Abstract
Background
Cognitive and motor function in ageing are intertwined, but whether slower motor response time (MRT) to a cognitive stimulus could herald accelerated mobility decline is unknown. Using data from The Irish Longitudinal Study on Ageing (TILDA), we examined whether slower MRT may predict a greater than expected increase in Time Up and Go (TUG) after 4 years.
Methods
Participants aged 50 years or older were divided into two groups based on their mean MRT (< 250 ms versus ≥ 250 ms). A repeated measures ANOVA compared TUG trajectories between groups, controlling for baseline age, sex, height, education level, mini mental-state examination (MMSE) score, self-reported vision and hearing, medical conditions (cardiovascular, cerebrovascular disease, diabetes), and number of medications.
Findings
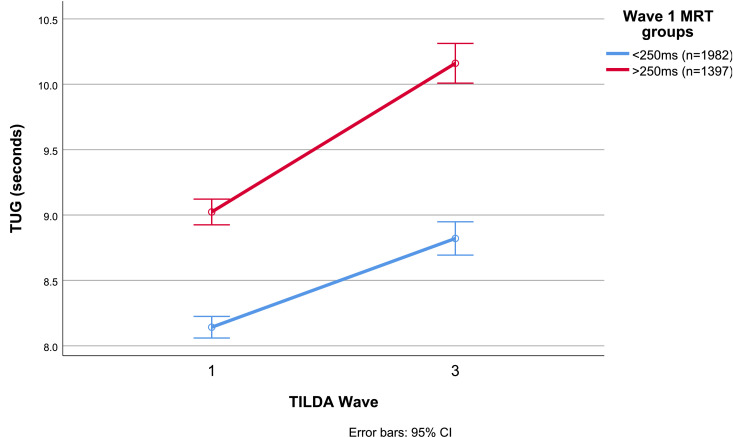
At Wave 1, 1982 (58.7%) had a mean MRT of < 250 ms, with a mean TUG of 8.1 s (SD 1.6); and 1397 (41.3%) had an MRT of ≥ 250 ms, with a TUG of 9.0 s (SD 2.2). At Wave 3, TUG increased to 8.8 s (SD 2.0) and 10.2 s (SD 3.9), respectively. The results of the adjusted repeated measures ANOVA suggested that there was a statistically significant interaction between MRT group and Wave (P = 0.023, η2p = 0.002).
Interpretation
TILDA participants in the slower MRT group seemed to have faster mobility decline, but this effect was statistically and clinically small.
Funding
TILDA is funded by Atlantic Philanthropies, the Irish Department of Health and Irish Life. Roman Romero-Ortuno is funded by Science Foundation Ireland (grant number 18/FRL/6188).
Keywords: Choice reaction time, Time Up and Go, Cognition, Mobility, Longitudinal study
Research in context.
Evidence before this study
We reviewed the PubMed database and found numerous studies reporting links between cognitive and motor function in ageing. From reviewing the literature and our own clinical experience, we hypothesised that impaired performance in an attention-based choice reaction time task may be predictive of a greater than expected decline in mobility.
Added value of this study
We were able to test our hypothesis in a large population-based study (TILDA), with large participant numbers and a 4-year follow-up period. With good statistical power, we found some evidence in support of our hypothesis, namely that slower choice reaction times may be an early maker of accelerated mobility decline in adults aged 50 or more years.
Implications of all the available evidence
Our findings are statistically significant, but the effect sizes are small, both statistically and clinically. Further research is required to clarify the significance of our findings and whether early cognitive interventions (e.g. training) might be able to prevent accelerated mobility decline and premature loss of independence, which would be of enormous human and economic importance in our ageing societies.
Alt-text: Unlabelled box
1. Introduction
Existing literature suggests that there may be a link between cognitive and motor function in the context of ageing[1]. For instance, postural control has been shown to be affected by access to attentional resources, as illustrated by the fact that response times to auditory tasks were increased when healthy older individuals were asked to concurrently achieve postural stability in the absence of visual and somatosensory cues [2]. This is supported by the finding that premotor time in the choice step reaction time task is longitudinally associated with falls risk in older adults [3].
Gait disorders are common amongst older persons with cognitive impairments, and motor slowing may precede and predict the onset of cognitive decline [4], as illustrated by the clinical manifestations of the so-called predementia motoric cognitive risk syndrome [5]. The Time Up and Go (TUG) test has been widely used as a standard measure of mobility in older adults, owing to its sensitivity, normal distribution and lack of ceiling effect. These properties are not shared by other mobility measures such as the Berg Balance Scale (BBS) or the Dynamic Gait Index (DGI) [6]. The TUG has also been shown to be a sensitive and specific measure of physical frailty, offering potential advantages over the application of Fried's criteria [7]. Previous studies have reported associations between TUG and cognitive performance, including tests of executive function [8], [9], [10]. Work on Wave 1 of The Irish Longitudinal Study on Ageing (TILDA) indicated that slower TUG was associated with poorer performance on sustained attention response, prospective memory and choice reaction time (CRT) tasks [11]. CRT latencies have been shown to increase significantly with age at a rate of 2.8 ms/year across all ages [12]. These associations would suggest that poor CRTs may be predictive of accelerated physical decline, but this hypothesis had not yet been tested longitudinally.
Data from TILDA have shown normative percentile values of TUG across different age groups [13]; with this information in mind, we hypothesised that impaired performance in an attention-based CRT task may be predictive of a greater than expected increase in TUG after a 4-year follow-up. Our aim was therefore to examine, using TILDA data, longitudinal TUG trajectories in two older population groups characterised by different baseline CRTs.
2. Methods
2.1. TILDA overview and dataset access
TILDA is a large prospective cohort study of the social, economic and health circumstances of community-dwelling people aged 50 years or more in Ireland. The study design has been described in detail by Kearney et al. [14]. and O'Donoghue et al. [15]. The sampling frame is the Irish Geodirectory, a listing of all residential addresses in the Republic of Ireland [15].
We accessed TILDA data via the Irish Social Science Data Archive (ISSDA) – www.ucd.ie/issda. The TILDA ISSDA dataset is an anonymised, publicly available subset of the data collected by TILDA. Both RC and RRO had access to the dataset over a period of approximately 6 months. Our study is based on data from the first two TILDA health assessment waves: Wave 1, which was collected between October 2009 and July 2011[16], and Wave 3, which was collected between March 2014 and October 2015[17].
At Wave 1, a clustered sample of addresses was randomly selected and household residents aged 50 and older and their spouses/partners (of any age) were eligible to participate. The household response rate at Wave 1 was 62%. In terms of data collection, TILDA fieldwork involved interviews using Computer-Aided Personal Interviewing (CAPI) techniques and a Self-Completion Questionnaire (SCQ). At Waves 1 and 3, participants were also invited to complete a health assessment, either at a TILDA Health Assessment Centre, where appropriate health measurement facilities were available, or in the respondents’ home, where a trained research nurse visited participants to take physical measurements and biological samples. TILDA's research nurses were fully trained to implement all Standard Operating Procedures relating to participants’ assessments.
Inclusion criteria for our study were being 50 or more years old at baseline and having valid TUG and CRT data at both Wave 1 and 3.
2.2. TUG
TUG was the only available measure of mobility in the TILDA ISSDA data. TUG was assessed only once using a chair with armrests and a seat of 46 cm height. Participants were asked to rise from the chair, walk 3 metres at normal pace on a straight line marked on the floor, turn around, walk back to the chair, and sit down again. Walking aids were allowed if required, and no instructions were given about the participants’ use of their arms. The time taken from the “Go” initial command to when the participant was sitting with his/her back resting against the back of the chair again was recorded using a stopwatch.
2.3. CRT
The CRT test used a computer-based program to assess concentration and processing speed. In the CRT test, participants were asked to depress a central button until a stimulus appeared on-screen: either the word YES or the word NO. Each time a stimulus appeared, respondents were required to press the corresponding button. A return to the central button was necessary after each response for the next word to appear on-screen. There were approximately 100 repetitions [18,19]. In our analyses, we used mean motor response time (MRT), in milliseconds (ms), as the mean time taken by participants during all the repetitions of the CRT test. Participants were then divided into two groups based on their mean MRT values – below 250 ms versus equal to or above 250 ms. This cut-off value was based on the median MRT in the total sample being approximately 250 ms and the fact that we aimed to have two MRT groups of balanced sizes for the multivariate analyses detailed below. This cut-off is also similar to CRT cut-off times used in previous studies [12,20].
2.4. Baseline characteristics
Variables used for characterisation of the sample at Wave 1 were: age (years), sex, height (cm), BMI (Kg/m2), highest education level (on a scale from 1 to 7 ranging from less than primary to postgraduate), Mini-Mental State Examination (MMSE) score [21], center for Epidemiology Studies Depression (CESD) score [22], the Hospital Anxiety and Depression Scale – Anxiety subscale (HADS-A) score [23], self-rated vision and hearing (both on a scale from 1 to 5; 1 = excellent; 2 = very good; 3 = good; 4 = fair; 5 = poor), history of hypertension, ischaemic heart disease (angina and/or heart attack), congestive heart failure, diabetes, stroke and/or TIA (transient ischaemic attack or ‘mini-stroke’), being on any antipsychotic medication, taking any antidepressant, and taking 5 or more regular medications (i.e. polypharmacy).
2.5. Statistical analyses
Statistical analyses were performed with IBM SPSS Statistics version 26. Descriptives were given as mean with standard deviation (SD) or count and percentage (%). Non-parametric tests (Mann-Whitney U test for continuous variables; Chi-square tests for dichotomous variables) were conducted to compare MRT groups across baseline (Wave 1) characteristics. Comparisons between continuous and ordinal variables were performed with the 2-sided Spearman correlation coefficient.
A repeated measures Analysis of Variance (ANOVA) was computed with a two-level within-subject factor (Wave 1 and Wave 3). Variables that showed statistically significant differences between the two baseline MRT groups were corrected for in the model. Categorical between-subject factors were MRT groups and sex. Covariates (potential confounders) were age, education score, MMSE score, self-reported vision and hearing score, number of medical conditions amongst the ones mentioned above, number of regular medications, and height. The TILDA release guide [24] states that respondents were encouraged to wear glasses if needed for the cognitive tests, and time was allowed to adjust a hearing aid if respondents had poor hearing; in addition, instructions were provided very clearly and more slowly than a normal conversation. Even though self-reported vision did not seem different between MRT groups (see below), it was included in the model as it was felt to be clinically important. Height was included due to Kenny et al.’s stratification of TUG normative values based on height, as well as age and sex [13]. A main effects [25] model was generated and a plot was created showing the interaction between Wave and MRT groups. Effect size estimates were based on the partial eta squared (η2p) statistic; this measures the proportion of the total variance in a dependant variable that is associated with the membership of different groups defined by an independent variable, and the effects of other independent variables and interactions are partialled out [26].
The level of statistical significance was set at P < 0.05 throughout.
2.5.1. Sensitivity analysis
As a sensitivity analysis, we repeated the adjusted model with MMSE groups (<24 versus 24 or more points) as the between-subject factor instead of focusing on MRT groups. This MMSE cut-off was used on the basis that it is the most widely used across previous studies for the detection of abnormal cognition in older people [27].
2.6. Ethical approval
Ethical approval was obtained from the Trinity College Dublin Research Ethics Committee and all participants provided written informed consent.
2.7. Role of funding source
TILDA is funded by Atlantic Philanthropies, the Irish Department of Health and Irish Life. Roman Romero-Ortuno is funded by a Grant from Science Foundation Ireland under Grant number 18/FRL/6188. None of the funding sources had any role in the study design; collection, analysis or interpretation of data; writing of the report; or the decision to submit the paper for publication. Both authors had full access to the full data in the study and accept responsibility to submit for publication.
3. Results
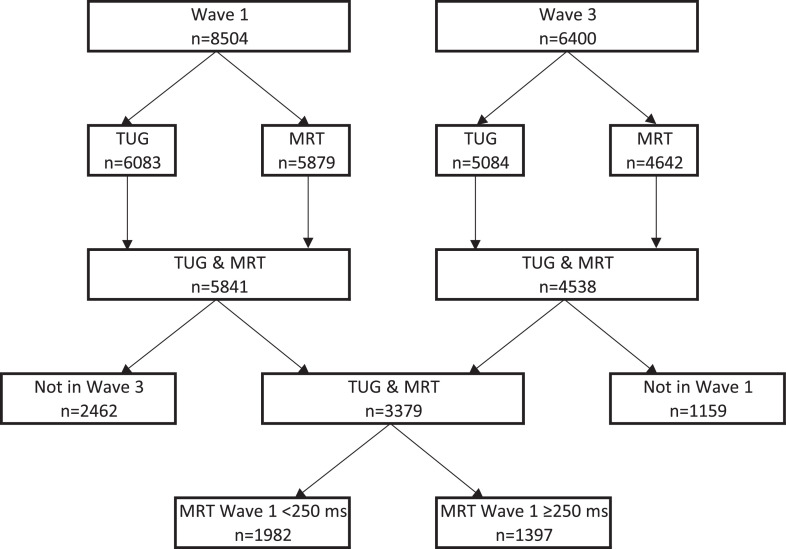
Out of 8504 participants in Wave 1 and 6400 participants in Wave 3, 3379 participants (44% males, 56% females) had both their TUG and MRT measured at Wave 1 and Wave 3. The flowchart in Fig. 1 outlines the breakdown of this cohort in terms of included and non-included numbers. In Wave 1, 2462 out of 5841 (42.2%) participants who had both TUG and MRT did not have TUG and MRT in Wave 3.
Fig. 1.
Participants’ flow chart.
TUG: Time Up and Go; MRT: motor response time.
At Wave 1, 1982 (58.7%) had a mean MRT of < 250 ms, with a mean TUG of 8.1 s (SD 1.6); and 1397 (41.3%) had an MRT of ≥ 250 ms, with a TUG of 9.0 s (SD 2.2). Baseline comparisons between MRT groups are shown in Table 1. Participants in the slower MRT group were older, more likely to be female, shorter, less educated, had lower MMSE score, more self-reported hearing impairment, more hypertension, diabetes and stroke/TIA, and were more likely to take antipsychotics, antidepressants and be on polypharmacy.
Table 1.
Comparison of baseline (Wave 1) characteristics between the two mean motor response time (MRT) groups.
| Mean MRT < 250 ms (N = 1982) |
Mean MRT ≥ 250 ms (N = 1397) |
P for difference | |
|---|---|---|---|
| Mean MRT, milliseconds (SD) | 189.1 (37.1) | 348.3 (130.4) | < 0.001* |
| Mean TUG, seconds (SD) | 8.1 (1.6) | 9.0 (2.2) | < 0.001* |
| Mean age, years (SD) | 58.9 (7.4) | 63.9 (8.7) | < 0.001* |
| Female Sex (%) | 52.0% | 60.6% | < 0.001^ |
| Mean height, cm (SD) | 167.4 (8.7) | 164.8 (8.9) | < 0.001* |
| Mean BMI, Kg/m2 (SD) | 28.2 (4.4) | 28.4 (4.5) | 0.065* |
| Mean education score (SD) | 4.2 (1.6) | 3.8 (1.5) | < 0.001* |
| Highest education level attained (%): | |||
| Less than primary | 1.3% | 2.2% | 0.044^ |
| Primary | 13.5% | 22.7% | < 0.001^ |
| Secondary | 62.4% | 59.7% | 0.120^ |
| Primary university degree | 13.2% | 10.3% | 0.009^ |
| Postgraduate | 9.6% | 5.2% | < 0.001^ |
| Mean MMSE score (SD) | 29.0 (1.3) | 28.5 (1.7) | < 0.001* |
| Mean CESD score (SD) | 5.4 (6.8) | 5.2 (6.6) | 0.314* |
| Mean HADS-A score (SD) | 5.5 (3.5) | 5.2 (3.5) | 0.073* |
| Poor self-rated vision or legally blind (%) | 0.7% | 1.1% | 0.254^ |
| Poor self-rated hearing (%) | 1.1% | 2.3% | 0.005^ |
| Hypertension (%) | 29.5% | 36.8% | < 0.001^ |
| Ischaemic heart disease (angina or heart attack) (%) | 5.5% | 7.1% | 0.059^ |
| Congestive heart failure (%) | 0.6% | 1.1% | 0.132^ |
| Diabetes (%) | 5.1% | 7.0% | 0.020^ |
| Stroke or ‘mini-stroke’ (TIA) (%) | 1.6% | 4.3% | < 0.001^ |
| Taking any antipsychotic (%) | 0.7% | 1.8% | 0.004^ |
| Taking any antidepressant (%) | 4.1% | 7.2% | < 0.001^ |
| Polypharmacy (≥ 5 medications) (%) | 4.3% | 8.7% | < 0.001^ |
MRT = motor response time; TUG: Time Up and Go; BMI = Body mass index; MMSE = Mini-mental state examination; CESD = Centre for Epidemiological Studies Depression Scale; HADS-A = Hospital anxiety and depression scale – anxiety subscale; TIA: transient ischaemic attack; SD: standard deviation.
2-sided Mann-Whitney U test;.
Chi-square test.
Further to the association between MRT groups and MMSE score in Wave 1, we also observed that the association between MRT (as a continuous variable) and MMSE tertiles in the total W1 analytical sample (N = 3379) was also statistically significant, with a 2-sided Spearman correlation coefficient of −0.22 (P < 0.001) (see Fig. 1 in the supplementary data).
At Wave 3, the unadjusted mean TUGs were 8.8 s (SD 2.0) and 10.2 s (SD 3.9) for the < 250 ms and ≥ 250 ms groups, respectively. The longitudinal trajectories of mean TUGs by baseline MRT group are shown in Fig. 2.
Fig. 2.
Unadjusted trajectories of Timed Up and Go (TUG) by baseline mean motor response time (MRT) group between TILDA Wave 1 (2009–2011) and Wave 3 (2014–2015). CI: confidence interval.
The results of the adjusted repeated measures ANOVA suggested that there was a significant main effect of MRT [F(1,3359) = 5.1, P = 0.023, η2p = 0.002] on TUG across waves. In the adjusted model, the estimated marginal means of TUG for those with an MRT of < 250 ms were 8.4 s (standard error, SE 0.04) at Wave 1, and 9.1 s (SE 0.05) at Wave 3. The estimated marginal means of TUG for those with an MRT of ≥ 250 ms were 8.7 s (SE 0.05) at Wave 1, and 9.7 s (SE 0.05) at Wave 3. Table 1 in the supplementary data outlines the main effects of all the variables included in the model. Other than MRT groups, the statistically significant effects were those of age (P < 0.001), MMSE score (P = 0.012) and education (P = 0.007).
The results of the sensitivity analysis are shown in Table 2 of the supplementary data and show that while MMSE groups did not have a significant main effect, MRT was still significant (P = 0.003) in that model.
4. Discussion
We hypothesised that TILDA participants aged 50 years or older who had a mean MRT of ≥ 250 ms at baseline would have, compared to those with a baseline MRT < 250 ms, a faster mobility decline as measured by the TUG approximately 4 years later. After adjustment for potential confounders, the results of our statistical model suggested that those with slower baseline cognitive responses had an average TUG increase of 1.0 s, compared to 0.7 s in the faster baseline cognitive response group.
Statistically, the model suggested that the two MRT groups exhibited divergent TUG trajectories, above the main significant effects of global cognition (MMSE), education and age. Our statistical findings do not exclude the possibility that slower baseline MRT could be a marker of accelerated mobility decline. Indeed, previous observations have suggested that mobility measures can be sensitive to subclinical variance in cognition [28]. In addition, in the Lifestyle Interventions and Independence for Elders (LIFE) study, processing speed at baseline seemed to be a significant predictor of subsequent major motor disability [29]. In this regard, some studies are suggesting that participation in cognitively stimulating activities may be beneficial not only for neuromotor performance, but also mobility in older adults [30,31]. Our results were significantly smaller in size than those obtained by Lord et al. [32], which used a light finger tap test as a reaction time measure, but this discrepancy could be justified by the fact that their study focused on those with a significant falls risk, whereas ours studied a relatively healthier community-based sample. However, the lack of simple reaction time (SRT) data prevents assessment of whether the results were biased by impaired sensorimotor rather than cognitive performance at baseline. This being said, Woods et al. [12]. reported that 80% of age-related CRT slowing could be accounted for by the central processing time (CPT), isolated by subtracting SRT's. The fact that the association between MRT (continuous variable) and MMSE tertiles was statistically significant may support the notion that CRT reflects participants’ global cognitive function. However, residual confounding by sensorimotor function is still possible.
Mechanisms underlying the shared relationship between cognition and physical function are not entirely understood and may represent an underlying ageing process that produces declines across various systems [33] – essentially a third process that is not captured in our study. For instance, disruption in the central nervous system involving white matter disease, beta amyloid and cerebral small-vessel injury, has been suggested to influence cognition and physical performance in older adults [34], [35], [36]. Such abnormalities may adversely affect motor function, gait and cognitive function; however, our study did not collect neuroimaging data to investigate this mechanism. Overall, physical and cognitive function may share a similar set of neural networks, but more research is needed to understand this complex interplay.
The significant effects of baseline age, education and cognition on mobility decline are as clinically expected and are further discussed below. As per Table 1 in the supplementary data, our model did not show any significant effects of polypharmacy and comorbidities on mobility decline. This may be seen as surprising in view of other studies suggesting that specific medications may increase the risk of impaired mobility by adversely affecting domains such as alertness, vision, and muscle strength [37,38]. In addition, cardiovascular morbidities may lead to earlier-onset vascular cognitive impairments which may also affect mobility. A reason why these effects were not significant in our analyses may be related to the relatively healthy, community-based nature of the TILDA sample.
Even though our findings may have biological plausibility, the significant P values attained in our statistical model should be understood in the context of the large sample sizes (and therefore, large statistical power) available in TILDA. Indeed, our η2p effect sizes can be classified as small [26], which is in keeping with the effect sizes found in the Canadian Longitudinal Study on aging, leading to their conclusion that mobility may not be, in isolation, a strong correlate of cognitive performance in middle and late-adulthood [39].
In terms of the clinical significance of our findings, the age of the participants differed markedly between the two MRT groups (Table 1), with a 5-year mean difference (59 vs. 64). It is therefore possible that even though the ANOVA model adjusted for age and other possible confounders, the apparent difference in TUG trajectories could be due to the older cohort being generally frailer, both physically and cognitively. To further explore the clinical significance of our results, we referred to Table 6 of Kenny et al.’s TILDA study on normative TUG values [13]; this suggests that on average (P50), TUG should increase from 8 to 9 s between age 60 and 65, but remain at 9 s between age 65 and 70. Our finding was that TUG increased by ~1 s for those in the < 250 ms group as expected, but also increased by ~1 s for the ≥ 250 ms group. According to this interpretation, the latter decline might be considered as more pronounced than clinically expected. Having said this, Donoghue et al. noted that a difference of 1.75 to 2.08 s in TUG between 2 assessments in the same individual can be expected by chance depending on the confidence interval used and when controlling for all other factors such as rater, time between assessments and time of day [40]. This suggests that overall, the effect of our findings is small, both statistically and clinically.
Frailty measures (e.g. frailty phenotype or index) may have been a good contribution towards characterising the cohort further. However, such measures were not available in the TILDA ISSDA dataset that we had access to. In addition, TUG has been reported to be a sensitive and specific measure of frailty [7]. On the other hand, since frailty measures incorporate a mix of subjective and objective elements, they could have obscured our analyses (e.g. had we considered change in frailty status as an outcome). Instead, mobility measured by TUG is a simple and objective measure, which we see as an asset of the study.
A limitation is that we did not analyse people who had TUG and CRT at Wave 1 and did not return to Wave 3 (42%), for instance those who died or withdrew. As dropouts in longitudinal studies of ageing are typically associated with higher risk profiles [41], it is possible that our findings are a conservative estimate. This is a potential source of result bias which may limit the generalisability of our work to the Irish older population and that of other countries. At the same time, it is possible that the divergence of motor trajectories could become stronger over a longer period of follow-up, which merits further research using the further waves of TILDA and other longitudinal cohorts where similar data are available. To facilitate comparability across studies, researchers have stated that it would be important to have a standardised assessment battery that captures shared characteristics of mobility and cognition seen in ageing [42].
Moreover, our method of analysis evaluates mean values and therefore presumes that change in function over time is relatively uniform. Longitudinal studies tracking function identify different trajectories of change (typically 5) which are not appreciated in our work unlike the analyses based on the PEP [43] or Boston RISE [44] studies.
In conclusion, TILDA participants aged 50 years or older who had a mean MRT of ≥ 250 ms at baseline seemed to have, compared to those with a baseline MRT < 250 ms, a faster mobility decline, as measured by the TUG, approximately 4 years later. However, this effect was both statistically and clinically small, and further research is required to clarify its significance. Residual confounding of our results by sensorimotor function is still possible. We recommend that further studies with longer follow-up attempt to establish if longer MRTs could be an early maker of accelerated mobility decline; if that were the case, early cognitive interventions (e.g. cognitive training) might be able to prevent accelerated mobility decline and premature loss of physical independence, which is of enormous human and economic importance in our ageing societies. Our study highlights the need for clinicians to be aware of the brain-body connection. Mobility and cognitive impairment dynamically unfold together across life, but the understanding of this relationship still needs more investigation [45].
Funding
TILDA is funded by Atlantic Philanthropies, the Irish Department of Health and Irish Life. Roman Romero-Ortuno is funded by a Grant from Science Foundation Ireland under Grant number 18/FRL/6188. The funders did not have any involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Authors’ contributions
RC had substantial contributions to the conception and design of the work, the acquisition, analysis, and interpretation of data for the work, revision of the work and its approval. RRO contributed to the conception and design of the work, analysis and interpretation of data for the work, revision of the work and its approval.
Data sharing statement
TILDA data was accessed via the Irish Social Science Data Archive - www.ucd.ie/issda. The publicly accessible dataset files are hosted by the Irish Social Science Data Archive based in University College Dublin, and the Interuniversity Consortium for Political and Social Research (ICPSR) based in the University of Michigan. Researchers wishing to access the data must complete a request form, available on either the ISSDA or ICPSR website. Both authors had full access to the full data in the study and accept responsibility to submit for publication.
Declaration of Competing Interest
The authors have no conflicts of interests.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100676.
Appendix. Supplementary materials
References
- 1.Demnitz N., Esser P., Dawes H. A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait Posture. 2016;50:164–174. doi: 10.1016/j.gaitpost.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shumway-Cook A., Woollacott M. Attentional demands and postural control: the effect of sensory context. J Gerontol A Biol Sci Med Sci. 2000;55(1):M10–M16. doi: 10.1093/gerona/55.1.m10. [DOI] [PubMed] [Google Scholar]
- 3.Wang D., Zhang J., Sun Y., Zhu W., Tian S., Liu Y. Evaluating the fall risk among elderly population by choice step reaction test. Clin Interv Aging. 2016;11:1075–1082. doi: 10.2147/CIA.S106606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verghese J., Lipton R.B., Hall C.B., Kuslansky G., Katz M.J., Buschke H. Abnormality of gait as a predictor of non-Alzheimer's dementia. N Engl J Med. 2002;347(22):1761–1768. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- 5.Verghese J., Wang C., Lipton R.B., Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci. 2013;68(4):412–418. doi: 10.1093/gerona/gls191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman T., Giladi N., Hausdorff J.M. Properties of the “timed up and go” test: more than meets the eye. Gerontology. 2011;57(3):203–210. doi: 10.1159/000314963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savva G.M., Donoghue O.A., Horgan F., O'Regan C., Cronin H., Kenny R.A. Using timed up-and-go to identify frail members of the older population. J Gerontol A Biol Sci Med Sci. 2013;68(4):441–446. doi: 10.1093/gerona/gls190. [DOI] [PubMed] [Google Scholar]
- 8.Van Uem J.M.T., Walgaard S., Ainsworth E. Quantitative timed-up-and-go parameters in relation to cognitive parameters and health-related quality of life in mild-to-moderate Parkinson's Disease. PLoS ONE. 2016;11(4) doi: 10.1371/journal.pone.0151997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kear B.M., Guck T.P., McGaha A.L. Timed up and go (TUG) test: normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health. 2017;8(1):9–13. doi: 10.1177/2150131916659282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGough E.L., Kelly V.E., Logsdon R.G. Associations between physical performance and executive function in older adults with mild cognitive impairment: gait speed and the timed “up & go” test. Phys Ther. 2011;91(8):1198–1207. doi: 10.2522/ptj.20100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donoghue O.A., Horgan N.F., Savva G.M., Cronin H., O'Regan C., Kenny R.A. Association between timed up-and-go and memory, executive function, and processing speed. J Am Geriatr Soc. 2012;60(9):1681–1686. doi: 10.1111/j.1532-5415.2012.04120.x. [DOI] [PubMed] [Google Scholar]
- 12.Woods D.L., Wyma J.M., Yund E.W., Herron T.J., Reed B. Age-related slowing of response selection and production in a visual choice reaction time task. Front Hum Neurosci. 2015;9 doi: 10.3389/fnhum.2015.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenny R.A., Coen R.F., Frewen J., Donoghue O.A., Cronin H., Savva G.M. Normative values of cognitive and physical function in older adults: findings from the Irish Longitudinal Study on Ageing. J Am Geriatr Soc. 2013;61(Suppl 2):S279–S290. doi: 10.1111/jgs.12195. [DOI] [PubMed] [Google Scholar]
- 14.Kearney P.M., Cronin H., O'Regan C. Cohort profile: the Irish longitudinal study on ageing. Int J Epidemiol. 2011;40(4):877–884. doi: 10.1093/ije/dyr116. [DOI] [PubMed] [Google Scholar]
- 15.Donoghue O.A., McGarrigle C.A., Foley M., Fagan A., Meaney J., Kenny R.A. Cohort profile update: the irish longitudinal study on ageing (TILDA) Int J Epidemiol. 2018;47(5):1398. doi: 10.1093/ije/dyy163. 1398l. [DOI] [PubMed] [Google Scholar]
- 16.Archive ISSD. Home, Irish Social Science Data Archive. Irish Social Science Data Archive. Accessed November 13, 2020. https://www.ucd.ie/issda/data/tilda/wave1/
- 17.Archive ISSD. Home, Irish Social Science Data Archive. Irish Social Science Data Archive. Accessed November 13, 2020. https://www.ucd.ie/issda/data/tilda/wave3/
- 18.Mosca I., Wright R.E. Effect of retirement on cognition : evidence from the Irish marriage bar. Demography. Published online June 7, 2018. Accessed November 13, 2020. 10.1007/s13524-018-0682-7 [DOI] [PMC free article] [PubMed]
- 19.O'Halloran A.M., Finucane C., Savva G.M., Robertson I.H., Kenny R.A. Sustained attention and frailty in the older adult population. J Gerontol B Psychol Sci Soc Sci. 2014;69(2):147–156. doi: 10.1093/geronb/gbt009. [DOI] [PubMed] [Google Scholar]
- 20.Hultsch D.F., MacDonald S.W.S., Dixon R.A. Variability in reaction time performance of younger and older adults. J Gerontol B Psychol Sci Soc Sci. 2002;57(2):P101–P115. doi: 10.1093/geronb/57.2.p101. [DOI] [PubMed] [Google Scholar]
- 21.Arevalo‐Rodriguez I., Smailagic N., Roqué i Figuls M. Mini‐Mental State Examination (MMSE) for the detection of Alzheimer's disease and other dementias in people with mild cognitive impairment (MCI) Cochrane Database Syst Rev. 2015;2015(3) doi: 10.1002/14651858.CD010783.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carleton R.N., Thibodeau M.A., Teale M.J.N. The center for epidemiologic studies depression scale: a review with a theoretical and empirical examination of item content and factor structure. PLoS ONE. 2013;8(3):e58067. doi: 10.1371/journal.pone.0058067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olssøn I., Mykletun A., Dahl A.A. The Hospital Anxiety and Depression Rating Scale: a cross-sectional study of psychometrics and case finding abilities in general practice. BMC Psychiatry. 2005;5:46. doi: 10.1186/1471-244X-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Documentation - the Irish longitudinal study on ageing (TILDA) - trinity college Dublin. Accessed November 13, 2020. https://tilda.tcd.ie/data/documentation/
- 25.Hox J.J. Lawrence Erlbaum Associates; 2002. Multilevel analysis: techniques and applications. [Google Scholar]
- 26.Richardson J.T.E. Eta squared and partial eta squared as measures of effect size in educational research. Educ Res Rev. 2011;6(2):135–147. doi: 10.1016/j.edurev.2010.12.001. [DOI] [Google Scholar]
- 27.Mini-Mental State Examination (MMSE) for the detection of dementia in people aged over 65. doi:10.1002/14651858.CD011145.pub2
- 28.Demnitz N., Zsoldos E., Mahmood A. Associations between mobility, cognition, and brain structure in healthy older adults. Front Aging Neurosci. 2017;9 doi: 10.3389/fnagi.2017.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Handing E.P., Chen H., Rejeski W.J. Cognitive function as a predictor of major mobility disability in older adults: results from the LIFE study. Innov Aging. 2019;3(2) doi: 10.1093/geroni/igz010. igz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marusic U., Verghese J., Mahoney J.R. Cognitive-based interventions to improve mobility: a systematic review and meta-analysis. J Am Med Dir Assoc. 2018;19(6):484–491. doi: 10.1016/j.jamda.2018.02.002. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Netz Y. Is there a preferred mode of exercise for cognition enhancement in older age?—a narrative review. Front Med. 2019;6 doi: 10.3389/fmed.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lord S.R., Fitzpatrick R.C. Choice stepping reaction time: a composite measure of falls risk in older people. J Gerontol A Biol Sci Med Sci. 2001;56(10):M627–M632. doi: 10.1093/gerona/56.10.m627. [DOI] [PubMed] [Google Scholar]
- 33.Christensen H., Mackinnon A.J., Korten A., Jorm A.F. The “common cause hypothesis” of cognitive aging: evidence for not only a common factor but also specific associations of age with vision and grip strength in a cross-sectional analysis. Psychol Aging. 2001;16(4):588–599. doi: 10.1037//0882-7974.16.4.588. [DOI] [PubMed] [Google Scholar]
- 34.Rosso A.L., Studenski S.A., Chen W.G. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci. 2013;68(11):1379–1386. doi: 10.1093/gerona/glt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prins N.D., van Dijk E.J., den Heijer T. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain J Neurol. 2005;128(Pt 9):2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- 36.Nadkarni N.K., Perera S., Snitz B.E. Association of Brain Amyloid-β with slow gait in elderly individuals without dementia: influence of cognition and apolipoprotein E ε4 Genotype. JAMA Neurol. 2017;74(1):82–90. doi: 10.1001/jamaneurol.2016.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thwaites J.H. Practical aspects of drug treatment in elderly patients with mobility problems. Drugs Aging. 1999;14(2):105–114. doi: 10.2165/00002512-199914020-00003. [DOI] [PubMed] [Google Scholar]
- 38.Mort J.R. Geriatric primer: implications and management of decline for the elderly patient. Consult Pharm J Am Soc Consult Pharm. 2009;24(8):611–625. doi: 10.4140/tcp.n.2009.611. [DOI] [PubMed] [Google Scholar]
- 39.Demnitz N., Hogan D.B., Dawes H. Cognition and mobility show a global association in middle- and late-adulthood: analyses from the Canadian Longitudinal Study on Aging. Gait Posture. 2018;64:238–243. doi: 10.1016/j.gaitpost.2018.06.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donoghue O.A., Savva G.M., Börsch-Supan A., Kenny R.A. Reliability, measurement error and minimum detectable change in mobility measures: a cohort study of community-dwelling adults aged 50 years and over in Ireland. BMJ Open. 2019;9(11) doi: 10.1136/bmjopen-2019-030475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ibrahim J.G., Molenberghs G. Missing data methods in longitudinal studies: a review. Test Madr Spain. 2009;18(1):1–43. doi: 10.1007/s11749-009-0138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montero-Odasso M., Almeida Q.J., Bherer L. Consensus on shared measures of mobility and cognition: from the canadian consortium on neurodegeneration in aging (CCNA) J Gerontol A Biol Sci Med Sci. 2019;74(6):897–909. doi: 10.1093/gerona/gly148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Z., Han L., Gahbauer E.A., Allore H.G., Gill T.M. Joint trajectories of cognition and frailty and associated burden of patient-reported outcomes. J Am Med Dir Assoc. 2018;19(4):304–309. doi: 10.1016/j.jamda.2017.10.010. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pedersen M.M., Holt N.E., Grande L. Mild cognitive impairment status and mobility performance: an analysis from the Boston RISE study. J Gerontol A Biol Sci Med Sci. 2014;69(12):1511–1518. doi: 10.1093/gerona/glu063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrucci L., Cooper R., Shardell M., Simonsick E.M., Schrack J.A., Kuh D. Age-related change in mobility: perspectives from life course epidemiology and geroscience. J Gerontol A Biol Sci Med Sci. 2016;71(9):1184–1194. doi: 10.1093/gerona/glw043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.