Abstract
Since the demonstration that microRNAs are deeply involved in the regulation of Cystic Fibrosis (CF) Transmembrane Conductance Regulator (CFTR) gene, a great attention has been dedicated to possible alteration of the CFTR gene expression by targeting miRNAs causing down-regulation of CFTR and CFTR-associated proteins. The data here presented are related to previously published studies on the effects of treatment of human bronchial cells of PNAs targeting miR-101-3p and miR-145-5p (microRNAs shown to regulate the CFTR mRNA). These data here presented are relative to two companion articles “Treatment of human airway epithelial Calu-3 cells with a Peptide-Nucleic Acid (PNA) targeting the microRNA miR-101-3p is associated with increased expression of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene” (published in European Journal of Medicinal Chemistry, 2020) and “Peptide Nucleic Acids for MicroRNA Targeting” (published in Methods in Molecular Biology, 2020). The data obtained indicate that, while the expression of most microRNAs is not affected by PNA treatment, some of them are strongly modulated. In particular, some microRNAs involved in CF and/or CFTR regulation are co-inhibited by miR-101-3p and miR-145-5p. Among them, miR-155-5p, miR-125b-5p, miR-132-3p and miR-6873-3p. This has been demonstrated by Next Generation Sequencing (NGS) followed by RT-qPCR and RT-ddPCR validation.
Keywords: Peptide nucleic acids, Cystic fibrosis, MicroRNAs, miR-101-3p, miR-145-5p, NGS, CFTR
Specifications Table
| Subject | Biochemistry, Genetics and Molecular Biology |
| Specific subject area | Molecular Medicine, Pulmonary and Respiratory Medicine, Drug Discovery, Pharmacology |
| Type of data | Chart Tables Graphs Figures Raw sequencing data |
| How data were acquired | NGS analysis has been performed using an Illumina NextSeq500 platform (Illumina, FC-404-2005). RT-qPCR reactions have been performed using the CFX96 Touch Real-Time PCR Detection System (BioRad, Hercules, CA, USA). |
| Data format | Raw and Analyzed |
| Parameters for data collection | For NGS, small RNA library pools were prepared, quantified and sequenced using Illumina NextSeq500 platform and NextSeq® 500/550 High Output Kit v2 (Illumina, FC-404-2005). The raw base-call data generated have been demultiplexed and converted to FASTQ format. The optimal read depth to analyse the miRNA transcriptome was determined at 10 million reads per sample. |
| Description of data collection | Human airway epithelial Calu-3 cells were treated with R8-PNA-a101 and R8-PNA-a145 for 72 h. Inhibition of miR-101-3p and miR-145-5p was obtained, associated with increase of CFTR expression. The PNAs were functionalized with an octo-arginine R8 peptide for maximizing cellular uptake. After treatment, the miRNome was analysed in untreated and PNA-treated cells by NGS, and the data obtained validated by RT-qPCR and RT-ddPCR. |
| Data source location | Department of Life Sciences and Biotechnology, University of Ferrara, Via Fossato di Mortara n.74, 44121 Ferrara, Italy |
| Data accessibility | Data are available within this article. The NGS raw data are available at the public expression database “European Nucleotide Archive” (ENA) (https://www.ebi.ac.uk/ena/browser/view/PRJEB39141). |
| Related research articles | E. Fabbri, A. Tamanini, T. Jakova, J. Gasparello, A. Manicardi, R. Corradini, A. Finotti, M. Borgatti, I. Lampronti, S. Munari, M.C. Dechecchi, G. Cabrini and R. Gambari, Treatment of human airway epithelial Calu-3 cells with a Peptide-Nucleic Acid (PNA) targeting the microRNA miR-101-3p is associated with increased expression of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene, Eur. J. Med. Chem. (2020) https://doi.org/10.1016/j.ejmech.2020.112876. R. Gambari, J. Gasparello, E. Fabbri, M. Borgatti, A. Tamanini, A. Finotti, Peptide Nucleic Acids for MicroRNA Targeting. Methods Mol Biol. 2105 (2020) 199-215. https://doi.org/10.1007/978-1-0716-0243-0_12. |
Value of the Data
-
•
This dataset provides information on the miRNomic profile obtained on cells modulated by peptide-nucleic acids (PNAs) targeting selected miRNAs (miR-101-3p and miR-145-5p).
-
•
Our dataset may be useful to all researchers interested in studying miR-101-3p and miR-145-5p regulated miRNAs, which is still a largely unexplored field of investigation.
-
•
The data further support the concept that pharmacologically down-regulation of a miRNA might be associated with dysregulation of other microRNAs.
-
•
The data can be used to further identify genes which are expected to be indirectly regulated by miR-101-3p and miR-145-5p.
-
•
The data have a translational value, since miR-101-3p and miR-145-5p are validated down regulator of CFTR in CF; accordingly, specific PNAs can be employed in protocols finalized to CFTR upregulation.
1. Data Description
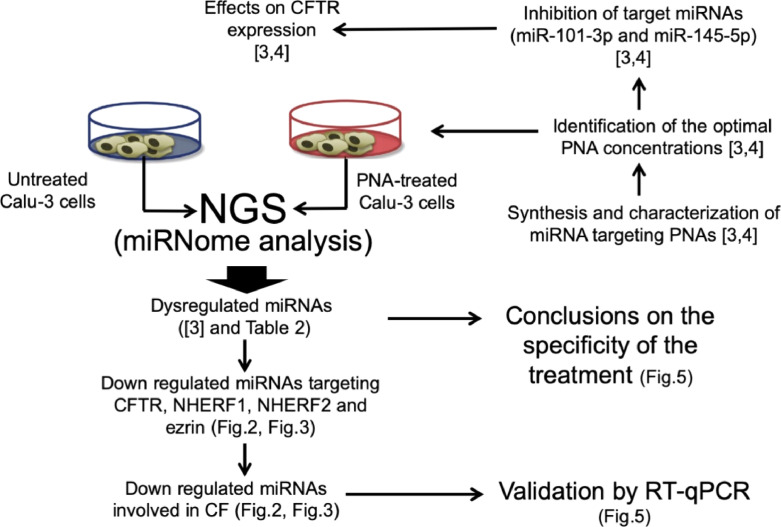
The experiments generating the data here reported have been performed on the human airway Calu-3 cell line [1] using peptide-nucleic acids (PNAs) [2] as gene expression modifiers. The description of these experiments can be found in Fabbri et al. [3] and Fabbri et al. [4]. The relevance of microRNAs for regulation of the Cystic Fibrosis (CF) Transmembrane Conductance Regulator (CFTR) gene can be found in several studies [3], [4], [5], [6], [7]. The possible use of miRNA modulators has been proposed elsewhere [8], [9]. Treatment of Calu-3 cells with R8-PNA-a101 and R8-PNA-a145 leads to a sharp inhibition of miR-101-3p and miR-145-5p hybridization signals, respectively [3], [4]. The R8-PNA-a101 and the R8-PNA-a145 were functionalized with an octo-arginine R8 peptide for maximizing cellular uptake [3], [4], [10]. The Flow chart diagram illustrating the steps in microRNA expression profiling analysis of Calu-3 cells treated with PNAs targeting miR-101-3p and miR-145-5p is depicted in Fig. 1.
Fig. 1.
A Flow chart diagram illustrating the steps in microRNA expression profiling analysis of Calu-3 cells treated with peptide nucleic acids targeting miR-101-3p and miR-145-5p.
1.1. miRNome profile of Calu-3 cells treated with R8-PNA-a101 and R8-PNA-a145: next-generation sequencing (NGS)
RNA from Calu-3 cells, either untreated or treated for 72 hours with 4 μM R8-PNA-a101 and 2 μM R8-PNA-a145, was analyzed by NGS, using the Illumina NextSeq500 platform and NextSeq® 500/550 High Output Kit v2. The NGS patterns obtained, using as parameter the inclusion of all dysregulated miRNAs exhibiting a Fold Change (FC) > 2, have been presented in Fabbri et al. for PNA-a101 [3] and in Gambari et al. for PNA-a145 [5]. Most of the miRNAs exhibited FC < 2: 403/479 (84.13%) and 393/465 (84.52%) for R8-PNA-a101 and R8-PNA-a145, respectively. On the contrary, a lower number of miRNAs were found either up- or down-regulated (see Table 1 for comparative analysis of the effects of R8-PNA-a101 and R8-PNA-a145 treatments). In particular, 7 and 5 miRNAs (up-regulated) and 22 and 19 miRNAs (down-regulated) exhibited a FC > 3 after Calu-3 cell treatment with R8-PNA-a101 and R8-PNA-a145, respectively. Cumulatively, the proportion of dysregulated miRNAs (either up- or down-regulated; FC>3) is low (6.05% and 5.17% for PNA-a101 and PNA-a145, respectively) (Table 1).
Table 1.
Dimension (numbers) of the cohorts of miRNAs exhibiting different FC values after treatment of Calu-3 cells with R8-PNA-a101 and R8-PNA-a145.
| Fold Change (FC) | Regulation | R8-PNA-a101 | R8-PNA-a145 |
|---|---|---|---|
| <2 | up or down | 403/479 (84.13%) | 393/465 (84.52%) |
| 2-3 | up | 12 (2.51%) | 18 (3.87%) |
| down | 35 (7.31%) | 30 (6.45%) | |
| >3 | up | 7 (1.46%) | 5 (1.08%) |
| down | 22 (4.59%) | 19 (4.09%) |
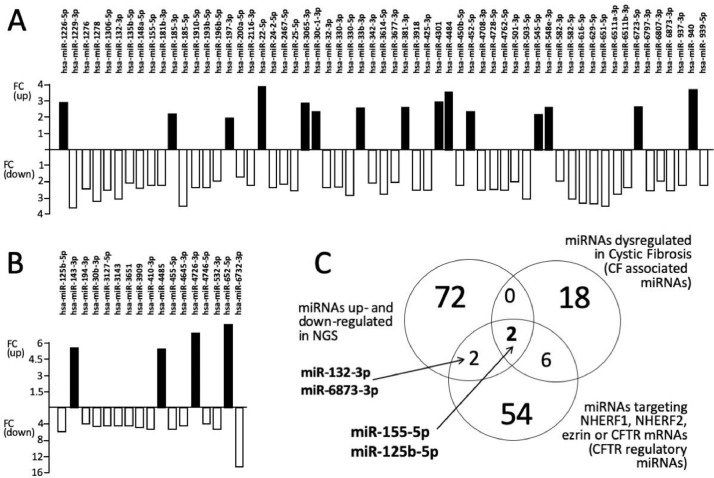
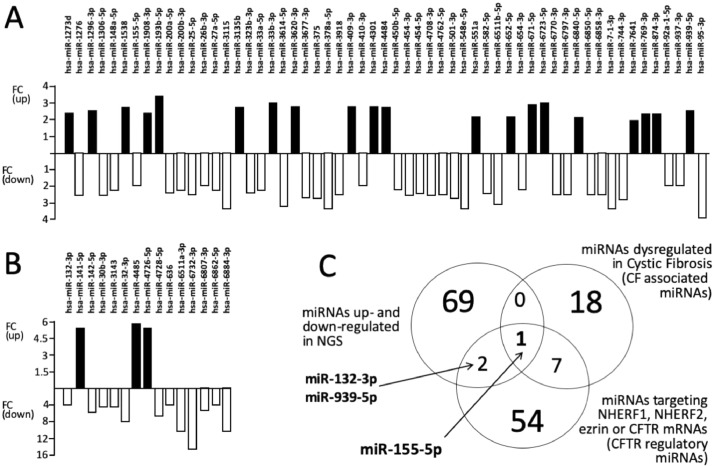
In panels A and B of Figs. 2 and 3 we report the detailed analysis of the fold changes of the dysregulated miRNAs found in Calu-3 cells treated with R8-PNA-a101 (Fig. 2) and R8-PNA-a145 (Fig. 3) in order to better identify and quantitate the changes found in up-regulated (black histograms) and down-regulated (white histograms) miRNAs (the complete file containing the calculated FC values can be found in Table 3 of the paper by Fabbri et al. [3] and in Table 2).
Fig. 2.
A,B. Fold Change (FC) values of the miRNAs found to be dysregulated following treatment of Calu-3 cells with the R8-PNA-a101. C. Venn diagram showing two miRNAs (miR-155-5p and miR-125b-5p) in common with the three lists (miRNAs up- and down-regulated in NGS, miRNAs dysregulated in CF and miRNA targeting NHERF1, NHERF2, ezrin or CFTR mRNAs).
Fig. 3.
A,B. Fold Change (FC) values of the miRNAs found to be dysregulated following treatment of Calu-3 cells with the R8-PNA-a145. C. Venn diagram showing one miRNA (miR-155-5p) in common with the three lists (miRNAs up- and down-regulated in NGS, miRNAs dysregulated in CF and miRNA targeting NHERF1, NHERF2, ezrin or CFTR mRNAs).
Table 3.
Raw RT-ddPCR data on the effects of PNA-a101 on miR-125b-5p, miR-101-3p and microRNA Let-7c-5p. For representative data and the final summary, see Fig. 5. N.a.= not applicable.
| Experiment | Treatment | Target | Concentration | Copies/20 ml well | Poisson Conf Max | Poisson Conf Min | Positives | Negatives | Accepted Droplets | Threshold | Treated/Untreated |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | Ctrl | miR-125b-5p | 6.7 | 134 | 8.2 | 5.4 | 95 | 16616 | 16711 | 4015 | n.a. |
| PNA-a101 | miR-125b-5p | 3.8 | 76 | 4.9 | 2.8 | 52 | 16181 | 16233 | 4015 | 0.57 | |
| Ctrl | miR-101-3p | 7.3 | 146 | 8.7 | 6.0 | 112 | 17885 | 17997 | 4015 | n.a. | |
| PNA-a101 | miR-101-3p | 2.0 | 40 | 2.9 | 1.4 | 29 | 16630 | 16659 | 4015 | 0.28 | |
| Ctrl | Let-7c-5p | 86.1 | 1722 | 91.0 | 81.3 | 1219 | 16045 | 17264 | 3513 | n.a. | |
| PNA-a101 | Let-7c-5p | 71.8 | 1436 | 76.2 | 67.4 | 1010 | 16048 | 17058 | 3513 | 0.83 | |
| #2 | Ctrl | miR-125b-5p | 3.2 | 64.2 | 3.5 | 2.9 | 47 | 17550 | 17597 | 4015 | n.a. |
| PNA-a101 | miR-125b-5p | 2.1 | 42.6 | 2.4 | 1.9 | 32 | 17487 | 17519 | 4015 | 0.66 | |
| Ctrl | miR-101-3p | 3.7 | 73 | 4.0 | 3.3 | 49 | 15996 | 16045 | 4015 | n.a. | |
| PNA-a101 | miR-101-3p | 0.9 | 17 | 1.0 | 0.7 | 11 | 15088 | 15099 | 4015 | 0.23 | |
| Ctrl | Let-7c-5p | 37.2 | 744 | 38.3 | 36.2 | 491 | 17616 | 18107 | 3513 | n.a. | |
| PNA-a101 | Let-7c-5p | 37.7 | 754 | 38.8 | 36.6 | 452 | 16067 | 16519 | 3513 | 1.01 | |
| #3 | Ctrl | miR-125b-5p | 3.2 | 64.4 | 3.4 | 3.0 | 48 | 18004 | 18052 | 4015 | n.a. |
| PNA-a101 | miR-125b-5p | 2.1 | 41.2 | 2.1 | 1.9 | 29 | 16649 | 16678 | 4015 | 0.64 | |
| Ctrl | miR-101-3p | 3.2 | 65 | 3.4 | 3.1 | 49 | 18502 | 18551 | 4015 | n.a. | |
| PNA-a101 | miR-101-3p | 0.9 | 19 | 1.0 | 0.8 | 14 | 18384 | 18398 | 4015 | 0.29 | |
| Ctrl | Let-7c-5p | 35.2 | 705 | 36 | 34.5 | 352 | 16345 | 16697 | 3513 | n.a. | |
| PNA-a101 | Let-7c-5p | 31.9 | 639 | 32.6 | 31.3 | 353 | 17535 | 17888 | 3513 | 0.91 | |
| #4 | Ctrl | miR-125b-5p | 4.5 | 90.4 | 4.7 | 4.4 | 57 | 16228 | 16285 | 4015 | n.a. |
| PNA-a101 | miR-125b-5p | 2.9 | 58.8 | 3.1 | 2.8 | 42 | 17606 | 17648 | 4015 | 0.65 | |
| Ctrl | miR-101-3p | 5.1 | 102 | 6.2 | 4.4 | 98 | 17754 | 17852 | 4015 | n.a. | |
| PNA-a101 | miR-101-3p | 1.5 | 30 | 2.1 | 0.9 | 24 | 16524 | 16548 | 4015 | 0.31 | |
| Ctrl | Let-7c-5p | 75.3 | 1506 | 84.8 | 69.9 | 1003 | 16053 | 17056 | 3513 | n.a. | |
| PNA-a101 | Let-7c-5p | 63.4 | 1268 | 65.9 | 59.7 | 754 | 15924 | 16678 | 3513 | 0.84 | |
Table 2.
List of miRNAs dysregulated after R8-PNA-a145 treatment of Calu-3 cells.
| microRNA | FC | Regulation | microRNA | FC | Regulation |
|---|---|---|---|---|---|
| miR-1273d | 2.37 | up | miR-450b-5p | 2.18 | down |
| miR-1276 | 2.52 | down | miR-454-3p | 2.52 | down |
| miR-1296-3p | 2.51 | up | miR-454-5p | 2.47 | down |
| miR-1306-5p | 2.52 | down | hsa-miR-4708-3p | 2.52 | down |
| miR-132-3p | 4.04 | down | hsa-miR-4726-5p | 5.57 | up |
| miR-141-5p | 5.57 | up | hsa-miR-4728-5p | 7.62 | down |
| miR-142-5p | 6.28 | down | hsa-miR-4762-5p | 2.52 | down |
| miR-148a-5p | 2.24 | down | hsa-miR-501-3p | 2.87 | down |
| hsa-miR-1538 | 2.60 | up | hsa-miR-548e-5p | 3.36 | down |
| miR-155-5p | 2.08 | down | hsa-miR-551a | 2.23 | up |
| miR-1908-3p | 2.23 | up | hsa-miR-582-5p | 2.69 | down |
| miR-193b-5p | 3.34 | up | hsa-miR-636 | 4,04 | down |
| miR-200a-5p | 2.39 | down | hsa-miR-6511a-3p | 9.23 | down |
| miR-200b-3p | 2.13 | down | hsa-miR-6511b-5p | 3.14 | down |
| miR-25-5p | 2.52 | down | hsa-miR-652-5p | 2.23 | up |
| miR-26b-3p | 2.05 | down | hsa-miR-654-3p | 2.27 | down |
| miR-27a-5p | 2.29 | down | hsa-miR-671-5p | 2.97 | up |
| miR-30b-3p | 4.19 | down | hsa-miR-6723-5p | 3.15 | up |
| miR-3115 | 3.36 | down | hsa-miR-6732-3p | 15.10 | down |
| miR-3135b | 2.67 | up | hsa-miR-6770-3p | 2.52 | down |
| miR-3143 | 4.19 | down | hsa-miR-6797-3p | 2.52 | down |
| miR-32-3p | 8.07 | down | hsa-miR-6807-3p | 5.03 | down |
| miR-323b-3p | 2.52 | down | hsa-miR-6840-5p | 2.23 | up |
| miR-33a-5p | 2.24 | down | hsa-miR-6850-5p | 2.52 | down |
| miR-33b-3p | 2.97 | up | hsa-miR-6858-3p | 2.52 | down |
| miR-3614-5p | 3.29 | down | hsa-miR-6862-5p | 4.04 | down |
| miR-3620-3p | 2.78 | up | hsa-miR-6884-3p | 6.71 | down |
| miR-3677-3p | 2.69 | down | hsa-miR-7-1-3p | 3.36 | down |
| miR-375 | 2.82 | down | hsa-miR-744-3p | 2.69 | down |
| miR-378a-5p | 3.36 | down | hsa-miR-7641 | 2.06 | up |
| miR-3918 | 2.52 | down | hsa-miR-769-3p | 2.23 | up |
| miR-409-3p | 2.79 | up | hsa-miR-874-3p | 2.23 | up |
| miR-410-3p | 2.02 | down | hsa-miR-92a-1-5p | 2.02 | down |
| miR-4301 | 2.79 | up | hsa-miR-937-3p | 2.10 | down |
| miR-4484 | 2.79 | up | hsa-miR-939-5p | 2.51 | up |
| miR-4485 | 5.92 | up | hsa-miR-95-3p | 3.36 | down |
In panels C of Figs. 2 and 3 the NGS data relative to dysregulated miRNAs in R8-PNA-a101 and R8-PNA-a145 cells were compared with a list of miRNAs previously reported to be dysregulated in cystic fibrosis (Table 2 of the study published by Fabbri et al. [3]) [11], [12], [13], [14], [15] and the list of miRNAs putatively able to target the 3’-UTR sequences of CFTR mRNA or mRNAs coding CFTR regulators (NHERF1, NHERF2 and ezrin) (Table 2 of the study published by Fabbri et al. [3], [14]). The objective of this comparison was to verify whether in the list of miRNAs dysregulated by treatments with PNA-a101 (Fig. 2) and PNA-a145 (Fig. 3), are present miRNAs already reported to be involved in CF or CFTR regulation.
The data obtained using R8-PNA-a101 indicate that only two miRNAs were found to be present in all the lists, namely miR-155-5p and miR-125b-5p, while miR-132-3p and miR-6873-3p were found in common with the NGS dysregulated list and the list of miRNAs targeting the 3’-UTR sequences of mRNA involved in CFTR expression (Fig. 2C). When R8-PNA-a145 was used, only one miRNA was found to be present in all the lists, miR-155-5p, while miR-132-3p and miR-939-5p were found in common with the NGS dysregulated list and the list of miRNAs targeting the 3’-UTR sequences of mRNA involved in CFTR expression (Fig. 3C).
For comparison, the list of miRNAs dysregulated after R8-PNA-a101 treatment of Calu-3 cells is available in Table 3 of the paper by Fabbri et al. [3]. The list of microRNAs dysregulated in Cystic Fibrosis and the list of microRNAs targeting CFTR, NHERF1, NHERF2, ezrin mRNAs are available in Table 2 and Table 1 of the same paper, respectively.
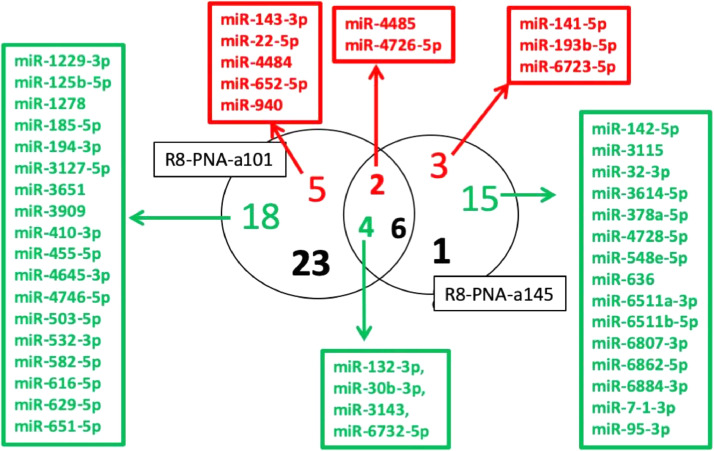
Fig. 4 reports a Venn-diagram comparing dysregulated miRNAs displaying FC>3 when miRNAs from cells treated with R8-PNA-a101 and R8-PNA-a145 are compared. This comparison was performed to verify the proportion of miRNAs highly dysregulated by both treatments. Interestingly, only 6 miRNAs (4 down-regulated and 2 up-regulated) were found in common, while the majority (23 for R8-PNA-a101 and 18 for R8-PNA-a145) were found specifically dysregulated for each PNA-based treatment, supporting the concept of a sequence-selectivity of the treatments. Fig. 4 also reports the list of down-regulated (in green) and up-regulated (in red) miRNAs.
Fig. 4.
A. Venn-diagram indicating the number of down-regulated (green) and up-regulated (red) miRNAs after treatment of Calu-3 cells with R8-PNA-a101 and R8-PNA-a145, as indicated (FC > 3). In black are the total numbers of dysregulated miRNAs. Only 6 miRNAs (<1.29%) were dysregulated in Calu-3 cells treated with both the two anti-miRNAs PNAs.
1.2. RT-ddPCR validation
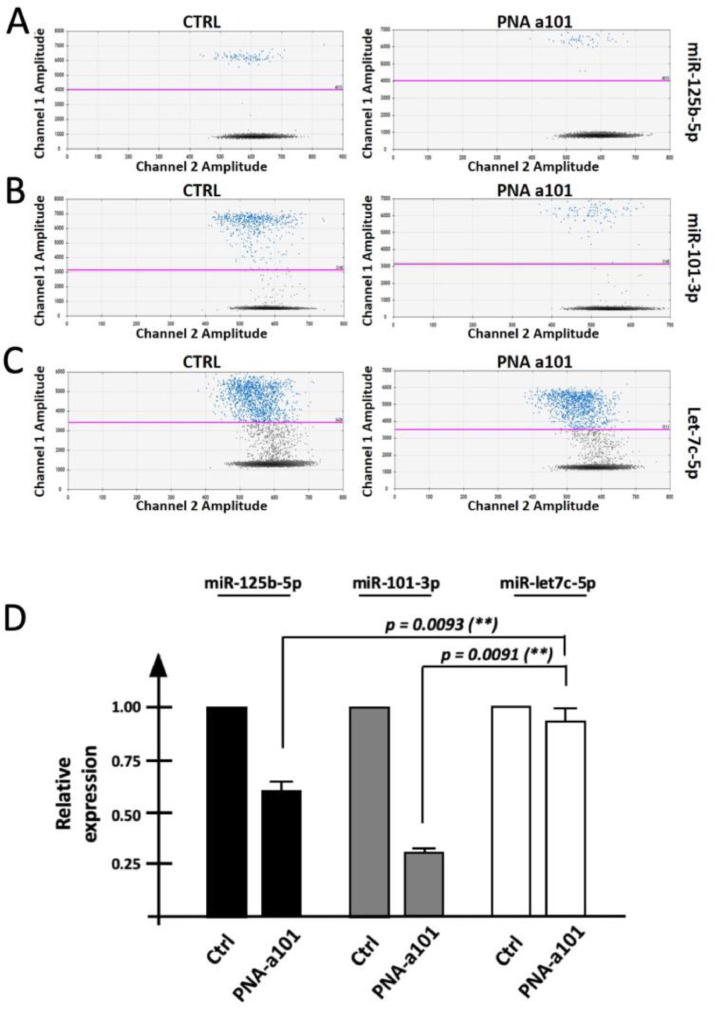
The down-regulation of miR-125b-5p following treatment of Calu-3 cells with R8-PNA-a101 has been validated by RT-qPCR [3] and by RT-ddPCR, as reported in Fig. 5. The data obtained by RT-ddPCR are in agreement with the RT-qPCR data and NGS data. Fig. 5A shows representative data depicting 2D plots obtained by RT-ddPCR analysis of miR-125b-5p, miR-101-3p and microRNA Let-7c-5p (used as not modulated control miRNA). Summary of the data obtained in three independent experiments is shown in Fig. 5B. Raw data of this experiment are reported in Table 3.
Fig. 5.
A. Representative 2D plots obtained by RT-ddPCR analysis of miR-125b-5p (A), miR-101-3p (B) and microRNA Let-7c-5p (C). Blue dots are relative to fluorescence positive droplets, black dots represent negative (no amplification) droplets. D. Summary of the data obtained in four independent experiments (average ± S.D). Raw data are reported in Table 3. Ctrl = control untreated cells. PNA-a101 = cells treated for 72 h with 4 μM R8-PNA-a101.
2. Experimental Design, Materials and Methods
2.1. Experimental design
The Flow Chart showing the experimental design is shown in Fig. 1. The synthesis of the R8-PNA-a101 and R8-PNA-a145 and their effects on miR-101-3p, miR-145-5p and CFTR have been presented in our related works by Fabbri et al. [3], [4].
2.2. Cell culture conditions
Calu-3 cells [1] were cultured in humidified atmosphere of 5% CO2/air in DMEM/F12 medium (Gibco, Grand Island, US) supplemented with 10% fetal bovine serum (Biowest, Nauillè, Francia), 100 units/ml penicillin and 100 μg/mL streptomycin (Lonza, Verviers, Belgio) and 1% NEEA (100X) (Non-Essential Amino Acids Solution; Gibco). Treatment with the employed R8-PNAs did not require transfection agents (such as lipofectamine), since the uptake was always nearly 100%, as reported by Brognara et al. [10] and Gambari et al. [5].
2.3. RNA extraction and quantitative analyses of miRNAs
Calu-3 cells were trypsinized and collected by centrifugation at 1500 rpm for 10 min at 4 °C. The cellular pellets were washed twice with PBS and lysed with the Tri-Reagent (Sigma Aldrich, St.Louis, Missouri, USA). RNA extraction was conducted following the manufacturer's instructions and the isolated RNA was washed once with cold 75% ethanol. After this washing step, the RNA was dried and dissolved in nuclease free pure water and considered ready for the use. For miRNA quantification, 300 ng of total RNA were reverse transcribed using the TaqMan™ miRNA Reverse Transcription Kit (Thermo Fisher Scientific) in a final volume of 30 µL, according with manufacturer's instruction. Obtained cDNA was stored at −80 °C until the moment of use. For miRNA RT-ddPCR, the reaction mix was prepared adding Supermix for Probes (no dUTP) 2X (Bio-Rad, Hercules, CA, USA), 20X TaqMan™ miRNA assay (Thermo Fisher Scientific) (assays ID were the following: hsa-miR-101-3p, TM:002253; hsa-miR-125b-5p, TM:000449) and cDNA. Droplets were automatically generated using Automated droplets generator (AutoDG) (Bio-Rad, Hercules, CA, USA) and generated emulsion was amplified using the following amplification program: 95 °C for 10 min, 95 °C for 15 s and 60 °C for 1 min. Steps two and three were repeated for 40 cycles and ramp rate was set at 2.5 °C/s. A final step at 98 °C for 10 min to inactivate polymerase enzyme activity was added. Amplified droplets were analyzed for the fluorescence content using QX200 Droplet Digital PCR system. Data analysis was performed using QuantaSoft software, version 1.7.4 (Bio-Rad). The microRNA, miR-let-7c-5p (assay ID hsa-miR-let-7c-5p, TM:001973) was employed to verify that the same amount of cDNA was loaded for each sample.
2.4. Next Generation Sequencing (NGS)
NGS experiments were performed at the Laboratory for Technologies of Advanced Therapies (LTTA) of Ferrara University. Small RNA libraries were prepared from total RNA using TruSeq® Small RNA Library PrepKit v2 (Illumina, RS-200-0012/24/36/48), according to manufacturer's indications. Briefly, 35 ng of purified RNA were linked to RNA 3’ and 5’ adapters, converted in cDNA and amplified using Illumina primers containing unique indexes for each sample. Libraries were quantified through Agilent Bioanalyzer, using High Sensitivity DNA kit (Agilent, 5067-4626); after size-selection and ethanol precipitation, the library pool was quantified through Agilent Bioanalyzer and High Sensitivity DNA kit, denatured and diluted to 1.8 pM and sequenced using Illumina NextSeq500 platform and NextSeq® 500/550 High Output Kit v2 (75 cycles) (Illumina, FC-404-2005). Raw base-call data generated from the Illumina NextSeq 500 system have been demultiplexed and converted to FASTQ format. After quality check, evaluated using FastQC tool (https://www.bioinformatics. babraham.ac.uk/projects/fastqc/), adapters sequences have been trimmed by Cutadapt (http://cutadapt.readthedocs.io/en/stable/index.html). In this step also sequences shorter than 10 nucleotides have been removed. Reads mapping has been performed using the STAR algorithm (https://www.ncbi.nlm.nih.gov/pubmed/23104886), and the reference genome was composed of human microRNAs sequences from the miRbase 22 (http://www.mirbase.org/). Count of raw mapped reads has been performed using the htseq-count script from the HTSeq tools (http://www-huber.embl.de/ HTSeq/doc/overview.html); raw counts have been normalized using DESeq2 bioconductor package (http://bioconductor.org/packages/release/bioc/html/DESeq2.html). The optimal read depth to analyse the miRNA transcriptome was determined at 10 million reads per sample.
2.5. Statistical analysis
In order to detect significance of the observed effects, the data obtained have been expressed as mean ± standard deviation (S.D.) and comparison among groups was made by using analysis of variances (ANOVA) with Dunnett's test for comparison with a single control. The numbers of replicates (both biological and technical) have been usually three (Fig. 4B) [3]. Statistical significance was defined as significant (*, p < 0.05) and highly significant (**, p < 0.01).
Ethics Statement
All the ethical requirements were followed. Human subjects and were not involved. The work did not involve animal experiments.
Author Contributions
R.G. and A.F. conceived and designed the experiments; E.F. performed the treatment of the cells with the R8-PNA-a101 and R8-PNA-a145; J.G. and A.F. performed the NGS experiments; J.G. performed the RT-qPCR and RT-ddPCR based experiments; R.G., A.F., E.F. and J.G. wrote the paper.
Declaration of Competing Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the data.
Acknowledgments
This work was supported by Fondazione Fibrosi Cistica (FFC), Projects “Revealing the microRNAs-transcription factors network in cystic fibrosis: from microRNA therapeutics to precision medicine (CF-miRNA-THER)” (FFC#7/2018) and “MicroRNA Therapeutics in CF: Targeting CFTR and inflammation networks (MICRORNA-CF)” (FFC#3/2016) to RG and and FAR 2019 from Ferrara University to AF.
References
- 1.Shen B.Q., Finkbeiner W.E., Wine J.J., Mrsny R.J., Widdicombe J.H. Calu-3: a human airway epithelial cell line that shows cAMP-dependent Cl- secretion. Am. J. Physiol. 1994;266:L493–L501. doi: 10.1152/ajplung.1994.266.5.L493. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen P.E., Egholm M., Berg R.H., Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991;254:1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- 3.Fabbri E., Tamanini A., Jakova T., Gasparello J., Manicardi A., Corradini R., Finotti A., Borgatti M., Lampronti I., Munari S., Dechecchi M.C., Cabrini G., Gambari R. Treatment of Calu-3 cells with a Peptide-Nucleic Acid (PNA) targeting the microRNA miR-101-3p is associated with increased expression of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene. Eur. J. Med. Chem. 2020 doi: 10.1016/j.ejmech.2020.112876. [DOI] [PubMed] [Google Scholar]
- 4.Fabbri E., Tamanini A., Jakova T., Gasparello J J., Manicardi A., Corradini R., Sabbioni G., Finotti A., Borgatti M., Lampronti I., Munari S., Dechecchi M.C., Cabrini G., Gambari R. A Peptide nucleic acid against MicroRNA miR-145-5p enhances the expression of the cystic fibrosis transmembrane conductance regulator (CFTR) in Calu-3 cells. Molecules. 2017;23:E71. doi: 10.3390/molecules23010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Megiorni F., Cialfi S., Dominici C., Quattrucci S., Pizzuti A. Synergistic post-transcriptional regulation of the Cystic Fibrosis Transmembrane conductance Regulator (CFTR) by miR-101 and miR-494 specific binding. PLoS One. 2011;6:e26601. doi: 10.1371/journal.pone.0026601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oglesby I.K., Chotirmall S.H., McElvaney N.G., Greene C.M. Regulation of cystic fibrosis transmembrane conductance regulator by microRNA-145, -223, and -494 is altered in ΔF508 cystic fibrosis airway epithelium. J. Immunol. 2013;190:3354–3362. doi: 10.4049/jimmunol.1202960. [DOI] [PubMed] [Google Scholar]
- 7.Lutful Kabir F., Ambalavanan N., Liu G., Li P., Solomon G.M., Lal C.V., Mazur M., Halloran B., Szul T., Gerthoffer W.T., Rowe S.M., Harris W.T. MicroRNA-145 antagonism reverses TGF-β inhibition of F508del CFTR correction in airway epithelia. Am. J. Respir. Crit. Care Med. 2018;197:632–643. doi: 10.1164/rccm.201704-0732OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gambari R., Gasparello J., Fabbri E., Borgatti M., Tamanini A., Finotti A. Peptide nucleic acids for MicroRNA targeting. Methods Mol. Biol. 2020;2105:199–215. doi: 10.1007/978-1-0716-0243-0_12. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A., Quijano E., Liu Y., Bahal R., Scanlon S.E., Song E., Hsieh W.C., Braddock D.E., Ly D.H., Saltzman W.M., Glazer P.M. Anti-tumor activity of miniPEG-γ-modified PNAs to inhibit MicroRNA-210 for cancer therapy. Mol. Ther. Nucleic Acids. 2017;9:111–119. doi: 10.1016/j.omtn.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brognara E., Fabbri E., E Bazzoli, Montagner G., Ghimenton C., Eccher A., Cantù C., Manicardi A., Bianchi N., Finotti A., Breveglieri G., Borgatti M., Corradini R., Bezzerri V., Cabrini G., Gambari R. Uptake by human glioma cell lines and biological effects of a peptide-nucleic acids targeting miR-221. J. Neurooncol. 2014;118:19–28. doi: 10.1007/s11060-014-1405-6. [DOI] [PubMed] [Google Scholar]
- 11.Gillen A.E., Gosalia N., Leir S.H., Harris A. MicroRNA regulation of expression of the cystic fibrosis transmembrane conductance regulator gene. Biochem. J. 2011;438:25–32. doi: 10.1042/BJ20110672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramachandran S., Karp P.H., Osterhaus S.R., Jiang P., Wohlford-Lenane C., Lennox K.A., Jacobi A.M., Praekh K., Rose S.D., Behlke M.A., Xing Y., Welsh M.J., McCray P.B. Post-transcriptional regulation of cystic fibrosis transmembrane conductance regulator expression and function by microRNAs. Am. J. Respir. Cell Mol. Biol. 2013;49:544–551. doi: 10.1165/rcmb.2012-0430OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKiernan P.J., Greene C.M. MicroRNA dysregulation in cystic fibrosis. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/529642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou C.H., Shrestha S., Yang C.D. miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018;46:D296–D302. doi: 10.1093/nar/gkx1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabbri E., Borgatti M., Montagner G., Bianchi N., Finotti A., Lampronti I., Bezzerri V., Dechecchi M.C., Cabrini G., Gambari R. Expression of microRNA-93 and Interleukin-8 during Pseudomonas aeruginosa-mediated induction of proinflammatory responses. Am. J. Respir. Cell Mol. Biol. 2014;50:1144–1155. doi: 10.1165/rcmb.2013-0160OC. [DOI] [PubMed] [Google Scholar]