Abstract
Chromatin is a highly dynamic structure whose plasticity is achieved through multiple processes including the posttranslational modification of histone tails. Histone modifications function through the recruitment of nonhistone proteins to chromatin and thus have the potential to influence many fundamental biological processes. Here, we focus on the function and regulation of lysine 20 of histone H4 (H4K20) methylation in multiple biological processes including DNA repair, cell cycle regulation, and DNA replication. The purpose of this review is to highlight recent studies that elucidate the functions associated with each of the methylation states of H4K20, their modifying enzymes, and their protein readers. Based on our current knowledge of H4K20 methylation, we critically analyze the data supporting these functions and outline questions for future research.
INTRODUCTION: THE H4K20 METHYLATION MARK
In eukaryotic organisms, genetic information is encoded within strands of DNA that must be compacted and organized to fit within the nucleus of the cell. The first level of compaction is the wrapping of DNA around an octamer of histone proteins to form the nucleosome, the core structural unit of chromatin. Within the nucleosome octamer exists two copies of each of the H2A, H2B, H3, and H4 histone proteins. N-terminal histone tails protrude from the nucleosome and can be posttranslationally modified to influence chromatin structure and accessibility. Modifications such as methylation, phosphorylation, and acetylation are an important way in which the cell can “mark” regions of the genome for differential and dynamic regulation. In this review, we focus on the methylation of lysine 20 of histone H4 (H4K20).
Methylation of H4K20 was one of the earliest histone posttranslational modifications discovered, and was found in both calf and pea extracts (1). Lysine in the histone H4 position 20 is evolutionarily conserved from yeast to humans (2–4). In addition, H4K20 is part of the group of residues of the H4 tail domain that interacts with the acidic patch, a specific region on the surface of nucleosomes that generates a negatively charged binding surface (5). This interaction mediates intrinsic chromatin condensation and the higher-order structure of chromatin in general, placing H4K20 central to the function of the H4 tail during chromatin remodeling (5). This methylation mark exists in four distinct states and can be un-, mono-, di-, or trimethylated (Fig. 1). The function of unmethylated H4K20 is currently unknown. Each of the methylated states of H4K20 is involved in different biological functions. Monomethylation (H4K20me) is involved in DNA replication. Dimethylation (H4K20me2) is involved in DNA damage repair. Trimethylation (H4K20me3) is considered a hallmark of silenced heterochromatin. H4K20me2 is the most abundant form of H4K20, existing on ∼80% of histones in Drosophila and human cells, and is enriched at sites of DNA damage (6–8). H4K20me1 and H4K20me3 are less abundant and are present on ∼10% and ∼5% of nucleosomes, respectively, in human and mouse cells (2, 9, 10). H4K20me1 has been implicated in transcriptional regulation and is preferentially enriched within the 5′ end of gene bodies (11). In contrast, H4K20me3 has been implicated in heterochromatin maintenance (4, 12) and localizes to telomeres (13, 14) and other repeat elements (15–18). More recently, H4K20me3 has been found to also localize to gene promoters, including the promoters of E2F responsive genes, inflammatory genes, and ribosomal genes (11, 19–21). In this report, we describe the functions associated with each of the methylation states of H4K20 and their respective modifying enzymes, summarize recent studies that dissect the functional role of H4K20 methylation, and critically analyze the data supporting these functions (Table 1).
Figure 1.
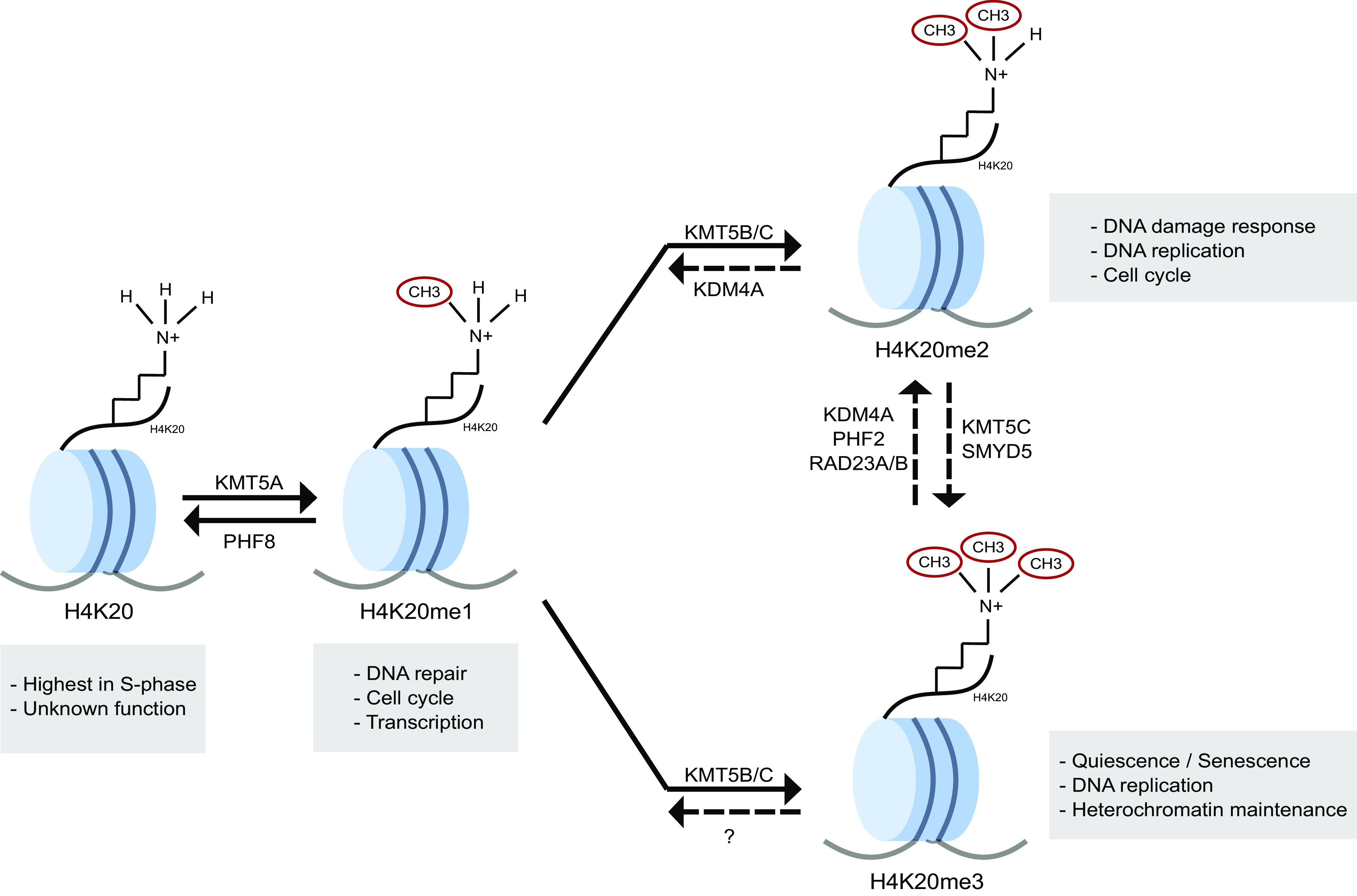
H4K20 methylation biology. Lysine 20 of histone H4 (H4K20) can be un-, mono-, di-, or trimethylated (red circle) each of which has distinct biological functions (gray boxes). Methyltransferase enzymes catalyze the conversion of H4K20 into these methylated states. Demethylase enzymes remove methyl groups from H4K20. Dashed lines represent recently reported enzymes that require more evidence to determine reproducibility.
Table 1.
H4K20 binding proteins
| Modification | Protein | Function | Reference |
|---|---|---|---|
| H4K20me1 | KMT5A (PR-SET7) | Methyltransferase | (22, 23) |
| PHF8 | Demethylase | (24) | |
| LSD1n | Demethylase | (25) | |
| RAD23A/B | Demethylase | (26) | |
| L3MBTL1 | Transcriptional repressor | (27) | |
| MSL3 | Transcription regulation | (28) | |
| H4K20me2 | KMT5B (Suv4-20h1) | Methyltransferase | (4) |
| MMSET | Methyltransferase | (6) | |
| DPY-21 | Demethylase | (29) | |
| RAD23A/B | Demethylase in vitro | (26) | |
| KDM4A (JMJD2A) | DNA damage response | (30, 31) | |
| ORC1 | DNA replication | (32) | |
| 53BP1 | DNA damage response | (33, 34) | |
| FANCD2 | DNA Repair | (35) | |
| L3MBTL1 | Transcriptional repressor | (27) | |
| H4K20me3 | KMT5C (Suv4-20h2) | Methyltransferase | (4) |
| SMYD3 | Methyltransferase | (36) | |
| SMYD5 | Methyltransferase | (21) | |
| PHF2 | Demethylase | (21) | |
| RAD23A/B | Demethylase | (26) | |
| KDM4A (JMJD2A) | DNA damage response | (30) | |
| ORCA/LRWD1 | DNA replication | (37) |
H4K20me1, Lysine 20 of histone H4 monomethylation; H4K20me2, Lysine 20 of histone H4 dimethylation; H4K20me3, Lysine 20 of histone H4 trimethylation.
H4K20 METHYLATION BIOLOGY
Three distinct lysine (K) methyltransferase (KMT) enzymes, each containing SET domains that configure methyltransferase activity, are responsible for the generation of the different methylation states of H4K20 (Fig. 1). The dynamic regulation of H4K20 methylation also includes demethylases, which remove methyl marks, and “readers” that are specialized effector proteins that bind the methylated residues. Together, all of these proteins contribute to the establishment and maintenance of the different H4K20 unmethylated and methylated states.
H4K20me1
Monomethylation of H4K20, the conversion of unmethylated H4K20 to H4K20me1, is catalyzed by KMT5A (also known as SET8 or PR-SET7) (22, 23). KMT5A displays robust specificity because the active site of the enzyme is unable to accommodate a lysine carrying more than a single methyl group (38, 39). In addition to H4K20, KMT5A also has enzymatic activity methylating other nonhistone targets. For example, KMT5A has been found to methylate proliferating cell nuclear antigen (PCNA), stabilizing the protein to promote its function in the DNA damage repair process, as well as the tumor suppressor p53, suppressing p53-mediated transcriptional activation of target genes (40–44). Drosophila genetically depleted of kmt5a have developmental defects and die in the late larval stage, whereas KMT5A knockout mouse embryos die before they reach the eight-cell stage (22, 23, 45). Mammalian cells lacking KMT5A activity showed reduced levels of all three methylated states of H4K20, cell cycle defects, DNA damage, and improper mitotic chromosome condensation (45–48). Although the severe phenotypes in response to KMT5A deletion are likely due to the loss of both H4K20 methylation and its function in nonhistone methylation, there are studies that suggest that H4K20 methylation specifically is responsible for some of the phenotypes associated with loss of KMT5A. For example, Houston et al. (46) found that HEK293T cells transfected with a catalytically dead, dominant negative form of KMT5A had reduced levels of H4K20me1, had increased levels of ɣH2A.X, and accumulated in G2/M, indicating that the methyltransferase function of KMT5A is required for mitotic entry and genomic stability. Notably, by knocking down KMT5A in wild-type and p53-null HCT116 cells, Houston et al. (46) found that these phenotypes occurred independently of KMT5A methylation of p53. Building on this, Shoaib et al. (48) generated U2OS cells containing an H4 mutant carrying a lysine to alanine substitution to understand the specific role KMT5A-mediated H4K20 methylation plays. Shoaib et al. (48) found that cells containing H4K20A had a significant decrease in the level of chromatin compaction and increased DNA damage, indicating that H4K20 methylation is important for these phenotypes associated with loss of KMT5A. An important caveat of these studies is that loss of KMT5A leads to not only reduced levels of monomethylated H4K20 but also a reduction in the levels of the di- and trimethylated forms while increasing the levels of the unmodified form. Similarly, the lysine to alanine substitution in histone H4 would abrogate not only the monomethylated form of the mark, but also the di- and trimethylated form of the mark. These limitations should be considered in determining the strength of the evidence that the monomethylated H4K20 is the crucial mark that determines the phenotypes associated with KMT5A deletion.
Additional evidence supporting an important role for the monomethylated form of H4K20 in the cell cycle and cell fate is derived from the analysis of the enzyme that demethylates H4K20me1, PHF8. PHF8 is a PHD and JUMONJI domain-containing protein that demethylates H4K20me1, as well as the transcriptionally repressive marks H3K9me1/2 and H3K27me2 (24, 49). Gene silencing of PHF8 leads to a delay in the G1-S transition during cell cycle progression in HeLa cells (24) and impairs neuronal differentiation in mouse embryonic carcinoma P19 cells (50). In vivo, functional studies have demonstrated that loss of PHF8 causes apoptosis of neural cells in zebrafish and learning and memory impairments in mice through suppression of mTOR signaling (51, 52). Although these studies have shown that the enzymatic function of PHF8 is important for these phenotypes, discerning which of its histone targets leads to these effects will be important in future work to better understand the potential role of H4K20me1 in these pathways.
Once H4K20me1 is formed, there are multiple different proteins that can bind to this mark and act as a reader or effector (Table 1). One such binding protein is L3MBTL1 [Lethal(3)mbt-like 1], a transcriptional repressor belonging to the polycomb group protein family (27). Biochemical analysis revealed that direct recruitment of L3MBTL1 by H4K20me1/2 is sufficient for chromatin compaction in purified protein-nucleosome complexes (53, 54). This chromatin compaction acts as a transcriptional repressor. L3MBTL1 is recruited to the promoters of p53-targeted genes where it represses their transcription through interactions with KMT5A-methylated-p53 in multiple human cell lines (55, 56). Consistent with this model, RNAi-mediated reduction of KMT5A in HeLa cells increases the expression levels of genes enriched with H4K20me1 in the gene body, and genomic regions enriched for H4K20me1 repress luciferase expression in an artificial promoter system, indicating the importance of KMT5A-mediated methylation of H4K20 for the transcriptional repression of H4K20me1-associated genes (11). In contrast, genome-wide ChIP analyses suggest that H4K20me1 correlates with transcriptional activation. ChIP-Seq analysis of human T cells found that H4K20me1 is one of the histone modifications most highly associated with active transcription (57). Similar to the HeLa cell study described above, H4K20me1 peaks were localized downstream from the transcription start site in T cells, yet in contrast, H4K20me1 correlated with highly transcribed genes (58, 59). Thus, the role of this modification in gene expression is still inconclusive, with evidence supporting both gene activation and repression. These conflicting experimental results may be due to variations in proliferation rates of different cell lines, given the cell-cycle variation of H4K20me1 (described below in H4K20 and the Cell Cycle), or may depend on the fate of H4K20me1 and whether it is bound by L3MBTL1, or another unidentified reader protein, or further methylated to H4K20me2/3.
H4K20me2
H4K20me1 can be further methylated to form H4K20me2 by KMT5B (Suv4-20h1) (Fig. 1). Deletion of this enzyme results in a decrease in levels of H4K20me2 and a concomitant increase in H4K20me1, without affecting H4K20me3 (4, 10). Suv4-20h1−/− mice produce pups at sub-Mendelian ratios, indicative of embryonic lethality. Suv4-20h1-null mutant mice that are born are smaller than wild-type littermates and die perinatally a few hours after birth. Together, these findings indicate an essential function for Suv4-20h1 during embryonic and postnatal development. These extreme phenotypes are likely because murine Suv4-20h1 is ubiquitously expressed during embryogenesis and in adult tissues (10). Knockdown of Suv4-20h1 in chronic myeloid leukemia cells with lentivirally introduced shRNAs resulted in growth inhibition, G1 arrest, and increased expression levels of p21 (60). In contrast to this, deletion of Suv4-20h1 in mouse muscle stem cells (MuSCs) via a complete knockout with Cre-lox technology or lentiviral shRNA knockdown promotes MuSC activation from a quiescent state, and induces transcriptional activation of the MyoD locus, suggesting that the role of Suv4-20h1 in the cell cycle may be cell-type specific (61). However, KMT5B has other reported targets in addition to H4K20me1, which complicates our understanding of whether H4K20 methylation plays an important role in the phenotypes associated with loss of KMT5B expression. ERK1 was identified as a nonhistone target of KMT5B (62), although a recent in vitro study detected no ERK1 methylation by KMT5B (63), so more evidence is needed to substantiate ERK1 as a KMT5B methylation target. Weirich et al. (63) identified CASZ1 (zinc finger protein castor homolog 1) and OSBPL1A (oxysterol-binding protein-related protein 1) as two nonhistone targets of KMT5B, although more work is needed to further validate these findings and to dissect the functional role of KMT5B methylation ofCASZ1 and OSBPL1A.
Recently, several potential demethylases of H4K20me2 have been identified (Table 1). DPY-21, an evolutionarily conserved subunit of the condensin-like dosage compensation complex (DCC), is responsible for demethylating H4K20me2, in turn, enriching H4K20me1, during X-chromosome inactivation in Caenorhabditis elegans (29, 64). Importantly, biochemical analysis of the mouse homolog Rsbn1 revealed that demethylase activity on H4K20me2 is evolutionarily conserved (29). Inactivation of the demethylase activity of DPY-21 eliminates H4K20me1 enrichment, increases X-linked gene expression, and reduces X-chromosome compaction, demonstrating the importance of H4K20 remodeling in the process of X-chromosome inactivation in C. elegans (29). A recent biochemical screen of over 2,000 nuclear proteins identified RAD23A/B as a demethylase for all three methylated states of H4K20 (26). Notably, demethylase activity on H4K20me2 was only detectable in vitro and in cells when the proteins were overexpressed, suggesting that RAD23A/B may not demethylate H4K20me2 under physiological conditions (26).
Similar to H4K20me1, H4K20me2 is read by an array of proteins that function in multiple types of cellular processes (Table 1). Here, we will focus our discussion on two, 53BP1 and ORC1. The double-strand break (DSB) repair protein p53-binding protein 1 (53BP1) is an important reader of H4K20me2 and functions as a coactivator of p53 (33, 65), a tumor suppressor that is frequently mutated in cancer (66) and is activated in response to DNA damage (33, 65). To facilitate repair of damaged DNA, the tandem Tudor domain of 53BP1 binds to H4K20me2 to act as a scaffold for additional DSB-responsive factors, and promote nonhomologous end-joining DNA repair (33, 34). Given that H4K20me2 is the most abundant of the H4K20 marks, its role in DNA repair is surprising because it would seem to lack the specificity that would be needed to recruit repair proteins to the needed genomic location. One mechanism that drives specificity is the role of other H4K20me2 readers, including the lysine demethylase KDM4A, in masking the mark to prevent 53BP1 binding in the absence of DNA damage (31, 67). The lysine demethylase KDM4A (also known as JMJD2A) has been shown to bind to multiple di- and trimethylated lysines, including both H4K20me2 and H4K20me3, although demethylase activity on H4K20 has not yet been detected (30, 31). Thus, it is not yet clear whether KDM4A acts as a demethylase for H4K20me2 or H4K20me3. Through an alternative function independent of its enzymatic activity, KDM4A plays a role in the DNA damage pathway by competing with 53BP1 for binding dimethylated H4K20 at sites of DNA damage (31, 67). Additional reported mechanisms to provide specificity for damaged DNA involve the recruitment of methyltransferases to DSBs to mediate de novo dimethylation of H4K20 (6, 68). The H3K36-histone methyltransferase, MMSET, has been shown to catalyze the dimethylation of H4K20 at sites of DSB to promote the recruitment of 53BP1 in human cell lines (6, 69). Other studies, in both human and mouse cell lines, have found that MMSET is dispensable for this mechanism (68, 70), and instead found that KMT5A and the subsequent recruitment of KMT5B play a key role (10, 42, 68, 70, 71). Cells depleted of KMT5A or KMT5B/C have reduced binding of 53BP1 and consequently impaired DSB repair (10, 68, 71), suggesting that modification of H4K20 could represent a target for new anticancer agents that prevent DNA repair. Further studies are required to clearly define the contributions of each methyltransferase to the role of H4K20me2 in DSB repair.
A second protein that can act as a reader of H4K20me2, ORC1, is a component of the origin of replication complex (ORC) that initiates the protein complex that licenses DNA for replication (32, 37). Interaction between ORC1 and H4K20me2 is required for efficient stabilization of ORC1 and other ORC components at chromatin to promote DNA replication and cell-cycle progression (32). How H4K20me2 is regulated at origins of replication is yet to be elucidated. One potential mechanism involves the targeting of KMT5A to origins of replication, by an unknown mechanism, to deposit H4K20me1 leading to the subsequent generation of H4K20me2, which facilitates ORC stabilization. Consistent with this model, loss of KMT5A enzymatic activity causes defects in proper firing at the origins of replication (37, 48, 72,73), and tethering KMT5A methyltransferase activity to a genomic locus promotes the loading of pre-replication complex proteins on chromatin (73). Notably, this effect is dependent on the presence and downstream activity of KMT5B (and KMT5C described in H4K20me3) (37). A recent study performed in human and mouse cells identified a novel mechanism by which nucleosomes containing the histone variant H2A.Z recruit and bind KMT5B, promoting H4K20me2 deposition, which, in turn, is required for ORC1 binding and stabilization (74). Further testing of these models and understanding the exact role of the H4K20me2-ORC1 interaction in replication regulation are key questions moving forward.
H4K20me3
Progressive methylation of H4K20me1 by KMT5C (Suv4-20h2) generates H4K20me3 (Fig. 1). KMT5B and KMT5C are related enzymes and share a high degree of sequence similarity in their catalytic SET domain (4). They have similar and overlapping functions as deletion of both enzymes in MEFs leads to loss of H4K20me2 and H4K20me3, with a concomitant increase in the levels of H4K20me1 (10, 75). However, deletion of the individual enzymes in MEFs identified clear differences between the enzymes with respect to H4K20 methylation; KMT5B deletion reduced H4K20me2 levels by ∼60% without affecting H4K20me3, whereas deletion of KMT5C largely eradicated H4K20me3 but did not have an impact on levels of H4K20me2 (10). Kmt5b−/−;Kmt5c−/− double knock-out mice are born at sub-Mendelian ratios, have perinatal death, and are smaller than control littermates upon birth (10). As described above in H4K20me2, a similar phenotype occurs with the single deletion of Kmt5b. Deletion of only Kmt5c is compatible with life, and the mice seem to develop normally, possibly due to the more restricted expression pattern of Kmt5c in the mouse embryo relative to Kmt5b, which is ubiquitously expressed in the mouse embryo and adult tissues, or through a compensatory effect by KMT5B (10). Downregulation of KMT5B combined with inactivation of KMT5C in brown adipose tissue (BAT) in mice resulted in complete abrogation of H4K20me3, activated BAT metabolism, and protection against obesity through regulation of the nuclear receptor PPAR-γ (76). In Drosophila, deletion of Kmt5c leads to longer telomeres and induces the expression of transposons (13, 77). Knockdown of KMT5C in human dermal fibroblasts results in decreased chromatin compaction, an increase of cells in the S-phase, and a defect in quiescence entry (2).
In addition to its SET domain, which performs its methyltransferase activity, the C-terminal domain of KMT5C has been shown to interact with cohesin and heterochromatin protein HP1 (4, 78). H4K20 trimethylation can be regulated via an interaction between KMT5C and HP1 (4). In this proposed model for H4K20 trimethylation, KMT5C is recruited to heterochromatic regions through its interactions with HP1, which also recognizes H3K9 methylation (4). Consistent with this model, deletion of H3K9 methyltransferases or HP1 causes decreased heterochromatic targeting of KMT5C, and a reduction in H4K20me3 (4). An alternative pathway that targets KMT5C to chromatin is through a lncRNA-based mechanism. In this pathway, the lncRNAs PAPAS, a heterogeneous population of lncRNAs, guide KMT5C to the promoters of rRNA genes (rDNA) to establish H4K20me3-mediated chromatin compaction and transcriptional repression (20).
Members of the RB1 family were shown to interact with both KMT5B and KMT5C (79). In RB1-family triple-knockout MEFs, H4K20me3 deposition was diminished at pericentric and telomeric chromatin, suggesting that members of the RB1 family are important for stabilizing H4K20me3 (79). In addition to its role in methylating H4K20, KMT5C was recently found to methylate three novel substrates in vitro: the zinc finger transcription factor CASZ1 and the centromeric proteins OIP5 and CENPU, although these interactions have not been confirmed in cells and the functional role of KMT5C-mediated methylation of these proteins is not yet understood (63).
Two other methyltransferases have been found to trimethylate H4K20, SMYD3 and SMYD5 (Table 1). SMYD3, known to mediate trimethylation of H3K4, has also been reported to trimethylate H4K20, as well as H4K5 (36, 80). The SMYD3 protein is frequently overexpressed in human cancers and has been found to regulate both human and mouse skeletal muscle differentiation through its methyltransferase activity (81). SMYD3 also regulates DNA repair via homologous recombination through H3K4 methylation in human breast cancer cell lines (82) and regulates cell cycle protein Cyclin D2 based on its methyltransferase activity toward H4K20 in human pancreatic cancer cell lines (83). SMYD5 is a component of the NCoR complex, which catalyzes trimethylation of H4K20 on a subset of proinflammatory promoters to repress expression of target genes in Drosophila and mouse primary macrophages (21). Recently, SMYD5 was found to mediate methylation of H4K20me2 to H4K20me3 at heterochromatin regions (84), regulate embryonic stem cell self-renewal (84), and play a crucial role in hematopoiesis during zebrafish embryogenesis (85). However, based on these studies, there is not sufficient evidence to prove that SMYD5 exerts these biological functions through its enzymatic activity, and this important distinction should be addressed in future studies. Although KMT5C is the primary methyltransferase of H4K20me3, KMT5C, SMYD3, and SMYD5 may direct the formation of H4K20me3 at different genomic locations, by various protein complexes. Alternatively, they may play redundant roles in the cell and have overlapping genomic targets. Given that expression of Kmt5c is limited during embryo development and detectable only in some adult tissues (10), it is also possible that the role of these methyltransferases is cell-type specific.
Multiple potential demethylases for H4K20me3 have been identified (Table 1). PHF2 was found to remove H4K20me3 in a nuclear factor-ϰB (NF-ϰB)-dependent manner on purified mononucleosomes and in primary macrophages grown in culture (21). Demethylation of H4K20me3 via PHF2 is a critical step in gene activation of inflammatory response genes, but more work is needed to understand whether PHF2 regulates H4K20me3 at additional genomic loci. In addition, several reports have demonstrated that PHF2 functions as a demethylase of additional histone modifications, including H3K4me3 and H3K9me1/2 (21, 86, 87). Recently, a screen of 2,500 nuclear proteins identified the human homologs of RAD23A/B (hHR23A/B) as demethylases of all three methylated forms of H4K20 in recombinant nucleosomes, although demethylase activity was detected on only H4K20me3 and H4K20me1 in cells (26). In mice, RAD23A and RAD23B have a fully redundant role in nucleotide excision repair, and inactivation of both RAD23A/B in mice causes embryonic lethality, indicating their importance for embryonic development (88). These proteins regulate the transcription of repetitive elements and mRNA by demethylating H4K20me3 (repetitive elements) and H4K20me1 (mRNA) (26). Further studies will provide important details of the mechanism by which these proteins are specifically recruited to H4K20 binding sites.
The primary known reader of H4K20me3 is ORCA/LRWD1, an important factor that interacts with components of the origin recognition complex (ORC), which binds to H4K20me3 via its WD repeat domain (37, 89). Although ORC1 described above in H4K20me2 as an H4K20me2 reader is part of the ORC complex, ORCA, the H4K20me3 reader, is not itself part of the complex, but instead stabilizes ORC onto chromatin. ORCA is required for the initiation of DNA replication, and it also plays crucial roles in heterochromatin organization, possibly via its interactions with repressive histone methyltransferases, including KMT5C (90). Depletion of ORCA results in loss of ORC binding to chromatin, diminished MCM2-7 loading, and an accumulation in the G1 phase, suggesting its importance in initiation of the pre-replication complex in G1 (89, 91). Consistent with this, Brustel et al. (92) demonstrated that the conversion of H4K20me1 to higher methylation states (H4K20me2/3) enhances pre-RC loading and replication activation through the recruitment of ORC-A at late-replicating heterochromatin domains. To prove that methylation of H4K20 is specifically required for this effect, Brustel et al. (92) replaced a large fraction of the pool of H4 histones with histone H4K20A mutants and silenced Suv4-20H with either a chemical inhibitor (human cell culture) or through genetic knockout (in MEFs). Notably, the effects of H4K20me1, H4K20me2, and H4K20me3 are indistinguishable in this model. Given that both H4K20me2 and H4K20me3 interact with different components of ORC, future studies should clarify which of these interactions is important for the recruitment of replication factors to late-replicating regions, as well as how that specificity is driven. Altogether, these findings suggest the H4K20me3-ORCA interaction may play an important role in the selection of replication initiation sites.
H4K20 AND THE CELL CYCLE
Levels of the different methylated forms of H4K20 vary widely across the cell cycle (2, 9). Levels of the unmodified form of H4K20 are the highest during the S-phase and decline after DNA replication as H4K20me1 begins to accumulate and cells move into G2/M. The cell cycle-dependent changes in H4K20me1 are due to parallel cell cycle changes in KMT5A protein levels (22, 23). KMT5A is actively targeted for proteasome-mediated degradation in S-phase and is later stabilized in G2 and M phases via M-phase specific phosphorylation at serine 29 (19, 93–95). Additionally, PHF8, the H4K20me1 demethylase, is removed from chromatin in prophase allowing for accumulation of H4K20me1 as cells continue through the M-phase (24). Notably, the newly constituted H4K20me1 in G2/M may be protected from further conversion by KMT5B/C until the cells have passed through mitosis (96). The mechanism by which this occurs is still unresolved but could involve a reader of H4K20me1 that physically blocks interactions with methyltransferases or posttranslational modifications to the KMT5B/C enzymes themselves that suppress enzymatic activity. Levels of dimethylated and trimethylated H4K20 are relatively stable through the cell cycle but increase in quiescence (2). This methylation pattern is associated with both reversible and irreversible cell cycle exit, as nondividing states, such as senescence, also show increased levels of H4K20me3 (2, 17, 61, 97). A reduction of KMT5C results in increased S-phase cell number and defects in cell cycle exit, whereas overexpression of KMT5C causes decreases in S-phase cell number and supports senescence (2, 17). The binding of H4K20me3 by ORCA suggests that a loss of H4K20me3 at critical regions of the genome may cause these proliferation defects by interfering with normal origin firing. The role of H4K20me2/3 in cell cycle exit may also be explained by currently undiscovered readers. Proper regulation of these dynamic fluctuations in H4K20 methylation throughout the cell cycle is important to preserve cellular homeostasis and further studies will highlight the specific role these marks play throughout the cell cycle.
H4K20 METHYLATION IN CANCER AND DEVELOPMENT
The role H4K20 methylation plays in proper regulation of cell cycle progression and cell cycle exit has implications in other aspects of biology, including cancer and development. Global loss of H4K20me3 has been observed in multiple types of cancer (98–102). In a comprehensive panel of normal tissues, cancer cell lines, and primary tumors, cancer cells consistently exhibited decreased H4K20me3 (99). In lung, bladder, colon, and liver cancer, H4K20me3 levels decreased with increasing tumor grade and H4K20me3 levels predicted survival (98, 101, 103, 104). In breast cancer cell lines, the loss of H4K20me3 was accompanied by a decrease in KMT5C, and ectopic expression of KMT5C suppressed cell invasiveness (100, 102, 105). In contrast, analysis of data from The Cancer Genome Atlas (TCGA) on KMT5C expression in multiple cancer types showed an elevation of KMT5C expression in tumors compared with matched healthy tissues (106). One possible explanation to accommodate these conflicting observations is that while levels of KMT5C increase in cancers, the simultaneous increase in expression of important demethylases may outcompete the methyltransferase, altogether resulting in lower levels of H4K20me3. Additionally, KMT5C may have other tumor-promoting functions in addition to H4K20 methylation. Recently, the microRNA mir-29 was identified as a negative regulator of KMT5C in breast cancer cells and mir-29 overexpression reduced KMT5C, promoted breast cancer cell epithelial-mesenchymal transition, migration, and invasion (107). These results are contradictory to a recent study showing that high levels of KMT5C in pancreatic cancer cells, and increased H4K20me3 at relevant genes, correlated with a loss of epithelial characteristics in progressively invasive cancer, suggesting the activity of KMT5C with regard to epithelial-mesenchymal transition and H4K20me3 is context-dependent (106). Together, these data suggest a complicated role for KMT5C-mediated H4K20 trimethylation in tumorigenesis.
CONCLUDING REMARKS
Methylation of H4K20 is essential for development, and alterations in H4K20 methylation biology are associated with a variety of diseases, including cancer and developmental disorders. Here, we highlight the diversity of functions associated with each methylation state, which are largely dictated by the binding of specific reader proteins. As an example, H4K20me2 is linked to either DNA repair or DNA replication based on whether it is bound by 53BP1 or ORC1. Understanding how distinct readers are differentially targeted will be important to address and will help clarify the mechanisms by which H4K20 methylation regulates different aspects of biology.
The abundance of modifications on histone tails makes cross-talk between modifications likely. For instance, cross-talk exists between methylation of H4K20 and acetylation of H4K16. H4K20me1 and H4K16ac are mutually restrictive in vitro and on Drosophila chromosomes, and acetylation of H4K16 prevents monomethylation of H4K20 by KMT5A (23). Additionally, H4K20me1 prevents the acetylation of H4K16 by the acetyltransferase p300 in vitro (23). However, proteomic analysis in HeLa cells showed that the most abundant form of H4K16ac occurs predominantly on nucleosomes which are also dimethylated at H4K20 (9). Importantly, acetylation at H4K16 antagonizes 53BP1 binding to H4K20me2 and thus affects DNA repair, suggesting that each mark can modulate the ability of reader domains to recognize and bind the other mark (108, 109). Finally, an antagonistic relationship between H4K20me3 and H4K16ac has been described in which KMT5C-mediated trimethylation of H4K20 blocks recruitment of hMOF and subsequent acetylation of H4K16ac, enforcing RNA Pol II pausing (110). Communication between histone modifications can also occur when the modifications are on different histone tails, such as between H3K9 and H4K20. Double knockout of the H3K9 methyltransferases Suv39h1/2 in MEFs depletes H3K9me3 and H4K20me3 (4). However, H3K9me3 signals are unaffected by the absence of KMT5B/C. Additionally, the C-terminal domain of KMT5C interacts with heterochromatin protein, HP1 which also recognizes H3K9me3 (78, 111). Together this suggests a model in which SUV39H-mediated H3K9me3 recruits HP1 which then brings in KMT5C to establish H4K20me3. Cross-talk between histone modifications provides a complex layer in the regulation and understanding of the genome.
Adding another layer of complexity is our assumption that each individual histone modification leads to biological consequences when the evidence to support such models are usually based on findings that may have multiple explanations. In truth, teasing apart the roles of individual methylation states is difficult. For example, in understanding the role of H4K20 and DNA replication, Shoaib et al. and others (48, 73) have shown that the absence of KMT5A or the H4K20 residue itself lead to improper recruitment and loading of the replication machinery and concluded that KMT5A and H4K20me1 were important for DNA licensing. However, preventing H4K20 monomethylation, in turn, prevents further di- and trimethylation, both of which have been shown to interact with crucial DNA replication licensing machinery (32, 37, 92). Thus, although H4K20me1 is important for this process, it is possible that the effect is also dependent on the di- and trimethylated forms of the lysine (37). Proving causality for a histone modification typically involves demonstrating that the catalytic activity of the enzyme is necessary for the biological response. But even this approach has caveats, as many histone-modifying enzymes have other nonhistone substrates. For instance, KMT5A has also been found to methylate not only H4K20, but also PCNA, to promote its function in the DNA damage response, and p53 to suppress its transcriptional activation of target genes (40, 42–44). Mammalian cells lacking KMT5A show reduced levels of H4K20me1, cell cycle defects, and DNA damage, suggesting that H4K20me1 plays an important role in these pathways (45–47). However, these phenotypes in response to KMT5A depletion are likely due to a combination of the absence of H4K20me1 as well as its function in nonhistone methylation pathways. Additionally, there may be redundancy such that more than one enzyme is capable of modifying a specific site, as in the case of H4K20me3. In this case, diminishing the levels of one enzyme may lead to a compensatory upregulation in the activity of another enzyme, masking the effect. An important and stringent test would be showing that mutation of the modified residue results in the same phenotype as mutation of the enzyme itself. However, this is not easily done in mammalian systems due to the many copies of histone genes present in the genome. Additionally, modification of the entire residue depletes all methylated forms of the mark, making the role of each methylation state indistinguishable. For all of these reasons, developing models about the functional role of a specific histone modification requires multiple lines of evidence, each of which may have caveats, so that together a strong argument can be made. Taken together, the available data at this point supports an important role for H4K20 in cell cycle, cancer, and cell fate, although there are many aspects of the mechanisms that contribute to its phenotypic roles that require further investigation.
GRANTS
This work was supported by National Institute of General Medical Science (NIGMS) Grant R01 GM081686 and R01 GM0866465, The National Institutes of Health (NIH) Grant R01 AR070245, National Cancer Institute (NCI) Grant RC1 CA 147961-02, PhRMA Foundation Grant 2007RSGl9572, National Cancer Institute Grant P50 CA092131, the Cancer Research Institute Clinical Laboratory Integration Program Award, a Melanoma Research Alliance Team Science Award; Rose Hills Foundation, Innovation, and Hal Gaba Awards from the UCLA Broad Stem Cell Center; the Jonsson Comprehensive Cancer Center Program Leader Vision Award; the University of California Cancer Research Coordinating Committee. This work was funded by the Cell and Molecular Biology Training Grant (NIH Ruth L. Kirschstein National Research Service Award GM007185; to A.Z.C.). A.Z.C was the recipient of a Whitcome Pre-Doctoral Fellowship.
DISCLOSURES
H.A.C is a member of the UCLA Broad Stem Cell Center, the Jonsson Comprehensive Cancer Center, the UCLA Molecular Biology Institute, and the UCLA Bioinformatics Interdepartmental Program. No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.Z.C. and H.A.C. analyzed data; A.Z.C. drafted manuscript; A.Z.C. and H.A.C. edited and revised manuscript; A.Z.C. and H.A.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the entire Coller laboratory for helpful discussion.
REFERENCES
- 1.DeLange RJ, Fambrough DM, Smith EL, Bonner J. Calf and pea histone IV. II. The complete amino acid sequence of calf thymus histone IV; presence of epsilon-N-acetyllysine. J Biol Chem 244: 319–334, 1969. [PubMed] [Google Scholar]
- 2.Evertts AG, Manning AL, Wang X, Dyson NJ, Garcia BA, Coller HA. H4K20 methylation regulates quiescence and chromatin compaction. Mol Biol Cell 24: 3025–3037, 2013. doi: 10.1091/mbc.e12-07-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanders SL, Portoso M, Mata J, Bähler J, Allshire RC, Kouzarides T. Methylation of histone H4 lysine 20 controls recruitment of Crb2 to sites of DNA damage. Cell 119: 603–614, 2004. doi: 10.1016/j.cell.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Schotta G, Lachner M, Sarma K, Ebert A, Sengupta R, Reuter G, Reinberg D, Jenuwein T. A silencing pathway to induce H3-K9 and H4-K20 trimethylation at constitutive heterochromatin. Genes Dev 18: 1251–1262, 2004. doi: 10.1101/gad.300704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalashnikova AA, Porter-Goff ME, Muthurajan UM, Luger K, Hansen JC. The role of the nucleosome acidic patch in modulating higher order chromatin structure. J R Soc Interface 10: 20121022, 2013. doi: 10.1098/rsif.2012.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pei H, Zhang L, Luo K, Qin Y, Chesi M, Fei F, Bergsagel PL, Wang L, You Z, Lou Z. MMSET regulates histone H4K20 methylation and 53BP1 accumulation at DNA damage sites. Nature 470: 124–128, 2011. doi: 10.1038/nature09658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H, Pesavento JJ, Starnes TW, Cryderman DE, Wallrath LL, Kelleher NL, Mizzen CA. Preferential dimethylation of histone H4 lysine 20 by Suv4-20. J Biol Chem 283: 12085–12092, 2008. doi: 10.1074/jbc.M707974200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young NL, DiMaggio PA, Plazas-Mayorca MD, Baliban RC, Floudas CA, Garcia BA. High throughput characterization of combinatorial histone codes. Mol Cell Proteomics 8: 2266–2284, 2009. doi: 10.1074/mcp.M900238-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pesavento JJ, Yang H, Kelleher NL, Mizzen CA. Certain and progressive methylation of histone H4 at lysine 20 during the cell cycle. Mol Cell Biol 28: 468–486, 2008. doi: 10.1128/MCB.01517-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schotta G, Sengupta R, Kubicek S, Malin S, Kauer M, Callén E, Celeste A, Pagani M, Opravil S, De La Rosa-Velazquez IA, Espejo A, Bedford MT, Nussenzweig A, Busslinger M, Jenuwein T. A chromatin-wide transition to H4K20 monomethylation impairs genome integrity and programmed DNA rearrangements in the mouse. Genes Dev 22: 2048–2061, 2008. doi: 10.1101/gad.476008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Congdon LM, Houston SI, Veerappan CS, Spektor TM, Rice JC. PR-Set7-mediated monomethylation of histone H4 lysine 20 at specific genomic regions induces transcriptional repression. J Cell Biochem 110: 609–619, 2010. doi: 10.1002/jcb.22570. [DOI] [PubMed] [Google Scholar]
- 12.Kourmouli N, Jeppesen P, Mahadevhaiah S, Burgoyne P, Wu R, Gilbert DM, Bongiorni S, Prantera G, Fanti L, Pimpinelli S, Shi W, Fundele R, Singh PB. Heterochromatin and tri-methylated lysine 20 of histone H4 in animals. J Cell Sci 117: 2491–2501, 2004. doi: 10.1242/jcs.01238. [DOI] [PubMed] [Google Scholar]
- 13.Benetti R, Gonzalo S, Jaco I, Schotta G, Klatt P, Jenuwein T, Blasco MA. Suv4-20h deficiency results in telomere elongation and derepression of telomere recombination. J Cell Biol 178: 925–936, 2007. doi: 10.1083/jcb.200703081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marión RM, Schotta G, Ortega S, Blasco MA. Suv4-20h abrogation enhances telomere elongation during reprogramming and confers a higher tumorigenic potential to iPS cells. PLoS One 6: e25680, 2011. doi: 10.1371/journal.pone.0025680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martens JH, O’Sullivan RJ, Braunschweig U, Opravil S, Radolf M, Steinlein P, Jenuwein T. The profile of repeat-associated histone lysine methylation states in the mouse epigenome. EMBO J 24: 800–812, 2005. doi: 10.1038/sj.emboj.7600545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montoya-Durango DE, Liu Y, Teneng I, Kalbfleisch T, Lacy ME, Steffen MC, Ramos KS. Epigenetic control of mammalian LINE-1 retrotransposon by retinoblastoma proteins. Mutat Res 665: 20–28, 2009. doi: 10.1016/j.mrfmmm.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson DM, Jaber-Hijazi F, Cole JJ, Robertson NA, Pawlikowski JS, Norris KT, Criscione SW, Pchelintsev NA, Piscitello D, Stong N, Rai TS, McBryan T, Otte GL, Nixon C, Clark W, Riethman H, Wu H, Schotta G, Garcia BA, Neretti N, Baird DM, Berger SL, Adams PD. Mapping H4K20me3 onto the chromatin landscape of senescent cells indicates a function in control of cell senescence and tumor suppression through preservation of genetic and epigenetic stability. Genome Biol 17: 158, 2016. doi: 10.1186/s13059-016-1017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhodes CT, Sandstrom RS, Huang S-WA, Wang Y, Schotta G, Berger MS, Lin C-HA. Cross-species analyses unravel the complexity of H3K27me3 and H4K20me3 in the context of neural stem progenitor cells. Neuroepigenetics 6: 10–25, 2016. doi: 10.1016/j.nepig.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbas T, Shibata E, Park J, Jha S, Karnani N, Dutta A. CRL4(Cdt2) regulates cell proliferation and histone gene expression by targeting PR-Set7/Set8 for degradation. Mol Cell 40: 9–21, 2010. doi: 10.1016/j.molcel.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bierhoff H, Dammert MA, Brocks D, Dambacher S, Schotta G, Grummt I. Quiescence-induced LncRNAs trigger H4K20 trimethylation and transcriptional silencing. Mol Cell 54: 675–682, 2014. doi: 10.1016/j.molcel.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 21.Stender JD, Pascual G, Liu W, Kaikkonen MU, Do K, Spann NJ, Boutros M, Perrimon N, Rosenfeld MG, Glass CK. Control of proinflammatory gene programs by regulated trimethylation and demethylation of histone H4K20. Mol Cell 48: 28–38, 2012. doi: 10.1016/j.molcel.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang J, Feng Q, Ketel CS, Wang H, Cao R, Xia L, Erdjument-Bromage H, Tempst P, Simon JA, Zhang Y. Purification and functional characterization of SET8, a nucleosomal histone H4-lysine 20-specific methyltransferase. Curr Biol 12: 1086–1099, 2002. doi: 10.1016/S0960-9822(02)00924-7. [DOI] [PubMed] [Google Scholar]
- 23.Nishioka K, Rice JC, Sarma K, Erdjument-Bromage H, Werner J, Wang Y, Chuikov S, Valenzuela P, Tempst P, Steward R, Lis JT, Allis CD, Reinberg D. PR-Set7 is a nucleosome-specific methyltransferase that modifies lysine 20 of histone H4 and is associated with silent chromatin. Mol Cell 9: 1201–1213, 2002. doi: 10.1016/S1097-2765(02)00548-8. [DOI] [PubMed] [Google Scholar]
- 24.Liu W, Tanasa B, Tyurina OV, Zhou TY, Gassmann R, Liu WT, Ohgi KA, Benner C, Garcia-Bassets I, Aggarwal AK, Desai A, Dorrestein PC, Glass CK, Rosenfeld MG. PHF8 mediates histone H4 lysine 20 demethylation events involved in cell cycle progression. Nature 466: 508–512, 2010. doi: 10.1038/nature09272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Telese F, Tan Y, Li W, Jin C, He X, Basnet H, Ma Q, Merkurjev D, Zhu X, Liu Z, Zhang J, Ohgi K, Taylor H, White RR, Tazearslan C, Suh Y, Macfarlan TS, Pfaff SL, Rosenfeld MG. LSD1n is an H4K20 demethylase regulating memory formation via transcriptional elongation control. Nat Neurosci 18: 1256–1264, 2015. doi: 10.1038/nn.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao X, Chen Y, Wu B, Wang X, Xue H, Yu L, Li J, Wang Y, Wang W, Xu Q, Mao H, Peng C, Han G, Chen CD. Histone H4K20 demethylation by two hHR23 proteins. Cell Rep 30: 4152–4164.e6, 2020. doi: 10.1016/j.celrep.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Boccuni P, MacGrogan D, Scandura JM, Nimer SD. The human L(3)MBT polycomb group protein is a transcriptional repressor and interacts physically and functionally with TEL (ETV6). J Biol Chem 278: 15412–15420, 2003. doi: 10.1074/jbc.M300592200. [DOI] [PubMed] [Google Scholar]
- 28.Kim D, Blus BJ, Chandra V, Huang P, Rastinejad F, Khorasanizadeh S. Corecognition of DNA and a methylated histone tail by the MSL3 chromodomain. Nat Struct Mol Biol 17: 1027–1029, 2010. doi: 10.1038/nsmb.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brejc K, Bian Q, Uzawa S, Wheeler BS, Anderson EC, King DS, Kranzusch PJ, Preston CG, Meyer BJ. Dynamic control of X chromosome conformation and repression by a histone H4K20 demethylase. Cell 171: 85–102.e23, 2017. doi: 10.1016/j.cell.2017.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J, Thompson JR, Botuyan MV, Mer G. Distinct binding modes specify the recognition of methylated histones H3K4 and H4K20 by JMJD2A-tudor. Nat Struct Mol Biol 15: 109–111, 2008. doi: 10.1038/nsmb1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mallette FA, Mattiroli F, Cui G, Young LC, Hendzel MJ, Mer G, Sixma TK, Richard S. RNF8- and RNF168-dependent degradation of KDM4A/JMJD2A triggers 53BP1 recruitment to DNA damage sites. EMBO J 31: 1865–1878, 2012. doi: 10.1038/emboj.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuo AJ, Song J, Cheung P, Ishibe-Murakami S, Yamazoe S, Chen JK, Patel DJ, Gozani O. The BAH domain of ORC1 links H4K20me2 to DNA replication licensing and Meier-Gorlin syndrome. Nature 484: 115–119, 2012. doi: 10.1038/nature10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Botuyan MV, Lee J, Ward IM, Kim J-E, Thompson JR, Chen J, Mer G. Structural basis for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair. Cell 127: 1361–1373, 2006. doi: 10.1016/j.cell.2006.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bunting SF, Callén E, Wong N, Chen H-T, Polato F, Gunn A, Bothmer A, Feldhahn N, Fernandez-Capetillo O, Cao L, Xu X, Deng C-X, Finkel T, Nussenzweig M, Stark JM, Nussenzweig A. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell 141: 243–254, 2010. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renaud E, Barascu A, Rosselli F. Impaired TIP60-mediated H4K16 acetylation accounts for the aberrant chromatin accumulation of 53BP1 and RAP80 in Fanconi anemia pathway-deficient cells. Nucleic Acids Res 44: 648–656, 2016. doi: 10.1093/nar/gkv1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foreman KW, Brown M, Park F, Emtage S, Harriss J, Das C, Zhu L, Crew A, Arnold L, Shaaban S, Tucker P. Structural and functional profiling of the human histone methyltransferase SMYD3. PLoS One 6: e22290, 2011. doi: 10.1371/journal.pone.0022290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beck DB, Burton A, Oda H, Ziegler-Birling C, Torres-Padilla M-E, Reinberg D. The role of PR-Set7 in replication licensing depends on Suv4-20h. Genes Dev 26: 2580–2589, 2012. doi: 10.1101/gad.195636.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Couture J-F, Collazo E, Brunzelle JS, Trievel RC. Structural and functional analysis of SET8, a histone H4 Lys-20 methyltransferase. Genes Dev 19: 1455–1465, 2005. doi: 10.1101/gad.1318405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao B, Jing C, Kelly G, Walker PA, Muskett FW, Frenkiel TA, Martin SR, Sarma K, Reinberg D, Gamblin SJ, Wilson JR. Specificity and mechanism of the histone methyltransferase Pr-Set7. Genes Dev 19: 1444–1454, 2005. doi: 10.1101/gad.1315905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huen MS, Sy SM-H, van Deursen JM, Chen J. Direct interaction between SET8 and proliferating cell nuclear antigen couples H4-K20 methylation with DNA replication. J Biol Chem 283: 11073–11077, 2008. doi: 10.1074/jbc.C700242200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milite C, Feoli A, Viviano M, Rescigno D, Cianciulli A, Balzano AL, Mai A, Castellano S, Sbardella G. The emerging role of lysine methyltransferase SETD8 in human diseases. Clin Epigenetics 8: 102, 2016. doi: 10.1186/s13148-016-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oda H, Hübner MR, Beck DB, Vermeulen M, Hurwitz J, Spector DL, Reinberg D. Regulation of the histone H4 monomethylase PR-Set7 by CRL4(Cdt2)-mediated PCNA-dependent degradation during DNA damage. Mol Cell 40: 364–376, 2010. doi: 10.1016/j.molcel.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi X, Kachirskaia I, Yamaguchi H, West LE, Wen H, Wang EW, Dutta S, Appella E, Gozani O. Modulation of p53 function by SET8-mediated methylation at lysine 382. Mol Cell 27: 636–646, 2007. doi: 10.1016/j.molcel.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veschi V, Liu Z, Voss TC, Ozbun L, Gryder B, Yan C, Hu Y, Ma A, Jin J, Mazur SJ, Lam N, Souza BK, Giannini G, Hager GL, Arrowsmith CH, Khan J, Appella E, Thiele CJ. Epigenetic siRNA and chemical screens identify SETD8 inhibition as a therapeutic strategy for p53 activation in high-risk neuroblastoma. Cancer Cell 31: 50–63, 2017. doi: 10.1016/j.ccell.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oda H, Okamoto I, Murphy N, Chu J, Price SM, Shen MM, Torres-Padilla ME, Heard E, Reinberg D. Monomethylation of histone H4-lysine 20 is involved in chromosome structure and stability and is essential for mouse development. Mol Cell Biol 29: 2278–2295, 2009. doi: 10.1128/MCB.01768-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Houston SI, McManus KJ, Adams MM, Sims JK, Carpenter PB, Hendzel MJ, Rice JC. Catalytic function of the PR-Set7 histone H4 lysine 20 monomethyltransferase is essential for mitotic entry and genomic stability. J Biol Chem 283: 19478–19488, 2008. doi: 10.1074/jbc.M710579200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Armstrong RL, Duronio RJ, MacAlpine DM. Methylation of histone H4 lysine 20 by PR-Set7 ensures the integrity of late replicating sequence domains in Drosophila. Nucleic Acids Res 44: 7204–7218, 2016. doi: 10.1093/nar/gkw333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shoaib M, Walter D, Gillespie PJ, Izard F, Fahrenkrog B, Lleres D, Lerdrup M, Johansen JV, Hansen K, Julien E, Blow JJ, Sørensen CS. Histone H4K20 methylation mediated chromatin compaction threshold ensures genome integrity by limiting DNA replication licensing. Nat Commun 9: 3704, 2018. doi: 10.1038/s41467-018-06066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng W, Yonezawa M, Ye J, Jenuwein T, Grummt I. PHF8 activates transcription of rRNA genes through H3K4me3 binding and H3K9me1/2 demethylation. Nat Struct Mol Biol 17: 445–450, 2010. doi: 10.1038/nsmb.1778. [DOI] [PubMed] [Google Scholar]
- 50.Qiu J, Shi G, Jia Y, Li J, Wu M, Li J, Dong S, Wong J. The X-linked mental retardation gene PHF8 is a histone demethylase involved in neuronal differentiation. Cell Res 20: 908–918, 2010. doi: 10.1038/cr.2010.81. [DOI] [PubMed] [Google Scholar]
- 51.Chen X, Wang S, Zhou Y, Han Y, Li S, Xu Q, Xu L, Zhu Z, Deng Y, Yu L, Song L, Chen AP, Song J, Takahashi E, He G, He L, Li W, Chen CD. Phf8 histone demethylase deficiency causes cognitive impairments through the mTOR pathway. Nat Commun 9: 114, 2018. doi: 10.1038/s41467-017-02531-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qi HH, Sarkissian M, Hu G-Q, Wang Z, Bhattacharjee A, Gordon DB, Gonzales M, Lan F, Ongusaha PP, Huarte M, Yaghi NK, Lim H, Garcia BA, Brizuela L, Zhao K, Roberts TM, Shi Y. Histone H4K20/H3K9 demethylase PHF8 regulates zebrafish brain and craniofacial development. Nature 466: 503–507, 2010. doi: 10.1038/nature09261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trojer P, Li G, Sims RJ 3rd, Vaquero A, Kalakonda N, Boccuni P, Lee D, Erdjument-Bromage H, Tempst P, Nimer SD, Wang Y-H, Reinberg D. L3MBTL1, a histone-methylation-dependent chromatin lock. Cell 129: 915–928, 2007. doi: 10.1016/j.cell.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 54.Trojer P, Reinberg D. Beyond histone methyl-lysine binding: how malignant brain tumor (MBT) protein L3MBTL1 impacts chromatin structure. Cell Cycle 7: 578–585, 2008. doi: 10.4161/cc.7.5.5544. [DOI] [PubMed] [Google Scholar]
- 55.Kalakonda N, Fischle W, Boccuni P, Gurvich N, Hoya-Arias R, Zhao X, Miyata Y, Macgrogan D, Zhang J, Sims JK, Rice JC, Nimer SD. Histone H4 lysine 20 monomethylation promotes transcriptional repression by L3MBTL1. Oncogene 27: 4293–4304, 2008. doi: 10.1038/onc.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.West LE, Roy S, Lachmi-Weiner K, Hayashi R, Shi X, Appella E, Kutateladze TG, Gozani O. The MBT repeats of L3MBTL1 link SET8-mediated p53 methylation at lysine 382 to target gene repression. J Biol Chem 285: 37725–37732, 2010. doi: 10.1074/jbc.M110.139527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, Cui K, Roh T-Y, Peng W, Zhang MQ, Zhao K. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet 40: 897–903, 2008. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barski A, Cuddapah S, Cui K, Roh T-Y, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell 129: 823–837, 2007. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 59.Cui K, Zang C, Roh T-Y, Schones DE, Childs RW, Peng W, Zhao K. Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell 4: 80–93, 2009. doi: 10.1016/j.stem.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu Y, Wang Y, Liu M, Nie M, Wang Y, Deng Y, Yao B, Gui T, Li X, Ma L, Guo C, Ma C, Ju J, Zhao Q. Suv4-20h1 promotes G1 to S phase transition by downregulating p21WAF1/CIP1 expression in chronic myeloid leukemia K562 cells. Oncol Lett 15: 6123–6130, 2018. doi: 10.3892/ol.2018.8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boonsanay V, Zhang T, Georgieva A, Kostin S, Qi H, Yuan X, Zhou Y, Braun T. Regulation of skeletal muscle stem cell quiescence by Suv4-20h1-dependent facultative heterochromatin formation. Cell Stem Cell 18: 229–242, 2016. doi: 10.1016/j.stem.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Vougiouklakis T, Sone K, Saloura V, Cho H-S, Suzuki T, Dohmae N, Alachkar H, Nakamura Y, Hamamoto R. SUV420H1 enhances the phosphorylation and transcription of ERK1 in cancer cells. Oncotarget 6: 43162–43171, 2015. doi: 10.18632/oncotarget.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weirich S, Kudithipudi S, Jeltsch A. Specificity of the SUV4-20H1 and SUV4-20H2 protein lysine methyltransferases and methylation of novel substrates. J Mol Biol 428: 2344–2358, 2016. doi: 10.1016/j.jmb.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 64.Bian Q, Anderson EC, Brejc K, Meyer BJ. Dynamic control of chromosome topology and gene expression by a chromatin modification. Cold Spring Harb Symp Quant Biol 82: 279–291, 2017. doi: 10.1101/sqb.2017.82.034439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang B, Matsuoka S, Carpenter PB, Elledge SJ. 53BP1, a mediator of the DNA damage checkpoint. Science 298: 1435–1438, 2002. doi: 10.1126/science.1076182. [DOI] [PubMed] [Google Scholar]
- 66.Rivlin N, Brosh R, Oren M, Rotter V. Mutations in the p53 tumor suppressor gene: important milestones at the various steps of tumorigenesis. Genes Cancer 2: 466–474, 2011. doi: 10.1177/1947601911408889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Acs K, Luijsterburg MS, Ackermann L, Salomons FA, Hoppe T, Dantuma NP. The AAA-ATPase VCP/p97 promotes 53BP1 recruitment by removing L3MBTL1 from DNA double-strand breaks. Nat Struct Mol Biol 18: 1345–1350, 2011. doi: 10.1038/nsmb.2188. [DOI] [PubMed] [Google Scholar]
- 68.Tuzon CT, Spektor T, Kong X, Congdon LM, Wu S, Schotta G, Yokomori K, Rice JC. Concerted activities of distinct H4K20 methyltransferases at DNA double-strand breaks regulate 53BP1 nucleation and NHEJ-directed repair. Cell Rep 8: 430–438, 2014. doi: 10.1016/j.celrep.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pei H, Wu X, Liu T, Yu K, Jelinek DF, Lou Z. The histone methyltransferase MMSET regulates class switch recombination. J Immunol 190: 756–763, 2013. doi: 10.4049/jimmunol.1201811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hartlerode AJ, Guan Y, Rajendran A, Ura K, Schotta G, Xie A, Shah JV, Scully R. Impact of histone H4 lysine 20 methylation on 53BP1 responses to chromosomal double strand breaks. PLoS One 7: e49211, 2012. doi: 10.1371/journal.pone.0049211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bromberg KD, Mitchell TR, Upadhyay AK, Jakob CG, Jhala MA, Comess KM, Lasko LM, Li C, Tuzon CT, Dai Y, Li F, Eram MS, Nuber A, Soni NB, Manaves V, Algire MA, Sweis RF, Torrent M, Schotta G, Sun C, Michaelides MR, Shoemaker AR, Arrowsmith CH, Brown PJ, Santhakumar V, Martin A, Rice JC, Chiang GG, Vedadi M, Barsyte-Lovejoy D, Pappano WN. The SUV4-20 inhibitor A-196 verifies a role for epigenetics in genomic integrity. Nat Chem Biol 13: 317–324, 2017. doi: 10.1038/nchembio.2282. [DOI] [PubMed] [Google Scholar]
- 72.Jørgensen S, Elvers I, Trelle MB, Menzel T, Eskildsen M, Jensen ON, Helleday T, Helin K, Sørensen CS. The histone methyltransferase SET8 is required for S-phase progression. J Cell Biol 179: 1337–1345, 2007. doi: 10.1083/jcb.200706150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tardat M, Brustel J, Kirsh O, Lefevbre C, Callanan M, Sardet C, Julien E. The histone H4 Lys 20 methyltransferase PR-Set7 regulates replication origins in mammalian cells. Nat Cell Biol 12: 1086–1093, 2010. doi: 10.1038/ncb2113. [DOI] [PubMed] [Google Scholar]
- 74.Long H, Zhang L, Lv M, Wen Z, Zhang W, Chen X, Zhang P, Li T, Chang L, Jin C, Wu G, Wang X, Yang F, Pei J, Chen P, Margueron R, Deng H, Zhu M, Li G. H2A.Z facilitates licensing and activation of early replication origins. Nature 577: 576–581, 2020. doi: 10.1038/s41586-019-1877-9. [DOI] [PubMed] [Google Scholar]
- 75.Pannetier M, Julien E, Schotta G, Tardat M, Sardet C, Jenuwein T, Feil R. PR-SET7 and SUV4-20H regulate H4 lysine-20 methylation at imprinting control regions in the mouse. EMBO Rep 9: 998–1005, 2008. doi: 10.1038/embor.2008.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pedrotti S, Caccia R, Neguembor MV, Garcia-Manteiga JM, Ferri G, de Palma C, Canu T, Giovarelli M, Marra P, Fiocchi A, Molineris I, Raso M, Sanvito F, Doglioni C, Esposito A, Clementi E, Gabellini D. The Suv420h histone methyltransferases regulate PPAR-γ and energy expenditure in response to environmental stimuli. Sci Adv 5: eaav1472, 2019. doi: 10.1126/sciadv.aav1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Phalke S, Nickel O, Walluscheck D, Hortig F, Onorati MC, Reuter G. Retrotransposon silencing and telomere integrity in somatic cells of Drosophila depends on the cytosine-5 methyltransferase DNMT2. Nat Genet 41: 696–702, 2009. doi: 10.1038/ng.360. [DOI] [PubMed] [Google Scholar]
- 78.Hahn M, Dambacher S, Dulev S, Kuznetsova AY, Eck S, Wörz S, Sadic D, Schulte M, Mallm J-P, Maiser A, Debs P, von Melchner H, Leonhardt H, Schermelleh L, Rohr K, Rippe K, Storchova Z, Schotta G. Suv4-20h2 mediates chromatin compaction and is important for cohesin recruitment to heterochromatin. Genes Dev 27: 859–872, 2013. doi: 10.1101/gad.210377.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gonzalo S, García-Cao M, Fraga MF, Schotta G, Peters AH, Cotter SE, Eguía R, Dean DC, Esteller M, Jenuwein T, Blasco MA. Role of the RB1 family in stabilizing histone methylation at constitutive heterochromatin. Nat Cell Biol 7: 420–428, 2005. doi: 10.1038/ncb1235. [DOI] [PubMed] [Google Scholar]
- 80.Van Aller GS, Reynoird N, Barbash O, Huddleston M, Liu S, Zmoos A-F, McDevitt P, Sinnamon R, Le B, Mas G, Annan R, Sage J, Garcia BA, Tummino PJ, Gozani O, Kruger RG. Smyd3 regulates cancer cell phenotypes and catalyzes histone H4 lysine 5 methylation. Epigenetics 7: 340–343, 2012. doi: 10.4161/epi.19506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Codato R, Perichon M, Divol A, Fung E, Sotiropoulos A, Bigot A, Weitzman JB, Medjkane S. The SMYD3 methyltransferase promotes myogenesis by activating the myogenin regulatory network. Sci Rep 9: 17298, 2019. doi: 10.1038/s41598-019-53577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Y-J, Tsai C-H, Wang P-Y, Teng S-C. SMYD3 promotes homologous recombination via regulation of H3K4-mediated gene expression. Sci Rep 7: 3842, 2017. doi: 10.1038/s41598-017-03385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vieira FQ, Costa-Pinheiro P, Almeida-Rios D, Graça I, Monteiro-Reis S, Simões-Sousa S, Carneiro I, Sousa EJ, Godinho MI, Baltazar F, Henrique R, Jerónimo C. SMYD3 contributes to a more aggressive phenotype of prostate cancer and targets Cyclin D2 through H4K20me3. Oncotarget 6: 13644–13657, 2015. doi: 10.18632/oncotarget.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kidder BL, Hu G, Cui K, Zhao K. SMYD5 regulates H4K20me3-marked heterochromatin to safeguard ES cell self-renewal and prevent spurious differentiation. Epigenetics Chromatin 10: 8, 2017. doi: 10.1186/s13072-017-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fujii T, Tsunesumi S-I, Sagara H, Munakata M, Hisaki Y, Sekiya T, Furukawa Y, Sakamoto K, Watanabe S. Smyd5 plays pivotal roles in both primitive and definitive hematopoiesis during zebrafish embryogenesis. Sci Rep 6: 29157, 2016. doi: 10.1038/srep29157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bricambert J, Alves-Guerra M-C, Esteves P, Prip-Buus C, Bertrand-Michel J, Guillou H, Chang CJ, Vander Wal MN, Canonne-Hergaux F, Mathurin P, Raverdy V, Pattou F, Girard J, Postic C, Dentin R. The histone demethylase Phf2 acts as a molecular checkpoint to prevent NAFLD progression during obesity. Nat Commun 9: 2092, 2018. doi: 10.1038/s41467-018-04361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wen H, Li J, Song T, Lu M, Kan P-Y, Lee MG, Sha B, Shi X. Recognition of histone H3K4 trimethylation by the plant homeodomain of PHF2 modulates histone demethylation. J Biol Chem 285: 9322–9326, 2010. doi: 10.1074/jbc.C109.097667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ng JM, Vermeulen W, van der Horst GT, Bergink S, Sugasawa K, Vrieling H, Hoeijmakers JH. A novel regulation mechanism of DNA repair by damage-induced and RAD23-dependent stabilization of xeroderma pigmentosum group C protein. Genes Dev 17: 1630–1645, 2003. doi: 10.1101/gad.260003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shen Z, Sathyan KM, Geng Y, Zheng R, Chakraborty A, Freeman B, Wang F, Prasanth KV, Prasanth SG. A WD-repeat protein stabilizes ORC binding to chromatin. Mol Cell 40: 99–111, 2010. doi: 10.1016/j.molcel.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giri S, Prasanth SG. Association of ORCA/LRWD1 with repressive histone methyl transferases mediates heterochromatin organization. Nucleus 6: 435–441, 2015. doi: 10.1080/19491034.2015.1102814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shen Z, Chakraborty A, Jain A, Giri S, Ha T, Prasanth KV, Prasanth SG. Dynamic association of ORCA with prereplicative complex components regulates DNA replication initiation. Mol Cell Biol 32: 3107–3120, 2012. doi: 10.1128/MCB.00362-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brustel J, Kirstein N, Izard F, Grimaud C, Prorok P, Cayrou C, Schotta G, Abdelsamie AF, Déjardin J, Méchali M, Baldacci G, Sardet C, Cadoret J-C, Schepers A, Julien E. Histone H4K20 tri-methylation at late-firing origins ensures timely heterochromatin replication. EMBO J 36: 2726–2741, 2017. doi: 10.15252/embj.201796541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jørgensen S, Eskildsen M, Fugger K, Hansen L, Larsen MS, Kousholt AN, Syljuåsen RG, Trelle MB, Jensen ON, Helin K, Sørensen CS. SET8 is degraded via PCNA-coupled CRL4(CDT2) ubiquitylation in S phase and after UV irradiation. J Cell Biol 192: 43–54, 2011. doi: 10.1083/jcb.201009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Julien E, Herr W. A switch in mitotic histone H4 lysine 20 methylation status is linked to M phase defects upon loss of HCF-1. Mol Cell 14: 713–725, 2004. doi: 10.1016/j.molcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 95.Wu S, Wang W, Kong X, Congdon LM, Yokomori K, Kirschner MW, Rice JC. Dynamic regulation of the PR-Set7 histone methyltransferase is required for normal cell cycle progression. Genes Dev 24: 2531–2542, 2010. doi: 10.1101/gad.1984210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jørgensen S, Schotta G, Sørensen CS. Histone H4 lysine 20 methylation: key player in epigenetic regulation of genomic integrity. Nucleic Acids Res 41: 2797–2806, 2013. doi: 10.1093/nar/gkt012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chicas A, Kapoor A, Wang X, Aksoy O, Evertts AG, Zhang MQ, Garcia BA, Bernstein E, Lowe SW. H3K4 demethylation by Jarid1a and Jarid1b contributes to retinoblastoma-mediated gene silencing during cellular senescence. Proc Natl Acad Sci U S A 109: 8971–8976, 2012. doi: 10.1073/pnas.1119836109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Benard A, Goossens-Beumer IJ, van Hoesel AQ, de Graaf W, Horati H, Putter H, Zeestraten EC, van de Velde CJ, Kuppen PJ. Histone trimethylation at H3K4, H3K9 and H4K20 correlates with patient survival and tumor recurrence in early-stage colon cancer. BMC Cancer 14: 531, 2014. doi: 10.1186/1471-2407-14-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fraga MF, Ballestar E, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G, Bonaldi T, Haydon C, Ropero S, Petrie K, Iyer NG, Pérez-Rosado A, Calvo E, Lopez JA, Cano A, Calasanz MJ, Colomer D, Piris MA, Ahn N, Imhof A, Caldas C, Jenuwein T, Esteller M. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet 37: 391–400, 2005. doi: 10.1038/ng1531. [DOI] [PubMed] [Google Scholar]
- 100.Tryndyak VP, Kovalchuk O, Pogribny IP. Loss of DNA methylation and histone H4 lysine 20 trimethylation in human breast cancer cells is associated with aberrant expression of DNA methyltransferase 1, Suv4-20h2 histone methyltransferase and methyl-binding proteins. Cancer Biol Ther 5: 65–70, 2006. doi: 10.4161/cbt.5.1.2288. [DOI] [PubMed] [Google Scholar]
- 101.Van Den Broeck A, Brambilla E, Moro-Sibilot D, Lantuejoul S, Brambilla C, Eymin B, Gazzeri S. Loss of histone H4K20 trimethylation occurs in preneoplasia and influences prognosis of non-small cell lung cancer. Clin Cancer Res 14: 7237–7245, 2008. doi: 10.1158/1078-0432.CCR-08-0869. [DOI] [PubMed] [Google Scholar]
- 102.Yokoyama Y, Matsumoto A, Hieda M, Shinchi Y, Ogihara E, Hamada M, Nishioka Y, Kimura H, Yoshidome K, Tsujimoto M, Matsuura N. Loss of histone H4K20 trimethylation predicts poor prognosis in breast cancer and is associated with invasive activity. Breast Cancer Res 16: R66, 2014. doi: 10.1186/bcr3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pogribny IP, Ross SA, Tryndyak VP, Pogribna M, Poirier LA, Karpinets TV. Histone H3 lysine 9 and H4 lysine 20 trimethylation and the expression of Suv4-20h2 and Suv-39h1 histone methyltransferases in hepatocarcinogenesis induced by methyl deficiency in rats. Carcinogenesis 27: 1180–1186, 2006. doi: 10.1093/carcin/bgi364. [DOI] [PubMed] [Google Scholar]
- 104.Schneider A-C, Heukamp LC, Rogenhofer S, Fechner G, Bastian PJ, von Ruecker A, Müller SC, Ellinger J. Global histone H4K20 trimethylation predicts cancer-specific survival in patients with muscle-invasive bladder cancer. BJU Int 108: E290– E296, 2011. doi: 10.1111/j.1464-410X.2011.10203.x. [DOI] [PubMed] [Google Scholar]
- 105.Shinchi Y, Hieda M, Nishioka Y, Matsumoto A, Yokoyama Y, Kimura H, Matsuura S, Matsuura N. SUV420H2 suppresses breast cancer cell invasion through down regulation of the SH2 domain-containing focal adhesion protein tensin-3. Exp Cell Res 334: 90–99, 2015. doi: 10.1016/j.yexcr.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 106.Viotti M, Wilson C, McCleland M, Koeppen H, Haley B, Jhunjhunwala S, Klijn C, Modrusan Z, Arnott D, Classon M, Stephan J-P, Mellman I. SUV420H2 is an epigenetic regulator of epithelial/mesenchymal states in pancreatic cancer. J Cell Biol 217: 763–777, 2018. doi: 10.1083/jcb.201705031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu Y, Shi W, Tang T, Wang Y, Yin X, Chen Y, Zhang Y, Xing Y, Shen Y, Xia T, Guo C, Pan Y, Jin L. miR-29a contributes to breast cancer cells epithelial-mesenchymal transition, migration, and invasion via down-regulating histone H4K20 trimethylation through directly targeting SUV420H2. Cell Death Dis 10: 176, 2019. doi: 10.1038/s41419-019-1437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hsiao K-Y, Mizzen CA. Histone H4 deacetylation facilitates 53BP1 DNA damage signaling and double-strand break repair. J Mol Cell Biol 5: 157–165, 2013. doi: 10.1093/jmcb/mjs066. [DOI] [PubMed] [Google Scholar]
- 109.Tang J, Cho NW, Cui G, Manion EM, Shanbhag NM, Botuyan MV, Mer G, Greenberg RA. Acetylation limits 53BP1 association with damaged chromatin to promote homologous recombination. Nat Struct Mol Biol 20: 317–325, 2013. doi: 10.1038/nsmb.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kapoor-Vazirani P, Kagey JD, Vertino PM. SUV420H2-mediated H4K20 trimethylation enforces RNA polymerase II promoter-proximal pausing by blocking hMOF-dependent H4K16 acetylation. Mol Cell Biol 31: 1594–1609, 2011. doi: 10.1128/MCB.00524-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lachner M, O’Carroll D, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410: 116–120, 2001. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]


