This cohort study assesses the association between maternal and neonatal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–specific antibody concentrations.
Key Points
Question
What is the association between maternal and neonatal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–specific antibody concentrations?
Findings
In this cohort study, SARS-CoV-2 IgG antibodies were transferred across the placenta in 72 of 83 pregnant women who were seropositive, and cord blood IgG concentrations were directly associated with maternal antibody concentrations, whereas IgM antibodies were not detected in any cord blood sera. Transfer ratios were associated with time elapsed from maternal infection to delivery and not associated with severity of maternal infection.
Meaning
Efficient transplacental transfer of SARS-CoV-2 IgG antibodies supports the potential for maternally derived antibodies to provide neonatal protection from SARS-CoV-2 infection.
Abstract
Importance
Maternally derived antibodies are a key element of neonatal immunity. Understanding the dynamics of maternal antibody responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during pregnancy and subsequent transplacental antibody transfer can inform neonatal management as well as maternal vaccination strategies.
Objective
To assess the association between maternal and neonatal SARS-CoV-2–specific antibody concentrations.
Design, Setting, and Participants
This cohort study took place at Pennsylvania Hospital in Philadelphia, Pennsylvania. A total of 1714 women delivered at the study site between April 9 and August 8, 2020. Maternal and cord blood sera were available for antibody measurement for 1471 mother/newborn dyads.
Exposures
SARS-CoV-2.
Main Outcomes and Measures
IgG and IgM antibodies to the receptor-binding domain of the SARS-CoV-2 spike protein were measured by enzyme-linked immunosorbent assay. Antibody concentrations and transplacental transfer ratios were analyzed in combination with demographic and clinical data.
Results
The study cohort consisted of 1714 parturient women, with median (interquartile range) age of 32 (28-35) years, of whom 450 (26.3%) identified as Black/non-Hispanic, 879 (51.3%) as White/non-Hispanic, 203 (11.8%) as Hispanic, 126 (7.3%) as Asian, and 56 (3.3%) as other race/ethnicity. Among 1471 mother/newborn dyads for which matched sera were available, SARS-CoV-2 IgG and/or IgM antibodies were detected in 83 of 1471 women (6%; 95% CI, 5%-7%) at the time of delivery, and IgG was detected in cord blood from 72 of 83 newborns (87%; 95% CI, 78%-93%). IgM was not detected in any cord blood specimen, and antibodies were not detected in any infant born to a seronegative mother. Eleven infants born to seropositive mothers were seronegative: 5 of 11 (45%) were born to mothers with IgM antibody only, and 6 of 11 (55%) were born to mothers with significantly lower IgG concentrations compared with those found among mothers of seropositive infants. Cord blood IgG concentrations were positively correlated with maternal IgG concentrations (r = 0.886; P < .001). Placental transfer ratios more than 1.0 were observed among women with asymptomatic SARS-CoV-2 infections as well as those with mild, moderate, and severe coronavirus disease 2019. Transfer ratios increased with increasing time between onset of maternal infection and delivery.
Conclusions and Relevance
In this cohort study, maternal IgG antibodies to SARS-CoV-2 were transferred across the placenta after asymptomatic as well as symptomatic infection during pregnancy. Cord blood antibody concentrations correlated with maternal antibody concentrations and with duration between onset of infection and delivery. Our findings demonstrate the potential for maternally derived SARS-CoV-2 specific antibodies to provide neonatal protection from coronavirus disease 2019.
Introduction
Newborn protection from infection is primarily dependent on neonatal innate immune responses and maternally derived, transplacentally acquired antibodies. The extent to which maternal antibodies produced in response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during pregnancy cross the placenta is important for understanding potential neonatal protection from coronavirus disease 2019 (COVID-19) and for developing appropriate maternal vaccination strategies when effective vaccines are widely available. To date and to our knowledge, studies of transplacental transfer of maternal SARS-CoV-2–specific antibodies to newborns are limited to case reports and small case series of women with symptomatic infection.1,2,3
Our center has previously reported on the prevalence of antibodies to SARS-CoV-2 among women presenting for delivery at 2 large birth centers in Philadelphia, Pennsylvania.4 In that study, we validated a SARS-CoV-2 spike protein receptor-binding domain (RBD) serological test using samples of prepandemic sera from nonpregnant and pregnant patients, as well as sera from COVID-19–recovered donors. We then used this validated assay to test sera routinely collected from parturient women on admission for delivery. Among 1293 samples collected from April 4 to June 3, 2020, we found that 80 parturient women (6.2%) possessed IgG and/or IgM SARS-CoV-2–specific antibodies at a level above assay background. We observed race/ethnicity differences in seroprevalence, with higher rates in Black/non-Hispanic and Hispanic/Latino women. In the current study, we use this assay to test sera from parturient women and cord blood collected from April 9 to August 8, 2020, at one of our birth centers to measure the incidence, efficiency, and dynamics of placental transfer of maternal antibodies to the newborn.
Methods
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. The institutional review board of the University of Pennsylvania approved this study as minimal risk that could not practicably be performed without waiver of consent.
Study Population
Women and their newborns delivered at Pennsylvania Hospital in Philadelphia between April 9, 2020, and August 8, 2020, were included in the study. During this period, women were routinely screened for SARS-CoV-2 by nasopharyngeal polymerase chain reaction (NP-PCR) testing when admitted to the hospital for delivery, and/or tested during pregnancy due to SARS-CoV-2 exposure or COVID-19 symptoms. Women have blood drawn for rapid plasma reagin at the time of delivery for routine syphilis screening, and newborns have cord blood stored for blood type and Coombs testing as clinically needed. Residual maternal and cord blood serum from this testing was collected for study purposes at the time it would otherwise be discarded by the hospital laboratories. Sera were fully deidentified prior to antibody measurements.
Study Definitions and Data Collection
Prior to full study participant and serum sample deidentification, demographic and clinical data, including information on SARS-CoV-2 testing prior to delivery, were collected from review of electronic medical records. Maternal illness severity was defined per definitions provided by the US Centers for Disease Control and Prevention: (1) asymptomatic: no history of COVID-19 symptoms at time of delivery or on review of prenatal history; (2) mild disease: symptoms that do not include shortness of breath or radiographic evidence of pneumonia, with normal oxygenation; and (3) moderate to critical disease: symptoms that include shortness of breath or radiographic evidence of pneumonia, with or without administration of supplemental oxygen, noninvasive respiratory support, or mechanical ventilation.5 Race and ethnicity were abstracted from documentation, which is typically self-reported at the time of admission. Prepregnancy body mass index was abstracted from documentation in the medical record or from the patient’s self-reported entry in birth registration. International Statistical Classification of Diseases and Related Health Problems, Tenth Revision diagnosis codes O24, E08 to E13, and Z79.4 were used to identify type 1 diabetes, type 2 diabetes, and gestational diabetes, and codes O10, O11, O13 to O16, I10 to I13, and I15 were used to identify hypertensive disorders, gestational hypertension, and preeclampsia. The accuracy of using these codes has been validated with medical record review.4 Preterm delivery was defined as less than 37 weeks’ gestation, and term was defined as 37 or more weeks’ gestation. Only the first twin from each pair was included in all analyses.
Antibody Measurement
Sera were tested using a validated enzyme-linked immunosorbent assay with plates coated with recombinant SARS-CoV-2 spike protein RBD.4 The RBD protein was produced in 293F cells using plasmid provided by Mt Sinai Hospital in New York, New York, and purified by nickel-nitrilotriacetic acid resin (Qiagen). This assay was validated using samples from COVID-19–recovered donors and samples collected prior to the COVID-19 pandemic, as previously described.4 Briefly, coated enzyme-linked immunosorbent assay plates (Immulon 4 HBX; Thermo Fisher Scientific) were stored overnight at 4 °C, then washed the following day with phosphate-buffered saline containing 0.1% Tween-20 (PBS-T) then blocked with PBS-T supplemented with 3% nonfat milk powder. Heat-inactivated serum samples were diluted in PBS-T supplemented with 1% nonfat milk powder; 50-μL diluted serum sample was added to each well, incubated at room temperature for 2 hours, and washed with PBS-T. Secondary antibodies were added (1:5000 diluted horseradish peroxidase labeled goat antihuman IgG (Jackson ImmunoResearch Laboratories) or 1:1000 diluted goat antihuman IgM–horseradish peroxidase (SouthernBiotech), and plates were incubated for 1 hour at room temperature followed by washing with PBS-T. SureBlue 3,3′,5,5′-tetramethylbenzidine substrate (KPL) was then added to each well, and plates were incubated for 5 minutes before 25 μL of 250 mM hydrochloric acid was added to each well to stop the reaction. Plates were read with the SpectraMax 190 microplate reader (Molecular Devices) at an optical density of 450 nm. All samples were also tested on plates coated with PBS only to obtain background optical density values, which were subtracted from the optical density values from plates coated with RBD. Monoclonal antibody CR3022 was included on each plate to adjust for plate-to-plate variation, and antibody concentrations were expressed in arbitrary units/mL relative to CR3022. Plasmids to express CR3022 were provided by Scripps.
All samples were first tested in duplicate at a 1:50 dilution. Samples with an IgG and/or IgM concentration above the lower limit of detection (0.20 arbitrary units/mL) were repeated in at least a 7-point dilution series to obtain quantitative results. Samples with IgG and/or IgM concentrations more than 0.48 arbitrary units/mL were considered seropositive. Samples with IgG and/or IgM concentrations below this cutoff were assigned a value of 0.24 arbitrary units/mL for statistical analysis.
Statistical Analyses
All antibody concentrations were log2-transformed for analysis and geometric mean concentrations with 95% CIs were reported unless stated otherwise. Transfer ratio was calculated as infant IgG concentration divided by maternal IgG concentration. Correlations between (1) maternal and infant IgG concentrations and (2) transfer ratio and days between NP-PCR testing and delivery were reported using the Pearson correlation coefficient. Standard descriptive analyses, including Fisher exact test, unpaired t test, analysis of variance, Mann-Whitney U test, and Kruskal-Wallis test, were used as appropriate to compare demographics, clinical characteristics, timing, and reason for maternal NP-PCR testing, antibody concentrations, and transfer ratios between analytic groups in Table 1 and Table 2. Statistical significance was set at P < .05. Stata version 16 (StataCorp) and Prism version 8 (GraphPad) were used for analyses.
Table 1. Maternal Illness Severity and Results of NP-PCR Testing and Antibody Concentrations.
| Characteristic | All (N = 83) | Asymptomatic (n = 50) | Disease | P value | |
|---|---|---|---|---|---|
| Mild (n = 25)a | Moderate to critical (n = 8) | ||||
| NP-PCR tested (mother), No. (%)b | 82 (99) | 49 (98) | 25 (100) | 8 (100) | >.99 |
| NP-PCR ever positive | 44 (54) | 15 (31) | 21 (84) | 8 (100) | <.001 |
| Reason for NP-PCR testing | |||||
| Routine admission screening | 52 (63) | 47 (96) | 5 (20) | 0 | <.001 |
| Symptoms | 27 (33) | 0 | 19 (76) | 8 (100) | |
| Otherc | 3 (4) | 2 (4) | 1 (4) | 0 | |
| Time between NP-PCR test and delivery, median (IQR), d | 1 (0-27) | 1 (0-1) | 27 (10-73) | 53 (44-73) | <.001 |
| Maternal IgM >0.48 arbitrary U/mL, No. (%) | 48 (58) | 28 (56) | 13 (52) | 7 (88) | .21 |
| Maternal IgM concentration, geometric mean (95% CI)d | 1.85 (1.43-2.39) | 1.39 (1.03-1.89) | 2.50 (1.42-4.39) | 3.33 (1.60-6.93) | .77 |
| Maternal IgG >0.48 arbitrary U/mL, No. (%) | 78 (94) | 46 (92) | 24 (96) | 8 (100) | .80 |
| Maternal IgG concentration, geometric mean (95% CI)d | 4.69 (3.57-6.14) | 3.92 (2.82-5.46) | 4.44 (2.67-7.38) | 15.27 (5.82-40.09) | .91 |
| Cord IgG >0.48 arbitrary U/mLe | 72 (92) | 44 (96) | 20 (83) | 8 (100) | .26 |
| Cord IgG concentration, geometric mean (95% CI)e,f | 4.23 (3.06-5.84) | 4.01 (2.77-5.83) | 3.09 (1.59-6.01) | 14.58 (4.26-49.84) | .44 |
| Transfer ratio, geometric mean (95% CI), %e,f | 0.90 (0.76-1.07) | 1.02 (0.85-1.23) | 0.70 (0.48-1.01) | 0.95 (0.45-2.01) | .34 |
Abbreviations: IQR, interquartile range; NP-PCR, nasopharyngeal polymerase chain reaction; U, units.
Mild disease in 21 of 25 cases was defined by symptoms reported in conjunction with NP-PCR testing. In the remaining 4 cases, women reported coronavirus disease 2019–consistent symptoms to obstetric caregivers prior to delivery, but testing was not done. In each case, routine NP-PCR screening results at the time of admission to labor floor were negative.
One asymptomatic woman declined routine admission NP-PCR screening.
Tested due to contact.
Only values >0.48 arbitrary U/mL included in the calculation of geometric mean.
Only includes infants born to IgG-seropositive mothers (maternal IgG >0.48 arbitrary U/mL).
Six of 78 infants born to IgG-seropositive mothers were seronegative (cord IgG ≤0.48 arbitrary U/mL); cord IgG concentration was set at 0.24 arbitrary U/mL for these 6 infants.
Table 2. Characteristics of Seropositive Mothers and Their Newbornsa.
| Characteristic | No. (%) | P value | |
|---|---|---|---|
| Seropositive (n = 72) | Seronegative (n = 11) | ||
| Maternal | |||
| Age, median (IQR), y | 29 (25-33) | 28 (22-32) | .33 |
| Race/ethnicity | |||
| Black/non-Hispanic | 32 (44) | 4 (36) | .17 |
| White/non-Hispanic | 10 (14) | 2 (18) | |
| Asian | 1 (1) | 1 (9) | |
| Hispanic | 28 (39) | 3 (27) | |
| Otherb | 1 (1) | 1 (9) | |
| Gravidity | |||
| 1 | 13 (18) | 1 (9) | .67 |
| 2 | 17 (24) | 4 (36) | |
| ≥3 | 42 (58) | 6 (55) | |
| Prepregnancy BMI | |||
| <18.0 | 3 (4) | 0 | .17 |
| 18.5-<25.0 | 18 (25) | 6 (55) | |
| 25.0-<30.0 | 26 (36) | 4 (36) | |
| ≥30.0 | 25 (35) | 1 (9) | |
| Diabetes | 6 (8) | 0 | >.99 |
| Hypertension | 15 (21) | 2 (18) | >.99 |
| Multiple gestation | 0 | 2 (18) | .02 |
| Cesarean delivery | 17 (24) | 2 (18) | >.99 |
| GA at delivery, median (IQR), wk:d | 39:3 (38:0-40:0) | 39:1 (37:3-39:5) | .39 |
| Preterm delivery at GA <37 wk | 7 (10) | 2 (18) | .34 |
| Infant sex (male) | 31 (43) | 2 (18) | .19 |
| Birth weight, median (IQR), g | 3315 (2940-3625) | 2990 (2500-3200) | .07 |
| Apgar score at 5 min <5c | 1 (1) | 0 | >.99 |
| Time between maternal sera sampling and delivery, median (IQR), h | 9 (4-20) | 14 (6-17) | .92 |
| NP-PCR tested during pregnancy | 71 (99) | 11 (100) | >.99 |
| NP-PCR positive | 38 (54) | 6 (55) | >.99 |
| Time between positive NP-PCR test result and delivery, median (IQR), d | 34 (1-72) | 6 (0-12) | .04 |
| Maternal IgG seropositive | 72 (100) | 6 (55) | <.001 |
| IgG concentration, geometric mean (95% CI)d | 5.22 (3.97-6.87) | 1.27 (0.57-2.82) | .005 |
| Maternal IgM seropositive | 41 (57) | 7 (64) | .75 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GA, gestational age; IQR, interquartile range; NP-PCR, nasopharyngeal polymerase chain reaction.
N = 83 paired maternal and neonatal cord sera.
Other included patient option to not define race/ethnicity (1) and to choose more than 1 race/ethnicity category (1).
Apgar score at 5 minutes was missing for 2 seropositive infants.
Only values >0.48 arbitrary units/mL included in the calculation of geometric mean.
Results
During the study period, 1714 women delivered. Thirty-one women had twin deliveries. Matched maternal-cord blood sera were available for 1471 mother/newborn dyads, including 21 twin deliveries (Figure 1). Of the 1471 dyads, 83 women (6%; 95% CI, 5%-7%) were SARS-CoV-2 IgG- and/or IgM-seropositive. Twenty-five of 83 women (30%) were previously identified as seropositive in our prior seroprevalence study.4 Among infants born to seropositive women, 72 (87%; 95% CI, 78%-93%) were seropositive and 11 (13%; 95% CI, 7%-22%) were seronegative. No infants who were seropositive were born to the 1388 seronegative women. All women who were seropositive were NP-PCR tested except for 1 who declined routine obstetric testing; 44 of 82 tested women (54%) were positive by NP-PCR testing at some point during pregnancy (Table 1). Most women who were seropositive (50 of 83 [60%]) were asymptomatic for COVID-19. Newborns were tested for SARS-CoV-2 by NP-PCR between 24 and 48 hours after birth only if the mother was NP-PCR positive and met clinical criteria for being contagious at the time of delivery. Among the 20 of 83 infants (24%) tested on the basis of these criteria, none were positive.
Figure 1. Study Flow Diagram.
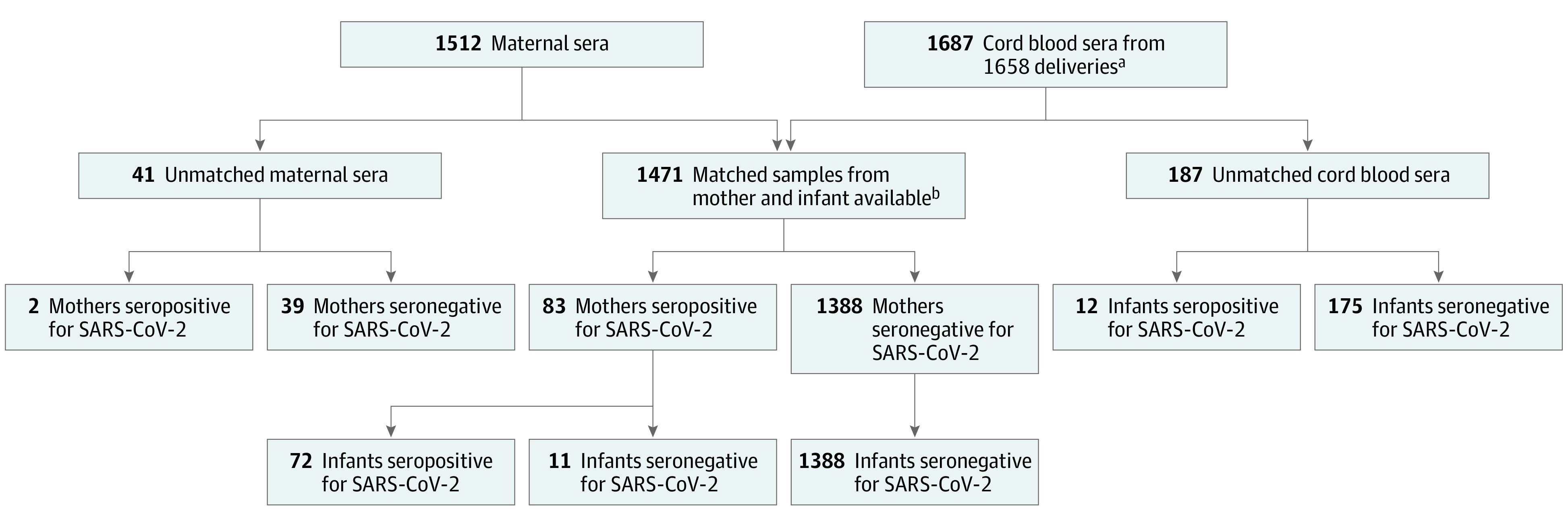
SARS-CoV-2 indicates severe acute respiratory syndrome coronavirus 2.
aIncludes 29 sets of twins; only the first twin is included in all analyses.
bIncludes 21 sets of twins.
There was a positive correlation between SARS-CoV-2 IgG concentrations in cord and maternal sera (r = 0.886; P < .001; Figure 2A). SARS-CoV-2 IgM antibodies were not detectable in any of the 72 infants who were seropositive. Of the 11 women who were seropositive with infants who were seronegative, 5 women were seropositive only by IgM, and the remaining 6 women had significantly lower geometric mean IgG concentrations compared with the 72 women with seropositive infants (1.27 vs 5.22 arbitrary units/mL; P = .005) (Table 2). IgM antibody concentrations were not significantly different in women with seronegative infants compared with women with seropositive infants (0.64 vs 0.81 arbitrary units/mL; P = .57; Figure 2B). We assessed the association between severity of maternal infection, maternal IgG concentration, and cord IgG concentration (Table 1). Women with moderate or critical illness had higher IgG and IgM concentrations, and infants born to these women had higher IgG concentrations, but these differences were not statistically significant.
Figure 2. Correlation Between Maternal and Neonatal Cord Sera Severe Acute Respiratory Syndrome Coronavirus 2–Specific Antibody Concentrations.
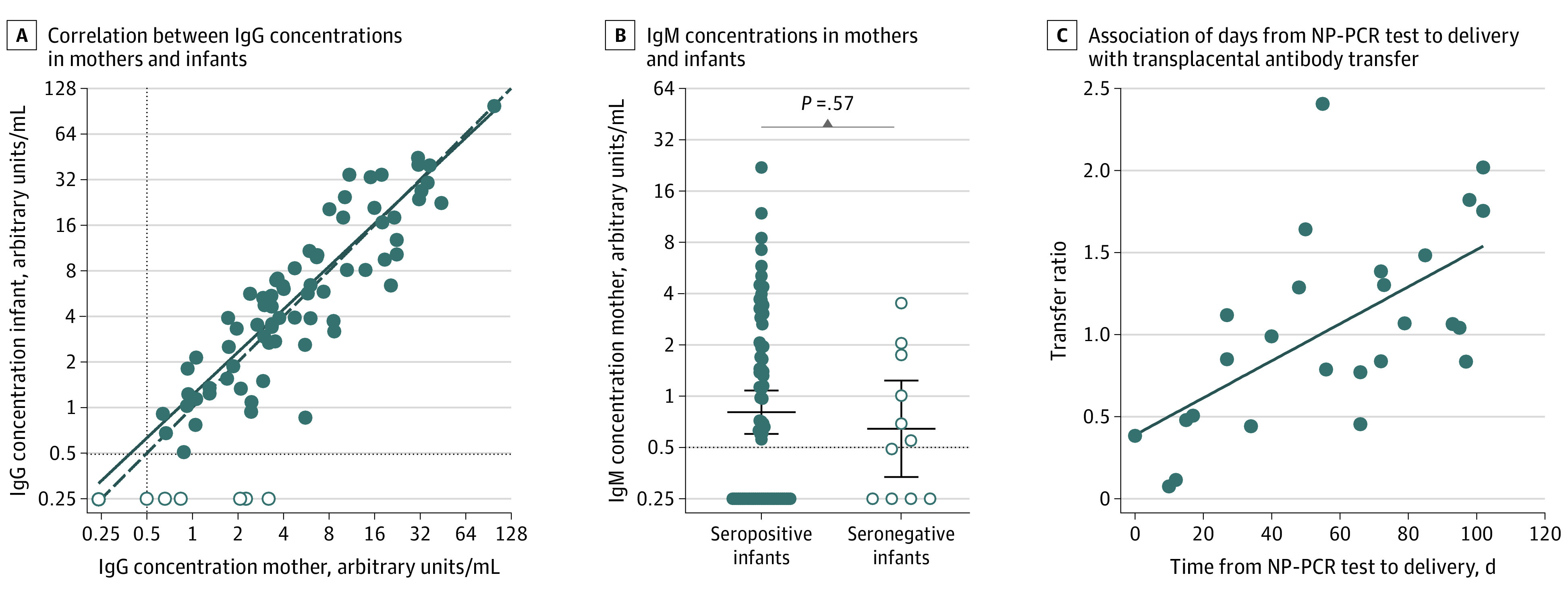
A, Correlation between IgG concentrations in sera from seropositive women and matched cord blood from seropositive (n = 72; filled circles) and seronegative (n = 11; open circles) infants. IgG concentrations in cord blood positively correlate with maternal IgG concentrations (r = 0.886; P < .001). B, IgM concentrations in sera from seropositive women with seropositive (n = 72; filled circles) and seronegative (n = 11; open circle) infants. Horizontal lines represent geometric mean titers and error bars indicate the 95% CI (P = .57 using an unpaired t test on log2-transformed IgM concentrations). In panels A and B, the horizontal dashed line indicates 0.48 arbitrary units/mL, which was the cutoff used to distinguish positive vs negative samples. Samples that were below this cutoff were assigned an antibody concentration of 0.24 arbitrary units/mL. C, Association of duration in days from nasopharyngeal polymerase chain reaction (NP-PCR) test to delivery with transplacental antibody transfer. Transfer ratio of IgG antibodies from mother to infant (n = 26 matched mother-infant dyads) is positively correlated with days from NP-PCR test to delivery (r = 0.620; P < .001).
Transfer ratios were not different among infants born to mothers with asymptomatic or symptomatic illness (Table 1). Excluding asymptomatic women whose onset of exposure or infection cannot be reliably determined, we used the timing of symptom-prompted viral testing as a surrogate for onset of infection. We assessed the association between transfer ratio and onset of maternal infection among a subset of 26 women with mild, moderate, or critical COVID-19 illness, who had a positive NP-PCR test result prior to delivery, and who delivered at term gestation. We observed a positive correlation between transfer ratio and increasing time between NP-PCR testing and delivery (r = 0.620; P < .001; Figure 2C). We further explored the association of maternal, fetal, and newborn characteristics with the infant’s serostatus among all mothers who were seropositive (n = 83) and with incremental transfer ratio among mothers who were IgG seropositive (n = 78) (Table 2 and Table 3). We found no association between maternal demographic and pregnancy health characteristics and cord seropositive status (Table 2). The geometric mean transfer ratio was not different comparing preterm (<37 weeks; n = 8) vs term (≥37 weeks; n = 70) gestation deliveries (0.53 [95% CI, 0.23-1.21] vs 0.96 [95% CI, 0.81-1.13]; P = .41).
Table 3. Characteristics Across Transfer Ratio Categories Among IgG-Seropositive Women (n = 78).
| Characteristic | No. (%) | |||||
|---|---|---|---|---|---|---|
| No transfer (n = 6) | Transfer ratioa | |||||
| <0.50 (n = 8) | 0.50-<1.00 (n = 24) | 1.00-<1.50 (n = 19) | 1.50-<2.00 (n = 14) | ≥2.00 (n = 7) | ||
| Preterm delivery at GA <37 wk | 1 (17) | 2 (25) | 2 (8) | 3 (16) | 0 | 0 |
| Concentration, geometric mean (95% CI) | ||||||
| Maternal IgG | 1.27 (0.57-2.82) | 7.11 (3.52-14.33) | 7.74 (4.45-13.48) | 3.43 (1.83-6.44) | 4.11 (2.74-6.15) | 4.82 (1.82-12.75) |
| Maternal IgMb | 0.44 (0.16-1.18) | 1.73 (1.13-2.66) | 1.44 (0.78-2.64) | 0.57 (0.33-0.99) | 0.38 (0.25-0.57) | 0.53 (0.19-1.51) |
| NP-PCR tested during pregnancy | 6 (100) | 8 (100) | 23 (96) | 19 (100) | 14 (100) | 7 (100) |
| NP-PCR positiveb | 5 (83) | 8 (100) | 11 (48) | 11 (58) | 5 (36) | 3 (43) |
| Time between positive NP-PCR test and delivery, median (IQR), d | 10 (0-12) | 4 (0-26) | 27 (2-66) | 72 (27-85) | 50 (0-98) | 55 (1-102) |
Abbreviations: GA, gestational age; NP-PCR, nasopharyngeal polymerase chain reaction; IQR, interquartile range.
Transfer ratio calculated as (cord IgG concentration)/(maternal IgG concentration).
After excluding 6 infants with no transfer, there was a significant difference between the transfer ratio categories for geometric mean maternal IgM concentration (P = .01) and number of NP-PCR–positive women (P = .04); all other characteristics were not significantly different (P ≥ .05) between the transfer ratio categories.
Discussion
We measured IgG and IgM antibody directed to the RBD of the SARS-CoV-2 spike protein in 1471 mother/newborn dyads at a single birth center in Philadelphia from April to August 2020 and detected IgG antibody in 83 maternal and 72 cord blood sera. Determination of correlates of maternal and neonatal immunity is a high priority area for SARS-CoV-2 research in maternal-child health domains,6 and our findings provide insight into the dynamics of maternally derived, potentially protective neonatal immunity.
Our findings align with current evidence that suggests that although placental and neonatal SARS-CoV-2 transmission may occur,1,2,3 such events are not common.7,8,9,10,11 We did not detect IgM antibodies in any cord blood serum samples even in cases of critical maternal illness or preterm delivery, supporting that maternal-fetal SARS-CoV-2 transmission is rare.2,3 Of greater concern is the potential for newborns to be infected postnatally from contagious mothers or other household contacts. We found efficient transfer of IgG antibodies from women who were SARS-CoV-2 seropositive (transfer ratios ≥1.0 in 40 of 72 infants who were seropositive), and a positive correlation between maternal and cord antibody concentrations. Our findings are aligned with studies of vaccine-elicited antibodies to pertussis, rubella, hepatitis B, and influenza, where cord sera/maternal sera transfer ratios ranging from 0.8 to 1.7 have been observed.12,13 Higher maternal antibody concentrations and a higher transfer ratio were associated with increasing duration between onset of maternal infection and time of delivery. Multiple other factors, such as antigen-elicited IgG subclass, maternal infections, maternal immunodeficiency, placental pathology, and gestational age at birth, are known to affect transfer efficiency and will require further study for SARS-CoV-2.14,15 We did not observe a significant difference in transfer efficiency comparing infants born preterm (defined as <37 weeks’ gestation), but this finding was likely affected by small numbers (n = 9) of preterm infants, with the earliest born at 31 weeks’ gestation. Further studies will be needed to define transplacental antibody dynamics at earlier gestational ages.
When vaccines are widely available, the optimal timing of maternal vaccination during pregnancy will need to consider maternal and fetal factors including the time needed to ensure neonatal protection. The majority of women in our study who were seropositive were asymptomatic, with uncertain timing of viral exposure. Among the subset of women in our study whose onset of infection could be estimated by symptoms prompting viral NP-PCR testing, all cord sera were seropositive if the maternal NP-PCR testing was 17 days or more prior to delivery.
Strengths and Limitations
The strengths of our study include a large cohort with access to available discarded specimens, allowing a focus on all women presenting for delivery throughout the study period, as opposed to studies targeting women with clinical infection identified during pregnancy or at the time of delivery. This study has several limitations, including single-site sample collection; small numbers of samples from preterm births; reliance on retrospective medical record review that limited our assessment of symptoms of COVID-19 during pregnancy; and a lack of information on postdelivery discharge outcomes. Because of the limited sample size from preterm births, we were not able to study the sole association of gestational age with transplacental transfer of antibodies, nor can we rule out that SARS-CoV-2 infection itself at specific times during pregnancy may be associated with the efficiency of transplacental antibody transfer.
Conclusions
Our findings demonstrate the potential for maternally derived antibodies to provide neonatal protection from SARS-CoV-2 infection and will help inform both neonatal management guidance and design of vaccine trials during pregnancy. Further studies are needed to determine if SARS-CoV-2 antibodies are protective against newborn infection; if so, at what concentration; and whether the transplacental kinetics of vaccine-elicited antibodies are similar to naturally acquired antibodies.
References
- 1.Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11(1):3572. doi: 10.1038/s41467-020-17436-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323(18):1846-1848. doi: 10.1001/jama.2020.4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;323(18):1848-1849. doi: 10.1001/jama.2020.4861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flannery DD, Gouma S, Dhudasia MB, et al. SARS-CoV-2 seroprevalence among parturient women in Philadelphia. Sci Immunol. 2020;5(49):eabd5709. doi: 10.1126/sciimmunol.abd5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention . SARS-CoV-2 illness severity criteria (adapted from the NIH COVID-19 Treatment Guidelines). Accessed December 22, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html#definitions
- 6.Muldoon KM, Fowler KB, Pesch MH, Schleiss MR. SARS-CoV-2: is it the newest spark in the TORCH? J Clin Virol. 2020;127:104372. doi: 10.1016/j.jcv.2020.104372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egloff C, Vauloup-Fellous C, Picone O, Mandelbrot L, Roques P. Evidence and possible mechanisms of rare maternal-fetal transmission of SARS-CoV-2. J Clin Virol. 2020;128:104447. doi: 10.1016/j.jcv.2020.104447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alzamora MC, Paredes T, Caceres D, Webb CM, Valdez LM, La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol. 2020;37(8):861-865. doi: 10.1055/s-0040-1710050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosier H, Farhadian SF, Morotti RA, et al. SARS-CoV-2 infection of the placenta. J Clin Invest. 2020;130(9):4947-4953. doi: 10.1172/JCI139569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma S, Bradshaw C, Auyeung NSF, et al. Outcomes of maternal-newborn dyads after maternal SARS-CoV-2. Pediatrics. 2020;146(4):e2020005637. doi: 10.1542/peds.2020-005637 [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809-815. doi: 10.1016/S0140-6736(20)30360-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Post AL, Li SH, Berry M, et al. Efficiency of placental transfer of vaccine-elicited antibodies relative to prenatal Tdap vaccination status. Vaccine. 2020;38(31):4869-4876. doi: 10.1016/j.vaccine.2020.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munoz FM, Patel SM, Jackson LA, et al. Safety and immunogenicity of three seasonal inactivated influenza vaccines among pregnant women and antibody persistence in their infants. Vaccine. 2020;38(33):5355-5363. doi: 10.1016/j.vaccine.2020.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fouda GG, Martinez DR, Swamy GK, Permar SR. The impact of IgG transplacental transfer on early life immunity. Immunohorizons. 2018;2(1):14-25. doi: 10.4049/immunohorizons.1700057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kachikis A, Englund JA. Maternal immunization: optimizing protection for the mother and infant. J Infect. 2016;72(suppl):S83-S90. doi: 10.1016/j.jinf.2016.04.027 [DOI] [PubMed] [Google Scholar]


