Abstract
Coronavirus disease 2019 (COVID-19) is an infection caused by a novel coronavirus (SARS-CoV-2) originated in China in December 2020 and declared pandemic by WHO. This coronavirus mainly spreads through the respiratory tract and enters cells through angiotensin-converting enzyme 2 (ACE2). The clinical symptoms of COVID-19 patients include fever, cough, and fatigue. Gastrointestinal symptoms (diarrhea, anorexia, and vomiting) may be present in 50% of patients and may be associated with worst prognosis. Other risk factors are older age, male gender, and underlying chronic diseases. Mitigation measures are essential to reduce the number of people infected. Hospitals are a place of increased SARS-CoV-2 exposure. This has implications in the organization of healthcare services and specifically endoscopy departments. Patients and healthcare workers safety must be optimized in this new reality. Comprehension of COVID-19 gastrointestinal manifestations and implications of SARS-CoV-2 in the management of patients with gastrointestinal diseases, under or not immunosuppressant therapies, is essential. In this review, we summarized the latest research progress and major societies recommendations regarding the implications of COVID-19 in gastroenterology, namely the adaptations that gastroenterology/endoscopy departments and professionals must do in order to optimize the provided assistance, as well as the implications that this infection will have, in particularly vulnerable patients such as those with chronic liver disease and inflammatory bowel disease under or not immunosuppressant therapies.
Keywords: chronic liver disease, coronavirus, coronavirus disease 2019, endoscopy, inflammatory bowel disease
Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by a novel coronavirus called SARS-CoV-2. This disease was first reported at the end of December 2019 in the province of Wuhan in mainland China; however, since then, it has spread rapidly around the globe, being considered pandemic by the WHO. SARS-CoV-2 main route of infectiousness is respiratory droplets and contact with infected surfaces [1]. Furthermore, SARS-CoV-2 RNA was found in the stool of infected patients, which could reflect a potential fecal-oral spread for this disease, although it is unclear if the viral concentration in the stool is sufficient for transmission [2]. The mechanism of cellular invasion is well documented. SARS-CoV-2 enters the body through the nose, mouth, and eyes and then attaches to the cells that have the protein angiotensin-converting enzyme 2 (ACE2) [3]. ACE-2 is found in alveolar cells of the lungs and gastrointestinal cells. The incubation period for COVID-19 is between 2 and 14 days [3]. Most common symptoms of COVID-19 are fever (40–80%), fatigue (40–60%), and dry cough (50–60%) [4–6]. The severity of this disease is mild to moderate in most cases, not requiring hospital care in about 80% of infected patients [6]. However, in Chinese population, up to 13.8% of patients presented with severe disease requiring hospitalization and an additional 4.7% present with critical condition requiring intensive care treatment with an overall fatality rate of 1–4% [4]. The major risk factors for severe illnesses and deaths identified so far are older age, male gender, and chronic underlying conditions such as arterial hypertension, diabetes mellitus, cardiovascular, and chronic kidney disease [6].
On average, the basic reproductive number (R0) for SARS-CoV-2 is 2–3 [7]. Given this R0, without mitigation measures, it is expected that 60% of the population would become infected. Furthermore, given the fact that around 20% of patients infected might need hospitalization, the pressure that COVID-19 can impose in the healthcare services capacity is alarming. Mitigation measures are essential to reduce the transmission chains. Healthcare services (includes outpatient, nursing homes/long-term care facilities, and inpatient) are no exception, and mitigation activities need to be implemented in this setting as well. Consequently, it is essential to understand the adaptations that gastroenterology departments and professionals must do in order to optimize the provided assistance. Furthermore, it is important to acknowledge the implications that this pandemic viral infection will have in particularly vulnerable patients such as those with chronic liver disease (CLD) and inflammatory bowel disease (IBD) under or not immunosuppressant therapies.
Impact of SARS-CoV-2 mitigation strategy in an endoscopy department
Hospitals and healthcare workers are at an increased risk of SARS-CoV-2. In the beginning of the epidemic in Wuhan, from 138 infected patients, 41.3% were presumed to be infected at the hospital, and, from those, up to 70% were healthcare providers [8]. With the mitigation of this pandemic in mind and in order to protect patients and healthcare providers, several changes in the organization of gastroenterology departments must be accomplished. However, these measures should be adequate to the rate of SARS-CoV-2 infection in each population. As more and more countries present substantial community transmission, especially in Europe and North America, suitable mitigation measures in this setting must be considered.
Recommendations from different international societies converge in their suggestions. The Center for Disease Control (CDC) recommends, in populations with substantial community transmission, that elective and nonurgent procedures should be canceled [9]. Given this, the activity of an endoscopy department should be restricted to urgent or emergent endoscopic procedures [10,11]. Several societies had defined ‘urgent endoscopic procedure’ as those related with acute upper or lower gastrointestinal bleeding, acute esophageal obstruction, or other gastrointestinal obstruction needing urgent decompression, acute cholangitis, acute biliary pancreatitis, infected pancreatic collections, jaundice secondary to malignant or benign biliary obstruction, and urgent inpatient nutrition support [10,12,13]. Cases where endoscopic procedures will urgently change management should also be considered [10]. Other procedures that should be considered are planned endoscopic mucosectomies or submucosal dissections for high-risk lesions, cancer staging endoscopy needed for subsequent treatment, new suspected IBD, small-bowel endoscopy in patients with active bleeding, or suspected malignancy [12]. Postponed patients should be reevaluated in 8 weeks [14]. Outpatient’s management should be made by telemedicine, and the use of telephone communications is encouraged [15].
All the healthcare staff must be trained on the adequate measures for infection prevention and control. These measures have been shown to dramatically decrease the risk of infection both for healthcare providers and for patients [16]. Knowledge about potential sources of contamination, correct hygiene measures, appropriate use of personal protection equipment (PPE), and timely identification of potential infected patients and high-risk interventions should be widespread (Table 1) [16]. CDC recommends that all healthcare providers should wear a facemask in the facility depending on supply of PPE [9]. It is also recommended that all patients use a surgical facemask during hospital stay [17]. Daily temperature checking should be performed for all staff before entering the working area [10,17]. The flow of staff and accompanying family members throughout the unit should be minimized [10,11]. Rotations both in intervention and general gastroenterology attendings should be considered, in order to minimize the contact and the eventual number of team members at the risk of simultaneous quarantine [10]. Only essential staff should be present during endoscopy [18].
Table 1.
Sequence for putting on and safely remove personal protective equipment
| Sequence for putting on personal protective equipment | |
|---|---|
| 1 | Remove every personal objects and adornments and hold the hair if necessary |
| 2 | Put on the interior pair of gloves |
| 3 | Put on the waterproof gown |
| 4 | Put on the mask or respirator (in accordance to risk of exposure) |
| 5 | Put on the waterproof hairnet |
| 6 | Put on the goggles or face shield |
| 7 | Put on the second pair of gloves |
| Sequence for removal of person protective equipment | |
| 1 | Remove the second pair of gloves |
| 2 | Use alcohol-based hand sanitizer |
| 3 | Remove goggles/face shield and hairnet in a singles gesture |
| 4 | Remove gown and the first pair of gloves |
| 5 | Use alcohol-based hand sanitizer |
Patients with necessary endoscopy procedures should have a prescreening evaluation, ideally 1 day before the procedure (by telephone) and on the day of endoscopy [11,17]. The assessment should include history of COVID-19 symptoms (fever, respiratory symptoms, or diarrhea), any contact with suspicious or confirmed cases of COVID19, and recent travel to high-risk areas [10–12]. All patients should have temperature measurements at arrival in the unit [10,17]. All endoscopic procedures, especially upper endoscopy, should be considered aerosol-generating procedures and proper PPE should be used [19]. Low-risk patients should be generally classified as patients without symptoms, no history of contact with COVID-19 patients, and no travel to endemic areas [11,20]. In these patients, PPE with surgical mask during endoscopy is advisable (Fig. 1) [10,11]. High-risk patients should be considered as: (1) patients with symptoms regardless of history of contact with COVID-19 or travel to endemic areas and (2) patients without symptoms but with contact with COVID-19 suspected or positive case or travel to high-risk areas 14 days before [11]. In these patients, PPE with N95/FFP2 should be used by a healthcare personal during endoscopic procedures (Fig. 1) [11]. When possible, endoscopic procedures should be made in negative-pressure room if performed in high-risk patients or patients positive for SARS-CoV-2 [20]. If an endoscopy unit is not equipped with a negative-pressure room, an operating room with negative-pressure can be an alternative. In substantial community transmission areas, as transmission via asymptomatic patients is well documented, prescreening and risk stratification is less useful [21]. In such situation, all patients proposed for endoscopic procedures should be considered high risk [17,18].
Fig. 1.
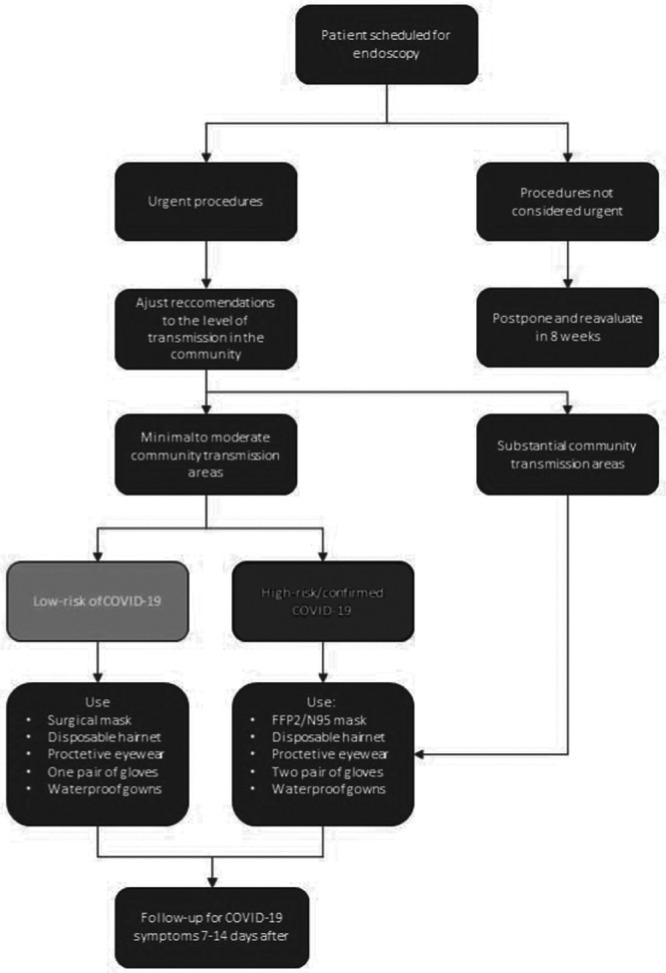
Decision-making for endoscopic procedures during COVID-19 mitigation strategy. PPE, personal protective equipment.
The amount of potentially contaminable surfaces like furniture should be minimized in an endoscopy room. Contaminated waste, especially from patients at high-risk or COVID-19 confirmed should be disposed using the local regulations related to high-risk waste [11]. Endoscopic equipment disinfection should be made in accordance to local recommendations [14]. Disposable equipment should not be reused; however, in the case of shortage of equipment, CDC presents guidance of extended or repeated use of respirators [10,22] with the possibility of use of a cleanable face shield or surgical mask over the N95/FFP2 respirator to reduce contamination and extend respirator use. Evidence on the efficacy of chemicals against SARS-CoV-2 is still not fully understood. However, reports of the use of 1:100 diluted bleach exist [20]. The cleaning process should include cleaning of all surface room followed by proper disinfection [23]. A follow-up, 7–14 days after the endoscopic procedure, should be made to ask about new diagnosis or development of symptoms of COVID-19 [24].
Gastrointestinal manifestations in patients with COVID-19
Even though the cardinal symptoms of SARS-CoV-2 infection include fever, fatigue, dry cough, and dyspnea, several gastrointestinal manifestations can be present as well in up to 50% of patients [6,25,26]. ACE-2 receptors present in the cell membrane are the mediator of SARS-CoV-2 entry and have been demonstrated to be highly expressed in gastric, duodenal, and rectal epithelia [27]. ACE-2 is involved in the control of intestinal inflammation and the disturbance of this control may lead to diarrhea [27]. Of note, RNA from SARS-CoV-2 has been detected in stool samples from patients with COVID-19 [28]. Moreover, the SARS-CoV-2 RNA can be detected in the stool sample even after symptoms resolution and negative nasopharyngeal samples. Many patients present initially with diarrhea, anorexia, and vomiting, without necessarily presenting respiratory symptoms. Pan et al. [25], in a cross-sectional multicenter study, reported the presence of gastrointestinal symptoms without respiratory manifestations in 3% of the patients. Furthermore, up to 48.5% of the patients presented with gastrointestinal symptoms, the most common being anorexia (40%) and diarrhea (29.3%) [25]. Patients with digestive symptoms have a longer time from symptom onset to admission when compared with patients without digestive symptoms (9.0 vs 7.3 days) [25]. This may be due to diagnostic delay because respiratory symptoms were not initially predominant. Adding to this, there have been reports of isolated diarrhea preceding respiratory symptoms and fever [29]. As such, COVID-19, especially if disseminated in the community, should be considered in patients with digestive symptoms in order to diagnose this disease in the early stage.
Abnormal liver function tests (LFTs), including aspartate aminotransferase, alanine aminotransferase, and total bilirubin, were identified in patients with COVID-19 and found in 14–53% of the patients [6,17,30–33]. So far, data suggest that liver injury is more prevalent in severe COVID-19 cases [33,34]. Direct liver injury via viral hepatitis is a possibility; however, LFT alterations are only mild in most patients and reports of liver biopsy in patients with COVID-19 present only microvesicular steatosis, which is a common finding in patients with sepsis [35]. Alternative explanations might be hypoxic hepatitis, drug-induced liver injury, or hepatic lesion from immune interactions involving intrahepatic cytotoxic T cells and Kupffer cells, commonly seen in other respiratory viral infections [36,37]. Liver insult in patients with mild COVID-19 is transient and does not require specific treatment; however, rare cases of severe acute liver injury were reported in patients with severe COVID-19 [5,33]. Low serum albumin in hospital admission is a marker of COVID-19 severity [5].
The American Association for the Study of Liver Disease (AASLD) released a list of recommendations regarding hepatology and COVID-19 [38]. A regular monitoring of LFT should be made in every patient hospitalized with COVID-19. Serologic testing of viral B and C hepatitis is warrant in patients with abnormal LFT. To limit transportation of COVID-19 patients, diagnostic imaging techniques should be reserved to patients with suspicion for biliary obstruction or venous thrombosis. Even though potential hepatotoxicity might exist, the presence of abnormal LFT should not be a contraindication to off-label treatments for COVID-19 (hydroxychloroquine, chloroquine, remdesivir, tocilizumab, or statins), if considered.
COVID-19 and chronic liver disease
CLD is an immunosuppressive condition that could theoretically increase the risk of worst COVID-19 outcomes. For this reason, CDC considers CLD as a medical condition at the risk of serious COVID-19 [9]. However, so far, CLD has not been identified as a condition at risk [6,39]. From 42 patients with CLD who have outcome data reported, mortality was 0–2% [4,6,8,30,32,33]. Guan et al. [6] reported 23 cases of COVID-19 in patients with hepatitis B infection. In this study, patients with severe COVID-19 were more likely to have hepatitis B infection (2.4% vs 0.6%). The effects of other etiologies of CLD on the prognosis of COVID-19 have not been evaluated.
Patients with autoimmune hepatitis or liver transplant recipient under immunosuppressive therapy are a group worth discussion. The effects of immunosuppression on COVID-19 are not well understood. The innate immune response may be the driver of pulmonary injury related to COVID-19 and immunosuppression may be protective [39]. Posttransplant immunosuppression was not a risk factor for mortality associated with other coronavirus like SARS or MERS [39]. Even so, CDC defines immunosuppressed patients as a group at risk for COVID-19 [9]. Reducing the dose or stopping immunosuppressants may lead to a flare of autoimmune hepatitis or precipitate acute rejection in a liver transplant recipient. AASLD recommends maintaining immunosuppression without prophylactic adjustments in patients without COVID-19 [38]. In patients with COVID-19, it is recommended to reduce the dose of prednisone but maintaining a dose of at least 10 mg/day; to reduce the dose of azathioprine or mycophenolate, especially in the case of lymphopenia, fever, or severe COVID-19; and to consider reducing but not stopping calcineurin inhibitor dosage.
There are currently no data regarding the effects of SARS-CoV-2 infection in patients with decompensated CLD or those waiting for liver transplant. AASLD recommends maintaining outpatient evaluation in patients with hepatocellular carcinoma (HCC) or those with severe disease and high model for end-stage liver disease score, as these patients are likely to benefit from immediate liver transplant listing [38]. Large-volume paracentesis should be continued.
It is yet unknown if patients with HCC are at an increased risk for severe COVID-19; however, other types of cancer have been associated with worst outcomes [40]. AASLD recommends maintaining HCC imaging surveillance and treatments during pandemic [38].
COVID-19 and inflammatory bowel disease
Limited evidence has been demonstrated in the behavior of COVID-19 in patients with IBD, especially regarding the role of immunosuppression. So far, the evidence suggests that patients with relative immunosuppression such as HIV patients and solid organ transplant do not appear to be at higher risk of complications [39]. Prior research has found that viral infections are more likely among IBD patients on thiopurines than in those on biologics, but this might not be extended to COVID-19 [41]. International organization for the study of IBD (IOIBD) states that the risk of infection with SARS-CoV-2 is not increased in patients with IBD, independent of treatment [42]. However, it is uncertain if active inflammation from IBD increases the risk of developing COVID-19. Furthermore, it is also uncertain if patients with IBD have a higher mortality from COVID-19. Similarly, the European Crohn’s and Colitis organization (ECCO) also states that IBD patients are not at an increased risk of developing COVID-19 infection; however, it defends that it is expected that patients under immunosuppressive or biological therapy are at a higher risk of severe infection [43]. By 2 April 2020, a total of 239 patients with IBD have been reported to have COVID-19 in the SECURE-IBD registry, mainly in Europe and USA [44]. So far, more cases of COVID-19 have been reported in patients with Crohn’s disease (n = 137) when compared with ulcerative colitis patients (n = 94). More male gender patients presented death as an outcome (6% vs 3%). The proportion of patients with severe COVID-19 outcomes (need of ICU and need of ventilator or death) increased with age. Patients with severe IBD activity presented a worst prognosis, with 30% presenting severe COVID-19 outcomes. As for treatment class, severe COVID-19 outcomes were present in 29% of patients with corticosteroid, 3% of patients with antitumor necrosis factor (TNF), 11% of patients with anti-TNF plus thiopurines or methotrexate, 14% of patients with janus kinase (JAK) inhibitor, 5% of patients with thiopurine monotherapy, 10% of patients with methotrexate monotherapy, and 20% of patients with sulfasalazine or mesalamine.
For patients under optimized immunosuppressive therapy with good control of IBD, it is recommended that the patient continues with its therapy, and also with daily activities as non-IBD individuals. It is considered well tolerated to continue infusions in a dedicated center and ECCO recommends that, even though delay in treatment can be considered, maintaining a regular schedule is the preferred strategy [15,45]. The switch from intravenous monoclonal antibodies to subcutaneous options is not advised because it can lead to an increased risk of loss of response [46]. Low CD4+ cells in blood has been associated with longer virus clearance time and a more severe disease [47]. As such, ECCO recommends that adding thiopurines to steroids or to monoclonal antibodies should be used cautiously [45]. JAK inhibitors can decrease the number of lymphocytes, as such ECCO recommends caution in its use [45]. The use of corticosteroids during the mitigation phase of COVID-19 pandemic is controversial and liberal use of corticosteroids is not advisable [48]. However, low-dose and short-term use of corticosteroids may not be associated with worst prognosis in patients with COVID-19 [49]. IOIBD, in patients taking prednisone therapy >20 mg/day, recommends a reduction in the dose to prevent SARS-CoV-2 infection [42]. Finally, all elective surgery and endoscopy should be postponed [42,50]. Table 2 resumes the group of patients with potential risk of severe COVID-19.
Table 2.
Patients with inflammatory bowel disease and potential high risk of severe COVID-19, based on SECURE-IBD database information on 1 April 2020 [43]
| IBD patients with potential risk of severe COVID-19 |
|---|
| Patients with comorbidities (hypertension, diabetes mellitus, cardiovascular and chronic kidney disease) |
| Patients with >60 years old |
| Patients with severe IBD activity |
| Patients with corticosteroid therapy (>20 mg/day of prednisone) |
| Patients with combination therapy (anti-TNF plus thiopurine or methotrexate) |
| Patients with JAK inhibitor therapy |
Anti-TNF, anti tumor necrosis factor; COVID-19, coronavirus disease 2019; IBD, inflammatory bowel disease; JAK, janus kinase.
In patients with IBD flare, ECCO and IOIBD recommend that in order to avoid hospitalizations and complications requiring surgery, all drugs indicated in the treatment of the flare should be used [42,45]. However, as previously stated, symptoms such as anorexia, diarrhea, nausea, vomiting, and abdominal pain are common in patients with SARS-CoV-2 infection [6]. As such, in areas with large-scale community transmission of SARS-CoV-2, it is important to recognize COVID-19 as a differential diagnosis of IBD flare. If a patient with IBD is confirmed to have COVID-19, ECCO suggests suspending all immunosuppressive therapy. Similarly, IOIBD suggest suspension of therapy with the exception of mesalazine. Currently, there are no evidences that support COVID-19 as a cause of IBD flare. However, such relation should not be unexpected, as even in patients without gastrointestinal symptoms, H1N1 influenza virus was associated with mild flares during the first week of viral infection, mostly in patients with ulcerative colitis [51].
Conclusion
The SARS-CoV-2 pandemic presents a challenge for the world healthcare authorities and clinicians, being essential to enforce and maintain mitigation measures for the next months. Gastroenterologists have additional responsibility to protect patients and themselves. The interplay between digestive conditions and SARS-CoV-2 remains largely unknown; however, several recommendations can be made with the information at hand. Recommendations about SARS-CoV-2 infection are under constant actualization and gastroenterologists should keep updated in order to provide the best healthcare services.
Acknowledgements
J.F.-S., A.P., and E.R.-P. drafted the manuscript and performed literature search. G.M. critically revised the manuscript.
Conflicts of interest
There are no conflicts of interest.
References
- 1.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020; 382:1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. ; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020; 382:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020; 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020; 80:388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng F, Liao C, Fan QH, Chen HB, Zhao XG, Xie ZG, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci. 2020; 40:275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020; 109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020; 323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prevention, UCfDCa. Implementation of mitigation strategies for communities with local COVID-19 transmission. 2020. https://www.cdc.gov/coronavirus/2019-ncov/downloads/community-mitigation-strategy.pdf. [Accessed April 1 2020].
- 10.New York Society for Gastrointestinal Endoscopy. New York Society for Gastrointestinal Endoscopy Guidelines for endoscopy units during the COVID-19 pandemic March 16. 2020. https://www.nysge.org//Files/NYSGE%20Guidelines%20for%20Endoscopy%20Units%20During%20the%20COVID-19%20Pandemic.pdf. [Accessed April 1 2020].
- 11.European Society of Gastrointestinal Endoscopy. ESGE and ESGENA position statement on gastrointestinal endoscopy and the COVID-19 pandemic. 2020. https://www.esge.com/assets/downloads/pdfs/general/ESGE_ESGENA_Position_Statement_gastrointestinal_endoscopy_COVID_19_pandemic.pdf. [Accessed April 1 2020]. [DOI] [PMC free article] [PubMed]
- 12.British Society of Gastroenterology. Advice for endoscopy teams during COVID-19. 2020. https://www.bsg.org.uk/covid-19-advice/endoscopy-activity-and-covid-19-bsg-and-jag-guidance/. [Accessed April 1 2020].
- 13.World Gastroenterology Organization. Gastroenterology Practice in COVID-19 Pandemic. 2020. https://www.worldgastroenterology.org/publications/e-wgn/gastroenterology-practice-in-covid-19-pandemic?utm_source=WGOBanner&utm_medium=Homepage&utm_campaign=COVID19Informationwebpage_COVID19_e-WGN_Article. [Accessed April 1 2020].
- 14.Sultan S, Lim JK, Altayar O, Davitkov P, Feuerstein JD, Siddique SM, et al. AGA institute rapid recommendations for gastrointestinal procedures during the COVID-19 pandemic. Gastroenterology. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiorino G, Allocca M, Furfaro F, Gilardi D, Zilli A, Radice S, et al. Inflammatory bowel disease care in the COVID-19 pandemic era: the humanitas, milan experience. J Crohns Colitis. 2020:jjaa058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/infection-control/infection-prevention-control-faq.html. [Accessed April 1 2020].
- 17.Zhang Y, Zhang X, Liu L, Wang H, Zhao Q. Suggestions of infection prevention and control in digestive endoscopy during current 2019-ncov pneumonia outbreak in Wuhan, Hubei Province. China.Endoscopy. 2020; 52:312–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American College of Gastroenterology. Joint Gastroenterology Society Message: COVID-19 use of personal protective equipment in GI endoscopy. 2020. https://webfiles.gi.org/links/media/JOINT_GI_SOCIETY_MESSAGE_PPE_FINAL_04012020.pdf.
- 19.Soetikno R, Teoh AY, Kaltenbach T, Lau JY, Asokkumar R, Cabral-Prodigalidad P, Shergill A. Considerations in performing endoscopy during the COVID-19 pandemic. Gastrointest Endosc. 2020; 27:S0016-5107(20)34033-5. [Accessed April 1 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Repici A, Maselli R, Colombo M, Gabbiadini R, Spadaccini M, Anderloni A, et al. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. Gastrointest Endosc. 2020; 4:S0016-5107(20)30245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai J, Sun W, Huang J, Gamber M, Wu J, He G. Indirect virus transmission in cluster of COVID-19 cases, Wenzhou, China, 2020. Emerg Infect Dis. 2020; 26:1343–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The National Institute for Occupational Safety and Health (NIOSH). NIOSH: Recommended guidance for extended use and limited reuse of N95 filtering facepiece respirators in healthcare settings. 2020. https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html. [Accessed April 1 2020].
- 23.Calderwood AH, Day LW, Muthusamy VR, Collins J, Hambrick RD, III, Brock AS, et al. ASGE guideline for infection control during GI endoscopy. Gastrointest Endosc. 2018; 87:1167–1179. [DOI] [PubMed] [Google Scholar]
- 24.American Society for Gastrointestinal Endoscopy. JOINT GI SOCIETY MESSAGE: COVID-19 clinical insights for our community of gastroenterologists and gastroenterology care providers. 2020. https://www.asge.org/home/joint-gi-society-message-covid-19. [Accessed April 1 2020].
- 25.Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). J Gen Intern Med. 2020; 35:1545–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang WZ, Feng S, Rao C, Xiao X, Xue Z, Lin Z, et al. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020; 69:1141–1143. [DOI] [PubMed] [Google Scholar]
- 28.Gu J, Han B, Wang J. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020; 158:1518–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Y, Liu P, Shi XL, Chu YL, Zhang J, Xia J, et al. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. 2020; 69:1143–1144. [DOI] [PubMed] [Google Scholar]
- 30.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020; 395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, et al. Clinical features of COVID-19-related liver damage. Clin Gastroenterol Hepatol. 2020:S1542-3565(20)30482-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020; 40:998–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W, Tao ZW, Wang L, Yuan ML, Liu K, Zhou L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020; 133:1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koskinas J, Gomatos IP, Tiniakos DG, Memos N, Boutsikou M, Garatzioti A, et al. Liver histology in ICU patients dying from sepsis: a clinico-pathological study. World J Gastroenterol. 2008; 14:1389–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams DH, Hubscher SG. Systemic viral infections and collateral damage in the liver. Am J Pathol. 2006; 168:1057–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020; 5:529–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Association for the Study of Liver Disease. Clinical insights for hepatology and liver transplant providers during the Covid-19 pandemic. 2020. https://www.aasld.org/sites/default/files/2020-03/AASLD-COVID19-ClinicalInsights-3.23.2020-FINAL-v2.pdf. [Accessed April 1 2020].
- 39.D’Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic. Liver Transpl. 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 40.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020; 21:335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology. 2018; 155:337–346.e10. [DOI] [PubMed] [Google Scholar]
- 42.International Organization for the study of Inflamatory Bowel Disease. IOIBD update on COVID19 for patients with Crohn’s disease and ulcerative colitis. 2020. https://www.ioibd.org/ioibd-update-on-covid19-for-patients-with-crohns-disease-and-ulcerative-colitis/. [Accessed April 1 2020].
- 43.European Crohn’s and Colitis Organization. 1st interview COVID-19 ECCO taskforce, published March 13. 2020. https://ecco-ibd.eu/images/6_Publication/6_8_Surveys/1st_interview_COVID-19%20ECCOTaskforce_published.pdf. [Accessed April 1 2020].
- 44.Suveillance epidemiology of coronavirus (COVID-19) under research exclusion, SECURE-IBD database. 2020. https://covidibd.org/current-data/. [Accessed April 1 2020].
- 45.European Crohn’s and Colitis Organization. 2nd Interview COVID-19 ECCO taskforce, published March 20. 2020. https://ecco-ibd.eu/images/6_Publication/6_8_Surveys/2nd_Interview_COVID-19_ECCO_Taskforce_published.pdf. [Accessed April 1 2020].
- 46.Hoentjen F, Haarhuis BJ, Drenth JP, de Jong DJ. Elective switching from infliximab to adalimumab in stable Crohn’s disease. Inflamm Bowel Dis. 2013; 19:761–766. [DOI] [PubMed] [Google Scholar]
- 47.Ling Y, Xu SB, Lin YX, Tian D, Zhu ZQ, Dai FH, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J. 2020; 133:1039–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020; 395:683–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou W, Liu Y, Tian D, Wang C, Wang S, Cheng J, et al. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct Target Ther. 2020; 5:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mao R, Liang J, Shen J, Ghosh S, Zhu LR, Yang H, et al. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol. 2020; 5:425–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rahier JF, Papay P, Salleron J, Sebastian S, Ellul P, Teich N, et al. Influenza A (H1N1)v infection in patients with inflammatory bowel disease: a case series. Aliment Pharmacol Ther. 2011; 33:499–500. [DOI] [PubMed] [Google Scholar]


