Abstract
In this study, a genetically diverse panel of 43 mouse strains was exposed to ammonia, and genome-wide association mapping was performed employing a single-nucleotide polymorphism (SNP) assembly. Transcriptomic analysis was used to help resolve the genetic determinants of ammonia-induced acute lung injury. The encoded proteins were prioritized based on molecular function, nonsynonymous SNP within a functional domain or SNP within the promoter region that altered expression. This integrative functional approach revealed 14 candidate genes that included Aatf, Avil, Cep162, Hrh4, Lama3, Plcb4, and Ube2cbp, which had significant SNP associations, and Aff1, Bcar3, Cntn4, Kcnq5, Prdm10, Ptcd3, and Snx19, which had suggestive SNP associations. Of these genes, Bcar3, Cep162, Hrh4, Kcnq5, and Lama3 are particularly noteworthy and had pathophysiological roles that could be associated with acute lung injury in several ways.
Keywords: acute lung injury, ARDS, chemical threat, functional genomics, transcriptomics
INTRODUCTION
Named for the Egyptian Amun-Ra worshipers (98), ammonia is used in the production of fertilizer, metals, and pharmaceuticals and as a refrigerant. Annual production exceeds 170 million tons (121), making it the second most manufactured chemical in the world. Ammonia gas is colorless, with a very distinct pungent aroma (36). Upon contact with the skin, eyes, and the mucosal surfaces of the respiratory tract, anhydrous ammonia gas rapidly reacts with water to form ammonium hydroxide. Ammonium hydroxide is caustic and causes necrosis of tissues through disruption of membrane lipids (saponification) and proteins, leading to cellular destruction. The large-scale production and use necessitate extensive storage and transport, which requires diligence to prevent intentional, e.g., terrorist attack on chemical facilities (4, 9, 36, 63, 99, 119), or accidental exposure to workers (6, 18, 25, 33, 37, 38, 43, 47, 52, 57, 60, 65, 68, 73, 75, 76, 82, 83, 94, 104, 107, 113, 117, 122, 125, 129, 141–143, 145, 147, 148, 154, 156) or to the general population (14, 15, 22, 60, 92, 139, 158, 165).
The degree of injury after inhalation of an irritant gas is affected by the duration of exposure, gas concentration, and depth of inhalation (110). The CDC’s Immediately Dangerous to Life or Health Concentration (IDLH) is 300 ppm (24), but this level is controversial (99). Short exposures to ammonia in low concentration can produce mild symptoms including eye, nasal, and throat irritation and signs including lacrimation and cough (137). The maximum short exposure tolerance has been estimated to be 300–500 ppm for 0.5–1 h (58). A change in respiration rate and moderate to severe irritation has been reported in seven subjects exposed to 500 ppm for 30 min (137). Moderate exposure (500–1,000 ppm) is estimated to cause edema and erythema of the lips and respiratory mucosal surfaces. Clinical findings in cases with moderate exposures include pharyngitis, laryngitis, productive cough, and bronchospasm. Patients may have rapid, shallow breathing, rhonchi, and blood-tinged sputum (33, 104, 113, 156).
Exposures to 2,500–6,000 ppm for 30 min have been estimated to be dangerous to life (140). Severe effects include second- and third-degree burns to the skin and to the nasal, oral, laryngeal, and respiratory mucosa. Airway obstruction, severe bronchospasm, and copious sputum production can occur. Patients who inhaled ammonia concentrations for a longer duration develop diffuse injury throughout the tracheobronchial tree because of increased depth of inhalation (33). Both epithelial damage and endothelial damage develop in alveolar injury, resulting in an alveolar capillary leak and the pathological changes of acute lung injury (aka acute respiratory distress syndrome) (6, 18, 25, 43, 52, 75, 76, 82, 113, 129, 154, 165). While exposure to anhydrous ammonia can lead to severe skin and eye injuries, the involvement of the lungs can be life threatening. The presence or absence of lung involvement has been found to be the best predicator of a fatal outcome (6, 52). Patients who survive the acute lung injuries may die later from respiratory complications such us bronchiectasis and bronchiolitis obliterans (37, 43, 60, 65, 76, 94, 129, 141–143). Although most case reports involve exposure of only a few people, patients with similar exposure can vary in their response, which suggests that an underlying individual susceptibility may also influence the clinical course.
Because of the similarity between the human and the mouse genomes, mice have been useful models to uncover genetic underpinnings of human physiology and pathophysiology. To better understand individual susceptibility to ammonia-induced acute lung injury, we exposed a panel of genetically diverse mouse strains and identified candidate genes. Whole genome sequencing has generated information on 17 mouse strains, which continues to strengthen single-nucleotide polymorphism (SNP) databases for the 43 mouse strains that were used in this study (11, 62, 67, 70, 86, 106, 128, 132, 150, 160, 161).Exploiting the genetic variability among inbred mouse strains, we previously developed functional genomic analyses of acrolein- (41, 79), chlorine- (80), and phosgene-induced acute lung injury (78). Here, we used genome-wide association mapping with a high-density SNP assembly and transcriptomic analysis to identify the genetic determinants of ammonia-induced acute lung injury.
MATERIALS AND METHODS
Experimental design.
Additional details of the methods are provided in Supplemental Material (see https://doi.org/10.6084/m9.figshare.12469043). Animal studies were reviewed and approved by and performed in accordance with the Institutional Animal Care and Use Committee of the University of Pittsburgh (Pittsburgh, PA). Inbred mouse strains (6–8 wk females; 43 strains) were obtained from Jackson Laboratory (Bar Harbor, ME) and housed under specific pathogen-free conditions. Mice were exposed to filtered air (control) or 1,500 ppm ammonia (Matheson Tri-Gas, Montgomery, PA) in a laminar-flow inhalation chamber. Ammonia concentrations were monitored (Polytron 3000; Drager Safety Inc., Pittsburgh, PA) during exposure. Time of death (survival time) was recorded during a 24-h exposure or after the mice had been returned to filtered room air.
Selection of mouse strains.
Mouse strains were selected from the Tier 1–3 priority strains of the Mouse Phenome Project (MPP) and based on previous studies of lung biology. The priority strains for the Mouse Phenome Database (MPD) (16) were classified based on strain availability, research trends, community input, and resources, including the release of the NIEHS Perlegen SNPs for 8.27 million genome-wide locations for a set of inbred strains (45). We selected 43 mouse strains that included 15 of the 16 NIEHS-Perlegen Tier 1 strains A/J, AKR/J, BALB/cByJ, BTBR t < +>tf/J, C3H/HeJ, C57BL/6J, CAST/EiJ, DBA/2J, FVB/NJ, KK/HIJ, MOLF/EiJ, NOD/ShiLtJ, NZW/LacJ PWD/PhJ, and WSB/EiJ. Tier 2 contains 10 strains that were used in this study: BUB/BnJ, C57BLKS/J, C57L/J, CBA/J, DDY/JcISidSeyFrkJ, MRL/MpJ, MSM/Ms, NZL/LtJ, SJL/J, and SM/J. These strains breed well, are widely used, and/or have unique phenotypes. Tier 3 consists of 10 strains, of which the following 9 were used in this study: BPL/1J, C58/J, CZECHII/EiJ, LP/J, MA/MyJ, NON/ShiLtJ, PL/J, RIIIS/J, and SWR/J. We selected eight additional strains that have been used in other investigations of ovalbumin hyperreactivity (BALB/cJ) and lung growth/cancer (129X1/SvJ, C57BL/10J, DBA/1J, I/LnJ, JF1/MsJ, LG/J, and PERA/EiJ,). These 43 strains are well distributed in the Mouse Family Tree (45, 123).
Strains were also selected based on the Petkov et al. (123) organization of 102 mouse strains into seven groups. We selected 43 strains of mice in order to have representative samples from each group. These included the following: Group 1: Bagg Albino derived (9 of 21 strains: A/J, AKR/J, BALB/cByJ, BALB/cJ, CBA/J, C3H/HeJ, LG/J, MRL/MpJ, and PL/J); Group 2: Swiss (7 of 16 strains: BUB/BnJ, FVB/NJ, MA/MyJ, NOD/LtJ, RIIIS/J, SJL/J, and SWR/J); Group 3: Japanese and New Zealand (5 of 10 strains: DDY/JclSidSeyFrkJ, KK/HIJ, NON/LtJ, NZL/tJ, and NZW/LacJ); Group 4: Little’s C57 and C58 (6 of 12 strains: C57BL/10J, C57BL/6J, C57BLKS/J, C57BR/cdJ, C57L/J, and C58/J); Group 5: Castle’s (4 of 11 strains: 129X1/SvJ BPL/1J, BTBR t < +>tf/tf, and LP/J); Group 6 Little’s DBA (4 of 8 strains: DBA/1J, DBA/2J, I/LnJ, and SM/J); and Group 7 Wild derived (8 of 26 strains: CAST/EiJ, CZECHII/EiJ, JF1/MsJ, MOLF/EiJ, MSM/Ms, PERA/EiJ, PWD/PhJ, and WSB/EiJ.
Genome-wide association analysis.
Employing the resulting strain distribution pattern, whole genome linkage disequilibrium analysis for survival time in ammonia was performed using single nucleotide polymorphisms (SNPs) from the National Institute of Environmental Health Sciences (NIEHS), Wellcome Trust Centre for Human Genetics (WTCHG), and the Broad Institute (which include SNPs from Roche and Genomic Institute of the Novartis Research Foundation). The NIEHS SNP database contains 8,272,574 SNPs, the WTCHG SNP database contains 13,348 SNPs, and the Broad SNP database contained 138,793 SNPs in commonly used mouse strains. The genomic positions of the SNPs for the two data sets were unified based on the latest NCBI mouse genome map (current build 37.1). These SNPs were edited to remove SNPs with <16 strains typed or without map information. The resulting informative (>1 million) SNPs span the mouse genome at an average density of >5 kb/SNP. The SNP genotyping accuracy is >99.8%.
A scatter (Manhattan) plot was generated by efficient mixed-models association (EMMA) corrected for confounding from population structure and genetic relatedness (11, 62). This method takes advantage of the specific nature of the optimization problem in applying mixed models for association mapping, which allows us to substantially increase the computational speed and reliability of the results. EMMA provided in silico whole genome association mapping of 43 inbred mouse strains using >4 million SNPs. EMMA has been validated through extensive simulation studies to estimate the statistical power of EMMA under various SNP effects, varying degrees of population structure, and differing numbers of multiple measurements per strain. An R package implementation and webserver of our EMMA method are publicly available. Previously, we determined that the conventional significance level LOD of 3.3 (corresponding to a pointwise P value of 1 × 10−4) for a genome-wide linkage study is not sufficiently rigorous and results in four to seven false positives in a genome-wide linkage disequilibrium (LD) scan (86). A more stringent threshold was used, in which the number of false positives for genome-wide association analysis at various nominal significance levels was estimated under different levels of trait heritability. This yielded one statistical association by chance in a genome-wide LD scan when the threshold is decreased to 1.6 × 10−5 or -log (P) = 4.8. Therefore, we used a significance threshold of –log (P) > 4.8 and a suggestive threshold of 4.8 ≥ –log (P) > 4.0.
Assessment of acute lung injury.
Phenotyping these strains, we identified JF1/MsJ as the most sensitive and LG/J as the most resistant mouse strain. Among the sensitive and resistant strains, we selected the BALB/cJ mice as sensitive and DBA/2J mice as resistant for further analysis because the genome of these mice is fully sequenced in contrast to the JF1/MsJ and LG/J mouse strains. In addition, BALB/cJ and DBA/2J mice are widely used and easily available from Jackson Laboratory compared with JF1/MsJ and LG/J mice, which are now cryopreserved.
To examine ammonia-induced changes in lung histology, additional BALB/cJ and DBA/2J mice were exposed to filtered air (0 h, control) or ammonia (1,500 ppm for 6 or 11 h). Mice were killed immediately after ammonia exposure by intraperitoneal injection of pentobarbital sodium (100 mg/kg Nembutal; Abbott Laboratories, Chicago, IL) and severing of the posterior abdominal aorta. To examine ammonia-induced changes in lung histology, sensitive BALB/cJ or resistant DBA/2J mice were exposed to filtered air (0 h, control) or ammonia (1,500 ppm) for 6 or 11 h (n = 3 mice/strain/group). Bronchoalveolar lavage fluid (BALF) was obtained in additional groups exposed to filtered air (0 h, control) or ammonia (1,500 ppm, 6 h) (n = 6 mice/strain/group).
To obtain tissue for mRNA analysis (n = 6 mice/strain/time), the diaphragm was punctured and the chest cavity opened. Lungs were excised, frozen in liquid nitrogen, and stored (−80°C). To obtain tissue for histology (n = 3 mice/strain/time), the chest wall was left intact, a cannula was inserted into the trachea, and the lung was instilled with phosphate-buffered saline containing 3.7% formaldehyde (pressure: 28 cmH2O, cat. no. SF100-4; Thermo Fisher Scientific, Pittsburgh, PA). The trachea was ligated, lungs were removed, and the inflated lung was immersed in fixative (24 h, 4°C). Fixed lungs were washed with Dulbecco’s phosphate-buffered saline containing Ca2+, Mg2+, 6.1 mM d-glucose, and 0.33 mM sodium pyruvate (DPBS; cat. no. 14287-080; Life Technologies, Carlsbad, CA), dehydrated through a series of graded ethanol solutions (30–70%), and processed in paraffin blocks (Hypercenter XP; Shandon, Ramsey, MN). The lung tissue was sectioned (5 μm) and stained with hematoxylin and eosin. To obtain bronchoalveolar lavage fluid, BALB/cJ and DBA/2J (n = 6 mice/strain/time) were exposed for 0 (control), 6, or 11 h, a cannula was inserted into the trachea, and the lung was instilled with Ca2+- and Mg2+-free Hanks’ balanced salt solution (cat. no. 14175-095, Life Technologies), and the recovered lavage fluid was placed on ice until protein analysis was performed. Total protein in cell-free supernatants was measured using a bicinchoninic acid assay (BCA; cat. no. 23325, Thermo Scientific) using bovine serum albumin (BSA) as a standard.
Selection of candidate genes.
SNPs in each of the identified candidate genes were evaluated for possible functional consequences. Candidates were prioritized by several criteria. The first criterion was whether the encoded protein has a function known to be associated with lung injury or inflammation. The second criterion was whether the encoded would be altered by a nonsynonymous SNP in a functional domain of the protein. Mice were grouped by nonsynonymous SNPs predicted to alter amino acid sequence, and the mean survival time was determined by Kaplan–Meier analysis. The difference in survival time between these groups was compared with the difference of the means of strains at the phenotypic extremes. The resulting SNPs were evaluated as to whether each could explain >10% of the phenotypic difference between the survival times of strains at the phenotypic extremes and had a minor allelic frequency of >10%. The third criterion was whether baseline lung transcripts or those following ammonia exposure varied in the resistant compared with sensitive mouse strain. These differences were evaluated by microarray and confirmed by qRT-PCR. Once genes with differential expression were identified, SNPs in 5′-untranslated region (UTR; promoter) that could alter putative transcription factor binding were evaluated. Regarding the latter two criteria, ≥10% of mice had to carry the minor allele, and ≥10% of the phenotypic difference between the strains at the phenotypic extremes could be accounted for by this allele.
Transcriptomic analysis.
Gene expression profiling was conducted using a hybridization microarray. Lung mRNA was analyzed from mice exposed 0 (filtered air control), 6, or 11 h using one microarray/mouse for the sensitive and resistant strain (n = 6–8 mice/strain/time). Samples obtained from these mice and additional mice were used for subsequent qRT-PCR. The sample size of six mice has the power = 0.8 (α = 0.05) to detect a log 2-fold change of 0.379 (i.e., 1.3-fold); thus, the set level for significance of log 2 ≥ 0.585-fold change (i.e.≥ 1.5-fold) was sufficiently stringent.
To perform a transcriptome-wide analysis of steady-state mRNA levels, total lung mRNA was analyzed by microarray and selected changes were confirmed by qRT-PCR. Lung mRNA was isolated (TRI Reagent), reverse transcribed, and fluorescently labeled. Each sample was hybridized to a microarray containing 31,769 murine 70-mer oligonucleotides. RNA quantity was initially assessed by spectrophotometer (Nanodrop ND-1000; Thermo Scientific, Wilmington, DE), and quality was assessed by electrophoresis (Agilent 2100 Bioanalyzer, Agilent Technologies). RNA (0.5 μg) was cyanine 3 labeled and cDNA synthesized (RNA Spike In – One Color, 5188-5282, Agilent Technologies). Labeled cRNA was transcribed from cDNA (Quick-Amp Labeling Kit – One Color, 5190-0442, Agilent Technologies). The labeled cRNA was quantified (Nanodrop ND-1000 and Agilent 2100 Bioanalyzer), and hybridized (65°C, 17 h; Gene Hybridization, 5188-5242, Agilent Technologies) onto the microarray (Whole Mouse Genome Kit 4x44K, G4122F, Agilent Technologies). Arrays were washed and scanned (DNA Microarray Scanner, G2505C, Feature Extraction v.10.7.3.1, Agilent Technologies). Six microarrays were obtained for each strain (BALB/cJ and DBA/2J) and each time (0, 6, or 11 h) to yield a total of 36 microarrays.
Data normalization was performed for each microarray separately by subtracting channel-specific local background intensities and centering the log-transformed intensities with a fitted local regression model (40). The statistical analysis was performed by fitting a mixed-effects linear model for each gene separately, with array effects assumed to be random while treatment and dye effects were assumed to be fixed (157). This model was fitted for each gene and statistical significance of the differential expression between strains after adjusting for the array effect. Results were assessed by calculating P values for the corresponding linear contrasts. Log 2-fold changes were assessed by ANOVA, and resulting t statistics from each comparison were modified for multiple hypotheses testing adjustment by calculating false discovery rate to determine statistical significance (10).
A total of 21 transcripts were assessed by qRT-PCR. Lung RNA (100 ng) from the divergent mouse strains (n = 8 mice/strain) was reverse transcribed into first-strand cDNA with the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) in a 100-μL reaction volume. cDNA (10 μL) was used in a subsequent PCR reaction using 25 μL of TaqMan Universal PCR Master Mix (Applied Biosystems), 2.5 μL of each primer mixture, and 12.5 μL of RNAse-free water. Validated primers for each gene were obtained from Applied Biosystems and normalized to RPL32 or RPS18 (Applied Biosystems). Analysis was performed with an Applied Biosystems 7900 hT system, and the following conditions: 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. For relative quantification of expression of each gene between the strains, the comparative cycle number threshold (CT) method (ΔΔCT) was used: ΔCT = CT (transcript of the gene of interest) – CT (e.g., rpL32), and this value was calculated for each sample. The comparative ΔΔCT calculation involved finding the difference between each sample’s ΔCT and the mean ΔCT for the most resistant strain. These values were then transformed to absolute values with a formula in which comparative expression level = 2 – ΔΔCT. In addition, transcripts and metabolites in biological pathways considered as hallmarks in lung injury were analyzed.
Identification of possible therapeutics.
The microarray results of the resistant DBA/2J mice were contrasted with the results of the sensitive BALB/cJ mice to identify therapeutics that might be protective in acute lung injury. Using the Library of Integrated Network-Based Cellular Signatures (LINCS) L1000, differentially expressed genes (DEGs) were assessed for enrichments that identified compounds previously associated with increasing (LINCS L1000 Up) or decreasing (LINCS L1000 Down) transcript levels. The list of identified compounds was then evaluated based on their mechanism of action. Priority was given to compounds previously associated with protection in models of acute lung injury or to compounds possessing protective activity (e.g., anti-inflammatory). Four groups were assessed. The first group, Group 1, used increased DEGs in DBA/2J mice assessed as LINCS L1000 Up and increased DEGs in BALB/cJ mice assessed as LINCS L1000 Down. We reasoned that the identified compound’s activity would augment DBA/2J beneficial increases while suppressing detrimental BALB/cJ increases in transcript levels. Group 2 was derived from DEGs that decreased in DBA/2J mice assessed as LINCS L1000 Down and decreased in BALB/cJ assessed as LINCS L1000 Up. Thus, the identified compound’s activity would augment DBA/2J beneficial decreases while suppressing detrimental BALB/cJ decreases in transcript levels. Group 3 was derived from DEGs that increased in DBA/2J mice assessed as LINCS L1000 Up or increased in BALB/cJ mice assessed as LINCS L1000 Down. The last group, Group 4, was identified by using DEGs decreased in DBA/2J assessed as LINCS L1000 Down, in which suppression might induce protection.
Cell lines and qRT-PCR analysis.
To examine breast cancer anti-estrogen resistance 3 (BCAR3) expression profile in lung cells and cells of monocytic origin, the cell lines CALU-1, H292, H226, H820, H441, and THP1 (ATCC, Manassas, VA) and human lung fibroblasts isolated from resected lungs were used for RNA preparation. Total RNA was extracted from cultured cells using TRI Reagent (cat. no. T9424; Sigma-Aldrich, St. Louis, MO). For each sample, 0.2 μg of DNase I-treated RNA was reverse transcribed into cDNA in a 12.5-μL reaction volume using an iScript cDNA synthesis kit (cat. no. 170-8891; Bio-Rad, Hercules, CA), diluted to 50 μL with water, and 2 μL of the resulting cDNA synthesis product was used for qRT-PCR analysis.
Breast cancer anti-estrogen resistant protein 3 (BCAR3) transcript expression profile in human lung cells.
One candidate identified in this study, BCAR3, was examined further in human cells. BCAR3 is a scaffold protein that interacts with multiple members of signaling pathways associated with cell cycle progression and cytoskeleton reorganization, tethering them into complexes (19, 115, 116, 145, 146). First, we examined BCAR3 transcript expression in various lung-related cell lines as determined by qRT-PCR analysis. Second, we investigated whether the differential pattern of expression was related to the BCAR3 splice variant expressed; the level of splice variants 1, 2, and 4 was determined. Third, we examined the possibility that BCAR3 gene expression is regulated by alternative promoters. Human BCAR3 gene spans over 285 kb, and exons 1, 2, and 3 encode the 5′-untranslated region of variant 1, whereas exon 4, which is ∼164 kb downstream of exon 1, encodes the 5′-untranslated region of variant 2 (19, 116, 146).
RESULTS
Ammonia induces lung injury sooner in BALB/cJ compared with DBA/2J mice.
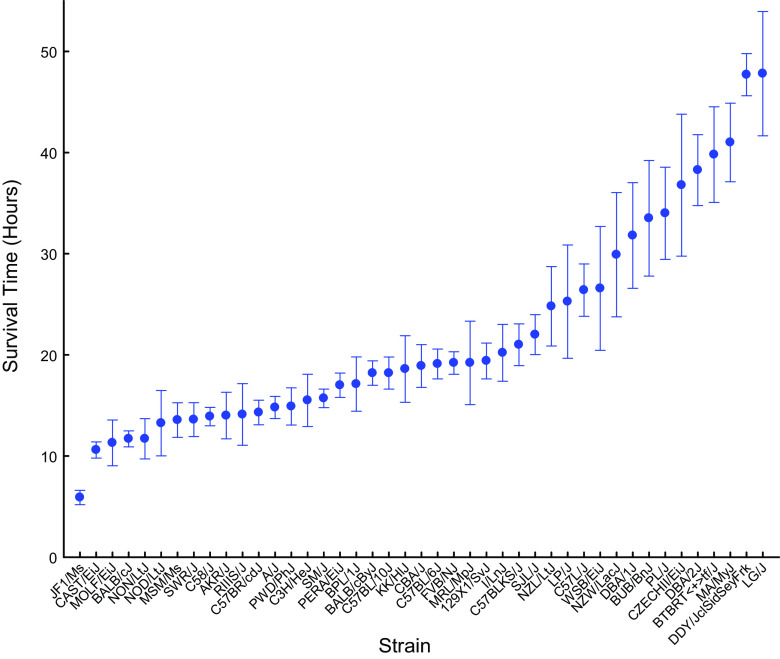
Mouse strains varied in sensitivity to ammonia-induced acute lung injury as determined by survival time (Fig. 1). The most sensitive JF1/MsJ strain was ∼8 times more sensitive than the resistant LG/J strain. Because more is known about the genome of sensitive BALB/cJ (mean survival time: 11.7 ± 0.8 h) and resistant DBA/2J mouse strains (mean survival time: 38.3 ± 3.5 h), they were selected for further analysis. The sensitive BALB/cJ and resistant DBA/2J mouse strains were exposed to either filtered air (control) or ammonia (1,500 ppm for 6 or 11 h) and anesthetized, and bronchoalveolar lavage was performed or lung tissue collected. Compared with untreated control, the sensitive BALB/cJ strain had increased BALF neutrophils (0.7 ± 0.2 to 7.4 ± 1.2%, P < 0.001) and protein (198 ± 37 to 344 ± 53 μg/mL, P < 0.05) at 6 h, whereas these variables were not different from control in the resistant DBA/2J strain. With time, the injury was evident in both strains, and at 11 h, both strains had increased BALF neutrophils compared with strain-matched controls, and the lung histology of BALB/cJ did not differ from that of DBA/2J (Fig. 2). Although these strains differed in survival time, the histological and BALF responses are consistent with acute lung injury.
Figure 1.
Mouse strains vary in sensitivity to ammonia-induced acute lung injury. Acute lung injury survival time of 43 mouse strains. Female mice (6–8 wk) were exposed to 1,500 ppm ammonia for up to 24 h and survival times were recorded hourly. Values are means ± SE (n = 10 mice/strain).
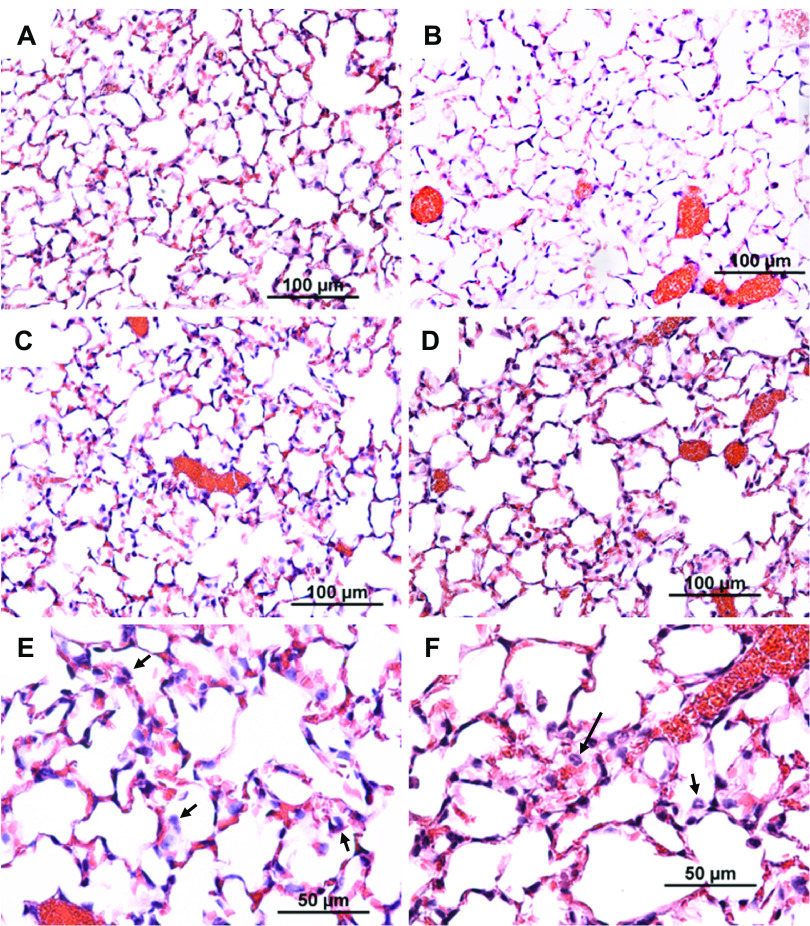
Figure 2.
Histological assessment of lung tissue from control BALB/cJ mice (A), control DBA/2J mice (B), ammonia-exposed BALB/cJ (C and E), or ammonia-exposed DBA/2J mice (D and F). Consistent with ammonia-induced acute lung injury, pulmonary edema and neutrophils (arrows in E and F) were evident in the sensitive BALB/cJ strain and in the resistant DBA/2J strain. Female mice were exposed to filtered air (Control) or to ammonia (1,500 ppm, 11 h) and anesthetized, and lung tissue was obtained. Tissues were fixed in formaldehyde and 5-µm sections were prepared with hematoxylin and eosin stain. Bars indicate magnification.
Candidate gene mapping of susceptibility to ammonia-induced acute lung injury.
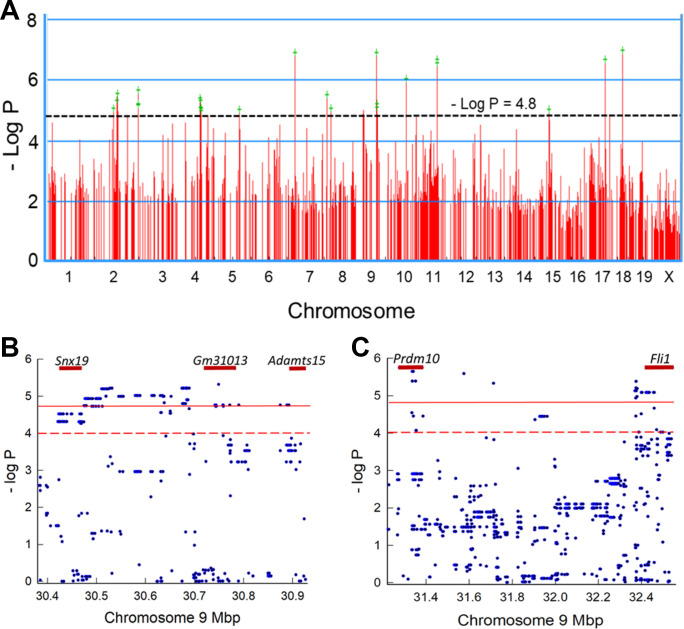
Using EMMA, we generated a Manhattan plot that identified SNPs associated with survival time (Fig. 3A). Only the top 1,000 SNPs of the 4 million measured are displayed in Fig. 3A. The SNP density is better displayed in two exemplary loci on chromosome 9. Within the gene boundaries of sorting nexin 19 (Snx19) 18 SNPs exceeded the suggestive threshold of 4.8 > −log P > 4.0 (Fig. 3B), and within the gene boundaries of PR domain-containing 10 (Prdm10), five SNPs exceeded the significance threshold of −log P > 4.8, and 10 other SNPs exceeded the suggestive threshold of 4.8 > −log P > 4.0 (Fig. 3C).
Figure 3.
Genome-wide association mapping of mouse strains that vary in sensitivity to ammonia-induced acute lung injury. A: genome-wide association map for ammonia-induced acute lung injury in mice. The scatter (Manhattan) plot was generated by efficient mixed-models association (EMMA) corrected for population structure and displays the corresponding –log (P) association probability for the top 1,000 region-wide single-nucleotide polymorphisms (SNPs) at the indicated chromosomal locations. B: exemplary genetic locus on chromosome 9 illustrating the SNP density. Within the gene boundaries of sorting nexin 19 (Snx19), for example, 18 SNPs exceed the suggestive linkage threshold of 4.8 ≥ −log P > 4.0. C: another exemplary genetic locus on chromosome 9 illustrating the SNP density. Within the gene boundaries of PR domain-containing 10 (Prdm10), for example, 5 SNPs exceeded significant threshold of −log P ≥ 4.8, and 10 SNPs exceeded the suggestive linkage threshold.
The EMMA analysis tested 4 million SNPs throughout the genome. The Sanger4 mouse genome analysis covered 80+ million SNPS (available at: https://phenome.jax.org/snp/retrievals). Thus, the Sanger 4 database was used for the secondary analysis of promoter and nonsynonymous SNPs. As an example, the EMMA analysis identified 38 SNPs within Snx19 and 8 SNPs within 2,000 bp in the 5′-UTR. In this chromosomal region, two significant and 28 suggestive SNPs were identified by EMMA. The Sanger4 provided strain variant data for 1079 SNPs within Snx19 and 106 SNPs with 2,000 bp in the 5′-UTR.
Overall, 16 genes that contained significant (Supplemental Table S1; see https://doi.org/10.6084/m9.figshare.12469049) and 22 genes that contained suggestive (Supplemental Table S2; see https://doi.org/10.6084/m9.figshare.12465935) SNP associations were identified throughout the mouse genome. To prioritize genes of interest, first we determined whether the protein encoded by the gene was previously associated with molecular function related to pulmonary edema or inflammation. Two such genes, (potassium voltage-gated channel, subfamily Q, member 5 (Kcnq5) and histamine receptor H4 (Hrh4), have been previously associated with pulmonary edema and inflammation, respectively (Table 1). The sequence difference in Kcnq5 encodes a nonsense-mediated decay variant that could lead to deleterious gain-of-function or dominant-negative activity of the resulting proteins. Similarly, the Hrh4 variant would result in a nonsynonymous Ser219Phe amino acid variant.
Table 1.
Genes with single nucleotide polymorphism associations linked to ammonia-induced acute lung injury
| Chr | Position | −Log P | SNP | Gene ID | Symbol | Description | Phenotype, % | Allele freq, % | Consequence | Protein Function |
|---|---|---|---|---|---|---|---|---|---|---|
| Function has been associated with pulmonary edema and inflammation | ||||||||||
| 1 | 32320573 | 4.6 | 8 | 226922 | Kcnq5 | Potassium voltage-gated channel Q 5 | 25 | 11 | Nonsense-mediated decay variant | Ion channel |
| 18 | 13009954 | 4.9 | 4 | 225192 | Hrh4 | Histamine receptor H4 | 11 | 17 | Ser 2i9 Phe 7 Transmembrane receptor | Receptor |
| Contains a nonsynonymous SNP in functional domain | ||||||||||
| 11 | 84456261 | 6.7 | 1 | 56321 | Aatf | Apoptosis antagonizing transcription factor | 13 | 40 | Glu 460 Gly TRAUB, AATF, C terminal | Transcription factor |
| 5 | 103775118 | 4.0 | 1 | 17355 | Aff1 | AF4/FMR2 family, member 1 | 11 | 26 | Leu 550 Pro AF-4 proto-oncoprotein | Transcription factor |
| 10 | 127014457 | 4.8 | 4 | 11567 | Avil | Advillin | 15 | 24 | Arg 15 Gly gelsolin_S1_like | Actin regulatory |
| 6 | 106161291 | 4.1 | 2 | 26784 | Cntn4 | Contactin 4 | 13 | 23 | Ile 64 Leu Immunoglobuiin I-set domain | Cell adhesion |
| 16 | 24 | Ile 493 Val Ig5 Contactin like | ||||||||
| 18 | 12525632 | 4.8 | 1 | 16774 | Lama3 | Laminin alpha 3 | 11 | 11 | Glu3174 Gly Laminin G domain | Basement membrane |
| 9 | 30464232 | 4.5 | 18 | 102607 | Snx19 | Sorting nexin 19 | 18 | 24 | His 883 Arg Sorting nexin C terminal | Exocytosis |
| 22 | 22 | Gly 652 Ala Phosphoinositide binding | ||||||||
| 25 | 10 | Ala 879 Pro Sorting nexin C terminal | ||||||||
| 9 | 86429419 | 6.9 | 1 | 70348 | Ube2cbp | Ubiquitin-conjugating enzyme E2C binding protein | 23 | 21 | Ile 232 Val HECT-like ubiquitin-conjugating enzyme (E2)-binding. | Protein ubiquitation |
| Contains a promoter SNP that alters putative transcription factor binding site and transcript levels differed between strains | ||||||||||
| 5 | 103775118 | 4.0 | 1 | 17355 | Aff1 | AF4/FMR2 family, member 1 | 32 | 14 | Loss EGR1 gain CEBPB | Transcription factor |
| 3 | 122487739 | 4.1 | 1 | 29815 | Bcar3 | Breast cancer anti-estrogen resistance 3 | 25 | 14 | Loss EGR1 | Signal transduction |
| 9 | 87262400 | 5.1 | 1 | 382090 | Cep162 | Centrosomal protein 162 | 21 | 43 | Gain KLF5 | Cilium assembly |
| 2 | 135971848 | 4.8 | 1 | 18798 | Plcb4 | Phospholipase C, beta 4 | 19 | 15 | Loss JUNB gain EGR1 | Signal transduction |
| 9 | 31335404 | 6.2 | 15 | 382066 | Prdm10 | PR domain containing 10 | 28 | 19 | Gain EGR1 | Transcription factor |
| 17 | 15 | Stop gained | ||||||||
| 6 | 71883737 | 4.1 | 1 | 69956 | Ptcd3 | Pentatricopeptide repeat domain 3 | 22 | 17 | Loss NR4A2 gain AHR | Mitochondrial translation |
Chr, chromosome; Position, base pair location of the single-nucleotide polymorphism (SNP) within the gene with highest –log P; –log P, highest negative log of probability for the SNP within the gene; SNPs, total number of significant –log P > 4.8 and suggestive SNP 4.0 < –log P < 4.8 observed in the gene; Symbol, official Entrez symbol; ID Entrez identification number, Description, official full gene name provided to Entrez by Mouse Genome Informatics (MGI) Database; Phenotype (%), the percent difference in mean survival time explained by the SNP; Allele Freq. (%), the frequency of the minor allele in the population that has been genotyped for the functional SNP; Consequence, the amino acid substitution with the functional domain or the change produced in a putative DNA transcription factor binding site in the promoter or gain of a stop codon. Protein Function, Action/Function of encoded protein. AHR, aryl hydrocarbon receptor, CEBPB, CCAAT/enhancer binding protein (C/EBP)β, EGR1, early growth response 1, JUNB, JunB proto-oncogene, AP-1 transcription factor subunit, KLF5, Kruppel-like factor 5, NR4A2, nuclear receptor subfamily 4, group A, member 2.
Phenotypic consequences of nonsynonymous SNPs.
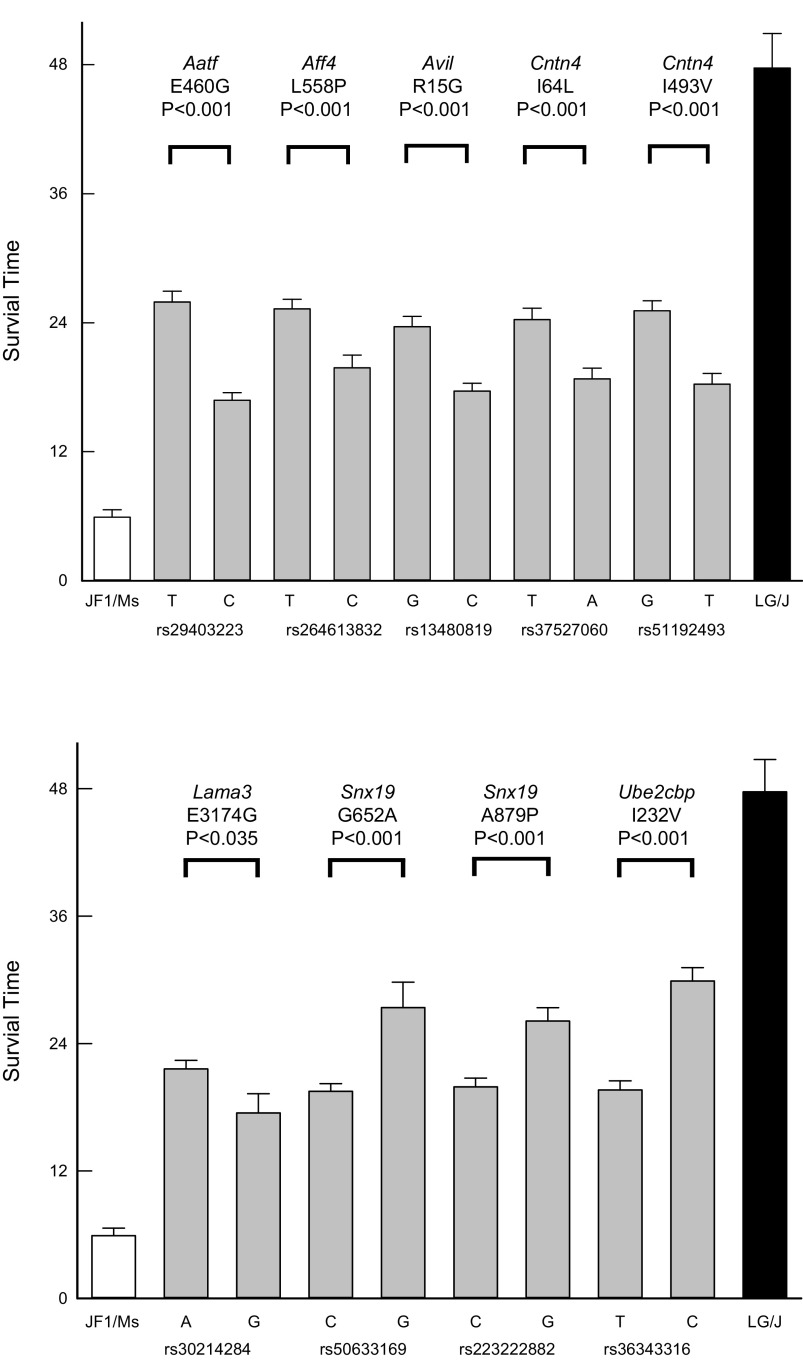
Nonsynonymous SNPs in the functional domain of the corresponding protein were determined in the remaining 36 identified genes. Mice were grouped by nonsynonymous SNPs, and the mean survival time was determined. The difference in survival time between these groups was compared with the difference of the means of strains at the phenotypic extremes. The mean survival time of the phenotypic extremes of mouse strains included the sensitive JF1/MsJ mice, which equaled 5.9 ± 0.7 h (n = 10 mice), and the resistant LG/J mice, which equaled 47.7 ± 3.2 h (n = 10 mice). The resulting SNPs were evaluated as to whether each could explain ≥10% of the phenotypic difference between the survival times of strains at the phenotypic extremes and had a minor allelic frequency of ≥10% (Table 1). For each SNP, the mean survival time was determined for mice in strains carrying one or the other of the two alleles (Fig. 4). The SNPs with predicted consequences of amino acid substitutions would alter hydropathy index (Aff1: rs264613832, Cntn4: rs37527060, Snx19: rs223222882), side-chain polarity (Aatf: rs29403223, Avil: rs13480819, Lama3: rs30214284), side-chain charge (Avil: rs13480819, Lama3: rs30214284), or contactin-immunoglobulin domain properties (Cntn4: rs51192493). For each gene or protein evaluated, DBA/2J carried the resistant variant and the BALB/cJ carried the sensitive variant.
Figure 4.
Assessment of the phenotypic difference in survival time between the strains produced by nonsynonymous single-nucleotide polymorphism (SNP) associations in functional domains of candidate genes. For each SNP, the mean survival time was determined for mice in strains carrying one or the other of the two alleles [e.g., the SNP rs29403223 in Aatf is associated with a mean survival time of 25.9 ± 1.0 h (n = 234 mice) for the T allele and 16.9 ± 0.7 h (n = 158 mice) for the C allele]. The difference between these allelic groups (9.0 h) was then compared with the difference of the mean survival time of the mouse strains at the phenotypic extremes (41.8 h), i.e., sensitive JF1/MsJ (5.9 ± 0.7 h, n = 10 mice) and resistant LG/J (47.7 ± 3.2 h, n = 10 mice) mouse strains. Thus, this analysis for Aatf SNP yielded a percent phenotype predicted: 9.0/41.8 × 100 = 22% and a minor allele frequency: 158/(158+234) × 100 = 40%. The predicted amino acid substitution (E460G: glutamic acid 460 glycine) in the corresponding AATF protein is contained in TRAUB (apoptosis-antagonizing transcription factor, COOH-terminal domain). The predicted amino acid substitutions for the remaining SNPs were: AFF1 (L558P: leucine 558 proline), AF-4 proto-oncoprotein; AVIL (R15G: arginine 15 glycine), gelsolin subdomain 1-like domain found in gelsolin, severin, villin, and related proteins; CNTN4 (I64L: isoleucine 64 leucine), immunoglobulin domain, CNTN4 (I493V: isoleucine 493 valine), fifth Ig domain of contactin; LAMA3 (E1568G: glutamic acid 1568 glycine), laminin B domain; SNX19 (G652A: glycine 652 alanine), Phosphoinositide-binding Phox homology domain of sorting nexin 19, SNX19 (A879P: alanine 879 proline), sorting nexin COOH terminal; and UBE2CBP (I232V: isoleucine 232 valine), uncharacterized conserved protein, respectively. The predicted consequences of amino acid substitutions would alter side-chain polarity (rs13480819, rs29883209, rs30214284), side-chain charge (rs13480819, rs30214284), or hydropathy index (rs264613832, rs37527060, rs29883209, rs223222882). The identifying reference SNP, i.e., the “rs number,” is indicated on the abscissa, with sample size within each bar. Values are means ± SE. All phenotypic differences were statistically different with indicated P values as determined by Kaplan–Meier survival analysis: log-rank analysis. Aatf, apoptosis-antagonizing transcription factor; Aff1, AF4/FMR2 family, member 1; Avil, advillin; Cntn4, contactin 4; Lama3, laminin-α3; Snx19, sorting nexin 19; Ube2cbp, ubiquitin-conjugating enzyme E2C-binding protein.
Genome-wide transcript analysis.
Microarray analysis was performed on BALB/cJ and DBA/2J mouse lung mRNA obtained at 0 (control), 6, and 11 h during 1,500-ppm ammonia exposure. The total number of protein-coding transcripts detected in the lung of either mouse was 18,644, which is ∼80% of all the 23,086 known protein-coding genes in the mouse genome. Transcripts that increased or decreased more than or equal to 1.5-fold in BALB/cJ mouse lung were compared between strains at 0 h or to strain-matched control (0 h) transcripts at 6 or 11 h.
Mouse lung transcripts increased or decreased more than or equal to 1.5-fold significantly in BALB/cJ control (0 h) compared with DBA/2J control mice (0 h) were analyzed first. Notably, 769 transcripts were statistically different between strains at baseline, which was ∼10% of the 7,463 transcripts significantly different in at least one comparison (Table 2 and Supplemental Table S3; see https://doi.org/10.6084/m9.figshare.12469124). BALB/cJ had 433 increased and 336 decreased lung transcripts compared with DBA/2J lung transcripts. Of note in the increased transcripts is mitogen-activated protein kinase kinase kinase-6 (MAP3K6 aka apoptosis signal-regulating kinase, ASK2), which regulates stress-induced caspase cleavage (118) and inflammasome activation (127). Of note among the decreased transcripts is retinoic acid early transcript 1E (RAET1E). The encoded protein is expressed on stressed cells and forms a ligand for natural killer group 2 member D (NKG2D) receptor, which is critical to natural killer T cell-mediated cytotoxicity (74).
Table 2.
Differences in transcriptomic response of BALB/cJ and DBA/2J mouse strains following ammonia exposure
| Hour | Change | BALB/cJ Total | BALB/cJ Not DBA/2J | DBA/2J Not BALB/cJ | DBA/2J Total |
|---|---|---|---|---|---|
| 6 | Increased | 1,007 | 555 | 427 | 879 |
| Decreased | 1,114 | 820 | 564 | 858 | |
| 11 | Increased | 1,402 | 692 | 829 | 1,539 |
| Decreased | 2,593 | 1,288 | 1,718 | 3,024 |
Next, the altered transcripts common in the DBA/2J and BALB/cJ mice following ammonia exposure were compared with strain-matched control. At 6 or 11 h, significantly different transcripts (i.e., changed ≥1.5-fold, P < 0.05) following ammonia exposure compared with strain-matched control (0 h) were determined (Fig. 5). To examine further the difference in transcript expression between the sensitive and resistant strains, pathway analyses were performed. Significant transcripts were analyzed using the Database for Annotation, Visualization, and Integrated Discovery (DAVID). The most enriched term in categories of Gene Ontogeny (GO) biological process, GO molecular function, or Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway were identified. At 6 h, enriched pathways with increased transcripts that are in common in the DBA/2J and BALB/cJ strains included Regulation of Apoptotic Process, Protein Kinase Activity, and Interleukin 17 (IL-17) Signaling (Fig. 5). The enriched pathways with decreased transcripts in both strains at 6 h included Heart Development, Transcription Factor/DNA Binding, and Herpes Simplex Virus 1 Infection. At 11 h, the enriched pathways with increased transcripts in both strains remained the same as at 6 h. The enriched pathways at 11 h with decreased transcripts in both strains included response to DNA Damage, Transcription Factor activity, and Peroxisome.
Figure 5.

Transcripts in enriched pathways that were altered in sensitive BALB/cJ and resistant DBA/2J mice, as determined by microarray analysis of lung mRNA following ammonia exposure. Microarray analysis was performed on mouse lung mRNA obtained at 0 h (filtered air control) or at 6 and 11 h during 1,500 ppm ammonia exposure (n = 6 arrays/strain/time), and significant differences were determined by Partek software (P < 0.05). Transcripts log 2 ≥ 0.58 (i.e., ≥1.5-fold) increased or log 2 ≤ −0.58 decreased in BALB/cJ and DBA/2J mouse lung compared with strain-matched control (0 h) were analyzed using Database for Annotation, Visualization, and Integrated Discovery (DAVID). The most enriched term in categories of Gene Ontogeny (GO) biological process, GO molecular function, or Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway were selected. Ten transcripts with the greatest difference in the identified pathways are displayed. At 6 h and at 11 h, the enriched pathways with increased transcripts (yellow) included Regulation of Apoptotic Process, Protein Kinase Activity and Interleukin 17 Signaling. At 6 h, the enriched pathways with decreased transcripts (purple) included Heart Development, Transcription Factor/DNA Binding, and Herpes Simplex Virus 1 Infection. At 11 h, the enriched pathways with decreased transcripts (purple) included Response to DNA Damage, Transcription Factor Activity, and Peroxisome pathways.
Following ammonia exposure, the lung transcriptomic response varied between the sensitive BALB/cJ compared with the DBA/2J mouse strain (Supplemental Table S3; see https://doi.org/10.6084/m9.figshare.12469124). Remarkably, ∼50% of the changed transcripts in one strain were unchanged in the other strain. For example, 555 of 1,007 (55%) transcripts increased at 6 h in BALB/cJ mouse lung were unique to that strain (i.e., not significantly changed in DBA/2J lung). Similarly, 427 of 879 (49%) transcripts increased at 6 h in DBA/2J mouse lung were unique to DBA/2J mice (i.e., not significantly changed in BALB/cJ).
At 6 h, the enriched biological process, molecular function, and KEGG pathways with increased transcripts in BALB/cJ mouse lung compared with BALB/cJ control (0 h), but not in DBA/2J mouse lung, included Response to Lipopolysaccharide (LPS), Regulation of Transcription, and Cell Receptor Signaling, respectively (Fig. 6A). The enriched pathways with decreased transcripts at 6 h only in BALB/cJ mouse lung included Regulation of Transcription, Phosphoprotein Phosphatase, and Transforming Growth Factor Beta 1 (TGF-β1) Signaling pathway. At 11 h, the enriched pathways with increased transcripts in BALB/cJ mouse lung included Cytosolic Ca2+ Regulation, G-Protein Receptor Signaling, and MAPK Cell Signaling. The enriched pathways with decreased transcripts at 11 h in BALB/cJ mouse lung included Cytokine-Mediated Signaling, Ubiquitin-Protein Transferase Activity, and WNT Signaling.
Figure 6.


Transcripts in enriched pathways that were altered more in sensitive BALB/cJ than resistant DBA/2J mice (A) or more in resistant DBA/2J than sensitive BALB/cJ mice (B) as determined by microarray analysis of lung mRNA following ammonia exposure. Microarray analysis was performed on mouse lung mRNA obtained at 0 (control), 6, and 11 h during 1,500 ppm ammonia exposure (n = 6 arrays/strain/time), and significance (P < 0.05) was determined by ANOVA. Transcripts log 2 ≥ 0.58 (i.e., ≥1.5-fold) increased or log 2 ≤ −0.58 decreased in BALB/cJ mouse lung at 6 or 11 h after exposure compared with BALB/cJ control (0 h) transcripts but not significantly different in DBA/2J mouse lung, were analyzed using Database for Annotation, Visualization, and Integrated Discovery (DAVID). The most significant enriched term in the categories of Gene Ontogeny (GO) biological process, GO molecular function, and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway were selected. Ten transcripts with the greatest difference between strains in these pathways are displayed. A: at 6 h, the enriched pathways with increased transcripts in BALB/cJ mouse lung (n = 1,007) included Response to Lipopolysaccharide, Regulation of Transcription, and Cell Receptor Signaling. The enriched pathways with decreased transcripts in BALB/cJ mouse lung (n = 1,114) included Regulation of Transcription, Phosphoprotein Phosphatase, and TGF (transforming growth factor) Beta Signaling. At 11 h, the enriched pathways with increased transcripts in BALB/cJ mouse lung (n = 1,402) included Cytosolic Ca2+ Regulation, G-protein Receptor Signaling, and MAPK Cell Signaling. The enriched pathways with decreased transcripts at 11 h in BALB/cJ mouse lung (n = 2,593) included Cytokine-Mediated Signaling, Ubiquitin-Protein Transferase Activity, and WNT Signaling. B: at 6 h, the enriched pathways with increased transcripts in DBA/2J mouse lung (n = 879) included Regulation of Cell Migration, Protein Kinase Activity, and MAPK Cell Signaling. Pathways with decreased transcripts at 6 h in DBA/2J mouse lung (n = 858) included DNA Damage Response, Protein Kinase Activity, and Ras Signaling Pathway. At 11 h, the enriched pathways with increased transcripts in DBA/2J mouse lung (n = 1,539) included Adenylate Cyclase (ADCY)-modulating G-protein Receptor (GPR) Coupling Signaling, Actin Binding, and MAPK Signaling. Pathway with decreased transcripts (n = 3,024) included Cell-Matrix Adhesion, DNA-dependent ATPase Activity, and Phosphoinositide 3-Kinase (PI3K)-Akt Signaling pathway.
Transcripts that changed more than or equal to 1.5-fold in DBA/2J mouse lung compared with DBA/2J control (0 h) but not significantly different in BALB/cJ mouse lung transcripts at 6 h included Regulation of Cell Migration, Protein Kinase Activity, and MAPK Cell Signaling (Fig. 6B). Pathways with decreased transcripts at 6 h in DBA/2J mouse lung included DNA Damage Response, Protein Kinase Activity, and Ras Signaling Pathway. At 11 h, the enriched pathways with increased transcripts in DBA/2J mouse lung included Adenylate Cyclase (ADCY)-Modulating G-protein Receptor (GPR) Coupling Signaling, Actin Binding, and MAPK Signaling. Pathways with decreased transcripts included Cell-Matrix Adhesion, DNA-Dependent ATPase Activity, and Phosphoinositide 3-Kinase (PI3K)-Akt Signaling Pathway.
Identification of possible therapeutics.
LINCS L1000 analyses involved four groups (Table 3). The first group contained inhibitors of the phosphoinositide 3-kinase/mammalian target of rapamycin (PI3K-mTOR) pathway, including GDC-0941 (pictilisib), GSK126458 (omipalisib), and torin-2. This group also included a proline-rich tyrosine kinase-2/focal adhesion kinase inhibitor (PYK2/FAK) PF-431396. The second group contained geldanamycin, radicicol, and tanespimycin (17-AAG), which inhibit heat shock protein-90 (HSP90). Two anti-inflammatory compounds, caffeic acid phenethyl ester (CAPE) and celastriol, were identified in the third group. This group also identified AS-601245, a mitogen-activated protein kinase 8/c-Jun NH2-terminal kinase (JNK) inhibitor, and NVP-AEW541, an insulin-like growth factor 1 receptor (IGF1R) inhibitor. The last group identified l-sulforaphane and chaetocin. The former is a nuclear factor, erythroid 2-like 2 (NFE2L2 aka Nrf2) activator that induced antioxidant response genes, and the latter is a methyltransferase/thioredoxin reductase-1 inhibitor.
Table 3.
Library of Integrated Network-based Cellular Signatures L1000 identified compounds using differentially expressed genes in resistant DBA/2J or sensitive BALB/cJ mouse lung following ammonia exposure
| Compound | P Vvalue | ALI Protection* | Mechanism |
|---|---|---|---|
| Group 1 (DBA/2J Increased DEGs & LINCS L1000 Up and BAL/cJ Increased DEGs & LINCS L1000 Down) | |||
| GDC-0941 (pictilisib) | 9.6E-04 | Yes | PI3K inhibitor |
| GSK126458 (omipalisib) | 8.3E-07 | No | PI3K and mTOR inhibitor |
| PF-431396 | 4.9E-04 | No | Pyk2/focal adhesion kinases inhibitor |
| Torin-2 | 9.3E-05 | No | mTOR inhibitor |
| Group 2 (DBA/2J Decreased DEGs & LINCS L1000 Down and BALB/cJ Decreased DEGs & LINCS L1000 Up) | |||
| Geldanamycin | 1.1E-03 | Yes | HSP90 inhibitor |
| Rradicicol | 3.4E-04 | Yes | HSP90 inhibitor |
| Tanespimycin (17-AAG) | 1.2E-05 | Yes | HSP90 inhibitor |
| AS-601245 | 2.3E-04 | No | c-Jun NH2-terminal kinase (JNK) inhibitor |
| NVP-AEW541 | 4.1E-04 | No | IGF1R inhibitor |
| Group 3 (DBA/2J Increased DEGs & LINCS L1000 Up or BALB/cJ Increased DEGs & L1000 Down) | |||
| Caffeic acid phenethyl ester | 3.2E-03 | Yes | Myeloid differentiation 2 inhibitor (anti-inflammatory) |
| Celastrol | 1.7E-03 | Yes | NF-kappa B inhibitor (anti-inflammatory) |
| Group 4 (DBA/2J Decreased DEGs & LINC L1000 Down) | |||
| l-Sulforaphane | 1.3E-02 | Yes | Nuclear factor, erythroid 2 like 2 (aka Nrf2) activator (antioxidant) |
| Chaetocin | 3.3E-02 | No | Methyltransferase/thioredoxin reductase 1 inhibitor |
ALI Protection indicates compound has been found to diminish differentially expressed genes (DEGs) in acute lung injury (ALI) in various rodent models.
LINCS, Library of Integrated Network-Based Cellular Signatures; PI3K, phosphoinositide 3-kinase; mTOR, mammalian target of rapamycin; Pyk2, protein tyrosine kinase 2; HSP90, heat shock protein 90; IGF1R, insulin-like growth factor 1.
Candidate gene transcript levels altered at baseline or following ammonia exposure.
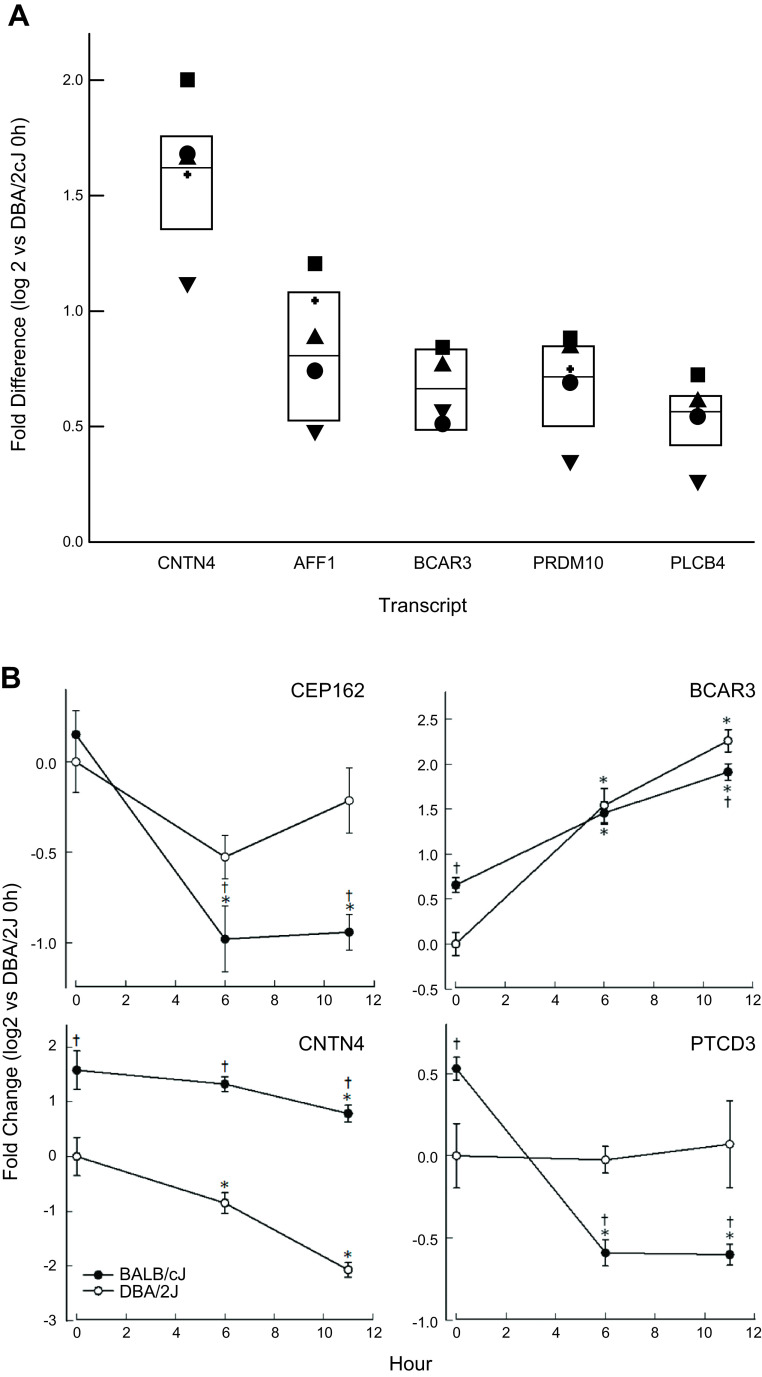
The genome-wide transcriptomics was further analyzed to determine whether lung transcripts differed between mouse strains before (0 h) and 6 or 11 h after ammonia exposure. Based on this initial analysis, transcript levels were determined by qRT-PCR for 12 candidate genes with significant SNP associations. Of these 12 genes, six had altered baseline transcripts in the DBA/2J mouse lung compared with the BALB/cJ mouse lung (Fig. 7A and Supplemental Table S3; see https://doi.org/10.6084/m9.figshare.12469124). For example, the baseline lung levels of CNTN4 transcript was increased 3.1-fold (log 2 = 1.6-fold) in BALB/cJ mice compared with the DBA/2J mice at 0 h. Based on the difference in transcript levels between the two strains, the six candidate genes were evaluated for SNPs contained in the 5′-UTR/promoter region to determine whether the sequence variation could alter a putative transcription factor binding site. These candidate genes included Cntn4, Aff1, breast cancer anti-estrogen resistance 3 (Bcar3), PR domain-containing 10 (Prdm10), phospholipase Cβ4 (Plcb4), and pentatricopeptide repeat domain 3 (Ptcd3).
Figure 7.
Transcript levels of candidate genes in BALB/cJ compared with DBA2/J mouse lung. A: baseline transcript levels for 6 candidate genes significantly increased in BALB/cJ mouse compared with DBA/2J mouse lung >1.5-fold (P < 0.05). Transcript levels of contactin 4 (CNTN4), AF/MR2 family, member 1 (AFF1), breast cancer anti-estrogen resistance 3 (BCAR3), PR domain-containing 10 (PRDM10), phospholipase Cβ4 (PLCB4), and pentatricopeptide repeat domain 3 (PTCD3) were determined by quantitative real time qPCR (n = 6 mice/strain). Each symbol represents a different mouse (i.e., the open or filled symbol represents the response of the same mouse for each transcript). Values are medians ± 25 or 75% (n = 6 mice/strain) normalized to ribosomal protein L32 (rpL32). All values are significantly different from DBA/2J as determined by ANOVA, with an all-pairwise multiple comparison procedure (Holm–Sidak method). B: transcript levels of candidate genes, centrosomal protein 162 (CEP162), BCAR3, CNTN4, and PTCD3 that were altered by ammonia exposure and differed between BALB/cJ and DBA/2J mouse lung. Values are medians ± 25 or 75% (n = 6 mice/strain) normalized to rpL32. *Significantly different (P < 0.05) from strain-matched control (0 h), as determined by ANOVA with an all-pairwise multiple comparison procedure (Holm–Sidak method). †Significant difference (P < 0.05) between the resistant DBA/2J and sensitive BALB/cJ mouse strains at the same time, as determined by ANOVA with an all-pairwise multiple comparison procedure (Holm-Sidak method).
Candidate gene transcript levels were also evaluated in BALB/cJ and DBA/2J mouse strains immediately after 6- or 11-h ammonia exposure (Fig. 7B). Of the 12 candidate genes, two transcripts, centrosomal protein-162 (CEP162) and pentatricopeptide repeat domain 3 (PTCD3) decreased more in the sensitive BALB/cJ mouse lung compared with the DBA/2J after ammonia exposure. In addition, BCAR3 increased and CNTN4 decreased more in the resistant DBA/2J compared with the sensitive BALB/cJ mouse strain after ammonia exposure (Fig. 7B).
BCAR3 transcript expression profile in human lung cells.
To further examine the possible role of genetic variants in BCAR3 transcript expression, human cell lines were examined. As determined by qRT-PCR analysis, BCAR3 transcript was expressed at variable levels in all the cells examined and increased in THP1 cells following phorbol 12-myristate 13-acetate (PMA) treatment (Fig. 8A). The occurrence of multiple BCAR3 transcript variants has been reported, but their significance and mode of regulation remain unclear. To investigate the expression profile of the BCAR3 mRNA variants, the level of splice variants 1, 2, and 4 was determined. Interestingly, the relative expression level of BCAR3 transcript variant 2 with respect to the total BCAR3 transcript is similar, whereas the expression level of variants 1 and 4 varies among the different cell types examined (Fig. 8B).
Figure 8.
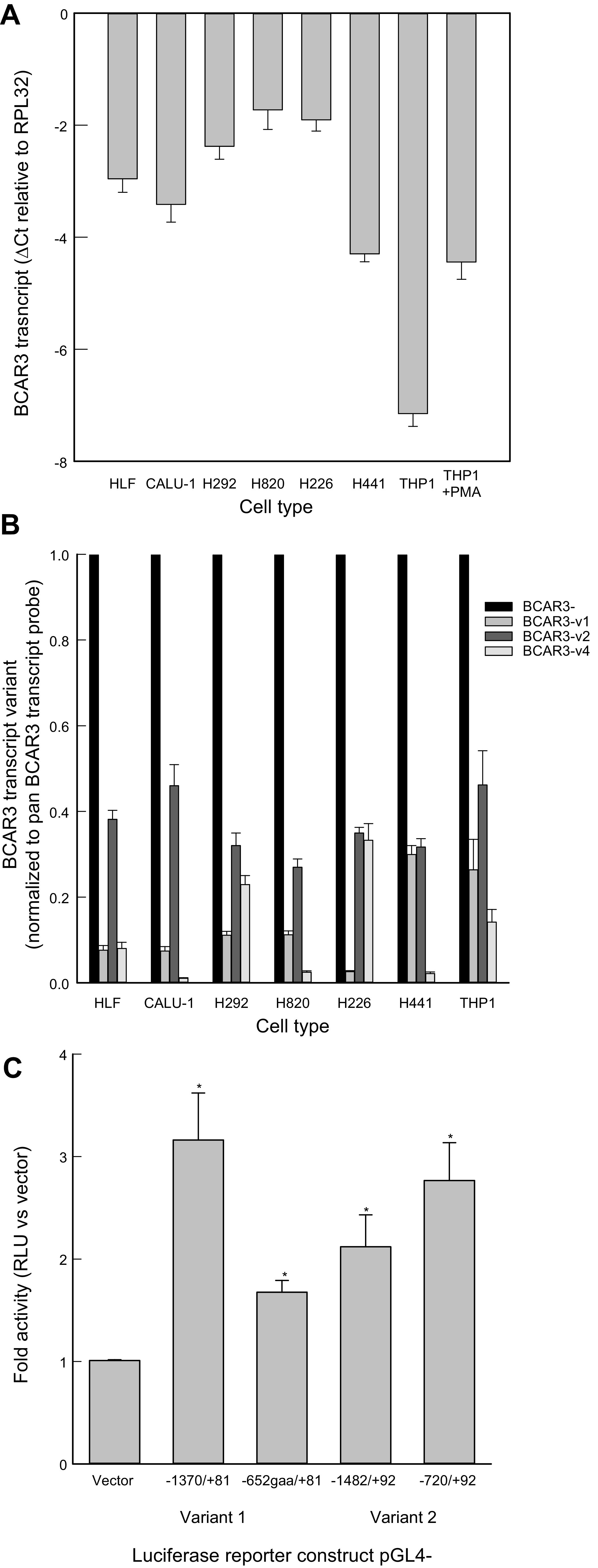
Breast cancer anti-estrogen resistance 3 (BCAR3) transcript expression profile varies among lung and THP1 cells. A: relative abundance of BCAR3 transcript expression in human lung fibroblasts (HLF), human lung cell lines (CALU-1, H292, H820, H226, H441), and untreated and phorbol 12-myristate 13-acetate (PMA)-treated THP1 cells. Transcript levels were determined by qRT-PCR analysis using a 3′ probe (Life Technologies, Hs00981962_m1) that detects the BCAR3 transcript variants 1, 2, 3, and 4. B: BCAR3 transcript variant 2 is widely expressed, whereas the expression profile of variants 1 and 4 varies among different cell types. The profile of BCAR3 transcript variants 1, 2, and 4 expression HLF, human lung cells lines (CALU-1, H292, H226, H820, H441), and THP1 cells was determined by real-time PCR analysis using probes specific to variants 1, 2, and 4 (Life Technologies, Hs00981963_m1, Hs00981968_m1, Hs00981954_m1, respectively) and expressed relative to BCAR3 transcript expression determined using a probe that detects variants 1, 2, 3, and 4 (Life Technologies, Hs00981962_m1). C: analysis of putative alternative BCAR3 promoter regions. The promoter regions overlapping BCAR3 transcript variants 1 and 2 exhibited higher luciferase reporter activity than the promoterless vector (n = 12–18, *P < 0.001), suggesting alternative promoter usage in BCAR3 gene regulation.
The human BCAR3 gene spans over 285 kb, and exons 1, 2, and 3 generate the 5′-untranslated region of variant 1, whereas exon 4, which is ∼164 kb downstream of exon 1, generates the 5′-untranslated region of variant 2 (19, 115, 116, 145, 146). Therefore, we tested the possibility that BCAR3 gene expression is regulated by alternative promoters. To identify and test putative BCAR3 promoter regions, DNA fragments overlapping exon 1 and exon 4 (i.e., nucleotide −1370 to +81 and 163830 to 165404, respectively, and deletion fragments (i.e., −652 to +81 and 164592 to 165404) were inserted in the pGL4-10 luciferase reporter vector (Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.12469043). The luciferase reporter activity of the constructs containing the putative promoter fragments increased compared with the promoterless reporter vector, suggesting that multiple promoters may regulate BCAR3 transcriptional activation at different transcription start sites (Fig. 8C).
DISCUSSION
Most reports of human exposure resulting in acute lung injury are anecdotal case reports and do not have estimates of exposure concentrations. Under accidental circumstances, concentrations may be unpredictable but are assumed to be high based on proximity to the source. Because of the sudden nature of accidents, only retrospective estimates of exposure levels are available. One estimate of an exposure level was a report of a fatality at a concentration >10,000 ppm (107). In this case, a victim was filling a tank wagon with a 25% ammonia solution. Ammonia exposure often leads to skin and ocular burns. Because ammonia is highly water soluble, when inhaled it mainly damages the upper respiratory tract and large-diameter airways. However, when inhaled in high concentrations, ammonia can penetrate to the alveolar region, which often is lethal. For example, Caplin (22) described the fate of 47 Londoners injured when an ammonia container was damaged during a World War II air raid. Eleven developed pulmonary edema at the time of exposure, and seven of these victims died over the next 48 h; three developed pulmonary edema within 6 h, and nine others developed acute lung injury several days later. In the 13 patients who died, autopsy revealed bronchial epithelial desquamation and moderate to severe pulmonary edema.
In this study, a genetically diverse panel of 43 mouse strains was exposed to 1,500 ppm ammonia to induce acute lung injury. A genome-wide association analysis using survival time as the trait of interest was performed, which revealed 38 genes with significant or suggestive linkage. Transcriptomic analysis of lung mRNA in two phenotypically divergent strains was used to further evaluate the identified genes. This integrative functional approach revealed 14 candidate genes that included Avil, Cep162, Hrh4, Kcnj1, Lama3, Plcb4, and Ube2cbp, which had significant SNP associations, and Aff1, Bcar3, Cntn4, Kcnq5, Prdm10, Ptcd3, and Snx19, which had suggestive SNP associations.
Acute lung injury is a complex syndrome and can be induced by numerous causes, including chemical irritants, near drowning, or infections. Currently, treatment is limited to managed ventilation. Although numerous clinical trials have been performed, no therapeutics have been approved for treatment. As evidenced by the present transcriptomic analyses, ammonia exposure induced changes in transcripts whose products regulate multiple signaling pathways. The LINCS L1000 analysis was used to identify drugs that reverse gene expression patterns that were altered by ammonia exposure. Identification of drugs that mitigate adverse response patterns could be protective against multiple agents that cause acute lung injury. Upon further study, such drugs could be repurposed for treatment of ammonia-induced lung injury as well. The LINCS L1000 analysis identified several drugs that could be useful in treating acute lung injury. GDC-0941 (pictilisib), a PI3K/mTOR inhibitor (44), previously has been found to protect from LPS-induced acute lung injury in CD-1 mice (30). Of note, “Response to LPS” was a pathway enriched with increased transcripts in the sensitive BALB/cJ mice (Fig. 6A). Other PI3K/mTOR inhibitors include GSK2126458 (omipalisib) (72), which has been proposed to treat idiopathic pulmonary fibrosis (89), PF-431396 (55), and torin-2 (87, 88).
The second LINCS L1000 group contained HSP90 inhibitors that also induce protection to HSP70. This group includes geldanamycin, which has been used in rodents to treat acute lung injury induced by hemorrhage (69, 126), ischemia-reperfusion (49), Pseudomonas aeruginosa (23), and H5N1 influenza virus (149). Other HSP90 inhibitors included radicicol and tanespimycin (17-AAG), which can diminish LPS-induced acute lung injury in mice (5, 27, 28).
Two anti-inflammatory compounds previously associated with acute lung injury protection in several rodent models of acute lung injury, CAPE (3, 21, 31, 42, 53, 64, 162) and celastriol (61, 85, 133, 152, 153) were identified in the third LINCS L1000 group. The last group identified l-sulforaphane and chaetocin. l-Sulforaphane is a NFE2L2 activator that induces antioxidant responses in the lung and protects against oxidative stress that is induced during acute lung injury (32, 90, 120, 131, 166).
Of the genes with significant SNP associations, Kcnq5, Lama3, Hrh4, Cep162, and Bcar3 are particularly noteworthy and have pathophysiological roles that could be associated with acute lung injury in several ways. A non-sense-mediated decay variant was identified in Kcnq5 that could explain 25% of the phenotypic differences in mouse strains. In humans, mutations in KCNQ5 (also known as Kv7.5) can result in a congenital neurological disorder with intellectual disability or epileptic encephalopathy (77, 134). During acute lung injury, fluid accumulates in the alveolar lumen. Lung fluid balance in the alveolus is maintained, in part, through active Na+ absorption initiated by Na+ entry across the apical membrane of airway epithelial cells (96). The intracellular Na+ concentration is low compared with extracellular Na+ and is maintained by basolateral Na+ extrusion via the Na+,K+-ATPase. Found in the apical membrane of airway epithelial cells (108), KCNQ5 forms a heteromeric channel with KCNQ3 (also known as Kv7.3), which contributes to K+ transport. The activity of K+ channels, which regulates K+ efflux, serves to maintain membrane potential. Inhibition of K+ efflux depolarizes the membrane potential and suppresses generation of the Na+ gradient, leading to a reduction in Na+ absorption (50). Diminished Na+ absorption could restrict fluid movement away from the alveolus and the resolution of pulmonary edema (95).
Additionally, KCNQ5 activation in airway smooth muscle can lead to reduction of histamine-induced- (26, 54) and methacholine-induced (17) bronchoconstriction. Recently, NO donors and riociguat have been found to enhance Kv7 currents, leading to pulmonary artery smooth muscle cell hyperpolarization (103), which, in turn, could contribute to NO/cGMP-induced pulmonary artery vasodilation. Interestingly, numerous hypotensive folk medicines from a genetically diverse range of plant species can selectively activate the vascular-expressed KCNQ5 potassium channel (93).
Here we identified laminin-α3 as a candidate gene and noted that a sequence variant could alter the side-chain polarity in the encoded protein’s laminin G domain. Migration of alveolar epithelial cells is necessary during the repair process of acute lung injury, requiring several hours for initiation and several days for completion (12, 46). Laminins are proteins present in the basement membranes of most organs (111) and are composed of an α-, β-, and γ-chain that form a heterotrimer coiled-coil domain protein (66, 111, 124). Expressed primarily in the epithelium, alveolar laminin-5 consists of LAMA3, LAMB3, and LAMC2 (124). Upon cleavage of LAMC2 by matrix metalloprotease (66, 100), laminin-5 enhances epithelial cell migration and repair following epithelial injury. LAMC2 cleavage fragments are increased in pulmonary edema and epithelial lining fluids of acute lung injury patients, and plasma levels are higher in nonsurviving compared with surviving patients (48, 66).
In addition, Lama3−/− gene-targeted mice die a few days after birth due to malnutrition and also develop skin blistering but have no obvious lung phenotype (135). In contrast, lung-specific Lama3 knockdown imparted resistance to mechanical ventilation-induced lung injury (VILI) in mice (144). These mice have improved lung mechanics, histology, alveolar capillary permeability, and increased survival following VILI. Surprisingly, Lama3 knockdown mice have increased bronchoalveolar fluid levels of TNF, cytokines, and chemokines following VILI. In contrast, mice with lung-specific Lama3 gene targeting had increased mortality, inflammation, and fibrosis after treatment with intratracheal bleomycin or adenovirus encoding active TGF-β (105).
A G protein-coupled receptor (GPCR), histamine H4 receptor (HRH4), is predominantly expressed in hematopoietic cells, and plays a role in T helper 2 (Th2)- and Th9-driven inflammation and allergy by stimulating the generation of cytokines and chemokines (1, 2, 56, 84, 114, 136, 164). In this study, we identified a nonsynonymous mutation at amino acid 219 (Ser to Phe; SIFT score = 0.02 deleterious) in the GCPR seven-membrane domain. In humans, genetic HRH4 variants in this domain of the protein are associated with infection-induced asthma (138), atopic dermatitis (29, 101, 163), and oral H1 antihistamine efficacy for allergic rhinitis (51). HRH4 receptor antagonist (2, 35, 114) or gene targeting in mice (35, 56) can diminish initiation and maintenance of inflammation. In addition, activation of HRH4 can lead to decreased intracellular cAMP levels (114), which decreases TGFB1 expression (20). Decreased TGFB1 diminishes its ability to decrease tumor necrosis factor (TNF) production (35, 56). TGFB1 signaling transcripts were decreased more in sensitive BALB/cJ compared with DBA/2J mouse lung 6 h following ammonia exposure (Fig. 6A).
Genetic variants in IL-17 have been associated with susceptibility and prognosis in acute lung injury (159). In this study, transcripts in the KEGG IL-17 signaling pathway increased following 6- or 11-h ammonia exposure in both strains (Fig. 5). At 6 h, sensitive BALB/cJ mice had increased transcripts in the biological process of response to LPS (Fig. 6A). In human CD4+ Th17 cells, HRH4 also can mediate IL-17 production (102, 159). In mouse mast cells, HRH4 can interact with Toll-like receptor 4 (TLR4) (114). In HRH4-deficient mice or mice treated with HRH4 antagonists, collagen-induced increased Th17 cell number and IL-17 production and LPS-induced TNF production were diminished (34).
A centrosomal protein, CEP162 (also called KIAA1009 or QN1) is a chromosome segregation 162-kDa ATPase (81) and an axoneme recognition protein that promotes ciliary transition zone assembly (151). The transition zone is an intracellular compartment adjacent to the centriole distal end, where axonemal microtubules cross-link to the surrounding membrane to form a barrier that gates the ciliary compartment. Using quantitative proteomics, Wang et al. (151) noted that CEP162 interacts with core transition zone components and mediates their association with microtubules. Loss of CEP162 arrests ciliogenesis at the stage of transition zone assembly. Abolishing its centriolar tethering allowed CEP162 to stay on the growing end of the axoneme and results in extra-long cilia with enlarged tips that actively release ciliary contents into the extracellular environment. In addition, in the absence of CEP162 the mitotic spindle is unable to organize correctly and leads to improper division of chromosomes, which eventually leads to cell death (13, 81, 109). In humans, genetic variants near CEP162 have been associated with diabetic retinopathy and coronary artery disease in patients with type 2 diabetes mellitus (7, 8).
Breast cancer anti-estrogen resistance gene 3 (BCAR3, also known as AND-34) was identified in the search for genes involved in the development of estrogen resistance in breast cancer cells (145). The gene encodes a component of intracellular signal transduction that causes estrogen-independent proliferation in breast cancer cells. The human BCAR3 gene spans over 285 kb, contains 17 exons, and produces multiple splice variant mRNAs. In the human lung, the main splice variants are BCAR3α1, with a 5′-untranslated region generated from exons 1, 2, and 3, and BCAR3α2, with a 5′-untranslated region generated from exon 4 (146). Transcripts result from alternative splicing of noncoding exons to the common exon 6, which contains the initial ATG for the BCAR3α variants. An additional splice variant, BCAR3β, can be generated by an initial ATG in exon 8, which also yields the 5′-untranslated region for this variant. Thus, BCAR3α and BCAR3β proteins have different amino termini. Exons 9 to 17 are common to all three transcripts. In mice, Bcar3 appears to contain only 13 exons generating one α- and one β-splice variant, but not in nonmalignant cells. The protein contains a putative src homology 2 (SH2) domain, common in tyrosine kinase signaling molecules, and has partial homology to the cell division cycle protein valosin-containing protein (also known as CDC48) (145). It also contains a carboxyl terminal domain homologous to GDP exchange factors (GEF), which can activate GTPases. BCAR3 mRNA and protein increases in response to IL-1β or TNF in thymic stromal cells (19). Also regulated by focal adhesion, BCAR3 protein can bind BCAR1 to form a scaffolding protein in a signaling network. This network is a component of integrin adhesion complexes that control cell proliferation and migration (19). Integrin activation of TGF-β is a critical event in acute lung injury (39, 78, 79, 155). After 6 h of ammonia exposure, transcripts in the KEGG TGF Beta Signaling pathway were decreased more in the sensitive BALB/cJ than in the resistant DBA/2J mouse lung (Fig. 6A).
Ammonia-induced lung injury, similar to other forms of chemical-induced lung injury, is a complex syndrome. Although the respiratory system represents a primary target of inhaled ammonia, other organs may also be impacted. In this study, we measured BALB/cJ and DBA/2J protein and neutrophils in lavage to establish that ammonia induced signs of acute lung injury. The phenotype used for genotyping was timed to development of lethal acute lung injury. In the past we examined the strain sensitivity to ozone-induced acute lung injury and measured survival time (130), whereas Steven Kleeberger’s group (71) measured ozone-induced neutrophils and protein in bronchoalveolar lavage. Interestingly, the strain patterns of these traits were dissimilar, suggesting that protein/neutrophils, while evidence of lung injury, do not predict survival. In addition, following hyperoxia, neonatal mice strains demonstrated a weak but statistically significant correlation between BALF neutrophils and BALF protein (112). Others and we have concluded that each of these phenotypes may have different genetic variants influencing the outcome.
Acute lung injury and subsequent respiratory failure can be precipitated by inhaled irritants, pneumonia, sepsis, aspiration of gastric contents, or major trauma (97). Determining the mechanisms responsible for the pathogenesis and the resolution of acute lung injury require a better understanding of environmental and genetic factors (59, 91). Currently, acute lung injury therapy is mainly supportive. The LINCS L1000 analysis identified several therapeutics that have been useful in animal models and thus might be worthy of future study. In humans, ammonia-induced acute lung injury is characterized by increased epithelial and endothelial permeability, pulmonary edema, severe arterial hypoxemia, and impaired carbon dioxide exhalation (22, 37, 47, 73). In this study, we applied a functional genomic approach to identify and evaluate candidate genes in a mouse model of ammonia-induced acute lung injury. Based on this analysis, candidates worthy of further analysis in clinical populations include KCNQ5, LAMA3, HRH4, CEP162, and BCAR3.
GRANTS
This study was supported by the NIH: National Institute of Environmental Health Sciences Grant ES015675, National Heart, Lung, and Blood Institute Grants HL077763 and HL085655 (G.D.L.); National Heart, Lung, and Blood Institute Grants HL084932 and HL095397 (N.K.); and Swedish Heart Lung Foundation (K.G.).
DISCLAIMERS
The contribution of L.J.V., who is currently employed at the National Institutes of Health, was performed at the time he was employed at the University of Pittsburgh School of Medicine and do not necessarily represent the views of the National Institutes of Health or the U.S. government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.B. and G.D.L. conceived and designed research; K.B., K.G., T.M.M., V.J.C., K.A.B., S.U., L.J.V., A.S.J., and G.D.L. performed experiments; K.B., K.G., T.M.M., V.J.C., K.A.B., S.U., L.J.V. N.K., E.K., A.S.J., and G.D.L. analyzed data; K.B., K.G., K.A.B., Y.P.P.D., J.P.F., N.K., E.K., E.E., D.R.P., and G.D.L. interpreted results of experiments; T.M.M., V.J.C., and G.D.L. prepared figures; K.B. and T.M.M. drafted manuscript; K.B., K.G., Y.P.P.D., J.P.F., D.R.P., A.S.J., and G.D.L. edited and revised manuscript; K.B., K.G., T.M.M., V.J.C., K.A.B., Y.P.P.D., S.U., J.P.F., L.J.V. N.K., E.K., E.E., D.R.P., A.S.J., and G.D.L. approved final version of manuscript.
REFERENCES
- 1.Akdis C, Jutel M, Akdis M. Regulatory effects of histamine and histamine receptor expression in human allergic immune responses. Chem Immunol Allergy 94: 67–82, 2008. doi: 10.1159/000154858. [DOI] [PubMed] [Google Scholar]
- 2.Akdis CA, Simons FE. Histamine receptors are hot in immunopharmacology. Eur J Pharmacol 533: 69–76, 2006. doi: 10.1016/j.ejphar.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 3.Akyol A, Ulusoy H, Imamoğlu M, Cay A, Yuluğ E, Alver A, Ertürk E, Koşucu M, Beşir A, Akyol A, Ozen I. Does propofol or caffeic acid phenethyl ester prevent lung injury after hindlimb ischaemia-reperfusion in ventilated rats? Injury 37: 380–387, 2006. doi: 10.1016/j.injury.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Anderson AR; Centers for Disease Control and Prevention (CDC) . Top five chemicals resulting in injuries from acute chemical incidents—Hazardous Substances Emergency Events Surveillance, nine states, 1999–2008. MMWR Suppl 64: 39–46, 2015. [PubMed] [Google Scholar]
- 5.Antonov A, Snead C, Gorshkov B, Antonova GN, Verin AD, Catravas JD. Heat shock protein 90 inhibitors protect and restore pulmonary endothelial barrier function. Am J Respir Cell Mol Biol 39: 551–559, 2008. doi: 10.1165/rcmb.2007-0324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arwood R, Hammond J, Ward GG. Ammonia inhalation. J Trauma 25: 444–447, 1985. doi: 10.1097/00005373-198505000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Awata T, Yamashita H, Kurihara S, Morita-Ohkubo T, Miyashita Y, Katayama S, Mori K, Yoneya S, Kohda M, Okazaki Y, Maruyama T, Shimada A, Yasuda K, Nishida N, Tokunaga K, Koike A. A genome-wide association study for diabetic retinopathy in a Japanese population: potential association with a long intergenic non-coding RNA. PLoS One 9: e111715, 2014. [Erratum in PLoS One 10: e0126789, 2015]. doi: 10.1371/journal.pone.0111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azzam SK, Osman WM, Lee S, Khalaf K, Khandoker AH, Almahmeed W, Jelinek HF, Al Safar HS. Genetic associations with diabetic retinopathy and coronary artery disease in Emirati patients with type-2 diabetes mellitus. Front Endocrinol (Lausanne) 10: 283, 2019. doi: 10.3389/fendo.2019.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagheri M, Verma M, Verter V. Transport mode selection for toxic gases: rail or road? Risk Anal 34: 168–186, 2014. doi: 10.1111/risa.12063. [DOI] [PubMed] [Google Scholar]
- 10.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57: 289–300, 1995. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 11.Bennett BJ, Farber CR, Orozco L, Kang HM, Ghazalpour A, Siemers N, Neubauer M, Neuhaus I, Yordanova R, Guan B, Truong A, Yang WP, He A, Kayne P, Gargalovic P, Kirchgessner T, Pan C, Castellani LW, Kostem E, Furlotte N, Drake TA, Eskin E, Lusis AJ. A high-resolution association mapping panel for the dissection of complex traits in mice. Genome Res 20: 281–290, 2010. doi: 10.1101/gr.099234.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berthiaume Y, Lesur O, Dagenais A. Treatment of adult respiratory distress syndrome: plea for rescue therapy of the alveolar epithelium. Thorax 54: 150–160, 1999. doi: 10.1136/thx.54.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bidou L, Crisanti P, Blancher C, Pessac B. A novel cDNA corresponding to transcripts expressed in retina post-mitotic neurons. Mech Dev 43: 159–173, 1993. doi: 10.1016/0925-4773(93)90033-T. [DOI] [PubMed] [Google Scholar]
- 14.Birken GA, Fabri PJ, Carey LC. Acute ammonia intoxication complicating multiple trauma. J Trauma 21: 820–822, 1981. doi: 10.1097/00005373-198109000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Bloom GR, Suhail F, Hopkins-Price P, Sood A. Acute anhydrous ammonia injury from accidents during illicit methamphetamine production. Burns 34: 713–718, 2008. doi: 10.1016/j.burns.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Bogue MA, Philip VM, Walton DO, Grubb SC, Dunn MH, Kolishovski G, Emerson J, Mukherjee G, Stearns T, He H, Sinha V, Kadakkuzha B, Kunde-Ramamoorthy G, Chesler EJ. Mouse phenome database: a data repository and analysis suite for curated primary mouse phenotype data. Nucleic Acids Res 48: D716–D723, 2020. doi: 10.1093/nar/gkz1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brueggemann LI, Haick JM, Neuburg S, Tate S, Randhawa D, Cribbs LL, Byron KL. KCNQ (Kv7) potassium channel activators as bronchodilators: combination with a β2-adrenergic agonist enhances relaxation of rat airways. Am J Physiol Lung Cell Mol Physiol 306: L476–L486, 2014. doi: 10.1152/ajplung.00253.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns TR, Mace ML, Greenberg SD, Jachimczyk JA. Ultrastructure of acute ammonia toxicity in the human lung. Am J Forensic Med Pathol 6: 204–210, 1985. doi: 10.1097/00000433-198509000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Cai D, Clayton LK, Smolyar A, Lerner A. AND-34, a novel p130Cas-binding thymic stromal cell protein regulated by adhesion and inflammatory cytokines. J Immunol 163: 2104–2112, 1999. [PubMed] [Google Scholar]
- 20.Cai WK, Hu J, Li T, Meng JR, Ma X, Yin SJ, Zhao CH, He GH, Xu GL. Activation of histamine H4 receptors decreases epithelial-to-mesenchymal transition progress by inhibiting transforming growth factor-β1 signalling pathway in non-small cell lung cancer. Eur J Cancer 50: 1195–1206, 2014. doi: 10.1016/j.ejca.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Çalikoglu M, Tamer L, Sucu N, Coskun B, Ercan B, Gul A, Calikoglu I, Kanik A. The effects of caffeic acid phenethyl ester on tissue damage in lung after hindlimb ischemia-reperfusion. Pharmacol Res 48: 397–403, 2003. doi: 10.1016/S1043-6618(03)00156-7. [DOI] [PubMed] [Google Scholar]
- 22.Caplin M Ammonia-gas poisoning: forty-seven cases in London shelter. Lancet 238: 95–96, 1941. doi: 10.1016/S0140-6736(00)72016-2. [DOI] [Google Scholar]
- 23.Carles M, Wagener BM, Lafargue M, Roux J, Iles K, Liu D, Rodriguez CA, Anjum N, Zmijewski J, Ricci JE, Pittet JF. Heat-shock response increases lung injury caused by Pseudomonas aeruginosa via an interleukin-10-dependent mechanism in mice. Anesthesiology 120: 1450–1462, 2014. doi: 10.1097/ALN.0000000000000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. The National Institute for Occupational Safety and Health (NIOSH) . Ammonia: Immediately Dangerous to Life or Health Concentrations (IDLH), May 1994 (Online). https://www.cdc.gov/niosh/idlh/7664417.html [25 Aug 2020].
- 25.Chao TC, Lo DS. Ammonia gassing deaths—a report on two cases. Singapore Med J 37: 147–149, 1996. [PubMed] [Google Scholar]
- 26.Chapman ID, Kristersson A, Mathelin G, Schaeublin E, Mazzoni L, Boubekeur K, Murphy N, Morley J. Effects of a potassium channel opener (SDZ PCO 400) on guinea-pig and human pulmonary airways. Br J Pharmacol 106: 423–429, 1992. doi: 10.1111/j.1476-5381.1992.tb14350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee A, Dimitropoulou C, Drakopanayiotakis F, Antonova G, Snead C, Cannon J, Venema RC, Catravas JD. Heat shock protein 90 inhibitors prolong survival, attenuate inflammation, and reduce lung injury in murine sepsis. Am J Respir Crit Care Med 176: 667–675, 2007. doi: 10.1164/rccm.200702-291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatterjee A, Snead C, Yetik-Anacak G, Antonova G, Zeng J, Catravas JD. Heat shock protein 90 inhibitors attenuate LPS-induced endothelial hyperpermeability. Am J Physiol Lung Cell Mol Physiol 294: L755–L763, 2008. doi: 10.1152/ajplung.00350.2007. [DOI] [PubMed] [Google Scholar]
- 29.Chen B, Ye T, Shao Y, Zhang J, Zhong Q, Hu X, Zhang W, Yu B. Association between copy-number variations of the human histamine H4 receptor gene and atopic dermatitis in a Chinese population. Clin Exp Dermatol 38: 295–300, 2013. doi: 10.1111/ced.12117. [DOI] [PubMed] [Google Scholar]
- 30.Chen C, Fang X, Wang Y, Li Y, Wang D, Zhao X, Bai C, Wang X. Preventive and therapeutic effects of phosphoinositide 3-kinase inhibitors on acute lung injury. Chest 140: 391–400, 2011. doi: 10.1378/chest.10-3060. [DOI] [PubMed] [Google Scholar]
- 31.Chen MF, Keng PC, Lin PY, Yang CT, Liao SK, Chen WC. Caffeic acid phenethyl ester decreases acute pneumonitis after irradiation in vitro and in vivo. BMC Cancer 5: 158, 2005. doi: 10.1186/1471-2407-5-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho HY, Miller-DeGraff L, Blankenship-Paris T, Wang X, Bell DA, Lih F, Deterding L, Panduri V, Morgan DL, Yamamoto M, Reddy AJ, Talalay P, Kleeberger SR. Sulforaphane enriched transcriptome of lung mitochondrial energy metabolism and provided pulmonary injury protection via Nrf2 in mice. Toxicol Appl Pharmacol 364: 29–44, 2019. doi: 10.1016/j.taap.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Close LG, Catlin FI, Cohn AM. Acute and chronic effects of ammonia burns on the respiratory tract. Arch Otolaryngol 106: 151–158, 1980. doi: 10.1001/archotol.1980.00790270015004. [DOI] [PubMed] [Google Scholar]
- 34.Cowden JM, Yu F, Banie H, Farahani M, Ling P, Nguyen S, Riley JP, Zhang M, Zhu J, Dunford PJ, Thurmond RL. The histamine H4 receptor mediates inflammation and Th17 responses in preclinical models of arthritis. Ann Rheum Dis 73: 600–608, 2014. doi: 10.1136/annrheumdis-2013-203832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cowden JM, Yu F, Challapalli M, Huang JF, Kim S, Fung-Leung WP, Ma JY, Riley JP, Zhang M, Dunford PJ, Thurmond RL. Antagonism of the histamine H4 receptor reduces LPS-induced TNF production in vivo. Inflamm Res 62: 599–607, 2013. doi: 10.1007/s00011-013-0612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davidson K, Ripple S. Ammonia In: Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 6. Washington, DC: National Academies Press, 2008, chapt 2. [PubMed] [Google Scholar]
- 37.de la Hoz RE, Schlueter DP, Rom WN. Chronic lung disease secondary to ammonia inhalation injury: a report on three cases. Am J Ind Med 29: 209–214, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 38.Derobert L, Proteau J, Caroff J. Etude anatomique de quatre cas d’intoxication aigue par inhalation de gaz ammoniac. [Anatomical study of 4 cases of acute poisoning by ammonia gas inhalation]. Ann Med Leg Criminol Police Sci Toxicol 44: 362–366, 1964. [PubMed] [Google Scholar]
- 39.Dhainaut JF, Charpentier J, Chiche JD. Transforming growth factor-beta: a mediator of cell regulation in acute respiratory distress syndrome. Crit Care Med 31, Suppl: S258–S264, 2003. doi: 10.1097/01.CCM.0000057901.92381.75. [DOI] [PubMed] [Google Scholar]
- 40.Duboit S, Yang YH, Callow MJ, Speed TP. Statistical methods for identifying differentially expressed genes in replicated cDNA microarray experiments. Statistica Sinica 12: 111–139, 2002. [Google Scholar]
- 41.Fabisiak JP, Medvedovic M, Alexander DC, McDunn JE, Concel VJ, Bein K, Jang ASB, Berndt A, Vuga LJ, Brant KA, Pope-Varsalona H, Dopico RA Jr, Ganguly K, Upadhyay S, Li Q, Hu Z, Kaminski N, Leikauf GD. Integrative metabolome and transcriptome profiling reveals discordant energetic stress between mouse strains with differential sensitivity to acrolein-induced acute lung injury. Mol Nutr Food Res 55: 1423–1434, 2011. doi: 10.1002/mnfr.201100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fidan H, Sahin O, Yavuz Y, Kilbas A, Cetinkaya Z, Ela Y, Ozen OA, Altuntas I. Caffeic acid phenethyl ester reduces mortality and sepsis-induced lung injury in rats. Crit Care Med 35: 2822–2829, 2007. doi: 10.1097/01.CCM.0000295588.86982.7D. [DOI] [PubMed] [Google Scholar]
- 43.Flury KE, Dines DE, Rodarte JR, Rodgers R. Airway obstruction due to inhalation of ammonia. Mayo Clin Proc 58: 389–393, 1983. [PubMed] [Google Scholar]
- 44.Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G, Chuckowree IS, Clarke PA, Depledge P, Eccles SA, Friedman LS, Hayes A, Hancox TC, Kugendradas A, Lensun L, Moore P, Olivero AG, Pang J, Patel S, Pergl-Wilson GH, Raynaud FI, Robson A, Saghir N, Salphati L, Sohal S, Ultsch MH, Valenti M, Wallweber HJ, Wan NC, Wiesmann C, Workman P, Zhyvoloup A, Zvelebil MJ, Shuttleworth SJ. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem 51: 5522–5532, 2008. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 45.Frazer KA, Eskin E, Kang HM, Bogue MA, Hinds DA, Beilharz EJ, Gupta RV, Montgomery J, Morenzoni MM, Nilsen GB, Pethiyagoda CL, Stuve LL, Johnson FM, Daly MJ, Wade CM, Cox DR. A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature 448: 1050–1053, 2007. doi: 10.1038/nature06067. [DOI] [PubMed] [Google Scholar]
- 46.Geiser T Mechanisms of alveolar epithelial repair in acute lung injury—a translational approach. Swiss Med Wkly 133: 586–590, 2003. [DOI] [PubMed] [Google Scholar]
- 47.George A, Bang RL, Lari AR, Gang RK, Kanjoor JR. Liquid ammonia injury. Burns 26: 409–413, 2000. doi: 10.1016/S0305-4179(99)00141-2. [DOI] [PubMed] [Google Scholar]
- 48.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 277: 225–228, 1997. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 49.Godzich M, Hodnett M, Frank JA, Su G, Pespeni M, Angel A, Howard MB, Matthay MA, Pittet JF, Godzich M, Hodnett M, Frank JA, Su G, Pespeni M, Angel A, Howard MB, Matthay MA, Pittet JF. Activation of the stress protein response prevents the development of pulmonary edema by inhibiting VEGF cell signaling in a model of lung ischemia-reperfusion injury in rats. FASEB J 20: 1519–1521, 2006. doi: 10.1096/fj.05-4708fje. [DOI] [PubMed] [Google Scholar]
- 50.Greenwood IA, Yeung SY, Hettiarachi S, Andersson M, Baines DL. KCNQ-encoded channels regulate Na+ transport across H441 lung epithelial cells. Pflugers Arch 457: 785–794, 2009. doi: 10.1007/s00424-008-0557-7. [DOI] [PubMed] [Google Scholar]
- 51.Gu J, Mao XH, Yang XZ, Ao HF, Zhang Z, Li Y. Histamine H4 receptor gene polymorphisms: a potential predictor of oral H1 antihistamine efficacy for allergic rhinitis. Int Forum Allergy Rhinol 7: 268–275, 2017. doi: 10.1002/alr.21870. [DOI] [PubMed] [Google Scholar]
- 52.Gueye L, Samb A, Ciss M, Ndoye O, Mbengue-Gaye A, Cisse F. [Ammonia-gas poisoning: respiratory troubles evaluated by functional exploration]. Dakar Med 46: 8–11, 2001. [PubMed] [Google Scholar]
- 53.Gurel A, Armutcu F, Hosnuter M, Unalacak M, Kargi E, Altinyazar C. Caffeic acid phenethyl ester improves oxidative organ damage in rat model of thermal trauma. Physiol Res 53: 675–682, 2004. [PubMed] [Google Scholar]
- 54.Haick JM, Brueggemann LI, Cribbs LL, Denning MF, Schwartz J, Byron KL. PKC-dependent regulation of Kv7.5 channels by the bronchoconstrictor histamine in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 312: L822–L834, 2017. doi: 10.1152/ajplung.00567.2016. [DOI] [PubMed] [Google Scholar]
- 55.Han S, Mistry A, Chang JS, Cunningham D, Griffor M, Bonnette PC, Wang H, Chrunyk BA, Aspnes GE, Walker DP, Brosius AD, Buckbinder L. Structural characterization of proline-rich tyrosine kinase 2 (PYK2) reveals a unique (DFG-out) conformation and enables inhibitor design. J Biol Chem 284: 13193–13201, 2009. doi: 10.1074/jbc.M809038200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hartwig C, Munder A, Glage S, Wedekind D, Schenk H, Seifert R, Neumann D. The histamine H4 -receptor (H4 R) regulates eosinophilic inflammation in ovalbumin-induced experimental allergic asthma in mice. Eur J Immunol 45: 1129–1140, 2015. doi: 10.1002/eji.201445179. [DOI] [PubMed] [Google Scholar]
- 57.Hatton DV, Leach CS, Beaudet AL, Dillman RO, Di Ferrante N. Collagen breakdown and ammonia inhalation. Arch Environ Health 34: 83–87, 1979. doi: 10.1080/00039896.1979.10667373. [DOI] [PubMed] [Google Scholar]
- 58.Henderson Y, Haggard HW. Noxious Gases (2nd ed.). New York: Reinhold Publishing Corp, 1943, p. 126. [Google Scholar]
- 59.Hernández-Beeftink T, Guillen-Guio B, Villar J, Flores C. Genomics and the acute respiratory distress syndrome: Current and future directions. Int J Mol Sci 20: 4004, 2019. doi: 10.3390/ijms20164004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoeffler HB, Schweppe HI, Greenberg SD. Bronchiectasis following pulmonary ammonia burn. Arch Pathol Lab Med 106: 686–687, 1982. [PubMed] [Google Scholar]
- 61.Hu M, Luo Q, Alitongbieke G, Chong S, Xu C, Xie L, Chen X, Zhang D, Zhou Y, Wang Z, Ye X, Cai L, Zhang F, Chen H, Jiang F, Fang H, Yang S, Liu J, Diaz-Meco MT, Su Y, Zhou H, Moscat J, Lin X, Zhang XK. Celastrol-induced Nur77 interaction with TRAF2 alleviates inflammation by promoting mitochondrial ubiquitination and autophagy. Mol Cell 66: 141–153.e6, 2017. doi: 10.1016/j.molcel.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kang HM, Zaitlen NA, Wade CM, Kirby A, Heckerman D, Daly MJ, Eskin E. Efficient control of population structure in model organism association mapping. Genetics 178: 1709–1723, 2008. doi: 10.1534/genetics.107.080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaplan E Targets for Terrorists: Chemical Facilities (Online). https://www.cfr.org/backgrounder/targets-terrorists-chemical-facilities [25 August 2020].
- 64.Karaboğa İ Caffeic acid phenethyl ester ameliorates pulmonary inflammation and apoptosis reducing Nf-κβ activation in blunt pulmonary contusion model. Ulus Travma Acil Cerrahi Derg 25: 433–439, 2019. doi: 10.5505/tjtes.2018.51694. [DOI] [PubMed] [Google Scholar]
- 65.Kass I, Zamel N, Dobry CA, Holzer M. Bronchiectasis following ammonia burns of the respiratory tract. A review of two cases. Chest 62: 282–285, 1972. doi: 10.1378/chest.62.3.282. [DOI] [PubMed] [Google Scholar]
- 66.Katayama M, Ishizaka A, Sakamoto M, Fujishima S, Sekiguchi K, Asano K, Betsuyaku T, Kotani T, Ware LB, Matthay MA, Hashimoto S. Laminin gamma2 fragments are increased in the circulation of patients with early phase acute lung injury. Intensive Care Med 36: 479–486, 2010. doi: 10.1007/s00134-009-1719-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, Heger A, Agam A, Slater G, Goodson M, Furlotte NA, Eskin E, Nellåker C, Whitley H, Cleak J, Janowitz D, Hernandez-Pliego P, Edwards A, Belgard TG, Oliver PL, McIntyre RE, Bhomra A, Nicod J, Gan X, Yuan W, van der Weyden L, Steward CA, Bala S, Stalker J, Mott R, Durbin R, Jackson IJ, Czechanski A, Guerra-Assunção JA, Donahue LR, Reinholdt LG, Payseur BA, Ponting CP, Birney E, Flint J, Adams DJ. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 477: 289–294, 2011. doi: 10.1038/nature10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kerstein MD, Schaffzin DM, Hughes WB, Hensell DO. Acute management of exposure to liquid ammonia. Mil Med 166: 913–914, 2001. doi: 10.1093/milmed/166.10.913. [DOI] [PubMed] [Google Scholar]
- 69.Kiang JG, Bowman PD, Lu X, Li Y, Ding XZ, Zhao B, Juang YT, Atkins JL, Tsokos GC. Geldanamycin prevents hemorrhage-induced ATP loss by overexpressing inducible HSP70 and activating pyruvate dehydrogenase. Am J Physiol Gastrointest Liver Physiol 291: G117–G127, 2006. doi: 10.1152/ajpgi.00397.2005. [DOI] [PubMed] [Google Scholar]
- 70.Kirby A, Kang HM, Wade CM, Cotsapas C, Kostem E, Han B, Furlotte N, Kang EY, Rivas M, Bogue MA, Frazer KA, Johnson FM, Beilharz EJ, Cox DR, Eskin E, Daly MJ. Fine mapping in 94 inbred mouse strains using a high-density haplotype resource. Genetics 185: 1081–1095, 2010. doi: 10.1534/genetics.110.115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kleeberger SR, Levitt RC, Zhang LY, Longphre M, Harkema J, Jedlicka A, Eleff SM, DiSilvestre D, Holroyd KJ. Linkage analysis of susceptibility to ozone-induced lung inflammation in inbred mice. Nat Genet 17: 475–478, 1997. doi: 10.1038/ng1297-475. [DOI] [PubMed] [Google Scholar]
- 72.Knight SD, Adams ND, Burgess JL, Chaudhari AM, Darcy MG, Donatelli CA, Luengo JI, Newlander KA, Parrish CA, Ridgers LH, Sarpong MA, Schmidt SJ, Van Aller GS, Carson JD, Diamond MA, Elkins PA, Gardiner CM, Garver E, Gilbert SA, Gontarek RR, Jackson JR, Kershner KL, Luo L, Raha K, Sherk CS, Sung CM, Sutton D, Tummino PJ, Wegrzyn RJ, Auger KR, Dhanak D. Discovery of GSK2126458, a highly potent inhibitor of PI3K and the mammalian target of rapamycin. ACS Med Chem Lett 1: 39–43, 2010. doi: 10.1021/mL900028r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lalić H, Djindjić-Pavicić M, Kukuljan M. Ammonia intoxication on workplace—case report and a review of literature. Coll Antropol 33: 945–949, 2009. [PubMed] [Google Scholar]
- 74.Lanier LL NKG2D receptor and its ligands in host defense. Cancer Immunol Res 3: 575–582, 2015. doi: 10.1158/2326-6066.CIR-15-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Latenser BA, Lucktong TA. Anhydrous ammonia burns: case presentation and literature review. J Burn Care Rehabil 21: 40–42, 2000. doi: 10.1097/00004630-200021010-00008. [DOI] [PubMed] [Google Scholar]
- 76.Leduc D, Gris P, Lheureux P, Gevenois PA, De Vuyst P, Yernault JC. Acute and long term respiratory damage following inhalation of ammonia. Thorax 47: 755–757, 1992. doi: 10.1136/thx.47.9.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lehman A, Thouta S, Mancini GMS, Naidu S, van Slegtenhorst M, McWalter K, Person R, Mwenifumbo J, Salvarinova R, Guella I, McKenzie MB, Datta A, Connolly MB, Kalkhoran SM, Poburko D, Friedman JM, Farrer MJ, Demos M, Desai S, Claydon T, Adam S, du Souich C, Elliott AM, Lehman A, Mwenifumbo J, Nelson TN, van Karnebeek C, Friedman JM, Adam S, Boelman C, Bolbocean C, Buerki SE, Candido T, Eydoux P, Evans DM, Gibson W, Horvath G, Huh L, Nelson TN, Sinclair G, Tarling T, Toyota EB, Townsend KN, Van Allen MI, van Karnebeek C, Vercauteren S; CAUSES Study; EPGEN Study . Loss-of-function and gain-of-function mutations in KCNQ5 cause intellectual disability or epileptic encephalopathy. Am J Hum Genet 101: 65–74, 2017. doi: 10.1016/j.ajhg.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leikauf GD, Concel VJ, Bein K, Liu P, Berndt A, Martin TM, Ganguly K, Jang AS, Brant KA, Dopico RA Jr, Upadhyay S, Cario C, Di YP, Vuga LJ, Kostem E, Eskin E, You M, Kaminski N, Prows DR, Knoell DL, Fabisiak JP. Functional genomic assessment of phosgene-induced acute lung injury in mice. Am J Respir Cell Mol Biol 49: 368–383, 2013. doi: 10.1165/rcmb.2012-0337OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leikauf GD, Concel VJ, Liu P, Bein K, Berndt A, Ganguly K, Jang AS, Brant KA, Dietsch M, Pope-Varsalona H, Dopico RA Jr, Di YP, Li Q, Vuga LJ, Medvedovic M, Kaminski N, You M, Prows DR. Haplotype association mapping of acute lung injury in mice implicates activin a receptor, type 1. Am J Respir Crit Care Med 183: 1499–1509, 2011. doi: 10.1164/rccm.201006-0912OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leikauf GD, Pope-Varsalona H, Concel VJ, Liu P, Bein K, Berndt A, Martin TM, Ganguly K, Jang AS, Brant KA, Dopico RA Jr, Upadhyay S, Di YP, Li Q, Hu Z, Vuga LJ, Medvedovic M, Kaminski N, You M, Alexander DC, McDunn JE, Prows DR, Knoell DL, Fabisiak JP. Integrative assessment of chlorine-induced acute lung injury in mice. Am J Respir Cell Mol Biol 47: 234–244, 2012. doi: 10.1165/rcmb.2012-0026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leon A, Omri B, Gely A, Klein C, Crisanti P. QN1/KIAA1009: a new essential protein for chromosome segregation and mitotic spindle assembly. Oncogene 25: 1887–1895, 2006. doi: 10.1038/sj.onc.1209215. [DOI] [PubMed] [Google Scholar]
- 82.Leung CM, Foo CL. Mass ammonia inhalational burns—experience in the management of 12 patients. Ann Acad Med Singap 21: 624–629, 1992. [PubMed] [Google Scholar]
- 83.Levy DM, Divertie MB, Litzow TJ, Henderson JW. Ammonia burns of the face and respiratory tract. JAMA 190: 873–876, 1964. doi: 10.1001/jama.1964.03070230009002. [DOI] [PubMed] [Google Scholar]
- 84.Lieberman P The basics of histamine biology. Ann Allergy Asthma Immunol 106, Suppl: S2–S5, 2011. doi: 10.1016/j.anai.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 85.Liu J, Liu J, Wang H, Bai M. Protective effect of celastrol for burn-induced acute lung injury in rats. Int J Clin Exp Pathol 12: 576–583, 2019. [PMC free article] [PubMed] [Google Scholar]
- 86.Liu P, Wang Y, Vikis H, Maciag A, Wang D, Lu Y, Liu Y, You M. Candidate lung tumor susceptibility genes identified through whole-genome association analyses in inbred mice. Nat Genet 38: 888–895, 2006. doi: 10.1038/ng1849. [DOI] [PubMed] [Google Scholar]
- 87.Liu Q, Wang J, Kang SA, Thoreen CC, Hur W, Ahmed T, Sabatini DM, Gray NS. Discovery of 9-(6-aminopyridin-3-yl)-1-(3-(trifluoromethyl)phenyl)benzo[h][1,6]naphthyridin-2(1H)-one (Torin2) as a potent, selective, and orally available mammalian target of rapamycin (mTOR) inhibitor for treatment of cancer. J Med Chem 54: 1473–1480, 2011. doi: 10.1021/jm101520v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Q, Xu C, Kirubakaran S, Zhang X, Hur W, Liu Y, Kwiatkowski NP, Wang J, Westover KD, Gao P, Ercan D, Niepel M, Thoreen CC, Kang SA, Patricelli MP, Wang Y, Tupper T, Altabef A, Kawamura H, Held KD, Chou DM, Elledge SJ, Janne PA, Wong KK, Sabatini DM, Gray NS. Characterization of Torin2, an ATP-competitive inhibitor of mTOR, ATM, and ATR. Cancer Res 73: 2574–2586, 2013. doi: 10.1158/0008-5472.CAN-12-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lukey PT, Harrison SA, Yang S, Man Y, Holman BF, Rashidnasab A, Azzopardi G, Grayer M, Simpson JK, Bareille P, Paul L, Woodcock HV, Toshner R, Saunders P, Molyneaux PL, Thielemans K, Wilson FJ, Mercer PF, Chambers RC, Groves AM, Fahy WA, Marshall RP, Maher TM. A randomised, placebo-controlled study of omipalisib (PI3K/mTOR) in idiopathic pulmonary fibrosis. Eur Respir J 53: 1801992, 2019. doi: 10.1183/13993003.01992-2018. [DOI] [PubMed] [Google Scholar]
- 90.Lv Y, Jiang H, Li S, Han B, Liu Y, Yang D, Li J, Yang Q, Wu P, Zhang Z. Sulforaphane prevents chromium-induced lung injury in rats via activation of the Akt/GSK-3β/Fyn pathway. Environ Pollut 259: 113812, 2020. doi: 10.1016/j.envpol.2019.113812. [DOI] [PubMed] [Google Scholar]
- 91.Lynn H, Sun X, Casanova N, Gonzales-Garay M, Bime C, Garcia JGN. Genomic and genetic approaches to deciphering acute respiratory distress syndrome risk and mortality. Antioxid Redox Signal 31: 1027–1052, 2019. doi: 10.1089/ars.2018.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maltau J, Samuelson G, Stakeberg H, Svedberg S, Ostergaard E. [Ammonia poisoning—from an ice rink accident]. Lakartidningen 76: 723–724, 1979. [PubMed] [Google Scholar]
- 93.Manville RW, van der Horst J, Redford KE, Katz BB, Jepps TA, Abbott GW. KCNQ5 activation is a unifying molecular mechanism shared by genetically and culturally diverse botanical hypotensive folk medicines. Proc Natl Acad Sci USA 116: 21236–21245, 2019. doi: 10.1073/pnas.1907511116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mao WJ, Xia W, Chen JY. [Lung transplantation for bronchiolitis obliterans after occupational ammonia poisoning: report of one case]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 30: 703–704, 2012. [PubMed] [Google Scholar]
- 95.Matalon S, Bartoszewski R, Collawn JF. Role of epithelial sodium channels in the regulation of lung fluid homeostasis. Am J Physiol Lung Cell Mol Physiol 309: L1229–L1238, 2015. doi: 10.1152/ajplung.00319.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis 142: 1250–1257, 1990. doi: 10.1164/ajrccm/142.6_Pt_1.1250. [DOI] [PubMed] [Google Scholar]
- 97.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, Herridge M, Randolph AG, Calfee CS. Acute respiratory distress syndrome. Nat Rev Dis Primers 5: 18, 2019. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.The Merriam-Webster New Book of Word Histories. Springfield, MA: Merriam Webster Inc, 1991, p. 12–13. [Google Scholar]
- 99.Michaels RA Emergency planning and the acute toxic potency of inhaled ammonia. Environ Health Perspect 107: 617–627, 1999. doi: 10.1289/ehp.99107617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miner JH Laminins and their roles in mammals. Microsc Res Tech 71: 349–356, 2008. doi: 10.1002/jemt.20563. [DOI] [PubMed] [Google Scholar]
- 101.Mommert S, Gschwandtner M, Gutzmer R, Werfel T. The role of the histamine H4 receptor in atopic dermatitis. Curr Allergy Asthma Rep 11: 21–28, 2011. doi: 10.1007/s11882-010-0162-7. [DOI] [PubMed] [Google Scholar]
- 102.Mommert S, Gschwandtner M, Koether B, Gutzmer R, Werfel T. Human memory Th17 cells express a functional histamine H4 receptor. Am J Pathol 180: 177–185, 2012. doi: 10.1016/j.ajpath.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 103.Mondéjar-Parreño G, Moral-Sanz J, Barreira B, De la Cruz A, Gonzalez T, Callejo M, Esquivel-Ruiz S, Morales-Cano D, Moreno L, Valenzuela C, Perez-Vizcaino F, Cogolludo A. Activation of Kv 7 channels as a novel mechanism for NO/cGMP-induced pulmonary vasodilation. Br J Pharmacol 176: 2131–2145, 2019. doi: 10.1111/bph.14662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Montague TJ, Macneil AR. Mass ammonia inhalation. Chest 77: 496–498, 1980. doi: 10.1378/chest.77.4.496. [DOI] [PubMed] [Google Scholar]
- 105.Morales-Nebreda LI, Rogel MR, Eisenberg JL, Hamill KJ, Soberanes S, Nigdelioglu R, Chi M, Cho T, Radigan KA, Ridge KM, Misharin AV, Woychek A, Hopkinson S, Perlman H, Mutlu GM, Pardo A, Selman M, Jones JC, Budinger GR. Lung-specific loss of α3 laminin worsens bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 52: 503–512, 2015. doi: 10.1165/rcmb.2014-0057OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, , et al. ; Mouse Genome Sequencing Consortium . Initial sequencing and comparative analysis of the mouse genome. Nature 420: 520–562, 2002. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 107.Mulder JS, Van Der Zalm HO. [Fatal case of ammonia poisoning]. Tijdschr Soc Geneeskd 45: 458–460, 1967. [Google Scholar]
- 108.Namkung W, Song Y, Mills AD, Padmawar P, Finkbeiner WE, Verkman AS. In situ measurement of airway surface liquid [K+] using a ratioable K+-sensitive fluorescent dye. J Biol Chem 284: 15916–15926, 2009. doi: 10.1074/jbc.M808021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Néron B, Marx M, Crisanti P. Role of QN1 protein in cell proliferation arrest and differentiation during the neuroretina development. Mech Dev 102: 107–117, 2001. doi: 10.1016/S0925-4773(01)00297-0. [DOI] [PubMed] [Google Scholar]
- 110.Newman LS, Bird KG. Toxic inhalational lung injury In: Clinical Respiratory Medicine (Silvestri GA, Agustí A, editors). (4th ed.). Philadelphia, PA: W.B. Saunders, 2012, chapt 56, p. 682–689. [Google Scholar]
- 111.Nguyen NM, Senior RM. Laminin isoforms and lung development: all isoforms are not equal. Dev Biol 294: 271–279, 2006. doi: 10.1016/j.ydbio.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 112.Nichols JL, Gladwell W, Verhein KC, Cho HY, Wess J, Suzuki O, Wiltshire T, Kleeberger SR. Genome-wide association mapping of acute lung injury in neonatal inbred mice. FASEB J 28: 2538–2550, 2014. doi: 10.1096/fj.13-247221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.O’Kane GJ Inhalation of ammonia vapour. A report on the management of eight patients during the acute stages. Anaesthesia 38: 1208–1213, 1983. doi: 10.1111/j.1365-2044.1983.tb12527.x. [DOI] [PubMed] [Google Scholar]
- 114.O’Mahony L, Akdis M, Akdis CA. Regulation of the immune response and inflammation by histamine and histamine receptors. J Allergy Clin Immunol 128: 1153–1162, 2011. doi: 10.1016/j.jaci.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 115.Oh MJ, van Agthoven T, Choi JE, Jeong YJ, Chung YH, Kim CM, Jhun BH. BCAR3 regulates EGF-induced DNA synthesis in normal human breast MCF-12A cells. Biochem Biophys Res Commun 375: 430–434, 2008. doi: 10.1016/j.bbrc.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 116.Oh MJ, Yi SJ, Kim HS, Kim JH, Jeong YH, van Agthoven T, Jhun BH. Functional roles of BCAR3 in the signaling pathways of insulin leading to DNA synthesis, membrane ruffling and GLUT4 translocation. Biochem Biophys Res Commun 441: 911–916, 2013. doi: 10.1016/j.bbrc.2013.10.161. [DOI] [PubMed] [Google Scholar]
- 117.Ortiz-Pujols S, Jones SW, Short KA, Morrell MR, Bermudez CA, Tilley SL, Cairns BA. Management and sequelae of a 41-year-old Jehovah’s Witness with severe anhydrous ammonia inhalation injury. J Burn Care Res 35: e180–e183, 2014. doi: 10.1097/BCR.0b013e318299d4d7. [DOI] [PubMed] [Google Scholar]
- 118.Ortner E, Moelling K. Heteromeric complex formation of ASK2 and ASK1 regulates stress-induced signaling. Biochem Biophys Res Commun 362: 454–459, 2007. doi: 10.1016/j.bbrc.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 119.Pałaszewska-Tkacz A, Czerczak S, Konieczko K. Chemical incidents resulted in hazardous substances releases in the context of human health hazards. Int J Occup Med Environ Health 30: 95–110, 2017. doi: 10.13075/ijomeh.1896.00734. [DOI] [PubMed] [Google Scholar]
- 120.Patel V, Dial K, Wu J, Gauthier AG, Wu W, Lin M, Espey MG, Thomas DD, Ashby CR Jr, Mantell LL. Dietary antioxidants significantly attenuate hyperoxia-Induced acute inflammatory lung injury by enhancing macrophage function via reducing the accumulation of airway HMGB1. Int J Mol Sci 21: 977, 2020. doi: 10.3390/ijms21030977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pattabathula V, Richardson J. Introduction to Ammonia Production. CEP: Chemical Engineering Progress. New York: American Institute of Chemical Engineers, 2016, p. 69–75. [Google Scholar]
- 122.Perry GF Jr A 23-year-old maintenance employee in a fertilizer manufacturing plant was exposed to a high concentration of ammonia vapor after a pipe ruptured. J Occup Environ Med 37: 1187–1188, 1995. doi: 10.1097/00043764-199510000-00001. [DOI] [PubMed] [Google Scholar]
- 123.Petkov PM, Ding Y, Cassell MA, Zhang W, Wagner G, Sargent EE, Asquith S, Crew V, Johnson KA, Robinson P, Scott VE, Wiles MV. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res 14: 1806–1811, 2004. doi: 10.1101/gr.2825804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pierce RA, Griffin GL, Mudd MS, Moxley MA, Longmore WJ, Sanes JR, Miner JH, Senior RM. Expression of laminin alpha3, alpha4, and alpha5 chains by alveolar epithelial cells and fibroblasts. Am J Respir Cell Mol Biol 19: 237–244, 1998. doi: 10.1165/ajrcmb.19.2.3087. [DOI] [PubMed] [Google Scholar]
- 125.Pirjavec A, Kovic I, Lulic I, Zupan Z. Massive anhydrous ammonia injury leading to lung transplantation. J Trauma 67: E93–E97, 2009. doi: 10.1097/TA.0b013e31817fd93f. [DOI] [PubMed] [Google Scholar]
- 126.Pittet JF, Lu LN, Geiser T, Lee H, Matthay MA, Welch WJ. Stress preconditioning attenuates oxidative injury to the alveolar epithelium of the lung following haemorrhage in rats. J Physiol 538: 583–597, 2002. doi: 10.1113/jphysiol.2001.013102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Place DE, Samir P, Karki R, Briard B, Vogel P, Kanneganti TD. ASK family kinases are required for optimal NLRP3 inflammasome priming. Am J Pathol 188: 1021–1030, 2018. doi: 10.1016/j.ajpath.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pletcher MT, McClurg P, Batalov S, Su AI, Barnes SW, Lagler E, Korstanje R, Wang X, Nusskern D, Bogue MA, Mural RJ, Paigen B, Wiltshire T. Use of a dense single nucleotide polymorphism map for in silico mapping in the mouse. PLoS Biol 2: e393, 2004. doi: 10.1371/journal.pbio.0020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Price SK, Hughes JE, Morrison SC, Potgieter PD. Fatal ammonia inhalation. A case report with autopsy findings. S Afr Med J 64: 952–955, 1983. [PubMed] [Google Scholar]
- 130.Prows DR, Shertzer HG, Daly MJ, Sidman CL, Leikauf GD. Genetic analysis of ozone-induced acute lung injury in sensitive and resistant strains of mice. Nat Genet 17: 471–474, 1997. doi: 10.1038/ng1297-471. [DOI] [PubMed] [Google Scholar]
- 131.Qi T, Xu F, Yan X, Li S, Li H. Sulforaphane exerts anti-inflammatory effects against lipopolysaccharide-induced acute lung injury in mice through the Nrf2/ARE pathway. Int J Mol Med 37: 182–188, 2016. doi: 10.3892/ijmm.2015.2396. [DOI] [PubMed] [Google Scholar]
- 132.Rau CD, Parks B, Wang Y, Eskin E, Simecek P, Churchill GA, Lusis AJ. High-density genotypes of inbred mouse strains: improved power and precision of association mapping. G3 (Bethesda) 5: 2021–2026, 2015. doi: 10.1534/g3.115.020784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ren R, Mao Y, Ruan Z, Wang Y, Zhang Y, Du J, Yu W. Celastrol attenuates ventilator induced lung injury in mouse through inhibition of MAPK pathway. Int J Clin Exp Pathol 10: 9302–9309, 2017. [PMC free article] [PubMed] [Google Scholar]
- 134.Rosti G, Tassano E, Bossi S, Divizia MT, Ronchetto P, Servetti M, Lerone M, Pisciotta L, Mancardi MM, Veneselli E, Puliti A. Intragenic duplication of KCNQ5 gene results in aberrant splicing leading to a premature termination codon in a patient with intellectual disability. Eur J Med Genet 62: 103555, 2019. doi: 10.1016/j.ejmg.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 135.Ryan MC, Lee K, Miyashita Y, Carter WG. Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J Cell Biol 145: 1309–1324, 1999. doi: 10.1083/jcb.145.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schaper-Gerhardt K, Wohlert M, Mommert S, Kietzmann M, Werfel T, Gutzmer R. Stimulation of histamine H4 receptors increases the production of IL-9 in Th9 polarized cells. Br J Pharmacol 177: 614–622, 2020. doi: 10.1111/bph.14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Silverman L, Whittenberger JL, Muller J. Physiological response of man to ammonia in low concentrations. J Ind Hyg Toxicol 31: 74–78, 1949. [PubMed] [Google Scholar]
- 138.Simon T, Semsei AF, Ungvári I, Hadadi E, Virág V, Nagy A, Vangor MS, László V, Szalai C, Falus A. Asthma endophenotypes and polymorphisms in the histamine receptor HRH4 gene. Int Arch Allergy Immunol 159: 109–120, 2012. doi: 10.1159/000335919. [DOI] [PubMed] [Google Scholar]
- 139.Singh S, Fadnis P, Sharma BK. Two fatalities following ammonia (NH3) gassing leaked during transport. J Assoc Physicians India 37: 481–482, 1989. [PubMed] [Google Scholar]
- 140.Smyth HF Jr Hygienic standards for daily inhalation. Am Ind Hyg Assoc Q 17: 129–185, 1956. [DOI] [PubMed] [Google Scholar]
- 141.Sobonya R Fatal anhydrous ammonia inhalation. Hum Pathol 8: 293–299, 1977. doi: 10.1016/S0046-8177(77)80026-9. [DOI] [PubMed] [Google Scholar]
- 142.Taplin GV, Chopra S, Yanda RL, Elam D. Radionuclidic lung-imaging procedures in the assessment of injury due to ammonia inhalation. Chest 69: 582–586, 1976. doi: 10.1378/chest.69.5.582. [DOI] [PubMed] [Google Scholar]
- 143.Tonelli AR, Pham A. Bronchiectasis, a long-term sequela of ammonia inhalation: a case report and review of the literature. Burns 35: 451–453, 2009. doi: 10.1016/j.burns.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 144.Urich D, Eisenberg JL, Hamill KJ, Takawira D, Chiarella SE, Soberanes S, Gonzalez A, Koentgen F, Manghi T, Hopkinson SB, Misharin AV, Perlman H, Mutlu GM, Budinger GR, Jones JC. Lung-specific loss of the laminin α3 subunit confers resistance to mechanical injury. J Cell Sci 124: 2927–2937, 2011. doi: 10.1242/jcs.080911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.van Agthoven T, van Agthoven TL, Dekker A, van der Spek PJ, Vreede L, Dorssers LC. Identification of BCAR3 by a random search for genes involved in antiestrogen resistance of human breast cancer cells. EMBO J 17: 2799–2808, 1998. doi: 10.1093/emboj/17.10.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vervoort VS, Roselli S, Oshima RG, Pasquale EB. Splice variants and expression patterns of SHEP1, BCAR3 and NSP1, a gene family involved in integrin and receptor tyrosine kinase signaling. Gene 391: 161–170, 2007. doi: 10.1016/j.gene.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Waheed I, Fuller A. Anhydrous ammonia pulmonary toxicity: a significant farming hazard. Southwest Respir Critical Care Chron 5: 41–44, 2017. doi: 10.12746/swrccc.v5i19.397. [DOI] [Google Scholar]
- 148.Walton M Industrial ammonia gassing. Br J Ind Med 30: 78–86, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang C, Liu P, Luo J, Ding H, Gao Y, Sun L, Luo F, Liu X, He H. Geldanamycin reduces acute respiratory distress syndrome and promotes the survival of mice infected with the highly virulent H5N1 influenza virus. Front Cell Infect Microbiol 7: 267, 2017. doi: 10.3389/fcimb.2017.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang JR, de Villena FP, Lawson HA, Cheverud JM, Churchill GA, McMillan L. Imputation of single-nucleotide polymorphisms in inbred mice using local phylogeny. Genetics 190: 449–458, 2012. doi: 10.1534/genetics.111.132381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang WJ, Tay HG, Soni R, Perumal GS, Goll MG, Macaluso FP, Asara JM, Amack JD, Tsou MF. CEP162 is an axoneme-recognition protein promoting ciliary transition zone assembly at the cilia base. Nat Cell Biol 15: 591–601, 2013. doi: 10.1038/ncb2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang YL, Lam KK, Cheng PY, Lee YM. Celastrol prevents circulatory failure via induction of heme oxygenase-1 and heat shock protein 70 in endotoxemic rats. J Ethnopharmacol 162: 168–175, 2015. doi: 10.1016/j.jep.2014.12.062. [DOI] [PubMed] [Google Scholar]
- 153.Wei Y, Wang Y. Celastrol attenuates impairments associated with lipopolysaccharide-induced acute respiratory distress syndrome (ARDS) in rats. J Immunotoxicol 14: 228–234, 2017. doi: 10.1080/1547691X.2017.1394933. [DOI] [PubMed] [Google Scholar]
- 154.Weiser JR, Mackenroth T. Acute inhalatory mass ammonia intoxication with fatal course. Exp Pathol 37: 291–295, 1989. doi: 10.1016/S0232-1513(89)80070-2. [DOI] [PubMed] [Google Scholar]
- 155.Wesselkamper SC, Case LM, Henning LN, Borchers MT, Tichelaar JW, Mason JM, Dragin N, Medvedovic M, Sartor MA, TomLinson CR, Leikauf GD. Gene expression changes during the development of acute lung injury: role of transforming growth factor beta. Am J Respir Crit Care Med 172: 1399–1411, 2005. doi: 10.1164/rccm.200502-286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.White ES A case of near fatal ammonia gas poisoning. J Occup Med 13: 549–550, 1971. [PubMed] [Google Scholar]
- 157.Wolfinger RD, Gibson G, Wolfinger ED, Bennett L, Hamadeh H, Bushel P, Afshari C, Paules RS. Assessing gene significance from cDNA microarray expression data via mixed models. J Comput Biol 8: 625–637, 2001. doi: 10.1089/106652701753307520. [DOI] [PubMed] [Google Scholar]
- 158.Woto-Gaye G, Mendez V, Boye IA, Ndiaye PD. [Death from ammonia poisoning: anatomo-pathologic features]. Dakar Med 44: 199–201, 1999. [PubMed] [Google Scholar]
- 159.Xie M, Cheng B, Ding Y, Wang C, Chen J. Correlations of IL-17 and NF-κB gene polymorphisms with susceptibility and prognosis in acute respiratory distress syndrome in a chinese population. Biosci Rep 39: BSR20181987, 2019. doi: 10.1042/BSR20181987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yalcin B, Wong K, Agam A, Goodson M, Keane TM, Gan X, Nellåker C, Goodstadt L, Nicod J, Bhomra A, Hernandez-Pliego P, Whitley H, Cleak J, Dutton R, Janowitz D, Mott R, Adams DJ, Flint J. Sequence-based characterization of structural variation in the mouse genome. Nature 477: 326–329, 2011. doi: 10.1038/nature10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yang H, Wang JR, Didion JP, Buus RJ, Bell TA, Welsh CE, Bonhomme F, Yu AH, Nachman MW, Pialek J, Tucker P, Boursot P, McMillan L, Churchill GA, de Villena FP. Subspecific origin and haplotype diversity in the laboratory mouse. Nat Genet 43: 648–655, 2011. doi: 10.1038/ng.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yildiz OG, Soyuer S, Saraymen R, Eroglu C. Protective effects of caffeic acid phenethyl ester on radiation induced lung injury in rats. Clin Invest Med 31: E242–E247, 2008. doi: 10.25011/cim.v31i5.4870. [DOI] [PubMed] [Google Scholar]
- 163.Yu B, Shao Y, Zhang J, Dong XL, Liu WL, Yang H, Liu L, Li MH, Yue CF, Fang ZY, Zhang C, Hu XP, Chen BC, Wu Q, Chen YW, Zhang W, Wan J. Polymorphisms in human histamine receptor H4 gene are associated with atopic dermatitis. Br J Dermatol 162: 1038–1043, 2010. doi: 10.1111/j.1365-2133.2010.09675.x. [DOI] [PubMed] [Google Scholar]
- 164.Zampeli E, Tiligada E. The role of histamine H4 receptor in immune and inflammatory disorders. Br J Pharmacol 157: 24–33, 2009. doi: 10.1111/j.1476-5381.2009.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang F, Zheng XF, Ma B, Fan XM, Wang GY, Xia ZF. Mass chemical casualties: treatment of 41 patients with burns by anhydrous ammonia. Burns 41: 1360–1367, 2015. doi: 10.1016/j.burns.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 166.Zhao B, Gao W, Gao X, Leng Y, Liu M, Hou J, Wu Y. Sulforaphane attenuates acute lung injury by inhibiting oxidative stress via Nrf2/HO-1 pathway in a rat sepsis model. Int J Clin Exp Pathol 10: 9021–9028, 2017. [PMC free article] [PubMed] [Google Scholar]