Abstract
Spag6 encodes an axoneme central apparatus protein that is required for normal flagellar and cilia motility. Recent findings suggest that Spag6 also plays a role in ciliogenesis, orientation of cilia basal feet, and planar polarity. Sensory cells of the inner ear display unique structural features that underlie their mechanosensitivity. They represent a distinctive form of cellular polarity, known as planar cell polarity (PCP). However, a role for Spag6 in the inner ear has not yet been explored. In the present study, the function of Spag6 in the inner ear was examined using Spag6-deficient mice. Our results demonstrate hearing loss in the Spag6 mutants, associated with abnormalities in cellular patterning, cell shape, stereocilia bundles, and basal bodies, as well as abnormally distributed Frizzled class receptor 6 (FZD6), suggesting that Spag6 participates in PCP regulation. Moreover, we found that the subapical microtubule meshwork was disrupted. Our observations suggest new functions for Spag6 in hearing and PCP in the inner ear.
Keywords: hair cell, hearing loss, inner ear, polarity, sperm-associated antigen 6
INTRODUCTION
The mammalian auditory sensory epithelium, the organ of Corti (OC) of the cochlea, is composed of three rows of outer hair cells (OHCs), a single row of inner hair cells (IHCs), and several types of interdigitating nonsensory supporting cells. These cells are characterized by unique morphologies, specializations of their actin and microtubule cytoskeletons, and complexity in cytoarchitectural organization as an epithelium (1). The hair cells are interdigitated with supporting cells (SCs) in the OC, forming a checkerboard-like cellular pattern. The actin-based stereocilia form a bilaterally symmetrical “V”-shaped stereocilia bundle, and microtubule-based kinocilium localize at the vertex of the “V”-shaped stereocilia bundle (2, 3), which is crucial for determining the orientation and morphology of the bundle. The uniform orientation of hair bundles at the surface of the OC—the vertex of each bundle pointing laterally—defines planar cell polarity (PCP) at the tissue level (4, 5). In addition, hair cells show cell-intrinsic polarity at the subcellular level, manifested as normally oriented bundles coupled with asymmetric bundle morphology. Studies have shown that the cytoskeleton elements, microtubules, and actin, are required for asymmetric subcellular localization of core PCP proteins, and keep basal bodies in a proper station within a cytoskeleton network below the surface in mouse tracheal epithelial cells, indicating important roles for cytoskeletal elements in PCP regulation (6).
The mouse sperm-associated antigen 6 (Spag6) gene, the ortholog of Chlamydomonas PF16, encodes a 55.3-kDa polypeptide that is a component of the axoneme central apparatus of flagella and motile cilia. It interacts with the central pair microtubules and is required for their stability in the axoneme (7, 8). Spag6 knockout leads to instability of microtubules in the axoneme and cilia/flagella dysfunction. Approximately 50% of Spag6-deficient mice die from hydrocephalus before adulthood because of abnormal function of ependymal cell cilia. Males surviving to maturity are infertile due to impaired sperm motility and disorganized flagella structures (8–11). Spag6 has been reported to play a role in the central nervous system of chickens and mice (8, 11, 12), the respiratory tract (11, 13), and various cancers (14–16). The expression of Spag6 in many tissues implies a broader function of the gene beyond its role in the axoneme central apparatus. Recent findings suggest that Spag6 also plays a role in ciliogenesis, orientation of cilia basal feet, and planar polarity (17).
Given the above information regarding Spag6, it is conceivable that Spag6 plays roles affecting the structure and/or function of the hair cells and support cells in the inner ear. To explore this possibility, we characterized auditory epithelial hair cell structure and function in Spag6 knockout mice.
ANIMALS AND METHODS
Genetically Modified Mice
Spag6 mutant mice were previously generated. Mice used in these studies had a 129/SvJ/C57BL/6J genetic background (8). To obtain homozygous (Spag6−/−) and wild-type (Spag6+/+) mice, male and female heterozygous mice were crossed, and offspring of these mice were genotyped by PCR, as described previously (8). Mice of either sex were used in this study. All animal procedures were performed according to protocols approved by the Ethics Committee of the Shandong Provincial ENT Hospital, Cheeloo College of Medicine, Shandong University.
Antibodies
The antibodies used were antiacetylated-α-tubulin (marker for acetylated microtubules, 1:100, mouse, Abcam; ab24610), Pericentrin (marker for basal bodies, 1:200, rabbit, Abcam; ab4448), FZD6 (1:100, mouse, R&D Systems; AF1526), Spag6 (1:100, rabbit, Abcam; Ab155653), Myosin 7a (marker for hair cells, 1:500, mouse, DSHB, myo7a 138-1), Dylight405 (1:100, EarthOx Life Science, Millbrae, CA; E032110-01), Dylight649 (1:100, EarthOx Life Science, Millbrae; E032620-01), and Alexa Fluor 488 phalloidin (1:1000; Invitrogen).
Auditory Brainstem Response and Cochlear Microphonics Recordings
Auditory brainstem response (ABR) and cochlear microphonics (CM) recordings were performed in a double-wall, sound-isolated chamber using a Tucker-Davis ABR workstation (Tucker-Davis Tech. Alachua, FL) (18). Mice of either sex (Spag6+/+, n = 9; Spag6−/−, n = 7) were anesthetized by intraperitoneal injection with 10% chloral hydrate (0.004 L/kg). Body temperature was maintained at 37–38°C by placing anesthetized mice on an isothermal pad. For ABR measurement, needle electrodes were placed under the dermis of the forehead, back, and mastoid. Mice were stimulated with clicks or 4-kHz, 8-kHz, 16-kHz, and 32-kHz pure tones. The results were recorded as sound pressure level (SPL), which is measured in decibels above a standard reference level (0.0002 dyn/cm2). A higher SPL or dB required to elicit a response under the same condition usually means diminished hearing. For CM measurement, the bulla was exposed, and a small window was made in the bullar bone to expose the round window niche under a dissecting microscope (LEICAL2, Leica Microsystems, Beijing CN-100044, China). A recording electrode, a silver ball electrode, was placed near the round-window membrane. The reference electrode was placed subcutaneously on the same side of the neck. CM was evoked by the clicks in the closed field. The response was filtered (300-3 kHz), and averaged 512 times. The thresholds to the stimulus were recorded.
Quantitative RT-PCR
RNA from the basilar membrane of cochlea samples at different time points was collected using TRIzol (lnvitrogen, Carlsbad, CA). Reverse transcription and quantitative PCR (qPCR) were performed as previously described (19). The primers used in this study were as followed: Spag6 forward 5′-AGTTCAGTAAGGTGCTGCCAC-3′ and reverse 5′-AGAAGTGTATCGGAGTATCCAG-3′. All samples were analyzed in triplicate.
Inner Ear Immunostaining and Imaging
Mice were euthanized by peritoneal injection of pentobarbital sodium (100 mg/kg). Dissected inner ear samples were fixed in fresh 4% paraformaldehyde in PBS (pH 7.2–7.4) for 6–8 h at 4°C. Tissues were incubated with primary antibodies in blocking solution (containing 3% goat serum and 3% Triton X-100) at 4° overnight. After being washed four times in PBS, the tissues were incubated with secondary antibodies in PBS at the room temperature for 1 h, and then stained with phalloidin for 20 min. Stained whole-mounted preparations were analyzed with a laser-scanning confocal microscope (DMI 4000B, Leica Microsystems, Beijing CN-100044, China).
Scanning Electron Microscopy
Inner-ear tissue samples were removed and quickly immersed in 2.5% glutaraldehyde for 6 h at 4°. The cochlea was dissected carefully to expose the basilar membrane. After being washed in PBS, tissues were stained with 1% osmium tetroxide, dehydrated, critical point dried, coated with ∼10 nm of platinum, and examined under a Hitachi S-900 scanning electron microscope (Hitachi, Tokyo, Japan) at 15 kV.
Statistical Methods
Values are reported as means ± SE. Significant differences were determined by the Student’s t-test. Values of P < 0.05 were considered to indicate statistical significance. Stereocilia bundle orientations were measured using ImageJ software (National Institutes of Health).
RESULTS
Spag6 Deficiency Impairs Auditory Function
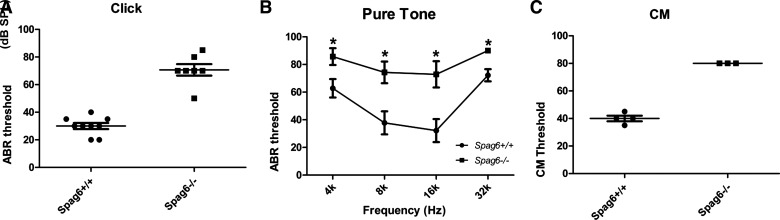
ABRs in 1-mo-old mice were recorded in response to click stimuli. We found that the Spag6−/− mice (n = 7) had severe hearing loss: the average ABR threshold of the Spag6-/- mice was 70.71 ± 4.14 dB SPL (sound pressure level), while in Spag6+/+ littermates (n = 9), it was 30.00 ± 2.21 dB SPL (Fig. 1A). Analysis of hearing function in response to pure tones (4, 8, 16, and 32 kHz) confirmed that hearing in Spag6−/− mice was diminished across the entire analyzed frequency spectrum (Fig. 1B). The SPLs required to elicit a response in the Spag6-deficient mice were significantly higher than that in the wild-type controls (*P < 0.05). Consistently, the CM response to 80 dB SPL could not be elicited in the Spag6−/− mice (n = 3), while the average CM threshold of Spag6+/+ littermates (n = 4) was 40.00 ± 4.08 dB SPL. The difference between the wild-type and mutant mice was significant (Fig. 1C, *P < 0.05). Our previous study found that SPAG6 is located in the cuticular plate and lateral wall of hair cells in the cochlea (20), interacting with the Prestin, which is in line with the abnormal auditory results.
Figure 1.
Spag6 deficiency affects auditory function. A and B: auditory brainstem response (ABR) thresholds (dB SPL) determined by click and pure tones in 1-mo-old wild-type (WT) and Spag6-deficient mice of either gender (Spag6+/+; n = 9; Spag6−/−; n = 7; means ± SE). Spag6−/− mice showed severe hearing loss, the average ABR threshold response to click was significantly higher than that in the wild-type mice (A). Analysis of hearing function in response to pure tones (4, 8, 16, and 32 kHz) also confirmed elevated thresholds at different frequencies in Spag6-deficient mice (B). The average cochlear microphonics (CM) threshold response to click of Spag6−/− mice was significantly higher than that in the wild-type mice (C) (Spag6+/+; n = 4; Spag6−/−; n = 3; means ± SE). *P < 0.05, Student’s t-test. SPL, sound pressure level.
Spag6 Inactivation Alters Cellular Patterning and Cell Shapes
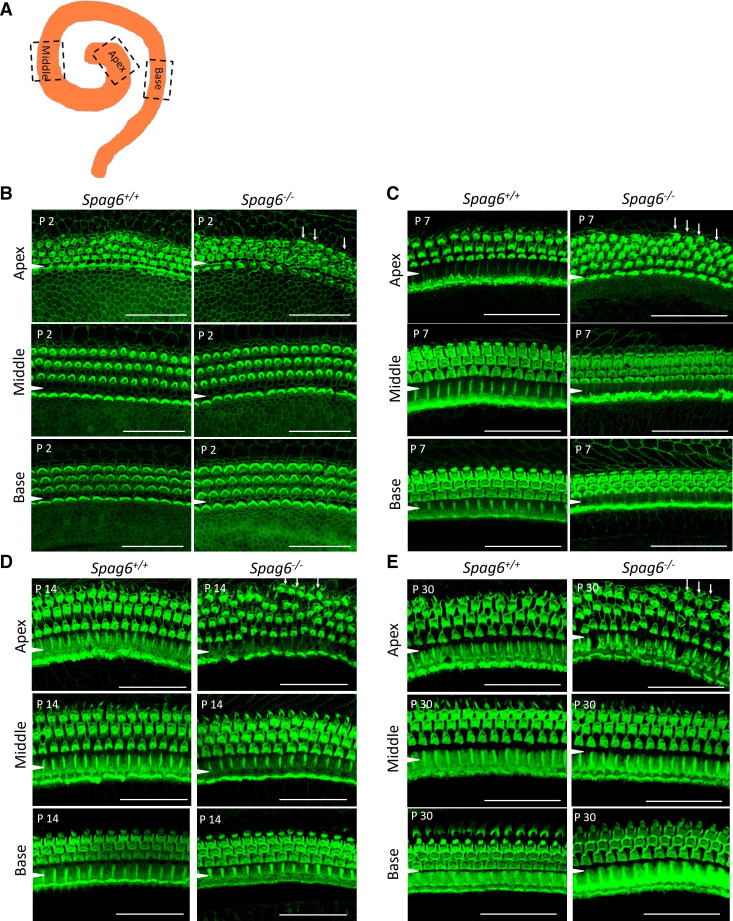
CM is generated primarily by the summed receptor currents of the outer hair cells (OHCs) responding to basilar membrane (BM) displacement (21). Abnormal CM results in mutant mice suggested defects in OHCs. Whole mount preparations at postnatal day 2 (P2), P7, P14, and P30 were analyzed, as illustrated in Fig. 2A. In the Spag6+/+ mice, irregularly arranged hair cells could be seen occasionally, because the mouse cochlea does not mature in morphology until P7–P10. At P2, there were more than three rows of OHCs in the apical turn in Spag6−/− mice (Fig. 2B, white arrows). At P7 and after that, the Spag6+/+ mouse HCs displayed a normal arrangement in apical, middle, and basal turns. However, in the P7, P14, and P30 Spag6−/− mice, there was an abnormal configuration of HCs, mainly in the apical turn (Fig. 2, C–E, white arrows).
Figure 2.
Spag6 inactivation alters cellular arrangement. Whole-mount preparations were prepared at postnatal day 2 (P2), P7, P14, and P30. A: diagram of observed apical, middle, and basal turn. B: at P2, in the Spag6+/+ mice, irregularly arranged hair cells could be seen occasionally because of immaturity. There were more than three rows of outer hair cells (OHCs) in the apical turn of Spag6−/− mice (white arrows). C−E: at P7 and after, the Spag6+/+ mouse inner hair cells (IHCs) displayed a normal arrangement in apical, middle, and basal turns. However, in the P7, P14, and P30 Spag6−/− mice, there still was a disrupted arrangement of OHCs, mainly in the apical turn indicating defects in development. (white arrows in C–E). A white arrowhead is located in the region between the IHCs and OHCs. Scale bars: 30 µm.
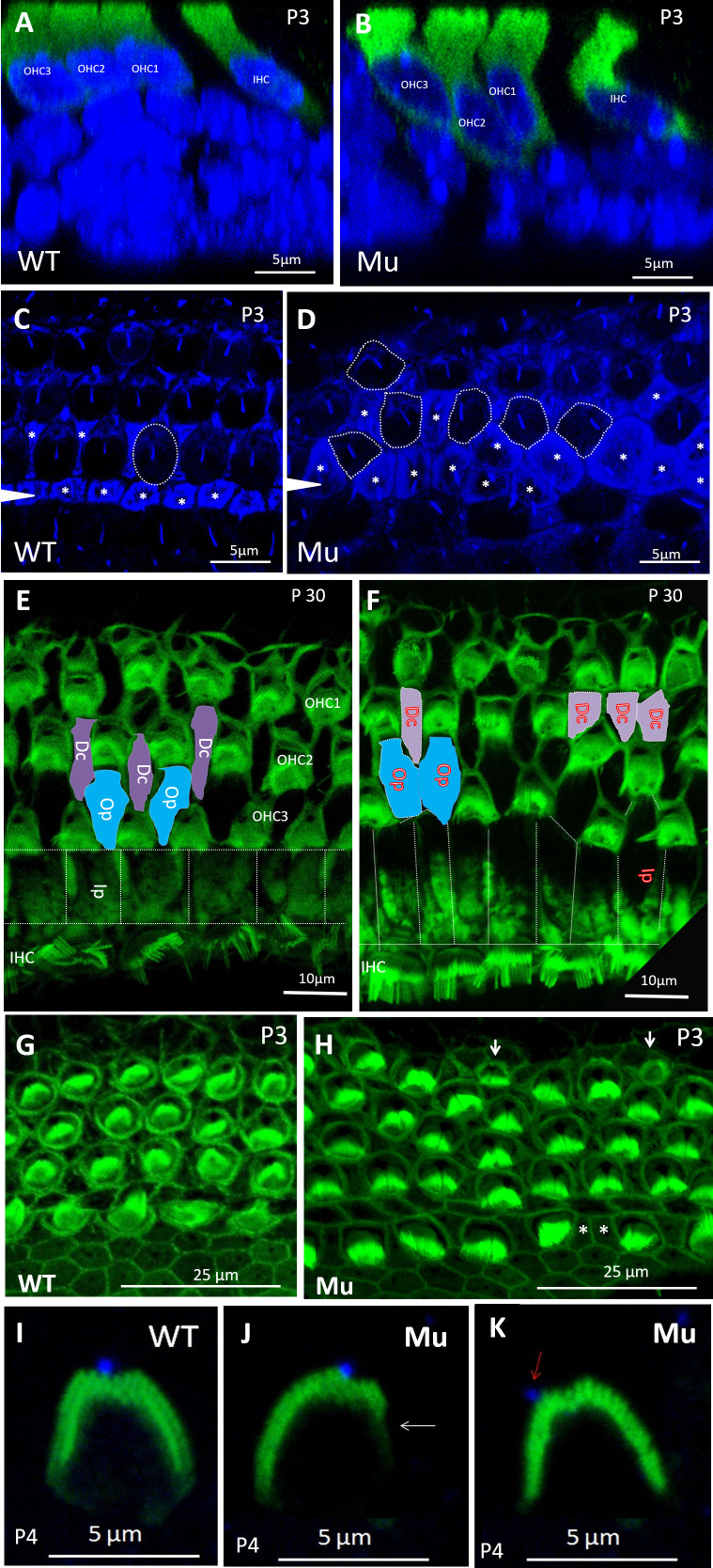
To study the possible involvement of Spag6 in cellular patterning, optical sectioning of whole-mount preparations was performed. OHCs of Spag6+/+ animals (Fig. 3A) had uniformly positioned nuclei. However, the nuclei of Spag6−/− OHCs at P3 were positioned at variable heights in the apico-basal axis of the sensory epithelium (Fig. 3B). Acetylated α-tubulin has been widely used as a marker for kinocilium. Staining for acetylated tubulin in pillar cells and the apical region of hair cells shows the support cells and hair cell patterns (22). Cochlear specimens from Spag6+/+ mice at P3 displayed precisely aligned hair cell rows. Hair cells had an oval shape (Fig. 3C, dotted circle), separated by regularly shaped supporting cells (Fig. 3C, asterisks). In contrast, cochlea of the Spag6−/− mice had a disordered arrangement and irregular shape of OHCs (Fig. 3D, dotted circle) and supporting cells (Fig. 3D, asterisks). Deiters’ cells (Dc), inner pillar (Ip) and outer pillar cells (Op) are different types of supporting cells. They form Corti’s organ and separate the hair cells, ensuring that the hair cells function properly. P30 cochlea of Spag6+/+ mice showed a normal arrangement of OHCs and supporting cells, as illustrated in Fig. 3E (blue block, Op cells; purple block, Dc cells). However, in the mutant mice, some supporting cells were adjacent to each other without HCs intervening (Fig. 3F, blue and purple block). These defects were seen in the apical coil of the cochlea and were mostly absent in the middle and basal coils.
Figure 3.
Spag6 inactivation alters cell shapes and cellular arrangement. Optical sectioning and whole-mount preparations were observed. Mice of any sex were used. A and B: nuclei (blue) of Spag6+/+ OHCs (green) at P3 were uniformly positioned (A); however, Spag6−/− OHCs were positioned at variable heights in the apical-basal axis of the sensory epithelium (B). HCs were stained by antimyosin 7a antibody. C and D: acetylated-α-tubulin (blue) marked microtubule-rich structures like supporting cells, kinocilia, and the cuticular plate of HCs. Cochlear specimens from Spag6+/+ mice at P3 displayed precisely aligned hair cell rows. Hair cells showed oval shape (C; dotted circle) separated by regularly shaped supporting cells (asterisks in C). In contrast, cochlea of the Spag6−/− mice showed a disordered arrangement and irregular shape of OHCs (dotted circles in D) and supporting cells (asterisks in D). E and F: cochlear apex (phalloidin, green) of Spag6+/+ mice at P30 showed a normal arrangement of OHCs and supporting cells, as illustrated (blue and purple block in E). However, in the Spag6−/− mouse, some supporting cells were adjacent without intervening HCs (blue and purple block in F). G and H: compared with the Spag6+/+ mice, cochlea of Spag6−/− mice showed abnormally shaped OHCs and supporting cells in the apex. Some smaller OHCs with or without stereocilia bundles could be found in the Spag6−/− mice (arrows in H). I, J, K: in the basal turn of Spag6+/+ cochlea at P4, the stereocilia bundle was “V”-shaped and basically symmetrical, the kinocilium (blue) was located at the apex of the “V” (I). In contrast, some Spag6−/− hair cell stereocilia bundles had a defective “V” shape and were asymmetrical (arrow in J), the kinocilium was not at the top of “V” (arrow in K). Dc, Deiter’s cell; IHC, inner hair cells; Ip, inner pillar cell; Mu, Spag6−/−; OHC, outer hair cells; Op, outer pillar cell; WT, Spag6+/+; WT, wild type.
In addition to patterning defects, cochlea of Spag6−/− mice showed abnormally shaped OHCs and supporting cells in the apex. Some much smaller OHCs with or without stereocilia bundles could be found in Spag6−/− mice (Fig. 3H, arrows). In the Spag6+/+mice, the stereocilia bundle was “V”-shaped and basically symmetrical, the kinocilium was located at the apex of the “V” (Fig. 3I). In contrast, some Spag6−/− stereocilia bundles had a defective “V” shape and were asymmetrical (Fig. 3J, arrow), and the kinocilium was not at the top of the stereocilia bundle (Fig. 3K, arrow). Similar to patterning defects, OHCs with smaller shape were concentrated in the apical turn and were intermingled with normal OHCs. Abnormal stereocilia morphology with normal OHC volume could also be observed in middle or basal turn. Thus, these two phenotypical changes of OHCs of the Spag6−/− mice can occur independently of each other.
Spag6 Inactivation Affects Stereocilia Bundle Polarity and Kinociliary Positioning
The kinocilia of HCs are required for development of stereocilia bundle polarity (23). In Spag6+/+ mice, three rows of OHCs and one row of IHCs were regularly aligned. The stereocilia form bilaterally symmetrical “V”-shaped stereocilia bundles (Fig. 4A). The position of the kinocilium predicts the orientation of the bundle. However, in the Spag6−/− mice, some hair cells showed a rotated orientation of stereocilia bundles and abnormal kinocilium positions relative to stereociliary bundles. Unlike Spag6+/+ mice, the kinocilium in Spag6−/− mice was not properly positioned and frequently deviated from the normal position (Fig. 4B).
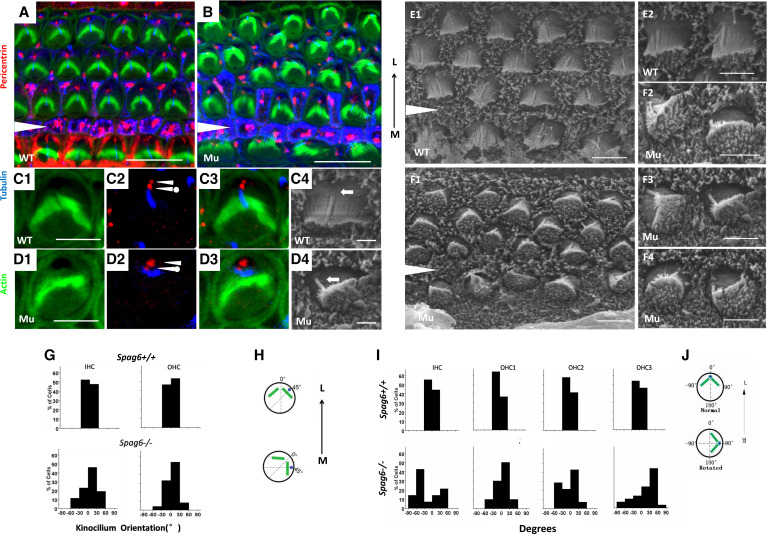
Figure 4.
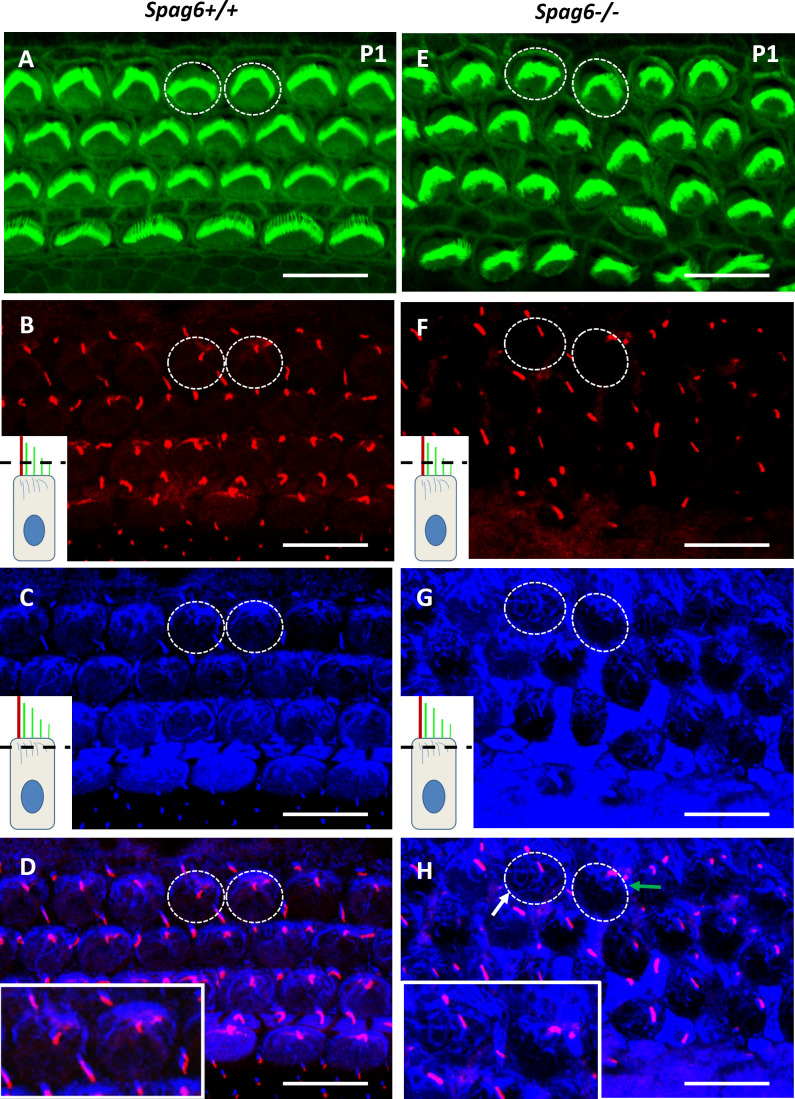
The position of kinocilia and basal bodies are affected in Spag6−/− mice. A–F: confocal projections of cochlear whole mounts and scanning electron micrographs of hair cells from control and Spag6−/− animals at postnatal day 1 of either sex. A and B, C1–C3, and D1–D3: cochlear whole mounts were stained with a pericentrin antibody (red) and an acetylated-α-tubulin antibody (blue) to mark centrioles within the basal bodies and kinocilium, respectively. The cytoskeleton was labeled with phalloidin (green). In a Spag6+/+ mouse, the maternal centriole (C2, arrowhead with a dot) was located at the base of the kinocilium (C2, blue), and the daughter centriole (C2, arrowhead) was positioned medially to the maternal centriole. This pair of centrioles was aligned along the mediolateral axis, and the kinocilium was at the vertex of the “V”-shaped stereocilia bundle (C4, arrow). In the Spag6−/− mice, the maternal centriole (D2, arrowhead with a dot) and daughter centriole (D2, arrowhead) were no longer aligned mediolaterally. The kinocilium (D2, blue) was apart from the vertex of the stereocilia bundle, disoriented, and no longer aligned along the mediolateral axis (D4, arrow). E and F: scanning electron microscopy (SEM) examination of wild-type (E1 and E2) and Spag6−/− mice (F1–F4) hair cells of apical turn at postnatal day 1. Spag6+/+ hair cells showed normal polarity (E1), and the kinocilium was in the vertex of the V-shaped stereocilia bundles (E2, arrows). In the Spag6−/− mice, the polarity of hair cells was disturbed (F1). The intrinsic polarity of some hair cells was affected, showing malformations (F2–F4), and kinocilia were positioned at the periphery in the same general direction as the disoriented bundles (F2–F4, arrows). The black arrowheads (in A, B, E1, F1) mark the support cell region between the inner hair cells (IHCs) and outer hair cells (OHCs). G−J: examination of the relationship between kinocilium position and bundle rotation in hair cells of the apical turn according to SEM observations at P1. In Spag6+/+ mice (G), there was a correlation between the kinocilia position and the apex of stereocilia bundles. In Spag6−/− mice (G), hair cell bundle and kinocilia were frequently mislocalized and distorted. The degrees between kinocilia position and the apex of stereocilia bundles varied from −60°to +60° (Spag6+/+, n = 160; Spag6−/−, n = 142). Diagrams (H) illustrate measurements of the relationship between kinocilium position and bundle rotation (degree). I: In Spag6−/− mice, the degrees of stereocilia bundle orientation were more than ±30°, compared with the Spag6+/+ mice. Diagrams (J) illustrate measurement of the bundle rotation (degrees). White triangle is located in the region between the outer and inner hair cells. L, lateral; M, medial. Scale bars: A and B, 10 μm; C−F, 5 μm.
The basal bodies, the base of the kinocilia, consist of two centrioles (24). The alignment and position of the two centrioles underlie not only the orientation of orderly arrays of microtubules in the apical cortex, but also the formation of the intrinsically polarized structure of stereocilia (23). On the basis of our observations of stereocilia bundle defects, we stained basal bodies for pericentrin to visualize the underlying stereocilia bundle defect. In Spag6+/+ hair cells, pairs of centrioles were aligned along the mediolateral axis (Fig. 4 C2, double arrowheads), and the maternal centriole was associated with the base of the kinocilium (23). However, in the Spag6−/− mice, the two centrioles of the basal body and kinocilium were dislocated (Fig. 4D2, double arrowheads); the centrioles were no longer aligned mediolaterally along the axis of bundle polarity.
Further observation by scanning electron microscopy was performed to analyze the kinocilum location, the stereocilia bundle location, and the relative location angle between the two. In the Spag6+/+ mice, the kinocilum orientation was no more than ±30° away from the vertex of the stereocilua bundle, as illustrated in Fig. 4H, while Spag6−/− mice had variable kinocilum orientation (Fig. 4G). Similarly, compared to the Spag6+/+ mice, Spag6−/− mice had scattered stereocilia bundles along the mediolateral axis (Fig. 4I), as illustrated in Fig. 4J.
Core PCP Proteins Are Abnormally Localized in Spag6-Deficient Mice
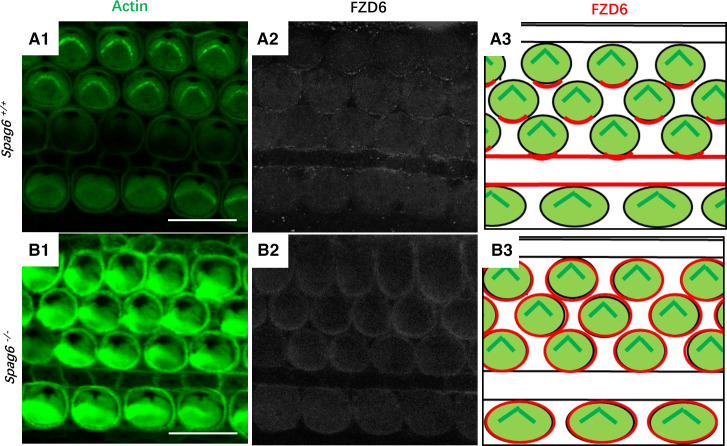
Given the evidence for abnormal PCP in the Spag6−/− mice, we wondered whether core PCP proteins would have an abnormal asymmetric localization. We stained cochlear whole mounts of P1 mice with phalloidin and antibody to Frizzled class receptor 6 (FZD6), a Wnt protein receptor involved in PCP. In Spag6+/+ mice, FZD6 was enriched along the apical medial borders of hair cells (Fig. 5, A1–A3); however, Spag6−/− mice showed an abnormal distribution of FZD6 protein, which lost its asymmetric and polarized distribution, but seemed to be distributed in the entire membrane in Spag6−/− mice (Fig. 5, B1–B3).
Figure 5.
Core planar cell polarity (PCP) proteins are localized abnormally in Spag6-deficient mice. A and B: whole-mount preparation staining of Frizzled class receptor 6 (FZD6) protein in the organ of Corti of Spag6+/+ mice (A) and Spag6−/− mice of either sex (B) at postnatal day 1. The tissues were double stained for FZD6 (white) and phalloidin (actin, green). The FZD6 protein was located at the borders of pillar cells and hair cells near the apical face of the cells in the Spag6+/+ mice (A2). In the Spag6−/− mice, the polarized distribution of FZD6 was lost (B2), and the protein was distributed along the entire membrane. A3 and B3 are diagrammatic drawings to show the FZD6 distribution (red) in Spag6+/+ and Spag6−/−, respectively. Scale bar: 10 μm.
Apical Microtubules Are Disturbed in Spag6-Deficient Mice
Whole mounts were immunostained to determine whether microtubules were disrupted in auditory epithelium of the mutants. Antiacetylated-α-tubulin staining was used to mark the kinocilium and apical microtubule system, and phalloidin to mark hair cells, respectively. Figure 6A shows a normal auditory epithelium of 1-day-old Spag6+/+ mice; two hair cells were randomly selected for observation (Fig. 6A, dotted circles). Different layers show kinocilium (Fig. 6B) and apical microtubules (Fig. 6C). Microtubules are normally anchored at the base of kinocilium by their minus ends, while the free plus ends emanate out to form an aster-like network (Fig. 6D). In the 1-day-old Spag6−/− littermates, hair cells (Fig. 6E, dotted circles), kinocilium (Fig. 6F), and apical microtubules (Fig. 6G) are shown in different layers in the same way. It is obvious that the kinocilium and apical microtubules of HCs were disordered in the cuticular plate, lost the aster-like network, with the center of the microtubules misoriented (Fig. 6H, white and green arrows).
Figure 6.
The apical cytoskeleton is altered in Spag6−/− mice. Whole-mount preparation were immunostained for actin (green) and acetylated-α-tubulin (white) to visualize stereocilia, kinocilia, and cytoplasmic microtubules. Confocal images were taken from the kinocilia level (see inset diagram, B and F) to the apical microtubule level (C and G). B and C, F, and G were overlapped respectively, to see the apical microtubule system and the kinocilium in each hair cell at the same time (D and H). The inset images in B, C, F, and G showed the scan level (black dotted line). Two hair cells were selected randomly in A and E appearing as a dotted-line circle and magnified for better visualization (D and H, see inset.). D: in the Spag6+/+ mice, the apical microtubules (blue) radiated along the periphery of the cell cortex from the base of kinocilium (red) where basal bodies were located. H: in the Spag6−/− mice, the apical microtubules were disturbed and distributed disorderly in the cuticular plate (white arrpw, left dotted circle). The kinocilium was dislocated (red), which is consistent with the misorientation of the microtubule network (green arrow, right dotted circle). Mice of either sex were used. Scale bar: A and B; 10 μm.
DISCUSSION
SPAG6 was first found in the central pair of “9 + 2” microtubule structures of motile cilia. The kinocilium in the cochlea lacks the central pair of microtubules, which is different from the motile cilia in the middle ear, sperm, or cilia in lung (8). Accordingly, we did not find that SPAG6 is localized in the cochlear kinocilium, but in the cuticular plate and lateral wall of HCs (20) (Supplemental Fig. S1, A and B). qPCR data showed a spike of Spag6 expression at P14, while a relative low expression at P1, P7, and P21 (Supplemental Fig. S1C). The onset of hearing in mice occurs at ∼P14, and full maturation occurs at P18 (25). Expression of certain proteins correlates well with the development of hearing. Prestin, the OHC motor protein, is expressed during the first postnatal week and rapidly reaches a mature level before the onset of hearing at P14 (26). SPAG6, as a microtubule-associated protein, has an interaction with prestin and prestin in its expression peaks within the onset of hearing at P14, and is speculated to be involved in hearing development.
Recent findings suggest that Spag6 plays a role in ciliogenesis, orientation of cilia basal feet, and planar polarity (17). The inner ear displays the most typical planar polarity form, which motivated us to explore a role for Spag6 in the cochlea.
Spag6 knockout mice were found to have profound hearing loss. To elucidate the underlying mechanism of the hearing loss, the morphology of sensory epithelium of cochlea was examined. We found that Spag6 inactivation alters cellular patterning, cell shape, stereocilia bundle polarity, and kinociliary positioning. The basal bodies in Spag6-deficeient mice did not align along the PCP axis, indicating that Spag6 mutation affects the tissue-level polarity and single-cell level polarity, or that the correct connections between the two were disturbed. We also found that the core PCP protein, FZD6, lost its normal polarized distribution in Spag6-deficient mice, consistent with disturbed polarity of the auditory epithelium at the tissue level. In a separate study, we found that the orientation of the central microtubules, as well as the orientation of basal feet, which determines cilia orientation, is random in Spag6-deficient brain ventricles and trachea epithelial cells. Furthermore, the distribution of the PCP regulator, Vangl2, was abnormal in Spag6-deficient trachea epithelial cells (17). In the middle ear of Spag6-deficient mice, we also found that FZD6 protein loses its polarized distribution, and the basal feet orientation is random (27). Collectively, these observations strongly suggest that SPAG6 protein is a regulator of PCP.
Microtubules play a very important role in hair cell function. First, it has been reported that rootlets of stereocilia and kinocilia anchor into the cuticular plate, which is significant for the morphology and function of cilia bundle. The cuticular plate is rich in microtubules. Second, microtubules that are connected to the cell cortex have a crucial role in defining cell shape and polarity (28). Interestingly, during the establishment of planar hair cell polarity, defects in cell-surface microtubules have been shown to lead to kinocilium/basal body mispositioning and defects in bundle morphology and orientation (29, 30). We found that the microtubule network at the apex of mutant OHCs was disorganized, suggesting that the abnormal hair cell intrinsic polarity phenotype could result from a perturbed microtubule network.
There are several possible mechanisms for the disrupted polarity in Spag6-deficient mice. Studies have shown that the polarized array of microtubules contributes to proper distribution of PCP regulators. For example, disruption of microtubules perturbs the localization of Frizzled in Drosophila wing epithelium. Therefore, polarized transport of core PCP proteins depends on the planar microtubule arrays (31). Our previous work found that SPAG6 is a microtubule-binding protein (7), and Spag6 knockout leads to instability of microtubules in the axoneme (8). In this study, a disturbed apical microtubule network of HCs and abnormal distribution of FZD6 protein were observed. It appears that the unstable apical microtubule network due to Spag6 inactivation caused the altered distribution of FZD6. It should be noted that Spag6 inactivation only leads to the improper distribution of FZD6, not deficiency of FZD6. The FZD6 protein, even though maldistributed, could provide partial information to direct polarity regulation, which could explain why the polarity phenotype in Spag6-deficient mice is less dramatic than that observed in other PCP gene knockout mice (32–34). Furthermore, it has been proposed that through planar polarized microtubules, directional information is transmitted from PCP proteins localized asymmetrically at adherens junctions, to basal bodies, orienting them in the direction of PCP signaling (6). In Spag6-deficient mice, unstable microtubules may not be able to communicate this polarity information.
In this study, CM, representing the function of hair cells, showed abnormalities in Spag6 mutant mice, suggesting that Spag6 mutation leads to hair cell dysfunction. It has been reported that Spag6 knockout caused decreased expression of prestin, which is a motor protein found in the outer hair cells of the cochlea, responsible for sound amplification in the mammalian hearing organ. The data imply that Spag6 is essential for mechanosensory function of OHCs (20). Although only the apical region of the cochlea has apparent cellular defects, hearing loss in Spag6 mutants is across the full frequency. This may be explained by the fact that spiral ganglion neuron apoptosis coexists in the KO mice, as we previously described (35). Therefore, hair cell abnormalities are one of the factors contributing to hearing loss in SPAG6 deficiency.
Conclusions
Our findings demonstrate a role for Spag6 in auditory function in mice, through a role in the regulation of PCP in the auditory epithelium. However, the exact molecular mechanisms by which Spag6 influences PCP require further study. It will be of interest to determine whether Spag6 mutations are associated with human hearing loss.
SUPPLEMENTAL DATA
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.13161260.v1.
GRANTS
This work was supported by the National Natural Science Foundation of China (Grants 81670932, 81900940, and 81800905), Taishan Scholars Program of Shandong Province (no. ts20130913), the Focus on Research and Development Plan in Shandong province (no. 2018GSF121027), Shandong Provincial Natural Science Foundation (ZR2019BH022), and the Key Project of Shandong Provincial Programs for Research and Development (2017CXGC1213), National Institutes of Health grant HD076257 (to Z.Z.), and the Wayne State University Start-Up Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.Z. and H.W. conceived and designed research; X.L., L.X. and Y.H. performed experiments; X.L., L.X. and W. Liu analyzed data; L.X. and Z.F. interpreted results of experiments; X.L. and D.Z. prepared figures; X.L. and L.X. drafted manuscript; Z.Z., H.W., W. Li., R.M.C., and J.F.S. edited and revised manuscript; X.L., D.Z., L.X., Y.H., W.Liu, W.Li, Z.F., R.M.C., J.F.S., Z.Z., and H.W. approved final version of manuscript.
REFERENCES
- 1.Raphael Y, Altschuler RA. Structure and innervation of the cochlea. Brain Res Bull 60: 397–422, 2003. doi: 10.1016/S0361-9230(03)00047-9. [DOI] [PubMed] [Google Scholar]
- 2.Cotanche DA, Corwin JT. Stereociliary bundles reorient during hair cell development and regeneration in the chick cochlea. Heart Res 52: 379–402, 1991. doi: 10.1016/0378-5955(91)90027-7. [DOI] [PubMed] [Google Scholar]
- 3.Tilney LG, Cotanche DA, Tilney MS. Actin filaments, stereocilia and hair cells of the bird cochlea. VI. How the number and arrangement of stereocilia are determined. Development 116: 213–226, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development 138: 1877–1892, 2011. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walck-Shannon E, Hardin J. Cell intercalation from top to bottom. Nat Rev Mol Cell Biol 15: 34–48, 2014. doi: 10.1038/nrm3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vladar EK, Bayly RD, Sangoram AM, Scott MP, Axelrod JD. Microtubules enable the planar cell polarity of airway cilia. Curr Biol 22: 2203–2212, 2012. doi: 10.1016/j.cub.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sapiro R, Tarantino LM, Velazquez F, Kiriakidou M, Hecht NB, Bucan M, Strauss JF 3rd. Sperm antigen 6 is the murine homologue of the Chlamydomonas reinhardtii central apparatus protein encoded by the PF16 locus. Biol Reprod 62: 511–518, 2000. doi: 10.1095/biolreprod62.3.511. [DOI] [PubMed] [Google Scholar]
- 8.Sapiro R, Kostetskii I, Olds-Clarke P, Gerton GL, Radice GL, Strauss IJ. Male infertility, impaired sperm motility, and hydrocephalus in mice deficient in sperm-associated antigen 6. Mol Cell Biol 22: 6298–6305, 2002. doi: 10.1128/MCB.22.17.6298-6305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horowitz E, Zhang Z, Jones BH, Moss SB, Ho C, Wood JR, Wang X, Sammel MD, Strauss JF. Patterns of expression of sperm flagellar genes: early expression of genes encoding axonemal proteins during the spermatogenic cycle and shared features of promoters of genes encoding central apparatus proteins. Mol Hum Reprod 11: 307–317, 2005. doi: 10.1093/molehr/gah163. [DOI] [PubMed] [Google Scholar]
- 10.Kiselak EA, Shen X, Song J, Gude DR, Wang J, Brody SL, Strauss JF, Zhang Z. Transcriptional regulation of an axonemal central apparatus gene, sperm-associated antigen 6, by a SRY-related high mobility group transcription factor, S-SOX5. J Biol Chem 285: 30496–30505, 2010. doi: 10.1074/jbc.M110.121590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z, Tang W, Zhou R, Shen X, Wei Z, Patel AM, Povlishock JT, Bennett J, Strauss JF. Accelerated mortality from hydrocephalus and pneumonia in mice with a combined deficiency of SPAG6 and SPAG16L reveals a functional interrelationship between the two central apparatus proteins. Cell Motil Cytoskeleton 64: 360–376, 2007. doi: 10.1002/cm.20189. [DOI] [PubMed] [Google Scholar]
- 12.Hamada T, Teraoka M, Imaki J, Ui-Tei K, Ladher RK, Asahara T. Gene expression of Spag6 in chick central nervous system. Anat Histol Embryol 39: 227–232, 2010. doi: 10.1111/j.1439-0264.2010.01000.x. [DOI] [PubMed] [Google Scholar]
- 13.Lonergan KM, Chari R, deLeeuw RJ, Shadeo A, Chi B, Tsao MS, Jones S, Marra M, Ling V, Ng R, MacAulay C, Lam S, Lam WL. Identification of novel lung genes in bronchial epithelium by serial analysis of gene expression. Am J Respir Cell Mol Biol 35: 651–661, 2006. doi: 10.1165/rcmb.2006-0056OC. [DOI] [PubMed] [Google Scholar]
- 14.Abe M, Watanabe N, McDonell N, Takato T, Ohira M, Nakagawara A, Ushijima T. Identification of genes targeted by CpG island methylator phenotype in neuroblastomas, and their possible integrative involvement in poor prognosis. Oncology 74: 50–60, 2008. doi: 10.1159/000139124. [DOI] [PubMed] [Google Scholar]
- 15.Mulaw MA, Krause A, Krause AJ, Deshpande AJ, Krause LF, Rouhi A, La Starza R, Borkhardt A, Buske C, Mecucci C, Ludwig W-D, Lottaz C, Bohlander SK. CALM/AF10-positive leukemias show upregulation of genes involved in chromatin assembly and DNA repair processes and of genes adjacent to the breakpoint at 10p12. Leukemia 26: 1012–1019, 2012. doi: 10.1038/leu.2011.307. [DOI] [PubMed] [Google Scholar]
- 16.Steinbach D. Identification of a set of seven genes for the monitoring of minimal residual disease in pediatric acute myeloid leukemia. Clin Cancer Res 12: 2434–2441, 2006. doi: 10.1158/1078-0432.CCR-05-2552. [DOI] [PubMed] [Google Scholar]
- 17.Teves ME, Sears PR, Li W, Zhang Z, Tang W, van Reesema L, Costanzo RM, Davis CW, Knowles MR, Strauss JF 3rd, Zhang Z. Sperm-associated antigen 6 (SPAG6) deficiency and defects in ciliogenesis and cilia function: polarity, density, and beat. PloS One 9: e107271, 2014. doi: 10.1371/journal.pone.0107271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang X, Zhu X, Ding B, Walton JP, Frisina RD, Su J. Age-related hearing loss: GABA, nicotinic acetylcholine and NMDA receptor expression changes in spiral ganglion neurons of the mouse. Neuroscience 259: 184–193, 2014. doi: 10.1016/j.neuroscience.2013.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun S, Zhang D, Sun G, Song Y, Cai J, Fan Z, Wang H. Solute carrier family 4 member 1 might participate in the pathogenesis of Meniere’s disease in a murine endolymphatic hydrop model. Acta Otolaryngol 139: 966–976, 2019. doi: 10.1080/00016489.2019.1663365. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Li X, Zhang Z, Wang H, Li J. Expression of prestin in OHCs is reduced in Spag6 gene knockout mice. Neurosci Lett 592: 42–47, 2015. doi: 10.1016/j.neulet.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dallos P, Cheatham MA. Production of cochlear potentials by inner and outer hair cells. J Acoust Soc Am 60: 510–512, 1976. doi: 10.1121/1.381086. [DOI] [PubMed] [Google Scholar]
- 22.Tannenbaum J, Slepecky NB. Localization of microtubules containing posttranslationally modified tubulin in cochlear epithelial cells during development. Cell Motil Cytoskeleton 38: 146–162, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 23.Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, Yoder BK, Chen P. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet 40: 69–77, 2008. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- 24.Dawe HR, Farr H, Gull K. Centriole/basal body morphogenesis and migration during ciliogenesis in animal cells. J Cell Sci 120: 7–15, 2006. doi: 10.1242/jcs.03305. [DOI] [PubMed] [Google Scholar]
- 25.Wu X, Gao J, Guo Y, Zuo J. Hearing threshold elevation precedes hair-cell loss in prestin knockout mice. Brain Res Mol Brain Res 126: 30–37, 2004. doi: 10.1016/j.molbrainres.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 26.Abe T, Kakehata S, Kitani R, Maruya S, Navaratnam D, Santos-Sacchi J, Shinkawa H. Developmental expression of the outer hair cell motor prestin in the mouse. J Membrane Biol 215: 49–56, 2007. doi: 10.1007/s00232-007-9004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Xu L, Li J, Li B, Bai X, Strauss JF 3rd, Zhang Z, Wang H. Otitis media in sperm-associated antigen 6 (Spag6)-deficient mice. PloS One 9: e112879, 2014. doi: 10.1371/journal.pone.0112879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li R, Gundersen GG. Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol 9: 860–873, 2008. doi: 10.1038/nrm2522. [DOI] [PubMed] [Google Scholar]
- 29.Ezan J, Lasvaux L, Gezer A, Novakovic A, May-Simera H, Belotti E, Lhoumeau AC, Birnbaumer L, Beer-Hammer S, Borg JP, Le Bivic A, Nürnberg B, Sans N, Montcouquiol M. Primary cilium migration depends on G-protein signalling control of subapical cytoskeleton. Nat Cell Biol 15: 1107–1115, 2013. doi: 10.1038/ncb2819. [DOI] [PubMed] [Google Scholar]
- 30.Sipe CW, Liu L, Lee J, Grimsley-Myers C, Lu X. Lis1 mediates planar polarity of auditory hair cells through regulation of microtubule organization. Development 140: 1785–1795, 2013. doi: 10.1242/dev.089763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimada Y, Yonemura S, Ohkura H, Strutt D, Uemura T. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev Cell 10: 209–222, 2006. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, Nolan PM, Steel KP, Brown SD, Gray IC, Murdoch JN. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol 13: 1129–1133, 2003. doi: 10.1016/S0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- 33.Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, Greer J, Kardos N, Wang J, Sussman DJ, Chen P, Wynshaw-Boris A. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet 4: e1000259, 2008. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 423: 173–177, 2003. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Xu L, Sun G, Wu X, Bai X, Li J, Strauss JF, Zhang Z, Wang H. Spag6 mutant mice have defects in development and function of spiral ganglion neurons. Sci Rep 7: 8638, 2017. doi: 10.1038/s41598-017-08739-8. [DOI] [PMC free article] [PubMed] [Google Scholar]