Abstract
Coordination-driven suprastructures have attracted much interest due to their unique properties. Among these structures, platinum-based architectures have been broadly studied due to their facile preparation. The resultant two- or three-dimensional (2D or 3D) systems have many advantages over their precursors, such as improved emission tuning, sensitivity as sensors, and capture and release of guests, and they have been applied in biomedical diagnosis as well as in catalysis. Herein, we review the recent related to platinum-based coordination-driven self-assembly (CDSA), and the text is organized to emphasizes both the synthesis of new metallacycles and metallacages and their various applications.
We review the recent developments in the growing field of platinum-based, coordination-driven self-assembly, and bringing together the key results.
Graphical Abstract
1. Introduction
Supramolecular interactions are key to the high complexity and functionality of intricate and functional molecular assemblies, and they have led to the emergence of novel materials.1–3 The self-organization of simple molecular building blocks into functional materials is a common strategy in the development of such suprastructures.4–6 Among various strategies for self-assembly, coordination-driven self-assembly (CDSA) is one of the most powerful and influential strategies.7–17 By choosing appropriate metal ions and via the sensible design of the building blocks, metal-organic complexes (MOCs) with various sizes, shapes and metal (M)/ligand (L) ratios have been obtained through CDSA.18–20
The first coordination macrocycle was made by Verkade,21 and several methodologies for the design and preparation of MOCs have been reported since then.22, 23 The directional bonding method,24 the molecular panelling approach,25 the symmetry interaction strategy,12 and the weak link strategy26 were introduced and developed by Fujita,8 Stang,9–11 Raymond,12 Mirkin,13 Newkome,14 and Nitschke.15–17 To date, crucial design principles have been established. The dynamic noncovalent interactions between metal centres and ligands as well as their shapes play essential roles in the controllable synthesis of target suprastructures.7–17
To date, a wide range of metallacycles and metallacages with various geometries and sizes have been reported.8–11 With such a diverse array of structures accessible, efforts have shifted from simply making new architectures to generating functional systems and exploiting their properties.17, 27, 28 MOCs with interesting biological29, 30 and photophysical properties31–33 have been developed. This review will focus on the recent advances in this field and our contributions of discrete multicomponent assembled systems based on platinum(II) metallacycles and metallacages. Both the construction of new structures and their properties are described in this review.
2. Design and synthesis
Recently, a wide variety of 2D systems (such as rhomboids,34–41 squares,42, 43 rectangles,44, 45 triangles,46 and double rhomboids47) and 3D systems (trigonal prisms,48–51 tetragonal prisms,52 cubes,53–57 hexagonal prisms,58, 59 adamantanoids,60, 61 and a variety of other cages) have been prepared. In this section, the synthetic and structural features of the assembled metallacycles and metallacages will be emphasized. Their functional properties will be discussed in a separate subsection.
1.1. Metallacycles
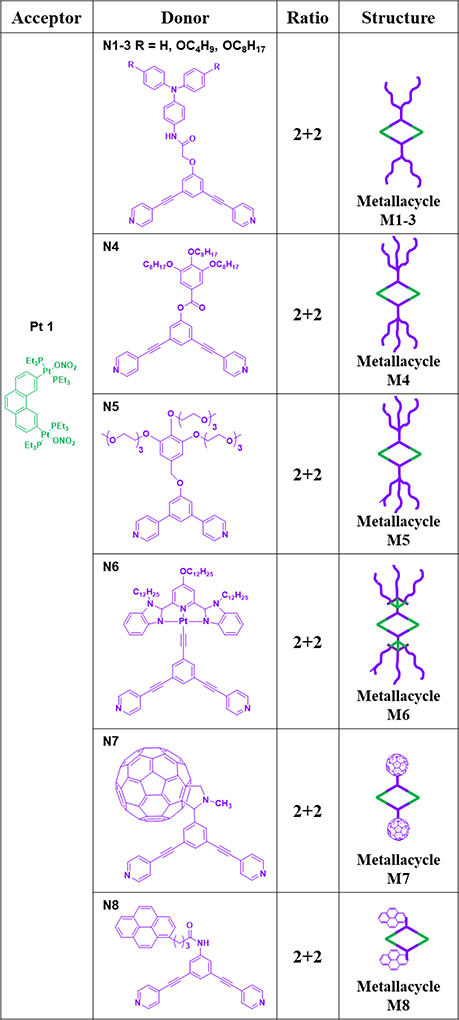
As reported before, molecular rhomboids can be prepared by the combination of 60° acceptor units and 120° donors (Table 1). For example, combinations of a 60° functional Pt(II) acceptor (Pt1) with 120° donor ligand modified with triphenylamine (TPA) derivatives with various alkyl chains (N1–3) resulted in the formation of a series of di-TPA rhomboidal metallacycles (M1–3)36 in excellent yields (Table 1). M4–5,47 possessing hydrophobic or hydrophilic dendrons, were prepared through the assembly of dipyridyl ligands N4 and N5 with Pt1.47 M6,62 containing two alkynyl platinum(II) bzimpy moieties, was prepared by mixing ligand N6 with Pt1 in a mixed solvent of acetone/water.62 Similarly, using a bis(pyridyl) subunit possessing a tethered C60 molecule (N7) with Pt1 gave a rhomboid (M7)63 with C60 units. Pyrene-functionalized 120° donor ligands N8 and Pt1 were used to prepare rhomboid M8.35
Table 1.
Pt1 (anion: ONO2−)-based rhomboids (M1–9).
 |
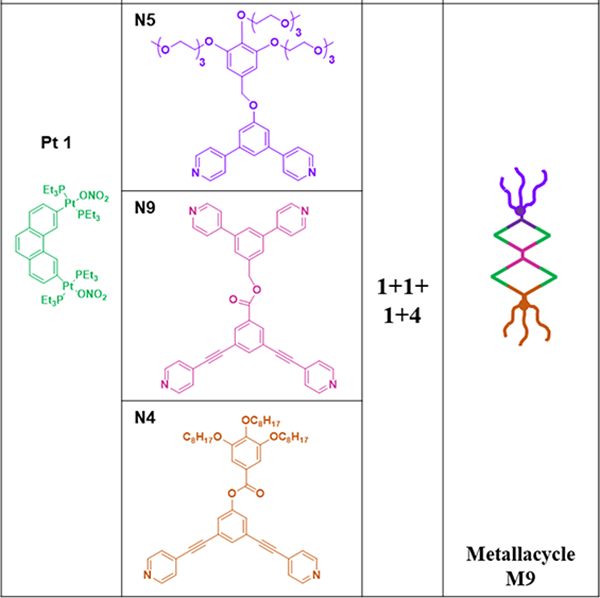 |
The self-sorting of N9, with dipyridyl ligands of distinct sizes, with N4, N5 and complementary organoplatinum(II) Pt1 lead to the formation of M947 (1:1:1:4 ratio).47 To study the effects of the counteranions on the functional properties, Pt2, possessing OTf− instead of ONO2−, was prepared.
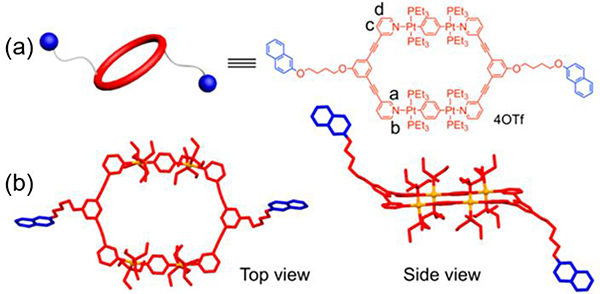
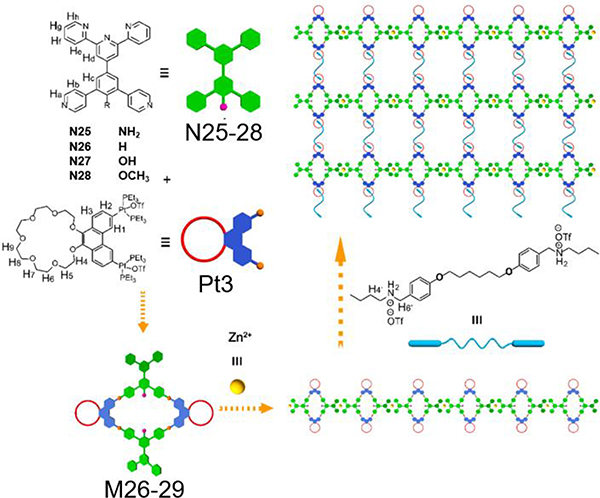
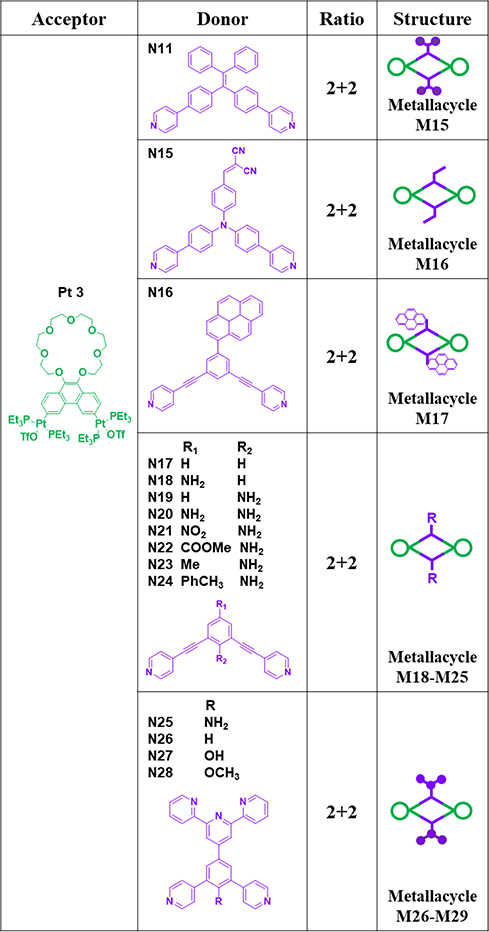
For example, the [2+2] assembly of 60° diplatinum acceptor Pt2 and 120° donor N11 gave rhomboid M11 (Table 2).64 Similarly, Pt1/2 and 120° TPE-based dipyridyl ligand N10 and pyrene functionalized ligand N12 were combined to furnish rhomboids M10/12.35, 65 To provide hydrogen bonding sites to drive further assembly, 2-ureido-4-pyrimidinone (UPy)-functionalized 120° N13 was used to construct M13.34 For biomedical applications, rhomboidal metallacycle M1438 was prepared by heating 120° dipyridyl donors N14 and Pt2 at 50 °C in methanol for 24 h. Recently, the modification of suprastructures via host–guest interactions has become an efficient and facile strategy for tuning the functions of the materials. Using this method, phenanthrene-21-crown-7 (P21C7)-containing acceptor Pt3 was prepared (Table 3). At room temperature, M15–1741 were prepared by mixing Pt3 with 120° dipyridyl donors N11, N15, or N16, respectively, in dichloromethane for 24 h.41 To prepare metallacycle-based fluorescent supramolecular polymers with designed properties, our group described the synthesis of metallacycles M18–25,37 which are composed of Pt3 and 120° dipyridyl donors N17–24.37 Subsequently, ligands possessing a terpyridyl moiety and other substituents were prepared, and Pt3 could assemble with 120° dipyridyl moieties (N25–28) to produce rhomboids M26–2966 to form heterometallic supramolecular polymers through CDSA and host–guest interactions.66
Table 2.
Pt1–2 (anion: ONO2−/OTf)-based rhomboids (M10–14).
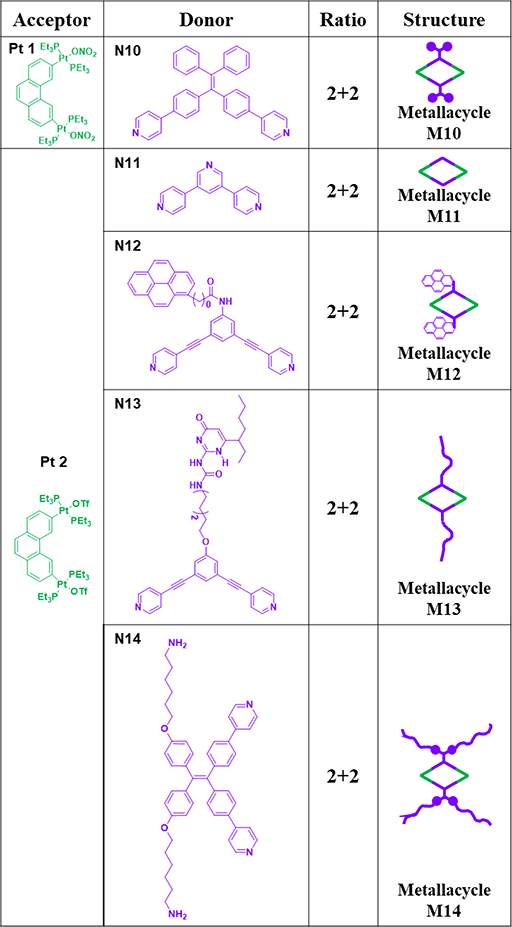 |
Table 3.
Pt3-based rhomboids (M15–29).
 |
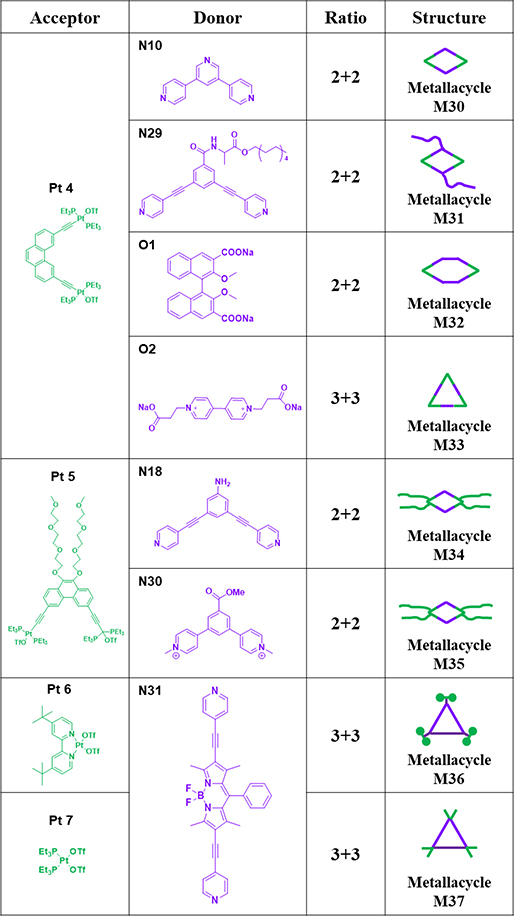
. As shown in Table 4, to obtain larger rhomboids, 60° diplatinum acceptor Pt4 was prepared. The [2+2] assembly of 120° donor N10 with Pt4 gave M30.64 M3167 was prepared by assembling 120° ligand N29 with Pt4 in a 1:1 molar ratio.67 M3259 was then prepared in one pot via two-component CDSA by mixing ligand O1 with Pt4 in a 1:1 ratio first in a solution of H2O/CD2Cl2 and then in CD2Cl2.59 The self-assembly of O2 with Pt4 yielded triangular M33.68 To investigate the biomedical applications of these structures, a platinum acceptor modified with PEG chains (Pt5) was synthesized. M34/3539, 40 were prepared in high yields by combining 120° precursor N18/30 and Pt5.39, 40 Treating Pt6/7 with N31 in CH2Cl/DMAc for 24 h at 60 °C resulted in the formation of triangular metallacycles M36/37.69
Table 4.
Pt4–7-based metallacycles (M30–37).
 |
As shown in Table 5, rhomboid M3870 was obtained by mixing donor N32 with acceptor Pt8.70 Products M38–42 were obtained by precipitation and redissolved in DMSO. A 120° TPE-based dipyridyl ligand, N11, and Pt8 were combined to furnish hexagonal M39,65 and its optical properties were evaluated.65 To obtain additional Pt-based compounds, a Pt(IV) prodrug was covalently conjugated to a 120° dipyridyl building block (N33). Subsequently, N33 self-assembled with Pt8 and generated supramolecular hexagonal M40.71
Table 5.
Pt8-based metallacycles (M38–42).
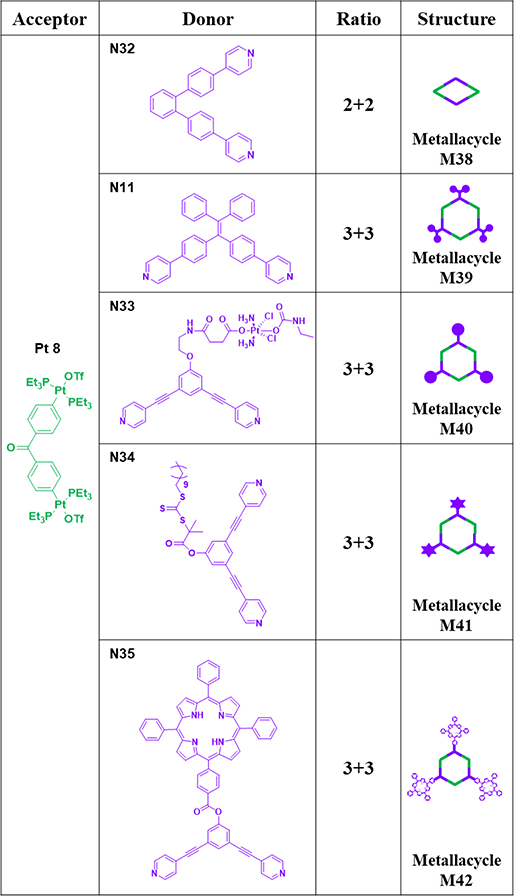 |
M4172 was prepared by mixing donor N34 with Pt8 at the appropriate stoichiometry.72 To enhance the photosensitization efficiencies and catalytic activities, N35 was designed and mixed with Pt8 to afford metallacycle M42.73
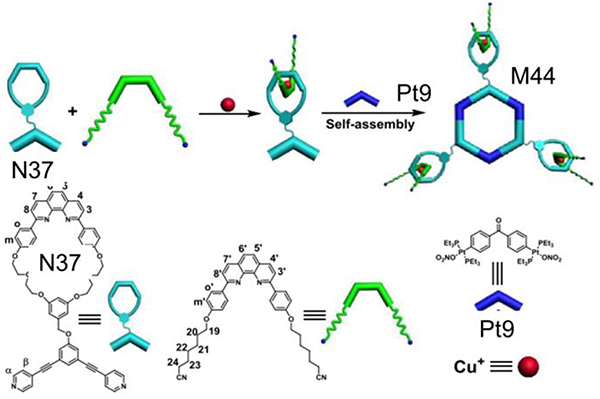
As shown in Table 6, stirring N36/N32 and Pt8 in CH2Cl2/acetone led to the formation of [1+2+4] double rhomboid M43.70 Tris-[2]pseudorotaxane metallacycle M4474 was obtained by the interaction between Pt9 and N37.74 M45/4675 were obtained by combining Pt9 and N17/38.75
Table 6.
Pt8–10-based metallacycles (M43–47).
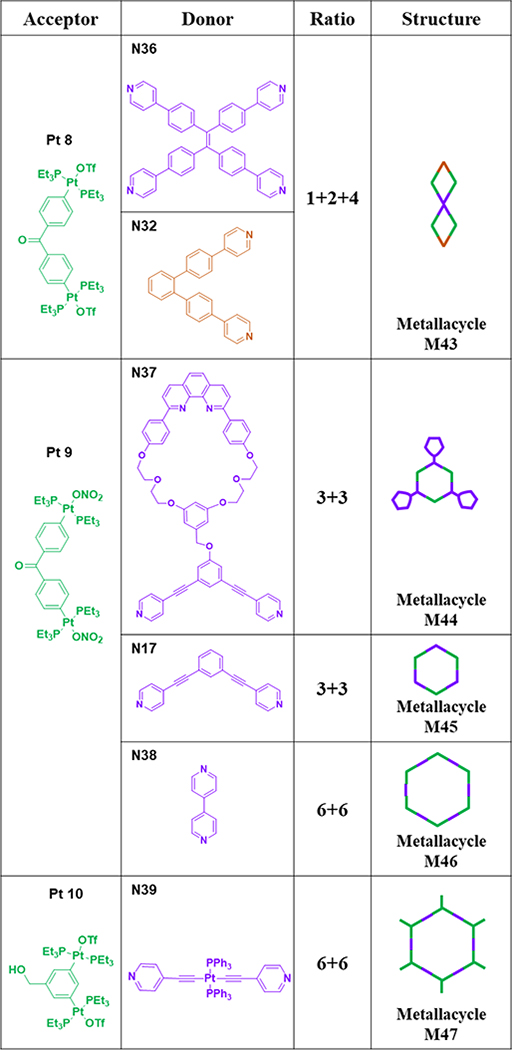 |
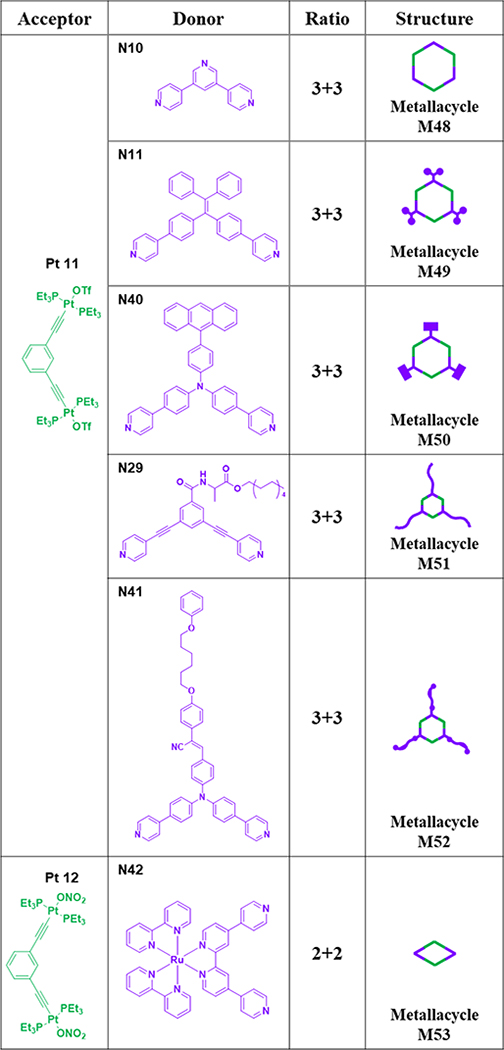
At room temperature, hexagonal M4776 was prepared by stirring a mixture of Pt10 and linear donor N39 in acetone-d6 for 8 h.76 As shown in Table 7, the [3+3] assembly of 120° diplatinum acceptor Pt11 with 120° donor N10/11 gave hexagonal M48/49.64, 65 Hexagonal metallacycle M5077 was prepared by stirring a mixture of N40 and Pt11 in CH2Cl2 and CH3OH.77 Hexagonal metallacycle M5167 was furnished by mixing alanine-based donor N29 and Pt11 in a 1:1 molar ratio.67 M5278 was obtained from N41 and Pt11 and was characterized by various spectroscopic techniques.78 Ditopic, Ru(II)-containing donor N42 was obtained by replacing one of the bpy groups of Ru(bpy)32+ with 4,4’:2’,2”:4”,4”’-quaterpyridine. The combination of N42 with Pt12 in CH2Cl2/CH3OH resulted in the formation of [2+2] rhomboid M53.79
Table 7.
Pt7–12-based rhomboids (M48–53).
 |
As shown in Table 8, hexagonal metallacycle M5472 was prepared via a combination of 120° donor N34 and acceptor subunit Pt13.72 M5580 was prepared by mixing methyl viologen-modified dipyridyl donor N43 and 120° platinum(II) acceptor Pt14 in a mixture of H2O and acetone at room temperature for 12 h followed by anion exchange.80 Stirring mixtures of coumarin-functionalized 120° dipyridyl donor N44, AgOTf and 120° di-Pt(II) acceptors Pt15/16 in CD2Cl2 at room temperature for 8 h led to the formation of hexagonal metallacycles M56/57, respectively.81
Table 8.
Pt13–18-based metallacycles (M54–61).
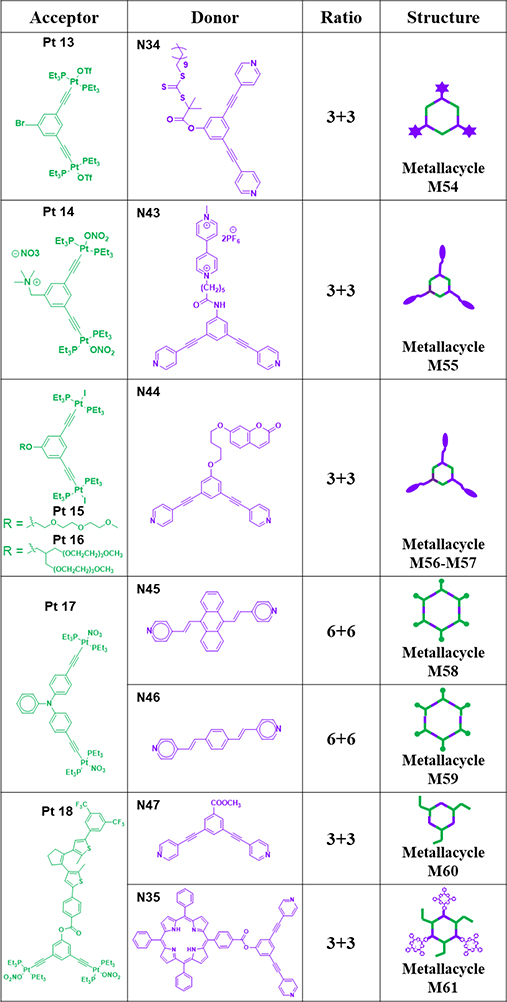 |
M5882 was obtained by mixing linear donor N45 with Pt acceptor Pt17.82 Analogous macrocycle M5982 was synthesized from donor 1,4-di(4-pyridylvinyl)benzene N46.82 N47/35, 120° donor ligands without and with a porphyrin moiety, are well-known 1O2 photosensitizers and were mixed with ligand Pt18 to afford discrete metallacycles M60/61.83
As shown in Table 9, M6284 was generated by stirring B21C7-tethered acceptor Pt19 and UPy-decorated donor N13 at a 1:1 molar ratio for 6 h in a mixture of CD2Cl2 and CD3NO2.84 Stirring a 1:1 mixture of methyl viologen-modified 120° donor N43 and 120° platinum(II) acceptor Pt20 in H2O and acetone mixture at room temperature, followed by anion exchange, resulted in the formation of hexagonal M63.85
Table 9.
Pt19–24-based metallacycles (M62–69).
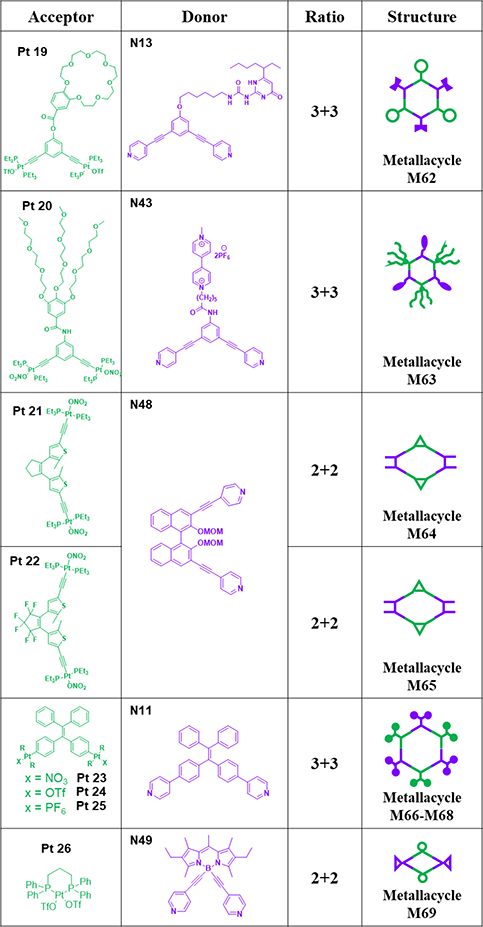 |
Chiral donor N48 and Pt(II) nitrate Pt21/22 were stirred in a mixed solvent for 8 h at 55 °C, and saturated KPF6 solution was added. Then, M64/6586 were prepared by recrystallization from a mixture of CH2Cl2 and ethyl ether.86 M66–6887, 88 were obtained by stirring a mixture of N11 with Pt23–25 in CD2Cl2 at room temperature.87 M6989 was prepared from BODIPY dye-based building blocks N49 and Pt26 and characterized.89
As shown in Table 10, organic ligand N50 and 180° acceptor Pt27 were mixed in acetone at 30 °C for 2 h to generate M70.90 Mixing ligands N51 and Pt27 in acetone for 4 h at room temperature afforded M71 with pendant pyrene moieties.91 M72 was obtained from mixing clip donors N7 and Pt27 at 55 °C.63 M7392 was prepared by mixing donor ligand N6 with Pt27 in a mixed solvent of acetone and water.92
Table 10.
Pt27–28-based metallacycles (M70–76).
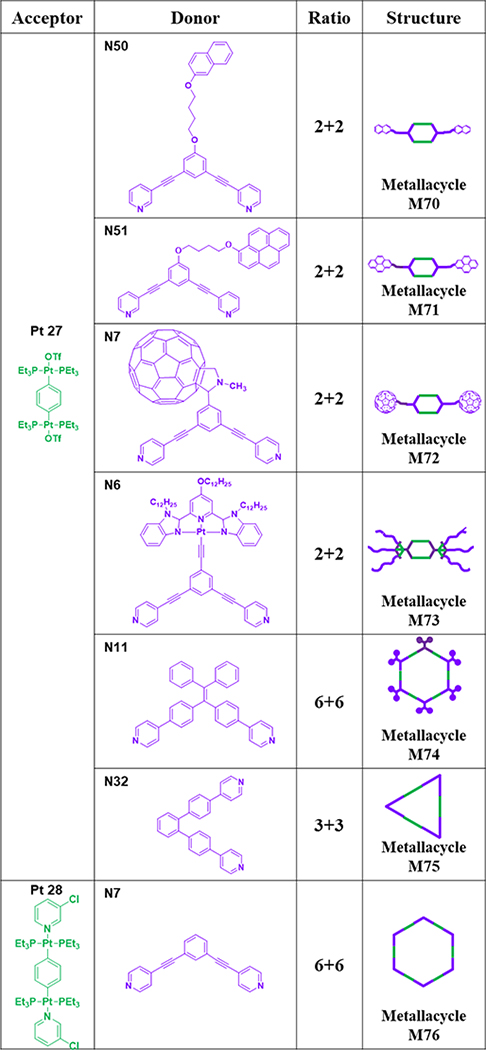 |
To study the influence of metallacycle shape on the optical properties, 120° TPE-based ligand N11 was combined with Pt27 to furnish rhomboid M74.65 To compare the shape effects of triangles and rhomboids, triangular M75 was obtained by mixing donor N32 with Pt27.70 M76 was synthesized by mixing donor N7 with Pt28.75 As shown in Table 11, N52 was mixed with 180 Pt(II) compound Pt27 in DMSO for 5 h at 80 °C to obtain M77.93 Mixing N36, N32 and Pt27 in CH2Cl2/acetone led to the formation of [1+4+6] double triangle M78.70
Table 11.
Pt27–31-based metallacycles (M77–82).
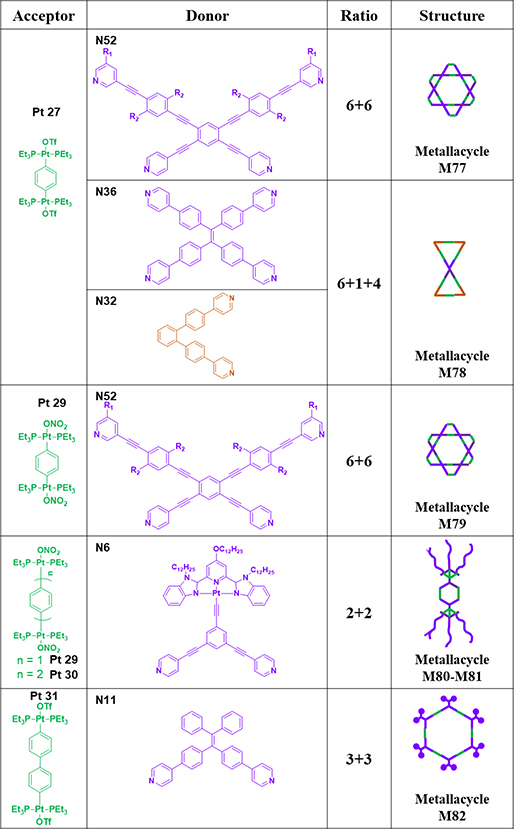 |
N52 was mixed with Pt29 in a 1:2 ratio in DMSO at 80 °C to obtain M79.93 Functionalized metallacycles M80/81,62 containing alkynyl-Pt(II) bzimpy moieties, were then prepared by mixing N6 with Pt29/30 in acetone/water at 55 °C.62 TPE-based ligand N11 was combined with Pt31 to furnish rhomboid M82.65
M83/8459 were prepared in one pot via a two-component CDSA by stirring ligand O1 with either 180° Pt-based acceptor Pt27 or Pt31 in a 1:1 ratio.59 M8577 was prepared by stirring a mixture of N40 and Pt32 in CH2Cl2/CH3OH for 10 h.77 M8678 was obtained from N41 and Pt-acceptor Pt32 in a mixed solvent system of dichloromethane-methanol for 6 h at room temperature.78
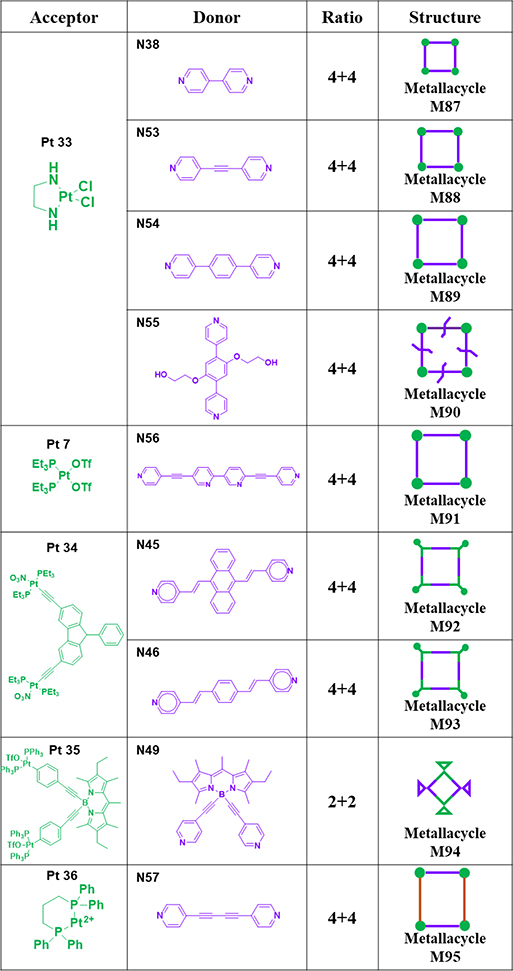
As shown in Table 13, molecular squares M87–9043 were obtained from 4,4′-bipyridine and N38, N53–55 and Pt33 by using the ball milling method.43 M9194 was obtained by treating Pt7 with an equimolar amount of donor N56 in freshly distilled dichloromethane under continuous stirring at 50 °C for 24 h.94 M92 was obtained by treating linear donor N45 with Pt34.82 Analogue M93 was generated by using 1,4-di(4-pyridylvinyl)benzene N46.82 M9489 was prepared from BODIPY dye-based building blocks N49 and Pt35 and characterized.89 M95 was fabricated from N57 and Pt36 and characterized.95
Table 13.
Pt33–36-based metallacycles (M87–95).
 |
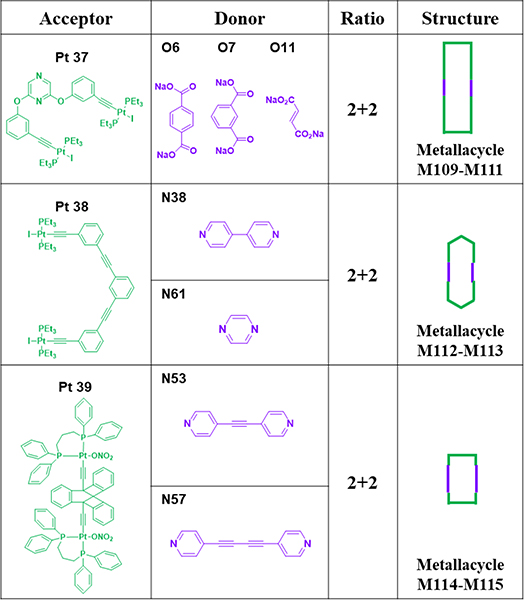
Rhomboid M96 was furnished by stirring ligand O1 with Pt7 in a solution of H2O/CD3COCD3 and then CD3COCD3.59 By stirring paraquat-derived carboxylate ligand O2 and Pt7 and dipyridyl ligands N54/58 in a 1:2:1 ratio in H2O/acetone, squares M97/98 were obtained.68 Rectangular compounds M99–101 were prepared via a three-component CDSA strategy.44 N59 and one of dicarboxylates O3–5 were mixed with Pt7 in acetone/water. The desired product was obtained by ethyl ether-induced precipitation. Huang reported the construction of two anthracene-based rectangles, M102 and 103,96 by means of CDSA with Pt7 and organic donor N45 and dicarboxylates O6 and 7, respectively.96 Ligand N60; dicarboxylate donors O4, O8, O9, O10 and O3 with different bite angles between the carboxylate groups; and Pt7 were mixed in a molar ratio of 1:1:2 to produce M104–108.46
As shown in Table 15, M109–111 were obtained by mixing Pt37 with O6, O7 and O11.97 M112–113 were obtained by mixing Pt38 and N38 and N61.98 Pyridine-terminated molecular rectangles M114–115 were obtained from Pt39 and N53 and 57.45
Table 15.
Pt37–39-based metallacycles (M109–115).
 |
1.2. Metallacages
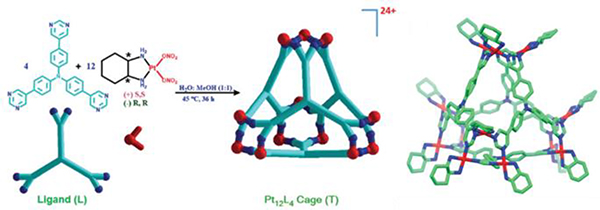
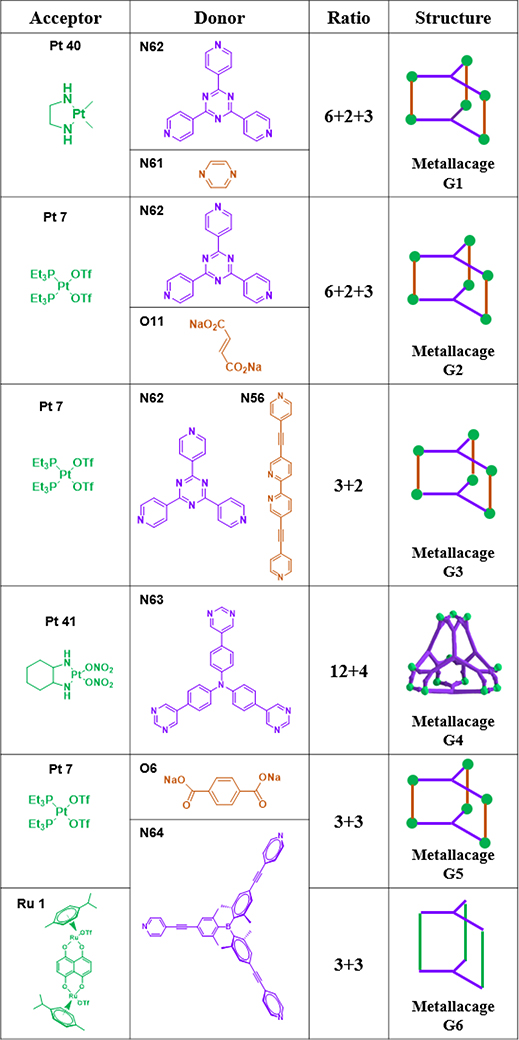
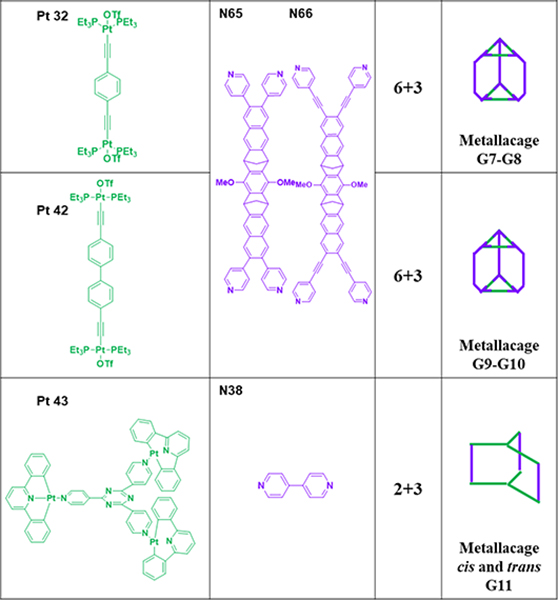
As shown in Table 16, metallacage G1 was prepared from N61/62 and Pt40.99 Metallacage G2 was obtained from N62/O11 and Pt7.50 Metallacage G3 was synthesized from N56/62 and Pt7.94 Chiral cis-[(1S,2S)-dch]Pt(NO3)2 (Pt41) and a hexadentate ligand (N63) assembled to form chiral M12L4 molecular tetrahedron G4.100 G4 possesses an internal nanocavity, which allows it to catalyse Michael addition reactions of nitrostyrene derivatives. The self-assembly of the tris(pyridyl)borane donor N64 with platinum (Pt7) and diruthenium (Ru1) electron acceptors furnished boron-containing trigonal prismatic supramolecular metallacages G5/6.48 Zhang described the preparation of molecular-clip-based tetrapyridyl donors (N65/66) and subsequently used them to synthesize four trigonal-prismatic cages (G7–10) by CDSA with 180° platinum(II) acceptors (Pt32/42).49 Hor and co-workers assembled a series of triangular metallaprisms (G11) with functional [Pt(HL)]-corner units (Pt43) using different coordination sequences.51
Table 16.
Trigonal prisms (G1–11).
 |
 |
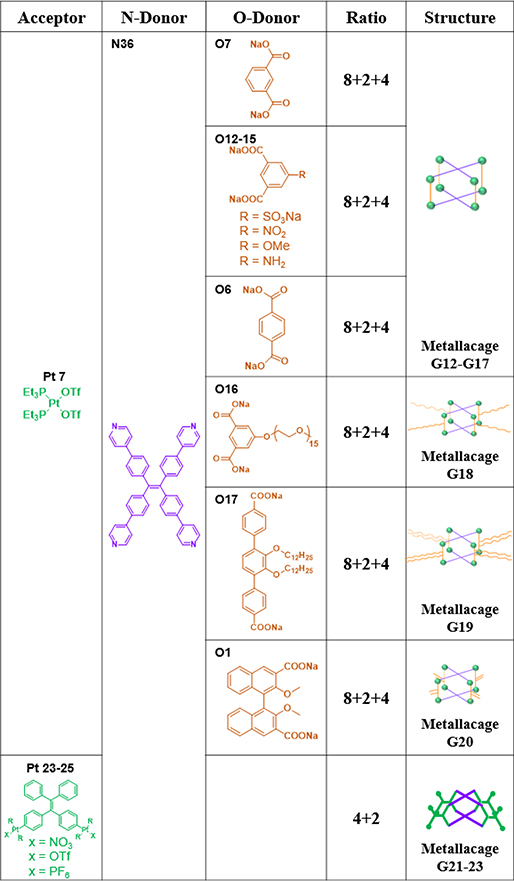
Yan et al. reported the fabrication of the tetragonal metallacage G12.53, 56, 101 Then, four metallacages (G13–16)54 with tuneable properties were prepared by the assembly of O12–15 bearing various substituents with N36, and Pt7.54 Upon mixing Pt7 and carboxylate ligand O6 with N36 in a specific ratio, an equilibrium with supramolecular prism (G17) as the predominant species was obtained.102 The combination of N36, poly(ethylene glycol) (PEG)-decorated dicarboxylate ligand O16 and Pt7 resulted in the formation of discrete tetragonal metallacage G18.56 When two donor building blocks (O17 and N36) were mixed with Pt7 in the appropriate solvents, metallacage G19 was formed.103 G20 was obtained from mixing O1, Pt7 and N36 in a ratio of 4:8:2 in H2O/CD3COCD3 followed by CD3COCD3.59
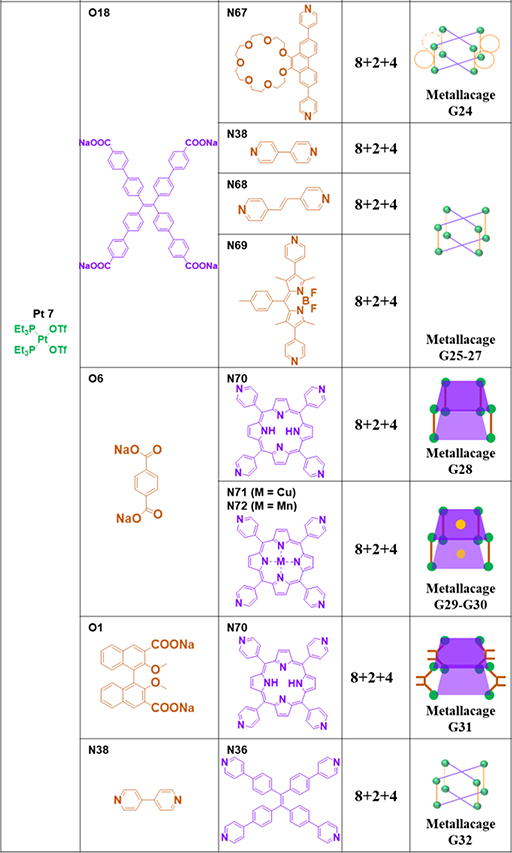
Metallacages G21/22 were obtained by stirring a mixture of Pt23–25 with N36 in a 2:1 ratio at 50 °C.87 The OTf− anion of cage G22 was exchanged by treatment with a saturated aqueous solution of KPF6 to yield G23.87 G24 was prepared from the self-assembly of TPE-based ligand O18, Pt7, and 21-crown-7 appendant dipyridyl ligand N67.55 To investigate the influence of pillars on emissive properties, Zhang synthesized three emissive tetragonal prisms, G25–27, consisting of eight Pt7 and three dipyridyl ligands (N38, 68 and 69).57 In addition to TPE, porphyrin can be used as faces to construct tetragonal prisms. For example, G28 was prepared by mixing Pt7, carboxylate ligand O6 and N70 in a ratio of 8:4:2.52, 104 Upon formation of G29–30,104 the intermolecular π–π stacking of TPP (5,10,15,20tetra(4-pyridyl)porphyrin) was effectively suppressed, and significant enhancements of the fluorescence and 1O2 generation quantum yield were realized.104 G31 was obtained by the CDSA of N70 with O1,59 and G32 was obtained by the CDSA of N36 with N38.95
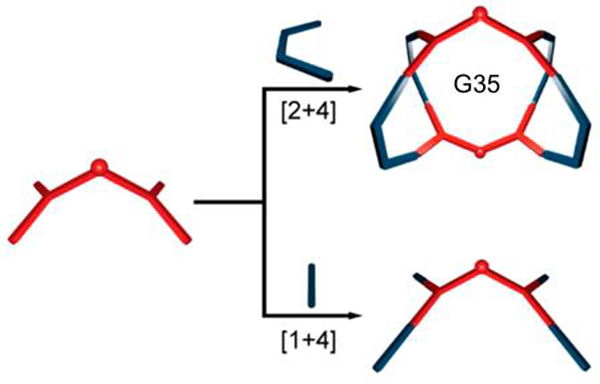
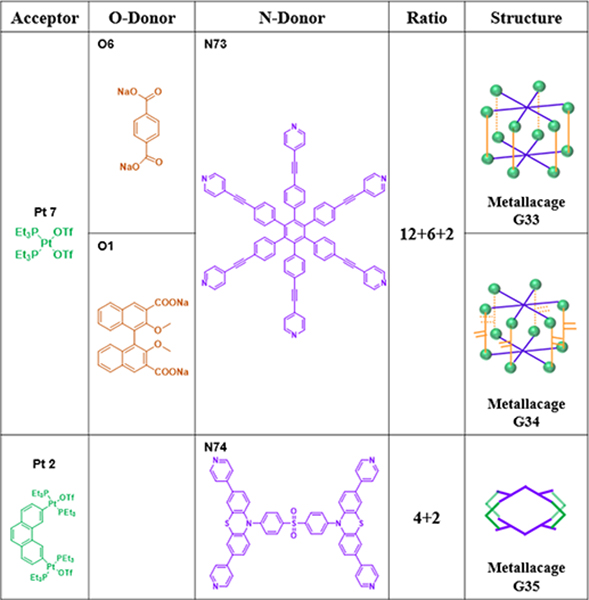
Chen and Yu loaded octaethylporphine (OEP) in to hexagonal metallacage (G33)58 to form multifunctional system G33⊃OEP, which was wrapped to afford nanoparticles (MNPs).58 As shown in Table 18, G3359 was quantitatively prepared from three species, namely, hexakis[4(4pyridylethynyl)phenyl]benzene (N73), disodium terephthalate (O6), and Pt7, in a 2:6:12 ratio.59 Hexagonal prism G34 was prepared from O1, Pt7 and hexapyridyl compound N73 in a ratio of 6:12:2. Taking advantage of the pyridyl groups, the CDSA of N74 with 60° diplatinum species Pt2 in an appropriate ratio led to the formation of G35.105
Table 18.
Hexagonal prisms and others (G33–35)
 |
He et al. described the preparation and characterization of G36–37 with potential light-harvesting properties (Table 19).106 These complexes were obtained from the self-assembly of Pt7 with 5,10,15,20-tetra(4-pyridyl)-21H,23H-porphine (N70) or 2,4,6-tris(4-pyridyl)-1,3,5-triazine (N62).
Table 19.
Trigonal, hexagon prisms (G36–45)
 |
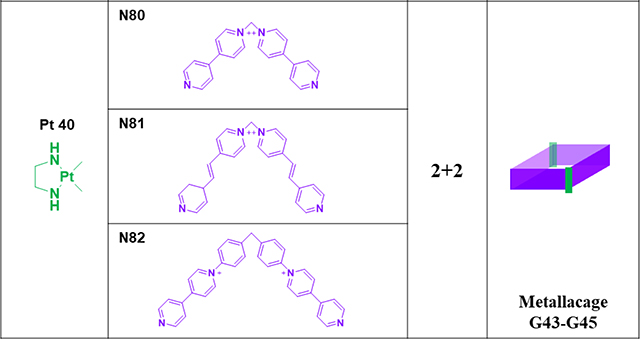 |
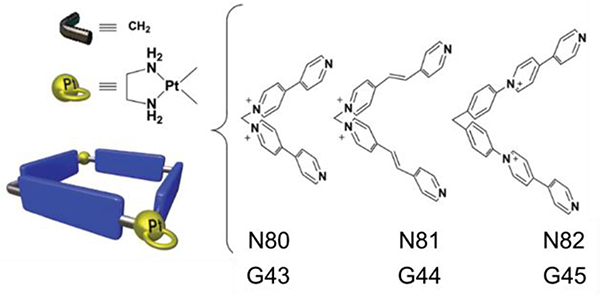
Hexagonal prismatic macrocycles G38–39107 were obtained from the [6+12] CDSA of ~120° donors (N75/76) with Pt7 in a 1:2 ratio.107 G40–42 were obtained by the [3+6] assembly of N77/78/79 with Pt44.95 To improve the binding affinity and selectivity towards specific DNA topologies, Terenzi reported three Pt(II) dinuclear squares (G43–45)108 and their constituent L-shaped 4,4′-bipyridine ligands N80-N82 as DNA binders.
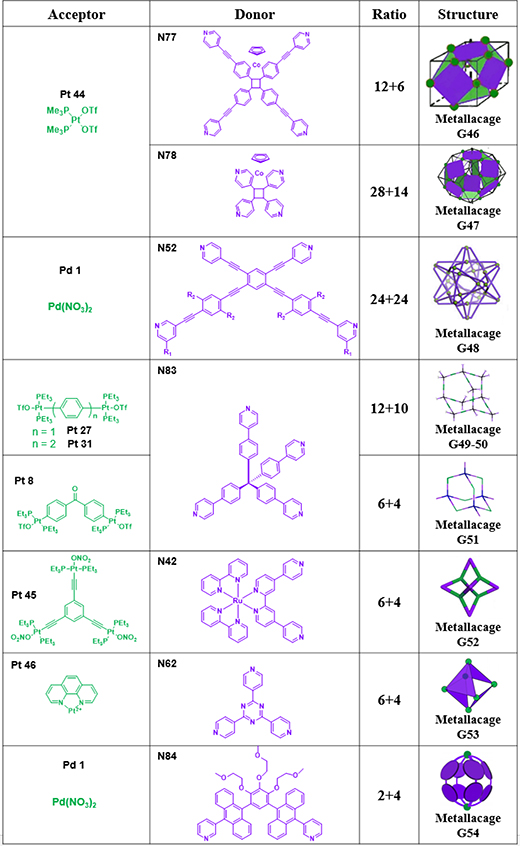
As shown in Table 20, G46 and 47 were obtained by the [3+6] assembly of N77/78 with Pt44.95 G48 was obtained by the [24+24] assembly of N52 with palladium.93 Cao also reported a direct method to construct two diamondoid based frameworks (G49–50) from N83 as a node and two linear platinum(II) acceptors (Pt27/31) as linkers,60 resulting in the formation of periodic and porous structures. G51 was obtained by the assembly of N83 with Pt8.61 Zhou described the use of molecular self-assembly to prepare a metallacage with strong 2-photon absorption by combining multiple Ru(II) complexes with Pt(II) building blocks via CDSA. The combination of N42 with tri-Pt(II) acceptor Pt45 in CH2Cl2/CH3OH (v/v = 1/1) resulted in the formation of [6+4] octahedral metallacage G52.79 Pt6L4 cage G53 was made by combining N62 with tri-Pt(II) acceptor Pt46.109 G54 was prepared by the coordination of N84 and palladium/platinum.110–112
Table 20.
Complicated cages (G46–54).
 |
2. Characterization
In addition to the routine use of NMR spectroscopy, X-ray crystallographic analysis and ion mobility mass spectrometry play an important role in CDSA.
3.1. X-ray crystallographic analysis
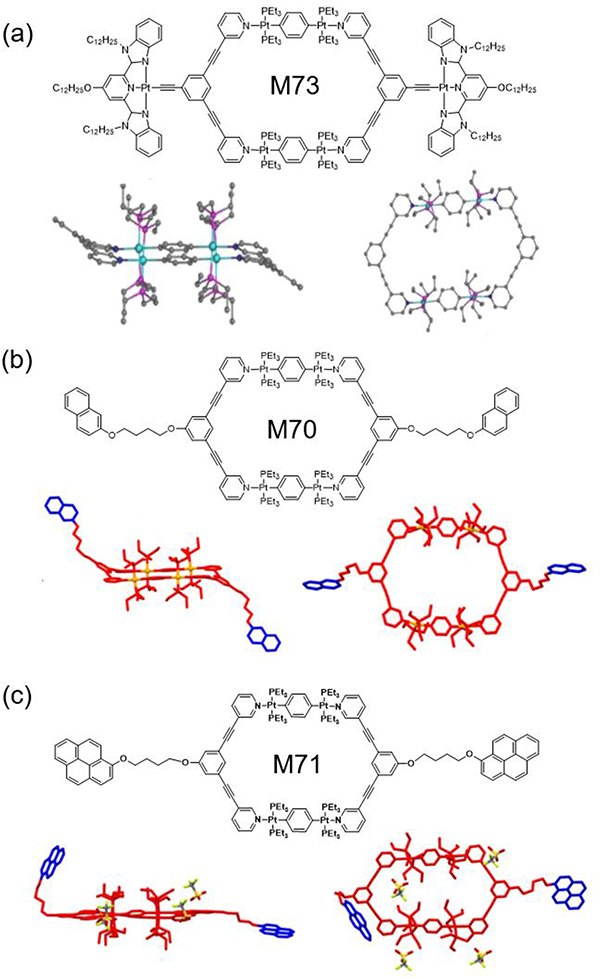
As shown in Fig. 1a, to confirm the conformation of M73,92 a model metallacycle (MM) was designed and prepared. Through the slow evaporation of MM CH2Cl2 solution, white crystals were obtained, revealing that MM adopted a chair conformation in the solid state. Crystals of M7090 were prepared by slow diffusion, and they were structurally characterized by X-ray diffraction (Fig. 1b). Slow diffusion of ether into an acetone solution of M71 afforded light-yellow single crystals of M7191 (Fig. 1c). Crystallographic analysis revealed the formation of an AA-BB-type monomer composed of two molecules of M71 connected by C−H···π interactions.
Fig. 1.
Chemical and crystal structures of (a) M73, (b) M70 and (c) M71. Figure adapted from ref. 90–92 with permission from the American Chemical Society, Copyright 2018 and 2019.
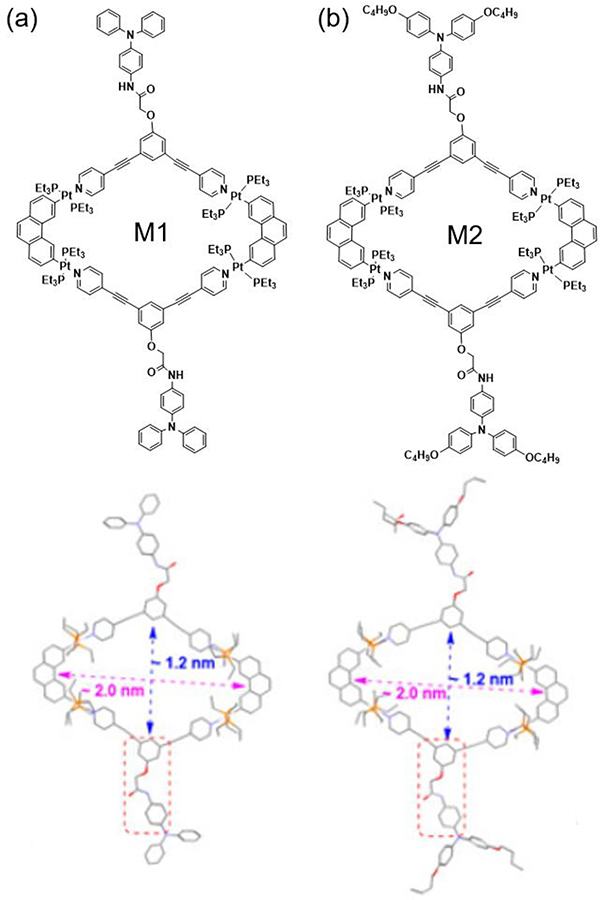
The slow diffusion method was also used to obtain single crystals of M1 and M2 (Fig. 2).36 The side view of the crystal showed that the metallacycle was essentially planar with four triethylphosphine ligands perpendicular to the metallacycle plane.
Fig. 2.
Chemical and crystal structures of (a) M1 and (b) M2. Figure adapted from ref. 36 with permission from the American Chemical Society, Copyright 2019.
The X-ray crystal structure of G4 is shown in Fig. 3.100 Vapor diffusion of acetone into a water/methanol solution of G4 afforded single crystals of G4. Chiral triangular subunits are present at the four corners of the tetrahedron, making G4 chiral.
Fig. 3.
Chemical and crystal structures of G4. Figure adapted from ref. 100 with permission from Royal Society of Chemistry, Copyright 2018.
X-ray crystal analysis clearly show the molecular structure and connectivity of G6.48 The cation of G6 was confirmed to be a hexanuclear ruthenium trigonal prismatic supramolecular cage consisting of six half-sandwich Ru units and two borane ligands with an interior pocket of approximately 400 Å3, as estimated using the six Ru vertices. Notably, the propeller-like conformation of the two triarylborane units prevented π-stacking interactions between these units.
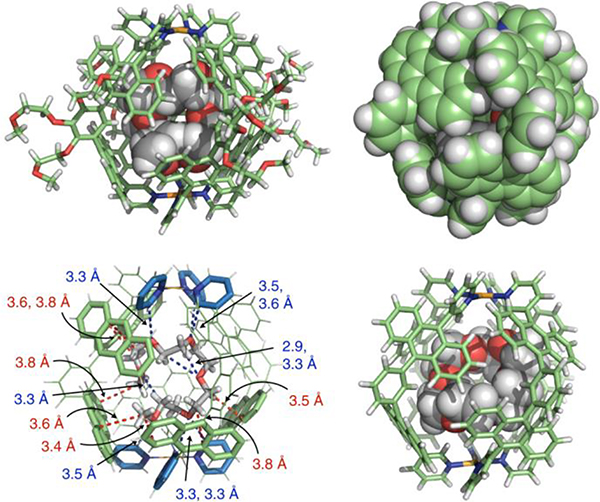
Pale-yellow crystals of G54•5EO (ethylene oxide) were prepared by slow concentration of the saturated H2O solution for 1 month at room temperature (Fig. 5a and b).112 The bound 5EO adopts an approximately coiled conformation in the cavity (Fig. 5c). The optimized structure of G54•CE indicates that cyclic CE adopts a bent conformation to fit in the spherical cavity (Fig. 5d).
Fig. 5.
Chemical and crystal structures of G54 and guest. Figure adapted from ref. 112 with permission from Springer Nature, Copyright 2018.
3.1. Ion mobility mass spectrometry
Although X-ray crystal analysis is a powerful tool for determining complicated structures, growing suitable crystals can be challenging.95 In this case, ion mobility mass spectrometry (IM-MS) can provide a direct measure of the size and stoichiometry of these complexes.113, 114
This approach obviates the need for product isolation and crystallization and allows the tracking of the product distribution over time. As shown in Fig. 6, the IM drift times do not change as a function of collision voltage, and each ion can be fit to a single Gaussian distribution throughout the collisional activation (CID) experiment; therefore, ions are observed to dissociate rather than rearrange or unfold in the gas phase.114
Fig. 6.
Gaussian peak-fitting IM spectra resulting from CID of the isobaric nitrate-free rhomboid and half-rhomboid. Figure adapted from ref. 114 with permission from The American Society for Mass Spectrometry, Copyright 2019.
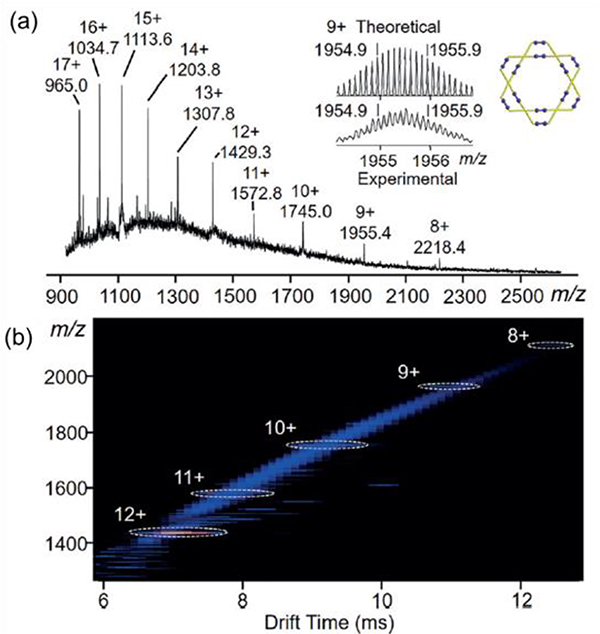
The ESI-MS data of M77 showed a series of peaks as a result of the loss of different numbers of triflate counterions (Fig. 7a).93 Furthermore, IM-MS showed a series of charge states with a narrow drift-time distribution, thus confirming the structure (Fig. 7b).
Fig. 7.
(a) ESI-MS and (b) IM-MS plot of M77. Figure adapted from ref. 93 with permission from Wiley-VCH, Copyright 2017.
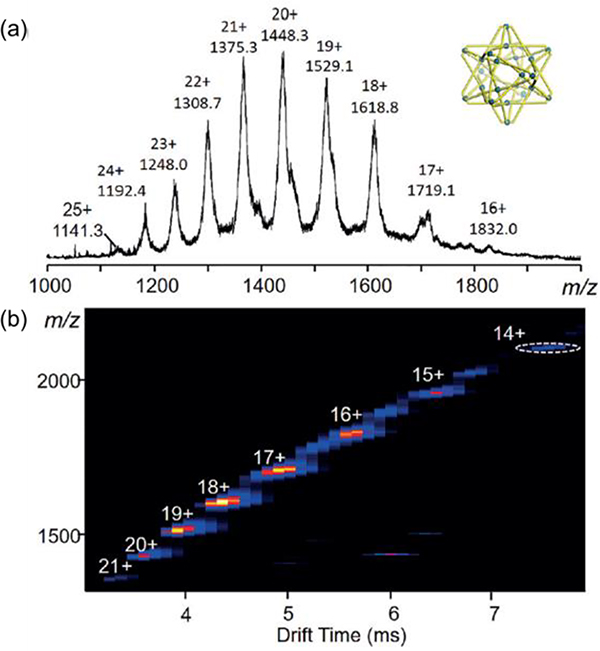
For G48, ESI-MS gave a set of peaks with sequential charges (Fig. 8).93 After deconvolution, the measured molecular weight of G48 was confirmed.
Fig. 8.
(a) ESI-MS and (b) IM-MS plot of M48. Figure adapted from ref. 93 with permission from Wiley-VCH, Copyright 2017.
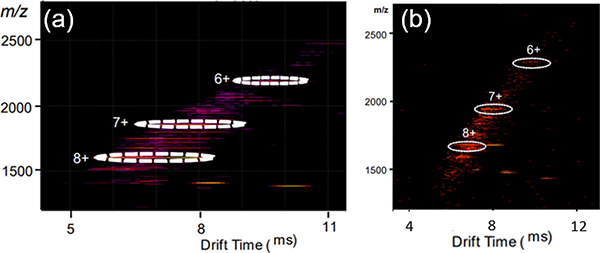
IM-MS showed one dominant series of bands for each charge state with a narrow drift time. Travelling wave ion mobility−mass spectrometry (TWIM-MS) of G38 and G39 provides further evidence for the formation of the target structures. As shown in Fig. 9, the TWIM-MS data of G38 and G39 show a prominent set of signals with multiple charged states.107
Fig. 9.
ESI-MS and IM-MS plots of G38/39. Figure adapted from ref. 107 with permission from American Chemical Society, Copyright 2019.
3. Properties
The design, synthesis, and characterization of MOCs have been discussed above. The photophysics and photochemistry of MOCs are highly diverse, and small modifications in the ligands can result in changes in the molecular congener systems.31–33 To better understand the resultant properties, the role of the bite angle, substituents, shape and size of the MOCs as well as the anions were investigated.
3.1. Optical properties
4.1.1. Bite angle and substituent effects
By systematically using dicarboxylate building blocks, phenazine-cored metallacycles M104–108 were obtained.46 As the metallacycle size decreased, the emission blueshifted (570 to 499 nm). The results indicate that the size of M104–108 dictated the constraint imposed on the excited-state planarization of the core, thereby altering their photophysical properties.
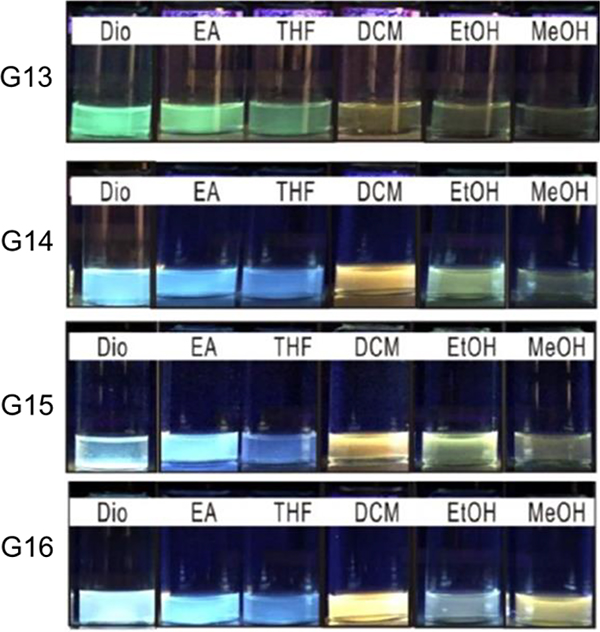
By selectively combining TPPE (N36), dicarboxylate moieties (O12–15), and Pt7, a series of metallacages (G13–16) with adjustable optical properties were prepared.54 When the substituents were changed from −SO3Na to −NH2, the cage emission was brighter (Fig. 11).
Fig. 11.
Photographs of G13–16 in different solvents. Figure adapted from ref. 54 with permission from American Chemical Society, Copyright 2018.
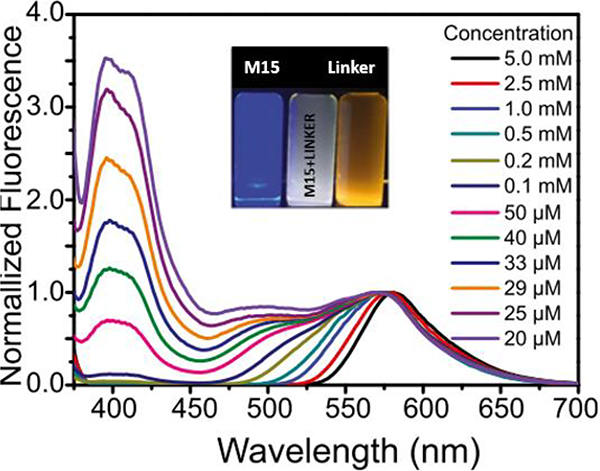
Three Pt(II) rhomboidal metallacycles with orange (M15), cyan (M16), and green (M17) emissions were prepared.41 As the concentration decreased, the emission of the assemblies formed by metallacycle M15 and a bis-ammonium linker shifted from orange to blue. White-light emission was obtained at a concentration of 29 μM (Fig. 12).
Fig. 12.
Emission spectra of M15 and a bis-ammonium linker at different concentrations; (inset) photograph of M15, bis-ammonium linker, and their mixture upon excitation (λex=365 nm). Figure adapted from ref. 41 with permission from the National Academy of Sciences, Copyright 2017.
Yin prepared supramolecular polymers with tuneable fluorescence by CDSA and host–guest interactions.37 By varying the substituents (N17–24) on the dipyridyl donors, the metallacycles exhibited emission wavelengths from 427 to 593 nm. White-light-emitting LED can be prepared by the combination of a thin film onto an ultraviolet LED and as-prepared MOCs (Fig. 13).
Fig. 13.
(a) LED off, (b) LED on, (c) lamp painted with M25 and bis-ammonium salt (LED off), and (d) lamp painted with M25 and bis-ammonium salt (LED on). Figure adapted from ref. 37 with permission from American Chemical Society, Copyright 2018.
White-light emission from a single chromophore was realized by dissolution of G18 in THF.56 The mixed yellow and blue emissions from the monomeric and aggregate ensembles resulted in overall white light (Fig. 14).
Fig. 14.
G18 in different solvents. Figure adapted from ref. 56 with permission from Springer Nature, Copyright 2015.
4.1.2. Effects of shape, size, and anion
In addition to the effects of bite angle and substituents, the shape of the metallacycles also plays an important role in tuning the emission.
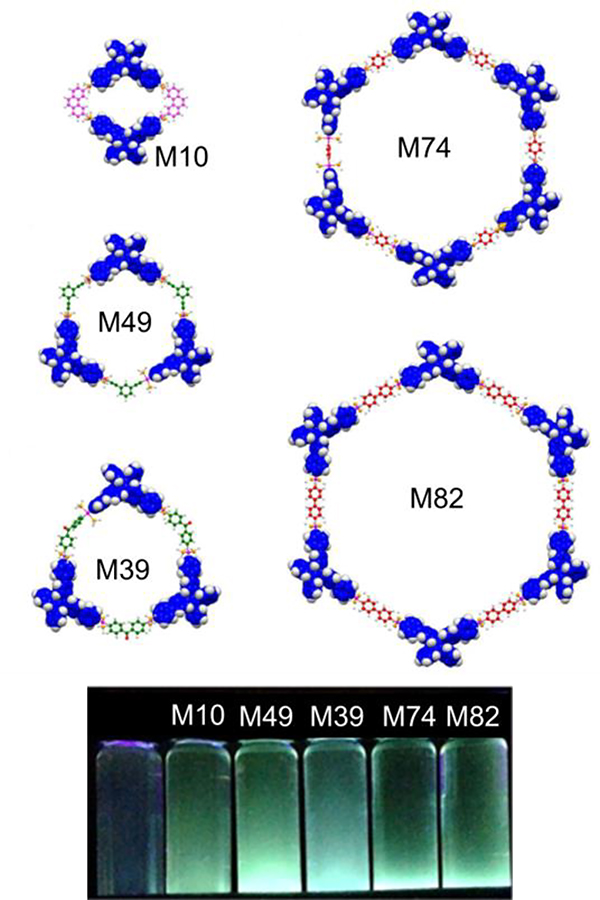
Yan prepared a suite of TPE-based metallacycles (M10, 49, 39, 74, and 82) and used them as chemosensors (Fig. 15).65 Because of their AIE characteristics, M10, M49 and M82 were explored for the detection of picric acid. Zhou described the selective formation of a metallarhomboid (M43) and a triangle (M75) by controlling the shape and stoichiometry of the ligand.70 Differences in the strength and shape result in different fluorescence enhancement behaviours for the double rhomboid (M43) and double triangle (M78). Fused M43 showed weaker emission in dilute solutions. However, a reversal of emission intensities was observed in the aggregated state.39 The different shapes result in dramatically different fluorescence efficiencies (Fig. 16).
Fig. 15.
M10, 49, 39, 74, and 82 in CH2Cl2. Figure adapted from ref. 65 with permission from American Chemical Society, Copyright 2015.
Fig. 16.
Scheme of (a) M43 and (n) M78. Figure adapted from ref. 39 with permission from National Academy of Sciences, Copyright 2019.
N36 were used to construct M66–68 and drum-like metallacages (G21–23) with three different counteranions via CDSA.87 A counterion effect was observed; compared with their NO3− counterion analogues, the species containing OTf− or PF6− counterions show stronger emission. The absorption coefficients, emission intensities, and quantum yield values in CH2Cl2 follow the order PF6− > OTf− > NO3− (Fig. 17).
Fig. 17.
Metallacycles M66–68 and metallacage G21–23 in CH2Cl2. Figure adapted from ref. 87 with permission from American Chemical Society, Copyright 2016.
Liu assembled G11 using different coordination sequences. By utilizing Pt-N bonds and the trans effect of a carbon donor, they successfully tuned the luminescence of geometric isomers of metallosupramolecules (Fig. 18).51
Fig. 18.
Photographs of G11 in CH2Cl2/hexane mixtures with different fractions of hexane upon excitation at 365 nm using a UV lamp. Figure adapted from ref. 51 with permission from American Chemical Society, Copyright 2019.
4.1.4. Observation and understanding of CDSA emission
Han prepared fullerene-functionalized metallacycle M72 (Fig. 19) and explored its excited-state decay pathways in a concentrated solution and showed that a radiative excimer with a long-lived triplet state can exist in a concentrated solution of fullerene−Pt complex.115
Fig. 19.
Excited-state decay pathways of C60-Pt complexes. Figure adapted from ref. 115 with permission from American Chemical Society, Copyright 2017.
Investigating the origin of changes in absorption and emission properties is important for understanding the photophysical nature and electronic structure of MOCs. As shown in Fig. 20, self-assembly into G35105 increases the quantum yield in solution 4-fold primarily due to the further twisting of the luminophore, which increases the radiative rate constant. The differences in quantum yields can be explained by the twist in the chromophore upon coordination to platinum or methylation of the pyridyl group, leading to intersystem crossing to a triplet state.105 This state then becomes more emissive with the addition of platinum, which increases the radiative rate constant via the heavy atom effect (Fig. 19).
Fig. 20.
Scheme showing (top) G35 and (bottom) the acyclic assembly. Figure adapted from ref. 105 with permission from American Chemical Society, Copyright 2019.
3.2. Host–guest properties
Due to the cyclic nature of these compounds, metallacycles and metallacages often have a central space capable of binding guests. As a result, a wide range of guest molecules with different properties, including metal ions, C60, pyrene, naphthalene, coronene, glucose, and oligo (ethylene oxides), have been successfully encapsulated in metallacycles and metallacages. As complex systems, the interactions between species give rise to one or more properties that cannot be attributed to their individual components.16, 116
3.2.1. Metallacage cavities used as hosts
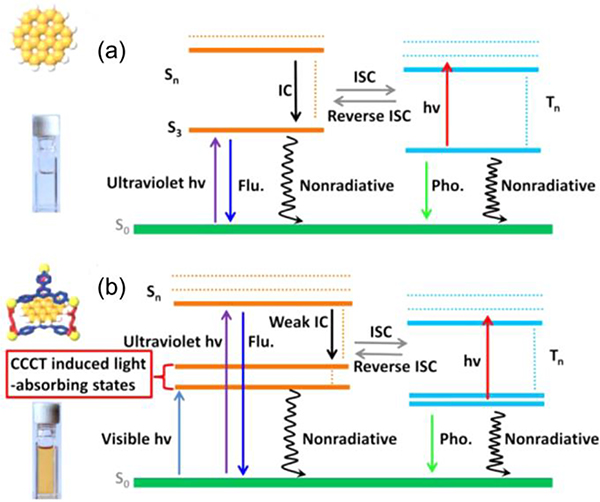
Han reported that the encapsulation of a chromophore by G2 dramatically enhances its photophysical properties (Fig. 21).50 Core-to-cage charge transfer (CCCT) arises from the electrostatic interactions between the delocalized electrons of the coronene and the positive charge associated with the metallacage. The Pt nodes of the host contribute to the excited-state frontier orbitals, further promoting intersystem crossing (ISC) and phosphorescence.
Fig. 21.
CCCT-induced light-absorbing states and mechanisms of the various excited-state decay pathways for (a) the coronene and (b) the cage-coronene system. Figure adapted from ref. 50 with permission from American Chemical Society, Copyright 2015.
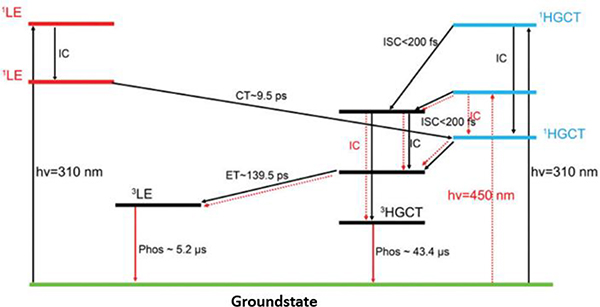
A similar G1-based system was investigated by combining several characterizations.99 A mechanism revealing the main excited-state decay pathways was proposed (Fig. 22).
Fig. 22.
Primary excited-state decay pathways in the host/guest cage G1. Figure adapted from ref. 99 with permission from Royal Society of Chemistry, Copyright 2018.
He described the design and synthesis of square-like M91 and trigonal prismatic G3 by precisely controlling the ratio between donor Pt7 and two different acceptors.94 M91 and G3 could selectively recognize divalent metal ions (Ni2+ and Zn2+) over monovalent (Ag+) or trivalent metal ions (Nd3+, Eu3+ and Tb3+), as shown in Fig. 23.
Fig. 23.
Schematic of the reorganization between M91/G3 and divalent metal ions. Figure adapted from ref. 94 with permission from the Royal Society of Chemistry, Copyright 2017.
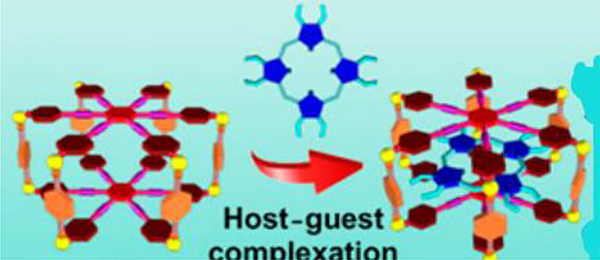
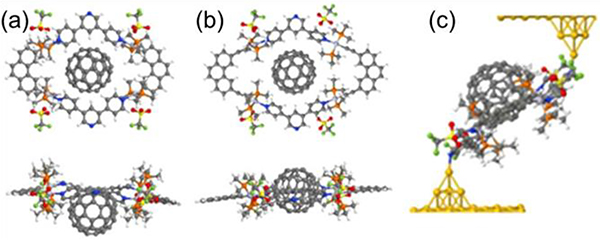
Zhang described the synthesis of four convex trigonal prisms G7–10 via CDSA with 180° platinum(II) acceptors Pt32/42.49 Because of the large flexible aromatic surfaces of these cages, their encapsulation of fullerene was investigated (Fig. 24). DOSY results suggested that encapsulation does not alter the overall size of the cage.
Fig. 24.
Representation of the formation of a host–guest complex by (a) stepwise and (b) one-pot self-assembly. Figure adapted from ref. 49 with permission from American Chemical Society, Copyright 2017.
Additionally, the host–guest interactions between G28 and pyrene were investigated.52 This recognition could be used to inhibit the liquid-crystalline behaviour of the nematic molecule containing cyanobiphenyl mesogens functionalized with a pyrenyl unit. Then, coronene was used as a competitive guest to recover the liquid-crystalline behaviour of the smectic material (Fig. 25).52
Fig. 25.
Schematic representation of the host–guest complexation between G28 and pyrene. Figure adapted from ref. 52 with permission from Wiley-VCH, Copyright 2016.
Chen and Yu utilized G33-containing therapeutic platinum(II) to encapsulate a photosensitizer, facilitating the codelivery of a chemotherapeutic agent and a photosensitizer (Fig. 26).58 Benefiting from the enhanced permeability and retention, the nanomedicine exhibited satisfactory antitumour performance against drug-resistant tumours.
Fig. 26.
Cartoon illustration of the preparation of G33 and octaethylporphine. Figure adapted from ref. 58 with permission from National Academy of Sciences, Copyright 2019.
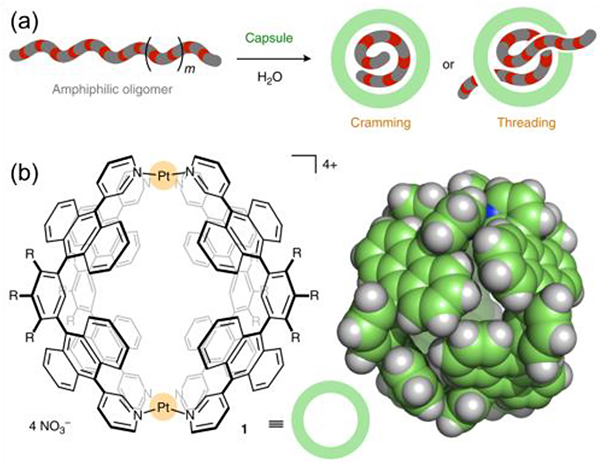
Yoshizawa reported the selective and efficient loading of D-sucrose within a molecular capsule (G54) from natural aqueous saccharide mixtures (Fig. 27).111
Fig. 27.
Recognition of D-glucose (a) in a hydrogen-bonding cavity and (b) in a polyaromatic cavity. (c) G54 and dD) its slice through the centre of the crystal structure. Figure adapted from ref. 111 with permission from AAAS, Copyright 2017.
Multiple CH-π interactions between the sucrose hydrocarbon backbone and the shape-complementary polyaromatic cavity endow G54 with unique selectivity, as demonstrated by theoretical calculations and control experiments. In addition, they disclosed unusual host–guest complexation between G54 and long amphiphilic oligomers.111 These unusual host–guest interactions occurred instantly, spontaneously, and quantitatively even in water at room temperature.112
3.2.2. Metallacycle cavities used as hosts
Three metallacycles (M11, 30, and 48) with controlled shapes and cavity sizes were generated by CDSA.64 Metallacycles with finely tuned cavity sizes possess different degrees of flexibility to accommodate C60. Upon host–guest complexation with C60, the molecular conductance of the metallacycle could be enhanced by an order of magnitude.
Shi prepared metallacycle M70 bearing two naphthalene units at its vertices, and it can be used to construct a linear supramolecular polymer through host−guest interactions between the cavity of the metallacycle and the naphthalene groups (Fig. 30).90
Fig. 30.
(a) Cartoon representation and chemical structure of M70; (b) top and side views of the single-crystal structure of M70. Figure adapted from ref. 90 with permission from American Chemical Society, Copyright 2019.
Then, metallacycle M71 containing two pyrene units was synthesized (Fig. 31).91 One of the pyrenes was encapsulated into the cavity of another pyrene of M71 to form a host−guest complex. Both in the solid state and in solution, M71 assembled into a supramolecular polymer.91
Fig. 31.
Assembled single crystal produced by the host−guest complexation. Figure adapted from ref. 91 with permission from American Chemical Society, Copyright 2019.
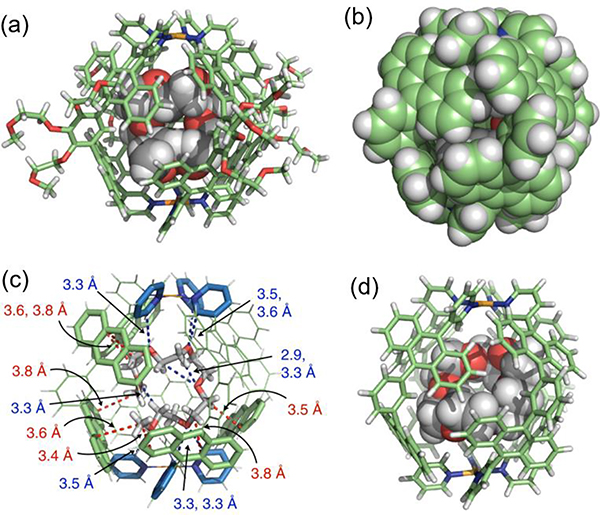
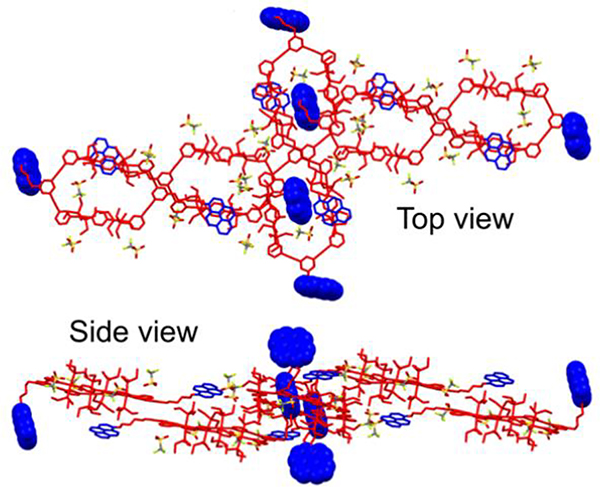
4.2.3. Functionalized pillars as hosts
Yin prepared new, bicyclic cross-linked supramolecular polymers by hierarchical unification of M26–29.66 The B21C7 units in the vertical direction provide a platform for incorporating the host−guest interactions with bis-ammonium, affording a cross-linked supramolecular polymer network (Fig. 32).
Fig. 32.
Supramolecular polymeric network. Figure adapted from ref. 66 with permission from American Chemical Society, Copyright 2019.
Cross-linked redox-responsive supramolecular polymers can be formed based on host–guest interaction between a guest and a metallacycle M44 (Fig. 33).74
Fig. 33.
Self-Assembled M44. Figure adapted from ref. 74 with permission from American Chemical Society, Copyright 2018.
Based on the host−guest interactions between G24 and ammonium salts, Yin prepared a supramolecular gel with G24 as the core by orthogonal self-assembly.55 Due to the dynamic nature of the noncovalent interactions in this system, its thermo- and cation-responsiveness is well preserved (Fig. 34).
Fig. 34.
Formation of a supramolecular polymer network from G24 and bisammonium Salt. Figure adapted from ref. 55 with permission from American Chemical Society, Copyright 2018.
Recently, interlocked molecular species have attracted considerable attention because of their application as nanoscale devices and molecular machines. As shown in Fig. 35. Based on the host−guest interactions that occur in catenanes and molecular necklaces, mechanically interlocked molecules consisting of two catenanes and one molecular necklace were efficiently constructed.117
Fig. 35.
Self-Assembly of Supramolecular Rectangles and [3]Catenanes. Figure adapted from ref. 117 with permission from American Chemical Society, Copyright 2015.
3.3. Hierarchical assembly
MOCs with controllable shapes and sizes have been employed to construct hierarchical systems with diverse functionalities.118–120 For example, by using G12 as building blocks, Sun prepared G12-based microneedle materials.53 The introduction of vitamin B12 into this platform could provide access to diverse artificial biological functions for mimicking natural synthesis systems (Fig. 36).
Fig. 36.
G12-based micrometre needles. Figure adapted from ref. 53 with permission from American Chemical Society, Copyright 2018.
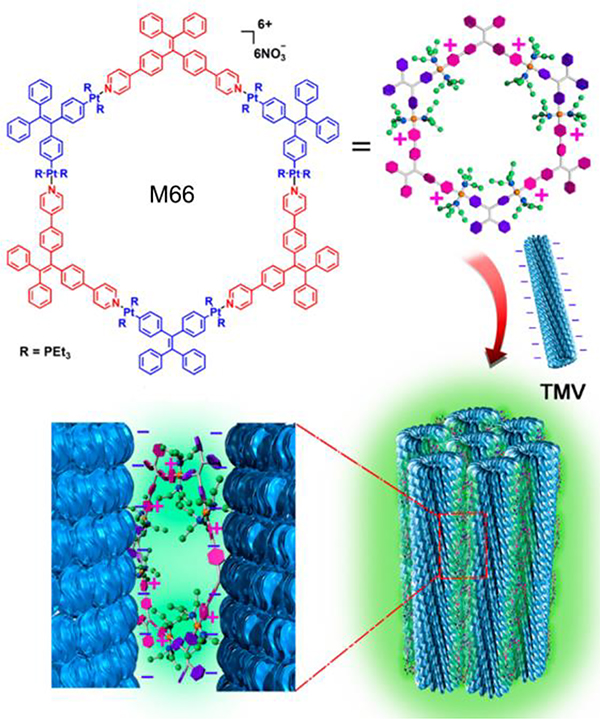
The hierarchical self-assembly of a rod-like TMV (tobacco mosaic virus) with M66 resulted in the formation of fibers.88 Due to the confinement effect in the resultant architectures, the AIE properties caused a notable enhancement in fluorescence (Fig. 37).
Fig. 37.
Coassembly of M66 with TMV. Figure adapted from ref. 88 with permission from American Chemical Society, Copyright 2016.
By using a coordination cage (G50) as the node, a supramolecular coordination framework (SCF) was prepared.61 This matterials exhibits an adamantanoid-to-adamantanoid substructure with two sets of cavities, namely, the interior cavity of the adamantanoid cage and the exterior adamantanoid space between G50 in the network (Fig. 38).
Fig. 38.
G50-based adamantoid materials. Figure adapted from ref. 61 with permission from American Chemical Society, Copyright 2018.
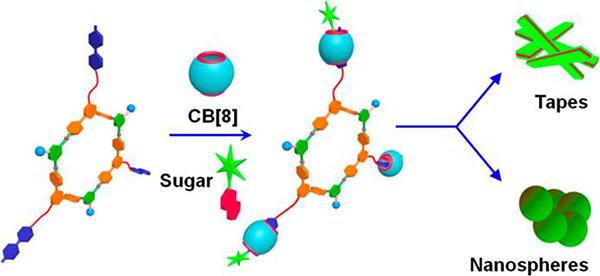
Datta combined CDSA with host−guest interactions to form various suprastructures, including nanospheres and tapes, depending upon the concentration.80 In these suprastructures, CDSA provides the first level of assembly, while host−guest interactions and hydrogen bonding drive the second level of organization (Fig. 39).
Fig. 39.
M55-based nanospheres and tapes. Figure adapted from ref. 80 with permission from American Chemical Society, Copyright 2018.
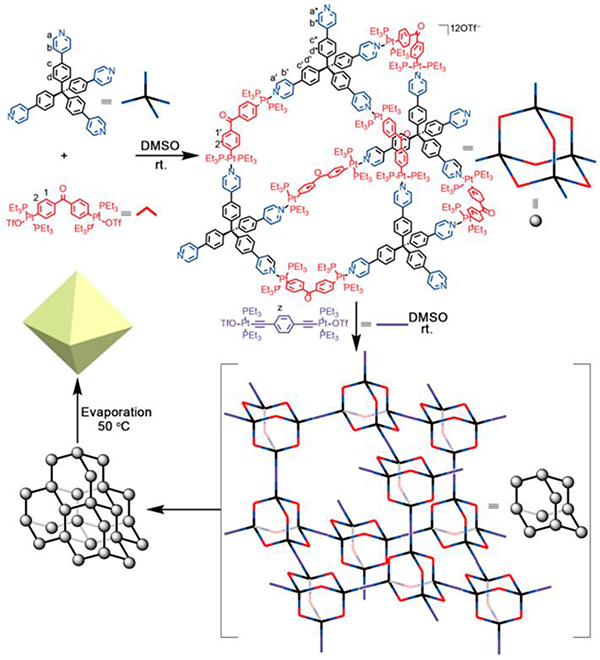
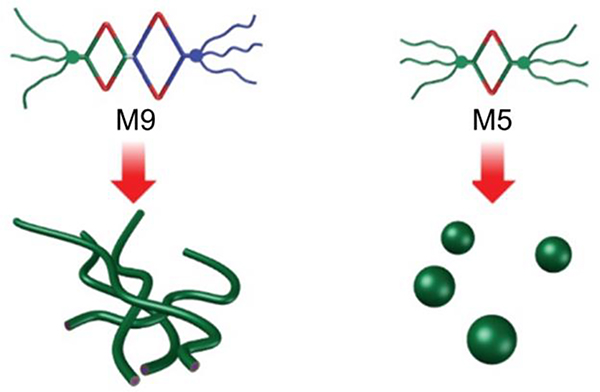
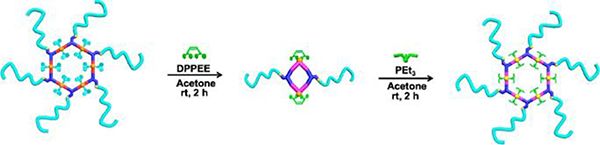
Stang prepared Janus metallacycle M9.47 In an aqueous solution, the hierarchical self-assembly of the supramolecular system was induced by a combination of CDSA and hydrophobic interactions, resulting in the formation of micrometre fibres (Fig. 40).
Fig. 40.
M9/5-based nanospheres and tapes. Figure adapted from ref. 47 with permission from American Chemical Society, Copyright 2019.
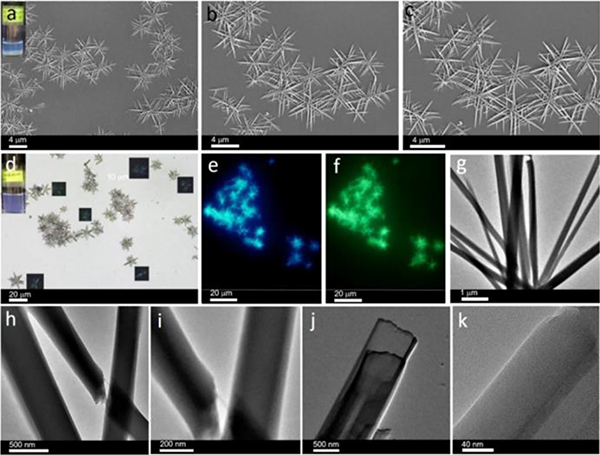
Triggered by visible light, stable triphenylamine (TPA) radical cations of M3 could be generated in both the solution and solid states.36 Furthermore, M3 was able to hierarchically self-assemble into nanovesicles in solution (Fig. 41); nanospheres can be formed in the solid phase mainly due to TPA radical interactions and aromatic stacking interactions. Sun used G13–16 as building blocks for hierarchical assembly.54 Depending on the substituents and solvents, diverse suprastructures (microwires, plates, and spheres) with tuneable emissions were prepared (Fig. 42).
Fig. 41.
M3-based nanovesicles. Figure adapted from ref. 36 with permission from the American Chemical Society, Copyright 2019.
Fig. 42.
G14-based micrometre-scale plates, and G15-based micrometre-scale hollow spheres. Figure adapted from ref. 54 with permission from the American Chemical Society, Copyright 2018.
Sun described the preparation of alanine-based chiral metallacycles M31/51.67 They assembled into nanospheres at low concentrations and generated chiral metallogels at high concentrations (Fig. 43). The molecular chirality of the metallacycles was transferred to the supramolecular architecture through the metallogelation process.
Fig. 43.
(a) M31 (b) M51-based metallogels. Figure adapted from ref. 67 with permission from American Chemical Society, Copyright 2017.
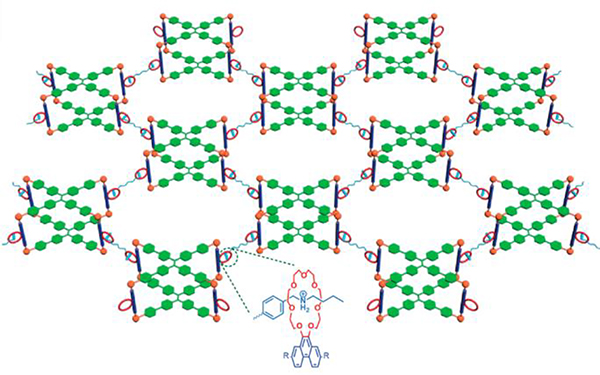
Cao reported the preparation of G49/50-based networks with tetra(4-(4- pyridinyl)phenyl)methane as the node and linear platinum(II) ligands as the linkers.60
These framework materials possess well-defined periodicity and porosity. These rigid frameworks can be directly converted into a metallogel when prepared in DMSO with the concentration increased (Fig. 44).60
Fig. 44.
(a) Synthesis and Disassembly of Diamondoid Frameworks and Tetrahedral G49/50; (b) Photographs of 1 (left) and 3 and 3+TBAB (right) in DMSO. Figure adapted from ref. 60 with permission from American Chemical Society, Copyright 2019.
As shown in Fig. 45, by combining metal−ligand coordination, hydrogen bonding, and host−guest interactions in a hierarchical fashion, M62-based supramolecular polymers were prepared.84
Fig. 45.
(a) Photograph of the M62-based metallogel. (b) SEM image of the xerogel of M62. Figure adapted from ref. 84 with permission from American Chemical Society, Copyright 2016.
Metallacycles M80/81 formed a yellow, translucent metallogel in cyclohexane and CH2Cl2 (v/v = 2/5) when the concentration was increased to 1.3 μM.62 It was proposed that the fibrous structures were first formed mainly through intermolecular Pt···Pt and π–π stacking interactions. Then, as the concentration was increased, a fibre-based network was formed because of the cross-linking of the fibres, which ultimately generated a stable supramolecular metallogel.
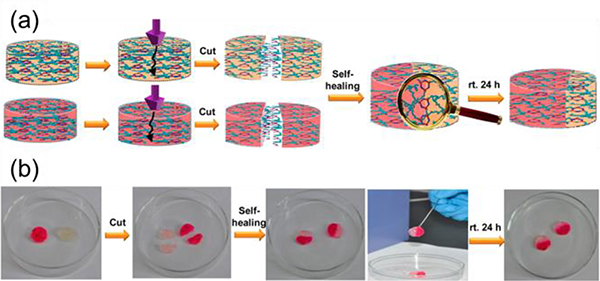
By using well-defined metallacycles (M41/54) as the cores, Yang prepared a family of star supramolecular polymers. In water, the obtained polymer behaved typically for a material with a lower critical solution temperature72 and formed a supramolecular hydrogel (Fig. 47).
Fig. 47.
(a) The mechanism of self-healing of a supramolecular polymeric hydrogel. (b) Photographs of the self-healing experiments. Figure adapted from ref. 72 with permission from American Chemical Society, Copyright 2016.
Yin prepared G24, dissolved it in acetone, and to that solution was added a solution of a bis-ammonium linker in acetone.55 A G24-based supramolecular gel was formed by mixing these two solutions. Both thermally induced and potassium-ion-induced gel−sol transitions can be observed by the naked eye (Fig. 48).
Fig. 48.
Optical and fluorescence photographs of the gel−sol transition of gel. Figure adapted from ref. 55 with permission from American Chemical Society, Copyright 2018.
Host−guest interactions between a bis-ammonium salt and M26–29 drive the formation of supramolecular polymers.66 By continuously increasing the concentration of polymer, a gel with self-healing properties was obtained.
4.4. Stimuli-triggered transformations
Stimuli such as solvents, anions, and changes in the component fractions have been extensively employed to trigger transformation processes of MOCs. For example, starting from functionalized metallacycle M47,76 a structural conversion of the transplatinum acetylide moiety can be triggered by simple phosphine ligand-exchange reactions.76
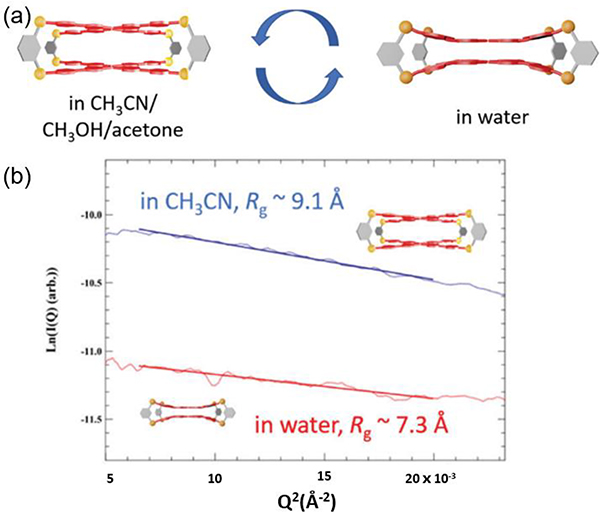
In response to solvent composition, negative solvatochromic emission (NSE) from a conformational change in a coordination cage was demonstrated by Liu. When the solvent was changed from acetone/acetonitrile/methanol to water, the emission of G12 gradually shifts to a shorter wavelength.101 Intramolecular π–π stacking and hydrophobic interactions between the TPPE planes drive molecular conformational change during this process.
4. Applications
Stimuli-responsive elements can be incorporated into MOCs using conventional techniques, resulting in profound implications for the development of smart materials,15 biomedicines,29, 30 catalysis,121 and so on.
5.1. MOC-based environmentally responsive materials
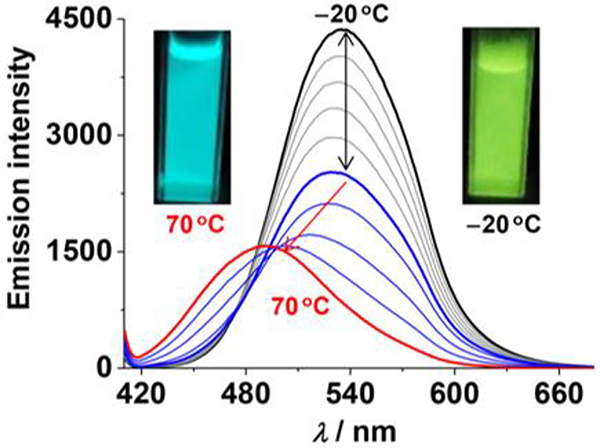
Hexagonal metallacycles M50 and M85, with three and six anthracene groups, respectively, were synthesized by Tang.77 Both M50 and M85 showed reversible changes in their absorption and emission spectra between −20 and 60 °C.
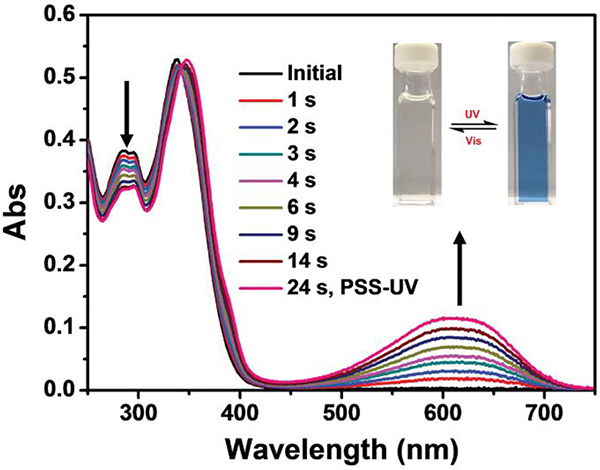
Chirality-tuneable metallacycles M64/6586 containing two light-responsive units and two chiral naphthol units were constructed by CDSA. Upon irradiation with ultraviolet and visible light, the conformations of these metallacycles underwent reversible transformations between ring-open and ring-closed forms, as demonstrated by an obvious change in their CD curves.86
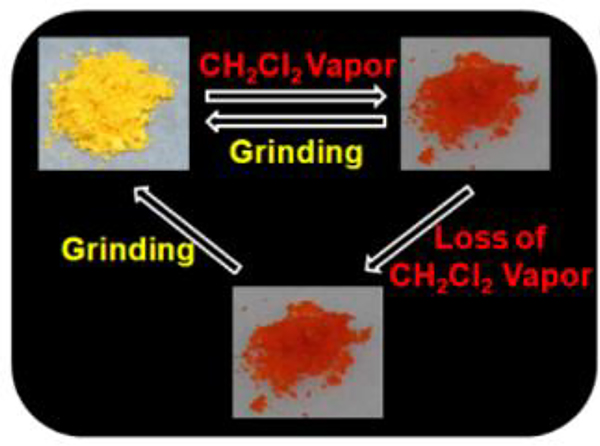
The M80-based vapochromic system features not only high selectivity for CH2Cl2 vapor molecules but also ultrastability.92 At room temperature, when exposed to CH2Cl2 vapour, M80 displayed a colour change from yellow to red. Furthermore, following exposure to CH2Cl2 vapour, M80 retained its red colour in air for several months.
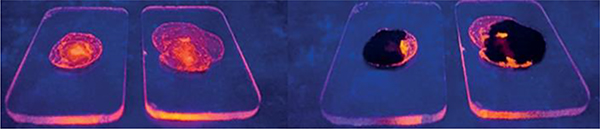
M59 and M92 have been used to detect nitroaromatic explosives.82 Based on fluorescence quenching, thin films of highly luminescent M59 and M92 showed efficient sensing of nitroaromatic compounds in the vapour phase.
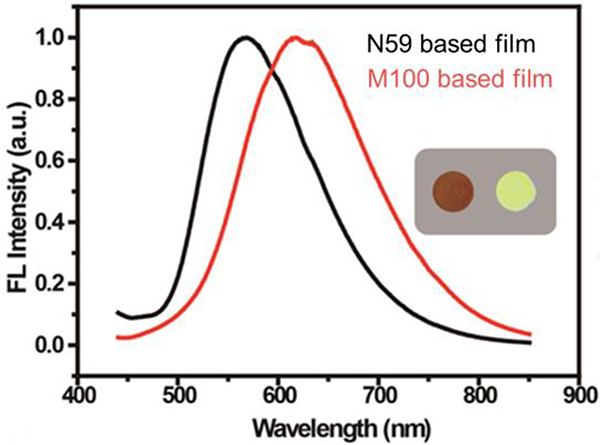
In the substrate-supported state, the quantum yield (QY) of M100 exceeded that of N59, which can be used for sensing.44 Because the metallacycle prevents the dense packing of the compound, the maximum emission of the M100-based film appears at 620 nm, but that of the N59-based film appears at 570 nm.44
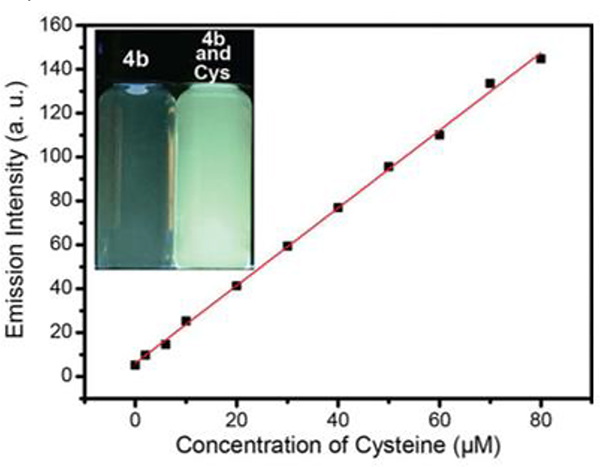
Zhang prepared three different tetragonal metallacages, G25–27.57 A self-destructive mechanism of G26 caused by thiol-containing amino acid was demonstrated in methanol/water.
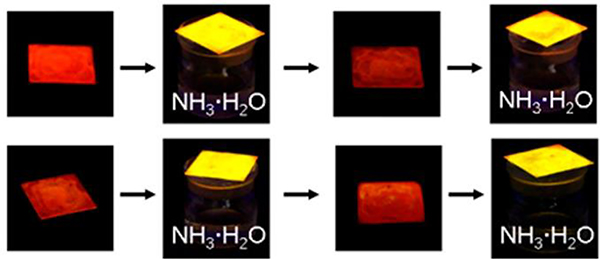
M102 and M103 films were used ammonia sensors as they changed colour from red to yellow upon exposure to ammonia and returned to their initial colour upon removal of the ammonia.96 When the M102 film was exposed to ammonia vapor, an obvious colour change from red to yellow was observed (Fig. 58).
Fig. 58.
Photographs of films of M102 when exposed to or removed from ammonia under a UV lamp at 365 nm. Figure adapted from ref. 96 with permission from American Chemical Society, Copyright 2017.
5.2. Biomedical applications
5.2.1. Metallacycles
TPE-containing fluorescent rhomboid M14 was used in cell imaging, and it showed significant enrichment in lung cells (Fig. 59).38
Fig. 59.
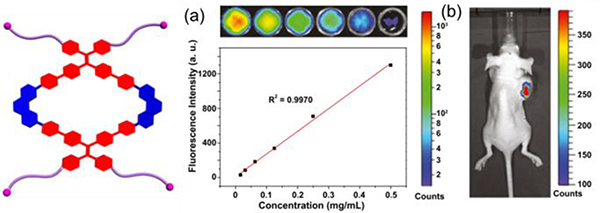
(Left) Schematic representation of M14. (a) Plot and fitting of the fluorescence intensity of M14 versus their concentration. (b) A treated with M14. Figure adapted from ref. 38 with permission from National Academy of Sciences, Copyright 2016.
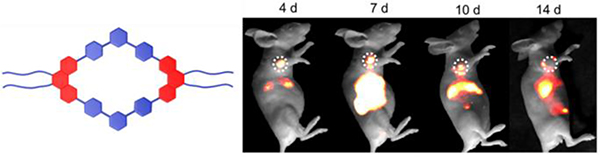
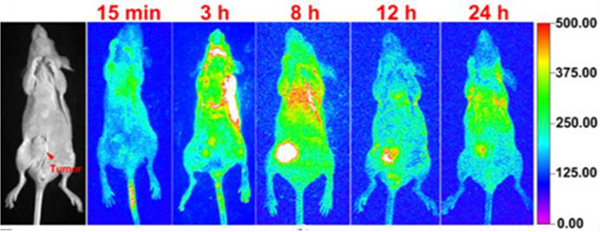
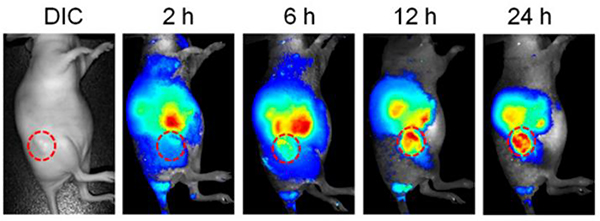
Sun designed and synthesized a NIR-II theranostic nanoprobe involving M34, which has a long emission wavelength.40 This design gives the nanoprobe good photostability and passive targeting ability, and therefore, it can facilitate the precise diagnosis of cancer with high resolution and selective delivery of M34 to the tumour region. Compared with probes in previous reports, this nanoprobe shows enhanced permeability and retention effects. In vitro and in vivo results further indicated that the nanoprobe has higher antitumour efficacy and fewer side effects than cisplatin. As shown in Fig. 60, the fluorescence signal at the tumour region was clearly observed even after 2 weeks.
Fig. 60.
NIR-II images of monitoring the M34 therapeutic response in HepG2 tumours. Figure adapted from ref. 40 with permission from National Academy of Sciences, Copyright 2019.
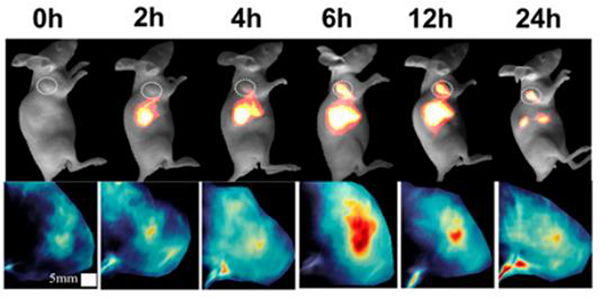
Sun engineered a dual-modal imaging and chemophotothermal therapeutic nanoagent that incorporates M35 and a fluorescent dye into multifunctional melanin dots that have photoacoustic and photothermal features.39 A combination of the antitumour activity of M35 and the inherent photothermal properties of melanin dots that have photoacoustic and photothermal features.39 A combination of the antitumour activity of M35 and the inherent photothermal properties of melanin dots synergistically enabled chemo-photothermal therapy to be precisely performed and gave the desired treatment effects with fewer side effects and increased lifetimes (Fig. 61).39
Fig. 61.
NIR-II fluorescence and PA (photoacoustic) images of U87MG tumour mice at different times after tail vein injection of M35. Figure adapted from ref. 39 with permission from National Academy of Sciences, Copyright 2019.
5.2.1. Metallacages
Terenzi and co-workers prepared three quadrangular boxes, G43–45 (Fig. 62), of distinct size that showed biological activity against cancer cells by influencing the expression of genes known to form guanine quadruplexes.108
Fig. 62.
Schematic representation of G43–45. Figure adapted from ref. 108 with permission from Royal Society of Chemistry, Copyright 2017.
Sleiman obtained platinum squares (M87–90) and examined their binding to DNA and RNA guanine quadruplexes (Fig. 63).43 M87–90 showed submicromolar binding affinities for the RNA telomeric sequence, which regulates telomere elongation in both telomerase-positive/negative cancer cells.
Fig. 63.
Schematic of the competitive fluorescence displacement assay. Figure adapted from ref. 43 with permission from American Chemical Society, Copyright 2017.
Chen used metallacage G19 to construct theranostic supramolecular nanoparticles.103 The fluorescence of localized MNPs in tumour tissues gradually increased with time (Fig. 64).
Fig. 64.
Fluorescence imaging of nude mice with HeLa cancer xenografts after treatment. Figure adapted from ref. 103 with permission from National Academy of Sciences, Copyright 2016.
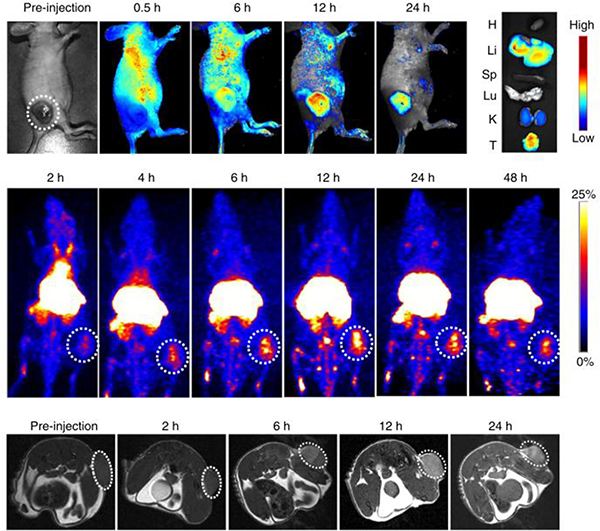
Yu prepared porphyrin-based metallacages, G28–30, and then used these cages for the fabrication of MNPs and studied their antitumour activity.104 By combining NIRFI, PET, and MRI, they achieved precise detection as well as therapy for some tumours. The combination of chemotherapy and PDT (photodynamic therapy) effectively ablated A2780CIS cells in mice without recurrence during the course of therapy. Combination of PDT (eliminated the primary tumour) and the chemotherapeutic drug (killed the residual cancer cells), inhibiting tumour recurrence (Fig. 65).
Fig. 65.
Mice before and after treatment. Figure adapted from ref. 104 with permission from Springer Nature, Copyright 2018.
Yu prepared platinum-based anticancer drug G33 and loaded it as cargo encapsulate a photosensitizer.58 The resulting assemblies could co-deliver chemotherapeutic agents and photosensitizers. The as-prepared MNPs could specifically deliver Pt7 and a photosensitizer to cancer cells. The MNPs accumulated at tumour sites. By combining chemotherapy and PDT, in vivo studies in a drug-resistant tumour model demonstrated that MNPs exhibited superior antitumour activity (Fig. 66).58
Fig. 66.
Time-dependent fluorescence images of A2780CIS tumour-bearing mice after in vivo injection of G33-loaded MNPs. Figure adapted from ref. 58 with permission from National Academy of Sciences, Copyright 2019.
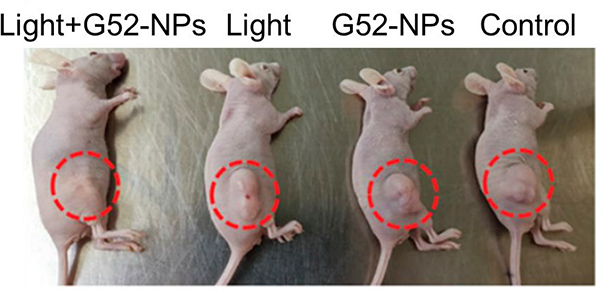
Zhou described the use of molecular self-assembly for accessing G52 with strong 2-photon absorption by combining multiple Ru(II) complexes with Pt(II) building blocks via coordination.79 G52 was encapsulated in a polymer to form nanoparticles that, upon internalization into cells, accumulate in the lysosomes, leading to high photocytotoxicity. The effect of 2-photon PDT on tumour growth was assessed by monitoring the tumour volumes in the mice over a period of 15 days (Fig. 67).
Fig. 67.
Representative photographs of A549 tumours in mice with different treatments. Figure adapted from ref. 79 with permission from National Academy of Sciences, Copyright 2019.
5.2.3. Antibacterial Activity
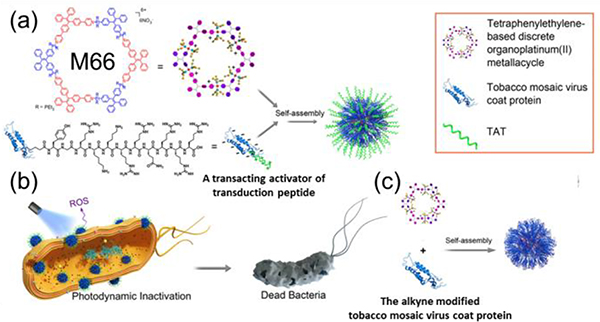
M66 acts as a photosensitizer with aggregation-induced emission.87 It self-assembles with a transacting activator of transduction peptide-decorated virus coat protein through electrostatic interactions. This assembly exhibits both ROS generation and a strong membrane-intercalating abilities, resulting in significantly enhanced PDI (photodynamic inactivation) efficiency against bacteria.122
5.3. Catalysis Activity
MOCs have attracted particular interest due to their structural modifiability and tuneable chemical properties, which are quite important in catalysis.123 Recently, Fe-,124 Co-,125, 126 Zn-,127 and Rh128-based tetrahedral cages and cubes have been used as catalysts. For example, Hooley and co-workers synthesized an Fe4L6 cage, which can serve as an effective supramolecular catalyst, providing up to a 1000-fold rate enhancement in acetal solvolysis.124 MOCs with defined cavities can catalyse chemical reactions with unprecedented selectivity under ambient conditions. Here, Pt(II)-based chiral tetrahedral G4 features an internal nanocavity,100 enabling it to catalyse Michael addition reactions of a series of nitrostyrene derivatives with an indole in a water/methanol mixture (Fig. 69). A series of reactions with various nitrostyrene derivatives and indoles in a water/methanol (9/1) mixture were investigated at room temperature.100
Fig. 69.
Schematic of the synthesis of tetrahedral G4. Figure adapted from ref. 100 with permission from the Royal Society of Chemistry, Copyright 2018.
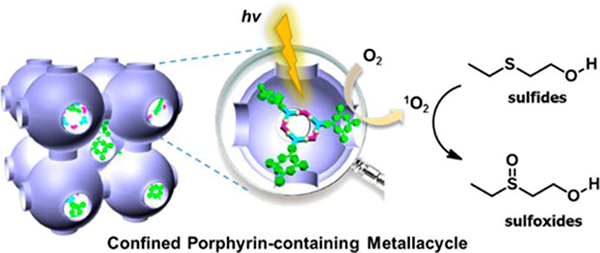
Yang presented the fabrication of M42 in the confined cavities of mesoporous carbon FDU-16.73 To demonstrate that the stability and activity of M42 can be enhanced in the confined cavity, the catalytic performance was evaluated (Fig. 70). Under the same reaction conditions, M42 and the composites exhibited 42% and 54% conversions, respectively, after 4 h (Fig. 70).73
Fig. 70.
Scheme for the photooxidation of sulfides. Figure adapted from ref. 73 with permission from American Chemical Society, Copyright 2018.
5. Conclusions
A large number of well-defined platinum organometallic species, ranging from metallacycles to metallacages, have been constructed via directional bonding, panelling, and dimetallic building block approaches. The applications of these structures for tuning emissions, host–guest interactions, sensing, biomedical and catalysis have been explored. The examples described in this review demonstrate that, through rational chemical manipulations, two-dimensional metallacycles and three-dimensional metallacages that can potentially serve as multifunctional systems, from sensing to catalysis, could be realized. Pt-containing metallacycles and metallacages will continue to be an active area of research and an important part of materials science. In the future, research in this area will likely be directed towards the formation and characterization of more complicated suprastructures and the exploitation of the functions of platinum metallacycles and metallacages in fields such as sensing, optics, catalysis and biomedical materials.
Fig. 4.
. Chemical and crystal structures of G6. Figure adapted from ref. 48 with permission from American Chemical Society, Copyright 2018.
Fig. 10.
(a) Self-assembly of M104–108, controlled by increasing steric hindrance from M104–108. (b) ground-state structures of titled molecules. Figure adapted from ref. 46 with permission from American Chemical Society, Copyright 2019.
Fig. 28.
(a) Schematic representation of the cramming or threading of long amphiphilic oligomers into a G54. (b) G54 and the X-ray crystal structure. Figure adapted from ref. 112 with permission from Springer Nature, Copyright 2018.
Fig. 29.
(a) M11+C60; (b) M30+C60; (c) [M11+C60] where the angles between the Au–N bond. Figure adapted from ref. 64 with permission from Springer Nature, Copyright 2019.
Fig. 46.
Proposed process for the formation of a supramolecular metallogel and the guest-induced gel-to-sol transformation. Figure adapted from ref. 62 with permission from Wiley-VCH, Copyright 2016.
Fig. 49.
Photographs of the self-healing process. Figure adapted from ref. 66 with permission from American Chemical Society, Copyright 2019.
Fig. 50.
Graphical representation of the synthesis of metallacycle and a metallacycle-linked star polymer. Figure adapted from ref. 76 with permission from American Chemical Society, Copyright 2019.
Fig. 51.
(a) Illustration of the proposed conformational change of G12 in different solvents. (b) Guinier analysis of G12 in aqueous (red) and acetonitrile solutions (blue). Figure adapted from ref. 101 with permission from Royal Society of Chemistry, Copyright 2019.
Fig. 52.
. Emission spectral changes (M50). Figure adapted from ref. 77 with permission from American Chemical Society, Copyright 2018.
Fig. 53.
Absorption spectral changes of M65 upon UV (365 nm) irradiation. Figure adapted from ref. 86 with permission from Wiley-VCH, Copyright 2018.
Fig. 54.
Photographs showing the dynamic colour changes under ambient light for the reversible vapochromic phenomenon of M80/81. Figure adapted from ref. 92 with permission from American Chemical Society, Copyright 2016.
Fig. 55.
. Fluorescence images of thin films of compounds M59 and M92 after exposure to NB (nitrobenzene). Figure adapted from ref. 82 with permission from Wiley-VCH, Copyright 2016.
Fig. 56.
Fluorescence spectra of M100 and N59-based silica gel plate-supported films. Figure adapted from ref. 44 with permission from American Chemical Society, Copyright 2019.
Fig. 57.
The emission intensities of G26 at 500 nm as a function of cysteine. Figure adapted from ref. 57 with permission from American Chemical Society, Copyright 2017.
Fig. 68.
Schematic illustration of the self-assembly of (a) M66 and a transacting activator of transduction peptide. (b) Antibacterial mechanism. (c) M66 and protein the alkyne-modified tobacco mosaic virus coat protein. Figure adapted from ref. 122 with permission from National Academy of Sciences, Copyright 2019.
Table 12.
Pt27–32-based metallacycles (M83–86).
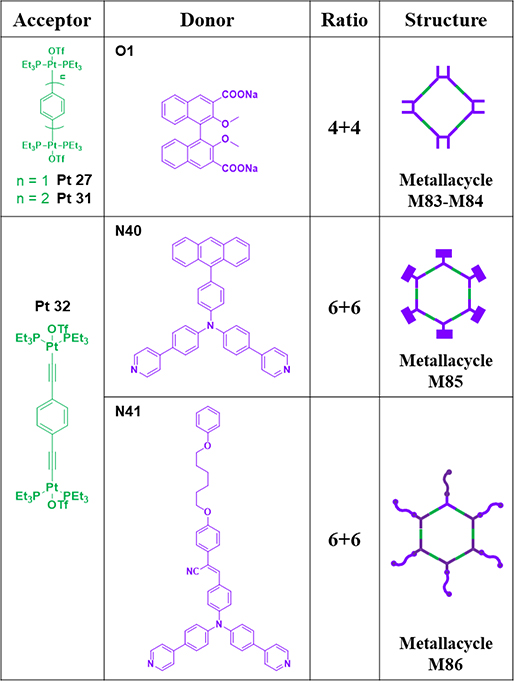 |
Table 14.
Pt7-based metallacycles (M96–108).
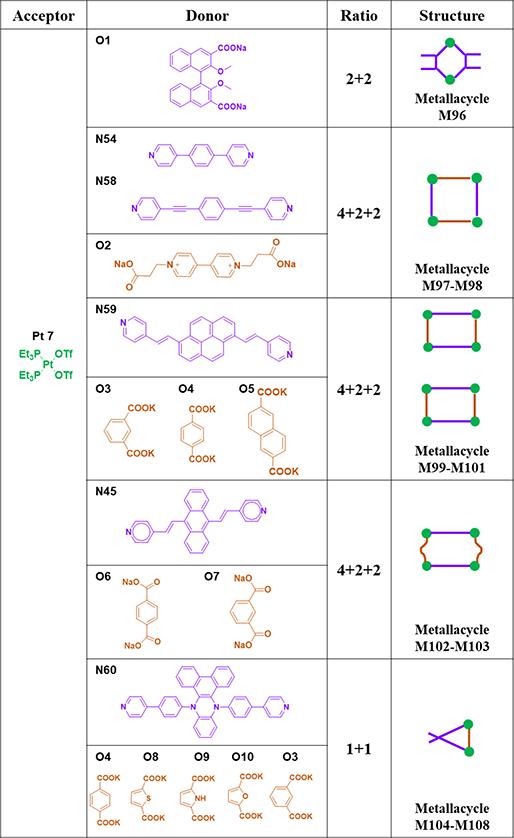 |
Table 17.
Tetragonal prisms (G12–32)
 |
 |
Acknowledgements
Y.S. thanks the National Natural Science Foundation of China (21503185, 21404062), Natural Science Foundation of Zhejiang Province (LY20B040003) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Biographies
 Yan Sun obtained her Ph.D. degree at the Institute of Chemistry, Chinese Academy of Sciences, in 2012. Her research interests mainly focus on the synthesis and application of novel functional materials, multi-level and higher-ordered assembly. She joined Peter J. Stang’s group at University of Utah in 2017, and now she is investigating metal-organic complexes (MOCs) and MOC-based multi-level assembly.
Yan Sun obtained her Ph.D. degree at the Institute of Chemistry, Chinese Academy of Sciences, in 2012. Her research interests mainly focus on the synthesis and application of novel functional materials, multi-level and higher-ordered assembly. She joined Peter J. Stang’s group at University of Utah in 2017, and now she is investigating metal-organic complexes (MOCs) and MOC-based multi-level assembly.
 Chongyi Chen obtained his BS degree in Chemistry in 2008 from the University of Science and Technology of China, his PhD degree in 2013 from Peking University and Institute of Chemistry, Chinese Academy of Sciences. He is currently an Associate Professor in the Department of Polymers at Ningbo University. His research interests include polypeptide-based chemistry and materials.
Chongyi Chen obtained his BS degree in Chemistry in 2008 from the University of Science and Technology of China, his PhD degree in 2013 from Peking University and Institute of Chemistry, Chinese Academy of Sciences. He is currently an Associate Professor in the Department of Polymers at Ningbo University. His research interests include polypeptide-based chemistry and materials.
 Jianbo Liu obtained his Ph.D. degree at the Shanghai institutes of Organic Chemistry, Chinese Academy of Science (SIOC) in 2016. He worked as a postdoctoral fellowship at University of Utah in 2017–2019. Then he continued his postdoc research in the University of California, San Francisco. His research interest is organic fluorine chemistry and asymmetric catalysis.
Jianbo Liu obtained his Ph.D. degree at the Shanghai institutes of Organic Chemistry, Chinese Academy of Science (SIOC) in 2016. He worked as a postdoctoral fellowship at University of Utah in 2017–2019. Then he continued his postdoc research in the University of California, San Francisco. His research interest is organic fluorine chemistry and asymmetric catalysis.
 Peter J. Stang is the David P. Gardner distinguished professor of Chemistry. He is a member of the US National Academy of Sciences, a foreign member of the Chinese Academy of Sciences, and the recipient of the Chinese Government “International Cooperation Award in Science and Technology” (2016), the ACS Priestley Medal (2013), and the US National Medal of Science (2011).
Peter J. Stang is the David P. Gardner distinguished professor of Chemistry. He is a member of the US National Academy of Sciences, a foreign member of the Chinese Academy of Sciences, and the recipient of the Chinese Government “International Cooperation Award in Science and Technology” (2016), the ACS Priestley Medal (2013), and the US National Medal of Science (2011).
Footnotes
Conflicts of interest
There are no conflicts to declare.
Notes and references
- 1.Vantomme G and Meijer EW, Science, 2019, 363, 1396–1397. [DOI] [PubMed] [Google Scholar]
- 2.Praetorius F, Kick B, Behler KL, Honemann MN, Weuster-Botz D and Dietz H, Nature, 2017, 552, 84. [DOI] [PubMed] [Google Scholar]
- 3.Tao K, Makam P, Aizen R and Gazit E, Science, 2017, 358, eaam9756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon AJ, Zhou Y, Ramasubramani V, Glaser J, Pothukuchy A, Gollihar J, Gerberich JC, Leggere JC, Morrow BR, Jung C, Glotzer SC, Taylor DW and Ellington AD, Nat. Chem, 2019, 11, 204–212. [DOI] [PubMed] [Google Scholar]
- 5.Rittle J, Field MJ, Green MT and Tezcan FA, Nat. Chem, 2019, 11, 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Y, Guo F, Zuo T, Hua J and Diao G, Nat. Commun., 2016, 7, 12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita D, Ueda Y, Sato S, Mizuno N, Kumasaka T and Fujita M, Nature, 2016, 540, 563–566. [DOI] [PubMed] [Google Scholar]
- 8.Ballester P, Fujita M and Rebek J, Chem. Soc. Rev, 2015, 44, 392–393. [DOI] [PubMed] [Google Scholar]
- 9.Cook TR and Stang PJ, Chem. Rev., 2015, 115, 7001–7045. [DOI] [PubMed] [Google Scholar]
- 10.Chakrabarty R, Mukherjee PS and Stang PJ, Chem. Rev., 2011, 111, 6810–6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook TR, Zheng Y-R and Stang PJ, Chem. Rev., 2013, 113, 734–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caulder DL and Raymond KN, Acc. Chem. Res, 1999, 32, 975–982. [Google Scholar]
- 13.Lifschitz AM, Rosen MS, McGuirk CM and Mirkin CA, J. Am. Chem. Soc, 2015, 137, 7252–7261. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborty S and Newkome GR, Chem. Soc. Rev., 2018, 47, 3991–4016. [DOI] [PubMed] [Google Scholar]
- 15.McConnell AJ, Wood CS, Neelakandan PP and Nitschke JR, Chem. Rev., 2015, 115, 7729–7793. [DOI] [PubMed] [Google Scholar]
- 16.Zarra S, Wood DM, Roberts DA and Nitschke JR, Chem. Soc. Rev., 2015, 44, 419–432. [DOI] [PubMed] [Google Scholar]
- 17.Zhang D, Ronson TK and Nitschke JR, Acc. Chem. Res., 2018, 51, 2423–2436. [DOI] [PubMed] [Google Scholar]
- 18.Sawada T, Saito A, Tamiya K, Shimokawa K, Hisada Y and Fujita M, Nat. Commun., 2019, 10, 921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clever GH and Punt P, Acc. Chem. Res., 2017, 50, 2233–2243. [DOI] [PubMed] [Google Scholar]
- 20.Gao Z, Han Y, Gao Z and Wang F, Acc. Chem. Res., 2018, 51, 2719–2729. [DOI] [PubMed] [Google Scholar]
- 21.Stricklen P and Verkade J, J. Am. Chem. Soc, 1983, 105, 2494–2495. [Google Scholar]
- 22.Zhang Y-Y, Gao W-X, Lin L and Jin G-X, Coord. Chem. Rev., 2017, 344, 323–344. [Google Scholar]
- 23.Bloch WM and Clever GH, Chem. Commun., 2017, 53, 8506–8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stang PJ and Olenyuk B, Acc. Chem. Res, 1997, 30, 502–518. [Google Scholar]
- 25.Fujita M, Tominaga M, Hori A and Therrien B, Acc. Chem. Res, 2005, 38, 369–378. [DOI] [PubMed] [Google Scholar]
- 26.Oliveri CG, Ulmann PA, Wiester MJ and Mirkin CA, Acc. Chem. Res, 2008, 41, 1618–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward MD, Hunter CA and Williams NH, Acc. Chem. Res, 2018, 51, 2073–2082. [DOI] [PubMed] [Google Scholar]
- 28.Chen L-J and Yang H-B, Acc. Chem. Res, 2018, 51, 2699–2710. [DOI] [PubMed] [Google Scholar]
- 29.Sepehrpour H, Fu W, Sun Y and Stang PJ, J. Am. Chem. Soc, 2019, 141, 14005–14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Judge N, Wang L, Ho YYL and Wang Y, Macromol. Res, 2018, 26, 1074–1084. [Google Scholar]
- 31.Rota Martir D and Zysman-Colman E, Chem. Commun., 2019, 55, 139–158. [DOI] [PubMed] [Google Scholar]
- 32.Jing X, He C, Zhao L and Duan C, Acc. Chem. Res, 2019, 52, 100–109. [DOI] [PubMed] [Google Scholar]
- 33.Saha ML, Yan X and Stang PJ, Acc. Chem. Res., 2016, 49, 2527–2539. [DOI] [PubMed] [Google Scholar]
- 34.Wei P, Yan X, Cook TR, Ji X, Stang PJ and Huang F, ACS Macro. Lett., 2016, 5, 671–675. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi S, Saha ML and Stang PJ, J. Organomet. Chem., 2017, 847, 294–297. [Google Scholar]
- 36.Huo G-F, Shi X, Tu Q, Hu Y-X, Wu G-Y, Yin G-Q, Li X, Xu L, Ding H-M and Yang H-B, J. Am. Chem. Soc, 2019, 141, 16014–16023. [DOI] [PubMed] [Google Scholar]
- 37.Xu L, Shen X, Zhou Z, He T, Zhang J, Qiu H, Saha ML, Yin S and Stang PJ, J. Am. Chem. Soc, 2018, 140, 16920–16924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang M, Li S, Yan X, Zhou Z, Saha ML, Wang Y-C and Stang PJ, Proc. Natl. Acad. Sci, 2016, 113, 11100–11105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun Y, Ding F, Chen Z, Zhang R, Li C, Xu Y, Zhang Y, Ni R, Li X, Yang G, Sun Y and Stang PJ, Proc. Natl. Acad. Sci. USA, 2019, 116, 16729–16735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Y, Ding F, Zhou Z, Li C, Pu M, Xu Y, Zhan Y, Lu X, Li H, Yang G, Sun Y and Stang PJ, Proc. Natl. Acad. Sci. USA, 2019, 116, 1968–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang M, Yin S, Zhang J, Zhou Z, Saha ML, Lu C and Stang PJ, Proc. Natl. Acad. Sci. USA, 2017, 114, 3044–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta V and Mandal SK, Dalton Trans., 2018, 47, 9742–9754. [DOI] [PubMed] [Google Scholar]
- 43.Garci A, Castor KJ, Fakhoury J, Do J-L, Di Trani J, Chidchob P, Stein RS, Mittermaier AK, Friscic T and Sleiman H, J. Am. Chem. Soc., 2017, 139, 16913–16922. [DOI] [PubMed] [Google Scholar]
- 44.Chang X, Zhou Z, Shang C, Wang G, Wang Z, Qi Y, Li Z-Y, Wang H, Cao L, Li X, Fang Y and Stang PJ, J. Am. Chem. Soc, 2019, 141, 1757–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plutnar J, Givelet C, Lemouchi C, Jaklová Dytrtová J, Císařová I, Teat SJ and Michl J, Organometallics, 2019, 38, 4633–4644. [Google Scholar]
- 46.Zhou Z, Chen D-G, Saha ML, Wang H, Li X, Chou P-T and Stang PJ, J. Am. Chem. Soc, 2019, 141, 5535–5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang W, Zhou Z, Zhou J, Shi B, Song B, Li X, Huang F and Stang PJ, Inorg. Chem, 2019, 58, 7141–7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryu JY, Lee JM, Nguyen Van N, Lee KM, Lee S, Lee MH, Stang PJ and Lee J, Inorg. Chem., 2018, 57, 11696–11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang M, Xu H, Wang M, Saha ML, Zhou Z, Yan X, Wang H, Li X, Huang F, She N and Stang PJ, Inorg. Chem., 2017, 56, 12498–12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, Chen J-S, Liu J-Y, Zhao G-J, Liu L, Han K-L, Cook TR and Stang PJ, J. Phys. Chem. Lett, 2015, 6, 1942–1947. [DOI] [PubMed] [Google Scholar]
- 51.Liu N, Lin T, Wu M, Luo H-K, Huang S-L and Hor TSA, J. Am. Chem. Soc., 2019, 141, 9448–9452. [DOI] [PubMed] [Google Scholar]
- 52.Yu G, Ye Y, Tong Z, Yang J, Li Z, Hua B, Shao L and Li S, Macromol. Rapid Commun, 2016, 37, 1540–1547. [DOI] [PubMed] [Google Scholar]
- 53.Sun Y, Zhang F, Jiang S, Wang Z, Ni R, Wang H, Zhou W, Li X and Stang PJ, J. Am. Chem. Soc, 2018, 140, 17297–17307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun Y, Yao Y, Wang H, Fu W, Chen C, Saha ML, Zhang M, Datta S, Zhou Z, Yu H, Li X and Stang PJ, J. Am. Chem. Soc., 2018, 140, 12819–12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu C, Zhang M, Tang D, Yan X, Zhang Z, Zhou Z, Song B, Wang H, Li X, Yin S, Sepehrpour H and Stang PJ, J. Am. Chem. Soc., 2018, 140, 7674–7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan X, Cook TR, Wang P, Huang F and Stang PJ, Nat. Chem, 2015, 7, 342–348. [DOI] [PubMed] [Google Scholar]
- 57.Zhang M, Saha ML, Wang M, Zhou Z, Song B, Lu C, Yan X, Li X, Huang F, Yin S and Stang PJ, J. Am. Chem. Soc., 2017, 139, 5067–5074. [DOI] [PubMed] [Google Scholar]
- 58.Yu G, Zhu B, Shao L, Zhou J, Saha ML, Shi B, Zhang Z, Hong T, Li S, Chen X and Stang PJ, Proc. Natl. Acad. Sci. USA, 2019, 116, 6618–6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ye Y, Cook TR, Wang S-P, Wu J, Li S and Stang PJ, J. Am. Chem. Soc, 2015, 137, 11896–11899. [DOI] [PubMed] [Google Scholar]
- 60.Cao L, Wang P, Miao X, Duan H, Wang H, Dong Y, Ma R, Zhang B, Wu B, Li X and Stang PJ, Inorg. Chem, 2019, 58, 6268–6275. [DOI] [PubMed] [Google Scholar]
- 61.Cao L, Wang P, Miao X, Dong Y, Wang H, Duan H, Yu Y, Li X and Stang PJ, J. Am. Chem. Soc., 2018, 140, 7005–7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang B, Zhang J, Zheng W, Chen L-J, Yin G-Q, Wang Y-X, Sun B, Li X and Yang H-B, Chem. Eur. J., 2016, 22, 14664–14671. [DOI] [PubMed] [Google Scholar]
- 63.Neti VSPK, Saha ML, Yan X, Zhou Z and Stang PJ, Organometallics, 2015, 34, 4813–4815. [Google Scholar]
- 64.Tang J-H, Li Y, Wu Q, Wang Z, Hou S, Tang K, Sun Y, Wang H, Wang H, Lu C, Wang X, Li X, Wang D, Yao J, Lambert CJ, Tao N, Zhong Y-W and Stang PJ, Nat. Commun, 2019, 10, 4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan X, Wang H, Hauke CE, Cook TR, Wang M, Saha ML, Zhou Z, Zhang M, Li X, Huang F and Stang PJ, J. Am. Chem. Soc, 2015, 137, 15276–15286. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Q, Tang D, Zhang J, Ni R, Xu L, He T, Lin X, Li X, Qiu H, Yin S and Stang PJ, J. Am. Chem. Soc, 2019, 141, 17909–17917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun Y, Li S, Zhou Z, Saha ML, Datta S, Zhang M, Yan X, Tian D, Wang H, Wang L, Li X, Liu M, Li H and Stang PJ, J. Am. Chem. Soc., 2018, 140, 3257–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ye Y, Wang S-P, Zhu B, Cook TR, Wu J, Li S and Stang PJ, Org. Lett., 2015, 17, 2804–2807. [DOI] [PubMed] [Google Scholar]
- 69.Zhou J, Zhang Y, Yu G, Crawley MR, Fulong CRP, Friedman AE, Sengupta S, Sun J, Li Q, Huang F and Cook TR, J. Am. Chem. Soc., 2018, 140, 7730–7736. [DOI] [PubMed] [Google Scholar]
- 70.Zhou Z, Yan X, Saha ML, Zhang M, Wang M, Li X and Stang PJ, J. Am. Chem. Soc, 2016, 138, 13131–13134. [DOI] [PubMed] [Google Scholar]
- 71.Yue Z, Wang H, Li Y, Qin Y, Xu L, Bowers DJ, Gangoda M, Li X, Yang H-B and Zheng Y-R, Chem. Commun., 2018, 54, 731–734. [DOI] [PubMed] [Google Scholar]
- 72.Zheng W, Chen L-J, Yang G, Sun B, Wang X, Jiang B, Yin G-Q, Zhang L, Li X, Liu M, Chen G and Yang H-B, J. Am. Chem. Soc., 2016, 138, 4927–4937. [DOI] [PubMed] [Google Scholar]
- 73.Chen L-J, Chen S, Qin Y, Xu L, Yin G-Q, Zhu J-L, Zhu F-F, Zheng W, Li X and Yang H-B, J. Am. Chem. Soc, 2018, 140, 5049–5052. [DOI] [PubMed] [Google Scholar]
- 74.Wu G-Y, Wang X-Q, Chen L-J, Hu Y-X, Yin G-Q, Xu L, Jiang B and Yang H-B, Inorg. Chem., 2018, 57, 15414–15420. [DOI] [PubMed] [Google Scholar]
- 75.Baba A, Kojima T and Hiraoka S, Chem. Eur. J., 2018, 24, 838–847. [DOI] [PubMed] [Google Scholar]
- 76.Zheng W, Wang W, Jiang S-T, Yang G, Li Z, Wang X-Q, Yin G-Q, Zhang Y, Tan H, Li X, Ding H, Chen G and Yang H-B, J. Am. Chem. Soc., 2019, 141, 583–591. [DOI] [PubMed] [Google Scholar]
- 77.Tang J-H, Sun Y, Gong Z-L, Li Z-Y, Zhou Z, Wang H, Li X, Saha ML, Zhong Y-W and Stang PJ, J. Am. Chem. Soc, 2018, 140, 7723–7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Acharyya K, Bhattacharyya S, Sepehrpour H, Chakraborty S, Lu S, Shi B, Li X, Mukherjee PS and Stang PJ, J. Am. Chem. Soc, 2019, 141, 14565–14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou Z, Liu J, Huang J, Rees TW, Wang Y, Wang H, Li X, Chao H and Stang PJ, Proc. Natl. Acad. Sci. USA, 2019, 116, 20296–20302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Datta S, Saha ML, Lahiri N, Yu G, Louie J and Stang PJ, Org. Lett, 2018, 20, 7020–7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang D, Li D, Li X and Jin W, Dyes Pigm., 2018, 152, 43–48. [Google Scholar]
- 82.Chowdhury A, Howlader P and Mukherjee PS, Chem.-Eur. J, 2016, 22, 7468–7478. [DOI] [PubMed] [Google Scholar]
- 83.Qin Y, Chen L-J, Dong F, Jiang S-T, Yin G-Q, Li X, Tian Y and Yang H-B, J. Am. Chem. Soc., 2019, 141, 8943–8950. [DOI] [PubMed] [Google Scholar]
- 84.Zhou Z, Yan X, Cook TR, Saha ML and Stang PJ, J. Am. Chem. Soc., 2016, 138, 806–809. [DOI] [PubMed] [Google Scholar]
- 85.Datta S, Misra SK, Saha ML, Lahiri N, Louie J, Pan D and Stang PJ, Proc. Natl. Acad. Sci. USA, 2018, 115, 8087–8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y-X, Zhou Q-F, Jiang S-T, Zhang Y, Yin G-Q, Jiang B, Li X, Tan H and Yang H-B, Macromol. Rapid Commun, 2018, 39, 1800454. [DOI] [PubMed] [Google Scholar]
- 87.Yan X, Wang M, Cook TR, Zhang M, Saha ML, Zhou Z, Li X, Huang F and Stang PJ, J. Am. Chem. Soc, 2016, 138, 4580–4588. [DOI] [PubMed] [Google Scholar]
- 88.Tian Y, Yan X, Saha ML, Niu Z and Stang PJ, J. Am. Chem. Soc., 2016, 138, 12033–12036. [DOI] [PubMed] [Google Scholar]
- 89.Martinou E, Seintis K, Karakostas N, Bletsou A, Thomaidis NS, Fakis M and Pistolis G, J. Phys. Chem. C 2017, 121, 5341–5355. [Google Scholar]
- 90.Shi B, Zhou Z, Vanderlinden RT, Tang J-H, Yu G, Acharyya K, Sepehrpour H and Stang PJ, J. Am. Chem. Soc, 2019, 141, 11837–11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi B, Liu Y, Zhu H, Vanderlinden RT, Shangguan L, Ni R, Acharyya K, Tang J-H, Zhou Z, Li X, Huang F and Stang PJ, J. Am. Chem. Soc, 2019, 141, 6494–6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiang B, Zhang J, Ma J-Q, Zheng W, Chen L-J, Sun B, Li C, Hu B-W, Tan H, Li X and Yang H-B, J. Am. Chem. Soc., 2016, 138, 738–741. [DOI] [PubMed] [Google Scholar]
- 93.Song B, Zhang Z, Wang K, Hsu C-H, Bolarinwa O, Wang J, Li Y, Yin G-Q, Rivera E, Yang H-B, Liu C, Xu B and Li X, Angew. Chem. Int. Ed., 2017, 56, 5258–5262. [DOI] [PubMed] [Google Scholar]
- 94.He Z, Li M, Que W and Stang PJ, Dalton Trans., 2017, 46. [DOI] [PubMed] [Google Scholar]
- 95.Givelet CC, Dron PI, Wen J, Magnera TF, Zamadar M, Cepe K, Fujiwara H, Shi Y, Tuchband MR, Clark N, Zboril R and Michl J, J. Am. Chem. Soc., 2016, 138, 6676–6687. [DOI] [PubMed] [Google Scholar]
- 96.Li Z, Yan X, Huang F, Sepehrpour H and Stang PJ, Org. Lett, 2017, 19, 5728–5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jana A, Bhowmick S, Kaur S, Kashyap HK and Das N, Dalton Trans., 2017, 46, 1986–1995. [DOI] [PubMed] [Google Scholar]
- 98.Bhowmick S, Jana A, Singh K, Gupta P, Gangrade A, Mandal BB and Das N, Inorg. Chem., 2018, 57, 3615–3625. [DOI] [PubMed] [Google Scholar]
- 99.Zhang R-L, Yang Y, Yang S-Q and Han K-L, Phys. Chem. Chem. Phys, 2018, 20, 2205–2210. [DOI] [PubMed] [Google Scholar]
- 100.Bhat IA, Devaraj A, Howlader P, Chi K-W and Mukherjee PS, Chem. Commun, 2018, 54, 4814–4817. [DOI] [PubMed] [Google Scholar]
- 101.Li H, Xie T-Z, Liang Z, Dahal D, Shen Y, Sun X, Yang Y, Pang Y and Liu T, Chem. Commun., 2019, 55, 330–333. [DOI] [PubMed] [Google Scholar]
- 102.Zhang M, Saha ML and Stang PJ, Struct. Chem., 2017, 28, 453–459. [Google Scholar]
- 103.Yu G, Cook TR, Li Y, Yan X, Wu D, Shao L, Shen J, Tang G, Huang F, Chen X and Stang PJ, Proc. Natl. Acad. Sci. USA, 2016, 113, 13720–13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu G, Yu S, Saha ML, Zhou J, Cook TR, Yung BC, Chen J, Mao Z, Zhang F, Zhou Z, Liu Y, Shao L, Wang S, Gao C, Huang F, Stang PJ and Chen X, Nat. Commun, 2018, 9, 4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou Z, Hauke CE, Song B, Li X, Stang PJ and Cook TR, J. Am. Chem. Soc, 2019, 141, 3717–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.He Z, Hou Z, Xing Y, Liu X, Yin X, Que M, Shao J, Que W and Stang PJ, Sci. Rep, 2016, 6, 29476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tang J-H, Ni R, He Y-Q, Vanderlinden RT, Li Y, Shi B, Li Z-Y, Wang H, Li X, Sun Y, Zhong Y-W and Stang PJ, Inorg. Chem, 2019, 58, 13376–13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Domarco O, Loetsch D, Schreiber J, Dinhof C, Van Schoonhoven S, Garcia MD, Peinador C, Keppler BK, Berger W and Terenzi A, Dalton Trans., 2017, 46, 329–332. [DOI] [PubMed] [Google Scholar]
- 109.Wang H, Qiu Z, Liu H, Jayawardhana AMDS, Yue Z, Daghlas H, Bowers DJ, Datta B and Zheng Y-R, Front. Chem, 2019, 7, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ahmedova A, Mihaylova R, Momekova D, Shestakova P, Stoykova S, Zaharieva J, Yamashina M, Momekov G, Akita M and Yoshizawa M, Dalton Trans., 2016, 45, 13214–13221. [DOI] [PubMed] [Google Scholar]
- 111.Yamashina M, Akita M, Hasegawa T, Hayashi S and Yoshizawa M, Sci. Adv, 2017, 3, e1701126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yamashina M, Kusaba S, Akita M, Kikuchi T and Yoshizawa M, Nat. Commun, 2018, 9, 4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kalenius E, Groessl M and Rissanen K, Nat. Rev. Chem, 2019, 3, 4–14. [Google Scholar]
- 114.Mallis C, Lal Saha M and Stang P, J. Am. Soc. Mass Spectrom, 2019, 30, 1654–1662. [DOI] [PubMed] [Google Scholar]
- 115.Zhang R-L, Yang Y, Yang S-Q, Neti VSPK, Sepehrpour H, Stang PJ and Han K-L, J. Phys. Chem. C, 2017, 121, 14975–14980. [Google Scholar]
- 116.Rizzuto FJ, von Krbek LKS and Nitschke JR, Nature Reviews Chemistry, 2019, 3, 204–222. [Google Scholar]
- 117.Ye Y, Wang S-P, Zhu B, Cook TR, Wu J, Li S and Stang PJ, Org. Lett, 2015, 17, 2804–2807. [DOI] [PubMed] [Google Scholar]
- 118.Hosono N and Kitagawa S, Acc. Chem. Res, 2018, 51, 2437–2446. [DOI] [PubMed] [Google Scholar]
- 119.Sun Y, Chen C and Stang PJ, Acc. Chem. Res., 2019, 52, 802–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li B, He T, Fan Y, Yuan X, Qiu H and Yin S, Chem. Commun, 2019, 55, 8036–8059. [DOI] [PubMed] [Google Scholar]
- 121.Zhao L, Jing X, Li X, Guo X, Zeng L, He C and Duan C, Coord. Chem. Rev., 2019, 378, 151–187. [Google Scholar]
- 122.Gao S, Yan X, Xie G, Zhu M, Ju X, Stang PJ, Tian Y and Niu Z, Proc. Natl. Acad. Sci, 2019, 116, 23437–23443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hong CM, Bergman RG, Raymond KN and Toste FD, Acc. Chem. Res., 2018, 51, 2447–2455. [DOI] [PubMed] [Google Scholar]
- 124.Holloway LR, Bogie PM, Lyon Y, Ngai C, Miller TF, Julian RR and Hooley RJ, J. Am. Chem. Soc., 2018, 140, 8078–8081. [DOI] [PubMed] [Google Scholar]
- 125.Oldacre AN, Friedman AE and Cook TR, J. Am. Chem. Soc., 2017, 139, 1424–1427. [DOI] [PubMed] [Google Scholar]
- 126.Oldacre AN, Crawley MR, Friedman AE and Cook TR, Chem. Eur. J., 2018, 24, 10984–10987. [DOI] [PubMed] [Google Scholar]
- 127.Jiao J, Li Z, Qiao Z, Li X, Liu Y, Dong J, Jiang J and Cui Y, Nat. Commun., 2018, 9, 4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nurttila SS, Brenner W, Mosquera J, van Vliet KM, Nitschke JR and Reek JNH, Chem. Eur. J., 2019, 25, 609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]