Abstract
Pulmonary fibrosis is one of the important causes of morbidity and mortality in fibroproliferative disorders such as systemic sclerosis (SSc) and idiopathic pulmonary fibrosis (IPF). Lysyl oxidase (LOX) is a copper-dependent amine oxidase whose primary function is the covalent crosslinking of collagens in the extracellular matrix (ECM). We investigated the role of LOX in the pathophysiology of SSc. LOX mRNA and protein levels were increased in lung fibroblasts of SSc patients compared with healthy controls and IPF patients. In vivo, bleomycin induced LOX mRNA expression in lung tissues, and LOX activity increased in the circulation of mice with pulmonary fibrosis, suggesting that circulating LOX parallels levels in lung tissues. Circulating levels of LOX were reduced upon amelioration of fibrosis with an antifibrotic peptide. LOX induced ECM production at the transcriptional level in lung fibroblasts, human lungs, and human skin maintained in organ culture. In vivo, LOX synergistically exacerbated fibrosis in bleomycin-treated mice. Further, LOX increased the production of interleukin (IL)-6, and the increase was mediated by LOX-induced c-Fos expression, the nuclear localization of c-Fos, and its engagement with the IL-6 promoter region. Our findings demonstrate that LOX expression and activity correlate with fibrosis in vitro, ex vivo, and in vivo. LOX induced ECM production via upregulation of IL-6 and nuclear localization of c-Fos. Thus, LOX has a direct pathogenic role in SSc-associated fibrosis that is independent of its crosslinking function. Our findings also suggest that measuring circulating LOX levels and activity can be used for monitoring response to antifibrotic therapy.
Keywords: extracellular matrix (ECM), fibrosis, idiopathic pulmonary fibrosis (IPF), lysyl oxidase (LOX), systemic sclerosis (SSc)
INTRODUCTION
Fibrosis is a pathological process characterized by fibroblast activation and proliferation and excess deposition of extracellular matrix (ECM) components such as fibronectin and collagen in an organ or tissue. Progressive fibrosis results in loss of organ function and is recognized to be one of the major causes of morbidity and mortality in individuals with a progressive pulmonary fibrotic disease such as idiopathic pulmonary fibrosis (IPF) and systemic fibrotic disease such as systemic sclerosis (SSc) (1, 12). SSc is a connective tissue disease of unknown etiology characterized by dermal and visceral organ fibrosis due to persistent overproduction of ECM (19, 37). Pulmonary involvement is currently the leading cause of morbidity and mortality in patients with SSc (36).
Lysyl oxidase (LOX) is a member of the LOX family of proteins that includes LOX, lysyl oxidase like-1 (LOXL1), LOXL2, LOXL3, and LOXL4. It is a copper-dependent amine oxidase whose primary function is to promote the covalent crosslinking of ECM proteins such as collagen and elastin, resulting in the establishment of the tensile strength of ECM (39). LOX activity is important in establishing the structural integrity and stability of the ECM in various organs. In addition to this traditional function, lesser known functions of LOX include regulation of gene transcription and modulation of cell signaling pathways (2). LOX levels are increased in SSc skin (3). LOX levels are also increased in sera of SSc patients compared with those of healthy controls, and circulating LOX concentrations correlate with the extent of skin fibrosis in SSc patients (32).
We have previously shown that a peptide derived from the C-terminus of endostatin, called E4, prevents and ameliorates dermal and pulmonary fibrosis in vitro, in vivo, and ex vivo (28, 40). We further reported that LOX is upregulated in normal human fibroblasts treated with transforming growth factor-β (TGF-β) and in lung tissues of bleomycin-treated mice and that the antifibrotic activity of E4 was accompanied by a decrease in LOX expression (40). Therefore, in the present study, we investigated whether circulating LOX can serve as a marker of lung fibrosis. We also sought to determine the role of LOX in fibrosis and whether LOX directly contributes to increased ECM production.
MATERIALS AND METHODS
Human lung and skin tissues.
Lung tissues were obtained from the explanted lungs of SSc and IPF patients who underwent lung transplantation at the University of Pittsburgh Medical Center, as previously reported (14, 29). All tissues were obtained under a protocol approved by the Institutional Review Board of the University of Pittsburgh and following written informed consent. Lung tissues were also obtained from organ donors (healthy controls; HC) whose lungs were not used for transplantation under a protocol approved by the Institutional Review Board of the University of Pittsburgh (14, 29). Human skin was obtained from corrective plastic surgery and maintained in organ culture, as we have previously reported (42). Skin punch biopsies were obtained from the clinically affected skin of patients with SSc or from the skin of their healthy twins from a cohort that was previously described (7, 31).
Primary fibroblast culture.
Primary human lung fibroblasts were cultured from lung tissues of patients with SSc and IPF undergoing lung transplantation following written consent as previously described (14, 29) under a protocol approved by the University of Pittsburgh Institutional Review Board. Primary fibroblasts were also cultured from the lung tissues of normal donors whose lungs were not used for transplantation (14, 29). Dermal fibroblasts were cultured as previously described (9). All fibroblasts were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Corning Incorporated Life Sciences, Tewksbury, MA) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO), penicillin, streptomycin, and antimycotic agent (Invitrogen, Carlsbad, CA). All cells were used between passages 3 and 7.
In vivo experiments.
All experiments were done under a protocol approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina. Pulmonary fibrosis was induced in mice as previously described with some modifications (28, 40). Briefly, bleomycin (1.2 mU/g body wt) in a total volume of 50 µL of PBS was intratracheally administered to 6–8-wk-old male CB57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME). Biotinylated-E4 or biotinylated-scrambled peptide (20 µg/mouse) in 100 µL of H2O was administered via oral gavage on the same day as bleomycin as well as 3 and 6 days posttreatment for a total of three doses. Mice were euthanized by CO2 asphyxiation, and lungs were harvested on days 3, 5, 10, and 21 for mRNA expression analysis and hydroxyproline assay. Sera were collected on day 21 for measurement of LOX levels and activity. In some experiments, lungs harvested on day 21 were fixed with 10% formalin and embedded in paraffin for hematoxylin and eosin (H&E) staining. To assess the potential fibrotic effect of LOX, 10 µg of recombinant LOX (LOX) (OriGene Technologies, Inc., Rockville, MD) or vehicle (PBS) was intratracheally administered 7 days after bleomycin treatment (1.0 mU/g body wt), and lungs were harvested on day 14.
In vitro fibroblast stimulation.
Actively growing primary human lung fibroblasts were stimulated as previously described with some modifications (27). Briefly, 2.0 × 105 primary fibroblasts were plated in six-well tissue culture plates in 10% FBS-containing DMEM. After 24 h, the cells were serum-starved in DMEM for 24 h before stimulation with recombinant LOX (LOX) or vehicle (VC) and harvested after 48 h for mRNA, chromatin immunoprecipitation (ChIP) assay, or nuclear fraction isolation using the Subcellular Protein Fractionation kit (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s protocol. Primary human lung fibroblasts were also treated with LOX in the presence or absence of IL-6-neutralizing antibody (R&D Systems Inc., Minneapolis, MN) or isotype control for 48 h for ECM extraction as previously described (29). In addition, primary human lung fibroblasts were infected with adenovirus-expressing mouse or human LOX or a control adenovirus at a multiplicity of infection (MOI) of 50. For studies of inhibition, primary lung fibroblasts were stimulated with 270 ng/mL human recombinant LOX following a 1-h pretreatment with 10 µg/mL β-aminopropionitrile monofumarate (BAPN), an irreversible inhibitor of lysyl oxidase activity (Sigma-Aldrich, St. Louis, MO), and 10 µg/mL T-5224, a transcription factor c-Fos/activator protein (AP)-1 inhibitor (Apexbio, Houston, TX), or vehicle (DMSO) for 48 h.
Adenovirus construct preparation.
Replication-deficient adenovirus serotype-5-expressing mouse LOX (LOX Ad) or no cDNA (control Ad) were obtained from the Vector Core Facility of the University of Pittsburgh. Adenovirus-expressing human LOX or control virus (Ad-CMV) were purchased from Vector BioLabs (Malvern, PA).
Ex vivo human lung culture.
Human lung tissue was cut into approximately 3-mm cores, and six cores were placed in each well of a six-well plate in serum-free DMEM. Two × 107 plaque-forming units (pfu) of LOX Ad, control Ad, LOX (270 ng/mL), or vehicle were added. In some experiments, lung tissue was treated with LOX in combination with BAPN (200 µg/mL) (Sigma-Aldrich). The tissues and media were harvested 96 h posttreatment. Collagen content in tissues was measured using hydroxyproline assay and mRNA expression levels were measured using quantitative PCR. The conditioned media were analyzed by immunoblotting and ELISA.
Ex vivo human skin culture.
Human skin tissue was cut into approximately 20-mm × 20-mm pieces and placed in each well of six-well plates in DMEM supplemented with 10% FBS. Recombinant LOX (810 ng/tissue) or vehicle in combination with anti-IL-6 antibody (5 µg/tissue) (R&D) or isotype control were injected. Tissues were harvested 120 h posttreatment. Tissues were evaluated for collagen content using hydroxyproline assay. Tissues were also fixed with 10% formalin and embedded in paraffin for H&E staining and measuring skin thickness as previously described (42).
Quantitative PCR.
Total RNA was extracted from human primary lung fibroblasts, human lung and skin tissues, and mouse lung tissues using a RNeasy mini kit (Qiagen Inc., Valencia, CA). First-strand cDNA was reverse-transcribed with an oligo (dT)12-15 primer (Invitrogen, Carlsbad, CA) and SuperScript IV Reverse Transcriptase (Invitrogen). Gene mRNA expression levels were evaluated by quantitative PCR using the TaqMan real-time PCR system (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. Gene expression levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Relative expression levels of fibroblasts were compared with RNA levels using the comparative CT method formula 2−ΔΔCt. The expression of each gene was measured as a ratio to GAPDH. Specific primers and probes for amplifying genes encoding human LOX (Hs00184700_m1), mouse LOX (Mm00495386_m1), human GAPDH (Hs02758991_g1), mouse Gapdh (Mm99999915_g1), human collagen 1A1 (Hs00164004_m1), human collagen 1A2 (Hs00164099_m1), human fibronectin (Hs00365052_m1), human IL-6 (Hs00985639_m1), and human c-Fos (Hs04194186_s1) were purchased from Applied Biosystems. Human B2M (Hs00187842_m1) was also used to confirm results obtained with GAPDH with no notable differences (data not shown).
Hydroxyproline assay.
To quantify crosslinked collagen content in human and mouse tissues, hydroxyproline levels were measured as previously described (28, 33).
LOX ELISA and activity assay.
Mouse sera and media conditioned by fibroblasts were collected and stored at −80°C. Levels of LOX in sera and conditioned media were measured using mouse LOX ELISA kit (Uscn Life Science Inc., Wuhan, China) according to the manufacturer’s protocol. Activity levels of LOX in sera and conditioned media were quantified using Amplite Fluorimetric Lysyl Oxidase Assay Kit (AAT Bioquest, Inc., Sunnyvale, CA) according to the manufacturer’s protocol.
Western blot analysis.
Conditioned media, ECM fractions, and cell lysates were analyzed by immunoblotting. The following antibodies were used: fibronectin (FN) monoclonal antibody (clone EP5) (Santa Cruz, Dallas, TX), collagen type I (Col) polyclonal antibody (Abnova, Taipei City, Taiwan), c-Fos monoclonal antibody (clone 9F6) (Cell Signaling Technology, Danvers, MA), and TATA-box binding protein (TBP) monoclonal antibody (Abcam, Cambridge, MA) as primary antibodies and horseradish peroxidase-conjugated antibody as a secondary antibody. Signals were detected using chemiluminescence on the FluorChem R System (ProteinSimple, San Jose, CA), and densitometry was analyzed with ImageJ software (NIH, Bethesda, MD).
Chromatin immunoprecipitation assay.
The chromatin immunoprecipitation (ChIP) assay was performed using an EZ ChIP chromatin immunoprecipitation kit (Upstate Biotechnology, Inc., Lake Placid, NY) as previously described (25). Briefly, human primary lung fibroblasts were treated with LOX (270 ng/mL) or vehicle (VC) for 48 h and crosslinked by the addition of 1% formaldehyde. Subsequently, the cells were lysed in SDS lysis buffer. Genomic DNA was extracted and fragmented into 200–1,000 bp segments using an ultrasonic processor. The sheared chromatin was precleared at 4°C for 1 h with rotation with protein G-agarose to avoid nonspecific binding. The protein G-agarose was pelleted by centrifugation, and an aliquot of 10 μL of the supernatant was saved for the input control. The remaining supernatants were incubated with 5 μg of anti-c-Fos monoclonal antibody (Cell Signaling Technology) or rabbit IgG (R&D systems) as an isotype control. After washing of the immunoprecipitated products, the chromatin was eluted from the agarose by incubation with elution buffer. The DNA-protein crosslinks were then reversed using a high-salt solution at 65°C for 4 h. Finally, the precipitated DNA was recovered using the provided spin column and eluted with 50 μL of elution buffer. PCR was performed using Taq DNA polymerase (Invitrogen) with 1 μL of the precipitated DNA as template. The primers used to detect the binding of c-Fos to the IL-6 promoter region are: forward, 5′-ATGCTAAAGGACGTCACATTGCACAA-3′; reverse, 5′-TGGCAGTTCCAGGGCTAAGGATTTCC-3′.
Statistical analysis.
All continuous variables are expressed as means ± SD. All statistical analyses were done using GraphPad Prism version 8 for Windows (GraphPad Software, La Jolla, CA). Comparisons between two groups were tested for statistical significance with the paired or unpaired t test, as appropriately indicated in the figure legends. Comparison among three or more groups was performed using ANOVA followed by Bonferroni’s test. P values < 0.05 were considered statistically significant.
RESULTS
LOX is increased in primary lung fibroblasts from patients with SSc.
We have previously reported that TGF-β induced LOX expression in normal human lung fibroblasts (40). We therefore examined LOX levels in primary lung fibroblasts from HC, SSc patients, and IPF patients. Expression levels of LOX in primary lung fibroblasts from HC (n = 9), SSc patients with pulmonary fibrosis (PF) (n = 12), SSc patients with pulmonary hypertension (PH) (n = 9), and IPF patients (n = 10) were examined by real-time PCR. As shown in Fig. 1A, LOX mRNA levels were significantly upregulated in lung fibroblasts from SSc patients compared with those from both HC and IPF patients. LOX mRNA levels were comparable in lung fibroblasts from IPF patients and HC. The highest levels of LOX mRNA were noted in SSc patients with PF. Thus, LOX expression was increased in lung fibroblasts cultured in vitro from SSc patients, especially patients with SSc-associated pulmonary fibrosis.
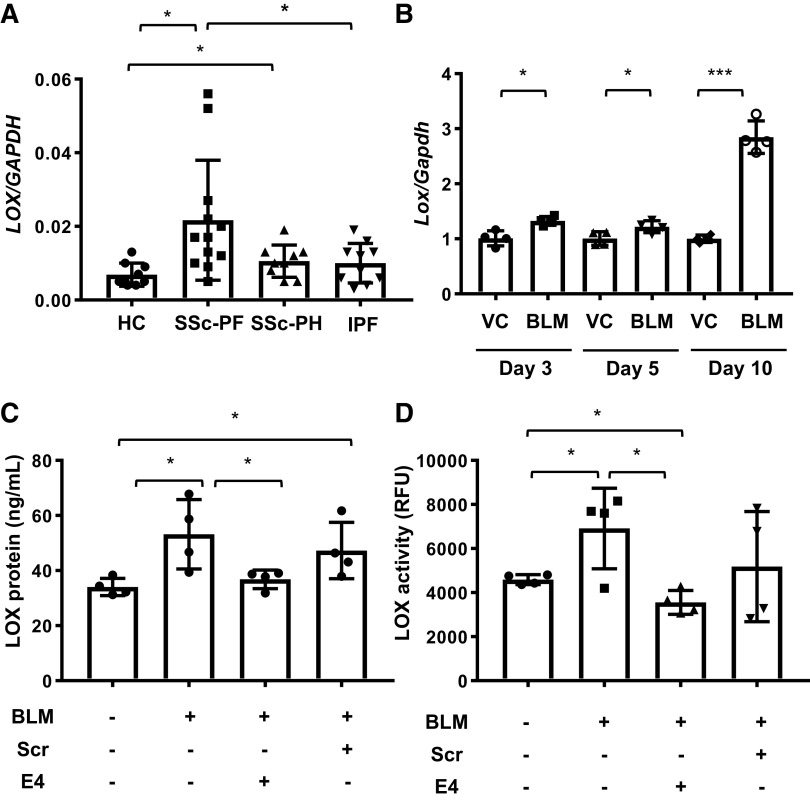
Figure 1.
Lysyl oxidase (LOX) is increased in human and murine systemic sclerosis (SSc) and serves as a biomarker of lung fibrosis in vivo. LOX mRNA levels are increased in lung fibroblasts of SSc patients. A: expression of LOX was evaluated in lung fibroblasts from 9 healthy controls (HC), 12 SSc patients with pulmonary fibrosis (SSc-PF), 9 SSc patients with pulmonary hypertension (SSc-PH), and 10 patients with idiopathic pulmonary fibrosis (IPF) using real-time PCR. Comparison among three or more groups was performed using ANOVA followed by Bonferroni’s test. Values represent means ± SD. *P < 0.05. B: lungs from male C57BL/6J mice intratracheally treated with PBS (n = 4) and bleomycin (BLM, n = 4) were harvested 3, 5, or 10 days posttreatment. Expression of Lox mRNA extracted from the lung tissues was evaluated using real-time PCR. Values represent means ± SD. Graphical presentation of the data analyzed using unpaired t test. *P < 0.05. ***P< 0.001. C: sera were harvested from mice treated with PBS (n = 4), bleomycin (BLM, n = 4), bleomycin and E4 (BLM + E4, n = 4), and bleomycin and scrambled control peptide (BLM + Scr, n = 4) 21 days posttreatment. LOX levels in sera were determined by ELISA. Comparison among three or more groups was performed using ANOVA followed by Bonferroni’s test. Values represent means ± SD. *P < 0.05. D: sera were harvested from mice treated with PBS (n = 4), bleomycin (BLM, n = 4), bleomycin and E4 (BLM + E4, n = 4), and bleomycin and scrambled control peptide (BLM + Scr, n = 4) 21 days posttreatment. LOX activity in sera was measured by activity assay. Comparison among three or more groups was performed using ANOVA followed by Bonferroni’s test. Values represent means ± SD. *P < 0.05. RFU, relative fluorescence units.
LOX is increased in vivo in the bleomycin-induced pulmonary fibrosis mouse model and correlates with fibrosis.
We previously demonstrated an increase in LOX mRNA and protein in mouse lungs 10 days following administration of bleomycin in vivo (28, 40). To assess how early LOX expression is induced as a result of bleomycin-induced lung fibrosis, we measured LOX mRNA expression in mouse lungs 3, 5, and 10 days after bleomycin treatment. On days 3 and 5, bleomycin induced Lox expression by 1.2-fold. Notably, bleomycin increased Lox mRNA expression in mouse lungs 10 days posttreatment by 2.8-fold (Fig. 1B). Our previous work also demonstrated that treatment of mice with the antifibrotic peptide E4 ameliorated fibrosis and reduced tissue levels of LOX (28). To determine if circulating LOX levels and activity can serve to monitor the response to antifibrotic therapy, we measured LOX protein and activity levels in sera of mice treated with bleomycin in combination with the E4 peptide or control scrambled peptide. Sera were collected from mice 21 days after bleomycin and peptide treatment. As shown in Fig. 1, C and D, bleomycin increased circulating levels of LOX protein and activity, respectively, in the sera of mice. The mean serum LOX level in mice treated with bleomycin was significantly higher (52.7 ± 12.0 ng/mL) than in mice treated with PBS (34.1 ± 3.1 ng/mL). The mean serum LOX activity level in mice treated with bleomycin was also significantly increased [5,718.6 ± 2,749.8 relative fluorescence unit (RFU)] compared with mice treated with PBS (4,208.9 ± 721.5 RFU). Furthermore, amelioration of bleomycin-induced pulmonary fibrosis by E4 peptide significantly reduced the protein (36.8 ± 3.4 ng/mL) and activity levels of LOX (3,358.9 ± 812.2 RFU). These data suggest that circulating LOX levels and activity are induced by bleomycin treatment and parallel the development of bleomycin-induced pulmonary fibrosis, while the levels are reduced when fibrosis is ameliorated as a result of E4 treatment. Thus, LOX is a suitable marker for monitoring response to therapy in the setting of fibrosis.
LOX promotes expression of ECM genes in in vitro, ex vivo, and in vivo pulmonary fibrosis models.
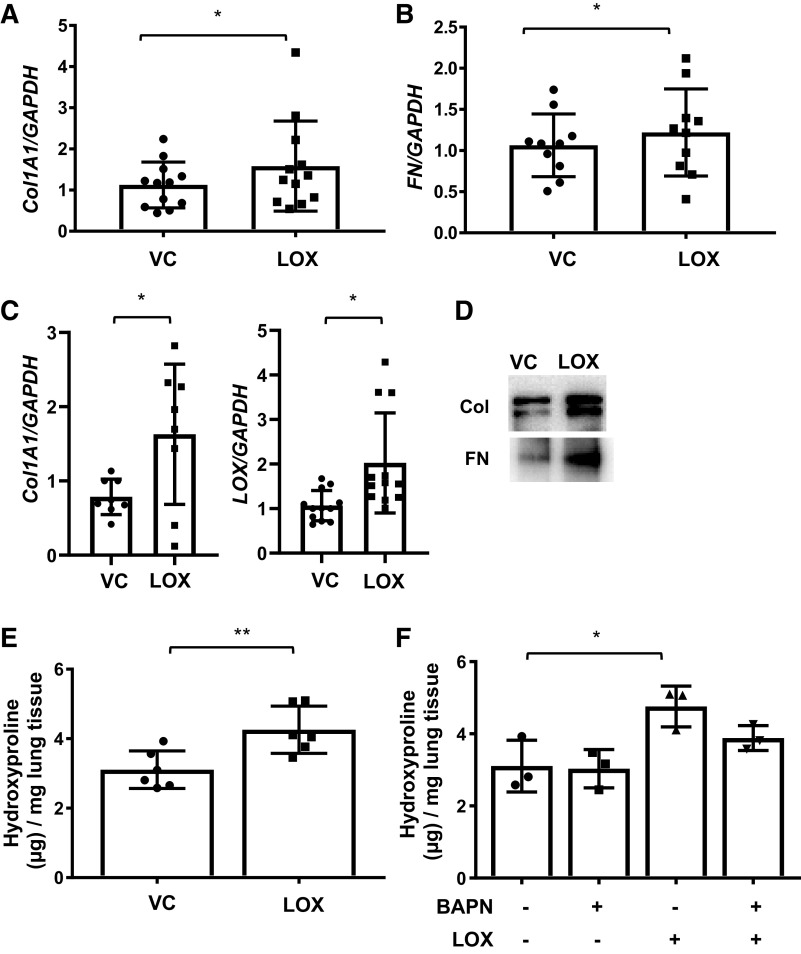
It is well established that LOX contributes to fibrosis via its crosslinking activity. To determine if LOX also contributes to the development of fibrosis by promoting ECM expression, we treated primary human lung fibroblasts from HC with recombinant LOX for 48 h and measured expression levels of ECM components. LOX treatment significantly increased collagen 1A1 (Col1A1) (Fig. 2A) and fibronectin (FN) (Fig. 2B) mRNA expression. We also examined the effects of LOX in human lung tissues maintained in organ culture. LOX significantly increased the transcription levels of Col1A1 (Fig. 2C), but not those of FN (data not shown) at the time point examined, and LOX increased production of collagen and fibronectin protein by lung tissues (Fig. 2D and Supplemental Fig. S1; all supplemental materials are available at https://doi.org/10.6084/m9.figshare.12797363.v1). LOX also increased its own expression in human lung tissues maintained in organ culture (Fig. 2C). In confirmation of its function, LOX increased hydroxyproline contents of human lung tissues in ex vivo organ culture (Fig. 2E). Interestingly, the inhibition of LOX catalytic activity by BAPN failed to completely abrogate LOX-induced ECM production (Fig. 2F), suggesting that induction of ECM transcription by LOX is independent of its crosslinking activity.
Figure 2.
Lysyl oxidase (LOX) promotes extracellular matrix production in vitro and ex vivo. A and B: mRNA were extracted from primary human lung fibroblasts treated with recombinant LOX (LOX) or vehicle (VC) for 48 h. Expression of collagen type1α1 (Col1A1) and fibronectin (FN) was evaluated using real-time PCR. The data were obtained from 10–12 different experiments using fibroblasts from lung tissues of 10–12 different individual normal donors. Values represent means ± SD. Graphical presentation of the data analyzed by paired t test. *P < 0.05. C: human lung tissues were treated with vehicle (VC) or recombinant LOX. Samples were harvested after 72 h for Col1A1 and 96 h for LOX of stimulation. Gene expression levels of Col1A1 and LOX were quantified using qPCR. The data were obtained from 8 to 12 different experiments using lung tissues of 8–12 different individual normal donors. Graphical presentation of the data analyzed by paired t test. Values represent means ± SD. *P < 0.05. D: the levels of Col1A1 and FN in the conditioned culture supernatants from human lung tissues were assessed by immunoblotting. The representative data were obtained from three different experiments using conditioned culture supernatants from lung tissues of three different individual healthy control (HC) donors. E: human lung tissues from healthy donors were treated with recombinant LOX (LOX) or vehicle (VC) for 96 h. The amount of collagen in the lung tissues was evaluated by hydroxyproline assay. The data were obtained from six different experiments using lung tissues of six different individual normal donors. Values represent means ± SD. Graphical presentation of the data analyzed by unpaired t test. **P < 0.01. F: human lung tissues were treated with vehicle (VC), LOX inhibitor BAPN (BAPN), recombinant LOX (LOX), LOX and LOX inhibitor BAPN (LOX + BAPN) for 96 h. The amount of collagen in the lung tissues was evaluated by hydroxyproline assay. The data were obtained from three different experiments using lung tissues of three different individual normal donors. Comparison among three or more groups was performed using ANOVA followed by Bonferroni’s test. Values represent means ± SD. *P < 0.05.
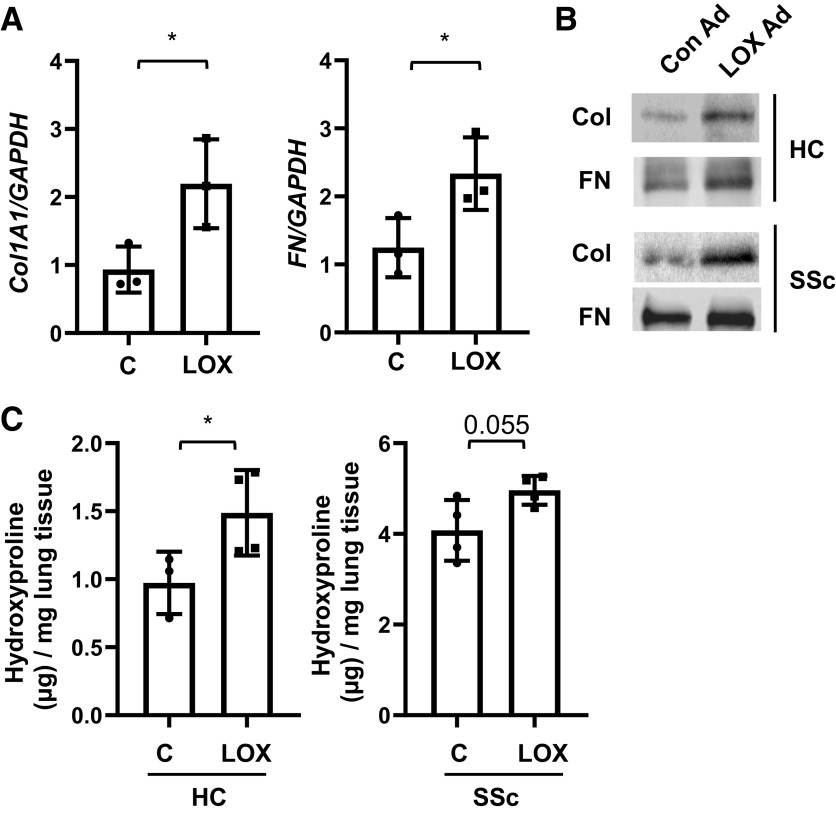
In a complementary approach, we expressed LOX via adenoviral vector. Human lung tissues obtained from healthy controls (HC) were treated with LOX Ad for 96 h. Real-time PCR revealed that mRNA expression levels of collagen 1A1 and fibronectin were significantly increased by LOX Ad (Fig. 3A). Similarly, collagen and fibronectin protein levels were also increased in the lungs from healthy donors (HC) and patients with SSc treated with LOX Ad compared with those treated with control Ad (Fig. 3B). Hydroxyproline assay revealed that the amount of collagen in lung tissues from SSc patients (4.1 ± 0.6 µg/mg) was higher than in lung tissues of healthy donors (1.0 ± 0.2 µg/mg) at baseline (Fig. 3C). LOX Ad treatment further increased the amount of collagen in lung tissues from both HC (1.5 ± 0.3 µg/mg) and SSc patients (5.0 ± 0.3 µg/mg) as compared with control Ad (Fig. 3C).
Figure 3.
Lysyl oxidase (LOX) overexpression increases collagen and fibronectin production in vitro and ex vivo. A: human lung tissues were infected with control adenovirus (C) or LOX-expressing adenovirus (LOX). Samples were harvested after 96 h. Gene expression levels of collagen 1A1 (Col1A1) and fibronectin (FN) were quantified using qPCR. The data were obtained from three different experiments using human lung tissues of three different individual normal donors. Graphical presentation of the data analyzed by paired t test. Values represent means ± SD. *P < 0.05. B: lung tissues from healthy control (HC) and systemic sclerosis (SSc) patients were infected with Con Ad or LOX Ad. The protein levels of Col1A1 and FN in the conditioned supernatants were assessed by immunoblotting. The data were obtained from three different experiments using conditioned culture supernatants from lung tissues of three different individual HC and SSc donors. C: lung tissues from HC and SSc patients (SSc) were cultured with Con Ad (C) and LOX Ad (LOX). The amount of collagen in the lung tissues was evaluated by hydroxyproline assay. Total collagen was expressed as μg collagen mg–1 wet tissue. The data were obtained from three to four different experiments using lung tissues of four different individual HC and SSc donors. Graphical presentation of the data analyzed by paired t test. Values represent means ± SD. *P < 0.05.
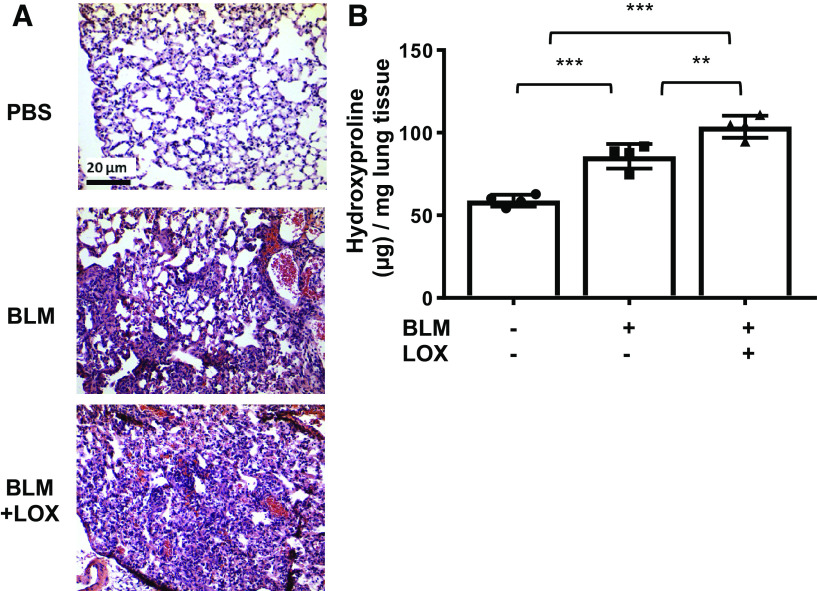
Having shown that recombinant LOX and adenovirally expressed LOX increase ECM gene expression and fibrosis in both human lung and skin tissues, we examined if exogenous LOX can exacerbate pulmonary fibrosis in vivo. Bleomycin (1.0 mU/g body wt) was intratracheally administered to mice with or without 10 µg of recombinant LOX. Mice were euthanized 14 days posttreatment, and harvested lung tissues were subjected to H&E staining and hydroxyproline assay. As shown in Fig. 4A, bleomycin in combination with LOX showed that LOX treatments worsened pulmonary fibrosis in mice. This result was confirmed by hydroxyproline assay where LOX administered with bleomycin synergistically exacerbated lung fibrosis (Fig. 4B). Thus, our data show that administration of LOX exacerbates bleomycin-induced pulmonary fibrosis in mice.
Figure 4.
Lysyl oxidase (LOX) promotes extracellular matrix production in vivo in pulmonary fibrosis model. A: lungs from male C57BL/6J mice were intratracheally administered with PBS, bleomycin (BLM), and bleomycin in combination with recombinant LOX (BLM + LOX). Lungs were harvested 14 days after administration. Representative H&E images of mouse lung tissues treated with LOX and bleomycin. Scale bar, 20 µm. B: the amount of collagen in the lung tissues was evaluated by hydroxyproline assay. Total collagen was expressed as μg collagen mg–1 wet tissue. The data were obtained from lung tissues of four different mice. Comparison among three or more groups was performed using ANOVA followed by Bonferroni’s test. Values represent means ± SD. **P < 0.01. ***P < 0.001.
Taken together, these results demonstrate that LOX can induce a fibrotic phenotype via induction of ECM expression in vitro in primary pulmonary fibroblasts, in human lung tissues ex vivo, and in mouse lungs in vivo.
LOX promotes ECM production in human skin tissues.
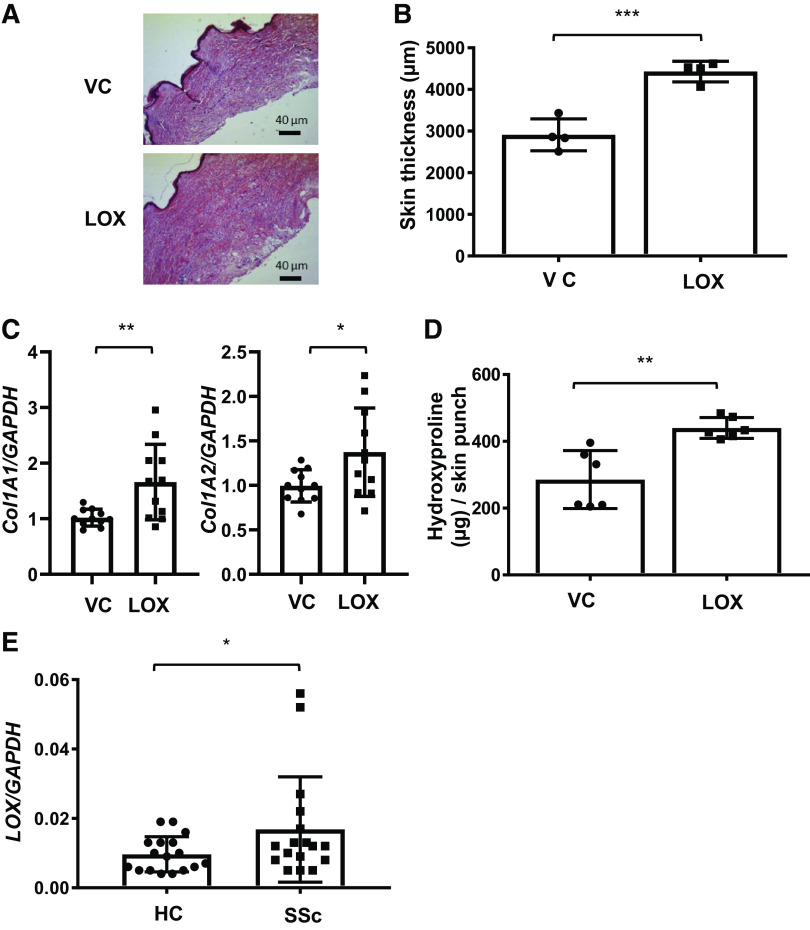
Dermal fibrosis is a hallmark of SSc, and the extent of dermal fibrosis has historically been used to monitor disease progression and response to treatment in clinical trials (37). Having shown that LOX promotes pulmonary fibrosis, we next sought to determine if LOX contributes to the development of fibrosis in another tissue—the skin. Human skin from healthy donors was maintained in organ culture as we previously reported (42). First, similar to our approach in human lung tissues, we confirmed the effects of overexpressed LOX in human skin tissues in organ culture. Human skin tissues were injected with adenoviral LOX (Ad-LOX) or a control (Ad-CMV), and the gene expression levels of LOX, Col1A1, Col1A2, and FN were measured using qPCR. As seen in Supplemental Fig. S2, A and B, overexpression of LOX in human skin tissues increased its own expression, as well as the expression of Col1A1, Col1A2, and FN, without a noticeable difference in proliferation (Supplemental Fig. S2C). In a parallel approach, we treated human skin with recombinant LOX for 48–120 h. Skin tissue was subjected to H&E staining and hydroxyproline assay. As shown in Fig. 5, A and B, the thickness of skin tissue, which is increased in fibrosis (40, 42), was increased by recombinant LOX treatment. In parallel, Col1A1 and Col1A2 mRNA levels were also increased in skin treated with LOX for 48 h (Fig. 5C). Induction of dermal fibrosis by LOX was further confirmed by hydroxyproline assay (Fig. 5D). Finally, similar to what was observed in SSc-PF lung fibroblasts, LOX expression was increased in dermal fibroblasts from twins discordant for SSc (Fig. 5E). Our findings in lung and skin tissues thus demonstrate that LOX can induce a fibrotic phenotype in more than one organ. Further, LOX can positively regulate its own expression, providing a positive feedback loop.
Figure 5.
Lysyl oxidase (LOX) promotes extracellular matrix production ex vivo in human skin tissues. A: human skin tissues from healthy donors were treated with recombinant LOX (LOX) or vehicle (VC) for 120 h. Representative H&E images of human skin tissues treated with LOX and VC. Scale bar, 40 µm. B: the thickness of skin tissues was measured from H&E stained sections. The data were obtained from four different experiments using skin tissues of four different individual normal donors. Values represent means ± SD. Graphical presentation of the data analyzed by unpaired t test. ***P < 0.001. C: mRNA levels of collagen 1A1 (Col1A1) and collagen 1A2 (Col1A2) were measured in skin tissues treated with VC or LOX for 48 h. The data were obtained from 11 different experiments using skin tissues of 11 different individual normal donors. Graphical presentation of the data analyzed by paired t test. Values represent means ± SD. *P < 0.05. **P < 0.01. D: the amount of collagen in the skin tissues was evaluated by hydroxyproline assay. Total collagen was expressed as μg collagen per 3-mm skin punch. The data were obtained from six different experiments using skin tissues of six different individual normal donors. Graphical presentation of the data analyzed by unpaired t test. Values represent means ± SD. **P < 0.01. E: dermal fibroblasts were cultured from the skin of patients with systemic sclerosis (SSc) and their healthy twins as controls (HC). LOX mRNA levels were measured using qPCR in passage 3 fibroblasts. The data were obtained from dermal fibroblasts of 17 different patients with SSc and their healthy twins. Graphical presentation of the data analyzed by paired t test. Values represent means ± SD. *P < 0.05.
LOX increases expression of IL-6 which mediates LOX induction of ECM.
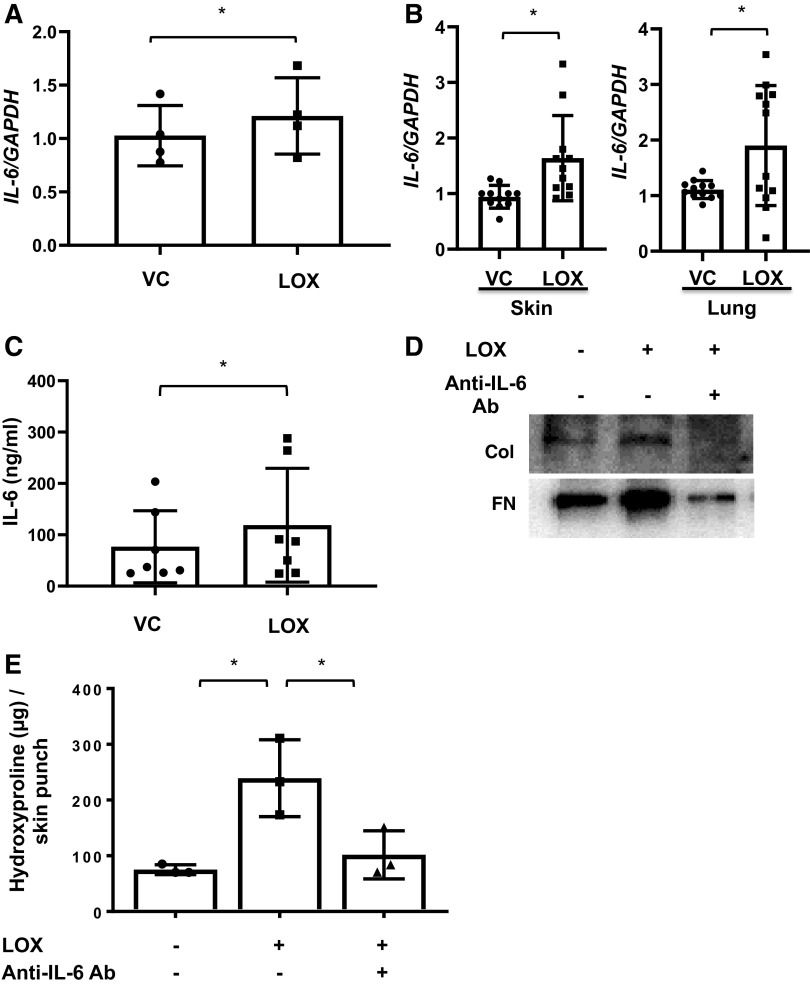
IL-6 is a proinflammatory and profibrotic cytokine, and its increased expression by SSc fibroblasts is one of the hallmarks of the disease (8). Since LOX regulated the transcription of ECM components, we examined its effect on IL-6 expression. LOX significantly increased IL-6 expression in primary lung fibroblasts (Fig. 6A). Recombinant LOX also increased IL-6 expression (Fig. 6B) and protein levels (Fig. 6C) in lung and skin tissues maintained in organ culture. Blocking IL-6 using neutralizing antibody reduced Col and FN levels induced by LOX in vitro in primary lung fibroblasts (Fig. 6D) and hydroxyproline levels ex vivo in human skin tissues (Fig. 6E). Neutralizing IL-6 also reduced Col1A1 expression in skin tissues (Supplemental Fig. S3A). Similarly, blocking IL-6 using neutralizing antibody in human fibrotic lung tissues obtained from the explanted lungs of SSc and IPF patients also showed reduction of Col1A1 and FN levels (Supplemental Fig. S3, B and C). These results indicate that LOX regulation of ECM production is in part via IL-6.
Figure 6.
Lysyl oxidase (LOX) induces IL-6 expression in vitro and ex vivo. A: primary human lung fibroblasts were treated with recombinant LOX (LOX) or vehicle (VC) for 48 h and IL-6 gene expression levels were measured using qPCR. The data were obtained from four different experiments using fibroblasts from lung tissues of four different individual donors. Graphical presentation of the data analyzed by paired t test. Values represent means ± SD. *P < 0.05. B: human skin and lung tissues in organ culture were treated with LOX or VC for 48 h for skin and 96 h for lung. IL-6 gene expression levels were measured using qPCR. The data were obtained from 11–12 different experiments using human skin and lung tissues of 11–12 different individual donors. Graphical presentation of the data analyzed by paired t test. Values represent means ± SD. *P < 0.05. C: media conditioned by human lung tissue cores treated with recombinant LOX (LOX) or vehicle (VC) for 96 h were analyzed for IL-6 protein levels using ELISA. The data were obtained from seven different experiments using culture supernatants from lung tissues of seven different individual donors. Graphical presentation of the data analyzed by paired t test. Values represent means ± SD. *P < 0.05. D: an equivalent number of primary human lung fibroblasts were treated with LOX in the presence or absence of IL-6-neutralizing antibody for 48 h. Collagen 1A1 (Col1A1) and fibronectin (FN) levels in the extracellular matrix fractions were assessed by immunoblotting. The representative data were obtained from three different experiments using primary human lung fibroblasts from lung tissues of three different individual normal donors. E: human skin tissues were treated with LOX in the presence of IL-6-neutralizing antibody or mouse IgG as control for 120 h. The amount of collagen in the skin tissues was evaluated by hydroxyproline assay. Total collagen was expressed as μg collagen per 3-mm skin punch. The data were obtained from three different experiments using skin tissues of three different individual donors. Comparison among three or more groups was performed using ANOVA followed by Bonferroni’s test. Values represent means ± SD. *P < 0.05.
LOX increases IL-6 expression via activation of c-Fos.
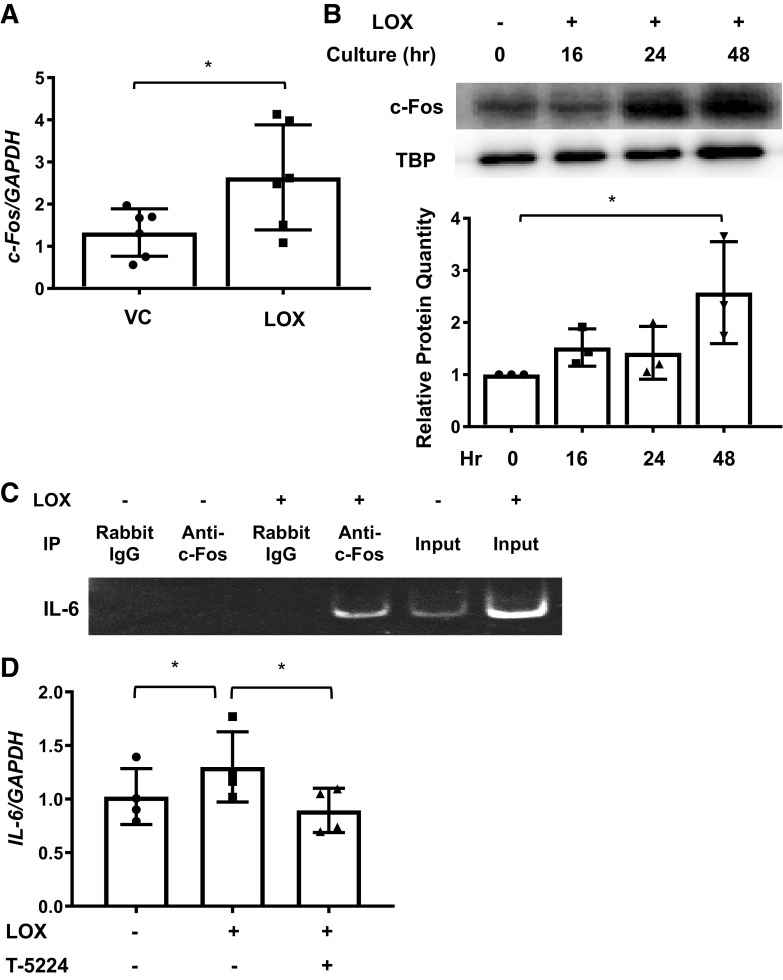
To identify the mechanism mediating LOX induction of IL-6, mRNA from fibroblasts treated with LOX for 16 h was subjected to PCR transcription factor array analysis (data not shown). Our array identified c-Fos as a transcription factor that was induced by LOX and had known binding sites in the IL-6 promoter. Using real-time PCR, we confirmed LOX induction of c-Fos expression in human primary lung fibroblasts from different donors (Fig. 7A). Further, immunoblotting analysis revealed that LOX induced the translocation of c-Fos into the nuclear fraction of fibroblasts (Fig. 7B and Supplemental Fig. S4, A and B). Next, using ChIP assay, we confirmed the binding of c-Fos to the IL-6 promoter following LOX treatment (Fig. 7C and Supplemental Fig. S4C). Finally, to confirm that LOX induction of IL-6 is via activation of c-Fos, primary human lung fibroblasts were cultured with LOX in the presence of the c-Fos inhibitor, T-5224. T-5224 significantly reduced LOX induction of IL-6 expression (Fig. 7D) but not gene expression levels of Col1A1 and FN at that time point (Supplemental Fig. S4D). These results demonstrate that LOX increases c-Fos expression and nuclear localization, which in turn mediates induction of IL-6 expression.
Figure 7.
Lysyl oxidase (LOX) induces c-Fos expression. A: mRNA were extracted from human primary lung fibroblasts treated with recombinant LOX (LOX) or vehicle (VC) for 16 h. Expression of c-Fos was evaluated using real-time PCR. The data were obtained from six different experiments using fibroblasts from lung tissues of six different individual donors. Graphical presentation of the data analyzed by paired t test. Values represent means ± SD. *P < 0.05. B: an equal number of human primary lung fibroblasts were treated with LOX at 0, 16, 24, and 48 h. Protein levels of c-Fos from the nuclear fraction were assessed by immunoblotting. Top: c-Fos in nuclear fractions from an equivalent number of fibroblasts was detected by immunoblotting and signals were normalized to TATA-box binding protein (TBP) at the bottom. Graphical presentation of the data from three different experiments using fibroblasts from lung tissues of three different individual donors and analyzed using ANOVA followed by Bonferroni’s test. Values represent means ± SD. *P < 0.05. C: an equal number of human primary lung fibroblasts were treated with LOX or vehicle (VC) for 48 h and coimmunoprecipitation of c-Fos and IL-6 promoter were performed. The samples were visualized on a polyacrylamide gel. D: primary human lung fibroblasts were treated with LOX in the presence or absence of c-Fos inhibitor (T-5224) for 48 h. Expression of IL-6 was evaluated using real-time PCR. The data were obtained from four different experiments using fibroblasts from lung tissues of four different individual donors. Comparison among three or more groups was performed using ANOVA followed by Bonferroni’s test. Values represent means ± SD. *P < 0.05.
DISCUSSION
Our results demonstrate that LOX levels and activity are increased in fibrosis as measured using in vitro, ex vivo, and in vivo models. LOX levels were increased in both lung tissues and the circulation of mice given bleomycin to induce pulmonary fibrosis, and increased circulating LOX paralleled fibrosis. Notably, LOX levels were decreased posttreatment with E4 peptide and correlated with amelioration of pulmonary fibrosis, suggesting that measuring LOX levels and activity is also useful for monitoring response to therapy in patients with SSc and possibly similar fibrotic disorders. These findings extend our previous research showing that E4 reduces LOX levels in pulmonary fibroblasts and in lung tissues of bleomycin-treated mice (40). Taken together, our findings further suggest that measuring LOX levels and activity can serve as a novel biomarker of fibroproliferative disorders such as SSc. Moreover, our findings show that LOX induces a fibrotic phenotype in vitro, ex vivo, and in vivo independently of its crosslinking activity. LOX induced fibrosis in both lung and skin tissues, suggesting that upregulation of LOX expression may be associated with fibrosis in different organs. Finally, LOX induced the binding of c-Fos to the IL-6 promoter and increased IL-6 levels, suggesting that its fibrotic effects are mediated, at least in part, via production of IL-6. This is further confirmed by the reduction of LOX-induced fibrosis observed in the presence of a neutralizing anti-IL-6 antibody.
Complete knockout of LOX is lethal (13, 24). In fact, Lox−/− mice die at birth or soon after birth due to cardiovascular malformations and diaphragm instability (21, 23). These studies indicate the importance of LOX during development (21). A role of LOX in wound repair is evident in fetal repair where scarless wounds were shown to have reduced LOX expression (5). The role of LOX is further supported by the observation that loss of LOX in dermal fibroblasts results in an 80% reduction in lysyl oxidase activity, suggesting that LOX is the major isoenzyme in these fibroblasts (24).
Several reports have described that LOX expression is associated with fibrosis. Cheng et al. (4) reported that LOX expression was upregulated in bleomycin-induced pulmonary fibrosis in mice and that knockdown of LOX expression or inhibition of LOX activity reduced collagen deposition and alleviated lung fibrosis. Yang et al. (41) also reported that knockdown of LOX expression led to a decrease in fibronectin abundance in uric acid-induced renal fibrosis in rats. Liu et al. (22) reported that inhibition of LOX accelerated the reversal of liver fibrosis. However, it was unclear how LOX regulates ECM production. LOX has been shown to exert nonenzymatic functions including cell proliferation and differentiation of osteoblast and adipocyte progenitor cells (39). Most recently, research has focused on the intracellular function of LOX as a transcriptional regulator in several cancer cell lines (2, 15). It is reported that intracellularly expressed LOX can regulate the transcription activity of the collagen type 3 gene through the binding of Ku antigen to the target DNA, which was dependent on LOX catalytic activity (10). Ku antigen also significantly increased the promoter activity of c-Jun, a transcriptional factor in the activator protein 1 (AP-1) family, through the binding to the promoter region of the c-Jun gene (16). In addition, Avouac et al. (1a) described that the AP-1 family of proteins, which includes c-Fos, is upregulated in mouse models of SSc and in the skin and dermal fibroblasts of patients with SSc, and that AP-1 inhibition reduced collagen synthesis in SSc fibroblasts and prevented the development of experimental dermal fibrosis (20). Thus, our data and reports from other groups suggest that LOX induces AP-1 transcription factors, resulting in an increase in ECM production and the development of fibrosis. These functions are likely due to intracellular LOX. In fact, LOX has been detected in the nuclei of cultured neonatal rat aortic smooth muscle cells and murine 3T3 fibroblasts. Intriguingly, once the extracellular form of LOX is processed, it is able to reenter the cells and localize in the nucleus. This localization is independent of the catalytic activity of the protein (15). Thus, intracellular LOX may promote AP-1 induction and transcriptional regulation of target genes such as collagen, fibronectin, and IL-6.
We examined the kinetics of LOX expression in a bleomycin-induced pulmonary fibrosis mouse model. Even though LOX expression was modestly upregulated in mouse lungs 3 and 5 days after bleomycin treatment, LOX mRNA levels were markedly increased in mouse lungs 10 days posttreatment. This result indicates that LOX expression is upregulated in the fibrotic phase in the bleomycin murine model of pulmonary fibrosis. Thus, targeting LOX is likely to impact the fibrotic, rather than inflammatory, phase of fibrosis. In fact, the benefit of targeting LOX in peritoneal fibrosis has been explored (11). Inhibition of LOX in abdominal peritoneal mesothelial cell-associated fibrosis and scarring prevented adhesion after surgery (11). Inhibition of LOX/LOXL crosslinking activity via the use of BAPN, a known inhibitor of collagen crosslinking, was tested in clinical trials for hypertrophic fibrotic scarring and keloidal scar treatment. However, the clinical trials were stopped due to toxicity (6). A humanized antibody to LOXL2, a member of the LOX family, was not effective at ameliorating IPF in a recent clinical trial (30). LOXL2 and LOX have identical enzymatic functions and promote crosslinking of collagen and elastin. In addition, they both have a “moonlighting” function as transcriptional regulators. However, it is not known if LOXL2 regulates ECM expression (15) as we observed in the current study for LOX. In addition, LOX has been shown to alter the transcriptome of lung fibroblasts (26). Specifically, LOX was shown to regulate the transcription of 134 genes in fibroblasts, although the reported perturbed genes did not include those investigated in our current study. These findings further support a “moonlighting” role for LOX in regulating gene expression. We thus propose that LOX may be a more effective therapeutic target for fibrosis than other members of the LOX family since its inhibition would reduce both its ECM-crosslinking activity and its induction of ECM and IL-6 gene expression. Recent clinical trials in SSc have included targeting the IL-6 receptor using tocilizumab. Patients with SSc receiving tocilizumab showed improvement in the severity of skin involvement (34, 35) and stabilization of lung function, although the changes in skin severity and lung function did not reach significance (18). Based on our findings, it would seem that concomitant targeting of LOX and IL-6 would be an effective strategy for improving fibrosis in more than one organ.
Copper ion is essential for the activity of LOX. d-Penicillamine is a strong chelator of copper (21). Increased expression of LOX in SSc skin has been documented (3). d-Penicillamine treatment reduces skin involvement and improves renal, cardiac, and pulmonary involvement in patients with diffuse cutaneous SSc (38), especially those positive for anti-RNA polymerase antibodies. Kazemi et al. (17) reported that d-penicillamine reduces the rate of liver fibrogenesis in patients with Wilson’s disease, a disorder whose hallmark is excess accumulation of copper in tissues. Thus, LOX inactivation via copper chelation may be one of the mechanisms mediating the beneficial effects of d-penicillamine treatment in these fibrotic disorders.
In summary, we have demonstrated that measuring LOX levels and activity serves as a novel biomarker of fibroproliferative disorders and for monitoring response to therapy and that LOX overexpression may play a pathogenic role in fibrosis by increasing expression of ECM components and IL-6. We propose that LOX is a novel therapeutic target for fibroproliferative disorders such as SSc and development of therapies, such as E4, that reduce LOX levels and activity are likely to improve organ fibrosis.
GRANTS
This publication was supported by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, through NIH/National Center for Advancing Translational Sciences (NCATS) Grant numbers TL1 TR001451 and UL1 TR001450, and by NIH National Heart, Lung, and Blood Institute Grants R01 HL121262, R01 HL153195, R01 HL133751, and R42 HL127802, and National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant K24 AR060297.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.N., A.D.B., and C.F.B. conceived and designed research; X.X.N., T.N., T.T., and L.M. performed experiments; X.X.N., T.N., T.T., and C.F.B. analyzed data; X.X.N., T.N., L.M., A.D.B., and C.F.B. interpreted results of experiments; X.X.N., T.N., and C.F.B. prepared figures; X.X.N., T.N., and C.F.B. drafted manuscript; X.X.N., T.T., L.M., A.D.B., and C.F.B. edited and revised manuscript; X.X.N., T.T., L.M., A.D.B., and C.F.B. approved final version of manuscript.
REFERENCES
- 1.Almeida I, Faria R, Vita P, Vasconcelos C. Systemic sclerosis refractory disease: from the skin to the heart. Autoimmun Rev 10: 693–701, 2011. doi: 10.1016/j.autrev.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 1a.Avouac J, Palumbo K, Tomcik M, Zerr P, Dees C, Horn A, Maurer B, Akhmetshina A, Beyer C, Sadowski A, Schneider H, Shiozawa S, Distler O, Schett G, Allanore Y, Distler JH. Inhibition of activator protein 1 signaling abrogates transforming growth factor β-mediated activation of fibroblasts and prevents experimental fibrosis. Arthritis Rheum 64: 1642–1652, 2012. doi: 10.1002/art.33501. [DOI] [PubMed] [Google Scholar]
- 2.Barker HE, Cox TR, Erler JT. The rationale for targeting the LOX family in cancer. Nat Rev Cancer 12: 540–552, 2012. doi: 10.1038/nrc3319. [DOI] [PubMed] [Google Scholar]
- 3.Chanoki M, Ishii M, Kobayashi H, Fushida H, Yashiro N, Hamada T, Ooshima A. Increased expression of lysyl oxidase in skin with scleroderma. Br J Dermatol 133: 710–715, 1995. doi: 10.1111/j.1365-2133.1995.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 4.Cheng T, Liu Q, Zhang R, Zhang Y, Chen J, Yu R, Ge G. Lysyl oxidase promotes bleomycin-induced lung fibrosis through modulating inflammation. J Mol Cell Biol 6: 506–515, 2014. doi: 10.1093/jmcb/mju039. [DOI] [PubMed] [Google Scholar]
- 5.Colwell AS, Krummel TM, Longaker MT, Lorenz HP. Early-gestation fetal scarless wounds have less lysyl oxidase expression. Plast Reconstr Surg 118: 1125–1129, 2006. doi: 10.1097/01.prs.0000221056.27536.db. [DOI] [PubMed] [Google Scholar]
- 6.Cox TR, Gartland A, Erler JT. Lysyl oxidase, a targetable secreted molecule involved in cancer metastasis. Cancer Res 76: 188–192, 2016. doi: 10.1158/0008-5472.CAN-15-2306. [DOI] [PubMed] [Google Scholar]
- 7.Feghali-Bostwick C, Medsger TA Jr, Wright TM. Analysis of systemic sclerosis in twins reveals low concordance for disease and high concordance for the presence of antinuclear antibodies. Arthritis Rheum 48: 1956–1963, 2003. doi: 10.1002/art.11173. [DOI] [PubMed] [Google Scholar]
- 8.Feghali CA, Bost KL, Boulware DW, Levy LS. Mechanisms of pathogenesis in scleroderma. I. Overproduction of interleukin 6 by fibroblasts cultured from affected skin sites of patients with scleroderma. J Rheumatol 19: 1207–1211, 1992. [PubMed] [Google Scholar]
- 9.Feghali CA, Wright TM. Identification of multiple, differentially expressed messenger RNAs in dermal fibroblasts from patients with systemic sclerosis. Arthritis Rheum 42: 1451–1457, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 10.Giampuzzi M, Botti G, Di Duca M, Arata L, Ghiggeri G, Gusmano R, Ravazzolo R, Di Donato A. Lysyl oxidase activates the transcription activity of human collagene III promoter. Possible involvement of Ku antigen. J Biol Chem 275: 36341–36349, 2000. doi: 10.1074/jbc.M003362200. [DOI] [PubMed] [Google Scholar]
- 11.Harlow CR, Wu X, van Deemter M, Gardiner F, Poland C, Green R, Sarvi S, Brown P, Kadler KE, Lu Y, Mason JI, Critchley HOD, Hillier SG. Targeting lysyl oxidase reduces peritoneal fibrosis. PLoS One 12: e0183013, 2017. doi: 10.1371/journal.pone.0183013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herzog EL, Mathur A, Tager AM, Feghali-Bostwick C, Schneider F, Varga J. Review: interstitial lung disease associated with systemic sclerosis and idiopathic pulmonary fibrosis: how similar and distinct? Arthritis Rheumatol 66: 1967–1978, 2014. doi: 10.1002/art.38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD. Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem 278: 14387–14393, 2003. doi: 10.1074/jbc.M210144200. [DOI] [PubMed] [Google Scholar]
- 14.Hsu E, Shi H, Jordan RM, Lyons-Weiler J, Pilewski JM, Feghali-Bostwick CA. Lung tissues in patients with systemic sclerosis have gene expression patterns unique to pulmonary fibrosis and pulmonary hypertension. Arthritis Rheum 63: 783–794, 2011. doi: 10.1002/art.30159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iturbide A, García de Herreros A, Peiró S. A new role for LOX and LOXL2 proteins in transcription regulation. FEBS J 282: 1768–1773, 2015. doi: 10.1111/febs.12961. [DOI] [PubMed] [Google Scholar]
- 16.Jiang D, Zhou Y, Moxley RA, Jarrett HW. Purification and identification of positive regulators binding to a novel element in the c-Jun promoter. Biochemistry 47: 9318–9334, 2008. doi: 10.1021/bi800285q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kazemi K, Geramizadeh B, Nikeghbalian S, Salahi H, Bahador A, Reza Nejatollahi SM, Dehghani SM, Dehghani M, Kakaei F, Malek-Hosseini SA. Effect of D-penicillamine on liver fibrosis and inflammation in Wilson disease. Exp Clin Transplant 6: 261–263, 2008. [PubMed] [Google Scholar]
- 18.Khanna D, Denton CP, Jahreis A, van Laar JM, Frech TM, Anderson ME, Baron M, Chung L, Fierlbeck G, Lakshminarayanan S, Allanore Y, Pope JE, Riemekasten G, Steen V, Müller-Ladner U, Lafyatis R, Stifano G, Spotswood H, Chen-Harris H, Dziadek S, Morimoto A, Sornasse T, Siegel J, Furst DE. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet 387: 2630–2640, 2016. doi: 10.1016/S0140-6736(16)00232-4. [DOI] [PubMed] [Google Scholar]
- 19.LeRoy EC The pathogenesis of systemic sclerosis. Clin Exp Rheumatol 7, Suppl 3: S135–S137, 1989. [PubMed] [Google Scholar]
- 20.Li W, Nellaiappan K, Strassmaier T, Graham L, Thomas KM, Kagan HM. Localization and activity of lysyl oxidase within nuclei of fibrogenic cells. Proc Natl Acad Sci USA 94: 12817–12822, 1997. doi: 10.1073/pnas.94.24.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipsky PE Immunosuppression by D-penicillamine in vitro. Inhibition of human T lymphocyte proliferation by copper- or ceruloplasmin-dependent generation of hydrogen peroxide and protection by monocytes. J Clin Invest 73: 53–65, 1984. doi: 10.1172/JCI111207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu SB, Ikenaga N, Peng ZW, Sverdlov DY, Greenstein A, Smith V, Schuppan D, Popov Y. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB J 30: 1599–1609, 2016. doi: 10.1096/fj.14-268425. [DOI] [PubMed] [Google Scholar]
- 23.Mäki JM, Räsänen J, Tikkanen H, Sormunen R, Mäkikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 106: 2503–2509, 2002. doi: 10.1161/01.CIR.0000038109.84500.1E. [DOI] [PubMed] [Google Scholar]
- 24.Mäki JM, Sormunen R, Lippo S, Kaarteenaho-Wiik R, Soininen R, Myllyharju J. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am J Pathol 167: 927–936, 2005. doi: 10.1016/S0002-9440(10)61183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mar AC, Chu CH, Lee HJ, Chien CW, Cheng JJ, Yang SH, Jiang JK, Lee TC. Interleukin-1 receptor type 2 acts with c-Fos to enhance the expression of interleukin-6 and vascular endothelial growth factor A in colon cancer cells and induce angiogenesis. J Biol Chem 290: 22212–22224, 2015. doi: 10.1074/jbc.M115.644823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mižíková I, Palumbo F, Tábi T, Herold S, Vadász I, Mayer K, Seeger W, Morty RE. Perturbations to lysyl oxidase expression broadly influence the transcriptome of lung fibroblasts. Physiol Genomics 49: 416–429, 2017. doi: 10.1152/physiolgenomics.00026.2017. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen XX, Muhammad L, Nietert PJ, Feghali-Bostwick C. IGFBP-5 promotes fibrosis via increasing its own expression and that of other pro-fibrotic mediators. Front Endocrinol (Lausanne) 9: 601, 2018. doi: 10.3389/fendo.2018.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimoto T, Mlakar L, Takihara T, Feghali-Bostwick C. An endostatin-derived peptide orally exerts anti-fibrotic activity in a murine pulmonary fibrosis model. Int Immunopharmacol 28: 1102–1105, 2015. doi: 10.1016/j.intimp.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilewski JM, Liu L, Henry AC, Knauer AV, Feghali-Bostwick CA. Insulin-like growth factor binding proteins 3 and 5 are overexpressed in idiopathic pulmonary fibrosis and contribute to extracellular matrix deposition. Am J Pathol 166: 399–407, 2005. doi: 10.1016/S0002-9440(10)62263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raghu G, Brown KK, Collard HR, Cottin V, Gibson KF, Kaner RJ, Lederer DJ, Martinez FJ, Noble PW, Song JW, Wells AU, Whelan TP, Wuyts W, Moreau E, Patterson SD, Smith V, Bayly S, Chien JW, Gong Q, Zhang JJ, O’Riordan TG. Efficacy of simtuzumab versus placebo in patients with idiopathic pulmonary fibrosis: a randomised, double-blind, controlled, phase 2 trial. Lancet Respir Med 5: 22–32, 2017. doi: 10.1016/S2213-2600(16)30421-0. [DOI] [PubMed] [Google Scholar]
- 31.Ramos PS, Zimmerman KD, Haddad S, Langefeld CD, Medsger TA Jr, Feghali-Bostwick CA. Integrative analysis of DNA methylation in discordant twins unveils distinct architectures of systemic sclerosis subsets. Clin Epigenetics 11: 58, 2019. doi: 10.1186/s13148-019-0652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rimar D, Rosner I, Nov Y, Slobodin G, Rozenbaum M, Halasz K, Haj T, Jiries N, Kaly L, Boulman N, Daood R, Vadasz Z. Brief report: lysyl oxidase is a potential biomarker of fibrosis in systemic sclerosis. Arthritis Rheumatol 66: 726–730, 2014. doi: 10.1002/art.38277. [DOI] [PubMed] [Google Scholar]
- 33.Santos AM, Jung J, Aziz N, Kissil JL, Puré E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest 119: 3613–3625, 2009. doi: 10.1172/JCI38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shima Y, Hosen N, Hirano T, Arimitsu J, Nishida S, Hagihara K, Narazaki M, Ogata A, Tanaka T, Kishimoto T, Kumanogoh A. Expansion of range of joint motion following treatment of systemic sclerosis with tocilizumab. Mod Rheumatol 25: 134–137, 2015. doi: 10.3109/14397595.2013.874749. [DOI] [PubMed] [Google Scholar]
- 35.Shima Y, Kuwahara Y, Murota H, Kitaba S, Kawai M, Hirano T, Arimitsu J, Narazaki M, Hagihara K, Ogata A, Katayama I, Kawase I, Kishimoto T, Tanaka T. The skin of patients with systemic sclerosis softened during the treatment with anti-IL-6 receptor antibody tocilizumab. Rheumatology (Oxford) 49: 2408–2412, 2010. doi: 10.1093/rheumatology/keq275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis 66: 940–944, 2007. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steen VD, Medsger TA Jr. Epidemiology and natural history of systemic sclerosis. Rheum Dis Clin North Am 16: 1–10, 1990. [PubMed] [Google Scholar]
- 38.Steen VD, Medsger TA Jr, Rodnan GP. D-Penicillamine therapy in progressive systemic sclerosis (scleroderma): a retrospective analysis. Ann Intern Med 97: 652–659, 1982. doi: 10.7326/0003-4819-97-5-652. [DOI] [PubMed] [Google Scholar]
- 39.Trackman PC Enzymatic and non-enzymatic functions of the lysyl oxidase family in bone. Matrix Biol 52–54: 7–18, 2016. doi: 10.1016/j.matbio.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamaguchi Y, Takihara T, Chambers RA, Veraldi KL, Larregina AT, Feghali-Bostwick CA. A peptide derived from endostatin ameliorates organ fibrosis. Sci Transl Med 4: 136ra71, 2012. doi: 10.1126/scitranslmed.3003421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Z, Xiaohua W, Lei J, Ruoyun T, Mingxia X, Weichun H, Li F, Ping W, Junwei Y. Uric acid increases fibronectin synthesis through upregulation of lysyl oxidase expression in rat renal tubular epithelial cells. Am J Physiol Renal Physiol 299: F336–F346, 2010. doi: 10.1152/ajprenal.00053.2010. [DOI] [PubMed] [Google Scholar]
- 42.Yasuoka H, Larregina AT, Yamaguchi Y, Feghali-Bostwick CA. Human skin culture as an ex vivo model for assessing the fibrotic effects of insulin-like growth factor binding proteins. Open Rheumatol J 2: 17–22, 2008. doi: 10.2174/1874312900802010017. [DOI] [PMC free article] [PubMed] [Google Scholar]