Keywords: hypertension, inflammation, lymphocytes, oxidative stress, preeclampsia, pregnancy, placental ischemia, T cell
Abstract
The reduced uterine perfusion pressure (RUPP) rat model and normal pregnant (NP) rat recipients of RUPP CD4+ T cells recapitulate many characteristics of preeclampsia such as hypertension and oxidative stress. We have shown an important hypertensive role for natural killer (NK) cells to cause mitochondrial dysfunction in RUPP rats; however, the role for RUPP CD4+ T cells to stimulate NK cells is unknown. Therefore, we hypothesized that RUPP-induced CD4+ T cells activate NK cells to cause mitochondrial dysfunction/reactive oxygen species (ROS) as mechanisms of hypertension during pregnancy. We tested our hypothesis by adoptive transfer of RUPP CD4+ T cells into NP rats or by inhibiting the activation of RUPP CD4+ T cells with Orencia (abatacept) and examining hypertension, NK cells, and mitochondrial function. RUPP was performed on gestation day (GD) 14, and splenic CD4+ T cells were isolated on GD 19 and injected into NP rats on GD 13. In a separate group of rats, Orencia was infused and the RUPP procedure was performed. Mean arterial pressure and placental and renal mitochondrial ROS increased in RUPP (n = 7, P < 0.05) and NP + RUPP CD4+ T-cell recipients (n = 13, P < 0.05) compared with control NP (n = 7) and NP + NP CD4+ T-cell recipients (n = 5) but was reduced with Orencia (n = 13, P < 0.05). Placental and renal respiration was reduced in RUPP (n = 6, P < 0.05) and NP + RUPP CD4+ T-cell recipients (n = 6, state 3 P < 0.05) compared with NP (n = 5) and NP + NP CD4+ T-cell recipients (n = 5) but improved with Orencia (n = 9, n = 8 P < 0.05). These data indicate that CD4+ T cells, independent of NK cells, cause mitochondrial dysfunction/ROS contributing to hypertension in response to placental ischemia during pregnancy.
INTRODUCTION
Preeclampsia (PE) is defined as new onset hypertension during pregnancy, affecting ∼5–7% of pregnancies worldwide (1). Typically, PE occurs after 20 wk of gestation, may be characterized by endothelial dysfunction in women without preexisting hypertension before pregnancy (2) and is a leading cause of maternal morbidity and mortality. Previous studies have shown that preeclampsia increases placental and plasma levels of inflammatory cytokines and an imbalance between regulatory T cells and effector T cells (subclasses of CD4+ T lymphocytes) (3, 4). PE is also associated with increased cytolytic natural killer (NK) cells, oxidative stress, and certain features of metabolic syndrome as shown by the upregulation of several mediators of endothelial cell dysfunction (2).
Reactive oxygen species (ROS) are highly reactive free radicals that cause damage to DNA/RNA and protein leading to cellular dysfunction and death (5, 6). Many studies have shown that ROS increases during normal pregnancy compared with the nonpregnant state, but an excess of ROS is reported in systemic, pathologic conditions of pregnancy such as preeclampsia (6, 7). Placental oxidative stress is strongly associated with inflammation and endothelial dysfunction during pregnancy, and we have identified NK cells as important mediators of ROS, specifically mitochondrial ROS (mtROS), which contributes to hypertension in response to placental ischemia (6–9). Moreover, we have shown that activating autoantibodies to the angiotensin II type 1 (AT1) receptor (AT1-AA), notoriously associated with PE (10, 11), mediate NK cell activation, mitochondrial dysfunction, and ROS in response to placental ischemia (6, 12).
We have shown that AT1-AA results from placental ischemia, cytokines associated with placental ischemia [IL-6, IL-17, or tumor necrosis factor-α (TNF-α)] or from T cell-B cell interactions in the reduced uterine perfusion pressure (RUPP) rat model of placental ischemia (13, 14). Moreover, adoptive transfer of CD4+ T-helper (Th)1 cells from the RUPP rat model of preeclampsia into normal pregnant (NP) rats causes increases in the circulating inflammatory cytokines TNF-α, IL-6, and IL-17, AT1-AA, and hypertension (4). In addition, studies conducted by the laboratories of LaMarca and Wallace (15, 16) have indicated that Orencia (abatacept), a fusion protein that binds to the extracellular domain of cytotoxic T-lymphocyte-associated protein 4, inhibited the costimulation necessary for T-cell activation and attenuates hypertension, T-cell proliferation, systemic inflammation, and blood-brain barrier (BBB) permeability in pregnant rats.
Although a role for T cells and Th17 cells to cause ROS is known, their role to stimulate NK cell-mediated mtROS still remains unknown. Vaka et al. (6) has recently shown the importance of renal and placental mt dysfunction in hypertension in response to placental ischemia in the RUPP model of PE. However, we do not know the effect of inhibiting T-cell activation with Orencia on NK cell-mediated mitochondrial dysfunction/ROS or hypertension in RUPP rats. Therefore, we sought to 1) examine the effect of adoptive transfer of RUPP CD4+ T cells to activate NK cells and cause mitochondrial dysfunction/ROS in NP rats and 2) examine the effect of inhibiting T-cell activation with Orencia on NK cell-mediated mitochondrial dysfunction/ROS or hypertension in RUPP rats.
METHODS
Pregnant female Sprague-Dawley rats purchased from Envigo (Indianapolis, IN) were used to conduct this study. Rats were housed in a temperature-controlled room (75°F) with a 12:12-h light-dark cycle with free access to standard chow and water. All experiments conducted were in compliance with the guidelines of the University of Mississippi Medical Center, and the animals were handled based on the approved and published principles in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee.
Reduction in Uterine Perfusion
The two control groups of rats consisted of NP; (n = 7) and RUPP; (n = 7) groups. The pregnant Sprague-Dawley rats weighing ∼200 g arrived on gestational day (GD) 10. The RUPP surgery was performed on day 14 pregnant rats under isoflourane anesthesia using a constrictive silver clip (0.203 mm) to the abdominal aorta superior to the iliac bifurcation through a midline laparotomy. Ovarian collateral circulation was reduced to the uterus with restrictive clips (0.100 mm) to the bilateral uterine arcades at the ovarian end, thereby reducing blood flow by ∼ 40% (11, 17).
The Hypertensive Role of CD4+ T Cells in Response to Placental Ischemia
Following our protocol outlined in previously published studies demonstrating the role of RUPP CD4+ T cells to mediate hypertension during pregnancy (4), spleens were isolated from NP and RUPP donor rats at the time of euthanasia on GD 19 and placed on ice-cold phosphate-buffered saline, pH 7.0, homogenized in culture dishes with RPMI medium containing 10% FBS, and filtered using a 100-μm cell strainer. CD4+ T cells were isolated from splenocytes using magnetic separation via CD4+ Dynabeads according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA). Upon release from the Dynabeads, CD4+ T cells were washed in PBS cultured in RPMI medium containing 25 mM HEPES, 2 mM glutamine, 100 U/ml penicillin-streptomycin, 1.022 ng/mL IL-2, and 4 ng/mL IL-12 for 24 h at 5% CO2 at 37°C. After centrifugation, cell pellets were washed with saline and 1 × 106 cells per 100 μL of saline were prepared for intraperitoneal injection into NP recipient rats on GD 13. These groups of rats were designated as NP rats injected with NP CD4+ T cells (NP + NP CD4+ T cells, n = 7) or NP rats injected with RUPP CD4+ T cells (NP + RUPP CD4+ T cells, n = 13). On day 18, indwelling carotid catheters were inserted into all groups of rats. On GD 19, rats were placed in restrainer cages and allowed to adjust and equilibrate for 1 h before measuring conscious MAP for 30 min as performed previously (6, 9, 11, 12, 18, 19). Pup and placenta weights were measured, and blood, placentas, and kidneys were collected for flow cytometry or for mitochondrial function analysis.
Infusion of Orencia
In a separate group of pregnant rats, Orencia (250 mg/kg) was infused intravenously via jugular catheter on day 13 and on day 14, these rats underwent the RUPP surgical procedure (RUPP + Orencia, n = 10). This dose of Orencia was previously shown to decrease circulating CD4+ T cells in RUPP rats (18). On day 18, indwelling carotid catheters were inserted into all groups of rats. ON GD 19, rats were placed in restrainer cages and allowed to adjust and equilibrate for 1 h before measuring conscious MAP for 30 min as performed previously (6, 9, 11, 12, 18, 19). Pup and placenta weights were measured, and blood, placentas, and kidneys were collected for flow cytometry or for mitochondrial function analysis.
Effect of RUPP CD4+ T Cells on Circulating and Placental NK Cells
On day 19, lymphocytes were isolated from blood and placenta by centrifugation on a cushion of Ficoll-Hypaque (Lymphoprep, Accurate Chemical & Scientific Corp., Westbury, NY) per the manufacturer’s instructions. Cells were incubated for 10 min at 1 × 106 cells at 4°C with antibodies rat anti-NK cell activation structures (ANK61) or rat anti-NK cell antibody (ANK44) (Abcam, Cambridge, MA). ANK61 binds to the killer cell activation structure expressed on NK cells, and ANK44 is expressed on stimulated, cytotoxic NK cells and was analyzed as previously published (20). The percentage of positive stained cells in the gated population was used for individual rat measurement, and the mean values for each experimental group were calculated.
Effect of RUPP CD4+ T Cells on Mitochondrial Function
Intact kidney or placenta mitochondria was isolated using the differential centrifugation method (6). In summary, tissues were rinsed and homogenized using a Dounce homogenizer and the homogenate was centrifuged at 4,000 rpm for 3 min at 4°C. The supernatant was centrifuged at 10,000 rpm for 10 min at 4°C, and the collected pellet was suspended in 1 mL of Mito I buffer (250 mM sucrose, 10 mM HEPES, 1 mM EGTA, and 0.1% BSA, pH 7.2) and centrifuged. The collected pellet was suspended in 1 mL of Mito II (250 mM sucrose, 10 mM HEPES, and 0.1% BSA, pH 7.2) and centrifuged at 10,000 rpm for 10 min at 4°C. The final pellet suspended in 200 µL of Mito II buffer and used for respiration and ROS experiments.
Respiration in isolated mitochondria was measured using Oxygraph 2 K. Basal, state 2, state 3, state 4, and uncoupled respiration rates were measured using glutamate/malate, ADP, oligomycin, and carbonyl cyanide-4-[trifluoromethoxy]phenylhydrazone, respectively (6). Rotenone and antimycin A were used to record nonmitochondrial respiration. The collected data were analyzed and expressed as picomoles of oxygen consumed per second per milligram of mitochondrial protein.
Mitochondrial H2O2 production in placental and renal mitochondria was determined by using amplex red assay (6, 21). Mitochondria (0.4 mg/mL) were incubated in a 96-well plate with respiration buffer, superoxide dismutase (40 U/mL), horseradish peroxidase (4 U/mL), and succinate (10 mM). Amplex red (10 µM) was added to the wells to start the reaction. The final volume of the wells in the microplate was 200 µL. The real-time production of H2O2 was recorded using a plate reader at 555/581-nm excitation/emission for 30 min at 25°C. Appropriate blanks without amplex red or mitochondrial protein were also included in the assay.
Statistical Analysis
All statistical analyses were performed with GraphPad Prism 7.02 software (GraphPad Software, San Diego, CA). All results are reported as means ± SE. Comparisons between groups were analyzed by a one-way ANOVA with Bonferroni multiple comparisons test as post hoc analysis or by a Student’s t test when comparisons were made between two groups. Results were considered as statistically significant when P < 0.05.
RESULTS
The Hypertensive Role of CD4+ T Cells in Response to Placental Ischemia
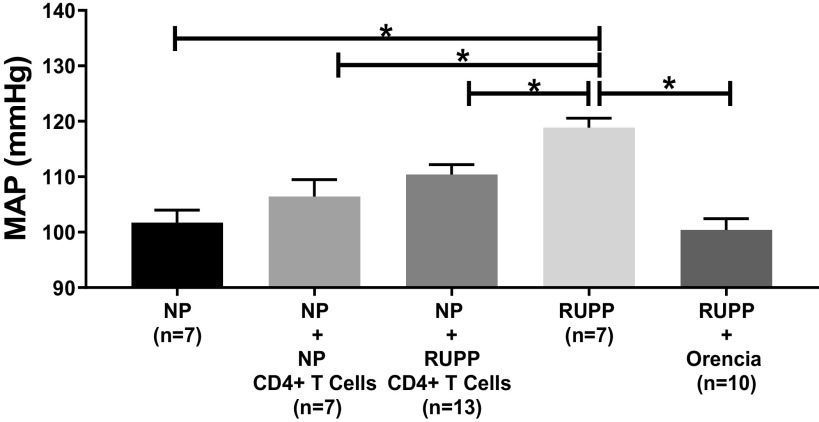
Statistical analyses for MAP and pup and placental weights were made between NP (n = 7), NP + NP CD4+ T cells (n = 7), NP + RUPP CD4+ T cells (n = 13), RUPP (n = 7), and RUPP + Orencia (n = 10) and are shown in Table 1. There was no difference in MAP between NP and NP + NP CD4+ T cells (102 ± 2 vs. 106 ± 32 mmHg) (Table 1 and Fig. 1). On day 19 of pregnancy, MAP was significantly elevated in RUPP and NP + RUPP CD4+ T cells compared with NP (Table 1 and Fig. 1). Moreover, blood pressure was significantly lower in RUPP + Orencia compared with RUPP controls (Table 1 and Fig. 1).
Table 1.
Physiological changes
| NP (n = 7) | NP + NP CD4+ T Cells (n = 7) | NP + RUPP CD4+ T Cells (n = 13) | RUPP (n = 7) | RUPP + Orencia (n = 10) | |
|---|---|---|---|---|---|
| Mean arterial pressure, mmHg | 102 ± 2 | 106 ± 3 | 110 ± 2* | 119 ± 2* | 100 ± 2 |
| Placental weight, g | 0.54 ± 0.03 | 0.51 ± 0.02 | 0.59 ± 0.01 | 0.54 ± 0.03 | 0.48 ± 0.02 |
| Fetal weight, g | 2.16 ± 0.06 | 2.28 ± 0.07 | 2.36 ± 0.03 | 2.00 ± 0.08+ | 1.92 ± 0.07 |
RUPP, reduced uterine perfusion pressure; NP, normal pregnant. *P < 0.05 compared with NP. +P < 0.05 compared with NP + NP CD4+ T cells.
Figure 1.
Mean arterial pressure (MAP) was elevated in reduced uterine perfusion pressure (RUPP) (n = 7) vs. normal pregnant (NP) (n = 7) (119 ± 2 vs. 102 ± 2 mmHg, P < 0.05), RUPP vs. NP + NP CD4+ T cells (n = 7) (119 ± 2 vs. 106 ± 3 mmHg, P < 0.05), and RUPP vs. RUPP + Orencia (n = 10) (119 ± 2 vs. 100 ± 2 mmHg, P < 0.05) rats. MAP decreased in RUPP rats administered Orencia vs. NP + RUPP CD4+ T-cell rats (100 ± 2 vs. 110 ± 2 mmHg, P < 0.05). The results represent means ± SE. *P < 0.05.
Effect of RUPP CD4+ T Cells on Circulating and Placental NK Cells
Circulating total NK cells were significantly increased in RUPP and NP + RUPP CD4+ T cells compared with NP (P < 0.05) and were reduced in RUPP + Orencia compared with RUPP (Table 2 and Fig. 2A). There was no change in circulating total NK cells in NP + NP CD4+ T cells compared with NP (Table 2 and Fig. 2A). Cytolytic circulating NK cells were significantly increased in NP + RUPP CD4+ T cells (P < 0.05) and RUPP compared with NP (n = 7) (Table 2 and Fig. 2B). Yet, there were no changes observed in RUPP versus RUPP + Orencia (Table 2 and Fig. 2B). Neither total nor placental activated NK cells changed with RUPP CD4+ T cells compared with NP controls or in RUPPs + Orencia compared with RUPP controls (Table 2 and Fig. 2, C and D).
Table 2.
NK cell changes
| NP | NP + NP CD4+ T Cells | NP + RUPP CD4+ T Cells | RUPP | RUPP + Orencia | |
|---|---|---|---|---|---|
| Circulating total NK cells, %gated | 44.62 ± 8.8 (n = 7) | 46.43 ± 10.14 (n = 5) | 59.06 ± 4.87* (n = 12) | 58.64 ± 4.25*# (n = 7) | 31.99 ± 6.76 (n = 10) |
| Cytolytic circulating NK cell, %gated | 0.32 ± 0.21 (n = 7) | 0.92 ± 0.85 (n = 5) | 2.84 ± 0.66* (n = 11) | 0.84 ± 0.59 (n = 7) | 0.81 ± 0.39 (n = 10) |
| Placental total NK cells, %gated | 43.8 ± 12.6 (n = 7) | 32.03 ± 15.2 (n = 5) | 27.8 ± 8.2 (n = 12) | 59.9 ± 15.9 (n = 4) | 27.7 ± 8.2 (n = 11) |
| Placental circulating NK cells, %gated | 1.5 ± 0.63 (n = 7) | 0.47 ± 0.47 (n = 5) | 0.99 ± 0.35 (n = 8) | 2.51 ± 2.43 (n = 6) | 1.69 ± 0.92 (n = 11) |
RUPP, reduced uterine perfusion pressure; NP, normal pregnant; NK, normal killer. *P < 0.05 compared with NP. #P < 0.05 compared with RUPP + Orencia.
Figure 2.
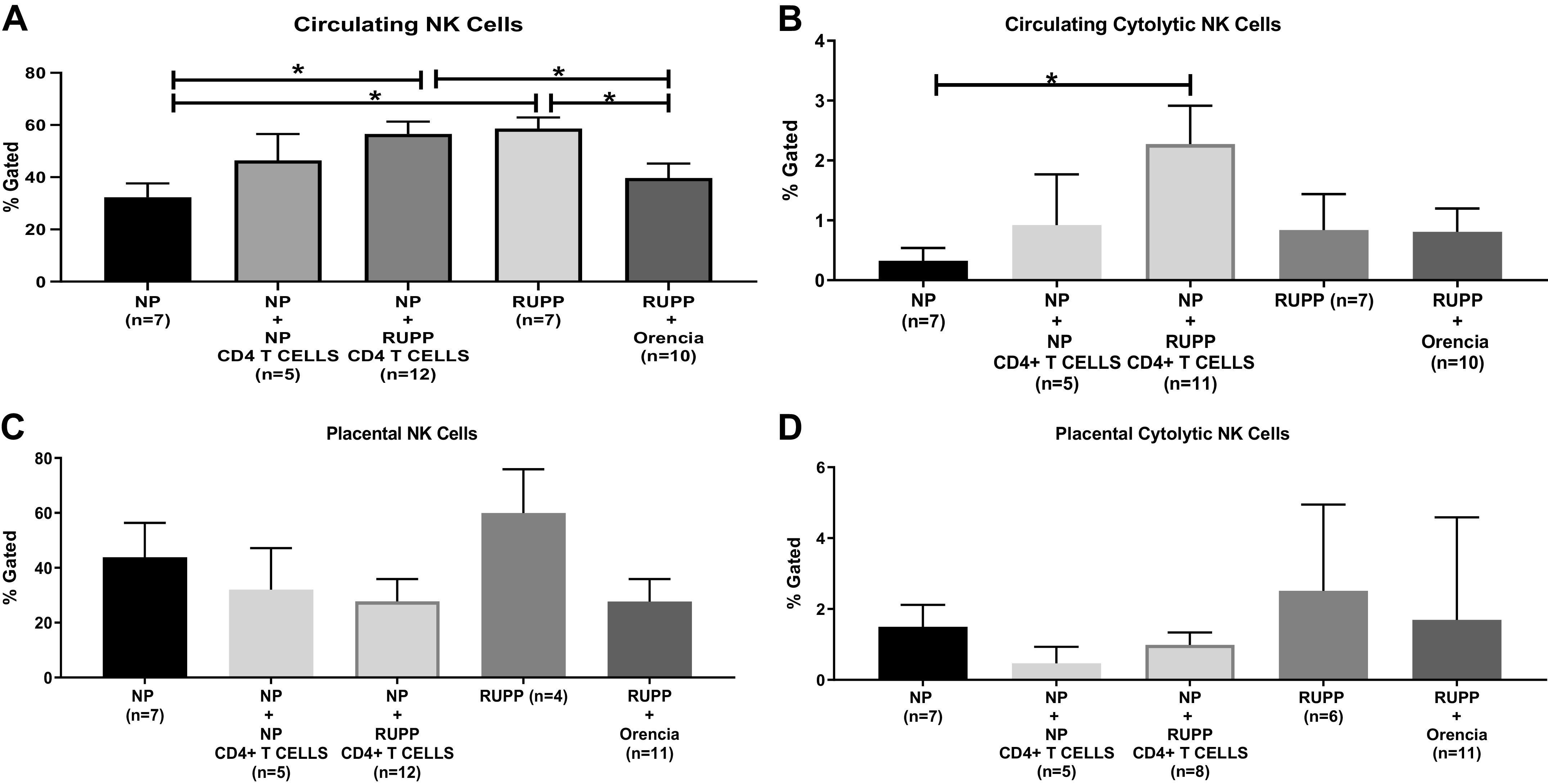
A: circulating total normal killer (NK) cells were significantly increased in reduced uterine perfusion pressure (RUPP) (58.64 ± 4.25% gated, n = 7; P < 0.05) and normal pregnant (NP) + RUPP CD4+ T cells (59.06 ± 4.87% gated, n = 12; P < 0.05) compared with NP (44.62 ± 8.8% gated, n = 7; P < 0.05) and were reduced in RUPP + Orencia (31.99 ± 6.76% gated, n = 10; P < 0.05) compared with RUPP. Circulating total NK cells were not changed in NP + NP CD4+ T cells (46.43 ± 10.14% gated; n = 5) compared with NP. The results represent means ± SE. *P < 0.05. B: cytolytic circulating NK cells were significantly increased in NP + RUPP CD4+ T cells (2.84 ± 0.66% gated, n = 11; P < 0.05) and RUPP rats (0.84 ± 0.59% gated, n = 7) compared with NP (0.32 ± 0.21% gated, n = 7). No changes were observed in RUPP vs. RUPP + Orencia (0.81 ± 0.39% gated, n = 10) and NP + NP CD4+ T cells (0.92 ± 0.85, n = 5). The results represent means ± SE. *P < 0.05. C: total NK cells in the placental were not changed in RUPP CD4+ T cells (27.8 ± 8.2% gated, n = 12) compared with NP controls (43.8 ± 12.6% gated, n = 7) and NP + NP CD4+ T Cells (32.03 ± 15.2% gated, n = 5) or in RUPPs + Orencia (27.7 ± 8.2% gated, n = 11) compared with RUPP control rats (59.9 ± 15.9% gated, n = 4). The results represent means ± SE. *P < 0.05. D: cytolytic NK cells in the placenta were not changed in RUPP CD4+ T cells (0.99 ± 0.35% gated, n = 8) compared with NP controls (1.5 ± 0.63% gated, n = 7) and NP + NP CD4+ T Cells (0.47 ± 0.47% gated, n = 5) or in RUPPs + Orencia (1.69 ± 0.92% gated, n = 11) compared with RUPP control rats (2.51 ± 2.43% gated, n = 6). The results represent means ± SE. *P < 0.05.
Effect of RUPP CD4+ T Cells on Mitochondrial Function
Importantly, placental mitochondrial ROS were significantly increased in real-time production of H2O2 in RUPP and NP + RUPP CD4+ T cells versus NP and NP + NP CD4+ T cells (Table 3 and Fig. 3A). Placental mtROS were significantly decreased in RUPP + Orencia (Table 3 and Fig. 3A). Renal mtROS increased in real-time production of H2O2 in RUPP and NP + RUPP CD4+ T cells versus NP and NP + NP T cells (Table 3 and Fig. 3B). Renal mtROS was significantly reduced in RUPP + Orencia compared with RUPP (Table 3 and Fig. 3B).
Table 3.
Mitochondrial ROS changes
| NP | NP + NP CD4+ T Cells | NP + RUPP CD4+ T Cells | RUPP | RUPP + Orencia | |
|---|---|---|---|---|---|
| Placental ROS, %gated | 100 ± 13.41 (n = 5) | 15.82 ± 1.69 (n = 3) | 196.9 ± 14.47*+ (n = 3) | 207.9 ± 13.98*+# (n = 5) | 60.33 ± 1.74 (n = 8) |
| Renal ROS, %gated | 100 ± 22.95 (n = 5) | 178.7 ± 5.2 (n = 3) | 200.2 ± 29.3* (n = 5) | 792.2 ± 163.9*+# (n = 4) | 265.9 ± 32.2 (n = 9) |
RUPP, reduced uterine perfusion pressure; NP, normal pregnant; ROS, reactive oxygen species. *P < 0.05 compared with NP. +P < 0.05 compared with NP + NP CD4+ T cells. #P < 0.05 compared with RUPP + Orencia.
Fig. 3.
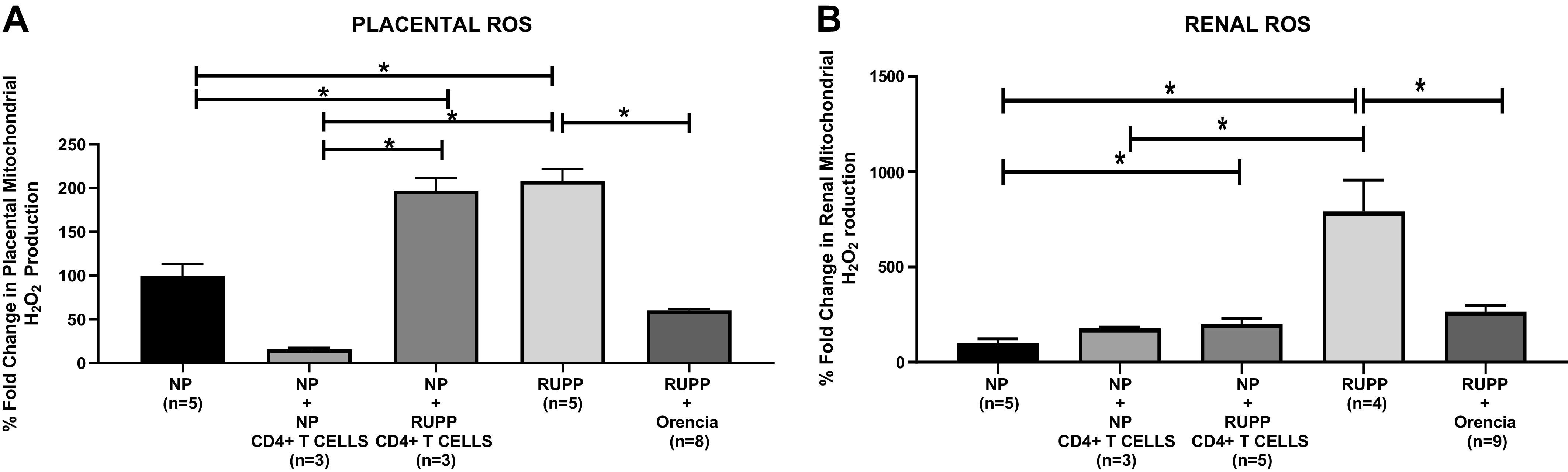
A: placental mitochondrial reactive oxygen species (ROS) significantly increased in reduced uterine perfusion pressure (RUPP) (n = 5) vs. normal pregnant (NP) (n = 5) (207.9 ± 13.98 vs. 100 ± 13.41% gated, P < 0.05) and RUPP vs. RUPP + Orencia (n = 8) (207.9 ± 13.98 vs. 60.33 ± 1.74% gated, P < 0.05). NP + RUPP CD4+ T cells (n = 3) (196.9 ± 14.47% gated, P < 0.05) underwent an elevation in mitochondrial placental ROS compared with NP controls (100 ± 13.41% gated). There was a significant decrease observed in placental mtROS in RUPP rats administered Orencia vs. NP + RUPP CD4+ T-cell rats (60.33 ± 1.74 vs. 196.9 ± 14.47% gated, P < 0.05). The results represent means ± SE. *P < 0.05. B: renal mitochondrial ROS significantly increased in RUPP (n = 4) vs. NP (n = 5) (792.2 ± 163.9 vs. 100 ± 22.95% gated, P < 0.05), NP + RUPP CD4+ T cells (n = 5) vs. NP (200.2 ± 29.3 vs. 100 ± 22.95% gated, P < 0.05), and RUPP vs. RUPP + Orencia (n = 9) (792.2 ± 163.9 vs. 265.9 ± 32.2% gated, P < 0.05). There was a decreased observed in renal mitochondrial ROS in RUPP rats administered Orencia vs. NP + RUPP CD4+ T-cell rats (265.9 ± 32.2 vs. 200.2 ± 29.3% gated). The results represent means ± SE. *P < 0.05.
Placental mitochondrial state 3 respiration significantly decreased in RUPP and NP + RUPP CD4+ T cells compared with NP controls and NP+ NP CD4+ T cells (Table 4 and Fig. 4A). State 3 placental respiration from RUPP + Orencia increased significantly compared with RUPP as shown in Fig. 4A. Placental mitochondrial uncoupled respiration decreased significantly in RUPP and NP + RUPP CD4+ T cells compared with NP controls and NP + NP CD4+ T cells (Table 4 and Fig. 4B). Uncoupled placental respiration increased in RUPP + Orencia versus RUPP as shown in Fig. 4B.
Table 4.
Placental respiration changes
| NP | NP + NP CD4+ T Cells | NP + RUPP CD4+ T Cells | RUPP | RUPP + Orencia | |
|---|---|---|---|---|---|
| Placental state 3 respiration, pmol O2/s/mg) | 422.7 ± 83.35 (n = 5) | 257.4 ± 37.9 (n = 5) | 209.3 ± 31.27* (n = 6) | 81.88 ± 36.46*+# (n = 6) | 193.7 ± 33.74 (n = 9) |
| Placental uncoupled respiration, pmol O2/s/mg | 229.7 ± 58.91 (n = 5) | 197.5 ± 13.18 (n = 5) | 152.1 ± 46.21 (n = 6) | 38.12 ± 21.27*+# (n = 6) | 228.4 ± 64.05 (n = 9) |
RUPP, reduced uterine perfusion pressure; NP, normal pregnant. *P < 0.05 compared with NP. +P < 0.05 compared with NP + NP CD4+ T cells. #P < 0.05 compared with RUPP + Orencia.
Fig. 4.
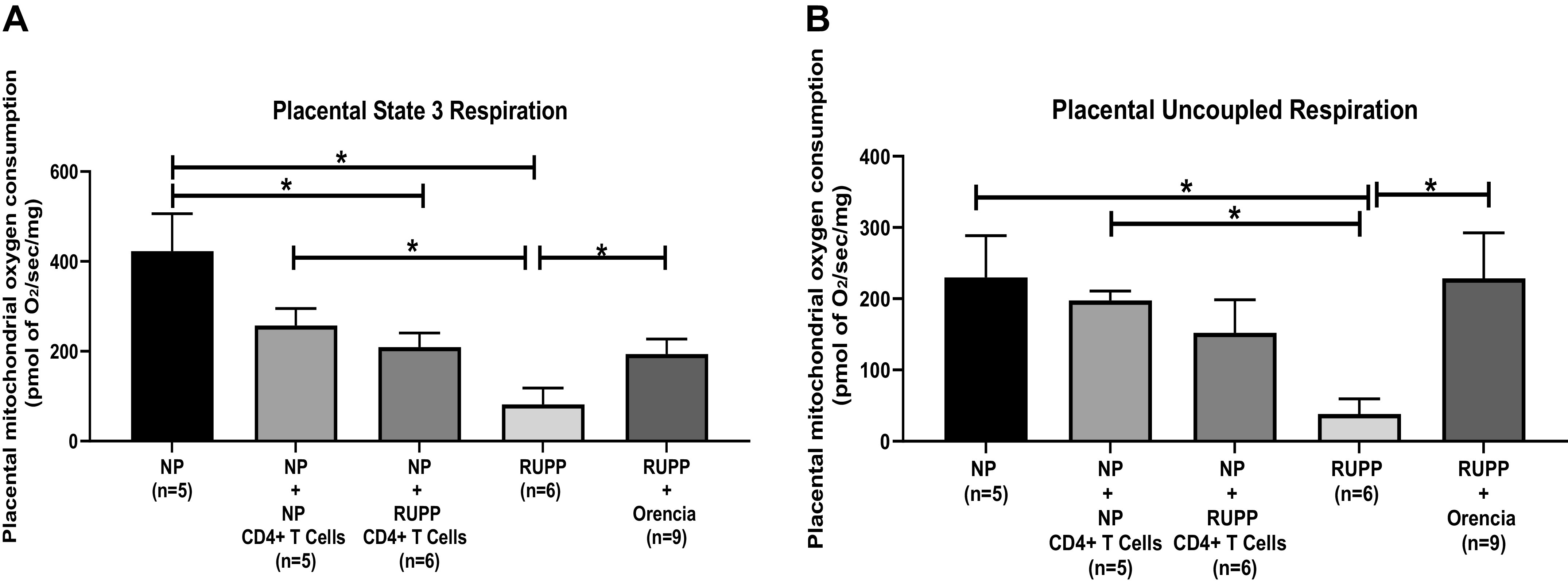
A: placental mitochondrial state 3 respiration significantly decreased in reduced uterine perfusion pressure (RUPP) (n = 6) (81.88 ± 36.46 pmol of O2/s/mg, P < 0.05) and normal pregnant (NP) + RUPP CD4+ T cells (n = 6) (209.3 ± 31.27 pmol of O2/s/mg, P < 0.05) compared with NP controls (n = 5) (422.7 ± 83.35 pmol of O2/s/mg). State 3 placental respiration RUPP + Orencia (n = 9) (193.7 ± 33.74 pmol of O2/s/mg, P < 0.05) increased significantly compared with RUPP. The results represent means ± SE. *P < 0.05. B: placental mitochondrial uncoupled respiration decreased significantly in RUPP (n = 6) (38.12 ± 21.27 pmol of O2/s/mg, P < 0.05) and NP + RUPP CD4+ T cells (n = 6) (152.1 ± 46.21 pmol of O2/s/mg) compared with NP controls (n = 5) (229.7 ± 58.91 pmol of O2/s/mg). Uncoupled placental respiration increased in. RUPP + Orencia (n = 9) (228.4 ± 64.05 pmol of O2/s/mg, P < 0.05) vs. RUPP. The results represent means ± SE. *P < 0.05.
Renal mitochondrial state 3 respiration significantly decreased in RUPP and NP + RUPP CD4+ T cells compared with NP controls and NP+ NP CD4+ T cells (Table 5 and Fig. 5A). State 3 renal respiration RUPP + Orencia increased significantly compared with RUPP as shown in Fig. 5A. Renal mitochondrial uncoupled respiration decreased significantly in RUPP and NP + RUPP CD4+ T cells compared with NP controls and NP + NP CD4+ T cell (Table 5 and Fig. 5B). Uncoupled renal respiration increased in RUPP + Orencia versus RUPP as shown in Fig. 5B.
Table 5.
Renal respiration changes
| NP | NP + NP CD4+ T Cells | NP + RUPP CD4+ T Cells | RUPP | RUPP + Orencia | |
|---|---|---|---|---|---|
| Renal state 3 respiration, pmol of O2/s/mg | 289.8 ± 43.40 (n = 5) | 343.4 ± 93.10 (n = 5) | 133.4 ± 21.40*+(n = 9) | 61.97 ± 24.63*+# (n = 6) | 196.5 ± 76.16 (n = 8) |
| Renal uncoupled respiration, pmol of O2/s/mg | 242.4 ± 27.67 (n = 5) | 282.6 ± 75.75 (n = 5) | 61.83 ± 18.00*+(n = 9) | 53.34 ± 22.76*+# (n = 6) | 235.6 ± 84.05 (n = 8) |
RUPP, reduced uterine perfusion pressure; NP, normal pregnant. *P < 0.05 compared with NP. +P < 0.05 compared with NP + NP CD4+ T cells. #P < 0.05 compared with RUPP + Orencia.
Fig. 5.
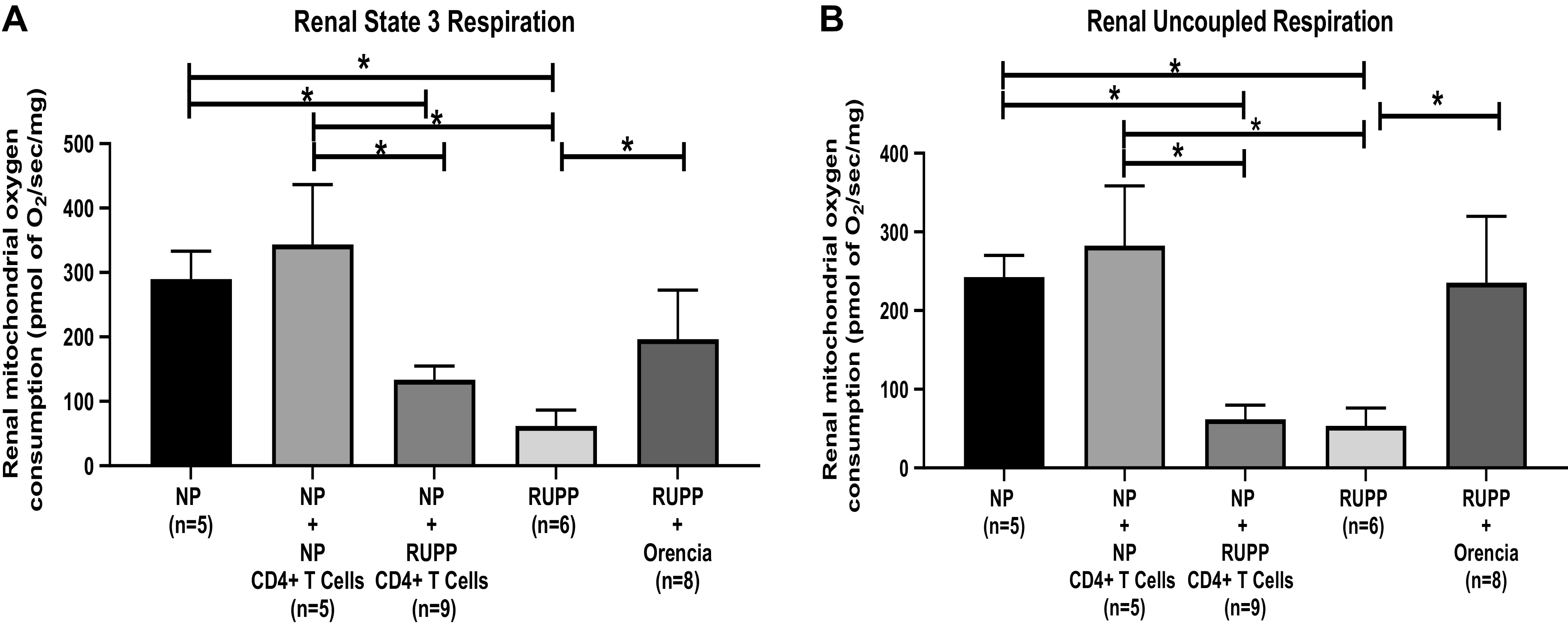
A: renal mitochondrial state 3 respiration significantly decreased in reduced uterine perfusion pressure (RUPP) (n = 6) (61.97 ± 24.63 pmol of O2/s/mg, P < 0.05) and NP + RUPP CD4+ T cells (n = 9) (133.4 ± 21.40 pmol of O2/s/mg, P < 0.05) compared with NP controls (n = 5) (289.8 ± 43.40 pmol of O2/s/mg). State 3 renal respiration RUPP + Orencia (n = 8) (196.5 ± 76.16 pmol of O2/s/mg, P < 0.05) increased significantly compared with RUPP. The results represent means ± SE. *P < 0.05. B: renal mitochondrial uncoupled respiration decreased significantly in RUPP (n = 6) (53.34 ± 22.76 pmol of O2/s/mg P < 0.05) and NP + RUPP CD4+ T cells (n = 9) (61.83 ± 18.00 pmol of O2/s/mg, P < 0.05) compared with NP controls (n = 5) (242.4 ± 27.67 pmol of O2/s/mg). Uncoupled renal respiration increased in RUPP + Orencia (n = 8) (235.6 ± 84.05 pmol of O2/s/mg, P < 0.05) vs. RUPP. The results represent means ± SE. *P < 0.05.
Placental respiratory control ratio (RCR; state 3/state 4) significantly decreased in RUPP and NP + RUPP CD4+ T cells compared with NP controls (Table 6). Placental RCR in RUPP + Orencia increased significantly compared with RUPP as shown in Table 6. Renal RCR was unchanged among the groups (Table 6).
Table 6.
RCR changes
| NP | NP + NP CD4+ T Cells | NP + RUPP CD4+ T Cells | RUPP | RUPP + Orencia | |
|---|---|---|---|---|---|
| Placental RCR (state 3/state 4) | 1.69 ± 0.19 (n = 5) | 1.51 ± 0.28 (n = 5) | 1.05 ± 0.02* (n = 6) | 0.73 ± 0.17*+# (n = 6) | 1.64 ± 0.32 (n = 9) |
| Renal RCR (state 3/state 4) | NP (n = 5) | 1.59 ± 0.49 (n = 5) | 0.98 ± 0.19 (n = 9) | 1.14 ± 0.29 (n = 6) | 1.24 ± 0.24 (n = 6) |
RCR, respiratory control ratio; RUPP, reduced uterine perfusion pressure; NP, normal pregnant. *P < 0.05 compared with NP. +P < 0.05 compared with NP + NP CD4+ T cells. #P < 0.05 compared with RUPP + Orencia.
DISCUSSION
We have previously shown the importance of CD4+ T cells from women with PE to cause hypertension during pregnancy (22). Moreover, we have demonstrated the importance of CD4+ T cells stimulated in response to placental ischemia in the RUPP rat to cause hypertension and the release of many factors associated with PE in pregnant women. This study supports our previous findings that CD4+ T cells from RUPP rats cause hypertension during pregnancy. In addition to hypertension, we demonstrate that RUPP CD4+ T cells stimulate circulating cytolytic NK cells and cause renal and placental mitochondrial dysfunction characterized by increased mitochondrial oxidative stress and decreased mitochondrial respiration, three factors that have more recently been highlighted as players in the pathophysiology of PE. This is further supported by the administration of Orencia (abatacept), an inhibitor of T-cell activation, to RUPP rats, which significantly decreases circulating cytolytic NK cells and renal and placental mitochondrial oxidative stress. Collectively, these data support the hypothesis that CD4+ T cells are important stimuli for circulating factors that contribute the pathophysiology of PE. However, it is important to note that adoptive transfer of RUPP CD4+ T cells into NP does not contribute to an increase placental NK cells nor did administration of Orencia significantly lower placental NK cells in RUPP. These data indicate that although T cells simulate circulating NK cells, there must be another regulating factor for placental NK cells in response to placental ischemia that was not identified in this study.
There are several characteristics of inflammation associated with PE, including an elevation in circulating inflammatory cytokines, an imbalance in T cells, NK cells, and oxidative stress. Data have shown that CD4+ T cells stimulated in response to placental ischemia induce a state similar to PE, without any additional stimulus (4, 11, 18). CD4+ T cells from placental ischemic rats have been reported to secrete elevated levels of inflammatory cytokines, cause a significant increase in MAP, and stimulate the release of sFlt-1 (4) and, therefore, it is not surprising that they also actively increase mitochondrial oxidative stress. As we have previously published, the adoptive transfer of RUPP CD4+ T cells into NP rats significantly increases MAP (4) as well as implicates CD4+ T cells as inflammatory factors in elevated MAP in RUPP rats (6, 18, 23). RUPP rats have previously been shown to have an increase in placental, circulating, and renal activated NK cells, which are very potent producers of oxidative stress as a mechanism of killing tumor cells or viral infected cells. However, it has not been shown if CD4+ T cells actively affect the levels of activated NK cells in circulation and placental tissue. Although it has become evident that endothelial dysfunction is largely associated with a generalized systemic inflammatory response involving the activation of leukocytes in the mother’s blood, the role of T-cell communication with NK cells in the circulation is largely unknown (24).
It has been shown that the immune cell profile is altered in women with PE and is characterized by an increase in cytolytic NK cells and a difference of CD4+ T cells subsets reflected by an increase in proinflammatory Th17 cells and a decrease in regulatory T cells compared with women with normal pregnancies (19). Furthermore, our group has previously demonstrated that the adoptive transfer of RUPP Th17 cells also causes hypertension and ROS and NK cell activation (4, 18,19). These novel findings indicate that CD4+ T cells are responsible for stimulating NK cells within the circulation and thus additionally the pathologic immune responses associated with the diagnosis of PE. However, we do not know if these are the stimulus of NK cell-mediated mtROS seen in the placenta and kidney in this study. Moreover, we do not know if Th17 or regulatory T cells were influenced by either abatacept or RUPP CD4+ T cells and if this could be the factor regulating the lack of change in placental NK cells seen in this study.
Mitochondrial dysfunction and ROS have been previously proven to be highly associated with the PE phenotype through the use of animal models of PE (6). Previously, researchers demonstrated that the adoptive transfer of placental ischemic CD4+ T cells from RUPP rats into NP rats increased placental and renal oxidative stress and MAP; however, the effect of CD4+ T cells on mitochondrial stress was unknown (4). Here, we transferred the collective RUPP CD4+ T-cell population, which will contain some regulatory T cells or Th17 cells. Since there were no changes with placental NK cells in this study, but we still saw increased placental mtROS, it may be that Th17 cells are responsible for causing the mitochondrial dysfunction in the placenta. Moreover, we have shown an important role for AT1-AA to stimulate NK cells and cause mtROS during pregnancy. Inhibition of T-cell costimulation was previously shown to attenuate the production of AT1-AA, TNF-α, and placental sFlt-1 in response to placental ischemia during pregnancy (18). AT1-AA is stimulated by RUPP CD4+ T cells, likely through their communication with B cells. Therefore, we hypothesize that AT1-AA directly causes mtROS and dysfunction independent of NK cells; however, additional studies are necessary to confirm this hypothesis. Importantly, the increase in mtROS could also be attributed to the increase in activated circulating NK cells. Previous studies have indicated that NK cell depletion administered to RUPP rats improved blood pressure and mitochondrial respiration/ROS and therefore further supports the link between NK cell activation and mtROS production (9). Although the exact stimuli for T lymphocytes during PE are unknown, the treatment of RUPP rats with abatacept appears to significantly mitigate some of the effects associated with PE.
Although adoptive transfer of RUPP CD4+ T cells increases placental mtROS to the same degree as that seen in RUPP rats, in this study, RUPP CD4+ T cells did not increase renal mtROS to the same level seen in RUPP kidneys. The profound increase in mtROS in the kidney of RUPP rats is similar to previous reports associated with the large decreases in neuronal nitric oxide synthase expression in the kidney as well as a reduction in glomerular filtration rate and renal plasma flow (25). Moreover, we have previously shown the increase in renal mtROS in RUPP kidneys to be significantly greater than in control pregnant rats; however, in this study the levels were higher than those previously published and this may be the reason that RUPP CD4+ T cells did not stimulate mtROS to the same degree as in RUPP controls. Nevertheless, it is possible that the kidneys exhibited an increased in oxidative stress attributable to the lack of bioavailable nitric oxide, circulating NK cells, and the deleterious effects on renal function.
Collectively, the data demonstrate an important role of RUPP CD4+ T cells in activating NK cells and causing mitochondrial dysfunction/ROS, which we know play an important role in hypertension in response to placental ischemia. This is further supported by the decreased hypertension and circulating NK cells and improved mitochondrial function and ROS with blockade of T-cell activation by Orencia in response to placental ischemia. The data identify CD4+ T cells as mediators of circulating factors, mediators of mitochondrial oxidative stress, and facilitators in the pathophysiology of PE and indicate that if T cells were not activated, hypertension and mitochondrial dysfunction/mtROS may not occur in response to PE.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01 HD 067541 06 (to B.L.) and P20 GM 121334 (to L.M.A. and B.L.), American Heart Association Early Career Award 19CDA34670055 (to L.M.A.), and NIH T32 Trainee Grant T32-HL105324 (to E.D.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.D.L. conceived and designed research; E.D., K.E.R., N.C., S.F., O.T.H., and T.I. performed experiments; E.D., D.C.C., B.D.L., K.E.R., L.M.A., V.R.V., M.F., and S.F. analyzed data; E.D., D.C.C., B.D.L., K.E.R., L.M.A., and V.R.V. interpreted results of experiments; E.D., B.D.L., and K.E.R. prepared figures; E.D., B.D.L., K.E.R., L.M.A., and M.F. drafted manuscript; E.D., D.C.C., B.D.L., K.E.R., L.M.A., V.R.V., and N.C. edited and revised manuscript; E.D., D.C.C., K.E.R., L.M.A., V.R.V., M.F., N.C., S.F., O.T.H., and T.I. approved final version of manuscript.
REFERENCES
- 1.Obstetricians American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obst Gynecol 122: 1122, 2013. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 2.Craici IM, Wagner SJ, Weissgerber TL, Grande JP, Garovic VD. Advances in the pathophysiology of pre-eclampsia and related podocyte injury. Kidney Int 86: 275–285, 2014. doi: 10.1038/ki.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, de St Groth BF, Nanan R. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol 183: 7023–7030, 2009. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- 4.Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, Wilson M, Martin JN Jr, LaMarca B. CD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension 57: 949–955, 2011. doi: 10.1161/HYPERTENSIONAHA.110.168344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granger JP. Inflammatory cytokines, vascular function, and hypertension. Am J Physiol Reg Integr Comp Physiol 286: R989–R990, 2004. doi: 10.1152/ajpregu.00157.2004. [DOI] [PubMed] [Google Scholar]
- 6.Vaka VR, McMaster KM, Cunningham MW Jr, Ibrahim T, Hazlewood R, Usry N, Cornelius DC, Amaral LM, LaMarca B. Role of mitochondrial dysfunction and reactive oxygen species in mediating hypertension in the reduced uterine perfusion pressure rat model of preeclampsia. Hypertension 72: 703–711, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsubara K, Higaki T, Matsubara Y, Nawa A. Nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. Int J Mol Sci 16: 4600–4614, 2015. doi: 10.3390/ijms16034600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sánchez-Aranguren LC, Prada CE, Riaño-Medina CE, Lopez M. Endothelial dysfunction and preeclampsia: role of oxidative stress. Front Physiol 5: 372, 2014. doi: 10.3389/fphys.2014.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaka VR, McMaster KM, Cornelius DC, Ibrahim T, Jayaram A, Usry N, Cunningham MW Jr, Amaral LM, LaMarca B. Natural killer cells contribute to mitochondrial dysfunction in response to placental ischemia in reduced uterine perfusion pressure rats. Am J Physiol Regul Integr Comp Physiol 316: R441–R447, 2019. doi: 10.1152/ajpregu.00279.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dechend R, Homuth V, Wallukat G, Müller DN, Krause M, Dudenhausen J, Haller H, Luft FC. Agonistic antibodies directed at the angiotensin II, AT1 receptor in preeclampsia. J Soc Gynecol Invest 13: 79–86, 2006. doi: 10.1016/j.jsgi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Novotny SR, Wallace K, Heath J, Moseley J, Dhillon P, Weimer A, Wallukat G, Herse F, Wenzel K, James N, Martin J, Dechend R, LaMarca B. Activating autoantibodies to the angiotensin II type I receptor play an important role in mediating hypertension in response to adoptive transfer of CD4+ T lymphocytes from placental ischemic rats. Am J Physiol Reg Integr Comp Physiol 302: R1197–R1201, 2012. doi: 10.1152/ajpregu.00623.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaka VR, Cunningham MW, Deer E, Franks M, Ibrahim T, Amaral LM, Usry N, Cornelius DC, Dechend R, Wallukat G, LaMarc BD. Blockade of endogenous angiotensin II type I receptor agonistic autoantibody activity improves mitochondrial reactive oxygen species and hypertension in a rat model of preeclampsia. Am J Physiol Reg Integr Comp Physiol 318: R256–R262, 2020. doi: 10.1152/ajpregu.00179.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelius DC, Wallace K, Kiprono L, Dhillon P, Moseley J, LaMarca B. Endothelin-1 is not a mechanism of IL-17 induced hypertension during pregnancy. Med J Obstet Gynecol 1: 1006, 2013. [https://pubmed.ncbi.nlm.nih.gov/25414910/]. [PMC free article] [PubMed] [Google Scholar]
- 14.LaMarca B, Cornelius DC, Harmon AC, Amaral LM, Cunningham MW, Faulkner JL, Wallace K. Identifying immune mechanisms mediating the hypertension during preeclampsia. Am J Physiol Reg Integr Comp Physiol 311: R1–R9, 2016. doi: 10.1152/ajpregu.00052.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bean C, Spencer SK, Bowles T, Kyle PB, Williams JM, Gibbens J, Wallace K. Inhibition of T‐cell activation attenuates hypertension, TNF α, IL‐17, and blood–brain barrier permeability in pregnant rats with angiogenic imbalance. Am J Reprod Immunol 76: 272–279, 2016. doi: 10.1111/aji.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace K, Bean C, Bowles T, Spencer SK, Randle W, Kyle PB, Shaffery J. Hypertension, anxiety, and blood-brain barrier permeability are increased in postpartum severe preeclampsia/hemolysis, elevated liver enzymes, and low platelet count syndrome rats. Hypertension 72: 946–954, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaMarca B, Amaral LM, Harmon AC, Cornelius DC, Faulkner JL, Cunningham MW. Placental ischemia and resultant phenotype in animal models of preeclampsia. Curr Hypertens Rep 18: 38, 2016. doi: 10.1007/s11906-016-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novotny S, Wallace K, Herse F, Moseley J, Darby M, Heath J, Gill J, Wallukat G, Martin JN, Dechend R, LaMarca B. CD4(+) T cells play a critical role in mediating hypertension in response to placental ischemia. J Hypertens 2: 38, 2013. [https://pubmed.ncbi.nlm.nih.gov/25401050/]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shields CA, McCalmon M, Ibrahim T, White DL, Williams JM, LaMarca B, Cornelius DC. Placental ischemia-stimulated T-helper 17 cells induce preeclampsia-associated cytolytic natural killer cells during pregnancy. Am J Physiol Reg Integr Comp Physiol 315: R336–R343, 2018. doi: 10.1152/ajpregu.00061.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elfarra JT, Cottrell JN, Cornelius DC, Cunningham MW Jr, Faulkner JL, Ibrahim T, Lamarca B, Amaral LM. 17-Hydroxyprogesterone caproate improves T cells and NK cells in response to placental ischemia; new mechanisms of action for an old drug. Preg Hypertens 19: 226–232, 2020. doi: 10.1016/j.preghy.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension 46: 82–86, 2005. doi: 10.1161/01.HYP.0000169152.59854.36. [DOI] [PubMed] [Google Scholar]
- 22.Harmon AC, Ibrahim T, Cornelius DC, Amaral LM, Cunningham MW Jr, Wallace K, LaMarca B. Placental CD4+ T cells isolated from preeclamptic women cause preeclampsia-like symptoms in pregnant nude-athymic rats. Preg Hypertens 15: 7–11, 2019. doi: 10.1016/j.preghy.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaMarca B, Cornelius D, Wallace K. Elucidating immune mechanisms causing hypertension during pregnancy. Physiology 28: 225–233, 2013. doi: 10.1152/physiol.00006.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sargent IL, Borzychowski AM, Redman CW. Immunoregulation in normal pregnancy and pre-eclampsia: an overview. Reprod Biomed Online 13: 680–686, 2006. doi: 10.1016/S1472-6483(10)60659-1. [DOI] [PubMed] [Google Scholar]
- 25.Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, Granger JP. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens 21: 1152–1156, 2008. doi: 10.1038/ajh.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]