Abstract
Background
Anatomical lung resection offers the best prospect of long-term survival in patients with non-small cell lung cancer (NSCLC). However, some patients with significant dyspnoea, impaired performance status (PS), borderline or poor pulmonary function are considered inoperable and instead referred for radiotherapy, chemotherapy or palliative care. The aims of the study were to determine whether pre-operative pulmonary physiotherapy (Prehab), by improving clinical parameters, (i) makes patients suitable for surgery who were considered inoperable on subjective criteria of dyspnoea >3 and PS >2, and objective criteria of diffusing capacity for carbon monoxide (DLCO) <50%; and (ii) thereby allows them to safely receive curative surgery with reduced morbidity and mortality.
Methods
From January 2017 to December 2018 a total of 306 patients were prospectively and sequentially assessed for Prehab and 216 patients with lung cancer studied. Their mean age (95% CI) was 71.7 ± 1.1 years, 50.5% (n = 109) were men and they received Prehab over 39.0 ± 7.0 days averaging 3.1 ± 0.6 sessions. Their dyspnoea scores, PS, level of activity, six minute walk test (6MWT) and frailty index prior to and following Prehab were determined. Following surgery the post-operative length of hospital stay (LOHS), complications and mortality at 30 days, 90 days and 1 year determined. Similar outcomes were determined for (i) high-risk patients with dyspnoea scores >3 and PS >2, and compared with low-risk patients having dyspnoea scores <2 and PS <2 (subjective criteria); and (ii) high-risk patients with DLCO <50% and compared with low-risk patients with DLCO >80% (objective criteria).
Findings
In the total cohort following Prehab, there was significant improvement in the dyspnoea scores <2 / ≥2 (40%/60% prior to Prehab vs. 65%/35% following Prehab, p = 0.00002), PS <2 / ≥2 (45%/55% prior to vs. 62%/38% following Prehab, p = 0.003), frailty index ≤3 / >3 (49%/51% vs 70%/30%, p = 0.0006), and 6MWT (306.6 ± 6.8 m vs 354.8 ± 52.7 m, p = 0.04). Post-operative major complication rates were 8.7%; median LOHS was 7 (IQR 6) days; hospital mortality at 30 days 1.3%, 90 days 4.7% and 1 year 16%. Using subjective criteria of dyspnoea scores >3 and PS >2, 100% of high-risk patients were considered inoperable. Following optimization with Prehab 84.2% of the high-risk patients were ready to proceed with radical treatment and 52.6% with surgery, and subsequently 42.8% of patients underwent surgery. Likewise, 78.8% of patients with DLCO <50% were considered inoperable. Following Prehab 86.5% of high-risk patients were ready to proceed with radical treatment and 59.1% with surgery, and 54.6% of high-risk patients underwent surgery. In each category there were no significant differences in complications, LOHS or mortality rates between the high-risk and low-risk patients.
Interpretation
Our prospective study showed that with Prehab there was clinical and statistically significant improvement in the dyspnoea scores, PS, level of activity and frailty, particularly in the high-risk group of patients. Importantly, Prehab made previously inoperable patients operable, allowing them to safely undergo curative lung resection. This strategy helps improve resection rates and may contribute to the long term survival of lung cancer patients.
Funding
This is a Welsh Health Specialised Services Committee (WHSSC) commissioned service.
Keywords: Pulmonary rehabilitation, Prehab, Lung cancer surgery, Optimization, Inoperable, Operable, DLCO, Dyspnoea, Performance status
Keywords: Abbreviations: 6MWT, Six minute walk test; COPD, Chronic obstructive pulmonary disease; DLCO, Diffusing capacity for carbon monoxide; FEV1, Forced expiratory volume in one second; HDU, High dependency unit; IQR, Interquartile range; LOHS, Length of hospital stay; NSCLC, Non-small cell lung cancer; Ppo, Predicted post-operative function; Prehab, Pre-operative pulmonary physiotherapy; PS, Performance status; VATS, Video assisted thoracoscopic surgery; WHO, World Health Organization
Research in context.
Evidence before this study
Published peer-reviewed literature informs us that anatomical lung resection offers the best prospect of long-term survival in patients with non-small cell lung cancer. In view of the dismal prognosis and long-term survival of un-resected bronchial cancer, surgical resection should be considered for every patient with anatomically resectable disease to improve prognosis and survival. However, the demographic of patients with resectable lung cancer is getting older and patients may have frailty and smoking related cardiopulmonary disease with reduced pulmonary function. Loss of lung tissue in such patients may grossly impair post-operative ventilatory function or diffusion capability predisposing them to dyspnoea, cardiopulmonary complications and death. Hence, some patients with significant dyspnoea, poor performance status, borderline or poor pulmonary function are considered in-operable and referred for radiotherapy, systemic anticancer treatment or palliative care instead.
Added value of this study
We report the positive impact of a standardised pulmonary prehabilitation program in optimising patients with diagnosed lung cancer and facilitating safe surgical resection. Patients who were considered high risk and inoperable on subjective criteria of high dyspnoea scores, impaired performance status, and inoperable on objective criteria of borderline or poor diffusing capacity for carbon monoxide, become suitable for surgery with Prehab and underwent curative surgery with outcomes, including mortality, no different to the low risk patients. Our study is unique in that it assesses and addresses frailty in lung cancer patients making patients with frailty less vulnerable to post-surgical adverse events.
Implications of all the available evidence
Lancet Oncology published the findings of the SURVMARK-2 project in November 2019 of cancer survival in high-income countries and observed the dismal survival of lung cancer patients in the UK. Innovative measures and a multi-pronged approach is no doubt required to improve survival of lung cancer patients. Our study suggests that optimizing patients who are considered inoperable due to significant dyspnoea, poor performance status, borderline or poor pulmonary function with a standardised Prehab program and providing them access to safe, curative surgery may contribute to the multi-disciplinary approach required to address a low survival of lung cancer patients in the UK.
Alt-text: Unlabelled box
1. Introduction
Anatomical lung resection offers the best prospect of long-term survival in patients with non-metastatic NSCLC [1,2]. In view of the dismal prognosis of un-resected bronchial cancer, surgical resection should be considered for every patient with anatomically resectable disease. The preoperative physiological assessment of patients undergoing anatomical lung resection includes forced expiratory volume in one second (FEV1) and the single breath predicted DLCO [3,4]. The predicted post-operative function (ppo) of each is calculated for each patient (appendix 1) [3], [4], [5]. However, the demographic of patients with resectable NSCLC is getting older and patients may have frailty, and smoking related underlying cardiopulmonary disease, for example, chronic obstructive pulmonary disease (COPD) or emphysema with reduced pulmonary function. Loss of lung tissue in such patients may grossly impair post-operative ventilatory function or diffusion capability, predisposing them to dyspnoea, cardiopulmonary complications, and death [3]. Hence, some patients with borderline or poor pulmonary function are considered in-operable and referred for radiotherapy, systemic anticancer treatment or palliative care instead [3], [4], [5].
The British Thoracic Society (BTS) guidelines outline criteria for assessing patients for surgery and recommends taking steps to improve cardiopulmonary function in those patients with borderline or poor pulmonary function and reassessing them prior to offering lung resection [3]. Preoperative pulmonary rehabilitation (Prehab) has shown to significantly improve symptoms of dyspnoea, exercise capacity and quality of life in patients with COPD; following lung volume reduction surgery; or lung transplantation [6], [7], [8], [9], [10], [11]. The benefit of Prehab for patients undergoing lung resection for NSCLC is being increasingly recognized and forms a part of the enhanced recovery pathway [11], [12], [13], [14], [15], [16]. It is expected that Prehab may improve preoperative pulmonary function. However, in high-risk patients considered inoperable due to symptoms of dyspnoea, impaired PS, frailty, and borderline or poor lung function, the beneficial impact of Prehab in optimizing patients to receive curative surgery with a reduced risk of pulmonary complications and mortality, has not been clearly demonstrated [12,[16], [17], [18], [19]]. In the current clinical environment Prehab is not standardized as there are different protocols in use [6,18]. Some centres do not apply routine Prehab due to concerns related to delaying of surgical resection as evidence for prehab in NSCLC, although cautiously optimistic from small and inconclusive studies, lacks the solid evidence to demonstrate the benefits of Prehab, especially in patients considered functionally inoperable [12,20]. At our institution we have a standardized Prehab protocol, which has been running successfully since 2017 [15]. All data is collected prospectively to monitor the impact of Prehab on patients being referred for surgery, especially patients considered inoperable on subjective criteria of dyspnoea, impaired PS, level of activity and frailty, and objective criteria of borderline or poor pulmonary function [3], [4], [5].
The aim of this study was to review our prospectively collected data and determine whether Prehab makes patients who were considered inoperable on subjective criteria of dyspnoea and impaired PS, and inoperable on objective criteria due to borderline or poor DLCO, become suitable for surgery. Following this, the aim was to assess whether these high risk patients could safely receive curative surgery with outcomes, namely post-operative complications and mortality, no different to the low risk patients.
2. Material and methods
Our two year prospective, observational, study was carried out between January 2017 and December 2018 at the Cardio-Thoracic Surgery Rehabilitation Centre at Morriston Hospital in Swansea, Wales, United Kingdom. We used the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting our observational study [21].
2.1. Ethics
The project was registered with the local NHS research and development department of the Health Board under the guidance of the NHS Research Ethics Centre [22], which confirmed that ethical approval was not required for this project. Subsequently, all research governance and ethical considerations were adhered to and the study was performed in accordance with the Declaration of Helsinki.
2.2. Study population
The service received 306 referrals for pre-treatment optimization of patients with Prehab from the lung cancer Multi-disciplinary Teams (MDTs) across South West Wales. 38.2% (n = 117) of the referrals were from the Swansea lung MDTs, 41.2% (n = 126) from the Hywel Dda lung MDTs and 20.6% (n = 63) from Princess of Wales lung MDT. The referral criteria were >1 Medical Research Council (MRC) dyspnoea; or >1 World Health Organization (WHO) performance status (PS); age > 70 years or frailty index >3; borderline or poor pulmonary function (FEV1 or DLCO); patients currently smoking; sedentary patients despite having adequate FEV1 or DLCO; or on clinical decision made by the treating physician who deemed that the patient would benefit from Prehab prior to treatment, for example, for psychological or other reasons.
2.2.1. Patients excluded
Of the 306 referred patients 8.5% (n = 26) of patients diagnosed with other cancers or benign diseases were excluded from the study (Fig 1). These were 5.2% (n = 16) with metastatic lung cancer; 0.6% (n = 2) with mesothelioma; 0.3% (n = 1) with sarcoma; 1.3% (n = 4) with mediastinal tumours, and 1.0% (n = 3) with a benign pathology (Fig 1). Of the remaining 280 patients with diagnosed or suspected lung cancer, 22.9% (n = 64) of patients were excluded from further analysis as 8.6% (n = 24) of patients declined the offer of Prehab; 6.8% (n = 19) did not attend for assessment for Prehab; 0.4% (n = 1) had a high cardiovascular risk for Prehab; 1.1% (n = 3) died before they could attend for assessment, and 6.1% (n = 17) proceeded straight to treatment or were referred to palliative care without Prehab (Fig 1).
Fig. 1.
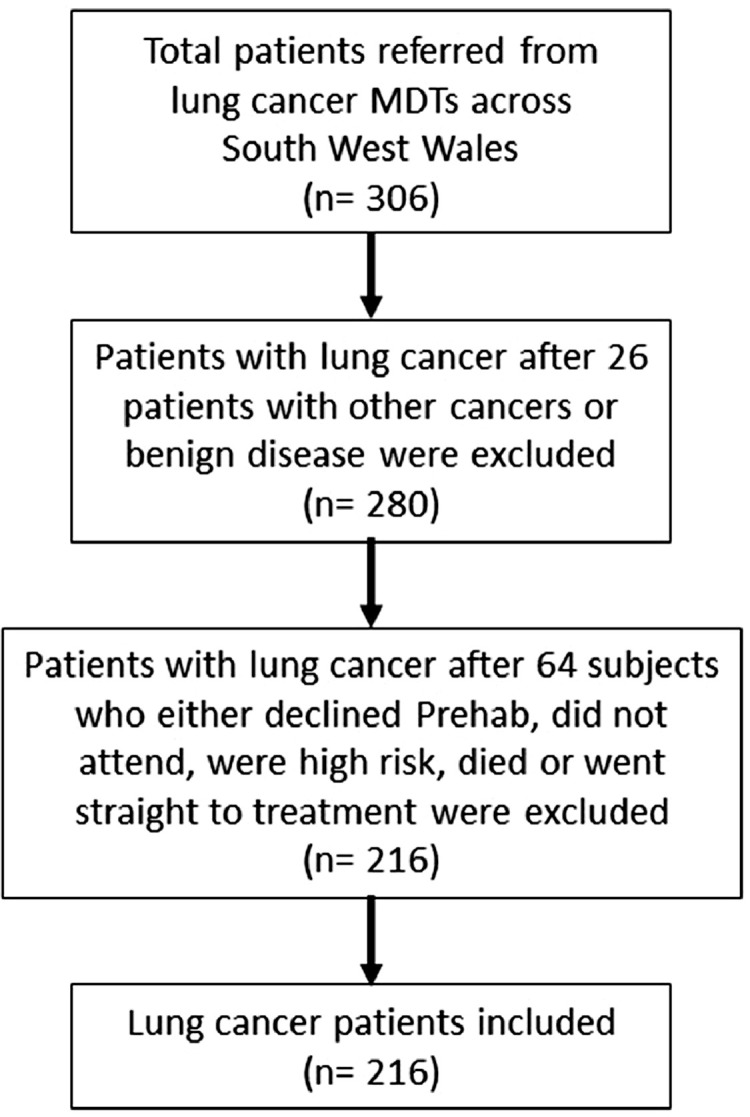
Study flow chart.
2.3. Participants
The baseline characteristics of the remaining 216 patients with a diagnosis of lung cancer are summarized in Table 1. For each patient the diagnosis and stage of lung cancer were validated with the data documented on the Welsh Clinical Portal. The IASLC (International Association for the Study of Lung Cancer) lung cancer staging system, 8th edition, was used for pre-treatment clinical staging of patients with suspected or diagnosed lung cancer (Table 1) [23]. Assessment of patients and data collection prior to commencing Prehab and on completion of Prehab were carried out by our cardiothoracic physiotherapists. These included recording the impact of Prehab on our patients’ WHO performance status, MRC dyspnoea score, six-minute walk test (6MWT), level of activity and frailty prior to and following Prehab. The duration of Prehab was recorded as number of sessions (mean ± 95% confidence interval), and our Prehab protocol is described below. Assessment for frailty were carried out using the Canadian 9-point Clinical Frailty Scale at first assessment and on completion of Prehab (Table 2) [24]. On this scale each level of frailty was ascribed an incremental number to grade the level of frailty as an index. Patients with an index of greater than 3 were considered as having clinically relevant frailty. Level of activity was measured using the Borg scale of perceived exertion and measurements recorded as sedentary, moderately active or active and supplemented by the 6MWT, which was performed according to ATS/ERS guidelines modified to a 10 m corridor instead of 30 m [7]. Data was collected at baseline and then after every second session to assess for improvement in activity levels. Assessment for risk of death following surgery were carried out using the recommended thoracoscore [3] and described previously [15]. Current smokers were provided with smoking cessation advice. Data on patients thus optimized with Prehab who then proceeded to surgery or for radical radiotherapy, systemic anticancer treatment or palliative care were collected. Different patients had different rehabilitation requirements. Hence, as patients demonstrated improvement in their dyspnoea score, PS and activity levels, thus meeting the criteria for rehabilitation, were taken for surgery. Patients with a greater risk of disease progression proceeded to treatment without completing their final assessment.
Table 1.
Baseline patients’ characteristics of 216 patients with lung cancer for Prehab.
| Parameter | Descriptor | n | % |
|---|---|---|---|
| Gender | Male | 109 | 50.5 |
| Female | 107 | 49.5 | |
| Age in years | Mean (± 95% CI) | 71.7 ± 1.1 | |
| Referral to assessment days | Mean (± 95% CI) | 15.4 ± 1.4 | |
| Stage of lung cancer | 1A1 | 29 | 13.4 |
| 1A2 | 38 | 17.6 | |
| 1A3 | 18 | 8.3 | |
| 1B | 52 | 24.1 | |
| IIA | 6 | 2.8 | |
| IIB | 24 | 11.1 | |
| IIIA | 33 | 15.3 | |
| IIIB | 10 | 4.6 | |
| IVA | 5 | 2.3 | |
| No stage | 1 | 0.5 | |
| Dyspnoea score | 0–2 (acceptable fitness for surgery) | 86 | 40.4 |
| 3–4 (high level of surgical risk) | 127 | 59.6 | |
| Performance status | 0–1 (acceptable fitness for surgery) | 96 | 45.1 |
| 2–4 (high level of surgical risk) | 117 | 54.9 | |
| Level of activity | Sedentary | 173 | 80.8 |
| Moderately active (acceptable fitness) | 39 | 18.2 | |
| Very active | 2 | 0.9 | |
| Frailty | 1–3 (acceptable fitness for surgery) | 105 | 49.5 |
| 4–7 (venerable for adverse events) | 107 | 50.5 | |
| Smoking habit | Current smoker | 43 | 19.9 |
| Ex- smoker | 144 | 66.7 | |
| Never smoked | 29 | 13.4 | |
| Smoking cessation advice | Accepted | 30 | 69.8 |
| Declined | 6 | 13.9 | |
| No advice | 7 | 16.3 | |
| FEV1 (litres) | Mean (± 95% CI) | 1.7 ± 0.1 | |
| DLCO (% predicted) | Mean (± 95% CI) | 66.4 ± 0.1 | |
| Thoracoscore (%) | Mean (± 95% CI) | 2.8 ± 0.5 | |
| Six minute walk distance (mts) | Mean (± 95% CI) | 306.6 ± 20.6 | |
| Not fit for | Surgery | 127 | 58.8 |
| Radiotherapy | 41 | 19.6 | |
| Assessment to surgery days | Mean (± 95% CI) | 39.0 ± 7.0 | |
| Sessions of Pre-hab | Mean (± 95% CI) | 3.1 ± 0.6 |
[Values are numbers (percentage) unless otherwise stated]. (CI= confidence interval). Level of activity was measured using the Borg scale of perceived exertion and assessments recorded as sedentary, moderately active or active.
Table 2.
Assessment tools: Canadian Study of Health and Ageing Clinical Frailty Scale [24] and Clavien-Dindo grading of complications according to severity of complications [25].
| Canadian study of health and ageing clinical frailty scale | ||
|---|---|---|
| Index | Fitness | Definition |
| 1 | Very fit | People who are robust, active, energetic and motivated. They tend to exercise regularly and are among the fittest for their age. |
| 2 | Fit | People who have no active disease symptoms but are less fit than category 1. Often, they exercise or are very active occasionally. |
| 3 | Managing well | People whose medical problems are well controlled, even if occasionally symptomatic, but often are not regularly active beyond routine walking. |
| 4 | Very mild frailty | Previously vulnerable, this category marks early transition from complete independence. While not dependent on others for daily help, often symptoms limit activities e.g., ‘slowed up’ and/or being tired during the day. |
| 5 | Mild frailty | People who often have more evident slowing, and need help with high order instrumental activities of daily living. Typically mild frailty impairs shopping and walking outside alone, meal preparation, medications and begins to restrict light housework. |
| 6 | Moderate frailty | People who need help with all outside activities and with keeping house. Inside, they often have problems with stairs and need help with bathing and might need minimal assistance with dressing. |
| 7 | Severe frailty | Completely dependent for personal care, from whatever cause (physical or cognitive). Even so, they seem stable and not at high risk of dying (within 6-months). |
| 8 | Very severe frailty | Completely dependent for personal care and approaching end of life. Typically they could not recover even from a minor illness |
| The Clavien-Dindo Classification [Grades 1 and 2 are minor complications and grades ≥ 3 major complications] | ||
|---|---|---|
| Grade | Description | |
| Grade I | Any deviation from the normal postoperative course without need for pharmacological treatment or surgical intervention. Allowed therapeutic regimens are antiemetics, antipyretics, diuretics, electrolytes, wound infection and physiotherapy. | |
| Grade II | Pharmacological treatment other than grade I. Atrial fibrillation, atelectasis, lower respiratory tract infection and blood transfusion are included. | |
| Grade III | Require surgical, bronchoscopic, endoscopic or radiological intervention. | |
| IIIa | Intervention not under general anaesthesia. | |
| IIIb | Intervention under general anaesthesia. | |
| Grade IV | Life threatening complication requiring intensive care management. | |
| IVa | Single organ dysfunction. | |
| IVb | Multi organ dysfunction. | |
| Grade V | Death of patient. | |
Surgical resection performed included lobectomy, segmental resection, pneumonectomy, wedge resection, endobronchial excision of tumour or an open and shut thoracotomy either via a minimally invasive approach or a standard thoracotomy (Table 1). Following surgery the safety parameters measured were major complications rates, length of hospital stay (LOHS), in-hospital mortality, and the 30 days, 90 days and 1 year mortality rates. In order to rank complications in order of severity for each patient in an objective and reproducible manner, the extended Clavien-Dindo classification for grading complications was used (Table 2) [25]. Grades 1 and 2 were considered minor complications and grades 3a to 5 major complications (Table 1). Adverse Cardio-respiratory events relating to the patients physical conditioning were additionally assessed. Outcomes were compared between patients who proceeded to Prehab and received subsequent treatment with those who declined or did not receive Prehab and went straight to treatment. Since patients in the latter group were not assessed, baseline parameters of this group were not available for comparison.
2.4. Study
To test our hypothesis patients were categorized subjectively as high-risk if their dyspnoea scores were > 3 and PS >2, or low-risk if their dyspnoea scores were <3 and PS <2, and compared. To assess meaningfully whether high risk patients thus categorized, could safely receive curative surgery with outcomes no different to the low risk ones, patients with intermediate risk and possibility of having overlapping subjective dyspnoea and PS scores namely, patients with dyspnoea scores 3 and PS 2 were excluded. These were patients who walked slower than most people on the level, or had to stop after 15 min or were capable of self-care but were unable to carry out any work activity. Likewise, those objectively considered as high-risk for adverse post-operative outcomes, as described by the BTS guidelines due to DLCO <50%, were compared with patients having a low-risk with DLCO >80% [3,5]. Patients with confounding DLCO between 50% and 80%, considered intermediate risk, were excluded. For each category the percentage of patients unfit to proceed with surgery at first assessment, and the percentage of patients suitable to proceed with surgery following Prehab were determined. Outcomes in terms of post-operative complications, LOHS and mortality were compared between the high and low risk patients in each category.
2.5. Pre-operative pulmonary rehabilitation protocol
Our Prehab program, delivered by trained cardiothoracic physiotherapists, comprised of four main elements [15], and were based on established guidelines [[7], [8], [9],18,19,[26], [27], [28], [29]]. Prehab was given over 2–4 weeks with supervised two weekly sessions of 70 min each at our Prehab centre or outreach units, along with exercises patients could carry out at home three times daily. The Prehab exercises were as follows.
2.5.1. Respiratory muscle training and breathing exercises
Comprised of strengthening the patients muscles of respiration; improving their diaphragmatic breathing and control; coordinating their breathing process; and re-training their respiratory muscles with the use of incentive spirometry. Incentive spirometry facilitates sustained maximal inspiration and improves the strength of respiratory muscles and sputum expectoration [26]. All patients performed these exercises three times daily for the duration of Prehab. Effective airway clearance techniques, to maintain a clear respiratory tract following surgery, were taught and performed three times daily. In addition, breathing exercises and re-training included techniques for muscle relaxation and control.
2.5.2. Cardiovascular exercises
To help improve generalized muscle weakness and pulmonary mechanics, stationary cycle ergometry and walking exercise training were also performed, whilst monitoring their heart rate, blood pressure and oxygen saturations, and were in keeping with The FITT (Frequency, Intensity, Type and Time) principles of training [29]. These were performed twice weekly, as a systemic program of aerobic exercise training. The Rate of Perceived exertion or Borg scale was used twice at every session to measure the intensity at which each patient was working and to guide them to increase or decrease their effort as required. Heart rate was also used using the Karvonen formula. We would calculate the maximum heart rate and then using their resting heart rate we calculated the patient's heart rate reserve. The heart rate reserve represented the cushion heartbeats available for exercise. We then calculated each patient's aerobic training heart rate range so each patient worked between 60% and 70%, which is considered ideal for cardiovascular conditioning by the British Cardiovascular Society (BACPR). The heart rate reserve was multiplied by 0.6 and 0.7 and then resting heart rate added to these numbers to create each individual patients training zones.
Patients were encouraged to carry out twice daily walking exercises and stair climbing exercises at home. To facilitate early post-operative ambulation, all patients received training enabling them to maintain activities of daily living following surgery. These included shoulder movement exercises, movements of upper and lower limb joints, standing up from a sitting position and walking.
2.5.3. Education
Health education and smoking cessation advice was given to all current smokers [27,28]. Where necessary nicotine replacement therapy was provided to patients if required. The duration of smoking cessation was at least two weeks prior to the date of surgery. Provision of smoking cessation support was maintained after surgery.
2.5.4. Pharmacology agents
Where necessary bronchodilator therapy was provided to the patients if required.
Following the initial evaluation of patients, Prehab was given addressing all four elements whilst being mindful of those patients with lung cancer the urgency of expediting their surgery in a timely manner. Hence, as patients met the criteria for fulfilling rehabilitation, they were taken for surgery. Patients who went straight to surgery were provided with advice and education regarding smoking cessation, physiotherapy and rehabilitation prior to surgery as described above [15].
2.6. Statistical analysis
The data are expressed as percentage (numbers), and means and ± 95% confidence interval (± 95% CI) or median and interquartile range (IQR). As this was not a randomized control trial for which odds ratio data would have been valuable, but a prospective case study with modest numbers, either the chi-square test with Yates correction, and where the sample size was small, the Fisher's exact test were used to evaluate differences in categorical clinical parameters and outcomes. For outcomes of normally distributed variables the Student's t-test was used for comparison of the mean values and standard deviation of two groups, otherwise the Mann-Whitney U nonparametric test was used. Paired samples t-test was used to compare the means of two sets of scores that were directly related to each other where sample size and available data allowed such comparison. For missing data, for example, where patients after attending initial few sessions had to proceed to surgery quickly or those patients exercising at home where they could not travel back for reassessment of their objective measures, improvement data was not available for paired sample t-test and improvement for the cohort or sub-groups were assessed. Statistical analysis of the data was performed using IBM SPSS Statistics, 2018 software.
2.6.1. Role of funding
Our Prehab program is commissioned by the Welsh Health Specialised Services Committee (WHSSC), which is responsible for the joint planning of Specialised and Tertiary Services in Wales. The funding source had no involvement in the study design, collection, analysis, interpretation or writing of the manuscript for publication.
3. Results
A total of 216 patients, mean age of 71.7 ± 1.1 years and 50.5% (n = 109) men, were assessed by the physiotherapy team to undergo Prehab. Their baseline characters are described in Table 1.
3.1. Baseline results for the whole cohort
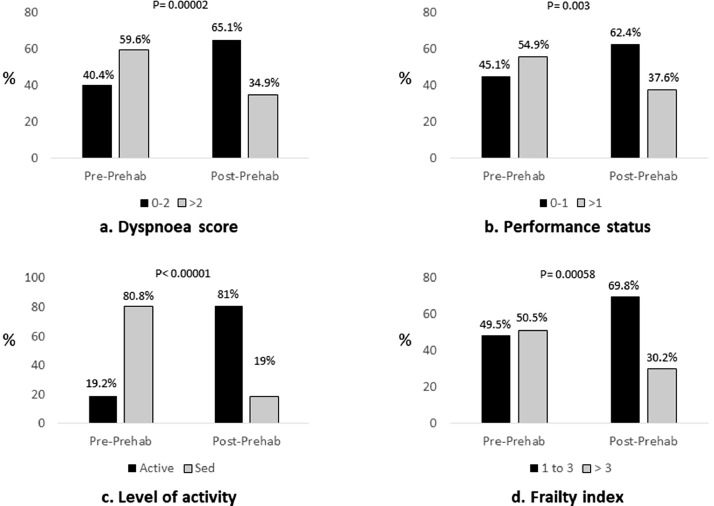
Following an average of 3.1 ± 0.6 Prehab sessions over 39.0 ± 7.0 days there was a significant improvement in parameters tested (Fig 2).
Fig. 2.
Impact of Prehab on (a) dyspnoea score, (b) performance status, (c) level of activity and (d) frailty.
Sed = sedentary. P values were calculated using Chi square test with Yate's correction and excluding unknown data.
3.1.1. Dyspnoea and PS
There was clinical and statistically significant improvement in the MRC dyspnoea scores of patients following Prehab (Fig 2a). Prior to Prehab 40.4% of patients had scores of ≤2 and 59.6% scores ≥3. Following Prehab 65.1% of patients had scores ≤2 and 34.9% scores ≥3 suggesting significant improvement in breathlessness (p = 0.00002) (Fig 2a). Likewise, 45.1% of patients were in PS of ≤1 and 54.9% with PS ≥2 prior to Prehab. Following Prehab there was a significant improvement in the PS with 62.4% of patients now in PS ≤1 vs. 37.6% in PS ≥ 2 (p = 0.003) (Fig 2b).
3.1.2. Level of activity
Lung cancer patients being considered for treatment often experience difficulties in being active. 80.8% of our patients were found to have a sedentary life style at assessment (Fig 2c). Following a period of Prehab there was clinical and statistically significant improvement in the level of activity of patients (Fig 2c) with 81% (n = 93) of patients becoming moderately active or active.
3.1.3. Frailty
Frailty is increasingly being recognized as an independent risk factor for adverse postoperative outcomes, namely, major complications, mortality and protracted length of hospital stay (LOHS) and institutional discharge [30,31]. At assessment 50.5% of patients were found to have a frailty index of greater than 3 (Fig 2d). Following Prehab there was clinical and statistically significant improvement in the frailty index of patients with 69.8% having an index of 1–3 (Fig 2d).
3.1.4. Six-minute walk test (6MWT)
The mean 6MWT distance prior to and following Prehab were 306.6 ± 6.8 m vs. 341 ± 113, p = 0.04) suggesting a significant improvement in the 6MWT of patients receiving Prehab.
3.1.5. Patients optimized and ready to commence treatment
At the time of assessment 48.2% of the total cohort of patients were considered unsuitable for any form of radical treatment namely, surgery or radiotherapy, and 58.8% not fit for surgery (Table 1). Following Prehab 93.1% vs. 48.2% (p<0.05) were ready to go forward for radical treatment compared to before Prehab respectively. Importantly, compared with patients prior to commencing with Prehab, there was a significant increase, from 41.2% patients not fit for surgery to 75.8% patients ready to proceed with surgery following Prehab (p = 0.00001). Following Prehab 69.4% (n = 150) of patients proceeded to surgery for lung cancer. Majority (70%) underwent anatomical lung resection namely, lobectomy (58.7%), segmental resection (9.3%) or pneumonectomy (2.0%) (Table 3). However, despite optimization with Prehab one patient, at the time of anaesthetic induction, failed a trial of one lung ventilation and therefore, could not proceed to surgical resection. Following Prehab 21.8% (n = 47) of patients proceeded to radiotherapy or systemic anti-cancer treatment.
Table 3.
Outcomes for the 216 patients with lung cancer following Prehab.
| Parameter | Descriptor | n | % |
|---|---|---|---|
| Outcome | Surgery | 150 | 69.4 |
| Radiotherapy | 26 | 12.1 | |
| Chemotherapy | 17 | 7.9 | |
| Palliative care | 8 | 3.7 | |
| Died before any treatment | 2 | 0.9 | |
| Declined treatment or none | 13 | 6.0 | |
| Surgery | Lobectomy | 88 | 58.7 |
| Pneumonectomy | 3 | 2.0 | |
| Segmentectomy | 14 | 9.3 | |
| Wedge resection | 39 | 26.0 | |
| Endobronchial excision | 2 | 1.3 | |
| Failed trial of OLV | 1 | 0.7 | |
| Thoracotomy (open and close) | 3 | 2.0 | |
| Surgical approach | Endobronchial excision | 2 | 1.3 |
| VATS | 18 | 12 | |
| Hybrid VATS | 74 | 49.3 | |
| Thoracotomy | 55 | 36.7 | |
| Failed OLV | 1 | 0.7 | |
| Post-operative cardiorespiratory adverse events | Cardiac: | ||
| Atrial fibrillation | 17 | 11.3 | |
| Major cardiac events | 3 | 2.0 | |
| DVT | 1 | 0.7 | |
| Hypotension/hypertension | 3 | 2.0 | |
| Respiratory: | |||
| Retained secretions | 24 | 16.0 | |
| Lobar collapse/consolidation | 9 | 6.0 | |
| Respiratory infection | 4 | 2.7 | |
| Air leak | 2 | 1.3 | |
| Prolonged chest tube drainage | 2 | 1.3 | |
| Surgical emphysema (mild) | 3 | 6.7 | |
| Pneumothorax | 1 | 2.2 | |
| Post-operative Clavien-Dindo Grade of complications for each patient | None | 73 | 48.6 |
| Minor: 1 | 33 | 22.0 | |
| 2 | 29 | 19.3 | |
| Major: 3a and 3b | 9 | 6.0 | |
| 4a | 4 | 2.7 | |
| 5 | 2 | 1.3 | |
| Post-operative mortality | Hospital mortality | 2 | 1.3 |
| 30 day mortality | 2 | 1.3 | |
| 90 day mortality | 7 | 4.7 | |
| 1 year mortality | 24 | 16 | |
| Recidivism (step up care) | 4 | 2.7 | |
| Post-operative length of stay in days | HDU days [median (IQR)] | 3 (3) | |
| Ward days [median (IQR)] | 3 (3) | ||
| Hospital days [median (IQR)] | 7 (6) |
Values are numbers (percentage) unless otherwise stated.
(HDU= high dependency unit; IQR= interquartile range; OLV= one lung ventilation; VATS= video assisted thoracoscopic surgery).
3.1.6. Post-operative complications
The post-operative cardiorespiratory adverse events namely, atrial fibrillation occurred in 11.3% of patients and 2.0% of patients experienced a major cardiac event (Table 3). 29.6% of patients developed respiratory complications (Table 3). Since patients may experience multiple events, in order to rank complications in an objective and reproducible manner, the post-operative complications were graded using the extended Clavien-Dindo grading [25]. Grades 1 and 2 were considered minor complications and grades 3a to 5 major complications. The major complication rate was 8.7% (n = 13/150) (Table 3).
3.1.7. Post-operative mortality
The in-hospital mortality was 1.3% (Table 3). The 30 day mortality was 1.3%, 90 day mortality 4.7%, and 1 year mortality 16% and in keeping with national UK standards.
3.1.8. Length of stay
The overall median length of hospital stay was 7 (IQR 6) days. The median length of stay in the HDU was 3 (IQR 3) days, and in the ward 3 (IQR 3) days (Table 3). Patients requiring stepped up care i.e., the post-operative recidivism rate in our study was 2.7% (n = 4) and low (Table 3).
3.2. Comparison between those who did and did not receive Prehab
A total of 60 patients (mean age 69.3 ± 2.7 years and 58% men) declined Prehab or did not attend Prehab and went straight to treatment or declined treatment (Table 4). In this group a significantly lower percentage of patients (30%) underwent surgery compared to 69.4% of patients who received Prehab (p<0.00001), and 38.9% of the surgical procedures were lobectomies. The median length of hospital stay was significantly longer for those who did not receive Prehab compared to those who did namely, 9 (IQR 4.5) days vs 7 (IQR 6) days (p = 0.03) and there was a trend towards higher rate of major post-operative complications in those who did not receive Prehab (Table 4).
Table 4.
Comparison between those who did and did not receive Prehab.
| Parameter | Did not receive Prehab | Received Prehab | P | |
|---|---|---|---|---|
| Gender | M:F | 35:25 | 109:107 | ns |
| Age (years) | Mean (± 95%CI) | 69.67 ± 2.7 | 71.7 ± 1.09 | 0.06 |
| Outcome | Surgery | 18 (30) | 150 (69.4) | 0.00001 |
| Radiotherapy | 14 (23.3) | 26 (12.0) | ||
| Chemotherapy | 12 (20) | 17 (7.9) | ||
| Palliative care | 1 (1.7) | 8 (3.7) | ||
| Died before treatment | 3 (5) | 2 (0.9) | ||
| Declined / no treatment | 12 (20) | 13 (6.0) | ||
| Type of surgery | Lobectomy | 7 (38.9) | 88 (58.7) | ns |
| Segmentectomy | 3 (16.7) | 14 (9.3) | ||
| Pneumonectomy | 1 (5.6) | 3 (2.0) | ||
| Wedge resection | 7 (38.9) | 39 (26.0) | ||
| Endobronchial excision | – | 2 (1.3) | ||
| Thoracotomy | – | 3 (2.0) | ||
| Failed trial of one lung ventilation | – | 1 (0.7) | ||
| Length of hospital stay | Median (IQR) | 9 (4.5) | 7 (6) | 0.03 |
| Post-operative Complications | Major | 4 (22.2) | 13 (8.7) | 0.09 |
| Nil | 7 (38.9) | 73 (48.7) | ||
| Post-operative Mortality | In hospital | 0 | 2 (1.3) | ns |
| 30 days | 0 | 2 (1.3) | ns | |
| 90 days | 0 | 7 (4.7) | ns | |
| 1 year mortality | 5 (27.8) | 24 (16.0) | ns |
Values are numbers (percentage) unless otherwise stated. P values were calculated using Fishers exact test for categorical data and Mann Whitney U test for continuous variables by excluding unknown data.
CI= confidence interval; ns= not significant.
3.3. Impact of Prehab on patients considered inoperable
3.3.1. Subjective criteria
Patients with dyspnoea scores of 4 and PS of 3 and 4 were considered as in-operable due to a prohibitively high risk of post-operative complications, poor quality of life and mortality. These patients are generally referred for radiotherapy, chemotherapy or palliative care instead. From a total of 216 patients assessed by the physiotherapy team to undergo Prehab, 12.9% (n = 28) of patients had dyspnoea scores of > 3 and PS of 3 or 4 (Table 5). For meaningful assessment to determine the impact of Prehab on in-operable patients the patients were divided into two groups (Table 5). (i) High-risk group of patients with high dyspnoea scores of > 3 and high PS of >2, and (ii) low-risk group of patients with low dyspnoea scores of <2 and low PS of < 2.
Table 5.
Baseline characteristics of high risk patients compared with low risk patients.
| Parameter | Dyspnoea <3 and PS <2 vs. dyspnoea >3 and PS >2 |
DLCO 〈50% vs. DLCO 〉 80% |
|||||
|---|---|---|---|---|---|---|---|
| Low risk [Dyspnoea <3 and PS <2] (n-64) |
High risk [Dyspnoea >3 and PS >2] (n = 28) |
P | Low risk [DLCO > 80%] (n = 31) |
High risk [DLCO <50%] (n = 33) |
p | ||
| Gender | M:F | 30:34 | 15:13 | ns | 19:12 | 18:15 | ns |
| Age (years) | Mean (± 95%CI) | 72.0 ± 2.08 | 73.8 ± 2.39 | ns | 72.4 ± 3.03 | 69.8 ± 2.77 | ns |
| Performance status | 0–1 | 61 (95.3) | 4 (14.3) | 0.00001 | 19 (53.3) | 15 (45.5) | ns |
| 2–4 | 3 (4.7) | 24 (85.7) | 11 (36.7) | 18 (54.5) | |||
| Dyspnoea score | 0–2 | 64 (100) | 0 | n/a | 17 (56.7) | 12 (36.4) | ns |
| 3–5 | 0 | 28 (100) | 13 (43.3) | 21 (63.6) | |||
| Level of activity | Sedentary | 33 (51.6) | 26 (92.9) | 0.0001 | 21 (70.0) | 27 (81.8) | ns |
| Active | 31 (48.4) | 2 (7.1) | 9 (30.0) | 6 (18.2) | |||
| Frailty | 1–3 | 58 (92.1) | 2 (7.1) | 0.00001 | 22 (75.9) | 14 (42.4) | 0.01 |
| 4–7 | 5 (7.9) | 26 (92.9) | 7 (24.1) | 19 (57.8) | |||
| Smoking habit | Current smoker | 7 (10.9) | 7 (25) | ns | 0 (0) | 6 (18.2) | 0.02 |
| Previous or never smoked | 57 (89.1) | 21 (75) | 31 (100) | 27 (81.8) | |||
| FEV1 (litres) | Mean (± 95%CI) | 1.79 ± 0.19 | 1.78 ± 0.29 | ns | 2.0 ± 0.19 | 1. 4 ± 0.21 | 0.000014 |
| DLCO (% predicted) | Mean (± 95%CI) | 71.4 ± 5.74 | 62.95 ± 5.19 | 0.03 | 94.5 ± 13.82 | 42.1 ± 2.29 | 0.00001 |
| Thoracoscore (%) | Mean (± 95%CI) | 2.06 ± 0.54 | 4.76 ± 1.86 | 0.0003 | 2.1 ± 0.8 | 3.3 ± 2.1 | Ns |
| 6MWT (mts) | Mean (± 95%CI) | 388.35 ± 29.19 | 139.61± 41.19 | 0.00001 | 376.5 ± 56.396 | 301 ± 44.695 | 0.023 |
| Assessment to surgery days | Mean (± 95%CI) | 35.3 ± 14.36 | 65.75 ± 51.85 | 0.03 | 39.75 ± 24.9 | 35.9 ± 14.6 | Ns |
| Considered not fit for | Surgery | 13 (20.3) | 28 (100) | 0.00001 | 11 (35.5) | 26 (78.8) | 0.0008 |
| Radiotherapy | 5 (7.8) | 10 (38.5) | 0.001 | 3 (9.7) | 11 (34.4) | 0.03 | |
Values are numbers (percentage) unless otherwise stated. P values were calculated using Fishers exact test for categorical data and t-test for independent continuous variables by excluding unknown data.
CI= confidence interval; IQR= interquartile range; ns= not significant, n/a = not applicable; 6MWT= six minute walk test.
High percentage of patients in the high-risk group led a sedentary life style compared to the low-risk group patients, had significantly high frailty indices, low DLCO, 6MWT and high thoracoscores, each suggesting higher risk for post-operative adverse events and mortality (Table 5). A significantly higher proportion of patients in the high-risk group were, therefore, not ready to proceed with radical treatment, including surgery, compared with patients in the low-risk group.
With Prehab there was clinical and statistically significant improvement in the dyspnoea, PS, frailty index and level of activity in the high-risk group of patients (Table 6). Following Prehab 84.2% of high-risk group patients were ready to proceed with radical treatment and 52.6% with surgery. Indeed, following optimization with Prehab 42.8% of high-risk patients, who were deemed subjectively to be in-operable, underwent surgery safely (Table 7). Following surgery there were no significant differences in the length of stay in the high dependency unit (HDU) or the ward, in major complications or mortality in the high-risk group of patients compared with the low-risk group of patients (Table 7). Thus, optimization with Prehab allowed a high percentage of high-risk in-operable patients to become operable and undergo curative surgery safely.
Table 6.
Optimization of high risk patients with Prehab.
| Parameter | Dyspnoea >3 and PS > 2 |
DLCO <50% |
|||||
|---|---|---|---|---|---|---|---|
| Prior to Prehab | Following Prehab | p | Prior to Prehab | Following Prehab | p | ||
| Performance status | 0–1 | 4 (14.3) | 7 (50) | 0.02 | 15 (45.4) | 14 (66.7) | ns |
| 2–4 | 24 (85.7) | 7 (50) | 18 (54.6) | 7 (33.3) | |||
| Dyspnoea score | 0–2 | 0 | 5 (35.7) | 0.008 | 12 (36.4) | 12 (57.1) | ns |
| >2 | 28 (100) | 14 (64.3) | 21 (63.6) | 9 (42.9) | |||
| Level of activity | Sedentary | 26 (92.9) | 6 (50) | 0.004 | 27 (81.8) | 2 (11.1) | 0.00001 |
| Active | 2 (7.1) | 6 (50) | 6 (18.2) | 16 (88.9) | |||
| Frailty | 1–3 | 2 (7.1) | 5 (35.7) | 0.03 | 14 (42.4) | 16 (76.2) | 0.02 |
| 4–7 | 26 (92.9) | 9 (64.3) | 19 (57.6) | 5 (23.8) | |||
| Considered not fit for | Surgery | 28 (100) | 9 (47.4) | <0.05 | 26 (78.8) | 9 (40.9) | 0.009 |
| Radiotherapy | 10 (38.5) | 3 (15.8) | ns | 11 (34.4) | 3 (13.6) | ns | |
Values are numbers (percentage) unless otherwise stated. P values were calculated using Fishers exact test for categorical data by excluding unknown data.
Table 7.
Outcomes following Prehab and surgery in the high risk patients compared with low risk patients.
| Parameter | Dyspnoea <3 and PS <2 vs. dyspnoea >3 and PS >2 |
DLCO 〈50% vs. DLCO 〉 80% |
|||||
|---|---|---|---|---|---|---|---|
| Low risk [Dyspnoea <3 and PS <2] (n-64) |
High risk [Dyspnoea >3 and PS >2] (n = 28) |
P | Low risk [DLCO > 80%] (n = 31) |
High risk [DLCO <50%] (n = 33) |
p | ||
| Outcome | Surgery | 55 (85.9) | 12 (42.8) | 0.0001 | 24 (77.4) | 18 (54.6) | 0.07 |
| Radiotherapy | 2 (3.1) | 6 (21.4) | 3 (9.7) | 7 (21.2) | |||
| Chemotherapy | 4 (6.25) | 2 (7.14) | 2 (6.5) | 5 (15.2) | |||
| Palliative care | 1 (1.56) | 5 (17.9) | 1 (3) | 3 (9.1) | |||
| Died before treatment | – | – | 1 (3) | – | |||
| Declined / no treatment | 2 (3.1) | 3 (10.7) | |||||
| Type of surgery | Lobectomy | 38 (70.4) | 5 (41.7) | 0.008 | 16 (66.7) | 6 (35.3) | ns |
| Segmentectomy | 6 (11.1) | 1 (8.3) | 1 (4.2) | 2 (11.8) | |||
| Pneumonectomy | 3 (5.6) | – | – | – | |||
| Wedge resection | 6 (11.1) | 5 (41.7) | 7 (29) | 9 (52.9) | |||
| Endobronchial excision | 1 (1.9) | – | – | – | |||
| Thoracotomy | – | 1 (8.3) | – | – | |||
| Surgical approach | Minimally invasive | 32 (59.3) | 9 (69.2) | ns | 16 (66.6) | 8 (47.1) | ns |
| Thoracotomy | 22 (40.7) | 4 (30.8) | 8 (33.4) | 9 (52.9) | |||
| Stay in HDU | Median (IQR) | 3 (2) | 3 (5) | ns | 3(2) | 4 (3.5) | 0.02 |
| Stay in Ward | Median (IQR) | 3 (3) | 3 (3) | ns | 3 (5) | 5 (5) | 0.03 |
| Length of hospital stay | Median (IQR) | 6 (5) | 7 (5.7) | ns | 6 (3.7) | 10 (8) | ns |
| Post- operative Complications | Major | 4 (7.3) | 2 (16.6) | ns | 3 (12.5) | 2 (11.8) | ns |
| Recidivism | 1 (1.8) | 1 (8.3) | ns | 1 (5.9) | 1 (4.2) | ns | |
| Post-operative Mortality | In hospital | 0 | 0 | ns | 0 | 0 | ns |
| 30 days | 0 | 0 | ns | 0 | 1 (5.9) | ns | |
| 90 days | 2 (3.6) | 1 (8.3) | ns | 0 | 2 (11.8) | ns | |
| 1 year | 7 (12.7) | 2 (16.7) | ns | 2 (8.3) | 5 (29.4) | ns | |
Values are numbers (percentage) unless otherwise stated. Minimally invasive approach included VATS and hybrid VATS. P values were calculated using Fishers exact test for categorical data and t-test for independent continuous variables by excluding unknown data.
CI= confidence interval; IQR= interquartile range; ns= not significant, n/a = not applicable; VATS= video assisted thoracoscopic surgery; 6MWT= six minute walk test.
3.3.2. Objective criteria
To determine the impact of Prehab on patients with significantly impaired lung function, suggested by DLCO < 50%, patients were divided into two groups (Table 5). (i) High-risk group of patients with DLCO < 50% and (ii) low-risk group of patients with DLCO > 80%. Whilst there were no significant differences in the dyspnoea scores or PS, patients with low DLCO had a significantly higher frailty index, lower 6MWT, FEV1, DLCO and high Thoracoscores, suggesting a high risk for post-operative complications and mortality. A significantly high proportion of patients (78.8%) were, therefore, not ready to proceed with surgery, compared with the low-risk group of patients (Table 5).
In the high-risk group of patients with low DLCO, following Prehab there was clinical and statistically significant improvement in the frailty index and level of activity (Table 6). 86.5% of high-risk group patients were ready to proceed with radical treatment and 59.1% with surgery (Table 6).
A significantly higher number of patients in the low-risk group underwent lung resection compared with patients in the high-risk group (Table 7). Nevertheless, following optimization with Prehab, 54.6% of high-risk patients previously deemed in-operable, underwent surgery safely (Table 7). Following surgery there were no significant differences in the major complication or mortality rates in the high-risk group of patients compared with the low-risk group of patients. Whilst the length of stay in the HDU for patients with low DLCO were significantly longer (Table 7), this did not affect their overall length of stay in hospital.
Our results confirm that optimization with Prehab allows a majority of in-operable patients with a low DLCO to become operable and undergo curative surgery safely.
4. Discussion
Our prospective study is in keeping with previous studies that demonstrate the beneficial impact of preoperative pulmonary rehabilitation or physiotherapy programs to improve exercise capacity in patients undergoing thoracotomy and lung resection [15,18,32]. Exercise and physical activity at an appropriate level have been shown to reduce symptoms of dyspnoea, improve PS, improve quality of life, and potentially reduce length of hospital stay and complications following surgery for lung cancer [15,32]. Thus, with a standardized Prehab program one can expect improvement in dyspnoea scores and PS as demonstrated in our study. In reality patients being considered for treatment for lung cancer often experience difficulties in being active [32]. Indeed, 80.1% of our patients were found to have a sedentary life style at assessment. Performance status, a measure of how well lung cancer patients are able to carry on ordinary daily activities, provides an estimate of what treatments a person may tolerate ([4,33,34]). 54.2% of our patients had high PS scores placing them at a high risk for post-operative morbidity and mortality. In keeping with the feasibility study by Jones et al. [34], and our pilot program [15], our study demonstrates that a structured Prehab program can improve activity levels and PS of patients prior to major thoracic surgery allowing patients to then safely undergo curative lung resection.
In the present study, 12.9% of patients had PS of 3 or 4 and dyspnoea scores of > 3 and were considered inoperable due to a prohibitively high risk of post-operative pulmonary complications, breathlessness and death. This was indicated by high thoracoscores for this group. With a standardized Prehab program 84.2% of the high-risk patients were optimized and ready to proceed with radical treatment, namely radiotherapy or surgery. Indeed 42.8% of patients proceeded to surgery with outcomes similar to patients considered to have low risks for adverse events or death, as indicated by low thoracoscores for the group [3,15]. Our study supports our hypothesis that by improving preoperative dyspnoea, PS, activity levels, and frailty, Prehab allows patients who were initially considered inoperable on subjective criteria of dyspnoea and PS, to become suitable for surgery. Prehab thus allows high-risk patients to safely receive curative surgery with a reduced risk of postoperative complications and mortality as demonstrated.
Impaired DLCO is currently considered one of the most important risk factors for postoperative complications after lung cancer surgery ([3,5,35,36]). Ferguson et al., showed that a predicted DLCO < 60% was associated with a 25% mortality and a 40% pulmonary morbidity [36]. We grouped patients with DLCOs of <50% as a high-risk group for developing post-operative pulmonary complications, breathlessness and death. In this high-risk group 78.8% were deemed unfit to proceed with safe surgery. With Prehab 59.1% of high-risk patients were optimized and 54.6% proceeded to surgery. Their outcomes were similar to patients considered to have low risks for adverse events or death. Our study supports our hypothesis that a standardized Prehab program improves preoperative clinical parameters in patients who were initially considered inoperable due to low DLCO values. By doing so Prehab allows this group of patients, who were deemed inoperable, to become suitable for surgery and safely receive curative surgery with a reduced risk of postoperative complications, LOHS and mortality.
Interestingly, in patients with low DLCO whilst the overall length of stay was no different to the low-risk patients, they required a longer duration of care in the HDU. In healthy subjects DLCO has the capacity to improve with exercise in response to the increase in pulmonary blood flow [35]. Its impairment during the preoperative workout has been correlated with the occurrence of postoperative complications after lung resection [37]. Despite Prehab and immediate post-operative rehabilitation, patients with a low baseline DLCO required a longer period of care in the HDU. This is consistent with the study by Novoa et al., who demonstrated that in patients with impaired DLCO, following lung resection the physiological increase in DLCO during exercise is still impaired on the third postoperative day [36].
Patient's ≥75 years-of-age have a significantly higher frequency of post-operative pulmonary complications [38]. Cancer Research informs us that the demographics of patients with lung cancer is getting older [39]. In this ageing population frailty is increasingly being recognized as an independent risk factor for adverse postoperative outcomes, namely, major morbidity, mortality and protracted LOHS [30,31]. Our study is unique in that we have used the Canadian 9-point Clinical Frailty Scale as an index to assess patients for levels of frailty and demonstrated that almost half the patients referred for Prehab had an index of greater than 3. Thus suggesting that a significant proportion of elderly patients are vulnerable to post-operative adverse events. By providing a standardized Prehab program to elderly patients with frailty and improving their frailty index, our study is the first of its kind to address frailty in surgical patients.
Our prospective study showed that a standardized Prehab program makes inoperable patient with potentially resectable disease operable, and safely undergo curative lung resection. This strategy we believe will help improve resection rates and may contribute to the long term survival of lung cancer patients. Lancet Oncology published the findings of the International Cancer Benchmarking Partnership (ICBP), Cancer Survival in High-Income Countries (SURVMARK-2) project in November 2019 [40]. Notably, the survival of patient with lung cancer amongst other cancers was poor. A multi-pronged approach is no doubt required to improve the survival of patients with lung cancer. Optimizing patients who are considered inoperable due to significant dyspnoea, impaired PS, frailty, borderline or poor pulmonary functions and providing them access to safe, curative surgery is one approach.
There were patients in all the groups studied who, despite Prehab were not able to proceed to surgery. Hence, avoiding giving a sense of false hope of surgery to patients who may not in the end proceed to surgery is important. When patients are referred for pre-treatment optimization with Prehab, it is our practice to provide counselling and careful explanation to all patients at the time of assessment that despite Prehab, they may not be suitable for surgery.
Our study has certain limitations. This is a pragmatic, real life provision of a standardized Prehab program to optimize lung cancer patients going forward for surgery and not a randomized trial, which would allow an objective comparison with a control population and adjust for potential confounding factors, residual confounding or unknown factors. The program is limited in its provision of service to this group of selected patients requiring optimization prior to surgical treatment. It is our intent for the program to be all inclusive and provide a standardized Prehab program to all lung cancer patients going for treatment, whether this be surgery, radiotherapy or systemic anticancer treatment.
We were unable to obtain a meaningful record of compliance with the home exercises program. Also the nature of patients doing exercises at home often meant they could not travel back for reassessment of their objective measures so we could not tell if these patients improved or not. This data was not included in our analysis including data of some patients who, after attending initial few sessions, had to proceed to surgery quickly. The study is also limited in its ability to routinely recheck pulmonary functions, especially DLCO and cardiopulmonary exercise test (CPEX) following Prehab [3,5]. Arranging physiological tests has a waiting time, and in our endeavour to provide prudent health care in a timely manner, we have limited our assessments to clinical parameters and the 6MWT. CPEX testing although available, was not routinely carried out and only a small number of patients underwent CPEX testing. Interestingly, with the significant improvements observed with Prehab in dyspnoea, PS, frailty, level of activity and the 6MWT, patients were able to proceed to surgery without having to undergo routine CPEX testing. Nevertheless, CPEX values measured prior to and following Prehab would provide strength to evaluating the impact of Prehab on high risk patients going forward to treatment.
The occurrence of post-operative pulmonary complications have a marked clinical and economic impact. Whilst the study shows an impact of Prehab on post-operative complications the impact on economic benefits and on the quality of life of patients were not assessed. Rehabilitation was provided to all patients following surgery and at home following their discharge from hospital. This data is not included. The strengths of the program were that patients were selected on set criteria and underwent a standardized Prehab program. There was also a broad uptake of the program from all lung cancer MDTs across South West Wales.
In summary, high-risk patients can be optimized with preoperative and postoperative pulmonary rehabilitation to reduce their frequency of post-operative pulmonary complications, hospital stay and improve postoperative outcomes including mortality. Our prospective study demonstrates that with a standardized Prehab program, high-risk patients for lung resection can be suitably optimized to proceed to safe surgical resection and have outcomes similar to low-risk patients. Therefore, at lung cancer MDTs the management plan for high-risk patients, who are otherwise deemed inoperable due to high dyspnoea scores, PS, frailty and borderline or poor lung function, should include consideration of referral for a period of structured Prehab. The duration of Prehab is guided by their stage of disease and risk of progression. Those suitably optimized with Prehab can be expected to safely proceed with curative lung resection. This strategy will help improve resection rates. Nevertheless, a suitably powered randomized control trial with long-term follow-up is required to confirm our observations and establish whether a structured Prehab program helps improve the long term survival of lung cancer patients.
Declaration of Interests
This is a Welsh Health Specialized Services Committee (WHSSC) commissioned service.
Acknowledgments
Acknowledgments
The Welsh Health Specialized Services Committee (WHSSC) – for their support in commissioning of the service and entrusting us with the delivery of this service across South West Wales. Our Health Boards’ senior management team for their unwavering support for the service and the hardworking dedicated Cardiothoracic Physiotherapy team for carrying out the program. The Physiotherapy managers of the Swansea bay University Health Board, the Hywel Dda University Health Board and Cwm Taff University Health Board for their help in setting up regional services locally nearer patients homes, and Tenovus Cancer Care, for the use of their mobile unit facilities to provide Prehab in the local community.
Funding
Authors IG, GT, and HT, received no financial compensation or other remuneration for data collection, analysis, or interpretation; trial design; patient recruitment; or any aspect pertinent to the study nor for writing of the manuscript or the decision to submit it for publication. All authors had full access to the full data in the study and accept responsibility to submit for publication.
Availability of data and materials
Access to individual-level data is governed by and processed under the United Kingdom's Data Protection Act (DPA) 2018, the EU General Data Protection Regulation (GDPR) and the Common Law Duty of Confidentiality (CLDC). Researchers may obtain the relevant approval and access data via the Swansea Bay University Health Board. Information pertaining to the study is available by contacting the corresponding author (IG). All authors consent to the publication.
Author contribution
IG planned the project and study design with GT. GT with HT and the physiotherapy department established the Pre-hab program. GT with HT and the physiotherapy team collected the data. IG and GT carried out the literature search, data verification (independently), data interpretation, data analysis. IG, GT and HT helped write the manuscript. GT registered the project with the NHS Research Ethics Centre. All authors have read and approved the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100663.
Contributor Information
Ira Goldsmith, Email: ira@doctors.org.uk, Ira.Goldsmith@nhs.wales.uk.
Gemma Chesterfield-Thomas, Email: Gemma.Thomas3@nhs.wales.uk.
Hannah Toghill, Email: Hannah.Toghill@wales.nhs.uk.
Appendix. Supplementary materials
References
- 1.Alberg A.J., Ford J.G., Samet J.M. Epidemiology of lung cancer. ACCP evidence-based clinical practice guidelines. Chest. 2007;132:29S–55S. doi: 10.1378/chest.07-1347. [DOI] [PubMed] [Google Scholar]
- 2.Reif M.S., Socinski M.A., Rivera M.P. Evidence-based medicine in the treatment of non-small cell lung cancer. Clin Chest Med. 2000;21:107–120. doi: 10.1016/s0272-5231(05)70011-3. [DOI] [PubMed] [Google Scholar]
- 3.Lim E., Baldwin D., Beckles M., Duffy J., Entwisle J., Faivre-Finn C., Kerr K., Macfie A., McGuigan J., Padley S., Popat S., Screaton N., Snee M., Waller D., Warburton C., Win T. Guidelines on the radical management of patients with lung cancer. Thorax. 2010;(Suppl 3):1–27. doi: 10.1136/thx.2010.145938. [DOI] [PubMed] [Google Scholar]
- 4.NICE guideline [NG122] 2019 Lung cancer: diagnosis and management. https://www.nice.org.uk/guidance/ng122/chapter/Recommendations.
- 5.Brunelli A., Kim A.W., Berger K.I., Addrizzo-Harris D.J. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: american college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e166S–e190S. doi: 10.1378/chest.12-2395. [DOI] [PubMed] [Google Scholar]
- 6.Mujovic N., Mujovic N., Subotic D. Preoperative pulmonary rehabilitation in patients with non-small cell lung cancer and chronic obstructive pulmonary disease. Arch Med Sci. 2014;10:68–75. doi: 10.5114/aoms.2013.32806. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spruit M.A. An official American thoracic society/European respiratory society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13–e64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 8.British Thoracic Society Standards of Care Subcommittee on Pulmonary Rehabilitation Pulmonary rehabilitation. Thorax. 2001;56(11):827–834. doi: 10.1136/thorax.56.11.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith K., Cook D., Guyatt G.H. Respiratory muscle training in chronic airflow limitation: a meta-analysis. Am Rev Respir Dis. 1992;145(3):533–539. doi: 10.1164/ajrccm/145.3.533. [DOI] [PubMed] [Google Scholar]
- 10.Mishra S.I., Scherer R.W., Snyder C., Geigle P.M., Berlanstein D.R., Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Datab Syst Rev. 2012;(Issue 8) doi: 10.1002/14651858.CD008465.pub2.14. Art. No.: CD008465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sommer M.S., Trier K., Vibe-Petersen J., Christensen K.B., Missel M., Christensen M., Larsen K.R., Langer S.W., Hendriksen C., Clementsen P.F., Pedersen J.H., Langberg H. Changes in health-related quality of life during rehabilitation in patients with operable lung cancer: a feasibility study (PROLUCA) Integr Cancer Ther. 2018 Jun;17(2):388–400. doi: 10.1177/1534735416668258. Epub 2016 Oct 3. PMID: 27698263; PMCID: PMC6041926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosero I.D., Ramírez-Vélez R., Lucia A., Martínez-Velilla N., Santos-Lozano A., Valenzuela P.L., Morilla I., Izquierdo M. Systematic review and meta-analysis of randomized, controlled trials on preoperative physical exercise interventions in patients with non-small-cell lung cancer. Cancers (Basel) 2019;11:944. doi: 10.3390/cancers11070944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benzo R., Wigle D., Novotny P. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer. 2011;74:441–445. doi: 10.1016/j.lungcan.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lotters F., van Tol B., Kwakkel G. Effects of controlled inspiratory muscle training in patients with COPD: a meta-analysis. Eur Respir J. 2002;20(3):570–576. doi: 10.1183/09031936.02.00237402. [DOI] [PubMed] [Google Scholar]
- 15.Chesterfield-Thomas Gemma, Goldsmith Ira. Impact of preoperative pulmonary rehabilitation on the thoracoscore of patients undergoing lung resection. Interact Cardiovasc Thorac Surg. 1 November 2016;23(Issue 5):729–732. doi: 10.1093/icvts/ivw238. [DOI] [PubMed] [Google Scholar]
- 16.Granger C.L., McDonald C.F., Berney S., Chao C., Denehy L. Exercise intervention to improve exercise capacity and health related quality of life for patients with Non-small cell lung cancer: a systematic review. Lung Cancer. 2011;72:139–153. doi: 10.1016/j.lungcan.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Langer Carsten Hendriksen, Clementsen Paul, Pedersen Jesper H, Langberg Henning. Perioperative rehabilitation in operation for lung cancer (PROLUCA) – rationale and design. BMC Cancer. 2014;14:404. doi: 10.1186/1471-2407-14-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley A., Marshall A., Stonehewer L. Pulmonary rehabilitation programme for patients undergoing curative lung cancer surgery. Eur J Cardiothorac Surg. 2013;44:266–271. doi: 10.1093/ejcts/ezt381. [DOI] [PubMed] [Google Scholar]
- 19.Saito H., Hatakeyama K., Konno H., Matsunaga T., Shimada Y., Minamiya Y. Impact of pulmonary rehabilitation on postoperative complications in patients with lung cancer and chronic obstructive pulmonary disease. Thorac Cancer. 2017;8(5):451–460. doi: 10.1111/1759-7714.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagarajan K., Bennett A., Agostini P., Naidu B. Is preoperative physiotherapy/pulmonary rehabilitation beneficial in lung resection patients? Interact Cardiovasc Thorac Surg. 2011;13:300–302. doi: 10.1510/icvts.2010.264507. [DOI] [PubMed] [Google Scholar]
- 21.von Elm Erik, G Altman Douglas, Matthias Egger, Pocock Stuart J., Gøtzsche Peter C., Vandenbroucke Jan P. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Patient Safety . National research ethics service – defining research. 2009. National patient safety agency.http://www.nres.npsa.nhs.uk [Online] Available from. [Accessed 12, January 2016] [Google Scholar]
- 23.Detterbeck F.C., Boffa D.J., Kim A.W., Tanoue L.T. The eighth edition lung cancer stage classification. Chest. 2017;151(1):193–203. doi: 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Canadian Study on Health & Aging A global clinical measure of fitness and frailty in elderly people. Rockwood K., editor. A global clinical measure of fitness and frailty in elderly peopleCMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. Revised 2008. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larson M., Kim M.J. Respiratory muscle training with the incentive spirometer resistive breathing device. Heart Lung. 1984 Jul;13(4):341–345. [PubMed] [Google Scholar]
- 27.Parsons A., Daley A., Begh R., Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. doi: 10.1136/bmj.b5569. –b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Institute for Health and Clinical Excellence . National Institute for Health and Clinical Excellence; London: UK: 2008. Smoking cessation services: guidance. (PH 10) [Google Scholar]
- 29.Katsukawa F. FITT principle of exercise in the management of lifestyle-related diseases. Clin Calcium. 2016;26(3):447–451. [PubMed] [Google Scholar]
- 30.Lee D.H., Buth K.J., Martin B.J., Yip A.M., Hirsch G.M. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010 Mar 2;121(8):973–978. doi: 10.1161/CIRCULATIONAHA.108.841437. [DOI] [PubMed] [Google Scholar]
- 31.Wilkinson K., Martin I.C., Gough M.J. NCEPOD; 2010. An age old problem. London: national confidential enquiry into patient outcome and death. [Google Scholar]
- 32.Wynes M.W. Physical activity benefits lung cancer patients and survivors. J Thoracic Oncol. 2015 April 1. [Google Scholar]
- 33.Kelly C.M., Shahrokni A. Moving beyond Karnofsky and ECOG performance status assessments with new technologies. J Oncol. 2016;2016 doi: 10.1155/2016/6186543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones L.W., Peddle C.J., Eves N.D., Haykowsky M.J., Courneya K.S., Mackey J.R., Joy A.A., Kumar V., Winton T.W., Reiman T. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer. 2007;110:590–598. doi: 10.1002/cncr.22830. pg. [DOI] [PubMed] [Google Scholar]
- 35.Novoa N.M, Esteban P., Hernández M.T.G., Fuentes M.G, Varela G., Jiménez M.F. Early exercise pulmonary diffusing capacity of carbon monoxide after anatomical lung resection: a word of caution for fast-track programmes. Eur J Cardio-Thoracic Surg. July 2019;56(Issue 1):143–149. doi: 10.1093/ejcts/ezz007. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson M.K., Little L., Rizzo L. Diffusing capacity predicts morbidity and mortality after pulmonary resection. J Thoracic Cardiovasc Surg. 1988;96:894–900. [PubMed] [Google Scholar]
- 37.Wang J.S., Abboud R.T., Evans K.G., Finley R.J., Graham B.L. Role of CO diffusing capacity during exercise in the preoperative evaluation for lung resection. Am J Respir Crit Care Med. 2000;162:1435–1444. doi: 10.1164/ajrccm.162.4.2001117. [DOI] [PubMed] [Google Scholar]
- 38.Agostini P., Cieslik H., Rathinam S. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax. 2010;65(9):815–818. doi: 10.1136/thx.2009.123083. [DOI] [PubMed] [Google Scholar]
- 39.Cancer Research. Lung cancer incidence by age. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/incidence#heading-One.
- 40.Arnold M., Rutherford M.J., Bardot A., Ferlay J., Andersson T.M.-.L., Myklebust T.A. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20:1493–1505. doi: 10.1016/S1470-2045(19)30456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to individual-level data is governed by and processed under the United Kingdom's Data Protection Act (DPA) 2018, the EU General Data Protection Regulation (GDPR) and the Common Law Duty of Confidentiality (CLDC). Researchers may obtain the relevant approval and access data via the Swansea Bay University Health Board. Information pertaining to the study is available by contacting the corresponding author (IG). All authors consent to the publication.