Abstract
Recent publications on the probable role of heparin-binding protein (HBP) as a biomarker in sepsis prompted us to investigate its diagnostic and prognostic performance in severe COVID-19. HBP and IL-6 were measured by immunoassays at admission and on day 7 in 178 patients with pneumonia by SARS-CoV-2. Patients were classified into non-sepsis and sepsis as per the Sepsis-3 definitions and were followed up for the development of severe respiratory failure (SRF) and for outcome. Results were confirmed by multivariate analyses. HBP was significantly higher in patients classified as having sepsis and was negatively associated with the oxygenation ratio and positively associated with creatinine and lactate. Logistic regression analysis evidenced admission HBP more than 18 ng/ml and IL-6 more than 30 pg/ml as independent risk factors for the development of SRP. Their integration prognosticated SRF with respective sensitivity, specificity, positive predictive value, and negative predictive 59.1%, 96.3%, 83.9%, and 87.8%. Cox regression analysis evidenced admission HBP more than 35 ng/ml and IL-6 more than 30 pg/ml as independent risk factors for 28-day mortality. Their integration prognosticated 28-day mortality with respective sensitivity, specificity, positive predictive value, and negative predictive value 69.2%, 92.7%, 42.9%, and 97.5%. HBP remained unchanged over-time course. A prediction score of the disposition of patients with COVID-19 is proposed taking into consideration admission levels of IL-6 and HBP. Using different cut-offs, the score may predict the likelihood for SRF and for 28-day outcome.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10096-020-04145-7.
Keywords: Heparin-binding protein, Interleukin-6, Respiratory failure, Mortality, Prognosis, COVID-19
Introduction
According to the new 2016 sepsis definition, sepsis is a life-threatening organ dysfunction that results from the dysregulated host response to an infection [1]. This definition does not distinguish between bacterial, fungal, and viral origin of an infection. With this in mind, severe lung infection caused by the new coronavirus SARS-CoV-2 (COVID-19) may well be a sepsis reaction. Indeed, recent findings from our group has shown that complex immune dysregulation in severe COVID-19 is dominated either by hyper-inflammatory responses or by modulation of the function of interleukin (IL)-6 receptor [2], thus supporting the concept that severe COVID-19 meets the Sepsis-3 definition.
With this in mind and considering the excessive mortality of severe COVID-19, it is reasonable to seek help from the available portfolio of biomarkers in traditional bacterial sepsis to identify at an early stage patients at risk of severe complications or of an unfavorable outcome. Heparin-binding protein (HBP) is secreted by the azurophilic granules of neutrophils and has been associated with organ dysfunction of sepsis, namely, acute respiratory dysfunction and acute kidney injury (AKI), and has been proposed to be used for the triage of patients at the emergency department (ED) [3]. Severe respiratory failure (SRF) is the most severe complication of pneumonia by SARS-CoV-2 [4, 5]. In the present study, we investigated if admission levels of HBP can be an early biomarker of organ dysfunction and of unfavorable outcome in a cohort of patients with pneumonia by SARS-CoV-2.
Patients and methods
Patients with infection by SARS-CoV-2 were enrolled in a prospective study during the period March to May 2020 in 10 study sites of the Hellenic Sepsis Study Group (seven departments of Internal Medicine and three Intensive Care Units). The study protocol has been approved by the Ethics Committees of the participating hospitals. Written informed consent was provided by the patients or first-degree relative in case of patients unable to consent. Inclusion criteria were as follows: (a) adults of both genders, (b) molecular detection of SARS-CoV-2 by RT-PCR using the material collected through one nasopharyngeal swab, (c) radiological signs compatible with lower respiratory tract infection in chest X-ray or chest computed tomography, (d) at least two signs of the systemic inflammatory response syndrome (SIRS), and (e) blood intake less than 24 h from the start of SIRS. Patients with infection by the human immunodeficiency virus and with neutropenia due to causes other than SIRS were excluded.
Blood sampling was performed at admission and after 7 days. At each time point, 5 ml of EDTA blood was sampled after venipuncture of one antecubital vein under aseptic conditions and centrifuged. HBP was measured in plasma by fluorescence dry quantitative immunoassay using the Jet-iStar 800 analyzer (JoinStar, Hangzhou, China); the lower limit of detection was 5.9 ng/ml. Interleukin (IL)-6 was also measured by an enzyme immunosorbent assay (Invitrogen, Carlsbad, CA, USA); the lower limit of detection was 10 pg/ml.
The following information were collected at baseline: (a) demographics and comorbidities allowing the measurement of Charlson Comorbidity Index (CCI); (b) severity scores of APACHE II (acute physiology and chronic health evaluation), pneumonia severity index (PSI), and SOFA (sequential organ failure assessment) score; (c) complete blood cell count and differential; and (d) biochemistry and arterial blood gasses; measurement of blood gasses was done using separate samples coming from arterial puncture. Patients were followed up on a daily basis for 28 days for the development of SRF and for survival. SRF was defined as a composite endpoint and included patients with pO2/FiO2 ratio less than 150 mmHg necessitating intubation and mechanical ventilation (MV). This definition contains all patients with real respiratory failure since it renders certain that low pO2/FiO2 is a condition that cannot be reversed without MV. Sepsis at admission was defined as any SOFA score equal to or more than 2 [1].
Results of HBP and IL-6 were expressed as medians and 95% confidence intervals (CIs). Comparisons were done between patients who developed SRF and those who did not develop SRF during follow-up by the Mann-Whitney U test. Similar comparisons were done for quantitative variables using the Student t test and for qualitative variables using the Fisher exact test. Quantitative variables were transformed into binomial variables using the Youden index by the coordinate points of the ROC (receiver operator characteristics) curve analysis. The CIs of percentages were calculated. Comparisons between groups were done using the Fisher exact test and Bonferroni correction for multiple comparisons. Forward step-wise logistic regression analysis was done between variables significant after univariate analysis in order to define variables independently associated with the development of SRF. Cox step-wise forward analysis was done between variables significant after univariate analysis in order to define variables independently associated with 28-day outcome. Correlation between variables was done using Spearman’s rank of order. Comparisons between admission and day 7 concentrations of HBPP and IL-6 were done by the Wilcoxon’s test. Any p value lower than 0.05 was considered significant.
Results
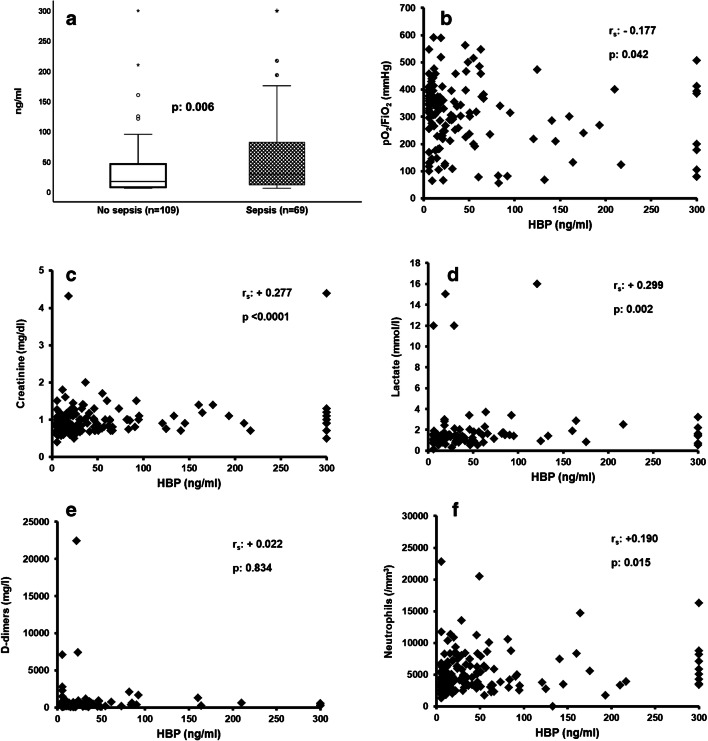
A total of 178 patients were enrolled during the study period. Their demographic baseline characteristics are provided in supplementary Tables 1 and 2. Those who were classified with sepsis at admission had higher levels of HBP (Fig. 1a). HBP was associated with organ dysfunction since it was negatively correlated with the pO2/FiO2 ratio (Fig. 1b); it was positively correlated with serum creatinine (Fig. 1c); and it was positively correlated with plasma lactate (Fig. 1d). HBP did not correlate with plasma D-dimers (Fig. 1e); and it was positively correlated with the absolute neutrophil count (Fig. 1f). Correlations with other biomarkers are provided in supplementary Fig. 1.
Fig. 1.
Association between heparin-binding protein (HBP) and organ dysfunction among patients with pneumonia by SARS-CoV-2. (a) Comparison of HBP between patients without sepsis by SARS-CoV-2 and sepsis by SARS-CoV-2; the p value of comparison by the Mann-Whitney U tests is provided. Circles denote outliers, and asterisk denotes extremes. (b) Correlation between HBP and pO2/FiO2; Spearman’s rank of correlation (rs) and the respective p value are provided. (c) Correlation between HBP and plasma creatinine; Spearman’s rank of correlation (rs) and the respective p value are provided. (d) Correlation between HBP and plasma lactate; Spearman’s rank of correlation (rs) and the respective p value are provided. (e) Correlation between HBP and plasma concentration of D-dimers; Spearman’s rank of correlation (rs) and the respective p value are provided. (f) Correlation between HBP and the absolute neutrophil count; Spearman’s rank of correlation (rs) and the respective p value are provided
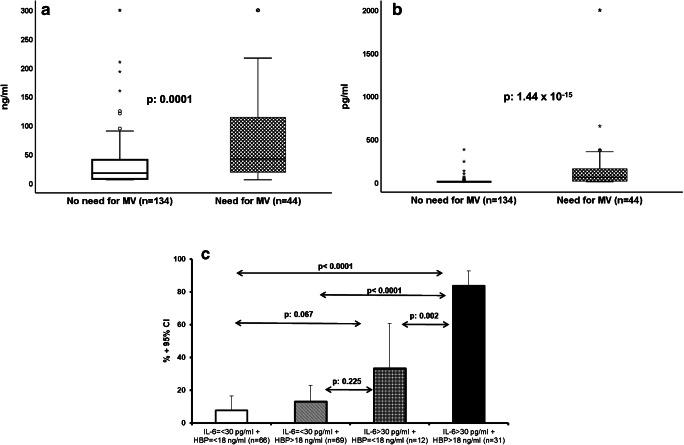
All patients who were treated with MV had pO2/FiO2 less than 150 mmHg before intubation. Admission levels of HBP and IL-6 were greater among patients with SRF compared to those without SRF (Fig. 2a and b). Univariate analysis showed eight variables that were associated with the development of SRF: male gender, CCI more than 2, SOFA score more than 3, PSI more than 87, history of solid malignancy, absolute neutrophil count at admission more than 4300/mm3, IL-6 > 30 pg/ml, and HBP > 18 ng/ml (supplementary Tables 1 and 3, supplementary Fig. 2 and Table 1). After step-wise forward logistic regression analysis, the only variables that were associated with the development of SRF were SOFA score more than 3, PSI more than 87, IL-6 > 30 pg/ml, and HBP > 18 ng/ml (Table 1). The output of the multivariate logistic regression analysis showing both increased IL-6 and HBP to be independent predictors of the development of SRF led to the assumption that both offer different information and should thus be integrated into one prediction model. Indeed (Fig. 2c), the probability of development of SRF when only IL-6 was elevated was 33.3%, and this was significantly increased when both IL-6 and HBP were elevated. The sensitivity, specificity, positive predictive value, and negative predictive values for SRF when both IL-6 and HBP were increased were 59.1%, 96.3%, 83.9%, and 87.8%, respectively.
Fig. 2.
Combination of heparin-binding protein (HBP) and interleukin (IL)-6 for the prognostication of the development of severe respiratory failure (SRF). (a) HBP admission concentrations among patients who will become in need or who will not become in need of mechanical ventilation (MV). The p value of comparison by the Mann-Whitney U test is provided. Circles denote outliers, and asterisk denotes extremes. (b) IL-6 admission concentrations among patients who will become or who will not become in need of mechanical ventilation (MV). The p value of comparison by the Mann-Whitney U test is provided. Circles denote outliers, and asterisk denotes extremes. (c) Risk of development of SRF among patients without any increase of admission HBP and IL-6, among patients with increase of only IL-6 or HBP, and among patients with increase of both HBP and IL-6. The p values of significance of the indicated comparisons are provided
Table 1.
Univariate and forward step-wise logistic regression analysis of parameters associated with the development of severe respiratory dysfunction (SRF) among patients with lower respiratory tract infection due to SARS-CoV-2
| Variable | Development of SRF | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| No (n = 134) | Yes (n = 44) | OR (95% CIs) | p value | OR (95% CIs) | p value | |
| Male gender (n, %) | 86 (64.2) | 40 (90.9) | 5.58 (1.88–16.55) | 0.0004 | ||
| CCI > 2 (n, %)# | 43 (32.1) | 25 (56.8) | 2.78 (1.39–5.59) | 0.004 | ||
| SOFA score > 3 (n, %)# | 17 (12.7) | 32 (72.7) | 18.53 (7.95–42.34) | 1.52 × 10−13 | 6.03 (1.98–18.42) | 0.002 |
| PSI > 87 (n, %)# | 16 (11.9) | 30 (68.2) | 15.80 (6.94–35.94) | 2.45 × 10−12 | 3.75 (1.20–11.72) | 0.023 |
| Solid tumor malignancy (n, %) | 1 (0.7) | 3 (6.8) | 9.73 (0.98–96.10) | 0.047 | ||
| Absolute neutrophil count > 4300/mm3 # (n, %) | 50 (37.3) | 31 (70.5) | 4.00 (1.92–8.37) | 0.0002 | ||
| IL-6 > 30 pg/ml # (n, %) | 13 (9.7) | 30 (68.2) | 19.94 (8.48–46.86) | 1.24 × 10−13 | 9.24 (3.23–26.42) | 1.06 × 10-14 |
| HBP on day 1 > 18 ng/ml# (n, %) | 65 (48.5) | 35 (79.5) | 4.12 (1.84–9.25) | 0.0003 | 3.08 (1.04–9.12) | 0.042 |
CCI Charlson Comorbidity Index, CI confidence intervals, HBP heparin-binding protein, OR odds ratio, PSI pneumonia severity index, SRF severe respiratory failure, SOFA sequential organ failure assessment
#Cut-off point was determined based on the coordinate point with the maximum value of the Youden index
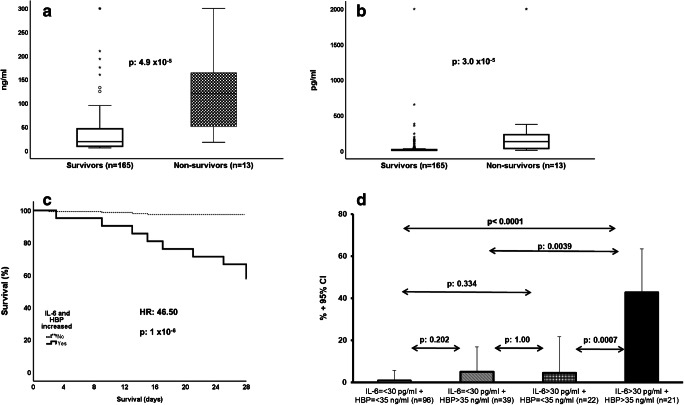
Admission levels of HBP and IL-6 were greater among the 28-day non-survivors compared to the 28-day survivors (Fig. 3a and b). Univariate analysis showed seven variables that were associated with 28-day mortality: CCI more than 2, SOFA score more than 3, PSI more than 87, history of COPD, absolute lymphocyte count at admission less than 915/mm3, IL-6 > 30 pg/ml, and HBP > 35 ng/ml (supplementary Tables 3 and 4, supplementary Fig. 3 and Table 2). After Cox step-wise forward analysis, the only variables that were associated with 28-day mortality were history of COPD, absolute lymphocyte count at admission less than 915/mm3, IL-6 > 30 pg/ml, and HBP > 35 ng/ml (Table 2). As per the development of SRF, the output of the multivariate Cox regression analysis showing both increased IL-6 and HBP to be independent predictors of unfavorable outcome led us to assume that both offer different information and should be integrated into one prediction model. The data demonstrated that the time to death was much shorter among patients with both elevated IL-6 and HBP concentrations (Fig. 3c). The sensitivity, specificity, positive predictive value, and negative predictive values for 28-day mortality when both IL-6 and HBP were increased were 69.2%, 92.7%, 42.9%, and 97.5%, respectively.
Fig. 3.
Combination of heparin-binding protein (HBP) and interleukin (IL)-6 for the early prediction of 28-day mortality. (a) HBP admission concentrations among survivors and non-survivors. The p value of comparison by the Mann-Whitney U test is provided. Circles denote outliers, and asterisk denotes extremes. (b) IL-6 admission concentrations among survivors and non-survivors. The p value of comparison by the Mann-Whitney U test is provided. Circles denote outliers, and asterisk denotes extremes. (c) Survival curves of patients with increase of both HBP and IL-6 at admission and all other patients. The hazard ratio (HR) and the p value of significance are provided. (d) Risk of 28-day mortality among patients without any increase of admission HBP and IL-6, among patients with increase of only IL-6 or HBP, and among patients with increase of both HBP and IL-6. The p values of significance of the indicated comparisons are provided
Table 2.
Univariate and multivariate Cox regression analysis of parameters associated with 28-day mortality among patients with lower respiratory tract infection due to SARS-CoV-2
| Variable | 28-outcome | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| Survivors (n = 165) | Non-survivors (n = 13) | HR (95% CIs) | p value | HR (95% CIs) | p value | |
| CCI > 2 (n, %)# | 59 (35.8) | 9 (69.2) | 3.78 (1.16–12.30) | 0.001 | ||
| SOFA score > 3 (n, %)# | 39 (23.6) | 10 (76.9) | 9.32 (2.57–33.91) | 0.001 | ||
| PSI > 87 (n, %)# | 37 (22.4) | 9 (69.2) | 6.81 (2.09–22.12) | 0.001 | ||
| History of COPD (n, %) | 7 (4.2) | 3 (23.1) | 6.13 (1.68–22.30) | 0.006 | 18.68 (3.31–105.27) | 0.001 |
| Absolute lymphocyte count < 915/mm3 # (n, %) | 55 (33.3) | 10 (76.9) | 8.55 (1.79–40.90) | 0.007 | 7.43 (1.81–30.48) | 0.005 |
| IL-6 > 30 pg/ml# (n, %) | 33 (20.0) | 10 (76.9) | 11.31 (3.11–41.15) | 0.0002 | 8.81 (2.06–37.58) | 0.003 |
| HBP on day 1 > 35 ng/ml# (n, %) | 49 (29.7) | 11 (84.6) | 11.62 (2.59–52.71) | 0.001 | 28.74 (4.19–197.22) | 0.001 |
CCI Charlson Comorbidity Index, CI confidence intervals, COPD chronic obstructive pulmonary disease, HBP heparin-binding protein, HR hazard ratio, PSI pneumonia severity index, SOFA sequential organ failure assessment
#Cut-off point was determined based on the coordinate point with the maximum value of the Youden index
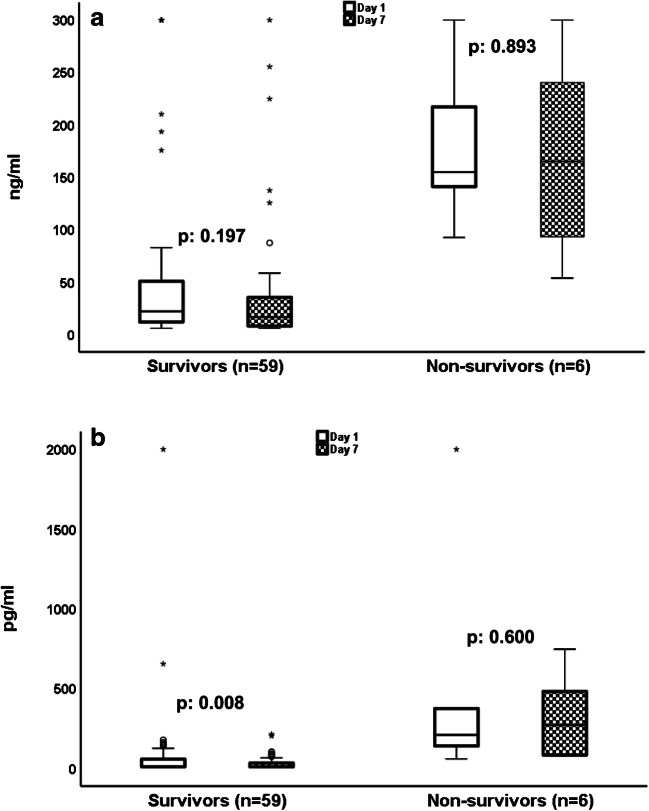
Day 7 samplings were available for 65 patients; 59 survivors and 6 non-survivors. The reason for not having samples for all patients on day 7 was either earlier hospital discharge or death before day 7. IL-6 was significantly decreased on day 7 among the survivors but not among the non-survivors. HBP remained stable among both survivors and non-survivors (Fig. 4).
Fig. 4.
Follow-up measurements of heparin-binding protein (HBP) and interleukin (IL)-6 in association with 28-day mortality. (a) HBP on admission day 1 and on follow-up day 7 among 28-day survivors and non-survivors. The p values of comparisons by the Wilcoxon’s test are provided. (b) IL-6 on admission day 1 and on follow-up day 7 among 28-day survivors and non-survivors. The p values of comparisons by the Wilcoxon’s test are provided
Discussion
The present study shows that HBP and IL-6 could be used together to predict the outcome of patients with pneumonia by SARS-CoV-2. We suggest that HBP is used in a two-stage approach. At a first stage, HBP should be used single to provide fair sensitivity of 79.5% and 84.6% for the prognostication of SRF and unfavorable outcome at the suggested cut-offs of 18 and 35 ng/ml, respectively. At a second stage, HBP should be used alongside IL-6 since their combination provides great rule-out validity since both specificity and negative predictive values are well above 90%. The lack of correlation of HBP with either the absolute lymphocyte count or with ferritin in spite of the existence of correlation between IL-6 and both the absolute lymphocyte count and ferritin suggests that HBP and IL-6 provide separate information as biomarkers.
Severe COVID-19 meets the Sepsis-3 criteria so as to be classified as viral sepsis. In this approach, the presented data on HBP are in line with several previous studies in patients with microbiologically documented or highly suspected sepsis of bacterial origin showing an early association of HBP with organ dysfunction. This was found in one cohort of 128 patients admitted at the ED [6] and also in another cohort of 93 patients with sepsis [7]. In a study of 674 patients admitted at the ED with infection, HBP more than 30 ng/ml had 78% sensitivity to predict progression into severe sepsis within the first 72 h [8]. In these studies, HBP levels ranging between 15 and 30 ng/ml were proposed as cut-offs for sepsis diagnosis. However, it needs to be outscored that in all studies published so far, HBP was measured by an enzyme immunosorbent assay and not by a fluorescence immunoassay like in our study.
Using a very interesting study design, Kahn et al. studied 524 patients admitted at the ED who were classified by experts into those with definitive bacterial infection, probable infection, definitive viral infection, probably not-infection, and definitive not-infection. HBP was increased in the first group significantly more than the other groups, and levels above 15 ng/ml were prognostic of organ dysfunction. However, HBP levels of viral infections of this study were below the levels reported here for SARS-CoV-2 [9]. This observation and the fact that the presented herein evidence is the first showing the ability to HBP to prognosticate 28-day outcome may bring two explanations: (a) severe COVID-19 resembles more to bacterial sepsis than to a traditional viral infection, and/or (b) HBP may be a biomarker more suitable for the prognosis of COVID-19 than bacterial sepsis.
It may be argued what the pathophysiological interpretation of these findings for severe COVID-19 may be. HBP is secreted by the azurophilic granules of circulating neutrophils. HBP and the absolute neutrophilic count were independently associated with SRF. Our findings are fully compatible with reports on sepsis patients where HBP is associated with hypoxia and shock, as shown by the negative correlation with the pO2/FiO2 ratio and the positive correlation with lactate. These correlations were described in patients with positive fluid balance in the lungs, i.e., increase of vascular endothelium leakage through an effect on protein kinase C of lung epithelial cells [10]. Although HBP is described to interact with the bradykinin-kallikrein system and activate the coagulation pathways [11], this does not seem to be the case in our study where HBP was not correlated with the levels of D-dimers.
The IL-6 pathway has been described to participate in the transition from mild and moderate to severe COVID-19 necessitating MV [2]. A recent analysis of 274 continuous patients showed increased IL-6 to be an independent risk factor for in-hospital mortality [12]. Another survey in only 40 patients split into those requiring MV and admission in an intensive care unit (n = 20) and into those with stable diseases showed similar increase of IL-6 among the most severe cases [13]. This was also the finding of a cohort comparing 17 patients with critical COVID-19 to 85 patients with mild or moderate disease [14]. The present study goes well beyond previous prospective cohorts since it demonstrates the importance of admission levels for the early prediction of the disease course. Another recent analysis by Herold et al. in 89 patients showed admission IL-6 to be predictive of the need for MV [15]. The suggested cut-off of 35 pg/ml is much close to the cut-off proposed by us.
Our results propose a novel prediction score for patients with COVID-19 taking into consideration admission levels of IL-6 and HBP. Using different cut-offs of these biomarkers, the score may predict the likelihood for the development of SRF and for 28-day outcome. Further studies are necessary to define the particular contribution of HBP in the pathophysiology of severe COVID-19.
Supplementary information
(DOCX 343 kb).
Authors’ contributions
MS, SM, SG, EV, MS, ML, KA, OT, and AG contributed to the collection of the clinical data, critically reviewed the manuscript for intellectual content, and gave final approval of the version to be published.
AV participated in study design, critically reviewed the manuscript for intellectual content, and gave final approval of the version to be published.
EJGB conceptualized the study design, contributed to the analysis of the data, wrote the manuscript, and gave final approval of the version to be published.
Funding
The study was funded in part by the Hellenic Institute for the Study of Sepsis and in part by JoinStar, Hangzhou, China.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Compliance with ethical standards
Conflict of interest
Anil Vasishta is consultant to JoinStar.
EJ Giamarellos-Bourboulis has received honoraria (paid to the University of Athens) from AbbVie USA, Abbott CH, Angelini Italy, Brahms GmbH, InflaRx GmbH, MSD Greece, Pfizer Greece, and XBiotech Inc. He has received independent educational grants from AbbVie, Abbott CH, Astellas Pharma Europe, AxisShield, bioMérieux Inc., InflaRx GmbH, Novartis Inc., and XBiotech Inc. He has received funding from the FrameWork 7 program HemoSpec, from the Horizon2020 Marie-Curie project European Sepsis Academy (granted to the National and Kapodistrian University of Athens), and from the Horizon2020 HemoSpec (granted to the Hellenic Institute for the Study of Sepsis).
All other authors have disclosed that they do not have any conflicts of interest relevant to this submission.
Ethics approval and consent to participate
Written informed consent was provided from all participants or their legal representatives. The study protocol was approved by the following Ethics Committee:
• ATTIKON University General Hospital
• AHEPA Thessaloniki University Hospital
• Sismanogleion Athens General Hospital
• Sotiria Athens General Hospital
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giamarellos-Bourboulis EJ, Netea MG, Ronina N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:92–100. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher J, Linder A. Heparin-binding protein: a key player in the pathophysiology of organ dysfunction in sepsis. J Intern Med. 2017;281:562–574. doi: 10.1111/joim.12604. [DOI] [PubMed] [Google Scholar]
- 4.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Br Med J. 2020;m1091:368. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu XW, Ma T, Liu W, et al. Sustained increase in angiopoietin-2, heparin-binding protein, and procalcitonin is associated with severe sepsis. J Crit Care. 2018;45:14–19. doi: 10.1016/j.jcrc.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y, Liu Z, Huang J, et al. Usefulness of the heparin-binding protein level to diagnose sepsis and septic shock according to Sepsis-3 compared with procalcitonin and C reactive protein: a prospective cohort study in China. BMJ Open. 2019;9:e02657. doi: 10.1136/bmjopen-2018-026527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linder A, Arnold R, Boyd JH, et al. Heparin-binding protein measurement improves the prediction of severe infection with organ dysfunction in the emergency department. Crit Care Med. 2015;43:2378–2386. doi: 10.1097/CCM.0000000000001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahn F, Tverring J, Mellhammar L, et al. Heparin-binding protein as a prognostic biomarker of sepsis and disease severity at the emergency department. Shock. 2019;52:e135–e145. doi: 10.1097/SHK.0000000000001332. [DOI] [PubMed] [Google Scholar]
- 10.Bentzer P, Fisher J, Kong HJ, et al. Heparin-binding protein is important for vascular leak in sepsis. Intensive Care Med Exp. 2016;4:33. doi: 10.1186/s40635-016-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenne E, Rasmuson J, Renné T, et al. Neutrophils engage the kallikrein-kinin system to open up the endothelial barrier in acute inflammation. FASEB J. 2019;33:2599–2609. doi: 10.1096/fj.201801329R. [DOI] [PubMed] [Google Scholar]
- 12.Zeng DX, Xu JL, Mao QX, Liu R, Zhang WY, Qian HY, Xu L (2020) Association of Padua prediction score with in-hospital prognosis in COVID-19 patients. QJM. 10.1093/qjmed/hcaa224 [DOI] [PMC free article] [PubMed]
- 13.McElvaney OJ, McEvoy N, McElvaney OF et al (2020) Characterization of the inflammatory response to severe COVID-19 illness. Am J Respir Crit Care Med. 10.1164/rccm.202005-1583OC [DOI] [PMC free article] [PubMed]
- 14.Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9:1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146:128–136. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 343 kb).
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.