Abstract
Purpose
To investigate chest computed tomography (CT) findings in asymptomatic patients tested positive for coronavirus disease (COVID-19) by reverse transcription-polymerase chain reaction (RT-PCR).
Material and methods
The chest CT images of 64 patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) who were RT-PCR test–positive but asymptomatic were retrospectively evaluated for the appearance and distribution of abnormal parenchymal findings.
Results
Of the 64 patients (mean age 59.4 ± 12; range 23–85), 42 (65%) were female, and 22 (35%) were male, and 16 (25%) of the patients had no abnormal findings on chest CT. Of the remaining 48 patients, lung involvement was bilateral in 32 (67%). Right upper lobe in 26 (54%), right middle lobe in 20 (42%), right lower lobe in 38 (79%), left upper lobe in 27 (56%), and left lower lobe were affected in 34 (71%) patients. The mean number of opacities detected in patients was 7.5 ± 5.7. The opacities were located only peripherally/subpleural in 22 (46%), only centrally/peribronchovascular in 5 (10%), and mixed in 21 (44%) patients. The frequency of pure ground glass opacities (GGO) was 63% GGO with a crazy-paving pattern or consolidation was 33%. Pure consolidation was detected in only two (4%) patients. Parenchymal opacities were only round in 27 (56%), only geographic demarcated in 3 (6%), only patchy in 2 (4%), and mixed in 16 (33%) patients.
Conclusion
Chest CT was normal in only one-quarter of the asymptomatic patients. CT findings in asymptomatic COVID-19 patients were often peripherally located, mostly round-shaped GGO.
Keywords: Asymptomatic, Computed tomography, Coronavirus disease, COVID-19, SARS-CoV-2
1. Introduction
The novel coronavirus disease 2019 (COVID-19) was first reported in Wuhan, Hubei province, China, spread rapidly to other parts of China and the world.1 The World Health Organization defined the COVID-19 disease as a pandemic on March 11, 2020. As of July 16, 2020, there have been 13,378,853 confirmed cases worldwide and 580,045 deaths.2 The cause of the disease is an RNA virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that belongs to the coronavirus family. For a definitive diagnosis, the SARS-CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) test from mucus or saliva sample must be positive. Another diagnostic method is the sequencing of the viral gene in blood and respiratory samples.3 However, RT-PCR sensitivity has been reported between 30% and 60% in the first test due to difficulties in sample acquisition, handling, and kit performance.4 Therefore, in COVID-19 diagnosis, a history of close contact with a positive patient and the presence of frequently defined COVID-19 clinical radiological findings gain importance.
Clinical findings in people infected with SARS-CoV-2 range from an asymptomatic course to severe pneumonia requiring mechanical ventilation. The most common symptoms of COVID-19 disease have been reported as fever, dry cough, sore throat, muscle pain, dyspnea, headache, and fatigue. Besides, loss of taste and smell, diarrhea, and vomiting are frequently reported symptoms.5 , 6 In a study conducted with 72,314 COVID-19 cases, the rate of RT-PCR positive asymptomatic patients was noticed as 1%.7 In a study examining 3711 cases, the rate of RT-PCR-positive asymptomatic patients was reported as 51.7%.8 Since the beginning of the epidemic, there have been studies describing chest computed tomography (CT) findings of COVID-19 disease. However, information about the presence and nature of radiological findings in asymptomatic people infected with the virus is limited. This study aimed to describe the CT findings of asymptomatic COVID-19 patients with RT-PCR-confirmed infection.
2. Materials and methods
2.1. Study design and patients
Approval for the study was obtained from the local faculty ethics committee. The study was retrospective, and the ethics committee did not require informed consent. When COVID-19 cases started to appear in our country, everyone from abroad was tested with RT-PCR and was quarantined for 14 days. Chest CT was obtained from patients who were positive for RT-PCR, whether symptomatic or not.
We retrospectively reviewed the RT-PCR test results of people isolated in quarantine between March 30, 2020, and May 1, 2020. Six hundred eighty-four patients with RT-PCR-positive were included in the study. Out of six hundred eighty-four individuals, 64 patients did not exhibit symptoms of the disease (fever, cough, sore throat, shortness of breath, weakness, muscle pain, fatigue, diarrhea, anosmia, and loss of taste). The patients who are initially (at the time of CT examination) or subsequently (during the 14 days in quarantine) symptomatic were excluded from the study (Fig. 1 ). Chest CT images of asymptomatic patients were evaluated, and the findings were recorded.
Fig. 1.
Flowchart of patient selection. RT-PCR, reverse transcription-polymerase chain reaction; CT, computed tomography.
2.2. CT scanning protocol
All chest CT images were obtained using the Somatom Definition Flash (Siemens Healthcare, Erlangen, Germany) scanner without contrast. Patients were scanned from the apex to the base while holding their breath after inspiration in the supine position. The chest CT parameters were as follows: tube voltage 100–120 kV; slice thickness 3 mm; pitch 0.8; automatic tube current (Care Dose 4D) 50–180 mA; and matrix 512 × 512.
2.3. CT image analysis
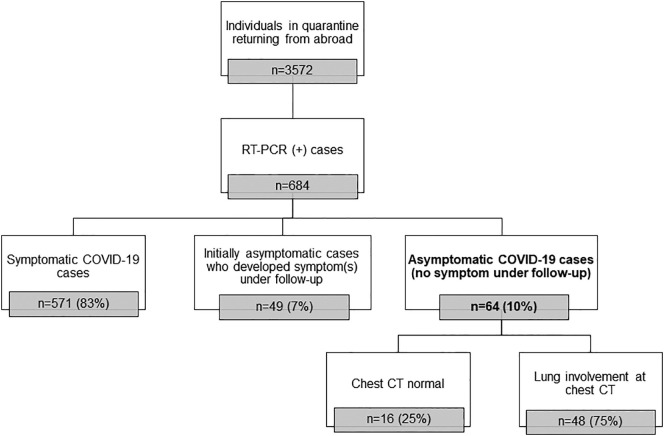
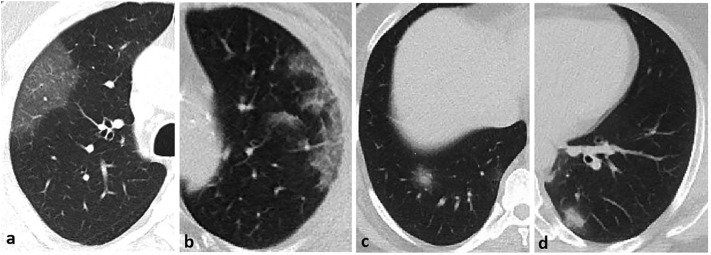
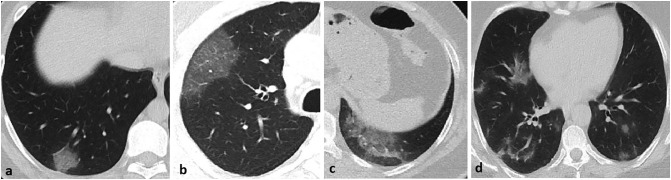
The CT images were independently examined by two radiologists with more than ten years' chest radiology experience. Final decisions were reached by consensus. In the inconsistency case, a third radiologist with 13 years' chest imaging experience evaluated the images and reached a common decision. CT images were evaluated for the appearance, localization, and distribution of abnormal parenchymal findings. The appearance of parenchymal changes was evaluated in the following sub-categories: a) pure ground glass opacity (GGO), b) interlobular and intralobular septal thickening with GGO (crazy paving pattern), c) consolidation with GGO, and d) pure consolidation (Fig. 2 ). GGO is described as increasing attenuation in the lung parenchyma with the preservation of vascular margins. Consolidation is defined as opacification in the lung parenchyma, causing the obscuring of the vessels. The shape of the parenchymal opacities was evaluated in four groups: round, geographic demarcated, patchy, and mixed (Fig. 3 ). The localization of parenchymal opacities was divided into three groups: central/peribronchovascular, peripheral/subpleural, or mixed (Fig. 4 ). Besides, the number of affected lobes in each patient and the total number of opacities observed in the parenchyma were recorded. Accompanying abnormalities, such as pleural effusion, emphysema, bronchiectasis, and mediastinal or hilar lymphadenopathy (lymph node short-axis > 10 mm), were noted.
Fig. 2.
The appearance of parenchymal opacities: a) pure ground glass opacity (GGO), b) interlobular and intralobular septal thickening with GGO (crazy paving pattern), c) consolidation with GGO, and d) pure consolidation.
Fig. 3.
The shape of parenchymal opacities: a) round, b) geographic demarcated, c) patchy, and d) mixed.
Fig. 4.
The localization of parenchymal opacities: a) peripheral/subpleural, b) central/peribronchovascular (arrow), c) mixed.
Chest CT findings of the asymptomatic patients were also categorized as ʻʻtypicalʼʼ, ʻʻindeterminateʼʼ, ʻʻatypicalʼʼ, and ʻʻnegativeʼʼ according to the Radiological Society of North America (RSNA) consensus statement for CT reporting of suspected COVID-19 pneumonia.9
2.4. Statistical analyses
The continuous variables of the study were expressed as mean ± standard deviation. The frequency of CT findings was expressed as numbers and percentages. The distribution of the opacities (lower vs. upper lobes) was compared with Fisher's exact test. Right and left lung involvement was compared with a Chi-squared test. The number of parenchymal opacities in males and females was compared using the Mann-Whitney U test. The Pearson correlation test was performed to evaluate the relationship between patient age and number of opacities. A P value of less than 0.05 was considered significant. SPSS version 23.0 (IBM Statistics) was used for the statistical analysis.
3. Results
The demographic and clinical data of the patients included in the study are shown in Table 1 . No abnormality was found in clinical and laboratory findings in all patients. Sixteen (25%) of the 64 patients had no abnormal findings on chest CT. The average age of these patients was 59 ± 11.4. Forty-eight patients (mean age 59.6 ± 12.3) had abnormal findings on chest tomography. There was no difference in mean age between patients with abnormal findings on chest CT and those without (p > 0.05).
Table 1.
Demographic and clinical features of asymptomatic COVID-19 patients (n = 64).
| Count | Percent | |
|---|---|---|
| Gender | ||
| Male | 22 | 35 |
| Female | 42 | 65 |
| Mean ± standard deviation (min–max) | |
|---|---|
| Age | 59.4 ± 12 (23–85) |
| Respiratory rate/min | 16 ± 2.4 (13−20) |
| Oxyhemoglobin saturation, % | 95 ± 1.2 (92–96) |
| White blood cell count, ×109/L | 6470 ± 1100 (4500–9850) |
| Lymphocyte count, ×109/L | 2800 ± 765 (1000–4500) |
| C-reactive protein, mg/L | 5.7 ± 1.4 (3–8) |
| D-dimer, μg/L | 280 ± 130 (120–450) |
3.1. Distribution of parenchymal abnormalities
Excluding the 16 patients with normal chest CT, forty-two (87.5%) patients had abnormal findings in the right lung parenchyma, while 39 (81.2%) patients had abnormal findings in the left lung parenchyma. Right and left lung involvement rates were very similar, and there was no significant difference (p > 0.05).
The number of affected lobes in 48 patients with abnormal findings on chest CT is shown in Table 2 . Bilateral lung involvement rate and the frequency of the lobes affected in both lungs are indicated in Table 2. Although the bilateral lower lobe percentage was higher, there was no significant difference between the lower and upper lobe involvement rates (p > 0.05). The mean number of opacities detected in patients was 7.5 ± 5.7 (range 1–20), while it was 8.8 ± 6.1 (1−20) in females and 5.3 ± 4.3 (1–15) in males. The number of parenchymal opacities was not significantly different between genders (p = 0.05). No correlation was found between the number of parenchymal opacities and age (Fig. 5 ).
Table 2.
Distribution of parenchymal opacities and number of affected lobes in asymptomatic COVID-19 patients (n = 48).
| No | Percent (%) | |
|---|---|---|
| Lung involvement | ||
| Unilateral | 16 | 33.3 |
| Bilateral | 32 | 66.7 |
| Frequency of lobe involvement | ||
| Right upper lobe | 26 | 54.2 |
| Right middle lobe | 20 | 41.7 |
| Right lower lobe | 38 | 79.2 |
| Left upper lobe | 27 | 56.2 |
| Left lower lobe | 34 | 70.8 |
| Number of affected lobes | ||
| One lobe | 15 | 31.3 |
| Two lobes | 5 | 10.4 |
| Three lobes | 9 | 18.8 |
| Four lobes | 4 | 8.3 |
| Five lobes | 15 | 31.3 |
Fig. 5.
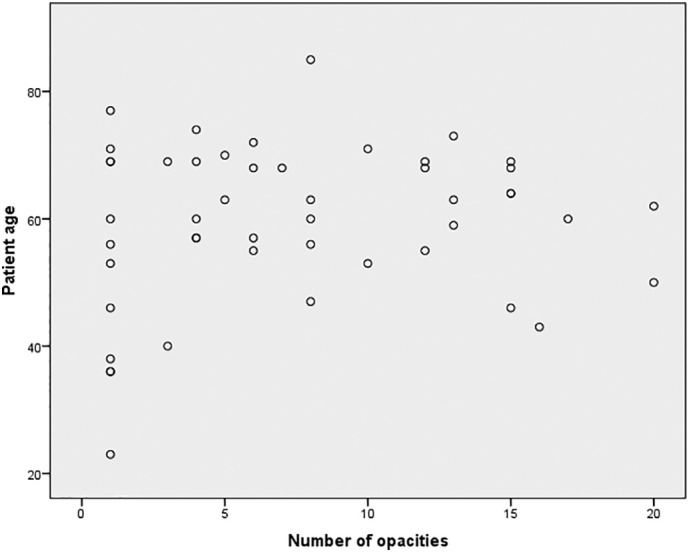
The scatter plot shows the relationship between the number of opacities and age in asymptomatic COVID-19 patients.
3.2. The appearance and localization of parenchymal abnormalities
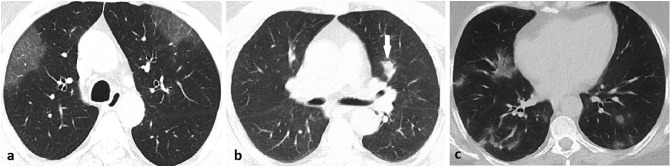
The appearance of lung opacities in patients is summarized in Table 3 . In our study group, the frequency of pure GGO was 62.5%, and GGO with crazy paving pattern or consolidation was 33.3%. Parenchymal involvement consisted of pure consolidation in only two (4.2%) patients. Also, four (8.4%) patients had a reverse halo sign (Fig. 6 ). The shape of lung parenchymal opacities in asymptomatic COVID-19 patients is shown in Table 3. Parenchymal opacities were only patchy in only 2 (4.1%) patients.
Table 3.
The appearance, shape, and localization of parenchymal opacities in asymptomatic COVID-19 patients (n = 48).
| No | Percent (%) | |
|---|---|---|
| Appearance of parenchymal opacities | ||
| Ground-glass opacity (only) | 30 | 62.5 |
| Ground-glass opacity with consolidation | 8 | 16.7 |
| Ground-glass opacity with interlobular and intralobular septal thickening (crazy paving pattern) | 8 | 16.7 |
| Consolidation (only) | 2 | 4.2 |
| Shape of parenchymal opacities | ||
| Round (only) | 27 | 56.2 |
| Geographic demarcated (only) | 3 | 6.2 |
| Patchy (only) | 2 | 4.1 |
| Mixed | 16 | 33.3 |
| Localization of parenchymal opacities | ||
| Peripheral/subpleural (only) | 22 | 45.8 |
| Central/peribronchovascular (only) | 5 | 10.4 |
| Mixed | 21 | 43.8 |
Fig. 6.
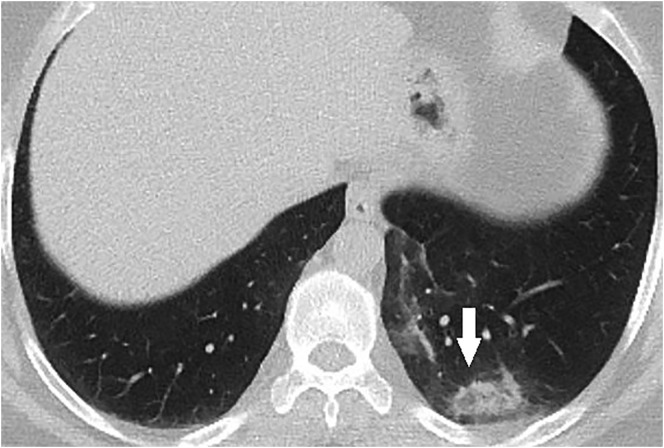
44-year-old male RT-PCR positive asymptomatic patient. Axial CT image shows the reverse halo sign in the lower lobe of the left lung (arrow).
The localization of parenchymal opacities in the lungs in patients is shown in Table 3. Lesions are only centrally/peribronchovascular located in 5 (10.4%) of 48 asymptomatic patients with abnormal CT findings.
Classification of findings according to the RSNA consensus statement for CT reporting of suspected COVID-19 pneumonia in asymptomatic COVID-19 patients indicated in Table 4 . None of the patients had an atypical appearance, according to the RSNA consensus statement.
Table 4.
Classification of chest CT findings of asymptomatic COVID-19 patients according to the Radiological Society of North America (RSNA) consensus statement for CT reporting of suspected COVID-19 pneumonia.
| COVID-19 pneumonia imaging classification | No | Percent (%) |
|---|---|---|
| Typical appearance | 33 | 51.5 |
| Indeterminate appearance | 15 | 23.5 |
| Atypical appearance | 0 | 0 |
| Negative for pneumonia | 16 | 25 |
No accompanying findings such as pleural effusion, emphysema, bronchiectasis, or mediastinal or hilar lymphadenopathy were found on the chest CT of any patient.
4. Discussion
COVID-19 pneumonia, caused by the novel coronavirus SARS-CoV-2, has spread around the world in a short time. Studies showing the radiological findings of COVID-19 pneumonia in symptomatic patients have been conducted. Hu et al. reported the clinical and radiological findings of 24 asymptomatic cases.10 However, the authors reported that 5 (20.8%) of 24 patients developed symptoms during hospitalization. Also, there is another study in which patients admitted to the hospital with symptoms not associated with COVID-19 were qualified as asymptomatic individuals, and their chest CTs were evaluated.11 In this study, 5.3% of asymptomatic individuals were incidentally identified as RT-PCR positive for COVID-19. However, we could not find any study reporting chest CT findings, especially by including RT-PCR positive and asymptomatic COVID-19 patients. In this study, we specifically investigated the chest CT findings of 64 patients who were laboratory-validated but had no specific symptoms of COVID-19 disease. Chest CTs were normal in 16 of 64 patients (25%). Similarly, Hu et al. reported the rate of asymptomatic patients with normal chest CT findings as 29.4%.10
In a study with twenty-one symptomatic patients, the bilateral lung parenchymal involvement rate was reported as 76%.12 Song et al., in their study of 51 symptomatic patients, reported the rate of bilateral involvement to be 86%.13 In a study investigating the differences between COVID-19 pneumonia and influenza pneumonia, the bilaterality rate was 85% in 122 COVID-19 patients.14 In our study, we determined that the rate of bilateral involvement was 67%. When our study results are compared with previous studies with symptomatic patients, we can say that the rate of bilateral lung involvement in asymptomatic patients is slightly lower than in symptomatic patients.[12], [13], 14. In studies conducted with symptomatic COVID-19 patients, no significant difference was reported between right and left lung involvement rates.15 , 16 Similarly, we found no significant difference between the two lung involvement rates in asymptomatic patients.
Song et al. reported a single-lobe involvement rate of 8% and the rate of five lobes' involvement as 39% in a series of 51 cases.13 Liu et al. reported a single lobe involvement rate of 8% and a five-lobe involvement rate of 43%.14 In our asymptomatic patient population, the rate of single lobe involvement was higher than in the studies mentioned at 31%. In 31% of our patients, all five lobes were affected, and this rate was lower compared to studies of symptomatic patients.13 , 14 As for which lobes are most affected, although the bilateral lower lobe was more affected, we did not find a significant difference between the rates for lower and upper involvement in asymptomatic patients. In some studies, similar to our study, there was no significant difference between the upper and lower lobes in terms of involvement in symptomatic patients, while the frequency of involvement of the lower zones was higher in some studies.12 , 14 , 16
As for the distribution of opacities, Han et al. reported the peripheral location rate of opacities as 90% in their 108 cases.17 Studies have reported, respectively, the frequency of peripheral locations as 84%, 62.1%, and 45%.13 , 14 , 16 In our study, 5 (10%) of 48 patients had opacities only in both lungs' central/peribronchovascular part. In the remaining 43 (90%) patients, peripherally/subpleural located (only or with central) opacities were present. Research to date shows that involvement is mostly in the peripheral areas of the lungs, as in our study, and the central parts of the lungs are less affected by this disease. Since the blood and lymph flow is more intense in the peripheral (subpleural) lung parenchyma, the inflammatory response to the virus is considered to be stronger in this region, so the lesions are often peripherally located.18
In a study conducted with 24 asymptomatic patients, the rate of GGO was reported to be 50%.10 Han et al. detected ground glass opacities in 80% of 108 symptomatic patients, consolidation with GGO in 41%, and crazy paving pattern in 40%.17 In another study of 101 symptomatic patients, the frequency of GGO was reported to be 86.1%; the frequency of consolidation was 43.6%.19 In their 130-case series, Wu et al. reported the rate of GGO to be 53.2%, and the rate of consolidation with GGO was 46.2%.18 We found GGO (pure or with consolidation or crazy paving pattern) in 96% of our patients. This rate was higher in our patient group compared to the studies of symptomatic patients. The rate of total patients with consolidation (pure or with GGO) was determined to be 20% in our study, and this rate is quite low compared to the rate for symptomatic patients. Since the virus primarily invades the interstitium and causes thickening and edema in the interlobular, intralobular, and peribronchovascular interstitium, GGO appears early parenchymal finding. Consolidation appears later and indicates that the virus has invaded the parenchyma. Li et al. reported that the consolidation rate was 88% in patients with severe disease and 53.4% in patients with milder symptoms in their study, comparing the chest CT findings of patients with severe and mild COVID-19 pneumonia.20 One of the reasons why our patients are asymptomatic may be the low rate of consolidation.
Ciccarese et al., in their study with 211 COVID-19 patients, reported that chest CT findings in 71.6% of patients were ʻʻtypicalʼʼ according to RSNA consensus statement.21 Chest CT findings were categorized as 36 (17.1%) patients as ʻʻindeterminateʼʼ, 7 (3.3%) as ʻʻatypicalʼʼ, and 17 (8%) as ʻʻnegativeʼʼ. In our study, chest CT findings of asymptomatic RT-PCR-positive patients were characterized as ʻʻtypicalʼʼ in 33 (52%), ʻʻindeterminateʼʼ in 15 (24%), and ʻʻnegativeʼʼ in 16 (25%). None of the patients had an atypical appearance, according to the RSNA consensus statement for CT reporting of suspected COVID-19 pneumonia.
Our study has some limitations. First, the number of patients included in the study was relatively small. The second limitation is that children are not included in the study. Third, none of the patients had a follow-up CT chest. Finally, no inter-rater correlation was obtained.
In conclusion, only one-fourth of the asymptomatic patients had no abnormal findings on chest CT. Three-quarters of asymptomatic COVID-19 patients had abnormal lung parenchymal findings on CT. GGO (pure or with crazy-paving pattern or consolidation) was the most common appearance of lung parenchymal opacities in asymptomatic COVID-19 patients. Peripheral/subpleural (with or without central/peribronchovascular) was the most common localization of the disease. Round (with or without patchy) was the most common shape of the opacities found in asymptomatic COVID-19 patients.
Declaration of competing interest
The authors declare that there is no conflict of interest.
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (July 16, 2020) Coronavirus disease 2019 (COVID-19) Geneva, Switzerland: WHO; 2020. Situation report-178. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200716-covid-19-sitrep-178.pdf?sfvrsn=28ee165b_2
- 3.Li X., Geng M., Peng Y., et al. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y., Yang M., Shen C. 2020. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. [DOI] [Google Scholar]
- 5.Cascella M., Rajnik M., Cuomo A., et al. Statpearls [internet] StatPearls Publishing; 2020. Features, evaluation and treatment coronavirus (COVID-19)https://www.ncbi.nlm.nih.gov/books/NBK554776/ [PubMed] [Google Scholar]
- 6.Stokes E.K., Zambrano L.D., Anderson K.N., et al. Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 8.Mizumoto K., Kagaya K., Zarebski A., et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson S., Kay F.U., Abbara S., et al. Radiological Society of North America expert consensus statement on reporting Chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA - Secondary Publication. J Thorac Imaging. 2020;35:219–227. doi: 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Z., Song C., Xu C., et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63:706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Smet K, De Smet D, Ryckaert T, et al. Diagnostic performance of chest CT for SARS-CoV-2 infection in individuals with or without COVID-19 symptoms. Radiology. 10.1148/radiol.2020202708. [DOI] [PMC free article] [PubMed]
- 12.Chung M., Bernheim A., Mei X., et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song F., Shi N., Shan F., et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295:210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu M, Zeng W, Wen Y, et al. COVID-19 pneumonia: CT findings of 122 patients and differentiation from influenza pneumonia. Eur Radiol 10.1007/s00330-020-06928-0. [DOI] [PMC free article] [PubMed]
- 15.Zhou S., Wang Y., Zhu T., et al. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR. 2020;214:1287–1294. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 16.Zhou S, Zhu T, Wang Y, et al. Imaging features and evolution on CT in 100 COVID-19 pneumonia patients in Wuhan, China, Eur Radiol 10.1007/s00330-020-06879-6. [DOI] [PMC free article] [PubMed]
- 17.Han R., Huang L., Jiang H., et al. Early clinical and CT manifestations of coronavirus disease 2019 (COVID-19) pneumonia. AJR. 2020;215:1–6. doi: 10.2214/AJR.20.22961. [DOI] [PubMed] [Google Scholar]
- 18.Wu J, Pan J, Teng D, et al. Interpretation of CT signs of 2019 novel coronavirus (COVID-19) pneumonia. Eur Radiol. 10.1007/s00330-020-06915-. [DOI] [PMC free article] [PubMed]
- 19.Zhao W., Zhong Z., Xie X., et al. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 20.Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed]
- 21.Ciccarese F, Coppola F, Spinelli D, et al. Diagnostic accuracy of North America expert consensus statement on reporting CT findings in patients with suspected COVID-19 infection: an Italian single center experience. Radiology. 10.1148/ryct.2020200312. [DOI] [PMC free article] [PubMed]