Abstract
Background and aims
Severe asthma is burdened by frequent exacerbations and use of oral corticosteroids (OCS) which worsen patients’ health and increase healthcare spending. Aim of this study was to assess the clinical and economic effect of adding mepolizumab (MEP) for the treatment of these patients.
Methods
Patients >18 years old, referred to 8 asthma clinics, starting MEP between May 2017 and December 2018, were enrolled and followed-up for 12 months. Information in the 12 months before mepolizumab were collected retrospectively. The evaluation parameters included: OCS use, number of exacerbations/hospitalizations, concomitant therapies, comorbidity, and annual number of working days lost due to the disease. The primary objective was to compare the annual total cost per patient pre- and post-MEP. Secondary outcomes included rates of exacerbations and number of OCS-dependent patients.
Results
106 patients were enrolled in the study: 46 male, median age 58 years. Mean annual cost pre- and post-MEP (cost of biologic excluded) was €3996 and €1,527, respectively. Total savings due to MEP resulted in €2469 (95%CI 1945–2993), 62% due to exacerbations reduction and 33% due to productivity increase. Such savings could fund about 22% of the total cost of MEP for one year. The introduction of MEP induced a clinical benefit by reducing both OCS-dependent patients (OR = 0.12, 95%CI 0.06–0.23) and exacerbation rate (RR = 0.19, 95%CI 0.15–0.24).
Conclusions
Patients with severe eosinophilic asthma experienced a clinical benefit in asthma control adding MEP to standard therapy. Biologic therapy can be, partially, funded by the savings produced by patients’ improvement.
Keywords: Severe asthma, Mepolizumab, Anti IL-5, Pharmacoeconomics, OCS, Comorbidities
Abbreviations: ACT, Asthma Control Test; CI, Confidence Intervals; COPD, chronic obstructive pulmonary disease; FeNO, fractional nitric oxide; GERD, gastroesophageal reflux disease; ICS, inhaled corticosteroids; IQR, interquartile range; LABA, long acting beta 2 agonist; LAMA, long acting muscarinic antagonist; LOS, Length of stay; MEP, Mepolizumab; OCS, Oral Corticosteroids; OR, Odds Ratio; RCTs, Randomized Controlled Trials; RR, Rate Ratio; SD, Standard Deviation
Take home messages
-
•
There was clear evidence of clinical and socio-economic benefits of mepolizumab in reducing exacerbation/hospitalization rates and OCS use during the first year of treatment.
-
•
The savings produced by patients' improvement can partially fund the cost of mepolizumab therapy.
Introduction
Asthma, together with chronic obstructive pulmonary disease (COPD), is the most frequent chronic airway disease, affecting more than 300 million people worldwide,1 with a 17.5% increase of diagnosis between 2006-2016.2 Among patients with asthma, a percentage varying from 5 to 10%3 suffers from a form, defined as “severe”,4 which cannot be controlled by the inhaled therapy (inhaled corticosteroids/bronchodilators; ICS/LABA/LAMA) administered at maximal doses. Furthermore severe asthma frequently requires the use of oral corticosteroids (OCS). The type of asthma, defined as severe, is burdened by serious clinical problems such as frequent exacerbations,3 hospitalizations,3 intubation, and in rare cases even death.5 Other clinically relevant comorbidities include: sleep disorders,6 intolerance to physical exercise, mood disorders,7 reduced productivity,8 and increased health care spending.9 The frequent use of OCS, even for short periods of time, adds a relevantly increased risk of comorbidities (ie, diabetes, osteoporosis, cataract, hypertension),10 which therefore inevitably become serious burdens from both clinical and pharmacoeconomic points of view.11
To achieve a better control of severe asthma, thus reducing exacerbations and OCS use, several biological drugs able to modulate the inflammatory characteristics of asthma were developed and marketed. Mepolizumab (MEP), an interleukin-5 antagonist, given monthly by subcutaneous administration, was the first biological agent specifically commercialized for uncontrolled severe eosinophilic asthma. Despite numerous randomized and real life trials that confirmed the efficacy and safety of MEP,12 there are still some unclear points, especially regarding the pharmacoeconomic aspects, since the drug is quite expensive.
In this study we analysed prospectively the effects of the introduction of MEP,13 for the treatment of patients with severe eosinophilic asthma, from an economic point of view, analysing the variation of direct and indirect costs related to the drug administration. Secondly, the effects of the drug were analysed with regard to the main outcomes, such as exacerbations and OCS spare.
Methods
Patients
Patients ≥18 years old, referred to 8 asthma clinics between May 1, 2017 and December 31, 2018, and treated with MEP (1 vial 100 mg subcutaneously/4 weeks), were enrolled in the study and followed-up for 12 months (Post period). All patients treated in the above mentioned period in involved clinics have been considered in the study; no patients have been discarded.
Asthma had been diagnosed clinically, functionally (with a documented bronchodilation/provocation) test, fractional nitric oxide (FeNO), Asthma Control Test (ACT); all patients met the criteria for severe uncontrolled asthma diagnosis according to American Thoracic Society.European Respiratory Society (ATS/ERS) guidelines4 as well as MEP prescription criteria according to the Italian Drug Agency. In particular, it was required an eosinophilic count of at least 300/μL in the year before MEP treatment, and of 150/μL before the first MEP injection.
The relevant clinical information were collected prospectively during the follow-up period, and the same data were collected retrospectively in the 12 months before starting mepolizumab treatment (Pre-MEP period). The main evaluated parameters included: OCS mean daily dose, number of exacerbations/hospitalizations, comorbidity, and annual number of working days lost due to the disease.
For the analysis of the clinical outcomes, exacerbation and hospitalization rates were defined as the number of events divided by the person-years (PY) of follow-up (pre- and after 1 year of MEP). Length of stay (LOS) in hospital was defined, for each episode, as the number of days of direct patient care (minimum 1 day) from admission to discharge. The total cost of resource consumption, drug therapies, and productivity loss were calculated both in the pre- and after 1 year period of treatment to estimate the impact due to introduction of MEP. All patients signed an informed consent for the treatment of personal data, which were anonymized.
Statistical analysis
Categorical variables are expressed as counts and percentages, continuous variables are summarized using median and interquartile range (IQR), mean and standard deviation (SD) were also reported if normally distributed (normality was tested using Shapiro-Wilk test), and number of events (exacerbation with or without hospitalization) are expressed as rates (ie, number of events per PY). Difference between pre- and post-MEP treatment were tested using Fisher exact test for categorical variables, Wilcoxon test for the continuous one (Welch test is also performed in case of normality) and Poisson test for rates. Furthermore, to quantify the agreement between inhaled corticosteroid/long-acting beta agonist (ICS/LABA) therapy prior to and after the introduction of mepolizumab, we used Cohen's kappa,14 in order to measure the agreement between the treatment categories pre- and post-MEP. Specific relative effect measures were also presented with 95% confidence intervals (CI): odds ratio (OR) for categorical outcomes, rate ratio (RR) for rates, and absolute difference for continuous outcomes (using a generalized linear model, family = gamma, link function = identity). All statistical analyses were performed using R (R Core Team 2018).
Economic evaluation
The impact of the MEP treatment was evaluated considering 1 subcutaneous 100 mg vial every 4 weeks.15 The cost for each treatment cycle was calculated according to the ex-factory price,16 applying the mandatory discount (5% + 5% reduction) and price reductions negotiated among the pharmaceutical company, Local Health Units, and Hospital Units.17 The cost of ICS/LABA, OCS, long-acting muscarinic antagonist (LAMA), antileukotrienes, and gastroesophageal reflux disease (GERD) therapies was calculated using the reference price established by negotiation for each product package18 and for the specific posology for each patient (Table A 1). The use of OCS was expressed in prednisone dose equivalents; hence, only the cost of prednisone was considered, assuming the best package fitting the daily dose. The cost of GERD therapy was calculated according to specific treatment reported in the database considering 4 weeks in acute phase (maximum dose) followed by maintenance with halved dose. When not specified, the mean of all reported treatments was considered as proxy.
The tariff of € 300 for an emergency room visit was applied for exacerbations not needing hospitalization,19 excluding additional drugs since all patients were already treated with OCS. The cost for each hospitalization was estimated in € 1939.18, calculated according to our Diagnosis Related Group 96 (Bronchitis and asthma, > 17 years with complications) and 97 (Bronchitis and asthma, > 17 years without complications) tariffs, weighted for the number of hospital admissions (both ordinary and day-hospital) in Italy.20
Each day lost due to the disease was valued using the cost of paid and unpaid (household activities, caring for family members and others, and volunteering) work, specific for age and sex21 updated to 201722 (Table A 2). For non-workers (students, retired, or unemployed) only the total length of stay in hospital was multiplied by the cost of unpaid work.
Results
A total of 106 patients was enrolled in the study: 46 men (43.4%) and 60 women (56.6%), mean age 57.35 years (SD = 10.56; median 58, IQR: 52.25–65). All patients were included in the analysis, and none was excluded due to missing data or clinical response to the drug. During the observation period we do not have any patients withdrawn from treatment.
The comparison between pre- and post-MEP periods for all clinical outcomes considered in the analysis is detailed in Table 1. The introduction of MEP showed a clinical benefit, significantly reducing both the proportion of patients treated with OCS (OR = 0.12, 95% CI 0.06–0.231), and mean daily OCS consumption in OCS-dependent patients (−4.73 mg, 95% CI -7.13 – −2.24). The annual exacerbation rate decreased from 4.085 events per PY pre-MEP to 0.774 with MEP (RR = 0.189; 95% CI 0.148–0.24); in both periods, exacerbations not needing hospitalization accounted for about 90% of all events. Mean hospital LOS per episode resulted slightly longer post-MEP (median pre = 5.5 vs median post 7 = days); however, the uncertainty in the absolute difference was high (1.41 days, 95% CI -2.65–10.06) (Table 2). Finally, MEP achieved a better control of disease symptoms since the proportion of patients losing working days due to hospitalizations or disease exacerbations decreased from 63.2% in the pre-MEP period to 31.2% in the post-MEP period (OR = 0.253, 95% CI 0.136–0.463). Furthermore, the number of working days lost decreased significantly after starting MEP (−11.87 days, 95% CI -16.87– −7.64).
Table 1.
Comparison of clinical outcome between the year before (pre-) and the year after (post-) MEP
| Pre period | Post period | Post vs pre (95% CI) | p-value | |
|---|---|---|---|---|
| OCS - dependent patients, N (%) | 84 (79.2%) | 33 (31.1%) | OR = 0.12 (0.06–0.231) | <0.0001 |
| OCS daily dosea, mg | 10 (5–13.75) | 5 (5–10) | Δ = −4.73 (−7.13– −2.24) | 0.0002 |
| Total exacerbation rate (events per PY) | 4.085 | 0.774 | RR = 0.189 (0.148–0.24) | <0.0001 |
| Not needing hospitalization | 3.699 | 0.717 | RR = 0.194 (0.15–0.248) | <0.0001 |
| Needing hospitalization | 0.387 | 0.057 | RR = 0.146 (0.051–0.347) | <0.0001 |
| LOS per episode (days) | 5.5 (3–8) | 7 (6–10) | Δ = 1.41 (−2.65–10.06) | 0.61 |
| Patients with lost working days N (%) | 67 (63.2%) | 32 (31.2%) | OR = 0.253 (0.136–0.463) | <0.0001 |
| Working days lost | 15 (10–23.5) | 4 (2–6.25) | Δ = −11.87 (−16.87– −7.64) | <0.0001 |
CI: confidence interval; LOS: length of stay; MEP: mepolizumab; OCS: oral corticosteroids; OR: odds ratio; PY: person-years; RR: rate ratio
Only OCS- dependent patients considered
Table 2.
Clinical patients’ characteristics at baseline
| Mean (SD) | |
|---|---|
| EXACERBATIONS 12 months | 4.1 (2.7) |
| HOSPITALIZATIONS 12 months | 0.4 (0.6) |
| FEV1% | 70 (20) |
| FEV1 L | 2.07 (0.78) |
| FVC % | 87 (18) |
| FVC L | 3.12 (1.02) |
| Bronchodilation test (FEV1 variation in ml vs baseline)b | 0.27 (0.85) |
| Eosinophilsa | 674 (433) |
| FeNO | 71 (52) |
| ACT | 17 (5) |
| OCS dependent (%) | 84 (79) |
| OCS dosec | 9.5 (8.5) |
Data are expressed as mean and standard deviation if not already specified.
(blood eosinophils/mcl) value expressed in geometric mean and SD
Bronchodilation test has been performed in 41 patients, the other have a provocation positivity test
mg of prednisone or equivalent
No differences were observed in the prevalence of comorbidities recorded in the study pre- and post-introduction of MEP (Table 3): rhinitis (OR = 0.807, 95% CI 0.435–1.493), polyposis (OR = 0.856, 95% CI 0.476–1.535), GERD (OR = 0.839, 95% CI 0.449–1.564), or dermatitis (OR = 1, 95% CI 0.181–5.523). As expected, the introduction of MEP did not influence the dose of inhaled therapy with ICS/LABA (Table A 3). There was an almost perfect concordance between the ICS/LABA therapy pre- and post-MEP (kappa = 0.837, 95% CI 0.758–0.917, p < 0.0001).
Table 3.
Comparison of main comorbidities between the year before and the year after starting MEP
| Pre period | Post period | OR post vs pre (95% CI) | p-value | |
|---|---|---|---|---|
| Rhinitis, N (%) | 74 (69.8%) | 69 (65.1%) | 0.807 (0.435–1.493) | 0.5578 |
| Polyposis, N (%) | 65 (61.3%) | 61 (57.5%) | 0.856 (0.476–1.535) | 0.6749 |
| GERD, N (%) | 35 (33.0%) | 31 (29.2%) | 0.839 (0.449–1.564) | 0.6565 |
| Dermatitis, N (%) | 4 (3.8%) | 4 (3.8%) | 1 (0.181–5.523) | >0.9999 |
CI: confidence interval; GERD: gastroesophageal reflux disease; MEP: mepolizumab; OR: odds ratio
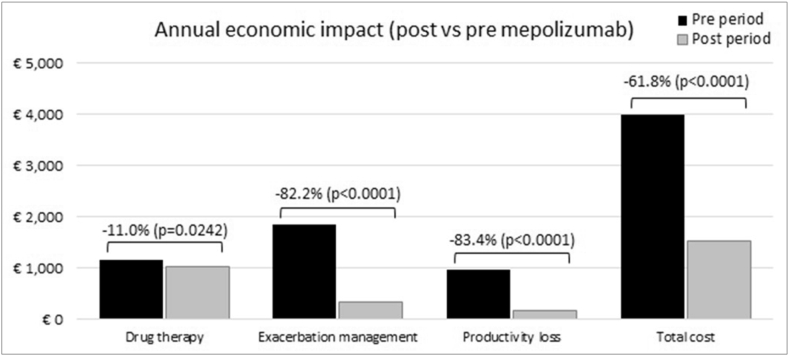
The mean annual cost pre- and post-MEP introduction is reported in Table 4, split in the 3 main domains considered in the analysis: drugs, exacerbation management, and productivity loss. Excluding the cost of MEP, total savings due to the added treatment resulted in € 2469 per patient corresponding to a relative reduction of −61.8% (Fig. 1). Most of the savings was due to exacerbation reduction (-€ 1529), followed by less productivity losses (-€ 812). Including MEP treatment, the total cost in the after period amounts to € 12,975 per patient, corresponding to an incremental cost (post vs pre period) of € 8979.
Table 4.
Total annual cost pre- and post-MEP, data are reported as mean (95% confidence interval)
| Pre period | Post period | Post vs pre | |
|---|---|---|---|
| Drug therapy | € 1163 (1087–1239) | € 1035 (952–1117) | -€ 128 (−239– −17)) |
| ICS/LABA | € 706 (676–737) | € 696 (645–747) | -€ 10 (−69–49) |
| OCS | € 75 (63–87) | € 20 (13–27) | -€ 55 (−69– −41) |
| LAMA | € 280 (223–338) | € 258 (201–315) | -€ 22 (−103–58) |
| Antileukotrienes | € 78 (58–98) | € 40 (24–56) | -€ 38 (−63– −13) |
| GERD | € 24 (16–31) | € 21 (14–29) | -€ 2 (−13–8) |
| Exacerbations management | € 1859 (1561–2158) | € 331 (210–451) | -€ 1529 (−1850– −1208) |
| Without hospitalizations | € 1109 (959–1260) | € 221 (150–291) | -€ 889 (-1065– −723) |
| With hospitalizations | € 750 (505–995) | € 110 (9–211) | -€ 640 (−904– −376) |
| Productivity loss | € 974 (724–1224) | € 162 (84–239) | -€ 812 (−1074– −551) |
| Paid work | € 598 (437–759) | € 97 (51–144) | -€ 500 (−668– −333) |
| Unpaid work | € 376 (265–487) | € 64 (30–99) | -€ 312 (−428– −196) |
| Total cost (MEP excluded) | € 3996 (3517–4476) | € 1527 (1312–1743) | -€ 2469 (−2993– −1945) |
| Total cost (MEP included) | € 3996 (3517–4476) | € 12,975 (12,759–13,191) | € 8979 (8455–9503) |
ICS: inhaled corticosteroids; GERD: gastroesophageal reflux disease; LABA: long-acting beta-adrenoceptor agonist; LAMA: long-acting muscarinic antagonist; MEP: mepolizumab; OCS: oral corticosteroids
Fig. 1.
Annual cost reduction per patients after mepolizumab introduction (MEP cost excluded)
Discussion
The results herein described, using the prospective and retrospective data collected in a real life setting, showed that a 1-year administration course of MEP in severe asthma could reduce the exacerbation rate, the dose of OCS taken by patients and the percentage of steroid-dependent subjects, confirming what previously demonstrated in randomized controlled trials (RCTs)23, 24, 25 and in real world studies.26,27 In fact, as already shown, the introduction of MEP in severe uncontrolled eosinophilic asthma. In particular we observed a reduction of exacerbations of 81%, after 1 year of therapy, and of OCS-dependent patients (from 84% to 33%). In patients who remained on chronic OCS, a significant reduction of the dose was seen (−4.73 mg (C.I. −7.13–2.24; p = 0.0002).
The effect of the drug in reducing exacerbations is relevant in pharmacoeconomics terms. The total mean annual cost per patient pre-MEP was estimated in € 3,996, where the management of exacerbations played a crucial role (€ 1859) corresponding per se to the 46.5% of the whole cost. The management of exacerbations includes not only the direct but also indirect costs related to lost working days, reduced productivity at work, loss in economic terms of activities not directly remunerative (voluntary work), estimated, in this analysis in a mean of € 974 per year for each patient (Table 4). The introduction in therapy of MEP, reduced by more than 80% both the costs of exacerbation management and the indirect costs, with a slight effect on the cost of drug therapy (−11%). The resulting cost savings per patient treated with MEP, not considering the cost of MEP, was € 2469 (95% CI 1945–2993), of which 62% attributable to the reduction in exacerbations and hospitalizations, and 33% to the reduction of productivity loss. This savings represents an economic return of about 22% on the starting investment ie, the cost of a year's treatment with MEP.
A secondary, but relevant observation, concerns OCS therapy. It is well known that prolonged OCS treatments are burdensome because of the side effects, such as cataracts, diabetes, osteoporosis, and hypertension.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 The use of OCS, therefore, has important delayed consequences from a pharmacoeconomic point of view, which could not be captured in this study.29 Although OCS are cheap per se, the cost associated with the morbidity induced by these drugs represents a very important shadow cost, particularly in patients exposed to an overload of corticosteroids. The analysis of an Italian pharmacoeconomic model aimed at predicting the cost of the adverse effects of corticosteroid events in individuals with severe asthma in real life30,31 showed that the total annual cost of OCS-related events is approximately € 242 million.10 In fact, compared to a rather high percentage of steroid-dependent patients (72%), before the introduction of MEP, with an average daily dosage of 10 ± 8.44 mg prednisone, the average cost for this therapy was € 75, reduced to € 20 after 12 months of therapy. In this case, the analysis of the one-year data alone is limiting with regard to the economic benefits of steroid reduction, in fact, analysing only the direct costs, related to what is saved in terms of expenditure for steroid therapy, an annual saving of just over € 50 per patient is quite evident, a relatively low gain if compared to an already described mean annual expenditure of around € 4000. This type of pharmacoeconomic analysis is limiting with regard to the real gain, both in clinical and economic terms, of reducing and/or suspending systemic steroid therapy. Although the OCS-induced damages appear already after short cycles,32 even at low dosages, and greater damages are caused by prolonged therapies over the years.10 Therefore, the effect, both from the clinical and the pharmacoeconomic point of view, could be captured using a longer perspective, projecting the savings related to the management of common comorbidities linked to continuous steroid treatment. Given this evidence, also international documents, starting with the Global Initiative for Asthma (GINA),3 strongly discourage the use of OCS in asthma, allowing their prescription only for short cycles or in patients for whom the administration of biological drugs has failed or is not possible.
The lifetime consequences of MEP were evaluated in a recent cost-effectiveness analysis33, 34, 35, 36, 37, 38 on a population of adults with severe uncontrolled asthma and eosinophilic inflammation. The authors found that adding MEP to standard ICS treatment resulted in an incremental cost per exacerbation averted of $ 24,626 with a clinical benefit of 23.96 exacerbations averted per patients and 1.53 quality-adjusted life years (QALYs) gained. However, while the cost offsets were more than $ 18,000, the treatment cost increased by about $ 600,000. Different scenario analyses were investigated and the authors concluded that adding MEP lowers exacerbation rates and improves quality of life, but its value was heavily influenced by the ability to identify responders and continue treatment for only those who respond.
The main advantage of this study is that it was conducted in real life, with retrospective and prospective data, and not using pharmacoeconomic mathematical models. The main limit is the small sample, due to the fact that severe eosinophilic asthma, eligible to MEP treatment is a rare disease per se.
Overall, the economic savings of the drug, in a real-life setting and in a limited observation over time (12 months), could be seen mainly in the reduction of exacerbation and hospitalization costs, and also in the indirect costs such as lost working days and savings in unpaid work activities. The introduction of the drug alone was, therefore, able to finance itself for a share of more than 20% of the total annual cost, a share that if we analyse the long-term effect, linked to the reduction in the need to take OCS and, subsequently, the lower comorbidities associated with it, can only increase. In the long term, it can be assumed that, if the drug maintains its stable efficacy over the years, further economic savings should be expected. In addition, by being able to treat patients before long periods of OCS intake, savings in comorbidities, another clinical and economical profit, would be possible.
Funding and conflict of interest
Lorenzo Pradelli is co-owner and employee of AdRes, which has received project funding from GSK. Massimiliano Povero is employee of AdRes, which has received project funding from GSK. None for other authors. SANI is supported by Unrestricted Grants from AstraZeneca, Glaxo Smith Kline, Novartis & Sanofi Genzyme.
Author contributions
DB conceived and planned the study, collected the data and contributed to write the paper. MP collected the data, performed the statistical analysis and contributed in the interpretation of the results. LP was involved in planning, interpretation of results and supervision of the work. GP contributed to planning, interpretation of results, supervised the study and contributed in writing the MS. All the remaining Authors did the clinical work, collected the data and contributed to the final manuscript.
Author consent to publication
All authors consented to the publication of this work.
Ethics considerations
Written informed consent was obtained, as discussed in the methods; data were anonymized.
Availability of data and materials
Partial data are available on request.
Author consent to publication
All authors consented to the publication of this work.
Written informed consent was obtained, as discussed in the methods; data were anonymized.
Partial data are available on request.
Acknowledgements
CIPRO (Centro Interprofessional Pneumologico di Ricerca ed Organization).
Footnotes
Full list of author information is available at the end of the article https://doi.org/10.1016/j.waojou.2021.100509
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2021.100509.
Contributor Information
Diego Bagnasco, Email: diego.bagnasco@dimi.unige.it.
SANI Network (Severe Asthma Network Italy):
Gabriella Guarnieri, Vincenzo Patella, Foschino Barbaro Maria Pia, Elisiana Carpagnano, Anna del Colle, Giulia Scioscia, Pelaia Gerolamo, Pierluigi Paggiaro, Manuela Latorre, Francesca Puggioni, Francesca Racca, Elisabetta Favero, Sandra Iannacone, Eleonora Savi, Marcello Montagni, Gianna Camiciottoli, Chiara Allegrini, Giuseppe Spadaro, Caterina Detoraki, Carla Galeone, Patrizia Ruggiero, Monna Rita Yacoub, Alvise Berti, Gisella Colombo, Nicola Scichilone, Carmen Durante, Maria Teresa Costantino, Chiara Roncallo, Mariachiara Braschi, Francesco Blasi, Alice D'Adda, Erminia Ridolo, Massimo Triggiani, Roberta Parente, D'Amato Maria, Maria Vittoria Verrillo, Zappa Maria Cristina, Marianna Lilli, Nunzio Crimi, Marco Bonavia, Angelo Guido Corsico, Amelia Grosso, Stefano Del Giacco, Margherita Deidda, Luisa Ricciardi, Stefania Isola, Francesca Cicero, Giuliana Amato, Federica Vita, Antonio Spanevello, Patrizia Pignatti, Francesca Cherubino, Dina Visca, Eleonora Aletti, Fabio Luigi Massimo Ricciardolo, Vitina Maria Anna Carriero, Francesca Bertolini, Pierachille Santus, Roberta Barlassina, Andrea Airoldi, Giuseppe Guida, Nucera Eleonora, Arianna Aruanno, Angela Rizzi, Cristiano Caruso, Stefania Colantuono, Alessandra Arcolaci, Andrea Vianello, Fulvia Chieco Bianchi, Maria Rita Marchi, Stefano Centanni, Simone Luraschi, Silvia Ruggeri, Rocco Rinaldo, Elena Parazzini, Cecilia Calabrese, Martina Flora, Lorenzo Cosmi, Linda Di Pietro, Enrico Maggi, Laura Pini, Luigi Macchia, Danilo Di Bona, Luca Richeldi, Carola Condoluci, Leonello Fuso, Matteo Bonini, Alessandro Farsi, Giulia Carli, Paolo Montuschi, Giuseppe Santini, Maria Elisabetta Conte, Elisa Turchet, Carlo Barbetta, Francesco Mazza, Simona D'Alo, Stefano Pucci, Maria Filomena Caiaffa, Elena Minenna, Luciana D'Elia, Carlo Pasculli, Vittorio Viviano, Paolo Tarsia, Joyce Rolo, Mariacarmela Di Proietto, Salvatore Lo Cicero, and Mariacarmela Di Proietto
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Loftus P.A., Wise S.K. Epidemiology and economic burden of asthma. Int Forum Allergy Rhinol. 2015;5(Suppl 1):S7–S10. doi: 10.1002/alr.21547. [DOI] [PubMed] [Google Scholar]
- 2.James S.L., Abate D., Abate K.H. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018 Nov 10;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GINA 2019 [Internet] 2019. https://ginasthma.org/wp-content/uploads/2019/04/GINA-2019-main-Pocket-Guide-wms.pdf Available from:
- 4.Chung K.F., Wenzel S.E., Brozek J.L. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 5.D'Amato G., Vitale C., Molino A. vol. 11. BioMed Central Ltd.; 2016. Asthma-related deaths; pp. 1–5. (Multidisciplinary Respiratory Medicine). [Google Scholar]
- 6.Sanz de Burgoa V., Rejas J., Ojeda P. Self-perceived sleep quality and quantity in adults with asthma: findings from the costeasma study. J Investig Allergol Clin Immunol. 2016;26(4):256–262. doi: 10.18176/jiaci.0044. [DOI] [PubMed] [Google Scholar]
- 7.Gold L.S., Thompson P., Salvi S., Faruqi R.A., Sullivan S.D. Level of asthma control and health care utilization in Asia-Pacific countries. Respir Med. 2014;108(2):271–277. doi: 10.1016/j.rmed.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Lee L.K., Obi E., Paknis B., Kavati A., Chipps B. Asthma control and disease burden in patients with asthma and allergic comorbidities. J Asthma. 2018;55(2):208–219. doi: 10.1080/02770903.2017.1316394. [DOI] [PubMed] [Google Scholar]
- 9.Zeiger R.S., Schatz M., Dalal A.A. Utilization and costs of severe uncontrolled asthma in a managed-care setting. J Allergy Clin Immunol Pract. 2016 Jan 1;4(1):120–129. doi: 10.1016/j.jaip.2015.08.003. e3. [DOI] [PubMed] [Google Scholar]
- 10.Heffler E., Bagnasco D., Canonica G.W. Strategies to reduce corticosteroid-related adverse events in asthma. Curr Opin Allergy Clin Immunol. 2019 Feb 1;19(1):61–67. doi: 10.1097/ACI.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 11.Canonica G.W., Colombo G.L., Bruno G.M. Shadow cost of oral corticosteroids-related adverse events: a pharmacoeconomic evaluation applied to real-life data from the Severe Asthma Network in Italy (SANI) registry. World Allergy Organ J. 2019;12(1):100007. doi: 10.1016/j.waojou.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagnasco D., Menzella F., Caminati M. Efficacy of mepolizumab in patients with previous omalizumab treatment failure: real-life observation. Allergy. 2019;74(12):2539–2541. doi: 10.1111/all.13937. [DOI] [PubMed] [Google Scholar]
- 13.Bagnasco D., Caminati M., Ferrando M. Anti-IL-5 and IL-5Ra: efficacy and safety of new therapeutic strategies in severe uncontrolled asthma. BioMed Res Int. 2018;2018:5698212. doi: 10.1155/2018/5698212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 15.SPC mepolizumab [Internet]. Available from: https://www.ema.europa.eu/en/documents/overview/nucala-epar-medicine-overview_it.pdf.
- 16.Informatory farmaceutico. 2019. www.codifa.it [Internet]. Available from:
- 17.Anagrafica SORESA [Internet]. Available from: https://www.soresa.it/imprese/Pagine/Anagrafica-Beni.aspx.
- 18.Liste di trasparenza farmaci equivalenti [Internet]. Available from: http://www.agenziafarmaco.gov.it/content/liste-di-trasparenza-e-rimborsabilità.
- 19.Delibera liguria [Internet]. Available from: http://www.asl4.liguria.it/ovinternet/resource/pronto_soccorso/Delibera_Regionale_OBI.pdf.
- 20.SDO. Available from: http://www.salute.gov.it/portale/temi/p2_6.jsp?lingua=italiano&id=1232&area=ricoveriOspedalieri&menu=vuoto.
- 21.Pradelli L., Ghetti G. A general model for the estimation of societal costs of lost production and informal care in Italy. Farmeconomia Heal Econ Ther pathways. 2017 Feb 21;18(1) [Google Scholar]
- 22.Harmonized Indices of Consumer Prices. European Commission EuroStat; 2018. https://ec.europa.eu/eurostat/web/hicp/data/main-tables [Internet]. Available from: [Google Scholar]
- 23.Pavord I.D., Korn S., Howarth P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 24.Bel E.H., Wenzel S.E., Thompson P.J. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 25.Ortega H.G., Liu M.C., Pavord I.D. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014 Sep 25;371(13):1198–1207. doi: 10.1056/NEJMoa1403290. [cited 2020 Jan 7] [DOI] [PubMed] [Google Scholar]
- 26.Bagnasco D., Milanese M., Rolla G. The North-Western Italian experience with anti IL-5 therapy and comparison with regulatory trials. World Allergy Organ J. 2018;11(1) doi: 10.1186/s40413-018-0210-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagnasco D., Caminati M., Menzella F. One year of mepolizumab. Efficacy and safety in real-life in Italy. Pulm Pharmacol Ther. 2019 Oct;58 doi: 10.1016/j.pupt.2019.101836. 101836. [DOI] [PubMed] [Google Scholar]
- 28.Bagnasco D, Brussino L, Caruso C, et al. Do the Current Guidelines for Asthma Pharmacotherapy Encourage Over-treatment? Ex pert Opinion On Pharmacotherapy. (in press). DOI: 10.1080/14656566.2020.1759551. [DOI] [PubMed]
- 29.Canonica G.W., Colombo G.L., Bruno G.M. Shadow cost of oral corticosteroids-related adverse events: a pharmacoeconomic evaluation applied to real-life data from the Severe Asthma Network in Italy (SANI) registry. World Allergy Organ J. 2019 Jan 1;12(1) doi: 10.1016/j.waojou.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senna G., Guerriero M., Paggiaro P.L. SANI-Severe Asthma Network in Italy: a way forward to monitor severe asthma. Clin Mol Allergy. 2017 Apr 10;15(1) doi: 10.1186/s12948-017-0065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heffler E., Blasi F., Latorre M. The severe asthma network in Italy: findings and perspectives. J Allergy Clin Immunol Pract. 2019 May 1;7(5):1462–1468. doi: 10.1016/j.jaip.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Volmer T., Effenberger T., Trautner C., Buhl R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J. 2018;52(4):1800703. doi: 10.1183/13993003.00703-2018. [DOI] [PubMed] [Google Scholar]
- 33.Whittington M.D., McQueen R.B., Ollendorf D.A. Assessing the value of mepolizumab for severe eosinophilic asthma: a corst-effectiveness analysis. Ann Allergy Asthma Immunol. 2017;118(2):220–225. doi: 10.1016/j.anai.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 34.Llanos J.P., Ortega H., Bogart M. Real-world effectiveness of mepolizumab in patients with severe asthma: an examination of exacerbations and costs. J Asthma Allergy. 2020;13:77–87. doi: 10.2147/JAA.S236609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bermejo I., Stevenson M., Cooper K. Mepolizumab for treating severe eosinophilic asthma: an evidence review Group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2018;36(2):131–144. doi: 10.1007/s40273-017-0571-8. [DOI] [PubMed] [Google Scholar]
- 36.Basu A., Dalal A., Canonica G.W. Economic analysis of the phase III MENSA study evaluating mepolizumab for severe asthma with eosinophilic phenotype. Expert Rev Pharmacoecon Outcomes Res. 2017;17(2):121–131. doi: 10.1080/14737167.2017.1298444. [DOI] [PubMed] [Google Scholar]
- 37.García-Mochón L., Gil-Sierra M.D., Alegre-Del Rey E.J., Alarcón de la Lastra-Romero C., Sánchez-Hidalgo M. Economic evaluation and budgetary burden of mepolizumab in severe refractory eosinophilic asthma. Evaluación económica y análisis de impacto presupuestario de mepolizumab en asma eosinofílica refractaria grave. Farm Hosp. 2019;43(6):187–193. doi: 10.7399/fh.11221. [DOI] [PubMed] [Google Scholar]
- 38.Ortega H., Hahn B., Tran J.N., Bell C., Shams S.A., Llanos J.P. Disease burden in patients with asthma before initiating biologics: a retrospective cohort database study. Allergy Asthma Proc. 2019;40(3):146–153. doi: 10.2500/aap.2019.40.4220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Partial data are available on request.