Abstract
Several recent reviews have suggested a role of oxidative stress in the pathophysiology of COVID-19, but its interplay with disease severity has not been revealed yet. In the present study, we aimed to investigate the association between the severity of COVID‐19 and oxidative stress parameters. Clinical data of 77 patients with COVID‐19 admitted to the hospital were analyzed and divided into moderate (n = 44) and severe (n = 33) groups based on their clinical condition. Production of oxidant (hydrogen peroxide) and defense antioxidants (total antioxidant capacity, reduced and oxidized glutathione, glutathione s-transferase), and oxidative damage (malondialdehyde, carbonyl, and sulfhydryl) were assessed using the serum samples. The results revealed that severe patients who presented high serum leukocyte count and CRP level stayed for a longer period in the hospital. However, there was no correlation observed between the oxidative stress parameters and degree of COVID-19 severity in the present study. In conclusion, these results indicate that the disease severity may not be a detrimental factor contributing to the changes in the redox profile of hospitalized patients with COVID-19.
Keywords: COVID-19, Coronavirus, Sars-Cov-2, Disease severity, Oxidative stress
Graphical abstract
1. Introduction
Acute respiratory syndrome (SARS) coronavirus 2 (SARS-CoV-2) is a pathogenic virus that is highly transmissible, and causes an infectious disease known as coronavirus disease 2019 (COVID-19) [1]. COVID-19 was first detected in late December 2019 in Wuhan, China. It rapidly spread across the world as a pandemic, and infected more than 90 million individuals causing 2 million deaths, according to the World Health Organization [2]. Most individuals who acquired the infection exhibited a mild condition and did not require hospitalization. Conversely, a subset of individuals progressed to the moderate or severe conditions [3]. The moderate and severe cases were driven by the host's response to the infection, leading to the activation of multisystemic inflammatory responses and respiratory dysfunction [1].
Elevated blood levels of cytokines and chemokines have been reported in patients with COVID-19 infection [[4], [5], [6], [7]] that trigger a pro-inflammatory environment and severe tissue damage, thereby contributing to the adverse outcomes of this disease [8]. Inflammatory disease progression is usually associated with the production of reactive oxygen species (ROS) followed by oxidative stress [9]. Previous studies have reported a link between the inflammatory response and oxidative stress in infectious diseases [[10], [11], [12], [13], [14]]. Moreover, several reviews have suggested a role of the redox system in the pathophysiology of COVID-19 infection [15,16]. Viruses use different strategies to alter the cell environment, including the modulation of the redox state [17]. An imbalance in the redox state towards antioxidant versus oxidant conditions has been observed in other infectious diseases [10,18,19], which increases the inflammation-dependent oxidative stress, and can be a key event during COVID-19 infection as well. In this scenario, the degree of ROS production and consequent oxidative stress during COVID-19 infection needs to be investigated. A spectrum of COVID-19 disease severity, ranging from asymptomatic or mild to moderate, severe, or critical has been established. Patients with mild disease symptoms exhibit rapid recovery, while those with moderate to severe disease exhibit high mortality and poor prognosis [20]. Previous evidence suggests that the severity and progression in hospitalized patients with COVID-19 may be related to the systemic changes in their redox profile [8,[14], [15], [16],21,22], but it has not been confirmed through experimental or descriptive studies. The information regarding the redox parameters in this disease may help better understand the natural history of COVID-19 and provide support for the clinical decision-making. Therefore, in this study, we aimed to evaluate the oxidative stress levels during the progression of hospitalized patients with COVID-19 and a possible interplay with disease severity.
2. Material and methods
2.1. Study design
This is a prospective cohort of hospitalized patients diagnosed with COVID-19 conducted in Curitiba, Paraná, Brazil, Curitiba, between June and July 2020. Seventy-seven patients of both sexes who were more than 18 years in age with clinical conditions and RT-PCR indicating COVID-19 were included in this study. Patients were admitted only after seeking the approval of the research ethics committee (number 31558020.8.0000.0103) and all patients signed the informed consent form. Blood samples from patients were collected regularly, and the serum was separated to evaluate the oxidative stress parameters. Clinical laboratory radiological data, and outcomes were recorded on a regular basis.
2.2. Inclusion and exclusion criteria
COVID-19 infection was defined using the clinical-radiological presentation along with a nasopharyngeal swab polymerase chain reaction (PCR) positive for SARS-CoV-2. In this study, we included only the hospitalized patients with moderate or severe COVID-19 infection. Patient with moderate disease was defined as an adult with clinical signs of pneumonia (fever, cough, dyspnea, and fast breathing) but no signs of severe pneumonia, such as SpO2 ≥ 90% on room air; and that with severe disease as an adult with clinical signs of pneumonia (fever, cough, dyspnea, fast breathing) along with the presentation of one of the following parameters: respiratory rate > 30 breaths/min; severe respiratory distress; or SpO2 < 90% on room air. Patients diagnosed with other viral infections, such as HIV, HCV, HBV, or other common respiratory viruses, were excluded along with those who underwent solid organ or hematological transplantation in the past. Patients who used tocilizumab were also excluded from this study.
2.3. Data and blood collection
Based on the medical records of the patients, data on sex, age, coexisting diseases, clinical symptoms, peripheral oxygen saturation, medicines for chronic use, and medicines for the treatment of COVID-19, length of stay in the hospital ward and ICU were collected. Using the data from the laboratory tests, we extracted the data regarding the white blood cell count, lymphocyte percentage, and CRP level. Blood samples (10 mL) were collected on the first day of hospitalization. Peripheral blood was collected in vacutainers without additives containing separating gel and kept at room temperature for 30 min to coagulate, then centrifuged at 1500 rpm for 10 min at 4 °C. The tubes then remained at rest for 1 h in a vertical position the serum was aliquoted and stored at −70 °C until biochemical tests were conducted. Haemolysed serum samples were discarded.
2.4. Oxidative stress assays
Serum H2O2 levels were measured using the Amplex® Red Hydrogen Peroxide/Peroxidase Assay Kit (ThermoFisher Scientific). Around 50 μL of serum in a total volume of 100 μL was used for the reaction, and after 30 min of incubation at room temperature, the reading was taken spectrophotometrically at 560 nm upon protecting the samples from light. The results were determined using a standard curve and expressed in μU H2O2/mL. Glutathione-S-Transferase (GST) activity was assessed using the procedure described by Habig et al. [23] and adapted by Habdous et al. [24] using 1-chloro-2,4-dinitrobenzene as a substrate. Enzyme activity was spectrophotometrically determined through continuously monitoring the changes in absorbance at 340 nm for 3 min at 25 °C. The glutathione total (GSHt) levels were assessed based on the reaction of GSH with 5,5-dithio-bis(2-nitrobenzoic acid) (DTNB; Ellman's Reagent, Sigma Aldrich Corporation, St. Louis, MO, USA), which generates an oxidized glutathione-TNB product that is later reduced by glutathione reductase in the presence of NADPH consequently generating GSH. The oxidized GSH (GSSG) was measured using the recycling of GSSG through spectrophotometric monitoring of NADPH in the presence of 2-vinylpyridine. The GSHt and GSSG concentrations were determined through a regression curve plotted using various GSH or GSSG standards [24]. The antioxidant equivalent concentrations were measured at 570 nm as a function of Trolox concentration according to the manufacturer's instructions. Serum total antioxidant capacity (TAC) was measured using the colorimetric assay kit (Sigma Aldrich Corporation, St. Louis, MO, USA). The antioxidant equivalent concentrations were measured at 570 nm as a function of Trolox concentration as described above. Sulfhydryl groups were measured as described previously [25]. Serum samples (50 μL) were diluted at 1:6 ratio in 0.1 M sodium phosphate containing 1 mM EDTA (pH 8.0), and 100 μL of this dilution was used to react with 50 μL (4 mg/mL) of DTNB. After an incubation period of 15 min at room temperature, sample absorbance was measured at 412 nm using a microplate reader (Versamax, Molecular Devices, EUA). Concentrations of sulfhydryl groups were determined through parallel measurements of an l-cysteine standard curve. Lipid peroxidation was determined from malondialdehyde (MDA-TBA) levels measured using high-performance liquid chromatography (Agilent 1220 Infinity LC, EUA) according to Tüközkan et al. [26]. MDA peaks were determined based on their retention time and confirmed through spiking with an exogenous standard. MDA concentrations were calculated using a standard curve prepared from 1,1,3,3-tetraethoxypropane. Protein carbonylation was determined through the reaction of 2,4-dinitrophenylhydrazine (DNPH) with carbonyl, generating an adduct, which is able to absorb light at 366 nm [27].
2.5. Statistical analysis
Categorical variables were expressed as absolute frequencies with proportions and analyzed using the chi-squared or Fisher's exact test. Continuous clinical variables were expressed as median values and interquartile range (IQR), and oxidative stress markers were expressed as mean ± standard deviation. The spearman's correlation between inflammatory and oxidative stress markers was determined. The normality of continuous variables was previously tested, and the Mann-Whitney U test was used to compare the moderate versus severe groups. p-value ≤ 0.05 was considered as significant. SPSS v23.0 (IBM, Chicago, IL) and GraphPad Prism v7 (GraphPad, San Diego, CA) were used for conducting the statistical analyses.
3. Results
As presented in Table 1 , a total of 77 patients with COVID-19 were included in this study, including 53 (69%) males and 24 (31%) females. Thirty-three (43%) patients were admitted to the ICU, and 15 patients (19%) required orotracheal intubation. Seventeen patients (22%) died while hospitalized. Most patients exhibited symptoms, including dyspnea, cough, and fever. Diarrhea, throat pain, and coryza were observed in less than 50% of patients. The most frequent comorbidities were systemic arterial hypertension (57%) and diabetes mellitus (29%). Other comorbidities observed were less than 20%. Forty patients (52%) presented more than one comorbidity. During admission, corticosteroids, antibiotics, and prophylactic heparin were administered for the management of patients.
Table 1.
Frequency of comorbidities and drugs in patients with COVID-19.
| Data | N | Frequency |
|---|---|---|
| General data | ||
| Male | 53 | 69% |
| Female | 24 | 31% |
| Intensive Care Unit admission | 33 | 43% |
| Orotracheal intubation | 15 | 19% |
| Death | 17 | 22% |
| Dyspnea | 43 | 62% |
| Cough | 54 | 78% |
| Fever | 37 | 54% |
| Diarrhea | 13 | 19% |
| Throat pain | 11 | 16% |
| Coryza | 3 | 4% |
| Comorbidity data | ||
| Systemic arterial hypertension | 39 | 57% |
| Diabetes mellitus | 20 | 29% |
| Chronic heart failure | 6 | 9% |
| Chronic coronary syndrome | 4 | 6% |
| Previous myocardial infarct | 3 | 4% |
| Previous stroke | 1 | 1% |
| Peripheral arterial disease | 1 | 1% |
| Arrhythmia | 5 | 7% |
| Asthma | 5 | 7% |
| Chronic obstructive pulmonary disease | 8 | 12% |
| Neoplasm | 2 | 3% |
| Dementia | 1 | 1% |
| Smoking | 4 | 6% |
| Drugs data | ||
| Vasoactive drug | 20 | 26% |
| Previous use of angiotensin-converting enzyme inhibitors | 12 | 17% |
| Previous use of angiotensin receptor blockers | 15 | 22% |
| Use of corticoid | 26 | 38% |
| Hydroxychloroquine | 11 | 16% |
| Low weight heparin | 48 | 71% |
The median age of patients was 60 years. There were 44 patients in the moderate group and 33 patients were diagnosed with severe COVID-19 and required admission in the ICU. Severe patients demonstrated higher leukocyte count (8520 cells/mm3 vs. 5540 cells/mm3; p < 0.001), longer duration of stay (12 d vs. 4 d; p < 0.001), higher CRP levels (20.6 mg/L vs. 6.3 mg/L), and lower O2 saturation (91% vs. 93%) than those in the moderate group. The differences between the other parameters were not statistically significant. The median number of days of hospitalization was 7 (range, 3–16) (Table 2 ).
Table 2.
Baseline data and clinical parameters in moderate and severe groups of COVID-19.
| General data | Moderate cases (n = 44) |
Severe cases (n = 33) |
P value | ||||
|---|---|---|---|---|---|---|---|
| Median | 25% quartile | 75% quartile | Median | 25% quartile | 75% quartile | ||
| Age (years) | 59 | 50 | 69 | 67 | 50 | 75 | 0.268 |
| Symptoms before admission (days) | 9.0 | 6.25 | 14.8 | 10.0 | 6.5 | 17.0 | 0.889 |
| Length of hospital stay | 4 | 1 | 9 | 12 | 6 | 19 | <0.001 |
| Temperature | 36.1 | 36 | 36.4 | 36.0 | 36 | 37 | 0.831 |
| Systolic arterial pressure (mmHg) | 130 | 120 | 140 | 130 | 120 | 146 | 0.293 |
| Diatolic arterial pressure (mmHg) | 80 | 80 | 89 | 80 | 70 | 88 | 0.637 |
| Heart rate (bpm) | 81 | 75 | 90 | 89 | 77 | 102 | 0.072 |
| Respiratory rate (bpm) | 19 | 16 | 21 | 20 | 18 | 22 | 0.361 |
| O2 saturation (%) | 93 | 92 | 96 | 91 | 87 | 96 | 0.027 |
| Hematocrit (%) | 38.6 | 35.5 | 42.6 | 37.9 | 34.0 | 42.1 | 0.675 |
| Leukocytes (/mm3) | 5540 | 4490 | 8060 | 8520 | 6300 | 13380 | <0.001 |
| Platelets (/mm3) | 195000 | 161500 | 240500 | 183000 | 134500 | 243500 | 0.390 |
| Creatinine (mg/dL) | 0.7 | 0.6 | 0.9 | 0.8 | 0.6 | 1.2 | 0.200 |
| C-reactive protein (mg/L) | 6.3 | 2.2 | 16.0 | 20.6 | 4.2 | 28.0 | 0.010 |
Data are expressed as median and interquartiles and analyzed using the student's t-test or Mann-Whitney U test when appropriate. CRP, C‐reactive protein.
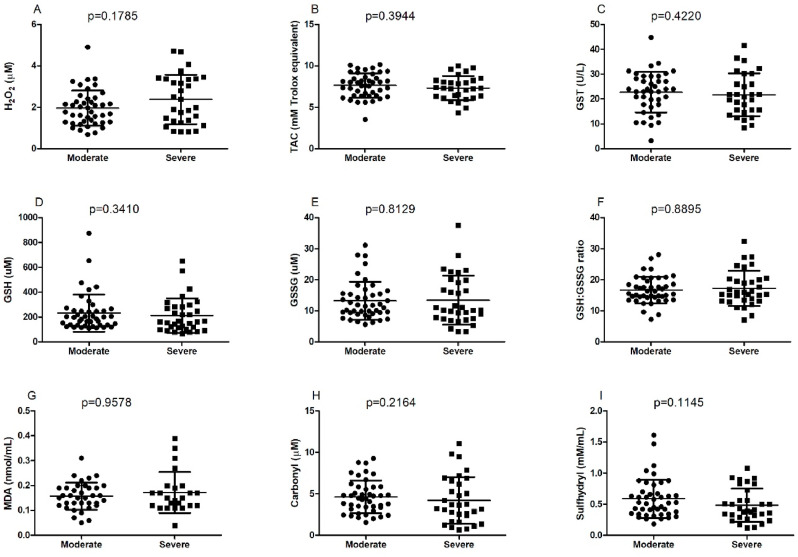
The oxidative stress parameters are shown in Fig. 1 . H2O2 level, TAC, GST activity, GSH content, GSSG content, GSH:GSSG ratio, MDA content, carbonyl content, and sulphydryl levels did not show statistical differences between the moderate and severe groups.
Fig. 1.
Classification of parameters of oxidative stress in patients with COVID-19 based on the severity of the disease (moderate, n = 33; severe, n = 44). (A) H2O2, (B) TAC, (C) GST, (D) GSH, (E) GSSG, (F) GSH:GSSG ratio, (G) MDA, (H) carbonyl, and (I) sulfhydryl. Data are expressed as median (minimum and maximum) ± standard deviation and analyzed using the student's t-test or Mann-Whitney U test when appropriate. The differences were considered to be significant at p < 0.05. SPSS v23.0 (IBM, Chicago, IL) and GraphPad Prism v7 (GraphPad, San Diego, CA) softwares were used for the statistical analyses. H2O2, hydrogen peroxide; TAC, total antioxidant capacity; GST, glutathione S-transferase; GSH, glutathione reduced; GSSG, glutathione dissulfide; MDA, malondealdehyde.
As shown in Table 3 , patients in moderate clinical condition showed a negative correlation between total antioxidant capacity and leukocyte levels (r = −0.360), and GSH:GSSG ration CRP levels (r = −0.360), whereas in severe patients, no correlation was observed.
Table 3.
Correlation between inflammatory and oxidative stress parameters.
| Variables | Moderate |
Severe |
|||
|---|---|---|---|---|---|
| Data | CRP | Leukocytes | CRP | Leukocytes | |
| H202 | Spearman's rho | 0.179 | −0.039 | −0.357 | −0.047 |
| p-value | 0.335 | 0.833 | 0.192 | 0.822 | |
| N | 31 | 32 | 15 | 26 | |
| TAC | Spearman's rho | −0.027 | −0.360 | 0.051 | 0.043 |
| p-value | 0.886 | 0.043* | 0.868 | 0.838 | |
| N | 31 | 32 | 14 | 25 | |
| GST | Spearman's rho | 0.137 | −0.050 | −0.433 | 0.070 |
| p-value | 0.478 | 0.797 | 0.140 | 0.739 | |
| N | 29 | 29 | 13 | 25 | |
| GSH | Spearman's rho | 0.099 | −0.105 | −0.376 | 0.021 |
| p-value | 0.598 | 0.568 | 0.151 | 0.917 | |
| N | 31 | 32 | 16 | 28 | |
| GSSG | Spearman's rho | 0.279 | −0.233 | −0.529 | −0.053 |
| p-value | 0.128 | 0.200 | 0.037 | 0.790 | |
| N | 31 | 32 | 16 | 28 | |
| GSH:GSSG | Spearman's rho | −0.356 | −0.014 | −0.032 | 0.307 |
| p-value | 0.049* | 0.939 | 0.908 | 0.113 | |
| N | 31 | 32 | 16 | 28 | |
| MDA | Spearman's rho | −0.030 | 0.180 | −0.171 | −0.021 |
| p-value | 0.887 | 0.369 | 0.577 | 0.926 | |
| N | 25 | 27 | 13 | 21 | |
| CARBONYL | Spearman's rho | −0.121 | −0.082 | 0.368 | 0.070 |
| p-value | 0.517 | 0.656 | 0.178 | 0.724 | |
| N | 31 | 32 | 15 | 28 | |
| SULFHYDRYL | Spearman's rho | 0.173 | −0.232 | −0.576 | −0.207 |
| p-value | 0.353 | 0.201 | 0.020 | 0.299 | |
| N | 31 | 32 | 16 | 27 | |
| MPO | Spearman's rho | 0.242 | 0.310 | 0.169 | 0.268 |
| p-value | 0.189 | 0.084 | 0.530 | 0.167 | |
| N | 31 | 16 | 28 | ||
Spearman's correlation between inflammatory and oxidative stress parameters in patients with COVID-19 based on the severity of the disease. The association was considered to be significant at p < 0.05. SPSS v23.0 (IBM, Chicago, IL) was used for statistical analyses. CRP, C reactive protein; H2O2, hydrogen peroxide; TAC, total antioxidant capacity; GST, glutathione S-transferase; GSH, glutathione reduced; GSSG, glutathione dissulfide; MDA, malondealdehyde.
4. Discussion
In previous studies, it has been revealed that ROS production and oxidative stress are associated with the replication and subsequent viral infection in this disease [[11], [12], [13], [14],28]. Many evidences also suggest that the overproduction of ROS and a deprived antioxidant system play a major role in the pathogenesis, progression, and severity of SARS-CoV-2 [8,15,16,21]. However, this evidence has mainly been supported by review manuscripts. There are no reports on the identification of oxidative stress markers in COVID-19 infected patients and whether there are differences in the moderate and severe redox profiles. In this prospective cohort, major symptoms presented in patients admitted to hospital facilities with COVID-19 were fever, cough, and dyspnea. Fever has been reported to be more common in moderate/severe patients (89%) than in mild patients (77%) [29]. However, there is considerable heterogeneity in fever symptoms if evaluated in the early phase of COVID-19 (32–95%) [29]. Cough and dyspnea vary according to the disease progression and severity. The prevalence of cough in moderate/severe patients is 63%, and that of dyspnea is 51% in severe patients [29]. Comorbidities are highly prevalent in patients who require intensive care. Several conditions have been demonstrated to be related to disease severity and poor outcomes, such as diabetes, cardiovascular diseases, and obesity [[30], [31], [32]]. Additionally, severity is also related to a high comorbidity index [33]. In our cohort, fever was the third most common symptom (50%), followed by cough (68%) and dyspnea (56%). The most common comorbidities were systemic arterial hypertension (50%) and diabetes mellitus (28%). Moreover, more than half of the patients (52%) presented at least two previous chronic health conditions.
Previous studies have shown that inflammatory parameters, such as CRP, procalcitonin, IL-6, and erythrocyte sedimentation rate, are positively correlated with the severity of COVID-19 [34,35]. Our results also showed elevated inflammatory markers in patients with severe COVID-19. Patients presented higher serum leukocyte cell count (p < 0.001), CRP level (p = 0.01), and length of stay (p < 0.001). Our group also demonstrated previously that these patients exhibit elevated levels of tumor necrosis factor-alpha, interleukin-4, interleukin-6, and interferon-gamma [36]. The inflammatory pattern response is still under investigation; however, it seems that the innate immune response contributes to the disease severity [35].
The inflammatory response has led researchers to believe that there might be possible differences in the redox profile of patients with COVID-19 [8,15,16], and thus, the use of antioxidants as a complementary therapeutic strategy in COVID-19 has been proposed [[37], [38], [39], [40]]. Oxidative stress affects the immune system through altering the immune cell function and inflammatory response [21]. One of the possible mechanisms involved in the production of ROS and oxidative stress in COVID-19 is related to the activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox) [41]. NADPH oxidase complex is activated from the chemotaxis of macrophages and neutrophils, which leads to ROS production, including superoxide radical anion and hydrogen peroxide production [42]. It also plays a potential pathological role during SARS-CoV-2 infection [8]. Therefore, understanding oxidative stress molecular mechanisms in COVID-19 progression to severe phenotypes is currently required clinically to improve the new therapies developed for the treatment of infected patients [21].
Several types of viral infections, such as hepatitis C [43], HIV [44], Zika [45], H1N1 [46], and influenza [14] have been shown to promote an elevated level of oxidative stress, and it has also been linked to the severity of disease [13,47]. In a recent study, Anticoli and colleagues showed that the induction of oxidative stress through auranofin led to a significant increase in the viral RNA titer, suggesting that a pro-oxidant state may favor the replication and/or persistence of hepatitis C virus [48]. Elevated oxidative stress induced by NOX4 activity with a decrease in GSH/GSSG ratio and an increase in oxidative damage has previously been described during the acute phase of SARS-Cov [49].
Evidence suggests that the overproduction of ROS and a reduced antioxidant capacity play a significant role in regulating the severity of SARS-CoV infection [15]. Interestingly, our results indicate that patients with more severe clinical symptoms exhibit similar oxidant production, antioxidant capacity, and oxidative damage compared to those with less severe symptoms. The results may be associated with sample collection time since both the groups were in the critical phase of infection. However, it is noteworthy that both the groups presented a surprisingly stable redox balance. The amount of GSH was higher (approximately seventeen times) than that of GSSG. Tomin and colleagues [50] suggest that serum values can be up to 6 times of those values found in the plasma due to the possible hemolysis that occurs during the coagulation process. In addition, the detection of other thiols in extracellular fluids (e.g., cysteine) may overestimate the concentration of GSH and oxidation artificial of GSH during sample deproteination with acids, which can lead to marked overestimation of GSSG [51].
A decrease in intracellular GSH concentration and/or in the GSH:GSSG ratio is often interpreted as evidence of cellular redox imbalance [[52], [53], [54]] and have been associated with COVID-19 [55]. For example, less availability of GSH will further increase oxidative stress, which favors S protein-ACE2 interaction and enhances the severity of COVID-19 infection [49]. Additionally, the decreased biosynthesis and/or increased depletion of GSH, along with the low GST activity, decreases the detoxification capacity, and could be related to ferroptosis, lipoperoxidation, and cell death [56]. Therefore, maintaining the redox balance is vital for both health and immunity. Unlike our findings, a previous publication showed the results of a case study carried out at Kursk State Medical University. Four patients with moderate and severe COVID-19 symptoms exhibited lower levels of GSH and higher ROS and ROS/GSH ratio in plasma than those with mild disease symptoms. The author suggested that GSH deficiency and oxidative stress in patients with COVID-19 are manifestations of severe infection [55]. However, these differences between studies may be related to the methodology used to measure GSH and GSSG, and the number of patients investigated in the case study.
The possible interplay of the COVID19 severity and oxidative stress parameters was not confirmed in severe patients. However, moderate patients showed a negative correlation between TAC and leukocyte levels and between GSH:GSSG ratio and CRP levels. Although these data are not conclusive, they may indicate that both antioxidant capacity and redox balance in serum of moderate patients with COVID-19 may depend on the inflammatory state. The correlation between inflammatory parameters and oxidative stress should be further investigated in future studies, including interleukin analyzes.
In conclusion, although these preliminary results do not represent all the parameters involved in regulating the redox profile in humans, they indicate that the disease severity may not be the detrimental factor contributing to the changes in the redox profile of the hospitalized patients with COVID-19.
5. Study limitations
In this study, there are some limitations that could compromise comparisons with other studies and also make it difficult to extrapolate results. This limitation is mainly due to the difficulties in collecting data during the pandemic of COVID-19 and also to the methodological conditions to carry out some experiments in our laboratory during the pandemic. 1) At the moment of collection, June and July 2020, we did not have all data from the patient's medical records as the amount of hospitalized patients impaired us with some information that was not included by health professionals, thus the incomplete medical records. 2) Our data collection was limited and dependent on the hospital routine. The samples obtained for this study were the same used by the hospital to monitor their patients, therefore we only had serum samples available to perform the analysis. 3) Some medical records had incomplete clinical information about the patients investigated.4) The MDA and GSH assay were conducted using standardized protocols for plasma and that can make the analysis of results harder. 5) We had difficulties in recruiting a control group. We initially tried patients who were no diagnosed with COVID-19, but we could not collect their blood samples. 6) This study did not verify the correlation of redox parameters according to gender, age, length of hospital stay, disease outcome and comorbidities.
We believe that these limitations are overshadowed by the benefits od the study since our data are very meaningful and contribute to elucidate the role of oxidative stress in the pathophysiology of COVID-19.
Author contributions
All authors contributed equally in writing and preparing the manuscript. All authors have approved the final version of the manuscript.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements and Funding Sources
We thank the National Council of Technological and Scientific Development-CNPq/Brazil, Coordination for the Improvement of Higher Education Personnel-CAPES/Brazil, Pontifícia Universidade Católica do Paraná and Banco Regional de Desenvolvimento do Extremo Sul (BRDE), Brazil for providing the financial support to conduct the research activities in the laboratory.
References
- 1.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin. Immunol. 2020;215:108427. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO, WHO Coronavirus Disease . WHO.Int; 2020. p. 1.https://covid19.who.int/ [Google Scholar]
- 3.Ayres J.S. A metabolic handbook for the COVID-19 pandemic. Nat. Metab. 2020;2:572–585. doi: 10.1038/s42255-020-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi C., Wang C., Wang H., Yang C., Cai F., Zeng F., Cheng F., Liu Y., Zhou T., Deng B., Vlodavsky I., Li J., Zhang Y. The potential of low molecular weight heparin to mitigate cytokine storm in severe COVID‐19 patients: a retrospective cohort study. Clin. Transl. Sci. 2020:12880. doi: 10.1111/cts.12880. cts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxidants Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vlahos R., Selemidis S. NADPH oxidases as novel pharmacologic targets against influenza A virus infection. Mol. Pharmacol. 2014;86:747–759. doi: 10.1124/mol.114.095216. [DOI] [PubMed] [Google Scholar]
- 11.Shin D.-H., Martinez S.S., Parsons M., Jayaweera D.T., Campa A., Baum M.K. Relationship of oxidative stress with HIV disease progression in HIV/HCV Co-infected and HIV mono-infected adults in miami. Int. J. Biosci. Biochem. Bioinforma. 2012;2:217–223. doi: 10.7763/ijbbb.2012.v2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dysangco A., Liu Z., Stein J.H., Dubé M.P., Gupta S.K. HIV infection, antiretroviral therapy, and measures of endothelial function, inflammation, metabolism, and oxidative stress. PloS One. 2017;12 doi: 10.1371/journal.pone.0183511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu M., Chen F., Liu T., Chen F., Liu S., Yang J. The role of oxidative stress in influenza virus infection. Microb. Infect. 2017;19:580–586. doi: 10.1016/j.micinf.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Checconi P., Salzano S., Bowler L., Mullen L., Mengozzi M., Hanschmann E.M., Lillig C.H., Sgarbanti R., Panella S., Nencioni L., Palamara A.T., Ghezzi P. Redox proteomics of the inflammatory secretome identifies a common set of redoxins and other glutathionylated proteins released in inflammation, influenza virus infection and oxidative stress. PloS One. 2015;10 doi: 10.1371/journal.pone.0127086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delgado-Roche L., Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch. Med. Res. 2020;51:384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fakhri S., Nouri Z., Moradi S.Z., Farzaei M.H. Astaxanthin, COVID-19 and immune response: focus on oxidative stress, apoptosis and autophagy. Phyther. Res. 2020;34 doi: 10.1002/ptr.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Checconi P., De Angelis M., Marcocci M.E., Fraternale A., Magnani M., Palamara A.T., Nencioni L. Redox-modulating agents in the treatment of viral infections. Int. J. Mol. Sci. 2020;21:1–21. doi: 10.3390/ijms21114084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amatore D., Sgarbanti R., Aquilano K., Baldelli S., Limongi D., Civitelli L., Nencioni L., Garaci E., Ciriolo M.R., Palamara A.T. Influenza virus replication in lung epithelial cells depends on redox-sensitive pathways activated by NOX4-derived ROS. Cell Microbiol. 2015;17:131–145. doi: 10.1111/cmi.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun K., Metzger D.W. Influenza infection suppresses NADPH oxidase–dependent phagocytic bacterial clearance and enhances susceptibility to secondary methicillin-resistant Staphylococcus aureus infection. J. Immunol. 2014;192:3301–3307. doi: 10.4049/jimmunol.1303049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shang W., Dong J., Ren Y., Tian M., Li W., Hu J., Li Y. The value of clinical parameters in predicting the severity of COVID-19. J. Med. Virol. 2020;92:2188–2192. doi: 10.1002/jmv.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beltrán-García J., Osca-Verdegal R., Pallardó F.V., Ferreres J., Rodríguez M., Mulet S., Sanchis-Gomar F., Carbonell N., García-Giménez J.L. Oxidative stress and inflammation in COVID-19-associated sepsis: the potential role of anti-oxidant therapy in avoiding disease progression. Antioxidants. 2020;9:936. doi: 10.3390/antiox9100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Habig W.H., Jakoby W.B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/S0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- 23.Habdous M., Vincent-Viry M., Visvikis S., Siest G. Rapid spectrophotometric method for serum glutathione S-transferases activity. Clin. Chim. Acta. 2002;326:131–142. doi: 10.1016/S0009-8981(02)00329-7. [DOI] [PubMed] [Google Scholar]
- 24.Rahman I., Kode A., Biswas S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2007;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 25.Aitken A., Learmonth M. Protein Protoc. Handb. Humana Press; 1996. Estimation of disulfide bonds using ellman's reagent; pp. 487–488. [DOI] [Google Scholar]
- 26.Tüközkan N., Erdamar H., Üniversitesi Tıp Fakültesi Biyokimya Anabilim Dalı G. Measurement of Total Malondialdehyde in Plasma and Tissues by High-Performance Liquid Chromatography and Thiobarbituric Acid Assay. www.firattipdergisi.com
- 27.Colombo G., Clerici M., Garavaglia M.E., Giustarini D., Rossi R., Milzani A., Dalle-Donne I. A step-by-step protocol for assaying protein carbonylation in biological samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016;1019:178–190. doi: 10.1016/j.jchromb.2015.11.052. [DOI] [PubMed] [Google Scholar]
- 28.De Flora S., Grassi C., Carati L. Attenuation of influenza-like symptomatology and improvement of cell- mediated immunity with long-term N-acetylcysteine treatment. Eur. Respir. J. 1997;10:1535–1541. doi: 10.1183/09031936.97.10071535. [DOI] [PubMed] [Google Scholar]
- 29.do Nascimento I.J.B., von Groote T.C., O'Mathúna D.P., Abdulazeem H.M., Henderson C., Jayarajah U., Weerasekara I., Pericic T.P., Gerald Klapproth H.E., Puljak L., Cacic N., Zakarija-Grkovic I., Meirelles Guimarães S.M., Atallah A.N., Bragazzi N.L., Marcolino M.S., Marusic A., Jeroncic A. Clinical, laboratory and radiological characteristics and outcomes of novel coronavirus (SARS-CoV-2) infection in humans: a systematic review and series of meta-analyses. PloS One. 2020;15 doi: 10.1371/journal.pone.0239235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie Y., Wang Z., Liao H., Marley G., Wu D., Tang W. Epidemiologic, clinical, and laboratory findings of the COVID-19 in the current pandemic: systematic review and meta-analysis. BMC Infect. Dis. 2020;20 doi: 10.1186/s12879-020-05371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Y., Lu Y., Huang Y.M., Wang M., Ling W., Sui Y., Zhao H.L. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020;113 doi: 10.1016/j.metabol.2020.154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pranata R., Huang I., Lim M.A., Wahjoepramono E.J., July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19–systematic review, meta-analysis, and meta-regression. J. Stroke Cerebrovasc. Dis. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebinge J.E., Achamallah N., Ji H., Clagget B.L., Sun N., Botting P., Nguyen T.T., Luong E., Ki E.H., Park E., Liu Y., Rosenberry R., Matusov Y., Zhao S., Pedraza I., Zaman T., Thompson M., Raedschelders K., Ber A.H., Grei J.D., Nobl P.W., Chug S.S., Merz C.N.B., Marbán E., Van Eyk J.E., Solomo S.D., Alber C.M., Chen P., Cheng S. Pre-existing traits associated with Covid-19 illness severity. PloS One. 2020;15 doi: 10.1371/journal.pone.0236240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu Z., Cai T., Fan L., Lou K., Hua X., Huang Z., Gao G. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int. J. Infect. Dis. 2020;95:332–339. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng F., Huang Y., Guo Y., Yin M., Chen X., Xiao L., Deng G. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int. J. Infect. Dis. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gadotti A.C., de Castro Deus M., Telles J.P., Wind R., Goes M., Garcia Charello Ossoski R., de Padua A.M., de Noronha L., Moreno-Amaral A., Baena C.P., Tuon F.F. IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. 2020;289 doi: 10.1016/j.virusres.2020.198171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang R., Wang X., Ni L., Di X., Ma B., Niu S., Liu C., Reiter R.J. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250 doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi Z., Puyo C.A. p>N-Acetylcysteine to combat COVID-19: an evidence review</p>. Therapeut. Clin. Risk Manag. 2020;16:1047–1055. doi: 10.2147/tcrm.s273700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Babaei F., Nassiri-Asl M., Hosseinzadeh H. Curcumin (a constituent of turmeric): new treatment option against COVID-19. Food Sci. Nutr. 2020;8:5215–5227. doi: 10.1002/fsn3.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carr A.C., Rowe S. The emerging role of vitamin c in the prevention and treatment of covid-19. Nutrients. 2020;12:1–8. doi: 10.3390/nu12113286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Violi F., Oliva A., Cangemi R., Ceccarelli G., Pignatelli P., Carnevale R., Cammisotto V., Lichtner M., Alessandri F., De Angelis M., Miele M.C., D'Ettorre G., Ruberto F., Venditti M., Pugliese F., Mastroianni C.M. Nox2 activation in covid-19. Redox Biol. 2020;36 doi: 10.1016/j.redox.2020.101655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belambri S.A., Rolas L., Raad H., Hurtado-Nedelec M., Dang P.M.C., El-Benna J. NADPH oxidase activation in neutrophils: role of the phosphorylation of its subunits. Eur. J. Clin. Invest. 2018;48 doi: 10.1111/eci.12951. [DOI] [PubMed] [Google Scholar]
- 43.Medvedev R., Ploen D., Hildt E. HCV and oxidative stress: implications for HCV life cycle and HCV-associated pathogenesis. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/9012580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ivanov A.V., Valuev-Elliston V.T., Ivanova O.N., Kochetkov S.N., Starodubova E.S., Bartosch B., Isaguliants M.G. Oxidative stress during HIV infection: mechanisms and consequences. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/8910396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ledur P.F., Karmirian K., da C., Pedrosa S.G., Souza L.R.Q., Assis-de-Lemos G., Martins T.M., de J., Ferreira C.C.G., de Azevedo Reis G.F., Silva E.S., Silva D., Salerno J.A., Ornelas I.M., Devalle S., Madeiro da Costa R.F., Goto-Silva L., Higa L.M., Melo A., Tanuri A., Chimelli L., Murata M.M., Garcez P.P., Filippi-Chiela E.C., Galina A., Borges H.L., Rehen S.K. Zika virus infection leads to mitochondrial failure, oxidative stress and DNA damage in human iPSC-derived astrocytes. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-57914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim J.Y., Oh E., Kim Y., Jung W.W., Kim H.S., Lee J., Sul D. Enhanced oxidative damage to DNA, lipids, and proteins and levels of some antioxidant enzymes, cytokines, and heat shock proteins in patients infected with influenza H1N1 virus. Acta Virol. 2014;58:253–260. doi: 10.4149/av_2014_03_253. [DOI] [PubMed] [Google Scholar]
- 47.Bolukbas C., Bolukbas F.F., Horoz M., Aslan M., Celik H., Erel O. Increased oxidative stress associated with the severity of the liver disease in various forms of hepatitis B virus infection_aptad 20050831. BMC Infect. Dis. 2005;5 doi: 10.1186/1471-2334-5-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anticoli S., Amatore D., Matarrese P., De Angelis M., Palamara A.T., Nencioni L., Ruggieri A. Counteraction of HCV-induced oxidative stress concurs to establish chronic infection in liver cell cultures. Oxid. Med. Cell. Longev. 2019 doi: 10.1155/2019/6452390. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suhail S., Zajac J., Fossum C., Lowater H., McCracken C., Severson N., Laatsch B., Narkiewicz-Jodko A., Johnson B., Liebau J., Bhattacharyya S., Hati S. Role of oxidative stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) infection: a review. Protein J. 2020;1:1. doi: 10.1007/s10930-020-09935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomin T., Schittmayer M., Birner-Gruenberger R. Addressing glutathione redox status in clinical samples by two-step alkylation with N-ethylmaleimide isotopologues. Metabolites. 2020;10:71. doi: 10.3390/metabo10020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giustarini D., Dalle-Donne I., Milzani A., Fanti P., Rossi R. Analysis of GSH and GSSG after derivatization with N-ethylmaleimide. Nat. Protoc. 2013;8:1660–1669. doi: 10.1038/nprot.2013.095. [DOI] [PubMed] [Google Scholar]
- 52.Jones D.P., Go Y.-M., Anderson C.L., Ziegler T.R., Kinkade J.M., Kirlin W.G. Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control. Faseb. J. 2004;18:1246–1248. doi: 10.1096/fj.03-0971fje. [DOI] [PubMed] [Google Scholar]
- 53.Giustarini D., Colombo G., Garavaglia M.L., Astori E., Portinaro N.M., Reggiani F., Badalamenti S., Aloisi A.M., Santucci A., Rossi R., Milzani A., Dalle-Donne I. Assessment of glutathione/glutathione disulphide ratio and S-glutathionylated proteins in human blood, solid tissues, and cultured cells. Free Radic. Biol. Med. 2017;112:360–375. doi: 10.1016/j.freeradbiomed.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 54.Meister A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988;263:17205–17208. https://europepmc.org/article/med/3053703 [PubMed] [Google Scholar]
- 55.Polonikov A. Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients. ACS Infect. Dis. 2020;6:1558–1562. doi: 10.1021/acsinfecdis.0c00288. [DOI] [PubMed] [Google Scholar]
- 56.Bjørklund G., Tinkov A.A., Hosnedlová B., Kizek R., Ajsuvakova O.P., Chirumbolo S., Skalnaya M.G., Peana M., Dadar M., El-Ansary A., Qasem H., Adams J.B., Aaseth J., Skalny A.V. The role of glutathione redox imbalance in autism spectrum disorder: a review. Free Radic. Biol. Med. 2020;160:149–162. doi: 10.1016/j.freeradbiomed.2020.07.017. [DOI] [PubMed] [Google Scholar]