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:934. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’’ in COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020;55:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Shi L., Xu J., Wang Y., Yang H. Elevated interleukin-6 and adverse outcomes in COVID-19 patients: a meta-analysis based on adjusted effect estimates. Immunogenetics. 2020;72:431–437. doi: 10.1007/s00251-020-01179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Li L., Shen A., Chen Y., Qi Z. Rational use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin. Drug Investig. 2020 doi: 10.1007/s40261-020-00917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X., Zeng X., Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the Perspectives of clinical immunologists from China. Clin. Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.-L., Hou Y.-L., Li D.-T., Li F.-Z. Laboratory findings of COVID-19: a systematic review and meta-analysis. Scand. J. Clin. Lab. Invest. 2020;80:441–447. doi: 10.1080/00365513.2020.1768587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.