Abstract
Background
Marijuana smoke contains some of the same toxicants present in tobacco smoke. Marijuana smoking is prevalent among HIV+ individuals, but few studies have characterized smoke-related toxicants or associated health outcomes in exclusive marijuana users.
Methods
This longitudinal study included 245 participants over age 40 (76% HIV+). 33 plasma and 28 urine metabolites of nicotine, ∆-9-trans-tetrahydrocannabinol, polycyclic aromatic hydrocarbons, and volatile organic compounds were assayed by liquid or gas chromatography/mass spectrometry. Exposures and health outcomes were assessed from surveys and medical records.
Findings
At baseline, 18% of participants were marijuana-only smokers, 20% tobacco-only smokers, and 24% dual marijuana-tobacco smokers (median (IQR) age 53 (47–60) years, 78% male, 54% white race). Marijuana smoking was independently associated with elevated plasma naphthalenes, 2-hydroxyfluorene sulfate, 4-vinylphenol sulfate, and o-cresol sulfate (p<0·05) and urine acrylonitrile and acrylamide metabolites (p<0·05), but levels were lower than those associated with tobacco smoking. Acrolein metabolite N-Acetyl-S-(3-hydroxypropyl)-l-cysteine (3HPMA) was significantly elevated in plasma and urine in tobacco-only and dual but not marijuana-only smokers, and correlated with nicotine metabolites (p<0·05). The highest tertile of 3HPMA was associated with increased cardiovascular disease diagnoses independent of tobacco smoking, traditional risk factors, and HIV status (odds ratio [95% CI] 3·34 [1·31–8·57]; p = 0·012).
Interpretation
Smoke-related toxicants, including acrylonitrile and acrylamide metabolites, are detectable in exclusive marijuana smokers, but exposures are lower compared with tobacco or dual smokers. Acrolein exposure is increased by tobacco smoking but not exclusive marijuana smoking in HIV+ and HIV- adults, and contributes to cardiovascular disease in tobacco smokers.
Funding
U.S. NIH.
Keywords: Marijuana, Tobacco, Smoke, Toxicants, Acrolein, Cardiovascular disease
Research in Context.
Evidence before this study
We searched PubMed for original research articles using combinations of terms “marijuana” or “cannabis” and “tobacco”; “biomarkers” or “exposure biomarkers” or “toxicants” or “metabolites” or “acrolein”; and “cardiovascular disease” until August 1, 2020. Many studies have characterized smoke-related toxicants in tobacco smokers, while few studies have detected some of the same combustion by-products in marijuana smokers. We found one article comparing smoke-related toxicants in urine between marijuana users and tobacco users, two articles comparing smoke-related toxicants between co-users of both substances and tobacco users, and no articles comparing acrolein and other smoke-related toxicant exposure between exclusive marijuana users and tobacco users in relation to health outcomes associated with these exposures. Acrolein exposure was previously associated with Framingham Risk Score in one cohort study and with cardiovascular toxicities in animal models; however, an association between acrolein and cardiovascular disease was not previously shown in human studies.
Added value of this study
In this longitudinal study of 245 HIV+ and HIV- participants, we showed that exclusive marijuana smokers had increased levels of some smoke-related toxicants, including naphthalene, acrylamide, and acrylonitrile metabolites, in plasma and urine compared to non-smokers. Concentrations of these toxicants were lower in exclusive marijuana smokers compared to tobacco smokers. Acrolein metabolites were increased in tobacco smokers but not marijuana smokers, and high acrolein levels were associated with cardiovascular disease after adjusting for tobacco smoking and other risk factors. This is the first longitudinal cohort study comparing exposure to acrolein and other smoke-related toxicants between exclusive marijuana smokers and tobacco smokers and evaluating cardiovascular outcomes associated with these exposures; these issues have particular relevance for HIV+ individuals given high rates of smoking and heightened risk of cardiovascular disease in this population.
Implications of all the available evidence
Marijuana smoking is associated with exposure to some smoke-related toxicants, including acrylamide and acrylonitrile metabolites, but exposures are reduced in comparison to tobacco smoking. Acrolein, a reactive aldehyde derived from combustion of tobacco and other sources, is increased by tobacco but not marijuana smoking, and contributes to cardiovascular disease in tobacco smokers independent of other risk factors. High acrolein levels may identify adults with increased cardiovascular risk, and reducing exposure may be a preventive strategy.
Alt-text: Unlabelled box
1. Introduction
Marijuana is the most frequently used illicit substance in the United States [1], and its medical and nonmedical use by adults is increasing with legalization [2,3]. Marijuana smoke contains some of the same toxic combustion products present in tobacco smoke [4,5], raising the possibility that exposure to some smoke-related toxicants could have adverse effects on health in heavy users. However, few studies have measured smoke exposure biomarkers in exclusive marijuana smokers or dual marijuana-tobacco smokers compared with exclusive tobacco smokers and non-smokers [6,7].
Smoke from tobacco combustion contains several hundred chemicals including carcinogens and toxicants, tobacco alkaloids, polycyclic aromatic hydrocarbons (PAHs), and volatile organic compounds (VOCs) linked to cardiovascular and lung disease and other adverse health effects including cancers [8], [9], [10], [11], [12], [13]. Among VOCs, the reactive aldehyde acrolein is notable for its toxicity and abundance in tobacco smoke [8,14,15]. Acrolein induces cardiovascular injury in animal models [16], [17], [18], [19], and has been linked to cardiovascular disease (CVD) risk factors in humans [20], yet its association with CVD diagnoses in humans is unknown.
The aims of this study were to compare plasma and urine levels of acrolein metabolites and other toxicants from smoke combustion between exclusive users of marijuana or tobacco, dual users of both substances, and non-smokers in cross-sectional and longitudinal analyses. The prevalence of marijuana and tobacco smoking [21,22] and smoking-related diseases such as CVD [23], [24], [25], [26] and chronic obstructive pulmonary disease (COPD) [27], [28], [29] in human immunodeficiency virus type 1 positive (HIV+) individuals is high compared with the general population. Therefore, we leveraged samples and data from three longitudinal cohort studies of HIV infection in the United States to select a longitudinal cohort of HIV+ and HIV- participants who were well-characterized for marijuana, tobacco, and other substance use, laboratory tests, and health outcomes. We then evaluated associations between the acrolein metabolite 3HPMA (N-Acetyl-S-(3-hydroxypropyl)-L-cysteine, also known as S- [3-hydroxypropyl] mercapturic acid) [10,30] and CVD diagnoses in analyses adjusted for tobacco smoking, HIV status, and other known risk factors.
2. Methods
2.1. Study design and participants
Participants (n = 245) were from the National NeuroAIDS Tissue Consortium (NNTC, n = 167), HIV Neurobehavioral Research Center (HNRC, n = 27), the Chicago component of the Multicenter AIDS Cohort Study (MACS, n = 42), and Bioreclamation IVT (n = 9 HIV- participants, Westbury, NY) (Supplementary Methods). De-identified data were obtained from study sites between 2016 and 2018 and accessed by DL, VM, and DG. The NNTC, HNRC, and MACS are ongoing prospective cohort studies of persons with HIV, or socioeconomic/behavioral risk factors associated with HIV acquisition, recruited at urban sites in the United States. Participants undergo standardized interviews detailing substance use, behavioral characteristics, medical conditions, medication use, physical examinations, and collection of biological specimens at biannual (MACS and HNRC) or at 6- or 12-month (NNTC) study visits (Fig. S1) [31], [32], [33]. All participants were enrolled with written informed consent, and Institutional Review Board approval was obtained at each site. CDC staff involvement was limited to coded specimen analysis/interpretation and thus CDC IRB review was not required. Eligible participants were age 40 and older with self-reported marijuana and tobacco smoking data at ≥2 study visits and one or more plasma and/or urine sample available from specimen biobanks, and antiretroviral therapy (ART) use with suppressed plasma viral load <200 HIV ribonucleic acid (RNA) copies/mL at baseline for HIV+ participants. Seventeen HIV+ participants had low-level viremia <1500 copies/mL at baseline, but suppressed viral load <200 copies/mL at prior and subsequent visits. Participants defined as marijuana smokers reported current daily or weekly use. Participants defined as tobacco smokers reported current daily cigarette smoking (minimum 0.1 packs per day). Participants were selected to maximize the number of marijuana smokers who did not smoke tobacco, while maintaining an overall balance of age and gender between smoking groups. Baseline and endpoint visits were defined as the first and last visits at age ≥40 with plasma and/or urine samples, respectively. Plasma and urine samples from ≥2 study visits (n = 2–6) were available for 165 (64%) and 42 (47%) participants, respectively. All blood and urine samples were collected from 2000 to 2016.
2.2. Covariate and outcome definitions
Participants were classified as marijuana or tobacco smokers based on self-reported current marijuana or tobacco smoking at ≥2 consecutive study visits, and detection of nicotine or THC metabolites in plasma and/or urine by metabolite profiling described below or urine toxicology testing for THC. A composite CVD outcome was comprised of the following diagnoses: myocardial infarction, coronary artery disease, cardiac stents, stroke, and atherosclerosis. Diagnoses were obtained from abstracted longitudinal clinical data (NNTC and HNRC cohorts) or International Classification of Diseases (ICD-9-CM) codes (MACS cohort) [34]. Traditional CVD risk factors were defined based on ≥2 visits with self-reported use of medications for these conditions or laboratory test values as follows: hypertension, systolic blood pressure >140 or diastolic blood pressure >90 mm Hg; hyperlipidemia, total cholesterol >240 mg/dl; diabetes, hemoglobin A1C ≥6·5%.
2.3. Urinary and plasma metabolomics profiling
Untargeted plasma metabolomic profiling was performed using ultra-high performance liquid chromatography/tandem mass spectrometry and gas chromatography/mass spectrometry (Metabolon, Inc. [Durham, NC]) [35,36] (Supplementary Methods). Data were normalized by median scaling, and log2-transformed. 1546 metabolites were measured, of which 1228 were identified and 28 metabolites of THC, nicotine, PAHs, VOCs, and other smoking-related metabolites were selected for further analysis.
For urine samples, 9 tobacco alkaloids and 24 VOC metabolites were analyzed using ultra-high performance liquid chromatography with electrospray tandem mass spectrometry [37,38] (Supplementary Methods). Metabolite levels were normalized to urine creatinine levels and log2-transformed.
2.4. Statistical analyses
All data preparation and analyses were performed in R (version 3.6.1) (see Supplementary Methods for additional details). Cross-sectional analyses were performed using Welch's t-test or Pearson's χ2-test where appropriate; cross-sectional analyses of metabolite values were performed at study endpoint. Hierarchical clustering and heatmaps were generated using the ComplexHeatmap package. Mixed-effects models were fit for each metabolite using either time-varying binary categorical marijuana and tobacco smoking variables or a full factorial variable of time-varying marijuana and tobacco smoking status. All models were adjusted by age and HIV status and included a random intercept and slope for each participant; plasma metabolite models were also adjusted for impaired renal function (estimated glomerular filtration rate [eGFR] <60). Associations between tobacco and marijuana smoking, selected plasma metabolite levels, and CVD diagnoses within 5 years prior to endpoint were assessed using logistic regression. Metabolite concentrations were modeled as both continuous and binary categorical (top vs. lower two tertiles) variables. All models were adjusted by age, HIV status, and number of traditional CVD risk factors (2–3 vs. 0–1); three participants with CVD diagnoses occurring >5 years prior to endpoint were excluded because we hypothesized that associations between smoke-related metabolites and CVD diagnoses were more likely to be detected for recent events. Gender was not included in these models because the number of CVD diagnoses (n = 30) limited the number of terms included in multivariable models and number of females was low (n = 54). Two-sided p values <0·05 were considered statistically significant in each analysis.
2.5. Role of the funding source
The study funders had no role in study design, data collection, analysis, or interpretation, or writing of this report. The corresponding author had full access to all study data and final responsibility for the decision to submit for publication.
3. Results
3.1. Baseline characteristics
Of 245 study participants who met eligibility requirements, the median baseline age [interquartile range (IQR)] was 53 [47–59] years, 78% were male, 54% were white race, and 76% were HIV+. At baseline visit, marijuana-only smokers (MJ+TS-) comprised 18% of participants, while tobacco-only smokers (MJ-TS+) and dual marijuana-tobacco smokers (MJ+TS+) comprised 20% and 24% of participants, respectively (Table 1). Among tobacco smokers (MJ-TS+ and MJ+TS+), the median [range] cigarette packs smoked per day was 0⋅75 [0⋅1–2⋅0], and the mean (standard deviation [SD]) pack-years for tobacco smokers and years use for marijuana smokers were 26 (21) and 15 (12) years at baseline, respectively. Marijuana and tobacco smokers (MJ+TS-, MJ-TS+, and MJ+TS+) were slightly younger at baseline compared with non-smokers (MJ-TS-) (median age [IQR] 52 [47, 58] and 57 [47, 72] years, respectively; p = 0⋅002), and more often male (83% and 69%, respectively; p = 0⋅014) and black race (34% and 23%, respectively; p = 0⋅101). HIV infection, heavy alcohol consumption, and heavy cocaine use were more common among marijuana and tobacco smokers compared with non-smokers (p<0·01), while other characteristics were similar between groups. Among HIV+ participants, 91% of participants had suppressed plasma viral load <200 copies/ml, and median duration of HIV infection and baseline CD4+ T-cell count were similar between exposure groups.
Table 1.
Baseline demographic and clinical characteristics of the study cohort.
| All participants (n = 245) | MJ-TS- (n = 90) | MJ+TS- (n = 45) | MJ-TS+ (n = 50) | MJ+TS+ (n = 60) | |
|---|---|---|---|---|---|
| Age, median [IQR] | 53·1 [47·0, 59·4] | 56·8 [47·0, 72·0] | 54·7 [47·6, 59·0] | 53·0 [48·1, 56·9] | 49·1 [44·0, 55·3] |
| Years of follow-up, median [IQR] | 1·5 [0·0, 2·1] | 1·8 [0·0, 2·1] | 1·4 [0·9, 2·2] | 1·6 [0·0, 2·3] | 1·5 [0·8, 2·1] |
| Race | |||||
| White | 133 (54·3) | 56 (62·2) | 27 (60·0) | 18 (36·0) | 32 (53·3) |
| Black | 74 (30·2) | 21 (23·3) | 10 (22·2) | 19 (38·0) | 24 (40·0) |
| Other | 38 (15·5) | 13 (14·4) | 8 (17·8) | 13 (26·0) | 4 (6·7) |
| Male gender | 191 (78·0) | 62 (68·9) | 41 (91·1) | 36 (72·0) | 52 (86·7) |
| Marijuana smoking (years), mean (SD) | – | – | 15·6 (12·2) | – | 14 (12·5) |
| Tobacco smoking | |||||
| Never | 98 (40·0) | 74 (82·2) | 24 (53·3) | – | – |
| Former | 37 (15·1) | 16 (17·8) | 21 (46·7) | – | – |
| Current | 110 (44·9) | – | – | 50 (100) | 60 (100) |
| Tobacco smoking (packs per day), mean (SD) | – | – | – | 0·53 (0·42) | 0·86 (0·41) |
| Tobacco smoking (pack-years), mean (SD)a | – | – | – | 22 (23·8) | 28 (19·8) |
| Cocaine useb | 43 (17·6) | 4 (4·4) | 7 (15·6) | 14 (28·0) | 18 (30·0) |
| Alcohol useb | 107 (43·7) | 23 (25·6) | 21 (46·7) | 19 (38·0) | 44 (73·3) |
| HIV positive | 186 (75·9) | 47 (52·2) | 42 (93·3) | 38 (76·0) | 59 (98·3) |
| Duration of HIV infection (years), median [IQR]c | 16·1 [11·7, 20·3] | 15·8 [11·8, 20·1] | 17·3 [13·1, 20·7] | 13·7 [10·9, 19·1] | 16·8 [11·8, 19·7] |
| CD4+ T-cell count (cells/μL), median [IQR]c | 440 [216, 667] | 440 [272, 659] | 347 [220, 525] | 446 [250, 668] | 473 [205, 708] |
| Plasma viral load <200 copies/mLc | 167 (90·2) | 43 (91·5) | 39 (95·1) | 31 (83·8) | 54 (91·5) |
| White blood cell count (x 109 cells/L), median [IQR] | 5·8 [4·5, 7·3] | 6·0 [4·6, 7·3] | 5·4 [4·3, 6·6] | 5·6 [4·4, 7·6] | 6·1 [4·5, 7·1] |
| Hypertension risk factord | 105 (42·9) | 30 (33·3) | 20 (44·4) | 27 (54·0) | 28 (46·7) |
| Hyperlipidemia risk factord | 63 (25·7) | 20 (22·2) | 12 (26·7) | 16 (32·0) | 15 (25·0) |
| Diabetesd | 31 (12·7) | 13 (14·4) | 5 (11·1) | 8 (16) | 5 (8·3) |
| 2–3 traditional cardiovascular risk factors | 56 (22·9) | 15 (16·7) | 11 (24·4) | 17 (34) | 13 (21·7) |
| Cardiovascular diagnosese | 30 (12·2) | 6 (6·7) | 5 (11·1) | 9 (18·0) | 10 (16·7) |
| COPD diagnosese | 23 (9·4) | 6 (6·7) | 4 (8·9) | 8 (16·0) | 5 (8·3) |
All data are n (%) unless otherwise indicated. Abbreviations: IQR, interquartile range; MJ-TS-, non-smoker; MJ+TS-, marijuana-only smoker; MJ-TS+, tobacco-only smoker; MJ+TS+, dual marijuana-tobacco smoker; SD, standard deviation.
- Estimated number of 20-cigarette packs smoked/day multiplied by years smoked.
- Dependence or abuse, or ≥2 study visits with heavy use (≥14 drinks/week of alcohol, or daily or weekly use of cocaine).
- Calculated for HIV positive participants only.
- ≥2 study visits with medication use for the indicated condition or ≥2 study visits with the following laboratory test values: hypertension, systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg; hyperlipidemia, total cholesterol >240 mg/dL; diabetes, hemoglobin A1C ≥6·5%.
- Diagnoses within 5 years prior to endpoint.
3.2. Metabolomic profiling of tobacco and marijuana smoke exposure biomarkers
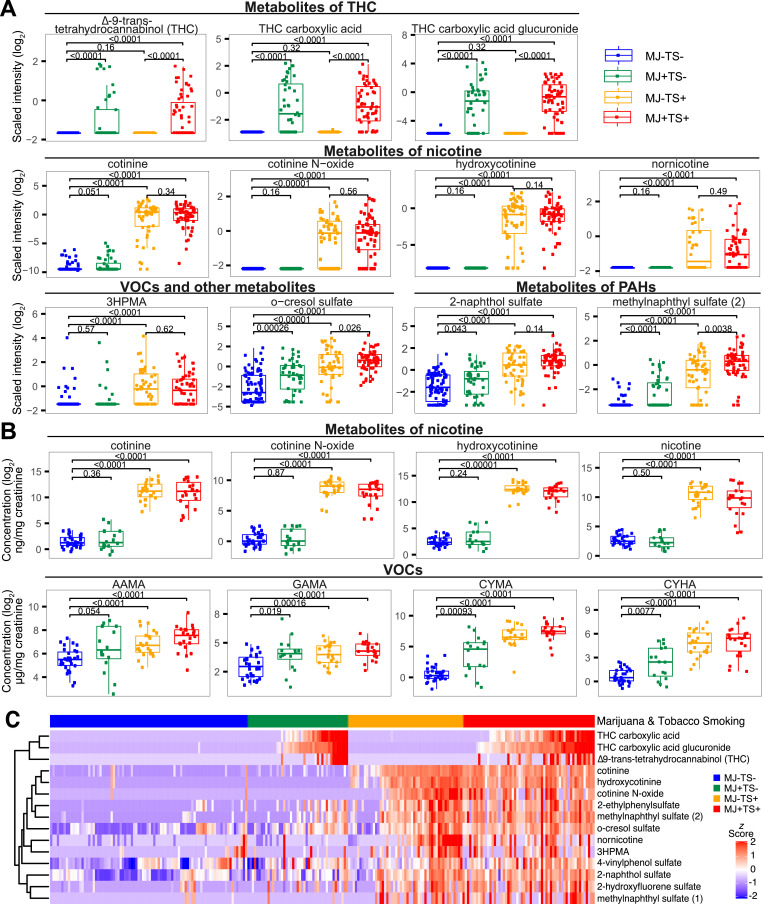
Geometric means with 95% CIs and for plasma and urine metabolites by marijuana and tobacco smoking at study endpoint are shown in Tables 2 and 3, respectively. Metabolites of nicotine and tobacco alkaloids were significantly increased in plasma and urine samples of tobacco smokers (MJ-TS+ and MJ+TS+) compared with nonsmokers (MJ-TS-) (range, 2·2–746·9 fold-increase, t-test, p<0·001), while THC metabolites were significantly increased in plasma samples of marijuana smokers (MJ+TS- and MJ+TS+) compared with nonsmokers (MJ-TS-) (3·7–86·9 fold-increase, p<0·001) (Fig. 1A-1B, Figs. S2–S3). In plasma samples, smoke exposure biomarkers including PAH metabolites 2-naphthol sulfate, methylnaphthyl sulfate (2), and 2-hydroxyfluorene sulfate, and VOC metabolites 2-ethylphenylsulfate, o-cresol sulfate (metabolite of toluene), and 4-vinylphenol sulfate (metabolite of styrene) were detected at higher levels in all three smoking groups (MJ+TS-, MJ-TS+, and MJ+TS+) compared with nonsmokers (MJ-TS-) (1·6–20·8 fold-increase, p<0·05), with highest levels detected in tobacco smokers (MJ-TS+ and MJ+TS+) (p<0·001). The acrolein metabolite 3HPMA was approximately two-fold higher in plasma and urine of tobacco smokers (MJ-TS+ and MJ+TS+) compared with nonsmokers (MJ-TS-) (p<0·001), but not significantly elevated in exclusive marijuana smokers (MJ+TS-) compared with nonsmokers (MJ-TS-). In urine samples, 3HPMA and CEMA (metabolites of acrolein), AAMA and GAMA (metabolites of acrylamide), CYMA and CYHA (metabolites of acrylonitrile), MHB3, and 34MH were significantly elevated in tobacco smokers (MJ-TS+ and MJ+TS+) compared with nonsmokers (MJ-TS-) (p<0·001). Acrylamide and acrylonitrile metabolites (AAMA, GAMA, CYMA, and CYHA) were elevated in marijuana-only smokers (MJ+TS-) compared with nonsmokers (MJ-TS-) (p<0·05), but levels were lower compared with those detected in tobacco-only smokers.
Table 2.
Geometric means (95% confidence intervals) of nicotine, THC, PAH, and VOC plasma metabolites at endpoint by marijuana and tobacco smoking.a
| Metabolite | MJ-TS- (n = 88) | MJ+TS- (n = 45) | MJ-TS+ (n = 51) | MJ+TS+ (n = 59) |
|---|---|---|---|---|
| Metabolites of nicotine | ||||
| cotinine | 0·00 (0·00, 0·00) | 0·00 (0·00, 0·00) | 0·57 (0·30, 1·06) | 0·81 (0·55, 1·18) |
| hydroxycotinine | 0·01 (0·01, 0·01) | 0·01 (0·01, 0·01) | 0·38 (0·22, 0·65) | 0·60 (0·44, 0·82) |
| 3-hydroxycotinine glucuronide | 0·78 (0·75, 0·82) | 0·76 (0·76, 0·76) | 1·66 (1·20, 2·28) | 1·43 (1·06, 1·92) |
| cotinine N-oxide | 0·16 (0·15, 0·17) | 0·15 (0·15, 0·15) | 0·66 (0·48, 0·91) | 0·75 (0·57, 0·97) |
| norcotinine | 0·40 (0·40, 0·40) | 0·40 (0·40, 0·40) | 0·55 (0·43, 0·71) | 0·56 (0·46, 0·68) |
| nornicotine | 0·35 (0·35, 0·35) | 0·35 (0·35, 0·35) | 0·72 (0·55, 0·96) | 0·65 (0·54, 0·77) |
| Metabolites of THC | ||||
| ∆−9-trans-tetrahydrocannabinol (THC) | 0·23 (0·23, 0·23) | 0·43 (0·31, 0·58) | 0·23 (0·23, 0·23) | 0·49 (0·38, 0·62) |
| THC carboxylic acid | 0·08 (0·08, 0·08) | 0·38 (0·24, 0·61) | 0·08 (0·08, 0·08) | 0·45 (0·32, 0·62) |
| THC carboxylic acid glucuronide | 0·02 (0·02, 0·02) | 0·28 (0·14, 0·53) | 0·02 (0·02, 0·02) | 0·49 (0·31, 0·77) |
| Metabolites of PAHs | ||||
| 2-naphthol sulfate | 0·26 (0·21, 0·33) | 0·41 (0·29, 0·58) | 1·27 (0·91, 1·77) | 1·94 (1·55, 2·44) |
| methylnaphthyl sulfate (1) | 0·18 (0·17, 0·19) | 0·19 (0·16, 0·21) | 0·49 (0·35, 0·70) | 0·72 (0·49, 1·04) |
| methylnaphthyl sulfate (2) | 0·07 (0·06, 0·07) | 0·13 (0·09, 0·18) | 0·52 (0·35, 0·76) | 1·05 (0·80, 1·40) |
| 2-hydroxyfluorene sulfate | 0·17 (0·15, 0·19) | 0·25 (0·19, 0·34) | 0·53 (0·38, 0·74) | 0·96 (0·73, 1·27) |
| VOCs and other metabolites | ||||
| N-Acetyl-S-(3-hydroxypropyl)-L-cysteine (3HPMA) | 0·43 (0·38, 0·49) | 0·46 (0·37, 0·57) | 0·92 (0·70, 1·22) | 0·85 (0·67, 1·07) |
| o-cresol sulfate | 0·16 (0·12, 0·21) | 0·40 (0·26, 0·62) | 0·92 (0·60, 1·43) | 1·62 (1·28, 2·04) |
| 3-acetylphenol sulfate | 0·40 (0·29, 0·55) | 0·55 (0·36, 0·86) | 1·01 (0·67, 1·52) | 0·95 (0·69, 1·31) |
| 4-vinylphenol sulfate | 0·46 (0·36, 0·57) | 0·76 (0·51, 1·14) | 0·98 (0·75, 1·28) | 1·60 (1·27, 2·01) |
| catechol sulfate | 0·54 (0·42, 0·70) | 0·81 (0·56, 1·17) | 0·78 (0·59, 1·03) | 0·95 (0·71, 1·26) |
| quinate | 0·25 (0·16, 0·38) | 0·57 (0·30, 1·07) | 0·55 (0·34, 0·90) | 0·61 (0·38, 0·97) |
| 2-ethylphenyl sulfate | 0·12 (0·11, 0·14) | 0·24 (0·17, 0·33) | 0·79 (0·54, 1·16) | 1·07 (0·81, 1·41) |
| 4-ethylphenyl sulfate | 0·61 (0·45, 0·83) | 0·93 (0·62, 1·40) | 1·54 (1·10, 2·17) | 1·42 (1·12, 1·79) |
| 3-hydroxy-2-methylpyridine sulfate | 0·34 (0·21, 0·58) | 0·55 (0·28, 1·08) | 0·75 (0·38, 1·50) | 0·66 (0·40, 1·09) |
| 3-hydroxypyridine glucuronide | 0·29 (0·20, 0·42) | 0·45 (0·27, 0·77) | 0·51 (0·30, 0·88) | 0·58 (0·37, 0·91) |
| 3-hydroxypyridine sulfate | 0·13 (0·09, 0·21) | 0·31 (0·17, 0·58) | 0·53 (0·34, 0·82) | 0·64 (0·44, 0·94) |
| 1,2,3-benzenetriol sulfate (2) | 0·44 (0·30, 0·65) | 0·81 (0·52, 1·27) | 0·81 (0·52, 1·28) | 0·79 (0·55, 1·14) |
| 4-vinylguaiacol sulfate | 0·20 (0·14, 0·28) | 0·40 (0·24, 0·65) | 0·80 (0·52, 1·22) | 0·90 (0·64, 1·27) |
| hydroquinone sulfate | 0·68 (0·53, 0·87) | 0·80 (0·53, 1·19) | 1·06 (0·76, 1·48) | 0·84 (0·64, 1·10) |
| isoeugenol sulfate | 0·29 (0·23, 0·37) | 0·25 (0·19, 0·32) | 0·96 (0·67, 1·39) | 0·94 (0·71, 1·24) |
Plasma metabolite data was available at endpoint for 243 participants. One MJ+TS+ participant changed to MJ-TS+ between baseline and endpoint visit. Abbreviations: MJ-TS-, non-smoker; MJ+TS-, marijuana-only smoker; MJ-TS+, tobacco-only smoker; MJ+TS+, dual marijuana-tobacco smoker; PAHs, polycyclic aromatic hydrocarbons; VOCs, volatile organic compounds.
- Values are scaled intensities.
Table 3.
Geometric means (95% confidence intervals) of nicotine and VOC urine metabolites at endpoint by marijuana and tobacco smoking.
| Metabolite | Abbreviation | MJ-TS- (n = 30) | MJ+TS- (n = 16) | MJ-TS+ (n = 23) | MJ+TS+ (n = 22) |
|---|---|---|---|---|---|
| Nicotine and tobacco alkaloids (ng/mg creatinine) | |||||
| Cotinine | COTT | 2·9 (2·3, 3·6) | 3·8 (2·1, 6·9) | 2166 (1419, 3306) | 1494 (782, 2854) |
| Hydroxycotinine | HCTT | 5·7 (4·6, 7·2) | 8·5 (4·4, 16·5) | 5392 (3747, 7759) | 3537 (2280, 5486) |
| Cotinine-n-oxide | COXT | 1·2 (1·0, 1·5) | 1·3 (0·8, 2·0) | 361 (254, 515) | 206 (130, 326) |
| Nicotine | NICT | 6·4 (5·1, 8·0) | 5·4 (3·5, 8·4) | 1485 (899, 2452) | 701 (310, 1587) |
| Nicotine-1′N-oxide | NOXT | 1·5 (1·2, 1·9) | 1·3 (0·8, 2·2) | 337 (201, 566) | 166 (87, 315) |
| Nornicotine | NNCT | 1·5 (1·2, 1·9) | 1·1 (0·8, 1·7) | 73·7 (49·0, 111·1) | 47·2 (23·2, 96·3) |
| Anabasine | ANBT | 0·31 (0·3, 0·4) | 0·23 (0·2, 0·4) | 7·30 (4·6, 11·7) | 4·22 (2·0, 8·8) |
| Anatabine | ANTT | 0·24 (0·2, 0·3) | 0·18 (0·1, 0·3) | 12·70 (7·7, 21·0) | 7·05 (3·1, 16·1) |
| 1-(3-Pyridyl)−1-butanol-4-carboxylic acid | HPBT | 1·29 (0·9, 1·8) | 3·12 (1·6, 6·1) | 703 (460, 1074) | 435 (255, 743) |
| VOCs (µg/g creatinine) | |||||
| 2-Methylhippuric acid | 2MHA | 29 (20, 42) | 46 (22, 97) | 108 (75, 155) | 117 (71, 192) |
| 3-Methylhippuric acid + 4-Methylhippuric acid | 34MH | 168 (128, 220) | 250 (152, 409) | 709 (499, 1008) | 616 (446, 849) |
| N-Acetyl-S-(2-carbamoylethyl)-L-cysteine | AAMA | 43 (35, 53) | 78 (44, 139) | 111 (87, 143) | 152 (117, 199) |
| N-Acetyl-S-(2-carbamoyl-2-hydroxyethyl)-L-cysteine | GAMA | 7·9 (6·4, 9·8) | 15·7 (9·3, 26·6) | 16·1 (12·1, 21·3) | 19·8 (15·7, 25·0) |
| N-Acetyl-S-(1-cyano-2-hydroxyethyl)-L-cysteine | CYHA | 1·61 (1·3, 2·0) | 4·9 (2·3, 10·4) | 26·9 (16·7, 43·3) | 30·8 (17·6, 53·7) |
| N-Acetyl-S-(2-cyanoethyl)-L-cysteine | CYMA | 1·47 (1·1, 2·0) | 12·47 (4·2, 37·4) | 102 (60, 172) | 180 (117, 277) |
| N-Acetyl-S-(2-carboxyethyl)-L-cysteine | CEMA | 115 (92, 142) | 148 (92, 239) | 384 (295, 501) | 335 (233, 481) |
| N-Acetyl-S-(3-hydroxypropyl)-L-cysteine | 3HPMA | 292 (252, 339) | 312 (195, 498) | 1180 (800, 1741) | 1111 (727, 1699) |
| N-Acetyl-S-(benzyl)-L-cysteine | BMA | 9·2 (6·3, 13·4) | 9·6 (6·1, 15·2) | 6·6 (4·5, 9·7) | 8·1 (4·6, 14·1) |
| N-Acetyl-S-(n-propyl)-L-cysteine | BPMA† | 4·6 (2·9, 7·1) | 2·0 (0·9, 4·3) | 1·4 (0·7, 2·8) | 1·9 (0·9, 4·2) |
| Mandelic acid | MADA | 99 (68, 145) | 160 (127, 203) | 257 (190, 347) | 282 (204, 391) |
| Phenylglyoxylic acid | PHGA | 214 (174, 264) | 244 (183, 325) | 362 (284, 462) | 381 (278, 523) |
| N-Acetyl-S-(phenyl)-L-cysteine | PMA | 0·9 (0·7, 1·2) | 0·6 (0·4, 0·9) | 0·8 (0·6, 1·1) | 1·0 (0·7, 1·4) |
| N-Acetyl-S- (2-hydroxypropyl)-L-cysteine | HPM2 | 32·7 (24·4, 43·7) | 26·4 (18·8, 37·2) | 65·5 (47·8, 89·9) | 72·3 (52·2, 100·2) |
| N-Acetyl-S-(phenyl-2-hydroxyethyl)-L-cysteine | PHEM† | 1·30 (0·9, 1·9) | 0·83 (0·54, 1·29) | 1·69 (1·27, 2·25) | 2·30 (1·6, 3·3) |
| trans, trans-Muconic acid | MUCA† | 108 (68, 170) | 63 (41, 95) | 161 (114, 226) | 143 (85, 239) |
| N-Acetyl-S-(N-methylcarbamoyl)-L-cysteine | AMCA | 145 (108, 195) | 201 (139, 290) | 469 (345, 637) | 505 (384, 664) |
| N-Acetyl-S-(2-hydroxyethyl)-L-cysteine | HEMA† | 0·9 (0·7, 1·2) | 0·7 (0·4, 1·0) | 1·3 (0·9, 1·9) | 2·1 (1·1, 3·9) |
| N-Acetyl-S-(3,4-dihydroxybutyl)-L-cysteine | DHBM | 439 (375, 513) | 277 (170, 449) | 526 (436, 635) | 511 (419, 624) |
| N-Acetyl-S-(4-hydroxy-2-buten-1-yl)-L-cysteine | MHB3 | 4·4 (3·5, 5·5) | 5·5 (3·3, 9·2) | 24·9 (14·7, 42·1) | 29·7 (20·7, 42·6) |
| N-Acetyl-S-(3-hydroxypropyl-1-methyl)-L-cysteine | HPMM | 667 (480, 927) | 498 (349, 709) | 2166 (1514, 3097) | 2222 (1617, 3051) |
| N-Acetyl-S-(4-hydroxy-2-methyl-2-buten-1-yl)-L-cysteine | IPM3 | 4·0 (3·0, 5·5) | 4·5 (2·6, 7·9) | 36·4 (21·2, 62·4) | 33·7 (21·0, 54·0) |
| 2-Thioxothiazolidine-4-carboxylic acid | TTCA | 14·9 (11·0, 20·1) | 25·2 (14·7, 43·3) | 16·4 (10·7, 25·2) | 22·8 (16·5, 31·4) |
| 2-Aminothiazoline-4-carboxylic acid | ATCA† | 186 (130, 267) | 93 (49, 175) | 53 (33, 87) | 146 (88, 241) |
Abbreviations: MJ-TS-, non-smoker; MJ+TS-, marijuana-only smoker; MJ-TS+, tobacco-only smoker; MJ+TS+, dual marijuana-tobacco smoker; VOCs, volatile organic compounds.
(ng/mg).
Fig. 1.
Plasma and urine biomarkers of exposure by marijuana and tobacco smoking. Box plots of selected plasma (A) or urine (B) exposure biomarkers at study endpoint. Horizontal bars denote medians, boxes span IQRs, whiskers extend to 1·5 X IQR, dots denote individual participant values. (C) Heatmap of selected plasma metabolites by marijuana and tobacco smoking. Metabolite intensities (rows) were standardized by z-scoring and clustered hierarchically. Individual participants (columns) were ordered by increasing THC or cotinine values within smoking groups (colored bar, top). MJ-TS- denotes non-smokers; MJ+TS-, marijuana-only smokers; MJ-TS+, tobacco-only smokers; MJ+TS+, dual marijuana-tobacco smokers. Abbreviations for metabolites: AAMA, N-acetyl-S-(2-carbamoylethyl)-L-cysteine; CYMA, N-acetyl-S-(2-cyanoethyl)-L-cysteine; CYHA, N-acetyl-S-(1-cyano-2-hydroxyethyl)-L-cysteine; GAMA, N-acetyl-S-(2-carbamoyl-2-hydroxyethyl)-L-cysteine; 3HPMA, N-Acetyl-S-(3-hydroxypropyl)-L-cysteine; PAH, polycylic aromatic hydrocarbons; THC, ∆−9-trans-tetrahydrocannabinol; VOC, volatile organic compound.
To investigate associations between metabolites of THC and nicotine with other metabolites of smoke exposure, we evaluated selected plasma metabolites at study endpoint by hierarchical clustering (Fig. 1C). As expected, THC metabolites in marijuana smokers (MJ+TS- and MJ+TS+) and nicotine metabolites in tobacco smokers (MJ-TS+ and MJ+TS+) clustered with other THC or nicotine metabolites, respectively. PAH metabolites 2-naphthol sulfate, 2-hydroxyfluorene sulfate, and methylnaphthyl sulfate (1) clustered together and were elevated in tobacco smokers (MJ-TS+ and MJ+TS+) and a subset of marijuana-only smokers (MJ+TS-) compared with nonsmokers. The acrolein metabolite 3HPMA clustered with nornicotine and correlated with metabolites of nicotine including nornicotine (Pearson's r = 0·58, p<0·0001), cotinine N-oxide (r = 0·37, p = 0·002), and cotinine (r = 0·33, p = 0·0066) among tobacco smokers (MJ-TS+ and MJ+TS+) with detectable 3HPMA levels (Fig. S4A). Methylnaphthyl sulfate (2), o-cresol sulfate, and 2-ethylphenylsulfate clustered together and were elevated in tobacco smokers (MJ-TS+ and MJ+ TS+) and subset of marijuana-only smokers (MJ+TS-) compared with nonsmokers, particularly those with detectable THC metabolites. O-cresol sulfate, 2-ethylphenylsulfate, and methylnaphthyl sulfate (2) showed a dose-dependent relationship and were significantly correlated with THC metabolites among exclusive marijuana smokers (MJ+TS-) (Pearson's r = 0·43–0·72, p<0·01; Fig. S5), suggesting these biomarkers are associated with recent THC exposure.
Among selected metabolites in urine, nicotine metabolites and tobacco alkaloids clustered together and exhibited high concentrations in tobacco smokers (MJ-TS+ and MJ+TS+) compared with exclusive marijuana smokers and nonsmokers (Fig. S6). Urine 3HPMA clustered near nicotine metabolites, and plasma and urine 3HPMA correlated with nicotine and tobacco alkaloid metabolites (Pearson's r = 0·44–0·83, p<0·05; Fig. S4B and S4C, respectively), suggesting acrolein metabolites are associated with tobacco smoking in a dose-dependent manner. Urine metabolites including AAMA, GAMA, CYMA, and CYHA displayed similar patterns within individuals and were significantly correlated with THC metabolites among marijuana-only smokers (Pearson's r = 0·48–0·90, p<0·05; Figure S7). These findings suggest that heavy marijuana smoking is associated with elevated VOCs, PAHs, and other smoke exposure biomarkers in plasma and urine compared with non-smokers, but levels of these biomarkers are lower in marijuana-only smokers compared with tobacco-only and dual smokers.
3.3. Longitudinal associations of marijuana and tobacco with smoke exposure biomarkers
Plasma and urine metabolomics profiling was performed at 2–6 timepoints within a median 1·5 years of follow-up for 165 (64%) and 42 (47%) of participants, respectively (mean [SD] 2·8 [1·0] and 2·9 [1·0] timepoints/participant in plasma and urine analyses, respectively). Within-subject intraclass correlation coefficients (ICCs) were highly significant for metabolites of nicotine and THC in plasma (ICCs 0·69–0·90; Table S1) and for nicotine and tobacco alkaloid metabolites in urine (ICCs 0·74–0·86; Table S2), indicating marijuana and tobacco smoking exposures remained consistent within individuals through follow-up. High ICCs (typically >0·6) were also observed for VOCs and other metabolites elevated in tobacco smokers (plasma and urine 3HPMA) or in subsets of exclusive marijuana smokers (plasma o-cresol sulfate, 2-ethylphenylsulfate, 4-vinylphenol sulfate; urine AAMA, GAMA, CYMA, CYHA).
We used mixed-effects models to further investigate longitudinal associations between marijuana or tobacco smoking and selected metabolites. In models adjusted for HIV status, age, and renal dysfunction (known to affect 3HPMA and potentially other metabolites [39]), marijuana smoking was associated with increased plasma PAH metabolites 2-naphthol sulfate (ß=0·82, p = 0·0016), methylnaphthyl sulfate (2) (ß=0·84, p<0·0001), 2-hydroxyfluorene sulfate (ß=0·47, p<0·001), and with increased o-cresol sulfate (ß=0·60, p = 0·0015), 4-vinylphenol sulfate (ß=0·49, p = 0·0036), catechol sulfate (ß=0·39, p = 0·021), 2-ethylphenyl sulfate (ß=0·44, p = 0·0028), 4-ethylphenyl sulfate (ß=0·41, p = 0·025), and 4-vinylguaiacol sulfate (ß=0·57, p = 0·014) (Table S3). Among urine metabolites, marijuana smoking was associated with increased acrylonitrile metabolites CYHA (ß=1·50, p = 0·001) and CYMA (ß=2·93, p<0·001) and increased acrylamide metabolites AAMA (ß=0·58, p = 0·0023) and GAMA (ß=0·39, p = 0·038) in adjusted models (Table S4). Consistent with cross-sectional analyses, tobacco smoking was associated with increased concentrations of most metabolites, with estimates three- to five-times higher than those for marijuana smoking. Interactions between marijuana and tobacco smoking were tested with separate models for all metabolites, and additional models including a stratified marijuana and tobacco smoking term where interactions were significant. While interactions were significant in models for four metabolites (p<0·05), there was no evidence for a substantial additive increase of metabolites in dual marijuana and tobacco smokers (MJ+TS+) for any metabolite tested. Together, these results confirm cross-sectional associations between marijuana smoking and increased plasma and urine smoke exposure biomarkers after adjusting for tobacco smoking and known risk factors, and suggest independent effects of tobacco and marijuana smoke on these exposure biomarkers.
3.4. Plasma acrolein metabolite 3HPMA is associated with cardiovascular disease
Given that tobacco smoking is a major risk factor for CVD and known toxicities of some combustion-related chemicals, we examined associations between plasma PAH and VOC biomarkers and CVD diagnoses within prior 5 years using logistic regression models. Acrolein metabolite 3HPMA concentrations were tested in these analyses given previous studies reporting associations between urinary 3HPMA and CVD risk factors [20]. We also tested o-cresol sulfate and methylnaphthyl sulfate (2), as these were non-THC metabolites showing the strongest associations with marijuana smoking in mixed-effects models. Elevated 3HPMA was associated with increased risk of CVD diagnoses within 5 years prior to endpoint in both univariate and multivariate models adjusted for age, tobacco smoking at endpoint, number of traditional CVD risk factors (hypertension, hyperlipidemia, and diabetes), and HIV status (odds ratio [OR] 3·48 [95% confidence interval CI 1·58–7·64]; p = 0·019; and OR 3·34 [95% CI 1·31–8·57]; p = 0·012, respectively, top vs. middle and lower tertiles) (Table 4). In separate models adjusted for the same factors, each two-fold 3HPMA increase was associated with 1·59-fold increased odds of a recent CVD diagnosis (95% CI 1·18–2·16; p = 0·0027). In sensitivity analyses restricted to HIV+ participants, the association between increased 3HPMA and odds of CVD diagnoses remained significant in adjusted models (OR 3·03 [95% CI 1·22–8·19]; p = 0·029, top vs. middle and lower tertiles) (Table S5). Among non-THC metabolites associated with marijuana smoking in mixed-effects models, increased plasma o-cresol sulfate and methylnaphthyl sulfate (2) were associated with increased odds of CVD diagnoses in unadjusted models (OR 2·53 [95% CI 1·17–5·49]; p = 0·0019; and OR 2·11 [95% CI 0·98–4·56]; p = 0·057; respectively, top vs. middle and lower tertiles) but not in adjusted models (Table S6), indicating these associations were weaker compared with other CVD risk factors, particularly the number of hypertension, diabetes, and hyperlipidemia risk factors. COPD diagnoses were higher in tobacco-only smokers compared with non-smokers (Table 1), but there were too few events to detect an association in adjusted models.
Table 4.
Association between plasma 3HPMA concentrations, tobacco smoking, and cardiovascular events.
| Unadjusted Models |
Multivariable Model 1 |
Multivariable Model 2 |
Multivariable Model 3 |
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Tobacco smoking | 2·28 (1·04–5·04) | 0·0408 | 2·04 (0·87–4·80) | 0·10 | 1·18 (0·44–3·15) | 0·74 | 1·31 (0·52–3·31) | 0·57 |
| Traditional CVD risk factors (2–3 vs. 0–1)a | 3·70 (1·66–8·19) | 0·0013 | 3·29 (1·43–7·58) | 0·0052 | 3·83 (1·62–9·04) | 0·0022 | 3·77 (1·58–8·96) | 0·0027 |
| 3HPMA concentration (top vs. middle and lowest tertiles, scaled intensity) | 3·48 (1·58–7·64) | 0·0019 | – | – | 3·34 (1·31–8·57) | 0·012 | – | – |
| 3HPMA concentration (per two-fold increase, scaled intensity) | 1·60 (1·23–2·08) | <0·001 | – | – | – | – | 1·59 (1·18–2·16) | 0·0027 |
Models were fit using logistic regression for 30 cardiovascular events occurring within 5 years prior to endpoint among 240 participants. Plasma metabolite data was available for 243 participants; 3 participants with events occurring more than 5 years prior to endpoint were excluded. The median duration between CVD diagnoses and endpoint was 1·08 years. Multivariable models also included terms for age and HIV status. Cardiovascular events included myocardial infarction, coronary artery disease, cardiac stents, stroke, and atherosclerosis. Abbreviations: CVD, cardiovascular disease, 3HPMA, plasma N-Acetyl-S-(3-hydroxypropyl)-L-cysteine.
- Number of traditional cardiovascular disease risk factors (hypertension, hyperlipidemia, diabetes risk factors - see Methods).
4. Discussion
In this longitudinal study of 245 participants (76% HIV+), we compared 28 plasma and 33 urine smoke-exposure biomarkers in tobacco-only smokers, marijuana-only smokers, dual marijuana-tobacco smokers, and non-smoking controls. To our knowledge, this is one of the largest studies to compare exposure biomarkers between marijuana and tobacco smokers. Consistent with previous studies [13,37,40,41], nicotine and tobacco alkaloids including cotinine, cotinine N-oxide, and hydroxycotinine were detected at significantly higher levels in both plasma and urine of tobacco smokers compared with non-smokers, while ∆9-THC, THC carboxylic acid, and THC carboxylic acid glucuronide were detected above background only in marijuana smokers. A subset of PAH, VOC, and other smoke exposure biomarkers detected in tobacco smokers were also detected in exclusive marijuana smokers, including 2-naphthol sulfate, methylnaphthyl sulfate (2), 2-hydroxyfluorene sulfate, 4-vinylphenol sulfate, and o-cresol sulfate in plasma, and acrylonitrile metabolites CYMA and CYHA and acrylamide metabolites AAMA and GAMA in urine, but concentrations of these chemicals were lower in marijuana-only compared with tobacco smokers. Among plasma metabolites associated with tobacco smoking, elevated acrolein metabolite 3HPMA was associated with increased CVD diagnoses in logistic regression models adjusted for tobacco smoking and traditional risk factors, while metabolites showing strongest associations with marijuana smoking in mixed-effects models (methylnaphthyl sulfate (2) and o-cresol sulfate) were not associated with increased CVD diagnoses in adjusted analyses. Taken together, these findings indicate marijuana smoke-exposure biomarkers reported in previous studies [5,6] are present at lower concentrations in exclusive marijuana smokers compared with tobacco smokers, and also show that acrolein exposure from tobacco smoke is associated with CVD.
Acrolein is a reactive aldehyde derived from combustion of tobacco, as well as other sources including heating of carbohydrates, animal fats, vegetable oils, and petroleum fuels [16,18,42]. Although potential sources of exposure include dietary sources (e.g., fried foods, charred meats), tobacco smoking is typically the largest source of acrolein exposure [8,14,30,42]. Consistent with previous studies [5,9,14,20,30], tobacco smokers showed elevated plasma and urine acrolein metabolite 3HPMA, which correlated positively with nicotine and metabolites of tobacco alkaloids among tobacco smokers. In contrast, 3HPMA concentrations were not elevated among exclusive marijuana smokers. Elevated plasma 3HPMA was associated with increased CVD diagnoses in adjusted logistic regression models among all subjects, and in sensitivity analyses limited to HIV+ participants. Participants with 3HPMA concentrations in the top tertile had increased CVD risk (OR 3·34 [95% CI 1·31–8·57]) comparable to participants with 2–3 vs. 0–1 traditional hypercholesterolemia, hypertension, or diabetes risk factors (OR 3·83 [95% CI 1·62–9·04]). These findings are consistent with a previous cohort study reporting higher urinary 3HPMA concentrations in smokers compared with non-smokers, and associations between increased 3HPMA and CVD risk measures including platelet-leukocyte aggregates and Framingham Risk score [20]. Previous studies have shown acrolein exposures can induce cardiovascular injury in animal models [17,19], induce or exacerbate dyslipidemia, and modify lipoproteins [43,44], and may contribute to neutrophil-mediated inflammation [45]. HIV infection is associated with increased inflammation [46,47] and renal dysfunction [48], which may further increase acrolein levels and its impact on cardiovascular disease [16,39,42,49]. Given its toxicity and environmental pervasiveness [16,50], future studies are needed to better characterize the mechanisms underlying acrolein-associated CVD risk.
While plasma and urinary biomarkers of smoke exposure have been well-characterized in tobacco smokers [9,10,13,40,41,[51], [52], [53]], only a few studies limited to analyses of urine samples have been reported in marijuana smokers [5,6,54]. Consistent with a previous study [5], the urine acrylonitrile metabolite CYMA (N-acetyl-S-(2-cyanoethyl)-L-cysteine) and acrylamide metabolites AAMA (N-acetyl-S-(2-carbamoylethyl)-L-cysteine) and GAMA (N-acetyl-S-(2-carbamoyl-2-hydroxyethyl)-l-cysteine) were significantly elevated in marijuana smokers in both cross-sectional comparisons and mixed-effects models adjusted for tobacco smoking and other confounders. Additionally, we identified increased acrylonitrile metabolite CYHA (N-acetyl-S-(1-cyano-2-hydroxyethyl)-L-cysteine) in marijuana smokers, which was associated with tobacco exposure in a previous study [9], but to our knowledge was not previously associated with marijuana smoking. In plasma, PAH metabolites methylnaphthyl sulfate (2) and 2-naphthol sulfate, and VOCs o-cresol sulfate and 4-vinylphenol sulfate were among the biomarkers most elevated in marijuana-only smokers compared with non-smokers in both cross-sectional and adjusted analyses. These biomarkers correlated positively with THC metabolite levels among exclusive marijuana smokers, suggesting marijuana smoking is a major source of exposure. While a small number of plasma PAH, VOC, and other metabolites, including methylnaphthyl sulfate (2), 2-hydroxyfluorene sulfate, 4-vinylphenol sulfate, and o-cresol sulfate were significantly elevated in cross-sectional analyses of dual marijuana and tobacco smokers compared with tobacco-only smokers, there were no significant positive marijuana-tobacco interactions in mixed-effects models, indicating independent effects of these exposures.
This study includes limitations, including the possibility that results may be specific to individuals recruited for the included research cohorts and may not be generalizable, and that the study design limits the ability to infer causality between exposures and outcomes. Smoking exposures were based on self-report, and as is typical of other cohort studies, there was insufficient information available regarding quantity or potency of marijuana smoked, use of products with high concentrations of THC versus cannabidiol, potential secondhand smoke exposures, and time between exposure and sample collection, which impacts detection of short half-life metabolites. However, we observed good agreement between self-reported smoking behavior and detected levels of nicotine or THC metabolites. The frequency of marijuana smoking was available for participants from the MACS cohort, of whom 21% reported daily or weekly smoking at baseline, which is comparable to proportions reported in larger cohort studies of HIV+ individuals in the United States [22,29]. The analyses focused on marijuana and tobacco smoking and possible use of other marijuana and tobacco products was not assessed. Five participants reported use of prescription oral THC (dronabinol), but this did not significantly affect the main findings. The cohort consisted of 76% HIV+ participants, which may influence applicability of findings to the general population. However, given consistency of our findings with prior studies, smoking is likely to be the major factor affecting metabolite levels and other findings in the study. There were too few HIV- participants to allow comparisons stratified by both HIV status and smoking, though there were no differences in metabolite levels between HIV+ and HIV- non-smokers in cross-sectional comparisons (data not shown). The number of CVD diagnoses (n = 30) limited the number of terms included in multivariable models, so possible effects of gender, body mass index, cocaine use, and other factors were not evaluated. Low representation of females is an important limitation of the study because it restricted our ability to assess the effects of gender. While high plasma acrolein metabolite 3HPMA was associated with increased CVD, the lack of association between other metabolites associated with marijuana smoking and CVD diagnoses should be interpreted with caution given sample sizes and limited number of diagnoses. While toxicant levels were typically lower in marijuana compared to tobacco smokers, previous studies of HIV+ individuals reported elevated CVD and COPD risk among tobacco or marijuana smokers [26,27,29]. Larger studies with longer follow-up are needed to fully assess potentially toxic effects of marijuana smoke constituents on health outcomes in men and women.
In conclusion, this study compares the effects of marijuana and tobacco smoking on plasma and urinary smoke-exposure biomarkers in a longitudinal cohort of HIV+ and HIV- individuals. Marijuana smoking was independently associated with elevated concentrations of some smoke-related toxicants, including urinary metabolites of acrylamide and acrylonitrile, and plasma PAHs and VOCs including methylnaphthyl sulfate (2), 2-hydroxyfluorene sulfate, 4-vinylphenol sulfate, and o-cresol sulfate, but levels were lower compared with those associated with tobacco smoking. Elevated acrolein metabolite 3HPMA correlated with metabolites of nicotine but not marijuana and was associated with increased CVD diagnoses independent of tobacco smoking and other risk factors. Thus, our findings together with all available evidence imply that high acrolein levels may identify adults at increased cardiovascular risk and reducing exposure may be a preventive strategy. The association between high acrolein levels and cardiovascular disease is particularly relevant for HIV+ individuals given high rates of tobacco smoking and heightened risk of CVD in this population. Overall, these findings identify potentially toxic combustion by-products associated with marijuana and tobacco smoking and suggest the need for further studies on health effects of acrolein and other smoke-related exposures that include participants more representative of the general population.
Author contributions
DG designed the study and analysis plan. DL, VM, SC, LW, BB, VDJ, BG, SM, SW, and DG contributed to acquisition, analysis, or interpretation of data. DL, VM, HU, and DG performed the statistical analysis. DL, VM, and DG wrote the first draft of the report. All authors contributed to critical review and writing of the article. All authors approved the final version of the report.
Declaration of Competing Interest
DL, VM, SC, HU, BG, SM, SW and DG report grants from the NIH during the conduct of the study. All other authors have nothing to disclose.
Acknowledgments
Funding
This study was supported by NIH grants to DG (R01 DA040391 and DA046203). NNTC sites were supported by National Institute of Mental Health (NIMH) and National Institute of Neurological Disorders and Stroke (NINDS) (grants U24MH100931, U24MH100930, U24MH100929, U24MH100928, U24MH100925). The HIV Neurobehavioral Research Center (HNRC) is supported by Center award P30 MH062512 from NIMH and CHARTER sites were supported by Award Number HHS-N-271-2010-00036C) and HHSN271201000030C from NIMH/NINDS. The MACS is funded by the National Institute of Allergy and Infectious Diseases (NIAID; U01-AI35039, U01-AI35040, U01-AI35041, U01- AI35042, and UM1-AI35043), with additional co-funding from the National Cancer Institute (NCI), National Institute on Drug Abuse (NIDA), and National Institute of Mental Health (NIMH) at the National Institutes of Health (NIH). MACS data collection is also supported by UL1-TR000424 (JHU CTSA).
Disclaimers
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Data sharing statement
De-identified participant data is available from the National NeuroAIDS Tissue Consortium [32], HIV Neurobehavioral Research Center [31], and Multicenter AIDS Cohort Study [33] to qualified researchers upon request. Plasma and urine biomarker data is available from the corresponding author upon request.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100697.
Appendix. Supplementary materials
References
- 1.SAMHSA. Key substance use and mental health indicators in the United States: results from the 2019 national survey on drug use and health. Available from https://www.samhsa.gov/data/sites/default/files/reports/rpt29393/2019NSDUHFFRPDFWHTML/2019NSDUHFFR1PDFW090120.pdf (Accessed 9 October 2020).
- 2.Hasin D.S., Saha T.D., Kerridge B.T. Prevalence of marijuana use disorders in the United States Between 2001-2002 and 2012-2013. JAMA Psychiatry. 2015;72(12):1235–1242. doi: 10.1001/jamapsychiatry.2015.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odani S., Soura B.D., Tynan M.A., Lavinghouze R., King B.A., Agaku I. Tobacco and marijuana use among US college and noncollege young adults, 2002-2016. Pediatrics. 2019;144(6) doi: 10.1542/peds.2019-1372. [DOI] [PubMed] [Google Scholar]
- 4.Moir D., Rickert W.S., Levasseur G. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol. 2008;21(2):494–502. doi: 10.1021/tx700275p. [DOI] [PubMed] [Google Scholar]
- 5.Wei B., Alwis K.U., Li Z. Urinary concentrations of PAH and VOC metabolites in marijuana users. Environ Int. 2016;88:1–8. doi: 10.1016/j.envint.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meier E., Vandrey R., Rubin N. Cigarette smokers vs. co-users of cannabis and cigarettes: exposure to toxicants. Nicotine Tob Res. 2020;22:1383–1389. doi: 10.1093/ntr/ntz199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith D.M., O'Connor R.J., Wei B., Travers M., Hyland A., Goniewicz M.L. Nicotine and toxicant exposure among concurrent users ("Co-users") of tobacco and cannabis. Nicotine Tob Res. 2020;22:1354–1363. doi: 10.1093/ntr/ntz122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodnar J.A., Morgan W.T., Murphy P.A., Ogden M.W. Mainstream smoke chemistry analysis of samples from the 2009 US cigarette market. Regul Toxicol Pharmacol. 2012;64(1):35–42. doi: 10.1016/j.yrtph.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Etemadi A., Poustchi H., Chang C.M. Urinary Biomarkers of carcinogenic exposure among cigarette, waterpipe, and smokeless tobacco users and never users of tobacco in the Golestan cohort study. Cancer Epidemiol Biomarkers Prev. 2019;28(2):337–347. doi: 10.1158/1055-9965.EPI-18-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregg E.O., Minet E., McEwan M. Urinary biomarkers of smokers' exposure to tobacco smoke constituents in tobacco products assessment: a fit for purpose approach. Biomarkers. 2013;18(6):467–486. doi: 10.3109/1354750X.2013.821523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris P.B., Ference B.A., Jahangir E. Cardiovascular effects of exposure to cigarette smoke and electronic cigarettes: clinical perspectives from the prevention of cardiovascular disease section leadership council and early career councils of the American college of cardiology. J Am Coll Cardiol. 2015;66(12):1378–1391. doi: 10.1016/j.jacc.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 12.Schick S.F., Blount B.C., Jacob P.R. Biomarkers of exposure to new and emerging tobacco delivery products. Am J Physiol Lung Cell Mol Physiol. 2017;313(3):L425–LL52. doi: 10.1152/ajplung.00343.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahab L., Goniewicz M.L., Blount B.C. Nicotine, carcinogen, and toxin exposure in long-term e-cigarette and nicotine replacement therapy users: a cross-sectional study. Ann Intern Med. 2017;166(6):390–400. doi: 10.7326/M16-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alwis K.U., deCastro B.R., Morrow J.C., Blount B.C. Acrolein exposure in U.S. tobacco smokers and non-tobacco users: NHANES 2005-2006. Environ Health Perspect. 2015;123(12):1302–1308. doi: 10.1289/ehp.1409251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minet E., Cheung F., Errington G., Sterz K., Scherer G. Urinary excretion of the acrylonitrile metabolite 2-cyanoethylmercapturic acid is correlated with a variety of biomarkers of tobacco smoke exposure and consumption. Biomarkers. 2011;16(1):89–96. doi: 10.3109/1354750X.2010.533287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henning R.J., Johnson G.T., Coyle J.P., Harbison R.D. Acrolein can cause cardiovascular disease: a review. Cardiovasc Toxicol. 2017;17(3):227–236. doi: 10.1007/s12012-016-9396-5. [DOI] [PubMed] [Google Scholar]
- 17.Ismahil M.A., Hamid T., Haberzettl P. Chronic oral exposure to the aldehyde pollutant acrolein induces dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2011;301(5):H2050–H2060. doi: 10.1152/ajpheart.00120.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moghe A., Ghare S., Lamoreau B. Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol Sci. 2015;143(2):242–255. doi: 10.1093/toxsci/kfu233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsakadze N.L., Srivastava S., Awe S.O., Adeagbo A.S., Bhatnagar A., D'Souza S.E. Acrolein-induced vasomotor responses of rat aorta. Am J Physiol Heart Circ Physiol. 2003;285(2):H727–H734. doi: 10.1152/ajpheart.00269.2003. [DOI] [PubMed] [Google Scholar]
- 20.DeJarnett N., Conklin D.J., Riggs D.W. Acrolein exposure is associated with increased cardiovascular disease risk. J Am Heart Assoc. 2014;3(4) doi: 10.1161/JAHA.114.000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frazier E.L., Sutton M.Y., Brooks J.T., Shouse R.L., Weiser J. Trends in cigarette smoking among adults with HIV compared with the general adult population, United States - 2009-2014. Prev Med. 2018;111:231–234. doi: 10.1016/j.ypmed.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Mimiaga M.J., Reisner S.L., Grasso C. Substance use among HIV-infected patients engaged in primary care in the United States: findings from the centers for AIDS research network of integrated clinical systems cohort. Am J Public Health. 2013;103(8):1457–1467. doi: 10.2105/AJPH.2012.301162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calvo M., Laguno M., Martinez M., Martinez E. Effects of tobacco smoking on HIV-infected individuals. AIDS Rev. 2015;17(1):47–55. [PubMed] [Google Scholar]
- 24.Freiberg M.S., So-Armah K. HIV and cardiovascular disease: we need a mechanism, and we need a plan. J Am Heart Assoc. 2016;4(3) doi: 10.1161/JAHA.116.003411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Islam F.M., Wu J., Jansson J., Wilson D.P. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med. 2012;13(8):453–468. doi: 10.1111/j.1468-1293.2012.00996.x. [DOI] [PubMed] [Google Scholar]
- 26.Lorenz D.R., Dutta A., Mukerji S.S., Holman A., Uno H., Gabuzda D. Marijuana use impacts midlife cardiovascular events in HIV-infected men. Clin Infect Dis. 2017;65(4):626–635. doi: 10.1093/cid/cix391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crothers K., Huang L., Goulet J.L. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183(3):388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzpatrick M.E., Kunisaki K.M., Morris A. Pulmonary disease in HIV-infected adults in the era of antiretroviral therapy. AIDS. 2018;32(3):277–292. doi: 10.1097/QAD.0000000000001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenz D.R., Uno H., Wolinsky S.M., Gabuzda D. Effect of marijuana smoking on pulmonary disease in HIV-infected and uninfected men: a longitudinal cohort study. EClinicalMedicine. 2019;7:55–64. doi: 10.1016/j.eclinm.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen M., Carmella S.G., Sipe C. Longitudinal stability in cigarette smokers of urinary biomarkers of exposure to the toxicants acrylonitrile and acrolein. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0210104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The HIV Neurobehavioral Research Center (HNRC). Information available from: https://hnrc.hivresearch.ucsd.edu/index.php/about-59 (Accessed 30 November 2020).
- 32.The national NeuroAIDS tissue consortium (NNTC). Information available from: https://nntc.org (Accessed 30 November 2020).
- 33.Detels R., Jacobson L., Margolick J. The multicenter AIDS cohort study, 1983 to. Public Health. 2012;126(3):196–198. doi: 10.1016/j.puhe.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Centers for Disease Control and Prevention. International classification of diseases, ninth revision, clinical modification (ICD-9-CM). Available from: https://www.cdc.gov/nchs/icd/icd9cm.htm (Accessed 30 November 2020).
- 35.Cassol E., Misra V., Morgello S., Kirk G.D., Mehta S.H., Gabuzda D. Altered monoamine and acylcarnitine metabolites in HIV-positive and HIV-negative subjects with depression. J Acquir Immune Defic Syndr. 2015;69(1):18–28. doi: 10.1097/QAI.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chettimada S., Lorenz D.R., Misra V. Exosome markers associated with immune activation and oxidative stress in HIV patients on antiretroviral therapy. Sci Rep. 2018;8(1):7227. doi: 10.1038/s41598-018-25515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alwis K.U., Blount B.C., Britt A.S., Patel D., Ashley D.L. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS) Anal Chim Acta. 2012;750:152–160. doi: 10.1016/j.aca.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei B., Feng J., Rehmani I.J. A high-throughput robotic sample preparation system and HPLC-MS/MS for measuring urinary anatabine, anabasine, nicotine and major nicotine metabolites. Clin Chim Acta. 2014;436:290–297. doi: 10.1016/j.cca.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakata K., Kashiwagi K., Sharmin S., Ueda S., Igarashi K. Acrolein produced from polyamines as one of the uraemic toxins. Biochem Soc Trans. 2003;31(2):371–374. doi: 10.1042/bst0310371. [DOI] [PubMed] [Google Scholar]
- 40.Gu F., Derkach A., Freedman N.D. Cigarette smoking behaviour and blood metabolomics. Int J Epidemiol. 2016;45(5):1421–1432. doi: 10.1093/ije/dyv330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prasad G.L., Jones B.A., Schmidt E., Chen P., Kennedy A.D. Global metabolomic profiles reveal differences in oxidative stress and inflammation pathways in smokers and moist snuff consumers. J. Metabol. 2015;1(2):1–11. [Google Scholar]
- 42.Stevens J.F., Maier C.S. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52(1):7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conklin D.J., Barski O.A., Lesgards J.F. Acrolein consumption induces systemic dyslipidemia and lipoprotein modification. Toxicol Appl Pharmacol. 2010;243(1):1–12. doi: 10.1016/j.taap.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pizzimenti S., Ciamporcero E., Daga M. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front Physiol. 2013;4:242. doi: 10.3389/fphys.2013.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noerager B.D., Xu X., Davis V.A. A potential role for acrolein in neutrophil-mediated chronic inflammation. Inflammation. 2015;38(6):2279–2287. doi: 10.1007/s10753-015-0213-2. [DOI] [PubMed] [Google Scholar]
- 46.Bahrami H., Budoff M., Haberlen S.A. Inflammatory markers associated with subclinical coronary artery disease: the multicenter AIDS cohort study. J Am Heart Assoc. 2016;5(6) doi: 10.1161/JAHA.116.003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kooij K.W., Wit F.W., Booiman T. Cigarette smoking and inflammation, monocyte activation, and coagulation in HIV-infected individuals receiving antiretroviral therapy, compared with uninfected individuals. J Infect Dis. 2016;214(12):1817–1821. doi: 10.1093/infdis/jiw459. [DOI] [PubMed] [Google Scholar]
- 48.Gameiro J., Jorge S., Lopes J.A. HIV and renal disease: a contemporary review. Int J STD AIDS. 2018;29(7):714–719. doi: 10.1177/0956462417750710. [DOI] [PubMed] [Google Scholar]
- 49.Anderson M.M., Hazen S.L., Hsu F.F., Heinecke J.W. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation. J Clin Invest. 1997;99(3):424–432. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haussmann H.J. Use of hazard indices for a theoretical evaluation of cigarette smoke composition. Chem Res Toxicol. 2012;25(4):794–810. doi: 10.1021/tx200536w. [DOI] [PubMed] [Google Scholar]
- 51.Goniewicz M.L., Smith D.M., Edwards K.C. Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA Netw Open. 2018;1(8) doi: 10.1001/jamanetworkopen.2018.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keith R.J., Fetterman J.L., Orimoloye O.A. Characterization of volatile organic compound metabolites in cigarette smokers, electronic nicotine device users, dual users, and nonusers of tobacco. Nicotine Tob Res. 2020;22(2):264–272. doi: 10.1093/ntr/ntz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rostron B.L., Corey C.G., Chang J.T., van Bemmel D.M., Miller M.E., Chang C.M. Associations of cigarettes smoked per day with biomarkers of exposure among U.S. adult cigarette smokers in the population assessment of tobacco and health (PATH) study wave 1 (2013-2014) Cancer Epidemiol Biomarkers Prev. 2019;28(9):1443–1453. doi: 10.1158/1055-9965.EPI-19-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei B., Smith D., O'Connor R., Travers M.J., Hyland A. Examining the association between body burdens of harmful chemicals and heaviness of marijuana smoking. Chem Res Toxicol. 2018;31(8):643–645. doi: 10.1021/acs.chemrestox.8b00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.