Abstract
Background
Degenerative signs on shoulder radiographs, including spur formation and narrow acromiohumeral intervals (AHIs), have been recognized as indicative of atrophic and fat-infiltrated rotator cuff muscles. Past studies have demonstrated that patients with poor quality muscles are prone to retraction of the supraspinatus tendon and failure to repair. However, the association between radiographic signs and tendon retraction has never been elucidated in previous literature. The present study aimed to investigate the association between the degenerative signs on shoulder radiographs and the severity of supraspinatus retraction.
Methods
Images of 67 individuals, who had undergone an arthroscopic rotator cuff repair, were retrospectively reviewed. The greater tuberosity (GT) morphology, subacromial spur, AHI, and acromial thickness were evaluated on the radiographs, whereas the retraction of the supraspinatus tendon was assessed via an MRI in accordance with the Patte classification. Simple regression analyses between the radiographic signs and Patte stages were performed, and factors reaching statistical significance were then included in the multiple ordinal logistic regression. Statistically significant predictors from the multiple regression analysis were constructed into combinations, for which the sensitivity and specificity were calculated.
Results
The GT morphology (P = .004), AHI (P = .083), subacromial spur (P = .008), and age (P = .004) were associated with supraspinatus retraction in the simple regression analyses. These four parameters were incorporated into the multiple ordinal logistic regression, where the GT spur (adjusted odds ratio 8.63, 95% confidence interval 2.16-34.53, P = .002) and AHI (AOR 0.79, 95% CI 0.63-0.98, P = .032) were demonstrated to be predictive of the Patte stage of supraspinatus retraction. The acromial spur implied a higher risk of severe retraction although this finding was not statistically significant (AOR 2.89, 95% CI 0.90-9.29, P = .075). The presence of concurrent GT spur and narrow AHI was highly specific (sensitivity 27.3% / specificity 91.1%) for advanced supraspinatus retraction.
Conclusion
The presence of a radiographic GT spur, narrow AHI, and subacromial spur indicated advanced retraction of the supraspinatus tendon. When patients with clinical suspicion of rotator cuff tear present with combinations of these radiographic signs, a prompt MRI examination and a referral to a shoulder specialist are recommended.
Keywords: Rotator cuff tear, radiography, greater tuberosity spur, tendon retraction
Associations between radiographic abnormalities of the greater tuberosity (GT) and pathology of the rotator cuff and were studied extensively in recent years. Radiographic GT spur and GT sclerosis indicate symptomatic rotator cuff tears24 and large-sized tears.18 Cyst formation in the anterior GT13 and a GT angle greater than 70 degrees6 have been found to be related to the incidence of rotator cuff tears as well. The combination of these radiographic degenerative signs is effective in ruling out rotator cuff tears when assessed by senior practitioners,3 with the combined sensitivity and negative predictive value being 91.7% and 80%, respectively.3 In patients with rotator cuff tears, the presence of a radiographic GT spur indicates atrophy and fatty infiltration of the supraspinatus muscle, as well as atrophy of the infraspinatus muscle,5 which are known to portend poor postoperative functional outcomes.11,16
The retraction of the supraspinatus tendon poses a negative impact on the reparability of rotator cuff tears. Patients whose preoperative magnetic resonance imaging (MRI) reveals concomitant supraspinatus retraction and fatty infiltration have been found to have a 92% rotator cuff repair failure rate,17 whereas absence of these degenerative changes is an indicator of repairable massive rotator cuff tears with a 96% specificity.14 The more advanced the supraspinatus retraction is, the more severe the supraspinatus muscle atrophy9 and fatty infiltration10 are. To our knowledge, the relation between radiographic degenerative signs and supraspinatus tendon retraction has not been elucidated.
The present study aimed to investigate the association between the degenerative signs on shoulder radiographs and the severity of supraspinatus retraction. We hypothesize that presence of GT spur, narrow acromiohumeral interval (AHI), and subacromial spur indicated advanced stages of supraspinatus retraction. Identifying radiographic markers and patient-related factors predictive of supraspinatus retraction can help physicians diagnose rotator cuff pathology early.
Patient enrollment
This was a retrospective case series comprising individuals who had undergone arthroscopic surgery for chronic rotator cuff repair, after failure of conservative treatment with rehabilitation and anti-inflammatory medications for at least 3 months. An a priori power analysis for the multiple regression was conducted using G∗Power. With the effect size = 0.3, alpha = 0.05, power = 0.9, and 3 predictors in the model, the projected sample size needed is approximately n = 52 for statistical comparison. The inclusion criteria required that every patient underwent shoulder radiography and MRI of the shoulder before surgery. All images were reviewed by two authors (H.-C.C. and W.-R.S.). The exclusion criteria included surgical indications other than rotator cuff tear (n = 16), partial supraspinatus tear (n = 2), a lack of either a radiograph (n = 3) or MRI (n = 6), and admission for revision surgery (n = 2). Of the 96 patients undergoing arthroscopic surgery, 67 patients, aged between 43 and 75 years (mean age, 58.7 years) met the inclusion criteria and were enrolled. The number (n = 67) will be more than adequate for the main objective of this study and should also allow for expected attrition
Assessment of radiographs
Standard true anteroposterior radiographs of the shoulder during external rotation were reviewed by a shoulder fellowship-trained orthopedic surgeon using a digital picture archiving and communication system. Four parameters of glenohumeral joint degeneration were studied (Figure 1).
-
(1)
Acromial spur was scored using a dichotomous outcome, namely presence or absence. Presence was defined as identifying any of the four types of coronal spur at the subacromion, in accordance with the classification proposed by Oh et al.21
-
(2)
Acromial thickness was evaluated as described by Oh et al.21 The thickness was measured at the widest portion of the acromion, typically lateral to the acromioclavicular joint. The spur was not included in the measurement.
-
(3)
AHI was defined as the shortest distance from the inferior cortex of acromion to the humeral head.
-
(4)
The GT morphology was graded as spurring, sclerotic, or normal, as depicted in the previous study.5 The grading was based on the degree of radiolucency and irregularity in the GT contour.
Figure 1.
Radiographic degenerative signs of the glenohumeral joint. (A) The presence of an acromial spur (broken line); (B) Acromial thickness ( ); (C) Acromiohumeral interval (
); (C) Acromiohumeral interval ( ); (D) Normal GT; (E) Sclerotic GT (
); (D) Normal GT; (E) Sclerotic GT ( ); (F) Spurring GT (
); (F) Spurring GT ( ). GT, greater tuberosity.
). GT, greater tuberosity.
Assessment of the MR images
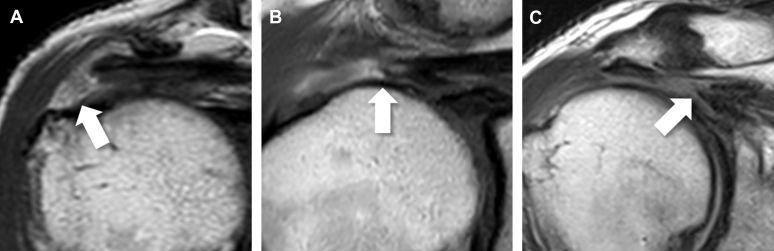
The MRI scans of each subject were reviewed to assess the rotator cuff tendon and muscle pathology. The coronal planes were reviewed to assess the degree of supraspinatus tendon retraction using the Patte classification23 (Figure 2).
Figure 2.
Retraction of supraspinatus tendon on MRI. (A) Patte stage 1, the stump is located near the insertion site at GT. (B) Patte stage 2, the stump is located at the humeral head. (C) Patte stage 3, the stump retracts to the level of the glenoid cavity. GT, greater tuberosity. The stump of torn supraspinatus tendon is indicated by an arrow (↑).
The MR images were independently reviewed by a senior shoulder orthopedic surgeon while blinded to the surgical findings and outcomes. All MRI scans were performed within 3 months before surgery. A 3.0-T (Achieva; Philips Medical Systems) or 1.5-T (Signa; GE Healthcare) imaging units equipped with a dedicated shoulder coil (4-channel SENSE shoulder coil for the 3.0-T system and a large shoulder array coil [receive only] for the 1.5-T system) was used.
Statistical analysis
The quantitative variables are shown as mean and standard deviation, and the qualitative variables are shown as frequency and percentage. Patient-related factors (age, gender, and the injured side) and the four radiographic signs mentioned previously first underwent simple regression with the severity of rotator cuff retraction. The categorical variables (gender, side, presence of acromial spur, and GT morphology) were analyzed using the chi-square or Fisher’s exact test, as appropriate. The quantitative variables (age, acromial thickness, and AHI) were analyzed using a simple ordinal logistic regression.
After the primary analyses, factors with P ≤ .1 were included in a univariate multiple ordinal logistic regression model, which examined the relative contribution of each potential predictor to the Patte classification stage. Adjusted odds ratios (AORs) and 95% confidence intervals (95% CIs) of the variables were calculated, and the significance of the AOR was tested using the Wald chi-square test. A two-tailed P-value of ≤ .05 was considered statistically significant in the multiple regression analysis. All data in this study were analyzed using SPSS version 17 (IBM, Armonk, NY, USA).
Predictors reaching statistical significance in the multiple regression analysis were constructed into combinations. We defined the Patte stage 3 retraction of the supraspinatus tendon as the outcome and computed the sensitivity and specificity of each combination using contingency tables. Youden’s indexes were calculated to capture the diagnostic performance of each combination.31 Rand accuracy, the proportion of true results among the total number of cases examined, was calculated to capture the diagnostic performance of each combination.26
Ethical issues
The study protocol was approved by the Institutional Review Board of National Cheng Kung University Hospital (A-ER-1U8-561). The requirement for written informed consent was waived considering the retrospective design.
Results
Basic characteristics
Examination of the 67 MRI scans revealed 25 cases (37.3%) with little retraction (Patte stage 1) and 31 cases (46.3%) with retraction to the humeral head (Patte stage 2); the remaining 11 cases (16.4%) had severe retraction to the glenoid cavity (Patte stage 3). Of the seven factors undergoing simple analyses, four factors reached statistical significance when associated with the Patte stage of the supraspinatus tendon. These were age (P = .004), presence of an acromial spur (P = .008), AHI (P = .083), and GT morphology (P = .003). The characteristics of the participants are shown in Table I.
Table I.
Characteristics of the study population
| Characteristic | Patte I | Patte II | Patte III | P value∗ |
|---|---|---|---|---|
| Gender | ||||
| Male | 13 | 15 | 6 | .929 |
| Female | 12 | 16 | 5 | |
| Age | 56.2 ± 12.1 | 58.8 ± 5.9 | 63.9 ± 5.3 | .004 |
| Side | ||||
| Left | 8 | 4 | 4 | .142 |
| Right | 17 | 27 | 7 | |
| Acromial spur | ||||
| No | 23 | 20 | 5 | .008 |
| Yes | 2 | 11 | 6 | |
| Acromial thickness | 8.95 ± 1.5 | 8.65 ± 1.5 | 9.36 ± 1.9 | .495 |
| AHI | 8.7 ± 2.2 | 7.9 ± 2.2 | 7.0 ± 3.0 | .083 |
| GT morphology | ||||
| Normal | 13 | 5 | 0 | .003 |
| Sclerotic | 6 | 6 | 3 | |
| Spurring | 6 | 20 | 8 |
AHI, acromiohumeral interval; GT, greater tuberosity.
P value using chi-squared test or ordinal logistic regression.
Multiple regression
The four significant factors from the simple analyses were incorporated into the multiple ordinal logistic regression model. The multiple regression revealed that GT spurs (P = .002) and AHI (P = .032) were predictive of the Patte stage and statistically significant, whereas the acromial spur showed a trend toward significance (P = .159) (Table II).
Table II.
Ordinal logistic regression analysis of predictive radiographic signs of Patte classification in patients with full thickness supraspinatus tears
| Predictor | Variable | B | SE | Wald chi-square | P value | AOR | 95% CI |
|---|---|---|---|---|---|---|---|
| GT morphology | Spurring | 2.155 | 0.7074 | 9.284 | .002 | 8.631 | 2.157-34.529 |
| Sclerotic | 1.330 | 0.7919 | 2.819 | .093 | 3.779 | 0.801-17.843 | |
| Normal | 0 | ||||||
| AHI | −0.238 | 0.1110 | 4.609 | .032 | 0.788 | 0.634-0.979 | |
| Acromial spur | Present | 1.060 | 0.5960 | 3.165 | .075 | 2.888 | 0.898-9.286 |
| Absent | 0 | ||||||
| Age | 0.053 | 0.0373 | 1.986 | .159 | 1.054 | 0.980-1.134 |
SE, standard error; P, P value; AOR, adjusted odds ratio; CI, confidence interval.
Using the normal group as a reference, the presence of a GT spur (AOR = 8.631, 95% CI 2.157-34.529) indicated that supraspinatus retraction tended to be more serious; the presence of GT sclerosis (AOR = 3.779, 95% CI 0.801-17.843) demonstrated a similar trend. When the AHI increased by 1 millimeter, the risk of developing advanced supraspinatus retraction decreased (AOR = 0.788, 95% CI 0.634-0.979). In other words, a narrow AHI implied the occurrence of severe supraspinatus tendon retraction. The presence of acromial spurs tended to be predictive of serious retraction (AOR = 2.888, 95% CI 0.898-9.286). The age was not a predictor of the degree of retraction, as revealed in the multiple ordinal logistic regression (P = .083).
Analysis of diagnostic performance
To improve the accuracy and applicability of prediction, combinations of the radiographic signs (GT spur, acromial spur, and narrow AHI ≤ 6 millimeters19) were constructed. The combinations were then statistically examined for their sensitivities and specificities in terms of predicting severe retraction (Patte stage 3) of the supraspinatus tendon. Among the combinations of two radiographic signs, the combination of a GT spur and acromial spur yielded the highest Youden’s index (0.294) with fair accuracy (77.6%), followed by the combination of a GT spur and narrow AHI (0.184, 80.6%). When the three signs were all combined, the accuracy showed marginal improvement (83.6%) while the Youden’s index (0.146) decreased (Table III).
Table III.
Diagnostic performance of combinations of radiographic signs
| Combination | Sensitivity | Specificity | Youden’s index | Accuracy |
|---|---|---|---|---|
| GT spur, acromial spur | 45.5% | 83.9% | 0.294 | 77.6% |
| GT spur, AHI ≤ 6 mm | 27.3% | 91.1% | 0.184 | 80.6% |
| Acromial spur, AHI ≤ 6 mm | 18.2% | 96.4% | 0.146 | 83.6% |
| GT spur, acromial spur, AHI ≤ 6 mm | 18.2% | 96.4% | 0.146 | 83.6% |
GT, greater tuberosity; AHI, acromiohumeral interval.
Discussion
Degenerative changes on a shoulder radiograph have been shown to indicate fatty infiltration and atrophic changes in rotator cuff muscles.5,13 In the present study, we found that radiographic signs, including GT spur formation, acromial spur formation, and narrow AHI, were associated with the stage of supraspinatus retraction. The combinations of these radiographic signs were highly specific and accurate in terms of predicting advanced retraction of the supraspinatus tendon. The results were clinically relevant, as they suggested that MRI examination shoulder be prioritized for patients presenting with radiographic degenerative signs of the glenohumeral joint.
Accumulating evidence has indicated that supraspinatus retraction and degeneration of rotator cuff muscles are among the primary determinants of the need for a complete rotator cuff repair. Patients with a supraspinatus muscle with severe fatty infiltration (Goutallier stage 2 or above) and severe retraction (the remaining tendon stump shorter than 15mm) have been found to have a failure rate as high as 92% two years after a rotator cuff repair, whereas those with little fatty infiltration and adequate tendon stump length were found to have a failure rate of only 25%.17 The combination of concomitant severe fatty infiltration of infraspinatus muscles and severe retraction of the supraspinatus tendon has been shown to be a highly specific predictor of irreparability of a rotator cuff tear.14 In addition, the degree of fatty infiltration correlates positively with the stage of retraction,10 which has been reported to equate negatively to postoperative function.8,11 Therefore, some authors have advocated early diagnosis and early intervention of rotator cuff tears, lest patients become susceptible to incomplete repairs after the degenerative rotator cuff cascade is initiated.12
To achieve early detection of supraspinatus retraction, in the present study, a preoperative MRI is suggested in patients with any two of the radiographic signs mentioned earlier. Although ultrasonography is as sensitive as MRI for the detection of tears15 and as useful for measuring the size of anteroposterior tears,29 its ability to measure the size of mediolateral tear is limited.29 On the other hand, computed tomography (CT) is also an effective diagnostic modality, but it has been reported to underestimate the degree of rotator cuff muscle degeneration among patients with concomitant glenohumeral arthritis.7 To conclude, MRI is superior regarding the visualization of rotator cuff muscle degeneration. The major disadvantage of an MRI is the inhibitory cost, which prevents some diagnostic algorithms from recommending it as a routine preoperative practice.20 This study demonstrated that plain radiographs of the shoulder could be a cost-effective means by which to help prioritize which patients should undergo an MRI examination. We recommend that patients with any two of the three radiographic signs mentioned earlier should undergo an MRI examination, regardless of whether their initial diagnostic tool is sonography or a CT.
Patients presenting with radiographically narrow AHI tend to be associated with advanced supraspinatus retraction, presumably because the reduced width reflects a loss of the cushioning effect of the supraspinatus tendon. When the supraspinatus tendon is torn, the AHI of the injured side is reduced compared with its healthy counterpart.25 The amount of reduction has also been shown to be positively correlated with the size of rotator cuff tears and the degree of infraspinatus fatty infiltration.28 The interval can be restored after the rotator cuff tendon is repaired,25 further highlighting the importance of the supraspinatus tendon in the maintenance of the subacromial space. In the present study, the decreased AHI is related to increased risk of severe supraspinatus retraction. This is reasonable, considering that the supraspinatus tendon can exert little cushion effect if the tendon is already retracted medially to the level of the glenoid cavity. A subacromial space unoccupied by the supraspinatus tendon allows the humeral head to migrate unopposed under a superiorly directed deltoid pull.28
The association between spur formation and retraction of the supraspinatus tendon can be attributed to, at least partially, the interaction between the GT and the coracoacromial arch. Oh et al proposed that heel-type acromial spurs are related to the impact of the humeral head on the acromion in the presence of superior-directed microinstability,21 which can occur in the absence of stabilizing forces from rotator cuff muscles.4 One previous study demonstrated that patients with GT spurs were prone to larger tears.5 The findings in the present study agree with past literature by showing that the coexistence of acromial and GT spurs is highly specific to supraspinatus retraction, which implied that osteophytes possibly form after the supraspinatus tendon retracts. Aside from direct impact from the humeral head, chronic tensile overload might also play a role. At the acromion, Burns and Whipple observed that anterior acromial traction spurs arose from repetitive stretching of the coracoacromial ligament by the GT in patients with chronic impingement syndrome.2 At the GT, the tensile overload and inhomogeneous distribution of strain in the supraspinatus tendon resulted in tearing and retraction.27 Increased load could injure Sharpey’s fibers at the insertion site and initiate both endochondral ossification of the fibrocarilaginous repair tissue and ligament ossification, initiating a spur generation cascade analogous to that of vertebral traction osteophyte formation.30
Interestingly, the simple ordinal logistic regression revealed a statistically significant association between age and retraction, whereas the multiple regression analysis indicated that age was not an independent predictor of the Patte stage. Previous literature has demonstrated that spur formation is detected more frequently among the elderly.1,22 Oh. et al examined shoulder radiographs from 208 individuals and concluded that acromial spurs were more prevalent in patients older than 65 years (80%) compared with those younger than 55 years (58%).21 In the present study, we isolated the age from spur formation and narrow AHI by calculating the adjusted odds ratio. Age was not an independent predictor, denoting that the predictability in the simple regression was largely derived from spur formation. This result agreed with conclusions from previous studies.
Limitations
There are several limitations to this study. The first was the available orientation of the radiographs. As axial and Y-view radiographs of the shoulder joint were not available in all of the patients included in the study, optimal classification of acromial spurs could not be achieved. Classification of acromial spurs based on morphologic characteristics could make it possible to gain an insight into the biomechanics of the shoulder joint. In addition, a prospective design instead of the present retrospective design may be more objective when it comes to evaluating the association between surgical outcomes and radiographic signs. The third limitation was that the outcome parameter was indirect, based on the retraction on MRI examinations. Correlation with the tendon mobility observed during arthroscopic surgery or the completeness of repair will be more straightforward.
Conclusions
The presence of a radiographic GT spur, narrow AHI, and subacromial spur portend advanced retraction of the supraspinatus tendon. When patients with clinical suspicion of a rotator cuff tear present with combinations of these radiographic signs, a prompt MRI examination and a referral to a shoulder specialist are recommended.
Disclaimer
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Acknowledgments
The authors thank Skeleton Materials and Bio-compatibility Core Lab, Research Center of Clinical Medicine, National Cheng Kung University Hospital, Tainan, Taiwan, for assistance with this project.
Footnotes
The study protocol was approved by the Institutional Review Board of National Cheng Kung University Hospital (B-ER-107-184). The requirement for written informed consent was waived considering the retrospective design.
References
- 1.Bonsell S., Pearsall AWt, Heitman R.J., Helms C.A., Major N.M., Speer K.P. The relationship of age, gender, and degenerative changes observed on radiographs of the shoulder in asymptomatic individuals. J Bone Joint Surg Br. 2000;82:1135–1139. doi: 10.1302/0301-620x.82b8.10631. [DOI] [PubMed] [Google Scholar]
- 2.Burns W.C., 2nd, Whipple T.L. Anatomic relationships in the shoulder impingement syndrome. Clin Orthop Relat Res. 1993;294:96–102. [PubMed] [Google Scholar]
- 3.Chin K., Chowdhury A., Leivadiotou D., Marmery H., Ahrens P.M. The accuracy of plain radiographs in diagnosing degenerate rotator cuff disease. Shoulder Elbow. 2019;11:46–51. doi: 10.1177/1758573217743942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chopp J.N., O'Neill J.M., Hurley K., Dickerson C.R. Superior humeral head migration occurs after a protocol designed to fatigue the rotator cuff: A radiographic analysis. Journal of Shoulder and Elbow Surgery. 2010;19:1137–1144. doi: 10.1016/j.jse.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Chuang H.C., Hong C.K., Hsu K.L., Kuan F.C., Lin C.L., Su W.R. The radiographic morphology of the greater tuberosity is associated with muscle degeneration in patients with symptomatic rotator cuff tears. J Shoulder Elbow Surg. 2019;28:1964–1970. doi: 10.1016/j.jse.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham G., Nicodeme-Paulin E., Smith M.M., Holzer N., Cass B., Young A.A. The greater tuberosity angle: a new predictor for rotator cuff tear. J Shoulder Elbow Surg. 2018;27:1415–1421. doi: 10.1016/j.jse.2018.02.051. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald M., Lawler S.M., Lowe J.T., Nelson R., Mantell M.T., Jawa A. Computed tomography underestimates rotator cuff pathology in patients with glenohumeral osteoarthritis. J Shoulder Elbow Surg. 2018;27:1451–1455. doi: 10.1016/j.jse.2018.02.034. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs B., Weishaupt D., Zanetti M., Hodler J., Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8:599–605. doi: 10.1016/s1058-2746(99)90097-6. [DOI] [PubMed] [Google Scholar]
- 9.Fukuta S., Tsutsui T., Amari R., Wada K., Sairyo K. Tendon retraction with rotator cuff tear causes a decrease in cross-sectional area of the supraspinatus muscle on magnetic resonance imaging. J Shoulder Elbow Surg. 2016;25:1069–1075. doi: 10.1016/j.jse.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert F., Meffert R.H., Schmalzl J., Weng A.M., Kostler H., Eden L. Grade of retraction and tendon thickness correlates with MR-spectroscopically measured amount of fatty degeneration in full thickness supraspinatus tears. BMC Musculoskelet Disord. 2018;19:197. doi: 10.1186/s12891-018-2096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gladstone J.N., Bishop J.Y., Lo I.K., Flatow E.L. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35:719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 12.Godeneche A., Elia F., Kempf J.F., Nich C., Berhouet J., Saffarini M. Fatty infiltration of stage 1 or higher significantly compromises long-term healing of supraspinatus repairs. J Shoulder Elbow Surg. 2017;26:1818–1825. doi: 10.1016/j.jse.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Gwark J.Y., Park T.S., Park H.B. Association between the location of tuberosity cysts and rotator cuff tears: A comparative study using radiograph and MRI. J Orthop Surg (Hong Kong) 2019;27 doi: 10.1177/2309499019825762. 2309499019825762. [DOI] [PubMed] [Google Scholar]
- 14.Kim J.Y., Park J.S., Rhee Y.G. Can Preoperative Magnetic Resonance Imaging Predict the Reparability of Massive Rotator Cuff Tears? Am J Sports Med. 2017;45:1654–1663. doi: 10.1177/0363546517694160. [DOI] [PubMed] [Google Scholar]
- 15.Lenza M., Buchbinder R., Takwoingi Y., Johnston R.V., Hanchard N.C., Faloppa F. Magnetic resonance imaging, magnetic resonance arthrography and ultrasonography for assessing rotator cuff tears in people with shoulder pain for whom surgery is being considered. Cochrane Database Syst Rev. 2013;2013:Cd009020. doi: 10.1002/14651858.CD009020.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McElvany M.D., McGoldrick E., Gee A.O., Neradilek M.B., Matsen F.A., 3rd Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43:491–500. doi: 10.1177/0363546514529644. [DOI] [PubMed] [Google Scholar]
- 17.Meyer D.C., Wieser K., Farshad M., Gerber C. Retraction of supraspinatus muscle and tendon as predictors of success of rotator cuff repair. Am J Sports Med. 2012;40:2242–2247. doi: 10.1177/0363546512457587. [DOI] [PubMed] [Google Scholar]
- 18.Mohammad Ghandour A., El Ghazaly S., El Ghandour T. Greater tuberosity sclerosis: Does it correlate with tear size? The Egyptian Journal of Radiology and Nuclear Medicine. 2017;48:425–429. doi: 10.1016/j.ejrnm.2017.02.005. [DOI] [Google Scholar]
- 19.Moor B.K., Bouaicha S., Rothenfluh D.A., Sukthankar A., Gerber C. Is there an association between the individual anatomy of the scapula and the development of rotator cuff tears or osteoarthritis of the glenohumeral joint?: A radiological study of the critical shoulder angle. Bone Joint J. 2013;95-b:935–941. doi: 10.1302/0301-620x.95b7.31028. [DOI] [PubMed] [Google Scholar]
- 20.Nazarian L.N., Jacobson J.A., Benson C.B., Bancroft L.W., Bedi A., McShane J.M. Imaging algorithms for evaluating suspected rotator cuff disease: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2013;267:589–595. doi: 10.1148/radiol.13121947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh J.H., Kim J.Y., Lee H.K., Choi J.A. Classification and clinical significance of acromial spur in rotator cuff tear: heel-type spur and rotator cuff tear. Clin Orthop Relat Res. 2010;468:1542–1550. doi: 10.1007/s11999-009-1058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panni A.S., Milano G., Lucania L., Fabbriciani C., Logroscino C.A. Histological analysis of the coracoacromial arch: correlation between age-related changes and rotator cuff tears. Arthroscopy. 1996;12:531–540. doi: 10.1016/s0749-8063(96)90190-5. [DOI] [PubMed] [Google Scholar]
- 23.Patte D. Classification of rotator cuff lesions. Clin Orthop Relat Res. 1990:81–86. [PubMed] [Google Scholar]
- 24.Pearsall AWt, Bonsell S., Heitman R.J., Helms C.A., Osbahr D., Speer K.P. Radiographic findings associated with symptomatic rotator cuff tears. J Shoulder Elbow Surg. 2003;12:122–127. doi: 10.1067/mse.2003.19. [DOI] [PubMed] [Google Scholar]
- 25.Pepe M., Kocadal O., Gunes Z., Calisal E., Aksahin E., Aktekin C.N. Subacromial space volume in patients with rotator cuff tear: The effect of surgical repair. Acta Orthop Traumatol Turc. 2018;52:419–422. doi: 10.1016/j.aott.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rand W.M. Objective Criteria for the Evaluation of Clustering Methods. Journal of the American Statistical Association. 1971;66:846–850. [Google Scholar]
- 27.Reilly P., Amis A.A., Wallace A.L., Emery R.J. Mechanical factors in the initiation and propagation of tears of the rotator cuff. Quantification of strains of the supraspinatus tendon in vitro. J Bone Joint Surg Br. 2003;85:594–599. doi: 10.1302/0301-620x.85b4.12062. [DOI] [PubMed] [Google Scholar]
- 28.Saupe N., Pfirrmann C.W., Schmid M.R., Jost B., Werner C.M., Zanetti M. Association between rotator cuff abnormalities and reduced acromiohumeral distance. AJR Am J Roentgenol. 2006;187:376–382. doi: 10.2214/ajr.05.0435. [DOI] [PubMed] [Google Scholar]
- 29.Tse A.K., Lam P.H., Walton J.R., Hackett L., Murrell G.A. Ultrasound determination of rotator cuff tear repairability. Shoulder Elbow. 2016;8:14–21. doi: 10.1177/1758573215585284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong S.H., Chiu K.Y., Yan C.H. Review Article: Osteophytes. J Orthop Surg (Hong Kong) 2016;24:403–410. doi: 10.1177/1602400327. [DOI] [PubMed] [Google Scholar]
- 31.Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]