Abstract
Background
Staphylococcal blood stream infections (SBSI) are a significant cause of morbidity and mortality, however there is little data on such infections in persons with HIV (PWH) in the combination antiretroviral therapy era, particularly when divided by species; methicillin-sensitive (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA) and coagulase-negative Staphylococcus (CoNS).
Methods
Using linked longitudinal clinical and microbiologic databases, all cases of SBSI in PWH accessing care at Southern Alberta Clinic were identified and demographic features and outcomes characterized. We compared participants with SBSI to those with no SBSI and determined the 1-year all-cause mortality following SBSI and longitudinally over the study period.
Findings
From 2000 to 2018, 130 SBSI occurred in 95 PWH over 21,526 patient-years follow-up. MSSA caused 38.4%, MRSA 26.1% and CoNS 35.3% of SBSI. Highest risks for SSBI were in Hepatitis C coinfection, low CD4 nadir, Indigenous/Metis ethnicity and in persons who use injection drugs (PWID). During follow-up, 423 deaths occurred in all PWH. Mortality rates for PWH with SBSI was 74.9/1000 patient-years (95% CI 59.2–94.9) compared with no SBSI 16.0/1000 patient-years (95% CI 14.4–17.7). The mortality Hazard Ratio was 2.61(95% CI 1.95–3.49, P= <0.001) for SBSI compared to no SBSI, following adjusting for confounding. Seventy deaths occurred in persons with SBSI with 40% in the first year. Higher 1-year mortality rates occurred in hospital-acquired infections.
Interpretation
Incidence rates of SBSI are high in PWH, with identified characteristics that further increase this risk. PWH who experience SBSI have a significant mortality risk within the first year of follow-up, however they also have greater long-term all-cause mortality compared to those with no SBSI. Further investigation is needed in PWH evaluating host, environment and pathogen differences that lead to differing rates of SBSI and mortality seen here.
Funding
No funding was received for this work.
Keywords: Bloodstream infections, HIV/AIDS, Staphylococcus aureus, Coagulase negative staphylococcus, Outcomes
Research in context.
Evidence before this study
Several studies have characterized staphylococcal blood stream infections (SBSI) in a general population, however few that have evaluated the specialized population of persons with HIV (PWH), particularly since the widespread usage of ART. We searched PubMed and MEDLINE for search concepts of “HIV/AIDS”, “Staphylococcus” and “Bacteremia” in addition to including many synonyms and relevant subject headings. The majority of data identified was based on Staphylococcus aureus both MSSA and MRSA, with minimal studies evaluating coagulase negative Staphylococcus (CoNS). Also, the majority of studies evaluated either community acquired, or hospital acquired infections, very few looked at total infections. Lastly, the majority of studies were done in the pre or early ART era with less studies evaluating SBSI in PWH during the combined ART era.
Added value of this study
The value of this study is in the comprehensive longitudinal databases that were utilized. We linked a clinical database of PWH with a microbiologic database overlapping in geographic region in order to capture and characterize all SBSI in PWH. This allowed us to evaluate and compare hospital-acquired and community-acquired cases as well as include MSSA, MRSA and CoNS over a longitudinal follow-up of 18 years. We were able to evaluate and compare clinical and epidemiologic characteristics as well as outcomes of our study population.
Implications of all the available evidence
Despite advances in HIV care, the incidence rates of SBSI are high in PWH and are associated with high mortality, particularly in the first-year of follow-up. There are epidemiologic and clinical characteristics that increase rates of SBSI and mortality in PWH. Further investigation is needed in PWH evaluating host, environment and pathogen differences that lead to increased rates of SBSI.
Alt-text: Unlabelled box
1. Introduction
Staphylococcal blood stream infection (SBSI) includes methicillin-susceptible (MSSA), methicillin-resistant Staphylococcus aureus (MRSA), and coagulase negative Staphylococcus (CoNS). SBSI has an annual population-based incidence rate of 20–30 cases/100,000 population in high-income countries [1]. Several studies have shown that Staphylococcus aureus is the second most common pathogen causing blood stream infection (BSI), after Escherichia coli [2, 3]. In the past 20 years with aggressive use of antibacterial therapy and modern source control strategies, the mortality rate for SBSI is estimated at ~25% [2, 4].
In North America, the rates of MSSA are relatively stable, while rates of MRSA are increasing, believed to be due to increased community-acquired (CA) cases [5, 6]. A study from the Calgary Health Region (CHR) recently reported on 840 episodes of Staphylococcus aureus bacteremia (SAB), demonstrating increased incidence rates from 23.5–32.0/100,000 from 2012 to 2014 [4]. There are limited studies evaluating CoNS BSI. Souvenir et al. demonstrated that CoNS accounted for 45–60% of all Staphylococcal isolates recovered from blood cultures, but these organisms are often considered contaminants [7]. Only 10–12% of all CoNS recovered from blood cultures are implicated in true SBSI [7].
Despite advances in HIV care, invasive bacterial infections continue to be a significant source of morbidity and mortality in persons with HIV (PWH) [8, 9]. Many studies evaluating SBSI in PWH were done in the pre or early antiretroviral therapy (ART) era, less information is available on the documented risk factors, demographics and outcomes in the combined ART era [9], [10], [11], [12], [13], [14]. Staphylococcus aureus is consistently reported as the most common cause of BSI in PWH [12, 13, 15, 16]. The prevalence of MRSA has been increasing in PWH and corresponds to the introduction of CA-infections [12]. The first Canadian outbreak of CA-MRSA was identified in the CHR in 2004, due to a local corrections facility outbreak [17, 18]. High risk populations for CA-MRSA are similar to those for HIV including; persons who use injection drug (PWID), homelessness and recent incarceration [18]. CoNS is frequently in the top three pathogens associated with BSI in PWH, however the clinical outcomes of these infections has not been studied [19].
Through this cohort study, we linked a longitudinal clinical database of adults with HIV living within our geographic region with a microbiologic database overlapping the same region to identify and characterize all SBSI cases in PWH. We aimed to identify characteristics of PWH which were associated with risk for SBSI, establish risk for recurrence and evaluate 1-year all-cause mortality following an episode of SBSI as well as longitudinally over the study period.
2. Methods
2.1. Population
All PWH in Southern Alberta, Canada receive HIV care through a centralized HIV program at the Southern Alberta Clinic (SAC), providing free access to all HIV services under universal health care. We included all PWH ≥18 years old accessing HIV care through SAC between January 1, 2000 and January 1, 2018. Patients were followed until they moved out of the region, were lost to follow-up (LTFU), died, or until the end of the study period. In order to evaluate 1-year mortality following SBSI, mortality data were collected until January 1, 2019.
2.2. Data collection
Through this retrospective cohort study, we identified all PWH with an SBSI during the study period through the centralized regional microbiology research database and matched these patients to the SAC database. During the study period all diagnostic microbiology services were provided for Calgary and surrounding rural areas (currently ~1.5 M people) by Calgary Laboratory Services (CLS). CLS is accredited by the College of Physicians and Surgeons of Alberta; all processes have quality assurance measures and standards, ensuring validity and accuracy of reported data.
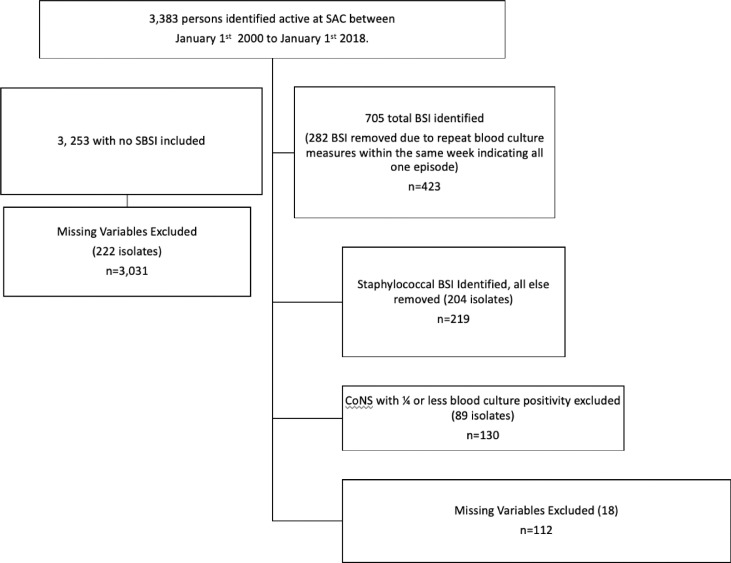
A standard adult protocol that routinely directs collection of a two-set, 4-bottle draw for each individual blood culture order was used [20]. The BacTAlert (bioMérieux, Laval, Quebec) automated system was used throughout the study and each bottle consisted of FAN Plus Media for optimal aerobic and anaerobic organism recovery. All blood cultures are routinely incubated for 5-days, which has been shown to improve the recovery of fastidious pathogens [21, 22]. Blood culture data included the date of sample collection, the location of the patient and the specific bacterial pathogen identified. Clinical and Laboratory Standard Institute guidelines were utilized to identify and determine the susceptibilities profiles of MSSA, MRSA and CoNS [23]. Blood cultures were evaluated by a physician (RL and DLC) and those with one set of two blood samples for CoNS were further evaluated for time to positivity and those with one out of four bottles positive for CoNS were considered contamination and excluded, resulting in the removal of 89 (40.6%) isolates (Fig. 1).
Fig. 1.
Flow diagram for inclusion into the study population.
The SAC database obtained sociodemographic and clinical data from patients with a documented SBSI (study patients) and without a documented SBSI (controls). Participant demographics included age, sex, ethnicity and HIV risk factors. Data on co-infections and comorbidities was recorded. HIV clinical characteristics included CD4 count, viral load and ART therapy and antimicrobial agents used for prophylaxis of opportunistic infections (OI). Clinical outcomes included; hospitalization, SBSI recurrence, and all-cause mortality. This study was approved by the Conjoint Health Research Ethics Board at the University of Calgary (REB17–2283) along with the microbiology research database (REB15–0629).
2.3. Definitions
Eligible participants had attended at least one regular SAC appointment in the year being evaluated. BSI that occurred in out-patients or within ≤72 h after hospital admission were categorized as community-acquired (CA), whereas BSIs that occurred >72 h after hospitalization was considered hospital-acquired (HA) [2]. For all SBSI episodes, hospitalization status was defined as being admitted at the time or within 48 h following the phlebotomy. Polymicrobial SBSI occurred when blood cultures yielded ≥2 different pathogenic organisms within 48 h of each other within the 5-day incubation period. Recurrence was defined as another SBSI more than 14 days after the first. CD4 counts and HIV viral load (VL) measurements collected most recent to diagnosis of a SBSI were used. Virologic suppression in plasma was defined as ≤200 RNA copies/mL [24, 25].
2.4. Statistical analysis
Descriptive analysis was performed using crude data. There were 132 missing values for ethnicity, 80 for CD4 nadir, 10 for age at HIV diagnosis and 18 for HIV risk factors. The number of participants with any missing values was low (n = 240, 7.0%). Univariate analysis was conducted using all data but observations with missing data were excluded for bivariate and multivariate analysis. Incidence rates for each type of BSI studied including MSSA, MRSA and CoNS, were calculated by dividing incident infections by the total number of patients attending SAC at year-end for each year of the study. Demographic data for those with each type of SBSI were compared using the chi-square test.
Unadjusted hazard ratios (HR) were calculated using a Poisson regression model to compare characteristics of PWH with SBSI versus those with no SBSI. Poisson regression was used as hazards appeared to be constant over time. Characteristics of participants with SBSI who died compared to those that survived 1-year following SBSI were compared using Cox proportional hazards model. A Poisson regression model was used to calculate HR for all-cause mortality over the 18-year follow-up for PWH with SBSI compared to those without. Confounding was assessed by sequential inclusion of each potential confounder in a regression model. Likelihood Ratio Tests (LRT) were used to test departures from linear effects. Variables included in the model as confounders a priori included; age, sex, CD4 nadir and PWID status [8], [9], [10], [11], [12], [13], [14], [15], [16]. All p-values are two-tailed tests with the statistical significance level set at p<0.05 including 95% confidence intervals. All analysis was performed using STATA version 15.0 (College Station, TX).
2.5. Role of funding
No funding was received for this work.
3. Results
From the 3383 PWH in this study, 130 episodes of SBSI identified amongst 95 PWH with 34 cases of recurrent infections. There were 50 (38.4%) MSSA, 34 (26.1%) MRSA and 46 (35.3%) CoNS BSI (Table 1). Persons included were initially diagnosed with HIV between 1982 and 2017, HIV testing was introduced in 1985, therefore prior to 1985, diagnosis date was based on a transfusion date. HIV was diagnosed <14 days prior to SBSI in 6 (4.5%) episodes, and 11 (8.5%) episodes within ≤2 months of an HIV diagnosis. The mean duration between HIV diagnosis and incident SBSI was 8.6 years (0–30.4 yrs.). The mean duration of follow-up for all enrolled participants was 7.8 ± 6.0 yrs. (0.01–18 yrs.); those with and without SBSI had similar follow-up at 7.7 yrs. and 8.1yrs, respectively. The overall rate of SBSI was 604/100,000 patient-years (PY) (Table 2).
Table 1.
Characteristics of PWH who experience SBSI compared to those who do not between 2000 and 2018.
| Characteristics | Total n (%) N = 3383 | Total n with no SBSI (%) n = 3253 | Total n with SBSI (%) n = 130 | CoNS (%) (n = 46) | MSSA (%) (n = 50) | MRSA (%) (n = 34) | P-value | *Rate of SBSI/1000 PY (95% CI) | Hazard ratio (HR) for SBSI (95% CI) | P-value for HR | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PWID | No | 2744 (81.1) | 2143 (65.8) | 64 (49.2) | 28 (60.9) | 22 (44.0) | 12 (35.3) | 0.062 | 2.78 (2.16–3.58) | 1.00 | |
| Yes | 580 (17.1) | 1051 (32.3) | 66 (50.8) | 18 (39.1) | 28 (56.0) | 22 (64.7) | 16.62 (13.08–21.12) | 5.97 (4.22–8.46) | <0.001 | ||
| Missing | 59 (1.7) | 59 (1.8) | 0 (0) | ||||||||
| Age at HIV diagnosis, years | ≤30 | 1275 (37.7) | 1233 (37.9) | 42 (32.3) | 12 (26.1) | 19 (38.0) | 11 (32.4) | 0.626 | 3.93 (2.95–5.41) | 1.00 | |
| 31–40 | 1190 (35.2) | 1137 (35.0) | 53 (40.8) | 20 (43.5) | 18 (36.0) | 15 (44.1) | 5.82 (4.45–7.62) | 1.48 (0.99–2.22) | 0.057 | ||
| 41–50 | 594 (17.6) | 570 (17.5) | 24 (18.5) | 8 (17.4) | 11 (22.0) | 5 (14.7) | 5.74 (3.85–8.57) | 1.46 (0.89–2.42) | 0.137 | ||
| >50 | 247 (7.3) | 239 (7.4) | 8 (6.2) | 4 (8.7) | 1 (2.0) | 3 (8.8) | 4.84 (2.42–9.69) | 1.22 (0.58–2.63) | 0.587 | ||
| Missing | 77 (2.3) | 74 (2.3) | 3 (2.3) | 2 (4.4) | 1 (2.0) | 0 (0) | |||||
| Sex | Male | 2582 (76.3) | 2480 (76.2) | 96 (73.9) | 34 (73.9) | 33 (66.0) | 29 (85.3) | 0.142 | 4.83 (3.94–5.91) | 1.00 | |
| Female | 801 (23.7) | 767 (23.8) | 34 (26.2) | 12 (26.1) | 17 (34.0) | 5 (14.7) | 5.33 (3.81–7.46) | 1.10 (0.74–1.63) | 0.624 | ||
| Missing | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Ethnicity | Caucasian | 1839 (54.4) | 1764 (54.2) | 75 (57.7) | 26 (56.5) | 30 (60.0) | 19 (55.9) | 0.210 | 4.84 (3.85–6.08) | 1.00 | |
| Indigenous/Metis | 395 (11.7) | 358 (11.0) | 37 (28.5) | 10 (21.7) | 16 (32.0) | 11 (32.4) | 16.15 (11.70–22.29) | 3.34 (2.25–4.95) | <0.001 | ||
| African/Caribbean/Black | 695 (20.5) | 686 (21.1) | 9 (6.9) | 4 (8.7) | 4 (8.0) | 1 (2.9) | 1.63 (0.85–3.13) | 0.34 (0.17–0.67) | 0.002 | ||
| Other | 322 (9.5) | 313 (9.6) | 9 (6.9) | 6 (13.0) | 0 (0) | 3 (8.8) | 3.31 (1.58–6.95) | 0.68 (0.32–1.49) | 0.338 | ||
| Missing | 132 (3.9) | 132 (4.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| CD4 Nadir | >200 cells/mm3 | 1983 (58.6) | 1949 (59.9) | 34 (26.2) | 7 (15.2) | 14 (28.0) | 12 (35.3) | 0.198 | 2.43 (1.73–3.40) | 1.00 | |
| ≤200 cells/mm3 | 1294 (38.3) | 1216 (37.4) | 78 (60.0) | 33 (71.7) | 27 (54.0) | 19 (55.9) | 6.91 (5.53–8.62) | 2.85 (1.90–4.26) | <0.001 | ||
| Missing | 106 (3.1) | 88 (2.7) | 18 (13.9) | 6 (13.0) | 9 (18.0) | 3 (8.8) | |||||
| HIV Risk Factor | gbMSM | 1548 (45.8) | 1512 (46.5) | 36 (27.7) | 15 (32.6) | 15 (30.0) | 6 (17.7) | 0.005 | 2.84 (2.05–3.94) | 1.00 | |
| Heterosexual | 1182 (34.9) | 1094 (33.6) | 88 (67.7) | 25 (54.4) | 35 (70.0) | 28 (82.4) | 10.77 (8.73–13.29) | 3.79 (2.57–5.59) | <0.001 | ||
| Other | 566 (16.7) | 563 (17.3) | 3 (2.3) | 3 (6.5) | 0 (0) | 0 (0) | 0.62 (0.20–1.94) | 0.22 (0.68–0.71) | 0.012 | ||
| Missing | 87 (2.6) | 84 (2.6) | 3 (2.3) | 3 (6.5) | 0 (0) | 0 (0) | |||||
| HCV | No | 2388 (70.6) | 2351 (72.3) | 37 (28.5) | 18 (39.1) | 11 (22.0) | 8 (23.5) | 0.081 | 1.89 (1.38–2.60) | 1.00 | |
| Yes | 460 (13.6) | 391 (12.0) | 69 (53.1) | 18 (39.1) | 28 (56.0) | 23 (67.7) | 19.15 (15.12–24.24) | 10.15 (6.80–15.13) | <0.001 | ||
| Missing | 535 (15.8) | 511 (15.7) | 24 (18.5) | 10 (21.7) | 11 (22.0) | 3 (8.8)_ | |||||
Rate of SBSI comparing different demographic characteristics. Hazard ratio calculated using Poisson regression analysis for SBSI adjusting by different characteristics.
Table 2.
Incident Rates of SBSI for PWH between 2000 and 2018, assessing rates of MSSA, MRSA and CoNS.
| Years | Total active patient-years of follow up at SAC | Total SBSI | Total Rate/100,000 years | CoNS | CoNS rate/100,000yr | MSSA | MSSA rate/100,000yr | MRSA | MRSA rate/100,000yr |
|---|---|---|---|---|---|---|---|---|---|
| 2000–2005 | 4468 | 34 | 761.0 | 14 | 313.3 | 19 | 425.2 | 1 | 22.4 |
| 2006–2011 | 7145 | 44 | 615.8 | 15 | 209.9 | 11 | 154.0 | 18 | 251.9 |
| 2012–2018 | 9913 | 52 | 524.6 | 17 | 171.5 | 20 | 201.8 | 15 | 151.3 |
| Totals: | 21,526 | 130 | 603.9 | 46 | 213.7 | 50 | 232.3 | 34 | 157.9 |
Number of total active patient at SAC were obtained from the database starting January 1st to December 31st.
Blood culture draws occurred on hospital wards in 58 (44.6%) SBSI episodes, 57 (43.9%) occurred in the Emergency Department and 15 (11.5%) were in a community-based setting. Most SBSI episodes (112, 86.2%) were CA with 18 (13.9%) being HA (Table 3). Most HA-SBSI were due to either MSSA (7, 38.9%) or CoNS (10, 55.6%), with only one case (5.6%) caused by MRSA. The first case of MRSA-BSI in this cohort was identified in 2005, and there has consistently been ≥1 case each year since. Central line associated BSI (CLABSI) accounted for 12 infections (4 MSSA, 3 MRSA, 5 CoNS).
Table 3.
Characteristics associated with 1-year mortality in PWH with SBSI, using Cox proportional hazards regression models.
| Characteristics | Total (%) | Alive at 1 year following SBSI episode (%) (n = 102) | Died within 1 year following SBSI episode (%) (n = 28) | P value | 1-year Mortality Rate/100 PY (95% CI) | Hazard ratio (HR) (95% CI) | P-value for HR | |
|---|---|---|---|---|---|---|---|---|
| Organism | CoNS | 46 (35.4) | 38 (37.3) | 8 (28.6) | 0.618 | 20.6 (10.3–41.3) | 1.00 | |
| MSSA | 50 (38.5) | 39 (38.2) | 11 (39.1) | 25.9 (14.3–46.8) | 1.27 (0.51–3.16) | 0.606 | ||
| MRSA | 34 (26.2) | 25 (24.5) | 9 (32.1) | 35.2 (18.3–67.6) | 1.67 (0.65–4.34) | 0.289 | ||
| Year of SBSI diagnosis | 2000–2005 | 34 (26.2) | 28 (27.5) | 6 (21.4) | 0.813 | 20.4 (9.1–45.3) | 1.00 | |
| 2006–2011 | 44 (33.9) | 34 (33.3) | 10 (35.7) | 26.9 (14.5–50.0) | 1.31 (0.47–3.59) | 0.605 | ||
| 2012–2017 | 52 (40.0) | 40 (39.2) | 12 (42.9) | 29.9 (17.0–52.6) | 1.41 (0.53–3.74) | 0.496 | ||
| Age at HIV diagnosis, years (n = 127) | ≤30 | 42 (33.1) | 39 (29.3) | 13 (46.4) | 0.105 | 37.5 (21.8–64.6) | 1.00 | |
| 31–40 | 53 (41.7) | 46 (46.5) | 7 (25.0) | 15.5 (7.4–32.6) | 0.42 (0.17–1.06) | 0.068 | ||
| >40 | 32 (25.2) | 24 (24.2) | 8 (28.6) | 33.2 (16.6–66.5) | 0.85 (0.35–2.06) | 0.724 | ||
| Sex | Male | 96 (73.9) | 73 (71.6) | 23 (82.1) | 0.259 | 30.4 (20.2–45.7) | 1.00 | |
| Female | 34 (26.2) | 29 (28.4) | 5 (17.8) | 16.1 (6.7–38.6) | 0.56 (0.21–1.47) | 0.239 | ||
| Ethnicity | Caucasian | 75 (57.7) | 57 (55.9) | 18 (64.3) | 0.827 | 30.6 (19.3–48.5) | 1.00 | |
| Indigenous/Metis | 37 (28.5) | 31 (30.4) | 6 (21.4) | 17.4 (7.8–38.7) | 0.60 (0.23–1.51) | 0.280 | ||
| African/Caribbean/Black | 9 (6.9) | 7 (6.9) | 2 (7.1) | 28.2 (7.0–112.6) | 0.94 (0.22–4.07) | 0.939 | ||
| Other | 9 (6.9) | 7 (6.9) | 2 (7.1) | 31.7 (7.9–126.9) | 1.01 (0.23–4.34) | 0.992 | ||
| CD4 Nadir (n = 112) | >200 cells/mm3 | 34 (30.4) | 27 (32.1) | 6 (21.4) | 0.281 | 22.4 (10.1–49.9) | 1.00 | |
| ≤200 cells/mm3 | 78 (69.6) | 57 (67.9) | 22 (78.6) | 35.0 (23.0–53.1) | 1.55 (0.63–3.81) | 0.345 | ||
| HIV risk factor | gbMSM | 36 (27.7) | 23 (22.6) | 13 (46.4) | 0.044 | 52.6 (30.5–90.6) | 1.00 | |
| Heterosexual | 88 (67.7) | 74 (72.6) | 14 (50.0) | 18.2 (10.8–30.7) | 0.39 (0.18–0.82) | 0.014 | ||
| Other | 6 (4.6) | 5 (4.9) | 1 (3.6) | 19.9 (2.8–141.0) | 0.42 (0.05–3.22) | 0.404 | ||
| PWID | No | 62 (47.7) | 46 (45.1) | 16 (57.1) | 0.258 | 32.7 (20.0–53.3) | 1.00 | |
| Yes | 68 (52.3) | 56 (54.9) | 12 (42.9) | 20.7 (11.8–36.5) | 0.66 (0.31–1.40) | 0.276 | ||
| Source | Hospital | 18 (13.9) | 10 (9.8) | 8 (28.6) | 0.011 | 70.4 (35.2–140.8) | 1.00 | |
| Community | 112 (86.2) | 92 (90.2) | 20 (71.4) | 20.9 (13.5–32.5) | 0.33 (0.15–0.76) | 0.009 | ||
| Hospitalized | No | 45 (34.6) | 38 (37.3) | 7 (25.0) | 0.227 | 17.8 (8.5–37.3) | 1.00 | |
| Yes | 85 (65.4) | 64 (62.8) | 21 (75.0) | 31.1 (20.3–47.7) | 1.74 (0.74–4.09) | 0.205 | ||
| Antimicrobial Prophylaxis | No Prophylaxis | 65 (50.0) | 56 (54.9) | 9 (32.1) | 0.033 | 16.3 (8.5–31.3) | 1.00 | |
| *Prophylaxis | 65 (50.0) | 46 (45.1) | 19 (67.9) | 36.9 (23.5–57.8) | 2.22 (1.00–4.91) | 0.049 | ||
| *On ART | No | 38 (29.2) | 37 (36.3) | 1 (3.6) | 0.001 | 2.8 (0.3–16.7) | 1.00 | |
| Yes | 92 (70.8) | 65 (63.7) | 27 (96.4) | 31.5 (21.6–45.9) | 12.62 (1.71–92.86) | 0.013 | ||
| *CD4 (n = 111) | >200 cells/mm3 | 46 (41.4) | 37 (44.6) | 9 (32.1) | 0.248 | 24.2 (12.6–46.5) | 1.00 | |
| ≤200 cells/mm3 | 65 (58.6) | 46 (55.4) | 19 (67.9) | 36.9 (23.5–57.8) | 1.52 (0.69–3.36) | 0.302 | ||
| *Viral Load (n = 114) | ≤200 cells/mm3 | 46 (40.4) | 32 (37.2) | 14 (50.0) | 0.231 | 40.4 (23.9–68.2) | 1.00 | |
| >200 cells/mm3 | 68 (59.7) | 54 (62.8) | 14 (50.0) | 24.5 (14.5–41.4) | 0.63 (0.30–1.32) | 0.218 | ||
3.1. Demographics
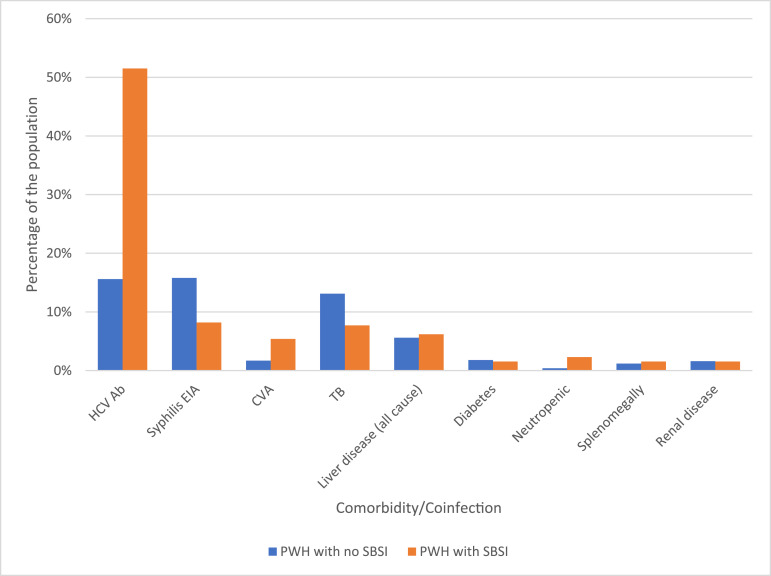
The mean age at HIV diagnosis was 35.6 ± 9.5 yrs. (16–63 yrs.), while the mean age at SBSI was 46.3 ± 9.2 yrs. (28–72 yrs.). The majority of those with SBSI were male (73.9%). PWH with SBSI episode were more likely to have HCV-coinfection compared to PWH with no SBSI (53.1% vs. 12.0% P <0.001). Due to small numbers of comorbidities in this cohort further associations could not be made (Fig. 2). Of HCV-coinfected persons, MSSA/MRSA accounted for most SBSI episodes (73.9%). Rates of SBSI were higher among PWID and heterosexual populations compared to gbMSM(gay, bisexual, men who have sex with men) (Table 1). Among those categorized other as their main HIV risk, the majority were from an endemic country followed by blood transfusion risk. Among cases of SBSI in gbMSM 57.1% were due to MSSA/MRSA, compared to 69.2% in the heterosexual population. In PWID, 49 (74.2%) were due to MSSA/MRSA, whereas 17 (25.8%) were due to CoNS (chi-square P = 0.020). Indigenous/metis persons had higher rates of SBSI 16.2/1000 PY compared to Caucasian 4.8/1000 PY and African/Caribbean/Black populations 1.6/1000 PY (Table 1).
Fig. 2.
Comorbidities/coinfections in PWH at SAC between 2000 and 2018 comparing those with SBSI to those without SBSI. HCV ab = hepatitis C antibody positive (n = 2824 no SBSI, n = 104 SBSI), Syphilis EIA = syphilis enzyme immunoassay (n = 1566 no SBSI, n = 49 SBSI), CVA = cerebrovascular event, TB=tuberculosis, defined as current disease or latent. Liver disease is defined as all cause; including persons with cirrhosis or hepatitis. Diabetes including both insulin dependent and not insulin dependent. Neutropenia defined as absolute neutrophil count <500/mm3. (n = 3253 no SBSI, n = 130 SBSI, unless otherwise specified).
3.2. HIV clinical characteristics
PWH with SBSI had a mean CD4 nadir of 159 cells/mm3, with 69.6% having a CD4 nadir of <200 cells/mm3. At the time of bacteremia, the most recent CD4 count averaged 228 cells/mm3 (0–959cells/mm3), with 58.6% of participants having a CD4 <200cells/mm3. HIV VL was undetectable (≤200 copies/mL) in 46 (40.4%) at the time of the initial SBSI. Most patients were being prescribed ART at the time of SBSI (92, 70.8%), and 65 (50%) were also on prophylactic antimicrobials for opportunistic infections (OIs) (Table 3). Use of trimethoprim-sulfamethoxazole and/or azithromycin prophylaxis had no association with SBSI (chi-square P = 0.230) (Table 4).
Table 4.
Characteristics associated with PWH with SBSI between 2000 and 2018, comparing the different staphylococcus species (MSSA, MRSA and CoNS).
| Characteristics associated with SBSI | Total with SBSI (%) n = 130 | CoNS (%) (n = 46) | MSSA (%) (n = 50) | MRSA (%) (n = 34) | *P value | |
|---|---|---|---|---|---|---|
| Year of SBSI diagnosis | 2000–2005 | 34 (26.2) | 14 (41.2) | 19 (55.9) | 1 (2.9) | 0.003 |
| 2006–2011 | 44 (33.9) | 15 (34.1) | 11 (25.0) | 18 (40.9) | ||
| 2012–2018 | 52 (40.0) | 17 (32.7) | 20 (38.5) | 15 (28.9) | ||
| Source | Hospital acquired | 18 (13.9) | 10 (55.6) | 7 (38.9) | 1 (5.6) | 0.055 |
| Community acquired | 112 (86.2) | 36 (32.1) | 43 (38.4) | 33 (29.5) | ||
| Hospitalized | No | 45 (34.6) | 19 (42.2) | 18 (40.0) | 8 (17.8) | 0.247 |
| Yes | 85 (65.4) | 27 (31.7) | 32 (37.7) | 26 (30.6) | ||
| 30-day mortality | 12 (9.2) | 2 (16.7) | 5 (41.7) | 5 (41.7) | 0.278 | |
| 1-year mortality | 28 (21.5) | 8 (35.4) | 9 (32.1) | 11 (39.3) | 0.618 | |
| Antimicrobial Prophylaxis | No Prophylaxis | 65 (50.0) | 19 (29.2) | 30 (46.2) | 16 (24.6) | 0.230 |
| Azithromycin | 2 (1.5) | 0 (0) | 2 (100.0) | 0 (0) | ||
| Septra | 35 (26.9) | 14 (40.0) | 10 (28.6) | 11 (31.4) | ||
| Azithromycin and Septra | 28 (21.5) | 13 (46.4) | 8 (28.6) | 7 (25.0) | ||
| On ART | No | 38 (29.2) | 12 (31.6) | 19 (50.0) | 7 (18.4) | 0.191 |
| Yes | 92 (70.8) | 34 (37.0) | 31 (33.7) | 27 (29.4) | ||
P-value obtained from chi-square test. n = 130.
3.3. Recurrent episodes of staphylococcal bacteremia
Twenty-two persons had recurrent infections; 13 persons had 2 episodes, 6 patients had 3 episodes, 2 patients had 4 episodes and 1 patient had 5 episodes. The average duration between each SBSI episode was 491 days (range 33–3121 days). In the person who had 5 episodes, these were all MRSA and separated by a minimum of 209 days. There was one case of polymicrobial SBSI due to MSSA followed 6 days later by CoNS-BSI. There were 11 instances where the recurrent BSI was with a different etiology all separated by at least 57 days. One PWH had a MSSA, MRSA and CoNS bacteremia all during the study period. The initial MSSA episode was followed by another MSSA-BSI 164 days later, an MRSA-BSI 82 days later and 273 days later had a CoNS-BSI. Most recurrent SBSI (60%) and the polymicrobial episode occurred in PWID.
3.4. Outcomes analysis
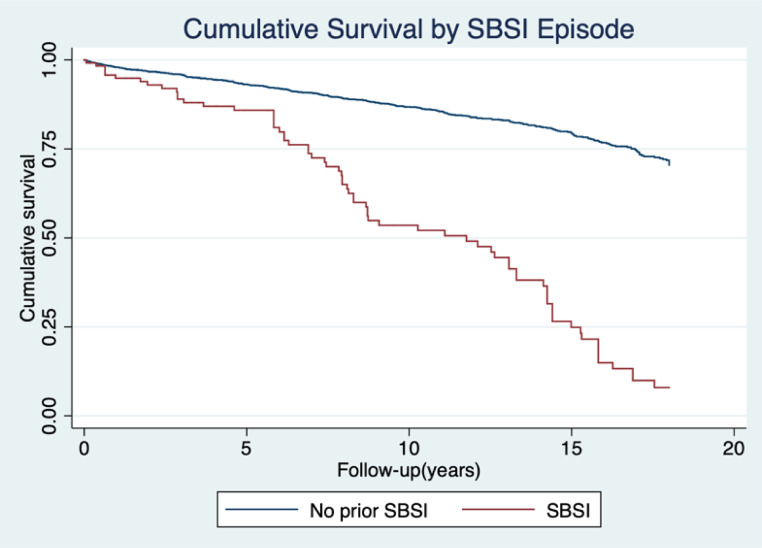
Overall, 423 deaths occurred over the 19-year follow-up period with a total mortality rate of 18.3/1000 patient-years (PY) (95% CI 16.6–20.1). The mortality rate was greater in those who had SBSI at 74.9/1000 PY (95% CI 59.2–94.9) compared to those with no SBSI at 16.0/1000 PY (95% CI 14.4–17.7) generating an unadjusted mortality Hazard Ratio (HR) for SBSI of 4.88 (95% CI 3.77–6.32 P= <0.001) (Fig. 3). The mortality for persons with SBSI remained higher HR=2.61 (95% CI 1.95–3.49, P= <0.001) following adjusting for age at HIV diagnosis, ethnicity, sex, CD4 nadir and HIV risk category when compared to PWH with no SBSI(Table 5).
Fig. 3.
Cumulative Survival Curve for PWH with SBSI compared to those without. The mortality rate was greater in those who had SBSI at 74.9/1000 PY (95% CI 59.2–94.9) compared to those with no SBSI at 16.0/1000 PY (95% CI 14.4–17.7) generating an unadjusted mortality Hazard Ratio (HR) for SBSI of 4.88 (95% CI 3.77–6.32 P <0.001).
Table 5.
Poisson regression model to assess confounders of the association between SBSI and all-cause mortality over 19 years of follow up, comparing the adjusted with unadjusted HR.
| Characteristic | Unadjusted HR | 95% CI | P-Value | ||
|---|---|---|---|---|---|
| Unadjusted HR of all-cause mortality based on SBSI | No SBSI | 1.00 | |||
| SBSI | 4.88 | 3.77–6.32 | <0.001 | ||
| Adjusted HR for all-cause mortality in SBSI by different characteristics | Adjusted HR | 95% CI | P-Value | ||
| SBSI | 2.61 | 1.95–3.49 | <0.001 | ||
| *Age at HIV diagnosis | 1.26 | 1.13–1.39 | <0.001 | ||
| Sex | Male | 1.00 | |||
| Female | 0.75 | 0.56–0.99 | 0.042 | ||
| Ethnicity | Caucasian | 1.00 | |||
| Indigenous/Metis | 1.23 | 0.93–1.62 | 0.144 | ||
| African/Caribbean/Black | 0.49 | 0.27–0.87 | 0.015 | ||
| Other | 0.37 | 0.21–0.67 | 0.001 | ||
| CD4 Nadir | >200 cells/mm3 | 1.00 | |||
| ≤200 cells/mm3 | 1.50 | 1.24–1.87 | <0.001 | ||
| PWID | No | 1.00 | |||
| Yes | 2.04 | 1.58–2.63 | <0.001 | ||
| HIV Risk Factor | gbMSM | 1.00 | |||
| Heterosexual | 1.33 | 1.03–1.71 | 0.027 | ||
| Other/Unknown | 0.75 | 0.38–1.45 | 0.388 | ||
Follow up duration is 19 years as morality data was collected until January 1st 2019 to ensure capture of 1 year mortality of all SBSI evaluated.
LRT done and shows no evidence that including separate effects for each group, therefore a linear trend was assumed and reported HR per group.
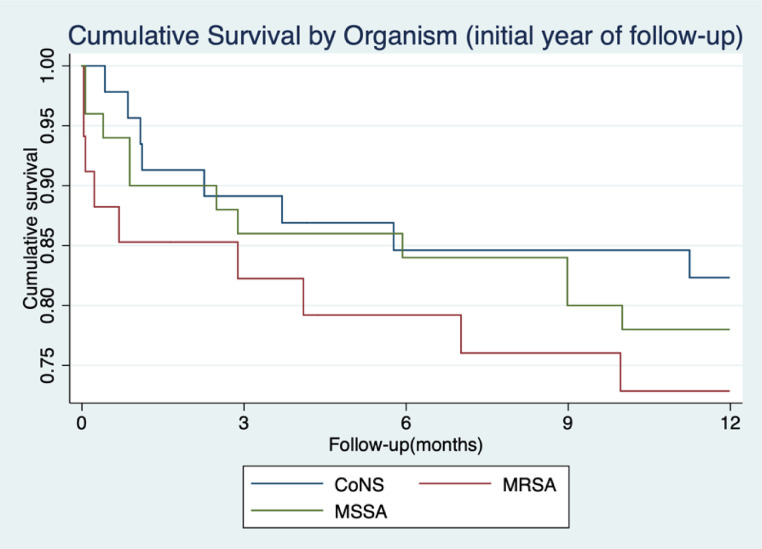
Seventy deaths occurred in persons with SBSI over the 19-year follow-up period with 28 (40%) occurring in the first year following SBSI. The overall 30-day mortality for all SBSI was 9.2% with 1-year mortality of 21.5%. The 30-day mortality rate was higher amongst those with MRSA (14.7%) compared to those with MSSA (10.0%), however likely due to low numbers this did not reach significance (P = 0.278). 1-year mortality rates for those with MSSA/MRSA was 23.8%. The rate was higher amongst MRSA (26.5%) compared to those with MSSA (22.0%), however this did not reach significance (P = 0.618) (Fig. 4). CoNS had the lowest 30-day mortality (4.3%). Those with recurrent bacteremia had 30-day mortality rates of 5.7%, with 1-year mortality being 20.0%.
Fig. 4.
Cumulative 1-year Survival Curve following SBSI episodes in PWH for MSSA, MRSA and CoNS. (n = 130).
Most patients with SBSI (65.4%) were hospitalized with 34.6% being managed in an ambulatory/community-based healthcare setting. Of outpatient cases, 40% had MSSA, 17% had MRSA and 42% had CoNS. While average duration of hospitalization was 31.6 days (range 2–209 days), those with CoNS bacteremia had longer length of stay (LOS) (36.9 days) compared to either types of SAB (LOS MRSA 29.8 days and MSSA 28.31 days). The most common reason for hospitalization for those who had MSSA BSI was due to cardiac disease or respiratory disease, MRSA was due to respiratory disease or the bacteremia itself and for CoNS-BSI was HIV related disease or psychiatric admission (Supplemental Figure 1). Hospitalized cases had a 15% 30-day mortality rate and 25% 1-year mortality. No deaths occurred in the community treated patients.
HA-SBSI had higher 30-day mortality compared to CA-SBSI, 22.2% vs 7.1%, (chi-square P = 0.040) as well as higher 1-year mortality 44.4% vs 17.9% (chi-square P = 0.011). Being a CA-pathogen was associated with improved 1-year mortality HR=0.33 (95% CI 0.15–0.76, P = 0.009) (Table 3). Of the 8 persons who died within one-year following HA-SBSI, 2 were MSSA, 1 MRSA and 5 were CoNS. Of persons with CLABSI, 5/12(41.7%) died within 1-year.
4. Discussion
The greatest association for SBSI among PHIV was seen in persons with PWID or heterosexual HIV risk factors, with CD4 nadirs less then 200 cells/mm3, of Indigenous/Metis ethnicity and coinfection with HCV. The rates of SBSI were greater among heterosexual populations compared to gbMSM, this is most likely explainable by PWID status, as those with self-reported IDU (injection drug use) were more likely to be heterosexual (79%) than gbMSM (21%). As previously described, rates of SBSI are greater among PWID as well as HCV-coinfection [12, 26, 27]. There were more episodes of MSSA and MRSA among PWID than CoNS. There was no significant difference between age at HIV diagnosis, sex and ethnicity among etiology of SBSI. A greater proportion of PWID and HCV-coinfected PWH had MSSA/MRSA rather than CoNS.
Indigenous/Metis populations were at higher risk of SBSI. This may be attributable to risk from IDU as 47% of Indigenous/Metis persons in our cohort had current or prior self-reported history of IDU compared to the entire cohort (21%). It is possible that other variables could be impacting the association such as comorbidities (i.e. Diabetes or CKD [28]) or sociodemographic factors. Other studies have also identified an association between incidence rates of Staphylococcal infections and ethnicity [29, 30, 31]. Further data is needed to evaluate specific reasons why differences among ethnicities are seen.
There were significant numbers of reinfection among this cohort, with 35 episodes being recurrent infections. A large population-based cohort study identified that SAB reinfection rates in PWH were six-fold higher than in HIV negative populations [32]. Other risk factors identified for SAB reinfection include; renal disease, diabetes, liver disease, peptic ulcer and paraplegia [32]. One study identified HIV and IDU as independent risk factors for SAB reinfection [9].
PWH with SBSI are at increased risk of mortality and particularly within the first year following SBSI. The mortality rates between MSSA, MRSA and CoNS were not significantly different, however this may be due to a small number of cases. In an earlier study looking at the entire Calgary Health Region population by Lam et al., the 30-day mortality rates were 30.6% for MRSA and 21.3% for MSSA [4, 33]. In our PWH cohort the 30-day mortality rates for MRSA was 14.7%, 10.0% for MSSA and 4.3% for CoNS. Age has been found to be associated with increased mortality in SBSI, the average age of our cohort at time of bacteremia was 46.3 yrs. compared to 62 yrs. in the study by Lam et al. [4, 34]. HA-SBSI was associated with higher mortality rates as previously shown in other studies [4, 33]. A small proportion of our cases were HA (13.9%) compared to the study by Lam et al. (26.1%) [4]. As PWH were younger at the time of SBSI as well as more cases were CA, this may explain the reduced mortality rates seen in PWH.
Prior to 2005, there were no cases of MRSA-BSI in our cohort, however since, there have been 34 cases. Only one was HA-MRSA. A recent study reported that 52.5% of positive MRSA infections in Calgary between 2004 and 2014 were CA-MRSA [17]. The prevalence increased substantially from 3.6/100,000 population in 2004 to 41.3 cases/100,000 in 2014 [17]. In our PWH cohort in 2014 the rate for MRSA bacteremia was 251/100,000 population, which is over 6 times greater than the general population. The rates of CoNS-BSI in PWH are decreasing with time and there has been relative stability with MSSA-BSI rates, one possible explanation for this is in the improvement in management of HIV and greater accessibility to ART. In our cohort of PWH the number of persons on ART increased from 57.9% in 2000 to 93.4% in 2017 and viral suppression of <200 copies/mL increased from 79.4% in 2000 to 96.8% in 2017 with HIV-related annual mortality rate declining from 11% in 1994 to 0.1% in 2017 [35]. Another explanation for the decline in rates of CoNS and MRSA is likely due to improvements in infection control strategies in hospital and outpatient settings [36], [37], [38].
CoNS was more likely to be HA when compared to MSSA and MRSA and this is likely explained as it is accepted as a common nosocomial pathogen. Declercq et al. found that out of 54 BSI in a cohort of PWH, the most common organism identified was CoNS accounting for 26% of cases [11]. CoNS are also the most common cause of pseudobacteremia [7]. We tried to include only true pathogens by excluding all cultures with growth of only one specimen from the blood sample collected. The 1-year mortality rates were higher for MSSA and MRSA, however there was no strong evidence to suggest this was significantly different compared to CoNS.
This work does have several limitations. Our population is geographically defined to Southern Alberta, therefore may not be generalizable to other populations. Due to small numbers of SBSI and deaths in our cohort, significance may not have been demonstrated in our analysis, especially when cases were subdivided into groups. It is possible that there are confounders of these associations that were not collected and evaluated in this study. Due to missing data for HCV status, this was not included in the Poisson regression model. Mortality outcomes were all cause, therefore direct linkage to SBSI cannot be made, future work should evaluate specific causes of mortality to identify associations among PWH that may benefit from prophylactic measures.
The strength of this study lies in the comprehensive, longitudinal clinical and microbiologic databases that were utilized. We identified all PWH with SBSI accessing HIV care and utilized the SAC database to provide a representative control population of PWH in our region to optimize the external validity of this study. The duration of cohort follow-up made it possible to calculate and compare mortality rates over time and in the different eras of HIV management strategies and ART availability.
While the incidence rate of SBSI is higher in PWH, the mortality rate is lower than in the past reported studies on SBSI in the same general population. PWH with SBSI were however at increased risk of mortality and particularly within the first year following SBSI. Higher 1-year mortality rates occurred in hospital-acquired infections and in gbMSM. While the mortality rates between MSSA, MRSA and CoNS, were not significantly different, the highest mortality was among MRSA and least among CoNS. The highest risk of SBSI in PWH was seen in those with Indigenous/Metis ethnicity, HCV-coinfection, low CD4 nadir and in PWID. Further investigation is needed in PWH evaluating host, environment and pathogen differences that lead to increased rates of SBSI.
Funding
No funding was received for this work.
Data sharing
Due to the confidential and identifiable nature of this dataset, data sharing will not be available. All authors have accessed the database and verified its accuracy.
Declaration of Competing Interest
MJ Gill has received honoraria as ad hoc member of national HIV advisory Boards to Merck, ViiV and Gilead. All other authors report no conflict.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100675.
Contributor Information
Raynell Lang, Email: Raynell.Lang@ahs.ca.
Deirdre Church, Email: dchurch@ucalgary.ca.
Appendix. Supplementary materials
References
- 1.Laupland K.B., Lyytikainen O., Sogaard M. The changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance study. Clin Microbiol Infect. 2013;19(5):465–471. doi: 10.1111/j.1469-0691.2012.03903.x. [DOI] [PubMed] [Google Scholar]
- 2.Laupland K.B., Church D.L. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin Microbiol Rev. 2014;27(4):647–664. doi: 10.1128/CMR.00002-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laupland K.B., Church D.L., Gregson D.B. Blood cultures in ambulatory outpatients. BMC Infect Dis. 2005;5:35. doi: 10.1186/1471-2334-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam J.C., Gregson D.B., Robinson S., Somayaji R., Conly J.M., Parkins M.D. Epidemiology and outcome determinants of staphylococcus aureus bacteremia revisited: a population-based study. Infection. 2019;47(6):961–971. doi: 10.1007/s15010-019-01330-5. [DOI] [PubMed] [Google Scholar]
- 5.David M.Z., Daum R.S. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23(3):616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allard C., Carignan A., Bergevin M. Secular changes in incidence and mortality associated with Staphylococcus aureus bacteraemia in Quebec, Canada, 1991-2005. Clin Microbiol Infect. 2008;14(5):421–428. doi: 10.1111/j.1469-0691.2008.01965.x. [DOI] [PubMed] [Google Scholar]
- 7.Souvenir D., Anderson D.E., Jr., Palpant S. Blood cultures positive for coagulase-negative staphylococci: antisepsis, pseudobacteremia, and therapy of patients. J Clin Microbiol. 1998;36(7):1923–1926. doi: 10.1128/jcm.36.7.1923-1926.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huson M.A., Stolp S.M., van der Poll T., Grobusch M.P. Community-acquired bacterial bloodstream infections in HIV-infected patients: a systematic review. Clin Infect Dis. 2014;58(1):79–92. doi: 10.1093/cid/cit596. [DOI] [PubMed] [Google Scholar]
- 9.Stammler Jaliff B., Dahl-Knudsen J., Petersen A., Skov R., Benfield T. Outcome and reinfection after Staphylococcus aureus bacteraemia in individuals with and without HIV-1 infection: a case-control study. BMJ Open. 2014;4(4) doi: 10.1136/bmjopen-2013-004075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer C.N., Skinhoj P., Prag J. Bacteremia in HIV-positive and AIDS patients: incidence, species distribution, risk-factors, outcome, and influence of long-term prophylactic antibiotic treatment. Scand J Infect Dis. 1994;26(6):635–642. doi: 10.3109/00365549409008630. [DOI] [PubMed] [Google Scholar]
- 11.Declercq S., De Munter P., Derdelinckx I. Characteristics, causes, and outcome of 54 episodes of bloodstream infections in a cohort of HIV patients. Infect Dis (Lond) 2015;47(9):611–617. doi: 10.3109/23744235.2015.1033002. [DOI] [PubMed] [Google Scholar]
- 12.Yehia B.R., Fleishman J.A., Wilson L. Incidence of and risk factors for bacteraemia in HIV-infected adults in the era of highly active antiretroviral therapy. HIV Med. 2011;12(9):535–543. doi: 10.1111/j.1468-1293.2011.00919.x. [DOI] [PubMed] [Google Scholar]
- 13.Ortega M., Almela M., Soriano A. Bloodstream infections among human immunodeficiency virus-infected adult patients: epidemiology and risk factors for mortality. Eur J Clin Microbiol Infect Dis. 2008;27(10):969–976. doi: 10.1007/s10096-008-0531-5. [DOI] [PubMed] [Google Scholar]
- 14.Burkey M.D., Wilson L.E., Moore R.D., Lucas G.M., Francis J., Gebo K.A. The incidence of and risk factors for MRSA bacteraemia in an HIV-infected cohort in the HAART era. HIV Med. 2008;9(10):858–862. doi: 10.1111/j.1468-1293.2008.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meynard J.L., Guiguet M., Fonquernie L. Impact of highly active antiretroviral therapy on the occurrence of bacteraemia in HIV-infected patients and their epidemiologic characteristics. HIV Med. 2003;4(2):127–132. doi: 10.1046/j.1468-1293.2003.00146.x. [DOI] [PubMed] [Google Scholar]
- 16.Afessa B., Morales I., Weaver B. Bacteremia in hospitalized patients with human immunodeficiency virus: a prospective, cohort study. BMC Infect Dis. 2001;1:13. doi: 10.1186/1471-2334-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill V.C., Ma I., Guo M., Gregson D.B., Naugler C., Church D.L. Sociodemographic and geospatial associations with community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) infections in a large Canadian city: an 11 year retrospective study. BMC Public Health. 2019;19(1):914. doi: 10.1186/s12889-019-7169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert M., MacDonald J., Gregson D. Outbreak in Alberta of community-acquired (USA300) methicillin-resistant Staphylococcus aureus in people with a history of drug use, homelessness or incarceration. CMAJ. 2006;175(2):149–154. doi: 10.1503/cmaj.051565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haddy R.I., Richmond B.W., Trapse F.M., Fannin K.Z., Ramirez J.A. Septicemia in patients with AIDS admitted to a university health system: a case series of eighty-three patients. J Am Board Fam Med. 2012;25(3):318–322. doi: 10.3122/jabfm.2012.03.110106. [DOI] [PubMed] [Google Scholar]
- 20.Miller J.M., Binnicker M.J., Campbell S. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the infectious diseases society of America and the American society for microbiology. Clin Infect Dis. 2018;67(6):e1–e94. doi: 10.1093/cid/ciy381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson M.L., Mirrett S., Reller L.B., Weinstein M.P., Reimer L.G. Recovery of clinically important microorganisms from the BacT/Alert blood culture system does not require testing for seven days. Diagn Microbiol Infect Dis. 1993;16(1):31–34. doi: 10.1016/0732-8893(93)90127-s. [DOI] [PubMed] [Google Scholar]
- 22.Kirn T.J., Weinstein M.P. Update on blood cultures: how to obtain, process, report, and interpret. Clin Microbiol Infect. 2013;19(6):513–520. doi: 10.1111/1469-0691.12180. [DOI] [PubMed] [Google Scholar]
- 23.Performance Standards for Antimicrobial Susceptibility Testing. Supplement M100. Wayne: clincal and Laboratory Standards Institute; 2017.Wayne: clincal and Laboratory Standards Institute; 2017.
- 24.Rodger A.J., Cambiano V., Bruun T. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet. 2019;393(10189):2428–2438. doi: 10.1016/S0140-6736(19)30418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC. HIV treatment as prevention. Division of HIV/AIDS prevention, national center for HIV/AIDS, viral hepatitis, STD, and TB prevention, centers for disease control and prevention. Available at: https://www.cdc.gov/hiv/risk/art/index.html. Accessed May 16, 2020.
- 26.Bassetti S., Battegay M. Staphylococcus aureus infections in injection drug users: risk factors and prevention strategies. Infection. 2004;32(3):163–169. doi: 10.1007/s15010-004-3106-0. [DOI] [PubMed] [Google Scholar]
- 27.Tumbarello M., Tacconelli E., Donati K.G. HIV-associated bacteremia: how it has changed in the highly active antiretroviral therapy (HAART) era. J Acquir Immune Defic Syndr. 2000;23(2):145–151. doi: 10.1097/00126334-200002010-00006. [DOI] [PubMed] [Google Scholar]
- 28.Canadian Diabetes Association Clinical Practice Guidelines Expert C., Harris S.B., Bhattacharyya O., Dyck R., Hayward M.N., Toth E.L. Type 2 diabetes in Aboriginal peoples. Can J Diabetes. 2013;37(Suppl 1):S191–S196. doi: 10.1016/j.jcjd.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 29.Irvine J., Canadian Paediatric Society F.N.I., Metis Health C. Community-associated methicillin-resistant Staphylococcus aureus in Indigenous communities in Canada. Paediatr Child Health. 2012;17(7):395–398. [PMC free article] [PubMed] [Google Scholar]
- 30.Tong S.Y., van Hal S.J., Einsiedel L., Currie B.J., Turnidge J.D. Australian New Zealand Cooperative on Outcomes in Staphylococcal S. Impact of ethnicity and socio-economic status on Staphylococcus aureus bacteremia incidence and mortality: a heavy burden in Indigenous Australians. BMC Infect Dis. 2012;12:249. doi: 10.1186/1471-2334-12-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serota D.P., Niehaus E.D., Schechter M.C. Disparity in quality of infectious disease vs addiction care among patients with injection drug use-associated staphylococcus aureus bacteremia. Open Forum Infect Dis. 2019;6(7):ofz289. doi: 10.1093/ofid/ofz289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiese L., Mejer N., Schonheyder H.C. A nationwide study of comorbidity and risk of reinfection after Staphylococcus aureus bacteraemia. J Infect. 2013;67(3):199–205. doi: 10.1016/j.jinf.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Laupland K.B., Ross T., Gregson D.B. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000-2006. J Infect Dis. 2008;198(3):336–343. doi: 10.1086/589717. [DOI] [PubMed] [Google Scholar]
- 34.van Hal S.J., Jensen S.O., Vaska V.L., Espedido B.A., Paterson D.L., Gosbell I.B. Predictors of mortality in Staphylococcus aureus Bacteremia. Clin Microbiol Rev. 2012;25(2):362–386. doi: 10.1128/CMR.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanhoff N., Vu Q., Lang R., Gill M.J. Impact of three decades of antiretroviral therapy in a longitudinal population cohort study. Antivir Ther. 2019;24(3):153–165. doi: 10.3851/IMP3287. [DOI] [PubMed] [Google Scholar]
- 36.Walz J.M., Ellison R.T., 3rd MackDA. The bundle "plus": the effect of a multidisciplinary team approach to eradicate central line-associated bloodstream infections. Anesth Analg. 2015;120(4):868–876. doi: 10.1213/ANE.0b013e3182a8b01b. [DOI] [PubMed] [Google Scholar]
- 37.Henderson D.M., Staiger T.O., Peterson G.N. A collaborative, systems-level approach to eliminating healthcare-associated MRSA, central-line-associated bloodstream infections, ventilator-associated pneumonia, and respiratory virus infections. J Healthc Qual. 2012;34(5):39–47. doi: 10.1111/j.1945-1474.2012.00213.x. quiz 8-9. [DOI] [PubMed] [Google Scholar]
- 38.Lawes T., Lopez-Lozano J.M., Nebot C.A. Effects of national antibiotic stewardship and infection control strategies on hospital-associated and community-associated meticillin-resistant Staphylococcus aureus infections across a region of Scotland: a non-linear time-series study. Lancet Infect Dis. 2015;15(12):1438–1449. doi: 10.1016/S1473-3099(15)00315-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.