Abstract
To understand how the microvasculature grows and remodels, researchers require reproducible systems that emulate the function of living tissue. Innovative contributions toward fulfilling this important need have been made by engineered microvessels assembled in vitro with microfabrication techniques. Microfabricated vessels, commonly referred to as “vessels-on-a-chip,” are from a class of cell culture technologies that uniquely integrate microscale flow phenomena, tissue-level biomolecular transport, cell–cell interactions, and proper three-dimensional (3-D) extracellular matrix environments under well-defined culture conditions. Here, we discuss the enabling attributes of microfabricated vessels that make these models more physiological compared with established cell culture techniques and the potential of these models for advancing microvascular research. This review highlights the key features of microvascular transport and physiology, critically discusses the strengths and limitations of different microfabrication strategies for studying the microvasculature, and provides a perspective on current challenges and future opportunities for vessel-on-a-chip models.
Keywords: angiogenesis, cellular microenvironment, microfluidics, tissue microfabrication, vascular remodeling
INTRODUCTION
Our bodies require efficient and simultaneous transport of fluids, nutrients, signaling molecules, waste products, and circulating cells between tissues and organs (1). The blood vasculature, or the tubular network composed of blood vessels, is tasked with coordinating this vital mass transport throughout the body (2–4). Improper formation of a functional vascular network during embryonic and fetal development is associated with a myriad of congenital disorders that cause severe morbidity or even mortality (5, 6). Dysfunction of the endothelial cells (ECs) that line the inside of all blood vessels contributes to many diseases such as atherosclerosis, cancer, stroke, and thrombosis (7–9). Consequently, considerable resources have been devoted to research into the molecular, cellular, and physicochemical determinants of blood vessel formation and function (10).
Traditionally, in vivo studies in both mammalian (e.g., transgenic mouse and rat) and nonmammalian (e.g., zebrafish) animal model systems have been at the forefront of many aspects of vascular research, such as genetic regulation (11, 12), vessel patterning during development (13, 14), and disease modeling (15). On the other hand, in vitro cell culture has been instrumental in advancing our understanding of EC biology and signaling mechanisms (16–18), morphogenesis coordinated by endogenous cell-generated forces (19, 20), and hemodynamic regulation of atherosclerosis (21–23). However, bridging in vivo and in vitro research has not always been successful and represents a major challenge in the field (24). Most conventional in vitro models lack the three-dimensional (3-D) complexity, blood flow, cell–cell interactions, or proper extracellular matrix (ECM) environment that are typical of living tissues (25, 26). Conversely, animal studies inherently provide physiological context but are limited in their ability to independently resolve aspects of the tissue environments that contribute to vascular diseases. In addition, several key anatomical differences in human versus animal models can lead to species-dependent discrepancies. One such example occurs during pregnancy, where the cellular events that coordinate the remodeling of the spiral arteries necessary to deliver nutrients to the placenta and fetus differ substantially in humans versus mice (27).
Microfabricated vessels, which we interchangeably refer to as “vessels-on-a-chip,” are engineered mimics or analogs of physiological blood vessels that offer promise to overcome some of the aforementioned limitations of conventional in vitro and in vivo models. The development of vessel-on-a-chip models has rapidly ascended in the past decade, which we believe has been bolstered by the convergence of principles from blood vessel physiology, transport phenomena, cell and matrix biology, and tissue microfabrication (Fig. 1). Tissue microfabrication itself may be viewed as an interdisciplinary field within bioengineering that integrates tissue engineering of 3-D scaffolds with design and fabrication of microfluidic ducts or channels (28). Therefore, this review does not cover detailed considerations of cell type, matrix type, and hydrogels used for engineered vessels, which have recently been featured elsewhere (29, 30), but instead presents an overview of microfabricated vessels that are inspired by the form and function of the microcirculation. To help understand the design considerations for microfabricated vessels, we first summarize important principles of microvascular transport and physiology and highlight the contributions made by in silico models toward this understanding. Next, we provide an overview of different state-of-the-art microfabrication strategies for engineering perfusable microvessels. Then we highlight the implementation of these models for advancing our understanding of microvascular physiology and pathophysiology. Finally, we discuss current challenges and future perspectives on the role of microfabricated vessels in helping bridge the technological gap between in vitro and in vivo vascular research.
Figure 1.
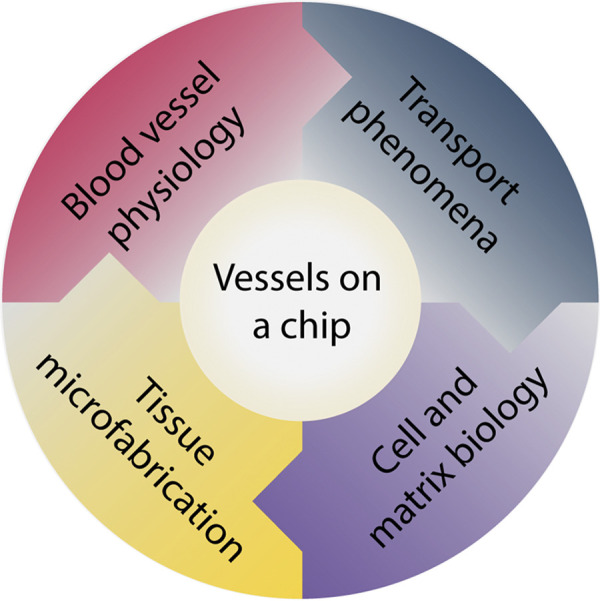
Integrative vessel-on-a-chip models. These engineered models are due to the convergence of principles from 1) blood vessel physiology, 2) transport phenomena, 3) cell and matrix biology, and 4) tissue microfabrication.
PHYSIOLOGICAL AND STRUCTURAL CHARACTERISTICS OF THE MICROCIRCULATION
The majority of oxygen (O2) transport occurs in the microcirculation (31–33), and a hierarchal branching vessel architecture is needed to fulfill cellular consumption demands while maintaining low resistance to flow (34). Altered spatial heterogeneity and temporal stability of network perfusion is associated with the reduced microvascular blood flow and tissue oxygenation that signal the onset and progression of microvascular diseases (35, 36). Consequently, the constituent blood vessels of the vasculature must be capable of rapid and dynamic remodeling in response to O2 deprivation (or hypoxia) and injury to tissue (37).
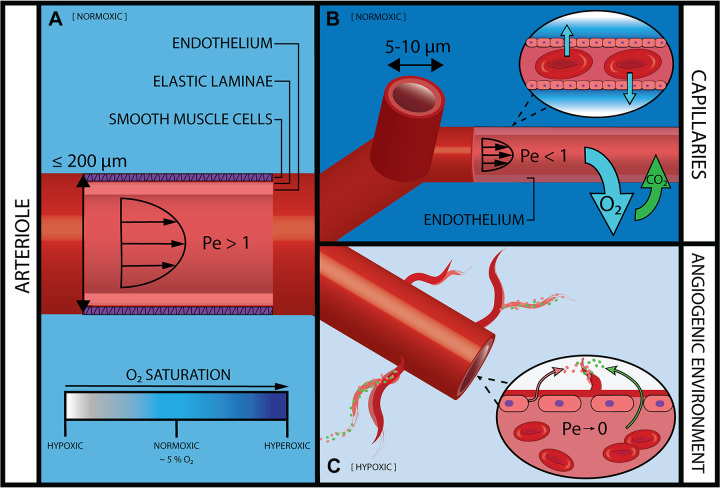
Arterioles and capillaries are small-diameter vessels of the microcirculation that facilitate the majority of O2 delivery to tissue cells (31–33). Arterioles range in diameter from 100 to 200 µm (Fig. 2A) (39), and their vessel walls are composed of an endothelial layer, inner and outer elastic lamina layers, and circumferentially arranged smooth muscle cells (SMCs). Arterioles are also known as “resistance vessels” because changes in their diameter due to contraction and relaxation of SMCs control the distribution of blood flow to specific organ capillary beds (40–42). In some organs and tissues, precapillary sphincters, located at the transition point from the arteriole to the capillary branch, direct flow to areas of higher metabolic demand. Previously believed to reside solely in the mesenteric system, these precapillary sphincters have recently been shown by Grubb et al. (42) to also exist in the brain. Inflammation in disease and injury disrupts normal arteriolar function, impacting capillary bed perfusion (41). Capillary vessel walls are made up of an endothelial layer and stabilized by pericytes embedded within the surrounding basement membrane (43). Capillaries are extremely narrow compared with other branches of the vascular network, some with diameters as narrow as 5 µm, such that red blood cells must pass single file (4). This progressive narrowing of vessels allows arterioles to serve as centrally located distributive channels for the far-reaching network of capillaries supplying nutrients to tissues (44). Nutrients and O2 exit the capillary through this thin wall to reach tissues, whereas waste products and carbon dioxide (CO2) diffuse into capillaries from tissues to eventually exit the body (Fig. 2B).
Figure 2.
Mass transport in the arterial microcirculation. A: the largest vessels in the arterial microcirculation are arterioles or “resistance vessels,” which supply blood to capillaries via convection (Pe > 1). A circumferential layer of smooth muscle cells enables precise mechanical control over blood flow and subsequent regional perfusion. In normal physiological environments, surrounding tissue and ECM is normoxic [partial pressure of oxygen () ∼ 5%] (38). B: capillaries are the smallest vessels in the microcirculation and the main site for gas and nutrient exchange. A thin endothelial vessel wall enables passive diffusion (Pe < 1). The surrounding ECM is normoxic, with a higher nearest the capillary as O2 is radially dispersed. C: tissue hypoxia ( < 5%) drives vessel angiogenesis in postcapillary venules. Increased vessel permeability causes leakage of plasma proteins (green) into surrounding interstitial fluid. Coupled with infiltration of endothelial cells (pink), these components assemble to form new vascular sprouts. (Drawing is not to scale.)
Vascular remodeling occurs through several primary mechanisms. Angiogenesis is the generation of new vessel segments from existing branches and occurs primarily in capillaries and postcapillary venules (45). The two basic modes of angiogenesis are vessel splitting (or intussusception) and vessel sprouting (Fig. 2C). Both of these angiogenic processes are tightly regulated under normal conditions and allow for the continued expansion of the vascular system to provide blood flow to avascular, ischemic, or growing tissue (46, 47). Angioadaptation describes the structural remodeling of existing vessels to optimize blood transport to surrounding tissues. This process is modulated by a combination of fluid dynamics through local shear forces on vessel walls and metabolic signal propagation (33, 48). Local changes in shear stress and metabolic signals also cause alterations to vessel diameter and permeability. Changes in vessel diameter alter the resistance to blood flow. Vessel dilation causes decreased flow resistance, allowing nutrients to be routed to areas of higher consumption (49). Vascular permeability describes vessel “leakiness” to fluid and micro- and macromolecules. Increased permeability is coincident with sprouting angiogenesis that is especially prevalent during wound healing, tumor growth, and chronic inflammation (50).
PRINCIPLES OF MASS TRANSPORT AND FLUID FLOW THROUGH VASCULATURE
Although some passive diffusion occurs as blood is pumped throughout the body, the diffusion limit of O2 in tissue is ∼100 µm because of low solubility. Consequently, the structural architecture of the microcirculation ensures that cells are always within 200 µm from the nearest capillary, and usually O2 must diffuse 30 µm or less to reach its destination (32). Here we outline the basic principles of mass transport through blood (diffusion and convection), as well as the fluid flow properties that must be considered in order to replicate physiological conditions in microfabricated vessel models. We note that this section does not describe the rheological basis of microvascular blood flow (49) because the perfusate used in microfabricated vessels is typically cell culture medium as opposed to whole blood.
Diffusion
Blood transports O2 and other small molecules through the body via the vascular system. The blood picks up O2 in the lungs, where O2 is transferred via diffusion as described by Fick’s law:
| (1) |
where D is the diffusion coefficient of the gas, and the differential describes the change in the gas concentration (c) over the change in position (x). In other words, the gas flux is directly proportional to the concentration gradient of the gas (4, 51).
The gaseous O2 travels in the blood primarily through binding to hemoglobin, the O2-carrying protein present in red blood cells. Hemoglobin consists of four protein subunits (globins), each of which can cooperatively bind O2. Hemoglobin’s affinity for O2 enables the O2-carrying capacity of blood to increase 70-fold compared to plasma (52). Hemoglobin has higher affinity for binding O2 in areas where the partial pressure of O2 () is higher, and lower affinity in areas where the is low. This enables hemoglobin to be an ideal carrier protein for O2, picking up O2 in the O2-rich lungs and delivering it to O2-consuming tissues.
As discussed above, most gas exchange occurs in microvessels, where O2 is delivered via diffusion (53). Since O2 is a small lipophilic solute, it is highly soluble in cell membranes and can easily diffuse across microvessels and into the surrounding tissue space (54). August Krogh developed the first mathematical tissue model of O2 delivery in the microcirculation. This cylinder model, in which O2 tension is a function of spatial position in a cylinder representing a single microvessel, is still widely referenced today and served as the basis for future development in the field of O2 transport (4, 55).
In addition to O2 and other gaseous molecules, transvascular transport of hydrophilic and/or large solutes, such as signaling proteins and other macromolecules, is required for tissue homeostasis (50, 56). In the capillaries of most tissue, narrow clefts between ECs are the main transport pathway for small hydrophilic solutes (57). Brain capillaries, on the other hand, are especially impermeable to small hydrophilic solutes (58). Conversely, microvessels in tumors and inflamed tissue typically exhibit large and frequent gaps between ECs (50). Consequently, the permeability of vessels in tumors and during inflammation is increased with reduced size selectivity to solutes compared with normal vessels (54, 59).
Convection
Convection enables the pressure-driven flow responsible for moving blood through the central bloodstream to the microcirculation, where most nutrient delivery occurs via diffusion across tissue barriers. Because of the limited tissue solubility of O2, convection is necessary to place O2 within reach of all tissues in the body. In addition to luminal transport, transvascular transport occurs by convection (or transendothelial filtration). The rate of transendothelial filtration is determined by the hydrostatic and osmotic pressure differences between blood vessels and the surrounding tissue (also known as Starling forces) and the hydraulic conductivity of blood vessels, which is a function of vessel permeability (56). Thus, transendothelial filtration is highly dependent on the state of the tissue (e.g., inflamed vs. normal) (60). The application of microscale culture technologies for studying transendothelial filtration has been reviewed more comprehensively elsewhere (61). However, we note here that when applying the Starling equation to in vitro microfabricated vessels, the oncotic effects are typically negligible because of the homogeneous cell culture medium composition (62).
Péclet Number
The Péclet number (Pe) is defined as the ratio of convective to diffusive transport and can be represented as follows:
| (2) |
where U represents the linear flow velocity in the control volume, L represents the length scale of flow, and D is the diffusion constant. The Pe is > 1 in convection-dominated transport and < 1 for diffusion-dominated transport (54, 63). The Pe is governed by changes in the physiological microenvironment and influences vessel formation. For instance, heightened interstitial flow (where Pe > 1) and regional hypoxia (where Pe < 1) can arise during inflammation, cancer, or exercise. Both of these physiological conditions independently promote angiogenesis (64–67).
Reynolds Number
The Reynolds number (Re) is a nondimensional quantity that describes a fluid’s flow behavior by providing the ratio between inertial and viscous forces. The equation for the Re can be written as follows (68):
| (3) |
where v is the average fluid velocity, ρ is the fluid density, L is the characteristic length, and µ is the fluid viscosity.
Fluid flow is described as laminar at low fluid velocities and as turbulent at high fluid velocities. The transition from laminar to turbulent flow occurs at Re ∼ 2,000 (69). Unlike in large arteries, blood flow in the microcirculation is primarily laminar, although laminar flow can be disturbed because of pathological changes in vessel geometry such as aortic stenosis in the coronary microcirculation (69–71). In such cases, blood flow may become turbulent downstream from the aberration. Another consequence of stenotic or occlusive disease in a major artery is the formation of tortuous or prominently twisted and curved collateral arteries (72). These so-called “corkscrew collaterals,” which can be < 1 mm in diameter (73), hinder collateral development that is necessary for restoring blood flow for tissue regeneration (74). Curved or tortuous microvessels have been frequently reported in retina, skeletal muscle, myocardium, and brain tissues (75–78). In addition, during pregnancy, coil-shaped maternal spiral arteries (∼200 µm in diameter at the onset of pregnancy) temporarily supply blood to the endometrium of the uterus (79). Tortuous capillaries are also associated with diseases such as hypertension, diabetes, and cancer (80–82). Curvature of microvessels can modify blood flow by introducing secondary flows known as Dean flows or vortices, even at low Re (Re ≤ 1) (83, 84).
THEORETICAL AND COMPUTATIONAL MODELS OF VASCULATURE
Similar to microfabricated vessels, theoretical and computational (i.e., in silico) models are an engineering-based approach for studying microvascular physiology (4) and pathophysiology (85). These in silico models predate the advent and widespread use of microfabricated vessels and have formed an important basis for our current understanding of the structural adaptation of vessel networks (33). Through this dynamic process, the vascular network is constantly redirected and pruned to fulfill the needs at the tissue and cellular levels to maintain function. Theoretical models can predict these effects (33). Simulations utilize in vivo data of microvascular networks with 300–1,000 vessel segments to investigate the response of the vascular network to specific stimuli. The results are then compared to experimental data (86). These models can be highly useful in determining the relationship between variables or identifying variables that have considerable effects on vessel structure and function.
A computational investigation of the relationship between vessel diameter and wall thickness as a response to circumferential wall stress or shear stress based on rat mesentery experimental measurements found that these variables are not separately controlled (87). Further investigations by Pries and Secomb have revealed the complementary roles of angioadaptive responses, such as pruning of existing vessel branches, and angiogenic sprouting in restructuring vasculature without disrupting blood flow (34). Reglin et al. (88) examined O2 sensing in tissues, vessel walls, and red blood cells to predict which had a greater effect on microvessel diameter adaptation and angiogenesis. In this work, only one metabolic signaling pathway was assumed active at a time, allowing them to be tested as independent variables. The study indicated that microvascular vessel wall signaling in response to O2 changes is centrally involved in the long-term, steady-state adjustment of vessel diameters in response to metabolic signals. Additionally, red blood cell signaling could play a minor role in adjustment of vessel tone to adapt total blood flow. Tissue signaling, meanwhile, did not appear to greatly impact microvessel diameter, although it may be vital in angiogenesis to supply hypoxic regions (88). Popel et al. have been involved in numerous studies of angiogenesis and blood flow dynamics through computational approaches that provide important insights into red blood cell aggregation and O2 transport in microscale blood flow, particularly in tortuous geometries such as tumor vasculature (89–94).
Computational simulations have been applied extensively for disease modeling, prolifically cancer, enabling elucidation into tumor architecture and targeted drug delivery (95–103). Such models are valuable for exploring how structural characteristics contribute to pathophysiological behavior and therapeutic response. For example, Welter et al. (101) found that in simulated breast cancer tumors, tissue hypoxia was induced by vascular compression that reduces or impairs blood flow. They concluded that perfusion-induced hypoxia in vivo is indicative of poorer prognosis as compressed vessels will impede chemotherapy (101).
Although computational models have greatly benefited our overall understanding of the vascular system, we must acknowledge that they cannot fully convey all of the underlying parameters. Moreover, experimental findings from these models can be difficult to validate in vivo, as it is nearly impossible to differentiate the effects of independent variables in the regulation of these intricate transport mechanisms. However, findings from computational studies can be applied to the design of robust in vitro microfluidic platforms. These on-chip models can allow for precise manipulation of multiple variables, enabling vigorous investigations of microcirculatory remodeling and mass transport.
DESIGN AND ASSEMBLY OF VESSEL-ON-A-CHIP MODELS
Microfabricated vessels-on-a-chip have emerged as versatile experimental platforms where different groups of vascular cells (e.g., ECs and SMCs) can be assembled, grown, and perfused under very defined conditions in vitro. In turn, long-standing questions regarding cell–cell (104–108) and cell–ECM (109–111) interactions can be interrogated in microfabricated vessels with high-resolution microscopy (112) and high-throughput analysis (113). Furthermore, microfluidic models have allowed researchers to obtain further insights into vessel dysfunction that underlies certain disease conditions (114–117). Such examples include but are not limited to sprouting angiogenesis (118, 119), vessel permeability (120, 121), and tumor cell–vessel interactions (122, 123). Aside from this, microfabricated vessels may provide a much more economical option for studying microvascular function because of reduced reagent consumption compared with established in vivo and in vitro assays (124). Below, we provide an overview of the microfabrication techniques that are commonly employed for engineering microvessels.
Microchannel-Based Vessels-on-a-Chip
Many of the approaches for constructing microfabricated vessels involve prepatterning ECs on a microchannel surface. Various techniques have been developed for rapid prototyping microchannels (125), yet easily the most widely adopted method is soft lithography of poly(dimethylsiloxane) (PDMS) (126), which is an optically clear and gas-permeable silicone elastomer. Soft lithography produces rectangular microchannels with cross-sectional scales on the order of tens to hundreds of micrometers, or comparable in dimensions to microvessels. These length scales are amenable to soluble factor signaling via paracrine and autocrine processes (124). Moreover, because of the small dimensions of microchannels, the predominant flow regime is laminar (127), as specified by Re (Eq. 3). Apart from manipulation of microchannel dimensions, certain microfabrication techniques enable in vitro recapitulation of in vivo geometric alterations to vascular structures caused by branching (or bifurcations), aneurysms, and stenosis (62, 128, 129).
The material properties of PDMS are conducive for rapid prototyping of microchannels with high fidelity and efficiency. In addition, PDMS possesses sufficient mechanical integrity to facilitate connections to an external pumping mechanism (e.g., programmable syringe pump) such that cellular responses due to controlled levels of perfusion inside microchannels can be assessed (130). However, one important limitation of PDMS is that it is prone to nonspecific absorption of proteins and hydrophobic drug molecules (131), especially compared with other synthetic polymers that are less commonly used for fabricating microchannels, such as polystyrene and cyclo-olefin polymers (132). These findings highlight the importance of material selection in microfluidic device design, particularly for drug and toxicity studies involving hydrophobic molecules (133). Moreover, since PDMS is rigid and impermeable to water, the physical and chemical properties of PDMS do not match those of native tissue (26). Therefore, two approaches are typically employed to improve the utility of PDMS-based microchannels for studying microvascular transport. One approach is to incorporate membrane filter inserts to create a semiporous planar surface in multilayer PDMS microchannel systems (134, 135). This configuration recapitulates distinct compartments for assessing functional tissue/vascular barriers. Another approach for making PDMS-based microchannels more tissuelike is to introduce a localized region of 3-D tissue scaffold that is laterally adjacent to EC-lined microchannels. The most commonly used tissue scaffold materials for this PDMS-hydrogel hybrid configuration are natural hydrogels such as fibrin or type I collagen (136), which are semiporous and can be readily remodeled by cells. Therefore, the material and biologic characteristics of the localized 3-D tissue scaffold enable mass transport of signaling molecules by diffusion and convection and observation of multicellular sprouting angiogenesis (137, 138).
Another method for creating prepatterned microfabricated vessels is to create a hydrogel-embedded lumen structure that is housed within a PDMS microchannel (139). This approach produces a more physiological circular lumen compared with the rectangular lumen of the PDMS-hydrogel hybrid design described above. The most commonly used method for creating hydrogel-embedded lumen structures is to cast around a stainless steel cylindrical needle (140). This method has been used to create vessels ranging in diameter from 150 to 300 µm (139). However, since the needle must be removed after scaffold polymerization, this method can only make a straight vessel geometry. Two techniques developed by Beebe’s group have demonstrated branched hydrogel-embedded lumen. One technique is viscous finger patterning inside branched PDMS microchannels (141). The second technique is casting hydrogel around a removable branched PDMS mandrel or rod (142). In addition, more complex vessel patterns can be fabricated in hydrogels with micropatterned PDMS stencils (119, 143) or a silicon master mold (144).
A recent study by Mandrycky et al. (145) developed a spiral microvessel model that evaluated the role of curvature and torsion in EC response to flow . This model was fabricated by casting either PDMS or collagen around a stainless steel spring (diameter of wire = 400 µm; diameter of spring = 3 mm), which was retracted with a custom two-axis microcontroller to yield a continuous and spiral microvessel. Although comparisons of EC responses to laminar versus disturbed flow have been widely reported (22), the effects of complex nondisturbed flows in curved vessels has not been well studied. Here it was shown convincingly that the secondary flows or Dean vortices that were introduced because of the curvature of the spiral microvessels (83, 84) altered EC phenotype and transcriptional profiles compared with ECs in straight microvessels of the same diameter. By employing advanced and creative microfabrication techniques, this study provided a previously unavailable tool for in vitro studies that identified unique changes in ECs due to complex and heterogeneous vascular structures found in vivo (72).
3-D Bioprinted Vasculature
In the past decade or so, 3-D bioprinting, or the application of additive manufacturing techniques for the biofabrication of living tissue, has rapidly ascended within bioengineering research (146). 3-D printing has been demonstrated to be a versatile and capable process for constructing in vitro complex tissues and organs, including vascular structures (147–151), with tailored biological components and mechanical properties. As such, many well-annotated review articles cover extensively the promising applications of bioprinting, as well as the various methods to bioprint (152–154). These methods include extrusion-based bioprinting (EBB), droplet-based bioprinting (DBB), laser-based bioprinting (LBB), and stereolithography. We direct readers to the aforementioned review papers for more detailed analysis of the different 3-D bioprinting methods.
One approach for bioprinting perfusable thick (>1 cm) vascularized tissue-on-chip was reported by Kolesky et al. (155). This study employed a multimaterial 3-D bioprinting method. To construct the vascular network, the authors used a sacrificial ink that was first printed and then removed inside a separately printed cell-laden ECM comprised of gelatin, fibrin, human mesenchymal stem cells (hMSCs), human neonatal dermal fibroblasts (hNDFs), and a matrix cross-linking agent. In this study, Pluronic F-127, a thermally responsive hydrogel, was the sacrificial ink. The Pluronic was dispensed through a tapered printing nozzle (410 µm in diameter) at room temperature and then cooled to 4°C, where it liquified and was washed away. Subsequently, the interconnected channels were lined with human umbilical vein ECs to form a perfusable vessel network that was symmetrically branched to ensure uniform perfusion throughout the tissue to provide the transport of nutrients, O2, and waste materials. The authors used this perfusable construct to deliver specific differentiation factors to the tissue in a more uniform manner than bulk delivery methods and demonstrated precise control and differentiation of the printed hMSCs toward an osteogenic lineage in situ.
Perfusable Vascular Beds Formed by Vasculogenic Self-Assembly
Another widely used approach for microengineering blood vessels in vitro is to apply principles of vasculogenic self-assembly. In these de novo vasculogenesis models, ECs are cocultured with either fibroblasts (104, 106) or mesenchymal stem cells (MSCs) (122) within a perfusable microtissue chamber composed of 3-D ECM (most commonly fibrin or type I collagen). Fibroblast-derived matrix proteins have been shown to be essential for stable EC lumen formation in vitro (156), and the resulting 3-D multicellular structures formed in EC-fibroblast cocultures in microtissues emulate the primitive vascular plexus generated by de novo vasculogenesis in vivo (1). Microvascular networks formed by self-assembly have been shown to remain viable through 40 days when perfused with interstitial flow (157). Self-assembled microvascular networks have been used to study anastomosis between vessel networks (104, 157) and cancer cell extravasation (122) and to screen for the efficacy of intravascular delivery of a chemotherapeutic agent (106), to name a few applications.
APPLICATION OF VESSEL-ON-A-CHIP MODELS FOR TRANSPORT STUDIES
O2 and Nutrient Transport
As demonstrated by the bioprinted example by Kolesky et al. (155) above, O2 and nutrient transport are crucial factors to consider when designing culture models that mimic physiological microenvironments that affect cell behavior. The approaches that have been used for generating either uniform hypoxia or oxygen gradients in microfluidic bioreactors (158) include supplying O2/N2/CO2 gases at desired ratios (159) and utilizing a chemical reaction of an O2 scavenger, such as sodium sulfite (Na2SO3) (160) or pyrogallol (161), that is spatially confined from the cell culture region. A benefit of the chemical scavenger is that O2 gradients can be generated without additional instrumentation (e.g., hypoxia chamber and gas controllers). These approaches have enabled studies on the effects of O2 conditions on 3-D microvascular network formation (162), endothelial sprouting (38), and vessel permeability (163) in microfabricated models.
Recently, Jomezadeh Kheibary et al. (164) developed a mathematical model that determined the O2 tension conditions needed within microfluidic bioreactors for normoxic environments. The conditions for their study involved one bioreactor, which comprised a microfluidic channel that contained an O2-permeable membrane and one with an impermeable membrane. The study examined how velocity, cell density, and microchannel length affected the O2 tension within the system. The group also analyzed O2 conditions within a single bioreactor and between two interconnected bioreactors. Results from the study demonstrated that all of the aforementioned parameters affected O2 tension. However, the group highlighted that by utilizing an oxygenator in bioreactors with fixed flow rates and varying the oxygenator’s length, the O2 concentration can be manipulated. Moreover, it was emphasized that future research should look into how O2 concentration varies in multicellular systems because of the different uptake rates between cells. This last point is especially important to consider because as researchers strive to make engineered models more complex and physiologically relevant, the use of multiple cell types should be implemented. Nonetheless, this study provided the research field with mathematical models to predict the variations in O2 conditions within microfluidic bioreactors.
A report by Hsu et al. (165) provided a well-structured, foundational study on physiological mass transport distribution within 3-D microtissue systems . Using the capacity of microfluidic systems to precisely control the mass transport parameters of Pe (Eq. 2), this study defined the ratio of convection versus diffusion within the cellular microenvironment that was conducive for vasculogenesis. The group’s model was a microfluidic device with a microtissue chamber containing 3-D fibrin gel seeded with normal human fibroblasts (NHLFs) and endothelial colony-forming cell-derived ECs (ECFC-ECs) as the vascular cell type. The results from this study showed that both interstitial flow (Pe > 10) and hypoxia (Pe < 0.1) independently stimulated vasculogenesis. However, intermediate flow conditions (0.1 < Pe < 10), which were representative of normal living tissue, resulted in no initiation of vasculogenesis. Collectively, this study demonstrated that vasculogenic response is a function of Pe and elaborated on how flow conditions as well as O2 transport conditions affect vessel network formation.
Much of the work on in silico approaches for studying microvascular remodeling and in vitro studies in microfabricated vessels have been conducted in parallel to each other. However, Kuzmic et al. (166) recently conducted a study in which they developed combined mathematical and computational models for studying EC migration and angiogenesis in microfluidic culture systems. Their models, which were based on two preexisting mathematical models (167, 168), were applied to predict the angiogenic responses due to a vascular endothelial growth factor (VEGF) chemical gradient in the defined microfluidic geometries. An important outcome from this study was that the data generated will help establish general design and operational guidelines for using microfluidic systems. Typically, microfluidic-based experiments rely heavily on trial-and-error methods with limited systematic optimization. Moreover, this study provided a theoretical basis for EC migration and angiogenesis observed in microfluidic experiments. The authors conclude that their approach can be applied to different microfluidic geometries and may provide a framework for designing more complex microfabricated tissues. Furthermore, this study is a demonstration of the benefit of integrating mathematical and computational modeling with microfluidic-based experiments toward providing insights into fundamental angiogenesis processes.
Modeling Functional Tissue/Vascular Barriers
As described above, the physiological and structural characteristics of the microcirculation make it a selective barrier for the exchange of nutrients and waste products between blood and surrounding tissue or organ. In addition, microvessel walls serve as an important barrier for drug transport to tissue-resident cells (54). Parenchymal, stromal, and mural cells confer tissue-specific properties of blood vessels through paracrine and juxtacrine interactions (46, 169). As such, microfabricated systems have been widely applied to model in vitro functional vascular/tissue barriers for evaluating tissue/organ-specific transport properties (170–172). These engineered systems are widely referred to as organs-on-a-chip and hold great promise for drug screening applications and creating patient-specific models for precision medicine (173). Here we highlight the application of organ-on-a-chip technology for studying tissue/vascular barrier function in the context of different human organ physiologies (Fig. 3).
Figure 3.
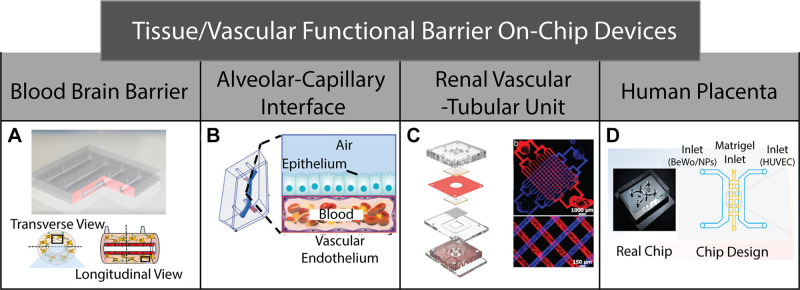
On-chip devices used to mimic various tissue/vascular functional barriers. A: blood brain barrier microfluidic model used to study nutrient exchange/waste removal in order to develop new drug delivery therapies. B: alveolar-capillary interface microfluidic model used to study pulmonary physiology in patients with thrombotic diseases. C: kidney-on-a-chip model utilized to study blood filtration and waste removal. D: human placenta-on-a-chip model designed to examine the effects of nanoparticles transferred from the mother to the fetus via the placenta. In this model, human placental trophoblast BeWo cells are cocultured with human umbilical vein endothelial cells (HUVECs). [Fig. 3A reproduced from Moya et al. (175), Fig. 3B from Jain et al. (174), Fig. 3C from Rayner et al. (176), and Fig. 3D from Yin et al. (177), with permission.]
A physiological barrier of great interest is the blood-brain barrier (BBB) because it is highly selective to both efficacious drug-loaded nanoparticles (NPs) and potential toxins (58). Over the past several years, numerous microfabricated BBB model systems have been developed (178–183). These models typically consist of specialized vascular and perivascular compartments composed of ECs and brain specific cells (e.g., astrocytes, brain pericytes), respectively, and aim to evaluate signaling mechanisms that underlie BBB function. One strategy for overcoming the transport obstacles posed by the BBB is to use the physiological process of receptor-mediated transcytosis (RMT) through brain ECs and toward the brain parenchyma (184). A recently developed microfabricated BBB model evaluated RMT mechanisms of functionalized NPs (178). This study also 3-D mapped NP distributions in the vascular and perivascular compartments. In addition, Moya et al. (175) recently developed a novel reconfigurable BBB model that allows for optimization of cellular orientation and physiologically relevant flow conditions. Most microfabricated BBB models are composed of multilayer rectangular channels that are separated by a filter insert. However, one advantage of the BBB model developed by Moya et al. is that it consists of a circular vessel lumen lined with human brain ECs that is surrounded circumferentially by an astrocyte-laden 3-D hydrogel (Fig. 3A). This configuration enables uniform intravascular wall shear stress levels where the ECs and astrocytes were cocultured for 28 days.
Another widely studied tissue/vascular barrier is the alveolar-capillary interface, which is the primary location for gas exchange in lungs (185). Numerous microfabricated models have been developed for evaluating changes in barrier integrity at this important interface due to toxic nanoparticles (186) and during pulmonary edema (187). It is noted that these studies pioneered the application of microfabricated systems as human cell culture models for reconstituting the tissue-tissue interfaces that are critical to organ function. More recently, Jain et al. (174) designed a lung alveolus-on-a-chip that investigated inflammation-induced thrombosis (Fig. 3B). These studies were enabled by perfusing through the vascular channels whole blood, which contains the necessary platelets and coagulation factors to form thrombi. This model enabled the study of platelet-endothelial interactions that underlie hemostasis regulation in a context that is highly relevant to pulmonary pathophysiology.
Other tissue/vascular barriers of great interest include the glomerular filtration unit of the kidney nephron and the placenta. Microfabricated models have successfully recreated these physiological interfaces in vitro. Recently, Rayner et al. (176) developed a kidney-on-a-chip model that allowed them to look into the physiology of the renal vascular-tubule unit, which is involved in blood filtration and nutrient exchange/waste removal (Fig. 3C) (188). The human renal vascular-tubule unit consists of a highly fenestrated endothelium (57) and a tubular lumen separated by a basement membrane layer. In the model developed by Rayner et al., microfabricated vascular and renal tubular compartments, composed of endothelial and kidney epithelial cells, respectively, were seeded against a solute permeable collagen membrane that can be remodeled by cells. This model was capable of performing kidney-specific functions, including reabsorption of albumin and glucose from the epithelial channel.
During pregnancy, nutrient exchange between a mother and a fetus is enabled by the placenta (189). Although the junctional adhesion molecules are known to regulate the paracellular permeability of placental microvessels (190), a poor understanding of the etiology of many pregnancy disorders has helped spur the development of microfabricated models for studying placental barrier function (191). Recently, Yin et al. (177) developed a 3-D placenta-on-a-chip model (Fig. 3D) to examine the consequences of environmental exposure to titanium dioxide nanoparticles (TiO2-NPs), which are manufactured in large quantities worldwide for a variety of applications (192). Specifically, this study evaluated the consequences of TiO2-NP exposure on the structural integrity of the placenta immune cell responses. This model featured both a maternal and a fetal side. TiO2-NPs were introduced through a centered Matrigel inlet, where they were allowed to flow toward the maternal side (containing placental trophoblasts) or toward the fetal side (containing human umbilical vein ECs). The configuration of this model allowed evaluation of how TiO2-NPs can bypass the placental barrier and enter toward the fetal bloodstream. Therefore, this model holds promise for studying pregnancy disorders that are characterized by impaired placental function.
CONCLUSIONS AND FUTURE OUTLOOKS
As an advanced cell culture technology, microfabricated vessels-on-a-chip have successfully recapitulated the multicellular, 3-D ECM, and structural components of blood vessels of the microcirculation under well-controlled conditions. Thus, as a collective, vessels-on-a-chip have proven to be a reliable and highly useful complement to in vivo studies of microvascular physiology and function. This standing has been propelled by tremendous advances in microfabrication and microfluidic techniques combined with a deep understanding of vascular biology and physiology. Further strengthening the integration between microscale engineering technology and methods from cell and molecular biology is imperative for the continued advancement of vessel-on-a-chip models.
The achievements of vessel-on-a-chip technology, particularly in elucidating the physicochemical determinants of sprouting angiogenesis and modeling tissue/vascular barriers, for example, are certainly worthy of commendation by the scientists in this research field. Yet despite this enthusiasm, numerous challenges—and opportunities—remain. With regard to angiogenesis, the majority of vessel-on-a-chip models have focused on EC autonomous responses underlying vessel sprouting and permeability. We still know less about the heterotypic cell–cell interactions between ECs and non-ECs (e.g., mural cells) that orchestrate angiogenesis and changes in vessel permeability. Yet, we believe strongly that our understanding of these processes is poised to advance rapidly with the continued improvements in fabricating more biologically complex and sophisticated vessel-on-a-chip models. Moreover, although sprouting angiogenesis is now readily studied in microfabricated vessels, cells and tissue in physiology can acquire vasculature by other means such as intussusceptive angiogenesis or vessel splitting (2). To our knowledge, intussusceptive angiogenesis has not yet been replicated in microfabricated vessels. Compared with vessel sprouting, much fewer experimental models are available for studying intussusception (10). Consequently, much less is known about the underpinnings of intussusception versus sprouting. Future vessel-on-a-chip models may help address this need to help improve the quality and availability of models for studying intussusception under well-controlled microenvironments.
Despite the very impressive advancements of vessel-on-a-chip models, we must surmise that at present the full pattern of vascular remodeling is still beyond reach. For example, current microfabricated vessels do not emulate the dynamic complexity of the structural remodeling processes of angioadaptation (i.e., vessel pruning, constriction/dilation) along different locations in the vascular plexus due to the local metabolic and hemodynamic states (33, 48). However, since the tissue oxygenation status is crucial for angioadaptation, incorporating the underlying fundamental transport properties of O2 would benefit studies in microfabricated vessels. One prospective approach for modulating the O2 environment is through intravascular delivery of O2-carrying nanoparticles that are capable of traversing inside engineered microvessels (193, 194). Yet we must also caution against racing to make vessel-on-a-chip models too complex, which may diminish experimental robustness and reproducibility. Furthermore, most vessel-on-a-chip models require microfluidic and microfabrication expertise that is not accessible to most biologists and physiologists. To address this need, important advancements have been made in reconfigurable “open microfluidic” systems that require only simple pipetting for operation. Such models have been used to study paracrine signaling mechanisms that facilitate angiogenesis in 3-D ECM with high throughput (195).
Computational models such as those described here have informed the design of microfluidic in vitro systems. For example, the work of Pries and Secomb has been widely applied to inspire the structure and function of fabricated microvessels. Their insights into the rheology and dynamics of blood flow, and of red blood cell behavior at vessel bifurcations, provide a basis of anticipated behavior in models that consider these effects (113, 196–200). However, it is notable that the hemodynamic stimuli included in these models are wall shear stress and intravascular pressure but exclude transvascular flow, which has been experimentally replicated with microfabricated vessels and other in vitro models, and is a potent morphogenetic mediator of the vasculature (67, 137, 139, 201–203). Computational models have also highlighted areas in which new methods of investigating the underlying mechanics are needed to deepen our understanding. Goldman and Popel’s simulations investigating the role of tortuous microvessel structure and vasomotion on O2 transport garnered interest in fabricating in vitro models capable of determining the role of blood rheology on blood flow oscillation independent from biological regulation in capillaries (90, 91, 204). Overall, these models have been important in enhancing our knowledge of vascular physiology, and they have potential to be applied to inform microfluidic design and fabrication. Increasing the communication and collaboration between these two areas may be a valuable way to augment both computational and microfluidic advances. This highlights just one of many avenues for growth in the near future as we seek to enhance the capabilities of in vitro microsystems in capturing physiological behavior.
GRANTS
This work was supported by funding awarded to J.W.S. from the American Heart Association (15SDG25480000), an NSF CAREER Award (CBET-1752106), NHLBI (R01 HL141941), and The Mark Foundation for Cancer Research (18-024-ASP). S.R.M. and A.F.P. are supported by funding from the Department of Defense (W81XWH1810059). J.J.A. is supported by funding from the GEM Fellowship, Ohio State University Discovery Scholars Fellowship, and the Gates Millennium Scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.R.M., J.J.A., and J.W.S. prepared figures; S.R.M., J.J.A., and J.W.S. drafted manuscript; S.R.M., J.J.A., A.F.P., and J.W.S. edited and revised manuscript; S.R.M., J.J.A., A.F.P., and J.W.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Don Belcher and Chia-Wen Chang for helpful discussions.
REFERENCES
- 1.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8: 464–478, 2007. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 473: 298–307, 2011. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldman D. Theoretical models of microvascular oxygen transport to tissue. Microcirculation 15: 795–811, 2008. doi: 10.1080/10739680801938289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Popel AS. Theory of oxygen transport to tissue. Crit Rev Biomed Eng 17: 257–321, 1989. [PMC free article] [PubMed] [Google Scholar]
- 5.Fleetwood IG, Steinberg GK. Arteriovenous malformations. Lancet 359: 863–873, 2002. doi: 10.1016/S0140-6736(02)07946-1. [DOI] [PubMed] [Google Scholar]
- 6.Timur AA, Driscoll DJ, Wang Q. Biomedicine and diseases: the Klippel-Trenaunay syndrome, vascular anomalies and vascular morphogenesis. Cell Mol Life Sci 62: 1434–1447, 2005. doi: 10.1007/s00018-005-4523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mourad JJ, des Guetz G, Debbabi H, Levy BI. Blood pressure rise following angiogenesis inhibition by bevacizumab. A crucial role for microcirculation. Ann Oncol 19: 927–934, 2008. doi: 10.1093/annonc/mdm550. [DOI] [PubMed] [Google Scholar]
- 8.Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, Murfee WL, Pacher P, Csiszar A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol 15: 555–565, 2018. doi: 10.1038/s41569-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. Endothelial dysfunction and vascular disease—30th anniversary update. Acta Physiol (Oxf) 219: 22–96, 2017. doi: 10.1111/apha.12646. [DOI] [PubMed] [Google Scholar]
- 10.Nowak-Sliwinska P, Alitalo K, Allen E, Anisimov A, Aplin AC, Auerbach R, , et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 21: 425–532, 2018. doi: 10.1007/s10456-018-9613-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelbrecht E, Levesque MV, He L, Vanlandewijck M, Nitzsche A, Niazi H, Kuo A, Singh SA, Aikawa M, Holton K, Proia RL, Kono M, Pu WT, Camerer E, Betsholtz C, Hla T. Sphingosine 1-phosphate-regulated transcriptomes in heterogenous arterial and lymphatic endothelium of the aorta. Elife 9: e52690, 2020. doi: 10.7554/eLife.52690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalén M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445: 776–780, 2007. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 13.Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol 248: 307–318, 2002. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 14.Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 134: 3317–3326, 2007. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Getz GS, Reardon CA. Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol 32: 1104–1115, 2012. doi: 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nachman RL, Jaffe EA. Endothelial cell culture: beginnings of modern vascular biology. J Clin Invest 114: 1037–1040, 2004. doi: 10.1172/JCI23284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan CC, Kumar S, Shah N, Bloodworth JC, Hawinkels LJ, Mythreye K, Hoyt DG, Lee NY. Endoglin regulation of Smad2 function mediates Beclin1 expression and endothelial autophagy. J Biol Chem 290: 14884–14892, 2015. doi: 10.1074/jbc.M114.630178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheitlin CG, Julian JA, Shanmughapriya S, Madesh M, Tsoukias NM, Alevriadou BR. Endothelial mitochondria regulate the intracellular Ca2+ response to fluid shear stress. Am J Physiol Cell Physiol 310: C479–C490, 2016. doi: 10.1152/ajpcell.00171.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson CM, Pirone DM, Tan JL, Chen CS. Vascular endothelial-cadherin regulates cytoskeletal tension, cell spreading, and focal adhesions by stimulating RhoA. Mol Biol Cell 15: 2943–2953, 2004. doi: 10.1091/mbc.e03-10-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinhart-King CA, Dembo M, Hammer DA. Endothelial cell traction forces on RGD-derivatized polyacrylamide substrata. Langmuir 19: 1573–1579, 2003. doi: 10.1021/la026142j. [DOI] [Google Scholar]
- 21.Ando J, Yamamoto K. Flow detection and calcium signalling in vascular endothelial cells. Cardiovasc Res 99: 260–268, 2013. doi: 10.1093/cvr/cvt084. [DOI] [PubMed] [Google Scholar]
- 22.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 91: 327–387, 2011. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gimbrone MA Jr, García-Cardeña G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc Pathol 22: 9–15, 2013. doi: 10.1016/j.carpath.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franco C, Gerhardt H. Tissue engineering: blood vessels on a chip. Nature 488: 465–466, 2012. doi: 10.1038/488465a. [DOI] [PubMed] [Google Scholar]
- 25.Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci 125: 3015–3024, 2012. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stroock AD, Fischbach C. Microfluidic culture models of tumor angiogenesis. Tissue Eng Part A 16: 2143–2146, 2010. doi: 10.1089/ten.tea.2009.0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter AM. Animal models of human placentation—a review. Placenta 28, Suppl: S41–S47, 2007. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Akbari E, Spychalski GB, Song JW. Microfluidic approaches to the study of angiogenesis and the microcirculation. Microcirculation 24: e12363, 2017. doi: 10.1111/micc.12363. [DOI] [PubMed] [Google Scholar]
- 29.Fleischer S, Tavakol DN, Vunjak‐Novakovic G. Engineered microvasculature: from arteries to capillaries: approaches to engineering human vasculature. Adv Funct Mater 30: 2070247, 2020. doi: 10.1002/adfm.202070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gold K, Gaharwar AK, Jain A. Emerging trends in multiscale modeling of vascular pathophysiology: organ-on-a-chip and 3D printing. Biomaterials 196: 2–17, 2019. doi: 10.1016/j.biomaterials.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Intaglietta M, Johnson PC, Winslow RM. Microvascular and tissue oxygen distribution. Cardiovasc Res 32: 632–643, 1996. doi: 10.1016/S0008-6363(96)00110-1. [DOI] [PubMed] [Google Scholar]
- 32.Place TL, Domann FE, Case AJ. Limitations of oxygen delivery to cells in culture: an underappreciated problem in basic and translational research. Free Radic Biol Med 113: 311–322, 2017. doi: 10.1016/j.freeradbiomed.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zakrzewicz A, Secomb TW, Pries AR. Angioadaptation: keeping the vascular system in shape. News Physiol Sci 17: 197–201, 2002. doi: 10.1152/nips.01395.2001. [DOI] [PubMed] [Google Scholar]
- 34.Secomb TW, Alberding JP, Hsu R, Dewhirst MW, Pries AR. Angiogenesis: an adaptive dynamic biological patterning problem. PLoS Comput Biol 9: e1002983, 2013. doi: 10.1371/journal.pcbi.1002983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chipperfield AJ, Thanaj M, Scorletti E, Byrne CD, Clough GF. Multi-domain analysis of microvascular flow motion dynamics in NAFLD. Microcirculation 26: e12538, 2019. doi: 10.1111/micc.12538. [DOI] [PubMed] [Google Scholar]
- 36.Frisbee JC, Goodwill AG, Frisbee SJ, Butcher JT, Wu F, Chantler PD. Microvascular perfusion heterogeneity contributes to peripheral vascular disease in metabolic syndrome. J Physiol 594: 2233–2243, 2016. doi: 10.1113/jphysiol.2014.285247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fung YC. Biomechanics: Mechanical Properties of Living Tissues. New York: Springer, 1993, p. 568. [Google Scholar]
- 38.Lam SF, Shirure VS, Chu YE, Soetikno AG, George SC. Microfluidic device to attain high spatial and temporal control of oxygen. PLoS One 13: e0209574, 2018. doi: 10.1371/journal.pone.0209574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pittman RN. Regulation of Tissue Oxygenation (2nd ed.). San Rafael, CA: Morgan & Claypool Life Science Publishers, 2016. [PubMed] [Google Scholar]
- 40.Fung YC, Zweifach BW. Microcirculation: mechanics of blood flow in capillaries. Annu Rev Fluid Mech 3: 189–210, 1971. doi: 10.1146/annurev.fl.03.010171.001201. [DOI] [Google Scholar]
- 41.Granger DN, Senchenkova E. Inflammation and the Microcirculation. San Rafael, CA: Morgan & Claypool Life Science Publishers, 2010, p. 1–87. [PubMed] [Google Scholar]
- 42.Grubb S, Cai C, Hald BO, Khennouf L, Murmu RP, Jensen AG, Fordsmann J, Zambach S, Lauritzen M. Precapillary sphincters maintain perfusion in the cerebral cortex. Nat Commun 11: 395, 2020. doi: 10.1038/s41467-020-14330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao H, Chappell JC. Microvascular bioengineering: a focus on pericytes. J Biol Eng 13: 26, 2019. doi: 10.1186/s13036-019-0158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmid-Schönbein GW, Woo SY, Zweifach BW. Frontiers in Biomechanics. New York: Springer, 1986, p. 395. [Google Scholar]
- 45.Folkman J, Shing Y. Angiogenesis. J Biol Chem 267: 10931–10934, 1992. [PubMed] [Google Scholar]
- 46.Jain RK. Molecular regulation of vessel maturation. Nat Med 9: 685–693, 2003. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 47.Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol 282: C947–C970, 2002. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- 48.Munn LL. Fluid mechanics and transport in tumors. In: Physical Sciences and Engineering Advances in Life Sciences and Oncology, edited by Janmey P, Fletcher D, Gerecht S, Levine R, Mallick P, McCarty O, Munn L, Reinhart-King C.. Cham, Switzerland: Springer International Publishing, 2016, p. 73–88. [Google Scholar]
- 49.Lipowsky HH. Microvascular rheology and hemodynamics. Microcirculation 12: 5–15, 2005. doi: 10.1080/10739680590894966. [DOI] [PubMed] [Google Scholar]
- 50.Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis 11: 109–119, 2008. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wayman M, Errington D. Respiration: gas transfer. Anaesth Intens Care Med 12: 481–484, 2011. doi: 10.1016/j.mpaic.2011.08.008. [DOI] [Google Scholar]
- 52.Endicott Thomas L. Transport of substances in the body Part 2: how oxygen and carbon dioxide are transported in the blood. AMWA J 33: 147–151, 2018. [Google Scholar]
- 53.Lücker A, Weber B, Jenny P. A dynamic model of oxygen transport from capillaries to tissue with moving red blood cells. Am J Physiol Heart Circ Physiol 308: H206–H216, 2015. doi: 10.1152/ajpheart.00447.2014. [DOI] [PubMed] [Google Scholar]
- 54.Dewhirst MW, Secomb TW. Transport of drugs from blood vessels to tumour tissue. Nat Rev Cancer 17: 738–750, 2017. doi: 10.1038/nrc.2017.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pittman RN. Oxygen transport in the microcirculation and its regulation. Microcirculation 20: 117–137, 2013. doi: 10.1111/micc.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res 87: 198–210, 2010. doi: 10.1093/cvr/cvq062. [DOI] [PubMed] [Google Scholar]
- 57.Claesson-Welsh L. Vascular permeability—the essentials. Ups J Med Sci 120: 135–143, 2015. doi: 10.3109/03009734.2015.1064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Engelhard HH, Arnone GD, Mehta AI, Nicholas MK. Biology of the blood-brain and blood-brain tumor barriers. In: Handbook of Brain Tumor Chemotherapy, Molecular Therapeutics, and Immunotherapy, edited by Newton HB. London: Academic Press, 2018, p. 113–125. [Google Scholar]
- 59.Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res 55: 3752–3756, 1995. [PubMed] [Google Scholar]
- 60.Swartz MA, Lund AW. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat Rev Cancer 12: 210–219, 2012. doi: 10.1038/nrc3186. [DOI] [PubMed] [Google Scholar]
- 61.Chang CW, Seibel AJ, Song JW. Application of microscale culture technologies for studying lymphatic vessel biology. Microcirculation 26: e12547, 2019. doi: 10.1111/micc.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akbari E, Spychalski GB, Rangharajan KK, Prakash S, Song JW. Flow dynamics control endothelial permeability in a microfluidic vessel bifurcation model. Lab Chip 18: 1084–1093, 2018. doi: 10.1039/C8LC00130H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Avendano A, Cortes-Medina M, Song JW. Application of 3-D microfluidic models for studying mass transport properties of the tumor interstitial matrix. Front Bioeng Biotechnol 7: 6, 2019. doi: 10.3389/fbioe.2019.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Egginton S, Zhou AL, Brown MD, Hudlická O. Unorthodox angiogenesis in skeletal muscle. Cardiovasc Res 49: 634–646, 2001. doi: 10.1016/S0008-6363(00)00282-0. [DOI] [PubMed] [Google Scholar]
- 65.Kwak SE, Lee JH, Zhang D, Song W. Angiogenesis: focusing on the effects of exercise in aging and cancer. J Exerc Nutrition Biochem 22: 21–26, 2018. doi: 10.20463/jenb.2018.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sierra H, Cordova M, Chen CJ, Rajadhyaksha M. Confocal imaging-guided laser ablation of basal cell carcinomas: an ex vivo study. J Invest Dermatol 135: 612–615, 2015. doi: 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song JW, Munn LL. Fluid forces control endothelial sprouting. Proc Natl Acad Sci USA 108: 15342–15347, 2011. doi: 10.1073/pnas.1105316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rapp BE. Fluids. In: Microfluidics: Modelling, Mechanics and Mathematics. Oxford, UK: Elsevier, 2017, p. 243–263. [Google Scholar]
- 69.Allan PL. Clinical Doppler Ultrasound. Oxford, UK: Elsevier Health Sciences, 2006. [Google Scholar]
- 70.Fukushima T, Azuma T, Matsuzawa T. Numerical analysis of blood flow in the vertebral artery. J Biomech Eng 104: 143–147, 1982. doi: 10.1115/1.3138328. [DOI] [PubMed] [Google Scholar]
- 71.Tovar-Lopez F, Thurgood P, Gilliam C, Nguyen N, Pirogova E, Khoshmanesh K, Baratchi S. A microfluidic system for studying the effects of disturbed flow on endothelial cells. Front Bioeng Biotechnol 7: 81, 2019. doi: 10.3389/fbioe.2019.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han HC. Twisted blood vessels: symptoms, etiology and biomechanical mechanisms. J Vasc Res 49: 185–197, 2012. doi: 10.1159/000335123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nas OF, Kandemirli SG, Erdemli Gursel B, Bilgin C, Korkmaz B, Yolgosteren A, Inecikli MF. Diagnostic utility of superb microvascular imaging in depiction of corkscrew collaterals in Buerger’s disease. J Clin Ultrasound, 2020. doi: 10.1002/jcu.22880. [DOI] [PubMed] [Google Scholar]
- 74.Helisch A, Schaper W. Arteriogenesis: the development and growth of collateral arteries. Microcirculation 10: 83–97, 2003. doi: 10.1080/mic.10.1.83.97. [DOI] [PubMed] [Google Scholar]
- 75.Batra S, Rakusan K. Capillary length, tortuosity, and spacing in rat myocardium during cardiac cycle. Am J Physiol Heart Circ Physiol 263: H1369–H1376, 1992. doi: 10.1152/ajpheart.1992.263.5.H1369. [DOI] [PubMed] [Google Scholar]
- 76.Cheung CY, Zheng Y, Hsu W, Lee ML, Lau QP, Mitchell P, Wang JJ, Klein R, Wong TY. Retinal vascular tortuosity, blood pressure, and cardiovascular risk factors. Ophthalmology 118: 812–818, 2011. doi: 10.1016/j.ophtha.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 77.Mathieu-Costello O, Potter RF, Ellis CG, Groom AC. Capillary configuration and fiber shortening in muscles of the rat hindlimb: correlation between corrosion casts and stereological measurements. Microvasc Res 36: 40–55, 1988. doi: 10.1016/0026-2862(88)90037-4. [DOI] [PubMed] [Google Scholar]
- 78.Thore CR, Anstrom JA, Moody DM, Challa VR, Marion MC, Brown WR. Morphometric analysis of arteriolar tortuosity in human cerebral white matter of preterm, young, and aged subjects. J Neuropathol Exp Neurol 66: 337–345, 2007. doi: 10.1097/nen.0b013e3180537147. [DOI] [PubMed] [Google Scholar]
- 79.Whitley GS, Cartwright JE. Cellular and molecular regulation of spiral artery remodelling: lessons from the cardiovascular field. Placenta 31: 465–474, 2010. doi: 10.1016/j.placenta.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hutchins PM, Marshburn TH, Maultsby SJ, Lynch CD, Smith TL, Dusseau JW. Long-term microvascular response to hydralazine in spontaneously hypertensive rats. Hypertension 12: 74–79, 1988. doi: 10.1161/01.HYP.12.1.74. [DOI] [PubMed] [Google Scholar]
- 81.Owen CG, Newsom RS, Rudnicka AR, Barman SA, Woodward EG, Ellis TJ. Diabetes and the tortuosity of vessels of the bulbar conjunctiva. Ophthalmology 115: e27–e32, 2008. doi: 10.1016/j.ophtha.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 82.Vakoc BJ, Lanning RM, Tyrrell JA, Padera TP, Bartlett LA, Stylianopoulos T, Munn LL, Tearney GJ, Fukumura D, Jain RK, Bouma BE. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat Med 15: 1219–1223, 2009. doi: 10.1038/nm.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Germano M. On the effect of torsion on a helical pipe flow. J Fluid Mech 125: 1–8, 1982. doi: 10.1017/S0022112082003206. [DOI] [Google Scholar]
- 84.Wang C. On the low-Reynolds-number flow in a helical pipe. J Fluid Mech 108: 185–194, 1981. doi: 10.1017/S0022112081002073. [DOI] [Google Scholar]
- 85.Jain RK. Determinants of tumor blood flow: a review. Cancer Res 48: 2641–2658, 1988. [PubMed] [Google Scholar]
- 86.Pries AR, Höpfner M, le Noble F, Dewhirst MW, Secomb TW. The shunt problem: control of functional shunting in normal and tumour vasculature. Nat Rev Cancer 10: 587–593, 2010. doi: 10.1038/nrc2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pries AR, Reglin B, Secomb TW. Remodeling of blood vessels: responses of diameter and wall thickness to hemodynamic and metabolic stimuli. Hypertension 46: 725–731, 2005. doi: 10.1161/01.HYP.0000184428.16429.be. [DOI] [PubMed] [Google Scholar]
- 88.Reglin B, Secomb TW, Pries AR. Structural adaptation of microvessel diameters in response to metabolic stimuli: where are the oxygen sensors? Am J Physiol Heart Circ Physiol 297: H2206–H2219, 2009. doi: 10.1152/ajpheart.00348.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bagchi P, Johnson PC, Popel AS. Computational fluid dynamic simulation of aggregation of deformable cells in a shear flow. J Biomech Eng 127: 1070–1080, 2005. doi: 10.1115/1.2112907. [DOI] [PubMed] [Google Scholar]
- 90.Goldman D, Popel AS. A computational study of the effect of capillary network anastomoses and tortuosity on oxygen transport. J Theor Biol 206: 181–194, 2000. doi: 10.1006/jtbi.2000.2113. [DOI] [PubMed] [Google Scholar]
- 91.Goldman D, Popel AS. A computational study of the effect of vasomotion on oxygen transport from capillary networks. J Theor Biol 209: 189–199, 2001. doi: 10.1006/jtbi.2000.2254. [DOI] [PubMed] [Google Scholar]
- 92.Kim E, Stamatelos S, Cebulla J, Bhujwalla ZM, Popel AS, Pathak AP. Multiscale imaging and computational modeling of blood flow in the tumor vasculature. Ann Biomed Eng 40: 2425–2441, 2012. doi: 10.1007/s10439-012-0585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsoukias NM, Goldman D, Vadapalli A, Pittman RN, Popel AS. A computational model of oxygen delivery by hemoglobin-based oxygen carriers in three-dimensional microvascular networks. J Theor Biol 248: 657–674, 2007. doi: 10.1016/j.jtbi.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang J, Johnson PC, Popel AS. Red blood cell aggregation and dissociation in shear flows simulated by lattice Boltzmann method. J Biomech 41: 47–55, 2008. doi: 10.1016/j.jbiomech.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cattaneo L, Zunino P. A computational model of drug delivery through microcirculation to compare different tumor treatments. Int J Numer Method Biomed Eng 30: 1347–1371, 2014. doi: 10.1002/cnm.2661. [DOI] [PubMed] [Google Scholar]
- 96.Fredrich T, Welter M, Rieger H. Dynamic vessel adaptation in synthetic arteriovenous networks. J Theor Biol 483: 109989, 2019. doi: 10.1016/j.jtbi.2019.109989. [DOI] [PubMed] [Google Scholar]
- 97.Freund JB, Shapiro B. Transport of particles by magnetic forces and cellular blood flow in a model microvessel. Phys Fluids 24: 051904, 2012. doi: 10.1063/1.4718752. [DOI] [Google Scholar]
- 98.Gardiner BS, Thompson SL, Ngo JP, Smith DW, Abdelkader A, Broughton BR, Bertram JF, Evans RG. Diffusive oxygen shunting between vessels in the preglomerular renal vasculature: anatomic observations and computational modeling. Am J Physiol Renal Physiol 303: F605–F618, 2012. doi: 10.1152/ajprenal.00186.2012. [DOI] [PubMed] [Google Scholar]
- 99.Kim M, Gillies RJ, Rejniak KA. Current advances in mathematical modeling of anti-cancer drug penetration into tumor tissues. Front Oncol 3: 278, 2013. doi: 10.3389/fonc.2013.00278. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kumar A, Henríquez Rivera RG, Graham MD. Flow-induced segregation in confined multicomponent suspensions: effects of particle size and rigidity. J Fluid Mech 738: 423–462, 2014. doi: 10.1017/jfm.2013.592. [DOI] [Google Scholar]
- 101.Welter M, Fredrich T, Rinneberg H, Rieger H. Computational model for tumor oxygenation applied to clinical data on breast tumor hemoglobin concentrations suggests vascular dilatation and compression. PLoS One 11: e0161267, 2016. doi: 10.1371/journal.pone.0161267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Welter M, Rieger H. Interstitial fluid flow and drug delivery in vascularized tumors: a computational model. PLoS One 8: e70395, 2013. doi: 10.1371/journal.pone.0070395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Welter M, Rieger H. Physical determinants of vascular network remodeling during tumor growth. Eur Phys J E 33: 149–163, 2010. doi: 10.1140/epje/i2010-10611-6. [DOI] [PubMed] [Google Scholar]
- 104.Kim S, Lee H, Chung M, Jeon NL. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 13: 1489–1500, 2013. [Erratum in Lab Chip 13: 4891, 2013]. doi: 10.1039/c3lc41320a. [DOI] [PubMed] [Google Scholar]
- 105.Kosyakova N, Kao DD, Figetakis M, López-Giráldez F, Spindler S, Graham M, James KJ, Won Shin J, Liu X, Tietjen GT, Pober JS, Chang WG. Differential functional roles of fibroblasts and pericytes in the formation of tissue-engineered microvascular networks in vitro. NPJ Regen Med 5: 1, 2020. doi: 10.1038/s41536-019-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Paek J, Park SE, Lu Q, Park KT, Cho M, Oh JM, Kwon KW, Yi YS, Song JW, Edelstein HI, Ishibashi J, Yang W, Myerson JW, Kiseleva RY, Aprelev P, Hood ED, Stambolian D, Seale P, Muzykantov VR, Huh D. Microphysiological engineering of self-assembled and perfusable microvascular beds for the production of vascularized three-dimensional human microtissues. ACS Nano 13: 7627–7643, 2019. doi: 10.1021/acsnano.9b00686. [DOI] [PubMed] [Google Scholar]
- 107.van der Meer AD, Orlova VV, ten Dijke P, van den Berg A, Mummery CL. Three-dimensional co-cultures of human endothelial cells and embryonic stem cell-derived pericytes inside a microfluidic device. Lab Chip 13: 3562–3568, 2013. doi: 10.1039/c3lc50435b. [DOI] [PubMed] [Google Scholar]
- 108.van Dijk CG, Brandt MM, Poulis N, Anten J, van der Moolen M, Kramer L, Homburg EF, Louzao-Martinez L, Pei J, Krebber MM, van Balkom BW, de Graaf P, Duncker DJ, Verhaar MC, Luttge R, Cheng C. New microfluidic model that allows monitoring of complex vascular structures and cell interactions in a 3D biological matrix. Lab Chip 20: 1827–1844, 2020. doi: 10.1039/D0LC00059K. [DOI] [PubMed] [Google Scholar]
- 109.Baker BM, Trappmann B, Stapleton SC, Toro E, Chen CS. Microfluidics embedded within extracellular matrix to define vascular architectures and pattern diffusive gradients. Lab Chip 13: 3246–3252, 2013. doi: 10.1039/c3lc50493j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bischel LL, Young EW, Mader BR, Beebe DJ. Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials 34: 1471–1477, 2013. doi: 10.1016/j.biomaterials.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chang CW, Seibel AJ, Avendano A, Cortes-Medina MG, Song JW. Distinguishing specific CXCL12 isoforms on their angiogenesis and vascular permeability promoting properties. Adv Healthcare Mater 9: e1901399, 2020. doi: 10.1002/adhm.201901399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang WY, Lin D, Jarman EH, Polacheck WJ, Baker BM. Functional angiogenesis requires microenvironmental cues balancing endothelial cell migration and proliferation. Lab Chip 20: 1153–1166, 2020. doi: 10.1039/C9LC01170F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Duinen V, Zhu D, Ramakers C, van Zonneveld AJ, Vulto P, Hankemeier T. Perfused 3D angiogenic sprouting in a high-throughput in vitro platform. Angiogenesis 22: 157–165, 2019. doi: 10.1007/s10456-018-9647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ayuso JM, Virumbrales-Munoz M, McMinn PH, Rehman S, Gomez I, Karim MR, Trusttchel R, Wisinski KB, Beebe DJ, Skala MC. Tumor-on-a-chip: a microfluidic model to study cell response to environmental gradients. Lab Chip 19: 3461–3471, 2019. doi: 10.1039/C9LC00270G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mathur T, Singh KA, Pandian NK, Tsai SH, Hein TW, Gaharwar AK, Flanagan JM, Jain A. Organ-on-chips made of blood: endothelial progenitor cells from blood reconstitute vascular thromboinflammation in vessel-chips. Lab Chip 19: 2500–2511, 2019. doi: 10.1039/C9LC00469F. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shelby JP, White J, Ganesan K, Rathod PK, Chiu DT. A microfluidic model for single-cell capillary obstruction by Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci USA 100: 14618–14622, 2003. doi: 10.1073/pnas.2433968100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Toh YC, Raja A, Yu H, van Noort D. A 3D microfluidic model to recapitulate cancer cell migration and invasion. Bioengineering (Basel) 5: 29, 2018. doi: 10.3390/bioengineering5020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nguyen DH, Stapleton SC, Yang MT, Cha SS, Choi CK, Galie PA, Chen CS. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc Natl Acad Sci USA 110: 6712–6717, 2013. doi: 10.1073/pnas.1221526110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, López JA, Stroock AD. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci USA 109: 9342–9347, 2012. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sato M, Sasaki N, Ato M, Hirakawa S, Sato K, Sato K. Microcirculation-on-a-chip: a microfluidic platform for assaying blood- and lymphatic-vessel permeability. PLoS One 10: e0137301, 2015. doi: 10.1371/journal.pone.0137301. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thomas A, Wang S, Sohrabi S, Orr C, He R, Shi W, Liu Y. Characterization of vascular permeability using a biomimetic microfluidic blood vessel model. Biomicrofluidics 11: 024102, 2017. doi: 10.1063/1.4977584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, Kamm RD. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc Natl Acad Sci USA 112: 214–219, 2015. [Erratum in Proc Natl Acad Sci USA 112: E818, 2015]. doi: 10.1073/pnas.1417115112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nagaraju S, Truong D, Mouneimne G, Nikkhah M. Microfluidic Tumor-vascular model to study breast cancer cell invasion and intravasation. Adv Healthc Mater 7: e1701257, 2018. doi: 10.1002/adhm.201701257. [DOI] [PubMed] [Google Scholar]
- 124.Young EW, Beebe DJ. Fundamentals of microfluidic cell culture in controlled microenvironments. Chem Soc Rev 39: 1036–1048, 2010. doi: 10.1039/b909900j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gale B, Jafek A, Lambert C, Goenner B, Moghimifam H, Nze U, Kamarapu S. A review of current methods in microfluidic device fabrication and future commercialization prospects. Inventions 3: 60, 2018. doi: 10.3390/inventions3030060. [DOI] [Google Scholar]
- 126.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in biology and biochemistry. Annu Rev Biomed Eng 3: 335–373, 2001. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 127.Kuo JS, Chiu DT. Controlling mass transport in microfluidic devices. Annu Rev Anal Chem (Palo Alto Calif) 4: 275–296, 2011. doi: 10.1146/annurev-anchem-061010-113926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mannino RG, Myers DR, Ahn B, Wang Y, Rollins M, Gole H, Lin AS, Guldberg RE, Giddens DP, Timmins LH, Lam WA. Do-it-yourself in vitro vasculature that recapitulates in vivo geometries for investigating endothelial-blood cell interactions. Sci Rep 5: 12401, 2015. doi: 10.1038/srep12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Westein E, van der Meer AD, Kuijpers MJ, Frimat JP, van den Berg A, Heemskerk JW. Atherosclerotic geometries exacerbate pathological thrombus formation poststenosis in a von Willebrand factor-dependent manner. Proc Natl Acad Sci USA 110: 1357–1362, 2013. doi: 10.1073/pnas.1209905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Young EW, Simmons CA. Macro- and microscale fluid flow systems for endothelial cell biology. Lab Chip 10: 143–160, 2010. doi: 10.1039/B913390A. [DOI] [PubMed] [Google Scholar]
- 131.Regehr KJ, Domenech M, Koepsel JT, Carver KC, Ellison-Zelski SJ, Murphy WL, Schuler LA, Alarid ET, Beebe DJ. Biological implications of polydimethylsiloxane-based microfluidic cell culture. Lab Chip 9: 2132–2139, 2009. doi: 10.1039/b903043c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Moore TA, Brodersen P, Young EW. Multiple myeloma cell drug responses differ in thermoplastic vs PDMS microfluidic devices. Anal Chem 89: 11391–11398, 2017. doi: 10.1021/acs.analchem.7b02351. [DOI] [PubMed] [Google Scholar]
- 133.Campbell SB, Wu Q, Yazbeck J, Liu C, Okhovatian S, Radisic M. Beyond polydimethylsiloxane: alternative materials for fabrication of organ-on-a-chip devices and microphysiological systems. ACS Biomater Sci Eng, 2020. doi: 10.1021/acsbiomaterials.0c00640. [DOI] [PubMed] [Google Scholar]
- 134.Chung HH, Mireles M, Kwarta BJ, Gaborski TR. Use of porous membranes in tissue barrier and co-culture models. Lab Chip 18: 1671–1689, 2018. doi: 10.1039/C7LC01248A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Song JW, Cavnar SP, Walker AC, Luker KE, Gupta M, Tung YC, Luker GD, Takayama S. Microfluidic endothelium for studying the intravascular adhesion of metastatic breast cancer cells. PLoS One 4: e5756, 2009. doi: 10.1371/journal.pone.0005756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bogorad MI, DeStefano J, Karlsson J, Wong AD, Gerecht S, Searson PC. Review: in vitro microvessel models. Lab Chip 15: 4242–4255, 2015. doi: 10.1039/C5LC00832H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Song JW, Daubriac J, Tse JM, Bazou D, Munn LL. RhoA mediates flow-induced endothelial sprouting in a 3-D tissue analogue of angiogenesis. Lab Chip 12: 5000–5006, 2012. doi: 10.1039/c2lc40389g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vickerman V, Blundo J, Chung S, Kamm R. Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Lab Chip 8: 1468–1477, 2008. doi: 10.1039/b802395f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Polacheck WJ, Kutys ML, Tefft JB, Chen CS. Microfabricated blood vessels for modeling the vascular transport barrier. Nat Protoc 14: 1425–1454, 2019. doi: 10.1038/s41596-019-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res 71: 185–196, 2006. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 141.Bischel LL, Lee SH, Beebe DJ. A practical method for patterning lumens through ECM hydrogels via viscous finger patterning. J Lab Autom 17: 96–103, 2012. doi: 10.1177/2211068211426694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jiménez-Torres JA, Peery SL, Sung KE, Beebe DJ. LumeNEXT: a practical method to pattern luminal structures in ECM gels. Adv Healthc Mater 5: 198–204, 2016. doi: 10.1002/adhm.201500608. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Choi NW, Cabodi M, Held B, Gleghorn JP, Bonassar LJ, Stroock AD. Microfluidic scaffolds for tissue engineering. Nat Mater 6: 908–915, 2007. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- 144.Qiu Y, Ahn B, Sakurai Y, Hansen CE, Tran R, Mimche PN, Mannino RG, Ciciliano JC, Lamb TJ, Joiner CH, Ofori-Acquah SF, Lam WA. Microvasculature-on-a-chip for the long-term study of endothelial barrier dysfunction and microvascular obstruction in disease. Nat Biomed Eng 2: 453–463, 2018. doi: 10.1038/s41551-018-0224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mandrycky C, Hadland B, Zheng Y. 3D curvature-instructed endothelial flow response and tissue vascularization. Sci Adv 6: eabb3629, 2020. doi: 10.1126/sciadv.abb3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ma X, Liu J, Zhu W, Tang M, Lawrence N, Yu C, Gou M, Chen S. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv Drug Deliv Rev 132: 235–251, 2018. doi: 10.1016/j.addr.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Grigoryan B, Paulsen SJ, Corbett DC, Sazer DW, Fortin CL, Zaita AJ, Greenfield PT, Calafat NJ, Gounley JP, Ta AH, Johansson F, Randles A, Rosenkrantz JE, Louis-Rosenberg JD, Galie PA, Stevens KR, Miller JS. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 364: 458–464, 2019. doi: 10.1126/science.aav9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A, Lewis JA. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep 6: 34845, 2016. doi: 10.1038/srep34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee A, Hudson AR, Shiwarski DJ, Tashman JW, Hinton TJ, Yerneni S, Bliley JM, Campbell PG, Feinberg AW. 3D bioprinting of collagen to rebuild components of the human heart. Science 365: 482–487, 2019. doi: 10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- 150.Liu W, Zhong Z, Hu N, Zhou Y, Maggio L, Miri AK, Fragasso A, Jin X, Khademhosseini A, Zhang YS. Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments. Biofabrication 10: 024102, 2018. doi: 10.1088/1758-5090/aa9d44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, Toro E, Chen AA, Galie PA, Yu X, Chaturvedi R, Bhatia SN, Chen CS. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater 11: 768–774, 2012. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M. 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact Mater 3: 144–156, 2018. doi: 10.1016/j.bioactmat.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kacarevic ZP, Rider PM, Alkildani S, Retnasingh S, Smeets R, Jung O, Ivanisevic Z, Barbeck M. An introduction to 3D bioprinting: possibilities, challenges and future aspects. Materials (Basel) 11: 2199, 2018. doi: 10.3390/ma11112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li J, Chen M, Fan X, Zhou H. Recent advances in bioprinting techniques: approaches, applications and future prospects. J Transl Med 14: 271, 2016. doi: 10.1186/s12967-016-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci USA 113: 3179–3184, 2016. doi: 10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CC. The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol Biol Cell 22: 3791–3800, 2011. doi: 10.1091/mbc.e11-05-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Moya ML, Hsu YH, Lee AP, Hughes CC, George SC. In vitro perfused human capillary networks. Tissue Eng Part C Methods 19: 730–737, 2013. doi: 10.1089/ten.tec.2012.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Byrne MB, Leslie MT, Gaskins HR, Kenis PJ. Methods to study the tumor microenvironment under controlled oxygen conditions. Trends Biotechnol 32: 556–563, 2014. doi: 10.1016/j.tibtech.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bakmiwewa SM, Heng B, Guillemin GJ, Ball HJ, Hunt NH. An effective, low-cost method for achieving and maintaining hypoxia during cell culture studies. Biotechniques 59: 223–224, 2015. doi: 10.2144/000114341. [DOI] [PubMed] [Google Scholar]
- 160.Wang L, Liu W, Wang Y, Wang JC, Tu Q, Liu R, Wang J. Construction of oxygen and chemical concentration gradients in a single microfluidic device for studying tumor cell-drug interactions in a dynamic hypoxia microenvironment. Lab Chip 13: 695–705, 2013. doi: 10.1039/C2LC40661F. [DOI] [PubMed] [Google Scholar]
- 161.Chang CW, Cheng YJ, Tu M, Chen YH, Peng CC, Liao WH, Tung YC. A polydimethylsiloxane-polycarbonate hybrid microfluidic device capable of generating perpendicular chemical and oxygen gradients for cell culture studies. Lab Chip 14: 3762–3772, 2014. doi: 10.1039/C4LC00732H. [DOI] [PubMed] [Google Scholar]
- 162.Olmedo-Suárez MÁ, Sekiguchi T, Takano A, Cañizares-Macías MP, Futai N. Integrated on-chip 3D vascular network culture under hypoxia. Micromachines (Basel) 11: 475, 2020. doi: 10.3390/mi11050475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Funamoto K, Yoshino D, Matsubara K, Zervantonakis IK, Funamoto K, Nakayama M, Masamune J, Kimura Y, Kamm RD. Endothelial monolayer permeability under controlled oxygen tension. Integr Biol (Camb) 9: 529–538, 2017. doi: 10.1039/C7IB00068E. [DOI] [PubMed] [Google Scholar]
- 164.Jomezadeh Kheibary N, Abolfazli Esfahani J, Mousavi Shaegh SA. Analysis of oxygen transport in microfluidic bioreactors for cell culture and organ‐on‐chip applications. Eng Rep 2: e12062, 2020. doi: 10.1002/eng2.12062. [DOI] [Google Scholar]
- 165.Hsu YH, Moya ML, Abiri P, Hughes CC, George SC, Lee AP. Full range physiological mass transport control in 3D tissue cultures. Lab Chip 13: 81–89, 2013. doi: 10.1039/C2LC40787F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kuzmic N, Moore T, Devadas D, Young EW. Modelling of endothelial cell migration and angiogenesis in microfluidic cell culture systems. Biomech Model Mechanobiol 18: 717–731, 2019. doi: 10.1007/s10237-018-01111-3. [DOI] [PubMed] [Google Scholar]
- 167.Connor AJ, Nowak RP, Lorenzon E, Thomas M, Herting F, Hoert S, Quaiser T, Shochat E, Pitt-Francis J, Cooper J, Maini PK, Byrne HM. An integrated approach to quantitative modelling in angiogenesis research. J R Soc Interface 12: 546, 2015. doi: 10.1098/rsif.2015.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gamba A, Ambrosi D, Coniglio A, de Candia A, Di Talia S, Giraudo E, Serini G, Preziosi L, Bussolino F. Percolation, morphogenesis, and burgers dynamics in blood vessels formation. Phys Rev Lett 90: 118101, 2003. doi: 10.1103/PhysRevLett.90.118101. [DOI] [PubMed] [Google Scholar]
- 169.Hughes CC. Endothelial–stromal interactions in angiogenesis. Curr Opin Hematol 15: 204–209, 2008. doi: 10.1097/MOH.0b013e3282f97dbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huh D, Torisawa YS, Hamilton GA, Kim HJ, Ingber DE. Microengineered physiological biomimicry: organs-on-chips. Lab Chip 12: 2156–2164, 2012. doi: 10.1039/c2lc40089h. [DOI] [PubMed] [Google Scholar]
- 171.Sakolish CM, Esch MB, Hickman JJ, Shuler ML, Mahler GJ. Modeling barrier tissues in vitro: methods, achievements, and challenges. EBioMedicine 5: 30–39, 2016. doi: 10.1016/j.ebiom.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yu F, Selva Kumar ND, Choudhury D, Foo LC, Ng SH. Microfluidic platforms for modeling biological barriers in the circulatory system. Drug Discov Today 23: 815–829, 2018. doi: 10.1016/j.drudis.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 173.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol 32: 760–772, 2014. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 174.Jain A, Barrile R, van der Meer AD, Mammoto A, Mammoto T, De Ceunynck K, Aisiku O, Otieno MA, Louden CS, Hamilton GA, Flaumenhaft R, Ingber DE. Primary human lung alveolus-on-a-chip model of intravascular thrombosis for assessment of therapeutics. Clin Pharmacol Ther 103: 332–340, 2018. doi: 10.1002/cpt.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Moya ML, Triplett M, Simon M, Alvarado J, Booth R, Osburn J, Soscia D, Qian F, Fischer NO, Kulp K, Wheeler EK. A reconfigurable in vitro model for studying the blood-brain barrier. Ann Biomed Eng 48: 780–793, 2020. doi: 10.1007/s10439-019-02405-y. [DOI] [PubMed] [Google Scholar]
- 176.Rayner SG, Phong KT, Xue J, Lih D, Shankland SJ, Kelly EJ, Himmelfarb J, Zheng Y. Reconstructing the human renal vascular-tubular unit in vitro. Adv Healthc Mater 7: e1801120, 2018. doi: 10.1002/adhm.201801120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yin F, Zhu Y, Zhang M, Yu H, Chen W, Qin J. A 3D human placenta-on-a-chip model to probe nanoparticle exposure at the placental barrier. Toxicol In Vitro 54: 105–113, 2019. doi: 10.1016/j.tiv.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 178.Ahn SI, Sei YJ, Park HJ, Kim J, Ryu Y, Choi JJ, Sung HJ, MacDonald TJ, Levey AI, Kim Y. Microengineered human blood-brain barrier platform for understanding nanoparticle transport mechanisms. Nat Commun 11: 175, 2020. doi: 10.1038/s41467-019-13896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Campisi M, Shin Y, Osaki T, Hajal C, Chiono V, Kamm RD. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 180: 117–129, 2018. doi: 10.1016/j.biomaterials.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cho H, Seo JH, Wong KH, Terasaki Y, Park J, Bong K, Arai K, Lo EH, Irimia D. Three-dimensional blood-brain barrier model for in vitro studies of neurovascular pathology. Sci Rep 5: 15222, 2015. doi: 10.1038/srep15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Elbakary B, Badhan RK. A dynamic perfusion based blood-brain barrier model for cytotoxicity testing and drug permeation. Sci Rep 10: 3788, 2020. doi: 10.1038/s41598-020-60689-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lee S, Chung M, Lee SR, Jeon NL. 3D brain angiogenesis model to reconstitute functional human blood-brain barrier in vitro. Biotechnol Bioeng 117: 748–762, 2020. doi: 10.1002/bit.27224. [DOI] [PubMed] [Google Scholar]
- 183.Prabhakarpandian B, Shen MC, Nichols JB, Mills IR, Sidoryk-Wegrzynowicz M, Aschner M, Pant K. SyM-BBB: a microfluidic blood brain barrier model. Lab Chip 13: 1093–1101, 2013. doi: 10.1039/c2lc41208j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pulgar VM. Transcytosis to cross the blood brain barrier. New advancements and challenges. Front Neurosci 12: 1019, 2018. doi: 10.3389/fnins.2018.01019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol 75: 593–615, 2013. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- 186.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science 328: 1662–1668, 2010. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, Hamilton GA, Thorneloe KS, McAlexander MA, Ingber DE. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med 4: 159ra147, 2012. doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jourde-Chiche N, Fakhouri F, Dou L, Bellien J, Burtey S, Frimat M, Jarrot PA, Kaplanski G, Le Quintrec M, Pernin V, Rigothier C, Sallée M, Fremeaux-Bacchi V, Guerrot D, Roumenina LT. Endothelium structure and function in kidney health and disease. Nat Rev Nephrol 15: 87–108, 2019. doi: 10.1038/s41581-018-0098-z. [DOI] [PubMed] [Google Scholar]
- 189.Pennington KA, Schlitt JM, Jackson DL, Schulz LC, Schust DJ. Preeclampsia: multiple approaches for a multifactorial disease. Dis Model Mech 5: 9–18, 2012. doi: 10.1242/dmm.008516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Leach L. The phenotype of the human materno-fetal endothelial barrier: molecular occupancy of paracellular junctions dictate permeability and angiogenic plasticity. J Anat 200: 599–606, 2002. doi: 10.1046/j.1469-7580.2002.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Blundell C, Yi YS, Ma L, Tess ER, Farrell MJ, Georgescu A, Aleksunes LM, Huh D. Placental drug transport-on-a-chip: a microengineered in vitro model of transporter-mediated drug efflux in the human placental barrier. Adv Healthc Mater 7: 10.1002/adhm.201700786, 2018. doi: 10.1002/adhm.201700786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shi H, Magaye R, Castranova V, Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol 10: 15, 2013. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Arifin DR, Palmer AF. Determination of size distribution and encapsulation efficiency of liposome‐encapsulated hemoglobin blood substitutes using asymmetric flow field‐flow fractionation coupled with multi‐angle static light scattering. Biotechnol Prog 19: 1798–1811, 2003. doi: 10.1021/bp034120x. [DOI] [PubMed] [Google Scholar]
- 194.Cuddington C, Moses S, Belcher D, Ramesh N, Palmer A. Next-generation polymerized human hemoglobins in hepatic bioreactor simulations. Biotechnol Prog 36: e2958, 2020. doi: 10.1002/btpr.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yu J, Berthier E, Craig A, de Groot TE, Sparks S, Ingram PN, Jarrard DF, Huang W, Beebe DJ, Theberge AB. Reconfigurable open microfluidics for studying the spatiotemporal dynamics of paracrine signalling. Nat Biomed Eng 3: 830–841, 2019. doi: 10.1038/s41551-019-0421-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kaliviotis E, Sherwood JM, Balabani S. Local viscosity distribution in bifurcating microfluidic blood flows. Phys Fluids 30: 030706, 2018. doi: 10.1063/1.5011373. [DOI] [Google Scholar]
- 197.Omori T, Imai Y, Kikuchi K, Ishikawa T, Yamaguchi T. Hemodynamics in the microcirculation and in microfluidics. Ann Biomed Eng 43: 238–257, 2015. doi: 10.1007/s10439-014-1180-8. [DOI] [PubMed] [Google Scholar]
- 198.Shen Z, Coupier G, Kaoui B, Polack B, Harting J, Misbah C, Podgorski T. Inversion of hematocrit partition at microfluidic bifurcations. Microvasc Res 105: 40–46, 2016. doi: 10.1016/j.mvr.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 199.Stauber H, Waisman D, Korin N, Sznitman J. Red blood cell dynamics in biomimetic microfluidic networks of pulmonary alveolar capillaries. Biomicrofluidics 11: 014103, 2017. doi: 10.1063/1.4973930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Thomas A, Tan J, Liu Y. Characterization of nanoparticle delivery in microcirculation using a microfluidic device. Microvasc Res 94: 17–27, 2014. doi: 10.1016/j.mvr.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Akbari E, Spychalski GB, Rangharajan KK, Prakash S, Song JW. Competing fluid forces control endothelial sprouting in a 3-D microfluidic vessel bifurcation model. Micromachines (Basel) 10: 451, 2019. doi: 10.3390/mi10070451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Song JW, Bazou D, Munn LL. Anastomosis of endothelial sprouts forms new vessels in a tissue analogue of angiogenesis. Integr Biol (Camb) 4: 857–862, 2012. doi: 10.1039/c2ib20061a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Vickerman V, Kamm RD. Mechanism of a flow-gated angiogenesis switch: early signaling events at cell-matrix and cell-cell junctions. Integr Biol (Camb) 4: 863–874, 2012. doi: 10.1039/c2ib00184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Forouzan O, Yang X, Sosa JM, Burns JM, Shevkoplyas SS. Spontaneous oscillations of capillary blood flow in artificial microvascular networks. Microvasc Res 84: 123–132, 2012. doi: 10.1016/j.mvr.2012.06.006. [DOI] [PubMed] [Google Scholar]