Abstract
This study characterizes conditions of N-nitrosodimethylamine production under simulated physiologic gastric states.
Introduction
N-nitrosodimethylamine (NDMA), a probable human carcinogen, was recently detected in ranitidine products,1 prompting widespread regulatory recalls.2 Ranitidine is a histamine2-receptor antagonist that inhibits gastric acid secretion and bears a molecular structure that putatively supports NDMA production under suitable reactive and storage conditions.3 We sought to further characterize conditions of NDMA production under simulated physiologic gastric states.
Methods
To characterize conditions under which ranitidine may yield NDMA, we used liquid chromatography–high resolution mass spectrometry (LC-HRMS)4 for NDMA detection on simulated gastric fluid (SGF) while varying pH and concentrations of ranitidine and nitrite (eMethods in the Supplement). Data analysis was conducted from June 1, 2019, through August 31, 2020. Because no patients were involved in this study, it was not under the purview of an institutional review board.
Briefly, SGF (2 g/L of sodium chloride in water) was prepared at various pH and nitrite concentrations. To initially evaluate NDMA formation at different human stomach–relevant pH conditions, 100 mL of SGF containing 50 mmol/L sodium nitrite was prepared at pH 1.2, 2.5, 3.5, 4.5, and 5.5 using hydrochloric acid. Commercially available ranitidine tablets of 150 and 300 mg were added to the SGF. To evaluate NDMA at variable nitrite concentrations, 100 mL of SGF containing 50, 25, 10, 5, 2.5, and 1 mmol/L of sodium nitrite was prepared at pH 2.5. A ranitidine cool mint 150 mg branded tablet (ie, Zantac) was added to the SGF under each condition. All experiments were conducted at 37 °C incubation, and samples were taken at the 2-hour time point. One milliliter of incubated SGF was transferred, centrifuged, and filtered by a 0.22-μm nylon filter into an LC vial. A known amount of 13C2D6-NDMA was spiked into the sample targeting the final internal standard concentration of 40 ng/mL. Ranitidine tablets were also directly diluted and run by LC-HRMS as control samples. The NDMA assays were performed per the US Food and Drug Administration–recommended LC-HRMS method for determination of nitrosamines in ranitidine drug substance and drug products.4
Results
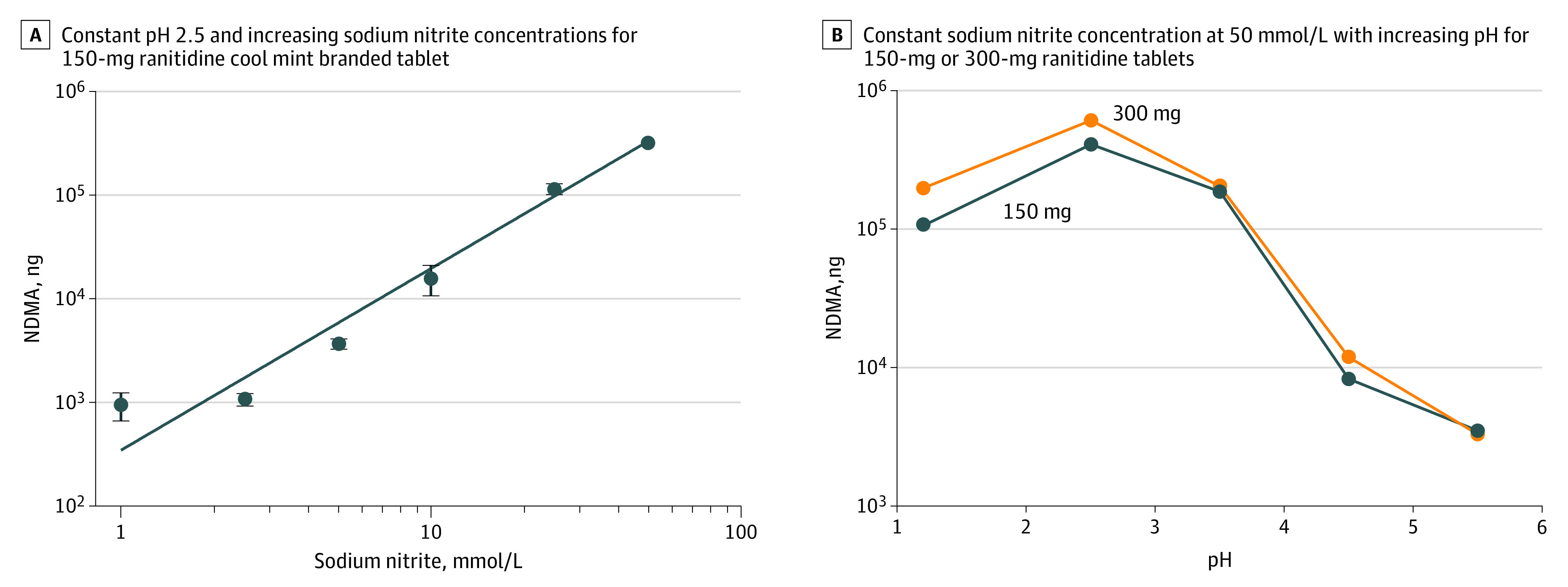
Using LC-HRMS, we detected NDMA formation from ranitidine in SGF across a range of physiologic conditions. At a constant pH of 2.5 and under increasing nitrite concentrations, 150 mg of cool mint branded ranitidine yielded increasing amounts of NDMA after 2 hours: a minimum of 947 ng of NDMA was detected at 1 mmol/L of sodium nitrite and a maximum of 320 000 ng at 50 mmol/L of sodium nitrite (Figure, A). Conversely, holding sodium nitrite constant at 50 mmol/L and varying pH from 1.2 to 5.5 in the presence of 150- or 300-mg ranitidine tablets demonstrated elevated NDMA production under acid conditions (198 000 ng of NDMA at pH 1.2) with diminishing NDMA yields at higher pH (3310 ng at pH 5.5) (Figure, B). Under optimally reactive physiologic conditions (pH 2.5 and 50 mmol/L of sodium nitrite), NDMA yield from a 300-mg ranitidine tablet increased to 0.612 mg (612 000 ng).
Figure. N-Nitrosodimethylamine (NDMA) Formation From Ranitidine in Simulated Gastric Fluid.
Discussion
The results of this study suggest that ranitidine may be a significant source of NDMA under a range of physiologically relevant conditions. Namely, under simulated gastric conditions, NDMA yield from a standard tablet of ranitidine was seen to increase with both increasing nitrite and decreasing pH to levels up to 3 orders of magnitude beyond established limits.
These analyses are not without limitation and encompass a broad range of reactive conditions, some of which exceed the usual gastric milieu. These findings should prompt investigation of other potential nitrosamine precursors in commercially available medications. Given that the putative carcinogenic mechanism of NDMA arises from DNA alkylation, studies of mutational signatures among tumors that may have arisen in the setting of ranitidine use could further inform the potential mechanistic cause of those lesions.5 Population-level epidemiologic analyses may characterize the extent to which these findings represent a public health concern. Although additional studies are ongoing, these data support recent regulatory actions to limit ranitidine availability.
eMethods. Supplementary Methods
References
- 1.Valisure Citizen Petition on Ranitidine . September 9, 2020. Accessed December 1, 2020. https://beta.regulations.gov/document/FDA-2019-P-4281-0001
- 2.FDA Requests Removal of All Ranitidine Products (Zantac) from the Market . 2020. Accessed August 24, 2020. https://www.fda.gov/news-events/press-announcements/fda-requests-removal-all-ranitidine-products-zantac-market
- 3.Mitch WA, Sharp JO, Trussell RR, Valentine RL, Alvarez-Cohen L, Sedlak DL. N-nitrosodimethylamine (NDMA) as a drinking water contaminant: a review. Environ Eng Sci. 2003;20:389-404. doi: 10.1089/109287503768335896 [DOI] [Google Scholar]
- 4.US Food and Drug Administration. Liquid chromatography-high resolution mass spectrometry (LC-HRMS) method for the determination of NDMA in ranitidine drug substance and drug product. Accessed November 13, 2019. https://www.fda.gov/media/130801/download
- 5.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. ; Australian Pancreatic Cancer Genome Initiative; ICGC Breast Cancer Consortium; ICGC MMML-Seq Consortium; ICGC PedBrain . Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415-421. doi: 10.1038/nature12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplementary Methods


