Abstract
The initiator caspase Dronc is the only CARD-domain containing caspase in Drosophila and is essential for apoptosis. Here, we report that homozygous dronc mutant adult animals are short-lived due to the presence of a poorly developed, defective and leaky intestine. Interestingly, this mutant phenotype can be significantly rescued by enteroblast-specific expression of dronc+ in dronc mutant animals, suggesting that proper Dronc function specifically in enteroblasts, one of four cell types in the intestine, is critical for normal development of the intestine. Furthermore, enteroblast-specific knockdown of dronc in adult intestines triggers hyperplasia and differentiation defects. These enteroblast-specific functions of Dronc do not require the apoptotic pathway and thus occur in a non-apoptotic manner. In summary, we demonstrate that an apoptotic initiator caspase has a very critical non-apoptotic function for normal development and for the control of the cell lineage in the adult midgut and therefore for proper physiology and homeostasis.
Subject terms: Cell biology, Genetics
Introduction
Caspases are important executioners of programmed cell death (apoptosis) in multicellular organisms. They encode highly specific Cys proteases which are produced as inactive zymogens in living cells. There are two different types of caspases. Initiator caspases (Caspase-8, Caspase-9 and Drosophila Dronc) act upstream of effector caspases (Caspase-3, Caspase-7 and Drosophila DrICE)1,2. Initiator caspases are distinguished from effector caspases by the presence of long prodomains that harbour protein/protein interaction domains such as the CARD (caspase activation and recruitment domain) in Caspase-9 and Dronc. In Drosophila, activation of Dronc proceeds through CARD/CARD interactions with the adaptor protein Dark3–6 which leads to formation of the apoptosome7–9. In the apoptosome, Dronc cleaves and activates effector caspases such as DrICE1,2,10. After activation, these effector caspases cleave a large number of cellular proteins initiating the demise of the cell.
Homozygous dronc mutants are strongly semi-lethal. Most of them die during pupal development, but at a very low frequency (< 1% of the expected offspring) homozygous escaper flies can be recovered. These escapers exhibit down-curved, opaque wings11. This phenotype is likely the result of failed apoptosis during wing maturation11. Other than the wing phenotype, any other obvious phenotype of homozygous adult dronc mutants have not been reported. However, they do have a very short life-span. They only live for about 3–4 days after eclosion. The cause for this short life-span is not known and will be examined in this study.
In addition to the apoptotic function of caspases, there is a growing list of non-apoptotic functions in basic cellular processes such as proliferation, differentiation, cell migration, sperm maturation and others12–14. When these processes require non-apoptotic caspase function, they are also referred to as caspase-dependent non-lethal cellular processes (CDPs)15. Very sensitive reporters of caspase activity have revealed that many cells experience caspase activity at one point in their life without triggering apoptosis16–18. How cells escape the potentially cell lethal activity of active caspases is currently subject of intense research.
The Drosophila midgut has emerged as a valuable model to study adult stem cells. It is composed of only 4 different cell types: intestinal stem cells (ISC), enteroblasts (EB), enterocytes (EC) and secretory enteroendocrine cells (EE)19–21 (reviewed in22). The ISCs are the only proliferating cells in the midgut. EBs are produced by asymmetric division of ISCs and are transient cells which differentiate into ECs or EEs19–21. ECs are absorptive epithelial cells and make up the majority of the cells in the midgut. More recently, it has also been suggested that EEs can be directly generated by asymmetric ISC division without going through an EB intermediate23–25.
The cell lineage in the midgut is under strict control to ensure proper function and homeostasis of the midgut (reviewed in22,26). ECs and EEs are regularly turned over and need to be replaced by new cells due to ISC mitosis. There is feedback from dying EC cells to control ISC activity27. Imbalances of this control can lead to malfunction of the intestine, dysplasia, premature aging and death of the animal. Because mammalian intestines are also subject to a similar cell lineage28, a clear understanding of the mechanisms involved in the control of this cell lineage may help to understand disease and identify potential targets for the cure of the disease.
Here, we show that the initiator caspase Dronc has essential functions for development and homeostasis of the Drosophila adult midgut. Interestingly, this function is primarily required in EBs and appears to be non-apoptotic in nature. Loss of dronc in EBs causes hyperplasia due to increased proliferation. Furthermore, there are significant differentiation defects. Specifically, loss of dronc results in an increased number of EBs and accumulation of cells that have features of both EBs and ECs. There is also a significant increase in the number of EEs. These data demonstrate that an apoptotic initiator caspase has a very critical function for the control of the cell lineage in the adult midgut and therefore for proper physiology and homeostasis.
Results
Homozygous dronc mutants die prematurely due to fragile and leaky intestines
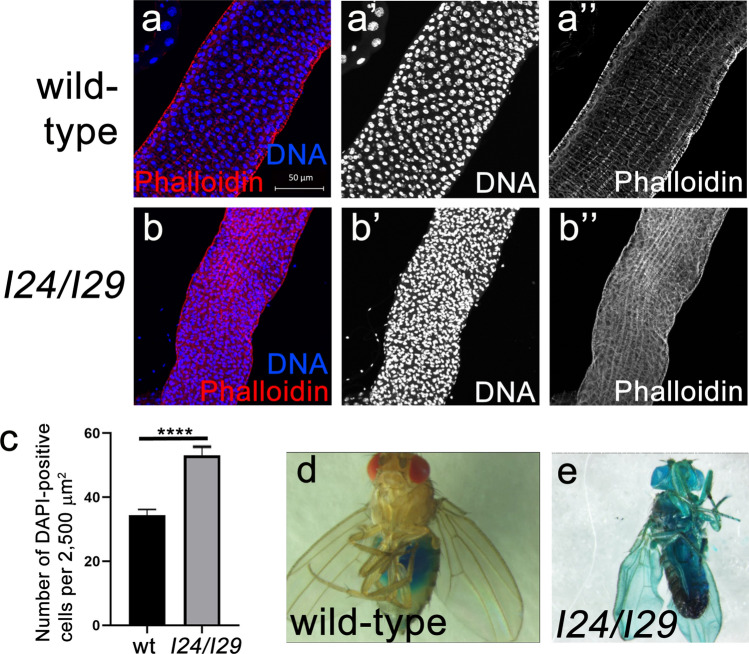
Homozygous dronc mutants are pupal lethal. However, at a very low frequency (less than 1% of the expected offspring), adult homozygous droncI24/droncI29 mutant flies can be recovered. These dronc alleles carry premature stop codons at codons 28 and 53 and encode strong, if not null, alleles of dronc11. With the exception of down-curved and opaque wings11, these mutant flies do not have any obvious phenotypic abnormalities. Nevertheless, they are very short-lived and die within 3 to 4 days after eclosion suggesting that they may have some internal defects. To identify the possible cause of this premature death, we examined the internal organs of these mutants. When dissecting the intestines of droncI24/droncI29 flies, we noticed that they are very fragile. By phallodin labelings, these intestines displayed structural irregularities (Fig. 1a,b). Furthermore, labelings with the nuclear dye DAPI show that the mutant intestines have a higher density of cells (Fig. 1a’,b’; quantified in c).
Figure 1.
Homozygous droncI24/droncI29 mutants have defective and leaky intestines. (a–c) DAPI- and Phalloidin-labeled wild-type (Canton S) and droncI24/droncI29 (I24/I29) intestines. The examples shown are taken from Region R4ab, but are representative for the entire intestine. Scale bar: 50 µm. (c) Quantification of the DAPI labelings in wt and droncI24/droncI29 intestines. The density of nuclei in 2500 μm2 fields was analysed by unpaired t test, two tailored and plotted ± SEM. ****p < 0.0001. n = 6 (wt), 5 (droncI24/droncI29). (d,e) Wild-type (Canton S) and droncI24/droncI29 mutant animals, subjected to a SMURF assay.
To examine if these structural defects cause a dysfunction of the intestine, we performed SMURF assays with homozygous droncI24/droncI29 flies. In a SMURF assay, a blue dye is mixed into the food and fed to the flies29,30. Flies with an intact intestine keep the blue food in the intestine which can be easily seen through the abdominal cuticle (Fig. 1d). However, in flies with an intestinal barrier dysfunction, the blue dye penetrates into every tissue of the fly, generating a SMURF phenotype30. We examined five homozygous droncI24/droncI29 mutants in the SMURF assay and all of them displayed the SMURF phenotype (Fig. 1e). Furthermore, while wild-type flies start feeding almost immediately, we noticed that droncI24/droncI29 mutant flies do not feed for the first 24 to 48 h after eclosion. These data suggest that dronc mutant intestines have structural defects and a defective barrier function causing a leaky gut.
EB-specific expression of dronc rescues semi-lethality and restores gut function of dronc mutants
To determine if the defective intestine causes the premature organismal lethality of homozygous droncI24/droncI29 mutants, we asked if cell-type specific expression of UAS-droncwt can rescue the strong semi-lethality and short lifespan as well as the defective and leaky gut phenotype of homozygous droncI24/droncI29 animals. As a control, we expressed the catalytic UAS-droncC318A mutant. Interestingly, expression of droncwt in EBs using the EB-specific driver Su(H)GBE-Gal4 (from now on Su(H)) gave the best rescue of the lethality (Fig. 2a; genotype 3). About 75% of the expected Su(H) > droncwt; droncI24/droncI29 progeny was recovered as adults. With the esg-Gal4 driver, which is expressed in ISCs and to some extent in EBs, a weaker rescue was recorded (Fig. 2a; genotype 2). The weakest rescue was scored when droncwt was expressed in mature ECs using NP1-Gal4 (Fig. 2a; genotype 4). Expression of the catalytic mutant droncC318A using all three Gal4 drivers was not able to rescue the lethality of droncI24/droncI29 animals (Fig. 2a; genotypes 5–7). These rescue crosses suggest that for development of the intestine, Dronc function is most critical in EBs and requires its catalytic activity.
Figure 2.
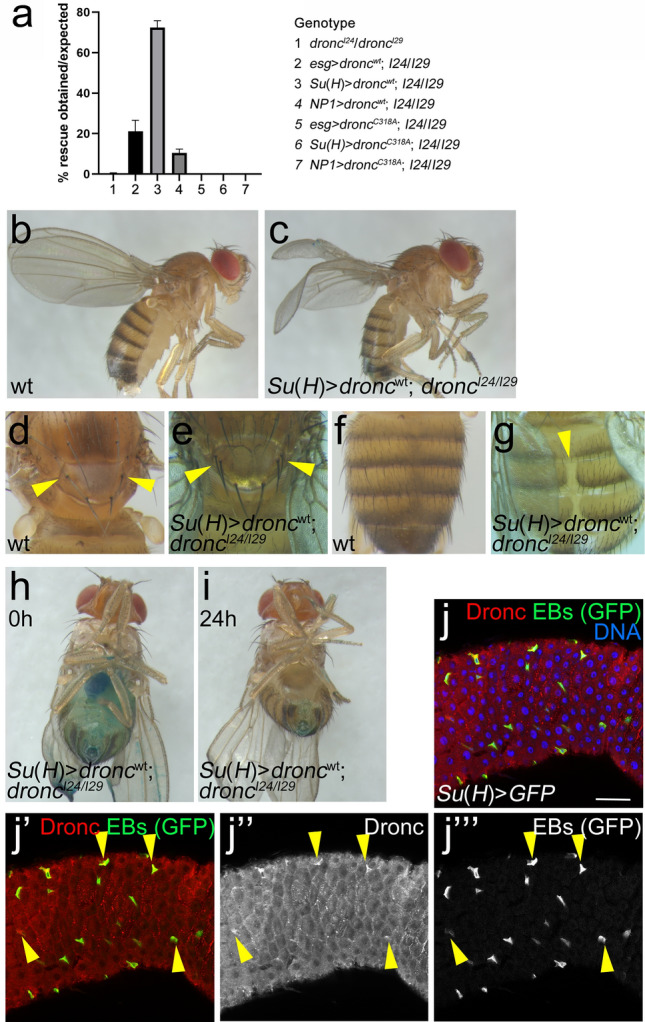
Enteroblast-specific expression of droncwt rescues the semi-lethality and leakage of homozygous dronc mutant animals. (a) esg-Gal4 (expression in ISCs), Su(H)-Gal4 (EBs) and NP1-Gal4 (ECs) were used to drive expression of UAS-droncwt or UAS-droncC318A in droncI24/droncI29 (I24/I29) mutants and survival was scored. droncC318A carries a mutation of the catalytic Cys318 residue to an Ala and represents a catalytic mutant. droncI24/droncI29 (genotype 1) is strongly pupal semi-lethal and less than 1% of the expected progeny can be recovered as adults. The obtained progeny in the rescue crosses is plotted as the percentage of the expected Mendelian progeny. Genotypes of expected rescued progeny are indicated on the right. The average of three independent experiments is shown. At least 200 flies were counted in each experiment. Rescue data were analyzed by one-way ANOVA with Holm-Sidak test for multiple comparisons. Error bars are SEM. (b,c) While the wings of wild-type (Canton S) flies are straight (b), the wings of Su(H) > droncwt; droncI24/droncI29 rescued flies are down-curved (c) similar to droncI24/droncI29 mutants 11. (d-g) Su(H) > droncwt; droncI24/droncI29 flies exhibit morphological phenotypes. Canton S (wt) flies contain 4 scutellar bristles (macrochaetae) (d) and have a closed abdomen (f). All Su(H) > droncwt; droncI24/droncI29 rescued flies have duplications of 1 or 2 macrochaetae (d, arrowheads) and about 50% display a split abdomen phenotype (g, arrowhead) (n > 50). (h,i) 25 out of 26 tested Su(H) > droncwt; droncI24/droncI29 show a wild-type SMURF phenotype (h) (compare to Fig. 1d,e). 24 h after removal from blue food, these flies have completely cleared the gut of blue food (i). (j) Labeling of Su(H) > GFP midguts with anti-Dronc antibodies (red in j and j’; grey in j’’). EBs are labelled by GFP (green in j and j’; grey in j’’’). Yellow arrows highlight several EBs containing Dronc protein. Scale bar: 50 µm. See also Supplementary Fig. S1.
However, these data have the caveat that the Gal4 drivers used are also expressed in other tissues during development. We can therefore not conclude, that the exclusive expression of dronc in EBs is sufficient for normal development and survival. Nevertheless, EB-specific expression of dronc does not rescue all known phenotypes of dronc mutants. The wings of Su(H) > droncwt; droncI24/droncI29 flies still have the reported dronc mutant phenotype (down-curved, opaque wings) (Fig. 2b,c). Furthermore, we observed one to two additional scutellar bristles (macrochaetae) with 100% penetrance (Fig. 2d,e) which had been reported for dark and cytochrome c-d mutants31,32 and represents a typical phenotype when cell death is blocked. We also recovered Su(H) > droncwt; droncI24/droncI29 flies with a split abdomen phenotype at reduced penetrance (Fig. 2f,g). The split abdomen phenotype is likely the result of reduced cell death of larval cells in the abdomen during pupal development33. Therefore, despite the caveat that Su(H)-Gal4 expression is not restricted to EBs in the midgut, Su(H)-driven expression of dronc cannot rescue all phenotypes of droncI24/droncI29 mutants, demonstrating that expression of dronc in select groups of cells is sufficient for development and survival of the animal.
To directly examine the effect of EB-specific expression of dronc on the physiology of the intestine, we performed SMURF assays with Su(H) > droncwt; droncI24/droncI29 rescued animals. Of 26 rescued flies which were tested in this assay, only one animal developed a SMURF phenotype. The intestines of the other 25 flies stayed intact and were also able to clear the blue food within 24 h after they were removed from the blue food (Fig. 2h,i). In contrast, 6 out of 7 tested esg > droncwt; droncI24/droncI29 flies (Fig. 2a, genotype 2) developed a SMURF phenotype.
Although we did not perform extended life span assays, Su(H) > droncwt; droncI24/droncI29 flies lived significantly longer (> 2 weeks) than droncI24/droncI29 flies which live only for 3–4 days after eclosion. The life span of esg > droncwt; droncI24/droncI29 also improved, but most of them died after about 1 week.
Given the critical role of Dronc in the adult intestine, in particular in EBs, we examined if Dronc is expressed in these cells using a Dronc-specific antibody. To identify EBs, we expressed GFP using the EB-specific driver Su(H)-Gal4. This analysis reveals that Dronc is expressed in EBs (Fig. 2j, yellow arrowheads) as well as in other cell types as demonstrated recently34. A specificity control of the anti-Dronc antibody is shown in Supplementary Fig. S1. Taken together, these results indicate that Dronc function in the intestine, in particular in EBs, is essential for intestinal integrity and organismal survival of the animal.
Down-regulation of dronc in EB cells causes hyperplasia
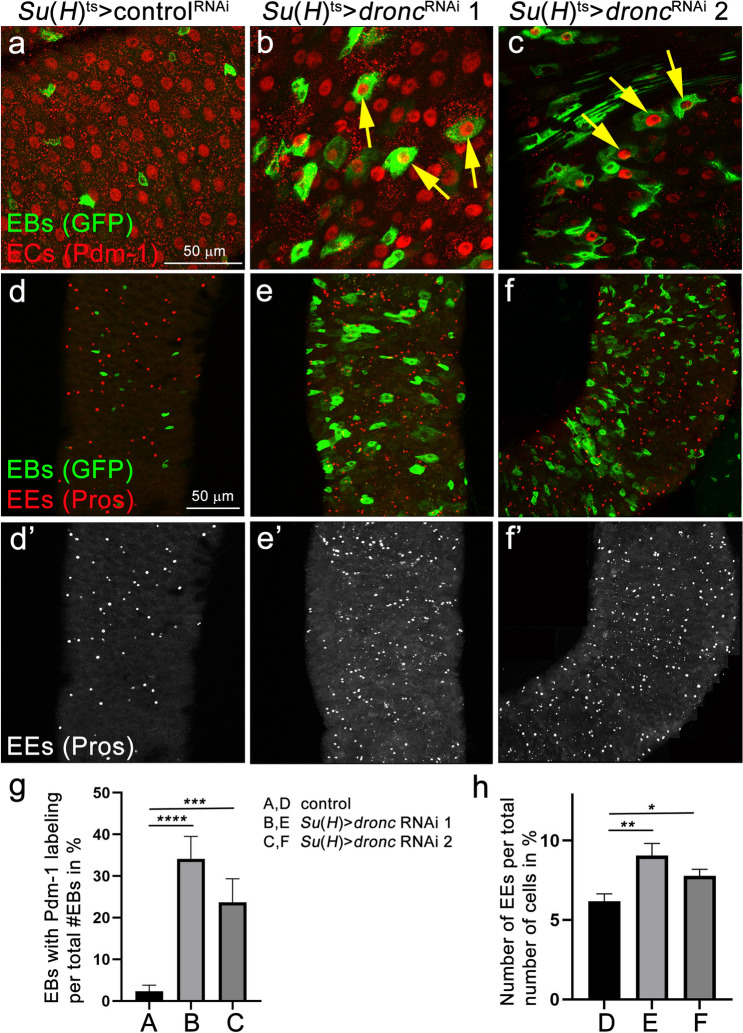
The above data indicate that dronc has a very important function in EB cells for proper formation of the intestine during development. Additionally, we examined if dronc also has an important role for homeostasis of the adult midgut. For that purpose, we down-regulated dronc in EB cells by RNAi, using Su(H)-Gal4ts. The ts in this annotation indicates the presence of Gal80ts which allows down-regulation of dronc using this Gal4 driver by temperature shift to 29 °C after the animals have fully developed and eclosed. 5 days old Su(H)ts > droncRNAi flies were shifted from 18 to 29 °C and their intestines were analysed 5–6 days later. Preferentially, region R4ab of the posterior midgut was examined in these assays35,36. There is a significant increase in the total number of cells in the midgut (as revealed by DAPI labelings (Fig. 3a–c’’, quantified in d)), and GFP-positive EB cells were overabundant compared to controls (Fig. 3a’–c’, quantified in e). Consistent results were obtained with 2 independent dronc RNAi lines (Fig. 3b–e). In addition to the EB overabundance of Su(H)ts > droncRNAi midguts, EBs (marked by GFP) also appear to change shape and are much larger than normal (Fig. 3a’–c’). This observation is also statistically significant (Fig. 3f).
Figure 3.
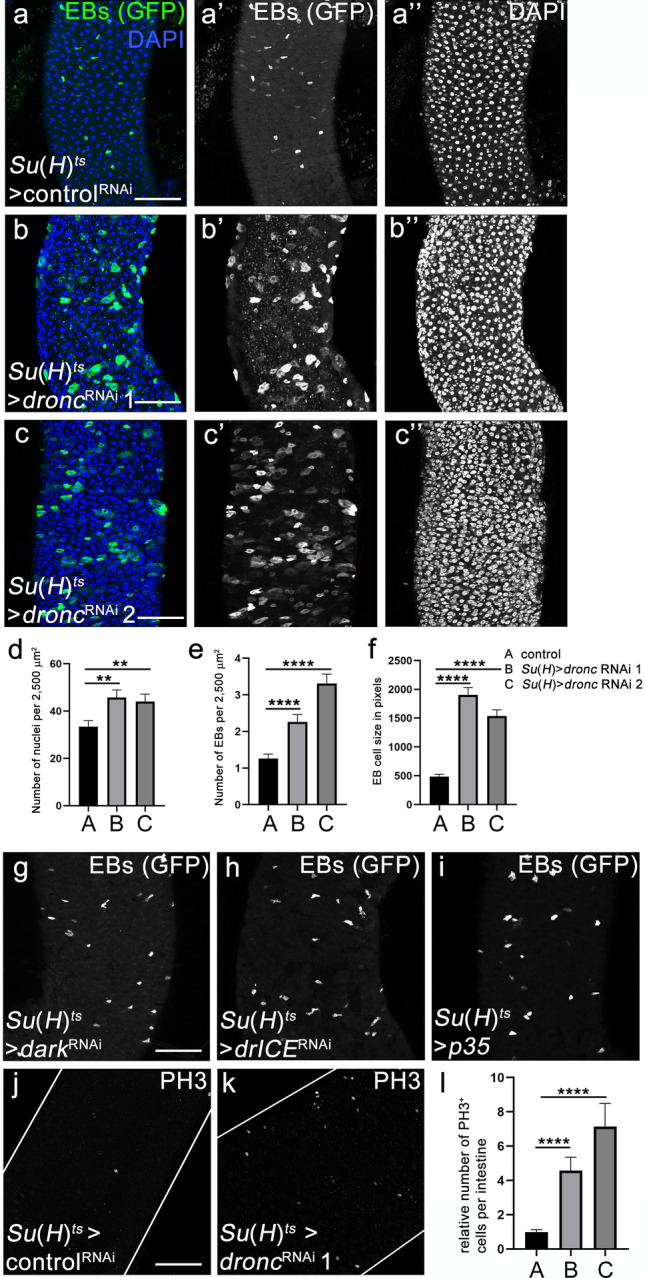
EB-specific knockdown of dronc causes accumulation of EBs and hyperplasia. In this and the following figure, crosses were raised at 18 °C until adults eclosed. 5 days old females of the indicated genotype were shifted to 29 °C and incubated for 5–6 days until dissection and labelling of the midguts. Two different dronc RNAi transgenes were used: line 1 = P{GD12376}v23033; line 2 = P{KK104278}v100424. Luciferase RNAi was used as a control in (a). (a–c) Control (a) and Su(H)ts > dronc RNAi 1 and 2 (b,c) midguts were labelled with GFP (green in a–c; grey in a’–c’) and DAPI (blue in a-c; grey in a’’–c’’). Note that in addition to an increased number of EBs in response to dronc RNAi, these cells also underwent morphological changes and appear much larger compared to control (a). Scale bar: 50 µm. Complete genotypes: (a) Su(H)-Gal4 UAS-GFP tub-Gal80ts; UAS-luciferase RNAi; (b) Su(H)-Gal4 UAS-GFP tub-Gal80ts; UAS-dronc RNAi 1; (c) Su(H)-Gal4 UAS-GFP tub-Gal80ts; UAS-dronc RNAi 2. (d,e) Quantification of the numbers of nuclei (DAPI) and EBs (GFP) of the genotypes in (a–c). To obtain DAPI counts, at least five random, but representative fields of 2500 μm2 each per posterior midgut were counted. In the case of EBs, all GFP-positive cells (corresponding to EBs) were counted in region R4ab and normalized to 2500 μm2 fields. Data were analyzed by one-way ANOVA with Holm-Sidak test for multiple comparisons. Error bars are SEM. ****p < 0.0001; **p < 0.01. Number of midguts analysed: n = 12 (control), 9 (dronc RNAi 1), 12 (dronc RNAi 2). (f) Quantification of the cell size of EBs of control and dronc RNAi midguts. Shape of EBs was outlined using the quick selection tool in Photoshop and the number of pixels in the selected area was recorded. Data were analyzed by one-way ANOVA with Holm-Sidak test for multiple comparisons. Error bars are SEM. ****p < 0.0001. n = 24 (control), 25 (dronc RNAi 1), 24 (dronc RNAi 2). (g–i) EB-specific knockdown of dark (g) and drICE (h) by RNAi or overexpression of p35 (i) does not phenocopy the phenotypes observed by dronc RNAi (compare to (b,c)). More examples and quantifications are shown in Supplementary Fig. S2. EBs were labelled with GFP. The conditions were the same as in (a–c). Scale bars: 50 µm. Complete genotypes: (g) Su(H)-Gal4 UAS-GFP tub-Gal80ts; UAS-dark RNAi; (h) Su(H)-Gal4 UAS-GFP tub-Gal80ts; UAS-drICE RNAi; (i) Su(H)-Gal4 UAS-GFP tub-Gal80ts; UAS-p35. (j,k) PH3 labelings of control and Su(H)ts > dronc RNAi midguts. Scale bar: 50 µm. Complete genotypes: (j) Su(H)-Gal4 UAS-GFP tub-Gal80ts; UAS-luciferase RNAi; (k) Su(H)-Gal4 UAS-GFP tub-Gal80ts; UAS-dronc RNAi (P{GD12376}v23033). (l) Quantification of PH3 labelings of control and Su(H)ts > dronc RNAi midguts. PH3 cells were manually counted across the entire intestine and analysed by one-way ANOVA with Holm-Sidak test for multiple comparisons. Error bars are SEM. ****p < 0.0001. n = 10 (control), 11 (dronc RNAi 1), 10 (dronc RNAi 2). Genotypes as in (a–c). See also Supplementary Figs. S2 and S3.
Given that dronc has an important function in apoptosis11,37–39, we considered the possibility that this EB-specific dronc phenotype is caused by loss of apoptosis. However, we did not observe a similar EB-overabundance phenotype in response to EB-specific (using Su(H)-Gal4ts) dark RNAi (Fig. 3g; see additional example and quantification in Supplementary Fig. S2), which encodes the adaptor protein for incorporation of Dronc into the apoptosome during apoptosis3,4,6. EB-specific RNAi targeting drICE, the most important effector caspase in Drosophila40,41, also did not phenocopy the dronc RNAi phenotype (Fig. 3h; Supplementary Fig. S2). Finally, EB-specific expression of the effector caspase inhibitor p35 in otherwise wild-type midguts using Su(H)-Gal4ts did not replicate the dronc RNAi phenotype (Fig. 3i; Supplementary Fig. S2). EB-specific darkRNAi, drICERNAi and p35 expression also did not phenocopy other aspects of the droncRNAi phenotype such as the increase in overall cell number and in EB cell size (Supplementary Fig. S2). Therefore, we can rule out that the Su(H)ts > droncRNAi phenotypes in adult midguts are caused entirely by loss of apoptosis. The functionality of the darkRNAi, drICERNAi and p35 transgenic lines was validated by the ability of these lines to suppress the GMR-reaper eye ablation phenotype, a commonly used apoptosis model42 (Supplementary Fig. S2).
It was previously shown that an accumulation of EBs can cause increased ISC proliferation43,44. Consistently, based on PH3-labeling experiments, we found that cell proliferation is increased in Su(H)ts > droncRNAi midguts (Fig. 3j,k). This was confirmed with two dronc RNAi lines (Fig. 3m). In contrast, EB-specific darkRNAi, drICERNAi and p35 expression did not phenocopy this phenotype (Supplementary Fig. S2) suggesting that dronc controls cell proliferation in a non-apoptotic manner. Combined, these observations suggest that EB-specific dronc RNAi triggers a hyperplastic phenotype in the intestine. Consistently also, mosaic analysis of adult intestines with droncI24 and droncI29 showed that dronc mutants form larger clones than control clones (Supplemental Fig. S3).
Down-regulation of dronc in EB cells causes differentiation defects
Given that ECs are much larger in size than EBs and that EBs in Su(H) > droncRNAi midguts are significantly enlarged (Fig. 3f), we considered that GFP-positive EBs in droncRNAi midguts also display features of EC cell fate. To examine this possibility, we labelled these midguts with Pdm-1 antibody, a marker for EC fate45,46. Consistently, in Su(H)ts > droncRNAi,GFP midguts we observed multiple examples where Su(H)ts-driven GFP overlaps with Pdm-1 labeling suggesting that these cells have properties of both EBs and ECs (Fig. 4a–c). While in control midguts, expression of Pdm-1 in EBs was also observed at a low frequency (~ 2% of total EBs), this number was significantly higher in Su(H)ts > droncRNAi midguts (Fig. 4g). These data imply that in normal midguts, Dronc is required for the appropriate differentiation of EBs into ECs.
Figure 4.
Down-regulation of dronc in EBs causes differentiation defects.(a-c) Shown are regions R4ab of control midguts (a) and of midguts in which dronc was down-regulated in EB cells using Su(H)-Gal4ts (b,c). These midguts were labelled with GFP (to visualize EBs) and Pdm-1 antibodies which labels nuclei of ECs. Yellow arrows in (b,c) point to GFP/Pdm-1 double positive cells. Two different dronc RNAi transgenes were used: line 1 = P{GD12376}v23033; line 2 = P{KK104278}v100424. Luciferase RNAi was used as a control in (a). Complete genotypes: (a) Su(H)-Gal4 UAS-GFP tub-Gal80ts; UAS-luciferase RNAi; (b,c) Su(H)-Gal4 UAS-GFP tub-Gal80ts/UAS-dronc RNAi line 1 (b)/line 2 (c). Scale bar: 50 µm. (d–f) Prospero (Pros) antibody (red in d–f; grey in d’–f’) was used to label enteroendocrine cells (EE) in regions R4ab of control midguts (d) and midguts in which dronc was down-regulated in EBs using Su(H)-Gal4ts (e,f). EBs are labelled by GFP (green in d–f) due to Su(H) > GFP expression. Genotypes as in (a–c). Scale bar: 50 µm. (g) Quantification of the data in (a–c). GFP/Pdm-1 double positive cells were normalized to the total number of GFP-positive cells and illustrated in %. GFP/Pdm-1 double positive and GFP-positive cells were manually counted and analysed by one-way ANOVA with Holm-Sidak test for multiple comparisons. Error bars are SEM. ****p < 0.0001; ***p < 0.001. Number of midguts analysed: n = 7 (control), 4 (dronc RNAi 1), 4 (dronc RNAi 2). (h) Quantification of the Pros labelings in (d’–f’). All Pros-positive cells (corresponding to EEs) were counted in region R4ab and normalized to the total number of cells. Data were analyzed by one-way ANOVA with Holm-Sidak test for multiple comparisons. Error bars are SEM. **p < 0.01; *p < 0.05. Number of midguts analysed: n = 12 (control), 7 (dronc RNAi 1), 6 (dronc RNAi 2).
We also examined enteroendocrine (EE) cells in Su(H)ts > droncRNAi midguts using the EE marker Prospero (Pros) and observed an increased number of Pros-positive cells (Fig. 4d’–f’; quantified in 4 h), indicating that the number of EEs is elevated. This EE overabundance phenotype was not observed in response to EB-specific darkRNAi, drICERNAi and p35 expression in adult midguts (Supplementary Fig. S2) suggesting that this droncRNAi-dependent phenotype is independent on its role in apoptosis. These observations further indicate that Dronc function in EBs is required for proper differentiation of intestinal cell types.
Discussion
In the absence of dronc during development, the adult intestine has structural defects, is fragile and leaky, limiting the lifespan of homozygous adult escapers to only 3–4 days. Interestingly, expression of dronc specifically in EBs can significantly rescue these phenotypes suggesting that dronc has a very important function in EBs for development of the intestine. Nevertheless, because Su(H)-Gal4 is also expressed in cells outside the intestine, we cannot exclude the possibility that other developmental defects contribute to the lethality of dronc mutant animals. Because the catalytic mutant droncC318A cannot rescue these phenotypes, Dronc likely requires its catalytic activity for proper function in the midgut. The observation that expression of dronc in ECs using NP1-Gal4 cannot significantly rescue the lethality of dronc mutants (Fig. 2a) does not mean that Dronc does not have a function in ECs. It only means that NP1-Gal4 is not expressed in those cells (such as EBs) where dronc has an essential function for survival.
EB-specific knockdown of dronc in adult intestines causes differentiation defects and hyperplasia due to increased proliferation. Therefore, Dronc function is critical for proper control of the cell lineage in the midgut and loss of dronc disrupts this homeostasis. The observation that loss of other genes in the apoptosis pathway (dark, drICE) or overexpression of the effector caspase inhibitor p35 do not replicate the EB-specific dronc phenotypes suggests that Dronc mediates this role in a non-apoptotic manner. Similar results were recently reported by Arthurton et al.47. This adds control of proper proliferation and differentiation of the adult midgut to the growing list of non-apoptotic functions of Dronc.
There are several interesting aspects of the EB-specific dronc phenotypes in the midgut. First, the observed hyperplasia is puzzling. In the Drosophila intestine, only ISCs are mitotically active, EBs are not19,20. In fact, EBs are the daughter cells of the asymmetric division of ISCs. The increased mitotic activity of ISCs in response to EB-specific dronc knockdown suggests that Dronc is involved in a feedback mechanism between EBs and ISCs. Elucidating the molecular mechanism of this feedback mechanism and the role Dronc plays in this will be an exciting avenue for future research.
Second, the increased number of EBs and accumulation of EBs expressing the EC marker Pdm-1 suggests that Dronc is involved in the differentiation process from EBs to ECs. The function of Dronc in this process can be explained in one of two opposite ways. Dronc may be required for an important step in the differentiation process from EBs to ECs. In the absence of dronc, while EBs are able to increase in size and induce expression of the EC marker Pdm-1, they cannot complete the differentiation program into ECs and get stuck along the way. This would result in an increased number of EBs in the intestine. However, the opposite explanation, that Dronc inhibits the differentiation of EBs into ECs under normal conditions, is formally also possible. In that case, the differentiation into ECs occurs so fast, that the EB-specific expression of GFP (which is Su(H)-Gal4 dependent) is not turned off early enough to avoid overlap of EB- and EC-specific markers. Future experiments will clarify by which mechanism Dronc controls the differentiation of EBs to ECs.
Third, the increase of EEs in response to EB-specific knockdown of dronc suggests that Dronc negatively controls the formation and differentiation of EEs. Because it is not clear whether EEs are also differentiating from EBs, as originally suggested19–21, or if they are direct descendants of ISCs23–25, it is not clear whether the role of Dronc in this process is autonomous or non-cell autonomous. Because we do not observe an overlap of EB-specific GFP expression (driven by Su(H)-Gal4) with EE-specific Pros labelling (Fig. 4d–f), suggest a non-cell autonomous control of EE fate by Dronc, but other explanations may be possible, too. In any case, what these data show is that under normal conditions, Dronc controls the number of EEs in an EB-specific manner.
Another important question for the future is how Dronc mediates the homeostatic effect in the adult intestine. There are several possibilities. It was shown that loss of the transcription factor escargot (esg) has similar phenotypes compared to dronc: increased differentiation into ECs and EEs48,49. Esg suppresses the expression of the differentiation-promoting factor Pdm-1 in progenitor cells48. Thus, Dronc may be involved in Esg-mediated control of Pdm-1 expression. Dronc may also be involved in the control of some of the signalling pathways that operate in the differentiation process, such as Notch (N) signalling. Given that N signalling is also controlled by esg48,49, it is possible that Dronc participates in this complex signalling network. However, because many signalling pathways are involved in the control of the cell lineage in the intestine (reviewed in22,26), Dronc may also control any of these pathways. Given that the only known biochemical function of Dronc is proteolytic activity, it will be a challenge in the future to identify the cleavage substrate(s) of Dronc in this process.
Finally, how is Dronc activated in this non-apoptotic context? During apoptosis, activation of Dronc occurs by incorporation into the Dark apoptosome7–9. However, in EBs of the intestine, this occurs in an apoptosome-independent manner because Dark is not involved (Fig. 3g; Supplementary Fig. S2). Dronc may be incorporated into a different protein complex for activation. For example, mammalian initiator caspases can be recruited into different complexes such as the apoptosome and the inflammosome50. A different complex may provide different properties to Dronc such that it does not cleave its apoptotic substrates and can act in a non-apoptotic manner. Alternatively, it is possible that a different protease may cleave and activate Dronc.
Impaired caspase function may also provide a contributing factor for the development of colon cancer in human patients. For example, Caspase-9, the Dronc ortholog in humans, is epigenetically silenced in almost 50% of colon cancer cases51,52. Other caspases, such as Caspase-7 are down-regulated in up to 85% of colon cancer cases51,52. While this silencing is likely a means for evading apoptosis, it could also trigger additional effects such as increased proliferation and differentiation defects, thus further supporting tumorigenesis. Therefore, revealing the mechanism by which Dronc expression in EBs maintains tissue homeostasis may also have important implications for understanding of the tumor-promoting effect of loss of caspase-9 in humans. Given that the function of Dronc for maintaining tissue homeostasis in the intestine is non-apoptotic, characterizing the role of Dronc in EBs does provide a convenient opportunity to study this function in the absence of its apoptotic function which may not be as simple for Caspase-9 in humans.
Materials and methods
Drosophila husbandry
All crosses were performed on standard cornmeal-molasses medium (60 g/L cornmeal, 60 ml/L molasses, 23.5 g/L bakers yeast, 6.5 g/L agar, 4 ml/L acid mix and 0.13% Tegosept). Crosses not involving conditional expression of transgenes were incubated at room temperature. Crosses involving conditional expression of transgenes including RNAi were incubated at 18 °C until adult offspring eclosed. Adults were kept at 18 °C for 5 days before they were incubated at 29 °C for another 5–6 days prior to dissection. Flies were provided fresh, yeasted food every day. Only female midguts were dissected and analysed. SMURF assays were performed as described29 except flies were incubated on blue food for 18 h.
Fly stocks and genetics
Canton S (BL64349) and luciferase RNAi (BL31603) were used as control stocks. The following dronc stocks were used: droncI24 and droncI29 (ref. 11); UAS-droncwt and UAS-droncC318A (ref.53); UAS-dronc RNAi: P{GD12376}v23033 and P{KK104278}v100424 from VDRC. Other stocks used were: UAS-p35 (BL5072); UAS-drICE RNAi (BL32403); UAS-dark RNAi (BL33924), esg-Gal4 (ref.54); Su(H)GBE-Gal4 = Su(H)-Gal4 (ref.55); NP1-Gal4 (ref.56); tub-Gal80ts (ref.57). The stock Su(H)GBE-Gal4 UAS-GFP/CyO; tub-Gal80ts/TM2 is a kind gift of Hsi-Yu Chen (Ip lab).
To induce dronc mosaics in the intestine (Supplemental Fig. S3), the MARCM58 (mosaic analysis with a cell repressible marker) technique was used which positively labels mutant clones with GFP. droncI24 and droncI29 were analysed which both carry FRT80 for mitotic recombination. 6 days old female flies of the correct genotype were heat shocked for 60 min at 37 °C in a water bath. Mosaic flies were kept at 25 °C for another 7 days before dissection.
Dissection and immunolabeling of adult guts
Intact female midguts were dissected using standard protocols59. Primary antibodies were: anti Dronc60 (1:200; a kind gift of Pascal Meier); PH3 (1:2,000; Millipore), Prospero (Pros, 1:20; DSHB; Prospero (MR1A) was deposited to the DSHB by C.Q. Doe); Pdm-161 (1:1,000; a kind gift of Yu Cai). DAPI was used to counterstain nuclei. Phalloidin labeling was used to assess the physical properties of the guts. Secondary antibodies were donkey Fab fragments from Jackson ImmunoResearch. If not noted otherwise, region R4ab35,36 in the posterior midgut was imaged and analysed. Images were obtained with a Zeiss LSM 700 confocal microscope, analysed with Zen 2012 imaging software (Carl Zeiss) and processed with Adobe Photoshop CS6.
Counts of DAPI-, Pdm-1-, Pros- and PH3-positive cells
DAPI-, Pdm-1- and Pros-positive cells were counted manually by detecting signal-positive cells as spots in region R4ab and normalized to areas of 2,500 µm2. PH3 counts were performed across the entire intestine. At least three independent experiments for every genotype were performed. Analysis and graph generation was done using GraphPad Prism 8.30. The statistical method used is indicated in the legends to the respective panels.
Supplementary Information
Acknowledgements
We would like to thank Yu Cai, Fernando Diaz-Benjumea, Tony Ip, Pascal Meier, the Bloomington Drosophila Stock Center (BDSC), the Vienna Drosophila Resource Center (VDRC) and the Developmental Studies Hybridoma Bank (DSHB) for reagents, fly stocks and antibodies. Hsi-Yu (Sylvie) Chen for help with midgut dissections. This work was funded by the National Institute of General Medical Science (NIGMS) under award number R35GM118330. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author contributions
Conceptualization, A.B.; Methodology, J.L.L., A.A.; Formal Analysis, J.L.L.; Investigation, J.L.L., M.T., A.A., A.S.; Resources, A.B.; Writing, A.B.; Supervision, A.B.; Funding Acquisition, A.B.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Code availability
All materials and fly stocks used in this study are available from the corresponding author on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-81261-0.
References
- 1.Salvesen GS, Hempel A, Coll NS. Protease signaling in animal and plant-regulated cell death. FEBS J. 2016;283:2577–2598. doi: 10.1111/febs.13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shalini S, Dorstyn L, Dawar S, Kumar S. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22:526–539. doi: 10.1038/cdd.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanuka H, et al. Control of the cell death pathway by Dapaf-1, a Drosophila Apaf-1/CED-4-related caspase activator. Mol. Cell. 1999;4:757–769. doi: 10.1016/S1097-2765(00)80386-X. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez A, et al. Dark is a Drosophila homologue of Apaf-1/CED-4 and functions in an evolutionarily conserved death pathway. Nat. Cell Biol. 1999;1:272–279. doi: 10.1038/12984. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava M, et al. ARK, the Apaf-1 related killer in Drosophila, requires diverse domains for its apoptotic activity. Cell Death Differ. 2007;14:92–102. doi: 10.1038/sj.cdd.4401931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou L, Song Z, Tittel J, Steller H. HAC-1, a Drosophila homolog of APAF-1 and CED-4 functions in developmental and radiation-induced apoptosis. Mol. Cell. 1999;4:745–755. doi: 10.1016/s1097-2765(00)80385-8. [DOI] [PubMed] [Google Scholar]
- 7.Cheng TC, et al. A near-atomic structure of the dark apoptosome provides insight into assembly and activation. Structure. 2017;25:40–52. doi: 10.1016/j.str.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorstyn L, Akey CW, Kumar S. New insights into apoptosome structure and function. Cell Death Differ. 2018;25:1194–1208. doi: 10.1038/s41418-017-0025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pang Y, et al. Structure of the apoptosome: Mechanistic insights into activation of an initiator caspase from Drosophila. Genes Dev. 2015;29:277–287. doi: 10.1101/gad.255877.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu D, Li Y, Arcaro M, Lackey M, Bergmann A. The CARD-carrying caspase Dronc is essential for most, but not all, developmental cell death in Drosophila. Development. 2005;132:2125–2134. doi: 10.1242/dev.01790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baena-Lopez LA, Arthurton L, Xu DC, Galasso A. Non-apoptotic Caspase regulation of stem cell properties. Semin. Cell Dev. Biol. 2018;82:118–126. doi: 10.1016/j.semcdb.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgon PG, Megeney LA. Caspase signaling, a conserved inductive cue for metazoan cell differentiation. Semin. Cell Dev. Biol. 2018;82:96–104. doi: 10.1016/j.semcdb.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima YI, Kuranaga E. Caspase-dependent non-apoptotic processes in development. Cell Death Differ. 2017;24:1422–1430. doi: 10.1038/cdd.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aram L, Yacobi-Sharon K, Arama E. CDPs: Caspase-dependent non-lethal cellular processes. Cell Death Differ. 2017;24:1307–1310. doi: 10.1038/cdd.2017.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang HL, Tang HM, Fung MC, Hardwick JM. In vivo CaspaseTracker biosensor system for detecting anastasis and non-apoptotic caspase activity. Sci. Rep. 2015;5:9015. doi: 10.1038/srep09015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baena-Lopez LA, et al. Novel initiator caspase reporters uncover previously unknown features of caspase-activating cells. Development. 2018;145:170811. doi: 10.1242/dev.170811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding AX, et al. CasExpress reveals widespread and diverse patterns of cell survival of caspase-3 activation during development in vivo. eLife. 2016 doi: 10.7554/eLife.10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 20.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 21.Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- 22.Miguel-Aliaga I, Jasper H, Lemaitre B. Anatomy and physiology of the digestive tract of Drosophila melanogaster. Genetics. 2018;210:357–396. doi: 10.1534/genetics.118.300224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salle J, et al. Intrinsic regulation of enteroendocrine fate by Numb. EMBO J. 2017;36:1928–1945. doi: 10.15252/embj.201695622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng X, Hou SX. Enteroendocrine cells are generated from stem cells through a distinct progenitor in the adult Drosophila posterior midgut. Development. 2015;142:644–653. doi: 10.1242/dev.113357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, et al. Transient Scute activation via a self-stimulatory loop directs enteroendocrine cell pair specification from self-renewing intestinal stem cells. Nat. Cell Biol. 2018;20:152–161. doi: 10.1038/s41556-017-0020-0. [DOI] [PubMed] [Google Scholar]
- 26.Gervais L, Bardin AJ. Tissue homeostasis and aging: new insight from the fly intestine. Curr. Opin. Cell Biol. 2017;48:97–105. doi: 10.1016/j.ceb.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Liang J, Balachandra S, Ngo S, O'Brien LE. Feedback regulation of steady-state epithelial turnover and organ size. Nature. 2017;548:588–591. doi: 10.1038/nature23678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang H, Edgar BA. Intestinal stem cell function in Drosophila and mice. Curr. Opin. Genet. Dev. 2012;22:354–360. doi: 10.1016/j.gde.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martins RR, McCracken AW, Simons MJP, Henriques CM, Rera M. How to catch a smurf? Ageing and beyond… In vivo assessment of intestinal permeability in multiple model organisms. Biol. Protoc. 2018;1:4. doi: 10.21769/BioProtoc.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rera M, et al. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 2011;14:623–634. doi: 10.1016/j.cmet.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanuka H, et al. Drosophila caspase transduces Shaggy/GSK-3beta kinase activity in neural precursor development. EMBO J. 2005;24:3793–3806. doi: 10.1038/sj.emboj.7600822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendes CS, et al. Cytochrome c-d regulates developmental apoptosis in the Drosophila retina. EMBO Rep. 2006;7:933–939. doi: 10.1038/sj.embor.7400773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakajima Y, Kuranaga E, Sugimura K, Miyawaki A, Miura M. Nonautonomous apoptosis is triggered by local cell cycle progression during epithelial replacement in Drosophila. Mol. Cell Biol. 2011;31:2499–2512. doi: 10.1128/MCB.01046-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amcheslavsky A, Lindblad JL, Bergmann A. Transiently, "Undead" enterocytes mediate homeostatic tissue turnover in the adult Drosophila midgut. Cell Rep. 2020;33:108408. doi: 10.1016/j.celrep.2020.108408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchon N, et al. Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Rep. 2013;3:1725–1738. doi: 10.1016/j.celrep.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Marianes A, Spradling AC. Physiological and stem cell compartmentalization within the Drosophila midgut. eLife. 2013;2:e00886. doi: 10.7554/eLife.00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kondo S, Senoo-Matsuda N, Hiromi Y, Miura M. DRONC coordinates cell death and compensatory proliferation. Mol. Cell Biol. 2006;26:7258–7268. doi: 10.1128/MCB.00183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chew SK, et al. The apical caspase dronc governs programmed and unprogrammed cell death in Drosophila. Dev. Cell. 2004;7:897–907. doi: 10.1016/j.devcel.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 39.Daish TJ, Mills K, Kumar S. Drosophila caspase DRONC is required for specific developmental cell death pathways and stress-induced apoptosis. Dev. Cell. 2004;7:909–915. doi: 10.1016/j.devcel.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 40.Xu D, et al. The effector caspases drICE and dcp-1 have partially overlapping functions in the apoptotic pathway in Drosophila. Cell Death Differ. 2006;13:1697–1706. doi: 10.1038/sj.cdd.4401920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muro I, et al. The Drosophila caspase Ice is important for many apoptotic cell deaths and for spermatid individualization, a nonapoptotic process. Development. 2006;133:3305–3315. doi: 10.1242/dev.02495. [DOI] [PubMed] [Google Scholar]
- 42.White K, Tahaoglu E, Steller H. Cell killing by the Drosophila gene reaper. Science. 1996;271:805–807. doi: 10.1126/science.271.5250.805. [DOI] [PubMed] [Google Scholar]
- 43.Zhai Z, et al. Accumulation of differentiating intestinal stem cell progenies drives tumorigenesis. Nat. Commun. 2015;6:10219. doi: 10.1038/ncomms10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J, Xu N, Huang H, Cai T, Xi R. A feedback amplification loop between stem cells and their progeny promotes tissue regeneration and tumorigenesis. eLife. 2016 doi: 10.7554/eLife.14330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beebe K, Lee WC, Micchelli CA. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev. Biol. 2010;338:28–37. doi: 10.1016/j.ydbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 46.Lee WC, Beebe K, Sudmeier L, Micchelli CA. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development. 2009;136:2255–2264. doi: 10.1242/dev.035196. [DOI] [PubMed] [Google Scholar]
- 47.Arthurton L, Nahotko DA, Alonso J, Wendler F, Baena-Lopez LA. Non-apoptotic caspase activation preserves Drosophila intestinal progenitor cells in quiescence. EMBO Rep. 2020 doi: 10.15252/embr.201948892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korzelius J, et al. Escargot maintains stemness and suppresses differentiation in Drosophila intestinal stem cells. EMBO J. 2014;33:2967–2982. doi: 10.15252/embj.201489072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loza-Coll MA, Southall TD, Sandall SL, Brand AH, Jones DL. Regulation of Drosophila intestinal stem cell maintenance and differentiation by the transcription factor Escargot. EMBO J. 2014;33:2983–2996. doi: 10.15252/embj.201489050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 51.Jager R, Zwacka RM. The enigmatic roles of caspases in tumor development. Cancers (Basel) 2010;2:1952–1979. doi: 10.3390/cancers2041952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmerini F, Devilard E, Jarry A, Birg F, Xerri L. Caspase 7 downregulation as an immunohistochemical marker of colonic carcinoma. Hum. Pathol. 2001;32:461–467. doi: 10.1053/hupa.2001.24328. [DOI] [PubMed] [Google Scholar]
- 53.Kamber Kaya HE, Ditzel M, Meier P, Bergmann A. An inhibitory mono-ubiquitylation of the Drosophila initiator caspase Dronc functions in both apoptotic and non-apoptotic pathways. PLoS Genet. 2017;13:e1006438. doi: 10.1371/journal.pgen.1006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yagi Y, Hayashi S. Role of the Drosophila EGF receptor in determination of the dorsoventral domains of escargot expression during primary neurogenesis. Genes Cells. 1997;2:41–53. doi: 10.1046/j.1365-2443.1997.d01-282.x. [DOI] [PubMed] [Google Scholar]
- 55.Zeng X, Chauhan C, Hou SX. Characterization of midgut stem cell- and enteroblast-specific Gal4 lines in drosophila. Genesis. 2010;48:607–611. doi: 10.1002/dvg.20661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang H, et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 58.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/S0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 59.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson R, et al. The DIAP1 RING finger mediates ubiquitination of Dronc and is indispensable for regulating apoptosis. Nat. Cell Biol. 2002;4:445–450. doi: 10.1038/ncb799. [DOI] [PubMed] [Google Scholar]
- 61.Yeo SL, et al. On the functional overlap between two Drosophila POU homeo domain genes and the cell fate specification of a CNS neural precursor. Genes Dev. 1995;9:1223–1236. doi: 10.1101/gad.9.10.1223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
All materials and fly stocks used in this study are available from the corresponding author on request.