Abstract
The high incidence of error reports received by the US Food and Drug Administration (FDA) involving 2-component vaccines led to collaboration between the United States Pharmacopeia (USP) and the Institute for Safe Medication Practices (ISMP). This collaborating group sought to further understand errors associated with all 2-component vaccines (i.e. vaccine components provided by the manufacturer in physically separate containers) and to provide safe practice strategies for storing, preparing, dispensing, and administering these vaccines as intended. Fourteen available 2-component vaccines were identified. The ISMP National Vaccine Errors Reporting Program (VERP) and the FDA Vaccine Adverse Event Reporting System (VAERS) were searched from the initiation of each respective reporting system through December 31, 2019. The three vaccines with the most reported reconstitution errors in the VERP and VAERS are Menveo® (meningococcal), Pentacel® (DTaP, Polio, Haemophilus influenzae type b), and ActHIB® [H. influenzae type b (Hib)]. Manufacturers should design labeling and packaging of vaccines to provide ease of storage and fail-safe preparation to prevent 2-component vaccine errors. Implementing risk reduction strategies, such as training healthcare professionals and affixing storage bin labels, remind healthcare professionals to mix the 2-components and facilitate appropriate administration.
Key Points
| A 2-component vaccine is any vaccine with two components (i.e. vaccine and specific diluent; vaccine liquid component and vaccine powder component) provided by the manufacturer in physically separate containers that require mixing prior to administration. |
| When 2-component vaccine errors occur, it results in only one of the two components being administered. This leads to lack of patient protection from preventable disease, need to revaccinate, and an increase in associated healthcare costs. |
| The three 2-component vaccines with the most reported reconstitution errors in the Institute for Safe Medication Practices (ISMP) National Vaccine Errors Reporting Program (VERP) and US Food and Drug Administration (FDA) Vaccine Adverse Event Reporting System (VAERS) are Pentacel® (DTaP, Polio, Haemophilus influenzae type b), Menveo® (meningococcal), and ActHIB® [H. influenzae type b (Hib)]. |
| Manufacturers should design labeling and packaging of vaccines to provide ease of storage and fail-safe preparation of vaccines to prevent 2-component vaccine errors. |
| Implementing risk-reduction strategies, such as training healthcare professionals and affixing storage bin labels, remind healthcare professionals to mix the 2-components and facilitate appropriate administration. |
Introduction
Vaccination, responsible for the prevention of serious diseases that are otherwise debilitating or deadly, is one of the most remarkable advancements in public health [1–3]. To sustain disease prevention, vaccine administration needs to be both widespread and performed correctly [2]. However, errors related to storage and use of vaccines continue to occur, including omission of a vaccine or vaccine component when administering 2-component vaccines [2, 4]. For the purpose of this article, a 2-component vaccine is defined as any vaccine with two components (i.e. vaccine and specific diluent; vaccine liquid component and vaccine powder component) provided by the manufacturer in physically separate containers.
The individual components of 2-component vaccines must be mixed together prior to administration, a step that introduces opportunity for errors [2]. For 2-component vaccines that include a vaccine and specific diluent, meaning a diluent supplied by the manufacturer specifically for reconstitution of that vaccine (Table 1), errors have occurred in which only the diluent was administered to the patient [2, 5, 6]. For these vaccines, the specific diluent must be used for reconstitution to achieve the intended effect for which the product was designed, studied, and approved [7]. For 2-component products that include two active vaccine components, one supplied as a powder and the other supplied as a liquid (Table 1), errors have occurred in which only a single liquid component has been administered. The two active vaccine components are either an antigen paired with an adjuvant or a polysaccharide paired with a protein carrier (i.e., conjugate vaccine) [13, 14]. Adjuvants and protein carriers enhance the immune response; therefore, administration of a sole component may render the vaccine less effective. Lack of familiarity with 2-component vaccines and container labeling and/or packaging factors contribute to these errors [2, 5].
Table 1.
| Brand name (manufacturer) | Component 1 (powder) | Component 1 container type | Component 1 storage requirements | Component 2 (liquid) [*contains active vaccine component] | Component 2 container type | Component 2 storage requirements |
|---|---|---|---|---|---|---|
| Vaccine components can be stored together | ||||||
|
ActHIB® (Sanofi Pasteur) |
Haemophilus influenzae type b, tetanus toxoid | Vial | 2° to 8 °C (35° to 46 °F) | 0.4% Sodium chloride | Vial | 2° to 8 °C (35° to 46 °F) |
|
Hiberix® (GlaxoSmithKline) |
H. influenzae type b, tetanus toxoid | Vial | 2° to 8 °C (36° to 46 °F) | 0.9% Sodium chloride | Vial | 2° to 25 °C (36° to 77 °F) |
|
Imovax® Rabies (Sanofi Pasteur) |
Rabies virus | Vial | 2° to 8 °C (35° to 46 °F) | Sterile water | Prefilled syringe | 2° to 8 °C (35° to 46 °F) |
|
M–M-R® II (Merck) |
Measles, mumps, rubella viruses | Vial | − 50 °C to + 8 °C (− 58 °F and + 46 °F) | Sterile water | Vial | − 49° to + 8 °C (− 56° to + 46 °F) |
|
Menveo® (GlaxoSmithKline) |
Meningococcal group A (MenA) | Vial | 2° to 8 °C (36° to 46 °F) | Meningococcal groups c, y, w-135 (MenCWY-135)* | Vial | 2° to 8 °C (36° to 46 °F) |
|
Pentacel® (Sanofi Pasteur) |
H. type b, tetanus toxoid (ActHIB) | Vial | 2° to 8 °C (35° to 46 °F) | Diphtheria, tetanus toxoids, acellular pertussis (DTaP-IPV)* | Vial | 2° to 8 °C (35° to 46 °F) |
|
RabAvert® (GlaxoSmithKline) |
Rabies virus | Vial | 2° to 8 °C (36° to 46 °F) | Sterile water | Prefilled syringe | 2° to 8 °C (36° to 46 °F) |
|
Rotarix® (GlaxoSmithKline) [ORAL VACCINE] |
Rotavirus | Vial | 2° to 8 °C (36° to 46 °F) | Calcium carbonate, sterile water, and xanthan | Prefilled oral applicator | 2° to 25 °C (36° to 77 °F) |
|
Shingrix® (GlaxoSmithKline) |
Recombinant varicella zoster virus surface glycoprotein E (gE) antigen | Vial | 2° to 8 °C (36° to 46 °F) | AS01B adjuvant* | Vial | 2° to 8 °C (36° to 46 °F) |
|
Vaxchora® (Paxvax) [ORAL VACCINE] |
Vibrio cholerae | Packet | − 25 °C to − 15 °C (− 13 °F to + 5 °F) | Buffer (sodium bicarbonate, sodium carbonate, ascorbic acid, and dried lactose) | Packet | − 25 °C to − 15 °C (− 13 °F to + 5 °F) |
|
YF-VAX® (Sanofi Pasteur) |
Yellow fever virus | Vial | 2° to 8 °C (35° to 46 °F) | 0.9% Sodium chloride | Vial | 2° to 8 °C (35° to 46 °F) |
| Vaccine components cannot be stored together | ||||||
|
ProQuad® (Merck) |
Measles, mumps, rubella, varicella viruses | Vial | − 50 °C to − 15 °C (− 58 °F and + 5 °F) | Sterile water | Vial | 2° to 25 °C (36° to 77 °F) |
|
Varivax® (Merck) |
Varicella virus | Vial | − 50° to − 15 °C (− 58° to + 5 °F) | Sterile water | Vial | 2° to 25 °C (36° to 77 °F) |
|
Zostavax® (Merck) |
Varicella-zoster virus | Vial | − 50 °C and −15 °C (− 58 °F and + 5 °F) | Sterile water | Vial | 2° to 25 °C (36° to 77 °F) |
In addition to omission errors, the risk of selecting and administering an incorrect diluent is also present and can prove fatal [6, 11, 15]. In 2014, fifteen children in Syria died after a neuromuscular blocking agent was used to reconstitute a measles vaccine instead of the appropriate diluent, the sterile water provided by the manufacturer [10]. The result of these errors is lack of patient protection from preventable disease, need to revaccinate, increased associated healthcare costs, and potential for adverse drug events (in some cases death) [5, 9, 11, 15]. Undetected errors result in failure to revaccinate, potentially propagating the spread of disease [5, 15]. Lack of protection from and spread of otherwise preventable disease due to incorrect preparation and administration of 2-component vaccines is a public health concern, which may be prevented with risk-reducing strategies [3, 5, 15, 16].
Reports of 2-component vaccine errors triggered an investigation of all 2-component vaccines marketed in the USA to identify improvement opportunities for storage, packaging, and labeling to facilitate appropriate preparation, dispensing, and administration of vaccines.
Identification of 2-Component Vaccines
First, a comprehensive list of all 2-component vaccines was compiled by reviewing package inserts of all vaccines marketed in the USA. Table 1 lists the 14 identified 2-component vaccines marketed in the USA and individual component storage requirements. ACAM2000® [vaccinia (smallpox)], was excluded because it is stockpiled by the US government for emergency use only and therefore not readily available for institutions to develop the safe practices outlined in this discussion [17]. The individual components of each vaccine were characterized by storage requirements and packaging and labeling to identify complexities that could potentially increase the risk for errors.
US Error Reporting for 2-Component Vaccines
The Institute for Safe Medication Practices (ISMP) Vaccine Error Reporting Program (VERP) and the US Food and Drug Administration (FDA) Vaccine Adverse Event Reporting System (VAERS) were used to identify error reports for 2-component vaccines. Underreporting is estimated to be 50–60% annually making the true extent of the number of errors impossible to determine [18]. VERP and VAERS are passive surveillance systems and do not calculate the number of times safety events are actually occurring but serve as a valuable signal to identify areas where errors occur and then implement strategies to prevent recurrence.
VERP and VAERS were searched from the initiation of each respective reporting system through December 31, 2019. The VERP database was searched on January 14, 2020 for the number of reconstitution error reports involving 2-component vaccines submitted from January 1, 2013 through December 31, 2019. Each error report was reviewed for omission of one component or reconstitution using an unintended diluent (e.g. sterile water, diluent from a different vaccine product) instead of the specific diluent provided. Table 2 represents the number of reconstitution error reports submitted to VERP. The most frequently reported 2-component vaccines were Pentacel® (n = 68), Menveo® (n = 44), and ActHIB® (n = 23). Pentacel® (DTaP, Polio, Haemophilus influenzae type b) is among the top 10 vaccines reported to VERP [2]. A 2017 analysis by ISMP provides a glimpse of why these errors occur. The top contributing factors for Pentacel® errors were unfamiliarity with process of mixing or preparing the product (18 reports), unfamiliarity with patient age for product (11 reports), and similar brand names (4 reports) [2].
Table 2.
Number of reconstitution error reports submitted to VERP for 2-component vaccines
| Vaccine | Year vaccinated | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | ||
| ACTHIB® | 1 | 6 | 5 | 3 | 0 | 4 | 4 | 23 |
| HIBERIX® | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 |
| IMOVAX® RABIES | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MMR® II | 0 | 1 | 1 | 0 | 0 | 1 | 2 | 5 |
| MENVEO® | 1 | 7 | 6 | 5 | 5 | 9 | 11 | 44 |
| PENTACEL® | 6 | 13 | 6 | 13 | 13 | 9 | 8 | 68 |
| PROQUAD® | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| ROTARIX® | 1 | 2 | 0 | 2 | 5 | 1 | 7 | 18 |
| RABAVERT® | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| SHINGRIX® | n/a | n/a | n/a | n/a | n/a | 6 | 9 | 15 |
| VARIVAX® | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 4 |
| YF-VAX® | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| ZOSTAVAX® | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| VAXCHORA® | n/a | n/a | n/a | 0 | 0 | 0 | 0 | 0 |
n/a vaccine not available on US market
The VAERS database was searched on February 25, 2020 for the number of reconstitution error reports involving 2-component vaccines submitted from January 1, 2007 through December 31, 2019. Search terms included: product preparation error; product preparation issue; product reconstitution issue; product reconstitution quality issue; wrong technique in product usage process; wrong technique in drug usage process. The number of reported reconstitution errors in VAERS for the 14 two-component vaccines is represented in Table 3. Similar to VERP, reconstitution errors were most frequently reported with the same 3 vaccines: Menveo® (n = 477), Pentacel® (n = 374), and ActHIB® (n = 198). Menveo® (meningococcal) reconstitution error reports have been published since 2014 by ISMP [19]. At the time, ISMP raised awareness of errors involving Menveo® by urging manufacturer label changes and educating healthcare professionals and the public. The original label for each Menveo® component bore identical barcodes and were difficult to differentiate due to similarity in label style, same red text, and small font size (Fig. 1a). After being acquired by another manufacturing company, labels of each component displayed a unique barcode, and color contrast of texts were enhanced by making the red warning “NOT TO BE USED ALONE” more visible (Fig. 1b). The consistent decline in error reports involving Menveo® (Table 3) suggests the positive impact of label improvements, although further research on variables are needed to confirm this relationship.
Table 3.
Number of reconstitution error reports submitted to VAERS for 2-component vaccines
| Vaccine | Year vaccinated | Unknown date | Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | |||
| ACTHIB® | 0 | 4 | 4 | 1 | 0 | 3 | 5 | 58 | 9 | 8 | 4 | 40 | 64 | 0 | 198 |
| HIBERIX® | n/a | n/a | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| IMOVAX® RABIES | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 |
| MMR® II | 0 | 4 | 5 | 1 | 0 | 0 | 1 | 6 | 7 | 13 | 15 | 8 | 17 | 0 | 77 |
| MENVEO® | n/a | n/a | n/a | 5 | 3 | 66 | 16 | 148 | 157 | 20 | 25 | 15 | 20 | 2 | 477 |
| PENTACEL® | n/a | 0 | 1 | 0 | 0 | 0 | 0 | 10 | 6 | 11 | 23 | 155 | 168 | 0 | 374 |
| PROQUAD® | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 2 | 5 | 4 | 7 | 0 | 24 |
| ROTARIX® | n/a | 0 | 0 | 3 | 0 | 1 | 0 | 2 | 12 | 2 | 3 | 10 | 2 | 0 | 35 |
| RABAVERT® | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 1 | 0 | 5 |
| SHINGRIX® | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 0 | 56 | 51 | 0 | 108 |
| VARIVAX® | 1 | 1 | 4 | 1 | 0 | 3 | 3 | 6 | 6 | 18 | 10 | 6 | 10 | 0 | 69 |
| YF-VAX® | 0 | 3 | 0 | 1 | 0 | 0 | 1 | 11 | 1 | 1 | 2 | 2 | 6 | 0 | 26 |
| ZOSTAVAX® | 1 | 2 | 3 | 1 | 0 | 8 | 9 | 16 | 13 | 7 | 10 | 4 | 0 | 0 | 74 |
| VAXCHORA® | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 0 | 0 | 1 | 2 | 0 | 3 |
n/a vaccine not available on US market
Fig. 1.
Menveo® Labels: (a) Previous Menveo® labels with identical barcodes and all red text [44] and (b) Modified Menveo® labels with only critical information in high contrast red text [23]
A search limitation is that multiple vaccine recipients may be represented in one report and duplicate reports may have been counted.
Packaging and Labeling Complexities
Not all 2-component vaccines are packaged with component 1 and 2 in the same carton. Nine of the 14 vaccines are supplied in the same carton, 4 of which are supplied as a unit-dose carton (Imovax® Rabies, RabAvert®, Shingrix®, Vaxchora®) [20–27, 29]. The national drug code (NDC) of the separately packaged sterile water diluents for M–M–R® II, ProQuad®, Varivax®, and Zostavax® is displayed on the carton but not clearly displayed in the prescribing information [30–33]. The specifically manufactured diluents for these vaccines are labeled “Sterile Diluent for Merck & Co. Inc Live Virus Vaccines (Sterile Water)” and do not reference the specific name(s) of the vaccine(s) they are used to reconstitute [30–33]. Look-alike labeling of both components can introduce the risk of misrepresenting the 2-component vaccine as a 1-component vaccine [19].
Storage Requirement Complexities
Unique and complex storage requirements exist for some of the vaccines, including multiple storage temperature ranges for one or more of the components [21, 25, 30–33]. Manufacturers provide the necessary storage requirements and time allowed between reconstitution and use to maintain drug stability. Eleven vaccines were found to have components that can be stored together. Three vaccines (ProQuad®, Varivax®, and Zostavax®) have components that cannot be stored together based on different required storage temperature ranges on the package insert [20–33]. Three vaccines with a lyophilized powder component (ProQuad®, Varivax®, and Zostavax®), specify that they can be moved from the freezer to the refrigerator for up to 72 h prior to reconstitution [31–33].
Labeling Strategies for Manufacturers
Safe labeling and packaging of vaccines mitigates harm by providing ease of storage and fail-safe preparation and administration of vaccines. These include designing products with larger labels to accommodate information without overcrowding to ensure the readability of important information. Consider assigning a unique NDC and barcode to each individual component of the vaccine. Barcodes are often scanned during drug administration to verify the correct product. If only one of the items is scanned, the electronic barcode administration system would alert the nurse that there is a second component missing for administration. Labeling each component with specific instructions for dispensing, reconstituting, and administering may also prevent errors. For example, when two vials are required, label one as “vial 1 of 2” and the other as “vial 2 of 2” utilizing different text colors to differentiate this from the general text [19]. Ensure warnings stand out on the label by employing text in colors that differ from the standard text on the label.
Packaging Strategies for Manufacturers
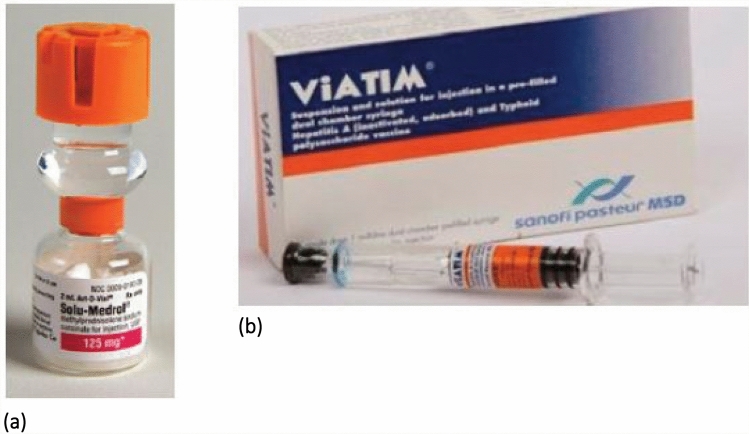
Differing storage requirements of components create opportunities for error such as inadvertent dispensing of only one component and/or incorrect storage, which can compromise the integrity of the vaccine. When stability and compatibility allow, manufacturers should consider designing packaging that forces the healthcare professional to mix the two components. Ready-to-use formulations, such as oral and injectable prefilled syringes, may be useful in preventing healthcare professional preparation errors [19, 34]. When possible, consider a dual-chambered vial or syringe that contains the powder component in one chamber and the liquid component in another and, upon breaking a barrier, the two components will mix prior to withdrawal for administration. Examples of such packaging are the products Solu-Medrol® (methylPREDNISolone) and ViATIM® (hepatitis A and typhoid polysaccharide), a vaccine only available in Europe (Fig. 2a, b). Consider reevaluating how the shelf-life is impacted by differing storage requirements. If all the ingredients of a vaccine can be packaged in one container, the final product will have only one expiration date (i.e. the date of the earliest expiring component) and storage requirement. If multiple containers are necessary, reconcile common storage requirements so all components may be stored together when possible.
Fig. 2.
Dual-chambered packaging: (a) Solu-Medrol® packaging of two components in one vial [19] and (b) ViATIM® packaging of two components in one syringe [19]
When storage requirements of both components are the same, but cannot be packaged in a single container, consider packaging the two components within a single unit-dose carton to facilitate selection and dispensing of all intended components.
Manufacturers should also consider investing in human factor testing to analyze the effectiveness and understanding of how multiple components and devices contribute to confusion and errors [19]. For example, the diluent of the oral 2-component vaccine, Rotarix, is supplied in an oral applicator syringe resembling a parenteral syringe [25]. Accidental injection of the Rotarix oral vaccine has occurred, which emphasizes the need to include administration devices that prompt administration by the appropriate route [35].
The FDA offers manufacturer guidance documents on considerations in labeling and product design, to assist in the prevention of errors with vaccines. Although these guidance documents are non-legally binding, they contain labeling and packaging design strategies drawing from past medication errors for pre-marketed drugs [36, 37].
Strategies for Healthcare Professionals
Continual education is a prerequisite for healthcare professionals; however, higher level risk-reduction strategies employed at the system level are fundamental in preventing medication errors. Safety mechanisms must be implemented within processes and workflow. A high leverage strategy, one that reduces risk for error by focusing on system enhancement rather than the individual’s vigilance, is a standard process for storing and dispensing 2-component vaccines in unit-dose kits. The components may also be held together by a band such as ElastiTag®, to prompt dispensing of both components together [38]. Healthcare professionals can utilize the US Center for Disease Control and Prevention (CDC) vaccine labels, further discussed below, for storage bins to facilitate the correct selection of vaccines and remind staff to reconstitute the contents of the vials [39]. Display reconstitution instructions on the medication administration record (MAR), patient label, pre-printed insert, and/or auxiliary label [19]. Upon administration, design the electronic health system to prompt users to document the NDC, lot number, and expiration date for each component of the vaccine used [19].
A search of organizations with experience in vaccine safety was conducted to identify available tools and resources. The CDC and Immunization Action Coalition (IAC) were searched for tools to assist healthcare settings and healthcare professionals design their workflow processes. The CDC provides free web-based training courses for healthcare professionals [40]. The CDC also provides labels which can be affixed to storage bins with critical reminders to reconstitute certain vaccines with the appropriate diluent prior to administration [39]. IAC provides guidance on what to do if the wrong liquid is used or if only the liquid was administered for Menveo®, Pentacel®, and Shingrix® [41]. If only the liquid is administered for Menveo®, and the patient plans to travel outside of the USA, revaccination must occur and can take place at any time. If only the liquid is administered for Pentacel®, the manufacturer must be contacted to obtain another liquid for the extra ActHIB® [H. influenzae type b (hib)] dose. With Shingrix® (herpes zoster), a correctly reconstituted dose should be administered 4 weeks after the invalid dose.
Report Events and Concerns
Reporting of events and concerns to both VERP and VAERS is confidential. ISMP is affiliated with ECRI. Together they operate the federally certified ECRI and ISMP Patient Safety Organization (PSO), which offers federal legal protection for certain patient safety information through vaccine-related error reports. Error reports represent only errors that have been reported, but the numbers of errors actually occurring may be more profound [42]. Unnoticed errors will not be reported. Healthcare professionals should be encouraged to report both internally and externally. According to a 2016 review article, among the 29 projects reviewed, interventions to encourage error reporting doubled the number of reports [43]. Error reports are important triggers for leadership to recognize that there are opportunities for mistakes to happen, which should catalyze improvements at both the healthcare setting level and at the manufacturing level. Sharing errors and concerns with reporting programs exposes issues and risks that can be analyzed to implement strategic systematic changes.
Conclusions
Preventing 2-component vaccine errors should be a multi-pronged effort. While manufacturers make the necessary changes, healthcare settings can still participate in safety initiatives by implementing high-leverage safety mechanisms within their processes. The responsibility to develop strategies for error prevention is not exclusive to one healthcare sector nor one discipline participating in the medication use process. Although costly for manufacturers to revamp their processes, packaging and labeling play foundational roles in medication safety. Healthcare settings will need to embed systematic strategies to supplement current manufactured product limitations and provide additional safety nets. A combination of different efforts will allow for safer use of 2-component vaccines, protecting patients throughout the world.
Declarations
Funding
No funding was received for this work. The open access fee was paid by the United States Pharmacopoeia.
Conflicts of interest/competing interests
The authors declare they have no conflicts or competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Yes.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
All authors contributed to the current opinion article. The first draft of the manuscript was written by Farzana Samad, Samantha J. Burton, and Diana Kwan. Material preparation, data collection and analysis were performed by Diana Kwan, Noah Porter, Michael R. Cohen, and Judy Smetzer. Jeanne Tuttle, Danial Baker, and Dennis E. Doherty provided critical review. All authors commented on previous versions of the manuscript and read and approved the final manuscript.
Footnotes
Jeanne Tuttle: Retired from Pharmacy Benefits Management Service.
The original version of this article was revised; the following sentence “Manufacturers are required to report errors to the FDA, but in the healthcare setting it is voluntary” has been deleted from Section 9: Report Events and Concerns.
Change history
1/6/2021
In the original publication of the article, the below sentence in the Report Events and Concerns paragraph was erroneously published.
References
- 1.Centers for Disease Control and Prevention. What would happen if we stopped vaccinations? 2017. https://www.cdc.gov/vaccines/vac-gen/whatifstop.htm. Accessed 16 Jan 2019.
- 2.ISMP. ISMP National Vaccine Errors Reporting Program 2017 analysis (part I): vaccine errors.continue with little change. ISMP Medicat Saf Alert! Acute Care. 2018;23(12):1–5.
- 3.ISMP. ISMP National Vaccine Errors Reporting Program part II: preparing for immunization activities and campaigns. ISMP Medicat Saf Alert! Acute Care. 2018;23(13):1–8.
- 4.Hibbs BF, Moro PL, Lewis P, Miller ER, Shimabukuro TT. Vaccination errors reported to the Vaccine Adverse Event Reporting System, (VAERS) United States, 2000–2013. Vaccine. 2015;33:3171–3178. doi: 10.1016/j.vaccine.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Shastay A. Administering just the diluent or one of two vaccine components leaves patients unprotected. Home Healthc Now. 2016;34(4):218–220. doi: 10.1097/NHH.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 6.ISMP. RabAvert needs better packaging. ISMP Medicat Saf Alert! Acute Care. 2013;18(21):1–2.
- 7.Wexler D. Use of vaccines with diluents. 2012. http://www.immunize.org/technically-speaking/20101101.asp. Accessed 27 Nov 2019.
- 8.Shimabukuro TT, Miller ER, Strikas RA, et al. Vaccine Administration errors involving recombinant zoster vaccine—United States, 2017-2018. Morb Mortal Wkly Rep. 2018;67(20):585–586. doi: 10.15585/mmwr.mm6720a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su JR, Miller ER, Duffy J, Baer BM, Cano MV. Administration error involving a meningococcal conjugate vaccine—United States, March 1, 2010-September 22, 2015. Morb Mortal Wkly Rep. 2016;65(6):161–162. doi: 10.15585/mmwr.mm6506a4. [DOI] [PubMed] [Google Scholar]
- 10.ISMP. ActHIB component of two-vial Pentacel is repeatedly missed. ISMP Medicat Saf Alert! Acute Care. 2011;16(10):1–2.
- 11.ISMP. Confusion abounds! 2-year summary of the ISMP national vaccine errors reporting program (part I). ISMP Medicat Saf Alert! Acute Care. 2014;19(24):1–6.
- 12.ISMP. Vaccine with two components. ISMP Medicat Saf Alert! Acute Care. 2009;14(16):1.
- 13.National Institute of Allergy and Infectious Diseases. What is a vaccine adjuvant? 2015. https://www.niaid.nih.gov/research/what-vaccine-adjuvant. Accessed 16 Jan 2019.
- 14.National Institute of Allergy and Infectious Diseases. Vaccine types. 2012. https://www.niaid.nih.gov/research/vaccine-types. Accessed 16 Jan 2019.
- 15.ISMP. ISMP outlines error-prevention strategies for two-component vaccines. Pharm Today. 2018;24(3):45. 10.1016/j.ptdy.2018.02.028.
- 16.McNeil MM, Hibbs BF, Miller ER, Cano MV. Errors in administration of an excess dosage of yellow fever vaccine-United States, 2017. Morb Mortal Wkly Rep. 2018;67(3):109–110. doi: 10.15585/mmwr.mm6703a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nalca A, Zumbrun EE. ACAM2000TM: the new smallpox vaccine for United States strategic national stockpile. Drug Des Devel Ther. 2010;4:71–79. doi: 10.2147/DDDT.S3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elden NM, Ismail A. The importance of medication errors reporting in improving the quality of clinical care services. Glob J Health Sci. 2016;8(8):54510. doi: 10.5539/gjhs.v8n8p243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ISMP. Administering just the diluent or one of two vaccine components leaves patients unprotected. ISMP Medicat Saf Alert! Acute Care. 2014;19(10):1–4.20.
- 20.ACTHIB [package insert]. Birdegewater, NJ; Sanofi Pasteur Inc; 1993.
- 21.HIBERIX [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2009.
- 22.IMOVAX RABIES [package insert]. Bridegewater, NJ: Sanofi Pasteur Inc; 1980.
- 23.MENVEO [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2019.
- 24.PENTACEL [package insert]. Bridegewater, NJ: Sanofi Pasteur Inc; 2008.
- 25.ROTARIX [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2008.
- 26.RABAVERT [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 1997.
- 27.SHINGRIX [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
- 28.YF-VAX [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 1953.
- 29.VAXCHORA [package insert]. Redwood City, CA: Paxvax, Inc; 2016.
- 30.M-M-R II [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp; 1971.
- 31.PROQUAD [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp; 2005.
- 32.VARIVAX [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp; 1995.
- 33.ZOSTAVAX [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp; 2006.
- 34.Vaccine Innovation Prioritisation Strategy (VIPS): VIPS Phase II executive summary: Dual-chamber delivery devices. 2020. https://www.gavi.org/sites/default/files/about/market-shaping/Phase-II/5_VIPS%20Phase%20II_Executive%20Summary_Dual%20Chamber%20Delivery%20Devices.pdf. Accessed 15 Sep 2020.
- 35.ISMP. Oral vaccine mistakenly given by injection. ISMP Medicat Saf Alert! Acute Care. 2014;19(3):1–3.
- 36.Food and Drug Administration. Safety considerations for container labels and carton labeling design to minimize medication errors. 2013. https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm349009.pdf. Accessed 27 Nov 2019.
- 37.Food and Drug Administration. Safety considerations for product design to minimize medication errors. 2016. https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm331810.pdf. Accessed 27 Nov 2019.
- 38.ElastiTag. https://www.elastitag.com/. Accessed 13 Feb 2019.
- 39.CDC. Vaccine Label Examples. 2020. https://www.cdc.gov/vaccines/hcp/admin/storage/guide/vaccine-storage-labels.pdf. Accessed 8 Aug 2019. Accessed 11 Sep 2020.
- 40.CDC. Web-based Training Course. 2020. https://www.cdc.gov/vaccines/ed/youcalltheshots.html. Accessed 11 Sep 2020.
- 41.Immunization Action Coalition. Don’t be Guilty of These Preventable Errors in Vaccine Administration! 2019. https://www.immunize.org/catg.d/p3033.pdf. Accessed 11 Sep 2020.
- 42.Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–396. doi: 10.2165/00002018-200629050-00003. [DOI] [PubMed] [Google Scholar]
- 43.Ribeiro-Vaz I, Silva AM, Costa Santos C, Cruz-Correia R. How to promote adverse drug reaction reports using information systems—a systematic review and meta-analysis. BMC Med Inform Decis Mak. 2016 doi: 10.1186/s12911-016-0265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vx Labels. Vaccine Information: Menveo. 2016. https://vxlabels.com/lib/vaccines/vax/menveo-2/page/6/. Accessed 11 Sep 2020.