Keywords: ammonia, androgen, proximal tubule, sex differences, testosterone
Abstract
Sexual dimorphic variations are present in many aspects of biology and involve the structure and/or function of nearly every organ system. Acid-base homeostasis is critical for optimal health, and renal ammonia metabolism has a major role in the maintenance of acid-base homeostasis. Recent studies have shown sex-dependent differences in renal ammonia metabolism with regard to both basal ammonia excretion and the response to an exogenous acid load. These sexual dimorphisms are associated with structural changes in the proximal tubule and the collecting duct and variations in the expression of multiple proteins involved in ammonia metabolism and transport. Studies using orchiectomy-induced testosterone deficiency and physiological testosterone replacement have shown that testosterone underlies much of the sex-dependent differences in the proximal tubule. This parallels the finding that the canonical testosterone target receptor, androgen receptor (AR), is present exclusively in the proximal tubule. Thus testosterone, possibly acting through AR activation, regulates multiple components of renal structure and ammonia metabolism. The lack of detectable AR in the remainder of the nephron and collecting duct suggests that some dimorphisms in renal structure and ammonia transporter expression are mediated through mechanisms other than direct testosterone-dependent AR activation. A better understanding of the mechanism and biological implications of sex’s effect on renal structure and ammonia metabolism is critical for optimizing our ability to care for both men and women with acid-base disturbances.
INTRODUCTION
Sex-dependent variations in the development and the regulation of structure and function are present in almost every organ in the mammalian body (1–3). These effects often lead to differences in disease prevalence, rates of progression, and response to treatment. Understanding these male- and female-specific variances can serve to identify new disease mechanisms and can lead to new and improved therapeutic opportunities. Thus there is an immense need to advance our understanding of these sex dimorphisms.
Sex affects many aspects of kidney function and disease. Sex differences in blood pressure are widely recognized, with more men having hypertension than women (4). As recently reviewed (5), this may be related, in part, to sex-specific variations in nitric oxide, the renin-angiotensin-aldosterone system, inflammation, and kidney function. There also are sexual dimorphisms in the expression of multiple renal epithelial cell Na+ transporters that correlate with altered responses to salt handling (6). A computational model of the rat proximal tubule has suggested that proximal tubule fractional Na+ reabsorption is lower in the female than male kidney and that this was due to a smaller transport area in combination with less Na+/H+ exchanger isoform 3 (NHE3) and claudin-2 expression (7). Another study has shown sex-specific variations in the role of medullary pericytes in ischemia-reperfusion injury (8). Diabetes mellitus is a major health concern, and there are numerous sex-dependent variations in the renal handling of glucose and in the response to diabetes mellitus (5, 9). For example, there are sex differences in renal arteriolar resistance in people with type 1 diabetes mellitus, which suggests the female kidney may be disproportionally affected by hyperfiltration early in this condition (9, 10). These sexual dimorphisms highlight the importance of better understanding the effect of sex on the kidney in health and disease.
A key function of the kidneys is to maintain acid-base homeostasis. The kidneys do so through the process of net acid excretion, which incorporates urinary ammonia,1 titratable acid, and bicarbonate excretion. Ammonia excretion is the predominant component of basal net acid excretion, and changes in ammonia metabolism account for >80% of the increased net acid excretion response to exogenous stimuli (11, 12). In patients with chronic kidney disease (CKD), loss of acid-base homeostasis, specifically metabolic acidosis, may affect as many as 40% (13, 14), and the metabolic acidosis appears largely due to inadequate ammonia excretion (15). Moreover, impaired ammonia excretion in patients with CKD correlates with worse clinical outcomes, including progressive CKD and death (16, 17). Intervention trials have shown correcting metabolic acidosis decreases CKD progression (18–20) and decreases the need for renal replacement therapy (18) and that it improves thyroid function (21), insulin sensitivity (22), muscle mass and strength (20), and physical functioning (23).
Renal ammonia excretion differs from that of almost all other urinary solutes. Urinary ammonia derives primarily from intrarenal generation coupled to carrier-mediated NH3 and transport (12, 14). Briefly, ammonia is produced primarily in the proximal tubule from metabolism of systemically derived glutamine. Phosphoenolpyruvate carboxykinase (PEPCK) is a key enzyme in ammonia generation, and glutamine synthetase mediates ammonia recycling. Ammonia produced in the proximal tubule is secreted preferentially into the luminal fluid via apical NHE3. In the thick ascending limb (TAL) of the loop of Henle, Na+-K+-2Cl− cotransporter (NKCC2) mediates ammonia reabsorption that creates the medullary interstitial concentration gradient. In the collecting duct, ammonia is then secreted primarily by collecting duct Rhesus (Rh) glycoproteins [Rh B glycoprotein (Rhbg) and Rh C glycoprotein (Rhcg)]. In response to metabolic acidosis, there is increased PEPCK, NHE3, Rhbg, and Rhcg expression in combination with decreased glutamine synthetase expression (for reviews, see Refs. 12 and 14).
Because ammonia has a critical role in acid-base homeostasis and impaired acid-base homeostasis leads to multiple adverse health outcomes, understanding the effect of sex on ammonia metabolism is important. The purpose of this review is to highlight recent work examining sexual dimorphisms in renal ammonia metabolism and the role of testosterone in these differences.
SEX DIFFERENCES IN RENAL AMMONIA METABOLISM AND TRANSPORT
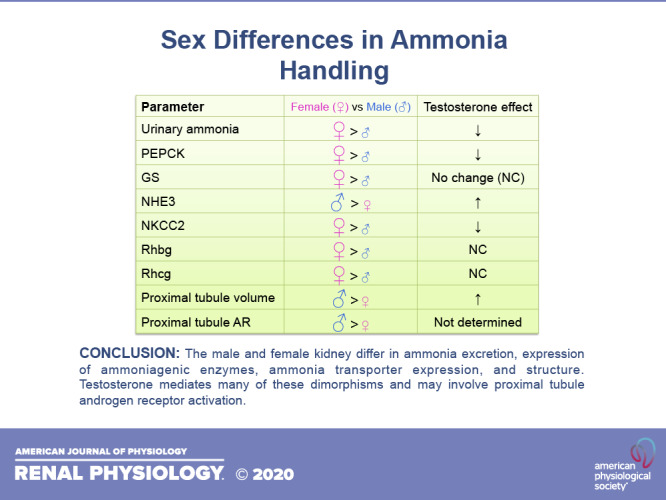
Several studies over the past few years have examined the effect of sex on renal ammonia handling. The initial study showing sexual dimorphisms identified that female mice excrete approximately twice as much urinary ammonia as do male mice (24). This occurred despite female mice being smaller in size, indicating that the sex-dependent alteration was even greater than twofold when considered on a per gram body weight basis. It was not due to variances in protein intake, the major determinant of endogenous acid production under basal conditions, as food intake did not differ significantly. Finally, female mice excreted more urinary ammonia despite statistically similar urine pH, indicating sexual dimorphism in ammonia excretion could not be attributed to variances in urine acidification (24). Thus there are sex-dependent effects on ammonia excretion that are not due to body size, diet, or urine acidification.
This sexual dimorphism in ammonia excretion was associated with altered expression of several critical proteins involved in ammonia generation and transport. PEPCK is a key protein in the proximal tubule ammonia-generating pathway, and glutamine synthetase is a key enzyme in the ammonia recycling pathway (11, 12, 25). Greater ammonia excretion in females was associated with greater expression of PEPCK and glutamine synthetase (Fig. 1A) (24). Expression of Na+-bicarbonate cotransporter, electrogenic, isoform 1 (NBCe1), which regulates proximal tubule ammoniagenic enzyme expression (27–29), did not differ, however.
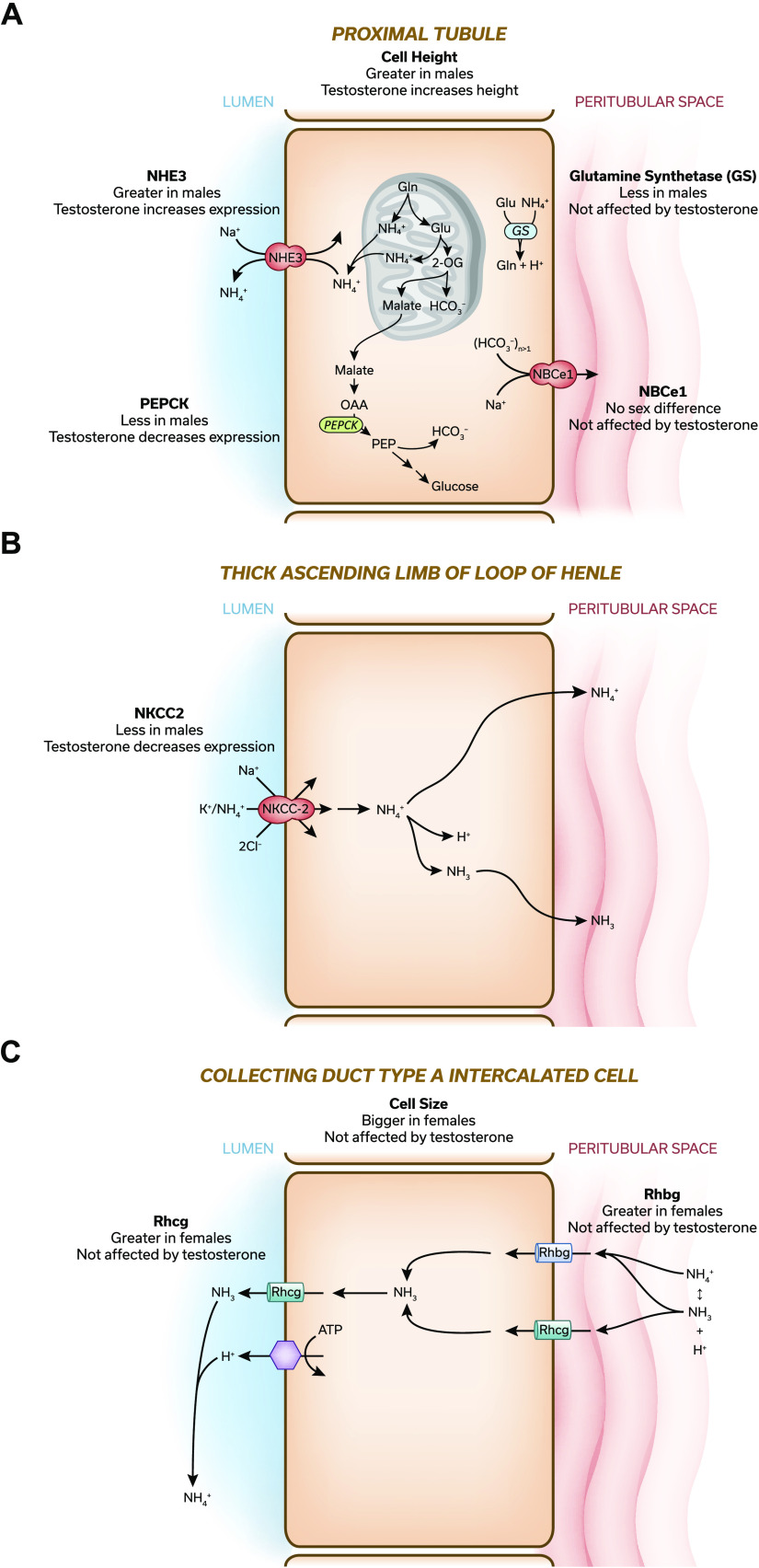
Figure 1.
Overview of sex differences in ammonia generation and transport. A−C: models of the proximal tubule (A), thick ascending limb of the loop of Henle (B), and collecting duct a type A intercalated cell (C). Key proteins involved in either ammonia metabolism or transport are shown, and sex-dependent variations in protein expression between male and female kidney are detailed. If orchiectomy-induced testosterone deficiency altered protein expression, and this effect was reversed by physiologic testosterone replacement, this is detailed as either “testosterone increases expression” or “testosterone decreases expression.” If there was no effect of either orchiectomy or physiological testosterone replacement, this is summarized as “testosterone does not alter expression.” Only selected proteins involved in ammonia generation or transport that have been shown to have sex-dependent expression are shown. Results are taken from Refs. 24, 32, 33. The interested reader is encouraged to see more in-depth reviews for a comprehensive description of renal ammonia handling (14, 26). NKCC, Na+-K+-2Cl− cotransporter; NHE3, Na+/H+ exchanger isoform 3; Rhbg, Rh B glycoprotein; Rhcg, Rh C glycoprotein; NBCe1, Na+-bicarbonate cotransporter, electrogenic, isoform 1. [Original figures from Ref. 11 were modified with permission to generate this figure.]
Ammonia excretion involves coordinated NH3 and transport by specific proteins in the proximal tubule, TAL, and collecting duct (14). NHE3 is the primary mechanism of proximal tubule ammonia secretion. Both our study in the mouse (24) and a previous study by Veiras et al. (6) in the rat found no sex difference in NHE3 expression (Fig. 1A). In contrast, Veiras et al. (6) found in the mouse significantly less NHE3 expression in the female kidney than in the male kidney. The reason for the inconsistent findings in Refs. 24 and 6 in the mouse kidney is unknown but could be related to overnight fasting being used in Ref. 6 but not in Ref. 24 or to variations in diet, time of kidney tissue collection, or other technical details.
The TAL reabsorbs luminal ammonia, which is critical for generating the medullary interstitial ammonia concentration gradient, through NKCC2-dependent mechanisms (14, 30). In the mouse kidney, both our study (24) and the study of Veiras et al. (6) showed that females had greater NKCC2 expression (Fig. 1B). In contrast, in the rat kidney, Veiras et al. (6) observed similar levels of NKCC2 expression (6).
Finally, collecting duct ammonia secretion involves Rhbg- and Rhcg-mediated ammonia transport (31). Greater ammonia excretion in female mice was associated with greater expression of both Rhbg and Rhcg (Fig. 1C) (24). Thus there are significant sex-dependent variations in several key proteins involved in ammonia transport. Some of these variations may also be species specific.
SEX DIMORPHISMS IN THE RESPONSE TO ACID LOADING
Sex also appears to alter the ammonia response to metabolic acidosis. Male mice, which excrete less ammonia then female mice under basal conditions, responded to exogenous acid loading with a significantly greater relative increase in ammonia excretion than did female mice, which enabled a similar absolute response (32). This occurred despite statistically similar ingested acid loads and similar plasma electrolyte and bicarbonate concentrations after acid loading. Sex-specific effects only involved ammonia excretion; titratable acid excretion did not differ significantly between male and female mice nor did the urine pH response.
The sexual dimorphic urinary ammonia response to acid-loading involves sex-specific response patterns for several critical proteins involved in ammonia homeostasis. Male mice had greater acid loading-induced increases than did female mice in PEPCK, Rhbg, and NBCe1 protein expression and they exhibited greater apical Rhcg polarization (32). Female mice responded to acid loading with significantly greater proximal tubule glutamine transporter, Na+-coupled neutral amino acid transporter 3 (SNAT3), than did male mice (32). Thus male and female mice generate a similar maximal ammonia excretion in response to an acid load but use sex-specific pathways to achieve this response.
TESTOSTERONE ALTERS RENAL AMMONIA METABOLISM
Testosterone appears to mediate several critical aspects of the sexual dimorphism in ammonia metabolism and transport. Orchiectomy (ORCH)-induced testosterone deficiency increased ammonia excretion in male mice by approximately twofold, and this effect was reversed by physiological testosterone replacement (33). This effect was not due to an altered dietary acid load, as food intake was not altered significantly. This approximately twofold ratio between hormonally intact and testosterone-deficient male mice mirrors the approximately twofold variance between male and female mice (24). This effect of testosterone on ammonia excretion appears to involve testosterone-dependent regulation of several critical proteins. ORCH increased expression of PEPCK and NHE3 in the proximal tubule (Fig. 1A) and NKCC2 (Fig. 1B) in the TAL, and replacement doses of testosterone reversed these effects (33). Testosterone deficiency and its replacement, however, did not alter expression of other proteins that exhibit sex-specific expression, such as proximal tubule glutamine synthetase and the collecting duct ammonia transporters Rhbg and Rhcg (Fig. 1, A and C, respectively) (33). Thus testosterone appears to have a major effect on ammonia handling and may explain much of the difference in ammonia excretion between male and female mice. However, there are also testosterone-independent effects of sex on ammonia metabolism.
RENAL ANDROGEN RECEPTOR LOCALIZATION
The canonical pathway through which testosterone mediates biological effects involves binding to the androgen receptor (AR) (34, 35). Expression of AR protein and mRNA has been observed in both male and female kidneys for decades but without identification of its specific cellular localization (36–40). We identified recently that AR protein is expressed specifically in the proximal tubule (33). AR was present in both the male and female mouse kidney, and expression was approximately twofold greater in males than in females (33). There was, in addition, an axial gradation in proximal tubule expression, with the greatest expression occurring in the proximal straight tubule in the outer medulla (33). AR protein not was detected in nonproximal tubule renal epithelial cells. Identification of proximal tubule-specific AR expression suggests that the effect of testosterone on proximal tubule size and on the expression of PEPCK and NHE3 may be mediated by AR activation.
SEXUAL DIMORPHISMS IN RENAL STRUCTURE
Conditions that alter ammonia excretion often alter renal size. Sex differences in renal structure have been known for decades, but they do not parallel ammonia excretion. As early as 1931, studies in rat models have shown the female kidney was significantly smaller than the male kidney (41), and this could be explained, at least in part, by a smaller size of the renal cortex and proximal tubules (for a review, see Ref. 3). Our more recent study (24) in the mouse similarly found fundamental structural variances in the proximal tubule and extended these findings by showing effects of sex on the collecting duct. We found that proximal tubules accounted for a significantly smaller proportion, ∼40%, of cortical volume, measured as volume density, in the female kidney than in the male kidney, where they accounted for ∼60% (Fig. 1A) (24). This correlated with decreased proximal tubule cell height, suggesting that smaller proximal tubule cells in the female kidney lead to the decreased volume density (24). Whether there are sex-specific variations in proximal tubule length has not been reported. The lesser proximal tubule volume density in the female kidney was accompanied by significantly greater collecting duct volume density and greater size of collecting duct type A intercalated cells (Fig. 1C) (24). Thus there are important effects of sex on baseline renal structure.
Renal and proximal tubule hypertrophy occur in response to chronic acid loading in both animal models and humans (42–44). We identified that chronic acid loading induced a greater degree of proximal tubule hypertrophy in male mice than it did in female mice (32). Additionally, this response was specific to the cortical proximal tubule; sex did not alter the response of the proximal straight tubule in the outer medulla nor did it alter the effect of acid loading on either collecting duct volume density or type A intercalated cell size. Thus there are both sex-dependent and -independent effects of acid loading on the proximal tubule and collecting duct structure.
ROLE OF TESTOSTERONE IN SEXUAL DIMORPHISM IN RENAL STRUCTURE
A large component of the sex-dependent differences in renal structure appears to reflect testosterone-dependent effects. Pharmacological androgen administration has been shown to increase kidney mass in mice, rats, and humans and to induce proximal tubule hypertrophy in the rat kidney (for a review, see Ref. 3). We have recently shown that ORCH-induced testosterone deficiency decreased proximal tubule volume density and that physiological testosterone replacement abrogated this effect (Fig. 1A) (33). ORCH also decreased cortical proximal tubule cell height, suggesting that the variance in volume density was due, at least in part, to decreased proximal tubule cell volume (33). It is intriguing to note that proximal tubule cortical volume density and height in the ORCH male kidney (33) are almost identical to that in the female kidney (24).
In contrast to the proximal tubule, testosterone does not appear to affect collecting duct structure. ORCH-induced testosterone deficiency did not alter either collecting duct volume density or type A intercalated cell size significantly (Fig. 1C) (33). Similarly, testosterone replacement had no effect (33). Sex dimorphisms in the collecting duct appear to result from a different mechanism than in the proximal tubule.
CONCLUSIONS
In summary, the male and female kidney differ in their ammonia generation, transport, and structure under baseline conditions, and they prioritize contrasting mechanisms to respond to acid loading. These variations occur in the proximal tubule, TAL, and collecting duct. Testosterone appears to mediate many of these dimorphisms, and this may involve proximal tubule AR activation. The lack of detectable AR expression in the TAL suggests that testosterone-dependent NKCC2 regulation is either an indirect effect of proximal tubule AR activation or is mediated through alternative signaling pathways. The lack of testosterone-dependent effects on collecting duct Rhbg and Rhcg expression or structure and the lack of collecting duct AR expression suggest that sex-dependent variances in the collecting duct are mediated by testosterone- and AR-independent signaling pathways.
Despite the recent advances in our understanding of the effect of sex on ammonia, additional studies are clearly needed. Studies defining the role of AR in the regulation of proximal tubule ammonia generation and transport are eagerly awaited. In addition, there are certainly testosterone-independent effects of sex, the mechanism of which is incompletely understood at present. Whether this involves direct effects of other sex steroid hormones, such as estrogens or progesterone, indirect effects of proximal tubule AR activation, or effects of sex chromosomes that are independent of gonadal hormones will be important issues for future studies to address.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Disease Grants R01-DK-107798 (to I.D.W. and J. W. Verlander) and K08-DK-120873 (to A.N.H.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.N.H. and I.D.W. conceived and designed research; A.N.H. performed experiments; A.N.H. and I.D.W. analyzed data; A.N.H. and I.D.W. interpreted results of experiments; A.N.H. prepared figures; A.N.H. drafted manuscript; A.N.H. and I.D.W. edited and revised manuscript; A.N.H. and I.D.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Jill W. Verlander for expertise in renal morphology and renal ammonia metabolism and transport in addition to mentorship and guidance.
Footnotes
Ammonia exists in two molecular forms, NH3 and , that are in equilibrium with each other. In this review, we use the term “ammonia” to referred to the combination of both molecular species.
REFERENCES
- 1.Karp NA, Mason J, Beaudet AL, Benjamini Y, Bower L, Braun RE, , et al. Prevalence of sexual dimorphism in mammalian phenotypic traits. Nat Commun 8: 15475, 2017. doi: 10.1038/ncomms15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardue ML, Wizemann TM. Exploring the biological contributions to human health: does sex matter? In: Institute of Medicine (US) Committee on Understanding the Biology of Sex and Gender Differences, edited by Wizemann TM, Pardue ML.. Washington, DC: National Academies Press, 2001. [PubMed] [Google Scholar]
- 3.Sabolić I, Asif AR, Budach WE, Wanke C, Bahn A, Burckhardt G. Gender differences in kidney function. Pflugers Arch 455: 397–429, 2007. doi: 10.1007/s00424-007-0308-1. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez LA, Sullivan JC. Sex differences in hypertension: where we have been and where we are going. Am J Hypertens 31: 1247–1254, 2018. doi: 10.1093/ajh/hpy148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Layton AT, Sullivan JC. Recent advances in sex differences in kidney function. Am J Physiol Renal Physiol 316: F328–F331, 2019. doi: 10.1152/ajprenal.00584.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veiras LC, Girardi AC, Curry J, Pei L, Ralph DL, Tran A, Castelo-Branco CC, Pastor-Soler N, Arranz CT, Yu AS, McDonough AA. Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J Am Soc Nephrol 28: 3504–3517, 2017. doi: 10.1681/ASN.2017030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q, Mcdonough AA, Layton HE, Layton AT. . Functional implications of sexual dimorphism of transporter patterns along the rat proximal tubule: modeling and analysis. Am J Physiol Renal Physiol 315: F692–F700, 2018. doi: 10.1152/ajprenal.00171.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crislip GR, O'Connor PM, Wei Q, Sullivan JC. Vasa recta pericyte density is negatively associated with vascular congestion in the renal medulla following ischemia reperfusion in rats. Am J Physiol Renal Physiol 313: F1097–F1105, 2017. doi: 10.1152/ajprenal.00261.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shepard BD. Sex differences in diabetes and kidney disease: mechanisms and consequences. Am J Physiol Renal Physiol 317: F456–F462, 2019. doi: 10.1152/ajprenal.00249.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Škrtić M, Lytvyn Y, Bjornstad P, Reich HN, Scholey JW, Yip P, Sochett EB, Perkins B, Cherneyl DZ. Influence of sex on hyperfiltration in patients with uncomplicated type 1 diabetes. Am J Physiol Renal Physiol 312: F599–F606, 2017. doi: 10.1152/ajprenal.00357.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiner ID, Mitch WE, Sands JM. . Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin J Am Soc Nephrol 10: 1444–1458, 2015. doi: 10.2215/CJN.10311013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiner ID, Verlander JW. Renal ammonia metabolism and transport. Compr Physiol 3: 201–220, 2013. doi: 10.1002/cphy.c120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moranne O, Froissart M, Rossert J, Gauci C, Boffa JJ, Haymann JP, Ben M'rad M, Jacquot C, Houillier P, Stengel B, Fouqueray B, NephroTest Study Group. Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol 20: 164–171, 2009. doi: 10.1681/ASN.2008020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiner ID, Verlander JW. Ammonia transporters and their role in acid-base balance. physiol Rev 97: 465–494, 2017. doi: 10.1152/physrev.00011.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraut JA, Madias NE. Metabolic acidosis of CKD: an update. Am J Kidney Dis 67: 307–317, 2016. doi: 10.1053/j.ajkd.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 16.Raphael KL, Carroll DJ, Murray J, Greene T, Beddhu S. . Urine ammonium predicts clinical outcomes in hypertensive kidney disease. J Am Soc Nephrol 28: 2483–2490, 2017. doi: 10.1681/ASN.2016101151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallet M, Metzger M, Haymann JP, Flamant M, Gauci C, Thervet E, Boffa JJ, Vrtovsnik F, Froissart M, Stengel B, Houillier P, NephroTest Cohort Study Group. Urinary ammonia and long-term outcomes in chronic kidney disease. Kidney Int 88: 137–145, 2015. doi: 10.1038/ki.2015.52. [DOI] [PubMed] [Google Scholar]
- 18.De Brito-Ashurst IM, Varagunam M, Raftery MJ, Yaqoob MM. . Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009. doi: 10.1681/ASN.2008111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Iorio BR, Bellasi A, Raphael KL, Santoro D, Aucella F, Garofano L, Ceccarelli M, Di Lullo L, Capolongo G, Di Iorio M, Guastaferro P, Capasso G, The UBI Study Group. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study. J Nephrol 32: 989–1001, 2019. doi: 10.1007/s40620-019-00656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubey AK, Sahoo B, Vairappan B, Haridasan S. Correction of metabolic acidosis improves muscle mass and renal function in chronic kidney disease stages 3 and 4: a randomized controlled trial. Nephrol Dial Transplant 35: 121–129, 2020. doi: 10.1093/ndt/gfy214. [DOI] [PubMed] [Google Scholar]
- 21.Disthabanchong S, Treeruttanawanich A. Oral sodium bicarbonate improves thyroid function in predialysis chronic kidney disease. Am J Nephrol 32: 549–556, 2010. doi: 10.1159/000321461. [DOI] [PubMed] [Google Scholar]
- 22.Bellasi A, Di Micco L, Santoro D, Marzocco S, De Simone E, Cozzolino M, Di Lullo L, Guastaferro P, Di Iorio B, UBI study investigators. Correction of metabolic acidosis improves insulin resistance in chronic kidney disease. BMC Nephrol 17: 158, 2016. doi: 10.1186/s12882-016-0372-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wesson DE, Mathur V, Tangri N, Stasiv Y, Parsell D, Li E, Klaerner G, Bushinsky DA. Veverimer versus placebo in patients with metabolic acidosis associated with chronic kidney disease: a multicentre, randomised, double-blind, controlled, phase 3 trial. Lancet 393: 1417–1427, 2019. doi: 10.1016/S0140-6736(18)32562-5. [DOI] [PubMed] [Google Scholar]
- 24.Harris AN, Lee HW, Osis G, Fang L, Webster KL, Verlander JW. Differences in renal ammonia metabolism in male and female kidney. Am J Physiol Renal Physiol 315: F211–F222, 2018. doi: 10.1152/ajprenal.00084.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curthoys NP, Moe OW. Proximal tubule function and response to acidosis. Clin J Am Soc Nephrol 9: 1627–1638, 2014. doi: 10.2215/CJN.10391012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiner ID, Verlander JW. Emerging features of ammonia metabolism and transport in acid-base balance. Semin Nephrol 39: 394–405, 2019. doi: 10.1016/j.semnephrol.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Handlogten ME, Osis G, Lee HW, Romero MF, Verlander JW, Weiner ID. NBCe1 expression is required for normal renal ammonia metabolism. Am J Physiol Renal Physiol 309: F658–F666, 2015. doi: 10.1152/ajprenal.00219.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HW, Harris AN, Romero MF, Welling PA, Wingo CS, Verlander JW, Weiner ID. . NBCe1-A is required for the renal ammonia and K+ response to hypokalemia. Am J Physiol Renal Physiol 318: F402–F421, 2020. doi: 10.1152/ajprenal.00481.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee HW, Osis G, Harris AN, Fang L, Romero MF, Handlogten ME, Verlander JW, Weiner ID. NBCe1-A regulates proximal tubule ammonia metabolism under basal conditions and in response to metabolic acidosis. JASN 29: 1182–1197, 2018. doi: 10.1681/ASN.2017080935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mount DB. Thick ascending limb of the loop of Henle. Clin J Am Soc Nephrol 9: 1974–1986, 2014. doi: 10.2215/CJN.04480413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol 69: 317–340, 2007. doi: 10.1146/annurev.physiol.69.040705.142215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris AN, Lee HW, Fang L ,Verlander JW, Weiner ID. . Differences in acidosis-stimulated renal ammonia metabolism in the male and female kidney. Am J Physiol Renal Physiol 317: F890–F905, 2019. doi: 10.1152/ajprenal.00244.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris AN, Lee HW, Verlander JW, Weiner ID. . Testosterone modulates renal ammonia metabolism. Am J Physiol Renal Physiol 318: F922–F935, 2020. doi: 10.1152/ajprenal.00560.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol 20: 3001–3015, 2002. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 35.Hunter I, Hay CW, Esswein B, Watt K, Mcewan IJ. . Tissue control of androgen action: The ups and downs of androgen receptor expression. Mol Cell Endocrinol 465: 27–35, 2018. doi: 10.1016/j.mce.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Grimont A, Bloch-Faure M, El Abida B ,Crambert G. Mapping of sex hormone receptors and their modulators along the nephron of male and female mice. FEBS Lett 583: 1644–1648, 2009. doi: 10.1016/j.febslet.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 37.Ruizeveld De Winter JA. Immunocytochemical localization of androgen receptor with polyclonal antibody in paraffin-embedded human tissues. J Histochem Cytochem 42: 125–126, 1994. doi: 10.1177/42.1.8263324. [DOI] [PubMed] [Google Scholar]
- 38.Takaoka M, Yuba M, Fujii T, Ohkita M, Matsumura Y. Oestrogen protects against ischaemic acute renal failure in rats by suppressing renal endothelin-1 overproduction. Clin Sci (Lond) 103, Suppl 48: 434S–437S, 2002. doi: 10.1042/CS103S434S. [DOI] [PubMed] [Google Scholar]
- 39.Takeda H, Chodak G, Mutchnik S, Nakamoto T ,Chang C. Immunohistochemical localization of androgen receptors with mono- and polyclonal antibodies to androgen receptor. J Endocrinol 126: 17–25, 1990. doi: 10.1677/joe.0.1260017. [DOI] [PubMed] [Google Scholar]
- 40.Wilson CM, Mcphaul MJ. A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol Cell Endocrinol 120: 51–57, 1996. doi: 10.1016/0303-7207(96)03819-1. [DOI] [PubMed] [Google Scholar]
- 41.Selye H. The effect of testosterone on the kidney. J Urol 42: 637–641, 1939. doi: 10.1016/S0022-5347(17)71560-1. [DOI] [Google Scholar]
- 42.Gluck SL, Underhill DM, Iyori M, Holliday LS, Kostrominova TY, Lee BS. Physiology and biochemistry of the kidney vacuolar H+-ATPase. Annu Rev Physiol 58: 427–445, 1996. doi: 10.1146/annurev.ph.58.030196.002235. [DOI] [PubMed] [Google Scholar]
- 43.Navaneethan SD, Schold JD, Arrigain S, Jolly SE, Wehbe E, Raina R, Simon JF, Srinivas TR, Jain A, Schreiber MJ Jr, Nally JV Jr.. Serum bicarbonate and mortality in stage 3 and stage 4 chronic kidney disease. Clin J Am Soc Nephrol 6: 2395–2402, 2011. doi: 10.2215/CJN.03730411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verlander JW, Chu D, Lee HW, Handlogten ME, Weiner ID. Expression of glutamine synthetase in the mouse kidney: localization in multiple epithelial cell types and differential regulation by hypokalemia. Am J Physiol Renal Physiol 305: F701–F713, 2013. doi: 10.1152/ajprenal.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]