Abstract
Treatment options for liver metastases (primarily colorectal cancer) are limited by high recurrence rates and persistent tumor progression. Surgical approaches to management of these metastases typically use heat energy including electrocautery, argon beam coagulation, thermal ablation of surgical margins for hemostasis, and preemptive thermal ablation to prevent bleeding or to effect tumor destruction. Based on high rates of local recurrence, these studies assess whether local effects of hepatic thermal injury (HTI) might contribute to poor outcomes by promoting a hepatic microenvironment favorable for tumor engraftment or progression due to induction of procancer cytokines and deleterious immune infiltrates at the site of thermal injury. To test this hypothesis, an immunocompetent mouse model was developed wherein HTI was combined with concomitant intrasplenic injection of cells from a well-characterized MC38 colon carcinoma cell line. In this model, HTI resulted in a significant increase in engraftment and progression of MC38 tumors at the site of thermal injury. Furthermore, there were local increases in expression of messenger ribonucleic acid (mRNA) for hypoxia-inducible factor-1α (HIF1α), arginase-1, and vascular endothelial growth factor α and activation changes in recruited macrophages at the HTI site but not in untreated liver tissue. Inhibition of HIF1α following HTI significantly reduced discreet hepatic tumor development (P = 0.03). Taken together, these findings demonstrate that HTI creates a favorable local environment that is associated with protumorigenic activation of macrophages and implantation of circulating tumors. Discrete targeting of HIF1α signaling or inhibiting macrophages offers potential strategies for improving the outcome of surgical management of hepatic metastases where HTI is used.
Keywords: colorectal cancer, hepatic metastases, hepatic thermal injury, hypoxia-inducible factor-1α, tumorigenesis
INTRODUCTION
Hepatic thermal injury (HTI) occurs during liver resection as a by-product from energy devices such as electrocautery or argon beam coagulation used to control bleeding. It also occurs intentionally as a result of radiofrequency ablation (RFA) or microwave ablation (MW) (1, 2) in an effort to destroy malignant cells (2–5). Thermal ablation modalities (RFA and MW) also have been used with increasing frequency to preemptively reduce surgical bleeding by precoagulating tissue planes before resection (3, 4, 6). Thermal ablation is designed to cause cell necrosis by heating tumors and surrounding normal hepatic parenchyma to ∼100°C or higher. These therapies are recommended for the treatment of colorectal liver metastases that are not amenable to resection because of anatomic location, functional liver reserve, or patient fitness for surgical resection (7–10).
Despite perceived clinical benefit, both liver resection and ablative therapy are plagued by high local recurrence rates (11–20). Surgical resection remains the standard of care for treating colorectal liver metastases, but recurrence rates of 40% or greater are reported among patients following hepatectomy, most occurring within 2 years of resection (15). The cellular and molecular mechanisms that contribute to tumor recurrence after surgical therapies are undoubtedly complex and remain incompletely understood (1, 16) but are thought to include local hypoxia (16) and changes in the microenvironment due to injury, repair, and inflammation (21). Processes creating a favorable tumor microenvironment are often associated with immune cell infiltrates, which also accompany the normal healing response of the liver to thermal ablation. These immune cells may be recruited either from the bone marrow or the peritoneal cavity (22). Accordingly, this healing response may contribute to increased susceptibility to hepatic metastases (1, 23, 24). These studies assess whether the healing process induced by surgical injury (thermal) inadvertently potentiates a prometastatic microenvironment at the site of injury.
A recent study by Medzhitov and colleagues (25) demonstrated that tumor-associated macrophages can be activated into a protumor phenotype, which is characterized by increased expression of hypoxia-inducible factor-1α (HIF1α), arginase-1 (Arg1), and vascular endothelial growth factor (VEGF). HIF1α activation is a critical upstream regulator of arginase-1 expression, and in mice, the genetic deletion of Arg1 (encoding for arginase-1) attenuates tumor growth in vivo (25, 26). Notably, tumor-promoting macrophages share functional characteristics with macrophages associated with wound healing and tissue remodeling, which also express VEGF and, especially, arginase-1 through an HIF1α-dependent pathway (25, 27–31). Furthermore, VEGF expression has been shown to be important in cancer progression and tissue remodeling (27–30, 32–34). Our laboratory has demonstrated that VEGF acts to recruit macrophages into the tumor microenvironment (35). These findings suggest that HTI-mediated tissue injury might result in activation of macrophages that contribute to local metastasis via an HIF1α-dependent pathway.
To test this hypothesis, we developed an immune-competent murine model, in which HTI was combined with an intrasplenic injection of murine colon carcinoma cells or murine pancreas carcinoma cells (36, 37). We examined the origin and activation state of HTI-associated macrophages and investigated the potential role of HIF1α signaling in promoting discrete hepatic metastases. Taken together, the findings suggest that therapeutic targeting of HTI-induced, macrophage-mediated hepatic metastatic disease presents one strategy for alleviating metastatic tumor engraftment or progression following therapeutic HTI of liver tumors.
EXPERIMENTAL PROCEDURES
Cell culture and materials.
MC38 colon adenocarcinoma cells and Pan02 pancreas adenocarcinoma cells (female and male) in derivation, respectively (38, 39), were received as a gift from Rolf Brekken, PhD (UT Southwestern Medical Center, Dallas, TX). Cells were maintained in RPMI culture media with additives of 10% fetal bovine serum and 1% penicillin/streptomycin (Cellgro, Manassas, VA). Cells were grown to 80% confluence before preparation for animal injection. We used both MC38 and Pan02 cells for proof of concept and demonstrated the same results for both cell lines; however, as there is no current clinical evidence to treat pancreatic cancer liver metastases, data are only shown for the MC38 colorectal primary cell line.
Animal procedures: hepatic thermal injury model.
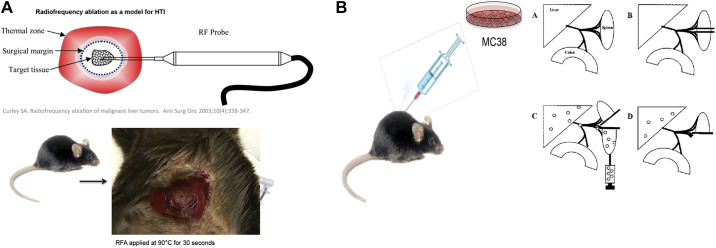
All procedures were performed in compliance with and approved by the University of Colorado Institutional Animal Care and Use Committee and the “Guide for the Care and Use of Laboratory Animals” as published by the National Institutes of Health. Male mice (n ≥ 3 per group) of strains C57BL/6 (Jackson Laboratory, Bar Harbor, ME) aged 8–10 wk were anesthetized using inhaled isoflurane. Mice then underwent either sham laparotomy or laparotomy with application of unipolar electrical current via use of a single-needle, radiofrequency probe (Radionics RFG-3C, Radionics Inc., Burlington, MA) to the midright lobe of the liver under sterile conditions. Thermal injury was accomplished by applying energy to normal hepatic parenchyma with a setting of 90°C and sustained exposure for 30 s. In the sham group, the probe was inserted into the right lobe of the liver for 30 s with no heat application (Fig. 1A). Metastatic tumor dissemination was modeled using a splenic injection of 50,000 MC38 colon adenocarcinoma cells suspended in 100 µL of phosphate-buffered saline (PBS) or 250,000 Pan02 cells in 100 µL PBS (data not shown), followed by hemisplenectomy to remove the primary injection site (Fig. 1B). A dose-response relationship of metastatic tumor engraftment was assessed via intrasplenic injection of either 1,000, 10,000, or 50,000 MC38 cells suspended in 100 µL of sterile PBS. One to three weeks after HTI or sham, mice were euthanized according to the University of Colorado IACUC recommendation by CO2 asphyxiation, followed by cervical dislocation to prepare for necropsy. Tumor burden was quantified by blinded observers and scored as number of discrete lesions as well as the presence of lesions at the HTI site. Tumor volume was calculated as (height × width2).
Fig. 1.
A: representative diagram for creating of hepatic thermal injury (HTI) in the mouse liver with representative picture of the HTI lesion. B: representative diagram for creation of the metastatic model of colon cancer metastases (MC38) [data for image derived from Soares et al. (37)].
Hepatic thermal injury-induced systemic cytokine elaboration.
We applied RF energy to the livers of immunocompetent mice (n = 5) to create a 3-mm HTI lesion. Mice were euthanized 24 h after thermal injury and cardiac puncture was performed to obtain blood. A ChemiArray panel was run on pooled serum to evaluate multiple inflammatory cytokines. Control was taken from mice that underwent sham surgery only, without hepatic thermal injury.
HIF1α inhibition in hepatic thermal injury model.
HTI and metastatic tumor dissemination were accomplished as described in the model. HIF1α inhibition was accomplished using YC-1 (Sigma Aldrich, St. Louis, MO) (32). Mice were given an intraperitoneal injection of 75 mg/kg of YC-1 on day 0 and day 1 postthermal injury and compared with mice that received vehicle control (10% dimethyl sulfoxide in phosphate-buffered saline; Sigma Aldrich, St. Louis, MO). Three weeks after HTI or sham, mice were euthanized as above for necropsy. Tumor burden was quantified by blinded observers and scored as number of discrete lesions as well as the presence of lesions at the HTI site. Tumor volume was calculated by (height × width2). At necropsy, HTI lesions, non-HTI hepatic metastases, and normal liver tissue samples were collected for the harvesting of intrahepatic mononuclear cells and examination of hepatic tissue by dissecting 50 mg samples around tumors and 50 mg samples of macroscopically normal-appearing liver from a separate hepatic lobe not receiving HTI for control. Liver samples containing tumor and not containing tumor were taken from each experimental group mouse. No pooled samples were used. Control mice had 50 mg liver samples taken.
Western blotting.
At the time of euthanasia, a portion from each tumor was preserved in 4% formalin for histology or snap frozen in liquid nitrogen and then homogenized in cOmplete lysis-M EDTA-free buffer (Roche, Basel, Switzerland) for Western blot analysis. Immunoblotting was performed to evaluate VEGFα, HIF1α, glucose transporter 1 (GLUT1), and GAPDH protein expression for each control and treatment as previously described (40).
RNA isolation and PCR.
Intrahepatic mononuclear cells (ihMNCs) were isolated from livers using gradient centrifugation as described previously (41, 42). Liver tissue samples were homogenized in the lysis buffer provided in the Qiagen RNeasy Plus Kit (Valencia, CA). Isolation of RNA was performed per protocol using the Qiagen RNeasy Plus Kit. Quality and concentration measurements were performed using the Nanodrop system (Thermo Fisher Scientific, Wilmington, DE). The qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD) was used to synthesize cDNA from the collected messenger ribonucleic acid (mRNA) for use with quantitative real-time polymerase chain reaction (RT-PCR). Probes for all examined target genes were obtained from Life Technologies (Grand Island, NY) as gene expression assays.
Immunohistochemistry.
Murine hepatic tissue was preserved in formalin, paraffin-embedded, cut onto slides, and stained by the Histology Core at the University of Colorado Anschutz Medical Center. IHC was performed using GATA-binding protein 6 (GATA6) specific antibody (Thermo Fisher Scientific) using the Ventana BenchMark XT automated stainer. Briefly, deparaffinized slides were pretreated with Borg solution (Biocare Medical) for 10 min and incubated with the primary antibody for 32 min at 37°C (dilution 1:25). The I-VIEW detection kit was used with the secondary antibody removed from the kit and substituted with Rabbit ImmPress (Vector Labs) diluted at 50% with PBS, followed by hematoxylin counterstaining. Nikon NIS Elements BR software (version 4.13) was used for image capture.
Single cell isolation and flow cytometry.
Tumor-containing tissues were collected from HTI sites, cut into small pieces, and resuspended in RPMI-1640 medium with Liberase (Roche Diagnostics Corporation, Indianapolis, IN). The tissues were digested for 50 min in a shaking incubator at 37°C. After centrifugation, digested tissues were passed through a 100-μm cell strainer to make single-cell suspensions. Single-cell suspensions were blocked with LEAF anti-mouse CD16/32 (clone 93) for 20 min on ice before staining with primary conjugated antibodies. Samples were analyzed by a CytoFLEX (Beckman Coulter, Indianapolis, IN). Data were analyzed using FlowJo software (Tree Star).
Quantitative and statistical analysis.
RT-PCR was quantified to determine fold-change in expression from control housekeeping gene Hprt using the 2-ddCT method as previously reported (29). Fold-change in expression is reported as means ± the standard error of the mean. Statistical significance was determined using Student’s t test for parametric comparisons and the Mann–Whitney test for nonparametric comparisons, with α = 0.05. All statistical analyses were conducted using GraphPad Prism 6.0 (La Jolla, CA).
RESULTS
Hepatic thermal injury and localized tumor progression.
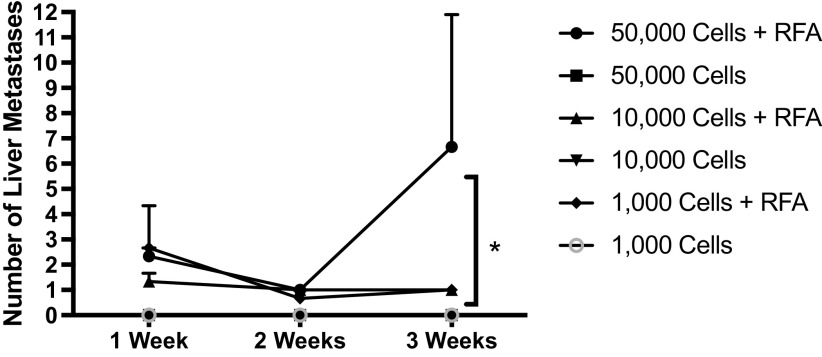
To assess whether HTI results in local changes that enhance tumor engraftment or progression, mice were subjected to HTI or sham surgery followed by immediate intrasplenic injection of murine colon cancer cells (HTI/MC38 mice). All mice undergoing HTI developed tumors at the site of thermal injury on necropsy performed at day 7 (Fig. 2A). In contrast, none of the mice undergoing a sham procedure (probe inserted without application of heat) had tumors at the site of probe insertion (nonthermal injury) (Fig. 2A). Notably, mice in the HTI/MC38 treatment group had additional hepatic tumors in noninjured sites as well, which was reflected by increased hepatic tumor volume (Fig. 2B). Only one mouse in the sham/MC38 group had a hepatic tumor, which was not at the nonthermal injury site (Fig. 2B). Mean hepatic tumor volume was 10.00 ± 10.00 mm3 in the sham group versus 238.0 ± 94.8 mm3 in the HTI/MC38 group (Fig. 2B). Hematoxylin and eosin staining of the HTI site with tumor formation in the HTI/MC38 group revealed a central necrotic lesion surrounded by tumor and infiltrating mononuclear cells (Fig. 2C). A modified dose-response curve demonstrates that use of even lower cell numbers is sufficient for engraftment and growth following HTI (Fig. 3).
Fig. 2.
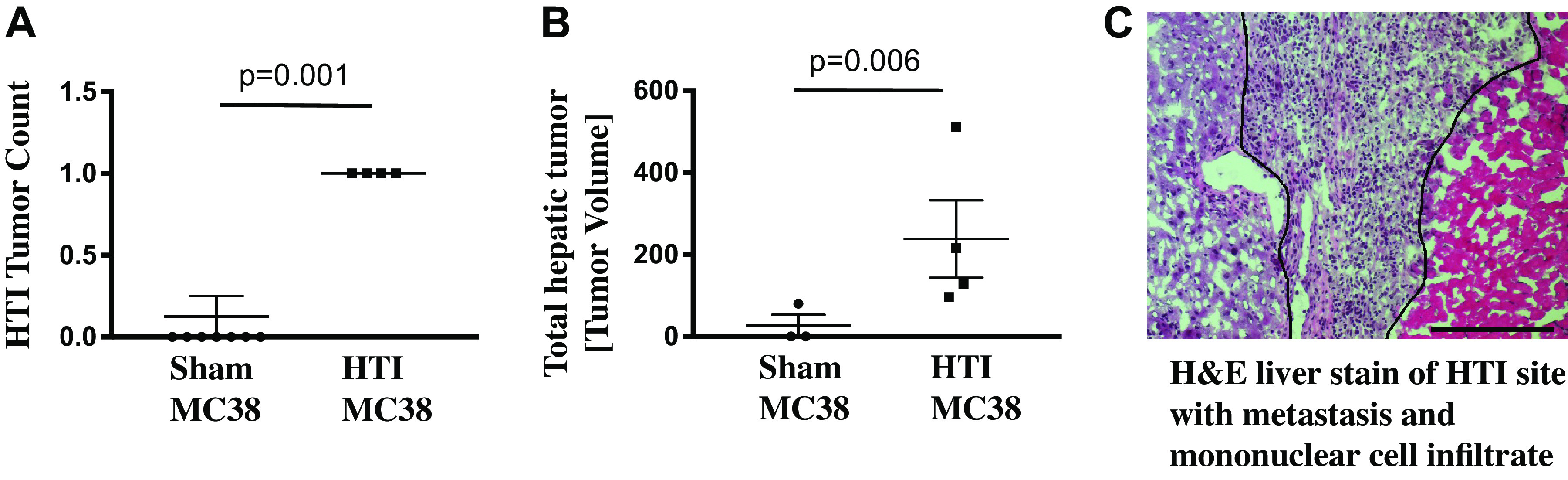
A: all mice that received hepatic thermal injury (HTI) developed a metastatic focus at the site of thermal injury. Conversely sham animals, who had a probe inserted into the liver but no thermal injury, did not develop any metastasis at the probe insertion site; mean of sham MC38 0 versus mean of HTI MC38 1, t test P = 0.001; standard deviation shown by error bars (n ≥ 4). B: mice that received HTI had significantly greater tumor volume. One mouse in the sham group developed a hepatic metastasis remote from the (nonpolar injury) probe placement site in this metastatic model; mean volume of sham MC38 10 mm3 versus mean of HTI MC38 238 mm3, t test P = 0.006; standard deviation shown by error bars (n ≥ 3). C: hematoxylin-eosin (H&E) staining of an HTI site demonstrates normal hepatic parenchyma bordering injured hepatic parenchyma with infiltrate of mononuclear cells and tumor cells. Bar = 1 μm.
Fig. 3.
At 3 wk after intrasplenic injection, all mice treated with radiofrequency ablation (RFA) demonstrated a dose-response relationship in the number of liver metastases seen at the time of necropsy (ANOVA *P value < 0.05 between all groups, standard deviation shown via error bars). No mice who were treated without hepatic thermal injury (HTI) via RFA had developed hepatic metastases at 3 wk after intrasplenic injection (n ≥ 3 for each time point and condition).
Hepatic thermal injury and systemic cytokine elaboration.
Hepatic thermal injury caused significant protumorigenic cytokine elevation. Of note, VEGF has the highest fold increase of cytokines examined when compared with control mice (Fig. 4).
Fig. 4.
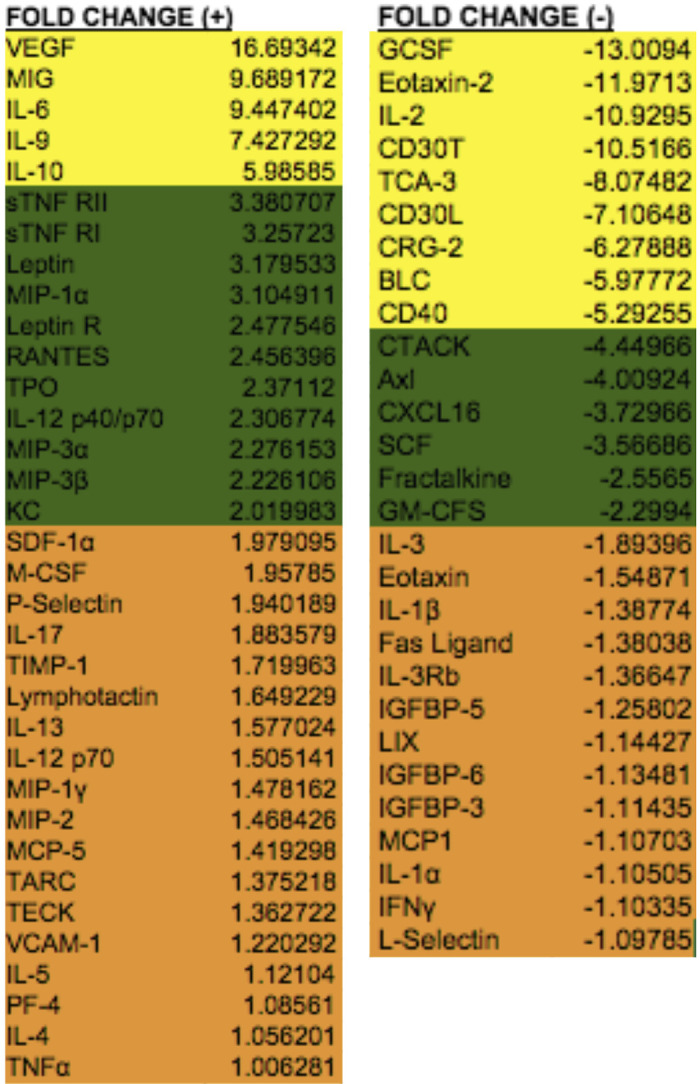
ChemiArray analysis for procancer cytokines performed on serum from five mice. Cytokines with >5-fold change are shown in yellow, cytokines with 2-fold to 5-fold change are shown in green, and cytokines with <2-fold change are shown in red. Serum taken 24 h posthepatic thermal injury demonstrates significant upregulation of multiple cytokines compared to control mice receiving sham surgery. Vascular endothelial growth factor (VEGF) showed greater than 16-fold increase compared to control.
Hepatic thermal injury and expression of protumorigenic factors.
We next evaluated whether HTI alone (in the absence of tumor cells) is associated with increased expression of protumorigenic factors. Using gene expression as one measure, there were significant increases in mRNA of HIF1α, glucose transporter 1 (GLUT1), and VEGFα at the HTI site relative to the unaffected liver from the same mouse. This upregulation was apparent as early as 1 h after thermal injury and continued to increase as long as 7 days postinjury (Fig. 5, A–C). Western blotting confirmed that HTI-induced upregulation of HIF1α mRNA resulted in increases in HIF1α protein as well (Fig. 5D).
Fig. 5.
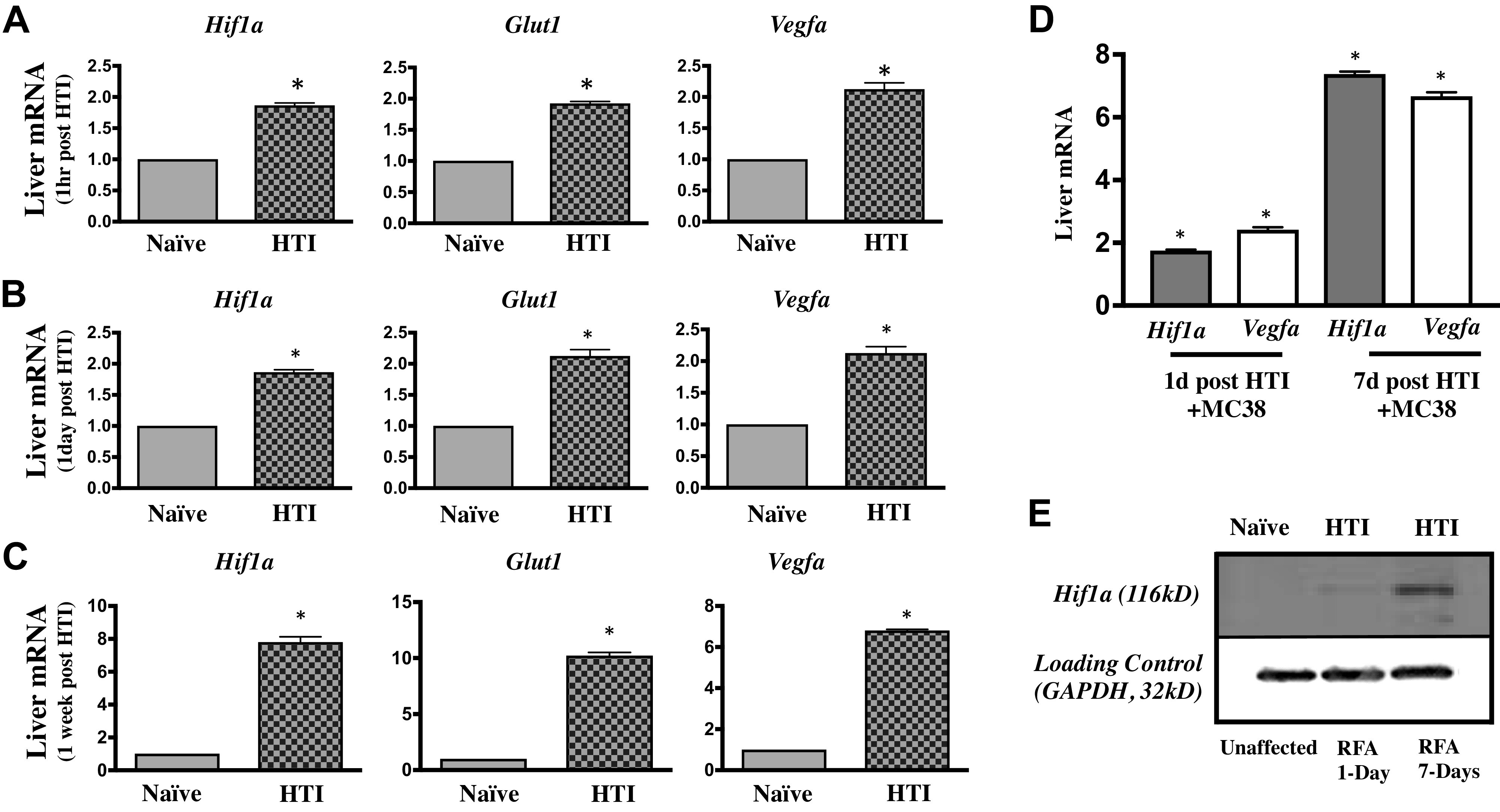
A: examination of the hepatic thermal injury (HTI) site, compared with uninjured (naïve) liver of equal volume from the same mouse reveals fold change upregulation of messenger ribonucleic acid (mRNA) for hypoxia-inducible factor-1α (HIF1α), glucose transporter 1 (GLUT1), and vascular endothelial growth factor α (VEGFα) at 1 h post-HTI (t test *P < 0.05, n ≥ 3). B: examination of the HTI site, compared to uninjured (naïve) liver of equal volume from the same mouse reveals fold change upregulation of mRNA for HIF1α, GLUT1, and VEGFα at 1 day post-HTI (t test *P < 0.05, n ≥ 3). C: examination of the HTI site, compared to uninjured (naïve) liver of equal volume from the same mouse reveals upregulation of mRNA for HIF1α, GLUT1, and VEGFα at 7 days post-HTI (t test *P < 0.05, n ≥ 3). D: examination of the HTI site for upregulation of HIF1α mRNA at 1 day and 7 days post-HTI combined with the injection of MC38 tumor cells shows similar upregulation as that of injury alone; [ANOVA, all compared to HTI naïve mice (data shown in Fig. 5, B and C) at either day 1 or day 7, *P < 0.05, n ≥ 3]. E: immunoblot confirms upregulated protein for HIF1α (116 kD) at the HTI site, both at day 1 and day 7; compared to loading control GAPDH (36 kD), (n ≥ 3). Blot condensed by protein size for ease of viewing with sample lanes unadulterated.
When HTI was used with MC38 intrasplenic injection, mRNA expression of HIF1α increased 1.87 ± 0.04 fold-change higher at the site of HTI as compared with uninjured liver tissue when measured after 24 h of injury/infusion. The magnitude of the mRNA response continued to increase over time, and 1 wk postinjury, the mRNA expression of HIF1α at the HTI application site was 7.8 ± 0.33 fold-change higher as compared with uninjured liver tissue. Similarly, mRNA expression of VEGFα increased from a 2.13 ± 0.10 fold-change increase on day 1 to 6.81 ± 0.05 fold-change increase after 7 days within the thermal lesion when compared with uninjured tissue liver tissue (Fig. 5E).
HIF1α signaling and tumorigenesis at the site of thermal injury.
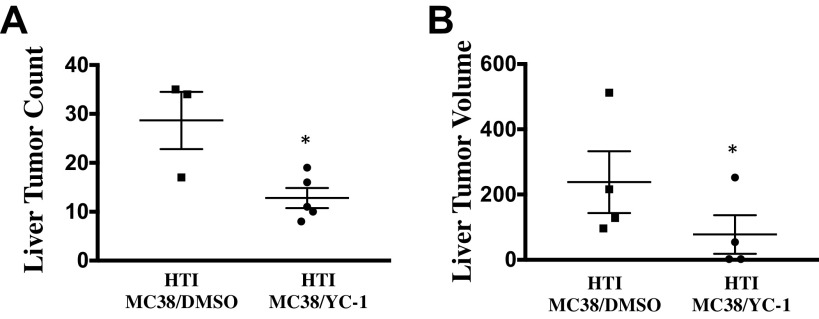
To evaluate the potential role of increased HIF1α signaling in promoting tumorigenesis following HTI, an analogous protocol was performed in the presence versus absence of a small molecule inhibitor of HIF1α (YC-1) (43, 44). Mice were treated by intraperitoneal injection of YC-1 (75 mg/kg) on day 0 (day of HTI and MC38 injection) and day 1 post-HTI. Seven days after the intrasplenic tumor injection, the total hepatic tumor burden was significantly reduced in mice that had received YC-1 treatment relative to vehicle control. The median number of total metastases was 4.0 (IQR = 2.0–4.5) in mice that received YC-1, versus 5.5 (IQR = 5.0–6.75) in mice that received vehicle control (P = 0.03, Fig. 6A). Similarly, tumor volume was significantly decreased in mice that received YC-1 as compared with vehicle control (Fig. 6B).
Fig. 6.
A: inhibition of hypoxia-inducible factor-1α (HIF1α) by YC-1 significantly decreases discrete liver metastases as denoted by lower liver tumor counts. The median number of total metastases was 4.0 (IQR = 2.0–4.5) in mice that received YC-1 versus 5.5 (IQR = 5.0–6.75) in mice that received vehicle control (t test; *P = 0.03, n ≥ 3). B: inhibition of HIF1α by YC-1 significantly decreases tumor volume of liver metastases (n ≥ 4). The median volume of metastases was 71 mm3 (IQR = 8–154) in mice that received YC-1 versus 239 mm3 (IQR = 167–355) in mice that received vehicle control (t test; *P = 0.03, n ≥ 4).
Hepatic thermal injury and activation of hepatic macrophages.
Based on the universal observation of mononuclear cells infiltrating the HTI/tumor site, the potential role of immune effector cells in HTI-associated tumor progression was assessed. We first determined the expression of a canonical macrophage chemoattractant C-C chemokine ligand-2 (CCL2) and C-C chemokine receptor-2 (CCR2) known to be expressed on recruited macrophages (42), in the HTI site relative to unaffected tissue. HTI promoted significant increases in mRNA expression of both CCL2 and CCR2 (Fig. 7A). We next isolated hepatic mononuclear cells isolated from the HTI site and compared these with hepatic mononuclear cells isolated from an equal-sized unaffected liver in the same mouse at day 7 post-HTI. Both, CCR2 and cluster of differentiation molecule-11b (CD11b, encoded by Itgam) mRNA amounts were significantly increased in hepatic mononuclear cells isolated from HTI site relative to ihMNCs isolated from unaffected tissue (Fig. 7B). These findings indicate that the HTI-associated mononuclear cells are enriched in macrophages. Furthermore, we found that HTI induced the infiltration of GATA6-positive ihMNCs, further supporting this finding (Fig. 7C).
Fig. 7.
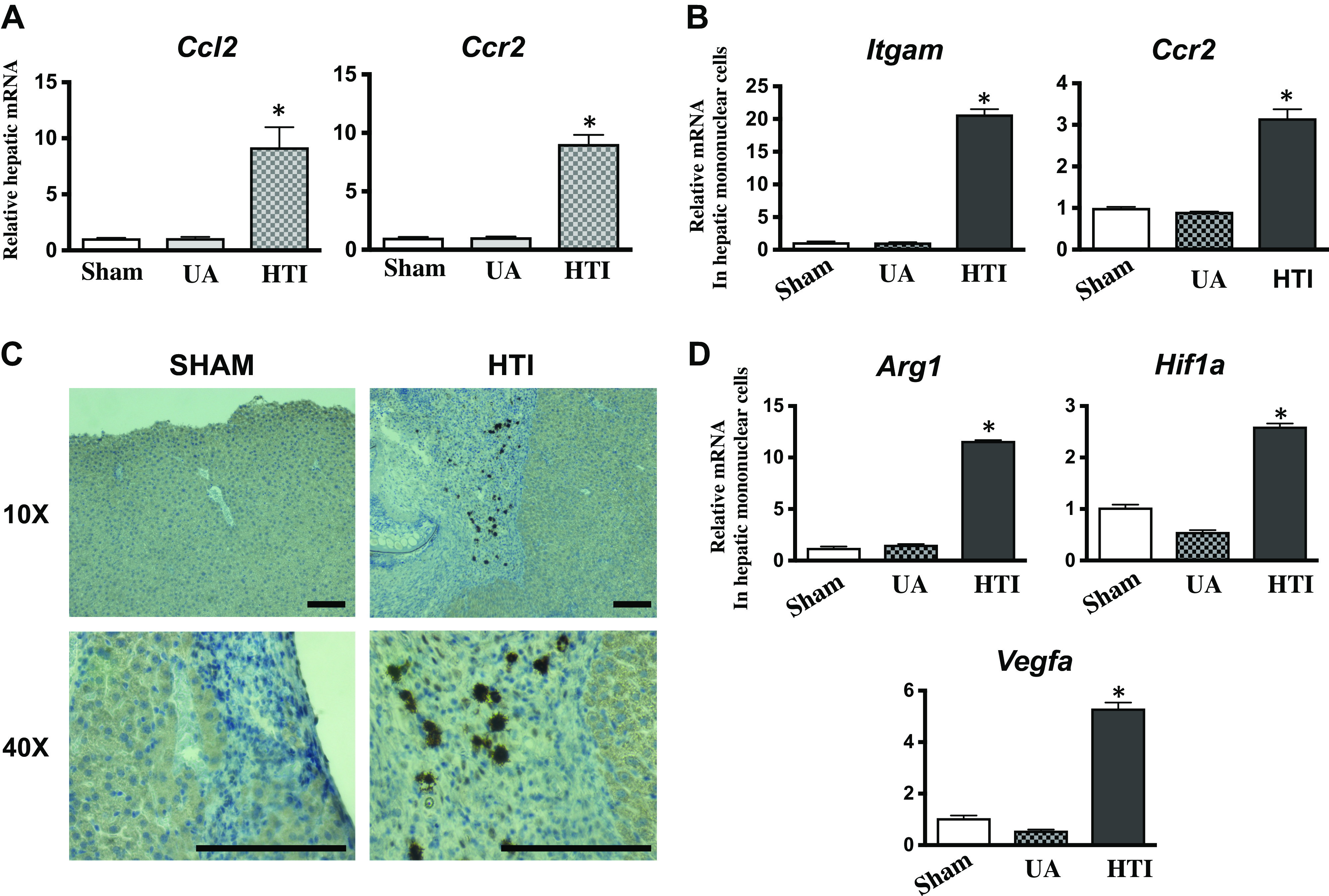
A: examination of the sham injury site, unaffected liver (UA) in mice receiving hepatic thermal injury (HTI), and the HTI site demonstrates significant upregulation for the messenger ribonucleic acid (mRNA) of C-C chemokine ligand-2 (CCL2) (8-fold) and C-C chemokine receptor-2 (CCR2) (8-fold) at the HTI site, indicating the presence of recruited macrophages (ANOVA P = 0.02, n ≥ 3, error bars indicate standard deviation). B: isolation of mononuclear cells from the sham injury site, unaffected liver (UA) in mice receiving HTI, and the HTI site demonstrates significant upregulation of the mRNA for Itgam (20-fold) and CCR2 (3-fold) at the HTI site indicating the presence of recruited macrophages (ANOVA P = 0.04, n ≥ 3, error bars indicate standard deviation). C: sections of liver revealed the presence of GATA-binding protein 6 (GATA6) positive cells in mice who received radiofrequency ablative injury to the liver but not the sham controls who had a sharp injury to the liver (RFA probe without ablative therapy). Sections are shown at both ×10 and ×40 magnification (n ≥ 3). Bar = 1 μm. D: isolation of mononuclear cells from the sham injury site, unaffected liver (UA) in mice receiving HTI, and the HTI site demonstrates significant fold change upregulation of the mRNA for arginase-1 (Arg1) (12-fold), hypoxia-inducible factor-1α (HIF1α) (2.5-fold), and VEGFα (5-fold) at the HTI site, indicating the polarization of these macrophages (ANOVA P = 0.01, n ≥ 3, error bars indicate standard deviation).
Expression of HIF1α, ARG1, and VEGFα in tumor-associated macrophages recently has been shown to be functionally important to tumor growth (12). Therefore, we assessed whether expression of these factors was increased in mononuclear cells isolated from the HTI/tumor site versus mononuclear cells isolated from an equal-sized unaffected liver in the same mouse at day 7 post-HTI. HTI/tumor-associated macrophages had significantly greater expression of mRNA for HIF1α, ARG1, and VEGFα when compared with mononuclear cells from the unaffected liver (Fig. 7D).
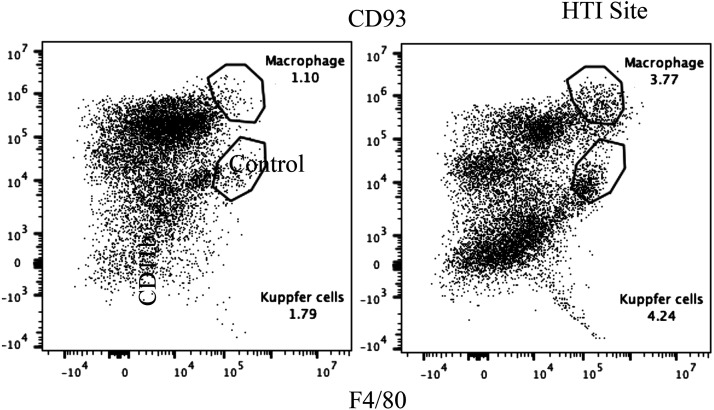
Flow cytometry confirmed the presence of non-Kupffer mononuclear cells consistent with macrophages within the tumor (Fig. 8).
Fig. 8.
Flow cytometry of tumor at engraftment at the site of hepatic thermal injury reveals recruitment of a macrophage cell population distinct from Kupffer cells.
DISCUSSION
In most abdominal malignancies, the appearance of hepatic metastases portends a poor prognosis. Colorectal cancer hepatic metastases, however, can potentially be cured with hepatic resection (11, 12, 15, 41, 45). This clinical finding has led to the expansion of treatment strategies to increase the frequency and safety of liver resection and to encourage clinical use of ablation devices to destroy liver tumors in situ (3, 4, 6, 46). Unfortunately, surgical treatments of hepatic metastases have a high rate of treatment failure due to local recurrence at the site of the colorectal cancer metastasis (47) or the development of new tumors remote from the local treatment site as a result of micrometastases (48).
In the current study, a mouse model was used to demonstrate that hepatic thermal injury promotes tumor engraftment or recurrence at not only the site of injury but also more remote sites not directly subjected to HTI. Furthermore, thermal injury was associated with upregulation of mRNA for HIF1α and its downstream products VEGF and arginase-1 as an early and persistent event following thermal injury (Fig. 5). Blockade of HIF1α with YC-1 inhibited tumor progression (Fig. 6). Histologically, hepatic thermal injury was associated with prominent recruitment of macrophages to the site of thermal injury. These macrophages also exhibited increased production of HIF1α as well as its downstream products. Taken together, these data suggest that surgical techniques using heat energy devices may inadvertently contribute to hepatic tumor recurrence.
Increased expressions of HIF1α and VEGF are hallmarks of wound healing and would be expected to result from thermal and other types of liver cell injury. Macrophages also are critical cellular mediators of wound healing, and we have recently reported that macrophages expressing HIF1α, VEGF, and arginase-1 are also critical components of chronic vascular remodeling (29). Analysis of gene expression in isolated intrahepatic mononuclear cells from within the HTI site and from unaffected liver tissue from the same animal demonstrated that expression of HIF1α, VEGF, and arginase-1 was largely restricted to macrophages within the injury site (29). Our findings suggest that hepatic thermal injury programs macrophages through similar or identical factors as those that condition macrophages in wound healing, chronic tissue remodeling, and cancer (22, 25, 28, 29, 34). These data further suggest that hepatic thermal injury conditions macrophages toward a wound-healing phenotype, which would be a normal and rapid response within the liver, particularly as the liver is known to have remarkable regenerative capacity (49). As noted earlier, Medzhitov et al. (25) reported an identical macrophage phenotype to that reported here, distinguished by expression of HIF1α, arginase-1, and VEGF in a murine model of melanoma, demonstrating that this macrophage phenotype was associated with tumor growth. Importantly expression of arginase-1 in an HIF1α-dependent pathway was shown to be critical for these macrophages in the promotion of tumor growth in vivo. Our findings of elevated mRNA for CCL2, CCR2, and Itgam reveal that recruited macrophages play a significant role in promoting a protumorigenic response to thermal injury and fit well with the work of Kubes et al. (22) In specifically examining the recruited immune cells within hepatic tumors, we found GATA6-positive macrophages as demonstrated by Kubes et al. (22), wherein thermal injury recruits peritoneal macrophages. The persistent presence of these immune cells within hepatic tumors suggests that these peritoneal macrophages may contribute to the creation of a favorable environment for subsequent tumor implantation and progression. This finding is of potential clinical interest, as it has been noted that extensive intraoperative peritoneal lavage improves patient survival for gastrointestinal malignancies (50).
Although there is enthusiasm for the concept that RFA might positively enhance the immune response via T-cell pathways (51), this response is likely insufficient to effect tumor clearance. Therefore, consideration of additional immunomodulation of macrophages holds promise for further augmentation of surgical therapy. We agree with Chu et al. (1) that heat ablative injury is a complex process, and undoubtedly, multiple immune pathways are invoked in response to therapeutic heat injury. Our observations are in line with the paradigm initially put forth by British surgeon Stephen Paget in 1889 (52), in which HTI-treated liver tissue becomes a “fertile soil” for circulating malignant cells shed from remote sites to then “seed” and grow anew. The effects of HTI are present early and they persist. Our findings demonstrate that the HIF1α-mediated pathway plays an important role in HTI-mediated tumor progression.
The orthotopic preclinical model developed for these studies enables control of the degree of hepatic injury, the mode of hepatic injury, and the tumor inoculum. Moreover, the use of syngeneic mice at the same age eliminates patient variability that confounds clinical observations. This model is unique as the concomitant creation of HTI with tumor inoculation within the spleen clearly shows the interrelation of tissue injury/healing and subsequent cancer engraftment and progression, yet fits well with the findings of heterotopic models with previously established tumors (53).
Despite the benefits of this model, the findings were unanticipated, as they relate to the demanding scenario of clinical management of patients with colon cancer. The degree to which all mice developed a metastasis at the site of thermal injury was not anticipated, and it is likely that there are interspecies differences in both signaling and response to circulating tumor cells. Furthermore, the immune system and cytokine response may vary by species. The model itself is designed to rapidly create metastases, and the circulating tumor volume at the time of the initial experiment may not reflect that seen in a typical patient, particularly if they have had systemic therapy. Although direct hepatic injection of tumor cells will produce tumors, reoperating on these animals to treat implanted liver tumors is difficult due to significant scarring and animal morbidity; thus, this model is a surrogate for surgical metastasectomy and thermal ablation. This current work has focused on the HIF1α-VEGFα axis, as our group has significant experience with this mechanism (23, 34, 35). We recognize that important contributions in tumor engraftment and progression may also be attributed to other proinflammatory cytokines such as IL-1ß and IL-6 but examining these is beyond the scope of this study. Other procancer cytokines should be examined to better understand the complex process of HTI in tumor progression.
Despite the potential limitations of this model, clinical consideration of regional or intrahepatic inhibition of HIF1α signaling and preventing specific macrophage polarization toward a wound-healing phenotype holds future therapeutic promise and, if supported by future clinical studies, may improve treatment outcomes of those affected by hepatic tumor metastasis (54, 55).
GRANTS
This study was supported in part by the National Institutes of Health/National Center for Advancing Translational Sciences Colorado Clinical and Translational Sciences Institute (CTSI) Grant Number UL1 TR001082 (to K. C. El Kasmi), the Glen O. Johnson Liver Tumor Research Grant, University of Colorado (to C. C. Barnett, Jr.), and the National Cancer Institute of the National Institutes of Health under Award Number P30CA046934 (to R. D. Schulick). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.L.H., J.G.F., Y.F., J.Y., A.L.A., Y.Z., R.D.S., K.C.E.K., and C.C.B. conceived and designed research; A.L.H., Y.F., J.Y., A.L.A., Y.Z., K.C.E.K., and C.C.B. performed experiments; A.L.H., Y.F., J.Y., A.L.A., Y.Z., R.D.S., K.C.E.K., and C.C.B. analyzed data; J.G.F., A.L.A., Y.Z., and C.C.B. interpreted results of experiments; Y.F., K.C.E.K., and C.C.B. prepared figures; A.L.H., J.G.F., J.Y., and C.C.B. drafted manuscript; A.L.H., J.G.F., Y.Z., R.D.S., and C.C.B. edited and revised manuscript; A.L.H., J.G.F., Y.F., J.Y., A.L.A., Y.Z., R.D.S., K.C.E.K., and C.C.B. approved final version of manuscript.
REFERENCES
- 1.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer 14: 199–208, 2014. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 2.Curley SA. Radiofrequency ablation of malignant liver tumors. Ann Surg Oncol 10: 338–347, 2003. doi: 10.1245/ASO.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Ayav A, Jiao LR, Habib NA. Bloodless liver resection using radiofrequency energy. Dig Surg 24: 314–317, 2007. doi: 10.1159/000103664. [DOI] [PubMed] [Google Scholar]
- 4.Pai M, Spalding D, Jiao L, Habib N. Use of bipolar radiofrequency in parenchymal transection of the liver, pancreas and kidney. Dig Surg 29: 43–47, 2012. doi: 10.1159/000335732. [DOI] [PubMed] [Google Scholar]
- 5.Sainani NI, Gervais DA, Mueller PR, Arellano RS. Imaging after percutaneous radiofrequency ablation of hepatic tumors: Part 1, normal findings. AJR Am J Roentgenol 200: 184–193, 2013. doi: 10.2214/AJR.12.8478. [DOI] [PubMed] [Google Scholar]
- 6.Strasberg SM, Drebin JA, Linehan D. Use of a bipolar vessel-sealing device for parenchymal transection during liver surgery. J Gastrointest Surg 6: 569–574, 2002. doi: 10.1016/s1091-255x(02)00030-6. [DOI] [PubMed] [Google Scholar]
- 7.Leung U, Kuk D, D'Angelica MI, Kingham TP, Allen PJ, DeMatteo RP, Jarnagin WR, Fong Y. Long-term outcomes following microwave ablation for liver malignancies. Br J Surg 102: 85–91, 2015. doi: 10.1002/bjs.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otto G, Düber C, Hoppe-Lotichius M, König J, Heise M, Pitton MB. Radiofrequency ablation as first-line treatment in patients with early colorectal liver metastases amenable to surgery. Ann Surg 251: 796–803, 2010. doi: 10.1097/SLA.0b013e3181bc9fae. [DOI] [PubMed] [Google Scholar]
- 9.Stättner S, Jones RP, Yip VS, Buchanan K, Poston GJ, Malik HZ, Fenwick SW. Microwave ablation with or without resection for colorectal liver metastases. Eur J Surg Oncol 39: 844–849, 2013. doi: 10.1016/j.ejso.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Weng M, Zhang Y, Zhou D, Yang Y, Tang Z, Zhao M, Quan Z, Gong W. Radiofrequency ablation versus resection for colorectal cancer liver metastases: a meta-analysis. PLoS One 7: e45493, 2012. doi: 10.1371/journal.pone.0045493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, Hess K, Curley SA. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 239: 818–825, 2004. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aloia TA, Vauthey JN, Loyer EM, Ribero D, Pawlik TM, Wei SH, Curley SA, Zorzi D, Abdalla EK. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 141: 460–466, 2006. doi: 10.1001/archsurg.141.5.460. [DOI] [PubMed] [Google Scholar]
- 13.Bozzetti F, Bignami P. Recommendation for surgical treatment of colorectal liver metastases. Ann Oncol 11: 243–244, 2000. doi: 10.1023/A:1008306400405. [DOI] [PubMed] [Google Scholar]
- 14.de Jong MC, Mayo SC, Pulitano C, Lanella S, Ribero D, Strub J, Hubert C, Gigot JF, Schulick RD, Choti MA, Aldrighetti L, Mentha G, Capussotti L, Pawlik TM. Repeat curative intent liver surgery is safe and effective for recurrent colorectal liver metastasis: results from an international multi-institutional analysis. J Gastrointest Surg 13: 2141–2151, 2009. doi: 10.1007/s11605-009-1050-0. [DOI] [PubMed] [Google Scholar]
- 15.de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg 250: 440–448, 2009. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 16.Govaert KM, Emmink BL, Nijkamp MW, Cheung ZJ, Steller EJ, Fatrai S, de Bruijn MT, Kranenburg O, Borel Rinkes IH. Hypoxia after liver surgery imposes an aggressive cancer stem cell phenotype on residual tumor cells. Ann Surg 259: 750–759, 2014. doi: 10.1097/SLA.0b013e318295c160. [DOI] [PubMed] [Google Scholar]
- 17.Govaert KM, van Kessel CS, Steller EJ, Emmink BL, Molenaar IQ, Kranenburg O, van Hillegersberg R, Borel Rinkes IH. Recurrence location after resection of colorectal liver metastases influences prognosis. J Gastrointest Surg 18: 952–960, 2014. doi: 10.1007/s11605-014-2461-0. [DOI] [PubMed] [Google Scholar]
- 18.Minami Y, Kudo M. Radiofrequency ablation of liver metastases from colorectal cancer: a literature review. Gut Liver 7: 1–6, 2013. doi: 10.5009/gnl.2013.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanis E, Nordlinger B, Mauer M, Sorbye H, van Coevorden F, Gruenberger T, Schlag PM, Punt CJA, Ledermann J, Ruers TJM. Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases. Analysis of the European Organisation for Research and Treatment of Cancer #40004 and #40983. Eur J Cancer 50: 912–919, 2014. 10.1016/j.ejca.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Viganò L, Capussotti L, Lapointe R, Barroso E, Hubert C, Giuliante F, Ijzermans JN, Mirza DF, Elias D, Adam R. Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol 21: 1276–1286, 2014. doi: 10.1245/s10434-013-3421-8. [DOI] [PubMed] [Google Scholar]
- 21.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 454: 436–444, 2008. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Kubes P. A reservoir of mature cavity macrophages that can rapidly invade visceral organs to affect tissue repair. Cell 165: 668–678, 2016.. doi: 10.1016/j.cell.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Benson DD, Beck AW, Burdine MS, Brekken R, Silliman CC, Barnett CC. Jr.. Accumulation of pro-cancer cytokines in the plasma fraction of stored packed red cells. J Gastrointest Surg 16: 460–468, 2012. doi: 10.1007/s11605-011-1798-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao D, Mittal V. The role of bone-marrow-derived cells in tumor growth, metastasis initiation and progression. Trends Mol Med 15: 333–343, 2009. doi: 10.1016/j.molmed.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513: 559–563, 2014. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chittezhath M, Dhillon MK, Lim JY, Laoui D, Shalova IN, Teo YL, Chen J, Kamaraj R, Raman L, Lum J, Thamboo TP, Chiong E, Zolezzi F, Yang H, Van Ginderachter JA, Poidinger M, Wong AS, Biswas SK. Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Immunity 41: 815–829, 2014. doi: 10.1016/j.immuni.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Bronte V, Murray PJ. Understanding local macrophage phenotypes in disease: modulating macrophage function to treat cancer. Nat Med 21: 117–119, 2015. doi: 10.1038/nm.3794. [DOI] [PubMed] [Google Scholar]
- 28.Campbell L, Saville CR, Murray PJ, Cruickshank SM, Hardman MJ. Local arginase 1 activity is required for cutaneous wound healing. J Invest Dermatol 133: 2461–2470, 2013. doi: 10.1038/jid.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El Kasmi KC, Pugliese SC, Riddle SR, Poth JM, Anderson AL, Frid MG, Li M, Pullamsetti SS, Savai R, Nagel MA, Fini MA, Graham BB, Tuder RM, Friedman JE, Eltzschig HK, Sokol RJ, Stenmark KR. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J Immunol 193: 597–609, 2014. doi: 10.4049/jimmunol.1303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qualls JE, Murray PJ. Tumor macrophages protective and pathogenic roles in cancer development. Curr Top Dev Biol 94: 309–328, 2011. doi: 10.1016/B978-0-12-380916-2.00010-3. [DOI] [PubMed] [Google Scholar]
- 31.Sica A, Invernizzi P, Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology 59: 2034–2042, 2014. doi: 10.1002/hep.26754. [DOI] [PubMed] [Google Scholar]
- 32.Lechner MG, Lade S, Liebertz DJ, Prince HM, Brody GS, Webster HR, Epstein AL. Breast implant-associated, ALK-negative, T-cell, anaplastic, large-cell lymphoma: establishment and characterization of a model cell line (TLBR-1) for this newly emerging clinical entity. Cancer 117: 1478–1489, 2011. doi: 10.1002/cncr.25654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 41: 49–61, 2014. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stenmark KR, Tuder RM, El Kasmi KC. Metabolic reprogramming and inflammation act in concert to control vascular remodeling in hypoxic pulmonary hypertension. J Appl Physiol (1985) 119: 1164–1172, 2015. doi: 10.1152/japplphysiol.00283.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dineen SP, Lynn KD, Holloway SE, Miller AF, Sullivan JP, Shames DS, Beck AW, Barnett CC, Fleming JB, Brekken RA. Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res 68: 4340–4346, 2008. doi: 10.1158/0008-5472.CAN-07-6705. [DOI] [PubMed] [Google Scholar]
- 36.Benson DD, Meng X, Fullerton DA, Moore EE, Lee JH, Ao L, Silliman CC, Barnett CC. Jr.. Activation state of stromal inflammatory cells in murine metastatic pancreatic adenocarcinoma. Am J Physiol Regul Integr Comp Physiol 302: R1067–1075, 2012. doi: 10.1152/ajpregu.00320.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, Wamwea A, Bigelow E, Lutz E, Liu L, Yao S, Anders RA, Laheru D, Wolfgang CL, Edil BH, Schulick RD, Jaffee EM, Zheng L. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother 38: 1–11, 2015. doi: 10.1097/CJI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corbett TH, Griswold DP Jr, Roberts BJ, Peckham JC, Schabel FM. Jr.. Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res 35: 2434–2439, 1975. [PubMed] [Google Scholar]
- 39.Corbett TH, Roberts BJ, Leopold WR, Peckham JC, Wilkoff LJ, Griswold DP Jr, Schabel FM. Jr.. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res 44: 717–726, 1984. [PubMed] [Google Scholar]
- 40.Howard K, Lo KK, Ao L, Gamboni F, Edil BH, Schulick R, Barnett CC. Jr.. Intercellular adhesion molecule-1 mediates murine colon adenocarcinoma invasion. J Surg Res 187: 19–23, 2014. doi: 10.1016/j.jss.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes K, Scheele J, Sugarbaker PH. Surgery for colorectal cancer metastatic to the liver. Optimizing the results of treatment. Surg Clin North Am 69: 339–359, 1989. doi: 10.1016/S0039-6109(16)44790-0. [DOI] [PubMed] [Google Scholar]
- 42.Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, Sugano G, Kato Y, Li J, Pollard JW. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med 212: 1043–1059, 2015. doi: 10.1084/jem.20141836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masoud GN, Wang J, Chen J, Miller D, Design LW. Synthesis and biological evaluation of novel HIF1alpha inhibitors. Anticancer Res 35: 3849–3859, 2015. [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao W, Li X, Li Z. Combination therapy with local radiofrequency ablation and YC-1 inhibits the proliferation and metastasis of hepatocellular carcinoma through activating β-catenin signaling. Pharmazie 71: 524–529, 2016. doi: 10.1691/ph.2016.6602. [DOI] [PubMed] [Google Scholar]
- 45.de Jong MC, van Vledder MG, Ribero D, Hubert C, Gigot JF, Choti MA, Schulick RD, Capussotti L, Dejong CH, Pawlik TM. Therapeutic efficacy of combined intraoperative ablation and resection for colorectal liver metastases: an international, multi-institutional analysis. J Gastrointest Surg 15: 336–344, 2011. doi: 10.1007/s11605-010-1391-8. [DOI] [PubMed] [Google Scholar]
- 46.Dong X, Sun Z, Wu T, Guo W, Yan S, Zheng S. 915-MHz microwave-assisted laparoscopic hepatectomy: a new technique for liver resection. Surg Endosc 33: 395–400, 2019. doi: 10.1007/s00464-017-5945-7. [DOI] [PubMed] [Google Scholar]
- 47.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G, Capussotti L, Vauthey JN. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 241: 715–722, 2005. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishioka Y, Shindoh J, Yoshioka R, Gonoi W, Abe H, Okura N, Yoshida S, Sakamoto Y, Hasegawa K, Fukayama M, Kokudo N. Clinical impact of preoperative chemotherapy on microscopic cancer spread surrounding colorectal liver metastases. Ann Surg Oncol 24: 2326–2333, 2017. doi: 10.1245/s10434-017-5845-z. [DOI] [PubMed] [Google Scholar]
- 49.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol 176: 2–13, 2010. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimada S, Tanaka E, Marutsuka T, Honmyo U, Tokunaga H, Yagi Y, Aoki N, Ogawa M. Extensive intraoperative peritoneal lavage and chemotherapy for gastric cancer patients with peritoneal free cancer cells. Gastric Cancer 5: 168–172, 2002. doi: 10.1007/s101200200029. [DOI] [PubMed] [Google Scholar]
- 51.Li G, Staveley-O'Carroll KF, Kimchi ET. Potential of radiofrequency ablation in combination with immunotherapy in the treatment of hepatocellular carcinoma. J Clin Trials 6: 257, 2016. doi: 10.4172/2167-0870.1000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ribatti D, Mangialardi G, Vacca A. Stephen Paget and the ‘seed and soil’ theory of metastatic dissemination. Clin ExperMed 6: 145–149, 2006. doi: 10.1007/s10238-006-0117-4. [DOI] [PubMed] [Google Scholar]
- 53.Shi L, Wang J, Ding N, Zhang Y, Zhu Y, Dong S, Wang X, Peng C, Zhou C, Zhou L, Li X, Shi H, Wu W, Long X, Wu C, Liao W. Inflammation induced by incomplete radiofrequency ablation accelerates tumor progression and hinders PD-1 immunotherapy. Nat Commun 10: 5421, 2019. doi: 10.1038/s41467-019-13204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hiroshima Y, Maawy A, Hassanein MK, Menen R, Momiyama M, Murakami T, Miwa S, Yamamoto M, Uehara F, Yano S, Mori R, Matsuyama R, Chishima T, Tanaka K, Ichikawa Y, Bouvet M, Endo I, Hoffman RM. The tumor-educated-macrophage increase of malignancy of human pancreatic cancer is prevented by zoledronic acid. PLoS One 9: e103382, 2014. doi: 10.1371/journal.pone.0103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, Mitchem JB, Plambeck-Suess SM, Worley LA, Goetz BD, Wang-Gillam A, Eberlein TJ, Denardo DG, Goedegebuure SP, Linehan DC. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res 19: 3404–3415, 2013. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]