Abstract
Purpose
Five different galenics were analyzed and compared concerning tissue breathability and gas exchange with the environment after an application period of 6 h on pig ear skin. Aim was to find the most suitable galenics for efficient moist treatment for everyday injuries (abrasions, lacerations and cuts) without influencing the transepidermal water loss.
Methods
A quantity of 0.1 g of the different test preparations was applied once topically to an area of 2 cm2. The analysis of the breathability was performed by TEWL (transepidermal water loss) measurements in the first hour after product application. The moisture retention effect was assessed by corneometry in the first 5 h after product application.
Results
The hydrogel preparations showed a higher breathability in contrast to a semi-occlusive ointment and petrolatum. The same applies to the moisture penetration of the skin. Here, all hydrogel formulations showed the highest tissue hydration. After 3 h an additional increase in moisture was observed for the areas treated with Tyrosur® CareExpert Wound Gel and the ointment.
Conclusion
In contrast to petrolatum and the semi-occlusive ointment, treatment with the hydrogels led to a preservation of the breathability and good moistening of the tissue, which is due to the galenics of the gels consisting of water, carbomer and propylene glycol. The increase in moisture after 3 h in areas treated with Tyrosur® CareExpert Wound Gel and the semi-occlusive ointment indicates a sustained moisturizing effect mediated by dexpanthenol.
Keywords: Hydrogel, Skin hydration, Breathability, Transepidermal water loss, Ointment
Hydrogel, skin hydration, breathability, transepidermal water loss, ointment
1. Introduction
Hydrogels are polymers that are able to absorb a high amount of water and whose molecules are either chemically or physically linked to form a three-dimensional network. On molecular level, hydrogels are characterized by mesh size, molecular weight of the polymer chains and by their specific composition. Through chemical modifications, hydrogels can be used in solid, semi-solid and liquid form, which makes them very flexible in use. Characterized by high versatility, permeability and their similarity to living tissue, hydrogels have been used in many biomedical applications the recent years [1]. However, they are still relatively rarely used in wound healing approaches, especially for superficial wounds such as abrasions or lacerations, although obvious advantages like high biocompatibility, biodegradability, very low immunogenicity, excellent drug delivery (e.g. antibiotics) and ease of use are well known [2].
In contrast to hydrogels, ointments are usually semi-solid, spreadable and homogeneous-looking bases that are used for external application on the skin or mucous membranes, although their galenic structure is dissimilar to living tissue. From a pharmaceutical point of view, ointments are clearly distinguishable from creams, pastes and gels, as they consist of only one phase in which solid or liquid substances may be dispersed [3]. Classic ointments are hydrophobic in nature, poorly absorbed and traditionally used for the local, topical application of drugs such as dexpanthenol as wound healing agents. Due to their (semi)-occlusive character, ointments are not suitable for all biomedical applications such as weeping dermatoses. Here, moist compresses must be used additionally. In contrast, anhydrous ointments are well suited for the treatment of chronic dermatoses paired with hyperkeratosis [4]. Even today, wound healing ointments are frequently used for superficial injuries, although alternative galenics are nowadays often more suitable.
Fast and efficient wound healing is of fundamental importance in order to quickly and completely restore the integrity of the damaged tissue and the protective function of the skin. Contrary to the still widespread opinion that everyday wounds heal best at dry air by scabbing, the current state of knowledge shows that the self-healing process can be supported therapeutically effective by an ideal moist wound management, which supports gas exchange and moisture supply, for example on the basis of modern hydrogels, which can help in all phases of healing [5, 6]. In the present study, ear skin from domestic pigs was used, which anatomically and physiologically has many properties in common with human skin and is therefore very well suited as model tissue [7, 8]. The aim of the study was to investigate and compare the breathability and moisturizing effect, elementary parameters of moist wound management, of five different preparations (three hydrogels, one semi-occlusive and one occlusive ointment). For this purpose, the transepidermal water loss (TEWL) and moisture were determined over a defined period of time by means of corneometry.
2. Material & methods
2.1. Pig ear skin
Pig ears (Sus scrofa domesticus) used in the present study were obtained from a local butcher. Pigs were exclusively slaughtered for food production. Ears (slaughterhouse waste) were purchased within 3 h after slaughtering. Bristles were carefully trimmed with scissors, pig ears were thoroughly washed with lukewarm water, gently blotted with cellulose wipes, and allowed to dry in an air-conditioned room at a constant temperature of 22 ± 1°C and 56 ± 2% humidity for 30 min. Weight of pig ears ranged from 61g to 67g with a thickness of 3 mm – 22 mm. Five 2 cm2 areas were drawn on pig ears on which the test products were subsequently randomly applied by means of a fingerstall. Substances were only applied in areas with a thickness of 8–10 mm to avoid area effects.
2.2. Test substances
Of each test substance, 0.1 g was applied once to an area of 2 cm2.
Tyrosur® Wound Healing Gel; Type: Hydrogel.
Ingredients: carbomer, propylene glycol, cetylpyridinium chloride, trometamol, ethanol tyrothricin, purified water.
Tyrosur® CareExpert Wound Gel; Type: Hydrogel.
Ingredients: carbomer, propylene glycol, xanthan gum, dexpanthenol, allantoin, vitamin E, cetylpyridinium chloride, trometamol, ethanol, citric acid, purified water.
Tyrosur® CareExpert Wound Gel without dexpanthenol; type: Hydrogel.
Ingredients: identical to Tyrosur® CareExpert Wound gel, only without dexpanthenol.
Bepanthen® Ointment; Type: Semi-occlusive ointment.
Ingredients: Cetyl alcohol, stearyl alcohol, white beeswax, wool fat, white soft paraffin, refined almond oil, liquid paraffin, Protegin X and purified water and 5% dexpanthenol.
White petrolatum; Type: occlusive ointment.
Ingredient: petrolatum.
2.3. Transepidermal water loss (TEWL)
The Tewameter® TM 300 (Courage & Khazaka, Cologne, Germany) was used to measure the TEWL. The TEWL is an indicator for the integrity of the skin barrier and the gas exchange of the tissue with the environment. Measurements were taken before (initial value) and 0, 10, 20, 30, 40, 50 and 60 min after product application. At each point in time, two separate measurements with 30 individual measurements each were performed for every area and values were averaged.
2.4. Corneometry
The skin moisture was determined by means of the Corneometer® CM 825 (Courage & Khazaka, Cologne, Germany) before (initial value) and 0, 1, 2, 3, 4, and 5 h after application of the product. Three independent measurements per test area were performed at each point in time and values were averaged.
All measurements were performed at constant temperature (21 ± 1°C) and humidity (54 ± 4%) after the tissue had acclimatized to the given conditions for 30 min. An untreated area served as control.
2.5. Statistics
Testing of the data for normal distribution was performed by Kolmogorov-Smirnov test. Analysis for significant differences was carried out using one way Anova and student's t-test. A p-value of <0.05 was considered statistically significant. The mean values and standard errors are shown.
3. Results
3.1. Transepidermal water loss
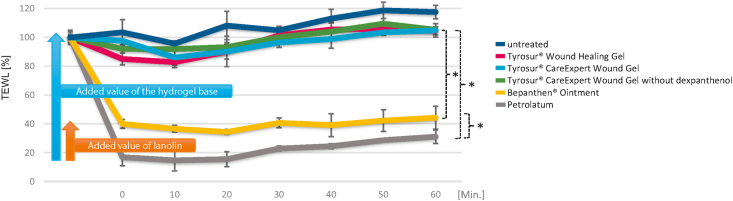
After application of the test substances, the TEWL was measured every 10 min over a period of 60 min (see Figure 1). As expected, petrolatum showed the most occlusive effect, which is in line with literature data on low gas permeability of such bases [9]. Directly after product application, the TEWL dropped to 16.9% of the initial value, which only rose again to 31.1% of the initial value in the course of the measurements. The treatment with the semi-occlusive ointment resulted in a reduction of the TEWL to 39.9% after product application, which did not change significantly during the course of the test series (44.3% after 60 min). In contrast, the skin areas treated with the hydrogels showed only a slight reduction in TEWL to 97.6% (Tyrosur® CareExpert Wound Gel), 85.1% (Tyrosur® Wound Healing Gel) and 92.0% (Tyrosur® CareExpert Wound Gel without dexpanthenol), respectively, compared to the initial value (100%), which, however, regenerated after about 30 min (see Table 1). An occlusive effect of the hydrogels was not observed over the entire measurement period. Likewise, no difference was detected between the different hydrogel bases. However, significant differences in breathability were measured between the hydrogel bases and petrolatum (p < 0.05) and the semi-occlusive ointment (p < 0.05) over the entire observation period. As expected, the untreated control showed the highest TEWL values.
Figure 1.
Transespidermal Water Loss (TEWL) measurements on pig ears with 5 different preparations over a period of 60 minutes. The 0-value refers to the first measurement directly after product application. The measured values were averaged and displayed as percentages. The standard deviation is given in each case. Significance level p < 0.05.
Table 1.
Average TEWL values over a period of 60 min for all tested preparations. All collected values were averaged and are given in percent. The values refer to the measured initial value for the respective skin area. The standard deviation is given in square brackets.
| Preparation | 0 min | 10 min | 20 min | 30 min | 40 min | 50 min | 60 min |
|---|---|---|---|---|---|---|---|
| untreated | 103.5 [± 8.8] | 95.7 [± 2.0] | 108.2 [± 9.8] | 104.94 [± 2.8] | 113.1 [± 6.5] | 118.7 {± 5.7] |
117.6 [± 4.6] |
| Tyrosur® Wound Healing Gel | 85.1 [± 4.1] | 82.8 [± 3.8] | 90.1 [± 5.2] | 101.4 [± 6.1] | 105.5 [± 5.6] | 106.0 [± 4.7] | 104.6 [± 2.7] |
| Tyrosur® CareExpert Wound Gel | 97.6 [± 6.8] | 86.1 [± 5.5] | 90.2 [± 10.5] | 96.4 [± 3.3] | 98.9 [± 6.5] | 103.5 [± 1.7] | 104.9 [± 4.6] |
| Tyrosur® CareExpert Wound Gel without dexpanthenol | 92.0 [± 4.9] | 91.9 [± 2.6] | 93.3 [± 5.0] | 100.1 [± 3.2] | 104.1 [± 4.7] | 109.5 [± 5.9] | 105.3 [± 7.9] |
| semi-occlusive ointment | 39.9 [± 3.1] | 36.6 [± 2.3] | 34.4 [± 1.7] | 40.7 [± 3.4] | 39.2 [± 7.9] | 42.3 [± 7.6] | 44.3 [± 8.0] |
| white petrolatum | 16.9 [± 6.0] | 14.7 [± 7.4] | 15.5 [± 5.2] | 22.9 [± 1.7] | 24.5 [± 1.7] | 28.7 [± 1.1] | 31.1 [± 4.7] |
Figure 1 shows the added value of the semi-occlusive ointment compared to petrolatum, as well as the hydrogel bases compared to petrolatum and the ointment. Due to the fat-free galenics of the hydrogels based on the combination of water, propylene glycol and carbomer, the tissue remains breathable.
3.2. Skin moisture
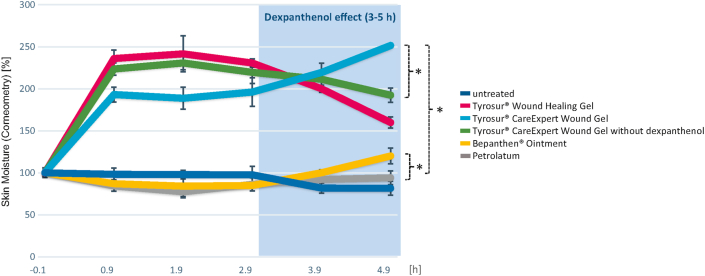
Following the TEWL measurements, the moisture of the ear skin was additionally analysed by means of a corneometer every hour for a period of 5h. The treatment with petrolatum and the semi-occlusive ointment initially resulted in slightly reduced measured values compared to the untreated control, which normalized after 4h (see Figure 2). However, it can be stated that petrolatum did not provide any moisture over the entire analysis period. All hydrogel-treated areas showed significantly increased skin moisture level (p < 0.05 vs. petrolatum and Bepanthen® Ointment) after only 1h, which was initially even more pronounced in the Tyrosur® Wound Healing Gel and the Tyrosur® CareExpert Wound Gel without dexpanthenol than in the Tyrosur® CareExpert Wound Gel with dexpanthenol (cf. Table 2). After 3h, however, moistening drops off again in the hydrogels without dexpanthenol, whereas in the dexpanthenol-containing gel and the semi-occlusive ointment a "surge of moisture" was visible after about 3h (cf. Figure 2). Five hours after product application a significant difference between the dexpanthenol-containing hydrogel and the two hydrogels without dexpanthenol was measured (p < 0.05). After 5h, the semi-occlusive ointment showed a significant difference to petrolatum (p < 0.05). These data indicate sustained tissue moistening by dexpanthenol, since only the two formulations with dexpanthenol (semi-occlusive ointment and Tyrosur® CareExpert Wound Gel) showed such an increase in moisture (dexpanthenol effect), whereas with otherwise identical formulations this effect was not observed with Tyrosur® CareExpert Wound Gel without dexpanthenol. Figure 2 shows the added moisturising value of the hydrogel bases compared with the semi-occlusive ointment (p < 0.05). The ointment only provided moisture (approx. 20 %) after 4h due to the dexpanthenol effect, whereas the hydrogels showed a clear moistening of the tissue already after 1h (between 93 % and 136 %).
Figure 2.
Corneometry measurements of 5 products on pig ears over a period of 5 hours after product application. The measured values were averaged and displayed as percentages. The standard deviation is given in each case. Significance level p < 0.05.
Table 2.
Average moisture development over a period of 5h of all tested preparations. All values were averaged and are given in percent. The values refer to the measured initial value for the respective skin area. The relative standard deviation is given in brackets.
| Preparation | 0 h | 1 h | 2 h | 3 h | 4 h | 5 h |
|---|---|---|---|---|---|---|
| untreated | 100 [± 5.5] | 98.2 [± 7.3] | 97.6 [± 5.1] | 97.6 [± 10.2] | 81.8 [± 6.1] | 81.6 [± 8.3] |
| Tyrosur® Wound Healing Gel | 100 [± 2.9] | 236.1 [± 10.0] | 241.6 [± 21.3] | 230.6 [± 4.9] | 200.1 [± 4.3] | 159.9 [± 6.6] |
| Tyrosur® CareExpert Wound Gel | 100 [± 5.7] | 193.17 [± 8.9] | 188.8 [± 12.9] | 196.2 [± 16.9] | 219.4 [± 11.1] | 251.6 [± 2.5] |
| Tyrosur® CareExpert Wound Gel without dexpanthenol | 100 [± 2.7] | 223.6 [± 7.3] | 230.7 [± 8.1] | 219.6 [± 13.3] | 211.4 [± 10.6] | [192.4] [± 8.6] |
| semi-occlusive ointment | 100 [± 3.4] | 87.0 [± 4.6] | 84.2 [± 12.7] | 84.9 [± 2.7]< | 100.0 [± 3.7] | 120.1 [± 9.4] |
| white petrolatum | 100 [± 5.5] | 84.4 [± 6.4] | 77.4 [± 7.1] | 86.3 [± 7.8] | 92.1 [± 7.3] | 94.1 [± 8.2] |
4. Discussion
In the present study, hydrogels showed the highest breathability compared to the semi-occlusive ointment and petrolatum as well as the strongest moisturizing effect over the entire experimental period (see Figures 1 and 2). The slightly higher breathability of the ointment (p < 0.05) in comparison to petrolatum only is probably due to the lanolin (wool wax) present in it. Both, the guarantee of gas exchange with the environment and the ability to moisturise tissue are important parameters of ideal moist wound management and are also decisive for rapid and physiological wound healing [5, 10]. The increase in moisture described in the results, in skin areas treated with Tyrosur® CareExpert Wound Gel and the semi-occlusive ointment, shown as a dexpanthenol effect (cf. Figure 2), ensures sustained, long-lasting moisturization of the tissue. The fact that dexpanthenol has many wound healing-promoting properties and a moisturizing effect during the wound healing process has already been well documented [11, 12]. Therefore, it is reasonable to assume that the observed effect is indeed attributable to dexpanthenol. Until about 60 years ago, dry wound healing was considered the best way to treat injuries. It was George Winter, however, who first demonstrated in 1962 that moist wound management achieved faster and more physiological healing results than traditional wound healing with crust formation [13, 14]. These findings have since been regarded as the basis of the principle of moist wound management. Although already in 2009 Alves et al. published a uniform recommendation in which ideal moist wound management was postulated as the standard therapeutic approach for all wounds, even minor injuries such as lacerations, cuts or abrasions [5], the view that wounds must heal in the air is still deeply rooted and still widespread in many patients today. However, the concept of moist wound treatment has important advantages for the patient over dry wound treatment. Clinical studies have repeatedly confirmed that moist conditions lead to faster wound contraction and can accelerate wound healing by up to 50% [13,15,16,]. Furthermore, an increased proliferation rate, accelerated cell migration into the wound tissue and increased and faster re-epithelialisation can be observed [17, 18]. Numerous publications also show a promotion of re-vascularisation and a significantly lower infection rate due to moist wound management [19, 20]. In addition, the concept offers the possibility of changing a wound dressing painlessly without destroying already regenerated tissue [6]. A further advantage is that less scarring and aesthetically better healing results can be achieved [21].
The advantages of modern moist wound treatment have been consistently proven to this day by a constantly growing number of scientific publications, clinical studies and in vitro analyses. Nevertheless, moist wound management must not be equated with wet wound care. A too wet environment can have a negative impact on wound healing [22]. Of great importance here is the balance between ideal wound moistening and the avoidance of tissue damage which can be caused by inadequate exudate management (maceration) [23]. Thus, newly formed epithelium can easily be confused with maceratively damaged tissue, as both can appear pale white at the wound margin. However, maceratively damaged tissue can be recognised by its odour, for example.
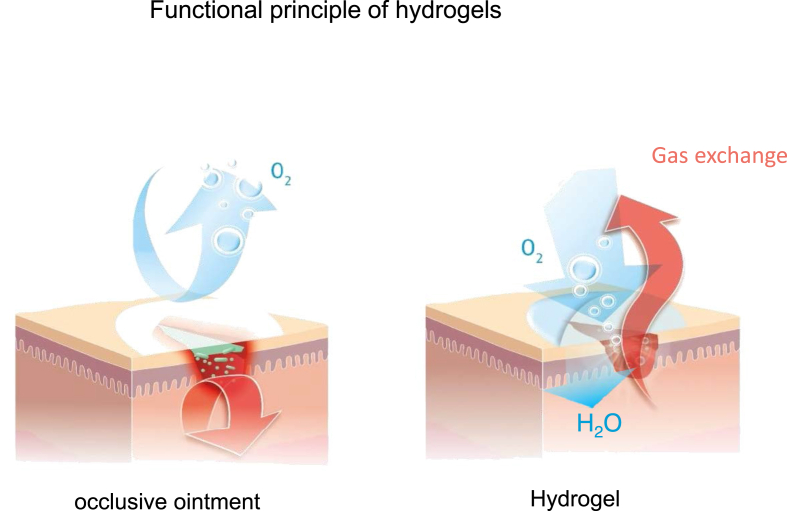
Moist wound management can be carried out in various ways, e.g. on the basis of hydrogels, if these have a high water content. Hydrogels consist of water-containing, water-soluble polymers which are linked to form a three-dimensional network [24]. They are breathable and can be used for different types of wounds (closed, open, weeping or dry). By contrast, occlusive ointments, which often have a fatty base, are considered rather unfavourable for open wounds, for example in chronic wound treatment, and are primarily suitable for use on irritated, unwounded skin. In contrast, modern hydrogels such as Tyrosur® can support all phases of wound healing. In the cleansing phase hydrogel bases support the removal of exudate, cell debris, foreign bodies and germs (autolytic debridement) [25]. In the granulation phase, hydrogels optimally moisturize the wound and thus provide an intensively hydrated matrix, which can promote cell migration into the wound [26]. In addition, collagen synthesis and cross-linking are supported. In the reparative phase the division of keratinocytes, which facilitate re-epithelialisation, is promoted [10]. In addition, the wound is optimally supplied with oxygen by the respiratory activity and wound contraction is promoted [[27] see Figure 3].
Figure 3.
In contrast to strongly occlusive bases such as Vaseline, which protects against dehydration but does not contain its own water, the hydrogel base allows extensive preservation of respiratory capacity and provides the wound with additional moisture.
Furthermore, hydrogels have a cooling and pain-relieving effect [1]. Hydrogel bases therefore offer great potential, as they can easily be combined with various substances that facilitate wound healing and/or have anti-inflammatory properties to promote healing in chronic wounds [28]. However, there is still a lot of educational work to be done by the treating physicians to make patients aware of these benefits and to establish ideal moist wound management as a standard approach even for minor wounds.
4.1. Outlook
Even today, further efficient and wound-healing-promoting substances are being sought, which can be incorporated into a hydrogel base, for example, in order to develop an optimal wound healing preparation. Clinical data in combination with tyrothricin are already available for the hydrogel base used in this study [29]. Luliconazole has recently been shown to be more effective against fungal infections when embedded in a hydrogel formulation [30]. Numerous publications have already described the mode of action and high efficiency of hydrogels in the healing of various wounds [31]. In general, the mode of action of hydrogels can be demonstrated during the entire wound healing process, starting with coagulation and ending with the modelling of the new tissue. Nevertheless, hydrogel-based preparations for therapeutic support of ideal moist wound management are still relatively little used. However, especially in the treatment of minor injuries, without being limited to this, patients seem to benefit from the manifold advantages of hydrogels.
4.2. Strength and limitations
The data obtained in the present study clearly show the advantages of the hydrogel formulations in terms of breathability in contrast to semi-occlusive or occlusive galenics. Additionally, the hydrogels were shown to provide significantly better tissue hydration. Likewise, an additional long-term moisturizing effect could be highlighted by the use of Dexpanthenol in both hydrogels and the semi-occlusive ointment. Nevertheless, despite the use of porcine skin, which closely resembles human skin in many properties, the results should be examined and confirmed with nativ human skin. Moreover, TEWL and skin hydration were measured on unwounded skin. If the higher breathability of hydrogels compared to (semi-) occlusive formulations is a key factor of faster wound healing should be addressed in clinical trials.
4.3. Practical relevance
The ideal moist wound management offers many advantages for the patient compared to the traditional approach of dry wound treatment. On the one hand, an intensively hydrated matrix is created which promotes wound healing, resulting in faster wound contraction and re-epithelisation of the tissue and consequently up to 50% faster and more physiological wound healing. On the other hand, the moist microclimate promotes angiogenesis, so that the risk of infection can be minimised. As there is no scab formation, wounds also heal with less pain and finer scar formation. Changing a wound dressing is also painless and does not destroy the newly formed tissue. The results increasingly described in the current literature suggest that for all wounds (even acute minor injuries) the still widespread dry wound treatment should be replaced by modern, moist wound management as firmly established in chronic wound treatment [32].
Declarations
Author contribution statement
L. Rüther: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
W. Voss: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare the following conflict of interests: This publication arose in the context of an ex vivo study carried out at Dermatest GmbH with the test substances. The sponsor of the study is Engelhard Arzneimittel GmbH & Co. KG, Germany.
Additional information
No additional information is available for this paper.
References
- 1.Bahram M., Mohseni N., Moghtader M. Intech Open; 2016. An Introduction to Hydrogels and Some Recent Applications; pp. 9–38. Chapter 2. [Google Scholar]
- 2.Aswathy S.H., Narendrakumar U., Manjubala I. Commercial hydrogel for biomedical applications. Heliyon. April 7 2020;6(4) doi: 10.1016/j.heliyon.2020.e03719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osborne D.W. Phase behaviour characterization of ointments containing lanolin or a lanolin substitute. Drug Dev. Ind. Pharm. 2008;19(11):1283–1302. [Google Scholar]
- 4.Uetsu N., Okamoto H., Jujii K. Treatment of chronic actinic dermatitis with tacrolimus ointment. J. Am. Acad. Dermatol. 2002 Dec;47(6):881–884. doi: 10.1067/mjd.2002.124703. [DOI] [PubMed] [Google Scholar]
- 5.Alves J.V.F., Angeloni A., Jawie A. MIMS Dermatology June Issue; 2009. Guidelines for the Treatment of Acute Minor Skin Wounds: a Consensus by Leading European experts. [Google Scholar]
- 6.Li J., Yu F., Chen G. Moist-retaining, self-recoverable, bioadhesive, and transparent in situ forming hydrogels to accelerate wound healing. ACS Appl. Mater. Interfaces. 2006 Jan 15;12(2):2023–2038. doi: 10.1021/acsami.9b17180. [DOI] [PubMed] [Google Scholar]
- 7.Jacobi U., Kaiser M., Mangelsdorf S. Porcine ear skin: an in vitro model for human skin. Skin Res. Technol. 2007 Feb;13(1):19–24. doi: 10.1111/j.1600-0846.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 8.Schmook F.P., Meingassner J.G., Billich A. Comparison of human skin or epidermal models with human and animal skin in in-vitro percutaneous absorption. Int. J. Pharm. 2001 Mar 14;215(1-2):51–56. doi: 10.1016/s0378-5173(00)00665-7. [DOI] [PubMed] [Google Scholar]
- 9.Ladizinsky D., Roe D. New insights into oxygen therapy for wound healing. Wounds. 2010;22(12):294–300. [PubMed] [Google Scholar]
- 10.Op´t Veld R.C., Walboomers X.F., Jansen J.A. Design considerations for hydrogel wound dressings; strategic and molecular advances. Tissue Eng. Part B. 2020;13 doi: 10.1089/ten.TEB.2019.0281. [DOI] [PubMed] [Google Scholar]
- 11.Baron J.M., Glatz M., Proksch E. Optimal support of wound healing: new insights. Dermatology. 2020 Jan 17:1–8. doi: 10.1159/000505291. [DOI] [PubMed] [Google Scholar]
- 12.Gehring W., Gloor M. Effect of topically applied dexpanthenol on epidermal barrier function and stratum corneum hydration. Results of a human in vivo study. Arzneimittelforschung. 2000 Jul;50(7):659–663. doi: 10.1055/s-0031-1300268. [DOI] [PubMed] [Google Scholar]
- 13.Winter G.D. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962 Jan 20;193:293–294. doi: 10.1038/193293a0. [DOI] [PubMed] [Google Scholar]
- 14.Winter G.D. Effect OF air exposure and occlusion ON experimental human SKIN wounds. Nature. 1963 Oct 26;200:377–378. doi: 10.1038/200378a0. [DOI] [PubMed] [Google Scholar]
- 15.Beam J.W. Occlusive dressings and the healing of standardized abrasions. J. Athl. Train. 2008 Oct-Dec;43(6):600–607. doi: 10.4085/1062-6050-43.6.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wigger-Alberti W., Kuhlmann M., Ekanayake T. Using a novel wound model to investigate the healing properties of products for superficial wounds. J. Wound Care. 2009 Mar;18(3):123–131. doi: 10.12968/jowc.2009.18.3.39813. [DOI] [PubMed] [Google Scholar]
- 17.Korting H.C., Schöllmann C., White R.J. Management of minor acute cutaneous wounds: importance of wound healing in a moist environment. J. Eur. Acad. Dermatol. Venereol. 2011;25(2):130–137. doi: 10.1111/j.1468-3083.2010.03775.x. [DOI] [PubMed] [Google Scholar]
- 18.Madden M.R., Nolan E., Finkelstein J.L. Comparison of an occlusive and semi-occlusive dressing and the effect of the wound exudate upon keratinocyte proliferation. J. Trauma. 1989 Jul;29(7):924–931. doi: 10.1097/00005373-198907000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Dowsett C., Ayello E. TIME principles of chronic wound bed preparation and treatment. Br. J. Nurs. 2004 Aug-12-Sep 8;13(15):S16–23. doi: 10.12968/bjon.2004.13.Sup3.15546. [DOI] [PubMed] [Google Scholar]
- 20.Kirsner R.S., Martin L.K. Drosou A Wound microbiology and the use of antibacterial agents. In: Rovee D.T., Maibach H.I., editors. The Epidermis in Wound Healing. CRC Press; Boca Raton, USA: 2004. pp. 155–182. [Google Scholar]
- 21.Hoeksema H., De Vos M., Verbelen J. Scar management by means of occlusion and hydration: a comparative study of silicones versus a hydrating gel-cream. Burns. 2013 Nov;39(7):147–148. doi: 10.1016/j.burns.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 22.Leaper D.J., Schultz G., Garville K. Extending the TIME concept: what have we learned in the past 10 years? Int. Wound J. 2012 Dec:9;(Suppl 2):1–19. doi: 10.1111/j.1742-481X.2012.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones M.L. An introduction to absorbent dressings. BR J Comm. Nurs. Dec. Suppul. Wound Care. 2014:S28–30. doi: 10.12968/bjcn.2014.19.Sup12.S28. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed M.E. Hydrogel: preparation, characterisation and applications: a review. J. Adv. Res. 2015 Mar;6(2):105–121. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Z., Golland B., Tronci G. A redox-responsive hyaluronic acid-based hydrogel for chronic wound management. J. Mater. Chem. B. 2019 Dec 21;7(47):7494–7501. doi: 10.1039/c9tb01683j. [DOI] [PubMed] [Google Scholar]
- 26.Ur Rehman S.R., Augustine R., Zahid A.A. Graphene oxide loaded hydrogel for enhanced wound healing in diabetic patients. Conf. Proc. IEEE Eng. Med. Biol. Soc. Jul 2019:3943–3946. doi: 10.1109/EMBC.2019.8857341. [DOI] [PubMed] [Google Scholar]
- 27.Vasconcelos M.S., Souza T.F.G., Figueiredo I.S. A phytomodulatory hydrogel with enhanced healing effects. Phytother Res. 2018 Apr;32(4):688–697. doi: 10.1002/ptr.6018. [DOI] [PubMed] [Google Scholar]
- 28.Xu Z., Han S., Gu Z. Advances and impact of antioxidant hydrogel in chronic wound healing. Adv. Healthc. Mater. 2020 Jan 24 doi: 10.1002/adhm.201901502. [DOI] [PubMed] [Google Scholar]
- 29.Wigger-Alberti W., Stauss-Grab M., Grigo K. Efficacy of a tyrothricin-containing wound gel in an abrasive wound model for superficial wounds. Skin Pharmacol. Physiol. 2012;25:52–56. doi: 10.1159/000343907. [DOI] [PubMed] [Google Scholar]
- 30.Kumar M., Shanthi N., Mahato S.K. Preparation of luliconazole nanocrystals loaded hydrogel for improvement of dissolution and antifungal activity. Heliyon. May 11 2019;5(5) doi: 10.1016/j.heliyon.2019.e01688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan H., Fan D., Cao W. Preparation and characterization of breathable hemostatic hydrogel dressings and determination of their effects on full-thickness defects. Polymers. 2017 Dec;9(12):727. doi: 10.3390/polym9120727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Institute for Health and Care Excellence (NICE) 30 March 2016. Chronic Wounds: Advanced Wound Dressing and Antimicrobial Dressings portal.https://www.nice.org.uk/advice/esmpb2/chapter/Key-points-from-the-evidence [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.