Abstract
Background
Hyperprogressive disease (HPD) is a new progressive pattern in patients with advanced hepatocellular carcinoma (HCC) treated with programmed cell death 1 (PD-1) inhibitors. We aimed to investigate risk factors associated with HPD in advanced HCC patients undergoing anti-PD-1 therapy.
Methods
A total of 69 patients treated with anti-PD-1 therapy between March 2017 and January 2020 were included. HPD was determined according to the time to treatment failure, tumour growth rate, and tumour growth rate ratio. Univariate and multivariate analyses were performed to identify clinical variables significantly associated with HPD. A risk model was constructed based on clinical variables with prognostic significance for HPD.
Findings
Overall, 10 (14·49%) had HPD. Haemoglobin level, portal vein tumour thrombus, and Child-Pugh score were significantly associated with HPD. The risk model had an area under the curve of 0·931 (95% confidence interval, 0·844–1·000). Patients with HPD had a significantly shorter overall survival (OS) than that of the patients with non-HPD (p < 0·001). However, there was no significant difference in OS between PD (progressive disease) patients with and without HPD (p = 0·05).
Interpretation
We identified three clinical variables as risk factors for HPD, providing an opportunity to aid the pre-treatment evaluation of the risk of HPD in patients treated with immunotherapy.
Funding
This study was funded by the National Natural Science Foundation of China (81571664, 81871323, and 81801665); National Natural Science Foundation of Guangdong Province (2018B030311024); Scientific Research General Project of Guangzhou Science Technology and Innovation Commission (201707010,328); and China Postdoctoral Science Foundation (2016M600145).
Keywords: Hyperprogressive disease, Programmed cell death 1, Immune checkpoint inhibitors, Hepatocellular carcinoma
Research in context.
Evidence before this study
For patients with advanced-stage and unresectable hepatocellular carcinoma (HCC), the survival benefit of the standard therapy of sorafenib is limited. Programmed cell death 1 (PD-1) inhibitors, which are emerging immune checkpoint inhibitors, have recently been proved effective in patients with HCC. However, some patients did not benefit from PD-1 therapy, and presented with a new and rapidly progressive pattern, namely, hyperprogressive disease (HPD).
Added value of this study
We found that three clinical variables were associated with HPD: haemoglobin level, portal vein tumour thrombus, and Child-Pugh score. Based on these, we constructed a risk model, which yielded an area under the curve of 0·931 (95% confidence interval, 0·844–1·000). After survival analysis, we found that patients with HPD have a shorter overall survival (OS) than do patients with non-HPD. However, there was no significant difference in OS between PD (progressive disease) patients with and without HPD.
Implications of all the available evidence
This study identified three risk factors to aid in the pre-treatment evaluation of the risk of HPD in patients treated with immunotherapy. This study confirmed that HPD have a higher growth ratio after immunotherapy, and is associated with a poor prognostic.
Alt-text: Unlabelled box
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third leading cause of cancer-related mortality, with 782,000 new cases annually worldwide [1,2]. The incidence of HCC is closely associated with chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) virus infections, heavy alcohol consumption, obesity, smoking, and diabetes [3]. For patients with early-stage disease, resection, transplantation, and radiofrequency ablation are recommended curative therapies; however, most patients are diagnosed with advanced-stage, unresectable disease [4]. The therapeutic options for these patients are limited. Sorafenib is the standard therapy for patients with advanced HCC; it prolongs median survival by 3 months [5], although patients frequently develop resistance, and the drug is poorly tolerated, leading to limited benefits. Therefore, there is an urgent need to identify available systemic therapies for patients with advanced HCC.
Programmed cell death 1 (PD-1) is an immune checkpoint molecule, and inhibitors that target PD-1 are promising therapeutic agents with encouraging efficacy in many advanced cancers, such as melanoma, non-small cell lung cancer (NSCLC), and head and neck squamous cell carcinoma (HNSCC). Scheiner et al. [6] reported that anti-PD-1 therapy showed a clear benefit in patients with both treatment-naive and previously treated advanced HCC. The disease control rate (DCR) was > 40% in their study, and the median survival was 11 months.
Notably, previous studies identified that a subset of patients treated with immunotherapy presented a paradoxical acceleration of tumour growth, defined as hyperprogressive disease (HPD) [7]. HPD has been reported to occur in approximately 13·8%, 29%, and 9% of patients with NSCLC, HNSCC, and uveal melanoma, respectively, who received immunotherapy [8]. Wong et al. and Scheiner et al. also reported that a subgroup of patients with advanced HCC treated with PD-1/programmed death-ligand-1 (PD-L1)-targeted therapy presented with rapid tumour growth, accounting for approximately 9% and 8% of their treated cohorts, respectively [6,9]. It has been hypothesised that anti-PD-1/PD-L1 therapy induces activated tumour-reactive T-cell apoptosis, resulting in local T-cell immune suppression, thus contributing to tumour growth and metastasis [10,11]. The median overall survival (OS) of patients with HPD in most previous studies was < 3 months. The differences in prognosis might be attributable to biological heterogeneity; hence, searching for risk factors associated with HPD to guide treatment decisions for patients is important. Great efforts have been made to identify risk factors associated with HPD in advanced cancers, such as older age, female sex, and more metastatic lesions before treatment [12]. However, these risk factors have not been used to analyse HPD in patients with advanced HCC treated with anti-PD-1 therapy.
Therefore, in this study, we aimed to analyse the association between clinical variables and HPD in patients with advanced HCC who underwent anti-PD-1 therapy and to investigate the risk factors associated with HPD. We thereby constructed a risk model to evaluate the risk of HPD in advanced HCC before treatment with anti-PD-1 therapy.
2. Materials and methods
This was a retrospective study of patients with histologically diagnosed advanced HCC treated with humanised, high-affinity, selective PD-1 inhibitors (nivolumab, pembrolizumab, and camrelizumab) at a single centre between March 2017 and January 2020. The inclusion criteria were as follows: (a) availability of PD-1 inhibitor infusion records and oncology clinic notes for chart review; (b) ongoing effective antiviral therapy and a viral load < 100 IU/mL at screening; (c) an Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 2; and (d) Child-Pugh score ≤ 9. The exclusion criteria were as follows: (a) previous treatment with immune checkpoint inhibitor therapy alone or in combination with any other treatment; (b) absence of any measurable tumour lesions; (c) absence of appropriate computed tomography (CT) scans before or after treatment; and (d) conditions necessitating treatment discontinuation (occurrence of Adverse Events [AEs] necessitating treatment withdrawal depending on the discretion of the investigator disease progression, development of a second malignant tumour, worsening of the ECOG PS to 4, and withdrawal of consent). The flow chart of patient selection is presented in Fig. 1. Additionally, we collected the data of patients treated with tyrosine kinase inhibitors (TKIs) as the control group to compare the pattern of tumour growth dynamics and HPD (n = 34).
Fig. 1.
Flowchart of patients included in the study.
The following data were collected: age, sex, alcohol consumption status, smoking history, body mass index (BMI), diabetes diagnosis, HBV infection, HCV infection, ECOG PS, Child-Pugh score, pre-/posttreatment alpha-fetoprotein (AFP) level, cancer antigen (CA) 125, CA 19–9, routine blood count (neutrophils, lymphocytes, haemoglobin, and platelets), prothrombin time, blood biochemistry (alanine aminotransferase, aspartate transaminase, lactate dehydrogenase, globulin, albumin, total albumin, total bilirubin, and direct bilirubin levels), number of targeted tumours, baseline diameter of targeted tumours, metastatic sites before PD-1 inhibitor therapy, number of metastases before PD-1 inhibitor therapy, portal vein tumour thrombus (PVTT), type of PVTT, type of treatment before PD-1 inhibitor therapy, and subsequent therapy. PVTT was categorised into four types, as previously described [13].
All patients underwent a contrast-enhanced CT scan before and after immunotherapy (the time interval was 6 weeks) in accordance with the liver protocol, which included unenhanced, arterial, portal venous, and washout phases. After obtaining a routine unenhanced scan, 1·5 mL/kg of contrast material (Ultravist 370, Bayer Schering Pharma, Berlin, Germany) was injected into an antecubital vein at a rate of 3·0–3·5 mL/s with a pump injector. The triple-phase (hepatic arterial, portal venous, and delayed phases) CT images were obtained at 30, 60, and 120 s, respectively. The pre-baseline scan was performed in the time interval between 6 weeks prior to treatment and baseline. The baseline scan was performed at the beginning of immunotherapy. The first evaluation scan was performed 6 weeks after initial immunotherapy. These CT scans were used to assess the treatment response according to the Response Evaluation Criteria in Solid Tumours (RECIST) 1·1 [14] and immune-related RECIST (irRECIST) [15]. Treatment response was evaluated and classified by an independent radiologist (L.J.) with 15 of experience in abdominal CT image interpretation and an independent treating medical oncologist (C.X.D.) with 20 years of experience.
The primary endpoint was OS, which was defined as the time from the date of the first PD-1 inhibitor treatment to the date of death or the most recent follow-up before June 2020. The secondary efficacy endpoint was development of a HPD prediction model in anti-PD1-treated patients. The objective response rate (ORR) (complete response [CR] + partial response [PR]) and DCR (CR + PR + stable disease [SD]) were also calculated.
Treatment-related AEs, including those occurring within 1 week after treatment, and other complications observed during the follow-up that were most likely associated with treatment were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4·0 [16].
2.1. PD-1 inhibitor doses
Overall, 32 patients were treated with a 2-week cycle of intravenous nivolumab at a dosage of 1–3 mg/kg body weight or a prescribed dose of 240 mg, and 23 patients received a 3-week cycle of intravenous pembrolizumab at a dose of 2 mg/kg body weight or a prescribed dose of 200 mg. The remaining 14 patients received intravenous camrelizumab at a dose of 3 mg/kg body weight in a 3-week cycle.
2.2. Definition of tumour growth rate and HPD
Tumour growth rate (TGR) is a tool that allows for the dynamic and quantitative assessment of tumour volume and burden. According to a previous study [17], TGR was defined as the monthly log-scale-calibrated change in the sum of the volumes of the target lesions according to RECIST 1·1. New lesions and non-measurable disease were excluded, and TGR was only quantified for the target lesions. TGR was calculated as previously described[17, 18].
HPD was defined as follows: (1) time to treatment failure (TTF) < 2 months; (2) disease progression at the first evaluation and > 50% increase in TGR; and (3) a TGR ratio (TGRR) > 2. TGRR was defined as the ratio of TGREXP (between baseline and the first tumour evaluation) to TGRPRE (before treatment onset). Further details can be found in the study by Ferte et al. [18].
2.3. Statistical analyses
We categorised patients according to treatment response into non-HPD (including PR, SD, and PD without HPD) and HPD (PD with HPD) groups. Associations between HPD and categorical or continuous variables were evaluated using the independent-samples t-test, χ2 test, or Mann-Whitney U test. Consistency tests were performed to analyse the consistency of evaluating treatment response based on RECIST 1·1 and irRECIST. The intra- and interobserver reliabilities of TGR from different measurements (radiologist L.J. and oncologist C.X.D.) were assessed by the intraclass correlation coefficient (ICC), with an ICC > 0·8 being regarded as excellent reliability. We calculated the reasons for progression between the PD without HPD and HPD groups. We performed univariate and multivariate analyses using logistic regression analysis to identify the clinical variables and AEs associated with HPD. A risk model was built based on the clinical variables that had prognostic significance for HPD using logistic regression analysis. We calculated the area under the curve (AUC) to assess the predictive ability of the model. We used a Cox proportional hazards model to calculate hazard ratios (HRs) and 95% confidence intervals [CIs]. Kaplan-Meier survival curves and the log-rank test were used to compare OS between the HPD and non-HPD groups. We also performed many subgroup survival analyses of OS between different groups. Landmark analyses were performed according to a landmark point at 1·5 months, with the p-values calculated separately for events that occurred up to and including 1·5 months and events that occurred between 1·5 months and the end of the follow-up period [19]. We also treated missing time-to-event data due to loss of the patient to follow-up or still alive in June 2020, which was the date of the last follow-up as censored data. All tests were two-sided, and a p-value < 0·05 was considered to indicate statistical significance. All statistical analyses were performed using Statistical Package for SPSS version 25·0 software (IBM Corp., Chicago, IL, USA) and STATA version 16·0 software (Stata Corporation, College Station, TX, USA).
2.4. Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of our hospital, and the study was performed following the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The requirement for written informed consent was waived by the Institutional Review Board due to the retrospective of our study.
2.5. Role of the funding sources
The funding sources had no role during the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication.
3. Results
3.1. Patient characteristics
A total of 69 patients treated with PD-1 inhibitors were included in this analysis. amongst them, 52 were treated with PD-1 inhibitors alone and 17 were treated with PD-1 inhibitors plus other therapies (four were treated with PD-1 inhibitors plus local therapy, five with PD-1 inhibitors plus systemic therapy, and eight with PD-1 inhibitors plus systemic therapy plus local therapy). The baseline clinical characteristics of the HPD and non-HPD groups are presented in Table 1. There were statistically significant differences in age, Child-Pugh score, posttreatment AFP, incidence of PVTT, and type of PVTT (all p < 0·05) between the two groups. amongst all patients, the mean age was 51 years; most patients were men (51, 81·4%), with normal BMI (mean, 20), high HBV infection (62, 89·9%), good ECOG PS (0 or 1: 65, 94·2%), and relatively substantial alcohol consumption and smoking history (41 and 36; 59·4% and 52·2%; respectively). Twenty-three patients experienced PVTT before PD-1 inhibitor therapy.
Table 1.
Baseline patient characteristics based on the occurrence of hyperprogressive disease.
| Variable | HPD N = 10 | Non-HPD N = 59 | p value |
|---|---|---|---|
| Mean age (year, range) | 42 (32–62) | 52 (24–83) | 0·04 |
| Sex, male/female | 9/1 | 48/11 | 0·51 |
| BMI, mean (kg/m2, range) | 21 (14–26) | 20 (14–26) | 0·39 |
| History | |||
| HBV | 9 | 53 | 0·99 |
| HCV | 1 | 6 | 0·99 |
| cirrhosis | 5 | 25 | 0·66 |
| alcohol consumption | 5 | 36 | 0·52 |
| smoking | 4 | 32 | 0·41 |
| diabetes | 4 | 14 | 0·28 |
| ECOG PS | 0·54 | ||
| ≤ 1 | 9 | 56 | |
| ˃ 1 | 1 | 3 | |
| Child-Pugh score | 0·001 | ||
| 5 | 1 | 33 | |
| 6 | 7 | 23 | |
| 7 | 2 | 3 | |
| Pre-treatment AFP (ng/mL) | 0·18 | ||
| ≤ 200 | 2 | 25 | |
| ˃ 200 | 8 | 34 | |
| Posttreatment AFP (ng/mL) | 0·04 | ||
| ≤ 200 | 1 | 27 | |
| ˃ 200 | 9 | 32 | |
| CA125 (U/mL) | 0·88 | ||
| ≤ 47 | 5 | 28 | |
| ˃ 47 | 5 | 31 | |
| CA199 (U/mL) | 0·96 | ||
| ≤ 27 | 5 | 30 | |
| ˃ 27 | 5 | 29 | |
| NLR | 0·92 | ||
| ≤ 3·57 | 8 | 48 | |
| ˃ 3·57 | 2 | 11 | |
| Haemoglobin level (g/dL) | 0·22 | ||
| ≤ 107 | 6 | 23 | |
| ˃ 107 | 4 | 36 | |
| Blood platelet count (k/μL) | 0·74 | ||
| ≤ 173 | 4 | 27 | |
| ˃ 173 | 6 | 32 | |
| Prothrombin time | 0·53 | ||
| ≤ 14·7 | 6 | 29 | |
| ˃ 14·7 | 4 | 30 | |
| ALT level (U/L) | 0·66 | ||
| ≤ 46 | 4 | 28 | |
| ˃ 46 | 6 | 31 | |
| AST level (U/L) | 0·36 | ||
| ≤ 41 | 7 | 32 | |
| ˃ 41 | 3 | 27 | |
| LDH level (U/L) | 0·81 | ||
| ≤ 210 | 5 | 32 | |
| ˃ 210 | 5 | 27 | |
| Globulin level (g/L) | 0·36 | ||
| ≤ 21 | 7 | 32 | |
| ˃ 21 | 3 | 27 | |
| Albumin level (g/L) | 0·35 | ||
| ≤ 37 | 4 | 33 | |
| ˃ 37 | 6 | 26 | |
| Total albumin level (g/L) | 0·59 | ||
| ≤ 58 | 6 | 30 | |
| ˃ 58 | 4 | 29 | |
| Total bilirubin level (mg/dL) | 0·41 | ||
| ≤ 37 | 7 | 33 | |
| ˃ 37 | 3 | 26 | |
| Direct bilirubin level (mg/dL) | 0·97 | ||
| ≤ 17 | 6 | 35 | |
| ˃ 17 | 4 | 24 | |
| PVTT | 0·008 | ||
| Yes | 7 | 16 | |
| No | 3 | 43 | |
| Type of PVTT | 0·03 | ||
| I | 4 | 0 | |
| II | 0 | 5 | |
| III | 3 | 7 | |
| IV | 0 | 3 | |
| Number of targets tumour | 0·36 | ||
| ≤ 2 | 7 | 32 | |
| ˃ 2 | 3 | 27 | |
| Mean baseline diameter of the target tumour (range) | 69·3 (29–175) | 89·4 (10–311) | 0·29 |
| Metastasis sites before PD-1 inhibitor therapy | 0·63 | ||
| Lung | 5 | 37 | |
| Bone | 2 | 9 | |
| Lymph node | 1 | 11 | |
| Other | 3 | 13 | |
| Metastatic number before PD-1 inhibitor therapy | 0·63 | ||
| ≤ 3 | 4 | 19 | |
| ˃ 3 | 6 | 40 | |
| Previous therapy | 0·86 | ||
| Surgical resection | 3 | 32 | |
| Radiotherapy | 1 | 2 | |
| TACE | 8 | 47 | |
| Radiofrequency ablation | 5 | 33 | |
| Systemic therapy | 5 | 36 | |
| Number of previous treatments | 0·88 | ||
| ≤2 | 5 | 28 | |
| ˃2 | 5 | 31 | |
| PD-1 inhibitors | 0·90 | ||
| Nivolumab | 5 | 28 | |
| Pembrolizumab | 3 | 19 | |
| Camrelizumab | 2 | 12 | |
| PD-1 inhibitor therapy | 0·23 | ||
| Monotherapy | 6 | 46 | |
| PD-1 inhibitor + other therapies | 4 | 13 | |
| PD-1 inhibitor + local therapy | 1 | 3 | |
| PD-1 inhibitor + systemic therapy | 3 | 2 | |
| PD-1 inhibitor + systemic therapy+ local therapy | 0 | 8 | |
| Subsequent therapy after the first PD | 0·44 | ||
| Yes | 2 | 19 | |
| no | 8 | 40 |
HPD: hyperprogressive disease; BMI: body mass index; ECOG PS: Eastern Cooperative Oncology Group performance status; AFP: alpha-fetoprotein; NLR: Neutrophil-to-lymphocyte ratio; ALT: alanine aminotransferase; AST: aspartate transaminase; LDH: lactate dehydrogenase; PVTT: portal vein tumour thrombus; TACE, transcatheter arterial chemoembolization; HAIC: Hepatic arterial infusion chemotherapy.
RECIST 1·1 and irRECIST were used to evaluate 69 patients. According to RECIST 1·1, six (8·7%) patients achieved PR, resulting in an ORR of 8·7%; 26 (37·7%) patients achieved SD; and 37 (53·6%) had PD at the first radiological evaluation, resulting in a DCR of 46·4%. According to irRECIST, 7 (10·1%) patients achieved PR, resulting in an ORR of 10·1%; 30 (43·5%) patients achieved SD; and 31 (44·9%) had PD at the first radiological evaluation, resulting in a DCR of 53·6%. The median time of OS was 7·9 (range, 1·3–28·8) months. The details are shown in Table 2. We found good consistency for evaluating treatment response based on RECIST 1·1 and irRECIST (Kappa = 0·85, p < 0·001).
Table 2.
Radiological response according to RECIST 1·1, irRECIST, and survival RECIST, Response Evaluation Criteria in Solid Tumours.
| All patients (n = 69,%) |
||
|---|---|---|
| RECIST 1·1 | irRECIST | |
| CR | 0 | 0 |
| PR | 6 (8·7) | 7 (10·1) |
| SD | 26 (37·7) | 30 (43·5) |
| PD without HPD | 27 (39·1) | 21 (30·4) |
| HPD | 10 (14·5) | 10 (14·5) |
| ORR (CR+PR) | 8·7% | 10·1% |
| DCR (CR+PR+SD) | 46·4% | 53·6% |
| OS, median (range) | 7·9 (1·3–28·8) | 7·9 (1·3–28·8) |
| 1‐year survival rate | 20·3% | 20·3% |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; HPD: Hyperprogressive disease; ORR, overall response rate; DCR, disease control rate; RECIST, Response Evaluation Criteria in Solid Tumours; irRECIST, immune-related RECIST; OS, overall survival.
Overall, 12 (17·4%) patients received immunotherapy as a first- or second-line treatment and 57 (82·6%) patients received immunotherapy as a third- or fourth-line treatment. There was no difference in treatment response based on RECIST 1·1 between patients who received immunotherapy as a first- or second-line treatment and those who received immunotherapy as a third- or fourth-line treatment (p = 0·24).
At least one AE was noted in 62 (89·9%) patients. Experiencing three or more AEs was more common in the HPD group than in the non-HPD group (80% vs. 39·3%, p = 0·005). The most common treatment-related AEs were decreased appetite (n = 19; 27·5%), rash (n = 18; 26·1%), diarrhoea (n = 17; 24·6%), fatigue (n = 16; 23·2%), ALT increase (n = 16; 23·2%), and bilirubinaemia (n = 16; 23·2%). AEs of higher grade (grade ≥ 3) developed in 13 (18·8%) patients. AEs observed in patients treated with immunotherapy are shown in Table 3. In patients who achieved PR or SD, the incidence of AEs was 75%, whereas in those with PD, the incidence was 92·1%. Further, amongst patients with PD, the incidence of AEs in patients without HPD was lower than that in those with HPD (91·4% vs. 100%, p = 0·01).
Table 3.
Adverse events.
| HPD (%) |
Non-HPD (%) |
|||
|---|---|---|---|---|
| Any grade | Grade 3/4 | Any grade | Grade 3/4 | |
| Treatment-related AEs | ||||
| Rash | 2(20·0) | 0 | 16(26·7) | 0 |
| Pruritus | 1(10·0) | 0 | 12(20·0) | 0 |
| Fatigue | 2(20·0) | 0 | 14(23·3) | 0 |
| Nausea | 2(20·0) | 0 | 5(8·3) | 0 |
| Diarrhoea | 5(50·0) | 0 | 12(20·0) | 0 |
| Decreased appetite | 2(20·0) | 0 | 17(28·3) | 0 |
| Hypothyroidism | 1(10·0) | 0 | 7(11·7) | 0 |
| Dry mouth | 2(20·0) | 0 | 8(13·3) | 0 |
| Insomnia | 1(10·0) | 0 | 6(10·0) | 0 |
| RCCEP | 2(20·0) | 0 | 8(13·3) | 0 |
| Laboratory treatment-related AEs | ||||
| ALT increase | 6(60·0) | 3(30·0) | 11(18·3) | 1(1·7) |
| AST increase | 5(50·0) | 2(20·0) | 10(20·0) | 3(5·0) |
| GGT increase | 4(40·0) | 3(30·0) | 9(15·0) | 1(1·7) |
| Bilirubinaemia | 6(60·0) | 2(20·0) | 10(16·7) | 1(1·7) |
| Lymphopenia | 1(10·0) | 0 | 0 | 0 |
| Leukopenia | 1(10·0) | 0 | 4(6·7) | 0 |
| Neutropenia | 1(10·0) | 0 | 7(11·7) | 0 |
| Thrombopenia | 1(10·0) | 0 | 9(15·0) | 0 |
| Anaemia | 1(10·0) | 1(10·0) | 6(10·0) | 1(1·7) |
| HBV reactivation | 1(10·0) | 0 | 5(8·3) | 0 |
HPD: hyperprogressive disease; RCCEP, reactive cutaneous capillary endothelial proliferation; AST, aspartate transaminase; ALT, alanine transaminase; GGT, gamma-glutamyl transpeptidase; HBV, hepatitis B virus.
3.2. Evaluation of HPD
We first examined the duration of TTF amongst the patients treated with PD-1 inhibitors. Of the 69 patients, 18 (26·08%) had a TTF < 2 months. The intra- and interobserver reliabilities of the measurement of TGR resulted in an ICC > 0.8, which indicates excellent reliability of the measurement (the result is shown in Supplementary sTable 1). We then evaluated disease progression at the first evaluation and the change in TGR (>50%) amongst the 18 patients. Only 10 (14·49%) patients had a TGREXP > 50%. The median TGREXP in patients with HPD was 106·39% (range, 75·42–136·17%). Finally, TGRR was calculated using the ratio of TGREXP to TGRPRE. A TGRR > 2 was observed in all 10 (14·49%) patients. The median TGRR in patients with HPD was 2·5 (range, 2·1–3·4). Therefore, according to our definition, we identified ten patients with HPD, and the incidence of HPD was 14·49%. A representative case of HPD is shown in Fig. 2. In contrast, amongst patients treated with TKIs, six (17·6%) patients had a TTF < 2 months, although their TGR was <50% (range: 18·3–33·7). Four (11·7) patients had a TGREXP > 50%, but TTF > 2 months and TGRR < 2. Only one (2·9%) patient had TGRR > 2, although TGREXP was 18·3.
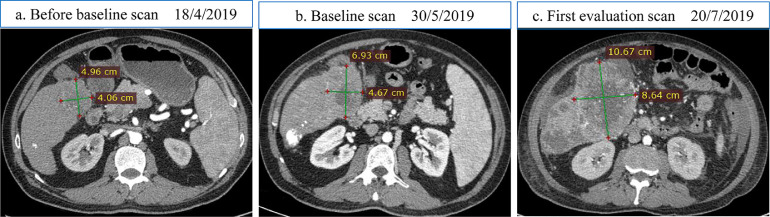
Fig. 2.
Example of a patient with hepatocellular carcinoma with hyperprogressive disease during treatment with PD-1 inhibitor. There is a significant radiological increase in the largest slice of liver lesion after two cycles of PD-1 inhibitor. TGR ratio was calculated to be 2·3.
We calculated the reasons for progression in patients with HPD, including the increase in size of target lesions (n = 9), new intrahepatic lesions (n = 5), new extrahepatic lesions (n = 5), new PVTT (n = 1), and new non-measurable lesions (n = 3). The primary reason for progression was the increase in size of target lesions, and the second cause was new intra-/extrahepatic lesions. Moreover, we also found that all patients with HPD had a progression of new intra-/extrahepatic lesions before immunotherapy; but the TGR was < 50%, and eight patients with HPD continually had new intra-/extrahepatic lesions combined with the increase in size of target lesions during immunotherapy.
3.3. Association between HPD and clinical variables
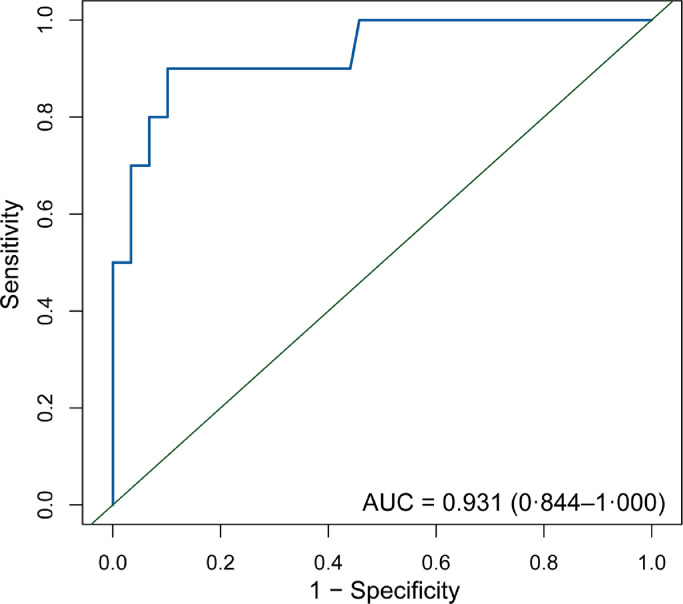
We investigated the clinical variables associated with HPD using univariate and multivariate analyses (Table 4). After the univariate analysis, age, pre-treatment AFP, haemoglobin level, PVTT, and Child-Pugh score were found to be significant. These variables were further included in the multivariate analyses. Haemoglobin level, PVTT, and Child-Pugh score were the clinical variables that were significantly associated with HPD in the multivariate analysis. We also analysed the difference between Child-Pugh scores of 5 and 6 points. There was a significant difference in the risk of HPD between these groups (p = 0·04). We also explored the association between the neutrophil-lymphocyte ratio (NLR) and HPD in different NLR cut-offs. No significant difference was found (cut-off was a continuous variable, 3·57, 4·125, 6.00: p = 0·37, 0·92, 0·51, 0·99). We included haemoglobin level, PVTT, and Child-Pugh score in the logistic regression analysis to evaluate the risk of HPD and built a risk model that yielded an AUC of 0·93 (95% CI, 0·84–1·00). The receiver operating characteristic (ROC) curve is presented in Fig. 3, and the performance of the risk model is presented in Table 5. According ROC analysis, the optimal cut-off value for dividing the patients into high- and low-risk groups was 0·24. We calculated the risk score for each patient using a formula resulting from these three clinical variables weighted by their regression coefficients:
Table 4.
Risk factors associated with the incidence of hyperprogressive disease in patients with hepatocellular carcinoma treated with PD-1 inhibitors.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 0·95 (0·89–0·99) | 0·05 | 0·93 (0·85–1·03) | 0·17 |
| Sex (male or female) | 2·06 (0·24- 18·02) | 0·51 | ||
| BMI | 1·08 (0·90–1·30) | 0·39 | ||
| HBV (yes or no) | 1·02 (0·11–9·49) | 0·99 | ||
| HCV (yes or no) | 0·98 (0·11–9·14) | 0·99 | ||
| Cirrhosis (yes or no) | 1·36 (0·36–5·35) | 0·65 | ||
| Alcohol consumption (yes or no) | 0·64 (0·17–2·45) | 0·51 | ||
| Smoking (yes or no) | 0·56 (0·14–2·20) | 0·41 | ||
| Diabetes (yes or no) | 2·14 (0·53–8·69) | 0·29 | ||
| ECOG PS (≤ 1 or ˃ 1) | 2·07 (0·19–22·19) | 0·55 | ||
| Child-Pugh score | 4·69 (1·45–15·19) | 0·01 | 12·87 (1·71–96·83) | 0·01 |
| Pre-treatment AFP | 1·00 (1·00–1·00) | 0·07 | 1.00 (1·00–1·00) | 0·39 |
| Posttreatment AFP | 1·00 (1·00–1·00) | 0·84 | ||
| CA125 | 0·99 (0·96–1·03) | 0·89 | ||
| CA199 | 1·01 (0·95–1·06) | 0·85 | ||
| NLR | 0·84 (0·58–1·22) | 0·37 | ||
| Haemoglobin level | 0·937 (0·88–0·99) | 0·02 | 0·89 (0·81–0·98) | 0·03 |
| Blood platelet count | 0·99 (0·98–1·01) | 0·48 | ||
| Prothrombin time | 0·96 (0·58–1·59) | 0·88 | ||
| ALT level | 1·02 (0·98–1·06) | 0·24 | ||
| AST level | 0·98 (0·94–1·02) | 0·36 | ||
| LDH level | 0·99 (0·98–1·01) | 0·64 | ||
| Globulin level | 0·90 (0·78–1·05) | 0·18 | ||
| Albumin level | 1·05 (0·86–1·29) | 0·63 | ||
| Total albumin level | 0·85 (0·68–1·05) | 0·13 | ||
| Total bilirubin level | 0·94 (0·76–1·16) | 0·56 | ||
| Direct bilirubin level | 0·99 (0·64–1·56) | 0·99 | ||
| PVTT (yes or no) | 11·73 (2·24–61·50) | 0·004 | 34·97 (2·58–474·30) | 0·008 |
| Number of targets tumour (≤ 2 or ˃ 2) | 0·54 (0·13–2·31) | 0·41 | ||
| Baseline diameter of the target tumour | 0·99 (0·98–1·01) | 0·29 | ||
| Number of metastatic sites (≤ 1 or ˃ 1) | 2·14 (0·53–8·69) | 0·29 | ||
| Metastatic number before PD-1 inhibitor therapy (≤ 3 or ˃ 3) | 0·71 (0·18–2·82) | 0·63 | ||
| PD-1 inhibitors | ||||
| Nivolumab | baseline | 0·96 | ||
| Pembrolizumab | 0·81 (0·17–3·79) | 0·79 | ||
| Camrelizumab | 0·90 (0·15–5·31) | 0·91 | ||
| Number of previous treatments (≤ 2 or ˃ 2) | 0·90 (0·23–3·45) | 0·88 | ||
OR: odds ratio; CI: confidence interval; BMI: body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; AFP: alpha-fetoprotein; NLR: Neutrophil-to-lymphocyte ratio; ALT: alanine aminotransferase; AST: aspartate transaminase; LDH: lactate dehydrogenase; PVTT: portal vein tumour thrombus.
Fig. 3.
ROC curves for a newly constructed risk model for hyperprogressive disease in advanced hepatocellular carcinoma treated with PD-1 inhibitor.
ROC: receiver operative characteristic; PD-1: programmed cell death 1.
Table 5.
Performance of the risk model.
| Index | Performance value |
|---|---|
| Area under the curve | 0·93 |
| Best threshold | 0·24 |
| Specificity | 0·89 |
| Sensitivity | 0·90 |
| Accuracy | 0·89 |
| Positive–likelihood ratio | 8·85 |
| Negative–likelihood ratio | 0·11 |
| Diagnose odds ratio | 79·50 |
| Number for diagnose | 1·25 |
| Positive-predictive value | 0·60 |
| Negative-predictive value | 0·98 |
3.4. Association between survival outcome, HPD, and clinical variables
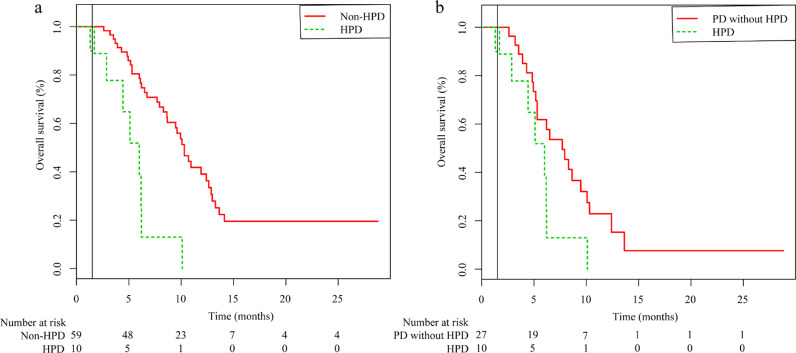
We investigated the association between HPD and survival outcomes. The OS of the HPD group was significantly lower than that of the non-HPD group for events that occurred between 1·5 months and the end of the follow-up period (median OS: 10·3 months vs. 6 months; HR = 4·79; 95% CI: 2·18–10·485; p < 0·001; Fig. 4a). We also analysed the difference in OS between PD patients with and without HPD. There was no significant difference in OS between these two groups for events that occurred between 1·5 months and the end of the follow-up period (HR = 2·50; 95% CI: 1·11–5·67; p = 0·05; Fig. 4b).
Fig. 4.
Kaplan-Meier curves for hyperprogressive disease in patients with advanced hepatocellular carcinoma treated with PD-1 inhibitors. (a) A survival analysis comparing the OS between the HPD and non-HPD groups. (b) Kaplan-Meier curves comparing the OS between PD patients with and without HPD. PD-1: programmed cell death 1; OS: overall survival; HPD: hyperprogressive disease.
A subgroup analysis of OS was performed between patients with Child-Pugh scores of 5 and 6 points; there was no significant difference in OS (HR = 0·93; 95% CI: 0·51–1·68; p ˃ 0·05). A subgroup analysis of OS was also performed based on the PD-1 inhibitor administered (nivolumab, pembrolizumab, and camrelizumab), and there was no significant difference in OS (HR = 1·17; 95% CI: 0·79–1·73; p ˃ 0·05). A significant difference in OS was observed in patients with immunotherapy administered as a first- or second-line treatment and those with immunotherapy administered as a third- or fourth-line treatment for events that occurred between 1.5 months and the end of the follow-up period (HR = 0·57; 95% CI: 0·27–1·18; p = 0·043). Additionally, there was no significant difference in OS between patients receiving PD-1 inhibitor monotherapy and those receiving PD-1 inhibitors plus other therapies (HR = 1·26; 95% CI: 0·58–2·75; p ˃ 0·05). After the first progression, 21 patients received subsequent therapy (included two patients with HPD), with one of them subsequently achieving an objective tumour response and exhibiting PR for approximately 8 months. There was no significant difference in OS between patients who received subsequent therapy and those who did not receive subsequent therapy (HR = 0·89; 95% CI: 0·47–1·68; p ˃ 0·05). The results of the landmark analysis are presented in Table 6.
Table 6.
Landmark analysis of overall survival in the HPD and subgroup survival analysis.
| Variables | p value |
|---|---|
| HPD vs. non-HPD | |
| ≤ 1·5 month | 0·02 |
| ˃ 1·5 month | < 0·001 |
| HPD vs. PD without HPD | |
| ≤ 1.5 month | 0·10 |
| ˃ 1.5 month | 0·05 |
| Child-Pugh score of 5 points vs. 6 points | |
| ≤ 1·5 month | 0·29 |
| ˃ 1·5 month | 0·68 |
| PD-1 inhibitor administered | |
| ≤ 1·5 month | 0·37 |
| ˃ 1·5 month | 0·06 |
| Immunotherapy as first-/second-line vs. as third-/fourth-line treatment | |
| ≤ 1·5 month | 0·34 |
| ˃ 1·5 month | 0·04 |
| PD-1 inhibitor monotherapy vs. PD-1 inhibitor plus other therapies | |
| ≤ 1·5 month | 0·08 |
| ˃ 1·5 month | 0·78 |
| Received subsequent therapy vs. not received subsequent therapy | |
| ≤ 1·5 month | 0·51 |
| ˃ 1·5 month | 0·81 |
HPD: Hyperprogressive disease; OS, overall survival.
4. Discussion
In this study, 10 (14·49%) patients with advanced HCC treated with anti-PD-1 immunotherapy developed HPD. After univariate and multivariate analyses, three variables were significantly associated with HPD, namely, haemoglobin level, PVTT, and Child-Pugh score. We used these risk factors to construct a risk model. In addition, we observed that HPD was closely associated with a poor OS in patients with HCC treated with immunotherapy.
Previous studies have reported that patients with advanced cancer treated with immunotherapy can develop HPD, which is not observed with other therapeutic regimens. Most studies examining NSCLC, such as that by Kato et al. [7], defined HPD as TTF < 2 months, a > 50% increase in tumour burden compared with pre-immunotherapy on imaging studies, and a more than two-fold increase in progression pace; the incidence of HPD was 6%. Kim et al. [20] also reported that the incidence of HPD was 12·7% in patients with HCC when HPD was defined as a four-fold increase in TGK and TGR ratios and a 40% increase in ΔTGR. In this study, when HPD was defined as TTF <2 months, a > 50% change in TGR at the first evaluation, and a TGRR > 2, the incidence of HPD was 14·27%. After analysing the reason for progression in patients with HPD, we found that the main reasons were the increase in size of target lesions and new intra-/extrahepatic lesions. Moreover, there were eight patients with HPD who presented with continually new intra-/extrahepatic lesions combined with the increase in size of target lesions before/during immunotherapy. Interestingly, the TGR of these patients was not > 50% before immunotherapy but was > 50% during immunotherapy. This result reflects that patients with HPD have a higher growth ratio after immunotherapy and usually present with continually new intra-/extrahepatic lesions concomitant with an increase in size of target lesions.
PVTT, haemoglobin level, and the Child-Pugh score were significantly associated with HPD in patients treated with anti-PD-1 therapy in this study. The haemoglobin level was negatively associated with an increased risk of HPD. Floridi et al. also suggested that higher pre-treatment haemoglobin values corresponded to a longer median OS in patients with HCC [21]. In contrast, PVTT was positively associated with an increased risk of HPD, which is consistent with the results of previous studies [22]. PVTT is one of the most important factors contributing to poor survival in HCC. The median OS of patients with HCC and PVTT has been reported to be only 3–6 months [23]. The Child-Pugh score was also positively associated with an increased risk of HPD. Sieghart et al. [24] and Adhoute et al. [25] reported that an increase in the Child-Pugh score was an independent negative prognostic factor for survival outcomes in patients with HCC. Additionally, a subgroup analysis showed a difference in the incidence of HPD in patients with a Child-Pugh score of 5 and in those with a Child-Pugh score of 6, although there was no significant difference in OS. However, previous studies reported that patients with a Child-Pugh score of 5 have a better prognosis than do those with a score of 6 [26,27].
Many factors associated with HPD in different cancers have been previously reported, such as age, sex, locoregional recurrence, poorer ECOG PS, NLR, C-reactive protein levels, and the number of metastatic lesions before treatment. A recent study reported that NLR is associated with HPD in patients with HCC treated with immunotherapy, and the optimal NLR cut-off value for predicting HPD is 4·125 [20]. However, our study showed that NLR is not associated with HPD when the NLR cut-off is a continuous variable, 3·57, 4·125, or 6·00. Champiat et al. [28] reported that the HPD group was older than the non-HPD group. In this study, age was significantly associated with HPD in the univariate analysis but not in the multivariate analysis. The mean ages were 51 years in our study, which was younger than that in the study by Champiat et al. (65 years). We found no significant association between the number of metastatic sites and HPD. This result was inconsistent with the results of the study by Ferrara et al. who reported that a high number of metastatic sites before PD-1/PD-L1 inhibitor treatment was associated with HPD [29]. HBV or HCV infection, alcohol consumption, high BMI, smoking, and diabetes, which have been reported to be closely associated with HCC, were also analysed. However, no significant association was found between these factors and HPD in our study. Furthermore, increased AFP level, which is observed in 70–80% of patients with HCC [30], was an important independent risk factor for HCC. Zhu et al. [31] and Butterfield et al. [32] showed that AFP could be recognised by human T cells and serve as a potential target, which redirects T cells to specifically recognise and kill HCC cells to achieve antitumor effects [31]. We also investigated the association between pre-/posttreatment AFP level and HPD. However, there was no significant difference in the AFP levels between the HPD and non-HPD groups. This may be attributable to the small sample size of the HPD group.
The PD-1 inhibitors nivolumab, pembrolizumab, and camrelizumab were used in this study, and no association was observed between the inhibitor type and the occurrence of HPD. In addition, there was a significant difference between patients who received immunotherapy as a first- or second-line treatment and as a third- or fourth-line treatment. These may be because previous treatments, such as sorafenib, transarterial chemoembolisation (TACE), and radiotherapy, may induce hypoxia in the tumour microenvironment, affecting sequence immunotherapy. A phase 3 trial of 413 patients with advanced HCC previously treated with sorafenib found that pembrolizumab, as a second-line treatment, had durable clinical efficacy and safety [33]. A phase 1/2 trial also reported that treatment with nivolumab was well-tolerated, encouraging antitumor efficacy in patients with advanced HCC previously treated with sorafenib [34].
Our data showed that patients in the HPD group had poorer survival outcomes than did those in the non-HPD group. This result was consistent with that of a previous study on NSCLC showing that HPD was associated with worse OS. Previous studies also reported that HCC patients with HPD were a special population of PD patients in OS, and these patients have a poor prognosis [20]. However, in this study, the OS of patients with HPD were similar with PD patients without HPD. Moreover, we found that patients with HPD had a higher risk of AEs and were more likely to develop more than three AEs. Accordingly, it is necessary to build a risk model to evaluate the initial risk of HPD. We proposed a risk score model based on significant clinical variables for the evaluation of the risk and management of therapeutic strategies for individual patients with advanced HCC. The results showed that the risk model had better performance in evaluating the risk of HPD than did the haemoglobin level, PVTT, or the Child-Pugh score alone. Additionally, the information on parameters that are used by the model can be easily acquired.
There are some limitations to this study. First, its retrospective nature, limited number of patients, and lack of external validation could result in some biases. To minimise the likelihood of overestimation of predictive ability and generalise our results to other populations, further larger samples, and external and prospective validation are still required. Additionally, some potential HPD could have been omitted because of the absence of any measurable tumour lesions or absence of appropriate CT scans before/after the treatment. Third, the same analysis should be tested in a control group treated with non-immunotherapeutic agents. This pattern of HPD was not exclusive to non-immunotherapeutic agents. Purcell et al. found that pre-treatment TGR could predict treatment response in patients treated with TACE [35]. We evaluated the data of patients treated with TKI as the control group but did not observe that this group satisfied the criteria for HPD. Fourth, in this study, we enrolled clinical variables that reflect the natural history of disease rather than therapy-related prognostic factors, and we did not document biomarkers and genomic data. Genomic data were confirmed to be associated with HPD in patients with NSCLC [36]; therefore, large-scale studies are necessary to identify immunologic drivers associated with HPD in patients with HCC treated with immunotherapy. Fifth, there were no significant differences in baseline characteristics and OS between PD-1 inhibitor monotherapy and combination therapy, and the identified risk factors were unchanged even after exclusion of the patients treated with PD-1 inhibitor plus other therapies (the result shown in Supplementary Table 2). Because of the small sample size of this study, further studies are needed to validate the risk model not only in patients treated with PD-1 inhibitor monotherapy but also in those treated with PD-1 inhibitor plus other therapies.
In conclusion, this study found three clinical variables associated with HPD. A risk model incorporating these variables was proposed to evaluate the risk of HPD in patients with advanced HCC treated with anti-PD-1 therapy. Additionally, this model still needs validation using larger samples of prospective data before it can be utilised as a tool that can evaluate the risk of HPD in patients treated with immunotherapy.
Author contributions
All authors contributed either to research design (Z.L.; W.L.G.; Z.J.Z. and Z.S.X.), and/or the data acquisition (C.Q.Y.; L.S.Y. and L.J.), analysis or interpretation (all authors) of data. Z.L. and W.L.G. drafted the manuscript, which was critically revised by all other authors. All authors approved the final version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (81571664,81871323, and 81801665); the National Natural Science Foundation of Guangdong Province (2018B030311024); the Scientific Research General Project of Guangzhou Science Technology and Innovation Commission (20107010328); and the China Postdoctoral Science Foundation (2016M600145). The funders had no role in the study design, collection, analysis, interpretation of data, writing of this report, and in the decision to submit the paper for publication.
Data sharing
The data that support the findings of this study are available on request from the corresponding author (S.Z.). The data are not publicly available due to information contained that could compromise the privacy of research participants.
Declaration of Competing Interest
The authors have no conflicts of interest to declare.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100673.
Contributor Information
Xudong Chen, Email: 871755741@qq.com.
Zejian Zhou, Email: zhzejian@126.com.
Shuixing Zhang, Email: shui7515@126.com.
Appendix. Supplementary materials
References
- 1.Forner A., Reig M., Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 2.Heimbach J.K., Kulik L.M., Finn R.S. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 3.Bray F., Ferlay J., Soerjomataram I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.European Association for Study of L, European Organisation for R, Treatment of C EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012;48(5):599–641. doi: 10.1016/j.ejca.2011.12.021. (Oxford, England: 1990) [DOI] [PubMed] [Google Scholar]
- 5.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P. Sorafenib in advanced hepatocellular carcinoma. New Eng J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Scheiner B., Kirstein M.M., Hucke F. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther. 2019;49(10):1323–1333. doi: 10.1111/apt.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato S., Goodman A., Walavalkar V. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23(15):4242–4250. doi: 10.1158/1078-0432.CCR-16-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champiat S., Ferrara R., Massard C. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol. 2018;15(12):748–762. doi: 10.1038/s41571-018-0111-2. [DOI] [PubMed] [Google Scholar]
- 9.Wong D.J., Lee J., Choo S.P. Hyperprogressive disease in hepatocellular carcinoma with immune checkpoint inhibitor use: a case series. Immunotherapy. 2019;11(3):167–175. doi: 10.2217/imt-2018-0126. [DOI] [PubMed] [Google Scholar]
- 10.Chen L., Gibbons D.L., Goswami S. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun. 2014;5:5241. doi: 10.1038/ncomms6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong H., Strome S.E., Salomao D.R. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 12.Lo Russo G., Facchinetti F., Tiseo M. Hyperprogressive disease upon immune checkpoint blockade: focus on non-small cell lung cancer. Curr Oncol Rep. 2020;22(5):41. doi: 10.1007/s11912-020-00908-9. [DOI] [PubMed] [Google Scholar]
- 13.Cheng S., Chen M., Cai J. Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus: 2016 edition. (1949-2553 (Electronic)). [DOI] [PMC free article] [PubMed]
- 14.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eu J Cancer (Oxford, England: 1990) 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Wolchok J.D., Hoos A., O'Day S. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 16.National Cancer Institute C. Common terminology criteria for adverse events (CTCAE) v 4.0.
- 17.Gomez-Roca C., Koscielny S., Ribrag V. Tumour growth rates and RECIST criteria in early drug development. Eu J Cancer (Oxford, England: 1990) 2011;47(17):2512–2516. doi: 10.1016/j.ejca.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Ferte C., Fernandez M., Hollebecque A. Tumor growth rate is an early indicator of antitumor drug activity in phase I clinical trials. Clin Cancer Res. 2014;20(1):246–252. doi: 10.1158/1078-0432.CCR-13-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson J.R., Cain K.C., Gelber R.D. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 20.Kim C.G., Kim C., Yoon S.E. Hyperprogressive disease during PD-1 blockade in patients with advanced hepatocellular carcinoma. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Floridi C., Pesapane F., Angileri S.A. Yttrium-90 radioembolization treatment for unresectable hepatocellular carcinoma: a single-centre prognostic factors analysis. Med Oncol. 2017;34(10):174. doi: 10.1007/s12032-017-1021-3. [DOI] [PubMed] [Google Scholar]
- 22.Wei X., Jiang Y., Zhang X., et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. (1527-7755 (Electronic)). [DOI] [PMC free article] [PubMed]
- 23.Kim P.A.-O., Choi S.A.-O., Kim J.A.-O., et al. Comparison of radioembolization and sorafenib for the treatment of hepatocellular carcinoma with portal vein tumor thrombosis: a systematic review and meta-analysis of safety and efficacy. (2005-8330 (Electronic)). [DOI] [PMC free article] [PubMed]
- 24.Sieghart W., Hucke F., Pinter M. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology. 2013;57(6):2261–2273. doi: 10.1002/hep.26256. [DOI] [PubMed] [Google Scholar]
- 25.Adhoute X., Penaranda G., Naude S. Retreatment with TACE: the ABCR SCORE, an aid to the decision-making process. J Hepatol. 2015;62(4):855–862. doi: 10.1016/j.jhep.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Okajima C., Arii S., Tanaka S. Prognostic role of child-pugh score 5 and 6 in hepatocellular carcinoma patients who underwent curative hepatic resection. Am J Surg. 2015;209(1):199–205. doi: 10.1016/j.amjsurg.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Hung H.-.H., Chao Y., Chiou Y.-.Y. A comparison of clinical manifestations and prognoses between patients with hepatocellular carcinoma and child–pugh scores of 5 or 6. Medicine (Baltimore) 2014;93(29):e348. doi: 10.1097/MD.0000000000000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Champiat S., Dercle L., Ammari S. Hyperprogressive disease is a new pattern of progression in cancer patients treated by Anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920–1928. doi: 10.1158/1078-0432.CCR-16-1741. [DOI] [PubMed] [Google Scholar]
- 29.Ferrara R., Mezquita L., Texier M. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4(11):1543–1552. doi: 10.1001/jamaoncol.2018.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao Y.Y., Lin Zz Fau - Hsu C., Hsu C Fau - Shen Y.-.C., et al. Early alpha-fetoprotein response predicts treatment efficacy of antiangiogenic systemic therapy in patients with advanced hepatocellular carcinoma. (0008-543X (Print)). [DOI] [PubMed]
- 31.Zhu W., Peng Y., Wang L., et al. Identification of α-fetoprotein-specific T-cell receptors for hepatocellular carcinoma immunotherapy. (1527-3350 (Electronic)). [DOI] [PMC free article] [PubMed]
- 32.Butterfield L.H., Ribas A., Dissette V.B. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res. 2006;12(9):2817–2825. doi: 10.1158/1078-0432.CCR-05-2856. [DOI] [PubMed] [Google Scholar]
- 33.Finn R.S., Ryoo B.Y., Merle P., et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: a Randomized, Double-Blind, Phase III Trial. (1527-7755 (Electronic)). [DOI] [PubMed]
- 34.El-Khoueiry A.B., Sangro B., Yau T., et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. (1474-547X (Electronic)). [DOI] [PMC free article] [PubMed]
- 35.Purcell Y., Sartoris R., Paradis V., et al. Influence of pretreatment tumor growth rate on objective response of hepatocellular carcinoma treated with transarterial chemoembolization. 2020;35(2):305–13. [DOI] [PubMed]
- 36.Kim C.G., Kim K.H., Pyo K.H. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol. 2019;30(7):1104–1113. doi: 10.1093/annonc/mdz123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.