Abstract
Social cognitive neuroscience is a novel field of interdisciplinary research that examines socio-emotional cognition and behavior by emphasizing the neural substrates of these processes. Insights from this biological perspective have established that socio-emotional processing does not happen in a sequential, but in a recursive and interlinked fashion; that individual brain regions are not associated with one, but multiple distinct social functions; and that brain regions are organized into dynamically interacting networks. These factors explain why it is difficult to pinpoint the neural substrates of particular social deficits in patients with brain diseases. With that said, there are specific brain regions that are highly specialized for the perception, regulation and modulation of emotion and behavior. This chapter will review key aspects of social processing beginning with their underlying neural substrates, including 1) perception of social signals, 2) social and emotional evaluation, and 3) behavioral response generation and selection. Case studies will be used to illustrate the real-life social deficits resulting from distinct patterns of neuroanatomic damage, highlighting the brain regions most critical for adequate social behavior.
Introduction
Social cognitive neuroscience is a novel field of interdisciplinary research that examines the emotional and cognitive processes necessary for navigating the human social world by emphasizing the neural substrates underlying these processes1. [KP1] Multiple distinct theories about the domains of social cognition exist, reflecting its complexity and interdisciplinary character.
These diverse theories agree that processes involved in social cognition do not happen in a sequential, but in a recursive and interlinked fashion; that brain regions are not associated with only one, but likely multiple social functions; and that brain regions are organized into networks, which interact dynamically with each other. These factors explain why it is difficult to pinpoint the neural substrates of particular social deficits in patients with brain diseases. While it is beyond the scope of this chapter to give a comprehensive review of the existing theories on the domains of social cognition and their underlying neural basis, we will provide a neuroanatomically-based overview of key social processes, including those implicated in the perception of socially relevant stimuli, their evaluation, and the generation of behavioral responses. We will also emphasize the critical roles emotional and motivational functioning play in healthy social behavior. The devastating impact of dysfunctional emotional processing on social behavior, sometimes in the presence of quite intact cognitive functions, will be also illustrated by case vignettes.
[KP1] Social cognitive neuroscience is a novel field of interdisciplinary research that examines socio-emotional cognition and behavior by emphasizing the neural substrates of these processes
I. Perception of Social Signals
Perception of socially relevant signals is central to successful navigation of the social world. Inputs relevant to social cognition arrive via vision, audition, touch, and smell. Of all the sensory systems involved in perception of social signals, we may understand vision the best. Social visual signals include information about the face, such as emotional expression and direction of gaze, as well as the body, in the form of socially communicative postures, gestures, and movements.2 Electrophysiological and neuroimaging studies in animals and humans converge to indicate that three major visual-association areas in the ventral occipitotemporal cortex and around the superior temporal sulcus are involved in the perceptual representation of these socially relevant visual signals.3-5 First, a region composed of the fusiform gyrus and its adjacent inferior temporal and occipital gyri with right-hemispheric dominance has been labeled as the fusiform face area, since it is preferentially activated by static facial features in functional neuroimaging studies.4 Its critical role in face recognition is supported by evidence that damage to this area and brain regions adjacent to it is associated with prosopagnosia,6 the inability to identify familiar faces. Second, an area of the right lateral occipitotemporal cortex, termed the extrastriate body area, responds preferentially to pictures of the human body, suggesting a specialized system for processing the visual appearance of the human body.5 Third, more anterior and dorsal regions of the temporal lobe, situated in and near the superior temporal sulcus, are preferentially involved in the perception of biological motion, such as gaze direction, as well as movements of the face (e.g., lips and mouth), head, hands, and body.3 These signals provide information about the actions, and by extension, the predicted action goals of others, an effect seen in several functional magnetic resonance imaging (fMRI) studies in which superior temporal sulcus regions were activated together with other brain regions when healthy individuals inferred the intentions of another person.7
In addition, anterior superior temporal sulcus regions, which some label the human-selective voice area, play an important role in voice perception, which is also of fundamental importance in social interactions.8 fMRI studies show that predominantly right-sided anterior superior temporal sulcus regions respond more to vocal than non-vocal sounds, more to human than animal vocalizations, and are involved in discriminating voices from different persons.8 All these reported [KP 2]brain regions in the temporo-occipital neocortex involved in encoding representations of socially relevant visual and auditory signals, likely play also a role in processing the emotional content of these signals in concert with other brain regions.9,10 We will discuss these processes in more detail in the next section.
Though few would argue that these regions are not fundamental to social perception, it is important to note that [KP 3]posterior lesions causing impaired perception of biological and non-biological signals do not necessarily cause inappropriate social behaviors. In fact, individuals with these posterior lesions may become even more sympathetic and friendly in social interactions (Case 5-1).
Case 5-1
A 56-year old nurse practitioner noticed that it was taking her progressively longer to read, and that her spelling was worsening. Her writing skills deteriorated also, although to a lesser degree. In addition, she became more hesitant in unfamiliar places and had difficulties finding routine landmarks such as restrooms. One to two years later she also started having difficulty reading the time on both digital and analog timepieces, and noted word-finding and multitasking problems. In the behavioral domain, the patient became more relaxed relative to the onset of the disease, showing less mental rigidity, although she got anxious when she experienced visual trouble. Her family history was significant for a father with onset of a dementia of unknown origin at the age of 82.
Examination revealed a pleasant, fully-oriented woman showing visual agnosia, simultanagnosia, severe acalculia and visuo-spatial deficits, and mild anomia and agraphia. There was mild executive dysfunction, whereas her memory appeared totally spared. Tasks assessing her ability to identify another person’s thoughts and feelings were normal. Caregiver-reports revealed no disease-associated changes in social graces, and the patient maintained an active social life with an extensive network of friends. MRI showed left greater than right atrophy of the temporo- and parieto-occipital cortices (Figure 5-1) and amyloid PET with Pittsburgh Compound-B (PIB) scan revealed amyloid deposits in the parietal and occipital lobes with relative sparing of the frontal lobe. Symptoms progressed within the next two years; in particular declines in visuo-spatial and executive functions, calculating, confrontation naming, and visual episodic memory were observed.
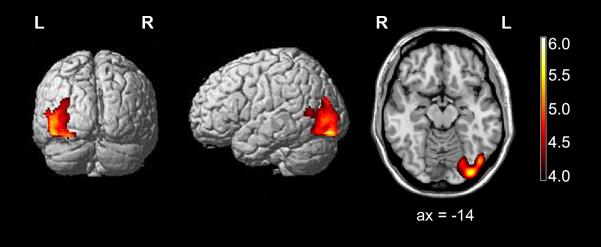
Figure 5-1.
Regions of patient’s gray matter loss relative to age-matched female healthy subjects using voxel based morphometry. Voxel based morphometry reveals atrophy of the left occipitotemporal cortex, predominantly involving the inferior and medial occipital gyri. Regions of grey matter loss are superimposed on rendered and sliced images of a standard brain from a single normal subject. The sliced image is displayed in radiologic convention (left is right); the rendered image with posterior view is displayed in neurologic convention (left is left), ax =axial.
Comment
This patient’s symptoms and findings are most consistent with a diagnosis of posterior cortical atrophy. Based on the positive amyloid PET scan Alzheimer’s disease is the most likely underlying neuropathology. The case highlights that affliction of brain regions involved in perception of social signals do not necessarily result in socially dysfunctional behavior. In some cases, to some degree also in the present case, patients with posterior brain lesions become even more sympathetic and friendly in social interactions, especially when the right hemisphere is less affected.
II. Evaluation of Social Signals
Recognizing the Salience of Environmental Stimuli
[KP 4]Upon perception of a social stimulus from the environment, the organism needs to automatically and rapidly recognize whether the stimulus has any personal relevance, essentially separating signal from noise. This initial step is necessary in order to focus cognitive resources on performing additional, more in-depth processing only on the stimuli deemed important. A number of structures play an essential role in this low-level salience detection process, all of which have been implicated in functional and lesion studies of social cognition.11,12
The amygdala is involved in at least three aspects of this salience-detection process relevant to social cognition. First, the amygdala is known to automatically, unconsciously assign a valence (i. e., emotional and motivational value associated with a stimulus) to biological stimuli,13 probably facilitating rapid processing of their potential reward or punishment value. For example, an fMRI study showed that the right amygdala is selectively sensitive to faces that had been associated with emotional descriptions compared to faces that had been associated with neutral descriptions, even when subjects were not consciously aware of the relationship between faces and description.14 Another study supported and extended these findings by showing that healthy people unconsciously preferred abstract images with high probability of food reward, whereas patients with anterior temporal lobe resections that included the amygdala were not influenced by these stimulus-valence associations.15 Second, it is well established that amygdala activity influences how more posterior occipitotemporal structures process emotional faces and bodies, in particular those faces with negative valence (e.g., fear).16 fMRI studies show that activations of face-selective brain regions such as the fusiform face area are enhanced in response to emotional faces compared to emotionally neutral faces,9 an effect likely due to modulatory feedback from the amygdala.13 Lesions studies support these findings showing decreased fusiform cortex activations in response to fearful faces in individuals with amygdala lesions.17 Similarly, the strength of amygdala response to emotional body postures correlates with the intensity of activations of body-selective brain regions such as the extrastriate body area.18 Third, the amygdala seems not only to be implicated in salience processing of biological stimuli, but also of stimuli that appear in an unpredictable fashion.19,20 Herry and colleagues demonstrated in mice and humans that the amygdala was more responsive to sequences with unpredictable tones than to sequences with predictable tones.19 Amygdala responses are also heightened in response to potentially threatening images, such as knives or guns 21 These findings suggest that [KP 5]the amygdala is involved in recognizing and assigning a valence to sensory stimuli that are potentially salient.20
Another critical brain structure for identifying the social salience of stimuli is the insula, which represents the physiological state of the body (interoceptive information) and brings it into awareness.22 Interoceptive information is mapped onto the posterior dorsal insula by way of the ventromedial thalamic nucleus. Cortical representations of the interoceptive information are then re-represented in the insula in a posterior-to-anterior fashion. As this gradient moves to the right anterior insula, higher-level information from the frontal lobes and anterior temporal structures interact with these representations to bring the physiological condition of the body into awareness (interoceptive awareness), likely subserving subjective experience of emotions (emotional awareness).22,23 These functions allow the insula to rapidly evaluate incoming stimuli for personal and social salience and to bring relevant stimuli to greater awareness. Often, the insula is coactivated with the anterior cingulate cortex during autonomic arousal in response to salient biological stimuli. Whereas [KP 6]the insula has been termed “limbic sensory cortex” based on its function in representing the physiological state of the body, the anterior cingulate cortex has been termed “limbic motor cortex” because of its role in autonomic control, aspects of performance monitoring such as error processing in effortful cognitive processes, and behavioral drive.12,22 Integrated with the insula, the anterior cingulate cortex assigns a motivational valence to stimuli, partly by modulating first-order autonomic centers such as the hypothalamus and motor centers such as the periaquaductal gray region in the brainstem.24 The orbitofrontal cortex (OFC), especially its lateral part, is involved in evaluating biological, and by extension, social stimuli for their potential for punishment. Functional neuroimaging studies in humans indicate that[KP 7] the lateral OFC is involved in evaluating the punishment value of stimuli (e. g., painful touch, angry facial expression), likely facilitating a change in behavior.25 The lateral OFC is also involved in the suppression and habituation to aversive stimuli such as a loud burst of noise.26 When these functions are disrupted by lateral OFC damage, as observed in patients with behavioral variant frontotemporal dementia and patients with OFC lesions, patients can overreact to unpredicted stimuli, or conversely, might be impaired in allocating attention to novel environmental stimuli, eventually resulting in impaired goal-directed behavior.27 Lastly, the lateral OFC inhibits activation of the amygdala during the recognition of socially salient stimuli such as emotional faces.28 This inhibitory function of the OFC probably resets the amygdala to ‘baseline’ function allowing the amygdala to resume its surveillance function for novel environmental signals.
[KP 8]The temporal pole, particularly on the right side, is not directly involved in autonomic arousal in response to salient stimuli, but does play a role in linking sensory representations with emotion and social memory, providing higher-level input into the decision about whether a stimulus is emotionally and socially salient.29 The functional role of the temporal pole is reflected by its structural connectivity. In monkeys, the temporal poles are highly interconnected with brain regions involved in autonomic arousal such as the hypothalamus, amygdala, insula, and OFC, as well as higher-order sensory regions in more posterior portions of the temporal lobes (30). Similarly to the amygdala, the temporal poles are responsive to emotional faces.31 In addition, humans who either have undergone surgical resection of the right anterior temporal lobe, or have sustained tissue loss in this area due to semantic dementia, sometimes fail to recognize personally familiar faces and/or voices,32,33 suggesting that the right temporal pole is implicated in coupling person-specific knowledge with polymodal perceptual representations.29 In contrast, prosopagnosic patients with more posterior temporal damage are solely impaired in recognizing familiar faces, but not voices, and can still identify emotional facial expressions.6 Decoupling of polymodal perceptual representations from their emotional and social contents might explain why lesion studies in animals and humans often associate right-sided or bilateral lesions of the anterior temporal lobes with abnormal social behavior. For example, female monkeys with lesions of the temporal poles, excluding the amygdala, lose their emotional attachment to peer monkeys and even to their own infants.34 Similarly, emotional detachment from close others has been observed in patients suffering from bilateral medial and anterior temporal lobe lesions due to herpes encephalitis,35 and in patients with semantic dementia showing predominantly atrophy of right anterior temporal regions.36
Neurodegeneration due to semantic dementia primary affects right or left anterior temporal regions, consistently including the temporal poles, and subsequently involves contralateral temporal regions and also insular and orbitofrontal regions,37 thus this condition provides important information about the role of the right temporal pole in human social behavior. [KP 9] Semantic dementia patients with right-sided involvement typically show abnormal social behavior including social withdrawal associated with emotional distance, bizarre facial expressions, irritability, impulsiveness, mental rigidity (including both obsessions and compulsions), and disruption of physiological drives (e. g, appetite, sleep) (Case 5-2).36,38 Interestingly, some of these patients exhibit fixed facial expressions, although they report that they feel happy, and have difficulties in posing different facial expressions. Another common feature of these patients is the dissociation between “cold” and “hot” reasoning about social situations. The cognitive functions required for evaluating and reacting to complex situations in life (e. g., knowing what measures to take in a medical emergency and acting accordingly) might be still quite intact, but their awareness of another person’s feelings (empathy) is often decimated.36 This dissociation may explain why these patients are often perceived as cold and arrogant in social interactions.39
Case 5-2
A 55 year-old accountant noted increasing difficulty recalling names of persons and objects. He also slowed down in reading and became impaired in comprehending and spelling common words. In addition, he had increasing difficulty recognizing familiar faces, up to the point that he was unable to differentiate between his two daughters. Around the same time, behavioral changes appeared, the first signs of which were a series of major life events within a short time period, including the loss of his job, likely due to interpersonal conflicts, and the divorce from his wife. At this time he was severely depressed and did have suicidal ideas. Based on his brother’s report he started to showed little respect for personal boundaries, especially with women, and developed rigid, obsessive eating behaviors based on books he read. Likely due to his diet regimen, he lost 15 to 20 pounds within 2 years. The patient also seemed impaired in perceiving other persons’ social signals, e.g., he would keep on talking even when the other person clearly appeared uncomfortable. He evidenced deficits in emotional control, in particular for positive emotions, e. g., when talking to a family member on the phone he sometimes became euphoric, and occasionally burst into tears. Family history was notable for a dementia syndrome in the Parkinson’s spectrum in his father, and early-onset Alzheimer’s disease in his paternal grandmother.
On examination he showed little eye contact and his affect was exceptionally flat. On a couple of occasions, though, for example when being asked to smile, the patient’s eyes became tearful, occasionally followed by laughing. He stated that he had a water problem in his head, which explained why he would cry too much when he was happy. During conversation he made several semantic paraphasic errors, and frequently used non-specific words such as ‘stuff’ and ‘thing’. He was severely impaired in naming and drawing objects, i. e., his drawing of a snake included two feet. He hardly recognized any famous faces and had almost no knowledge about famous people. He evidenced some mild executive dysfunction, whereas verbal and non-verbal episodic memory and visuospatial functions were preserved. He was severely impaired at labeling the emotions he saw in brief videos, and was unable to recognize when someone spoke sarcastically, though he had good comprehension of sincere social exchanges. Caregiver-reports on his interpersonal behavior revealed decreased extraversion and assertiveness, and greater coldness relative to his premorbid personality. MRI showed moderate right greater than left atrophy of the anterior temporal lobes, the insula and the anterior cingulate cortex, and mild right-sided orbitofrontal and caudate atrophy (Figure 5-2). Amyloid PET-PIB scan was negative, and FDG-PET showed predominantly right-sided anterior temporal hypometabolism. Within the next two years symptoms further increased, with further losses of caregiver-rated empathy. In addition, his hygiene deteriorated and he got impaired in activities of daily living, i. e., brother started managing his finances.
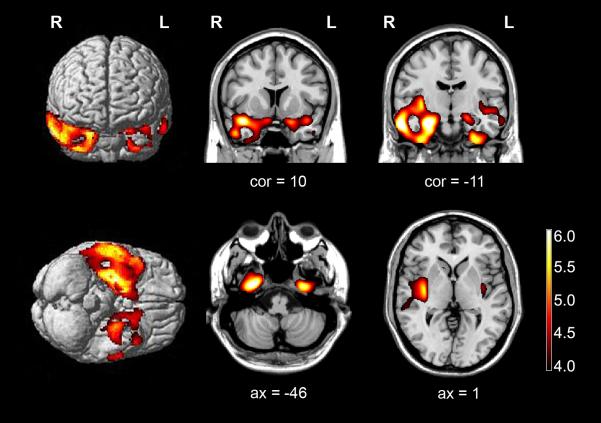
Figure 5-2.
Regions of patient’s grey matter loss relative to age-matched male healthy subjects using voxel based morphometry. Voxel based morphometry reveals predominantly right-sided anterior temporal lobe atrophy, including the temporal pole, amygdala, anterior fusiform and parahippocampal cortices, and the inferior temporal gyrus and predominantly right-sided insular atrophy. Regions of grey matter loss are superimposed on rendered and sliced images of a standard brain from a single normal subject. Images are displayed in radiologic convention (left is right).
cor = coronal, ax = axial,
Comment
This patient’s syndrome is most consistent with a diagnosis of semantic dementia, the temporal variant of frontotemporal lobar degeneration, with features of temporal lobe involvement on the right (emotional blunting, impaired perception of social cues, and prosopagnosia) and left side (anomia and semantic loss). In this case, symptoms related to right temporal involvement started somewhat earlier than left temporal symptoms, and this dissociation is reflected by the right predominance of his temporal atrophy. Typically, predominantly left-sided semantic dementia patients, who are more often reported in literature than right-sided semantic dementia patients,38 initially have near-normal social behavior, while their behavior becomes increasingly disordered with the advent of right-sided involvement.
Viewed individually, it is clear from the established functions of these key cortical and subcortical brain regions that they are implicated in recognizing the salience of environmental stimuli, and have relevance to social and emotional functioning. Furthermore, recent evidence unequivocally supporting the tight functional integration of these structures comes from functional connectivity analyses of healthy human brains.40 In a task-free state, Seeley and colleagues showed that blood oxygen level-dependant (BOLD) signal fluctuations of the amygdala, anterior insula, dorsal anterior cingulate cortex, and portions of the right temporal pole, together with brain regions mostly implicated in homeostatic regulation and emotion such as brainstem regions or the ventral striatum, covary across time, indicating an intrinsically connected functional network of these brain regions. This [KP 10]“salience network”, which likely serves as a gateway to emotional states and emotional awareness, has further been shown to share structural covariance in normal brains, and to be preferentially damaged in behavioral variant frontotemporal dementia.41
Emotion Recognition / Subjective Experience of Emotion
Once a stimulus is identified as personally relevant, it immediately takes on an emotional and motivational valence. Because of this, the[KP 11] cortical and subcortical brain regions implicated in identifying the salience of environmental stimuli are also key regions in emotion recognition and the subjective experience of emotion. These overlapping functions have been highlighted by Seeley and colleagues, who showed that subjects’ self-ratings of anxiety correlate with the level of functional connectivity of the dorsal anterior cingulate cortex of the resting-state “salience network”.40 The role of this intrinsic connectivity network in emotion recognition and emotional states is corroborated by other studies showing functional or structural involvement of these structures in social and emotional cognition. In individuals experiencing both autonomic and emotional arousal in response to various socially salient stimuli such viewing faces of loved ones 42 or social rejection,43 the anterior cingulate cortex and anterior insula are often coactivated. Internally inducing states of emotion by recalling personally relevant emotional events also coactivate the anterior cingulate cortex and insula.44
This link between the perception and experience of emotions is one mechanism by which observing another’s emotional state can automatically induce the same emotion internally. Electrophysiological and functional neuroimaging studies in animals and humans converge to indicate that regions that are activated by observing another person experiencing pain, emotion, or action largely overlap with regions that are activated by experiencing those phenomena, an occurrence sometimes referred to as “shared representations”.45 For example, parts of the cortical network (i. e., inferior parietal, inferior frontal pars opercularis, and premotor regions) activated when one is observing another person performing a motor action are also activated while oneself is performing that action.46 This “mirror” neuron system allows us to automatically and covertly simulate another person’s actions, and may provide a basis for understanding another person’s intentions, since this system seems to encode the goal of an observed action. A similar role for mirror neurons in emotion sharing has been suggested, because similar brain regions including the anterior insula and anterior cingulate cortex are activated when observing another person experiencing pain or expressing an emotion as also when experiencing one’s own pain or emotion.47 While shared representations may be important for optimal socio-emotional functioning, studies have not established whether these regions are critical for understanding another person’s intentions or emotional experiences, or merely play a supportive role. In addition, as we will discuss later, this [KP 12]automatic overlapping of actions and emotions belonging to the self and other is not sufficient to fully infer the other’s mental state, which requires additional processes such as correct attribution of agency and effortful, high-level executive operations.
Human lesion studies also support the interconnection of salience, arousal, and emotion. Insula lesions interfere with the ability to process aversive sensory stimuli, in particular facial and vocal expressions of disgust,48 and anterior cingulate cortex lesions (including medial prefrontal regions (Brodmann area 9) alter patients’ capacity for both identifying and experiencing emotions.49 Furthermore, decreased grey matter volume of the subgenual anterior cingulate cortex is observed in patients with emotion regulation disorders like major depression and bipolar disorder.50 Importantly, rostro-ventral portions of the anterior cingulate cortex and the right > left anterior insula are among the earliest and most consistently affected brain regions in patients with behavioral variant frontotemporal dementia.51 [KP 13] Behavioral variant frontotemporal dementia, a variant of frontotemporal lobar degeneration, is characterized by early, striking social behavior changes, which typically precede major cognitive deficits (Case 5-3). Early behavioral symptoms include apathy, loss of social inhibitions, changes in personality toward introversion and passivity, impaired insight into behavioral changes, and poor judgment..52
Case 5-3
A 62-year old physician became progressively more aloof, exhibiting increased insensitivity to others. On one occasion he abandoned his two 3-year-old grandchildren at night a block from their house, believing they could return home on their own. One to two years later, he started to behave in a sexually inappropriate manner towards different women, to eat voraciously (subsisting on junk food, pizza, and ice cream), to drink wine heavily, and to misuse medications such as Valium (up to 30mg a day). On several occasions, even after being explicitly told not to do so, he entered his neighbor’s garage and stole liquor. The patient lacked any insight into the inappropriateness of his actions. Family members reported that he also showed impaired decision-making and problem-solving in daily life situations, e. g., shuffling boxes around without purpose during a family move. Family history revealed no neurological or psychiatric disorders apart from myasthenia gravis of the patient’s father.
Neurological exam was normal apart from a proximal symmetrical weakness of the upper and lower extremities. Scores of standard neuropsychological tests were within average apart from mild impairments in verbal generation and in a complex executive task requiring a combination of set-shifting and verbal response inhibition. Despite his quite preserved cognitive skills, his emotion recognition for faces and voices was impaired, as was his ability to detect sarcasm and deception and to adopt another person’s perspective. Based on caregiver-reports, his empathic concern for others was abnormally low and had decreased substantially since disease onset. MRI revealed right greater than left medial prefrontal, orbitofrontal, insular, and anterior temporal atrophy (Figure 5-3).
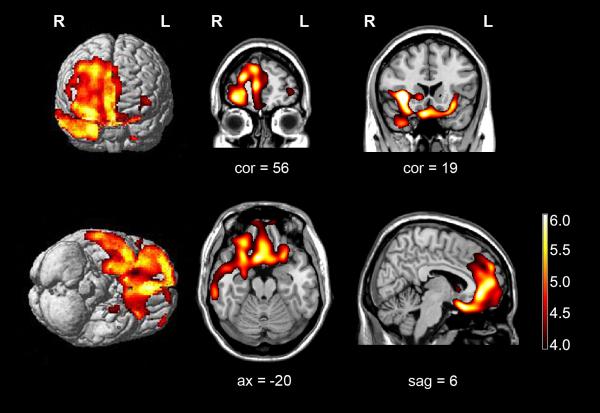
Figure 5-3.
Regions of patient’s grey matter loss relative to age-matched male healthy subjects using voxel based morphometry. Voxel based morphometry reveals predominantly right-sided atrophy of the frontal lobe, including the orbitofrontal cortex and the medial prefrontal and dorsolateral prefrontal cortices. In addition, there is atrophy of the right anterior caudate nucleus, right anterior insula, and the right anterior temporal lobe, in particular the temporal pole. Regions of grey matter loss are superimposed on rendered and sliced images of a standard brain from a single normal subject. Images are displayed in radiologic convention (left is right).
The patient died 12 months after the first evaluation as a result of a sepsis, probably due to an invasive chondrosarcoma. The neuropathological pattern was consistent with Pick’s disease, showing tau positive neurons especially within the hippocampus and the temporal lobe, ballooned achromatic neurons in the cingulate gyrus, and scattered tau positive astrocytes within the cerebral cortex and subcortical white matter.53
Comment
This patient suffered from Pick’s disease, and his clinical presentation met diagnostic criteria for behavioral variant frontotemporal dementia. Despite quite intact executive functioning, he was impaired in high-level socio-cognitive tasks involving his ability to adopt another person’s thoughts and feelings. This discrepancy has been observed frequently in other patients with behavioral variant frontotemporal dementia, suggesting that while impairment in executive functions as measured by standard neuropsychological tests is not necessary for failure of these high-level socio-cognitive tasks, the ability to concurrently recognize and experience emotions plays an essential role.
Self-Other Distinction / Attribution of Agency
In the previous section we described the neural mechanisms underlying shared representations, and suggested that others’ fundamental emotional and motivational states can be automatically mirrored in one’s own internal state. While this is an important initial step towards understanding others, additional cognitive processes are required to recognize where the self ends, and the other begins. [KP 14]Without self-other distinction, our interpretation of others’ behavior remains egocentric and inaccurate.54 Self-other distinctions set the stage for perspective-taking, and allow an internal emotional state generated by perceiving another’s emotion to transcend the level of primitive emotional contagion and lead to a mature empathic response (e. g, “It makes me sad that her dog died, but it was her dog, not mine, so I should be comforting her.”) Neuroanatomically, the right inferior parietal cortex at the junction of the posterior temporal cortex [KP 15](the temporoparietal junction), plays an important role in identifying who initiated an emotion or action intention, and provides a basis for distinguishing the self from the other.55 Functional neuroimaging studies indicate that the right temporoparietal junction is critical for attributing a sense of agency, i.e., comparing self-generated and other-generated actions.56 Transcranial magnetic stimulation applied over the right inferior parietal cortex to generate transient ‘functional lesions’ causes impaired discrimination between one’s own face and other familiar faces.57 Similarly, electrical stimulation of this region causes out-of-body experiences (i. e. the experience that one’s body is no longer one’s own),58 and damage to this region is associated with unawareness of paresis, and misidentification of one’s own limbs. The temporoparietal junction is an essential part of the right-lateralized ventral attention network, which reorients attention to salient, novel stimuli in both visual and auditory modalities.59 In a social context, it seems to function in part as an “other-detector,” interrupting ongoing cognitive processes to alert one to the presence of an agency that is not one’s own, and diverting attentional resources towards this potentially important stimulus. While many studies have shown activations of the temporoparietal junction in the context of social perspective-taking, they suggest that it coactivates with medial frontal structures. While the right temporoparietal junction is involved in more low-level aspects of making self-other distinctions, right dorsomedial prefrontal regions perform the more abstract functions involved in mental state attribution.55
III. High-Level Social-Cognitive Processing and Behavioral Response Selection
The socio-emotional functions described in the previous two sections are comparatively primitive and are hard-wired into the human brain, meaning that they are fairly robust, show little variability across individuals, and do not require much education, effort, or general intelligence. Clearly, however, many aspects of social cognition are susceptible to environmental factors such as developmental milieu and cultural training as well as intrinsic factors such as general fluid intelligence and temperament. Individual differences in capacity for these higher-level social functions account for the tremendous variability in social skills and personality across normal persons, and only part of this can be explained by neural factors. However, some higher-order components of social functioning, such as the ability to perform complex reasoning about another’s perspective, do rely on specific neural networks.
Adapting Another’s Perspective
One’s ability to create shared representations and simulate others’ emotions provides a basis for understanding others; however, one’s representations of the other are rooted in one’s personal knowledge, attitudes, and beliefs derived from previous life experiences. This means that one’s own perspective is the ‘default mode of the human mind’, thus [KP 16]successful social interactions require one to make an effort to identify where the other’s perspective differs from self-perspective.45 Even one’s own self-perspective differs across time, such that one’s current desires and drive states can influence what one believes one might have thought or done in the past, or would think or do in the future.54 Thus, the ability to accurately estimate even one’s own perspective, much less another’s perspective, can require elements of mental time travel as well as self-regulation. First, the ability to project oneself into the past or the future is partly mediated by a network of structures often called the “memory network” or the “default mode network”, which includes medial frontal as well as parietal and hippocampal regions.60 Notably, patients with autistic disorder, who are impaired in adopting another’s mental or emotional perspective, show decreased activity in this “time travel” network when simply lying in the scanner without performing any task, i.e., in the resting-state.60 Second, effortful self-regulation is also required for accurate high-level perspective-taking, because the ability to set aside one’s own current perspective requires both mental flexibility and active inhibitory processes,45 abilities which to some degree overlap with traditional executive functions, and which may be mediated by frontopolar, dorsolateral frontal, and parietal structures.
Functional neuroimaging studies of self- and other-perspective taking reveal a variety of activations, usually including dorsal and rostral medial prefrontal cortex and adjacent paracingulate cortex, the right posterior superior temporal sulcus, right temporoparietal junction, and the temporal poles.61 Of these regions the [KP 17]medial prefrontal cortex and adjacent paracingulate gyrus are the most consistently activated regions when adapting another person’s perspective. Results from lesion studies, however, are less consistent. While Shamay-Tsoory and colleagues found that right medial prefrontal lesions were the region most likely to be affected in frontal lesion patients with abnormal perspective taking,62 other studies have pointed out that focal lesions of the dorsomedial prefrontal cortex and/or anterior cingulate cortex do not necessarily result in impaired perspective taking of mental states such as thoughts and intentions.63,64 These findings suggest that perspective taking, or more broadly, the ability to correctly infer others’ thoughts and intentions, does not rely solely on dorsomedial prefrontal structures, but probably also on the other brain regions of the network, i.e., right posterior superior temporal sulcus, right temporoparietal junction, and the temporal poles. While these other regions seem not uniquely associated with mental state inference, they provide supportive information by recognizing goal-directed behavior (posterior superior temporal sulcus), distinguishing between one’s own and others’ agency and intentions (right temporoparietal junction), and retrieving semantic and autobiographical knowledge (temporal poles). While both can share these “time travel” and “executive functioning” elements, cognitive perspective taking (the capacity to attribute mental states such as thoughts and intentions, or cognitive theory of mind) can be distinguished from emotional perspective taking (the capacity to attribute emotional experiences; emotional theory of mind; empathy). Emotional perspective taking is based on the more fundamental capacity to automatically and covertly simulate another’s emotions, utilizing the ventromedial frontal circuits described earlier. A recent fMRI study revealed that the [KP 18]medial orbitofrontal cortex (OFC) is far more recruited in emotional than in cognitive perspective-taking.65 Clinical dissociations between cognitive and emotional perspective taking deficits abound. For example both patients with behavioral variant frontotemporal dementia and with Alzheimer’s disease (AD) are impaired on inferring mental states,66 though in AD patients impaired cognitive perspective taking ability is likely due primarily to cognitive deficits such as impaired working memory and set-shifting. In contrast, patients with behavioral variant frontotemporal dementia and patients with OFC-lesions, but not AD patients, are impaired in inferring emotional experience.67
While the ability to imagine what another person thinks is important, it should not be considered a marker for healthy social skills. Cognitive perspective taking can remain intact in patients with dysfunctional social behavior68 suggesting that the ability to understand another person’s intentions is not adequate to prevent social deficits. In contrast, [KP 19]loss of emotional perspective taking or lack of empathy is consistently associated with dysfunctional social behavior, highlighting the crucial role of intact emotion processing on social behavior.
Behavioral Regulation
Having understood the other persons’ thoughts and feelings, one must regulate one’s behavioral response in a manner appropriate to the context. Behavioral regulation involves top-down control processes, including both emotion regulation and integration of attitudes with external social context. These processes are primarily mediated by a dorso- and ventrolateral prefrontal network, but also by dorsomedial prefrontal regions, including the dorsal anterior cingulate cortex.27 The capacity for emotion regulation has a particularly critical influence on maintaining adequate social behavior. One important strategy for emotion regulation is known as cognitive reappraisal, and involves the conscious reinterpretation of the meaning of an emotional experience in order to change the emotional response. Effective reappraisal is associated with better interpersonal functioning and psychological and physical well-being.69 Interestingly activations of brain regions involved in emotion reappraisal modulate the activity of emotion-processing regions such as the amygdala, subgenual anterior cingulate cortex, ventromedial prefrontal cortex, and the insula.70 These relationships imply that [KP 20]primarily neocortical, dorsal prefrontal regions exert control over limbic/paralimbic, ventral prefrontal and anterior temporal regions to modulate emotional experience and autonomic arousal. Brain regions involved in cognitive control of emotion are important for attenuating emotional distress, thus facilitating goal-oriented interpersonal behavior, but they seem not as critical for adequate emotional and social behavior as the phylogenetically older emotion - processing regions situated in the ventromedial prefrontal and anterior temporal cortices.
Future Directions
[KP 21]There is a growing trend away from reducing social and emotional cognition down to the functions of single brain regions, and a greater emphasis on identifying the interactive neural networks that underpin social and emotional functioning. One recent technical development in fMRI has made it possible to identify these networks by showing which brain regions functionally covary in task-free, resting-states. This approach, applied to healthy individuals, has revealed a number of highly reproducible intrinsically connected networks that are involved in functions from vision and sensory-motor processing to attentional control and memory. These functional networks closely match structural networks in humans, as revealed by diffusion tensor imaging tractography71 and by structural covariance studies41 Identifying how these intrinsically connected networks work together to support different aspects of social cognition may provide a new level of explanation to existing theories about brain-behavior relationships.
While extraordinary advances have been made in social cognitive neuroscience thanks to task-based functional imaging studies, [KP 22] studies of humans with brain lesions are still required to demonstrate causality in neuroanatomic models of emotion and social cognition. One alternative to patient-based research, which by necessity has relied on observational rather than experimental research designs, might be the application of the non-invasive trancranial magnetic stimulation (TMS) to healthy humans. TMS, which induces transient changes in brain activity by rapidly changing magnetic fields, has already been used to isolate neuroanatomic structures underlying higher social cognitive processes such as decision-making 72 and perspective-taking 73. In combination with observational studies of brain damaged patients showing aberrant social behavior, such as patients with behavioral variant frontotemporal dementia, the temporary lesions created by this experimental intervention can more precisely characterize the neural basis of social and emotional processes.
KEY POINTS.
[KP 1] Social cognitive neuroscience is a novel field of interdisciplinary research that examines socio-emotional cognition and behavior by emphasizing the neural substrates of these processes
[KP 2] brain regions in the temporo-occipital neocortex involved in encoding representations of socially relevant visual and auditory signals, likely play also a role in processing the emotional content of these signals in concert with other brain regions
[KP 3] posterior lesions causing impaired perception of biological and non-biological signals do not necessarily cause inappropriate social behaviors
[KP 4] Upon perception of a social stimulus from the environment, the organism needs to automatically and rapidly recognize whether the stimulus has any personal relevance
[KP 5] the amygdala is involved in recognizing and assigning a valence to sensory stimuli that are potentially salient
[KP 6] the insula has been termed “limbic sensory cortex” based on its function in representing the physiological state of the body, the ACC has been termed “limbic motor cortex” because of its role in autonomic control, aspects of performance monitoring and behavioral drive
[KP 7] the lateral OFC is involved in evaluating the punishment value of stimuli likely facilitating a change in behavior
[KP 8] The temporal pole does play a role in linking sensory representations with emotion and social memory
[KP 9] SemD patients with right-sided involvement typically show abnormal social behavior including social withdrawal associated with emotional distance, mental rigidity (including both obsessions and compulsions), and disruption of physiological drives
[KP 10] ]“salience network”, which likely serves as a gateway to emotional states and emotional awareness, has been shown to be preferentially damaged in behavioral variant frontotemporal dementia
[KP 11] cortical and subcortical brain regions implicated in identifying the salience of environmental stimuli are also key regions in emotion recognition and the subjective experience of emotion
[KP 12] automatic overlapping of actions and emotions belonging to the self and other is not sufficient to fully infer the other’s mental state
[KP 13] bvFTD, a variant of frontotemporal lobar degeneration (FTLD), is characterized by early, striking social behavior changes, which typically precede major cognitive deficits
[KP 14] Without self-other distinction, our interpretation of others’ behavior remains egocentric and inaccurate
[KP 15] ]the temporoparietal junction, plays an important role in identifying who initiated an emotion or action intention, and provides a basis for distinguishing the self from the other
[KP 16] successful social interactions require one to make an effort to identify where the other’s perspective differs from self-perspective
[KP 17] medial prefrontal cortex and adjacent paracingulate gyrus are the most consistently activated regions when adapting another person’s perspective
[KP 18] medial OFC is far more recruited in emotional than in cognitive perspective-taking
[KP 19] loss of emotional perspective taking is consistently associated with dysfunctional social behavior
[KP 20] primarily neocortical, dorsal prefrontal regions exert control over limbic/paralimbic, ventral prefrontal and anterior temporal regions to modulate emotional experience and autonomic arousal
[KP 21] There is a greater emphasis on identifying the interactive neural networks that underpin social and emotional functioning
[KP 22] human lesion studies are still required to demonstrate causality in neuroanatomic models of emotion and social cognition
REFERENCES
- 1.Ochsner KN, Lieberman MD. The emergence of social cognitive neuroscience. Am Psychol. 2001;56(9):717–734. [PubMed] [Google Scholar]
- 2.Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4(3):165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 3.Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. 2000;4(7):267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- 4.Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci. 2006;361(1476):2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Downing PE, Jiang Y, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293(5539):2470–2473. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- 6.De Renzi E. Disorders of visual recognition. Semin Neurol. 2000;20(4):479–485. doi: 10.1055/s-2000-13181. [DOI] [PubMed] [Google Scholar]
- 7.Gallagher HL, Frith CD. Functional imaging of “theory of mind”. Trends Cogn Sci. 2003;7(2):77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 8.Belin P. Voice processing in human and non-human primates. Philos Trans R Soc Lond B Biol Sci. 2006;361(1476):2091–2107. doi: 10.1098/rstb.2006.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30(3):829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 10.Beaucousin V, Lacheret A, Turbelin MR, et al. FMRI study of emotional speech comprehension. Cereb Cortex. 2007;17(2):339–352. doi: 10.1093/cercor/bhj151. [DOI] [PubMed] [Google Scholar]
- 11.Adolphs R. The social brain: neural basis of social knowledge. Annu Rev Psychol. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493(1):154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 13.Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11(11):489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Somerville LH, Wig GS, Whalen PJ, Kelley WM. Dissociable medial temporal lobe contributions to social memory. J Cogn Neurosci. 2006;18(8):1253–1265. doi: 10.1162/jocn.2006.18.8.1253. [DOI] [PubMed] [Google Scholar]
- 15.Johnsrude IS, Owen AM, White NM, et al. Impaired preference conditioning after anterior temporal lobe resection in humans. J Neurosci. 2000;20(7):2649–2656. doi: 10.1523/JNEUROSCI.20-07-02649.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41(1):88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- 17.Vuilleumier P, Richardson MP, Armony JL, et al. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 2004;7(11):1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- 18.Peelen MV, Atkinson AP, Andersson F, Vuilleumier P. Emotional modulation of body-selective visual areas. Soc Cogn Affect Neurosci. 2007;2(4):274–283. doi: 10.1093/scan/nsm023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herry C, Bach DR, Esposito F, et al. Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 2007;27(22):5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whalen PJ. The uncertainty of it all. Trends Cogn Sci. 2007;11(12):499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Kensinger EA, Garoff-Eaton RJ, Schacter DL. How negative emotion enhances the visual specificity of a memory. J Cogn Neurosci. 2007;19(11):1872–1887. doi: 10.1162/jocn.2007.19.11.1872. [DOI] [PubMed] [Google Scholar]
- 22.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 23.Critchley HD, Wiens S, Rotshtein P, et al. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 24.Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- 25.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Rule RR, Shimamura AP, Knight RT. Orbitofrontal cortex and dynamic filtering of emotional stimuli. Cogn Affect Behav Neurosci. 2002;2(3):264–270. doi: 10.3758/cabn.2.3.264. [DOI] [PubMed] [Google Scholar]
- 27.Hooker CI, Knight RT. The role of lateral orbitofrontal cortex in the inhibitory control of emotion. In: Zald DH, Rauch SL, editors. The Orbitofrontal Cortex. Oxford University Press; New York: 2006. pp. 307–324. [Google Scholar]
- 28.Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11(1):43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- 29.Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130(pt 7):1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- 30.Mesulam MM. Behavioral neuroanatomy. In: Mesulam MM, editor. Principles in Behavioral and Cognitive Neurology. Oxford University Press; New York: 2000. [Google Scholar]
- 31.Blair RJ, Morris JS, Frith CD, et al. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122(pt 5):883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- 32.Gainotti G, Barbier A, Marra C. Slowly progressive defect in recognition of familiar people in a patient with right anterior temporal atrophy. Brain. 2003;126(pt 4):792–803. doi: 10.1093/brain/awg092. [DOI] [PubMed] [Google Scholar]
- 33.Glosser G, Salvucci AE, Chiaravalloti ND. Naming and recognizing famous faces in temporal lobe epilepsy. Neurology. 2003;61(1):81–86. doi: 10.1212/01.wnl.0000073621.18013.e1. [DOI] [PubMed] [Google Scholar]
- 34.Kling A, Steklis HD. A neural substrate for affiliative behavior in nonhuman primates. Brain Behav Evol. 1976;13(2-3):216–238. doi: 10.1159/000123811. [DOI] [PubMed] [Google Scholar]
- 35.Lilly R, Cummings JL, Benson DF, Frankel M. The human Kluver-Bucy syndrome. Neurology. 1983;33(9):1141–1145. doi: 10.1212/wnl.33.9.1141. [DOI] [PubMed] [Google Scholar]
- 36.Mychack P, Kramer JH, Boone KB, Miller BL. The influence of right frontotemporal dysfunction on social behavior in frontotemporal dementia. Neurology. 2001;56(11 suppl 4):S11–15. doi: 10.1212/wnl.56.suppl_4.s11. [DOI] [PubMed] [Google Scholar]
- 37.Brambati SM, Rankin KP, Narvid J, et al. Atrophy progression in semantic dementia with asymmetric temporal involvement: a tensor-based morphometry study. Neurobiol Aging. 2009;30(1):103–111. doi: 10.1016/j.neurobiolaging.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seeley WW, Bauer AM, Miller BL, et al. The natural history of temporal variant frontotemporal dementia. Neurology. 2005;64(8):1384–1390. doi: 10.1212/01.WNL.0000158425.46019.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sollberger M, Stanley CM, Wilson SM, et al. Neural basis of interpersonal traits in neurodegenerative diseases. Neuropsychologia. 2009;47(13):2812–2827. doi: 10.1016/j.neuropsychologia.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seeley WW, Crawford RK, Zhou J, et al. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21(3):1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302(5643):290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 44.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 45.Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev. 2004;3(2):71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- 46.Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends Cogn Sci. 2004;8(9):396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24(3):771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Calder AJ, Lawrence AD, Young AW. Neuropsychology of fear and loathing. Nat Rev Neurosci. 2001;2(5):352–363. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- 49.Hornak J, Bramham J, Rolls ET, et al. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126(pt 7):1691–1712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- 50.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13(8):663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seeley WW, Crawford R, Rascovsky K, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65(2):249–255. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang PN, Miller BL. Clinical aspects of frontotemporal dementia. In: Miller BL, Cummings JL, editors. The Human Frontal Lobes. Guilford Press; New York: 2007. pp. 365–381. [Google Scholar]
- 53.Narvid J, Gorno-Tempini ML, Slavotinek SJ, et al. Of brain and bone: the unusual case of Dr. A. Neurocase. 2009;15(3):190–205. doi: 10.1080/13554790802632967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Boven L, Loewenstein G. Social projection of transient drive states. Pers Soc Psychol Bull. 2003;29(9):1159–1168. doi: 10.1177/0146167203254597. [DOI] [PubMed] [Google Scholar]
- 55.Decety J, Sommerville JA. Shared representations between self and other: a social cognitive neuroscience view. Trends Cogn Sci. 2003;7(12):527–533. doi: 10.1016/j.tics.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 56.Farrer C, Frith CD. Experiencing oneself vs another person as being the cause of an action: the neural correlates of the experience of agency. Neuroimage. 2002;15(3):596–603. doi: 10.1006/nimg.2001.1009. [DOI] [PubMed] [Google Scholar]
- 57.Uddin LQ, Molnar-Szakacs I, Zaidel E, Iacoboni M. rTMS to the right inferior parietal lobule disrupts self-other discrimination. Soc Cogn Affect Neurosci. 2006;1(1):65–71. doi: 10.1093/scan/nsl003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blanke O, Arzy S. The out-of-body experience: disturbed self-processing at the temporoparietal junction. Neuroscientist. 2005;11(1):16–24. doi: 10.1177/1073858404270885. [DOI] [PubMed] [Google Scholar]
- 59.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 60.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 61.Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358(1431):459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shamay-Tsoory SG, Tomer R, Berger BD, Aharon-Peretz J. Characterization of empathy deficits following prefrontal brain damage: the role of the right ventromedial prefrontal cortex. J Cogn Neurosci. 2003;15(3):324–337. doi: 10.1162/089892903321593063. [DOI] [PubMed] [Google Scholar]
- 63.Bird CM, Castelli F, Malik O, et al. The impact of extensive medial frontal lobe damage on “Theory of Mind” and cognition. Brain. 2004;127(pt 4):914–928. doi: 10.1093/brain/awh108. [DOI] [PubMed] [Google Scholar]
- 64.Baird A, Dewar BK, Critchley H, et al. Social and emotional functions in three patients with medial frontal lobe damage including the anterior cingulate cortex. Cogn Neuropsychiatry. 2006;11(4):369–388. doi: 10.1080/13546800444000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hynes CA, Baird AA, Grafton ST. Differential role of the orbital frontal lobe in emotional versus cognitive perspective-taking. Neuropsychologia. 2006;44(3):374–383. doi: 10.1016/j.neuropsychologia.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 66.Fernandez-Duque D, Baird JA, Black SE. False-belief understanding in frontotemporal dementia and Alzheimer’s disease. J Clin Exp Neuropsychol. 2009;31(4):489–497. doi: 10.1080/13803390802282688. [DOI] [PubMed] [Google Scholar]
- 67.Gregory C, Lough S, Stone V, et al. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease: theoretical and practical implications. Brain. 2002;125(pt 4):752–764. doi: 10.1093/brain/awf079. [DOI] [PubMed] [Google Scholar]
- 68.Blair RJ, Cipolotti L. Impaired social response reversal. A case of “acquired sociopathy”. Brain. 2000;123(pt 6):1122–1141. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- 69.Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- 70.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 71.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knoch D, Fehr E. Resisting the power of temptations: the right prefrontal cortex and self-control. Ann N Y Acad Sci. 2007;1104:123–134. doi: 10.1196/annals.1390.004. [DOI] [PubMed] [Google Scholar]
- 73.Kalbe E, Schlegel M, Sack AT, et al. Dissociating cognitive from affective theory of mind: A TMS study. Cortex. 2009 doi: 10.1016/j.cortex.2009.07.010. [DOI] [PubMed] [Google Scholar]