Abstract
Background
Chagas disease (CD) remains an important endemic disease in Latin America. However, CD became globalized in recent decades. The majority of the chronically infected individuals did not receive etiologic treatment for several reasons, among them the most conspicuous is the lack of access to diagnosis. The impact of trypanocidal treatment on CD chronic phase, without cardiac involvement (indeterminate form ICF), is yet to be determined. We aimed to evaluate the effect of trypanocidal treatment with benznidazole (BZN) on the rate of progression to Chagas heart disease in patients with ICF.
Methods
This is a retrospective cohort observational study including patients with ICF treated with BZN and compared to a group of non-treated patients matched for age, sex, region of origin, and the year of cohort entry. We reviewed the medical charts of all patients followed from May 1987 to June 2020 at the outpatient center of the Evandro Chagas National Institute of Infectious Diseases (INI) of the Oswaldo Cruz Foundation (Fiocruz), Rio de Janeiro, Brazil. Patients’ follow-up included at least one annual medical visit and one annual electrocardiogram (ECG). Echocardiographic exams were performed at baseline and during the follow-up. Disease progression from ICF to cardiac form was defined by changes in baseline ECG. Cumulative incidence and the incidence rate were described in the incidence analysis. Cox proportional hazards models were used to estimate hazard ratios and 95% confidence intervals for the association between BZN and CD progression, cardiovascular events or death.
Findings
One hundred and fourteen treated patients met the study inclusion criteria. A comparison group of 114 non-treated patients matched for age, sex, region of origin, and the year of cohort entry was also included, totalizing 228 patients. Most patients included in the study were male (70.2%), and their mean age was 31.3 (+7.4) years. Over a median follow-up of 15.1 years (ranging from 1.0 to 32.4), the cumulative CD progression incidence in treated patients was 7.9% vs. 21.1% in the non-treated group (p = 0.04) and the CD progression rate was 0.49 per 1.000 patients/year in treated patients vs. 1.10 per 1.000 patients/year for non-treated patients (p = 0.02). BZN treatment was associated with a decreased risk of CD progression in both unadjusted (HR 0.46; 95%CI 0.21 to 0.98) and adjusted (HR 0.43; 95%CI 0.19 to 0.96) models and with a decreased risk of occurrence of the composite of cardiovascular events only in the adjusted (HR 0.15; 95%CI 0.03 to 0.80) model. No association was observed between BZN treatment and mortality.
Interpretation
In a long-term follow-up, BZN treatment was associated with a decreased incidence of CD progression from ICF to the cardiac form and also with a decreased risk of cardiovascular events. Therefore, our results indicate that BZN treatment for CD patients with ICF should be implemented into clinical practice.
Keywords: Chagas disease, Benznidazole, Indeterminate form, Disease progression, Electrocardiogram
Research in context.
Evidence before this study
We searched PubMed and Google Scholar on September 2020, for articles published in English, Portuguese or Spanish, without publication time constraints, using the terms (''benznidazole'') AND (''therapy''). Although the use of benznidazole in the chronic phase of Chagas disease is already indicated in guidelines for some defined situations, including the indeterminate form, and we already have some studies that prove its effectiveness, the impact of treatment on clinical evolution from indeterminate to cardiac form in Chagas disease still needs more evidence. The few studies in the literature that evaluate the efficacy of benznidazole in relation to the progression of the clinical condition of individuals with chronic Chagas' disease come up against a relevant aspect, which is the follow-up time after the etiological treatment. This condition is due to the natural history of Chagas disease, in which chronic carriers of the disease take decades to manifest clinical changes.
Added value of this study
This study covers a long post-treatment follow-up period, in which patients with the indeterminate form of Chagas disease treated with benznidazole progressed less to the cardiac form and presented fewer clinical events related to Chagas disease when compared to untreated patients.
Implications of all the available evidence
According to the World Health Organization, about 6 to 7 million people are infected with Trypanosoma cruzi worldwide, with a significant proportion of whom may develop chronic Chagas heart disease, one of the main determinants of morbidity and mortality. Therefore, in light of the findings of this study, we corroborate the current guidelines that recommend offering etiological treatment to individuals up to 50 years of age, who have the indeterminate form of Chagas disease.
Alt-text: Unlabelled box
1. Introduction
Chagas disease (CD) remains an important endemic disease and public health problem in Latin American countries and has become globalized in recent decades [1]. After acute infection, patients not treated with trypanocidal drugs usually progress to a chronic phase that consists of four well-defined clinical forms: the indeterminate form (ICF), characterized by the absence of electrocardiographic (ECG) abnormalities and normal radiological exams of the chest, esophagus, and colon; the cardiac form, which presents ECG abnormalities with or without global or segmental left ventricular systolic dysfunction; the digestive form, with esophagus and/or intestine peristalsis dysfunction, which may lead to megasyndromes, and cardiodigestive form, an association of cardiac and digestive disorders [2]. CD treatment and clinical management are directed according to the phase and clinical presentation of the disease. Specific treatment with trypanocidal drugs is mandatory in the acute phase, congenital cases or reactivation due to immunosuppression. In the chronic phase, the trypanocidal treatment is indicated in children and adolescents, recent infection, and women of childbearing age [3].
One century after its discovery, there are still questions about which drug is safe and effective in the various clinical forms of chronic CD. In the 1960s and 1970s, the first trypanocidal drugs were described, nifurtimox and benznidazole (BZN) [4], which showed impressive results with healing of 70% of treated individuals in the CD acute phase. However, the evidence accumulated since then is not enough to guarantee that these drugs prevent CD clinical progression from the ICF to the cardiac form [5].
The most relevant question about prescribing trypanocidal treatment for CD chronic patients is whether they will have a clinical benefit. Until the mid-1990s, the role of the parasite in the pathogenesis of chronic Chagas heart disease was considered secondary, with cardiac complications essentially assumed to be caused by autoimmune disorders [6]. After a study by Viotti et al. [7] that demonstrated a BZN beneficial effect on the evolution of the CD chronic phase, the paradigm began to change [8,9]. Some authors who previously stood for the exclusive autoimmune hypothesis changed their view to accept that a low-grade, incessant, systemic infection is essential for the pathogenesis of chronic Chagas heart disease [10]. In addition, the striking correlation between the presence of Trypanosoma cruzi antigen and the severity of the inflammatory infiltrate within the myocardium supports a direct role for the parasite in the perpetuation of myocardial inflammation in CD [11]. Currently, there is a consensus that the persistence of parasites, together with an unbalanced immune response, triggers a sustained inflammatory response that lead to chronic CD progression [12]. Therefore, the eradication of Trypanosoma cruzi (T.cruzi) may be a prerequisite for slowing the progression from ICF to Chagas heart disease [13]. This paradigm was also used to justify a clinical trial to test etiological treatment of patients with chronic Chagas heart disease. However, this trial could not demonstrate a clinical benefit [14].
The use of trypanocidal drugs in murine models of chronic T.cruzi infection showed a concomitant decrease of the parasite load and of the cardiac dysfunction and myocarditis, indicating that parasite persistence may play an important role in the pathogenesis of chronic chagasic cardiomyopathy [15,16]. In anima nobile, Viotti et al. [7] were pioneers in showing that chronic CD patients treated with BZN present a lower incidence of new ECG changes than non-treated patients. However, the indication of the etiological CD treatment in the chronic phase remains controversial, largely due to a difficult evaluation in terms of efficacy [17]. Factors such as the low prevalence of detectable parasitemia, persistence of antibodies that results in positive serological tests after treatment, and clinical outcomes which require a long-term follow-up, are significant limitations to the evaluation of therapeutic efficacy. A meta-analysis by Pérez-Molina et al. [18] showed that the effect of trypanocidal treatment in late chronic infection is doubtful. Although data generally pointed to a beneficial effect, this was marginal. This uncertainty is greater in asymptomatic patients aged over 50 years, in whom the risk-benefit of treatment does not seem favorable. The results obtained also vary according to the phase of the disease, time of treatment and dosage, age, geographical origin of the patients, and susceptibility of the T.cruzi lineage [19,20]. Moreover, the use of different evaluation protocols, which include different outcomes, increases the complexity of evaluating the efficacy of a specific treatment for chronic CD [21,22].
Studies performed in the 1990s and 2000s showed that trypanocidal treatment may promote some positive results on parasitemia, serology, and heart disease progression [23,24]. More recently, most clinical trials tested trypanocidal treatment efficacy by means of polymerase chain reaction (PCR) as the main parasitological outcome [25], [26], [27], [28], [29]. Similarly, observational studies followed the same outcome [30,31]. Therefore, the focus of the current studies involving etiologic treatment is the antiparasitic effect, achieved by a persistent negative PCR after treatment. However, the clinical significance of an antiparasitic effect is still controversial and few studies in recent years have used disease progression as the main outcome of trypanocidal treatment, either in patients with the ICF or Chagas heart disease [32,33].
Thus, the objective of the present study is to evaluate the effect of trypanocidal treatment with BZN on the rate of progression to heart disease in patients with ICF.
2. Methods
This is a retrospective cohort observational study including patients with ICF of CD, treated with BZN (TP) and compared to a group of non-treated patients (NTP) matched for prognostic variables of CD progression: age (5 years’ range), sex, region of origin, and the year of cohort entry. Clinical and epidemiological data were retrieved from the medical records. The NTP were chosen from a spreadsheet containing only the baseline characteristics, with no information about studied outcomes in order to minimize selection bias. All patients were followed at the outpatient center of the Evandro Chagas National Institute of Infectious Diseases (INI) of the Oswaldo Cruz Foundation (Fiocruz), located at the state of Rio de Janeiro, Brazil from May 1987 to June 2020. Serological diagnosis of CD was confirmed when two simultaneous serological techniques were reactive as follows: indirect immunofluorescence (titer >1/40) and enzyme-linked immunosorbent assay, whose cutoff value is described in Lapa et al. [34]. None of the patients had previous clinical findings of acute Chagas disease. Patients who had less than one year of follow-up or without at least two paired ECGs during the follow-up, or those who interrupted BZN treatment within less than 30 days were excluded from the study. During the period in which patients were diagnosed with CD and admitted for follow-up, there were no established criteria for the use of BZN in the chronic CD phase. Despite the lack of guidelines, young patients (< 30 years old) with any positive parasitological test were treated with BZN at the discretion of the attending physician. The dosage of BZN usually varied between a fixed dose of 200 mg, regardless of weight, for 30 to 60 days, and 5 mg/kg/day, for 30 to 60 days. The region of origin was classified according to the morbidity of CD categorized by a national ECG survey [35] and defined as low (normal ECGs > 50%), high (altered ECGs > 50%), and non-endemic areas (Rio de Janeiro and Espírito Santo states). During the study follow-up, all patients were living in the state of Rio de Janeiro.
In the present study, we considered a pragmatic ICF definition based on positive serological tests, absence of cardiac and/or digestive symptoms and/or signs, normal conventional ECG or with the presence of nonspecific abnormalities, and normal radiological findings of the heart. Patients without symptoms compatible with megasyndromes did not undergo contrasted examinations of the esophagus and colon. This pragmatic definition of ICF, based on the 2nd Brazilian consensus on Chagas disease [36], considers only the presence of cardiac and/or digestive symptoms and ECG findings, not including the echocardiographic evaluation.
The primary outcome was progression from ICF to the cardiac form. Secondary outcomes included a composite outcome of cardiovascular events (heart failure, stroke, or device implantation: pacemaker or implantable cardioverter defibrillator) and death. Progression was defined by ECG changes during the follow-up. The follow-up time was measured from the time of first ECG until the occurrence of study outcomes (CD progression, composite of cardiovascular events or death) or the last medical appointment before the administratively censored date on June 2020 for those who were still followed or lost to follow-up.
Patients underwent an initial evaluation, which included epidemiological history, clinical anamnesis, physical examination focused on CD-related cardiovascular signs and symptoms, chest X-ray examination, 12-lead ECG and two-dimensional echocardiogram with Doppler (ECHO). ECG was performed on admission and repeated annually in all patients. The Minnesota Code Manual of Electrocardiographic Findings (modified for CD) was used to standardize the ECG interpretation [37]. The ECG abnormalities compatible with Chagas heart disease according to the criteria recommended by the 2nd Brazilian Consensus on Chagas Disease were: 2nd- and 3rd-degree right bundle-branch block, associated or not to left anterior fascicular block, frequent ventricular premature beats (VPBs >1 by ECG), polymorphous or repetitive nonsustained ventricular tachycardia, 2nd- and 3rd-degree atrioventricular block, sinus bradycardia with heart rate less than 50 bpm, sinus node dysfunction, 2nd- and 3rd-degree left bundle-branch block, atrial fibrillation, electrical inactive area, or primary ST-T wave changes. All ECG evaluations were performed exclusively by the same single physician until 1998 and by two cardiologists from this date on. In case of disagreement, cases were determined by consensus. The ECHO examination included parasternal and cross-sectional views and 2-, 4- and 3- chamber apical views and variations to identify wall motion abnormalities. Left ventricular global systolic function was assessed by the Simpson or Teicholz method and classified as normal, mild, moderate, or severe depressed [38]. Patients’ follow-up included at least one annual medical visit and one annual ECG in which cardiovascular events and CD progression were ascertained. ECHO exams were performed at baseline and during the follow-up based on signs and symptoms presented by each patient and at the physicians’ discretion. The cardiovascular outcomes of interest included CD progression, a composite of cardiovascular events (heart failure, stroke, or device implantation), and death that were identified by reviewing the medical records. The classification of Chagas heart disease followed the criteria adopted in the 2nd Brazilian Consensus on Chagas disease. Serological CD tests were performed on admission and repeated at any visit during the follow-up with intervals of at least one year. Serological outcome was defined by persistent negative serology in indirect immunofluorescence assay.
2.1. Statistical analysis
Descriptive statistics was presented as means (standard deviations) for continuous and absolute frequencies (percentages) for categorical variables. Comparisons of patients’ characteristics according to BZN treatment were performed using t-test (continuous) or chi-squared test (categorical) at baseline and at the end of follow-up. Cumulative incidence (expressed by the number of patients that presented the outcome divided by the total number of patients during the follow-up) and the incidence rate (expressed as the number of patients that presented the outcome divided by the time of exposure of each individual) were described in the incidence analysis. Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CI) for the association between BZN and study outcomes. Models were fitted without adjustments, as well as, adjusted for potential confounders including age, sex, region of origin, left ventricular ejection fraction, number of comorbidities (arterial hypertension, diabetes mellitus and dyslipidemia), presence of non-chagasic cardiomyopathy, and non-specific ECG abnormalities. Additional analyses to examine the differential responses of BZN doses (fixed dose of 200 mg vs. other doses) were also performed. The Schoenfeld residuals test did not demonstrate substantial deviation from proportional hazard assumptions. Kaplan-Meier survival curves for CD progression stratified according to BZN treatment were constructed and compared using the log-rank test. Data analyses were performed using Stata 13.0 software (College Station, Texas) and statistical significance was set at a 2-tailed p-value of <0.05 for all analyses.
2.2. Ethics committee approval
The study was approved by the Evandro Chagas National Institute of Infectious Diseases Research Ethics Committee (Number: 054/2008 - May 5, 2009). The Ethics committed waived informed consent due to the retrospective nature of the study.
2.3. Role of the funding source
None.
3. Results
Out of 2177 patients followed at the outpatient center of INI-Fiocruz from May 1987 to June 2020, 1085 presented ICF. From these, 159 patients were treated with BZN. Among the patients who were treated with BZN, 38 were excluded because treatment was interrupted within less than 30 days due to adverse drugs reactions and seven for not having at least two paired ECG during the follow-up, leaving a sample of 114 TP. A comparison non-treated group of 114 patients matched for age, sex, region of origin, and the year of cohort entry was also included, totalizing 228 patients.
The baseline clinical and demographic characteristics of patients included in the study are described in Table 1 (total and according to TP and NTP). Basically, most patients included in the study were male (70.2%), came from an area with low CD mortality rates (52.6%), and had a mean age of 31.3 years-old. One-third of patients had non-specific ECG abnormalities and 5.6% had echo abnormalities with a mean of left ventricular ejection fraction of 66.9%. Overall, no major differences were observed between TP and NTP, except for a greater percentage of first-degree right bundle-branch block and sinus bradycardia with heart rate 50–60 bpm in TP and NTP, respectively.
Table 1.
Baseline characteristics of patients (total and stratified by BZN treatment) (n = 228).
| Variable | Mean (standard deviation) or Frequency (%) |
p-value | ||
| Total (n = 228) | NTP (n = 114; 50%) | TP (n = 114; 50%) | ||
| Age (years) | 31.3 (+7.4) | 32.7 (7.4) | 29.8 (7.1) | 0.003 |
| Female sex | 68 (29.8) | 34 (29.8) | 34 (29.8) | 1.00 |
| Region of origin | ||||
| Low CD mortality area | 120 (52.6) | 62 (54.4) | 58 (50.9) | 0.79 |
| High CD mortality area | 79 (34.7) | 37 (32.5) | 24 (36.8) | |
| Non-endemic area | 29 (12.7) | 15 (13.2) | 14 (12.3) | |
| Non-specific ECG abnormalities | 76 (33.3) | 36 (31.6) | 40 (35.1) | 0.57 |
| First-degree right bundle-branch block | 19 (8.3) | 4 (3.5) | 15 (13.2) | 0.008 |
| Left anterior fascicular block | 7 (3.1) | 3 (2.6) | 4 (3.5) | 0.70 |
| Isolated atrial or ventricular premature beats | 5 (2.2) | 1 (0.9) | 4 (3.5) | 0.18 |
| Sinus bradycardia with heart rate 50–60 bpm | 30 (13.2) | 20 (17.5) | 10 (8.8) | 0.05 |
| Low QRS voltage | 5 (2.2) | 2 (1.8) | 3 (2.6) | 0.65 |
| Secondary ST-T wave changes | 12 (5.3) | 6 (5.3) | 6 (5.3) | 1.00 |
| First degree atrioventricular block | 3 (1.3) | 2 (1.8) | 1 (0.9) | 0.56 |
| Fist-degree left bundle-branch block | 6 (2.6) | 3 (2.6) | 3 (2.6) | 1.00 |
| Left ventricular ejection fraction (n = 215)* | 66.9 (5.9) | 67.0 (5.9) | 66.8 (5.8) | 0.76 |
| Echocardiogram abnormalities (n = 215)* | 12 (5.6) | 7 (6.7) | 5 (4.5) | 0.48 |
| Diastolic dysfunction | 1 (0.5) | 1 (1.0) | 0 (0.0) | 0.30 |
| Segmental dysfunction | 9 (4.2) | 5 (4.8) | 4 (3.6) | 0.66 |
| Global systolic dysfunction | 3 (1.4) | 2 (1.9) | 1 (0.9) | 0.52 |
*Left ventricular ejection fraction (n = 215, 104 NTP and 111 TP).
*Echocardiogram abnormalities (n = 215, 104 NTP and 111 TP).
Low/moderate mortality area: Brazilian States - Ceará, Paraíba, Pernambuco, Piaui, Sergipe, Alagoas, Rio Grande do Norte, Paraná, Rio Grande do Sul, São Paulo, Mato Grosso do Sul - and Bolivia.
High mortality area: Brazilian States - Minas Gerais, Bahia e Goiás.
Non-endemic area: Brazilian States – Rio de Janeiro and Espírito Santo.
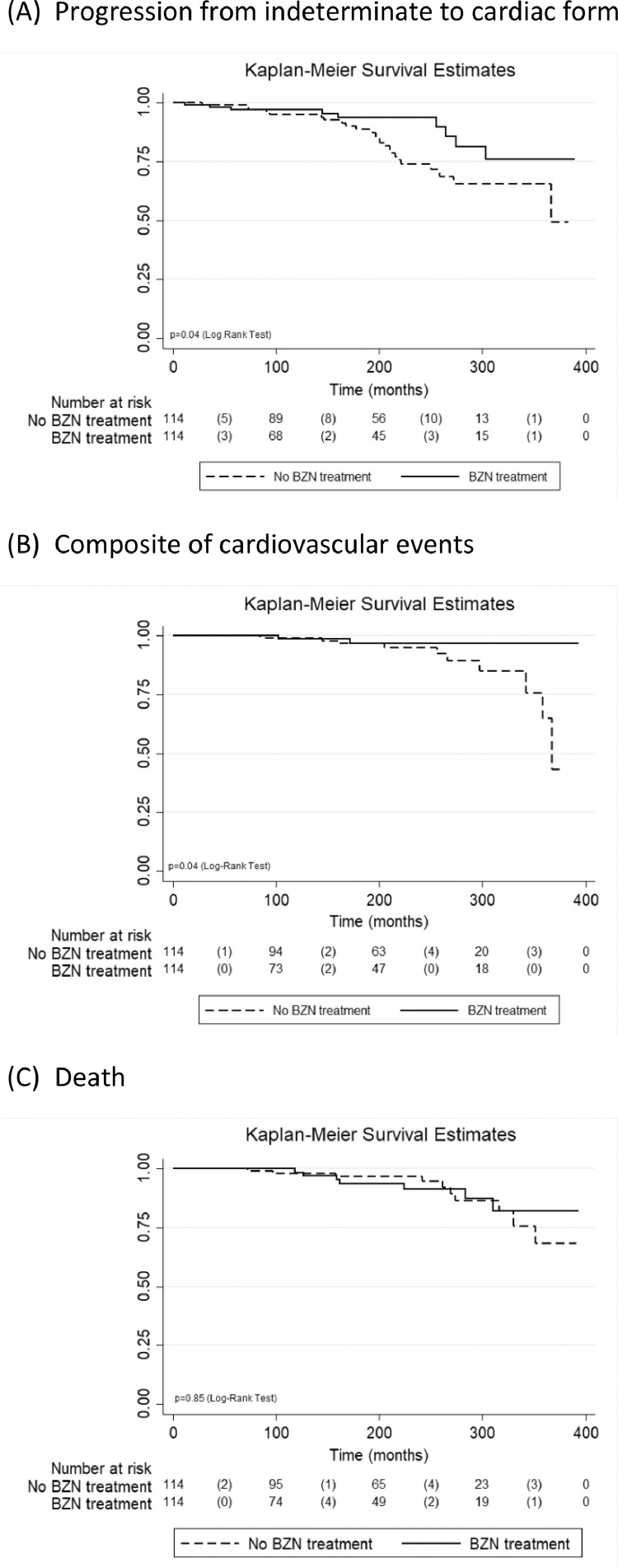
The association between BZN treatment with CD progression, composite of cardiovascular events and death are depicted in Table 2. Over a median follow-up period of 15.1 years (ranging from 1.0 to 32.4), 33 patients progressed from ICF to the cardiac form, resulting in a 14.5% cumulative incidence (21.1% for NPT vs. 7.9% for TP; p = 0.04) and 0.82 per 1000 patients/year incidence rate (1.10 for NPT vs. 0.49 for TP; p = 0.02). BZN treatment was associated with a decreased risk of CD progression in both unadjusted (HR 0.46; 95%CI 0.21 to 0.98) and adjusted (HR 0.44; 95%CI 0.20 to 0.99) models. Twelve patients (5.3%) presented the composite of cardiovascular events (including heart failure, stroke, and/or device implantation) during the median follow-up of 16.1 years (ranging from 1.5 to 32.7), of those 10 NTP (8.8%) and 2 TP (1.8%). BZN treatment was associated with a decreased risk of occurrence of the composite of cardiovascular events only in the adjusted (HR 0.15; 95%CI 0.03 to 0.77) model. There were 17 deaths during the median follow-up of 16.6 years (ranging from 1.5 to 32.7). Four deaths were directly related to CD, three due to sudden death and one due to heart failure. All three patients that presented sudden death had arterial hypertension and none of them progressed to Chagas heart disease. The patient who died due to heart failure was the only one who progressed to the cardiac form. However, no association was observed for BZN treatment and death in both unadjusted and adjusted models. Fig. 1 illustrates the survival curves free of CD progression (A), the composite of cardiovascular events (B), and death (C) according to BZN treatment. No differences for schemes of BZN dose (fixed dose of 200 mg vs. other doses) were observed for any of the studied outcomes (p>0.20).
Table 2.
Survival estimates for progression from indeterminate to cardiac form of Chagas disease and death according to BZN treatment (n = 228).
| Number of events | Cumulative incidence | Incidence rate (95%CI) (per 1000 person-years) |
Unadjusted |
Adjusted* |
|||
| HR (95%CI) | p-value | HR (95%CI) | p-value | ||||
| BZN treatment | Progression from indeterminate to cardiac form of Chagas disease | ||||||
| No | 24 | 21.1% | 1.10 (0.74 to 1.64) | 1.00 (Reference) | 0.04 | 1.00 (Reference) | 0.04 |
| Yes | 9 | 7.9% | 0.49 (0.25 to 0.95) | 0.46 (0.21 to 0.98) | 0.44 (0.20 to 0.99) | ||
| BZN treatment | Composite of cardiovascular events (heart failure, stroke, or device implantation) | ||||||
| No | 10 | 8.8% | 0.42 (0.23 to 0.79) | 1.00 (Reference) | 0.06 | 1.00 (Reference) | 0.02 |
| Yes | 2 | 1.8% | 0.10 (0.03 to 0.42) | 0.23 (0.05 to 1.07) | 0.15 (0.03 to 0.77) | ||
| BZN treatment | Death | ||||||
| No | 10 | 8.8% | 0.41 (0.22 to 0.77) | 1.00 (Reference) | 0.85 | 1.00 (Reference) | 0.95 |
| Yes | 7 | 6.1% | 0.35 (0.17 to 0.74) | 0.91 (0.35 to 2.39) | 1.04 (0.37 to 2.89) | ||
*Model adjusted for age, sex, Left ventricular ejection fraction, number of comorbidities, presence of non-chagasic cardiomyopathy, non-specific ECG abnormalities, and region of origin.
Fig. 1.
Kaplan Meier curves for progression from indeterminate to cardiac form (A) and death (B) by BZN treatment.
Table 3 depicts the clinical characteristics of patients at the end of follow-up. The prevalence of comorbidities was 32.0% for arterial hypertension (39.5 in NTP vs. 24.6 in TP; p = 0.02), 12.3% for diabetes mellitus (13.2 in NTP vs. 11.4 in TP; p = 0.69) and 33.3% for dyslipidemia (43.0 in NTP vs. 23.7 in TP; p = 0.002). Only few patients (2.6%) had non-chagasic cardiomyopathy (2.6% in both groups; p = 1.00) and 16.7% presented other comorbidities including cancer, chronic obstructive pulmonary disease, hypothyroidism, depression, alcohol abuse and co-infection viral hepatitis (22.8 in NTP vs. 10.5 in TP; p = 0.01). The incidence of megaviscera during the follow-up was 6.6%. There was a higher incidence of CD progression, 10.1% progressed to stage A (14.0% in NTP vs. 6.1% in TP), 2.2% to stage B1 (2.6% in NTP vs. 1.8% in TP), and 2.2% to stage C (4.4% in NTP vs. 0.0% in TP; p = 0.02). Among the nine patients who presented segmental LV dysfunction at the baseline, two maintained the segmental LV dysfunction, two developed global systolic dysfunction, three no longer presented the segmental LV dysfunction and two did not undergo the control echocardiogram. ECG and ECHO abnormalities were more frequent in the NTP than in the TP at the end of follow-up (p = 0.003 for both). Serological negativation was observed in 11.4% of TP with no cases in NTP (p<0.001). The median time for serological negativation was 9.5 years (IQR 25%−75% 7.4 to 21.3), ranging from 1.1 to 28.3 years. A more detail description including the characteristics of patients that progressed from the ICF to the cardiac form are presented in Supplemental Tables 1 and 2.
Table 3.
Clinical characteristics of patients at the end of follow-up (n = 228).
| Mean (standard deviation) or Frequency (%) |
p-value | |||
| Variable | Total (n = 228) |
NTP (n = 114; 50%) |
TP (n = 114; 50%) |
|
| Digestive form | 15 (6.6) | 9 (7.9) | 6 (5.3) | 0.42 |
| Arterial Hypertension | 73 (32.0) | 45 (39.5) | 28 (24.6) | 0.02 |
| Diabetes Mellitus | 28 (12.3) | 15 (13.2) | 13 (11.4) | 0.69 |
| Dislipidemia | 76 (33.3) | 49 (43.0) | 27 (23.7) | 0.002 |
| Non-chagasic cardiomyopathy | 6 (2.6) | 3 (2.6) | 3 (2.6) | 1.00 |
| Other comorbidities* | 38 (16.7) | 26 (22.8) | 12 (10.5) | 0.01 |
| Clinical presentation of Chagas disease | ||||
| Indeterminate | 195 (85.5) | 90 (78.9) | 105 (92.1) | 0.02 |
| Cardiac stage A | 23 (10.1) | 16 (14.0) | 7 (6.1) | |
| Cardiac stage B1 | 5 (2.2) | 3 (2.6) | 2 (1.8) | |
| Cardiac stage B2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Cardiac stage C | 5 (2.2) | 5 (4.4) | 0 (0.0) | |
| CD specific ECG abnormalities | 34 (14.9) | 25 (21.9) | 9 (7.9) | 0.003 |
| Second or third-degree right bundle-branch block | 14 (6.1) | 11 (9.7) | 3 (2.6) | 0.03 |
| Frequent atrial or ventricular premature beats | 5 (2.2) | 3 (2.6) | 2 (1.8) | 0.65 |
| Primary ST-T wave abnormalities | 13 (5.7) | 11 (9.7) | 2 (1.8) | 0.01 |
| Electrical inactive area | 4 (1.8) | 2 (1.8) | 2 (1.8) | 1.00 |
| Second or three-degree left bundle-branch block | 2 (0.9) | 1 (0.9) | 1 (0.9) | 1.00 |
| Atrial flutter | 1 (0.4) | 1 (0.9) | 0 (0.0) | 0.32 |
| Atrial tachycardia | 1 (0.4) | 1 (0.9) | 0 (0.0) | 0.32 |
| Echocardiogram abnormalities (n = 172) | 56 (32.6) | 40 (42.1) | 16 (20.8) | 0.003 |
| Diastolic dysfunction | 42 (24.4) | 32 (33.7) | 10 (13.0) | 0.002 |
| Segmental dysfunction | 19 (11.1) | 14 (14.8) | 5 (6.5) | 0.09 |
| Global systolic dysfunction | 13 (7.6) | 10 (10.5) | 3 (3.9) | 0.10 |
| Serological cure | 13 (5.7) | 0 (0.0) | 13 (11.4) | <0.001 |
Other comorbidities included cancer, asthma, chronic obstructive pulmonary disease, hypothyroidism, depression, alcohol abuse, and co-infection (HIV, HTLV, and viral hepatitis).
4. Discussion
Many factors may be involved in the risk of developing chronic Chagas heart disease and/or megasyndromes including age, sex, geographical origin, parasite load, genetic heterogeneity of T. cruzi and its discrete typing units (DTU, TcI–TcVI), immunological aspects of the host, severity of the initial acute infection, transmission route, exposure to T. cruzi reinfection in areas with sustained vector transmission, nutritional status, presence of comorbidities, and the social context and quality of life of CD patients [39,40].
The global annual rate of progression from ICF to Chagas heart disease is estimated at 1.9% [41] and can justify the etiologic treatment to prevent CD progression [42]. Longitudinal and observational studies conducted in the last decade helped to determine the efficacy of etiologic treatment on clinical progression of ICF, as they indicated that adult patients treated with trypanocidal drugs progressed less to the cardiac form than non-treated patients [32,33,[43], [44], [45], [46]].
Our study identified that BZN TP presented a lower progression to heart disease when compared to NTP. Moreover, we also determined that BZN TP had a lower risk of cardiovascular events. Other studies that used ECG changes as an outcome had reported similar results regarding the cumulative incidence of progression. For example, Viotti et al. [7] reported 4.1% in TP and 18.7% in NTP, Fabbro et al. [45] reported 3.7% in TP and 24.6% in NTP, and Fragata-Filho et al. [46] reported 20.9% in TP and 53.1% in NTP. One factor that justifies these different progression rates is the different range of patients’ age between those studies, as age is directly associated with the time of infection and disease progression in the chronic phase. Trypanocidal treatment studies reported a higher prevalence of young patients in TP group than in the NTP control group, which tended to be older. Younger individuals have a higher potential to develop heart disease during their life span and, therefore, a higher probability of presenting ECG changes during follow-up [47]. In the present study, pairing both groups by age removed this potential bias from the final analysis of the BZN effect.
Another factor that should be considered in the analysis of results is the follow-up time. A retrospective study with healthy blood donors with CD not treated with trypanocidal drug reported that a follow-up time of ten years would help to identify the incidence of Chagas heart disease [48]. In the present study, the progression curves of both groups begin to differentiate after around 13 years of follow-up, after which, there was greater progression to heart disease in NTP. Considering the natural history of CD, a long follow-up time would be ideal to evaluate the rate of progression to heart disease. In this sense, the results of this study are based on an extended follow-up time of up to 33 years, with a median of 15.1 years, similar between patients who progressed or did not progressed.
All patients lived in urban areas of the state of Rio de Janeiro and had been away from their hometown for at least 20 years and were not subject to possible T.cruzi reinfection. Although most patients came from CD nonendemic or low/medium morbidity and mortality regions (65.3%), those from regions with high CD morbidity and mortality, such as Bahia, Minas Gerais and Goiás states, predominated among those who progressed, reinforcing the findings of a Brazilian national survey that showed a higher prevalence of chronic heart disease in these regions [35].
In our study, most patients used a fixed dose of 200 mg/day (79.8%), regardless of weight, for a mean period of 30 to 60 days. The BZN beneficial effect was not related to treatment period or daily dose, although this dosage is considered sub-dose by the current guidelines. Few studies addressed different BZN dose regimens, some with drugs association, but all of them used parasite load as a surrogate for efficacy and did not analyze patients’ clinical progression [25,28,29,33]. Also, changes in post-treatment serological curve occur only after a long-term follow-up. In our study, the long-term follow-up time allowed the observation that serological change.
The most frequent ECG changes that defined CD progression were second or third-degree right bundle-branch block and primary ST-T wave abnormalities. These findings are compatible with the literature that addresses electrocardiographic changes in Chagas disease [49]. It is important to emphasize that ECG abnormalities related to CD progression are well defined and relatively easy to be identified by a specialized cardiologist. Furthermore, patients who progressed are still followed at our institution and the new ECG findings that defined progression were still present in subsequent ECGs performed at our outpatient clinics.
Of the 2177 patients diagnosed with Chagas' disease followed up at the outpatient center, 52% are women. Therefore, it is surprising that the majority of patients treated with benznidazole in our study were men. Unfortunately, we do not have a reasonable explanation for this. However, we can hypothesize that the fact that women are at risk of having a higher frequency of adverse events with benznidazole may have led to treatment discontinuation before 30 days [50], which determined a greater presence of men in this study.
At the onset of the study, ICF classification was based solely in the absence of typical Chagas heart disease alterations in the ECG. The abnormalities found in other cardiologic exams were not taken into account for ICF classification. We were also unable to retrospectively exclude asymptomatic digestive Chagas disease. Although previous articles have linked segmental changes on the echocardiogram to a worse prognosis when compared to normal ECHO [51] and changes in the global systolic function of the left ventricle already constitute the presence of heart disease, in our study these changes, in the presence of a normal ECG at the beginning of the follow-up, did not influence the progression to Chagas heart disease. Regarding the low QRS voltage, it is considered a non-specific electrocardiographic alteration of CD [36]. Only one study identified the presence of low QRS voltage as a predictor of death but not confirmed in other cohorts [49]. In our study, patients had low QRS voltage at baseline (2NTP / 3TP), and this variable was included as a potential confounder in the adjusted survival model as a non-specific ECG abnormality.
The present study is as far as we know the first report of decreased risk of subsequent cardiovascular events in ICF patients treated with BZN. We were not able to demonstrate a reduced mortality risk, but the mortality among ICF patients is quite small and similar to the overall population and our study was underpowered to evaluate this event. Furthermore, most deaths were not due to causes related to CD and, probably, would not be avoided by BZN treatment. In fact, Catalioti and Acquatella compared TP with NTP and reported no mortality differences in a five-year follow-up [52].
This study has some limitations. The pragmatic definition of ICF considering only the presence of symptoms and the ECG findings may have misclassified some patients at baseline. However, this approach seems to be more suitable to the reality of CD patients, that usually resides in low socioeconomic areas with limited health care resources. As it is a retrospective observational study, the patients were not randomly allocated to the treatment group, which allows potential confounding factors. However, the use of matching strategy minimized this potential bias, although matching for all potential confounders is not feasible and there is still the possibility of residual confounding. To account for residual confounders, we included some additional variables in our survival models and the results were consistent even after adjustments, reinforcing the strength of our findings. However, the lack of a more detailed information on sociodemographic factors is also a limitation that can implicate in potential residual confounders. Another limitation is that studied patients were young (77% < 35 years), which limits the applicability of the results to the treatment of older patients. The changes over time in factors that could influence the study outcomes (e.g. availability and access of healthcare, behavioral and sociodemographic risk factors, alternative treatments and point of intervention) were not evaluated. However, we believe that those changes would have occurred in a similar degree in both groups, therefore a non-differential error. Despite these limitations, a major advantage of this study is that it has a long clinical follow-up period that better evaluates the disease progression outcome
In conclusion, this long-term follow-up study identified a beneficial effect of BZN on CD progression as TP present 54% decreased risk of progression from ICF to cardiac form. We also identified a protective effect of BZN on cardiovascular events. Therefore, our results give further support for the trypanocidal treatment of patients with CD ICF. Future prospective randomized studies should be conducted to definitively establish the role of BZN in the treatment of chronic CD.
Declaration of Competing Interests
The authors declare no conflict of interest.
Acknowledgments
Authors contribution
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. AMH-M was responsible for study concept. SSX, ASS, RMS, LHCS, ARC, MTH, HHV, FSNS, MNB, PEAAB, FMC and GMSS were responsible for acquisition, analysis or interpretation of data. AMH-M was responsible for drafting of the manuscript. RMS was responsible for reviewing of the manuscript. FACC and MFFM were responsible for statistical analysis. All authors have read and agreed to the published version of the manuscript.
Data sharing statement
The data that support the findings of this study are available from the corresponding author upon request.
Funding
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100694.
Appendix. Supplementary materials
References
- 1.Conners E.E., Vinetz J.M., Weeks J.R., Brouwer K.C. A global systematic review of Chagas disease prevalence among migrants. Acta Tropica. 2016;156:68–78. doi: 10.1016/j.actatropica.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Echeverria L.E. Morillo CA.American trypanosomiasis (Chagas disease) Infect. Dis. Clin. North Am. 2019;33:119–134. doi: 10.1016/j.idc.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Pan American Health Organization. PAHO New guide for diagnosis and treatment of Chagas disease. 2019 [Internet]. Available in: https://www.paho.org/hq/index.php?option=com_content&view=article&id=14906:paho-issues-new-guide-for-diagnosis-and-treatment-of-chagas-disease<emid=135&lang=pt
- 4.Pérez-Molina J.A., Crespillo-Andújar C., Bosch-Nicolau P., Molina I. Trypanocidal treatment of Chagas disease. Enfermedades Infecciosas y Microbiología Clínica. 2020 doi: 10.1016/j.eimc.2020.04.011. S0213005X20301932. [DOI] [PubMed] [Google Scholar]
- 5.Matta Guedes P.M., Gutierrez F.R.S., Nascimento M.S.L., Do-Valle-Matta M.A., Silva J.S. Antiparasitical chemotherapy in Chagas’ disease cardiomyopathy: current evidence. Tropical Med Int Health. 2012;17(9):1057–1065. doi: 10.1111/j.1365-3156.2012.03025.x. [DOI] [PubMed] [Google Scholar]
- 6.Cunha-Neto E., Duranti M., Gruber A., Zingales B., Messias I., Stolf N. Autoimmunity in Chagas disease cardiopathy: biological relevance of a cardiac myosin-specific epitope crossreactive to an immunodominant Trypanosoma cruzi antigen. Proc Nat Acad Sci. 1995;92:3541–3545. doi: 10.1073/pnas.92.8.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viotti R., Vigliano C., Armenti H., Segura E. Treatment of chronic Chagas’ disease with benznidazole: clinical and serologic evolution of patients with long-term follow-up. Am Heart J. 1994;127(1):151–162. doi: 10.1016/0002-8703(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 8.Tarleton R.L., Zhang L. Chagas disease etiology: autoimmunity or parasite persistence? Parasitology Today. 1999;15(3):94–99. doi: 10.1016/s0169-4758(99)01398-8. [DOI] [PubMed] [Google Scholar]
- 9.Viotti R., Alarcón de Noya B., Araujo-Jorge T., Grijalva M.J., Guhl F., López M.C. Towards a paradigm shift in the treatment of chronic chagas disease. Antimicrob Agents Chemother. 2014;58(2):635–639. doi: 10.1128/AAC.01662-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marin-Neto J.A., Cunha-Neto E., Maciel B.C., Simões M.V. Pathogenesis of chronic chagas heart disease. Circulation. 2007;115(9):1109–1123. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- 11.Higuchi M., de L., De Brito T., Martins Reis M., Barbosa A., Bellotti G., Pereira-Barreto A.C. Correlation between Trypanosoma cruzi parasitism and myocardial inflammatory infiltrate in human chronic chagasic myocarditis: light microscopy and immunohistochemical findings. Cardiovascular Pathology. 1993;2(2):101–106. doi: 10.1016/1054-8807(93)90021-S. [DOI] [PubMed] [Google Scholar]
- 12.Urbina J.A. Recent clinical trials for the etiological treatment of chronic chagas disease: advances, challenges and perspectives. J Eukaryot Microbiol. 2015;62(1):149–156. doi: 10.1111/jeu.12184. [DOI] [PubMed] [Google Scholar]
- 13.Urbina J.A. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Tropica. 2010;115(1–2):55–68. doi: 10.1016/j.actatropica.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Rassi Jr A., Marin Neto J.A., Rassi A. Chronic Chagas cardiomyopathy: a review of the main pathogenic mechanisms and the efficacy of aetiological treatment following the BENznidazole Evaluation for Interrupting Trypanosomiasis (BENEFIT) trial. Mem Inst Oswaldo Cruz. 2017;112(3):224–235. doi: 10.1590/0074-02760160334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia S., Ramos C.O., Senra J.F.V., Vilas-Boas F., Rodrigues M.M., Campos-de-Carvalho A.C. Treatment with benznidazole during the chronic phase of experimental Chagas’ disease decreases cardiac alterations. Antimicrob. Agents Chemother. 2005;49:1521–1528. doi: 10.1128/AAC.49.4.1521-1528.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bahia M.T., Andrade I.M., Martins T.A.F., Nascimento ÁF da S do, Diniz L., de F., Caldas I.S. Fexinidazole: a potential new drug candidate for chagas disease. Pollastri MP, organizador. PLoS Negl Trop Dis. 2012;6(11):e1870. doi: 10.1371/journal.pntd.0001870. de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez S.J., Romano P.S., Engman D.M. Precision health for Chagas disease: integrating parasite and host factors to predict outcome of infection and response to therapy. Front Cell Infect Microbiol. 2020;10:210. doi: 10.3389/fcimb.2020.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pérez-Molina J., Pérez-Ayala A., Moreno S., Fernández-González M., Zamora J., López-Velez R. Use of benznidazole to treat chronic Chagas’ disease: a systematic review with a meta-analysis. J Antimicrobial Chemotherapy. 2009;64(6):1139–1147. doi: 10.1093/jac/dkp357. [DOI] [PubMed] [Google Scholar]
- 19.Sguassero Y., Cuesta C.B., Roberts K.N., Hicks E., Comandé D., Ciapponi A. Course of Chronic trypanosoma cruzi infection after treatment based on parasitological and serological tests: a systematic review of follow-up studies. Cunha-Neto E, organizador. PLoS ONE. 2015;10(10) doi: 10.1371/journal.pone.0139363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caldas I.S., Santos E.G., Novaes R.D. An evaluation of benznidazole as a Chagas disease therapeutic. Expert Opin Pharmacotherapy. 2019;20(15):1797–1807. doi: 10.1080/14656566.2019.1650915. [DOI] [PubMed] [Google Scholar]
- 21.Villar JC, Perez JG, Cortes OL, Riarte A, Pepper M, Marin-Neto JA, et al. Trypanocidal drugs for chronic asymptomatic Trypanosoma cruzi infection. Cochrane Heart Group, organizer. Cochrane Database Systemat Rev. 2014 [Internet]. Available in: http://doi.wiley.com/10.1002/14651858.CD003463.pub2 [DOI] [PMC free article] [PubMed]
- 22.Espinoza M R.A. Criterios de cura en la enfermedad de Chagas: interpretación de hallazgos parasitológicos, serológicos y clínicos. Revista del Instituto Nacional de Higiene Rafael Rangel. 2003;34(2):27–34. [Google Scholar]
- 23.Sgambatti de Andrade A.L.S., Zicker F., de Oliveira R.M., Almeida e Silva S., Luquetti A., Travassos L.R. Randomized trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet. 1996;348:1407–1413. doi: 10.1016/s0140-6736(96)04128-1. [DOI] [PubMed] [Google Scholar]
- 24.Machado-de-Assis G.F., Diniz G.A., Montoya R.A., Dias J.C.P., Coura J.R., Machado-Coelho G.L.L. A serological, parasitological and clinical evaluation of untreated Chagas disease patients and those treated with benznidazole before and thirteen years after intervention. Mem Inst Oswaldo Cruz. 2013;108(7):873–880. doi: 10.1590/0074-0276130122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molina-Morant D., Fernández M.L., Bosch-Nicolau P., Sulleiro E., Bangher M., Salvador F. Efficacy and safety assessment of different dosage of benznidazol for the treatment of Chagas disease in chronic phase in adults (MULTIBENZ study): study protocol for a multicenter randomized Phase II non-inferiority clinical trial. Trials. 2020;21(1):328. doi: 10.1186/s13063-020-4226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morillo C.A., Waskin H., Sosa-Estani S., del Carmen Bangher M., Cuneo C., Milesi R. Benznidazole and posaconazole in eliminating parasites in asymptomatic T. Cruzi Carriers. J Am College Cardiol. 2017;69(8):939–947. doi: 10.1016/j.jacc.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 27.Molina I., Gómez i Prat J., Salvador F., Treviño B., Sulleiro E., Serre N. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N Engl J Med. 2014;370(20):1899–1908. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- 28.Torrico F., Gascon J., Ortiz L., Alonso-Vega C., Pinazo M.-.J., Schijman A. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: a proof-of-concept, randomised, placebo-controlled trial. Lancet Infect Dis. 2018;18(4):419–430. doi: 10.1016/S1473-3099(17)30538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciapponi A., Barreira F., Perelli L., Bardach A., Gascón J., Molina I. Fixed vs adjusted-dose benznidazole for adults with chronic Chagas disease without cardiomyopathy: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2020;14(8) doi: 10.1371/journal.pntd.0008529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sulleiro E., Silgado A., Serre-Delcor N., Salvador F., Tavares de Oliveira M., Moure Z. Usefulness of real-time PCR during follow-up of patients treated with Benznidazole for chronic Chagas disease: experience in two referral centers in Barcelona. PLoS Negl Trop Dis. 2020;14(2) doi: 10.1371/journal.pntd.0008067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murcia L., Carrilero B., Ferrer F., Roig M., Franco F., Segovia M. Success of benznidazole chemotherapy in chronic Trypanosoma cruzi-infected patients with a sustained negative PCR result. Eur J Clin Microbiol Infect Dis. 2016;35(11):1819–1827. doi: 10.1007/s10096-016-2733-6. [DOI] [PubMed] [Google Scholar]
- 32.Bertocchi G.L., Vigliano C.A., Lococo B.G., Petti M.A., Viotti R.J. Clinical characteristics and outcome of 107 adult patients with chronic Chagas disease and parasitological cure criteria. Trans R Soc Trop Med Hygiene. 2013;107(6):372–376. doi: 10.1093/trstmh/trt029. [DOI] [PubMed] [Google Scholar]
- 33.Álvarez M.G., Ramírez J.C., Bertocchi G., Fernández M., Hernández Y., Lococo B. New scheme of intermittent benznidazole administration in patients chronically infected with Trypanosoma cruzi: clinical, parasitological and serological assessment after three years of follow-up. Antimicrob Agents Chemother. 2020 doi: 10.1128/AAC.00439-20. AAC00439-20v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lapa J.S., Saraiva R.M., Hasslocher-Moreno A.M., Georg I., Souza A.S., Xavier S.S. Dealing with initial inconclusive serological results for chronic Chagas disease in clinical practice. Eur J Clin Microbiol Infect Dis. 2012;31(6):965–974. doi: 10.1007/s10096-011-1393-9. [DOI] [PubMed] [Google Scholar]
- 35.Gonçalves J.G.F., Prata A., Dias J.C.P., Macêdo V. The electrocardiographic survey. Rev. Soc. Bras. Med. Trop. 2011;44:40–46. doi: 10.1590/s0037-86822011000800007. [DOI] [PubMed] [Google Scholar]
- 36.Dias J.C.P., Ramos Jr A.N., Gontijo E.D., Luquetti A., Shikanai-Yasuda M.A., Coura J.R. 2nd Brazilian consensus on chagas disease. 2015. Epidemiol Serv Saúde. 2016;25:7–86. doi: 10.5123/S1679-49742016000500002. [DOI] [PubMed] [Google Scholar]
- 37.Maguire J.H., Mott K.E., Souza J.A.A., Almeida E.C., Ramos N.B., Guimarâes A.C. Eletrocardiographic classification and abbreviated lead system for population-based studies of Chagas disease. Bull Pan Am Health Organ. 1982;16(1):47–58. [PubMed] [Google Scholar]
- 38.Cerqueira M., Weissman N., Dilsizian V., Jacobs A., Kaul S., Laskey W. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the american heart association. Circulation. 2002;105(4):539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 39.Macedo V. Influência da exposição à reinfecção na evolução da doença de Chagas. Estudo longitudinal de 5 anos. Revista de Patologia Tropical. 1976;5:33–116. [Google Scholar]
- 40.Echeverría L.E., Rojas L.Z., López L.A., Rueda-Ochoa O.L., Gómez-Ochoa S.A., Morillo C.A. Myocardial involvement in chagas disease and insulin resistance: a non-metabolic model of cardiomyopathy. Global Heart. 2020;15(1):36. doi: 10.5334/gh.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chadalawada S., Sillau S., Archuleta S., Mundo W., Bandali M., Parra-Henao G. Risk of chronic cardiomyopathy among patients with the acute phase or indeterminate form of chagas disease: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(8) doi: 10.1001/jamanetworkopen.2020.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coura J.R., Borges-Pereira J. Chronic phase of Chagas disease: why should it be treated? A comprehensive review. Mem Inst Oswaldo Cruz. 2011;106(6):641–645. doi: 10.1590/s0074-02762011000600001. [DOI] [PubMed] [Google Scholar]
- 43.Soverow J., Hernandez S., Sanchez D., Forsyth C., Flores C.A., Viana G. Progression of baseline electrocardiogram abnormalities in chagas patients undergoing antitrypanosomal treatment. Open Forum Infect Dis. 2019;6(2):1–5. doi: 10.1093/ofid/ofz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viotti R., Vigliano C., Lococo B., Bertocchi G., Petti M., Alvarez M.G. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med. 2006;144(10):724–734. doi: 10.7326/0003-4819-144-10-200605160-00006. [DOI] [PubMed] [Google Scholar]
- 45.Fabbro D.L., Streiger M.L., Arias E.D., Bizai M.L., del Barco M.L., Amicone N.A. Trypanocide treatment among adults with chronic Chagas disease living in Santa Fe City (Argentina), over a mean follow-up of 21 years: parasitological, serological and clinical evolution. Rev Soc Bras Med Trop. 2007;40(1):1–10. doi: 10.1590/s0037-86822007000100001. [DOI] [PubMed] [Google Scholar]
- 46.Fragata-Filho A.A., França F.F., Fragata C da S, Lourenço A.M., Faccini C.C., Costa C.A. Evaluation of parasiticide treatment with Benznidazol in the electrocardiographic, clinical, and serological evolution of chagas disease. PLoS Negl Trop Dis. 2016;10(3) doi: 10.1371/journal.pntd.0004508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dias J.C.P. História natural da doença de Chagas. Arq Bras Cardiol. 1995;65(4):359–366. [PubMed] [Google Scholar]
- 48.Sabino E.C., Ribeiro A.L., Salemi V.M., Oliveira C.D.L., Antunes A.P., Menezes M.M. Ten-year Incidence of Chagas cardiomyopathy among asymptomatic, T. Cruzi seropositive former blood donors. Circulation. 2013;127(10):1105–1115. doi: 10.1161/CIRCULATIONAHA.112.123612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brito B.O., de F., Ribeiro A.L.P. Electrocardiogram in Chagas disease. Rev Soc Bras Med Trop. 2018;51(5):570–577. doi: 10.1590/0037-8682-0184-2018. [DOI] [PubMed] [Google Scholar]
- 50.Sperandio da Silva G.M., Mediano M.F.F., Alvarenga Americano do Brasil P.E., da Costa Chambela M., da Silva J.A., de Sousa A.S. A clinical adverse drug reaction prediction model for patients with chagas disease treated with benznidazole. Antimicrob Agents Chemother. 2014;58(11):6371–6377. doi: 10.1128/AAC.02842-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt A., Romano M.M.D., Marin-Neto J.A., Rao-Melacini P., Rassi Jr A., Mattos A. Effects of trypanocidal treatment on echocardiographic parameters in chagas cardiomyopathy and prognostic value of wall motion score index: a benefit trial echocardiographic substudy. J Am Soc Echocardiogr. 2019;32(2):286–295. doi: 10.1016/j.echo.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Catalioti F., Acquatella H. Comparacion de mortalidad durante seguimiento por 5 años en sujetos con enfermedad de Chagas crónica con y sin tratmiento de benzonidazol. J Tropic Pathol. 1998;27(1):29–31. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.