Abstract
Polychlorinated biphenyls (PCBs) are ubiquitous in the environment and exposure to them is associated with immune, endocrine and neural dysfunction. Effects of PCBs on inflammation and immunity are best described in spleen and blood, with fewer studies on neural tissues. This is an important gap in knowledge, as molecules typically associated with neuroinflammation also serve neuromodulatory roles and interact with hormones in normal brain development. The current study used Sprague-Dawley rats to assess whether gestational PCB exposure altered hypothalamic gene expression and serum cytokine concentration in neonatal animals given an immune challenge. Dams were fed wafers containing a mixture of PCBs at an environmentally relevant dose and composition (20 ug/kg, 1:1:1 Aroclor 1242:1248:1254) or oil vehicle control throughout their pregnancy. One day old male and female offspring were treated with an inflammatory challenge (lipopolysaccharide, LPS, 50 ug/kg, sc) or saline vehicle control approximately 3.5 hours prior to tissue collection. Across both basal and activated inflammatory states, PCB exposure caused greater expression of a subset of inflammatory genes in the hypothalamus and lower expression of genes involved in dopamine, serotonin, and opioid systems compared to oil controls. PCB exposure also altered reactions to inflammatory challenge: it reversed the normal decrease in Esr2 hypothalamic expression and induced an abnormal increase in IL-1b and IL-6 serum concentration in response to LPS. Many of these effects were sex specific. Given the potential long-term consequences of neuroimmune disruption, our findings demonstrate the need for further research.
Keywords: Endocrine disrupting chemicals, sex difference, dopamine, estrogen receptor beta, cytokine, lipopolysaccharide
Introduction
Communication between neural, endocrine, and immune systems is essential for an organism’s ability to respond to, and interact with, its environment. While the neuroimmune system was initially described in mediating behavioral and pathological responses to inflammatory challenges, we are now appreciating its active role in normal, hormone-sensitive developmental processes (Bilbo et al., 2012). Numbers of microglia, the resident immune cells in the brain, and their expression of cytokine signaling molecules are developmentally regulated and sexually dimorphic (Gendron et al., 1991; Young et al., 1995; Ortega et al., 2011; Bilbo et al., 2012; Schwarz et al., 2012; Crain et al., 2013; Hanamsagar et al., 2017). They mediate basic processes like synaptic pruning and formation, neuronal survival, differentiation, and programmed cell death (Hanamsagar and Bilbo, 2017). These processes drive learning and cognition in the hippocampus (Zhao et al., 2015; Torres et al., 2016), later sexual behavior in the hypothalamus (Amateau and McCarthy, 2002; Amateau and McCarthy, 2004; Lenz et al., 2013) and social behavior in frontal cortex (Filiano et al., 2016; Nelson and Lenz, 2017b). As such, disruption by perinatal immune challenges can result in an increased incidence of schizophrenia, depression, bipolar disorder, epilepsy, and autism spectrum disorders (Bilbo and Schwarz, 2012; Knuesel et al., 2014; Reisinger et al., 2015; Hanamsagar et al., 2017).
Neuroimmune signaling functions during development can also be disrupted by environmental challenges other than inflammatory ones, including effects of maternal stress, high fat diet, and diesel exhaust on microglial action (Bolton et al., 2013; Bolton et al., 2014; Bolton et al., 2017; Hanamsagar and Bilbo, 2017). Effects of hormone-active environmental contaminants are just beginning to be characterized (Rebuli et al., 2016; Meadows et al., 2017; Takahashi et al., 2018). One group of widespread environmental contaminants with great potential to affect developing neuroimmune systems are polychlorinated biphenyls (PCBs). PCBs are present in the food chain due to prior industrial contamination (Anderson et al., 1998; Kostyniak et al., 1999; Turyk et al., 2006; McGraw and Waller, 2009) and exposure can be high during early development: fetuses are exposed to PCBs via placental transfer (Newsome et al., 1995), infants are exposed to concentrated PCBs via breastmilk, and biological detoxification systems are still immature in developing individuals (Hansen, 1998; Landrigan, 2001; Blake et al., 2005; de Zwart et al., 2008). Indeed, negative correlations are found between degrees of exposure to PCBs in utero and fetal and childhood growth and neurodevelopmental milestones in populations from Michigan, New York, Netherlands, Germany, and the Yu-cheng disaster (Fein et al., 1984; Jacobson et al., 1990; Gladen and Rogan, 1991; Chen et al., 1994; Guo et al., 1995; Darvill et al., 2000; Schantz et al., 2003; Stewart et al., 2003; Stewart et al., 2008; Berghuis et al., 2013; Nowack et al., 2015)
The possible mechanisms of PCB action on neuroimmune processes are potentially diverse. PCBs are well-recognized as dopaminergic cell neurotoxicants (Seegal et al., 1986; Bell, 2014) and endocrine-disrupting chemicals (EDCs) (Soto et al., 1995; Portigal et al., 2002; Gore et al., 2015). Given that neural and peripheral immune cell populations are affected by estradiol (Lenz et al., 2013; Rebuli et al., 2016) and dopamine (Yan et al., 2015), they are possible targets of PCB action. PCBs are also suspected peripheral immunotoxicants (Weisglas-Kuperus, 1998). For example, exposure to PCBs at 18 months of age is associated with blunted vaccine efficacy at 5 and 7 years of age in children (Heilmann et al., 2010). In peripheral tissues of adult rodents, exposure to industrial mixtures of different PCBs, called Aroclors (A) 1242, 1248, and 1254, suppressed adaptive immune responses (Exon et al., 1985; Davis and Safe, 1989; Silkworth et al., 1989; Hamers et al., 2011), while other specific PCB congeners increase innate immune responses (Choi et al., 2012). PCBs may also affect inflammatory molecules in the neural tissues, but this phenomenon has not been well-studied (Voie and Fonnum, 2000; Mariussen et al., 2002; Sipka et al., 2008). Most recently, (Hayley et al., 2011) demonstrated that gestational exposure to a PCB mixture increased select cytokine expression in adult female brains. Importantly, the above studies were on adult animals, in vitro, or with just one sex. As sex differences are present in peripheral and neural immune responses to an bacterial endotoxin in rodents and humans (Moxley et al., 2002; Marriott et al., 2006; Loram et al., 2012; Everhardt Queen et al., 2016), neuroimmune developmental patterns (Schwarz et al., 2012; Crain et al., 2013; Lenz et al., 2013; Crain and Watters, 2015; Nelson and Lenz, 2017a), and neuroimmune responses to stressors and drugs (Pyter et al., 2013; Hudson et al., 2014; Doyle et al., 2017), it is essential to compare males and females directly.
Given the importance of inflammatory molecules in the brain for normal development and later health status, we sought to determine neonatal effects of PCBs on the hypothalamus, a region previously demonstrated to be PCB sensitive (Bell, 2014) and essential for the inflammatory fever response. We quantified basal and lipopolysaccharide (LPS)-challenge-induced expression of genes related to inflammatory signaling, nuclear receptors, and neurotransmitter systems that are sensitive to environmental contaminants and/or hormones (Grossman, 1984; Safe et al., 1985; Hestermann et al., 2000; Vegeto et al., 2003; Beischlag et al., 2008; Warner et al., 2012; Busbee et al., 2013). This study tests the hypothesis that early life PCB exposure will alter basal neuroimmune signaling and reactivity to an immune challenge, which could be mechanisms by which PCBs affect a range of hormone-sensitive social behaviors and neurodevelopmental outcomes.
Materials and Methods
Animals and Husbandry
All animal protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by The University of Texas at Austin Institutional Animal Care and Use Committee. Sprague Dawley rats were used, as is common in studies of PCBs and neuroimmune effects in early development. They were purchased from Harlan Laboratories (Houston, Texas) and were housed in a humidity- and temperature- controlled room with a 12-hour light / 12-hour dark light cycle, lights off at noon, at 21–23 °C. Two to three animals were group housed in polycarbonate cages (43 × 21 × 25 cm) with aspen bedding (PJ Murphy Forest Products, Sani-Chip), a PVC tube for enrichment, and weekly cage changes. Rats were fed low phytoestrogen Harlan-Teklad 2019 Global Diet (Harlan-Teklad, Indianapolis, Indiana) ad libitum for the duration of the experiment. While not tested for PCB content, no fish meal is used in production thereby reducing the chance of contamination. Upon arrival, rats were handled daily to acclimate them to their new housing conditions, and mating began at least two weeks later.
Females (3–4 month old, virgin) were mated with sexually experienced male rats (~6 months old) overnight after confirming receptivity. The morning after successful mating, as indicated by a sperm positive vaginal smear and termed embryonic day (E) 1, dams were singly housed. Dams were provided with nesting materials several days prior to expected parturition on E23. On the day after birth [postnatal day (P) 1], pups were weighed, anogenital distances were measured, and tissue was collected.
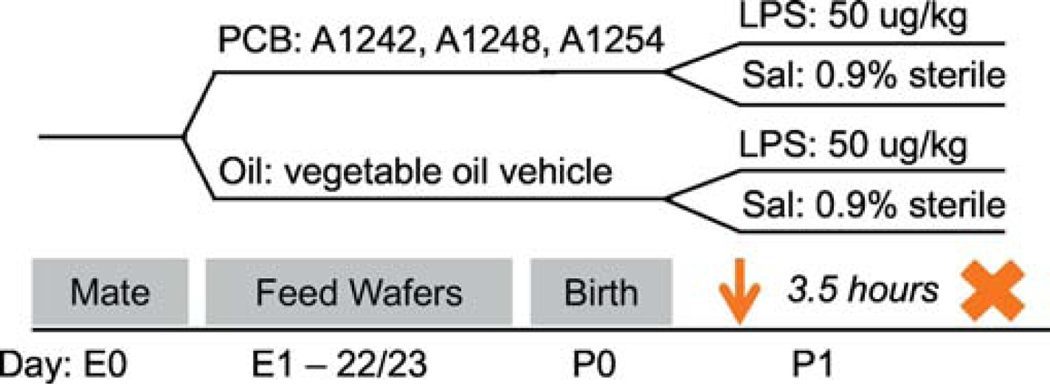
During gestation, dams were exposed to either Oil vehicle (n = 12) or PCB mixture, described below (n = 11). After birth, male and female offspring were injected with either lipopolysaccharide (LPS) or saline (Sal) vehicle (see below). This created four experimental groups per sex, in a 2×2 design (Figure 1). Each litter contributed no more than one male and female per treatment group. However, not all litters were large enough to contribute one animal to every group, as some pups were also assigned to other experiments. Number of pups per group were as follows: female Oil-Sal (n = 11); female Oil-LPS (n = 11); female PCB-Sal (n = 8); female PCB-LPS (n = 8); male Oil-Sal (n = 10); male Oil-LPS (n = 10); male PCB-Sal (n = 11); male PCB-LPS (n = 10). Litter treatments were evenly represented across three cohorts run over the course of a year. Cohort did not affect expression of genes significantly altered by PCBs or LPS when tested as a fixed variable or covariate. The experimenters were blind to treatment throughout the duration of the experiment.
Figure 1.
Experimental Design. Pregnant dams were fed pieces of wafers treated with ~100ul of either Oil (n=12) or PCBs (n = 11) throughout their pregnancy, embryonic day (E)1 to E22 or 23. One male and one female from each litter was injected (i.p.) with lipopolysaccharide (LPS) or saline (Sal) on postnatal day (P) 1 and brains were collected 3.5 hours later.
PCB Treatment
A mixture of PCBs (1:1:1 ratio of Aroclor 1242, 1248, and 1254) was used. They were all purchased from AccuStandard, New Haven, CT, and catalog, lot, and CAS numbers are as follows: C-242-N-50MG, Lot# 01141, CAS# 53469–21-9; C-248N-50MG, Lot# F-110, CAS# 12672–29-6; C-254N-50MG, Lot# 5428, CAS# 11097–69-1. Together, these commercially created blends of PCBs are composed of predominately noncoplanar congeners (only ~10% coplanar and ~6% dioxin like) and lightly- and heavily-chlorinated PCBs, per the authors calculations from (Frame et al., 1996) and United States Environmental Protection Agency congener classification (2003), and were chosen to mimic the range of mixtures found in natural populations in the US (Hites et al., 2004; Kostyniak et al., 2005). PCBs were diluted in vegetable oil vehicle, chosen because the fatty acid profile matches that of the regular chow (10% palmitic, 4% stearic, 23% oleic, 51% linoleic, 7% linolenic). Approximately 100 μl of vehicle or PCB oil (500 μg fat per oil dose) was applied to a quarter of palatable wafer (‘Nilla Wafer, Nabisco, ~0.9 g wafer; 180 μg fat and 330 μg sugar per wafer serving), adjusted to provide a dose of 20 μg PCB / kg body weight (BW) per individual dam. Given high concentrations of PCBs in breastmilk and other studies indicating that lactational transfer is actually higher than placental (Takagi et al., 1976; Takagi et al., 1986), this exposure route, timing, and dose mimics exposure of a breastfeeding human baby in North American populations exposed to moderately high amounts of PCBs. This is estimated based on estimates of transfer of PCBs from dam to offspring and additional routes of PCB exposure, and is generally lower than many other studies of this same nature (Grandjean et al., 1995; Lanting et al., 1998; Stellman et al., 1998; Dewailly et al., 1999; Dekoning and Karmaus, 2000). Dams were randomly assigned to either Oil or PCB groups in a counterbalanced design. Dams were fed oil or PCB treated wafers every weekday morning from E1 until parturition. Prior to mating, dams were trained to take the wafer out of the experimenter’s hand so that they generally consumed wafers immediately upon receipt. PCBs were handled with necessary personal protective equipment in a ventilation hood, and chemical and animal waste is disposed of via the Environmental Health and Safety office on campus. No effects of PCBs were seen on birth outcomes such as P1 body weights or anogenital index, and there were no effects on number of pups on P1 (live or dead). Dam body weight gain over pregnancy was also not affected.
LPS Treatment
Lipopolysaccharide (LPS, E. coli 0111:B4, Sigma, L4391, Lot 014M4019V) was used to induce a temporary and non-infectious inflammatory response. LPS binds toll-like receptors on immune cells, which then activates nuclear factor of kappa light poly-peptide gene enhancer in B-cells (NFκB), induces cytokine expression, and results in sickness behaviors. Within two hours before lights out, pups were injected (~0.1 ml, i.p., 28-gauge needle) with sterile normal saline (Sal) vehicle or LPS (50 μg/kg as per (Schwarz and Bilbo, 2011)) and immediately returned to their litter or home cage. The litters were observed to confirm that the injected pups remained in the nest.
Tissue Collection
Rats were euthanized and brains were collected three to four hours after injection, at the peak of the LPS-induced cytokine expression (Ortega et al., 2011) and in the first third of the dark period. Animals were taken from their home cage and rapidly decapitated in an adjacent room under dim red light. Brains were quickly removed from the skull, chilled on ice, and the whole hypothalamus dissected out with razor blades. Trunk blood samples were collected and allowed to clot for 30 minutes before centrifugation (1500 × g for 5 minutes) and serum stored for subsequent cytokine and hormone assays. All samples were transferred into individual RNase-free eppendorf tubes, and stored at −80C.
Gene Expression
RNA was isolated as previously described (Bell et al., 2016). Briefly, frozen tissue was lysed and homogenized using 22 gauge needles and syringes. RNA was extracted using an RNeasy Mini Kit (Qiagen Product Number 74104, Lot # 154011565) with RNase Free-DNase set (Qiagen Product Number 79254, Lot # 151045293). RNA quantity was assessed via Promega QuantiFluor Systems on the Glomax Multi + Detection System (RNA: Cat No E3310, Lot # 0000156608), according to manufacturer instructions, and RNA quality was assessed by randomly selecting ~10% of the samples to run on a Bioanalyzer 2100 (Agilent RNA 6000 Pico Kit, Cat No 5067–1513, Agilent Technologies, Santa Clara, California); all tested samples had RNA integrity numbers of 9 and above. RNA samples (200 ng) were converted to cDNA in 20 μl reactions using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Cat No 4368814, Lot # 00372558) with RNase inhibitor (Applied Biosystems, Cat No N8080119, Lot # 1634300) according to the manufacturer directions. Samples were held at 25°C for 10 minutes, 37°C for 120 minutes, and 85°C for 5 minutes on Applied Biosystems 2720 Thermocycler.
Quantitative PCR was completed with cDNA samples on custom-designed microfluidic 48-gene Taqman Low Density Array (TLDA) cards (Applied Biosystems, Cat No 4342253), using Taqman Gene Expression Mastermix (Applied Biosystems, Cat No 4369016) according to manufacturer’s directions. Targets were selected for their involvement in xenobiotic responses, inflammation, hormone signaling, and neurotransmitter signaling (listed in Table 1 and described in Supplemental Table 1). qPCR was carried out on an Applied Biosystems ViiA 7 (Software version 1.2.4) with the following run parameters: 50°C for 2 minutes, 95°C for 10 minutes, 45 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Quantification cycle (Cq) was determined automatically by the software and used to determine relative expression. A geometric mean of 18s, Rpl13a and Gapdh was determined and used as the reference Cq and subtracted from target Cqs to determine delta Cq within each sample. The median Delta Cq of the same sex Veh-Veh group was subtracted to determine fold change in expression for each individual. Any outlier within each group was identified by a Grubbs test, but no more than one animal was removed per group. Five animals were deemed outliers from all gene expression analysis due to hypothalamic brain sections being slightly too rostral or caudal. Ten other individual data points (out of 3552 data points from 48 genes per 74 remaining animals) were removed because they were highly significant outliers (p < 0.01) on a gene-by-gene basis. Of these ten, only three were removed from genes where a significant effect was found, and their removal actually reduced the likelihood of an effect being detected.
Table 1.
Effects of LPS or PCB exposure to increase (↑) or decrease (↓) expression of hypothalamic genes in females (left) and males (right).
| Female | Male | |||||
|---|---|---|---|---|---|---|
| Gene | effect of: | LPS | PCB | LPS | PCB | |
| Xenobiotic signaling | ||||||
| AhR | aryl hydrocarbon receptor | |||||
| Arnt | aryl hydrocarbon receptor nuclear translocator | |||||
| Cyp1a1 | cytochrome P450, family 1, subfamily a, polypeptide 1 | |||||
| Rela | v-rel reticuloendotheliosis viral oncogene homolog A (avian) | |||||
| Inflammatory signaling | ||||||
| Ccl22 | chemokine (C-C motif) ligand 22 | PCB é ** | ||||
| Cxcl9 | chemokine (C-X-C motif) ligand 9 | LPS é ** | LPS é ** | |||
| Cybb | cytochrome b-245, beta polypeptide | |||||
| Ifna1 | interferon-alpha 1 | |||||
| Ikbkb | inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase beta | PCB é * | LPS ê * | |||
| Il1a | interleukin 1 alpha | |||||
| Il1b | interleukin 1 beta | LPS é ** | ||||
| Il6 | interleukin 6 | |||||
| Il7r | interleukin 7 receptor | |||||
| Itgam | integrin, alpha M | |||||
| Itgb2 | integrin, beta 2 | |||||
| Myd88 | myeloid differentiation primary response gene 88 | |||||
| Nfkb1 | nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 | |||||
| Ptgs2 | prostaglandin-endoperoxide synthase 2 | LPS é ** | LPS é ** | |||
| Ptges | prostaglandin E synthase | LPS é ** | ||||
| Tlr4 | toll-like receptor 4 | |||||
| Tnf | tumor necrosis factor | LPS é ** | PCB é * | |||
| Inflammatory modulators | ||||||
| Anrtl | aryl hydrocarbon receptor nuclear translocator-like | LPS ê ** | ||||
| Arrb1 | arrestin, beta 1 | LPS ê * | ||||
| Map3k7 | mitogen activated protein kinase kinase kinase 7 | |||||
| Tgfb2 | transforming growth factor, beta 2 | LPS ê * | ||||
| Hormones, enzyme, and receptors | ||||||
| Ar | androgen receptor | |||||
| Crh | corticotropin releasing hormone | |||||
| Cyp19a1 | cytochrome P450, family 19, subfamily a, polypeptide 1 | |||||
| Esr1 | estrogen receptor 1 | LPS ê * | ||||
| Esr2 | estrogen receptor 2 | PCB × LPS* | LPS ê * | |||
| Opioid precursors and receptors | ||||||
| Oprk1 | opioid receptor, kappa 1 | LPS ê * | ||||
| Oprm1 | opioid receptor, mu 1 | LPS ê * | LPS ê * | |||
| Pdyn | prodynorphin | |||||
| Pomc | proopiomelanocortin | LPS ê * | PCB ê * | |||
| Dopamine enzymes, receptors, and transporter | ||||||
| Drd1a | dopamine receptor D1A | |||||
| Drd2 | dopamine receptor D2 | |||||
| Th | tyrosine hydroxylase | PCB ê * | PCB ê * | |||
| Slc6a3 | solute carrier family 6, member 3 | PCB ê * | PCB ê * | |||
| Serotonin enzymes, receptors, and transporter | ||||||
| Htr1a | 5-hydroxytryptamine (serotonin) receptor 1A | LPS ê * | ||||
| Htr2a | 5-hydroxytryptamine (serotonin) receptor 2A | |||||
| Ido1 | indoleamine 2,3-dioxygenase 1 | |||||
| Tph1 | tryptophan hydroxylase 1 | |||||
| Slc6a4 | solute carrier family 6, member 4 | PCB ê * | PCB ê * | |||
p < 0.05
p < 0.01.
Serum Cytokines
Serum samples were thawed on ice and diluted in assay buffer. The Milliplex Cytokine / Chemokine Hormone assay (RECYTMAG-65K, Cat No) was run according to manufacturer directions. This assay contains Interleukin (IL)- 1a, 4, 1b, 6, 10, interferon gamma (IFNγ), and tumor necrosis factor (TNF, also known as TNFα), which were selected based on literature and availability in the assay. Samples (25 μl) were run in duplicate across two plates. Quality controls fell within appropriate range; intraassay variabilities for the seven assays were between 0.9 and 7.4; interassay variability of QCs and standards were 14.2 (IL-1a), 8.9 (IL-4), 24.2 (IL-1b), 8.9 (IL-6), 7.5 (IL-10), 32.6 (IFNγ), 12.2 (TNF). While the interassay variability is high for IL-1b and IFNγ, groups were represented evenly across the plates and there were not significant differences in serum concentration between the two runs. Two animals were identified as outliers with a Grubbs test across several serum cytokine analysis and were removed from all cytokine analysis; eight other data points were removed on an individual basis because they were also highly significant outliers.
Serum Corticosterone
Corticosterone was determined via radioimmunoassay according to manufacturer instructions (ImmuChem Double Antibody 125I RIA Kit, MP Biomedicals LLC, Orangeburg NY) in duplicate 100 μl serum samples. Intra-assay CV was 2.18% and minimum detectability was 7.7 ng/ml. One outlier was identified with a Grubbs test and was removed from analysis.
Statistics
Analysis was completed using SPSS (Version 23, IBM) and GraphPad Prism, with significant differences demarcated in figures and tables as *, p < 0.05 and **, p < 0.01. Hypothalamic gene expression, serum cytokine concentrations, and serum corticosterone concentrations were analyzed using a two-way analysis of variance (ANOVA) to determine main effects of PCB treatment, LPS treatment, and interactions between PCB and LPS treatment within a sex. Males and females are treated as independent units of analysis because of known sex differences in neuroimmune outcomes, which may overwhelm any PCB or LPS treatment effects within a statistical model. Accordingly, qPCR data was normalized to same sex-controls. Appropriate follow-up t-tests were used to identify the source of detected interactions. If measures failed to meet normality or homogeneity assumptions (as indicated by Shapiro Wilks and Levene’s tests) even after square root transformation, non-parametric Mann-Whitney U tests were used within a sex, and are indicated in the results as a ‘MW’. The non-parametric equivalent of a main effect of PCB or LPS was identified by collapsing across the other treatment levels, and an interaction was identified by testing for effects of one treatment within the other and vice versa.
For four genes (Il6, Ifna1, Cyp1a1, and Cxcl9) and two serum cytokines (IL-1α and IFNγ), at least one group had fewer than 30% of its samples fail to amplify (defined as Cq < 35) or reach detectable levels; as such, group differences were identified with a χ2 goodness of fit test. As with non-parametric tests, the equivalent of main effects and interactions were tested by collapsing across or testing within the other treatment levels. Il4 failed to amplify in all hypothalamic samples. Because of the large number of genes analyzed, the Benjamini-Hochberg False Discovery Rate (Benjamini and Hochberg, 1995) correction was applied. For these calculations, p values for effects of PCBs were drawn together from 2×2 ANOVAs, nonparametric tests, or χ2 analysis within a sex. No outcomes survived the correction. However, because each target was chosen based on an a priori hypothesis, group differences were considered statistically significant at p < 0.05 level.
To determine relationships among gene expression patterns and serum hormones and cytokines, hierarchical cluster analysis and heatmaps were generated within a sex using R (version 3.0.3). Dependent variable columns with 20 or fewer observations per sex (Cxcl9, Cxcl22, Cyp1a, Idol, Ifna1, Il4, Il6, serum IL-1a, and serum IFNγ, due to low levels of expression) were removed and data were transformed into z-scores. A correlation matrix was created using Pearson’s correlations with pairwise complete functions to deal with missing data. This was transformed into a dissimilarity matrix by subtracting the absolute value of the pairwise correlation from one, and then a distance matrix by removing self-correlations. The distance matrix was used to build hierarchical clusters with the R package hclust, with each object beginning as its own cluster and iteratively adding the two most similar clusters with a complete agglomerative method. The quality of the clusters was tested with the R package pvclust via multiscale bootstrap resampling of the same distance measures, and 10,000 bootstrap replications. The package calculates an ‘appropriately unbiased’ p-value for each cluster; when p > 0.95, the hypothesis that “the cluster does not exist” is rejected with significance level 0.05 (Shimodaira, 2004). A similar process was used to quantify relationships between groups across all measures; z-score transformed averages of target levels were determined per group, and hclust and pvclust were again used to determine p values for clusters of groups within a sex.
Results
Gene Expression
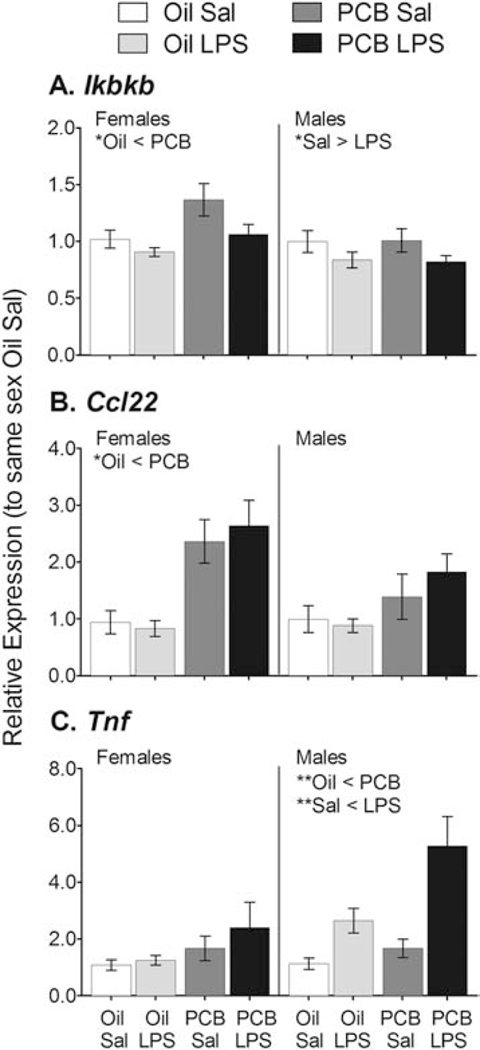
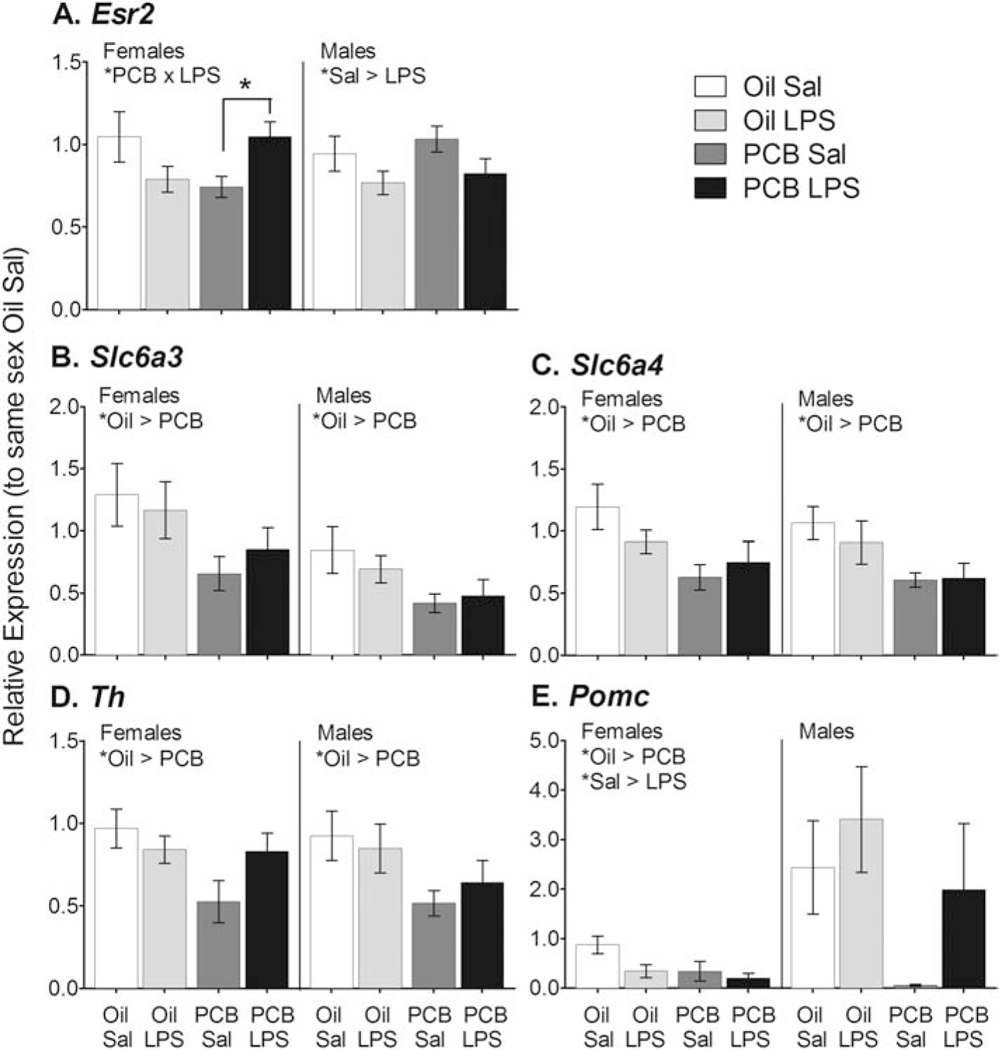
A summary of PCB and LPS effects within each sex on gene expression are presented in Table 1, with full gene names. Animals exposed to PCBs showed higher expression of several proinflammatory genes compared to Oil exposed controls, independent of LPS challenge (Figure 2). These genes included Ikbkb in females (MW, p < 0.05); Ccl22 in females (F(1,16) = 28.58, p < 0.01); and Tnf in males (square root transformed, F(1,32) = 6.91, p < 0.05). PCB exposure also affected genes that modulate neurotransmission (Figure 3). For one gene, Esr2, PCBs altered the effect of LPS in females, as indicated by a PCB × LPS interaction (F(1,33) = 6.24, p < 0.05). Expression was greater in LPS- than Sal- exposed animals, but only in females exposed to PCBs (F(1,14) = 7.66, p < 0.05). This is opposite of the pattern observed in control females, as well as the main treatment effect in males (F(1,33) = 4.93, p < 0.05), in which LPS exposure reduced expression. Animals exposed to PCBs also showed lower expression of Slc6a3 in females (F(1,27) = 4.68, p < 0.05) and males (F(1,29) = 5.73, p < 0.05); Slc6a4 in females (MW, p < 0.05) and males (F(1,32 = 7.50, p = 0.01); Th in females (F(1,33) = 4.15, p = 0.05) and males (F(1,33) = 5.41, p < 0.05); and Pomc in females (F(1,27) = 4.54, p < 0.05).
Figure 2.
PCBs increased gene expression of inflammatory molecules in the hypothalamus. Expression of Ikbkb in females (A), Ccl22 in females (B), and Tnf in males (C); were all greater in PCB than Oil exposed rats. LPS also reduced expression of Ikbkb (A) and increased expression of Tnf (C) in males relative to Sal controls. Data shown are mean +/− SEM. Within-sex main effects of PCB or LPS treatment are noted in each subtitle, *p < 0.05 and **p < 0.01.
Figure 3.
PCBs reduced gene expression of neurotransmission modulators in the hypothalamus. PCB exposed females showed an increase in Esr2 expression in response to LPS that was not present in controls, while LPS reduced expression in males (A). PCBs also downregulated expression of Slc6a3 in males and females (B); Slc6a4 in males and females (C); Th in females and males (D); and Pomc in females (E). Data shown are mean +/− SEM. Within-sex main effects of PCB or LPS treatment and PCB × LPS interaction are noted in each subtitle, *p < 0.05.
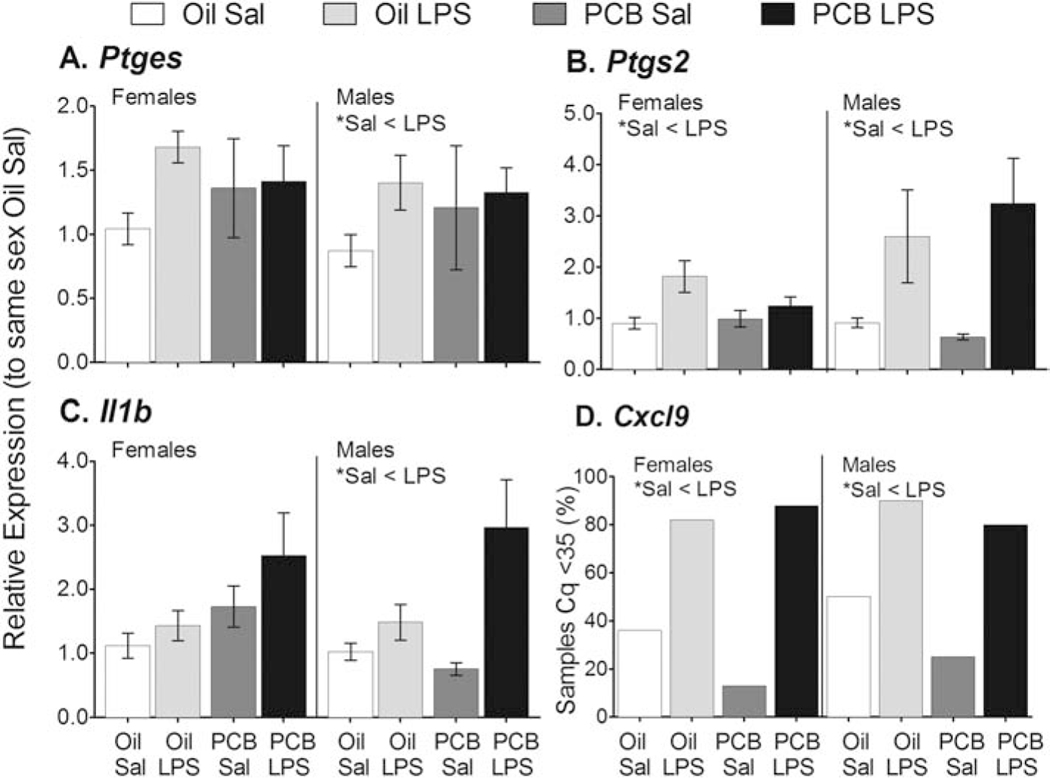
The LPS challenge produced the expected increases in proinflammatory molecules, independent of PCB exposure, and more so in males than females (Figure 4). Animals exposed to LPS showed greater expression of Ptges in males (F(1,30 = 8.86, p < 0.01); Ptgs2 in females (F(1,32) = 7.40, p = 0.01) and males (MW, p < 0.01); Il1b in males (MW, p < 0.01); and Tnf in males (see Figure 2A, MW, p < 0.01). Additionally, Cxcl9 expression was detectable in more LPS (84%) than Sal (26%) treated females (χ2 = 14.01, p < 0.01); and LPS (85%) than Sal (36%) treated males (χ2 = 11.91, p < 0.01). LPS also caused small but significant decreases in expression of molecules known to modulate, but not directly mediate, inflammatory responses, and only in males (data not shown): Arntl (F(1,33) = 7.82, p < 0.01); Arrb1 (F(1,33) = 4.45, p < 0.05); and Tgfb2 (F(1,32) = 4.57, p < 0.05).
Figure 4.
LPS increased expression of four inflammatory molecules in the hypothalamus. Expression of Ptges in males (A); Ptgs2 in females and males (B); and Il1b in males (C); were all greater in LPS than Sal treated rats. Data shown are mean +/− SEM. LPS exposure also increased the percent of samples with detectable levels of Cxcl9 in males and females (D). No effects of PCBs were observed in these targets. Within-sex main effects of LPS treatment are noted in each subtitle, *p < 0.05.
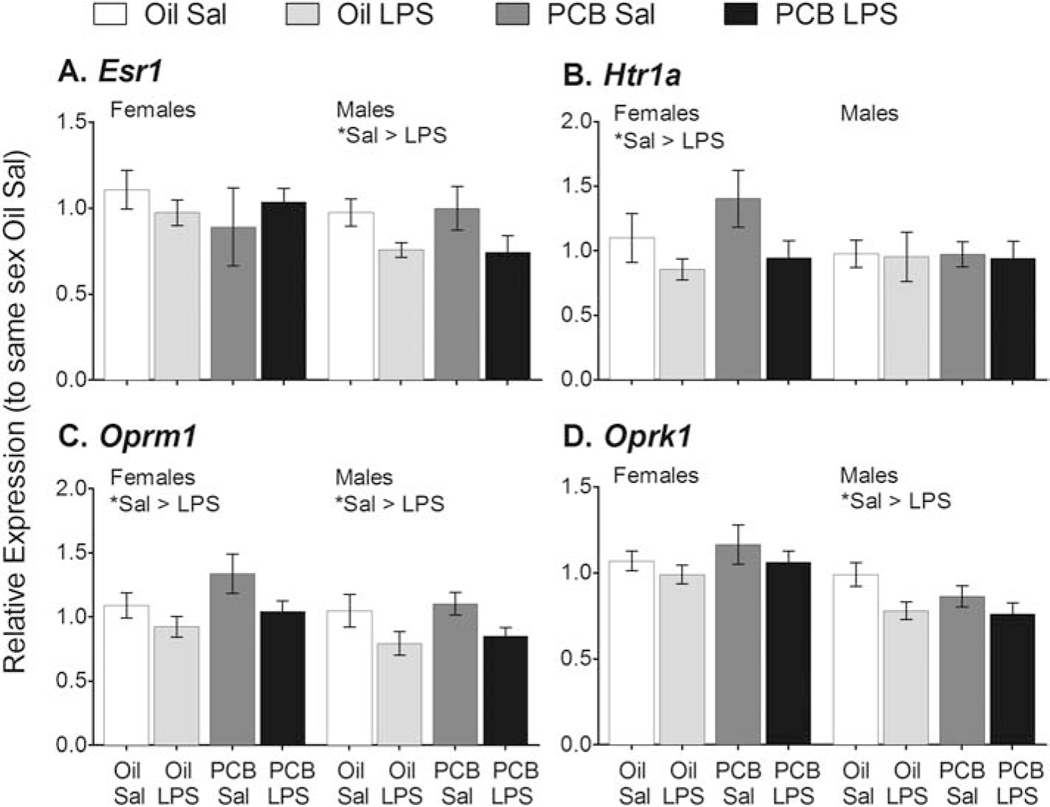
The inflammatory challenge also reduced expression of some modulators of neurotransmission and hormone signaling (Figure 5). These genes included Esr1 in males (F(1,33) = 6.93, p < 0.05); Esr2 in males (F(1,33) = 4.93, p < 0.05, see Figure 3A); Htr1a in females (F(1,32) = 4.60, p < 0.05); Oprm1 in females (F(1,33) = 4.83, p < 0.05) and males (F(1,33) = 6.91, p < 0.05); Oprk1 in males (F(1,33) = 6.50, p < 0.05); and Pomc in females (see Figure 3E, F(1,27) = 4.41, p < 0.05).
Figure 5.
LPS reduced gene expression of four neurotransmission modulators in the hypothalamus. Expression of Esr1 in males (A); Htr1a in females (B); Oprm1 in females and males (C); and Oprk1 in males (D); were all lower in LPS than Sal counterparts. No effects of PCBs were detected on these targets. Data shown are mean +/− SEM. Within-sex main effects of LPS treatment are noted in each subtitle, *p < 0.05.
Serum cytokines
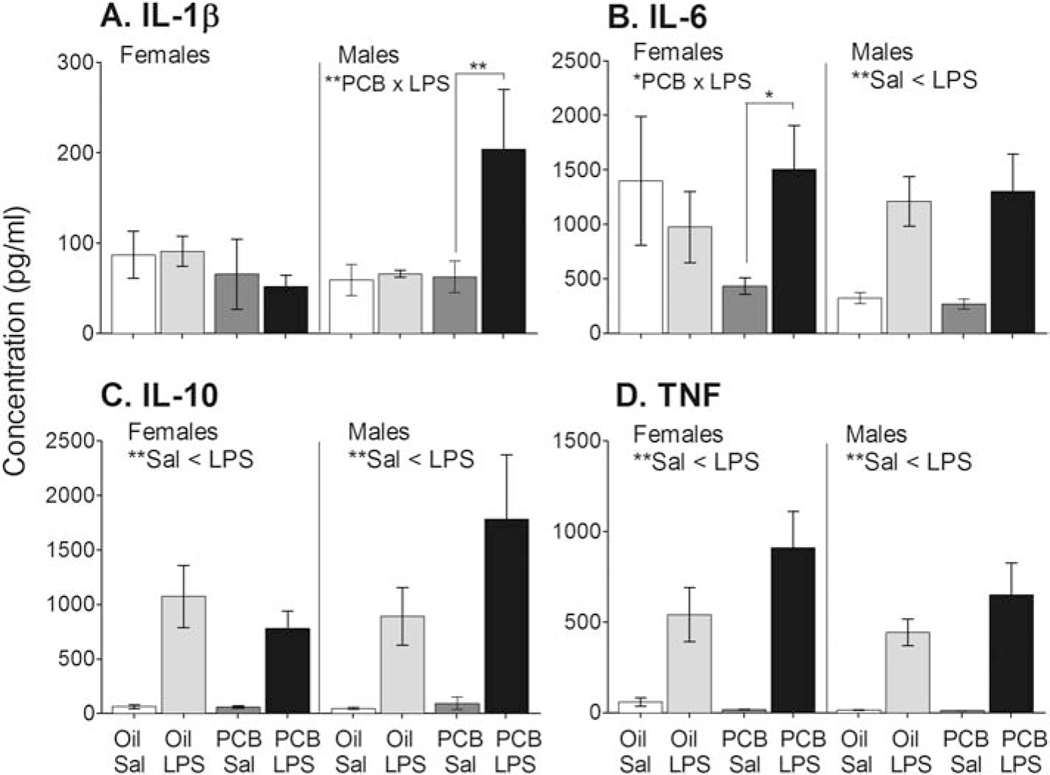
There was a significant main effect of LPS on concentrations of the cytokines IL-10 and TNF in neonatal females and males; both were greater in LPS than Sal exposed rats (MW, all p < 0.01) (Figure 6). IL-6 was also significantly higher in LPS than Sal treated males. In males exposed to PCBs but not Oil, IL-1b was significantly elevated in LPS compared to Sal (MW, p < 0.01). In females exposed to PCBs, there was also an IL-6 response to LPS (MW, p < 0.01) that was not present in Oil group. No effects were detected for IL-4 or IL-1a. IFNγ had some groups in which fewer than 30% of the samples were detectable and so were analyzed with a χ2 instead of ANOVA. For IFNγ, a greater proportion of male LPS treated samples (65%) were above levels of detection than Sal treated samples (21%, χ2 = 7.653, df = 1, p < 0.01). No effects of LPS on IFNγ were detected in females. No main effects of PCBs were detected for any serum cytokine.
Figure 6.
Serum concentrations are shown for four cytokines significantly affected by treatments. LPS increased interleukin-1β in PCB exposed males only (A), and interleukin-6 in PCB exposed females only (B). A significant main effect of LPS (greater than Sal) was found for IL-6 in males (B), and for interleukin-10 (C) and tumor necrotic factor (D) in all groups. No main effects of PCBs were observed. Data shown are mean +/− SEM. Within-sex main effects of LPS treatment are noted in each subtitle, and sources of significant PCB × LPS interactions are shown by bars. *p < 0.05 and **p < 0.01.
Serum hormones
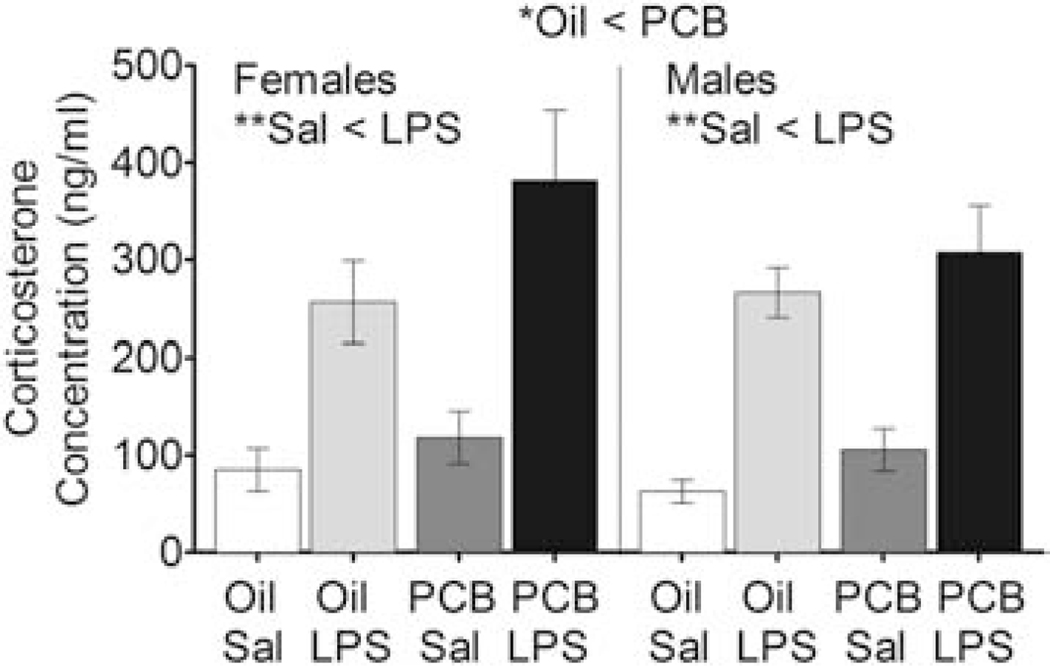
Corticosterone concentrations were higher in LPS-challenged females and males than in Sal-exposed females (F(1,32) = 27.28, p < 0.01) and males (F(1,36) = 30.95, p < 0.01). No effect of PCB exposure on corticosterone was detected when analyzed within a sex with a two-way ANOVA, but one was detected when analysis was collapsed across sex: Corticosterone concentrations were higher in animals exposed to PCBs than Oil (F(1,68) = 6.02, p < 0.05) (Figure 7).
Figure 7.
Serum Hormone Results. A) Corticosterone was greater in LPS- than Sal-treated males and females, and in PCB- than Oil-exposed animals (but only when analyzed across sexes but not within). Data shown are mean (bar height or line) +/− SEM. Within sex main effects of PCB or LPS treatment are noted in each subtitle, *p < 0.05 and **p < 0.01.
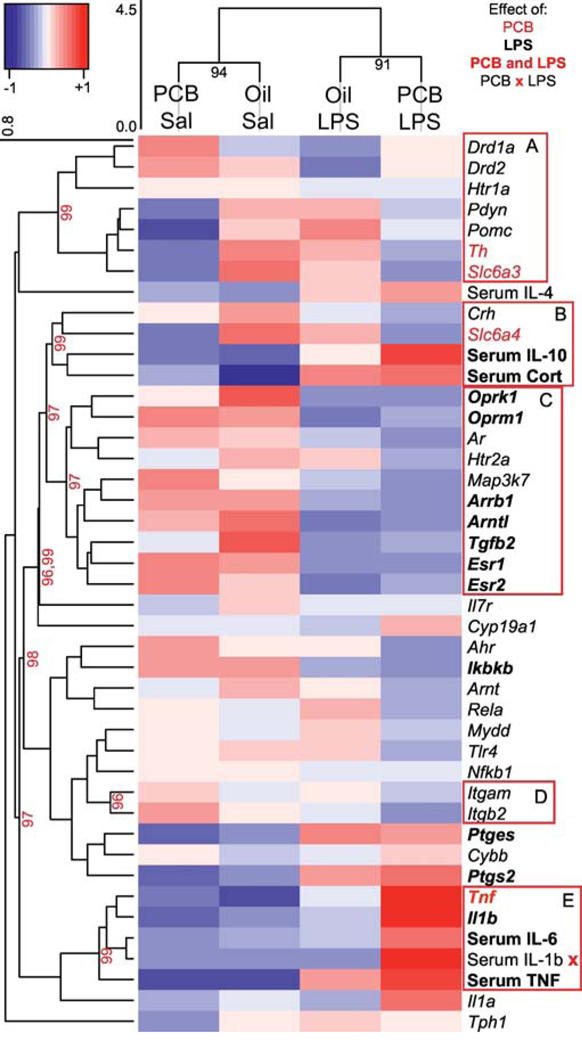
Hierarchical Cluster Analysis
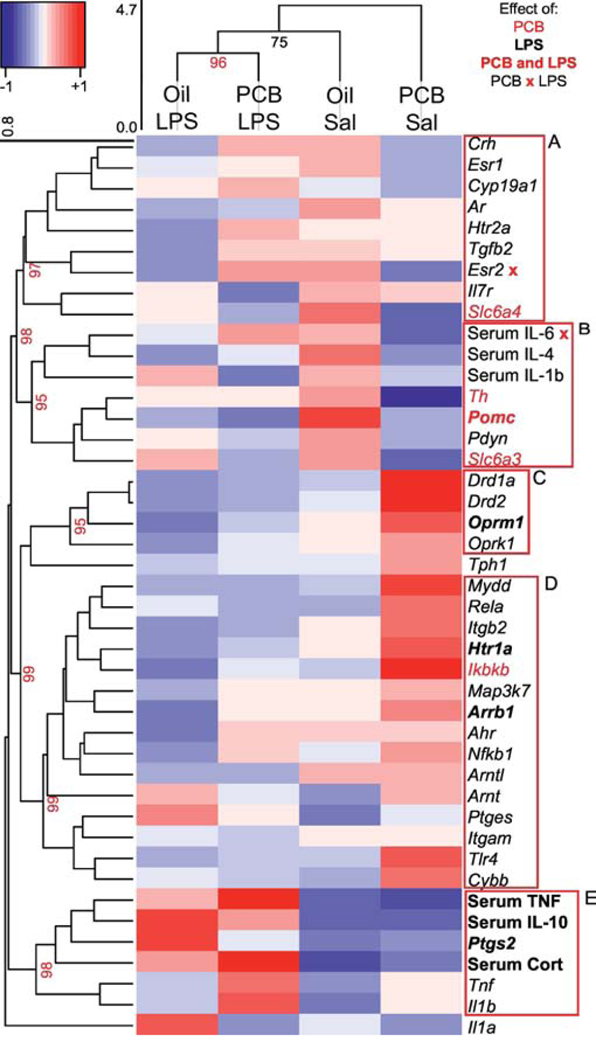
Hierarchical cluster analysis was conducted separately for the sexes. In females, Oil LPS and PCB LPS groups clustered together (p = 0.96), but Oil and PCB groups treated with saline did not (Figure 8). In males, Sal and LPS treated groups appeared to cluster together, independent of PCB treatment (Figure 9), however these clusters are just approaching statistical validation (p = 0.94 and 0.91). In females (Figure 8), clusters A and B included targets related to hormones and neurotransmitters in which PCB and LPS exposure interacted to affect expression. By contrast, expression of targets in cluster E was predominantly increased by LPS and included inflammatory molecules and corticosterone. In males (Figure 9), clusters A and B again included targets related to neurotransmission modulation that were decreased by PCBs, whereas expression of genes and concentrations of serum cytokines in clusters C and E were decreased and increased by LPS, respectively.
Figure 8.
Female Heatmap. Hierarchical cluster analysis using correlation coefficients of hypothalamic gene expression, serum hormones, and serum cytokines was used to create dendrograms showing relationships within the targets and groups. The height of the clusters indicates the distance between measures, and a p value (>95 is significant) and red box indicates validated clusters. For visual clarity, only highest tier significant target clusters are shown, and some large clusters are broken down into similarly significant and more functionally relevant groups. Dendrograms are linked to a heatmap showing z-scores of target expression, with red indicating highest expression and blue indicating lowest. Target labels are red if targets are significantly affected by PCBs, bold if targets are significantly affected by LPS, and both red and bold if targets are affected by both PCBs and LPS. Interactions between PCB and LPS treatments on Esr2 and Serum IL-6 are indicated by an x.
Figure 9.
Male Heatmap. Hierarchical cluster analysis using correlation coefficients of hypothalamic gene expression, serum hormones, and serum cytokines was used to create dendrograms showing relationships within the targets and groups. The height of the clusters indicates the distance between measures, and a p value (>95 is significant) and red box indicates validated clusters. For visual clarity, only highest tier significant target clusters are shown, and some large clusters are broken down into similarly significant and more functionally relevant groups. Dendrograms are linked to a heatmap showing z-scores of target expression, with red indicating highest expression and blue indicating lowest. Target labels are red if targets are significantly affected by PCBs, bold if targets are significantly affected by LPS, and both red and bold if targets are affected by both PCBs and LPS. Interactions between PCB and LPS treatments on serum IL-1b are indicated by an x.
Discussion
Neuroimmune processes are important mediators of normal brain development, and perturbations in their developmental trajectories have been implicated in later behavioral alterations and disease outcomes (Hanamsagar et al., 2017). Previous work has demonstrated that PCBs can influence peripheral immune systems and neurodevelopment, independently. However, this study is one of the first to indicate that exposure to environmentally relevant concentrations and mixtures of PCBs can affect neuroimmune systems, which may be a mechanism of the effects on hormone-sensitive and sexually differentiated behavior. These effects of PCBs are generally independent of LPS exposure. However, PCB exposure also induced a response to LPS that was not present otherwise: heightened release of serum IL-1b and IL-6, and expression of hypothalamic Esr2. Immune challenge with LPS affected expression of genes related to neuroimmune signaling, and both PCBs and LPS affected expression of genes involved in serotonin and opioid neurotransmission, but there was minimal overlap in molecular targets between the two insults. These results may suggest that PCBs and LPS affect neuroimmune and neuromodulator signaling via distinct intracellular cascades that converge at a few key targets.
Importantly, many of the effects of both PCBs and LPS were sex-specific. This may be the result of known sex differences in peripheral and neural immune systems. In the hypothalamus, there are more overall and activated microglia in males compared to females in the preoptic area at this age in an estradiol-sensitive manner (Lenz et al., 2013; Pyter et al., 2013). As such, exposure to perinatal hormones could alter responses to LPS and/or be affected by PCB exposure. Peripheral immune cells are also hormone sensitive and show sex-differences in expression of TLR4 proteins that could explain sex-specific responses to LPS in the current study (Marriott et al., 2006; Temple et al., 2008; Aomatsu et al., 2013; Nelson and Lenz, 2017a). However, discrete populations of cells at different developmental states could show unique responses to LPS; indeed, dendritic and macrophages show dramatic changes during neonatal periods (Kuper et al., 2016). Clearly, more research is needed to follow-up on this interesting possibility.
PCB effects on inflammation
Independent of LPS challenge, PCBs affected expression of a limited set of genes involved in the transmission of neuroimmune signaling molecules. Because of the importance of Ikbkb and Ccl22 in a host of inflammatory responses, the PCB-induced upregulation in females could have major consequences on health. Ikbkb encodes a kinase subunit (IKKβ) that responds to pro-inflammatory stimuli, such as LPS, IL-1, and TNF, to release NFκB from inhibition by IκB (Hacker and Karin, 2006). NFκB is a critical mediator of transcriptional responses to LPS and cytokines in innate immunity and inflammation (Schutze et al., 1995). The PCB-induced upregulation of Ikbkb in the current study is in agreement with a suggestion that PCB153 effects on cytokine induction may be mediated by degradation of IkB to activate NFκB (Kwon et al., 2002).
In contrast to Ikbkb, chemokine (C-C motif) ligand 22 (CCL22, the product of Ccl22) is associated with M2-polarized macrophages that promote tissue remodeling (Biswas and Mantovani, 2010) and the fever response to prostaglandins in the hypothalamus (Osborn et al., 2011). Ccl22 undergoes dramatic shifts in sexually differentiated expression across development within the hippocampus and cortex (Schwarz et al., 2012) and this dynamic regulation might make it a more vulnerable target to perturbation. Exposure to diesel exhaust particles reduced concentrations of CCL22 in M2 polarized human blood derived macrophages (Jaguin et al., 2015), while the current study demonstrates that PCBs increased expression of Ccl22 in hypothalamic tissues; these differences could result from unique cell populations, developmental periods, and/or contaminant mechanism of action, but highlight CCL22 as a molecule of continued interest for future study.
PCB exposure induced a distinct, yet also pro-inflammatory response in males: greater basal hypothalamic expression of Tnf than oil exposed controls. Tnf encodes tumor necrosis factor (TNF), a proinflammatory signaling molecule that acts as a cytokine and induces apoptosis (Sedger and McDermott, 2014). Beyond this, TNF is also involved in neural developmental processes, including synaptic plasticity and pruning (McCoy and Tansey, 2008). If TNF protein levels are indeed disrupted in the current model, altered synaptic plasticity could be a mechanism for potential long-term deficits from developmental PCB exposure. This PCB-induced Tnf upregulation is in agreement with previous studies of acute PCB exposure on circulating concentrations in adult male rodents (Choi et al., 2012; Abliz et al., 2016).
LPS effects on inflammation and interactions with PCBs
In validation of our experimental model, LPS treatment induced the expected inflammatory responses in both Oil and PCB treated animals, including greater expression of proinflammatory hypothalamic genes in LPS than Sal exposed animals, including Cxcl9, Il1b (males only) and Tnf (males only). This was accompanied by elevated concentrations of serum cytokines IL-6 (males only), IL-10, TNF, and the stress hormone corticosterone. As such, males appear to be more sensitive to LPS at this developmental period, in direct agreement with studies on serum cytokine responses to LPS (Marriott et al., 2006; Everhardt Queen et al., 2016) and analogous to prefrontal cortex responses to an acute stressor (Hudson et al., 2014) in adult mice. Effects of LPS to increase expression of genes involved in prostaglandin E2 could also be a mechanism by which early life LPS treatment alters hormone sensitive behaviors in adulthood (Nelson and Lenz, 2017a).
LPS challenge also revealed peripheral proinflammatory effects of PCBs that were otherwise undetected at basal states and sex-specific. In females, PCB exposure exacerbated the serum IL-6 response to LPS, such that the female-typical response was now male-like. IL-6 is thought of as a classic M1 monocyte activation marker (Mantovani et al., 2004) and is also important in activating T cells and differentiating B cells (Hunter and Jones, 2015). These findings are in agreement with other studies demonstrating effects of PCBs on IL-6 in rat serum (Miller et al., 2010) and human mast cells in vitro (Kwon et al., 2002) but not in brain (Hayley et al., 2011).
An interaction between PCB exposure and LPS challenge on a different serum cytokine was also observed in males: the LPS challenge induced greater serum concentrations of IL-1b, but only in PCB exposed animals. A similar response pattern is observed in gene expression (Figure 4c), however only a main effect of LPS was detected statistically. IL-1b is a prototypic pro-inflammatory cytokine involved in the fever response, hypothalamic-pituitary-adrenal axis activation, and sickness behaviors (Shaftel et al., 2008) and implicated in autoinflammatory syndrome (Guarda et al., 2011) and neurodegeneration (Shaftel et al., 2008). The male-specific PCB-induced response is in agreement with findings on PCB 153 from adult male rat serum (Abliz et al., 2016), human mast cells (Kwon et al., 2002), and P1 microglia primary culture (Loram et al., 2012).
PCB and LPS effects on neuromodulators
PCBs and LPS interacted to potentiate expression of a single gene: Esr2. Esr2, which encodes estrogen receptor beta (ERβ), was upregulated in response to LPS, but only in PCB-exposed females. This is in contrast to effects observed in other studies where LPS reduced Esr2 in endothelial cell culture, (Holm et al., 2010) and in male animals in the current study, but similar to effects in estradiol treated primary microglia from rats of undisclosed sex (Liu et al., 2005). Estrogens and their receptors can have both pro- or anti-inflammatory effects depending on the dose, duration of treatment, and target tissues and cell types (Arevalo et al., 2015; Kovats, 2015; Nelson and Lenz, 2017a); therefore the PCB-induced change could have important effects on downstream responses to immune challenge. ERβ is also affected by other EDCs including chlorpyrifos (Venerosi et al., 2015), BPA (Cao et al., 2014), and PCBs (Warner et al., 2012; Qu et al., 2014), however PCB effects are somewhat inconsistent (Salama et al., 2003; Dickerson et al., 2011; Walker et al., 2014). Expression of Esr1 or Ahr, potential receptors for PCBs, was not affected by PCBs in this study.
PCBs and LPS both affected dopaminergic, serotonergic, and opioidergic pathways, but in non-interacting ways. PCB-exposed male and female animals showed lower expression of genes encoding tyrosine hydroxylase (Th), the rate-limiting enzyme in dopamine production, and dopamine transporter (Slc6a3), but not receptors. Striatal dopamine is a well-established target of PCBs (Bell, 2014) and these data indicate that the hypothalamic population is also sensitive. PCB exposed male and female animals also showed a reduction in expression of Slc6a4, the gene encoding the serotonin transporter, adding to the list of mechansism by which PCBs can affect serotonergic regulation (Boix and Cauli, 2012). In contrast to PCB-responsive amines, the opioid system appears to be less sensitive to PCBs, with the only effects being a slight decrease in Pomc expression in females. Instead, this system responded to LPS, as expected from our understanding of endogenous opioids role in inflammation (Hua, 2016).
Questions for continued study
Whether PCBs act peripherally and/or centrally is an open question. PCBs do accumulate in lipophilic brain tissue, and could therefore have direct actions at hypothalamic receptors. Of interest is the concept that PCBs acutely alter the permeability of the blood brain barrier in adult male mice (Seelbach et al., 2010; Choi et al., 2012), thus increasing effects of peripheral inflammatory signals in the brain. As such, PCBs could indirectly affect hypothalamic gene expression by affecting peripheral signals which then reach brain to induce slightly different responses to LPS. This possibility is supported by the clustering of serum IL-6 and hypothalamic Esr2 in females (Figure 9, clusters A and B) and serum IL-1b and hypothalamic Tnf in males (Figure 10, cluster E). While these correlations certainly do not indicate a causal relationship between serum cytokine levels and neural gene expression, they do indicate potential targets for continued investigation of sex-specific PCB effects.
Effects of PCBs were observed in only a small portion of targets assessed, which could indicate a limited and specific effect of PCBs on inflammation. Alternatively, it could be a result of our experimental design, as PCB effects might be localized to a subset of specific cell types (Loram et al., 2012) or subregions within the hypothalamus (Pintado et al., 2011), or become detectable later post-LPS injection. The 3.5 hour delay between LPS challenge and tissue collection was chosen as a compromise between the time when the effects of LPS on gene expression peaks in the rodent brain between neonatal, adolescent, and adult animals (Ortega et al., 2011), as these ages were assessed in a separate study. Ongoing studies using immunohistochemistry are seeking to determine if the effects in gene expression translate to alterations in protein levels, and numbers and localization of cells expressing those proteins in the brain.
Overall, this study demonstrated that neonatal exposure to PCBs can induce limited, but potentially important, basal and LPS challenge-induced alterations in the brain and body’s inflammatory response. These findings reinforce the concept that the study of exposure to chemicals in isolation, without additional environmental challenges, may be insufficient to assess their full effects (Desaulniers et al., 2013). Early life inflammation, either in utero or postnatally, is associated with neurological disorders and other adverse long-term consequences (Nawa and Takei, 2006; Fatemi and Folsom, 2009). Therefore, understanding effects of PCBs during this early developmental period is essential in appreciating potential long-lasting effects into adulthood in accordance with the concept of developmental origins of health and disease (Barker, 1990).
Supplementary Material
Highlights.
Perinatal polychlorinated biphenyls (PCBs) alter neonatal hypothalamic gene expression
PCBs reduce expression of dopamine-related targets in males and females
PCBs alter serum cytokine responses to inflammatory LPS differently between sexes
PCBs increase expression of selected neuroimmune signals independent of LPS effect
Acknowledgements
The authors wish to thank Spurthi Tarugu, Tejaswi Marri, Michael Reilly, Dr. Weiling Yin, Dr. Alexandra Garcia and Lindsay Thompson for their assistance with tissue collection and molecular work.
Funding Information
This work was supported by the National Institutes of Environmental Health and Science [R01 ES020662 and R01 ES023254 to ACG; T32 ES07247 and F32 ES023291 to MRB] and DePaul University to MRB.
Footnotes
Supplementary Data Description
Supplementary Table 1 includes a list of all the gene targets, including a brief description of the protein they encode, the NCBI reference sequence, Life Technologies Assay IDI, and Probe Sequence.
Conflict of Interest Statement
• None of the authors (M Bell, A Dryden, R Will, or A Gore) have any conflicts of interests regarding any of the following items:
• third-party financial support for the work in the submitted manuscript.
• financial relationships with any entities that could be viewed as relevant to the general area of the submitted manuscript.
• sources of revenue with relevance to the submitted work who made payments to us, or to our institution on our behalf, in the 36 months prior to submission.
• interactions with the sponsor of outside of the submitted work.
• relevant patents or copyrights (planned, pending, or issued).
• relationships or affiliations that may be perceived by readers to have influenced, or give the appearance of potentially influencing, what we wrote in the submitted work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abliz A, Chen C, Deng W, Wang W, Sun R, 2016. NADPH Oxidase Inhibitor Apocynin Attenuates PCB153-Induced Thyroid Injury in Rats. International Journal of Endocrinology 2016, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM, 2002. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J. Neurosci 22, 8586–8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM, 2004. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat. Neurosci 7, 643–650. [DOI] [PubMed] [Google Scholar]
- Anderson HA, Falk C, Hanrahan L, Olson J, Burse VW, Needham L, Paschal D, Patterson D, Hill RH, 1998. Profiles of Great Lakes critical pollutants: a sentinel analysis of human blood and urine. The Great Lakes Consortium. Environmental Health Perspectives 106, 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aomatsu M, Kato T, Kasahara E, Kitagawa S, 2013. Gender difference in tumor necrosis factor-α production in human neutrophils stimulated by lipopolysaccharide and interferon-γ. Biochem Bioph Res Co 441, 220–225. [DOI] [PubMed] [Google Scholar]
- Arevalo M-A, Azcoitia I, Garcia-Segura LM, 2015. The neuroprotective actions of oestradiol and oestrogen receptors. Nature Reviews Neuroscience 16, 17–29. [DOI] [PubMed] [Google Scholar]
- Barker DJP, 1990. The Fetal And Infant Origins Of Adult Disease: The Womb May Be More Important Than The Home. BMJ: British Medical Journal 301, 1111–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beischlag TV, Morales JL, Hollingshead BD, Perdew GH, 2008. The aryl hydrocarbon receptor complex and the control of gene expression. Crit. Rev. Eukaryot. Gene Expr 18, 207–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, 2014. Endocrine-disrupting actions of PCBs on brain development and social and reproductive behaviors. Current Opinion in Pharmacology 19, 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, Hart BG, Gore AC, 2016. Two-hit exposure to polychlorinated biphenyls at gestational and juvenile life stages: 2. Sex-specific neuromolecular effects in the brain. Mol. Cell. Endocrinol 420, 125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 57, 289–300. [Google Scholar]
- Berghuis SA, Soechitram SD, Hitzert MM, Sauer PJJ, Bos AF, 2013. Prenatal exposure to polychlorinated biphenyls and their hydroxylated metabolites is associated with motor development of three-month-old infants. Neurotoxicology 38, 124–130. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM, 2012. The immune system and developmental programming of brain and behavior. Front Neuroendocrinol 33, 267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Smith SH, Schwarz JM, 2012. A lifespan approach to neuroinflammatory and cognitive disorders: a critical role for glia. J Neuroimmune Pharmacol 7, 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SK, Mantovani A, 2010. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11, 889–896. [DOI] [PubMed] [Google Scholar]
- Blake MJ, Castro L, Leeder JS, Kearns GL, 2005. Ontogeny of drug metabolizing enzymes in the neonate. Semin Fetal Neonatal Med 10, 123–138. [DOI] [PubMed] [Google Scholar]
- Boix J, Cauli O, 2012. Alteration of serotonin system by polychlorinated biphenyls exposure. Neurochem. Int 60, 809–816. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Auten RL, Bilbo SD, 2014. Prenatal air pollution exposure induces sexually dimorphic fetal programming of metabolic and neuroinflammatory outcomes in adult offspring. Brain, Behavior, and Immunity 37, 30–44. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Huff NC, Smith SH, Mason SN, Foster WM, Auten RL, Bilbo SD, 2013. Maternal stress and effects of prenatal air pollution on offspring mental health outcomes in mice. Environmental health perspectives 121, 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Marinero S, Hassanzadeh T, Natesan D, Le D, Belliveau C, Mason SN, Auten RL, Bilbo SD, 2017. Gestational Exposure to Air Pollution Alters Cortical Volume, Microglial Morphology, and Microglia-Neuron Interactions in a Sex-Specific Manner. Frontiers in Synaptic Neuroscience 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busbee PB, Rouse M, Nagarkatti M, Nagarkatti PS, 2013. Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders. Nutr. Rev 71, 353–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Joyner L, Mickens JA, Leyrer SM, Patisaul HB, 2014. Sex-specific Esr2 mRNA expression in the rat hypothalamus and amygdala is altered by neonatal bisphenol A exposure. Reproduction 147, 537–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Yu ML, Rogan WJ, Gladen BC, Hsu CC, 1994. A 6-year follow-up of behavior and activity disorders in the Taiwan Yu-cheng children. American journal of public health 84, 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JJ, Choi YJ, Chen L, Zhang B, Eum SY, Abreu MT, Toborek M, 2012. Lipopolysaccharide potentiates polychlorinated biphenyl-induced disruption of the blood-brain barrier via TLR4/IRF-3 signaling. Toxicology 302, 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain JM, Nikodemova M, Watters JJ, 2013. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. Journal of Neuroscience Research 91, 1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain JM, Watters JJ, 2015. Microglial P2 Purinergic Receptor and Immunomodulatory Gene Transcripts Vary By Region, Sex, and Age in the Healthy Mouse CNS. Transcriptomics: open access 3, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvill T, Lonky E, Reihman J, Stewart P, Pagano J, 2000. Prenatal exposure to PCBs and infant performance on the fagan test of infant intelligence. Neurotoxicology 21, 1029–1038. [PubMed] [Google Scholar]
- Davis D, Safe S, 1989. Dose-response immunotoxicities of commercial polychlorinated biphenyls (PCBs) and their interaction with 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin. Toxicol. Lett 48, 35–43. [DOI] [PubMed] [Google Scholar]
- de Zwart L, Scholten M, Monbaliu JG, Annaert PP, Van Houdt JM, Van den Wyngaert I, De Schaepdrijver LM, Bailey GP, Coogan TP, Coussement WC, Mannens GS, 2008. The ontogeny of drug metabolizing enzymes and transporters in the rat. Reproductive Toxicology 26, 220–230. [DOI] [PubMed] [Google Scholar]
- Dekoning EP, Karmaus W, 2000. PCB exposure in utero and via breast milk. A review. 10, 285–293. [DOI] [PubMed] [Google Scholar]
- Desaulniers D, Xiao GH, Cummings-Lorbetskie C, 2013. Effects of lactational and/or in utero exposure to environmental contaminants on the glucocorticoid stress-response and DNA methylation of the glucocorticoid receptor promoter in male rats. Toxicology 308, 20–33. [DOI] [PubMed] [Google Scholar]
- Dewailly E, Mulvad G, Pedersen HS, Ayotte P, Demers A, Weber JP, Hansen JC, 1999. Concentration of organochlorines in human brain, liver, and adipose tissue autopsy samples from Greenland. Environmental Health Perspectives 107, 823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SM, Cunningham SL, Gore AC, 2011. Prenatal PCBs disrupt early neuroendocrine development of the rat hypothalamus. Toxicol. Appl. Pharmacol 252, 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle HH, Eidson LN, Sinkiewicz DM, Murphy AZ, 2017. Sex Differences in Microglia Activity within the Periaqueductal Gray of the Rat: A Potential Mechanism Driving the Dimorphic Effects of Morphine. The Journal of Neuroscience 37, 3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA, 2003. Table of Polychorinated Biphenyl (PCB) Congeners, www.epa.gov, pp. [Google Scholar]
- Everhardt Queen A, Moerdyk-Schauwecker M, McKee LM, Leamy LJ, Huet YM, 2016. Differential expression of inflammatory cytokines and stress genes in male and female mice in response to a lipopolysaccharide challenge. PloS one 11, e0152289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exon JH, Talcott PA, Koller LD, 1985. Effect of lead, polychlorinated biphenyls, and cyclophosphamide on rat natural killer cells, interleukin 2, and antibody synthesis. Fundamental and Applied Toxicology 5, 158–164. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, 2009. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophrenia bulletin 35, 528–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein GG, Jacobson JL, Jacobson SW, Schwartz PM, Dowler JK, 1984. Prenatal exposure to polychlorinated biphenyls: Effects on birth size and gestational age. The Journal of Pediatrics 105, 315–320. [DOI] [PubMed] [Google Scholar]
- Filiano AJ, Xu Y, Tustison NJ, Marsh RL, Baker W, Smirnov I, Overall CC, Gadani SP, Turner SD, Weng Z, 2016. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame GM, Wagner RE, Carnahan JC, Brown JJF, May RJ, Smullen LA, Bedard DL, 1996. Comprehensive, quantitative, congener-specific analyses of eight aroclors and complete PCB congener assignments on DB-1 capillary GC columns. Chemosphere 33, 603–623. [Google Scholar]
- Gendron RL, Nestel FP, Lapp WS, 1991. Expression of tumor necrosis factor alpha in the developing nervous system. Int. J. Neuroscience 60, 129–136. [DOI] [PubMed] [Google Scholar]
- Gladen BC, Rogan WJ, 1991. Effects of perinatal polychlorinated biphenyls and dichlorodiphenyl dichloroethene on later development. The Journal of pediatrics 119, 58–63. [DOI] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT, 2015. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev 36, E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, Needham LL, Burse VW, Patterson DG, Sampson EJ, Jorgensen PJ, Vahter M, 1995. Relation of a seafood diet to mercury, selenium, arsenic, and polychlorinated biphenyl and other organochlorine concentrations in human milk. Environ. Res 71, 29–38. [DOI] [PubMed] [Google Scholar]
- Grossman CJ, 1984. Regulation of the Immune System by Sex Steroids*. Endocr. Rev 5, 435–455. [DOI] [PubMed] [Google Scholar]
- Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Förster I, Farlik M, Decker T, Du Pasquier RA, Romero P, 2011. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34, 213–223. [DOI] [PubMed] [Google Scholar]
- Guo YL, Lambert GH, Hsu C-C, 1995. Growth abnormalities in the population exposed in utero and early postnatally to polychlorinated biphenyls and dibenzofurans. Environmental health perspectives 103, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker H, Karin M, 2006. Regulation and function of IKK and IKK-related kinases. Sci Stke 2006, re13. [DOI] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Cenijn PH, Pencikova K, Palkova L, Simeckova P, Vondracek J, Andersson PL, Stenberg M, Machala M, 2011. In Vitro Toxicity Profiling of Ultrapure Non-Dioxin-like Polychlorinated Biphenyl Congeners and Their Relative Toxic Contribution to PCB Mixtures in Humans. Toxicol. Sci 121, 88–100. [DOI] [PubMed] [Google Scholar]
- Hanamsagar R, Alter MD, Block CS, Sullivan H, Bolton JL, Bilbo SD, 2017. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia 65, 1504–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamsagar R, Bilbo SD, 2017. Environment matters: microglia function and dysfunction in a changing world. Current Opinion in Neurobiology 47, 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LG, 1998. Stepping backward to improve assessment of PCB congener toxicities. Environmental Health Perspectives 106, 171–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayley S, Mangano E, Crowe G, Li N, Bowers WJ, 2011. An in vivo animal study assessing long-term changes in hypothalamic cytokines following perinatal exposure to a chemical mixture based on Arctic maternal body burden. Environ Health 10, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann C, Budtz-Jørgensen E, Nielsen F, Heinzow B, Weihe P, Grandjean P, 2010. Serum Concentrations of Antibodies Against Vaccine Toxoids in Children Exposed Perinatally to Immunotoxicants. Environmental Health Perspectives 118, 1434–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestermann EV, Stegeman JJ, Hahn ME, 2000. Relative contributions of affinity and intrinsic efficacy to aryl hydrocarbon receptor ligand potency. Toxicol. Appl. Pharmacol 168, 160–172. [DOI] [PubMed] [Google Scholar]
- Hites RA, Forest C, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ, 2004. Global assessment of organic contaminants in farmed salmon. Science (New York, N.Y.) 303, 226–229. [DOI] [PubMed] [Google Scholar]
- Holm A, Andersson KE, Nordström I, Hellstrand P, Nilsson B-O, 2010. Down-regulation of endothelial cell estrogen receptor expression by the inflammation promoter LPS. Mol. Cell. Endocrinol 319, 8–13.20079402 [Google Scholar]
- Hua S, 2016. Neuroimmune Interaction in the Regulation of Peripheral Opioid-Mediated Analgesia in Inflammation. Frontiers in Immunology 7, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson SP, Jacobson-Pick S, Anisman H, 2014. Sex differences in behavior and proinflammatory cytokine mRNA expression following stressor exposure and re-exposure. Neuroscience 277, 239–249. [DOI] [PubMed] [Google Scholar]
- Hunter CA, Jones SA, 2015. IL-6 as a keystone cytokine in health and disease. Nat Immunol 16, 448–457. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Humphrey HEB, 1990. Effects of in utero exposure to polychlorinated biphenyls and related contaminants on cognitive functioning in young children. The Journal of Pediatrics 116, 38–45. [DOI] [PubMed] [Google Scholar]
- Jaguin M, Fardel O, Lecureur V, 2015. Exposure to diesel exhaust particle extracts (DEPe) impairs some polarization markers and functions of human macrophages through activation of AhR and Nrf2. PloS one 10, e0116560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S, Prinssen EP, 2014. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol 10, 643–660. [DOI] [PubMed] [Google Scholar]
- Kostyniak P, Stinson C, Greizerstein H, Vena J, Buck G, Mendola P, 1999. Relation of Lake Ontario fish consumption, lifetime lactation, and parity to breast milk polychlorobiphenyl and pesticide concentrations. Environ. Res 80, S166–S174. [DOI] [PubMed] [Google Scholar]
- Kostyniak PJ, Hansen LG, Widholm JJ, Fitzpatrick RD, Olson JR, Helferich JL, Kim KH, Sable HJK, Seegal RF, Pessah IN, Schantz SL, 2005. Formulation and characterization of an experimental PCB mixture designed to mimic human exposure from contaminated fish. Toxicol. Sci 88, 400–411. [DOI] [PubMed] [Google Scholar]
- Kovats S, 2015. Estrogen receptors regulate innate immune cells and signaling pathways. Cellular immunology 294, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuper CF, van Bilsen J, Cnossen H, Houben G, Garthoff J, Wolterbeek A, 2016. Development of immune organs and functioning in humans and test animals: Implications for immune intervention studies. Reproductive Toxicology 64, 180–190. [DOI] [PubMed] [Google Scholar]
- Kwon O, Lee E, Moon TC, Jung H, Lin CX, Nam K-S, Baek SH, Min H-K, Chang HW, 2002. Expression of Cyclooxygenase-2 and Pro-inflammatory Cytokines Induced by 2,2’,4,4’,5,5’-Hexachlorobiphenyl (PCB 153) in Human Mast Cells Requires NF-kappaB Activation. Biological and Pharmaceutical Bulletin 25, 1165–1168. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, 2001. Pesticides and polychlorinated biphenyls (PCBs): an analysis of the evidence that they impair children’s neurobehavioral development. Molecular genetics and Metabolism 73, 11–17. [DOI] [PubMed] [Google Scholar]
- Lanting CI, Huisman M, Muskiet FAJ, van der Paauw CG, Essed CE, Boersma ER, 1998. Polychlorinated Biphenyls in Adipose Tissue, Liver, and Brain from Nine Stillborns of Varying Gestational Ages. Pediatr Res 44, 222–225. [DOI] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, Haliyur R, McCarthy MM, 2013. Microglia are essential to masculinization of brain and behavior. J. Neurosci 33, 2761–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Fan X-L, Zhao Y, Luo G-R, Li X-P, Li R, Le W-D, 2005. Estrogen provides neuroprotection against activated microglia-induced dopaminergic neuronal injury through both estrogen receptor-α and estrogen receptor-β in microglia. Journal of Neuroscience Research 81, 653–665. [DOI] [PubMed] [Google Scholar]
- Loram LC, Sholar PW, Taylor FR, Wiesler JL, Babb JA, Strand KA, Berkelhammer D, Day HE, Maier SF, Watkins LR, 2012. Sex and estradiol influence glial proinflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology 37, 1688–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M, 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology 25, 677–686. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Myhre O, Reistad T, Fonnum F, 2002. The polychlorinated biphenyl mixture aroclor 1254 induces death of rat cerebellar granule cells: the involvement of the Nmethyl-D-aspartate receptor and reactive oxygen species. Toxicol. Appl. Pharmacol 179, 137–144. [DOI] [PubMed] [Google Scholar]
- Marriott I, Bost KL, Huet-Hudson YM, 2006. Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: A possible mechanism for gender-based differences in endotoxic shock susceptibility. Journal of Reproductive Immunology 71, 12–27. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG, 2008. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation 5, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw JE, Waller DP, 2009. Fish ingestion and congener specific polychlorinated biphenyl and p, p’-dichlorodiphenyldichloroethylene serum concentrations in a great lakes cohort of pregnant African American women. Environ Int 35, 557–565. [DOI] [PubMed] [Google Scholar]
- Meadows JR, Parker C, Gilbert KM, Blossom SJ, DeWitt JC, 2017. A single dose of trichloroethylene given during development does not substantially alter markers of neuroinflammation in brains of adult mice. Journal of Immunotoxicology 14, 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V, Kahnke T, Neu N, Sanchez-Morrissey S, Brosch K, Kelsey K, Seegal R, 2010. Developmental PCB exposure induces hypothyroxinemia and sex-specific effects on cerebellum glial protein levels in rats. International Journal of Developmental Neuroscience 28, 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxley G, Posthuma D, Carlson P, Estrada E, Han J, Benson LL, Neale MC, 2002. Sexual dimorphism in innate immunity. Arthritis & Rheumatism 46, 250–258. [DOI] [PubMed] [Google Scholar]
- Nawa H, Takei N, 2006. Recent progress in animal modeling of immune inflammatory processes in schizophrenia: implication of specific cytokines. Neuroscience research 56, 2–13. [DOI] [PubMed] [Google Scholar]
- Nelson LH, Lenz KM, 2017a. The immune system as a novel regulator of sex differences in brain and behavioral development. Journal of Neuroscience Research 95, 447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LH, Lenz KM, 2017b. Microglia depletion in early life programs persistent changes in social, mood-related, and locomotor behavior in male and female rats. Behav. Brain Res 316, 279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WH, Davies D, Doucet J, 1995. PCB and organochlorine pesticides in Canadian human milk — 1992. Chemosphere 30, 2143–2153. [DOI] [PubMed] [Google Scholar]
- Nowack N, Wittsiepe J, Kasper-Sonnenberg M, Wilhelm M, Schölmerich A, 2015. Influence of Low-Level Prenatal Exposure to PCDD/Fs and PCBs on Empathizing, Systemizing and Autistic Traits: Results from the Duisburg Birth Cohort Study. PLoS ONE 10, e0129906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega A, Jadeja V, Zhou H, 2011. Postnatal development of lipopolysaccharide-induced inflammatory response in the brain. Inflamm Res 60, 175–185. [DOI] [PubMed] [Google Scholar]
- Osborn O, Sanchez-Alavez M, Dubins JS, Gonzalez AS, Morrison B, Hadcock JR, Bartfai T, 2011. Ccl22/MDC, is a prostaglandin dependent pyrogen, acting in the anterior hypothalamus to induce hyperthermia via activation of brown adipose tissue. Cytokine 53, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintado C, Revilla E, Vizuete ML, Jiménez S, García-Cuervo L, Vitorica J, Ruano D, Castaño A, 2011. Regional difference in inflammatory response to LPS-injection in the brain: Role of microglia cell density. Journal of Neuroimmunology 238, 44–51. [DOI] [PubMed] [Google Scholar]
- Portigal CL, Cowell SP, Fedoruk MN, Butler CM, Rennie PS, Nelson CC, 2002. Polychlorinated biphenyls interfere with androgen-induced transcriptional activation and hormone binding. Toxicol. Appl. Pharmacol 179, 185–194. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Kelly SD, Harrell CS, Neigh GN, 2013. Sex differences in the effects of adolescent stress on adult brain inflammatory markers in rats. Brain, Behavior, and Immunity 30, 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J, Liu W, Huang C, Xu C, Du G, Gu A, Wang X, 2014. Estrogen receptors are involved in polychlorinated biphenyl-induced apoptosis on mouse spermatocyte GC-2 cell line. Toxicology in Vitro 28, 373–380. [DOI] [PubMed] [Google Scholar]
- Rebuli ME, Gibson P, Rhodes CL, Cushing BS, Patisaul HB, 2016. Sex differences in microglial colonization and vulnerabilities to endocrine disruption in the social brain. Gen. Comp. Endocrinol 238, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisinger S, Khan D, Kong E, Berger A, Pollak A, Pollak DD, 2015. The Poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol. Ther 149, 213–226. [DOI] [PubMed] [Google Scholar]
- Safe S, Bandiera S, Sawyer T, Robertson L, Safe L, Parkinson A, Thomas PE, Ryan DE, Reik LM, Levin W, 1985. PCBs: structure-function relationships and mechanism of action. Environmental health perspectives 60, 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama J, Chakraborty TR, Ng L, Gore AC, 2003. Effects of polychlorinated biphenyls on estrogen receptor-beta expression in the anteroventral periventricular nucleus. Environmental health perspectives 111, 1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ, Rice DC, 2003. Effects of PCB exposure on neuropsychological function in children. Environmental Health Perspectives 111, 357–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutze S, Wiegmann K, Machleidt T, Kronke M, 1995. Tnf-Induced Activation of Nf-Chi-B. Immunobiology 193, 193–203. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD, 2011. LPS elicits a much larger and broader inflammatory response than Escherichia coli infection within the hippocampus of neonatal rats. Neurosci Lett 497, 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD, 2012. Sex differences in microglial colonization of the developing rat brain. J. Neurochem 120, 948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedger LM, McDermott MF, 2014. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants–past, present and future. Cytokine Growth F R 25, 453–472. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Brosch KO, Bush B, 1986. Polychlorinated biphenyls produce regional alterations of dopamine metabolism in rat brain. Toxicol. Lett 30, 197–202. [DOI] [PubMed] [Google Scholar]
- Seelbach M, Chen L, Powell A, Choi YJ, Zhang B, Hennig B, Toborek M, 2010. Polychlorinated biphenyls disrupt blood–brain barrier integrity and promote brain metastasis formation. Environmental health perspectives 118, 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaftel SS, Griffin WST, O’Banion MK, 2008. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimodaira H, 2004. Approximately unbiased tests of regions using multistep-multiscale bootstrap resampling. Ann. Statist 32, 2616–2641. [Google Scholar]
- Silkworth J, Cutler D, Sack G, 1989. Immunotoxicity of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin in a complex environmental mixture from the Love Canal. Fundamental and Applied Toxicology 12, 303–312. [DOI] [PubMed] [Google Scholar]
- Sipka S, Eum SY, Son KW, Xu S, Gavalas VG, 2008. Oral administration of PCBs induces proinflammatory and prometastatic responses. Environ. Toxicol. Pharmacol 25, 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO, 1995. The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environmental health perspectives 103, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellman SD, Djordjevic MV, Muscat JE, Gong L, Bernstein D, Citron ML, White A, Kemeny M, Busch E, Nafziger AN, 1998. Relative abundance of organochlorine pesticides and polychlorinated biphenyls in adipose tissue and serum of women in Long Island, New York: Cancer Epidemiology Biomarkers & Prevention 7, 489–496. [PubMed] [Google Scholar]
- Stewart PW, Lonky E, Reihman J, Pagano J, Gump BB, Darvill T, 2008. The Relationship between Prenatal PCB Exposure and Intelligence (IQ) in 9-Year-Old Children. Environmental Health Perspectives 116, 1416–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PW, Reihman J, Lonky EI, Darvill TJ, Pagano J, 2003. Cognitive development in preschool children prenatally exposed to PCBs and MeHg. Neurotoxicology and Teratology 25, 11–22. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Aburada S, Hashimoto K, 1986. Transfer and distribution of accumulated (14C) polychlorinated biphenyls from maternal to fetal and suckling rats. Arch. Environ. Contam. Toxicol 15, 709–715. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Otake T, Kataoka M, Murata Y, Aburada S, 1976. Studies of the transfer and distribution of [14C]polychlorinated biphenyls from maternal to fetal and suckling rats. Toxicol. Appl. Pharmacol 38, 549–558. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Komada M, Miyazawa K, Goto S, Ikeda Y, 2018. Bisphenol A exposure induces increased microglia and microglial related factors in the murine embryonic dorsal telencephalon and hypothalamus. Toxicol. Lett 284, 113–119. [DOI] [PubMed] [Google Scholar]
- Temple SE, Pham K, Glendenning P, Phillips M, Waterer GW, 2008. Endotoxin induced TNF and IL-10 mRNA production is higher in male than female donors: Correlation with elevated expression of TLR4. Cellular Immunology 251, 69–71. [DOI] [PubMed] [Google Scholar]
- Torres L, Danver J, Ji K, Miyauchi JT, Chen D, Anderson ME, West BL, Robinson JK, Tsirka SE, 2016. Dynamic microglial modulation of spatial learning and social behavior. Brain, Behavior, and Immunity 55, 6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk M, Anderson HA, Hanrahan LP, Falk C, Steenport DN, Needham LL, Patterson DG, Freels S, Persky V, Consortium GL, 2006. Relationship of serum levels of individual PCB, dioxin, and furan congeners and DDE with Great Lakes sport-caught fish consumption. Environ. Res 100, 173–183. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A, 2003. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc. Natl. Acad. Sci. U.S.A 100, 9614–9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venerosi A, Tait S, Stecca L, Chiarotti F, De Felice A, Cometa MF, Volpe MT, Calamandrei G, Ricceri L, 2015. Effects of maternal chlorpyrifos diet on social investigation and brain neuroendocrine markers in the offspring–a mouse study. Environmental Health 14, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voie Ø, Fonnum F, 2000. Effect of polychlorinated biphenyls on production of reactive oxygen species (ROS) in rat synaptosomes. Arch. Toxicol 73, 588–593. [DOI] [PubMed] [Google Scholar]
- Walker DM, Goetz BM, Gore AC, 2014. Dynamic postnatal developmental and sex-specific neuroendocrine effects of prenatal polychlorinated biphenyls in rats. Mol. Endocrinol 28, 99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J, Osuch JR, Karmaus W, Landgraf JR, Taffe B, O’Keefe M, Mikucki D, Haan P, 2012. Common classification schemes for PCB congeners and the gene expression of CYP17, CYP19, ESR1 and ESR2. Sci. Total Environ 414, 81–89. [DOI] [PubMed] [Google Scholar]
- Weisglas-Kuperus N, 1998. Neurodevelopmental, immunological and endocrinological indices of perinatal human exposure to PCBs and dioxins. Chemosphere 37, 1845–1853. [DOI] [PubMed] [Google Scholar]
- Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, Zhou R, 2015. Dopamine Controls Systemic Inflammation through Inhibition of NLRP3 Inflammasome. Cell 160, 62–73. [DOI] [PubMed] [Google Scholar]
- Young MRI, Farietta T, Crayton JW, 1995. Production of nitric oxide and transforming growth factor-β in developing and adult rat brain. Mechanisms of ageing and Development 79, 115–126. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Xie X, Fan Y, Zhang J, Jiang W, Wu X, Yan S, Chen Y, Peng C, You Z, 2015. Phenotypic dysregulation of microglial activation in young offspring rats with maternal sleep deprivation-induced cognitive impairment. Scientific reports 5, 9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.