Abstract
Diabetes mellitus is a global health concern associated with significant morbidity and mortality. Inadequate control of diabetes leads to chronic complications and higher mortality rates, which emphasizes the importance of achieving glycemic targets. Although glycated hemoglobin (HbA1c) is the gold standard for measuring glycemic control, it has several limitations. Therefore, in recent years, along with the emergence of continuous glucose monitoring (CGM) technology, glycemic control modalities have moved beyond HbA1c. They encompass modern glucometrics, such as glycemic variability (GV) and time-in-range (TIR). The key advantage of these newer metrics over HbA1c is that they allow personalized diabetes management with person-centric glycemic control. Basal insulin analogues, especially second-generation basal insulins with properties such as longer duration of action and low risk of hypoglycemia, have demonstrated clinical benefits by reducing GV and improving TIR. Therefore, for more effective and accurate diabetes management, the development of an integrated approach with second-generation basal insulin and glucometrics involving GV and TIR is the need of the hour. With this objective, a multinational group of endocrinologists and diabetologists reviewed the existing recommendations on TIR, provided their clinical insights into the individualization of TIR targets, and elucidated on the role of the second-generation basal insulin analogues in addressing TIR.
Keywords: Clinical insights, Continuous glucose monitoring, Diabetes, Glycemic variability, Hypoglycemia, Insulin degludec, Insulin detemir, Insulin glargine 100 U/mL, Insulin glargine 300 U/mL, Insulin therapy, Second-generation basal insulin, Time-in-range
Key Summary Points
| Although glycated hemoglobin (HbA1c) is the gold standard for measuring glycemic control, it has several limitations. Glycemic control modalities have moved beyond HbA1c and encompass modern glucometrics, such as glycemic variability (GV) and time-in-range (TIR). |
| The key advantage of these newer metrics over HbA1c is that they allow personalized diabetes management with person-centric glycemic control. |
| Basal insulin analogues, especially second-generation basal insulins with properties such as longer duration of action and low risk of hypoglycemia, have demonstrated clinical benefits by reducing GV and improving TIR. |
| A multinational group of endocrinologists and diabetologists provided their clinical insights for guiding clinicians on the initiation of insulin therapy in people with diabetes, with emphasis on the role of the second-generation basal insulin analogues. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13247288.
Introduction
Optimal management of diabetes necessitates frequent and precise measurement of blood glucose levels [1]. Landmark trials in diabetes, including UKPDS (UK Prospective Diabetes Study) and DCCT (Diabetes Control and Complications Trial), demonstrated that glycated hemoglobin (HbA1c) is linked to vascular complications [2, 3]. Thereafter, HbA1c was widely accepted as a biomarker of glycemia and emerged as the benchmark for diabetes management. Despite its importance as an indicator for the development for diabetes-related complications, many studies have also revealed the limitations of this biomarker of glycemia [4]. Apart from inaccurate test results in the presence of several medical conditions, HbA1c neither provides any information on glucose dynamics nor captures glucose fluctuations [4].
Self-monitoring of blood glucose (SMBG) serves as the primary technique to evaluate and manage glycemic control [5]. Self-monitoring of blood glucose provides immediate blood glucose values through intermittent finger-stick measurements [5]. The glucose values derived from SMBG are useful in monitoring the glycemic effects of antidiabetic medications, food, and exercise [4]. However, the use of SMBG is limited as a result of physical discomfort, inconvenience, inaccuracy of results in a few instances, and errors in capillary blood glucose readings and data recording [5]. Secondly, the most important drawback of capillary blood glucose readings is the limited amount of information retrieved on the basis of measurements at few specific time points per day (such as before meals and at bedtime) compared to 5–10-min sampling by a continuous glucose sensor [6].
Clinical observations have revealed that glucose profiles can greatly differ even in individuals with well-controlled diabetes. While some people with diabetes have small or moderate glucose excursions with rare hypoglycemia events, others have significantly large glucose fluctuations with frequent episodes of hypoglycemia. Such fluctuations in glucose levels over time within the same day, or between different days at the same time points, are reflected as GV. GV is one of the major components of dysglycemia in people with diabetes [4]. Accumulating evidence indicates that apart from chronic hyperglycemia, the chronic complications of diabetes also result from hypoglycemia and GV [1]. Besides, GV is also a major determinant of hypoglycemia, which gives another reason to target GV for intervention [7, 8]. However, GV is not accurately reflected by both widely used tools for monitoring glycemia, namely HbA1c and SMBG [5, 9]. Therefore, apart from reducing the glycemic burden, as recorded by HbA1c, minimizing GV is an appropriate treatment goal and the need of the hour [10].
A reliable measurement of GV is provided by continuous glucose monitoring (CGM), which allows for 24-h real-time measurement of glucose levels [10], and it can be performed in real time or intermittently. Intermittently viewed monitoring (I-CGM) shows continuous glucose numbers retrospectively. This method of monitoring is an intermediate between a CGM system and a normal blood glucose meter. Unlike I-CGM, real-time CGM (RT-CGM) offers real-time measurement of glucose trends, direction, and the rate of glycemic change. It must be noted that CGM provides results based on measurements of the interstitial fluid and not of blood monitoring; therefore, “real-time” may be a misnomer and delays are a part of this process as well [11]. The development of CGM technology has permitted the creation of new metrics for monitoring glucose, such as GV and the percentage of time spent in target glucose range—called TIR (time-in-range). These metrics provide clinical insights into short-term glycemic control. CGM also provides key inputs to allow algorithm-based insulin delivery [9].
As a result of the limitations of HbA1c in monitoring short-term glycemic changes and the emergence of new metrics for monitoring glucose, the term “beyond HbA1c” has emerged in the field of diabetes research over the past few years. The field of “beyond HbA1c” includes glucometrics, such as TIR, GV, and the percentage of time in hyperglycemia and hypoglycemia, and person-reported outcomes, i.e., quality-of-life (QoL) assessments. With the increasing accuracy of CGM, blood glucose monitoring in people with diabetes is entering a new era [9].
Even though there is evidence favoring early initiation of insulin therapy in individuals with poorly controlled diabetes by oral antidiabetic drugs (OADs), the optimal use of insulin therapy is delayed by several physician- and person-related barriers. The second-generation basal insulin analogues glargine 300 U/mL (Gla-300) and insulin degludec evidently provide more therapeutic benefits, have lower risk of hypoglycemia, and also possess longer action profiles (> 24 h) compared to first-generation insulin analogues [12]. As compared to first-generation insulin analogues, second-generation analogues offer lower GV and comparable TIR [13]. Further, emerging evidence indicates that these second-generation basal insulin analogues help overcome barriers associated with early insulin use and effectively address parameters involved in moving “beyond HbA1c” [12].
With this background, an effort was undertaken to develop clinical evidence-based expert opinions and views for delineating appropriate basal insulin strategies for the treatment of diabetes, based on modern glucose metrics such as GV and TIR. This document summarizes the background evidence-based discussion and key opinions and views of multinational experts on basal insulin strategies for better accuracy in diabetes management.
Methods
A group of multinational clinical experts comprising diabetologists and endocrinologists from 15 countries, namely India, Nepal, Indonesia, Ghana, UAE, Bangladesh, Egypt, Malaysia, Philippines, Nigeria, Sri Lanka, Qatar, Maldives, South Africa, and Singapore, met virtually on 31 May 2020. The expert panel thoroughly reviewed available literature evidence and discussed the importance of TIR and basal insulin strategies in diabetes management, with emphasis on the role of second-generation basal insulin analogues. The clinical insights into TIR provided were based on the review of the Advanced Technologies and Treatments for Diabetes (ATTD) international consensus on the use of CGM 2017 and TIR 2019. Additionally, a review of published literature comparing the TIR profiles of the basal insulins was conducted on the basis of databases searches from PubMed and Google Scholar using the terms “time in range” in combination with “glargine,” “degludec,” and “detemir.” Further, a gray literature search was performed using the same search terms. Articles published in journals resulting from these searches in the last 5 years and relevant references cited in those articles were examined. Only the relevant articles published in English were included.
On the basis of key opinions, review of scientific evidence, and clinical judgment, the panel provided their clinical insights into individualizing TIR and basal insulin strategies in diabetes management, which are summarized in this article. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Emerging Glucose Metrics and Measures: Impact on Diabetes Outcomes
Moving “Beyond HbA1c”: Need of the Hour
Measurement of HbA1c is considered the gold standard method for assessing chronic glycemic control in people with diabetes. However, the measurement of HbA1c has its limitations, including its inability to measure acute glucose fluctuations. Further HbA1c is an unreliable measure in patients with anemia, iron deficiency, hemoglobinopathies, and during pregnancy; moreover, it does not provide information about hypoglycemia and on how to adjust the treatment regimen when HbA1c levels are elevated. Therefore, although HbA1c is a valuable indicator of glycation, as well as an indicator of population health and diabetic complications, some limitations do exist for this parameter as well [14].
Lack of Reliability of HbA1c in Estimating Prevailing Glycemia: Concept of Glycation Gap
Absolute dependence on HbA1c for assessment of glycemic control in diabetes may lead to an error in clinical assessment and management owing to either under- or overestimation of prevailing glycemia. The deviation of HbA1c from serum fructosamine-predicted HbA1c is defined as the glycation gap (GGap), and depicts an individually consistent difference between other measures of mean glycemia and HbA1c [15]. A glycation gap is found in around 40% of people with diabetes and is associated with diabetes-related complications and mortality [15]. Individuals with a positive GGap may receive uptitration of glycemia treatment, putting them at undue risk for hypoglycemia. However, individuals with a negative GGap may be falsely reassured with a measured HbA1c level that is lower than actual glycemic status. This does not result in appropriate therapy intensification to improve glycemia, putting such individuals at risk for diabetes-related complications [15]. Therefore, for therapeutic purposes, using HbA1c levels alone is not completely reliable for evaluating glycemic status [15].
SMBG: Limitations in Filling Glycation Gap
Although SMBG has gained broad application with the widespread availability of glucometers, this traditional method only measures single glucose values at time points determined by the user. Therefore, SMBG only provides a snapshot of the whole glucose picture—ignoring rapid glycemic changes occurring between SMBG measurements [4]. It does not provide any indication of the rate or direction of glucose level changes [14]. As a result, even frequent SMBG does not adequately reflect the GV or GGap in people with diabetes, since it provides only serial, single time-point measurements in 24-h glucose dynamics [5].
Continuous Glucose Monitoring
The field of daily management of diabetes with glucose concentration monitoring was revolutionized with the introduction of minimally invasive needle CGM sensors in 1999 [16]. CGM sensors measure interstitial glucose concentrations. A transmitter is linked to the sensor, which sends signals to an insulin pump or handheld receiver or smartphone supported with an appropriate application. CGM is commonly used in two ways: real-time CGM by patients and retrospective CGM by clinicians [17].
CGM sensors provide almost continuous glucose measurement, delivering readings every 5 min. Additionally, the use of CGM reveals hypo- and hyperglycemic events that are not detected by SMBG. This system improves glycemic control and reduces GV [18, 19]. Besides the real-time availability of glucose concentration, CGM also provides users with visual/acoustic hypoglycemic alerts, which help mitigate or avoid hypoglycemia [16].
Unlike SMBG, which measures glucose levels in the blood, CGM measures glucose changes in interstitial fluid (ISF). There are differences between blood glucose and ISF glucose: while blood transfers glucose to all parts of the body, ISF transfers glucose to cells. Further, ISF glucose is measured continuously every few minutes, while SMBG is performed a few times every day [20].
Compared to HbA1c, CGM is a more direct and integrated method for estimating mean glucose levels [21]. This method helps in mean glucose calculations through a better reflection of glycemic peaks and troughs along with providing high-density data, thereby addressing the GGap for individual patients. Therefore, CGM potentially complements HbA1c for estimating glycemic control [15].
Systematic analyses of CGM data enable visualization of glucose changes over time and also help identify factors responsible for such changes, thereby providing indications for optimizing diabetes therapy [20]. However, implantation of the CGM system is associated with some inconvenience and discomfort, as well as requiring higher levels of medical intervention [22]. The advantages and disadvantages of the CGM system are summarized in Table 1.
Table 1.
Advantages and limitations of CGM
| Advantages [22, 23] | Limitations |
|---|---|
| Detailed assessment of glycemic control | Safety issues: Site reactions; rashes due to adhesives; falling off, pulling off, sweating (all significantly improved with recent modifications); losing receiver or transmitter; various transmission issues at night; sensors malfunctioning, and silencing of alarms if the smartphone is in silent or vibration mode [24]. |
| Provides comprehensive glucose data |
Technical issues: Adjustment of initial calibration and regular daily recalibrations required for some of the currently available devices; episodic differences in the performance of the sensor in the same individual; sensors can be used for only varying lengths of time, with implantable sensors lasting the longest [24]. Sensor accuracy issues: In extreme glucose ranges, such as < 60 mg/dL or > 200 mg/dL, observed during prolonged aerobic exercise, sensor accuracy may be compromised. This disadvantage continues to be a challenge and calls for patient awareness [25]. |
| Alerts for hypoglycemia and hyperglycemia | Privacy and data concerns: There are risks to the privacy of data of patients and their ownership and ability to control how their information is collected, stored, and used [26]. However, privacy and patient-data-related concerns are associated with cloud data storage and the risk for hacks, and not technically with the sensor per se. |
| No missed recordings |
User/patient-related issues: Burden of wearing a device continuously; a skin puncture is done each time for insertion of the glucose sensor into the subcutaneous part of skin tissue; complex and difficult to personalize the user interface [24]. However, the user interface, displays, user-friendliness, data management, and data analysis software have significantly improved over time [27]. Impact of sleep positions: “Compression lows”—Specific sleep positions might result in aberrant glucose readings, such as a sudden decrease in reported glucose values, presumably due to decreased local blood flow caused by tissue compression. In some cases, aberrant CGM readings display elevated values [28]. |
Improved Glycemic Outcomes with CGM: Clinical Evidence
In Type 1 Diabetes Mellitus: Although conventional intensive insulin therapy leads to good glycemic control, it is associated with the risk of hypoglycemia [29]. In an open-label, randomized crossover trial (Glycaemic control & Optimisation of Life quality in type 1 Diabetes [GOLD] study) conducted among adults with type 1 diabetes mellitus (T1DM), it was observed that intensive insulin therapy with multiple daily insulin injections (MDI) was more effective using CGM as compared to conventional treatment (HbA1c, 7.92% vs. 8.35%) [18]. The GOLD 3 study assessed the effects of CGM on nocturnal and daytime hypoglycemia in people with T1DM treated with MDI. The time with nocturnal glucose levels below 70 mg/dL decreased by 48%, while glucose levels below 54 mg/dL reduced by 65% with CGM. Time with daytime glucose levels below 70 mg/dL reduced by 40% and below 54 mg/dL by 54%. The study concluded that CGM reduced both nocturnal and daytime hypoglycemia in people with T1DM treated with MDI and improved hypoglycemia-related confidence, thereby improving the QoL [29].
In Type 2 Diabetes Mellitus: In people with type 2 diabetes mellitus (T2DM) using insulin, CGM is a monitoring tool and influences the choice of insulin regimen. This method can be used to motivate patients to choose earlier insulin treatments, in case of oral antidiabetic drug failure, and for selecting the most appropriate insulin regimen [30].
CGM may be useful in predicting hypoglycemia in high-risk populations such as elderly people using insulin. Klimontov and Myakina used blinded CGM in 83 insulin-treated elderly inpatients (65–80 years old) to assess the risk of nocturnal hypoglycemia. The study observed nocturnal hypoglycemia in 39% of the 24-h CGM recordings [31].
A prospective randomized trial by Ehrhardt et al. comparing SMBG and RT-CGM demonstrated a significant improvement in HbA1c following RT-CGM in people with T2DM not taking prandial insulin. While the mean decline in HbA1c at 12 weeks was 0.5 ± 0.8% in the SMBG group, it was 1.0 ± 1.1% in the RT-CGM group (p = 0.006) [32]. In the study by Zick et al., 72 h of CGM was used in people with T2DM on MDI. It was reported that CGM detected 56.9% of individuals with hypoglycemia, compared to 26.4% detected by conventional SMBG. Moreover, in people with T2DM treated with a variety of insulin regimens, CGM revealed a more comprehensive picture of hypoglycemia, even during a period of stable therapy [33]. As per a study by Pazos-Couselo et al., significantly higher percentages of hyperglycemic (p = 0.047) and hypoglycemic episodes (p = 0.016) were detected by CGM compared to SMBG. Further CGM detected nocturnal hypoglycemia in 33% of participants. It was also observed that 19% of participants who reportedly had no hypoglycemia by SMBG measurements had hypoglycemia when assessed using CGM measurements [34].
Optimal utilization of CGM may also help in motivating or avoiding diabetes burnout in people with T2DM. Evidence indicates that improved lifestyle and behavior changes, i.e., reduced diabetes burnout associated with RT-CGM use, result in reduced HbA1c levels in these people [35].
Recent Techniques for Measuring Blood Glucose Fluctuations: Time-in-Range and Glycemic Variability
GV refers to fluctuations in blood glucose levels. Fluctuations in blood glucose levels can lead to intraday and interday variations, which can increase both the risk of hypoglycemia and glycemic excursions to the hyperglycemic range [36]. For people using CGM, TIR is defined as the time spent in the target glucose range (70–180 mg/dL) while aiming at reducing the time spent in hypoglycemia. TIR serves as an outcome measure for glycemic control in clinical trials, for complementing other components such as HbA1c and blood glucose levels [37].
GV and Its Implications in Diabetes Mellitus
Several studies have elucidated the relationship between GV and hypoglycemia. Hypoglycemia is more common in individuals with increased GV [36]. A pooled analysis of six randomized controlled trials (RCTs) conducted among people with T2DM treated with 24 weeks of Gla-100 or comparators demonstrated that all measures of GV were significantly associated with poor glycemic control and the development of hypoglycemia during the trial [38]. Moreover, intraday GV is associated with a higher risk of nocturnal and overall hypoglycemia, and a decrease in GV is strongly correlated with a reduction in both hyperglycemic and hypoglycemic episodes [36].
Studies have highlighted the key role played by GV in T1DM as well. Besides reinforcing the relationship between GV and the risk of hypoglycemia, these studies have also shown a link between GV and the development of cardiovascular autonomic dysfunction in adults with T1DM. Further the increased inflammation as a result of GV could lead to endothelial damage and vascular complications in adults with T1DM [39].
Analysis of data from the double-blind Trial Comparing Cardiovascular Safety of Insulin Degludec versus Insulin Glargine (Gla-100) in Patients with Type 2 Diabetes at High Risk of Cardiovascular Events (DEVOTE) evaluated the link between GV and the occurrence of severe hypoglycemia and subsequent mortality. An increased risk of severe hypoglycemia (hazard ratio [HR] 4.11, 95% confidence interval [CI] 3.15, 5.35) and all-cause mortality (HR 1.58, 95% CI 1.23, 2.03) was observed in individuals with interday fasting GV [40]. People experiencing severe hypoglycemia have a greater than twofold higher risk of cardiovascular death and all-cause mortality [41].
GV also has implications for diabetic complications. It is associated with markers of cardiovascular and endothelial damage. High GV is associated with cognitive impairment in people with T2DM [36]. GV has also been associated with the prediction of mortality: a higher risk of mortality is observed in individuals with higher visit-to-visit GV [36]. Besides, it has been observed that GV has implications for patients’ QoL and psychological well-being. In insulin-treated people, high GV is associated with poor QoL, as well as increased length of hospital stay. When diabetes treatment is associated with the perception of reduced GV, there is a simultaneous improvement in QoL parameters, such as treatment satisfaction, work productivity, and absenteeism [36].
To avoid hypoglycemia, providing treatment that can control blood glucose levels and at the same time minimize interday and intraday GV is important. Mori et al. conducted a study to assess the actual state of interday GV and identify factors affecting GV in diabetic outpatients on insulin therapy. Interday GV was seen in 93% of outpatients, and 62% of patients showed inconsistent interday GV. Interday variability was associated with the following factors: gender (higher in women compared to men, p = 0.04), diabetes type (higher in people with T1DM compared to those with T2DM, p < 0.001), dose of insulin (mean of the daily differences [MODD] correlated positively with total insulin dose, bolus insulin dose, and basal insulin but not with bolus dose to basal dose ratio; p = 0.001, 0.016, 0.001, and 0.361, respectively), and treatment regimen (MODD value highest in individuals treated with basal and bolus insulin and lowest in those treated with mixed preparations). The study indicated that treatment and instructions based on individuals’ characteristics, interday GV, and lifestyle are important for people receiving insulin therapy [42].
Connecting the Dots: Time-in-Range and Impact on Diabetes Outcomes
Advancements in CGM technology have provided access to alternative indices and new metrics of glucose control. Among these metrics, TIR has emerged as a preferred measure among individuals with diabetes. Also, emerging evidence indicates that TIR can predict long-term complications of diabetes and pregnancy outcomes. However, in the case of physicians who are mostly familiar with HbA1c and blood glucose measurements, it might be difficult to understand and explain TIR goals to patients, position TIR in comparison to other glucose metrics, and interpret TIR [43]. The Advanced Technologies & Treatments for Diabetes (ATTD) international consensus on the use of CGM 2017 standardized the use of CGM to promote therapy adjustments in people with both type 1 and type 2 diabetes mellitus, especially those with frequent hypoglycemia. Before the 2017 consensus, different studies used different time-in-target ranges, and hence one study could not be compared with another. To overcome such variations and discrepancies, the ATTD consensus categorized hypoglycemia into three levels:
-
(i)
Level 1 (54 to < 70 mg/dL)
-
(ii)
Level 2 (< 54 mg/dL)
-
(iii)
Level 3 (severe hypoglycemia causing cognitive impairment, not defined by definite glucose value)
Hyperglycemia was also categorized into three levels:
-
(i)
Level 1 (alert level, > 180 mg/dL to ≤ 250 mg/dL)
-
(ii)
Level 2 (clinically significant, > 250 mg/dL)
-
(iii)
Level 3 (clinical diagnosis: hyperosmolar hyperglycemic or state ketoacidosis)
Categorization of the time spent in the three levels of hypoglycemia and hyperglycemia allows a more accurate assessment of severity and facilitates initiation of the most appropriate response [14, 37].
On the basis of the categorization of hypo- and hyperglycemia into different levels based on glucose values, the ATTD consensus panel defined TIR as the time spent in an individual’s target glucose range of 70–180 mg/dL (3.9–10 mmol/L) [14]. The TIR metric includes three CGM measurements: percentage of readings and time per day above target glucose range (TAR), within target glucose range (TIR), and time below target glucose range (TBR). As per the recent ATTD international consensus on TIR (2019), most people with T1DM and T2DM should aim to spend more than 70% of time per day (approx. > 17 h) in TIR (70–180 mg/dL), with TBR (< 70 mg/dL) less than 4% and TAR (> 180 mg/dL) being less than 25% of time per day (Fig. 1) [44].
Fig. 1.
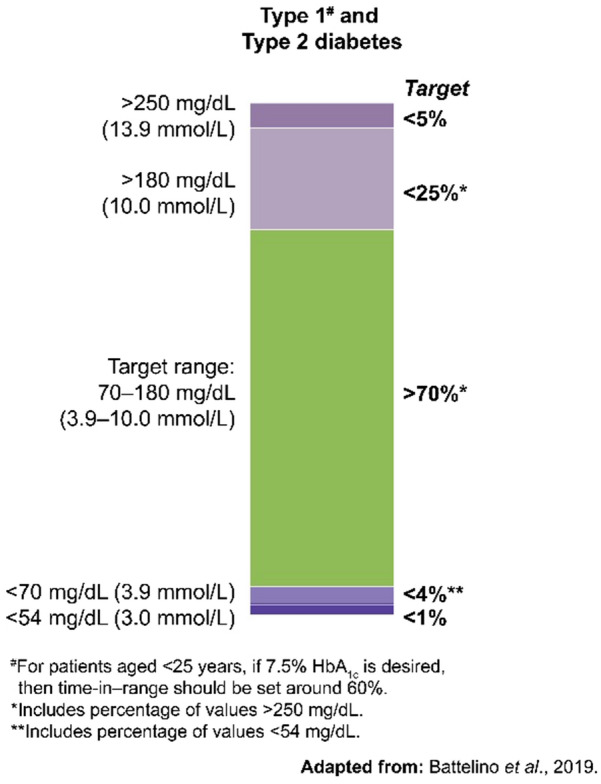
Continuous glucose monitoring-based time-in-range targets for patients with diabetes [44]
Petersson et al. evaluated the relationship between time-in-target range (TIT) and HbA1c through CGM in children and adolescents with T1DM. While TIR was defined as 70–180 mg/dL, TIT was defined as 70–140 mg/dL. Over 60 days, the mean TIR was 60.8% (± 13.1%), while the mean TIT was 40.9% (± standard deviation [SD], 12.2%). Moreover, a significant nonlinear relation was observed between TIT and HbA1c during the study period. The study concluded that in addition to HbA1c, time spent in TIT could be a useful metric to assess glycemic control [45].
Beck et al. conducted analyses utilizing datasets from four randomized trials including 545 adults with type 1 diabetes who had central laboratory measurements of HbA1c at baseline and 6 months. TIR (70–180 mg/dL [3.9–10.0 mmol/L]) of 70% and 50% corresponded with HbA1c values of approximately 7% and 8%, respectively. An increase in TIR of 10% (2.4 h per day) corresponded to a decrease in HbA1c of approximately 0.6%, establishing an inverse relationship between the two [46].
Vigersky and McMahon also analyzed the relationship between TIR and HbA1c across multiple studies (n = 1137), including people with T2DM. The study revealed that for target HbA1c of ≤ 7% and ≤ 6.5%, the %TIR targets should be approximately 65% and 70%, respectively. The study reported that for achieving a target decrease in HbA1c of 0.8% (9 mmol/mol), there was a 10% change in %TIR [47].
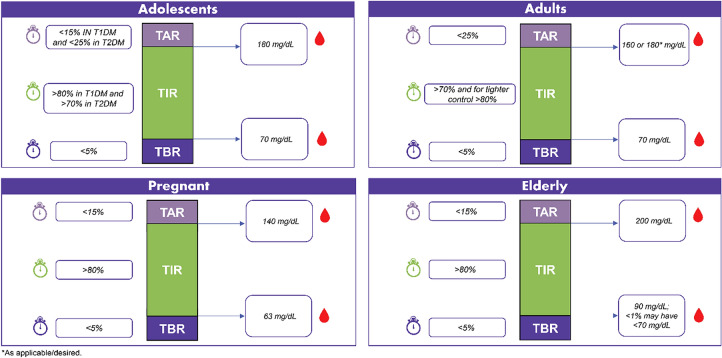
Apart from measuring glycemic control in patients, TIR may also be used as an appropriate clinical endpoint in diabetes research [48]. For example, TIR serves as a valid clinical outcome for evaluating the risk of vascular complications of diabetes. Evidence suggests that TIR is inversely correlated with the risk of developing vascular complications in people with diabetes [48]. On the basis of the above discussion points, the expert panel put forward recommendations for TIR management based on population type and the phase of life. The recommendations are captured herein and summarized in Fig. 2.
Fig. 2.
Panel recommendations for TIR based on age group
TIR Recommendations for Different Populations and Life Phases
TIR is a useful metric in OAD inadequacy in adults, as OAD inadequacy is usually seen within 5 years in two-thirds of cases.
In T1DM, the lower cutoff may be 80 mg/dL to prevent hypoglycemia.
Adults with T2DM without complications deserve tight control: > 80% in 70–160 mg/dL, as this group is mostly on metformin and the risk of hypoglycemia is low; hence, 70 mg/dL is the acceptable lower limit and TAR < 15%, TBR < 5%.
The definition of TIR needs to be individualized particularly in organ dysfunction, pregnancy, and the elderly.
TIR recommendations based on the four phases of life can be adolescents T1DM/T2DM (10–18 years), adults (> 18 years), pregnancy, and elderly.
- For adolescents: A target range of 70–180 mg/dL is acceptable in T1DM and T2DM. Setting the lower limit to a value above 70 mg/dL will suspend insulin too early, which is not recommended. Higher TIR is recommended for T1DM, as they are more prone to GV due to reduced endogenous insulin.
- (i) In T1DM: > 80% TIR in a target blood glucose of 70–180 mg/dL; < 15% TAR and < 5% TBR
- (ii) In T2DM: > 70% TIR in a target blood glucose of 70–180 mg/dL; < 25% TAR and < 5% TBR
- For adults: As most people with diabetes fall into this category, it is better to strive for tighter control in adults. Hence, two recommendations can be considered for TIR in adults and individualized as needed:
- (i) Same as ATTD consensus recommendations for T1DM and T2DM: > 70% TIR in a target blood glucose of 70–180 mg/dL; < 25% TAR and < 5% TBR, or
- (ii) Tighter control: > 80% TIR in a target blood glucose of 70–160 mg/dL; < 15% TAR and < 5% TBR. Tighter control is recommended in people who are highly motivated, newly diagnosed, have a long-life expectancy, and do not have co-morbidities.
For pregnancy: Considering the target range similar to ATTD consensus recommendations, the time spent in the target range should be increased by 10%, as it has the potential to lead to better outcomes: > 80% TIR in a target blood glucose of 63–140 mg/dL; < 15% TAR and < 5% TBR.
For elderly: Targets need to be relaxed with more time spent in the relaxed range, as this group is more susceptible to hypoglycemia; > 80% TIR in a target blood glucose of 90–200 mg/dL; < 15% TAR, < 5% TBR (< 90 mg/dL) < 1% (< 70 mg/dL).
80–90% children aged < 10 years have hypoglycemia unawareness. Thus, the lower limit of TIR should be relaxed.
CGM reading differences with increase in temperature should be taken in account both during physiological (e.g., during summer) and pathological (medication intake such as antipyretic, e.g., acetaminophen) conditions.
The variation between CGM and SMBG is most evident during times when blood glucose levels are rapidly changing.
Relationship Between Time-in-Range and Microvascular Outcomes
A strong association between HbA1c levels and the risk of diabetes-associated chronic vascular complications was first established in the landmark DCCT trial [2]. Using the dataset of the DCCT trial, Beck et al. evaluated the association between TIR and development and/or progression of microalbuminuria, and diabetic retinopathy. For every 10 percentage-point decrease in TIR, the hazard rate for the development of microalbuminuria was increased by 40% (95% CI 25–56), while a 64% (95% CI 51–78) increase in retinopathy progression was observed (Fig. 3). This indicates a strong association between TIR and the risk of microvascular complications [48]. Another study investigated the association between diabetic retinopathy and TIR assessed by CGM in people with T2DM. Persons with more advanced diabetic retinopathy had significantly lower TIR and higher measures of GV. Moreover, with increasing TIR, the prevalence of diabetic retinopathy decreased, based on severity. TIR was negatively associated with diabetic retinopathy; people with T2DM with vision-threatening retinopathy had the lowest TIR [49].
Fig. 3.
Impact of TIR on microvascular outcomes [46]
Another common microvascular complication is diabetic peripheral neuropathy (DPN). In a study on the association between TIR and DPN, Mayeda et al. demonstrated that DPN prevalence was 43% and 74% among participants who were within the target range > 70% and < 70% of the time, respectively. For every 10% decrease in TIR, there was a 25% increase in the risk of DPN. However, there was no significant association between HbA1c and DPN symptoms. Therefore, the study concluded that the prevalence of DPN is inversely correlated with TIR [50].
Effect of Time-in-Range on Macrovascular Outcomes
Carotid intima-media thickness (CIMT) serves as a biomarker of subclinical atherosclerosis and can be used to predict incident cardiovascular events. A recent study investigated the use of TIR, measured by CGM and CIMT, as a surrogate marker of cardiovascular disease. It was observed that people with T2DM and abnormal CIMT had significantly lower TIR compared to those with normal CIMT (p < 0.001). These findings suggest a link between TIR and macrovascular disease [51].
Daily Life Measures Based on Time-in-Range
An online survey was conducted to understand patients’ perspectives regarding the success of diabetes therapies, factors having the greatest impact on their daily lives, and the drivers of diabetes and mindset improvement. The study revealed TIR as the most common factor that had a great impact on daily life across all three study groups (people with T1DM [T1], people with T2DM on insulin therapy [T2I], and people with T2DM not on insulin therapy [T2NI]). Further, 36% of T2I respondents, 54% of T1 respondents, and 22% of T2NI respondents reported TIR as the key factor for a positive mindset [52].
Individualizing TIR Goals for Improving Outcomes
In patients undergoing cardiac surgery, poor perioperative control of blood glucose levels is associated with poor outcomes. Various glycemic targets have been prescribed to reduce wound infection and overall mortality rates. A prospective study by Omar et al. evaluated glucose control in patients with or without diabetes, after cardiac surgery. The glycemic target of the patients was 6.0–8.1 mmol/L, as determined by TIR. The patients with > 80% TIR had better outcomes in terms of wound infection (p = 0.05), length of hospital and intensive care unit (ICU) stay (p = 0.03 and p = 0.04, respectively), and length of ventilation (p = 0.03) as compared to those with < 80% TIR [53].
Randomized trial data confirm that CGM leads to improvement in both neonatal health outcomes and maternal glucose control. The common target throughout pregnancy is to increase TIR while reducing TAR and TBR. During the second and third trimesters, 5% lower TIR and 5% higher TAR is associated with an increased risk of neonatal hypoglycemia, large-for-gestational age infants, and neonatal ICU admission. Therefore, a TIR of > 70% (16 h, 48 min) and a TAR of < 25% (6 h) should be aimed for optimal neonatal outcomes as early as possible during pregnancy [54].
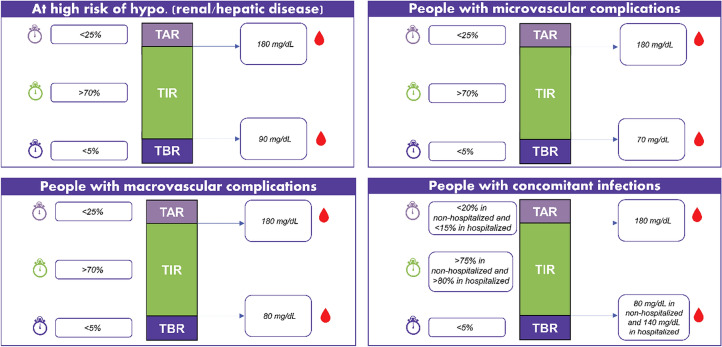
The proposed clinical insights for TIR management in the presence of complications are presented herein and in Fig. 4.
Fig. 4.
Panel recommendations for TIR based on complications profile
Clinical Insights on TIR Recommendations for Different Complications
CGM should be considered in pregnancy, renal failure, discordance between HbA1c, and clinical features/SMBG.
TIR is an “additional” measure (along with other glycemic parameters), mainly if more accessible parameters are inconclusive.
There is a need for more longitudinal studies (cross-sectional) to establish TIR as a surrogate marker of complications.
For individuals at high risk of hypoglycemia (renal/hepatic disease): A relaxed target range with a high lower limit is recommended; > 70% TIR in a target blood glucose of 90–180 mg/dL; < 25% TAR and < 5% TBR.
-
With micro-/macrovascular complications:
(i) Microvascular: Same as adults; > 70% TIR in a target blood glucose of 70–180 mg/dL; < 25% TAR and < 5% TBR or > 80% TIR in a target blood glucose of 70–160 mg/dL; < 15% TAR and < 5% TBR.
(ii) Macrovascular: Same target as microvascular complications or relaxed as following ≥ 70% TIR in a target blood glucose of 80–180 mg/dL; < 25% TAR and < 5% TBR. While stringent control has shown microvascular benefits, people with macrovascular complications may be at a higher risk of hypoglycemia, and hence relaxed targets are recommended.
-
With concomitant acute infections:
(i) Non-hospitalized: ≥ 75% TIR in a target blood glucose of 80–180 mg/dL; < 20% TAR and < 5% TBR.
(ii) Hospitalized: > 80% TIR in a target blood glucose of 140–180 mg/dL; < 15% TAR and < 5% TBR. The cutoffs are kept in this range, assuming that use of antipyretics such as acetaminophen in acute infections can have an effect on CGM readings, giving a false higher value.
Those with fever taking antipyretics and on CGM should undergo cross-check with finger stick method.
In patients with sepsis in ICU: TAR (> 180 mg/dL) should be minimized to < 5% and the lower limit of 70 mg/dL should be pushed to 80 mg/dL to minimize mortality.
Basal Insulin Strategies for Time-in-Range and GV: Emerging Position of Second-Generation Basal Insulins
Management of GV as the Stepping-Stone in Diabetes Management
As intraday and interday GV are risk factors in people with diabetes [55], several factors have been proposed to help reduce GV in such individuals who are on insulin treatment, such as [55-60]:
Timely insulinization
Choice of insulin
Initiation dose
Proper timing of injection of prandial insulin (for regular or rapid-acting analogue)
Systematic and adequate titration
Prompt intensification (using prandial insulin) when indicated
Stepwise intensification
Simple regimen
Dosing flexibility
Role of Basal Insulin Analogues in Controlling GV in People with Diabetes
Different basal insulin analogues with varied time–action profiles have been developed over the years. These include first-generation basal insulin analogues, viz. Gla-100 and insulin detemir, second-generation basal insulin analogues, viz. Gla-300 and insulin degludec. Understanding the differences between first- and second-generation basal insulin analogues helps healthcare providers in making the most appropriate treatment decisions according to individual needs of people with diabetes [58].
Advantages of Second-Generation Over First-Generation Basal Insulin Analogues
Both first-generation and second-generation basal insulin analogues have similar efficacy in terms of reducing HbA1c. However, compared to first-generation analogues, newer analogues have longer and more stable pharmacokinetics (PK) and pharmacodynamics (PD) profiles. Second-generation basal insulin analogues are associated with low variability, with predictable and stable glycemic control beyond 24 h, thus, providing clinically meaningful benefits compared to first-generation basal insulin analogues, including longer duration of action (> 24-h coverage), reduced GV due to a more stable PK profile, and better injection time flexibility [12]. Furthermore, the second-generation basal insulin analogues have a lower risk of all-day hypoglycemia and nocturnal hypoglycemia compared to first-generation basal insulin analogues [12]. These analogues are associated with a greater improvement in treatment satisfaction, patient-related outcomes, and QoL [61, 62]. Taken together, second-generation basal insulin analogues provide new treatment options to physicians for achieving target glycemic levels [12].
Improved Control Over Glucose Variability: Comparing First-Generation and Second-Generation Basal Insulin Analogues in T1DM and T2DM
A study by Yu evaluated the real-world benefits of Gla-300 in lowering nocturnal fluctuations in blood glucose levels and nocturnal hypoglycemia in people with T2DM. The mean nocturnal glucose level achieved during the Gla-300 (121 ± 31.23 mg/dL) treatment phase was similar to that achieved during the Gla-100 (126.1 ± 35.11 mg/dL) treatment phase. Variability in mean nocturnal glucose levels (SD and CV) and the mean amplitude of glucose excursions between nights were lower during the Gla-300 treatment period compared to the Gla-100 treatment period. The study concluded that Gla-300 is comparable to Gla-100 in providing tight glycemic control. Besides, it was observed that Gla-300 had a greater potential to reduce the frequency of nocturnal hypoglycemia vs. Gla-100 [63].
A two-period crossover study compared glucose control with Gla-300 and Gla-100 in people with T1DM. Both Gla-300 and Gla-100 had a comparable percentage of time within the target glucose range. For Gla-300, a significantly lower increase in CGM-based glucose measurement was observed during the last 4 h of the 24-h injection interval as compared to Gla-100 (least-squares mean difference − 14.7 mg/dL [95% CI − 26.9 to − 2.5]; p = 0.0192). Irrespective of morning or evening injection, mean 24-h glucose curves for the Gla-300 group were smoother than for the Gla-100 group—indicating lower glycemic excursions. Compared to Gla-100, the nocturnal hypoglycemia rate was lower with Gla-300 (4.0 vs. 9.0 events per participant-year: rate ratio 0.45 [95% CI 0.24–0.82]) [13].
An open-label, multicenter, prospective, observational study by Yamamoto et al. enrolled 21 participants with T1DM to investigate the differences in GV between Gla-100 and IDeg using CGM. The results showed that the mean amplitude of glycemic excursions was significantly reduced with degludec (p = 0.028), as was the area under the curve for daily blood glucose level < 70 mg/dL (p = 0.046). The authors concluded that degludec confers reduced hypoglycemia and daily blood glucose variability in participants with T1DM as compared to Gla-100 [64].
Another study by Iga et al. involving 20 patients with T1DM reported that fasting interstitial GV on the CGM curves was significantly less with IDeg than Gla-100 (25.9 ± 22.0 vs. 43.8 ± 30.1 mg/dL, p = 0.04). The authors concluded that while the hypoglycemic episodes were similar with both IDeg and Gla-100, treatment with IDeg was associated with lower GV [65].
Second-Generation Basal Insulins: Gla-300 vs. Insulin Degludec
Studies have compared the safety and efficacy of the second-generation basal insulin analogues Gla-300 and insulin degludec in adults with T1DM or T2DM. A study comparing the PK/PD profiles of Gla-300 vs. IDeg-100 demonstrated an evenly distributed PK profile and better steady-state PD profile with Gla-300 (20% lower within-day variability) in people with T1DM, with a once-daily dosing regimen of 0.4 U/kg/day [66]. However, an earlier study reported IDeg-200 to have lower intraday variability (37% lower) and interday variability (ca. four times lower) compared to Gla-300 [67]. The BRIGHT study was the first head-to-head clinical trial evaluating the efficacy and safety of two second-generation basal insulin analogues (Gla-300 and IDed-100) in insulin-naïve adults with T2DM inadequately controlled with OADs (± GLP-1RA). Both insulins provided similar glycemic control, with relatively low and comparable hypoglycemia incidence and rates during the overall study period. However, during the titration period, hypoglycemia was lower with Gla-300 as compared to IDeg [68]. Another head-to-head trial, the Trial Comparing the Efficacy and Safety of Insulin Degludec 200 units/mL and Insulin Glargine 300 units/mL in Subjects with Type 2 Diabetes Mellitus Inadequately Treated with Basal Insulin and Oral Antidiabetic Drugs (CONCLUDE), reported that incidence of overall symptomatic hypoglycemia was not significantly different between Gla-300 and IDeg-200 during the maintenance period in individuals with T2DM. Since the study could not meet its primary endpoint, the secondary endpoints were considered exploratory and not conclusive [69]. A small-scale study used CGM for comparing the safety and efficacy of Gla-300 and insulin degludec. The mean percentage of TIR was similar with both insulin types (p = 0.848), but the percentage of time in hypoglycemia was significantly lower with Gla-300 as compared to insulin degludec (p = 0.002). The percentage of time spent with nocturnal or severe hypoglycemia was also significantly lower with Gla-300 than with insulin degludec (1.1 ± 2.4 vs. 4.2 ± 5.8 and 0.04 ± 0.18 and 1.8 ± 3.0, respectively). The study showed that during insulin degludec treatment, the mean percentage of time with hypoglycemia, especially nocturnal hypoglycemia, significantly correlated negatively with albumin level, possibly as a result of the reversible binding of insulin degludec to albumin [70]. Another study by Yamabe et al. showed that although both these insulins were comparable in terms of efficacy, nocturnal hypoglycemia was significantly lower with Gla-300 compared to insulin degludec (p = 0.021). The study indicated that although both these long-acting insulin analogues are comparable in terms of efficacy, Gla-300 has lesser risk of nocturnal hypoglycemia than insulin degludec [71].
Although the use of CGM allows better assessment of glycemic control taking TIR into consideration, large-scale studies based on CGM to compare the safety and efficacy of Gla-300 and IDeg are lacking. The InRange is an ongoing multicenter phase IV study that will collect CGM data over 20 days in adults with T1DM receiving either Gla-300 or IDeg-100. Although the study is aimed at showing the noninferiority of Gla-300 to IDeg-100 in terms of glycemic control and TIR, upon completion, it is also expected to provide valuable insights into the utility of CGM in clinical practice [72].
With this background, the experts delineated recommendations for the management of TIR in different patient populations. The use of second-generation basal insulins was emphasized by the expert members. The clinical insights regarding the choice of treatment modality for TIR management are summarized below.
Clinical Insights on TIR Recommendations, Based on Modality of Treatment
Second-generation basal insulin scores better than insulin regimens in terms of increased duration of action, lesser GV, improved quality of life, reduction of diabetes distress, better adherence, prevention of cognitive dysfunction, and long-term complications.
In practice, second-generation basal insulin brings down blood glucose with lower hypoglycemia compared to first-generation insulin for equivalent glucose levels.
TIR recommendations remain the same regardless of the modality of treatment. The treatment should be directed at achieving TIR. This can be achieved with basal insulins, especially second-generation basal insulins.
TIR targets remain the same regardless of whether the patient was on insulin or OADs (taking into consideration specific population, age group, and associated complications).
Conclusion and Future Perspectives
On the basis of clinical evidence, the expert panel suggests the use of CGM-based glucose metrics, such as TIR and GV, in addition to HbA1c for effective diabetes management and decreasing the risk of both micro- and macrovascular complications. In this review, the panel individualized TIR for specific populations and complications in the context of diabetes. As personalized diabetes management moves from theory to practice, it is hoped that these recommendations can help practitioners effectively refine patient care models. Finally, person-centric glycemic control with CGM and second-generation basal insulin analogues is an option for more effective and accurate diabetes management, along with improved adherence and QoL measures.
Acknowledgements
The topic of using a patient-centric (with regard to time-in-range) approach to manage type 2 diabetes was discussed at a multi-region, virtual expert committee meeting held in June 2020 which was logistically supported by Sanofi. This article was subsequently developed as an entirely separate exercise, based on the experts’ identified need for literature conveying the regional perspective on this topic. The authors have not received any funding from Sanofi for writing and reviewing this manuscript. Sanofi and/or its affiliates did not exercise any selective influence over the opinions expressed by other authors in this article. The contents of this article and the opinions expressed within are those of the authors, and it is the decision of the authors to submit the manuscript as a clinical insight for publication. All authors had full access to the articles reviewed in this manuscript, take responsibility for the writing of this manuscript, including critical review and editing of each draft, approval of the submitted version, and take complete responsibility for the integrity and accuracy of this manuscript. Before submission to the journal, Sanofi was given the opportunity to review the manuscript. However, the authors remain responsible for all content and editorial decisions. The content published herein solely represents the views and opinions of the authors and does not necessarily represent the views or opinions of sponsor Sanofi and/or its affiliates. The details published herein are intended for informational, educational, academic and/or research purposes and are not intended to substitute for professional medical advice, diagnosis or treatment.
Funding
Medical writing and the journal's Rapid Service fee were paid for by Sanofi India. The authors received no honoraria from Sanofi directly or indirectly related to the development of this publication.
Medical Writing and Editorial Assistance
Medical writing and editorial support were provided by Dr. Rajshri Mallabadi and Dr. Dhanya Mukundan of BioQuest Solutions Pvt. Ltd. and was paid for by Sanofi India.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Disclosures
Sanjay Kalra is on the Editorial Board of Diabetes Therapy. The remaining authors Shehla Shaikh, Gagan Priya, Manas P. Baruah, Abhyudaya Verma, Ashok K. Das, Mona Shah, Sambit Das, Deepak Khandelwal, Debmalya Sanyal, Sujoy Ghosh, Banshi Saboo, Ganapathi Bantwal, Usha Ayyagari, Daphne Gardner, Cecilia Jimeno, Nancy E. Barbary, Khadijah A. Hafidh, Jyoti Bhattarai, Tania T. Minulj, Hendra Zufry, Uditha Bulugahapitiya, Moosa Murad, Alexander Tan, Selim Shahjada, Mijinyawa B. Bello, Prasad Katulanda, Gracjan Podgorski, Wajeeha I. AbuHelaiqa, Rima Tan, Ali Latheef, Sedeshan Govender, Samir H. Assaad-Khalil, Cecilia Kootin-Sanwu, Ansumali Joshi, Faruque Pathan, and Diana A. Nkansah have nothing to disclose.
References
- 1.Wright LA, Hirsch IB. Metrics beyond hemoglobin A1c in diabetes management: time in range, hypoglycemia, and other parameters. Diabetes Technol Ther. 2017;19:S16–26. doi: 10.1089/dia.2017.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. [DOI] [PubMed]
- 3.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohnert KD, Heinke P, Vogt L, Salzsieder E. Utility of different glycemic control metrics for optimizing management of diabetes. World J Diabetes. 2015;6:17–29. doi: 10.4239/wjd.v6.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma BD, Bansal P. The utility and pitfalls of the currently used measures of glycaemia in the diagnosis and management of diabetes mellitus. JIACM. 2015;16:227–235. [Google Scholar]
- 6.Gómez AM, Umpierrez GE, Muñoz OM, et al. Continuous glucose monitoring versus capillary point-of-care testing for inpatient glycemic control in type 2 diabetes patients hospitalized in the general ward and treated with a basal bolus insulin regimen. J Diabetes Sci Technol. 2015;10:325–339. doi: 10.1177/1932296815602905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rama Chandran S, Tay WL, Lye WK, et al. Beyond HbA1c: comparing glycemic variability and glycemic indices in predicting hypoglycemia in type 1 and type 2 diabetes. Diabetes Technol Ther. 2018;20:353–362. doi: 10.1089/dia.2017.0388. [DOI] [PubMed] [Google Scholar]
- 8.Monnier L, Colette C, Wojtusciszyn A, et al. Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care. 2017;40:832–838. doi: 10.2337/dc16-1769. [DOI] [PubMed] [Google Scholar]
- 9.Vigersky RA. Going beyond HbA1c to understand the benefits of advanced diabetes therapies. J Diabetes. 2019;11:23–31. doi: 10.1111/1753-0407.12846. [DOI] [PubMed] [Google Scholar]
- 10.Ajjan R, Slattery D, Wright E. Continuous glucose monitoring: a brief review for primary care practitioners. Adv Ther. 2019;36:579–596. doi: 10.1007/s12325-019-0870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chehregosha H, Khamseh ME, Malek M, Hosseinpanah F, Ismail-Beigi F. A view beyond HbA1c: role of continuous glucose monitoring. Diabetes Ther. 2019;10:853–863. doi: 10.1007/s13300-019-0619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mauricio D, Hramiak I. Second-generation insulin analogues - a review of recent real-world data and forthcoming head-to-head comparisons. Eur Endocrinol. 2018;14:2–9. doi: 10.17925/EE.2018.14supp1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergenstal RM, Bailey TS, Rodbard D, et al. Comparison of insulin glargine 300 units/mL and 100 units/mL in adults with type 1 diabetes: continuous glucose monitoring profiles and variability using morning or evening injections. Diabetes Care. 2017;40:554–560. doi: 10.2337/dc16-0684. [DOI] [PubMed] [Google Scholar]
- 14.Danne T, Nimri R, Battelino T. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631–1640. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nayak AU, Singh BM, Dunmore SJ. Potential clinical error arising from use of HbA1c in diabetes: effects of the glycation gap. Endocr Rev. 2019;40:988–999. doi: 10.1210/er.2018-00284. [DOI] [PubMed] [Google Scholar]
- 16.Cappon G, Vettoretti M, Sparacino G, Facchinetti A. Continuous glucose monitoring sensors for diabetes management: a review of technologies and applications. Diabetes Metab J. 2019;43:383–397. doi: 10.4093/dmj.2019.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood A, O'Neal D, Furler J, Ekinci EI. Continuous glucose monitoring: a review of the evidence, opportunities for future use and ongoing challenges. Intern Med J. 2018;48:499–508. doi: 10.1111/imj.13770. [DOI] [PubMed] [Google Scholar]
- 18.Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317:379–387. doi: 10.1001/jama.2016.19976. [DOI] [PubMed] [Google Scholar]
- 19.Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317:371–378. doi: 10.1001/jama.2016.19975. [DOI] [PubMed] [Google Scholar]
- 20.Siegmund T, Heinemann L, Kolassa R, Thomas A. Discrepancies between blood glucose and interstitial glucose—technological artifacts or physiology: implications for selection of the appropriate therapeutic target. J Diabetes Sci Technol. 2017;11:766–772. doi: 10.1177/1932296817699637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen RM, Franco RS, Smith EP, Higgins JM. HbA1c and blood glucose do not match: how much is determined by race, by genetics, by differences in mean red blood cell age? J Clin Endocrinol Metab. 2019;104:707–710. doi: 10.1210/jc.2018-02409. [DOI] [PubMed] [Google Scholar]
- 22.Schnell O, Barnard K, Bergenstal R. Role of continuous glucose monitoring in clinical trials: recommendations on reporting. Diabetes Technol Ther. 2017;19(7):391–399. doi: 10.1089/dia.2017.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melki V, Ayon F, Fernandez M, et al. Value and limitations of the continuous glucose monitoring system in the management of type 1 diabetes. Diabetes Metab. 2006;32:123–129. doi: 10.1016/S1262-3636(07)70258-6. [DOI] [PubMed] [Google Scholar]
- 24.Petrie JR, Peters AL, Bergenstal RM, Holl RW, Fleming GA, Heinemann L. Improving the clinical value and utility of CGM systems: issues and recommendations: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care. 2017;40:1614–1621. doi: 10.2337/dci17-0043. [DOI] [PubMed] [Google Scholar]
- 25.Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21:313–321. doi: 10.1089/dia.2018.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Britton KE, Britton-Colonnese JD. Privacy and security issues surrounding the protection of data generated by continuous glucose monitors. J Diabetes Sci Technol. 2017;11:216–219. doi: 10.1177/1932296816681585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chawla M, Saboo B, Jha S, et al. Consensus and recommendations on continuous glucose monitoring. J Diabetol. 2019;10:4–14. doi: 10.4103/jod.jod_48_18. [DOI] [Google Scholar]
- 28.Mensh BD, Wisniewski NA, Neil BM, Burnett DR. Susceptibility of interstitial continuous glucose monitor performance to sleeping position. J Diabetes Sci Technol. 2013;7:863–870. doi: 10.1177/193229681300700408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ólafsdóttir AF, Polonsky W, Bolinder J, et al. A randomized clinical trial of the effect of continuous glucose monitoring on nocturnal hypoglycemia, daytime hypoglycemia, glycemic variability, and hypoglycemia confidence in persons with type 1 diabetes treated with multiple daily insulin injections (GOLD-3). Diabetes Technol Ther. 2018;20:274–84. [DOI] [PMC free article] [PubMed]
- 30.Monnier L, Colette C, Boegner C, Pham TC, Lapinski H, Boniface H. Continuous glucose monitoring in patients with type 2 diabetes: Why? When? Whom? Diabetes Metab. 2007;33:247–252. doi: 10.1016/j.diabet.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Klimontov VV, Myakina NE. Glucose variability indices predict the episodes of nocturnal hypoglycemia in elderly type 2 diabetic patients treated with insulin. Diabetes Metab Syndr. 2017;11:119–124. doi: 10.1016/j.dsx.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 32.Ehrhardt NM, Chellappa M, Walker MS, Fonda SJ, Vigersky RA. The effect of real-time continuous glucose monitoring on glycemic control in patients with type 2 diabetes mellitus. J Diabetes Sci Technol. 2011;5:668–675. doi: 10.1177/193229681100500320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zick R, Petersen B, Richter M, Haug C, SAFIR Study Group. Comparison of continuous blood glucose measurement with conventional documentation of hypoglycemia in patients with type 2 diabetes on multiple daily insulin injection therapy. Diabetes Technol Ther. 2007;9:483–92. [DOI] [PubMed]
- 34.Pazos-Couselo M, García-López JM, González-Rodríguez M, et al. High incidence of hypoglycemia in stable insulin-treated type 2 diabetes mellitus: continuous glucose monitoring vs. self-monitored blood glucose. Observational prospective study. Can J Diabetes. 2015;39:428–33. [DOI] [PubMed]
- 35.Allen NA, Fain JA, Braun B, Chipkin SR. Continuous glucose monitoring counseling improves physical activity behaviors of individuals with type 2 diabetes: a randomized clinical trial. Diabetes Res Clin Pract. 2008;80:371–379. doi: 10.1016/j.diabres.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Umpierrez GE, Kovatchev B. Glycemic variability: how to measure and its clinical implication for type 2 diabetes. Am J Med Sci. 2018;356:518−27. [DOI] [PMC free article] [PubMed]
- 37.Gabbay MAL, Rodacki M, Calliari LE, et al. Time in range: a new parameter to evaluate blood glucose control in patients with diabetes. Diabetol Metab Syndr. 2020;12:22. doi: 10.1186/s13098-020-00529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilmot EG, Choudhary P, Leelarathna L, et al. Glycaemic variability: the under-recognized therapeutic target in type 1 diabetes care. Diabetes Obes Metab. 2019;21:2599–2608. doi: 10.1111/dom.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inzucchi SE, Umpierrez G, DiGenio A, Zhou R, Kovatchev BP. Association of measures of glycemic variability with glycemic control and hypoglycemic events. Diabetes. 2012;61:A291. [Google Scholar]
- 40.Zinman B, Marso SP, Poulter NR, et al. Day-to-day fasting glycaemic variability in DEVOTE: associations with severe hypoglycaemia and cardiovascular outcomes (DEVOTE 2) Diabetologia. 2018;61:48–57. doi: 10.1007/s00125-017-4423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pieber TR, Marso SP, McGuire DK, et al. DEVOTE 3: temporal relationships between severe hypoglycemia, cardiovascular outcomes and mortality. Diabetologia. 2018;61:58–65. doi: 10.1007/s00125-017-4422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mori H, Okada Y, Kurozumi A, Narisawa M, Tanaka Y. Factors influencing inter-day glycemic variability in diabetic outpatients receiving insulin therapy. J Diabetes Investig. 2017;8:69–74. doi: 10.1111/jdi.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Advani A. Positioning time in range in diabetes management. Diabetologia. 2020;63:242–252. doi: 10.1007/s00125-019-05027-0. [DOI] [PubMed] [Google Scholar]
- 44.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petersson J, Åkesson K, Sundberg F, Särnblad S. Translating glycated hemoglobin A1c into time spent in glucose target range: a multicenter study. Pediatr Diabetes. 2019;20:339–344. doi: 10.1111/pedi.12817. [DOI] [PubMed] [Google Scholar]
- 46.Beck RW, Bergenstal RM, Cheng P, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol. 2019;13:614–626. doi: 10.1177/1932296818822496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vigersky RA, McMahon C. The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther. 2019;21:81–85. doi: 10.1089/dia.2018.0310. [DOI] [PubMed] [Google Scholar]
- 48.Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400–405. doi: 10.2337/dc18-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. 2018;41:2370–2376. doi: 10.2337/dc18-1131. [DOI] [PubMed] [Google Scholar]
- 50.Mayeda L, Katz R, Ahmad I, et al. Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diabetes Res Care. 2020;8:e000991. doi: 10.1136/bmjdrc-2019-000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu J, Ma X, Shen Y, et al. Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther. 2020;22:72–78. doi: 10.1089/dia.2019.0251. [DOI] [PubMed] [Google Scholar]
- 52.Runge AR, Kennedy L, Brown AS, et al. Does time-in-range matter? Perspectives from people with diabetes on the success of current therapies and the drivers of improved outcomes. Clin Diabetes. 2018;36:112–119. doi: 10.2337/cd17-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Omar AS, Salama A, Allam M, et al. Association of time in blood glucose range with outcomes following cardiac surgery. BMC Anesthesiol. 2015;15:14. doi: 10.1186/1471-2253-15-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphy HR. Continuous glucose monitoring targets in type 1 diabetes pregnancy: every 5% time in range matters. Diabetologia. 2019;62:1123–1128. doi: 10.1007/s00125-019-4904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iuchi H, Sakamoto M, Matsutani D, Suzuki H, Horiuchi R, Utsunomiya K. The durability of basal insulin affects day-to-day glycemic variability assessed by continuous glucose monitoring in type 2 diabetes patients: a randomized crossover trial. Diabetes Technol Ther. 2017;19:457–462. doi: 10.1089/dia.2017.0028. [DOI] [PubMed] [Google Scholar]
- 56.Becker RH, Nowotny I, Teichert L, Bergmann K, Kapitza C. Low within- and between-day variability in exposure to new insulin glargine 300 U/ml. Diabetes Obes Metab. 2015;17:261–267. doi: 10.1111/dom.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garber AJ. Will the next generation of basal insulins offer clinical advantages? Diabetes Obes Metab. 2014;16:483–91. [DOI] [PubMed]
- 58.Cheng AYY, Patel DK, Reid TS, Wyne K. Differentiating basal insulin preparations: understanding how they work explains why they are different. Adv Ther. 2019;36:1018–1030. doi: 10.1007/s12325-019-00925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schiavon M, Dalla Man C, Cobelli C. Insulin sensitivity index-based optimization of insulin to carbohydrate ratio: in silico study shows efficacious protection against hypoglycemic events caused by suboptimal therapy. Diabetes Technol Ther. 2018;20:98–05. [DOI] [PMC free article] [PubMed]
- 60.Bell KJ, Smart CE, Steil GM, Brand-Miller JC, King B, Wolpert HA. Impact of fat, protein, and glycemic index on postprandial glucose control in type 1 diabetes: implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care. 2015;38:1008–1015. doi: 10.2337/dc15-0100. [DOI] [PubMed] [Google Scholar]
- 61.American Diabetes Association Insulin administration. Diabetes Care. 2004;27:S106–S109. doi: 10.2337/diacare.27.2007.S106. [DOI] [PubMed] [Google Scholar]
- 62.Grelle JL, Kutter SN, Giruzzi ME, Tawwater JC. Impact of insulin detemir administration time on hypoglycemia rates in hospitalized patients. Pharmacotherapy. 2017;37:1523–1529. doi: 10.1002/phar.2045. [DOI] [PubMed] [Google Scholar]
- 63.Yu CN. Nocturnal glycemic control with new insulin glargine 300 U/mL. Adv Med. 2019;2019:8587265. doi: 10.1155/2019/8587265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamamoto C, Miyoshi H, Fujiwara Y, et al. Degludec is superior to glargine in terms of daily glycemic variability in people with type 1 diabetes mellitus. Endocr J. 2016;63:53–60. doi: 10.1507/endocrj.EJ15-0438. [DOI] [PubMed] [Google Scholar]
- 65.Iga R, Uchino H, Kanazawa K, et al. Glycemic variability in type 1 diabetes compared with degludec and glargine on the morning injection: an open-label randomized controlled trial. Diabetes Ther. 2017;8:783–792. doi: 10.1007/s13300-017-0269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bailey TS, Pettus J, Roussel R, et al. Morning administration of 0.4 U/kg/day insulin glargine 300 U/mL provides less fluctuating 24-hour pharmacodynamics and more even pharmacokinetic profiles compared with insulin degludec 100 U/mL in type 1 diabetes. Diabetes Metab. 2018;44:15–21. [DOI] [PubMed]
- 67.Heise T, Nørskov M, Nosek L, Kaplan K, Famulla S, Haahr HL. Insulin degludec: lower day-to-day and within-day variability in pharmacodynamic response compared with insulin glargine 300 U/mL in type 1 diabetes. Diabetes Obes Metab. 2017;19:1032–1039. doi: 10.1111/dom.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenstock J, Cheng A, Ritzel R, et al. More similarities than differences testing insulin glargine 300 units/mL versus insulin degludec 100 units/mL in insulin-naive type 2 diabetes: The randomized head-to-head BRIGHT trial. Diabetes Care. 2018;41:2147–2154. doi: 10.2337/dc18-0559. [DOI] [PubMed] [Google Scholar]
- 69.Philis-Tsimikas A, Klonoff DC, Khunti K, et al. Risk of hypoglycaemia with insulin degludec versus insulin glargine U300 in insulin-treated patients with type 2 diabetes: the randomised, head-to-head CONCLUDE trial. Diabetologia. 2020;63:698–710. doi: 10.1007/s00125-019-05080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawaguchi Y, Sawa J, Sakuma N, Kumeda Y. Efficacy and safety of insulin glargine 300 U/mL vs insulin degludec in patients with type 2 diabetes: a randomized, open-label, cross-over study using continuous glucose monitoring profiles. J Diabetes Investig. 2019;10:343–351. doi: 10.1111/jdi.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamabe M, Kuroda M, Hirosawa Y, Kamino H, Ohno H, Yoneda M. Comparison of insulin glargine 300 U/mL and insulin degludec using flash glucose monitoring: a randomized cross-over study. J Diabetes Investig. 2019;10:352–357. doi: 10.1111/jdi.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Battelino T, Bosnyak Z, Danne T, et al. InRange: comparison of the second-generation basal insulin analogues glargine 300 U/mL and degludec 100 U/mL in persons with type 1 diabetes using continuous glucose monitoring-study design. Diabetes Ther. 2020;11(4):1017–1027. (Published corrections appear in Diabetes Ther. 2020;11(7):1607–8 and 11(8):1907–8). [DOI] [PMC free article] [PubMed]