Abstract
Hemoglobinopathies are major health problems among Iraqi Kurds, who are a distinct ethnic group inhabiting North and Northeastern Iraq. We reviewed published literature on these disorders in this part of the world, and it was revealed that the most prevalent is β-thalassemia with carrier rates of 3.7–6.9%. Alpha thalassemia is less prevalent with carrier rates of 0.03-1.22%, while the sickle cell gene is variably distributed with carrier rates of 0.06–1.2%. Other structural hemoglobinopathies and δβ-thalassemia are sporadic. Twenty-seven different β-thalassemia mutations were identified, with seven constituting 82% of 1039 chromosomes characterized, namely: IVS-II-1 (G>A), IVS-I-6 (T>C), IVS-I-I (G>A), codon 8 (−AA), codon 8/9 (+G), IVS-I-110 (G>A), and codon 5 (-CT). There were notable regional variations in the distribution of β-thalassemia mutations, with Cd44 being mainly prevalent in the North, while IVS-I-110 is mainly prevalent in the East. In relevance to α-thalassemia, ten different mutations were detected, with the four most frequent constituting 92.4% of 262 alleles characterized being: −α3.7, --MED, α−5ntα, and αPolyA1α. In relevance to sickle cell gene, it is seen in the northern part of the region bordering Turkey, with comparable prevalence rates, and is associated, similar to Turkey, mainly with the Benin haplotype, unlike that in Southern Iraq where it is associated with the Arab-Indian haplotype, similar to Eastern Arabian Peninsula. Given the high prevalence of hemoglobinopathies in the region, and the high rates of consanguineous marriages, a preventive program was initiated in 2008, and results of its first 5 years were promising, though there are still many outstanding challenges that require addressing.
Keywords: Hemoglobinopathies, Thalassemia, Sickle cell disease, Kurdistan, Iraq
Introduction
Hemoglobinopathies are disorders affecting globin chain synthesis and constitute the commonest autosomal recessive inherited diseases worldwide, but particularly so in the Eastern Mediterranean including Iraq (De Sanctis et al. 2017). They could be classified into either quantitative or qualitative subtypes. In the former, it is the quantity of the normal globin chains that are affected, and they are labeled according to the deficient chain, e.g., α-, β-, and δβ-thalassemias. Qualitative hemoglobinopathies, on the other hand, are due to changes in the structure of the globin chains (also called structural hemoglobinopathies) and include among others: hemoglobin S, C, E, and D disorders (Weatheral and Clegg 2001). These disorders were first reported from Iraq in the mid-1960s of the last century (Baker and Al-Quasi 1964;Taj-Eldin et al. 1968), and since then, they emerged as important health problems, and with the increasing number of cases, centers were established through the country, including the Kurdistan region, for their care and management. Much of the research focusing on these disorders in the Kurdistan region occurred in the past 15 years, and the current study aims to provide an updated overview of the genetic epidemiology of these disorders in the region.
Historical preview
Kurds constitute the fourth largest ethnic and linguistic group in the Middle East after Arabs, Turks, and Persians. Most Kurds inhabit a loosely defined region called Kurdistan extending over contiguous parts of Iraq, Iran, Turkey, and Syria. In Iraq, the Kurds constitute the bulk of the population in its Northern and Northeastern regions, contrary to other parts of the country where Arabs predominate. The origin of Kurds has long been a subject for speculation. It is believed, by some historians and based on cultural and linguistic evidence, that Kurds are most likely an Indo-European ethnic group, which had immigrated from the East and settled in the region more than 2500 years ago (Arshi and Zabihi 1990). Furthermore, once they settled in this region, Kurds did not live in isolation, but should have interacted throughout their history with other populations including Persians, Assyrians, Greeks, Romans, Arabs, and Turks (Izady 1992). These interactions may have contributed to variable degrees to the genetic makeup of present-day Kurds.
Methods
A comprehensive literature review was performed through PubMed, and WHO Index Medicus for Eastern Mediterranean Region databases, as well as Google Scholar. The keywords used were Iraq, Kurds, Kurdistan, combined with thalassemia, sickle cell, hemoglobinopathy, hemoglobinopathies, premarital screening, and preventive program.
Prevalence of hemoglobinopathies in Iraqi Kurds
The Iraqi Kurdistan region is currently served mainly by three provincial thalassemia centers, one in each of its three main provinces, namely, Duhok (north), Erbil (center), and Sulaimani (east) (Fig. 1), with a total number of registered symptomatic hemoglobinopathy patients in excess of 3200, which constitutes around one-fourth of the registered patients in the country (Kadhim et al. 2017). Prevalence rates of β-thalassemia minor observed as deduced from premarital screening programs in the three main cities in the region range from 3.7 to 6.9%, with the higher figure coming from Erbil province at the center of the region, while the least comes from Duhok at its North (Table 1) (Al-Allawi and Al-Dousky 2010; Al-Allawi et al. 2013b; Jalal et al. 2008; Polus 2017). These rates are generally comparable with reports from other parts of the country, where rates of 4.4% and 4.6% have been reported from Baghdad (the country’s capital and at its center) and Basrah (at the country’s extreme South), respectively (Hassan et al. 2003; Yahya et al. 1996). The prevalence of β-thalassemia trait in neighboring countries is more or less comparable with the Iraqi figures including 2.0–3.0% in Lebanon (Charafeddine et al. 2008), 3.0–3.5% in Jordan (Bashir et al. 1991; Bashir et al. 1992), 2.6–3.7% in Turkey (Yıldız et al. 2005), 5.0% in Syria (Galanello et al. 2003), and 5.0–10.0% in Iran (Karimi and Rasekhi 2002). The relatively high frequency of β-thalassemia in Eastern Mediterranean countries is expected since these countries all lie along the Malaria belt, and individuals heterozygous to β-thalassemia have a selective advantage against malaria(De Sanctis et al. 2017; Durand and Coetzer 2008). It is important to note here that malaria had been highly endemic and for centuries in Northern Iraq, including the Kurdistan region, up to the 1960s (Abul-Hab 1969; Niazi 1968).
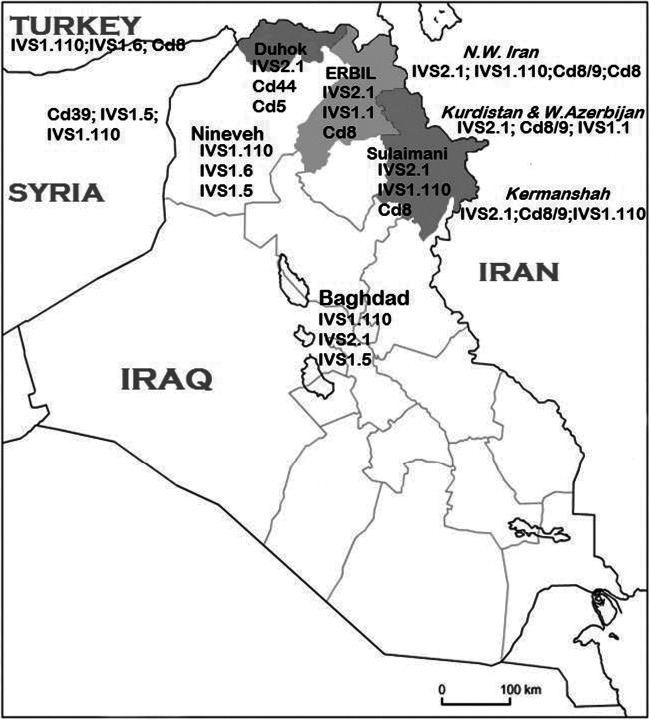
Fig. 1.
A map of Iraq and its surrounding countries, with the three provinces of Iraqi Kurdistan shaded, showing the most frequent β-thalassemia mutations in each region
Table 1.
Premarital screening studies on hemoglobinopathies in three Iraqi Kurdish provinces
| Author (year) | Province | No. of individuals screened | β-thal carriers (%) | α-thal carriers (%) | δβ-thal carriers (%) | Sickle cell carriers (%) |
|---|---|---|---|---|---|---|
| Al-Allawi and Al-Dousky (2010) | Duhok | 1182 | 3.7 | NR | 0.1 | 1.2 |
| Al-Allawi et al. ( 2013b ) | Sulaimani | 108264 | 3.98 | 1.22 | 0.11 | 0.07 |
| Polus (2017) | Erbil | 12448 | 6.9 | 0.032 | NR | 0.064 |
NR not reported
The estimated frequency of α-thalassemia among premarital screened individuals varied in different provinces of the region, with reported rates of 0.032–1.22% (Al-Allawi et al. 2013b; Polus 2017). The rate from the country’s capital Baghdad was reported as 1.0% (Yahya et al. 1996). Rates of α-thalassemia trait vary in neighboring Arab countries between < 1 and 5% (Hamamy and Al-Allawi 2013). While frequencies of 3.6–6.3% were reported by Turkish newborn alpha thalassemia molecular screening studies (Canatan et al. 2002; Fei et al. 1989) and 10.1% in Iranian newborns, though the prevalence varies in different parts of Iran (Valaei et al. 2018).
Delta-beta thalassemia was only sporadically reported in premarital screened individuals, constituting 0.1–0.14% of an aggregate of 2654 individuals studied in Duhok and Sulaimani provinces (Al-Allawi and Al-Dousky 2010; Al-Allawi et al. 2013b; Jalal et al. 2008).
The frequencies of sickle cell trait are quite low in eastern and central provinces of the region (Sulaimani and Erbil) at 0.06–0.07%, but it is seen in polymorphic frequency in its Northern province (Duhok), where it reaches 1.2% of those attending the premarital centers for screening (Al-Allawi and Al-Dousky 2010; Al-Allawi et al. 2013b; Polus 2017). Interestingly, the only other part of Iraq with high frequencies of the sickle cell gene is Basrah at the extreme South at 6.5% (Hassan et al. 2003). The East Anatolia region of Turkey bordering the Duhok province shows comparable frequencies of sickle cell gene (1.7%) (Tadmouri et al. 1998), while and similar to Sulaimani province, sickle cell gene is quite sporadic at 0.025% in Western Iran bordering the latter Iraqi province (Rahimi et al. 2019).
Other structural hemoglobinopathies were only reported sporadically including Hb C (0.06%), D Punjab (0.04%), Hb Lepore (0.01%), Hb G (< 0.001%), and Hb J (< 0.001%) (Al-Allawi et al. 2013b). Furthermore, few alpha structural variants including Hb Kurdistan and Hb Q have been encountered occasionally (unpublished observations).
Molecular basis of β-thalassemia among Kurds
A total of 27 different β-thalassemia mutations were identified among 1039 thalassemia chromosomes characterized up to date. The most common seven mutations, constituting nearly 82% of all characterized mutations, were IVS-II-1 (G>A), IVS-I-6 (T>C), IVS-I-I (G>A), codon 8 (−AA), codon 8/9 (+G), IVS-I-110 (G>A), and codon 5 (−CT). Other less frequent mutations include codon 39 (C>T), codon 44 (−C), IVS-I-5 (G>C), codon 82/83(−G), and IVS-I-128 (T>G). Table 2 lists the frequencies and origins of these 12 mutations among the characterized alleles. It also shows that the bulk of the mutations was Mediterranean, with some Turkish, Kurdish, Saudi Arabian, Azerbaijani, and Asian Indian origins. A further 15 mutations were reported infrequently or sporadically (< 1.0%) that include codon 36/37 (−T), IVS-I-130 (G>C), IVS-II-745 (C>G), IVS-II-850 (G>T), codon 22 (−7 bp), codon 30 (G>C), codon 15 (G>A), codon 127 (CAG>CGG), − 101 (C>T), − 87 (C>G), − 30 (T>A), − 28 (A>C), poly-A (AATAAA>AATAAG), 5′ UTR + 22 (G>A), and CAP + 1(A>C) (Al-Allawi et al. 2010a, 2014, 2006; Amin et al. 2020; Jalal et al. 2010; Shamoon et al. 2015). The spectrum of mutations noted among Kurds is quite consistent with their history and interactions with surrounding Mediterranean populations, while the presence of Asian Indian mutations could be attributed to the region being along the ancient silk road route, which connected China and Europe through the Indian subcontinent and the Middle East.
Table 2.
The frequencies of the 12 most frequent β-thalassemia mutations among 1039 characterized chromosomes among Kurds
| Mutation | Origin | Number of alleles (%) (total 1039) |
|---|---|---|
| IVS-II-1 (G>A) | Eastern Mediterranean | 340 (32.7) |
| IVS-I-6 (T>C) | Mediterranean | 182 (17.5) |
| IVS-I-1 (G>A) | Mediterranean | 84 (8.1) |
| Codon 8 (−AA) | Turkish | 80 (7.7) |
| Codons 8/9 (+G) | Asian Indian | 65 (6.3) |
| IVS-I-110 (G>A) | Mediterranean | 51 (4.9) |
| Codon 5 (−CT) | Mediterranean | 49 (4.7) |
| Codon 39 (C>T) | Western Mediterranean | 41 (3.9) |
| Codon 44 (−C) | Kurdish | 40 (3.8) |
| IVS-I-5 (G>C) | Asian Indian | 25 (2.4) |
| IVS-I-128 (T>G) | Saudi Arabian | 20 (1.9) |
| Codon 82/83 (−G) | Azerbaijani | 14 (1.3) |
When we evaluated the distribution of the mutations in the subcategories of thalassemia major (TM) and intermedia (TI), several notable differences were observed (Table 3), though in both subcategories, IVS-II-1 was the most frequent. It is worth noting that the mild β+ IVS-I-6 was significantly more frequent in TI (P< 0.001), while the more severe β0 alleles, namely: IVS-I-1, codon 39, codon 8/9, codon 44, codon 5, were all significantly more frequent in TM. Studies on TI among Iraqi Kurds also documented that besides milder β-thalassemia alleles, XmnI polymorphism (rs7482144) contributed significantly to the less severe phenotype in patients with severe β-thalassemia alleles, while coinheritance of alpha thalassemia did not (Al-Allawi et al. 2014; Shamoon et al. 2015).
Table 3.
A comparison between the frequencies of various β-thalassemia mutations in thalassemia major and Intermedia
| Mutation | Thalassemia major (n 244) | Thalassemia intermedia (n 571) | P value |
|---|---|---|---|
| IVS-II-1 (G>A) | 73 (29.9) | 217 (38.0) | 0.027 |
| IVS-I-1 (G>A) | 45 (18.4) | 25 (4.4) | < 0.001 |
| Codon 8 (−AA) | 23 (9.4) | 35 (6.1) | 0.081 |
| Codon 39 (C>T) | 23 (9.4) | 7 (1.2) | < 0.001 |
| Codons 8/9 (+G) | 23 (9.4) | 19 (3.3) | < 0.001 |
| Codon 44 (−C) | 21 (8.6) | 4 (0.7) | < 0.001 |
| Codon 5 (−CT) | 16 (6.6) | 13 (2.3) | 0.002 |
| IVS-I-5 (G>C) | 6 (2.4) | 2 (0.3) | 0.203 |
| IVS-I-6 (T>C) | 6 (2.4) | 162 (28.4) | < 0.001 |
| IVS-I-110 (G>A) | 3 (1.2) | 22 (3.9) | 0.083 |
| IVS-I-128 (T>G) | - | 20 (3.5) | - |
| Codon 82/83 (−G) | - | 11( 1.9) | - |
| − 101 (C>T) | - | 6 (1.0) | - |
| Others | 15 (6.1) | 37 (6.5) | - |
Looking at the spectrum of mutations within each of the three provinces of Iraqi Kurdistan, notable differences were noted as shown in Table 4, though all three shared the fact that IVS-II-1 was the most prevalent mutation. The latter mutation is the most frequent in neighboring Iran, including the regions bordering Iraq, and it has been suggested that this Mediterranean mutation may have originated in this country since its prevalence is highest in Central Iran and it decreases as we move west into Iraq and Turkey (Najmabadi et al. 2001). Among the notable differences in the distribution of β-thalassemia mutations in the three provinces of the region are those related to codon 44, which is the second in frequency in Duhok, while it was only sporadic in the other two provinces. Furthermore, the frequency of the latter mutation is higher than that reported in any other population worldwide, except among Kurdish Jews who have immigrated out of this same area in the mid-twentieth century (Rund et al. 1991). This supports the notion that it may have originated in the above-mentioned province rather recently, i.e., after the Kurds have migrated and settled there nearly 2500 years ago (Al-Allawi et al. 2010a, 2006; Jalal et al. 2010; Rund et al. 1991). The other important observation was that IVS-I-110, which was infrequently encountered in Erbil and Duhok, is the second in frequency in Sulaimani. The latter mutation is a Mediterranean mutation and is the most frequent mutation in central Iraq as well as neighboring Middle Eastern countries. Furthermore, IVS-I-110 is among the common mutations in the mainly Kurdish populated Iranian provinces of Azerbaijan, Kermanshah, and Kurdistan bordering the Sulaimani province (Al-Allawi et al. 2013a; Haghi et al. 2009; Hamamy and Al-Allawi 2013; Hosseinpour Feizi et al. 2008; Jalal et al. 2010; Mehrabi et al. 2013). Another notable example of regional variation is relevant to codon 8, which shows its highest frequencies in Sulaimani at the East, and decreases in frequency to the West and North of the region. Codon 8 (−AA), labeled a Turkish/Mediterranean mutation, has its highest frequency worldwide in the country of Azerbaijan, where it constitutes nearly one-third of β-thalassemia alleles (Aliyeva et al. 2020). It is also a common mutation in Iranian Azerbaijan province bordering the Sulaimani province on the East (Hosseinpour Feizi et al. 2008), and it may have been spread by gene flow from the latter Iranian region.
Table 4.
The variation in the frequencies of various β-thalassemia mutations in the three main provinces of Kurdistan region
| Mutation | Relative frequency (%) | ||
|---|---|---|---|
| Duhok | Erbil | Sulaimani | |
| IVS-II-1 (G>A) | 18.3 | 28.4 | 25.2 |
| IVS-I-6 (T>C) | 8.7 | 3.4 | 4.1 |
| IVS-I-1 (G>A) | 8.7 | 23.0 | 4.1 |
| Codon 8 (−AA) | 2.9 | 12.8 | 15.4 |
| Codons 8/9 (+G) | 7.7 | 10.1 | 12.2 |
| IVS-I-110 (G>A) | 1.9 | 2.0 | 19.5 |
| Codon 5 (−CT) | 10.6 | 5.4 | 7.3 |
| Codon 39 (C>T) | 8.7 | 4.7 | 1.6 |
| Codon 44 (−C) | 12.5 | 1.3 | 1.6 |
| IVS-I-5 (G>C) | 6.7 | 0.7 | 8.1 |
| Reference | Al-Allawi et al. (2006) | Al-Allawi et al. (2010a) | Jalal et al. (2010) |
| No. of alleles | 104 | 148 | 123 |
Notable differences have also been documented between the spectrum of β-thalassemia mutations among Iraqi Kurds and the Iraqi Arabs (Fig. 1). A study from Iraq’s capital Baghdad, at the country’s center, and where Arabs constitute the vast majority, revealed that IVS-I-110 constituted nearly a third of the alleles, while IVS-II-1 came second, followed by IVS-I-5, IVS-I-1, and codon 8/9 (Al-Allawi et al. 2013a). Likewise, a study from Nineveh province at Iraq’s Northwest bordering the Kurdistan region of Iraq, and where Arabs also constitute the majority, revealed that IVS-I-110 constituted around a third of the mutations, followed by IVS-I-6, IVS-I-5, codon 44, and codon 39, while IVS-II-1 constituted a mere 6.4 % of the characterized mutations (Eissa et al. 2015).
Several studies reported on the spectrum of β-thalassemia mutations in the Kurdish populations of the neighboring countries. Haghi et al. reported a more or less comparable spectrum among Iranian Kurds with that reported among Iraqi Kurds, with IVS-II-1, codon 8/9, IVS-I-1, codon 5, codon 8, and IVS-I-110 being the most frequent. Likewise, Rahimi and coworkers reported that IVS-II-1 mutation was the most frequent, followed by codon 8/9, IVS-I-110, and codon 36/37 (-T) in the Western Iranian Kurdish population (Haghi et al. 2009; Rahimi et al. 2010) (Fig. 1). Studies from the Diyarbakir region of Southeastern Turkey, an area well known for its ethnic Kurd majority, revealed a rather different mutation distribution than that seen among Iraqi Kurds, with IVSI.110 being the most frequent, followed by IVS-I-6, codon 8, and IVS-II-1 (Ince et al. 2003). Similarly, Tadmouri et al. showed that IVS-1-110, IVS-I-6, codon 8, − 30 (T>A), and IVS-I-1 were the most frequent in East Southern region of Turkey bordering Kurdistan region of Iraq (Tadmouri et al. 1998). On the other hand, Murad et al. (2018) documented that codon 39, followed by IVS-I-5, IVS-I-110, IVS-1-1, and IVS-II-1, were the most frequent mutations in Northeastern Syria bordering Iraq, where there is a Kurdish ethnic presence, which is again quite different from that detailed above among Iraqi Kurds. However, unlike studies from Iraq and Iran, none of the studies from Turkey or Syria focused on the ethnic origin of the studied populations. Studies from the Arabian Peninsula, on the other hand, revealed that while IVS-II-I is the most frequent mutation in Kuwait and second in frequency in Eastern Saudi Arabia, other common mutations including codon 39 and IVS-I-5 are much more frequently encountered in these Arab Peninsular countries than they are among Kurds (Adekile et al. 1994; Al-Sultan et al. 2011).
Molecular basis of Alpha thalassemia among Iraqi Kurds
Molecular characterization of 262 chromosomes for α-thalassemia in 219 Iraqi Kurds with unexplained hypochromia and/or microcytosis revealed 10 different mutations (Al-Allawi et al. 2009, 2013c; Shamoon 2020). The most frequently encountered were −α3.7 (rightwards deletion), --MED (Mediterranean double deletion), α−5ntα (α1 IVS-I 5 nucleotide deletion), αPoly A1α (polyadenylation signal (Poly A1) mutation [AATAAA>AATAAG]), and α1 codon 59 (Hb Adana) seen in 62.6%, 16.4%, 9.2%, 4.2%, and 2.7%, respectively (Table 5). These 10 mutations were arranged in 15 different genotypes (Table 6). The most common α-thalassemia allele identified in each of the three provinces is the rightward deletion (−α3.7), which is consistent with reports from Turkey, Iran, as well as several other Eastern Mediterranean countries (Guvenc et al. 2010; Hadavi et al. 2007; Hellani et al. 2009; Qaddoumi et al. 2008). A notable difference in the distribution of mutations between Erbil and the other two provinces is that the α−5ntα was second in frequency following −α3.7 mutation, surpassing the --MED double deletion in relative frequency. The α−5ntα, which involves 5 nucleotide deletion in IVS-1 of α1 gene, was first reported from Italy, is also a frequent mutation in Western Iran, and several Middle Eastern and Mediterranean countries (Baysal et al. 1995; Dehbozorgian et al. 2015; Hadavi et al. 2007; Jassim et al. 2001; Kanavakis et al. 2000). The (--MED) is the second most frequent mutation in Duhok and Sulaimani, but not in Erbil; this mutation is a frequent mutation in Southern Turkey, one study from Western Iran, and Mediterranean countries like Cyprus and Greece (Baysal et al. 1995; Guvenc et al. 2010; Hadavi et al. 2007; Kanavakis et al. 2000). The polyadenylation signal (poly A1) mutation was first reported from Saudi Arabia, and hence Saudi PolyA1 mutation (Higgs et al. 1983), and it was reported in high frequencies in Saudi Arabia, UAE, Bahrain, and Jordan, but was sporadic or infrequent in Iran and Turkey (Baysal 2011; Guvenc et al. 2010; Hadavi et al. 2007; Hellani et al. 2009; Jassim et al. 2001; Qaddoumi et al. 2008). In the latter two countries, it is the Turkish polyadenylation signal mutation (poly A2) [AATAAA>AATGAA] that is the more frequent poly-A mutation encountered (Bozdogan et al. 2015; Guvenc et al. 2010; Hadavi et al. 2007). In their recent study on Kurds of Western Iran, Alibakhshi and coworkers further affirmed the latter observation regarding PolyA2 mutation distribution and found it second in frequency after −α3.7, followed by −α4.2 (leftward) deletion and codon 59 (or Hb Adana) (G>A) (ααcodon 59) (Alibakhshi et al. 2020). The latter study noted that unlike the Kurds of Iraq, the --MED double deletion was not as frequent, which would require further scrutiny. Hb Adana is an unstable hemoglobin variant due to a mutation in the α1 gene, first described in Turkey (Cürük et al. 1993), and had been encountered in studies from Western Iran and Southern Turkey (Alibakhshi et al. 2020; Guvenc et al. 2010; Hadavi et al. 2007).
Table 5.
The distribution of various α-thalassemia alleles, overall and in the three provinces of Iraqi Kurdistan among 219 individuals with unexplained hypochromia and microcytosis
| Mutation | Number of alleles (%) | |||
|---|---|---|---|---|
| Duhok | Sulaimani | Erbil | Cumulative no. | |
| −α3.7 | 36 (67.9) | 62 (59.6) | 66 (62.9) | 164 (62.6) |
| --MED | 12 (22.6) | 24 (23.1) | 7 (6.7) | 43 (16.4) |
| αPoly A1α | 1(1.9) | 4 (3.8) | 6 (5.7) | 11(4.2) |
| −α4.2 | 2 (3.8) | 2 (1.9) | 2 (1.9) | 6 (2.3) |
| ααAdana | 1 (1.9) | 3 (2.9) | 3 (2.9) | 7 (2.7) |
| −(α)20.5 | - | 3 (2.9) | - | 3 (1.1) |
| α−5ntα | - | 3(2.9) | 21(20) | 24 (9.2) |
| αCSα | - | 2 (1.9) | - | 2 (0.8) |
| αPolyA2α | - | 1 (1.0) | - | 1 (0.4) |
| ααEvanson | 1(1.9) | - | - | 1(0.4) |
| Total no. of alleles characterized | 53 | 104 | 105 | 262 |
Table 6.
The relative frequency of characterized α thalassemia genotypes among 219 individuals with otherwise unexplained hypochromia and/or microcytosis
| Genotype | Sulaimani | Erbil | Duhok | Total (%) |
|---|---|---|---|---|
| −α3.7/αα | 33 | 39 | 26 | 98 (44.7) |
| --MED/αα | 24 | 7 | 12 | 43 (19.6) |
| −α3.7/−α3.7 | 14 | 9 | 5 | 28 (12.8) |
| αPoly A1α/αα | 4 | 6 | 1 | 11 (5.0) |
| α−5ntα/αα | 1 | 9 | - | 10 (4.6) |
| α−5ntα/−α3.7 | - | 6 | - | 6 (2.7) |
| −α4.2/αα | 1 | 2 | 2 | 5 (2.3) |
| ααAdana/αα | 3 | - | 1 | 4 (1.8) |
| α−5ntα/α−5ntα | 1 | 3 | - | 4 (1.8) |
| −(α)20.5/αα | 3 | - | - | 3 (1.4) |
| ααAdana/−α3.7 | - | 3 | - | 3 (1.4) |
| −α3.7/−α4.2 | 1 | - | - | 1 (0.46) |
| αCSα/ αCSα | 1 | - | - | 1 (0.46) |
| αPolyA2α/αα | 1 | - | - | 1 (0.46) |
| ααEvanson/αα | - | - | 1 | 1 (0.46) |
| Total no. | 87 | 84 | 48 | 219 |
The clinically significant phenotype of alpha thalassemia, namely, Hb H disease, is uncommon in the Iraqi Kurds in general, the first study published reported on 11 Hb H disease cases which were all characterized with −α3.7/--MED genotype (Al-Allawi et al. 2009). Another recent study reported on an additional 44 cases (Shamoon et al. 2020), and if the molecular results from these 55 cases are pooled together, the most frequent genotype would be −α3.7/--MED documented in 67.3%, followed by αPolyA1α/αPolyA1α, and −α4.2/--MED in 10.9% and 5.4%, respectively. These observations are more or less consistent with reports from Turkey and Iran where −α3.7/--MED is the most frequent genotype associated with Hb H disease (Cürük 2007; Paridar et al. 2019), but are strikingly different from reports from Saudi Arabia and other Peninsular Arab countries where the main genotype associated with Hb H is αPolyA1α/αPolyA1α (Adekile et al. 1994; Al-Awamy 2000; Al-Riyami et al. 2020; Al Moamen et al. 2018).
Hemoglobin Bart’s hydrops fetalis, the most severe phenotype of alpha thalassemia, which is due to homozygosity to a double alpha gene deletion, is quite rare among Iraqi Kurds and it was reported on only one occasion and molecularly characterized as a (--MED/--MED) defect (Al-Allawi et al. 2010b).
Molecular and clinical aspects of sickle cell disease among Iraqi Kurds
Of the three main Kurdish provinces, sickle cell gene is only present in polymorphic frequencies in the Northern Duhok province; thus, it is expected that the majority of registered sickle cell disease (SCD) cases (87%) reside in the latter province, while they are sporadic/infrequent in the two other provinces of the region. Furthermore, it is quite intriguing to note that in the whole country of Iraq, SCD cases cluster in two localities at the opposite ends of the country, one in the Kurds at the extreme Northern province of Duhok, the other among the Arabs at the extreme Southern Basrah province. However, looking at the β-globin haplotypes associated with the sickle cell gene in these two clusters reveals two different origins. Among Kurds, it is the Benin haplotype that predominates (~ 70%), while the Arab Indian haplotype constitutes a mere 12.5% (Al-Allawi et al. 2012). The Benin haplotype is also the most frequently encountered haplotype in neighboring Turkey (Aluoch et al. 1986), and the predominantly Kurdish population of Southeastern Turkey shares the same dialect as the population of Duhok, which is different from the dialect of other parts of Iraqi Kurdistan (Izady 1992), and it is plausible that the sickle mutation has been introduced to Duhok region through their interactions with their Turkish counterparts, and thereafter, its frequency was amplified through selective advantage offered by malaria, which was endemic until the mid of the twentieth century (Abul-Hab 1969; Aidoo et al. 2002; Niazi 1968). This is in contrast to the sickle cell gene that is seen among Arabs in Southern Iraq Basrah province, where the Arab Indian haplotype predominates at ~ 55%, while the Benin haplotype is seen in 19.4% (Yaseen et al. 2020). The Arab population of Basrah has close links and interactions throughout their history with the population of the Eastern Arabian Peninsula, where the Arab-Indian haplotype is the predominant haplotype associated with the sickle cell gene (Adekile 2001; Al-Ali et al. 2020).
A study on 103 unrelated Kurdish patients with sickle cell disease revealed that the most common genotype encountered was sickle cell anemia (HbSS) in 68%, while sickle/β0 thalassemia and sickle/β+ thalassemia were encountered in another 24.2% and 7.8%, respectively. The β mutations encountered were consistent to some extent with their frequencies in the population, with IVS-II-1 being the most frequent, followed by IVS-I-110, codon 8, codon 44, codon 22, IVS-I-1, codon 30, and IVS-I-6. It was also noted that in those homozygous for sickle cell genes, 10% had concomitant alpha thalassemia. Only one mutation was identified in these patients, which is nothing else than the most common of alpha mutations among the Kurds, the rightward single-gene deletion (−α3.7) (Al-Allawi et al. 2012).
The phenotypic heterogeneity of SCD has been well documented in many populations and it is attributed to a variety of genetic and non-genetic factors (Kutlar 2007). One of the major modifiers of disease severity in SCD is hemoglobin F; the latter is modulated mainly by three major quantitative trait loci (QTL) on chromosomes 11, 2, and 6 (Thein et al. 2009). A recent study enrolled 128 Kurdish SCD patients to address the role of these QTLs in the phenotypic variability of this disorder (Al-Allawi et al. 2019). The study documented that HBG2 rs7482144, BCL11A rs1427407, and HMIP rs9399137 contributed significant 18.1%, 14.3%, and 8.8% to HbF variability in Kurdish Iraqi SCD. Furthermore, the cumulative numbers of minor alleles of these polymorphisms were positively linked to hemoglobin concentration but negatively so to LDH, reticulocyte counts, leukocyte counts, transfusion, and pain frequencies. The study concluded that the cumulative number of minor alleles at the three aforementioned polymorphisms may serve as a better predictor of phenotypic variability of SCD, than each one on its own (Al-Allawi et al. 2019).
The prevention program
In addition to the high prevalence of hemoglobinopathies among Iraqi Kurds, the latter also show a high rate of consanguineous marriage ranging between 24.3 and 27% (Al-Allawi and Al-Dousky 2010; Jalal et al. 2008), which is consistent with its overall rate in Iraq of 30% (Hamamy and Alwan 1994). Thus, it would be expected that the frequency of homozygous births for these autosomal recessive disorders to increase (Galanello et al. 2003). Accordingly, the need to institute a preventive program for these hemoglobin disorders became a necessity rather than an option (Al-Allawi 2008). Following a series of pilot studies performed in various provinces of the region (Al-Allawi and Al-Dousky 2010; Al-Allawi et al. 2006; Jalal et al. 2008), such a program was initiated and supported by the local government which made premarital screening for hemoglobin disorders mandatory throughout the Kurdistan autonomous region in 2008. The program was inspired and based upon the successful model employed by Cyprus (Angastiniotis et al. 1986) and is briefly based on mandatory premarital screening to identify hemoglobinopathy carriers and couples at risk, followed by genetic counseling to the latter couples. Thereafter, prenatal diagnosis by chorionic villus sampling was offered to those who wished to pursue their marriage plans, with the prospect of the termination of affected fetuses before 16-week gestation (Al-Allawi et al. 2015; Al-Allawi et al. 2013b; Angastiniotis et al. 1986).
In its first 5 years, a total of 105,409 couples (210,818 individuals) were screened in two provincial centers, namely Sulaimani and Duhok. A total of 353 (3.3/1000) were identified as couples at risk (both partners were carriers of a hemoglobinopathy), of whom 86.4% were available for extended follow-up. Among the latter couples, 93.1% proceeded with their marriage with a total of 335 registered pregnancies, Prenatal diagnosis (PND) was sought in 117. Affected fetuses were identified in 16.2% by chorionic villus sampling, of whom 94.7% sought to terminate the pregnancy (Al-Allawi et al. 2013b, 2015).
The main reason for not seeking PND was the cost (61.2%), and this was quite evident from different rates of uptake of PND in Duhok and Sulaimani. In the latter where PND was financially supported fully by the local government, the rate of uptake of PND was 76%, compared with 15% in Duhok where it was not (Al-Allawi et al. 2013b, 2015). The limiting effect of cost on the uptake of PND has also been reported from other Middle Eastern countries like Turkey and Lebanon (Tosun et al. 2006; Zahed and Bou-Dames 1997). Another reason for not seeking PND was the traditional cultural belief in fate (23.9%), the latter is not unique to Iraqi Kurds but is a common cultural attitude in other Middle Eastern countries (Alswaidi and O’Brien 2009). A further, but rather a minor reason for opting out of PND, was religious beliefs (7.5%), i.e., refraining from PND because the couple considered abortion religiously unacceptable. Part of the success of the program could be attributed to the fact that a good number of local religious scholars supported the program and the regional council of Islamic scholars issued a fatwa allowing termination of gestations affected by severe diseases, including thalassemia major (Al-Allawi et al. 2015). Furthermore, it was found that 77.9% of newly registered affected babies during the first 5 years of the preventive program were to those who were married before its initiation (Al-Allawi et al. 2015).
These reports revealed that most couples at risk chose to proceed with their marriage despite counseling due to social obligations and norms, and that in the presence of financial support, the majority of at-risk couples would choose to resort to PND. Moreover, it was demonstrated that almost all those who got affected fetuses chose to terminate their pregnancies in the first 16 weeks of gestation. The program managed to reduce the affected birth rate among couples at risk by more than one third over the 5 years (Al-Allawi et al. 2013b, 2015). Based on the data in the first 5 years of the program, the need to upgrade educational programs through various media outlets and schools to target the younger sector of the population probably before marriage; provision of financial support for local PND programs; and offering parallel antenatal screening programs seem to be viable options to reduce affected birth rates further.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study does not contain any studies with human participants.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abul-Hab J. Malaria vector survey in North Iraq. I. Provinces of Naynawah and Dhook. Bull Endem Dis (Baghdad) 1969;11:117–133. [PubMed] [Google Scholar]
- Adekile AD. Sickle cell disease in Kuwait. Hemoglobin. 2001;25:219–225. doi: 10.1081/hem-100104030. [DOI] [PubMed] [Google Scholar]
- Adekile AD, Gu LH, Baysal E, Haider MZ, al-Fuzae L, Aboobacker KC, al-Rashied A, Huisman TH. Molecular characterization of alpha-thalassemia determinants, beta-thalassemia alleles, and beta S haplotypes among Kuwaiti Arabs. Acta Haematol. 1994;92:176–181. doi: 10.1159/000204216. [DOI] [PubMed] [Google Scholar]
- Aidoo M, Terlouw DJ, Kolczak MS, McElroy PD, ter Kuile FO, Kariuki S, Nahlen BL, Lal AA, Udhayakumar V. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet. 2002;359:1311–1312. doi: 10.1016/S0140-6736(02)08273-9. [DOI] [PubMed] [Google Scholar]
- Al Moamen NJ, Thabet A, Mahdi F, Newton H, Salman E. Various α-thalassemia genotype combinations of the Saudi-type polyadenylation signal mutation (α(T-Saudi)α) in the population of Bahrain: an update of genotype-phenotype analyses. Hemoglobin. 2018;42:166–170. doi: 10.1080/03630269.2018.1499523. [DOI] [PubMed] [Google Scholar]
- Al-Ali AK, Alsulaiman A, Alzahrani AJ, Obeid OT, Vatte CB, Cyrus C, Alnafie AN, Alali RA, Alfarhan M, Mozeleski B, Steinberg MH. Prevalence and diversity of haplotypes of sickle cell disease in the Eastern Province of Saudi Arabia. Hemoglobin. 2020;44:78–81. doi: 10.1080/03630269.2020.1739068. [DOI] [PubMed] [Google Scholar]
- Al-Allawi N. The preventive program for haemoglobinopathies in Duhok: an option or a necessity. Duhok Med J. 2008;2:1–4. [Google Scholar]
- Al-Allawi NA, Al-Dousky AA. Frequency of haemoglobinopathies at premarital health screening in Dohuk, Iraq: implications for a regional prevention programme. East Mediterr Health J. 2010;16:381–385. [PubMed] [Google Scholar]
- Al-Allawi NA, Jubrael JM, Hughson M. Molecular characterization of beta-thalassemia in the Dohuk region of Iraq. Hemoglobin. 2006;30:479–486. doi: 10.1080/03630260600868097. [DOI] [PubMed] [Google Scholar]
- Al-Allawi NA, Badi AI, Imanian H, Nikzat N, Jubrael JM, Najmabadi H. Molecular characterization of alpha-thalassemia in the Dohuk region of Iraq. Hemoglobin. 2009;33:37–44. doi: 10.1080/03630260802626053. [DOI] [PubMed] [Google Scholar]
- Al-Allawi NA, Hassan KM, Sheikha AK, Nerweiy FF, Dawood RS, Jubrael J. β-thalassemia mutations among transfusion-dependent thalassemia major patients in Northern Iraq. Molecular biology international. 2010;2010:479282. doi: 10.4061/2010/479282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Allawi NA, Shamdeen MY, Rasheed NS. Homozygosity for the Mediterranean a-thalassemic deletion (hemoglobin Barts hydrops fetalis) Ann Saudi Med. 2010;30:153–155. doi: 10.4103/0256-4947.60523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Allawi NA, Jalal SD, Nerwey FF, Al-Sayan GO, Al-Zebari SS, Alshingaly AA, Markous RD, Jubrael JM, Hamamy H. Sickle cell disease in the Kurdish population of northern Iraq. Hemoglobin. 2012;36:333–342. doi: 10.3109/03630269.2012.692344. [DOI] [PubMed] [Google Scholar]
- Al-Allawi NA, Al-Mousawi BM, Badi AI, Jalal SD. The spectrum of β-thalassemia mutations in Baghdad, Central Iraq. Hemoglobin. 2013;37:444–453. doi: 10.3109/03630269.2013.810641. [DOI] [PubMed] [Google Scholar]
- Al-Allawi NA, Jalal SD, Ahmed NH, Faraj AH, Shalli A, Hamamy H. The first five years of a preventive programme for haemoglobinopathies in Northeastern Iraq. J Med Screen. 2013;20:171–176. doi: 10.1177/0969141313508105. [DOI] [PubMed] [Google Scholar]
- Al-Allawi NA, Jalal SD, Rasheed NS, Bayat N, Imanian H, Najmabadi H, Faraj A. The spectrum of α-thalassemia mutations in the Kurdish population of Northeastern Iraq. Hemoglobin. 2013;37:56–64. doi: 10.3109/03630269.2012.749490. [DOI] [PubMed] [Google Scholar]
- Al-Allawi NA, Jalal SD, Mohammad AM, Omer SQ, Markous RS. β-thalassemia intermedia in Northern Iraq: a single center experience. Biomed Res Int. 2014;2014:262853. doi: 10.1155/2014/262853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Allawi NA, Al-Doski AA, Markous RS, Mohamad Amin KA, Eissa AA, Badi AI, Asmaro RR, Hamamy H. Premarital screening for hemoglobinopathies: experience of a single center in Kurdistan, Iraq. Public Health Genomics. 2015;18:97–103. doi: 10.1159/000368960. [DOI] [PubMed] [Google Scholar]
- Al-Allawi N, Qadir SMA, Puehringer H, Chui DHK, Farrell JJ, Oberkanins C. The association of HBG2, BCL11A, and HMIP polymorphisms with fetal hemoglobin and clinical phenotype in Iraqi Kurds with sickle cell disease. Int J Lab Hematol. 2019;41:87–93. doi: 10.1111/ijlh.12927. [DOI] [PubMed] [Google Scholar]
- Al-Awamy BH. Thalassemia syndromes in Saudi Arabia. Meta-analysis of local studies. Saudi Med J. 2000;21:8–17. [PubMed] [Google Scholar]
- Alibakhshi R, Moradi K, Aznab M, Dastafkan Z, Tahmasebi S, Ahmadi M, Omidniakan L. The spectrum of α-thalassemia mutations in Kurdistan Province, West Iran. Hemoglobin. 2020;44:156–161. doi: 10.1080/03630269.2020.1768863. [DOI] [PubMed] [Google Scholar]
- Aliyeva G, Asadov C, Mammadova T, Gafarova S, Guliyeva Y, Abdulalimov E. Molecular and geographical heterogeneity of hemoglobinopathy mutations in Azerbaijanian populations. Ann Hum Genet. 2020;84:249–258. doi: 10.1111/ahg.12367. [DOI] [PubMed] [Google Scholar]
- Al-Riyami AZ, Daar S, Kindi SA, Madhani AA, Wali Y, Rawahi MA, Zadjali SA. α-Globin genotypes associated with Hb H disease: a report from Oman and a review of the literature from the Eastern Mediterranean Region. Hemoglobin. 2020;44:20–26. doi: 10.1080/03630269.2020.1720709. [DOI] [PubMed] [Google Scholar]
- Al-Sultan A, Phanasgaonkar S, Suliman A, Al-Baqushi M, Nasrullah Z, Al-Ali A. Spectrum of β-thalassemia mutations in the eastern province of Saudi Arabia. Hemoglobin. 2011;35:125–134. doi: 10.3109/03630269.2011.553567. [DOI] [PubMed] [Google Scholar]
- Alswaidi FM, O’Brien SJ. Premarital screening programmes for haemoglobinopathies, HIV and hepatitis viruses: review and factors affecting their success. J Med Screen. 2009;16:22–28. doi: 10.1258/jms.2008.008029. [DOI] [PubMed] [Google Scholar]
- Aluoch JR, Kilinç Y, Aksoy M, Yüregir GT, Bakioglu I, Kutlar A, Kutlar F, Huisman TH. Sickle cell anaemia among Eti-Turks: haematological, clinical and genetic observations. Br J Haematol. 1986;64:45–55. doi: 10.1111/j.1365-2141.1986.tb07572.x. [DOI] [PubMed] [Google Scholar]
- Amin SS, Jalal SD, Ali KM, Mohammed AI, Rasool LK, Osman TJ. Beta-thalassemia intermedia: a single thalassemia center experience from Northeastern Iraq. Biomed Res Int. 2020;2020:2807120. doi: 10.1155/2020/2807120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angastiniotis M, Kyriakidou S, Hadjiminas M. How thalassemia was controlled in Cyprus. World Health forum. 1986;7:291–297. [Google Scholar]
- Arshi Z, Zabihi K. Kurdistan. Östersund: Oriental art publications; 1990. [Google Scholar]
- Baker F, Al-Quasi M. Sickle cell anemia in Iraq: first case report. 3. J Fac Med Baghdad. 1964;6:26–31. [Google Scholar]
- Bashir N, Barkawi M, Sharif L. Prevalence of haemoglobinopathies in school children in Jordan Valley. Ann Trop Paediatr. 1991;11:373–376. doi: 10.1080/02724936.1991.11747532. [DOI] [PubMed] [Google Scholar]
- Bashir N, Barkawi M, Sharif L, Momani A, Gharaibeh N. Prevalence of hemoglobinopathies in north Jordan. Trop Geogr Med. 1992;44:122–125. [PubMed] [Google Scholar]
- Baysal E. α-Thalassemia syndromes in the United Arab Emirates. Hemoglobin. 2011;35:574–580. doi: 10.3109/03630269.2011.634698. [DOI] [PubMed] [Google Scholar]
- Baysal E, Kleanthous M, Bozkurt G, Kyrri A, Kalogirou E, Angastiniotis M, Ioannou P, Huisman TH. alpha-Thalassaemia in the population of Cyprus. Br J Haematol. 1995;89:496–499. doi: 10.1111/j.1365-2141.1995.tb08354.x. [DOI] [PubMed] [Google Scholar]
- Bozdogan ST, Yuregir OO, Buyukkurt N, Aslan H, Ozdemir ZC, Gambin T. Alpha-thalassemia mutations in adana province, southern Turkey: genotype-phenotype correlation. Indian J Hematol Blood Transfus. 2015;31:223–228. doi: 10.1007/s12288-014-0406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canatan D, Oğuz N, Güvendik İ, Yıldırım S. The incidence of alpha-thalassemia in Antalya- Turkey. Turk J Haematol. 2002;19:433–434. [PubMed] [Google Scholar]
- Charafeddine K, Isma’eel H, Charafeddine M, Inati A, Koussa S, Naja M, Taher A. Survival and complications of beta-thalassemia in Lebanon: a decade’s experience of centralized care. Acta Haematol. 2008;120:112–116. doi: 10.1159/000171088. [DOI] [PubMed] [Google Scholar]
- Cürük MA. Hb H (beta4) disease in Cukurova, Southern Turkey. Hemoglobin. 2007;31:265–271. doi: 10.1080/03630260701297279. [DOI] [PubMed] [Google Scholar]
- Cürük MA, Dimovski AJ, Baysal E, Gu LH, Kutlar F, Molchanova TP, Webber BB, Altay C, Gürgey A, Huisman TH. Hb Adana or alpha 2(59)(E8)Gly-->Asp beta 2, a severely unstable alpha 1-globin variant, observed in combination with the -(alpha)20.5 Kb alpha-thal-1 deletion in two Turkish patients. Am J Hematol. 1993;44:270–275. doi: 10.1002/ajh.2830440410. [DOI] [PubMed] [Google Scholar]
- De Sanctis V, Kattamis C, Canatan D, Soliman AT, Elsedfy H, Karimi M, Daar S, Wali Y, Yassin M, Soliman N, Sobti P, Al Jaouni S, El Kholy M, Fiscina B, Angastiniotis M. β-Thalassemia distribution in the Old World: an ancient disease seen from a historical standpoint. Mediterr J Hematol. Infect Dis Ther. 2017;9:e2017018. doi: 10.4084/MJHID.2017.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehbozorgian J, Moghadam M, Daryanoush S, Haghpanah S, Imani Fard J, Aramesh A, Shahsavani A, Karimi M. Distribution of alpha-thalassemia mutations in Iranian population. Hematology. 2015;20:359–362. doi: 10.1179/1607845414Y.0000000227. [DOI] [PubMed] [Google Scholar]
- Durand PM, Coetzer TL. Hereditary red cell disorders and malaria resistance. Haematologica. 2008;93:961–963. doi: 10.3324/haematol.13371. [DOI] [PubMed] [Google Scholar]
- Eissa AA, Kashmoola MA, Atroshi SD, Al-Allawi NA. Molecular characterization of β-thalassemia in Nineveh Province illustrates the relative heterogeneity of mutation distributions in Northern Iraq. Indian J Hematol Blood Transfus. 2015;31:213–217. doi: 10.1007/s12288-014-0369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei YJ, Kutlar F, Harris HF, 2nd, Wilson MM, Milana A, Sciacca P, Schiliro G, Masala B, Manca L, Altay C, et al. A search for anomalies in the zeta, alpha, beta, and gamma globin gene arrangements in normal black, Italian, Turkish, and Spanish newborns. Hemoglobin. 1989;13:45–65. doi: 10.3109/03630268908998052. [DOI] [PubMed] [Google Scholar]
- Galanello R, Eleftheriou A, Traaeger-Synedions J, Petrou M, Angastiniotis M. Prevention of thalassemias and other hemoglobin disorders. Nicosia: TIF Publications; 2003. [Google Scholar]
- Guvenc B, Yildiz SM, Tekinturhan F, Dincer S, Akyuzluer I, Okten S, Erkman H. Molecular characterization of alpha-Thalassemia in Adana, Turkey: a single center study. Acta Haematol. 2010;123:197–200. doi: 10.1159/000302203. [DOI] [PubMed] [Google Scholar]
- Hadavi V, Taromchi AH, Malekpour M, Gholami B, Law HY, Almadani N, Afroozan F, Sahebjam F, Pajouh P, Kariminejad R, Kariminejad MH, Azarkeivan A, Jafroodi M, Tamaddoni A, Puehringer H, Oberkanins C, Najmabadi H. Elucidating the spectrum of alpha-thalassemia mutations in Iran. Haematologica. 2007;92:992–993. doi: 10.3324/haematol.10658. [DOI] [PubMed] [Google Scholar]
- Haghi M, Khorshidi S, Hosseinpour Feizi MA, Pouladi N, Hosseinpour Feizi AA. beta-Thalassemia mutations in the Iranian Kurdish population of Kurdistan and West Azerbaijan provinces. Hemoglobin. 2009;33:109–114. doi: 10.1080/03630260902862020. [DOI] [PubMed] [Google Scholar]
- Hamamy HA, Al-Allawi NA. Epidemiological profile of common haemoglobinopathies in Arab countries. J Community Genet. 2013;4:147–167. doi: 10.1007/s12687-012-0127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamy H, Alwan A. Hereditary disorders in the Eastern Mediterranean Region. Bull World Health Organ. 1994;72:145–154. [PMC free article] [PubMed] [Google Scholar]
- Hassan MK, Taha JY, Al-Naama LM, Widad NM, Jasim SN. Frequency of haemoglobinopathies and glucose-6-phosphate dehydrogenase deficiency in Basra. East Mediterr Health J. 2003;9:45–54. [PubMed] [Google Scholar]
- Hellani A, Fadel E, El-Sadadi S, El-Sweilam H, El-Dawood A, Abu-Amero KK. Molecular spectrum of alpha-thalassemia mutations in microcytic hypochromic anemia patients from Saudi Arabia. Genet Test Mol Biomark. 2009;13:219–221. doi: 10.1089/gtmb.2008.0123. [DOI] [PubMed] [Google Scholar]
- Higgs DR, Goodbourn SE, Lamb J, Clegg JB, Weatherall DJ, Proudfoot NJ. Alpha-thalassaemia caused by a polyadenylation signal mutation. Nature. 1983;306:398–400. doi: 10.1038/306398a0. [DOI] [PubMed] [Google Scholar]
- Hosseinpour Feizi MA, Hosseinpour Feizi AA, Pouladi N, Haghi M, Azarfam P. Molecular spectrum of beta-thalassemia mutations in Northwestern Iran. Hemoglobin. 2008;32:255–261. doi: 10.1080/03630260802004145. [DOI] [PubMed] [Google Scholar]
- Ince HH, Ayyildiz O, Kalkanli S, Batun S, Muftuoglu E. Molecular basis of beta-thalassemia mutations in Diyarbakir in the southeastern region of Turkey. Hemoglobin. 2003;27:275–278. doi: 10.1081/hem-120026055. [DOI] [PubMed] [Google Scholar]
- Izady M. The Kurds: a concise handbook. Washington: Taylor and Francis International Publishers; 1992. [Google Scholar]
- Jalal SD, Al-Allawi NA, Faraj AH, Ahmed NH. Prevalence of haemoglobinopathies in Sulaimani–Iraq. Duhok Med J. 2008;2:71–97. [Google Scholar]
- Jalal SD, Al-Allawi NA, Bayat N, Imanian H, Najmabadi H, Faraj A. β-Thalassemia mutations in the Kurdish population of northeastern Iraq. Hemoglobin. 2010;34:469–476. doi: 10.3109/01676830.2010.513591. [DOI] [PubMed] [Google Scholar]
- Jassim N, Al-Arrayed S, Gerard N, Al-Mukharraq H, Al-Ajami A, Ducrocoq R, Nagel R, Krishnamoorthy R. Molecular basis of α-thalassemia in Bahrain. Bahrain Med Bull. 2001;23:3–7. [Google Scholar]
- Kadhim KA, Baldawi KH, Lami FH. Prevalence, incidence, trend, and complications of thalassemia in Iraq. Hemoglobin. 2017;41:164–168. doi: 10.1080/03630269.2017.1354877. [DOI] [PubMed] [Google Scholar]
- Kanavakis E, Papassotiriou I, Karagiorga M, Vrettou C, Metaxotou-Mavrommati A, Stamoulakatou A, Kattamis C, Traeger-Synodinos J. Phenotypic and molecular diversity of haemoglobin H disease: a Greek experience. Br J Haematol. 2000;111:915–923. [PubMed] [Google Scholar]
- Karimi M, Rasekhi AR. Efficiency of premarital screening of beta-thalassemia trait using MCH rather than MCV in the population of Fars Province, Iran. Haematologia (Budap) 2002;32:129–133. doi: 10.1163/156855902320387961. [DOI] [PubMed] [Google Scholar]
- Kutlar A. Sickle cell disease: a multigenic perspective of a single gene disorder. Hemoglobin. 2007;31:209–224. doi: 10.1080/03630260701290233. [DOI] [PubMed] [Google Scholar]
- Mehrabi M, Alibakhshi R, Fathollahi S, Farshchi MR. The spectrum of β-thalassemia mutations in Kermanshah Province in West Iran and its association with hematological parameters. Hemoglobin. 2013;37:544–552. doi: 10.3109/03630269.2013.814036. [DOI] [PubMed] [Google Scholar]
- Murad H, Moasses F, Dabboul A, Mukhalalaty Y, Bakoor AO, Al-Achkar W, Jarjour Rami A, RA, Geographical distribution of β-globin gene mutations in Syria. Hematology. 2018;23:697–704. doi: 10.1080/10245332.2018.1461291. [DOI] [PubMed] [Google Scholar]
- Najmabadi H, Karimi-Nejad R, Sahebjam S, Pourfarzad F, Teimourian S, Sahebjam F, Amirizadeh N, Karimi-Nejad MH. The beta-thalassemia mutation spectrum in the Iranian population. Hemoglobin. 2001;25:285–296. doi: 10.1081/hem-100105221. [DOI] [PubMed] [Google Scholar]
- Niazi AD. Malaria situation in Zakho Qadha--Mosul Liwa. Bull Endem Dis (Baghdad) 1968;10:185–194. [PubMed] [Google Scholar]
- Paridar M, Azizi E, Keikhaei B, Takhviji V, Baluchi I, Khosravi A. Iranian patients with hemoglobin H disease: genotype-phenotype correlation. Mol Biol Rep. 2019;46:5041–5048. doi: 10.1007/s11033-019-04955-9. [DOI] [PubMed] [Google Scholar]
- Polus R. Prevalence of Hemoglobinopathies among marrying couples in Erbil province of Iraq. Iraqi J Hematol. 2017;6:90–93. [Google Scholar]
- Qaddoumi AA, Kamal N, Shbailat T. Molecular spectrum of alpha-thalassemia in Jordan. J R Med Serv. 2008;5:23–27. [Google Scholar]
- Rahimi Z, Muniz A, Parsian A. Detection of responsible mutations for beta thalassemia in the Kermanshah Province of Iran using PCR-based techniques. Mol Biol Rep. 2010;37:149–154. doi: 10.1007/s11033-009-9560-0. [DOI] [PubMed] [Google Scholar]
- Rahimi Z, Najafi S, Moghofehie L, Amiri E, Vaisi-Raygani A, Rahimi Z. The prevalence of hemoglobinopathies in reference laboratory of Kermanshah, Western Iran. Iran J Public Health. 2019;48:359–361. [PMC free article] [PubMed] [Google Scholar]
- Rund D, Cohen T, Filon D, Dowling CE, Warren TC, Barak I, Rachmilewitz E, Kazazian HH, Jr, Oppenheim A. Evolution of a genetic disease in an ethnic isolate: beta-thalassemia in the Jews of Kurdistan. Proc Natl Acad Sci U S A. 1991;88:310–314. doi: 10.1073/pnas.88.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamoon RP. Molecular spectrum of α-thalassemia mutations in Erbil province of Iraqi Kurdistan. Mol Biol Rep. 2020;47:6067–6071. doi: 10.1007/s11033-020-05681-3. [DOI] [PubMed] [Google Scholar]
- Shamoon RP, Al-Allawi NA, Cappellini MD, Di Pierro E, Brancaleoni V, Granata F. Molecular basis of β-thalassemia intermedia in Erbil Province of Iraqi Kurdistan. Hemoglobin. 2015;39:178–183. doi: 10.3109/03630269.2015.1032415. [DOI] [PubMed] [Google Scholar]
- Shamoon RP, Yassin AK, Polus RK, Ali M. Genotype-phenotype correlation of HbH disease in northern Iraq. BMC Med Genet. 2020;21:203. doi: 10.1186/s12881-020-01141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadmouri GO, Tüzmen S, Ozçelik H, Ozer A, Baig SM, Senga EB, Başak AN. Molecular and population genetic analyses of beta-thalassemia in Turkey. Am J Hematol. 1998;57:215–220. doi: 10.1002/(sici)1096-8652(199803)57:3<215::aid-ajh6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Taj-Eldin S, al-Rabii H, Jawad J, Fakhri O. Thalassaemia in Iraq. Ann Trop Med Parasitol. 1968;62:147–153. doi: 10.1080/00034983.1968.11686542. [DOI] [PubMed] [Google Scholar]
- Thein SL, Menzel S, Lathrop M, Garner C. Control of fetal hemoglobin: new insights emerging from genomics and clinical implications. Hum Mol Genet. 2009;18:R216–R223. doi: 10.1093/hmg/ddp401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun F, Bilgin A, Kızılok A, Arpacı A, Yüreğir GT. Five-year evaluation of premarital screening program for hemoglobinopathies in the province of Mersin, Turkey. Turk J Haematol. 2006;23:84–89. [PubMed] [Google Scholar]
- Valaei A, Karimipoor M, Kordafshari A, Zeinali S. Molecular basis of α-thalassemia in Iran. Iran Biomed J. 2018;22:6–14. doi: 10.22034/ibj.22.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatheral D, Clegg J. Thalassemia syndromes. Oxford: Blackwell Publishers; 2001. [Google Scholar]
- Yahya H, Khalel K, Allawi NA, Hilmi FF. Thalassaemia genes in Baghdad-Iraq. East Mediterr Health J. 1996;2:315–319. [Google Scholar]
- Yaseen N, Al-Mamoori H, Hassan M. Sickle β-globin haplotypes among patients with sickle cell anemia in Basra, Iraq: a cross sectional study. Iraqi J Hematol. 2020;2020:9. [Google Scholar]
- Yıldız S, Atalay A, Bağcı H, Atalay E. Beta-thalassemia mutations in Denizli province of Turkey. Turk J Haematol. 2005;22:19–23. [PubMed] [Google Scholar]
- Zahed L, Bou-Dames J. Acceptance of first-trimester prenatal diagnosis for the haemoglobinopathies in Lebanon. Prenat Diagn. 1997;17:423–428. doi: 10.1002/(sici)1097-0223(199705)17:5<423::aid-pd68>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]