Abstract
The measurement of fluid compartmentalization, or the distribution of fluid volume between extracellular (ECF) and intracellular (ICF) spaces, historically requires complicated, burdensome, and often terminal methodologies that do not permit repeated or longitudinal experiments. New technologies including time-domain nuclear magnetic resonance (TD-NMR)-based methods allow for highly accurate measurements of total body water (TBW) within minutes in a noninvasive manner, but do not permit dissection of ECF versus ICF reservoirs. In contrast, methods such as bioimpedance spectroscopy (BIS) allow dissection of ECF versus ICF reservoirs but are hampered by dependence on many nuanced details in data collection that undermine confidence in experimental results. Here, we present a novel combinatorial use of these two technologies (NMR/BIS) to improve the accuracy of BIS-based assessments of ECF and ICF, while maintaining the advantages of these minimally invasive methods. Briefly, mice undergo TD-NMR and BIS-based measures, and then fat masses as derived by TD-NMR are used to correct BIS outputs. Mice of the C57BL/6J background were studied using NMR/BIS methods to assess the effects of acute furosemide injection and diet-induced obesity on fluid compartmentalization, and to examine the influence of sex, body mass and composition, and diet on TBW, ECF, and ICF. We discovered that in mice, sex and body size/composition have substantial and interactive effects on fluid compartmentalization. We propose that the combinatorial use of NMR/BIS methods will enable a revisioning of the types of longitudinal, kinetic studies that can be performed to understand the impact of various interventions on body fluid homeostasis.
Keywords: compartments, furosemide, hydration, obesity
INTRODUCTION
Historically, body composition analysis has been cumbersome, involving technically challenging and/or cost-prohibitive methodologies, and often the additional limitation that endpoints must be assessed terminally. One low-cost methodology that has been forwarded to allow serial assessments of body composition (such as fat mass) but also provides unique insights into body water compartmentalization is bioimpedance spectroscopy (BIS). Compared with less-sophisticated but related single-frequency bioimpedance analysis (BIA) or multifrequency bioimpedance analysis (MF-BIA), BIS has been demonstrated to provide superior testing characteristics for analyzing body composition in a minimally invasive manner, and when used in rodents, to provide results that are close to those obtained using 3H for total body water (TBW) and 35S for extracellular fluid (ECF) (1). Nonetheless, there remains some skepticism among researchers with regard to the use of BIS and related technologies for analyzing body composition in rodents. For example, some investigators have demonstrated a level of uncertainty and some failure in reproducibility with regard to fat mass assessments using these methods (2).
With increasing global interest in metabolic disorders such as obesity and diabetes, many research institutions have started providing access to sophisticated equipment within “metabolic phenotyping” core facilities, such as time-domain nuclear magnetic resonance (TD-NMR), magnetic resonance imaging (MRI), and dual X-ray absorptiometry (DXA, or DEXA), which can provide rapid, accurate, precise, and noninvasive assessments of body composition including fat and lean or fat-free masses (3). Thus, the somewhat controversial use of BIS to assess fat mass has undoubtedly contributed to its low rate of use compared to TD-NMR, MRI, and DXA-based methodologies. Unfortunately, this has resulted in the secondary consequence of reduced utilization of BIS to assess body water compartmentalization. As BIS is arguably the only method to easily, noninvasively, and serially/longitudinally track body water compartmentalization, this represents a missed opportunity for researchers interested in water and electrolyte homeostasis physiology. The availability of a minimally invasive or noninvasive method to repeatedly/longitudinally assess body fluid compartmentalization in small species such as mice, which have small blood volumes that preclude widespread use of more traditional tracer/dilution-based methods, is of particular interest to researchers using genetically modified mouse models.
Here, we tested the concept that the combined utilization of widely accepted TD-NMR-based analyses of fat mass could be used to correct BIS-based assessments of body water compartmentalization in mice, and thereby provide increased accuracy and precision for this rapid, noninvasive approach to tracking difficult-to-track aspects of fluid homeostasis such as the distribution of fluid between extracellular (ECF) and intracellular (ICF) spaces. We propose that the addition of BIS-based assessments of body water compartmentalization will add significant value to insights gained by existing and more widely accepted body composition analysis methods (e.g., TD-NMR, MRI, and DXA), and that the combinatorial use of these methods will improve the accuracy and precision of BIS-based assessments of body water compartmentalization.
MATERIALS AND METHODS
Animals
All animal use complied with the American Physiological Society’s Guiding Principles in the Care and Use of Laboratory Animals, the National Research Council’s Guide for the Care and Use of Laboratory Animals (4), and received prior approval from the Medical College of Wisconsin’s Institutional Animal Care and Use Committee.
Nontransgenic littermate control mice of both sexes, aged 9–11.5 wk obtained from various transgenic lines backcrossed to and maintained on the C57BL/6J background strain at the Medical College of Wisconsin, and wild-type C57BL/6J mice aged from 9 to 26 wk and previously purchased from the Jackson Laboratories, were used for all studies. Animals were maintained on soy-free Teklad 2920X chow or a high-fat diet (HFD) delivering 45% kcal from fat (OpenSource D12451) with ad libitum access to hyperchlorinated tap water, and were housed in a vivarium maintained at 22°C with a 14:10-h light:dark cycle.
Time-Domain Nuclear Magnetic Resonance
Animals were weighed to the nearest 0.01 g. Body composition (fat mass, lean mass, and free water mass) were determined within minutes before animals were studied by BIS, using Time-Domain Nuclear Magnetic Resonance (TD-NMR) via a Bruker LF110 system with mouse probe assembly, essentially as previously described (3). The TD-NMR system was calibrated by the manufacturer’s representative using mixes of oil for “fat,” lean chicken breast meat for “lean,” and saline or water for “free fluid” similar to previous descriptions by other authors (5). Fat-free mass (FFM) was calculated as the total body mass minus fat mass as determined by TD-NMR. Total body water was calculated as 73.2% of FFM (6).
Bioimpedance Spectroscopy
BIS was performed using an ImpediMed ImpediVET BIS1 system. Mice were anesthetized by an isoflurane inhalant (2%) before electrodes were placed subcutaneously in locations recommended by the manufacturer and as previously described by others (1). Fluid compartmentalization was then determined using manufacturer’s recommended constants for mice (density = 1.05; proportion = 1; hydration = 0.732; males: RhoE = 998.9, RhoI = 1220.2/females: RhoE = 586.9, RhoI = 756.8). A single successful recording from each animal was reanalyzed multiple times within the supplied software package by changing the “length” constant, which is meant to represent the distance between emitting and recording electrodes, such that several theoretical values bracketing the measured distance were analyzed for each animal.
TD-NMR-Based Correction of BIS Results
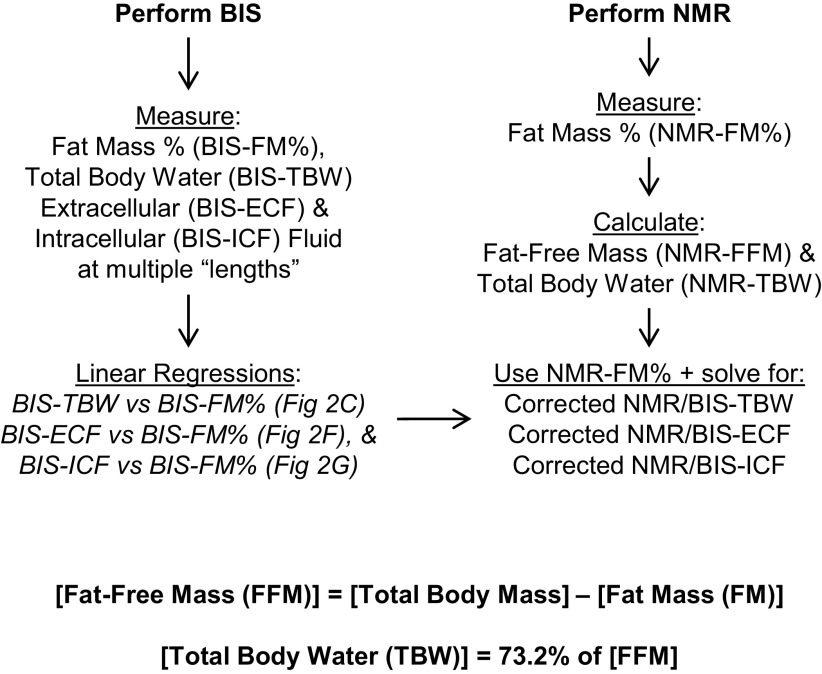
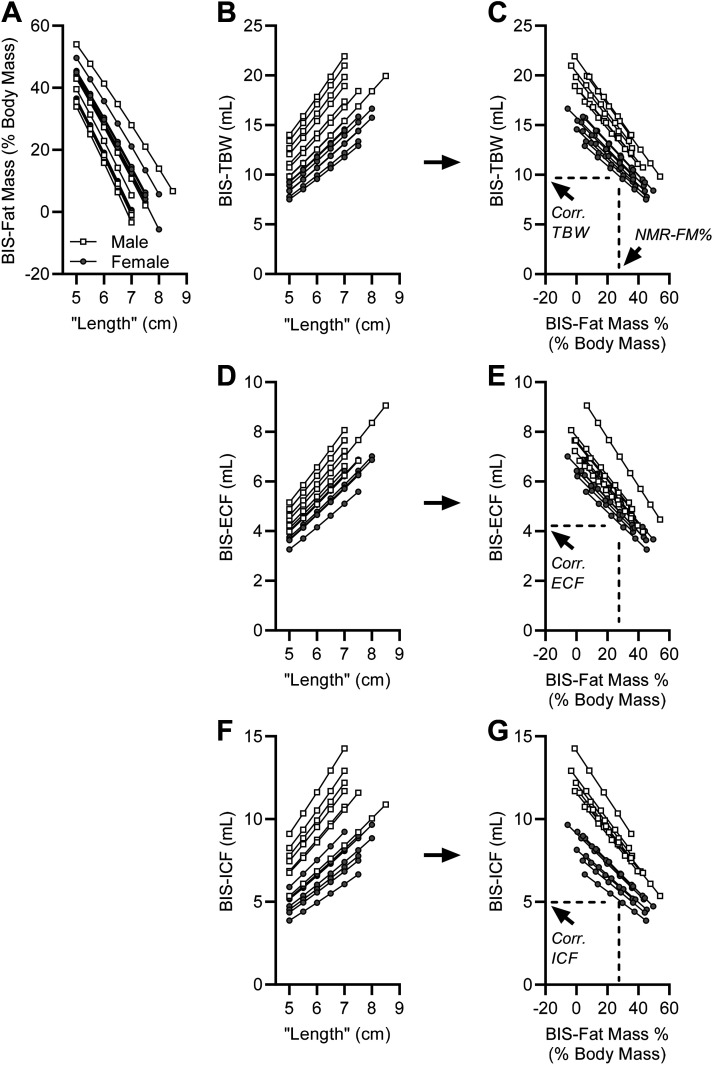
Data collected from TD-NMR and BIS methods from each individual animal were processed as outlined in Fig. 1. Briefly, BIS-based assessments of fat mass (BIS-FM), total body water (BIS-TBW), extracellular (BIS-ECF), and intracellular (BIS-ICF) fluid volumes were calculated for each animal using multiple hypothetical “length” values. These various calculated endpoints were then related to “length” by simple linear regression, as illustrated in Fig. 2. As BIS-FM, BIS-TBW, BIS-ECF, and BIS-ICF were all linearly related to “length,” BIS-FM (Fig. 2A) was then substituted for “length” within each animal to calculate linear relationships between BIS-TBW (Fig. 2C), BIS-ECF (Fig. 2E), and BIS-ICF (Fig. 2G) versus BIS-FM. Finally, TD-NMR-based assessments of fat mass (NMR-FM%) were then used to interpolate corrected NMR/BIS-TBW, NMR/BIS-ECF, and NMR/BIS-ICF values for each individual animal.
Figure 1.
Workflow of combined NMR/BIS methodology. Fluid compartmentalization is analyzed using BIS, and body composition is analyzed using time-domain NMR. From BIS analyses, fat mass, total body water, extracellular, and intracellular fluid volumes are determined at multiple theoretical “lengths.” Linear regressions are then performed to associate calculated fluid compartment sizes and fat mass against theoretical “length” values. Because these relationships are demonstrably linear (i.e., see Fig. 2, A, B, D, and F), substitution methods are then used to associate calculated fluid compartment sizes with calculated fat mass values within each animal (i.e., see Fig. 2, C, E, and G). NMR-based assessments of body composition, including fat mass, fat-free mass, and total body water, are then used to solve for corrected fluid compartment sizes for each animal (i.e., see Fig. 2, E and G). BIS, bioimpedance spectroscopy; NMR, nuclear magnetic resonance.
Figure 2.
Illustration of linear correlations among fluid compartments and theoretical “lengths” between emitting and recording BIS electrodes, and correction of BIS-derived TBW, ECF, and ICF values using NMR-derived fat mass values. A: linear correlations between BIS-calculated fat mass and theoretical “lengths” between BIS electrodes for n = 7 male and n = 8 female nontransgenic mice backcrossed onto the C57BL/6J background strain. B: linear correlations between BIS-calculated total body water (BIS-TBW) and theoretical “lengths” between BIS electrodes. C: linear correlation between BIS-calculated TBW and fat mass. D: linear correlations between BIS-calculated extracellular fluid (BIS-ECF) and theoretical “lengths” between BIS electrodes. E: linear correlation between BIS-calculated ECF and fat mass. F: linear correlations between BIS-calculated intracellular fluid (BIS-ICF) and theoretical “lengths” between BIS electrodes. G: linear correlation between BIS-calculated ICF and fat mass. In C, E, and G, dotted lines illustrate the insertion of TD-NMR-based assessments of fat mass (NMR-FM%) to solve for corrected NMR/BIS-TBW (Corr. TBW), NMR/BIS-ECF (Corr. ECF), and NMR/BIS-ICF (Corr. ICF) on an individual animal basis. BIS, bioimpedance spectroscopy; ECF, extracellular fluid; ICF, intracellular fluid; TBW, total body water.
Example Application: Acute Volume Depletion by Furosemide
In one example application of this methodology, we examined the effect of acute injection of furosemide (a sodium-potassium-chloride cotransporter inhibitor that is used clinically as a loop diuretic) on fluid retention and compartmentalization. During the light phase (between 9 AM and 2 PM) mice were removed from their home cage (i.e., ad libitum access to 2920X chow diet and water) and underwent NMR and BIS-based measures as outlined in Fig. 1. Mice were then immediately injected with 100% dimethylsulfoxide (DMSO) vehicle or furosemide (30 mg/mL in 100% DMSO) into the peritoneal cavity using a 25-µL glass syringe (Hamilton) and 30-gauge needle, at a volume of 0.33 µL/g body mass. This resulted in delivery of a total of 0 or 10 mg/kg furosemide treatments, respectively. Mice were immediately placed individually into single-mouse metabolic cages (Nalgene) with no access to food or water for the remainder of the study, to enable quantification of urine production over time. Body composition by TD-NMR, fluid compartmentalization by BIS, and urine masses were then reassessed at 10, 30, and 60 min after drug injection. At the conclusion of the experiment, mice were immediately euthanized by CO2 asphyxiation and body mass was assessed using a high-resolution balance (i.e., 0.0001 g). The body was then bisected longitudinally to increase surface area and expose internal organs, and placed into a laboratory oven maintained at 60°C for 4–5 days until masses stabilized. Desiccated bodies were then reweighed to determine total body water mass (i.e., total “wet” body mass at euthansia minus total desiccated “dry” body mass yields “desiccated” TBW mass).
Example Application: Prolonged Diet-Induced Obesity
Adult littermate control mice from various colonies, all backcrossed at least seven generations onto the C57BL/6J background and maintained on 2920X chow diet, or wild-type C57BL/6J mice that had been purchased from the Jackson Laboratories and maintained on a 45% HFD (D12451) for 10 wk underwent TD-NMR and BIS assessments as outlined in Fig. 1. Data were then analyzed as described above to calculate corrected NMR/BIS-TBW, NMR/BIS-ECF, and NMR/BIS-ICF values.
Statistics
Throughout, data are reported from individual animals where possible, and summary data are presented as means ± SE. Data were analyzed by simple linear regression or by two-way repeated-measures ANOVA followed by the Sidak multiple-comparisons procedure, as indicated in individual figure legends, using GraphPad Prism v8.4. Differences were considered significant with P < 0.05.
RESULTS
Preliminary Experiments
Preliminary studies to compare the outputs of BIS- and NMR-based methods were carried out on C57BL/6J mice of both sexes between 6 and 15 wk of age (n = 69). Comparisons of measured lengths between implanted electrodes, assessed by a standard laboratory ruler, and optimized “lengths” calculated using the workflow in Fig. 1 were then carried out. Regardless of sex, uninterpretable relationships were observed between physically measured “lengths” between electrodes and the calculated values for “length,” highlighted by an R2 value from simple linear regression of only 0.000057, and slope of zero (P value vs. 0 = 0.951) (data not shown). These findings prompted our development and application of the new NMR/BIS method as described herein, which does not rely on a physically measured distance between electrodes.
Acute Volume Depletion by Furosemide
To demonstrate the NMR/BIS method described in Fig. 1, we first examined the effect of acute volume depletion induced by the loop diuretic furosemide on TBW and fluid compartments. To minimize the volume of fluid injected for this experiment, we dissolved furosemide at its maximal solubility in a common vehicle. Using DMSO as the vehicle, this permitted administration of furosemide at a 30 mg/mL concentration. By injecting only 0.33 µL/g of body mass, this yielded the commonly utilized 10 mg/kg dose while minimizing injection volumes. In the current cohort of animals (DMSO, n = 8: 26.27 ± 0.69 g, vs. furosemide, n = 8: 26.10 ± 0.72 g total body mass), this resulted in very small total injection volumes into the intraperitoneal space (DMSO: 8.8 ± 0.7 µL vs. furosemide: 8.7 ± 0.2 µL).
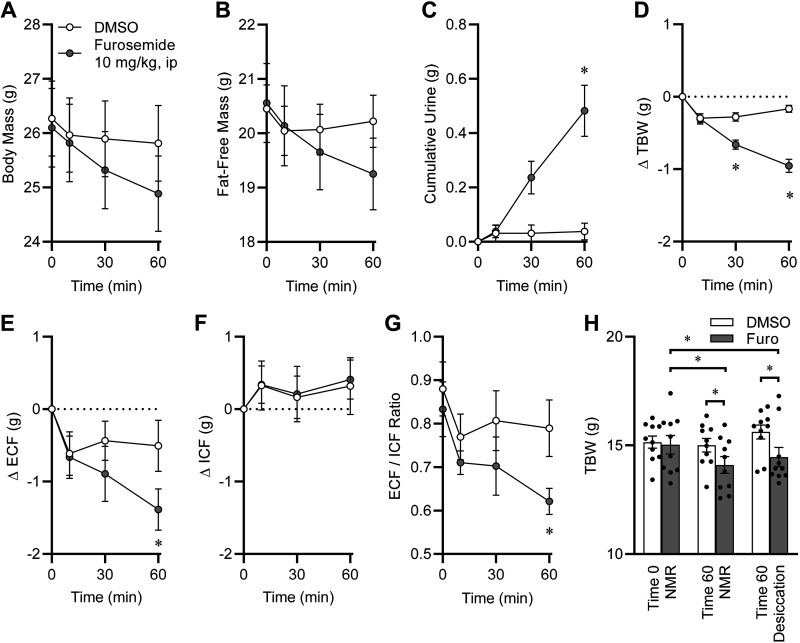
Compared with DMSO vehicle treatment and as expected, furosemide caused significant changes in body mass (Fig. 3A) and fat-free mass (Fig. 3B). Also as expected, furosemide caused a significant increase in urine production (Fig. 3C). Total body water, determined by TD-NMR, was significantly reduced by furosemide (Fig. 3D). After correction by TD-NMR using the workflow illustrated in Fig. 1, NMR/BIS-ECF was significantly reduced by furosemide (Fig. 3E), whereas NMR/BIS-ICF was unchanged (Fig. 3F), resulting in a significant reduction in the ECF-to-ICF ratio (Fig. 3G).
Figure 3.
Example application of the NMR/BIS method, with acute volume depletion by furosemide. A: body mass assessed by electronic balance immediately before, and at 10, 30, and 60 min after acute intraperitoneal injection of 100% dimethylsulfoxide (DMSO) or furosemide, 10 mg/kg, delivered via injection of 30 mg/mL furosemide dissolved in 100% DMSO solution at 0.33 µL/g body mass. Drug × time interaction P < 0.0001. B: fat-free mass, determined by TD-NMR. Drug × time interaction P < 0.0001. C: cumulative urine mass. Drug × time interaction P = 0.0002. D: change in total body water (TBW), calculated as 73.2% of fat-free mass as determined by TD-NMR. Drug × time interaction P < 0.0001. E: change in NMR/BIS-corrected extracellular fluid (ECF) mass. F: change in NMR/BIS-corrected intracellular fluid (ICF) mass. G: ratio of ECF/ICF. H: comparison of TBW mass as determined at time 0 by TD-NMR, and 60 min postinjection by TD-NMR and by desiccation. For A–H, DMSO n = 8 and furosemide n = 8 male C57BL/6J mice purchased at 10 wk of age from the Jackson Laboratories, aged to 12 wk and maintained on 2920X chow diet. Data are presented as means ± SE and were analyzed using two-way repeated-measures ANOVA followed by Sidak’s multiple-comparisons procedure. *P < 0.05 between treatment groups at indicated timepoint, by Sidak’s multiple-comparisons procedure. BIS, bioimpedance spectroscopy; ECF, extracellular fluid; ICF, intracellular fluid; NMR, nuclear magnetic resonance; TD-NMR, time-domain nuclear magnetic resonance.
We then performed control experiments to assess the validity of TD-NMR-based assessments of TBW in these animals. Immediately following the 60-min postinjection time point, TBW values as assessed by TD-NMR were confirmed by desiccation of the body. TBW values determined by TD-NMR at time zero (e.g., immediately before injection of drugs) were indistinguishable between groups that would subsequently receive either DMSO or furosemide (Fig. 3H). Relative to DMSO and relative to its preinjection baseline values, furosemide injection caused a significant reduction in TBW at 60 min as determined by both TD-NMR- and desiccation-based methods. Importantly, within either treatment group (i.e., within the furosemide group or within the DMSO group), there was no significant difference in TBW at 60 min postinjection as determined using TD-NMR- versus desiccation-based methods (R2 = 0.492), confirming the accuracy of TBW determination by TD-NMR. As TBW determined by the combined NMR/BIS method is mathematically derived from the TBW content as calculated by TD-NMR, these methods yielded indistinguishable results (R2 > 0.999).
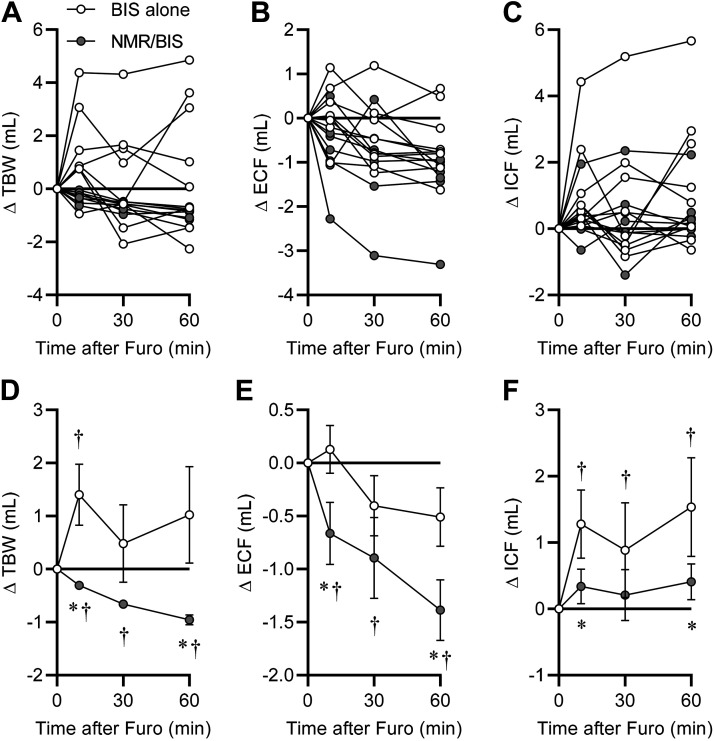
Comparison of the changes in TBW, ECF, and ICF spaces following furosemide injection as determined by BIS alone using the manufacturer’s recommended protocol (i.e., analyzing BIS outcomes using the physically measured “length” between electrodes as determined with a laboratory ruler for each individual animal) versus the new combined NMR/BIS method (i.e., determining BIS-derived values at multiple theoretical “length” values within each individual animal, solving for a calculated “length” value by substitution of NMR-derived fat mass, and subsequently correcting fluid compartment sizes as in Figs. 1 and 2) provides a critical demonstration of the increased precision provided by the combined NMR/BIS method versus the use of BIS alone. Volumes of TBW (Fig. 4A), ECF (Fig. 4B), and ICF (Fig. 4C) from individual animals highlight the gross differences in group variance when using BIS alone versus the combined NMR/BIS method. Comparison of the effect of furosemide at each timepoint after injection highlights that the two methods detect significantly different, and indeed opposite, effects of furosemide on TBW at 10 and 60 min after furosemide injection, and that NMR/BIS is able to detect the significant reduction in TBW already at 10 min after injection (means ± SE: −0.31 ± 0.07 mL, P < 0.05) (Fig. 4D). Whereas BIS alone failed to detect a significant change in ECF at any timepoint, NMR/BIS detected a significant reduction in ECF (−0.66 ± 0.29 mL, P < 0.05) already at 10 min after the injection (Fig. 4E). BIS alone indicated significant increases in ICF at all timepoints, but NMR/BIS failed to detect a significant increase in ICF at any timepoint (Fig. 4F). Combining these observations, NMR/BIS failed to indicate a significant difference in ICF at 60 min (+0.41 ± 0.27 mL), yet the significant difference detected in ECF at the 10 min-timepoint was characterized by a similar variance; this supports the conclusion that when applied to sample sizes of n = 8/group, that the combined NMR/BIS method provides sufficient precision to reliably detect differences in ECF and ICF compartments between 0.4 and 0.6 mL. Larger group sizes would of course be expected to further resolve this range, and may provide detection of differences with even smaller effect sizes.
Figure 4.
Comparison of BIS alone versus NMR/BIS method to detect changes in fluid compartment volumes. A–C: changes in total body water (TBW), extracellular fluid (ECF), and intracellular fluid (ICF) volumes for each individual animal as determined using BIS alone versus NMR/BIS method, from the furosemide experiment presented in Fig. 3. D–F: data from A–C presented as group means ± SE. *P < 0.05 between methods at timepoint indicated, and †P < 0.05 vs. time 0 within the method, by Sidak’s multiple-comparisons procedure. BIS, bioimpedance spectroscopy; NMR, nuclear magnetic resonance.
Effect of Prolonged Diet-Induced Obesity on Fluid Compartments
In a second application of our new NMR/BIS method, we next examined fluid compartmentalization in animals maintained on chow and high-fat diets. Mice of both sexes fed either 2920X chow diet or D12451 45% HFD for 10 wk underwent NMR/BIS analysis under ad libitum food and water conditions.
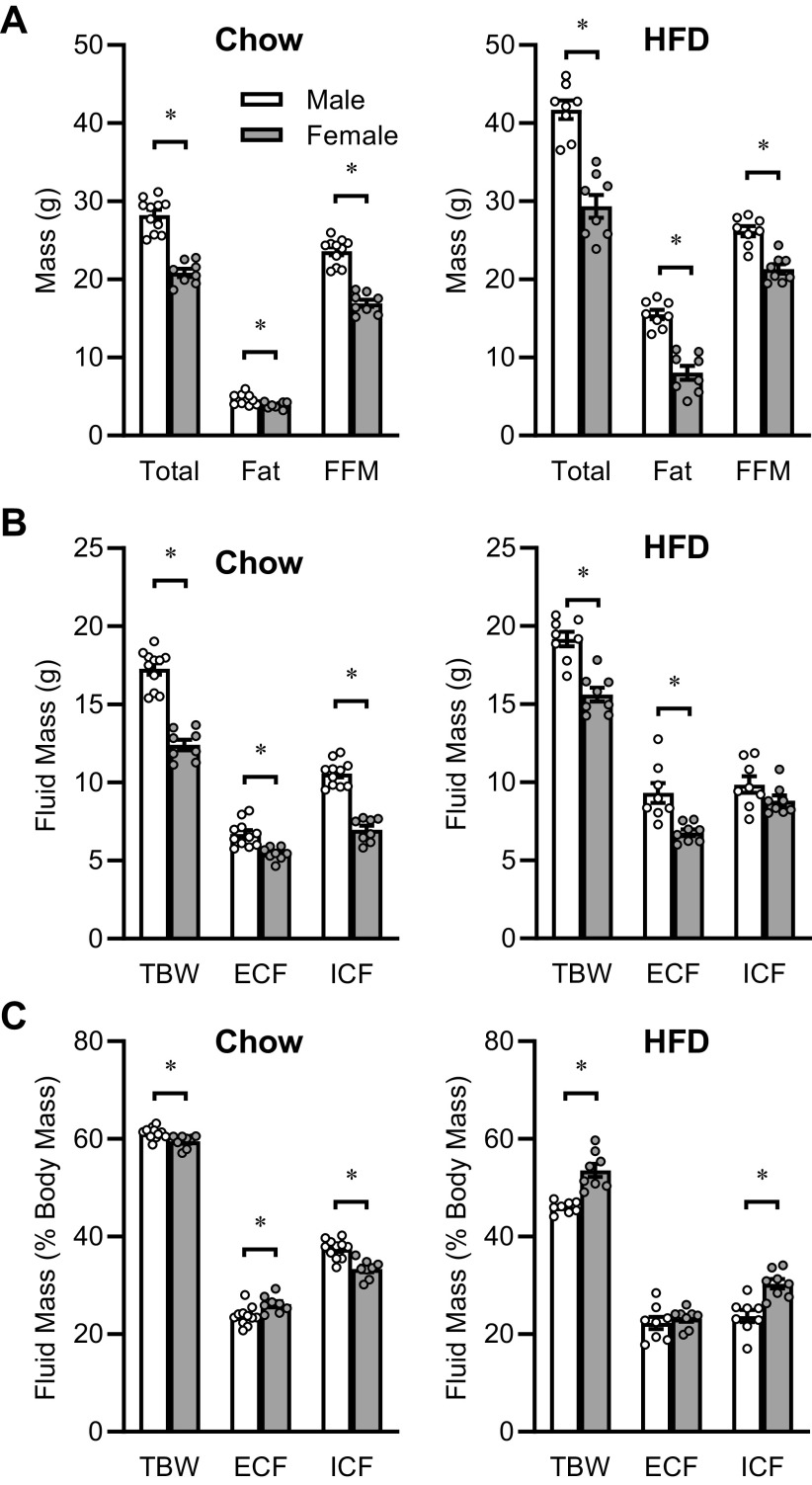
Animals fed soy-free 2920X chow diet (aged 9–12 wk) exhibited sex-dependent differences in total body mass, fat mass, and fat-free mass, with males exhibiting increases in all three types of mass (Fig. 5A). Similarly, total body water as determined by TD-NMR, and ECF and ICF masses as determined by the NMR/BIS method were greater in males (Fig. 5B). Males exhibited a much smaller yet still statistically significant increase in TBW after normalization to total body mass (Fig. 5C). Although ICF remained significantly greater per total body mass in males, ECF per body mass was significantly decreased in males.
Figure 5.
Example application of the NMR/BIS method, with mice fed chow versus mice with prolonged high-fat diet exposure. A: total body mass (Total), fat mass (Fat), and fat-free mass (FFM) determined by TD-NMR in mice maintained on different diets. Nontransgenic mice (n = 7 male and n = 8 female) backcrossed onto the C57BL/6J background strain were maintained on 2920X “Chow” diet and studied between 9 and 12 wk of age. Wild-type C57BL/6J mice (n = 8 male and n = 8 female) obtained from the Jackson Laboratories and maintained on a 45% high-fat diet (“HFD”) for 10 wk were studied at 26.5 wk of age. B: total body water (TBW) determined by TD-NMR, and extracellular (ECF) and intracellular (ICF) fluid masses determined by the combined NMR/BIS method. C: TBW, ECF, and ICF values normalized within animal by total body mass. *P < 0.05 by Tukey’s multiple comparisons procedure. BIS, bioimpedance spectroscopy; NMR, nuclear magnetic resonance.
Animals that were maintained on a 45% HFD for 10 wk also exhibited sex-dependent differences in fluid compartmentalization, however, these differences were distinct from those observed in chow-fed animals. Total body mass, fat mass, and fat-free masses were all significantly greater in males maintained on HFD (Fig. 5A). TBW and ECF masses were greater in males, though ICF mass was similar between males and females fed HFD (Fig. 5B). Interestingly, relative to females, males fed HFD exhibited a significantly lower TBW and ICF mass after normalization to total body mass (Fig. 5C), which was opposite to the effects observed in chow-fed mice.
Total body hydration is often expressed as TBW normalized to total body mass. Comparison of this ratio in chow-fed versus HFD-fed mice indicated that prolonged HFD feeding results in significant dehydration in both sexes, though the effect appears to be much more robust in males (Fig. 6A). To explore whether this change in hydration was due to the diet per se, versus changes in body mass or composition, we performed regression analyses. Linear regression of TBW per body mass versus log10-transformed total body mass (Fig. 6B), fat-free mass (Fig. 6C), or fat mass (Fig. 6D) illustrates significant (i.e., nonzero) relationships between TBW and each of these assessments of mass or composition. Interestingly, the regressions differed significantly due to sex, indicating that the relationships between total body hydration and body size or composition are dependent on sex.
Figure 6.
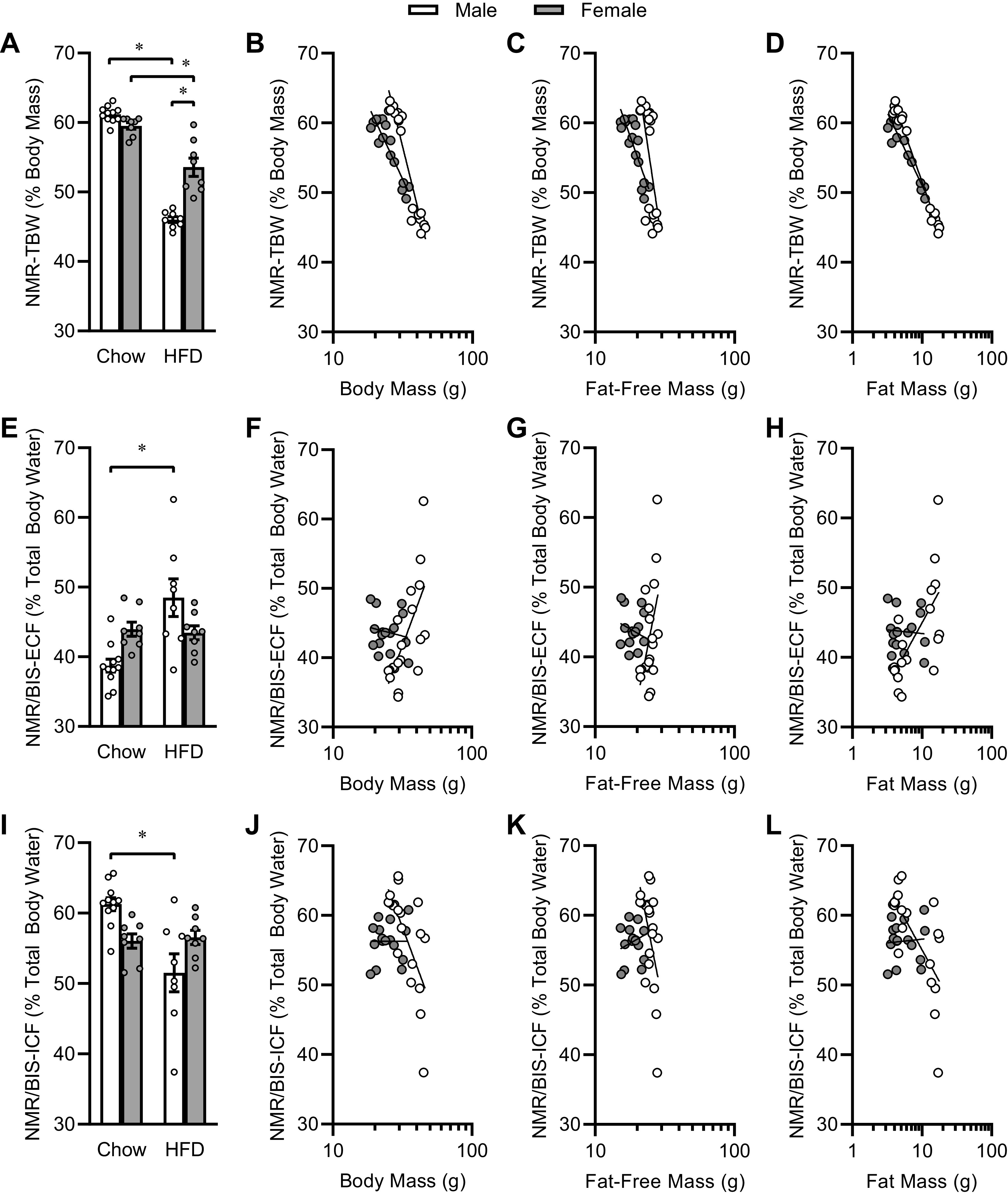
Total body hydration and compartmentalization with increasing adiposity. A: TBW normalized to total body mass, in mice maintained on chow or HFD. B: correlation of NMR-TBW with body mass. Linear regression versus Log10(body mass) reveals distinct nonzero correlations (P < 0.05 for each sex vs. zero slope; P < 0.0001 between sexes) for both sexes; males R2 = 0.92 and females R2 = 0.86 between hydration and total body mass. C: correlation of NMR-TBW with fat-free mass. Linear regression versus Log10(fat-free mass) reveals distinct nonzero correlations (P < 0.05 for each sex vs. zero slope; P = 0.0034 between sexes) for both sexes; males R2 = 0.43 and females R2 = 0.72 between hydration and fat-free mass. D: correlation of NMR-TBW with fat mass. Linear regression versus Log10(fat mass) reveals distinct nonzero correlations (P < 0.05 for each sex vs. zero slope; P < 0.0001 between sexes) for both sexes; males R2 = 0.99 and females R2 = 0.90 between hydration and fat mass. E: ECF normalized to total body water. F: correlation of ECF per TBW versus total body mass. Linear regression vs. Log10(body mass) reveals distinct correlations (males: P = 0.0012 vs. zero slope; females P = 0.5322 vs. zero slope; P = 0.0009 between sexes) between sexes; males R2 = 0.47 and females R2 = 0.03. G: correlation of ECF per TBW versus fat-free mass. Linear regression reveals distinct correlations (males: P = 0.0180 vs. zero slope; females P = 0.3443 vs. zero slope; P = 0.0089 between sexes) between sexes; males R2 = 0.29 and females R2 = 0.06. H: correlation of ECF per TBW vs. fat mass. Linear regression reveals distinct correlations (males: P = 0.0012 vs. zero slope; females P = 0.7912 vs. zero slope; P = 0.0129 between sexes) between sexes; males R2 = 0.47 and females R2 = 0.01. I: ICF normalized to total body water. J: correlation of ICF per TBW vs. total body mass; statistics are identical to F. K: correlation of ICF per TBW vs. fat-free mass; statistics are identical to G. L: correlation of ICF per TBW vs. fat mass; statistics are identical to H. For all panels, n = 11 male and n = 8 male mice fed chow, and n = 8 each sex fed HFD. BIS, bioimpedance spectroscopy; ECF, extracellular fluid; HFD, high-fat diet; ICF, intracellular fluid; NMR, nuclear magnetic resonance; TBW, total body water; TD-NMR, time-domain nuclear magnetic resonance. *P < 0.05 by Tukey multiple comparisons procedure.
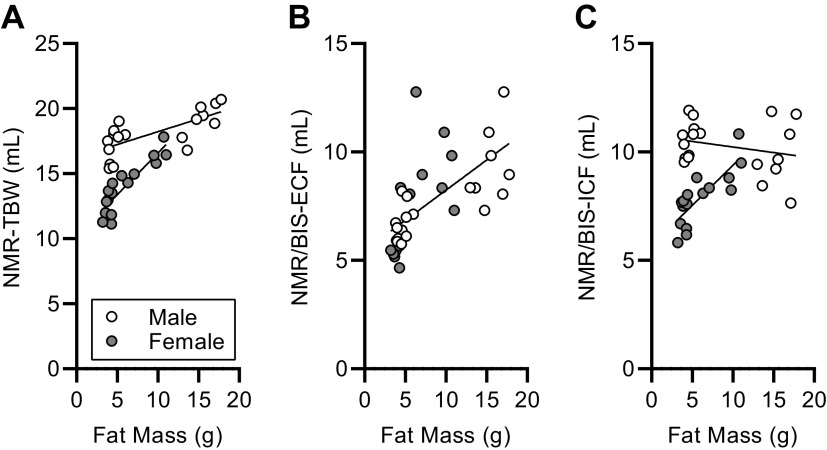
To explore how body water compartmentalization differs in animals maintained on different diets, we compared the fraction of TBW that was present in the ECF compartment. Simple comparison of ECF/TBW ratios among groups demonstrates that HFD significantly shifted water from the ICF space to the ECF space in males, whereas diet had no effect in females (Fig. 6E). Again, to explore whether this effect is due to diet per se, versus body composition, we used regression analyses to compare ECF/TBW ratios versus body mass (Fig. 6F), fat-free mass (Fig. 6G), or fat mass (Fig. 6H). Similarly, ICF/TBW ratios were affected by diet in males (Fig. 6I), and regression analyses were applied to ICF/TBW ratios versus body mass (Fig. 6J), fat-free mass (Fig. 6K), and fat mass (Fig. 6L). Regardless of diet, body size and composition had no significant effect (i.e., slopes are indistinguishable from zero) on this relative fluid compartmentalization in females. In contrast, body size and composition metrics all exhibited significant, positive relationships with ECF/TBW ratio (and equivalent/reciprocal negative relationships with ICF/TBW) in males. Interestingly, analysis of the relationships of absolute TBW, ECF, and ICF volumes versus fat mass (Fig. 7, A–C) indicate that TBW and ECF both generally increase with increasing fat mass in each sex, but although ICF volumes increase with increasing fat mass in females, this effect is not observed in males. Together, these data support a significant effect of diet on distribution of fluid between the ECF versus ICF spaces but illustrate that body composition changes secondary to dietary intervention may underlie this effect. Future studies that are appropriately controlled and powered will be required to dissociate the effects of diet versus body composition, and to these ends, the current study provides critical initial assessments of effect sizes and variance in C57BL/6J mice of both sexes.
Figure 7.
Fluid compartments with increasing fat mass. A: correlation of TBW with fat mass. Linear regression reveals distinct correlations (males: P = 0.0007 vs. zero slope; females P < 0.0001 vs. zero slope; P < 0.0001 between sexes) between sexes; males R2 = 0.50 and females R2 = 0.80. B: correlation of ECF with fat mass. Linear regression reveals similar correlations (males: P = 0.0001 vs. zero slope; females P = 0.0083 vs. zero slope; P = 0.1324 between sexes) between sexes; males R2 = 0.58 and females R2 = 0.40. C: correlation of ICF with fat mass. Linear regression reveals distinct correlations (males: P = 0.3114 vs. zero slope; females P = 0.0002 vs. zero slope; P < 0.0001 between sexes) between sexes; males R2 = 0.06 and females R2 = 0.65. For all panels, n = 11 male and n = 8 male mice fed chow, and n = 8 each sex fed HFD. ECF, extracellular fluid; HFD, high-fat diet; ICF, intracellular fluid; TBW, total body water.
NMR-Derived Free Fluid versus NMR/BIS-ECF
Finally, others have recently proposed the use of NMR-based measures of “free fluid” to estimate ECF (5). Therefore, we compared the outcomes of NMR-based free fluid mass versus NMR/BIS-ECF values for the animals examined in the current cohorts. We determined that the correlation of these variables within each sex was respectable {i.e., females: R2 = 0.7888, [free fluid = 1.184 ± 0.164 (NMR/BIS-ECF) – 5.115 ± 1.009]; males: R2 = 0.5482, [free fluid = 0.906 ± 0.143 (NMR/BIS-ECF) – 4.389 ± 1.072]}, and that while the slopes of these regressions were indistinguishable (P = 0.4380), that the elevation of the lines was significantly different between the sexes (P = 0.0071).
DISCUSSION
Here, we sought to build upon recent advances in technology and the increasing availability of TD-NMR and BIS to explore the hypothesis that the integration of these two approaches would enable improved approaches to the assessment of body fluid compartmentalization in rodents. We used classic desiccation-based methods to validate measures of TBW by TD-NMR, then used TD-NMR-based assessments of TBW to correct BIS-based measures of ECF and ICF and overcome methodological dependence on tenuous measures of interelectrode distances that are otherwise required for BIS-based assessments.
To validate this new NMR/BIS-based method of assessing ECF and ICF, we first performed a study of the effects of acute administration of the loop diuretic furosemide on fluid compartments. The acute effects of furosemide on body water compartments have been reported in other species using various classic measurement techniques. Using deuterated water, sodium thiocyanate, and Evans blue dye dilution methods to measure TBW, ECW, and plasma volumes, respectively, Forro et al. (7) demonstrated in horses that intravenous furosemide resulted in a significant decrease in ECF in 2 h. The ECF loss was twofold greater than that of TBW, suggesting a shift of fluid to the intracellular space. Similarly, O’Donovan et al. (8) measured TBW, ECF, and plasma volume using deuterium oxide, sodium bromide, and Evans blue dye, respectively, following a single intravenous dose of furosemide (1 mg/kg) in human infants with bronchopulmonary dysplasia. Four hours after furosemide, TBW and ECF were significantly decreased, associated with a nonsignificant increase in ICF. Within the ECF compartment, interstitial water but not plasma volume was decreased. Furosemide has also been shown to acutely decrease ECF by bioimpedance analysis within several hours of administration in adult humans with pulmonary edema (9). Using BIS and TD-NMR, we identified similar decreases in TBW and ECF within 30–60 min of furosemide administration in C57BL/6J mice. In contrast to the above findings, we did not see any significant effect on ICF, however, nonsignificant positive deflections in ICF were observed. This minor difference may be related to the different time courses of the studies, the rate at which the translocation of fluid between body compartments resulting from changes in body water compartment oncotic and hydrostatic pressures occur, and possible species-specific differences in compartment exchange kinetics. Therefore, our findings in mice are generally consistent with the effects of acute furosemide administration in the literature, and support our use of this new approach to study fluid compartmentalization.
Direct pairwise comparison of the use of BIS alone, which has previously been supported as a generally accurate method with fair precision when used for assessments of body fluid compartmentalization in mice (1), against the combined NMR/BIS method (Fig. 4), provides clear illustration that our new combined method provides increased precision. Chapman et al. (1) previously suggested that BIS alone is capable of detecting differences in roughly the 0.5–1 mL range. Here, we demonstrate that combined NMR/BIS methods are capable of detecting changes in TBW of less than 0.3 mL, and changes in ECF less than 0.66 mL (but likely in the range of 0.4–0.6 mL) when applied to cohorts of n = 8 mice. We suspect that even smaller effect sizes may be reliably detected when the method is applied to larger cohorts of animals, but regardless, these volumes already represent small changes in TBW (2%) and ECF (12%) compartment volumes. We conclude that the combined NMR/BIS method will provide greater resolution and precision to study fluid compartmentalization, which will enable our future studies of progressively smaller rodents such as newly weaned mice, or in neonatal and pre-weanling rats.
Changes in fluid homeostasis are associated with changes in body size and composition, and thus, there is increasing interest in the impact of obesity on fluid balance physiology plus the possible role for changes in fluid handling in observed associations of obesity with many types of cardiometabolic disease. Studies in human adults demonstrate that obesity is associated with altered distribution of body water, with a greater ratio of ECF to ICF when compared with normal-weight individuals (10, 11), and similar results were noted in mice in the current study. Additionally, studies examining NHANES data demonstrate that inadequate hydration and increased plasma tonicity are significantly associated with increased body mass index in humans (12, 13). Therefore, a greater ECF/ICF ratio may reflect hypertonic stress on cells, with a redistribution of water from the intracellular to extracellular space. Review of randomized clinical trials similarly reveals that water intake is associated with elevated energy expenditure and fat oxidation in metabolically inflexible obese humans, highlighting a potential bidirectional relationship between obesity and dehydration (14). It has been proposed that chronic ECF reductions are associated with obesity through changes in the renin-angiotensin system (RAS) (15), and our recent studies examining the antagonistic interplay between the local brain RAS versus the circulating and local adipose RAS in the control of energy expenditure in mice support this hypothesis (16–18). Further, the arginine vasopressin (AVP) system is undoubtedly involved in fluid homeostasis, and its V1A receptor (Avpr1a) has been implicated in hepatic function and glycemic control (19). Thus, an increased understanding of the impact of body size, body composition, and diet on fluid dynamics is needed.
We therefore used the new NMR/BIS methodology to examine changes in body water compartments during diet-induced obesity. To enable these studies, we examined fluid compartmentalization in mice maintained on a standard laboratory chow diet, versus mice maintained for 10 wk on a moderate HFD that delivers 45% of its caloric content in the form of fats. As we recently published, this model causes significant increases in body mass, fat mass, and clinically relevant alterations in energy homeostasis including resistance to the central actions of leptin, alterations in feeding behavior, and alterations in energy expenditure that parallel observations in humans experiencing prolonged obesity (20). Similar to studies described above, HFD feeding resulted in a significant dehydration and redistribution of fluid compartmentalization, however, unique to this study was the characterization of sex-specific differences in the redistribution of body water associated with obesity. While male and female mice experienced a reduction in TBW (expressed as percent of total body mass) following a high-fat diet, a relative redistribution of fluid from ICF to ECF was only observed in males.
Little information exists regarding sex-specific differences in body water distribution, especially in the context of obesity. Using BIA, Ritz et al. (21) found that the ratio of TBW per total body mass is reduced with increasing body mass index (BMI) in humans, and we observed similar effects in mice (Fig. 6). In contrast, whereas Ritz et al. (21) reported that the proportion of ICF to TBW decreased with increasing BMI, and more steeply in women than in men, we observed no significant effect of total body mass, fat mass, or fat-free mass on the relative distribution of fluid between ECF versus ICF spaces in females. In contrast, increasing total body mass, fat mass, and fat-free mass were each correlated in males with a general redistribution of fluid from the ICF space toward the ECF space. Reasons for this difference remain unclear. In our study, mice on chow and HFD were of different ages. We did not explore an effect of age on body water distribution, but in humans, hydration of fat-free mass does not change during healthy aging from 38 to 66 yr of age (22). Additionally, we relied on TD-NMR for determination of TBW, whereas Ritz relied on bioelectrical impedance analysis (BIA). Other methodological biases may also exist. Regardless, based on our findings from the HFD- versus chow-fed cohorts, we conclude that TBW content varies by body mass in a complex manner that is influenced by diet (or, more likely, body mass and composition). Based on the large amount of scatter in ECF and ICF endpoints, especially in the male mice fed HFD, plus the wide range of body masses examined, we posit that these correlational calculations are likely underpowered to detect more subtle (and perhaps combinatorial) effects of diet and sex on ECF and ICF compartmentalization. Future studies of mass-matched mice of each sex fed various diets are warranted to more fully clarify the interactions among sex, diet, and body size in the regulation of body fluid compartmentalization.
In addition to sex differences in body fluid compartmentalization, increasing evidence supports the concept that fluid volumes within females vary across the menstrual cycle. For example, Aguree et al. (23) recently demonstrated small but significant (∼2%) plasma volume changes occur between the midluteal and early follicular phases in healthy women. In the current study, we did not track cycle status, but future studies should be aware of this potential rhythm. Indeed, the increased precision provided by NMR/BIS may allow for detection of small but regular rhythms in fluid compartmentalization.
Underscoring the importance of developing new approaches to assess fluid compartmentalization, other groups are working to develop methods that are similarly minimally invasive for use in rodents. Morla et al. (5) recently demonstrated that TD-NMR-based methods can be used to estimate fluid some aspects compartmentalization in mice. The authors demonstrated that TD-NMR-based assessments of “free fluid,” which they argue is reflective of the ECF compartment, could be used to assess fluid compartment changes with acute and chronic interventions even if “free fluid” underestimates the total ECF as determined by other methods. The authors used the Bruker LF50 and LF90 models of TD-NMR, which are closely related to the Bruker LF110 model used in our study, and the authors challenged fluid homeostasis in their mice using fluid injections, metabolic caging, fluid restriction, and deoxycorticosterone pivalate (DOCP)-salt challenges; these challenges resulted in changes in TBW and free fluid that are consistent with patterns that are expected based on prior literature—highlighting the utility of this rapid and noninvasive approach. Notably and of direct relevance to our development of the NMR/BIS method reported herein, the authors acknowledged that NMR “free fluid” measures do not quantitate the total ECF or ICF masses, and therefore explicitly concluded in their manuscript that “… bioimpedance spectroscopy, the dilution technique or other terminal investigations, should be used after a TD-NMR study to draw kinetics of body compartment behaviors following stimuli” (5). Building upon the recommendations of these authors, we correlated NMR-based assessments of “free fluid” and NMR/BIS-ECF values in the current study. This correlation demonstrated, as Morla et al. suspected, that NMR “free fluid” is strongly correlated with ECF, but also that a significant difference in that correlation exists according to the sex of the animal studied. We conclude that the use of TD-NMR alone represents a powerful tool for exploring changes in TBW and possibly ECF, but that the simultaneous adoption of the BIS method (i.e., the combined NMR/BIS approach presented herein) is likely to uncover previously unappreciated effects of sex and perhaps diet or body mass on fluid compartmentalization.
In summary, we conclude that the combination of TD-NMR and BIS methods will allow for vastly improved assessment of fluid compartmentalization. This combined method is very fast, largely noninvasive and can be safely performed serially over very short periods to evaluate the kinetics of fluid redistribution in response to acute (or chronic) interventions. Application of this combined method is poised to greatly increase our understanding of the bidirectional interactions between hydration and development, aging, obesity, hypertension, and other pathological states.
GRANTS
This work was supported by grants from the National Institutes of Health (HL134850 and HL084207) and the American Heart Association (18EIA33890055), the MCW Clinical & Translational Science Institute “Obesity” Ensemble (UL1TR001436), and the Advancing a Healthier Wisconsin Endowment to MCW.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.L.S. and J.L.G. conceived and designed research; J.L.S., K.B., J.J.R., C.C.G., and J.L.G. performed experiments; J.L.S., K.B., J.J.R., C.C.G., C.M.L.B., and J.L.G. analyzed data; J.L.S., C.C.G., C.M.L.B., and J.L.G. interpreted results of experiments; J.L.S. and J.L.G. prepared figures; J.L.S. and J.L.G. drafted manuscript; J.L.S., C.C.G., C.M.L.B., and J.L.G. edited and revised manuscript; J.L.S., K.B., J.J.R., C.C.G., C.M.L.B., and J.L.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Kelsey Wackman and Ko-Ting Lu, along with the MCW Biomedical Resource Center, for the technical assistance on the project.
REFERENCES
- 1.Chapman ME, Hu L, Plato CF, Kohan DE. Bioimpedance spectroscopy for the estimation of body fluid volumes in mice. Am J Physiol Renal Physiol 299: F280–F283, 2010. doi: 10.1152/ajprenal.00113.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubertin G, Sayeh A, Dillenseger JP, Ayme-Dietrich E, Choquet P, Niederhoffer N. Comparison of bioimpedance spectroscopy and X-ray micro-computed tomography for total fat volume measurement in mice. PloS One 12: e0183523, 2017. doi: 10.1371/journal.pone.0183523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grobe JL. Comprehensive assessments of energy balance in mice. Methods Mol Biol 1614: 123–146, 2017. doi: 10.1007/978-1-4939-7030-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals (8th ed.). Washington, DC: National Acadamies Press (US), 2011. [Google Scholar]
- 5.Morla L, Shore O, Lynch IJ, Merritt ME, Wingo CS. A noninvasive method to study the evolution of extracellular fluid volume in mice using time-domain nuclear magnetic resonance. Am J Physiol Renal Physiol 319: F115–F124, 2020. doi: 10.1152/ajprenal.00377.2019. [DOI] [PubMed] [Google Scholar]
- 6.Sheng HP, Huggins RA. A review of body composition studies with emphasis on total body water and fat. Am J Clin Nutr 32: 630–647, 1979. doi: 10.1093/ajcn/32.3.630. [DOI] [PubMed] [Google Scholar]
- 7.Forro M, Lindinger MI. Frusemide results in an extracellular to intracellular fluid shift in horses. Equine Vet J Suppl 38: 245–253, 2006. doi: 10.1111/j.2042-3306.2006.tb05547.x. [DOI] [PubMed] [Google Scholar]
- 8.O'Donovan BH, Bell EF. Effects of furosemide on body water compartments in infants with bronchopulmonary dysplasia. Pediatr Res 26: 121–124, 1989. doi: 10.1203/00006450-198908000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Ng Kam Chuen MJ, Lip GY, Macfadyen RJ. Performing repeated noninvasive bedside measures of volume response to intravenous furosemide in acute pulmonary edema: a feasibility assessment. Cardiovasc Ther 27: 89–95, 2009. doi: 10.1111/j.1755-5922.2009.00080.x. [DOI] [PubMed] [Google Scholar]
- 10.Steijaert M, Deurenberg P, Van Gaal L, De Leeuw I. The use of multi-frequency impedance to determine total body water and extracellular water in obese and lean female individuals. Int J Obes Relat Metab Disord 21: 930–934, 1997. doi: 10.1038/sj.ijo.0800497. [DOI] [PubMed] [Google Scholar]
- 11.Waki M, Kral JG, Mazariegos M, Wang J, Pierson RN Jr, Heymsfield SB. Relative expansion of extracellular fluid in obese vs. nonobese women. Am J Physiol 261: E199–E203, 1991. doi: 10.1152/ajpendo.1991.261.2.E199. [DOI] [PubMed] [Google Scholar]
- 12.Chang T, Ravi N, Plegue MA, Sonneville KR, MM. avis. Inadequate hydration, BMI, and obesity among US adults: NHANES 2009–2012. Ann Fam Med 14: 320–324, 2016. doi: 10.1370/afm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stookey JD, Barclay D, Arieff A, Popkin BM. The altered fluid distribution in obesity may reflect plasma hypertonicity. Eur J Clin Nutr 61: 190–199, 2007. doi: 10.1038/sj.ejcn.1602521. [DOI] [PubMed] [Google Scholar]
- 14.Stookey JJ. Negative, null and beneficial effects of drinking water on energy intake, energy expenditure, fat oxidation and weight change in randomized trials: a qualitative review. Nutrients 8: 19, 2016. doi: 10.3390/nu8010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thornton SN. Increased hydration can be associated with weight loss. Front Nutr 3: 18, 2016. doi: 10.3389/fnut.2016.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claflin KE, Sandgren JA, Lambertz AM, Weidemann BJ, Littlejohn NK, Burnett CM, Pearson NA, Morgan DA, Gibson-Corley KN, Rahmouni K, Grobe JL. Angiotensin AT1A receptors on leptin receptor-expressing cells control resting metabolism. J Clin Invest 127: 1414–1424, 2017. doi: 10.1172/JCI88641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Littlejohn NK, Grobe JL. Opposing tissue-specific roles of angiotensin in the pathogenesis of obesity, and implications for obesity-related hypertension. Am J Physiol Regul Integr Comp Physiol 309: R1463–R1473, 2015. doi: 10.1152/ajpregu.00224.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Littlejohn NK, Keen HL, Weidemann BJ, Claflin KE, Tobin KV, Markan KR, Park S, Naber MC, Gourronc FA, Pearson NA, Liu X, Morgan DA, Klingelhutz AJ, Potthoff MJ, Rahmouni K, Sigmund CD, Grobe JL. Suppression of resting metabolism by the angiotensin AT2 receptor. Cell Rep 16: 1548–1560, 2016. doi: 10.1016/j.celrep.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taveau C, Chollet C, Waeckel L, Desposito D, Bichet DG, Arthus M-F, Magnan C, Philippe E, Paradis V, Foufelle F, Hainault I, Enhorning S, Velho G, Roussel R, Bankir L, Melander O, Bouby N. Vasopressin and hydration play a major role in the development of glucose intolerance and hepatic steatosis in obese rats. Diabetologia 58: 1081–1090, 2015. doi: 10.1007/s00125-015-3496-9. [DOI] [PubMed] [Google Scholar]
- 20.Deng G, Morselli LL, Wagner VA, Balapattabi K, Sapouckey SA, Knudtson KL, Rahmouni K, Cui H, Sigmund CD, Kwitek AE, Grobe JL. Single-nucleus RNA sequencing of the hypothalamic arcuate nucleus of C57BL/6J mice after prolonged diet-induced obesity. Hypertension 76: 589–597, 2020. doi: 10.1161/HYPERTENSIONAHA.120.15137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritz P, Vol S, Berrut G, Tack I, Arnaud MJ, Tichet J. Influence of gender and body composition on hydration and body water spaces. Clin Nutr 27: 740–746, 2008. doi: 10.1016/j.clnu.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Ritz P. Body water spaces and cellular hydration during healthy aging. Ann NY Acad Sci 904: 474–483, 2000. doi: 10.1111/j.1749-6632.2000.tb06502.x. [DOI] [PubMed] [Google Scholar]
- 23.Aguree S, Bethancourt HJ, Taylor LA, Rosinger AY, Gernand AD. Plasma volume variation across the menstrual cycle among healthy women of reproductive age: A prospective cohort study. Physiol Rep 8: e14418, 2020. doi: 10.14814/phy2.14418. [DOI] [PMC free article] [PubMed] [Google Scholar]