To the Editor:
We read the recent publication in Pharmacological Research on the impact of pre-admission antithrombotic therapy in coronavirus disease 2019 (COVID-19) patients with great interest [1]. A recent retrospective cohort study demonstrated that the use of heparin improved the 28-day mortality of severe COVID-19 patients at risk of sepsis-induced coagulopathy. [2] Another study found that patients who received treatment-dose anticoagulants were more likely to require invasive mechanical ventilation [3]. However, few population-based studies have examined the effects of prophylactic antithrombotic therapy on the risk of severe COVID-19. Thus, the present study aims to evaluate whether anticoagulant and anti-platelet use is associated with a lower risk of severe COVID-19 infection through a territory-wide, propensity score-matched cohort study.
Ethics approval for this study was obtained from the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster. The inclusion criteria were patients who tested positive for the severe acute respiratory syndrome coronavirus-2 under reverse-transcriptase polymerase chain reaction (RT-PCR) between January 1st to August 22nd, 2020 in Hong Kong. The patient data were extracted from the Clinical Data Analysis and Reporting System (CDARS), a Hong Kong-wide electronic health record database that compiles data from all public hospitals to establish a comprehensive and accessible medical record for each patient, and has been used by our team and other groups to conduct population-based studies in the past [4]. The following information was extracted from CDARS: (1) demographics; (2) prior comorbidities; (3) hospitalization characteristics before the COVID-19 related admission; (4) medications prescribed; (5) laboratory tests (complete blood count, biochemical tests, cardiac function tests, C-reactive protein, and arterial blood gas); (6) the need for intensive care unit (ICU) admission and intubation. The codes from the International Classification of Disease-Ninth Edition (ICD-9) documenting the comorbidities and intubation procedure are shown in Supplementary Tables 1 and 2, respectively. Patient mortality was extracted from the Hong Kong Death Registry, a governmental registry compiling the registered death records of Hong Kong citizens. The primary outcome is the need for admission to the intensive care unit (ICU), intubation, or death followed-up until September 8th, 2020. Detailed information on the statistical analyses is shown in the Supplementary Appendix.
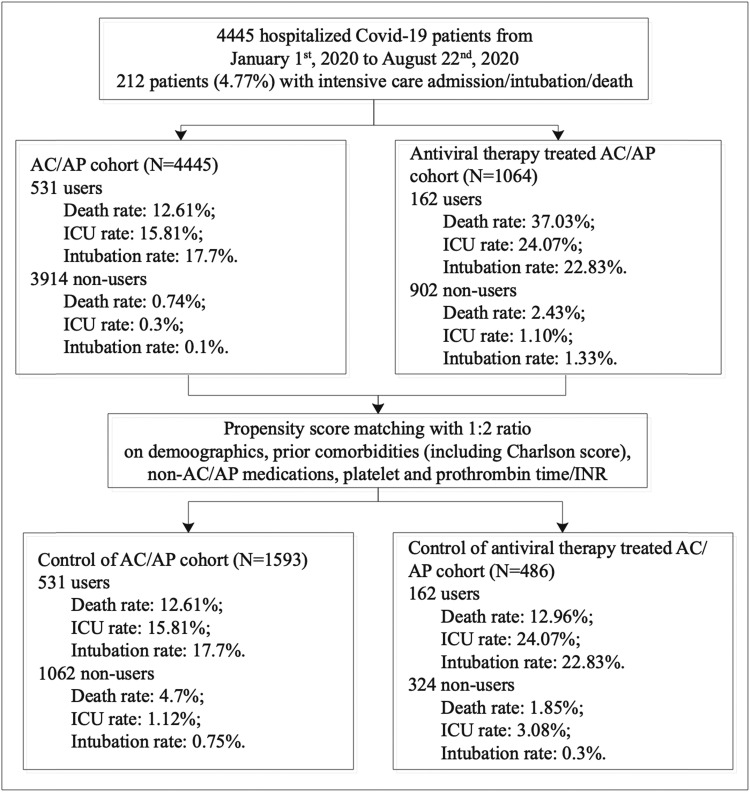
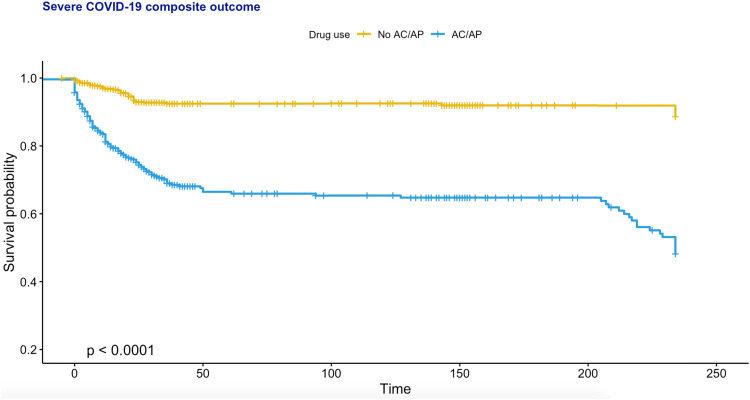
The procedures of data processing are detailed in Fig. 1. The present study consists of 4445 consecutive patients who have been tested positive for the severe acute respiratory syndrome coronavirus-2 (median age 44.8 years old, 95% CI: [28.9, 60.8]; 50% male). Of these, 212 met the primary outcome (Supplementary Table 3). The baseline clinical characteristics of the cohort stratified by anticoagulant or antiplatelet use before and after propensity score matching for baseline demographics, past medical comorbidities (including the Charlson Comorbidity Index score), medication history, platelet and prothrombin time/international normalized ratio (INR) are shown in Supplementary Table 4. The percentage of COVID-19 patients meeting the primary outcome was significantly higher in anticoagulants users compared to non-users, both before (n = 145/292, 41.7% vs. n = 67/4097, 1.6%; P < 0.0001) and after propensity score matching (n = 145/292, 41.7% vs. n = 46/696, 6.6%; P < 0.0001). Similarly, antiplatelet users also showed a higher percentage compared to non-users before (n = 70/292, 24.0% vs. n = 142/4153, 3.4%; P < 0.0001) and after matching (n = 70/292, 24.0% vs. n = 91/584, 15.6%; P < 0.0001). Kaplan-Meier curves stratified by anticoagulants or antiplatelets use for the unmatched and matched cohorts are shown in Fig. 2 and Fig. 3, respectively. Based on the propensity score-matched cohort, univariable Cox regression showed that the use of anticoagulants (hazard ratio [HR]: 4.65, 95% confidence interval [CI]: [3.36, 6.43], P < 0.0001) or antiplatelets (HR: 1.54, 95% CI: [1.13, 2.10], P = 0.0061) was associated with a higher risk of the primary outcome (Supplementary Table 5). For anticoagulant/antiplatelet users vs. non-users, the HR was 5.19 (95% CI: [3.89–6.90] P < 0.0001). Regarding individual drug classes, the use of dabigatran, enoxaparin, clopidogrel or aspirin was a significant predictor. Multivariate Cox analyses showed that anticoagulant/antiplatelet use remained a significant predictor after adjusting for age, past comorbidities and/or medication classes (Supplementary Table 6).
Fig. 1.
Procedures of data processing.
Fig. 2.
Kaplan-Meier curve for the severe disease outcome in PCR-positive COVID-19 patients stratified by anticoagulant (AC)/ antiplatelet (AP) use after 1:2 propensity score matching.
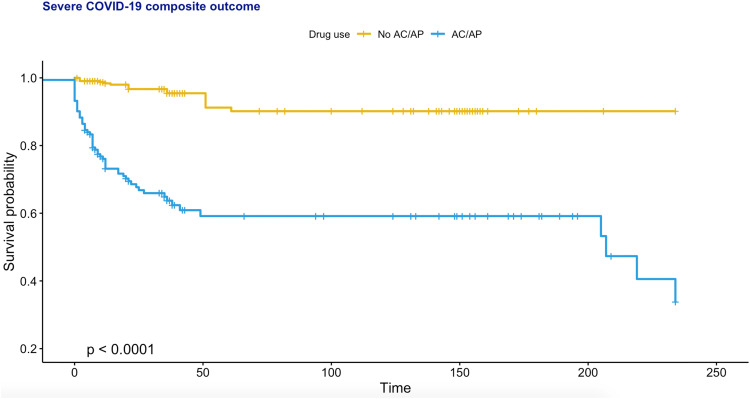
Fig. 3.
Kaplan-Meier curve for the severe disease outcome in PCR-positive COVID-19 patients receiving antiviral or steroid therapy, stratified by anticoagulant (AC)/ antiplatelet (AP) use after 1:2 propensity score matching.
As the inclusion of PCR-positive asymptomatic cases may introduce bias. Subgroup analysis including only COVID-19 patients receiving anti-viral or steroid therapy (lopinavir/ritonavir, ribavirin, interferon beta-1b, hydroxychloroquine, steroids) was performed. This subgroup cohort consists of 1064 patients, in whom 82 (7.7%) met the primary outcome (Supplementary Table 7). Their unmatched and matched baseline characteristics are shown in Supplementary Table 8. After propensity score matching, univariate Cox regression showed that the use of antiplatelets/anticoagulants was a significant predictor of severe disease (HR: 8.88, 95% CI: [5.11, 15.43]; P < 0.0001; Supplementary Table 9). Regarding individual drug classes, only the use of edoxaban or enoxaparin was a significant predictor. The relationship between anticoagulant/antiplatelet use remained significant in multivariate Cox analyses adjusting for age, past comorbidities and/or medication classes (Supplementary Table 10).
Finally, 1:1 propensity score matching was performed between anticoagulant and antiplatelet users receiving antiviral/steroid therapy (n = 63 for each group, 28 patients prescribing both anticoagulants and antiplatelets were excluded; Supplementary Table 11). Univariate Cox regression showed that the use of antiplatelets was associated with a lower risk of severe disease compared to anticoagulant users (HR: 0.17, 95% CI: [0.07, 0.44]; P < 0.0001; Supplementary Table 12). The lower risk observed for antiplatelet users remained significant in multivariate Cox analyses adjusting for age, past comorbidities and/or medication classes (Supplementary Table 13).
COVID-19 has placed a significant burden on healthcare systems worldwide. It particularly affects patients with existing comorbidities more severely [5], [6], [7], complicated by important effects of individual drug classes [8], [9], [10] or drug-drug interactions [11]. Our data indicate that the use of anticoagulants or antiplatelets is associated with a higher risk of severe COVID-19 disease after propensity score matching in a Chinese cohort. However, antiplatelet use was associated with lower risk of severe disease compared to anticoagulant use. Our findings should be validated in future studies.
Funding
This study was supported by the National Natural Science Foundation of China (NSFC) Grant Nos. 72042018, 71972164 and 71672163, in part by the Health and Medical Research Fund Grant (HMRF), the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region No. 16171991, and in part by The Theme‐Based Research Scheme of the Research Grants Council of Hong Kong Grant No. T32‐102/14N.
Conflicts of Interest
The authors declare no potential or actual conflicts of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.phrs.2021.105473.
Appendix A. Supplementary material
Supplementary material.
.
References
- 1.Russo V., Di Maio M., Attena E., Silverio A., Scudiero F., Celentani D., Lodigiani C., Di Micco P. Clinical impact of pre-admission antithrombotic therapy in hospitalized patients with COVID-19: a multicenter observational study. Pharmacol. Res. 2020;159 doi: 10.1016/j.phrs.2020.104965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paranjpe I., Fuster V., Lala A., Russak A.J., Glicksberg B.S., Levin M.A., Charney A.W., Narula J., Fayad Z.A., Bagiella E., Zhao S., Nadkarni G.N. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients With COVID-19. J. Am. Coll. Cardiol. 2020;76(1):122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou J., Wang X., Lee S., Wu W.K.K., Cheung B.M.Y., Zhang Q., Tse G. Proton pump inhibitor or famotidine use and severe COVID-19 disease: a propensity score matched territory-wide study. Gut. 2020 doi: 10.1136/gutjnl-2020-323668. [DOI] [PubMed] [Google Scholar]
- 5.Li X., Guan B., Su T., Liu W., Chen M., Bin Waleed K., Guan X., Gary T., Zhu Z. Impact of cardiovascular disease and cardiac injury on in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis. Heart. 2020;106(15):1142–1147. doi: 10.1136/heartjnl-2020-317062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y., Wang Z., Tse G., Zhang L., Wan E.Y., Guo Y., Lip G.Y.H., Li G., Lu Z., Liu T. Cardiac arrhythmias in patients with COVID-19. J. Arrhythmia. 2020;36(5):827–836. doi: 10.1002/joa3.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shao M., Li X., Liu F., Tian T., Luo J., Yang Y. Acute kidney injury is associated with severe infection and fatality in patients with COVID-19: a systematic review and meta-analysis of 40 studies and 24,527 patients. Pharmacol. Res. 2020;161 doi: 10.1016/j.phrs.2020.105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y., Tse G., Li G., Lip G.Y.H., Liu T. ACE inhibitors and angiotensin II receptor blockers may have different impact on prognosis of COVID-19. J. Am. Coll. Cardiol. 2020;76(17):2041. doi: 10.1016/j.jacc.2020.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alamer A., Abraham I. Mortality in COVID-19 patients treated with ACEIs/ARBs: re-estimated meta-analysis results following the Mehra et al. retraction. Pharmacol. Res. 2020;160 doi: 10.1016/j.phrs.2020.105053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y., Xie C., Chen X., Hong Q., Huang H. Would ACEIs/ARBs be beneficial for COVID-19 patients without hypertension? Pharmacol. Res. 2020;159 doi: 10.1016/j.phrs.2020.104959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awortwe C., Cascorbi I. Meta-analysis on outcome-worsening comorbidities of COVID-19 and related potential drug-drug interactions. Pharmacol. Res. 2020;161 doi: 10.1016/j.phrs.2020.105250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.