Abstract
Bacterial wilt as a soil-borne disease was caused by Ralstonia solanacearum, and seriously damages the growth of tobacco. Integrated biocontrol method was explored to control bacterial wilt. Nevertheless, the long-term effects of the integrated biocontrol method on soil bacterial community, soil physicochemical properties and the incidence of bacterial wilt are not well understood. In this study, B. amyoliquefaciens ZM9, calcium cyanamide and rice bran were applied to tobacco fields in different ways. The disease index and incidence of tobacco bacterial wilt (TBW), soil physicochemical properties, colonization ability of B. amyoliquefaciens ZM9, and rhizopshere bacterial community were investigated. The results showed that the integrated application of B. amyoliquefaciens ZM9, rice bran and calcium cyanamide had the highest control efficiency of TBW and bacteria community diversity. Additionally, the integrated biocontrol method could improve the colonization ability of B. amyoliquefaciens ZM9. Furthermore, the integrated biocontrol method could effectively suppress TBW by regulating soil physicochemical properties, promoting beneficial bacteria and antagonistic bacteria of rhizopshere soil. This strategy has prospect of overcoming the defects in application of a single antagonistic bacteria and provides new insights to understand how to improve the colonization capacity of antagonistic bacteria and control efficacy for TBW.
Subject terms: Soil microbiology, Microbial communities, Microbe
Introduction
Bacterial wilt is a typical soil-borne disease caused by Ralstonia solanacearum1. Due to the high lethality and wide host range, it is widely distributed worldwide, which seriously affects the yield and quality of crops2,3. Some researchers believed that the rhizosphere microecological imbalance was the main reason for bacterial wilt4. The interdependent ecosystem formed by plants soil microorganisms was destroyed, while the imbalance of rhizosphere microecological further deteriorated the soil microenvironment, unhealthy growth of plants, a large number of pathogenic bacteria multiplying, and eventually lead to outbreaks of soil-borne diseases5,6.
Biological control is widely used for prevention and control of soil-borne diseases due to its broad-spectrum, persistent and environmentally friendly characteristics7,8. The most common method of biological control is to control the abundance of pathogenic bacteria through antagonistic bacteria, so as to reduce the harm of pathogens to plants, and antagonistic bacteria are also beneficial to plant growth9,10. Until now more than 100 antagonists of bacterial wilt include Bacillus spp., Pseudomonas spp., Streptomyces spp. and so on were isolated and identified11,12. However, in practical application, these antagonists were affected by environmental conditions and crops. Moreover, the control efficacy is unstable, the adaptability to the environment is poor, and the colonization ability is decreased13. Since the colonization of antagonistic in soil is difficult after the application of antagonistic bacteria alone, the improvement of the colonization of antagonistic bacteria in the soil rhizosphere has become the research focus of biological control. Previous studies demonstrated that antagonistic bacteria could better colonize plant roots when they were applied to the soil with organic fertilizers or soil amendments14,15. At the same time, plant root exudates also have a positive induction effect on microbial biofilm formation and chemotactic, thus inhibiting the growth of pathogenic bacteria or improving plant resistance16,17. Therefore, organic fertilizers or soil amendments assistance of antagonistic bacteria should be considered as an effective integrated biocontrol method to improve the colonization ability of antagonistic bacteria and control bacterial wilt. However, most trials about the integrated biocontrol have been carried over short time periods18. Since the quality of the soil, such as the physicochemical properties, microbial community, etc. is extremely important for the growth of plants, changing the physicochemical properties of the soil is a long process that could take several years. However, the long-term effects of the combined application of antagonistic bacteria, organic fertilizers or soil amendments on soil bacterial community, soil physicochemical properties and the incidence of bacterial wilt had been poorly understood in field experiment15.
Our previous study showed that B. amyloliquefaciens ZM9 as an antagonistic bacterium of bacterial wilt was isolated through its ability to colonize tobacco and rhizosphere soil, which can improve the microbial abundance of rhizosphere soil5. Studies have evidenced soil amendment calcium cyanamide can improve soil ecological environment, and increase crop yield19,20. Additionally, rice bran, as an effective organic fertilizer, provides soil microorganisms with carbon, nitrogen and other basic nutrients21. In this study, the experiment were carried out in infected tobacco fields for three years, and antagonistic bacteria B. amyloliquefaciens ZM9, rice bran and calcium cyanamide were applied to the fields in different ways. The main aims of this study were to (1) compare the control efficacy of different treatment groups for TBW, (2) assess the colonization ability of B. amyloliquefaciens ZM9, and (3) explore the dynamic changes of physicochemical properties and rhizosphere bacterial community. To provide new insights to understand how to improve the colonization ability of antagonistic bacteria and control efficacy for TBW.
Results
Control of TBW by different treatments
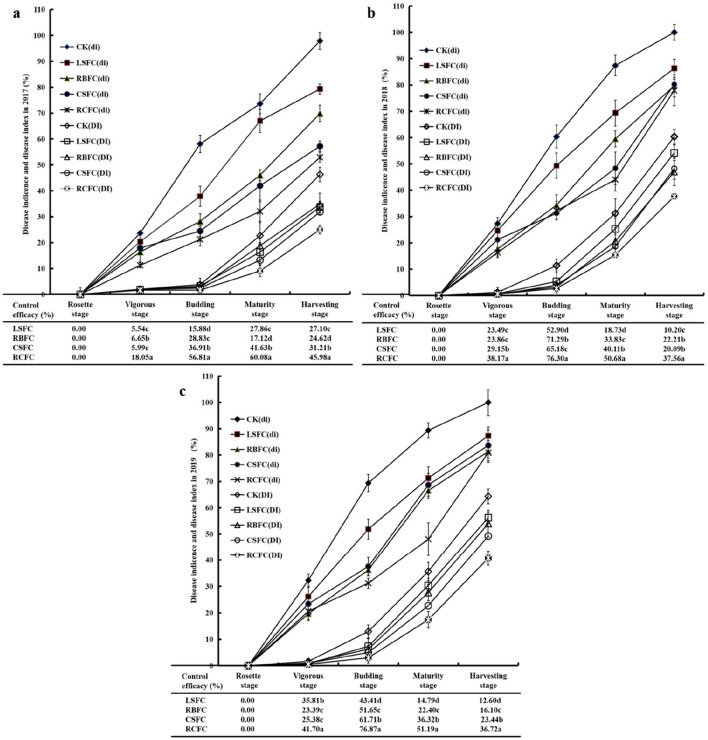
Disease incidence and index with five treatments at different tobacco growth stages in different culture years were calculated. In 2017, the disease incidence and index of LSFC (the group with the application of alone liquid fermentation inoculants), RBFC (the group with the application of solid fermentation inoculants and rice bran), CSFC (the group with the application of solid fermentation inoculants and calcium cyanamide) and RCFC (the group with the application of solid fermentation inoculants, rice bran and calcium cyanamide) groups were lower than those of the CK group (the control group). Moreover, the disease incidence of RCFC group was the lowest in the five treatment groups. In 2018 and 2019, the variation tendency of disease incidence and index in CK, LSFC, RBFC, CSFC and RCFC groups was similar to that of 2017. Furthermore, the control efficacy was significantly higher in the RCFC group than in the LSFC, RBFC and CSFC groups at vigorous, budding, maturity and harvesting stages (Fig. 1).
Figure 1.
Disease incidence, disease index and control efficacy of five treatment groups at different stages in 2017 (a), 2018 (b) and 2019 (c). (The different letters in the same column indicate significant differences as determined by LSD test. p < 0.05).
Physicochemical properties
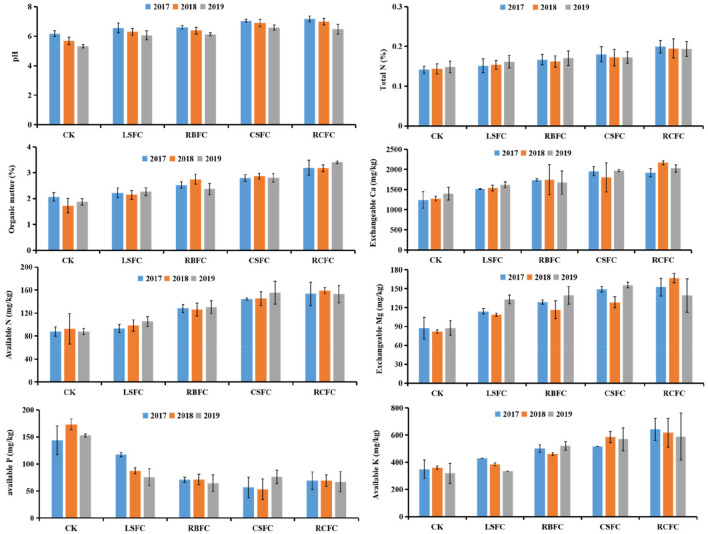
The physicochemical properties of different treatments were analyzed. The contents of organic matter (OM), alkaline nitrogen (alkaline N), total nitrogen (TN), available potassium (available K), exchangeable calcium (exchangeable Ca) and exchangeable magnesium (exchangeable Mg) in the CK, LSFC, RBFC, CSFC and RCFC groups increased successively, and the value of pH increased successively too. These soil physicochemical properties of the RCFC groups were slightly higher than other treatment groups. While, the contents of available P in the CK, LSFC, RBFC, CSFC and RCFC groups decreased successively (Fig. 2). In addition, the pH of different treatments showed decreased with the years from 2017 to 2019. What’s more, OM (Pearson = − 0.713, p = 0.003), pH (Pearson = − 0.745, p = 0.001), TN (Pearson = − 0.524, p = 0.045), alkaline N (Pearson = − 0.524, p = 0.045), available K (Pearson = − 0.734, p = 0.002), exchangeable Ca (Pearson = − 0.720, p = 0.002) and exchangeable Mg (Pearson = − 0.758, p = 0.001) showed significantly negative correlation with the incidence of TBW (Table S1).
Figure 2.
Soil physiochemical properties in different treatments from 2017 to 2019. Values are means of SD.
Diversity analysis of soil bacterial community in the tobacco rhizosphere
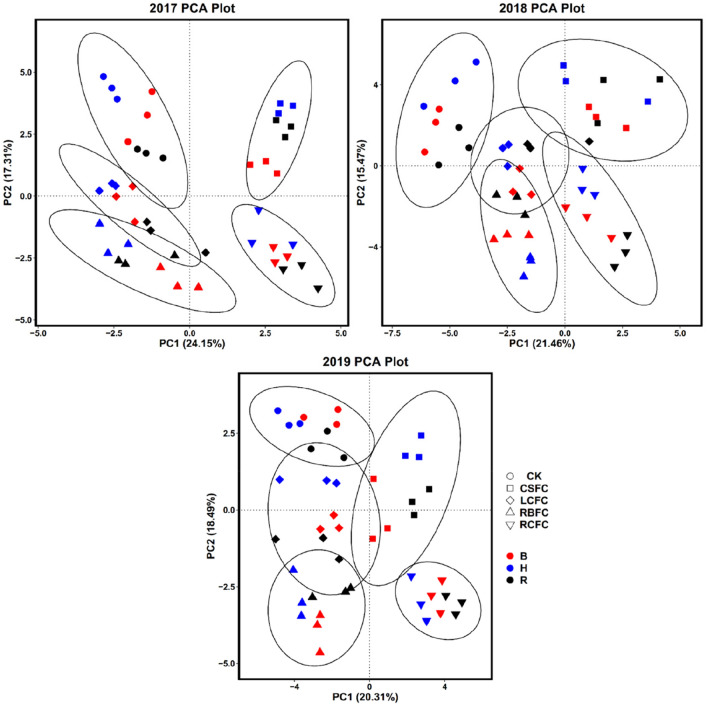
In total 712,691 high-quality raw sequences with the average length of 252 bps for bacteria were obtained from rhizosphere soil samples after quality filtering. The difference of OTUs, Sobs, Shannon, Simpson and Chao1 of bacteria richness and diversity were analyzed (Table S2). The number of OTUs in all samples ranged from 3852 to 6515, and the OTUs, Sobs and Chao 1 were higher in the rhizophere soil of the LSFC, RBFC, CSFC and RCFC groups than the CK group. Comparing with other treatment groups, the numbers of OTUs and Sobs of bacteria in the RCFC group was slightly higher. From 2017 to 2019, the rhizosphere bacterial community of different treatments changed with different trends at rosette, budding and harvesting stage. Pearson correlation showed the OTUs, Sobs, and Chao1 had significant negative correlation with the incidence of TBW (Table S3). Principal components analysis (PCA) were carried out using OTUs in the different treatments. In 2017, 41.46% of total variance was explained by the first two axes with 24.15% and 17.31% explanations in PC1 and PC2. In 2018, PC1 and PC2 explained 36.93% of total bacterial community. In 2019, 38.8% of total variance was explained by PC1 and PC2. From 2017 to 2019, the samples collected during different tobacco developmental stages within one treatment showed close distances, but distribution among different treatments was relatively discrete (Fig. 3), which indicated that the structure of bacterial community in the same sampling period was similar, and different treatments played important impact on the formation of bacterial community in the rhizosphere soil.
Figure 3.
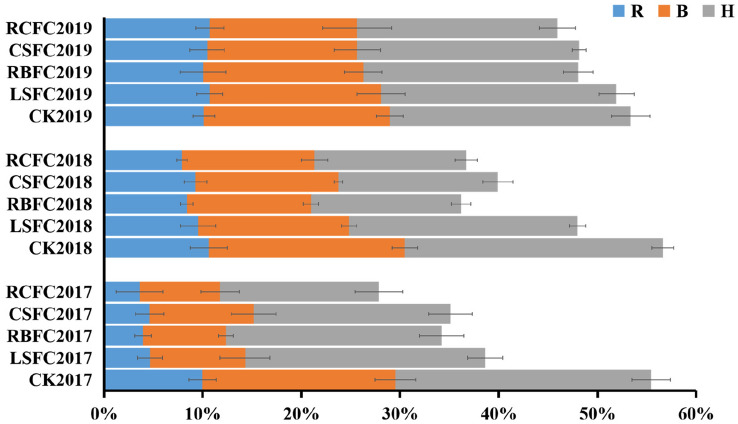
Two-dimensional plot of the principal components analysis (PCA) for the top ten abundant bacterial phyla in the different treatments at rosette (R), budding (B) and harvesting (H) stage in 2017, 2018 and 2019.
The composition and structure of rhizospheric bacterial community in different treatments
The top ten abundant bacterial phyla were selected to compare the changes of bacterial community in rhizosphere soil of five treatment groups during tobacco growth period from 2017 to 2019 were Proteobacteria, Acidobacteria, Actinobacteria, Chloroflexi, Gemmatimonadetes, Firmicutes, Bacteroidetes, Planctomycetes, Verrucomicrobia and Nitrospirate. In 2017, the abundance of the phylum Proteobacteria included the pathogen R. solanacearum during harvesting stage in the CK, LSFC, RBFC, CSFC and RCFC groups were 58.15%, 56.33%, 43.91%, 44.60% and 37.61%, respectively. Furthermore, the abundance of Proteobacteria in the RCFC group was lower than that in the CK group. The abundance of the phylum Firmicutes included B. amyloliquefaciens ZM9 during harvesting stage in the CK, LSFC, RBFC, CSFC and RCFC groups were 2.02%, 2.65%, 3.07%, 5.28% and 6.53%, respectively (Fig. S1). The top ten abundant of bacterial phyla in five treatments changed irregularly during different tobacco growth stages from 2017 to 2019. For example, In the CK, the abundance of Proteobacteria was low at the rosette stage (R), increased at the budding stage (B), then decreased at the harvesting stage (H) from 2017 to 2019. In the CSFC and RCFC groups, the abundance of Proteobacteria were decreased at the budding stage (B), and increased at the harvesting stage (H). However, there are no regular changes in other treatment groups (Fig. S1). In addition, the top ten abundant phyla showed significantly negative correlation with the incidence of TBW, including Chloroflexi (Pearson = − 0.627, p = 0.000), Firmicutes (Pearson = − 0.455, p = 0.008), Planctomycetes (Pearson = − 0.467, p = 0.006) and Nitrospirae (Pearson = − 0.858, p = 0.000) (Table S4).
Dynamic variations of B. amyloliquefaciens ZM9 and R. solanacearum
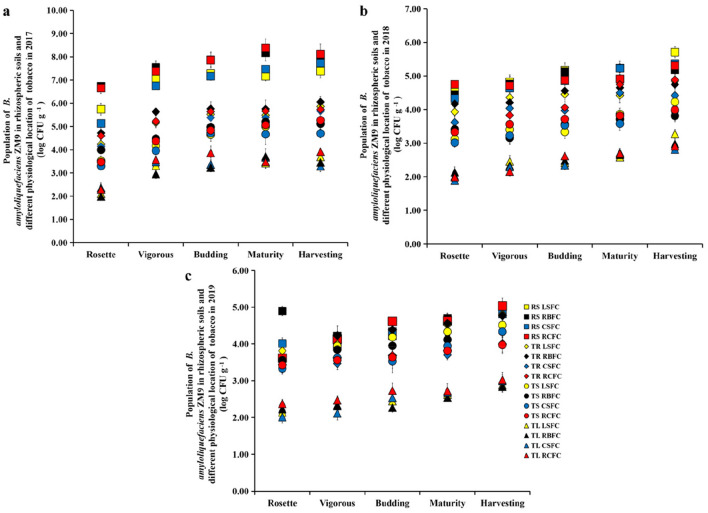
The abundances of B. amyloliquefaciens ZM9 in rhizospheric soil, tobacco root, stem and leaf of four treatment groups (LSFC, RBFC, CSFC and RCFC) at different tobacco growth stages were calculated by the plated count method. The abundances of B. amyloliquefaciens ZM9 in rhizospheric soil were obviously higher than that in tobacco root, stem and leaf, and the abundances of B. amyloliquefaciens ZM9 in tobacco root, stem and leaf decreased successively. Additionally the abundances of B. amyloliquefaciens ZM9 in rhizospheric soil, tobacco root, stem and leaf of four treatment groups increased with the growth stages of tobacco (Fig. 4). From 2017 to 2019, the abundances of B. amyloliquefaciens ZM9 in rhizospheric soil decreased with years, and the difference in the abundances of B. amyloliquefaciens ZM9 in rhizospheric soil, tobacco root and stem gradually decreased with years (Fig. 3). As we focused on the TBW caused by R. solanacearum, we investigated the abundance of R. solanacearum in rhizospheric soil. In 2017, the abundance of R. solanacearum decreased significantly (p < 0.05) in the LSFC, RBFC, CSFC and RCFC groups, and the abundance of this pathogen in RCFC groups was the lowest among all treatment groups (Fig. 5). From 2017 to 2019, the abundance of R. solanacearum increased with the tobacco growth stages and years. Compared with the control group, the abundance of R. solanacearum in LSFC, RBFC, CSFC and RCFC groups decreased (Fig. 4). What’s more, the abundance of R. solanacearum showed significantly positive correlation with the incidence of TBW (p = 0.001). However, there was no significantly correlation between the abundance of R. solanacearum and bacterial community (Table S5). The linear regression analysis showed that the abundance of R. solanacearum was negatively correlated with the abundances of B. amyloliquefaciens ZM9 (Fig. S2).
Figure 4.
Population dynamic of B. amyloliquefaciens ZM9 in rhizospheric soil (RS), tobacco root (TR), stem (TS) and leaf (TL) of four treatment groups (LSFC, RBFC, CSFC and RCFC) at different tobacco growth stages in 2017 (a), 2018 (b) and 2019 (c).
Figure 5.
Population dynamics of the R. solanacearum in five treatment groups (CK, LSFC, RBFC, CSFC and RCFC) at rosette (R), budding (B) and harvesting (H) stage from 2017 to 2019.
Relationships between bacterial community and soil physicochemical properties
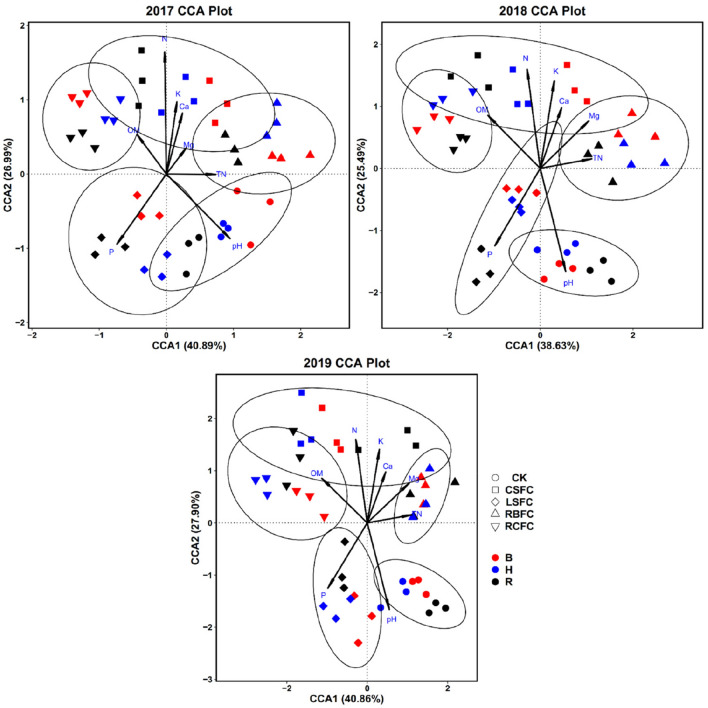
The relationships between bacterial community structure and soil physicochemical properties were analysed with canonical correspondence analysis (CCA) from 2017 to 2019. Eight factors including pH, OM, alkaline N, TN, available K, available P, exchangeable Ca and exchangeable Mg were selected for CCA, and results showed that the treatments of CK, LSFC, RBFC, CSFC and RCFC were separated from each other (Fig. 6). These variables explained 67.88%, 64.12% and 68.76% of bacterial community variation in 2017, 2018 and 2019, respectively. The bacterial community composition in CK and LSFC groups were positively correlated with P and pH, but CSFC, RBFC and RCFC groups show negatively correlation with available P and pH. OM, available K, alkaline N, exchangeable Ca and exchangeable Mg positively correlated with CSFC, RBFC and RCFC groups, and negatively correlated with CK and LSFC groups (Fig. 6). The longer arrows of environmental factors showed that pH, OM, alkaline N, available K and available P play major roles in the formation of soil bacterial community structure, and pH, available P and available K best explained the differences between treatments, which was validated by Monte Carlo test (Table S6).
Figure 6.
Canonical correspondence analysis (CCA) of the relationship between rhizospheric bacterial community and soil physicochemical properties in five treatment groups (CK, LSFC, RBFC, CSFC and RCFC) at rosette (R), budding (B) and harvesting (H) stage in 2017, 2018 and 2019. The soil properties are indicated with arrows, including soil pH, organic matter (OM), total nitrogen (TN), alkaline nitrogen (N), available phosphorous (P), available potassium (K), exchangeable calcium (Ca), exchangeable magnesium (Mg) content. The percentage of variation is explained by each axis.
Beneficial bacteria variation in response to different treatments
In this long-term field experiment, the relative abundance of beneficial bacteria in the tobacco rhizosphere in five treatment groups were compared. The relative abundance of beneficial bacteria in LSFC, RBFC and RCFC groups were significantly higher than that in the CK (Fig. S3). In 2017, among the beneficial bacteria in the tobacco rhizosphere, the relative abundance of Acinetobacter, Azospirillum, Bradyrhizobium, Chthonomonas, Granulicella, Hyphomicrobium, Mesorhizobium, Pseudomonas, Psychrobacter and Stenotrophomons in the LSFC, RBFC, CSFC and RCFC groups were higher than in the CK. Moreover, the relative abundance of Acinetobacter, Bradyrhizobium, Pseudomonas and Psychrobacter increased significantly in the LSFC, RBFC, CSFC and RCFC groups, and these beneficial bacteria were slightly higher in RCFC (Fig. S3b). In 2018 and 2019, the variation tendency of the beneficial bacteria in different treatment groups was similar to that in 2017 (Fig. S3). What’s more, Pearson analysis indicated that Acinetobacter, Azospirillum, Bradyrhizobium, Chthonomonas, Granulicella, Hyphomicrobium, Mesorhizobium, Pseudomonas, Psychrobacter and Stenotrophomons showed strong negative correlation with the incidence of TBW (Table S7).
Antagonistic bacteria variation in response to different treatments
For further exploring the distribution of the antagonistic bacteria in different treatments, the abundances of antagonistic bacteria were calculated (Fig. S4). The relative abundance of antagonistic bacteria in LSFC, RBFC and RCFC groups were higher than in the CK. Furthermore, the relative abundance of Actinospica, Bacillus, Burkholderia, Catenulispora increased significant in the LSFC, RBFC, CSFC and RCFC groups (Fig. S4). These antagonistic bacteria had significant negative correlation with the incidence of TBW (Table S8). In addition, antagonistic bacteria in LSFC, RBFC and CSFC groups decreased with years from 2017 to 2019 (Fig. S4).
Discussion
In previous investigation, B. amyoliquefaciens ZM9 as an efficient biocontrol agent could suppress TBW by producing lipopeptides to suppress R. solanacearum and regulating the tobacco rhizosphere microbial community in a field trial5. However, the long-term effects of B. amyloliquefaciens ZM9 with organic fertilizers or soil amendments on rhizopshere soil physicochemical properties, microbial community and the incidence of bacterial wilt is still unclear. In this study, B. amyloliquefaciens ZM9, rice bran and calcium cyanamide were applied to tobacco fields in different ways. Through long-term monitoring for three years, we found that rice bran and calcium cyanamide assitant with B. amyloliquefaciens ZM9 could effectively suppressed TBW through changing soil physicochemical properties and bacteria abundances.
Through three years of long-term monitoring, there was no significant change in the control efficacy of TBW by the combined application of B. amyloliquefaciens ZM9, rice bran and calcium cyanamide (Fig. 1). The result of present study revealed that the integrated biocontrol method is sustainable for the prevention and treatment of TBW. Since researchers indicated that the combined application of antagonistic bacteria and organic fertilizer or soil amendments can be effective in the prevention and control of various soil-borne diseases15,18. In this study, a significantly negative relationship between some soil properties (OM, alkaline N, available K, exchangeable Ca, exchangeable Mg and pH) and the incidence of TBW was well documented (Table S1). We infer that the integrated biocontrol measure may reduce the incidence of TBW by regulating soil physicochemical properties. Calcium cyanamide can increase soil pH, total N, alkaline N and exchangeable Ca22. Additionally, rice bran provides soil with C, N, P and other nutrients21. C may increase the soil OM, and OM improves soil fertility and crop yields23. At the same time, OM is an important source of microbial nutrients24. Increase pH is important for inhibited the survival of R. Solanacearum, increase N and P meet the need of plant growth20,23. However, after 3 years of long-term monitoring, the pH values of different treatments decreased with the years (Fig. 2), which consistently with previous reports6,25. That may attribute to the accumulation of allelochemicals. Moreover, the soil physicochemical properties were related to the microbial community. As reported, pH may stress microbial cells to select specific microbial groups and affect the composition of soil microbial community26. P application can cause an increase in bacterial diversity, with increase Actinobacteria and decreased Acidobacteria in soils27. In this study, CCA results also showed that soil physicochemical properties relevant to the bacterial community structure (Fig. 6). Here, the available P in the treatment groups were decreased compared with control group (Fig. 2). It maybe the treatment groups plants absorb much more effective P, and the abundance of microorganisms were higher in the treatment groups than the control group, these further promoted the treatment groups consumption of P.
The incidence of TBW was also associated with soil microbial community diversity, as indicated by OTUs, Sobs and Chao1. Bacterial OTUs, Sobs and Chao1 were more diverse in biocontrol treatment groups than in the control group (Table S2). Additionally, OTUs and Sobs had significant negative correlation with the incidence of TBW (Table S3). This is similar to previous report that the highly rhizosphere soil microbial community diversity would decrease the incidence of TBW28. Shen et al.29 found that organic fertilizer could increase soil microbial activity, enhance competition for nutrients for pathogens. A recent report indicated that soil amendment calcium cyanamide could supply sufficient N, and increase soil pH, microbial diversity30. Our findings also demonstrated that both the alone application rice bran, calcium cyanamide or B. amyoliquefaciens ZM9 and the combined application of rice bran, calcium cyanamide and B. amyoliquefaciens ZM9 (RCFC group) could effect on the variation of bacterial community, while the combined application would bring higher bacteria community diversity than the other treatments. We also hypothesized that the rhizospheric bacterial community varies at different plant growth stages. The most abundant phyla bacterial detected in this study were Proteobacteria, Acidobacteria, Actinobacteria and Chloroflexi (Fig. S1), supporting the findings of others4,31,32. Proteobacteria paly important roles in C, N and S cycles23,33. Actinobacteria as the third most prominent was negative correlation with the incidence of TBW. Liu et al.34 revealed that antgonistic bacteria Actinobacteria can produce a diverse range of antibiotics and induce plant resistance to control plant bacterial diseases. These bacterial can rapidly respond to environmental perturbations leading to dynamic changes in abundance, activity and composition35. It is noted that these rhizospheric bacterial changed with different trends during different tobacco growth stages from 2017 to 2019 (Fig. S1).
In this study, we have successfully isolated B. amyoliquefaciens ZM9 through its ability to colonize the tobacco root, stem, leaf, and rhizosphere soil, and the results were consistent with our previous findings5. This indicated that B. amyloliquefaciens ZM9 had colonization ability. Also, we found that the abundances of B. amyloliquefaciens ZM9 in rhizospheric soil gradually decreased under continuous cropping (Fig. 4). We suspected that the reason why colonization of B. amyloliquefaciens ZM9 strain was decreased with years from 2017 to 2019 was that the concentration of B. amyloliquefaciens ZM9 in the medium was decreased continuously with the years. Linear fitting showed that the abundance of R. solanacearum was negatively correlated with the abundances of B. amyloliquefaciens ZM9 (Fig. S2). Corresponding with reality, the decreased abundance of B. amyloliquefaciens ZM9 can lead the increased R. solanacearum, because the antagonistic effect will become weaker and weaker, and this also proves the antagonistic effect of B. amyloliquefaciens ZM9 on R. solanacearum from another aspect.
In the current study, during three years of long-term monitoring, the variation tendency of the beneficial bacteria in different treatment groups was similar, and many beneficial bacteria involved in element cycling and promoted plant growth. Some beneficial bacteria in the rhizosphere soil were higher abundances in the LSFC, RBFC, CSFC and RCFC groups than in the CK, including Acinetobacter, Azospirillum, Bradyrhizobium, Mesorhizobium, Pseudomonas, Psychrobacter and Stenotrophomons (Fig. S3). This indicated that biocontrol inoculants might stimulate beneficial bacteria. Some of these beneficial bacteria can produce hormones to stimulate plant growth, increase plant resistance to stress5. While, Mesorhizobium can assist plant to survive by producing biofilm36. Moreover, some species of Granulicella were found to use different substances as carbon sources, and several species of Chthonomonas have a special effect on the transition of organic carbon in the rhizosphere soil37,38. The bacteria Hyphomicrobium involved in nitrogen cycling and can perform partial or complete denitrification39. We also found that these beneficial bacteria showed strong negative correlation with the incidence of TBW. These beneficial bacteria can directly and effectively control TBW by competing for nutrients, space, and inducing systemic resistance40,41.
It has been well documented that Actinospica, Bacillus, Burkholderia, Catenulispora, Chryseobacterium, Clostridium, Flavobacterium, Haliangium, Microbispora, Paenibacillus, Rhodopseudomonas, Sphingomonas, Staphyloccus, Stenotrophomonas and Streptomyces as antagonistic bacteria can mitigate many soil-borne diseases42–47. Antagonistic bacteria Actinospica, Catenulispora, Haliangium, Microbispora can form a alliance of antibiotic producers and change bacterial community structure35. In addition, there were several endophytic or rhizobacteria such as Burkholderia, Sphingomonas, Staphyloccus, Bacillus and Rhodopseudomonas can promote plant growth and inhibit pathogenic bacteria44,48–51. These antagonistic bacteria were higher abundances in LSFC, RBFC and RCFC groups than in the CK (Fig. S4). Moreover, these antagonistic bacteria were negatively related to the incidence of TBW (Table S8), revealing that biocontrol teatment groups may improve the growth of antagonistic bacteria and contribute to pathogen inactivation. We also found antagonistic bacteria in LSFC, RBFC and CSFC groups decreased with years from 2017 to 2019 (Fig. S4), indicating the antagonistic bacteria decreased under continuous cropping.
Conclusions
The combined application of rice bran and calcium cyanamide could promote the colonization ability of B. amyoliquefaciens ZM9 and effectively suppress TBW by regulating soil physicochemical properties, and enriching the beneficial bacteria and antagonistic bacteria in the soils. Our results provide a promising strategy for TBW control by adding soil amendment calcium cyanamide and organic fertilizer rice bran.
Materials and methods
Field experiment
Field experiment were performed in a 15 years history of continuous cropping tobacco field in Xuan’en County (109°26ʹ20ʺ E, 29°59 ʹ 55 ʺ N), Enshi City, Hubei province, China from April to September in 2017, 2018 and 2019. The incidence of TBW in this field had been above 95% every year for the past five years before this study. Tobacco seedings (Yunyan87) were grown according to our previous study5. B. amyloliquefaciens ZM9 (Genbank: KF906355.1) was used as the antagonistic bacteria in this study. Five treatments were established: (1) the control group (CK, without any pesticide); (2) the group with the application of alone liquid fermentation inoculants (LSFC): B. amyloliquefaciens ZM9 was incubated and irrigated into the tobacco root as previous study5,21; (3) the group with the application of solid fermentation inoculants and rice bran (RBFC): 300 g of rice bran was mixed with the 100 mL of the diluted B. amyloliquefaciens ZM9 culture (1.0 × 107 CFU/mL) and fermented for 3 days, adjusted to a final concentration to 2.5 × 106 CFU/g, and then 40 g was applied to soil for each plant when the tobacco was transported; (4) the group with the application of solid fermentation inoculants and calcium cyanamide (CSFC): 150 kg/hm2 of calcium cyanamide were applied during soil preparation process, and then irrigated the diluted B. amyloliquefaciens ZM9 culture into the tobacco roots; and (5) the group with the application of solid fermentation inoculants, rice bran and calcium cyanamide (RCFC): 150 kg/hm2 of calcium cyanamide were applied during soil preparation process, and then loaded same amount of B. amyloliquefaciens ZM9 as described in LSFC. All treatments were performed only in the first year and then monitored 3 years continuously. There were 180 tobacco plants per treatment, three replicates (60 plants in each replicate). All treatments and replicates were randomly placed in the field, and groups were irrigated with the same amount of initial water.
Disease incidence, index and control efficacy calculation
The TBW disease index (DI) based on severity scale of 0–9 was described in a previous study23. Briefly, “0” represents the plants without visible symptoms; “1” represents the presence of occasional chlorotic spots on stems, or less than half of the leaves wilted on unilateral stems; “3” represents the presence of a black streak less than half the height of the stem, or between half to two-thirds of the leaves wilted on unilateral stems; “5” represents the presence of a black streak over half the length of the stem, but not reaching the top of the stem, or more than two-thirds of the leaves wilted on unilateral stems; “7” represents the presence of a black streak reaching the top of the stem, or all leaves wilted; and “9” represents the dead plant. Based on the number of plants in each rating scale, disease incidence (di) and disease index (DI) of TBW were calculated as di = n′/ N × 100% and DI = ∑(r × n)/(N × 9) × 100. where n′ is the total number of infected tobacco plants, r is the rating scale of disease severity, n is the number of infected tobacco plants with a rating of r, and N is the total number of plants. Control efficacy = [(di of control − di of treatment)/di of control] × 100%. The di, DI and control efficacy were measured at 1 week, 3 weeks, 6 weeks, 9 weeks and 15 weeks after irrigation in 2017, 2018 and 2019, respectively.
Rhizosphere soil sampling and physicochemical properties analysis
The rhizosphere soil was collected by five-spot-sampling method from 2017 to 2019, respectively. Then transported to the laboratory, stored at − 80 °C for microbiological analysis, and at room temperature for physicochemical properties analysis. Soil samples were air-dried and ground (< 2 mm) to determine available nutrients and soil characteristics. pH was determined by potentiometric method, organic matter (OM) was determined by potassium dichromate volumetric method, total nitrogen (TN) was determined by Kjeldahl method, alkaline nitrogen (alkaline N) was determined by alkali solution diffusion method, available phosphorus (available P) was determined by the sodium bicarbonate method, available potassium (available K) was determined by flame spectrophotometer, and exchangeable calcium (exchangeable Ca), exchangeable magnesium (exchangeable Mg) was determined by atomic absorption spectrophotometry24.
DNA extraction
Soil DNA was extracted from 0.5 g rhizosphere soil using the FastDNA Spin Kit (MP NA gene. The V4 region of 16S rRNA Biomedicals, USA) following the manufacture’s protocol. The integrity of DNA samples were determined by 1% agarose gel electrophoresis. Then the concentration and purity of the DNA were determined using a Nanodrop ND-1000 Spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA).
DNA sequence data collection and analysis
The extracted soil DNA was used as template to amplify 16S rRNA gene were amplified with the primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′)5. All PCR reactions were performed on Illumina HiSeq platforms (Illumina Inc., USA) at Novogene Bioinformatics Technology Co., Ltd (Beijing, China). The library quality was assessed on the Qubit@ 2.0 Fluorometer (Termo Scientifc) and Agilent Bioanalyzer 2100 system. Sequences analysis was performed by Uparse software (Version 7.0.1001, http://drive5.com/uparse/). Sequences with ≥ 97% similarity were assigned to the same OTUs. Each representative sequence was screened for further taxonomic information annotation. FASTX Toolkit 0.0.13 software package was used to preliminarily filtrate the raw sequence data with removing the low mass base at the tail of the sequence (Q value less than 20) and the sequences with lengths less than 35 bp. The sequence quality was statistically analyzed by CASAVA1.8, and finally, the length of the valid reads was approximately 250 bp. The operational taxonomic units (OTUs), observed-species (Sobs), Shannon, Simpson and Chao1 were calculated to evaluate richness and diversity of soil microbial community.
Dynamic variation of B. amyloliquefaciens ZM9 and R. solanacearum in field experiments
Tobacco plants were uprooted from four treatment groups (LSFC, RBFC, CSFC and RCFC) at different growth stages to evaluate the population dynamic of B. amyloliquefaciens ZM9 in the rhizosphere soil (RS), tobacco root (TR), stem (TS) and leaf (TL) by the plate count method with three biological replicates5 (Fig. S5). The media used in this study for B. amyloliquefaciens ZM9 plate count was the LB selective media with 200 mg/mL rifampin. The abundances of R. solanacearum in rhizospheric soil of five treatment groups (CK, LSFC, RBFC, CSFC and RCFC) at different tobacco growth stages were calculated based on 16S rRNA gene sequencing data.
Statistical analysis
The data were analyzed with Microsoft Excel 2007 and SPSS version 18.0 (IBM, USA). Differences between treatments were assessed by one-way analysis of variance (ANOVA) and least significant difference (LSD) test (p < 0.05). Correlation analysis was conducted by Pearson (2- tailed). Principal components analyzed (PCA) with the weighted UniFrac distance and canonical correspondence analysis (CCA) were carried out using the vegan package in R (Version 2.15.3). Monte Carlo test were used to construct dissimilarity matrices of bacterial community and soil physicochemical properties.
Supplementary Information
Acknowledgements
This work was supported by the key technology projects of China National Tobacco Corporation (CNTC) (No. 110201502018 and No. 110201901042(LS-05)) and the key technology projects of Hubei tobacco companies (No. 027Y2018-038).
Author contributions
Y.Y., Y.L. and Y.H. initiated and designed the research. X.Y., C.L., L.W., J.F. performed the experiments and collected the data. Y.H. and Y.L. analyzed the data and wrote the manuscript, X.L., Y.Y. and S.C. revised the manuscript. All authors reviewed and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yun Hu and Yanyan Li.
Contributor Information
Xihong Li, Email: lxh885@126.com.
Yong Yang, Email: yangyong@hubu.edu.cn.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-82060-3.
References
- 1.Tanskersten J, Huang H, Allen C. Ralstonia solanacearum needs motility for invasive virulence on tomato. J. Bacteriol. 2011;183:3597–3605. doi: 10.1128/JB.183.12.3597-3605.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mansfied J, Genin S, Magor S, Citovsky V, Sriariyanum M, Ronald P, Dow M, Verdier V, Beer S, Machado MA, Toth I, Salmond G, Foster GD. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012;13:614–629. doi: 10.1111/j.1364-3703.2012.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nioni YA, Toyota K. Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes Environ. 2015;30:1–11. doi: 10.1264/jsme2.ME14144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang R, Zhang HC, Sun LG, Qi GF, Chen S, Zhao XY. Microbial community composition is related to soil biological and chemical properties and bacterial wilt outbreak. Sci. Rep. 2017;7:343. doi: 10.1038/s41598-017-00472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu B, Wang X, Yang L, Yang H, Zeng H, Qiu Y, Wang CJ, Yu J, Li JP, Xu DH, He ZL, Chen SW. Effects of Bacillus amyloliquefaciens ZM9 on bacterial wilt and rhizosphere microbial community of tobacco. Appl. Soil Ecol. 2016;103:1–12. doi: 10.1016/j.apsoil.2016.03.002. [DOI] [Google Scholar]
- 6.Xiong W, Li ZG, Liu HJ, Xue C, Zhang RF, Wu HS, Li R, Shen QR. The effect of long-term continuous cropping of black pepper on soil bacterial community as determined by 454 pyrosequencing. PLoS ONE. 2015;10:e0136946. doi: 10.1371/journal.pone.0136946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whipps JM, Notes A. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2011;52:487–511. doi: 10.1093/jxb/52.suppl_1.487. [DOI] [PubMed] [Google Scholar]
- 8.Grosch R, Dealtry S, Schreiter S, Berg G, Mendonca-Hagler L, Smalla K. Biocontrol of Rhizoctonia solani: complex interaction of biocontrol strains, pathogen and indigenous microbial community in the rhizosphere of lettuce shown by molecular methods. Plant Soil. 2012;361:343–357. doi: 10.1007/s11104-012-1239-y. [DOI] [Google Scholar]
- 9.Alabouvettec C, Olivain C, Migheli Q, Steinberg C. Microbiological control of soil-brone phytopathogenic fungi with special emphasis on wilt-inducing Fusarium oxysporum. New Phytol. 2009;184:529–544. doi: 10.1111/j.1469-8137.2009.03014.x. [DOI] [PubMed] [Google Scholar]
- 10.Igiehon NO, Babalola OO. Biofertilizers and sustainable agriculture: Exploring arbuscular mycorrhizal fungi. Appl. Microbiol. Biot. 2017;101:4871–4884. doi: 10.1007/s00253-017-8344-z. [DOI] [PubMed] [Google Scholar]
- 11.Pal KK, Tilak KVBR, Saxcna AK, Dey R, Singh CS. Suppression of maize root diseases caused by Macrophomina phaseolina, Fusarium moniliforme and Fusarium graminearum by plant growth promoting rhizobacteria. Microbiol. Res. 2001;156:209–223. doi: 10.1078/0944-5013-00103. [DOI] [PubMed] [Google Scholar]
- 12.Wang XB, Liang GB. Control efficacy of an endophytic Bacillus amyloliquefaciens strain BZ6–1 against peanut bacterial Wilt Ralstonia solanacearum. Biomed Res. Int. 2014;465435:1–11. doi: 10.1155/2014/465435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thurman JH, Crowder DW, Northfield TD. Biological control agents in the Anthropocene: Current risks and future options. Curr. Opin. Insect. Sci. 2017;23:59–64. doi: 10.1016/j.cois.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Zhang N, Wu K, He X, Li SQ, Zhang ZH, Shen B, Yang XM, Zhang RF, Huang QW, Shen QR. A new bioorganic fertilizer can effectively control banana wilt by strong colonization with Bacillus subtilisN11. Plant Soil. 2011;344:87–97. doi: 10.1007/s11104-011-0729-7. [DOI] [Google Scholar]
- 15.Wu K, Yuan SF, Wang LL, Shi JX, Zhao J, Shen B, Shen QR. Effects of bio-organic fertilizer plus soil amendment on the control of tobacco bacterial wilt and composition of soil bacterial community. Biol. Fertil. Soils. 2014;50:961–971. doi: 10.1007/s00374-014-0916-9. [DOI] [Google Scholar]
- 16.Mendes R, Garbeva P, Raaijmakers JM. The rhizosphere microbiome: Signifificance of plant benefificial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013;37:634–663. doi: 10.1111/1574-6976.12028. [DOI] [PubMed] [Google Scholar]
- 17.Jin,Y. Q, Zhu, H. F., Luo, S., Yang, W. W., Zhang, L., Li, S.S., Jin, Q., Cao, Q., Sun, S. R. & Xiao, M. Role of maize root exudates in promotion of colonization of bacillusvelezensis strain s3–1 in rhizosphere soil and root tissue. Curr. Microbiol. 76, 855–862 (2019). [DOI] [PubMed]
- 18.Liu YX, Shi JX, Feng YG, Yang XM, Li X, Shen QR. Tobacco bacterial wilt can be biologically controlled by the application of antagonistic strains in combination with organic fertilizer. Biol. Fert. Soils. 2013;49:447–464. doi: 10.1007/s00374-012-0740-z. [DOI] [Google Scholar]
- 19.Shi K, Wang L, Zhou YH, Yu YL, Yu JQ. Effects of calcium cyanamide on soil microbial community and Fusarium oxysporum f. sp. Cucumberinum. Chemosphere. 2009;75:872–877. doi: 10.1016/j.chemosphere.2009.01.054. [DOI] [PubMed] [Google Scholar]
- 20.He K, Yang S, Li H, Wang H, Li Z. Effects of calcium carbonate on the survival of Ralstonia solanacearum in soil and control of tobacco bacterial wilt. Eur. J. Plant Pathol. 2014;140:665–675. doi: 10.1007/s10658-014-0496-4. [DOI] [Google Scholar]
- 21.Harada KM, Tanaka K, Fukuda Y, Hashimoto W, Murara K. Paenibacillus sp. strain HC1 xylanases responsible for degradation of rice bran hemicellulose. Microbiol. Res. 2008;163:293–298. doi: 10.1016/j.micres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Choi HW, Chung IM, Sin MH, Kim YS, Sim JB, Kim JW, Kim KD, Chun SC. The effect of spent mushroom sawdust compost mixes, calcium cyanamide and solarization on basal stem rot of the cactus Hylocereus Trigonus caused by Fusarium oxysporum. Crop Prot. 2007;26:162–168. doi: 10.1016/j.cropro.2006.04.017. [DOI] [Google Scholar]
- 23.Chen S, Qi GF, Ma GQ, Zhao XY. Biochar amendment controlled bacterial wilt through changing soil chemical properties and microbial community. Microbiol. Res. 2020;231:126–373. doi: 10.1016/j.micres.2019.126373. [DOI] [PubMed] [Google Scholar]
- 24.Cookson WR, Abaye DA, Marschner P, Murphy DV, Stockdale EA, Goulding KWT. The contribution of soil organic matter fractions to carbon and nitrogen mineralization and microbial community size and structure. Soil Biol. Biochem. 2005;37:1726–1737. doi: 10.1016/j.soilbio.2005.02.007. [DOI] [Google Scholar]
- 25.Zheng BX, Hao XL, Ding K, Zhou GW, Chen QL, Zhang JB, Zhu YG. Long-term nitrogen fertilization decreased the abundance of inorganic phosphate solubilizing bacteria in an alkaline soil. Sci. Rep. 2017;7:42284. doi: 10.1038/srep42284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimitriu PA, Grayston SJ. Relationship between soil properties and patterns of bacterial β-diversity across reclaimed and natural boreal forest soils. Microb. Ecol. 2010;59:563–573. doi: 10.1007/s00248-009-9590-0. [DOI] [PubMed] [Google Scholar]
- 27.Leff JW, et al. Consistent responses of soil microbial community to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA. 2015;112:10967–10972. doi: 10.1073/pnas.1508382112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang HW, Li J, Xiao YH, Gu YB, Liu HW, Liang YL, Liu XD, Hu J, Meng DL, Yin HQ. An Integrated insight into the relationship between soil microbial community and tobacco bacterial wilt disease. Front. Microbiol. 2017;8:2179. doi: 10.3389/fmicb.2017.02179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen ZZ, Wang DS, Ruan YZ, Xue C, Zhang J, Li R, Shen QR. Deep 16S rRNA pyrosequencing reveals a bacterial community associated with banana Fusarium wilt disease suppression induced by bio-organic fertilizer application. PLoS ONE. 2014;9:e98420. doi: 10.1371/journal.pone.0098420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu YP, Wu JX, Ma YJ, Lian Y, Sun H, Xie DC, Li YY, Brookes PC, Yao HY. Dynamic changes in soil chemical properties and microbial community structure in response to different nitrogen fertilizers in an acidified celery soil. Soil Ecol. Lett. 2019;1:105–113. doi: 10.1007/s42832-019-0012-z. [DOI] [Google Scholar]
- 31.Kinkel LL, Schlatter DC, Bakker MG, Arenz BE. Streptomyces competition and co-evolution in relation to plant disease suppression. Res. Microbiol. 2012;163:490–499. doi: 10.1016/j.resmic.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Xu CY, Bai SH, Hao Y, Rachaputi RCN, Xu Z, Wallace HM. Peanut shell biochar improves soil properties and peanut kernel quality on a red Ferrosol. J. Soil. Sedim. 2015;15:2220–2231. doi: 10.1007/s11368-015-1242-z. [DOI] [Google Scholar]
- 33.Saijai S, Ando A, Inukai R, Shinohara M, Ogawa J. Analysis of microbial community and nitrogen transition with enriched nitrifying soil microbes for organic hydroponics. Biosci. Biotechnol. Biochem. 2016;80:2247–2254. doi: 10.1080/09168451.2016.1200459. [DOI] [PubMed] [Google Scholar]
- 34.Liu XT, Bolla K, Ashforth EJ, Zhuo Y, Gao H, Huang P, Stanley SA, Huang DT, Zhang LX. Systematics-guided bioprospecting for bioactive microbial natural products. Antonie Van Leeuwenhoek. 2012;101:55–66. doi: 10.1007/s10482-011-9671-1. [DOI] [PubMed] [Google Scholar]
- 35.Li S, Wu FZ. Diversity and co-occurrence patterns of soil bacterial and fungal communities in seven intercropping systems. Front. Microbiol. 2018;9:1521–1533. doi: 10.3389/fmicb.2018.01521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen W, Teng Y, Li ZG, Liu WX, Ren WJ, Luo YM, Christie P. Mechanisms by which organic fertilizer and effective microbes mitigate peanut continuous cropping yield constraints in a red soil of south China. Appl. Soil Ecol. 2008;28:23–34. [Google Scholar]
- 37.Sakai M, Hosoda A, Ogura K, Ikenaga M. The growth of Steroidobacter agariperforans sp nov., a novel agar-degrading bacterium isolated from soil, is enhanced by the diffusible metabolites produced by bacteria belonging to rhizobiales. Microb. Environ. 2014;29:89–95. doi: 10.1264/jsme2.ME13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee KCY, Morgan XC, Dunfield PF, Tamas I, McDonald IR, Stott MB. Genomic analysis of Chthonomonas calidirosea, the first sequenced isolate of the phylum Armatimonadetes. ISME J. 2014;8:1522–1533. doi: 10.1038/ismej.2013.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albuquerque L, Costa MS. The family Gaiellaceae. In: Rosenberg E, editor. The Prokaryotes Actinobacteria. Berlin: Springer; 2014. pp. 357–360. [Google Scholar]
- 40.Qihui H, Ilana KG. Harvesting the complex pathways of antibiotic production and resistance of soil bacilli for optimizing plant microbiome. FEMS Microbiol. Ecol. 2020;96:142. doi: 10.1093/femsec/fiaa142. [DOI] [PubMed] [Google Scholar]
- 41.Marian M, Nishioka T, Koyama H, Suga H, Shimizu M. Biocontrol potential of Ralstonia sp. TCR112 and Mitsuaria sp. TWR114 against tomato bacterial wilt. Appl. Soil Ecol. 2018;128:71–80. doi: 10.1016/j.apsoil.2018.04.005. [DOI] [Google Scholar]
- 42.Liu HX, Li SM, Luo MY, Luo LX, Li JQ, Guo JH. Biological control of Ralstonia wilt, Phytophthora blight, Meloidogyne root-knot on bell pepper by the combination of Bacillus subtilis AR12, Bacillus subtilis SM21 and Chryseobacterium sp. R89. Eur. J. Plant Pathol. 2014;139:107–116. doi: 10.1007/s10658-013-0369-2. [DOI] [Google Scholar]
- 43.Ramesh R, Joshi AA, Ghanekar MP. Pseudomonads: major antagonistic endophytic bacteria to suppress bacterial wilt pathogen, Ralstonia solanacearum in the eggplant (Solanum melongena L.) World J. Microbiol. Biotechnol. 2009;25:47–55. doi: 10.1007/s11274-008-9859-3. [DOI] [Google Scholar]
- 44.Badri DV, Weir TL, van der Lelie D, Vivanco JM. Rhizosphere chemical dialogues: Plant-microbe interactions. Curr. Opin. Biotech. 2009;20:642–650. doi: 10.1016/j.copbio.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 45.Gupta RS, Gao B. Phylogenomic analyses of clostridia and identification of novel protein signatures that are specific to the genus Clostridium sensu stricto (cluster I) Int. J. Syst. Evol. Microbiol. 2009;59:285–294. doi: 10.1099/ijs.0.001792-0. [DOI] [PubMed] [Google Scholar]
- 46.Xu ZH, Shao JH, Li B, Yan X, Shen QR, Zhang RF. Contribution of bacillomycin D in Bacillus amyloliquefaciens SQR9 to antifungal activity and biofilm formation. Appl. Environ. Microbiol. 2013;79:808–815. doi: 10.1128/AEM.02645-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raza W, Ling N, Yang LD, Huang QW, Shen QR. Response of tomato wilt pathogen Ralstonia solanacearum to the volatile organic compounds produced by a biocontrol strain Bacillus amyloliquefaciens SQR-9. Sci. Rep. 2016;6:24856. doi: 10.1038/srep24856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Innerebner G, Knief C, Vorholt JA. Protection of Arabidopsis thaliana against leaf-pathogenic Pseudomonas syringae by Sphingomonas strains in a controlled model system. Appl. Environ. Microbiol. 2011;77:3202–3210. doi: 10.1128/AEM.00133-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nawangsih AA, Damayanti I, Wiyono S, Kartika JG. Selection and characterization of endophytic bacteria as biocontrol agents of tomato bacterial wilt disease. HAYATI J. Biosci. 2011;18:66–70. doi: 10.4308/hjb.18.2.66. [DOI] [Google Scholar]
- 50.Xue QY, Ding GC, Li SM, Yang Y, Lan CZ, Guo JH, Smalla K. Rhizocompetence and antagonistic activity towards genetically diverse Ralstonia solanacearum strains an improved strategy for selecting biocontrol agents. Appl. Microbiol. Biotechnol. 2013;97:1361–1371. doi: 10.1007/s00253-012-4021-4. [DOI] [PubMed] [Google Scholar]
- 51.Luo LY, Wang P, Zhai ZY, Su P, Tan XQ, Zhang DY, Zhang Z, Liu Y. The effects of Rhodopseudomonas palustris PSB06 and CGA009 with different agricultural applications on rice growth and rhizosphere bacterial community. AMB Expr. 2019;9:173. doi: 10.1186/s13568-019-0897-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.