Abstract
Non-invasive prenatal testing (NIPT), is a prenatal screening test for chromosomal aneuploidies (trisomy 21, trisomy 18, and trisomy 13). While women under 35 years of age with no other risk factors are considered low risk for pregnancies with aneuploidy, most babies with aneuploidy are born to low-risk women. Across the USA, including Wisconsin, many private insurances do not cover initial NIPT for low-risk women, creating a potential financial burden that may limit patient selection of NIPT. Low-risk women with public insurance in Wisconsin are covered for NIPT. This pilot study determined if a difference exists in NIPT uptake based on insurance type in low-risk pregnant women in their first trimester. It also explored genetic counselor perspectives on how insurance coverage for NIPT is addressed with patients. Women with public insurance were 3.43 times more likely to have NIPT as an initial screen for aneuploidy than women with private insurance, indicating that insurance coverage may present a barrier to care. Additionally, analysis showed no evidence of different demographic variables interacting with another to impact outcome after allowing for insurance coverage (X214 = 14.301, p = 0.428). Our data also suggests that more genetic counselors would recommend NIPT to patients if insurance coverage was not a barrier and were more likely to discuss financial risks associated with NIPT when a patient had private insurance. We conclude that some women cannot choose one of the safest and most sensitive prenatal aneuploidy screening tests due to financial barriers put into place by the lack of insurance coverage.
Keywords: NIPT, Insurance coverage, Health disparities, FTS, Genetic counseling, Prenatal
Introduction
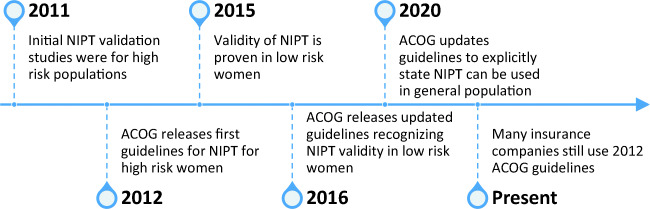
In 2012, the American College of Obstetrics and Gynecology (ACOG) recommended that all pregnant women be offered the option of aneuploidy screening or diagnostic testing for fetal genetic conditions (American College of Obstetrics and Gynecology 2012). Chromosomal aneuploidies most commonly screened for during prenatal care are Trisomy 21, Trisomy 18, and Trisomy 13. Women considered at high risk for aneuploidy include those 35 years of age or older at delivery (a.k.a. advanced maternal age (AMA)), fetal ultrasound findings indicating an increased risk for aneuploidy, history of prior pregnancy with aneuploidy, positive maternal screening (e.g., first trimester screen or quad screen), or parental balanced Robertsonian translocation (American College of Obstetrics and Gynecology 2012). Those considered low risk do not meet any of the previously mentioned criteria. Initially, the 2012 ACOG guidelines stated non-invasive prenatal testing (NIPT) should be used for women with high-risk factors. In 2016, ACOG updated their guidelines and did not limit clinical use of NIPT with specific criteria (American College of Obstetrics and Gynecology 2016). Most recently in 2020, ACOG released a new practice bulletin explicitly stating that all patients should be offered both screening and diagnostic testing options regardless of risk status (American College of Obstetrics and Gynecology 2020). Fig. 1 depicts a timeline of NIPT validity studies and ACOG guidelines for NIPT.
Fig. 1.
A timeline of validity studies and ACOG guidelines. Oftentimes, there is a lag between validity studies, updated guidelines, and payor response
Diagnostic testing via amniocentesis, where fetal cells in the amniotic fluid are sampled, or chorionic villus sampling (CVS), where cells from the fetal-derived part of the placenta are sampled, are inherently invasive, elevating the risk of fetal loss compared to a blood-based screening test. Screening tests, such as non-invasive prenatal testing (NIPT) and First Trimester Screening (FTS), provide risk assessments using markers found in maternal blood, are considered non-invasive, and require confirmation through diagnostic testing for any abnormal findings.
NIPT, also known as cell-free fetal DNA testing, is a screening test that quantifies maternal and placental DNA fragments within the maternal bloodstream rather than sampling fetal tissues to detect imbalances in the quantity of DNA associated with the three most common chromosomal aneuploidies. NIPT was introduced in 2011 and was initially used to screen pregnancies with high risk for aneuploidy. Since NIPT is performed with maternal blood rather than sampling fetal tissue, it offers a non-invasive and safer option for testing than diagnostic testing while maintaining a high level of detection.
Another non-invasive screening test, First Trimester Screening (FTS), combines information from a maternal blood test that looks at hormones and metabolite levels, an ultrasound around 11–14 weeks gestation, and information about the pregnant women (e.g., weight and gestational age), to determine a personalized risk assessment for the woman to have a fetus with trisomy 21, trisomy 18, or trisomy 13.
NIPT, however, can screen for additional genetic conditions that FTS cannot. In addition to the three common chromosomal aneuploidies, it can also screen for imbalances in DNA associated with the sex chromosomes (e.g., 45, X, 47, XXY, 47, XXX, 47, XYY), additional trisomies (e.g., trisomy 16, trisomy 22), and select microdeletion syndromes (e.g., 22q11.2 deletion syndrome, Cri du Chat Syndrome (5p deletion)) (Lefkowitz et al. 2016). With more recent advances, there is also the ability to screen for de novo autosomal dominant conditions caused by single gene mutations (e.g., craniosynostosis syndromes, Noonan Syndrome, achondroplasia). When focusing on the conditions that both FTS and NIPT can screen, NIPT has increased sensitivity and specificity compared to FTS, with a 99.1% sensitivity and 99.9% specificity for trisomy 21, compared to a 93% sensitivity for FTS. For trisomy 18, NIPT has a > 99.9% sensitivity and 99.6% specificity compared to a 95% sensitivity for FTS. NIPT for trisomy 13 has a 91.7% sensitivity and 99.7% specificity, while FTS has a 95% sensitivity (Integrated Genetics 2019; NTD-Eurofins 2019). As for the conditions that NIPT alone has the capability to screen for, NIPT can detect sex chromosome aneuploidies with a sensitivity of 96.2% and specificity of 99.7% (Integrated Genetics 2019), and microdeletion syndromes sensitivity ranges from 86.2 to 99.9% and specificity ranges from 99.4 to 100% (Lefkowitz et al. 2016).
Despite the safety, sensitivity, and specificity of NIPT, it is not always used consistently in prenatal care. Past research has demonstrated that patients have an enthusiastic interest in the information NIPT offers and for increasing access to NIPT (Vanstone et al. 2019). Common themes among research studies across numerous countries with a variety of healthcare system models showed that patients preferred NIPT due to the increased detection rate, lower false positive and negative rates, minimal risk to the fetus, and the early availability compared to FTS and diagnostic testing (Allyse et al. 2014; Farrell et al. 2014; Tiller et al. 2015). A major concern of women in past studies was the cost and potential inequitable access to NIPT (Vanstone et al. 2019). There is little quantitative data that explores these barriers and disparities that exist in offering and/or selecting NIPT.
Some studies have shown that there has been a significant increase of NIPT selection since the introduction of the test in 2011. One study that explored NIPT in high-risk women, largely due to AMA, found that the use of invasive diagnostic testing significantly decreased in patients that first underwent NIPT. This suggested that NIPT was a desirable testing option to many women and enabled them to make value-based decisions to avoid complications of invasive testing such as miscarriage (Beamon et al. 2014). More recently in 2018, a study comparing the selection of NIPT in women over 35 years old (i.e., high-risk population) versus women under 35 years old (i.e., low-risk population) found an increase in the selection of NIPT in the low-risk population compared to selection rates in previous years. In fact, more women under 35 years of age had NIPT done compared to women over 35 years old, suggesting that women of any age desired NIPT and that their values aligned with having NIPT instead of FTS. In both age groups, the selection of NIPT has increased over time (Chen et al. 2019). From an ethical purview, questions have been discussed about whether NIPT should be part of public health services and if costs should be reimbursed by health insurance companies, a controversial topic worldwide. They noted that many women are limited by financial barriers when choosing prenatal screening options, especially NIPT. In 2016, costs of NIPT had the potential to be equal to or exceed the average monthly income per household in low- and middle-income countries (Rolfes and Schmitz 2016).
In the USA, there are two main methods for health insurance coverage: publicly funded insurance (e.g., Medicaid, Medicare) and commercial private insurance. In order to qualify for public insurance, certain criteria need to be met. Federal laws require states to provide coverage to low-income families defined as income at or below 133% of the federal poverty level, qualifying pregnant women and children, and individuals receiving Supplemental Security Income (Medicaid 2020). Individuals with public insurance do not incur a cost for having insurance coverage like a premium and often times do not receive medical bills due to the federal and state tax contributions. Private insurance, on the other hand, can be obtained through an employer or the Affordable Care Act Marketplace. There are many different private insurance companies and policies, with different premiums and coverage allotted.
There has been little published quantitative research to date on how the selection of NIPT differs between different demographic groups of patients, including different socioeconomic groups where health disparities are known to be prevalent. Typically, health disparities largely impact individuals with limited resources due to factors such as insurance coverage, low socioeconomic status, unemployment, lack of proximity to or transportation to hospitals and clinics, language or cultural barriers, or other sociocultural factors (Health Disparities 2020). Many individuals with limited resources do have public insurance, but there remains a large number of individuals who do not qualify for public insurance and still face socioeconomic struggles. Perhaps paradoxically, the financial burden of uncovered costs or co-pays for patients who have private insurance coverage is a disparity in terms of NIPT. The cost to a patient to have NIPT can vary depending on billing practices (i.e., patient self-pay vs. institutional bill with contracted prices vs. direct bill to insurance). As an example, self-pay prices for NIPT can range from $299 to 349, yet list prices can range from $1100 to 1590 (Integrated Genetics Laboratory 2020; Natera Laboratory 2020). Some laboratories offer financial assistance and lower patient self-pay prices compared to the list price, which may be affordable for some. For others, their self-pay prices could still present a barrier to equitable care between women with private insurance and women with public insurance.
In 2016, ACOG stated that NIPT could be offered to the general obstetric population given it has similar sensitivity and specificity as the high risk population (American College of Obstetrics and Gynecology 2016). However, many insurance companies still use the 2012 ACOG guidelines for determination of insurance coverage for NIPT. In Wisconsin, low-risk women with public insurance (e.g., Medicaid) do not incur a financial burden for NIPT ordered by their provider, regardless of whether they meet the clinical criteria for NIPT laid out by ACOG in 2012. This effectively eliminates any financial barrier for these women considering NIPT as they will not incur a cost for testing. Thus, it may be assumed that women who elect not to undergo NIPT would be due to personal values and not because of a financial burden of testing, although there has not been research done on this rationale to date.
On the other hand, low-risk women with private insurance find that NIPT is often not covered in their insurance plan since many insurance companies still follow the 2012 ACOG guidelines. Instead, multiple national private insurance companies consider FTS medically necessary for any pregnant woman who wants it, regardless of risk factors and so they will authorize this screening. Yet, NIPT is only considered medically necessary by many insurance companies when high-risk criteria are met (Aetna 2020). The regional private insurance company that a majority of women in our study have has a similar policy that is based on the 2012 ACOG guidelines (Quartz 2019). While private insurance companies typically do not cover NIPT, they often cover other less sensitive and specific screens, such as FTS or maternal serum quad screening, or they approve and cover diagnostic, invasive procedures such as amniocentesis and CVS for any woman, regardless of risk factors. These low-risk women with private insurance may have a financial barrier to NIPT; the option to have NIPT may rely on the woman’s ability to pay out-of-pocket or self-pay options, rather than full coverage benefits from their private insurance policy. This creates a barrier to equitable care between women with private insurance and public insurance.
Given that NIPT is a simple blood test, it can be ordered by a wide range of providers at any local medical provider’s office and does not require a woman to travel to a specialty clinic. The 2016 and 2020 ACOG guidelines state that “screening for aneuploidy should be an informed patient choice, with an underlying foundation of shared decision making that fits the patient’s clinical circumstances, values, interests, and goals” (American College of Obstetrics and Gynecology 2016; American College of Obstetrics and Gynecology 2020). Past studies have also shown that a major concern of NIPT is not receiving the proper informed consent for the test and the need for a formal consent process (Cernat et al. 2019; Farrell et al. 2014). Prenatal genetic counselors educate women on their different testing options and help facilitate the process of making an informed choice that is in alignment with their values. In this study, we focused on women who had met with a genetic counselor prior to deciding what prenatal testing option they desired (i.e., FTS, NIPT, or a diagnostic test) and the financial impact of each. This study also surveyed practicing prenatal genetic counselors in the state of Wisconsin about their perspectives on discussing insurance coverage for NIPT with patients.
Based on past literature demonstrating that women preferred NIPT to FTS and diagnostic testing (Allyse et al. 2014; Vanstone et al. 2019) and the idea that cost of testing presents a barrier to equitable care, we hypothesized that low-risk women with private insurance elect whether or not to have NIPT based on factors of financial burden (e.g., whether their insurance will pay for the test). Our study design was limited to retrospective chart review and we did not survey patients to directly evaluate their rationale behind testing choice. As a proxy outcome, we assessed whether decisions to undergo NIPT during the first trimester of pregnancy in low-risk women were associated with a difference in insurance coverage.
Methods
Patient data
Patient records of women who had received genetic counseling services during the 2018 calendar year at UnityPoint Health-Meriter, Madison, WI, USA were reviewed. UnityPoint Health-Meriter is a University of Wisconsin affiliated hospital that provided prenatal ultrasounds for approximately 12,000 women in 2018 and is the largest delivering hospital in the state of Wisconsin. This study was approved by the UnityPoint Health-Meriter Institutional Review Board (Study #2019-027).
Women who have met with their primary care obstetrics provider and have decided to pursue some form of aneuploidy screening are referred to UnityPoint Health-Meriter between 10 and 14 weeks gestation for a first trimester screening ultrasound and are also seen by a prenatal genetic counselor at the time of the first trimester ultrasound to discuss testing options including FTS, NIPT, as well as diagnostic testing (CVS and amniocentesis), so they can make an informed decision best for them, which is considered standard of care. All prenatal ultrasounds and clinical consults, including genetic counseling, use GE Viewpoint™ software for reporting and storage. As such, patients who met with a prenatal genetic counselor were identified via the ViewPoint™ database using Current Procedural Terminology (CPT) codes associated with first trimester screening ultrasound visits during the timeframe of January 1, 2018 through December 31, 2018. Data from all women seen by prenatal genetic counselors in 2018 who were low risk for aneuploidy based on age (i.e., under the age of 35) and less than 14 weeks gestation were initially considered. If a woman meeting the initial criteria had also received an ultrasound diagnosis that would classify them as high risk, they were subsequently excluded from our study. Additionally, we only focused on women that selected either NIPT or FTS. We did not track or collect data on women who declined all testing or who decided to pursue a diagnostic test as a first-tier test. The assumptions that private insurance would not cover NIPT was based on the most common regional insurance provider’s policy and that patients would incur a financial burden, as well as the fact that patients with public insurance would not incur a financial burden for NIPT based on the billing structure of public insurance were used.
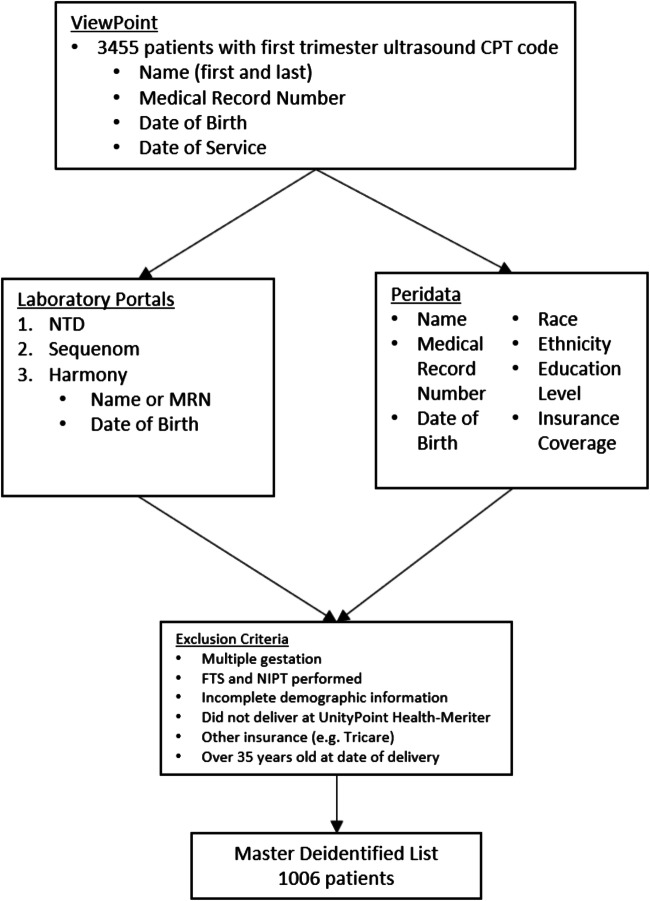
Using this criteria, 3455 records were initially pulled, with the goal of identifying a sample size of at least 1001 patients for the desired statistical power for the study. Sample size was determined assuming that the uptake of NIPT for low-risk women with private insurance would be low (15%), while low-risk women with public insurance would have an acceptance rate that was at least 10 percentage points higher. If assumptions were correct, a total sample size of 1001 would provide a 95% chance of detecting the effect at the 0.05 level of significance.
As displayed in Fig. 2, patients from the preliminary list were then referenced through Peridata (Perinatalweb.org), a statewide platform used by hospitals in Wisconsin to track birth record information. Using the timeframe of January 1, 2018 through July 31, 2019, patient’s date of delivery was confirmed, as Perdiata sorts information based on date of delivery. The validity of patient information was also verified using medical records numbers (MRN). Information on race, ethnicity, education level, and insurance type was collected. Insurance type was documented at time of delivery. While it is possible that insurance coverage could have changed from the first trimester of pregnancy when testing took place to time of delivery, there was no way to elucidate those differences through the databases used. We cross-referenced the list from GE ViewPoint™ with Peridata to provide demographic information. All samples sent for FTS are processed through NTD-Eurofins Laboratory, and samples for NIPT during this time frame were sent to either Ariosa Laboratory or Integrated Genetics Laboratory. All FTS or NIPT ordered from these labs from January 1, 2018 through December 31, 2018 were imported into the master patient list curated from GE ViewPoint™. This master dataset was cross-checked for exclusion criteria prior to being deidentified. Full exclusion criteria included (1) women equal to or older than 35 years old at delivery, (2) women who received both FTS and NIPT in their pregnancy (as this would imply abnormal FTS leading to follow up with NIPT), (3) multiple gestations, (4) women with incomplete demographic information, (5) women who delivered outside of the UnityPoint Health-Meriter Hospital (as this would lead to incomplete demographic data obtained from Peridata), and (6) other insurances such as Tricare, given that this policy differs from Medicaid and private companies. Once all exclusion criteria had been applied, 1006 patients remained as our study cohort.
Fig. 2.
Flow of data from different databases to final patient list
Statistical analysis of patient data
The dataset was divided into four different categories for analysis: public insurance and NIPT, public insurance and FTS, private insurance and NIPT, and private insurance and FTS. An odds ratio was performed using R (version 3.6.3) to determine if there was statistical significance between women with public insurance who underwent NIPT and women with private insurance who did not undergo NIPT. A binomial logistic regression was used to model the odds of accepting NIPT as a function of education, race, ethnicity, and type of insurance. Likelihood ratio tests were performed to determine if the variables, either in combination or individually, were associated with testing choice, and if any of the variables interacted with each other.
Genetic counselor survey
An online electronic Qualtrics (Qualtrics, Provo, UT) survey of genetic counselors practicing prenatal genetic counseling in the state of Wisconsin was developed and conducted. To identify eligible individuals for the survey, a recruitment email was sent in February 2020 to the Wisconsin Genetic Counselor Association (WIGCA) list maintained by WIGCA. To be eligible for the survey, individuals must have self-identified as a genetic counselor who provided prenatal genetic counseling services as part of their job within the past 2 years in the state of Wisconsin. Not all of the genetic counselors in this survey participated in the care of our patient population. Consent was obtained from all survey participants. A total of 17 individuals participated in the survey. Although the WIGCA has a total of 120 genetic counselor members, 51 genetic counselors self-identified with WIGCA as providing prenatal genetic counseling. Assuming that there were 51 potential participants meeting the criteria, the response rate was 33%.
The prenatal genetic counselor survey had 16 items that included a mixture of five-point Likert style questions and multiple-choice questions. Data collected included demographic information (e.g., percentage of practice spent in a prenatal setting, number of years of experience, region of state). Questions about their experience with chromosomal aneuploidy testing and screening (FTS, NIPT, CVS, or amniocentesis), such as what type of screening, and how often, they routinely offer screening to pregnant women less than 14 weeks gestation and under 35 years of age, how often they discuss financial risks with patients, when do they determine a patient’s insurance coverage, as well as their perception on how much insurance coverage influences their counseling approach were asked. A copy of the survey questions can be found in Appendix 1. The survey data was then compiled and analyzed, and responses were grouped into two different categories: all/most or sometimes/rarely/never depending on the question asked (e.g., when they offered NIPT and when they looked at a patient’s insurance coverage).
Results
Demographic information for patient data was recorded (Table 1). Data comparing uptake of NIPT versus FTS by type of insurance, public or private, were analyzed using a likelihood ratio test for 2 × 2 tables (Table 2), with the test inverted to produce a 95% confidence interval for the odds ratio. The odds of accepting NIPT among low-risk women with public insurance are 3.43 (95% CI: 2.50–4.71; p < 0.001) times the odds of accepting NIPT for low -risk women with private insurance. Phrased another way, the proportion of women on public insurance who chose NIPT is 26.6 (95% CI: 19.4–33.7; p < 0.001) percentage points higher than the proportion of women with private insurance who chose NIPT.
Table 1.
Patient demographics
| Category | Total (n = 1006) | Percentage of total | Public (%) | Private (%) |
|---|---|---|---|---|
| Insurance type | ||||
| Private insurance | 787 | 78% | – | – |
| Public insurance | 219 | 22% | – | – |
| Race | ||||
| Non-White | 215 | 21% | 90 (42%) | 125 (58%) |
| American Indian or Alaskan Native | 2 | < 1% | 0 (0%) | 2 (100%) |
| Asian | 53 | 5% | 7 (13%) | 46 (87%) |
| Asian Indian | 35 | 3% | 4 (11%) | 31 (89%) |
| Black or African American | 73 | 7% | 53 (73%) | 20 (27%) |
| Other | 17 | 2% | 10 (59%) | 7 (41%) |
| Not reported | 35 | 3% | 16 (46%) | 19 (54%) |
| White | 791 | 79% | 129 (16%) | 662 (84%) |
| Ethnicity | ||||
| Hispanic | 107 | 11% | 62 (58%) | 45 (42%) |
| Not Hispanic | 893 | 89% | 156 (17%) | 737 (83%) |
| Unknown | 6 | < 1% | 1 (17%) | 5 (83%) |
| Education | ||||
| No College | 207 | 20% | 128 (62%) | 79 (38%) |
| 8th grade or less | 6 | 1% | 6 (100%) | 0 (0%) |
| 9th–12th grade; no diploma | 45 | 4% | 36 (80%) | 9 (20%) |
| High school degree or GED | 155 | 15% | 86 (55%) | 69 (45%) |
| Unknown | 1 | < 1% | 0 (0%) | 1 (100%) |
| Some college | 799 | 80% | 91 (11%) | 708 (89%) |
| Some college credit; no degree | 129 | 13% | 44 (34%) | 85 (66%) |
| Associate degree | 103 | 10% | 24 (23%) | 79 (77%) |
| Bachelor’s degree | 345 | 34% | 16 (5%) | 329 (95%) |
| Master’s degree | 146 | 15% | 4 (3%) | 142 (97%) |
| Doctorate or professional degree | 76 | 8% | 3 (4%) | 73 (96%) |
*Percentages may not add up to exactly 100% due to rounding at .5 to the next whole number
Table 2.
Individuals with private insurance versus public insurance and their choice of testing
| NIPT (n (%)) | FTS (n (%)) | Total | |
|---|---|---|---|
| Private Insurance | 163 (.207) | 625 (.793) | 788 |
| Public Insurance | 103 (.472) | 115 (.518) | 218 |
| Total | 256 | 740 | 1006 |
We also cross-compared if education, race, and ethnicity played a role in determining whether NIPT was selected by a patient (Table 3). The data showed no evidence that the four different variables interact with one another (X211 = 12.631, p = 0.318). There is no indication that education, race, or ethnicity has a significant association with the odds ratio of NIPT after the allowance was made for type of insurance (X214 = 14.301, p = 0.428). Although each variable is separately associated with NIPT, the associations disappear once the allowance is made for type of insurance.
Table 3.
Race, education, ethnicity, and insurance and choice of NIPT
| Chi-square (df) | p value | OR (95% CI) | |
|---|---|---|---|
| Insurance alone (private/public) | 57.249 (1) | < 0.001 | 3.43 (2.50, 4.71) |
| Education (none/some) | |||
| Ignoring insurance | 18.525 (1) | < 0.001 | 2.06 (1.49, 2.85) |
| Adjusting for insurance | 0.324 (1) | 0.569 | 1.12 (0.76, 1.64) |
| Race (non-white/white) | |||
| Ignoring insurance | 6.637 (1) | 0.011 | 1.54 (1.11, 2.13) |
| Adjusting for insurance | 0.370 (1) | 0.543 | 1.12 (0.78, 1.58) |
| Ethnicity (Hispanic/Non-Hispanic) | |||
| Ignoring insurance | 10.606 (1) | 0.001 | 2.02 (1.33, 3.06) |
| Adjusting for insurance | 0.979 (1) | 0.322 | 1.26 (0.80, 1.97) |
| Insurance (public/private) | |||
| Ignoring others | 58.260 (1) | < 0.001 | 3.46 (2.52, 4.75) |
| Adjusting for others | 32.296 (1) | < 0.001 | 3.06 (2.08, 4.51) |
Results from the survey sent to prenatal genetic counselors in Wisconsin (Table 4) demonstrated that they typically offer both FTS and NIPT to their low-risk patients. While not statistically significant due to the small number of participants, our data suggests that more genetic counselors would recommend NIPT to their patients if insurance was not a barrier compared to FTS (88% vs 64%). Genetic counselors were more likely to discuss the financial risks associated with NIPT when a patient had private insurance versus when a patient had public insurance (82% vs 53%). Lastly, while genetic counselors stated they were likely to look at a patient’s insurance coverage before or during the appointment, a majority of genetic counselors agreed that a patient’s insurance coverage did not impact their counseling based on what testing choices were offered.
Table 4.
Genetic counselor survey results
| Question | Response | n | %* |
|---|---|---|---|
| How often do you routinely offer FTS to “low risk” pregnant women? | All/most | 16 | 94% |
| Some/rarely/never | 1 | 6% | |
| How often do you routinely offer NIPT to “low risk” pregnant women? | All/most | 15 | 88% |
| Some/rarely/never | 2 | 12% | |
| How often do you routinely offer diagnostic testing (amniocentesis and CVS) to “low risk” pregnant women? | All/most | 13 | 77% |
| Some/rarely/never | 4 | 24% | |
| When considering the “low risk” pregnant patient that desires aneuploidy screening, would you recommend FTS if insurance coverage was not a barrier and there would be no financial risk to the patient? | All/most | 9 | 64% |
| Some/rarely/never | 5 | 36% | |
| When considering the “low risk” pregnant patient that desires aneuploidy screening, would you recommend NIPT if insurance coverage was not a barrier and there would be no financial risk to the patient? | All/most | 14 | 88% |
| Some/rarely/never | 2 | 13% | |
| When considering the “low risk” pregnant patient that desires aneuploidy screening, would you recommend diagnostic testing (amniocentesis and CVS) if insurance coverage was not a barrier and there would be no financial risk to the patient? | All/most | 7 | 54% |
| Some/rarely/never | 6 | 45% | |
| When thinking about a “low risk” pregnant patient, how often do you look at insurance coverage before the appointment? | Always/mostly | 12 | 71% |
| Half/sometimes/never | 5 | 30% | |
| When thinking about a “low risk” pregnant patient, how often do you look at insurance coverage during the appointment? | Always/mostly | 8 | 50% |
| Half/sometimes/never | 8 | 50% | |
| When thinking about a “low risk” pregnant patient, how often do you look at insurance coverage after the appointment? | Always/mostly | 3 | 19% |
| Half/sometimes/never | 13 | 81% | |
| When thinking about the “low risk” pregnant patient who is considering NIPT, how often do you discuss financial risks when the patient asks? | All/most | 17 | 100% |
| Some/rarely/never | 0 | 0% | |
| When thinking about the “low risk” pregnant patient who is considering NIPT, how often do you discuss financial risks when the patient seems concerned about the cost? | All/most | 17 | 100% |
| Some/rarely/never | 0 | 0% | |
| When thinking about the “low risk” pregnant patient who is considering NIPT, how often do you discuss financial risks when the patient has public insurance? | All/most | 9 | 53% |
| Some/rarely/never | 8 | 47% | |
| When thinking about the “low risk” pregnant patient who is considering NIPT, how often do you discuss financial risks when the patient has private insurance? | All/most | 14 | 82% |
| Some/rarely/never | 3 | 18% | |
| If a “low risk” pregnant patient has public insurance, when would you tell her what test her insurance will cover? | During appointment | 12 | 71% |
| If they ask | 5 | 29% | |
| If a “low risk” pregnant patient has private insurance, when would you tell her what test her insurance will cover? | During appointment | 15 | 88% |
| If they ask | 2 | 12% | |
| A patient’s insurance coverage changes my approach to counseling | Agree/mostly agree | 4 | 24% |
| Neither agree nor disagree | 3 | 18% | |
| Disagree/mostly disagree | 10 | 59% |
*Percentages may not add up to exactly 100% due to rounding at .5 to the next whole number
Discussion
This study shows that women with public insurance are 3.43 times more likely to undergo NIPT than women in the same low-risk group with private insurance. Additionally, the data shows that education, ethnicity, and race do not have a significant association with choice of testing; insurance coverage is significantly associated with whether a woman elects to undergo NIPT. Prenatal genetic counselors at UnityPoint Health-Meriter follow the 2016 ACOG guidelines, but that conflicts with insurance companies that follow the 2012 ACOG guidelines. This supports our hypothesis that women with private insurance may encounter a financial barrier when making decisions on which chromosomal aneuploidy screening test to choose. Additionally, our data provides important confirmation that financial barriers drive decisions in prenatal screening choice that has been outlined by past qualitative studies (Allyse et al. 2014; Farrell et al. 2014; Tiller et al. 2015; Vanstone et al. 2019). Given study design limitations of not directly surveying patients about testing decisions, there may be other explanations for the observed phenomenon. For example, the data could also suggest women are not offered the same prenatal screening options based on their insurance. While our study focused on finding the difference between these two groups and not reasons for this difference, other studies have suggested reasons as to why this difference may exist.
We have found that there is an inequity for women with private insurance who want NIPT but are unable to endure the financial costs associated with testing. Health equity is usually discussed in terms of individuals having limited or no access to care related to social determinants of health. The group of women with private insurance presents a population that is different from what is typically thought of in terms of health equity. These women have private insurance plans but are still faced with the burden of costs associated with healthcare due to deductibles, co-insurance, co-pays, or lack of coverage for particular tests or indications. Regional private insurance companies may not cover NIPT in the low-risk population but do cover FTS based on their use of the 2012 ACOG guidelines (Quartz 2019). Although the public insurance program in the state of Wisconsin does list the high-risk criteria established by ACOG in 2012, providers may elect to offer this screen, and those women who select NIPT do not receive a bill. Each state policy differs in regard to coverage of NIPT for the general population.
Healthcare costs in the USA remain higher than other developed countries. During the study period in 2018, U.S. healthcare spending was $3.6 trillion, or $11,172 per person (US Healthcare Spending 2020), and accounted for 17.7% of the gross domestic product (GDP) (Tipirneni et al. 2018). Additionally, medical bills account for more than half of debts sent to collection agencies (Hamel et al. 2016). In a study done on citizens who filed for bankruptcy, the majority of respondents very much or somewhat agreed that medical expenses contributed to their bankruptcy (Himmelstein et al. 2019). Past studies have shown that many patients have delayed or forgone both preventative and non-preventative care due to associated health care costs (Tipirneni et al. 2018). In cancer genetic testing, it was shown that many women who are offered testing do not end up having the test done, possibly for financial reasons, as those who did undergo testing had better affordability and insurance coverage than those who did not undergo testing (Kieran et al. 2007). These data suggest that cost of testing and financial risks are a barrier to receiving equitable care. Women in our study with public insurance typically experience more healthcare inequities like lack of access to care, difficulties supporting a child with or without special health needs (Anderson et al. 2007; Knoll 1992; Rubeis and Steger 2019) due to various socioeconomic factors, but in this particular case, they do not incur a cost for NIPT and appear to have more ability to select a screening test based on other factors outside of financial factors. However, women with private insurance that does not cover NIPT, who are typically not thought to have major barriers to healthcare, likely do face an inequity as suggested by this study. This juxtaposition should be considered with other aspects of healthcare and addressed when discussing health equity, namely those with private insurance that are finding the financial burden of cost-sharing to impact uptake of certain care.
Decisions about NIPT could also be influenced by how genetic counselors present the information regarding associated financial risks for each testing option. Our survey suggests that there is a small difference in what genetic counselors discuss between women with public insurance and women with private insurance. During appointments with women with private insurance, genetic counselors indicated they were more likely to discuss financial risks with the patients compared to similar appointments with women who have public insurance. This could be a contributing factor toward the difference observed in test selection between the two groups. Since genetic counselors discuss financial implications more with patients with private insurance, patients may be more aware of the cost and perhaps feel that genetic counselors are warning them of the price of the test. The women with public insurance perhaps do not feel the same pressure or anxiety that women with private insurance face because the genetic counselors either do not discuss the cost or the cost is negligible since no bill will be received by the patient. Genetic counselors across different regions in Wisconsin indicated that if insurance coverage had no influence, more would offer NIPT compared to FTS and diagnostic testing, indicating 88% would offer NIPT for their patients based on question 11 in our survey (Appendix 1), compared to 64% offering FTS. This finding supports our hypothesis that insurance coverage does play a role in whether or not women with private insurance choose NIPT due to a potential financial burden. Without insurance coverage acting as a barrier, genetic counselors would offer NIPT at a higher rate than FTS and diagnostic testing, so they do need to alter how they present information to patients based on their insurance coverage. The majority of genetic counselors discuss testing costs regardless of insurance; however, presentation bias cannot be eliminated as a possible study limitation based on the low number of respondents. Further studies would be needed to explore these perceptions.
All genetic counselors spend a portion of their appointments discussing insurance related concerns with patients given that many genetic tests may not be fully covered or covered at all by insurance. A study from 2017 showed that almost all genetic counselors discussed costs and insurance coverage for genetic testing, and that insurance coverage had some influence over a patient’s decision on testing, and a majority of genetic counselors believed their practice was influenced by insurance coverage (Brown et al. 2018). Our data mirrors these findings because most genetic counselors said that they look at the patient’s insurance coverage as well as bring up costs associated with NIPT and FTS to a patient. However, a majority of our respondents stated that insurance coverage does not change their approach to counseling, implying that counselors feel that they are providing balanced information about all options. A major reason that genetic counselors feel it is necessary to discuss cost and insurance coverage is to allow patients to make an informed decision. Some genetic counselors feel burdened by having to discuss the details of cost and insurance and feel that this information can impact a session and ability to build rapport with patients (Brown et al. 2018). Genetic counseling training programs typically do not incorporate insurance coverage training into their curriculums, so many recent graduates may feel uncomfortable and underprepared to discuss this information with patients (Accreditation Council for Genetic Counseling 2019; Brown et al. 2018). Additionally, this raises questions concerning standardization between genetic counselors across the country.
By comparison, other healthcare providers such as physicians may not face the dilemma of frequently having to discuss insurance coverage of treatments or procedures that genetic counselors have to face. It is either not an issue that arises or they have support staff in place to assist with these matters. One past study showed that physicians may even lack knowledge and skills associated with discussing financial risks and lack cost health literacy (Fischer et al. 2020). Education on this topic, not only for genetic counselors but for physicians and other healthcare providers, could benefit patients and ensure their ability to make informed decisions. While few genetic counselors work for insurance companies to develop coverage policies (National Society of Genetic Counselors 2018), the need for education presents a unique opportunity for the profession. As the field of genetic counseling grows, more genetic counselors intersecting with public health and health insurance companies are needed for the best care of patients.
Future research at additional institutions nationally is needed. Additional exploration could be done to explore the differences between major private insurance companies’ policies regarding first trimester aneuploidy screening options should also be pursued. Additionally, it would be intriguing to interview patients directly for insight into their considerations when electing prenatal testing options. Furthermore, interviewing program directors to explore how insurance coverage factors are incorporated into their training curriculum could provide insight into next steps. As a result, the National Society of Genetic Counselors may find that a consensus for standard of care in delivery of cost information risk may be warranted to ensure national quality and standard of care.
In summary, we hypothesized that insurance coverage and out of pocket costs played a role in whether a woman chose NIPT. Our study found that low-risk women with private insurance were 3.43 times less likely to choose NIPT compared to women with public insurance. Therefore, we conclude that some women cannot choose the prenatal chromosomal aneuploidy screening test of their choice due to financial barriers put into place by the lack of complete insurance coverage. NIPT has higher sensitivity, lower false positive rates, and can screen for more conditions than other first trimester chromosomal aneuploidy screening options. Additionally, it is standard of care that when a first trimester screening test is abnormal or shows an increased risk for aneuploidy, NIPT is offered as a next tier test before invasive diagnostic testing like amniocentesis or chorionic villus sampling. If private insurance companies covered NIPT initially for the low-risk population of women, that could eliminate this disparity and allow women to choose a test that reflects their values.
Acknowledgments
Thank you to Michael Lasarev MS, Natalie Berger, MS, CGC, Anna Zakas, MS, MPH, CGC, Hannah Wand, MS, CGC, UWSMPH Masters of Genetic Counselor Studies Program.
Appendix
Survey to Wisconsin Genetic Counselors that provide prenatal genetic counseling.
The following questions are based on scenarios involving a “low risk” pregnant woman < 14 weeks of gestation and < 35 years of age. Over the past year, in general…
- How often do you routinely offer First Trimester Screening to “low risk” pregnant woman <14weeks of gestation and < 35 years of age?
- All of the time, most of the time, some of the time, rarely, never
- How often do you routinely offer Non-Invasive Prenatal Testing (NIPT) to “low risk” pregnant woman < 14 weeks of gestation and < 35 years of age?
- All of the time, most of the time, some of the time, rarely, never
- How often do you routinely offer Invasive Diagnostic testing to “low risk” pregnant woman < 14 weeks of gestation and < 35 years of age?
- All of the time, most of the time, some of the time, rarely, never
- How often do you discuss financial risks associated with NIPT with a “low risk” pregnant patient < 14 weeks gestation and < 35 years of age?
- All of the time, most of the time, some of the time, rarely, never
- When thinking about the “low risk”, < 14 weeks gestation, < 35 years of age patient that is considering NIPT, how often do you discuss financial risks?
- When a patient asks
-
i)All of the time, most of the time, some of the time, rarely, never
-
ii)What influences this decision? (Free Type Box)
-
i)
- When a patient seems concerned about the cost
-
i)All of the time, most of the time, some of the time, rarely, never
-
ii)What influences this decision? (Free Type Box)
-
i)
- When a patient has public insurance (i.e. MA, BadgerCare, etc.)
-
i)All of the time, most of the time, some of the time, rarely, never
-
ii)What influences this decision? (Free Type Box)
-
i)
- When a patient has private insurance, how often do you discuss the financial risks to the patient?
-
i)All of the time, most of the time, some of the time, rarely, never
-
ii)What influences this decision? (Free Type Box)
-
i)
- If a “low risk” pregnant woman < 14 weeks of gestation and < 35 years of age has public insurance (i.e. Medicaid), when do you specifically tell the patient what test their insurance covers?
- During the appointment, outside the appointment, if they ask, never
- If a “low risk” pregnant woman < 14 weeks of gestation and < 35 years of age has private insurance, when do you specifically tell the patient what test their insurance covers?
- During the appointment, outside the appointment, if they ask, never
- A patient’s insurance coverage changes my approach to counseling
- Agree, mostly agree, neither agree nor disagree, mostly disagree, disagree
- When thinking about a “low risk” pregnant patient < 14 weeks gestation and < 35 years of age, how often do you look at a patient’s insurance coverage:
- Before an appointment?
-
i)Always, most times, sometimes, rarely, never
-
i)
- During an appointment?
-
i)Always, most times, sometimes, rarely, never
-
i)
- After an appointment?
-
i)Always, most times, sometimes, rarely, never
-
i)
- Information covered about aneuploidy screening during a routine first trimester appointment is consistent between prenatal counselors in my clinic
- Agree, mostly agree, neither agree nor disagree, mostly disagree, disagree
- When considering the “low risk”, < 14 weeks gestation, < 35 year old pregnant patient that desires aneuploidy screening, what test would you recommend if insurance coverage was not a barrier?
- First trimester screen
-
i)All of the time, most of the time, some of the time, rarely, never
-
i)
- Non-invasive prenatal testing (NIPT)
-
i)All of the time, most of the time, some of the time, rarely, never
-
i)
- Diagnostic testing
-
i)All of the time, most of the time, some of the time, rarely, never
-
i)
Demographics
- Do you provide prenatal genetic counseling?
- Yes
- No
- What estimate of your job duties is spent providing prenatal genetic counseling?
- Sliding scale from 0 to 100%?
-
What region of Wisconsin do you work? Use the map below for regional designations.
(List each region for subject selection)- Northern Region
- Western Region
- Southern Region
- Northeastern Region
- Southeastern Region
- How long have you been a practicing genetic counselor?
- < 5 years
- 5–10 years
- 11–15 years
- 16–20 years
- 20+ years
- Do not wish to report
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Accreditation Council for Genetic Counseling. Standards of Accreditation for Graduate Programs in Genetic Counseling. October 2019
- Aetna. Serum and Urine Marker Screening for Fetal Aneuploidy 2020 [cited 2020 April 14]
- Allyse M, Sayres LC, Goodspeed TA, Cho MK. Attitudes towards non-invasive prenatal testing for aneuploidy among US adults of reproductive age. J Perinatol. 2014;34(6):429–434. doi: 10.1038/jp.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetrics and Gynecology. Committee Opinion No. 545: noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol 2012;120(6):1532–1534. 10.1097/01.AOG.0000423819.85283.f4 [DOI] [PubMed]
- American College of Obstetrics and Gynecology. Committee on Practice Bulletins—Obstetrics CmoG, and the Society for Maternal-Fetal Medicine. Practice Bulletin No. 163: screening for fetal aneuploidy. Obstet Gynecol 2016;127(5):e123–e137. 10.1097/AOG.0000000000001406 [DOI] [PubMed]
- American College of Obstetrics and Gynecology. Committee of Practice Bulletins – Obstetrics, Committee on Genetics, and Society for Maternal-Fetal Medicine. Practice Bulletin No. 226: Screening for Fetal Chromosomal Abnormalities. 2020
- Anderson D, Dumont S, Jacobs P, Azzaria L. The personal costs of caring for a child with a disability: a review of the literature. Public Health Rep. 2007;122(1):3–16. doi: 10.1177/003335490712200102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamon CJ, Hardisty EE, Harris SC, Vora NL. A single center's experience with noninvasive prenatal testing. Genet Med. 2014;16(9):681–687. doi: 10.1038/gim.2014.20. [DOI] [PubMed] [Google Scholar]
- Brown S, Puumala S, Leonhard J, Bell M, Flanagan J, Dean LW, Stein Q. Genesurance counseling: genetic counselors’ roles and responsibilities in regards to genetic insurance and financial topics. J Genet Couns. 2018;27(4):800–813. doi: 10.1007/s10897-017-0180-x. [DOI] [PubMed] [Google Scholar]
- Cernat A, De Freitas C, Majid U, Trivedi F, Higgins C, Vanstone M. Facilitating informed choice about non-invasive prenatal testing (NIPT): a systematic review and qualitative meta-synthesis of women’s experiences. BMC Pregnancy Childbirth. 2019;19(1):27. Published 2019 Jan 14. 10.1186/s12884-018-2168-4 [DOI] [PMC free article] [PubMed]
- Chen KM, White K, Shabbeer J, Schmid M. Maternal age trends support uptake of non-invasive prenatal testing (NIPT) in the low-risk population. J Matern Fetal Neonatal Med. 2019;32(23):4039–4042. doi: 10.1080/14767058.2018.1481033. [DOI] [PubMed] [Google Scholar]
- Farrell RM, Agatisa PK, Nutter B. What women want: lead considerations for current and future applications of noninvasive prenatal testing in prenatal care. Birth. 2014;41(3):276–282. doi: 10.1111/birt.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer KA, Walling A, Wenger N, Glaspy J. Cost health literacy as a physician skill-set: the relationship between oncologist reported knowledge and engagement with patients on financial toxicity [published online ahead of print, 2020 Mar 19]. Support Care Cancer. 2020;10.1007/s00520-020-05406-z. [DOI] [PubMed]
- Hamel L, Norton, M, Pollitz K, Levitt L, Claxton G, Brodie M. Results from the Kaiser Family Foundation/New York times medical bills survey 2016
- Health Disparities. MedlinePlus 2020 [cited 2020 April 20]
- Himmelstein DU, Lawless RM, Thorne D, Foohey P, Woolhandler S. Medical bankruptcy: still common despite the affordable care act. Am J Public Health. 2019;109(3):431–433. doi: 10.2105/AJPH.2018.304901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Integrated Genetics. MaterniT 21 plus 2019 [cited 2020 March 2]
- Integrated Genetics Laboratory. Phone Interview. March 2020
- Kieran S, Loescher LJ, Lim KH. The role of financial factors in acceptance of clinical BRCA genetic testing. Genet Test. 2007;11(1):101–110. doi: 10.1089/gte.2006.9999. [DOI] [PubMed] [Google Scholar]
- Knoll J. Being a family. The experience of raising a child with a disability or chronic illness. Monogr Am Assoc Ment Retard. 1992;18:9–56. [PubMed] [Google Scholar]
- Lefkowitz RB, Tynan JA, Liu T, et al. Clinical validation of a noninvasive prenatal test for genomewide detection of fetal copy number variants. Am J Obstet Gynecol. 2016;215(2):227.e1–227.e16. doi: 10.1016/j.ajog.2016.02.030. [DOI] [PubMed] [Google Scholar]
- Medicaid. Medicaid eligibility. [Cited 2020 November 4]
- Natera Laboratory. Phone interview. March 2020
- National Society of Genetic Counselors. 2018 Professional Status Survey. [cited 2020 April 14]
- NTD-Eurofins. First Trimester Screen. 2019 [cited 2020 March 2]
- Quartz. Prenatal/Preconception Genetic Testing 2019 [cited 2020 April 14]
- Rolfes V, Schmitz D. Unfair discrimination in prenatal aneuploidy screening using cell-free DNA? Eur J Obstet Gynecol Reprod Biol. 2016;198:27–29. doi: 10.1016/j.ejogrb.2015.12.023. [DOI] [PubMed] [Google Scholar]
- Rubeis G, Steger F. A burden from birth? Non-invasive prenatal testing and the stigmatization of people with disabilities. Bioethics. 2019;33(1):91–97. doi: 10.1111/bioe.12518. [DOI] [PubMed] [Google Scholar]
- Tiller GE, Kershberg HB, Goff J, Coffeen C, Liao W, Sehnert AJ. Women’s views and the impact of noninvasive prenatal testing on procedures in a managed care setting. Prenat Diagn. 2015;35(5):428–433. doi: 10.1002/pd.4495. [DOI] [PubMed] [Google Scholar]
- Tipirneni R, Politi MC, Kullgren JT, Kieffer EC, Goold SD, Scherer AM. Association between health insurance literacy and avoidance of health care services owing to cost. JAMA Netw Open. 2018;1(7):e184796. doi: 10.1001/jamanetworkopen.2018.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Healthcare Spending. CMS.gov 2020 [cited 2020 April 14]
- Vanstone M, Cernat A, Majid U, Trivedi F, De Freitas C. Perspectives of pregnant people and clinicians on noninvasive prenatal testing: a systematic review and qualitative meta-synthesis. Ont Health Technol Assess Ser. 2019;19(5):1–38. [PMC free article] [PubMed] [Google Scholar]