Abstract
We tested the hypothesis that inducible nitric oxide synthase (iNOS) contributes to reduced nitric oxide (NO)-dependent vasodilation in non-Hispanic Blacks and prehypertensive non-Hispanic Whites. Twenty Black and twenty White participants (10 normotensive, 10 prehypertensive per group; n = 40 total) participated in this study. Participants were instrumented with two microdialysis fibers, and each site was randomized as control (lactated Ringer) or iNOS inhibition (0.1 mM 1400W). Laser-Doppler flow probes and local heaters were used to measure skin blood flow and heat the skin to induce vasodilation, respectively. Each site was heated from 33°C to 39°C (rate: 0.1°C/s). Once a plateau was established, 20 mM nitro-l-arginine methyl ester (l-NAME), a nonspecific NOS inhibitor, was infused at each site to quantify NO-dependent vasodilation. At control sites, %NO-dependent vasodilation was reduced in prehypertensive Whites (47 ± 10%NO) and in both normotensive and prehypertensive Blacks (39 ± 9%NO and 28 ± 5%NO, respectively) relative to normotensive Whites (73 ± 8%NO; P < 0.0001 for all comparisons). Compared with respective control sites, iNOS inhibition increased NO-dependent vasodilation in prehypertensive Whites (68 ± 8%NO) and in both normotensive and prehypertensive Blacks (78 ± 8%NO and 55 ± 6%NO, respectively; P < 0.0001 for all comparisons). We failed to find an effect for normotensive Whites (77 ± 7%NO). After iNOS inhibition, %NO-dependent vasodilation was similar between normotensive Whites, prehypertensive Whites, and normotensive Blacks. Inhibition of iNOS increased NO-dependent vasodilation to a lesser extent in prehypertensive Blacks. These data suggest that iNOS contributes to reduced NO-dependent vasodilation in prehypertension and in Black participants.
NEW & NOTEWORTHY Inducible nitric oxide synthase (iNOS) is typically upregulated in conditions of increased oxidative stress and may have detrimental effects on the vasculature. Endothelial nitric oxide (NO), which is cardioprotective, is reduced in prehypertensive non-Hispanic Whites and in non-Hispanic Blacks. We found that inhibition of iNOS can increase endothelial NO-dependent vasodilation in prehypertensive White participants and in both normotensive and prehypertensive Black participants.
Keywords: human, microdialysis, microvascular, nitric oxide
INTRODUCTION
Non-Hispanic Blacks demonstrate enhanced vasoconstrictor and blunted vasodilator responses to pharmacological interventions, in both conduit vessels and microvessels, compared with non-Hispanic Whites (1–10). These enhanced vasoconstrictor and blunted vasodilator responses may partly explain the higher incidence rates of cardiovascular disease and hypertension in the Black population (11, 12). Recently, our laboratory demonstrated that blunted sensory nerve-mediated and endothelium-dependent vasodilation occurs in young, healthy Blacks and is associated with a decrease in nitric oxide (NO) bioavailability (13, 14). Research suggests that the decrease in NO bioavailability may be due, in part, to an increase in oxidative stress and a decrease in antioxidant capacity (15, 16). Thus, examining treatments to improve NO bioavailability by reducing the prooxidant environment is a current area of research. For example, local infusion of l-arginine, a precursor to NO, improved the cutaneous microvascular response to local heating in young, healthy Blacks (17). Additionally, upregulation of NADPH oxidase and xanthine oxidase activity in endothelial cells of Blacks compared with Whites increases superoxide production and decreases NO production (4, 18). Inhibition of NADPH oxidase (4, 19), inhibition of xanthine oxidase(4), and administration of a superoxide dismutase mimetic (2) are all capable of restoring the NO contribution to cutaneous microvascular vasodilation in response to local heating in Blacks.
Endothelial dysfunction associated with increased oxidative stress and reduced antioxidant capacity can trigger other injurious pathways in the vasculature. For example, inducible nitric oxide synthase (iNOS) expression is upregulated in hypertension (20), and iNOS activity stimulates an overproduction of NO. In cellular environments where there is already heightened oxidative stress, this overproduction of NO, which itself is a free radical, is readily converted to superoxide and peroxynitrite and can further reduce endothelial NO-dependent vasodilation (20–22). In addition, iNOS can uncouple NO production from endothelial nitric oxide synthase (eNOS) because of an upregulation of arginase, which competes for the NO precursor l-arginine. Collectively, the overproduction of NO via iNOS and the uncoupling of eNOS can result in reduced NO-dependent vasodilation. As discussed above, increased oxidative stress and reduced antioxidant capacity are present in Blacks and are partly responsible for reduced eNOS-mediated vasodilation. Whether the increase in oxidative stress stimulates an overproduction of NO via iNOS and, subsequently, uncoupling of eNOS in Blacks with or without hypertension (22) is unknown.
The cutaneous microcirculation offers a representative surrogate for otherwise less accessible microvascular beds (23–27). Importantly, local heating of the skin allows for simultaneous assessment of sensory nerve-mediated and eNOS-dependent microvascular vasodilation. The purpose of this study was to investigate the role of iNOS in cutaneous sensory nerve-mediated and endothelial NO-dependent vasodilation in normotensive and prehypertensive Blacks and Whites. We hypothesized that inhibition of iNOS would augment microvascular NO-dependent vasodilation in prehypertensive Whites and in both normotensive and prehypertensive Blacks.
METHODS
Ethical Approval
All procedures were approved by the Advarra Institutional Review Board and the Georgia State University Institutional Review Board (Pro00024265). Approval for all drugs was obtained from the Food and Drug Administration (IND 138231). According to NIH guidelines, this was not a clinical trial and was not registered in a clinical trial database. Participants were verbally instructed of the purposes, procedures, and risks involved in the study before providing their informed written and verbal consent. The study obeyed the regulations set forth by the Declaration of Helsinki.
Participants
Twenty individuals who identified as Black (10 women, 10 men) and 20 individuals who identified as White (13 women, 7 men), all between the ages of 18 and 40 yr, participated in the study. Participants were divided into groups of 10 based on race and blood pressure status, normotensive or prehypertensive, following the average of three manual blood pressure measurements. Blood pressure groupings were based on the 2017 American Heart Association (AHA) guidelines (normotensive: systolic blood pressure <120 mmHg and diastolic blood pressure <80 mmHg; prehypertensive: systolic blood pressure between 120 and 129 mmHg or diastolic blood pressure between 80 and 89 mmHg) (28). All participants were nonsmokers, with no history of known cardiovascular, metabolic, or neurological diseases as confirmed by a medical history questionnaire.
Female participants were required to have a negative urine pregnancy test (McKesson hCG Combo Test Casssette; Consult Diagnostics, Richmond, VA) on the day of the experimental protocol. Phase of menstrual cycle or oral contraceptive use was documented, but not controlled, in the present study for two reasons: 1) only testing women during the early follicular/placebo phase when estrogen and progesterone are low reduces external validity, and 2) to our knowledge, there are data demonstrating effects of oral contraceptives, but not the normal menstrual cycle, on cutaneous vascular responses to local heating (29,30). Furthermore, there are data demonstrating no effect of the menstrual cycle on vascular function in either the conduit vessels or the microvasculature, suggesting that testing women during all phases of the menstrual cycle/oral contraceptives does not significantly increase variability in the data (31–35). Of the 10 Black female participants, 5 were taking oral contraceptives and 3 were tested during the placebo/low-hormone phase; 2 of 5 normally menstruating Black female participants were tested during the menstrual/early follicular phase. Of the 13 White female participants, 9 were taking oral contraceptives and 5 were tested during the placebo/low-hormone phase; 1 of 4 naturally menstruating White female participants was tested during the menstrual/early follicular phase. Experiments were conducted throughout the day, and participants were asked to avoid alcohol, caffeine, and strenuous exercise for 8 h before the trial. Additionally, participants were instructed to avoid high-fat meals for 8–12 h before experiments. All trials were conducted in a temperature-controlled laboratory at Georgia State University (∼24°C and ∼40% relative humidity).
Instrumentation
Upon arrival at the laboratory, participants were weighed and had their height measured with a digital scale with a platform stadiometer (Healthometer Professional; Pelstar, Alslip, IL). Participants sat in a semirecumbent position for the remainder of the experimental trial. Intermittent blood pressure measurements were assessed every 8–12 min with a digital sphygmomanometer (Vital Signs Series 6000; Welch Allyn, Skaneateles Falls, NY). Mean arterial pressure was calculated as one-third pulse pressure plus diastolic pressure.
Two microdialysis fibers (CMA 31 Linear Microdialysis Probe, 55-kDa cutoff membrane; CMA Microdialysis AB, Kista, Sweden) were inserted intradermally into the skin on the dorsal side of the left forearm. An ice pack was used to numb the skin (∼5 min) before the microdialysis fibers were placed (36). The skin was cleaned with sterile alcohol wipes, and a 23-gauge needle was inserted into the dermal layer of the skin. Each site was placed at least 4 cm apart, with entry and exit points at each site ∼2.5 cm apart. Microdialysis fibers were then threaded through the lumen of each needle until the semipermeable membrane was next to the needle. The needle and fiber were then moved until the membrane was below the dermis. Finally, the needle was completely removed, and the fibers were taped to the skin to ensure positioning. After placement, fibers were perfused, via microinfusion pump (BASi Bee Hive, West Lafayette, IN), with lactated Ringer solution (Baxter Healthcare USP; Deerfield, IL) at 2.0 µL/min. Cutaneous red blood cell flux, an index of skin blood flow, was measured over each microdialysis fiber with a laser-Doppler flow probe (VP7 A/T with Moor VMS-LDF2; Moor Instruments, Wilmington, DE). Each laser-Doppler probe was placed within the center of a local heating element (Moor Instruments).
Blood samples were obtained at the beginning of each experimental trial, after an overnight fast, from the antecubital vein, and samples were stored in two Vacutainers with 10.8 mg of K2EDTA. One of the samples was then centrifuged (Beckman GS-6KR, Beckman Coulter Centrifuge; Indianapolis, IN) at 1,000 rpm and 3°C for 10 min. The centrifuged sample was then split between plasma and hematocrit, and both whole blood and centrifuged samples were stored in a −80°C freezer (Revco UxF; Thermo Fisher Scientific, Waltham, MA) until subsequent analysis. Blood samples were analyzed for glutathione peroxidase and superoxide dismutase activities (measures of antioxidant capacity) and 8-isoprostane concentration (a measure of oxidative stress) with commercially available ELISA kits (Cayman Chemical Company, Ann Arbor, MI).
Experimental Protocol
After placement of microdialysis fibers, skin blood flow was monitored until trauma resolution from the needle insertion had dissipated (∼45–60 min). Next, the microdialysis fibers were randomly assigned to receive a lactated Ringer solution (control) or 0.1 mM 1400W (20) (AdipoGen, San Diego, CA), an iNOS inhibitor, at a rate of 2.0 µL/min. To ensure adequate inhibition of iNOS, 1400W was infused for at least 45 min and for the duration of the protocol.
Local heaters were set to 33°C during baseline. Skin blood flow was monitored until a stable baseline was achieved (∼10–15 min). After baseline, local heaters were raised to 39°C at a rate of 0.1°C/s (9). During local heating the skin exhibits a biphasic, hyperemic response that is facilitated by two mechanisms. First, an initial peak occurs within the first ∼1–2 min of heating and is primarily mediated by cutaneous sensory nerves, with a moderate contribution from endothelium-dependent and -independent mechanisms (37–45). Over time (15–30 min), the vasodilation to local heating achieves a plateau, which is primarily mediated (∼60–80%) by endothelial NO (37–39, 42–50). When a plateau in skin blood flow in response to local heating was achieved (∼30–40 min), 20 mM of the nonspecific NOS inhibitor nitro-l-arginine methyl ester (l-NAME) (EMD Millipore Corp., Burlington, MA), was perfused to quantify the contribution of NO to the plateau in skin blood flow. Once a post-l-NAME plateau was achieved, the temperature of the local heaters was increased to 43°C and 54 mM sodium nitroprusside (SNP) (EMD Millipore Corp., Burlington, MA) was infused to induce maximal cutaneous vasodilation.
Data Analysis
Skin blood flow and local heater temperature were continuously sampled at 40 Hz (PowerLab 16/35; ADInstruments, Colorado Springs, CO) with commercially available software (LabChart 8; ADInstruments). Cutaneous vascular conductance (CVC) was calculated as red blood cell flux divided by mean arterial blood pressure (MAP). All CVC data are presented as a percentage of maximal CVC (%CVCmax).
Statistical Analysis
Sample size was determined with an a priori power analysis. Effect sizes were specified based on preliminary data from pilot studies completed in our laboratory. Assuming an α level of 0.05, 80% power, and mean %NO-dependent vasodilation of 47% [standard deviation (SD) = 8%] and 70% (SD = 5%) for Blacks and Whites, respectively, the needed sample size to detect this difference in means was 10 per group. Skin blood flow (%CVCmax) data were analyzed by a three-way ANOVA with factors of race (Black and White), blood pressure status (normotensive and prehypertensive), and microdialysis treatment (control and 1400W). For the three-way ANOVA, we began with a model of main effects and explored additive interaction terms for each two-way interaction. Differences in glutathione peroxidase activity, superoxide dismutase activity, and concentration of 8-isoprostane between the four groups were analyzed by a two-way ANOVA with factors of race and blood pressure status. For all ANOVAs, Tukey correction factors were used to account for multiple pairwise comparisons. All data were analyzed and graphed with commercially available software (SAS, Cary, NC and GraphPad Prism 8, San Diego, CA). All data are presented as means ± SD. The level of significance was set at 0.05.
RESULTS
Anthropometrics, Baseline %CVCmax, and Maximal CVC
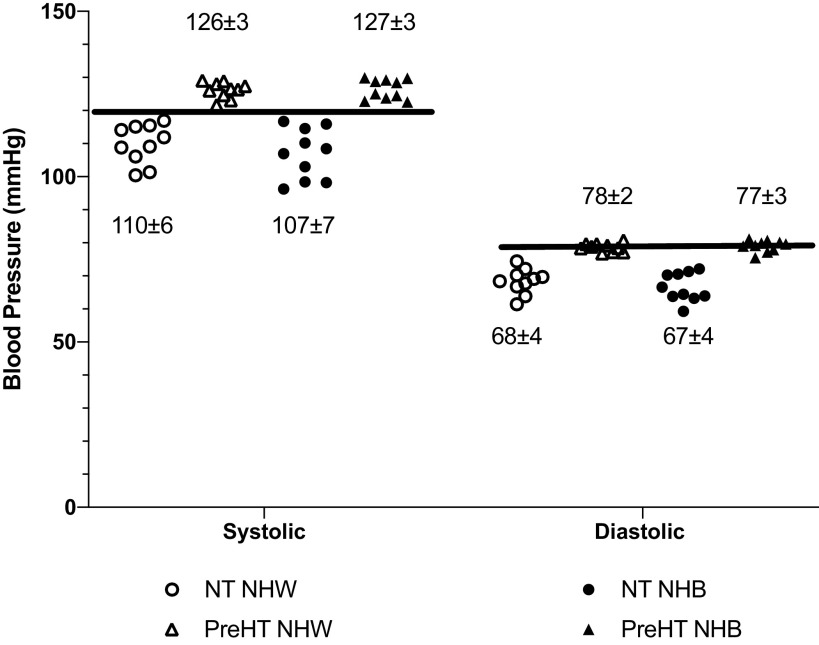
Anthropometric data and hemodynamic variables are shown in Table 1, and baseline %CVCmax and maximal CVC data are shown in Table 2. Individual systolic, diastolic, and mean arterial blood pressures for each participant are shown in Fig. 1. All normotensive participants were within normotensive ranges for both systolic and diastolic blood pressure; all prehypertensive participants had a systolic blood pressure in excess of 120 mmHg, but diastolic blood pressure ranged between 77 and 81 mmHg.
Table 1.
Participant demographics
| Non-Hispanic White Normotensive | Non-Hispanic Black Normotensive | Non-Hispanic White Prehypertensive | Non-Hispanic Black Prehypertensive | |
|---|---|---|---|---|
| n, male, female | 7, 3 | 5, 5 | 6, 4 | 5, 5 |
| Age, yr | 26 ± 5 | 23 ± 3 | 23 ± 2 | 21 ± 3 |
| Height, m | 1.72 ± 0.10 | 1.72 ± 0.09 | 1.69 ± 0.13 | 1.90 ± 0.06 |
| Mass, kg | 73.11 ± 13.04 | 73.57 ± 9.95 | 71.29 ± 13.32 | 75.07 ± 9.57 |
| BMI, kg/m2 | 24.72 ± 2.7 | 25.00 ± 3.41 | 25.00 ± 4.50 | 20.91 ± 2.96 |
| Systolic BP, mmHg | 110 ± 6 | 107 ± 8 | 126 ± 2 | 127 ± 3 |
| Diastolic BP, mmHg | 68 ± 4 | 67 ± 4 | 79 ± 2 | 78 ± 3 |
| Mean arterial pressure, mmHg | 82 ± 4 | 80 ± 6 | 89 ± 7 | 93 ± 4 |
| Heart rate, beats/min | 63 ± 5 | 70 ± 12 | 73 ± 8 | 70 ± 12 |
Data are shown as means ± SD; n, number of participants. BMI, body mass index; BP, blood pressure.
Table 2.
Baseline (%CVCmax) and maximal (CVC) values
| n, male, female | Baseline (%CVCmax) |
Maximal (CVC) |
|||
|---|---|---|---|---|---|
| Control | 1400W | Control | 1400W | ||
| Non-Hispanic White normotensive | 7, 3 | 17 ± 9 | 17 ± 7 | 3.01 ± 0.51 | 2.74 ± 0.92 |
| Non-Hispanic Black normotensive | 5, 5 | 12 ± 5 | 17 ± 12 | 2.92 ± 0.60 | 2.72 ± 0.68 |
| Non-Hispanic White prehypertensive | 6, 4 | 19 ± 10 | 17 ± 9 | 3.01 ± 0.85 | 3.19 ± 0.91 |
| Non-Hispanic Black prehypertensive | 5, 5 | 21 ± 15 | 23 ± 16 | 2.44 ± 0.58 | 2.70 ± 0.63 |
Data are shown as means ± SD; n, number of participants. CVC, cutaneous vascular conductance; CVCmax, maximum CVC.
Figure 1.
Individual blood pressure responses from the experimental trial. The horizontal lines indicate the threshold for prehypertension for both systolic and diastolic blood pressure. All normotensive participants had systolic and diastolic blood pressures below the threshold, and all prehypertensive participants had a systolic blood pressure above the threshold. Although the mean diastolic blood pressure for the prehypertension group was below the threshold, most participants were at, or very near, the diastolic threshold. NT NHW, normotensive non-Hispanic White (n = 10 participants); PreHT NHW, prehypertensive non-Hispanic White (n = 10 participants); NT NHB, normotensive non-Hispanic Black (n = 10 participants); PreHT NHB, prehypertensive non-Hispanic Black (n = 10 participants).
Initial Peak Data
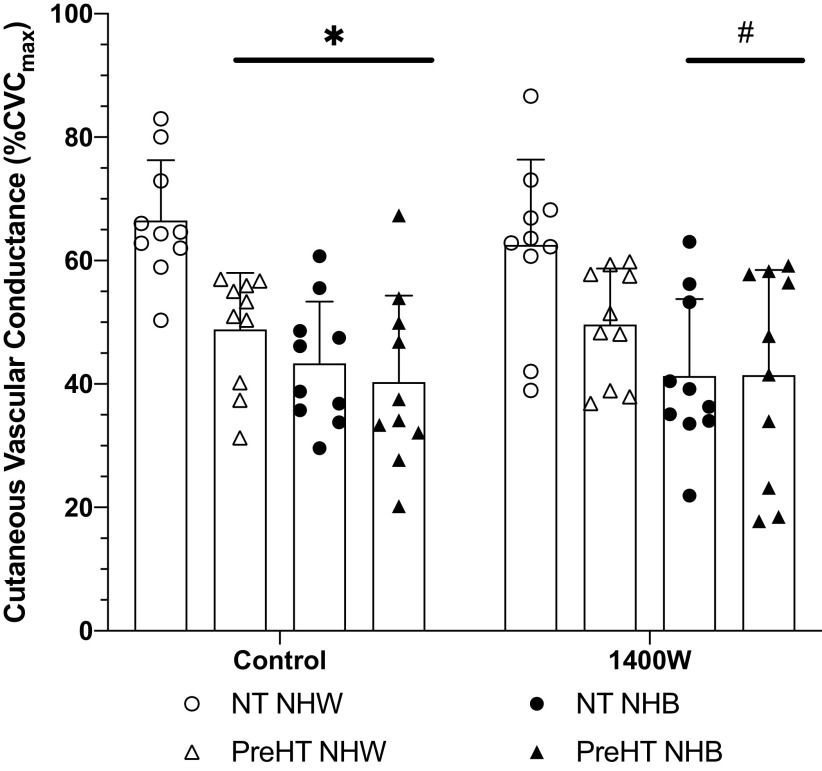
The initial peak response to local heating is shown in Fig. 2. Initial peak values at the control site for normotensive Whites (67 ± 10%CVCmax) were statistically different compared with prehypertensive Whites (49 ± 9%CVCmax; P = 0.03), normotensive Blacks (43 ± 10%CVCmax; P < 0.01), and prehypertensive Blacks (40 ± 14%CVCmax; P < 0.01).
Figure 2.
Initial peak % of maximum cutaneous vascular conductance (%CVCmax) responses. Group responses are shown as means ± SD and were compared with a 3-way ANOVA. At control sites, initial peak responses were reduced in prehypertensive non-Hispanic White (PreHT NHW, n = 10 participants), normotensive non-Hispanic Black (NT NHB, n = 10 participants), and prehypertensive non-Hispanic Black (PreHT NHB, n = 10 participants) relative to normotensive non-Hispanic White (NT NHW; n = 10 participants). At 1400W sites, the initial peak was reduced in both NT NHB and PreHT NHB relative to both NT NHW and PreHT NHW. *P < 0.05 vs. NT NHW at control sites; #P < 0.05 vs. NHW NT at 1400W sites.
Compared with control, there was not a meaningful effect of iNOS inhibition on the initial peak for normotensive Whites (63 ± 14%CVCmax; P = 0.99), prehypertensive Whites (50 ± 9%CVCmax; P > 0.99), normotensive Blacks (41 ± 12%CVCmax; P > 0.99), or prehypertensive Blacks (41 ± 17%CVCmax; P > 0.99). At iNOS-inhibited sites, initial peak responses for normotensive Whites were statistically different compared with normotensive Blacks and compared with prehypertensive Blacks (P < 0.01 for both comparisons).
Plateau Data
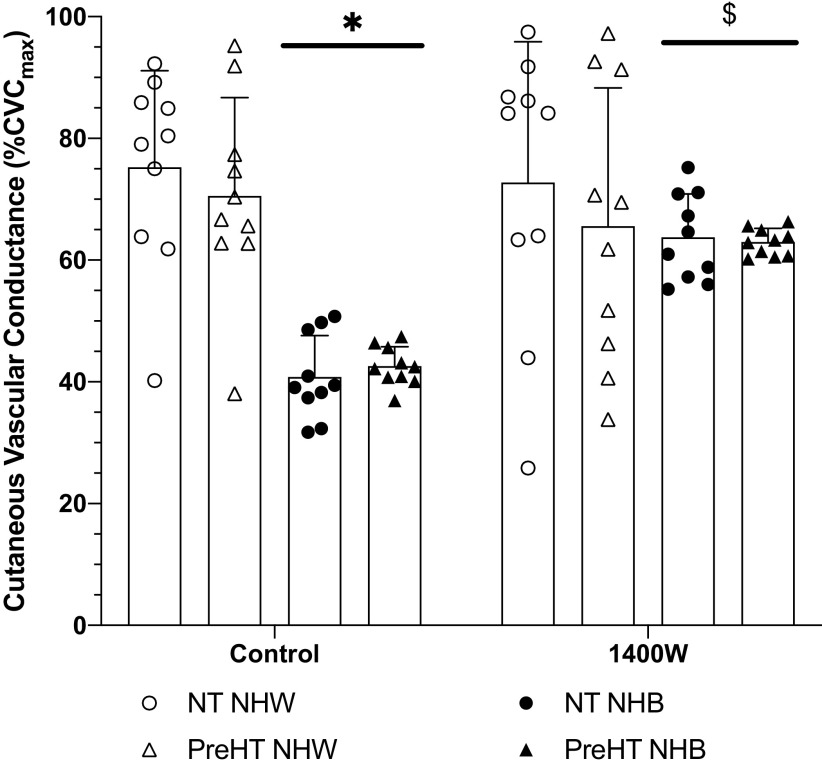
The plateau phase of the local heating response is displayed in Fig. 3. At control sites, plateau values were statistically different between normotensive Whites (75 ± 16%CVCmax) and both normotensive (41 ± 7%CVCmax; P < 0.01) and prehypertensive (43 ± 3%CVCmax; P < 0.01) Blacks. Observed differences also occurred between prehypertensive Whites (71 ± 16%CVCmax) and both normotensive (P < 0.01) and prehypertensive (P < 0.01) Blacks.
Figure 3.
Plateau % of maximum cutaneous vascular conductance (%CVCmax) responses. Group responses are shown as means ± SD and were compared with a 3-way ANOVA. At control sites, plateau responses were reduced in normotensive (NT NHB, n = 10 participants) and prehypertensive (PreHT NHB, n = 10 participants) non-Hispanic Blacks relative to normotensive (NT NHW, n = 10 participants) and prehypertensive (PreHT NHW, n = 10 participants) non-Hispanic Whites. At 1400W sites, there were no differences between groups. Compared with control sites, 1400W significantly increased the plateau in NT NHB and PreHT NHB. *P < 0.05 vs. NT NHW and PreHT NHW at control sites; $P < 0.05 vs. respective control sites.
We failed to find a difference between control and 1400W sites for normotensive Whites (73 ± 23%CVCmax; P > 0.99) or prehypertensive Whites (66 ± 22%CVCmax; P = 0.99). Compared with respective control sites, 1400W augmented the plateau in normotensive (64 ± 9%CVCmax; P = 0.01) and prehypertensive (63 ± 6%CVCmax; P = 0.04) Blacks.
Post l-NAME Plateau Data
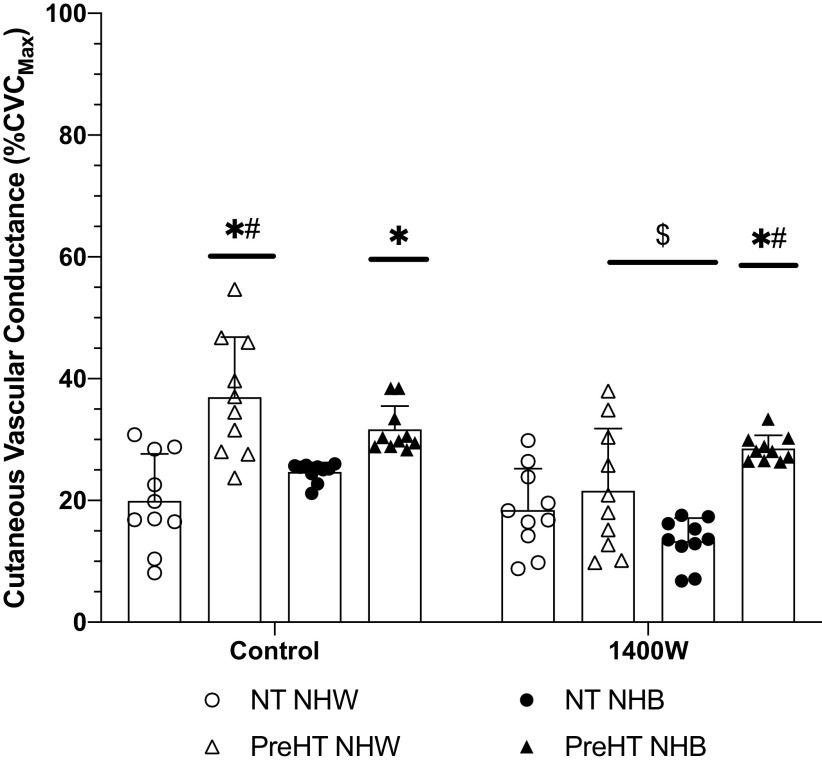
The post l-NAME plateau is displayed in Fig. 4. At control sites, differences in post-l-NAME plateau responses were observed between normotensive Whites (20 ± 8%CVCmax) and prehypertensive Whites (37 ± 10%CVCmax; P < 0.01) and prehypertensive Blacks (32 ± 4%CVCmax; P < 0.01). There was also an observed difference between prehypertensive and normotensive Blacks (P < 0.01). The post-l-NAME plateau in normotensive Blacks averaged 25 ± 5%CVCmax.
Figure 4.
Post-nitro-l-arginine methyl ester (l-NAME) plateau % of maximum cutaneous vascular conductance (%CVCmax) responses. Group responses are shown as means ± SD and were compared with a 3-way ANOVA. At control sites, the post-l-NAME plateau was greater in prehypertensive non-Hispanic Whites (PreHT NHW, n = 10 participants) and prehypertensive non-Hispanic Blacks (PreHT NHB, n = 10 participants) relative to normotensive non-Hispanic Whites (NT NHW, n = 10 participants). The PreHT NHW post-l-NAME plateau at control sites was significantly greater than that for normotensive non-Hispanic Blacks (NT NHB, n = 10 participants). There was no difference between NT NHW and NT NHB at control sites. At 1400W sites, the post-l-NAME plateau was significantly reduced compared to the respective control sites for PreHT NHW and NT NHB, but there was no observed effect of 1400W for NT NHW or PreHT NHB. The 1400W post-l-NAME plateau was significantly greater in PreHT NHB relative to both NT NHW and NT NHB. *P < 0.05 vs. NT NHW within a treatment site; #P < 0.05 vs. NT NHB within a treatment site; $P < 0.05 vs. respective control site.
Compared with control, the post-l-NAME plateau at 1400W sites was significantly reduced (i.e., there was a greater response to nitric oxide synthase inhibition) in prehypertensive Whites (22 ± 10%CVCmax; P < 0.01) and normotensive Blacks (13 ± 6%CVCmax; P < 0.05). There was no statistical difference between control and 1400W sites for normotensive Whites (20 ± 8%CVCmax vs. 18 ± 7%CVCmax; P = 0.99) or for prehypertensive Blacks (32 ± 4%CVCmax vs. 29 ± 9%CVCmax; P = 0.96). Between-group comparisons at 1400W sites revealed a statistical difference between normotensive Whites and prehypertensive Blacks (P = 0.02) as well as between normotensive and prehypertensive Blacks (P < 0.01).
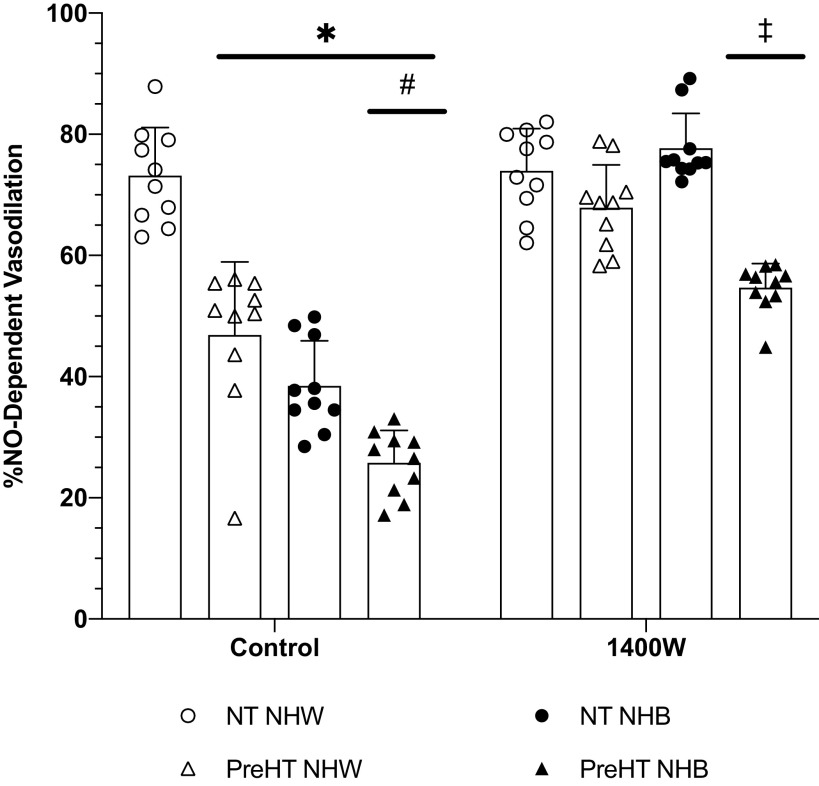
Percent NO-Dependent Vasodilation
The percent contribution of NO to the plateau, an estimate of microvascular endothelial NO-dependent vasodilation, is shown in Fig. 5. At control sites, the percent NO-dependent vasodilation in normotensive Whites (73 ± 8%NO) was significantly greater compared with prehypertensive Whites (47 ± 10%NO; P < 0.01), normotensive Blacks (39 ± 9%NO; P < 0.01), and prehypertensive Blacks (28 ± 5%NO; P < 0.01). There were also observed differences at control sites between prehypertensive Whites and Blacks (P < 0.01) and between normotensive and prehypertensive Blacks (P < 0.01).
Figure 5.
Calculated % nitric oxide (%NO)-dependent vasodilation. Group responses are shown as means ± SD and were compared with a 3-way ANOVA. At control sites, %NO-dependent vasodilation was significantly reduced in prehypertensive non-Hispanic Whites (PreHT NHW; n = 10 participants), normotensive non-Hispanic Blacks (NT NHB; n = 10 participants), and prehypertensive non-Hispanic Blacks (PreHT NHB; n = 10 participants) relative to normotensive non-Hispanic Whites (NT NHW; n = 10 participants). The %NO-dependent vasodilation at control sites in PreHT NHB was significantly reduced compared to PreHT NHW and NT NHB. At 1400W sites, %NO-dependent vasodilation was restored in both PreHT NHW and NT NHB. The %NO-dependent vasodilation at 1400W sites was increased in PreHT NHB, but this was reduced relative to all other groups. *P < 0.05 vs. NT NHW; #P < 0.05 vs. PreHT NHW and NT NHB; ‡P < 0.05 vs. NT NHW, PreHT NHW, and NT NHB.
Compared with control sites, inhibition of iNOS with 1400W augmented percent NO-dependent vasodilation in prehypertensive Whites (68 ± 8%NO; P < 0.01), normotensive Blacks (78 ± 8%NO; P < 0.01), and prehypertensive Blacks (55 ± 6%NO; P < 0.01); however, we failed to find a difference between control and 1400W sites in normotensive Whites (73 ± 8%NO vs. 77 ± 7%NO; P > 0.99). There were statistical differences at 1400W sites between prehypertensive Blacks and normotensive Whites (P < 0.01), prehypertensive Whites (P < 0.01), and normotensive Blacks (P < 0.01).
Glutathione Peroxidase Activity, Superoxide Dismutase Activity, and 8-Isoprostane Concentration
Glutathione peroxidase activity (Table 3) was statistically greater in normotensive and prehypertensive Blacks compared with prehypertensive Whites (P = 0.02). We failed to find a difference in superoxide dismutase activity between any of the groups (Table 3). 8-Isoprostane (Table 3) was greater in prehypertensive Blacks relative to both normotensive (P = 0.04) and prehypertensive (P = 0.02) Whites.
Table 3.
Glutathione peroxidase, superoxide dismutase, and 8-isoprostane data
| Non-Hispanic White |
Non-Hispanic Black |
|||
|---|---|---|---|---|
| Normotensive | Prehypertensive | Normotensive | Prehypertensive | |
| n, male, female | 7, 3 | 6, 4 | 5, 5 | 5, 5 |
| Glutathione peroxidase, nmol/min/mL | 719.76 ± 548.92 | 520.57 ± 176.81 | 1,449.15 ± 792.40* | 1,429.50 ± 886.73* |
| Superoxide dismutase, U/mL | 0.65 ± 0.49 | 0.89 ± 0.85 | 0.68 ± 0.47 | 1.01 ± 0.37 |
| 8-Isoprostane, pg/mL | 96.06 ± 75.89 | 87.71 ± 50.65 | 129.33 ± 53.30 | 183.98 ± 89.02*# |
Values are means ± SD; n, number of participants. *P < 0.05 vs. prehypertensive non-Hispanic White; #P < 0.05 vs. normotensive non-Hispanic White.
DISCUSSION
The primary finding of this study is that inhibition of iNOS with 1400W in prehypertensive Whites and normotensive Blacks increased cutaneous endothelial NO-dependent vasodilation to levels similar to those observed in normotensive Whites. Inhibition of iNOS also improved endothelial NO-dependent vasodilation in prehypertensive Blacks but to a lesser extent. The mechanisms by which iNOS inhibition improved cutaneous endothelial NO-dependent vasodilation appear to differ for each group (discussed in detail below).
Initial Peak Response to Local Heating
The rapid, initial peak response is mediated predominantly by sensory nerve mechanisms, with a minor contribution from NO. The data from the present study are consistent with recent findings from our laboratory suggesting that sensory nerve-mediated cutaneous vasodilation is reduced in prehypertensive Whites and in normotensive and prehypertensive Blacks relative to normotensive Whites (13, 14). Although our previous studies demonstrated both reduced sensory nerve-mediated and NO-dependent components of the initial peak in these groups, the experimental design in our previous studies did not allow us to investigate mechanisms contributing to reduced responses (13, 14). The data from the present study indicate that iNOS is not responsible for any reduction in the initial peak response to local heating, as evidenced by the lack of effect at iNOS-inhibited sites relative to control sites for prehypertensive Whites or for normotensive and prehypertensive Blacks (Fig. 2). It thus appears that other mechanisms are responsible for the reduced initial peak response; however, those mechanisms remain unknown at this time.
Plateau Response to Local Heating and Calculated %NO-Dependent Vasodilation
Data from this study demonstrate that inhibition of iNOS restores cutaneous vasodilation independent of blood pressure status in Blacks. The present data also suggest that increased iNOS activity occurs in individuals, regardless of race, with subclinical increases in blood pressure. These data support the hypothesis that increased iNOS activity contributes to reduced endothelial NO-dependent vasodilation and that non-Hispanic Blacks may be more susceptible to the effects of iNOS.
Under optimal conditions, expression of iNOS is minimal; however, during pathophysiological conditions such as burn wounds (51), infection, chronic inflammation (52), and tumor proliferation, iNOS continuously and erratically produces NO (53). Expression of iNOS requires interferon regulatory factor 1 and nuclear factor κ-light chain by activated B cells, thus explaining why iNOS upregulation is indicative of a proinflammatory environment (16, 54, 55). Research suggests that upregulation of iNOS expression in the endothelium impairs vasomotor function by limiting the availability of the cofactor tetrahydrobiopterin for eNOS, which can be attributed to the inflammatory response (56). Additionally, iNOS upregulation may impair vascular smooth muscle contractions via soluble guanylate cyclase and thus activation of the NO/cGMP pathway (56). Chronic iNOS-induced inflammation can lead to a host of vascular disorders including hypertension (20) and arterial stiffness (56, 57), which may play a part in the onset and progression of cardiovascular disease.
The plateau and post-l-NAME plateau data indicate that mechanisms of reduced microvascular endothelial NO-dependent vasodilation, and subsequent improvement with iNOS inhibition, differ between prehypertensive Whites, normotensive Blacks, and prehypertensive Blacks (Figs. 3–5) relative to normotensive Whites. In prehypertensive Whites, there was a blunted delta response (i.e., difference between the plateau and post-l-NAME plateau), but the overall plateau response was similar to normotensive Whites. The blunted delta response to l-NAME in prehypertensive Whites is an interesting result. On the basis of the present data, it is unclear as to why the delta response was blunted in prehypertensive Whites. It is possible that under conditions of prehypertension there is compensation or upregulation of NO-independent mechanisms, such as endothelium-derived hyperpolarizing factors (58), that preserve the magnitude of the plateau. Since these modulating mechanisms would be independent of NO, they would be insensitive to NO inhibition and would manifest as a blunted delta response. In normotensive Blacks the post-l-NAME plateau was similar to normotensive Whites, but the overall plateau response was reduced. In prehypertensive Blacks, there is a reduced delta response. The relative response to iNOS inhibition within group mimicked the differences displayed at control sites. Inhibition of iNOS augmented the response to l-NAME only in prehypertensive Whites (i.e., a greater reduction in the post-l-NAME plateau) but augmented both the response to l-NAME and the overall plateau in normotensive Blacks. In prehypertensive Blacks, inhibition of iNOS only augmented the overall plateau response. Regardless of mechanism of action, iNOS inhibition augmented the overall delta response such that endothelial NO-dependent cutaneous vasodilation was increased in prehypertensive Whites, normotensive Blacks, and prehypertensive Blacks.
The reasons for these differential responses at both control and iNOS inhibition sites are unknown, but they clearly indicate different mechanisms of vascular control. The almost complete restoration of endothelial NO-dependent vasodilation following iNOS inhibition in both prehypertensive Whites and normotensive Blacks suggests that reductions in endothelial NO-dependent vasodilation in these populations may be reversed by interventions that reduce the influence of iNOS activity. Conversely, reducing iNOS activity in prehypertensive Blacks does not appear to be sufficient to completely reverse any observed reductions in endothelial NO-dependent vasodilation.
Antioxidant Capacity and Oxidative Stress
Oxidative stress is a result of an imbalance between prooxidants and antioxidants. Oxidative stress leads to endothelial dysfunction and promotes a proinflammatory environment within the vasculature, which may lead to atherosclerosis and other cardiovascular disease (CVD) comorbidities (2, 3, 15, 19, 59). Here, our results suggest that the ability of antioxidant defense mechanisms to sequester the elevation in oxidants in Blacks is altered. For example, levels of 8-isoprostanes, a surrogate marker of oxidant stress, were increased in prehypertensive Blacks. Interestingly, 8-isoprostane was elevated despite increased activity of glutathione peroxidase in this population. Because glutathione is a powerful antioxidant and promotes a healthy vasculature, we contend that elevated glutathione peroxidase activity in the Black population serves as a protective mechanism in response to the increase in 8-isoprostanes. It is important to note that we only measured one biomarker of oxidative stress (8-isoprostanes). It is possible that other biomarkers of oxidative stress may be upregulated in prehypertensive Whites and/or normotensive and prehypertensive Blacks.
We failed to find any differences in superoxide dismutase activity between groups. Previous data suggest that superoxide production is higher in the Black population compared with the White population (18, 60). In the cutaneous circulation, recent data from Hurr et al. (2) and Patik et al. (4) suggest that microdialysis infusion of TEMPOL (superoxide dismutase mimetic), allopurinol (xanthine oxidase inhibition), or apocynin (NADPH oxidase inhibition) improves endothelial NO-dependent vasodilation in young, healthy Black participants, suggesting that the reactive oxygen species superoxide reduces NO-dependent vasodilation in the Black population. Data from Kim and Brothers (61) further suggest that consumption of flavanol, which may have antioxidant properties, can improve cutaneous microvascular function in young, healthy Black participants . The present superoxide dismutase biomarker data are not necessarily at odds with the findings from these previous studies. First, infusion of TEMPOL via microdialysis introduces a relatively high concentration of superoxide dismutase that may counteract any superoxide in the local interstitial space. Second, there was high variability in the superoxide dismutase data, which may be, in part, attributed to the acute induction and tight regulation of the activity of this enzyme.
Limitations
There are a few limitations that need to be addressed. First, recruitment of non-Hispanic Black and White participants was done via self-identification. We excluded potential participants who self-identified as having multiracial or Hispanic backgrounds. Second, blood pressure was measured three times before enrollment and throughout the experimental protocol. Blood pressure is known to fluctuate throughout the 24-h day-night cycle, but the blood pressure measurements collected in this study define a limited part of the day (∼4 h); however, all participants met the AHA requirements defining normotensive and prehypertensive blood pressure status. Third, we did not analyze blood samples for lipids, glucose, HbA1C, or other makers related to CVD risk factors. Therefore, it is unknown whether participants had dyslipidemia, hypercholesterolemia, or prediabetes; however, no participants reported type 1 or 2 diabetes, and the magnitudes of responses in the two normotensive groups are similar to those previously reported in studies with similar populations (4, 61). Fourth, we only assessed two biomarkers of antioxidant capacity and one biomarker of oxidative stress, and there was a large amount of variability in our biomarker analyses. There are other biomarkers of antioxidant capacity and/or oxidative stress that may yield more conclusive data. As such, the biomarker data should be considered with caution. Fifth, it should be noted that there were differences in the number of men and women tested in both of the White participant groups, and women from all groups were tested during all phases of the menstrual cycle and, as such, it is possible some of the differences may be influenced by sex hormones. Recent data from Patik et al. (4) suggest that oxidative stress contributes more to reduced cutaneous NO-dependent vasodilation in Black men than women. The findings in the present study may therefore be sex specific; however, this study was not designed or powered to detect differences between sexes, and any such differences would be expected to be minimal as there were, at most, four more women than men in the normotensive White group and phase of menstrual cycle was heterogeneous across all groups.
Conclusions
Our data suggest that iNOS activity may be increased in Blacks and prehypertensive Whites, and inhibition of iNOS with 1400W appears to increase endothelial NO-dependent vasodilation in prehypertensive Whites and normotensive Blacks to levels observed in normotensive Whites. Inhibition of iNOS with 1400W increases endothelial NO-dependent vasodilation in prehypertensive Blacks but to a lesser extent. These data collectively suggest that interventions or strategies that reduce iNOS activity may be beneficial at improving the bioavailability of cardioprotective endothelial NO.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grant R01 HL-141205-03 (to B. J. Wong).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.S.O., A.A.Q., and B.J.W. conceived and designed research; J.T.M. and C.G.T. performed experiments; J.T.M., J.S.O., Y.S., M.J.H., and B.J.W. analyzed data; J.T.M., J.S.O., Y.S., M.J.H., and B.J.W. interpreted results of experiments; B.J.W. prepared figures; J.T.M. drafted manuscript; J.T.M., C.G.T., J.S.O., Y.S., M.J.H., A.A.Q., and B.J.W. edited and revised manuscript; J.T.M., C.G.T., J.S.O., Y.S., M.J.H., A.A.Q., and B.J.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all of the participants for volunteering time in this study and Joseph Hwang, Demetria Walker, Brittany McLeod, and Justin West for assistance with data collection.
REFERENCES
- 1.Cardillo C, Kilcoyne CM, Cannon RO 3rd, Panza JA. Racial differences in nitric oxide-mediated vasodilator response to mental stress in the forearm circulation. Hypertension 31: 1235–1239, 1998. doi: 10.1161/01.HYP.31.6.1235. [DOI] [PubMed] [Google Scholar]
- 2.Hurr C, Patik JC, Kim K, Christmas KM, Brothers RM. Tempol augments the blunted cutaneous microvascular thermal reactivity in healthy young African Americans. Exp Physiol 103: 343–349, 2018. doi: 10.1113/EP086776. [DOI] [PubMed] [Google Scholar]
- 3.Lang CC, Stein CM, Brown RM, Deegan R, Nelson R, He HB, Wood M, Wood AJ. Attenuation of isoproterenol-mediated vasodilatation in blacks. N Engl J Med 333: 155–160, 1995. doi: 10.1056/NEJM199507203330304. [DOI] [PubMed] [Google Scholar]
- 4.Patik JC, Curtis BM, Nasirian A, Vranish JR, Fadel PJ, Brothers RM. Sex differences in the mechanisms mediating blunted cutaneous microvascular function in young black men and women. Am J Physiol Heart Circ Physiol 315: H1063–H1071, 2018. doi: 10.1152/ajpheart.00142.2018. [DOI] [PubMed] [Google Scholar]
- 5.Perregaux D, Chaudhuri A, Rao S, Airen A, Wilson M, Sung BH, Dandona P. Brachial vascular reactivity in blacks. Hypertension 36: 866–871, 2000. doi: 10.1161/01.HYP.36.5.866. [DOI] [PubMed] [Google Scholar]
- 6.Ray CA, Monahan KD. Sympathetic vascular transduction is augmented in young normotensive blacks. J Appl Physiol (1985) 92: 651–656, 2002. doi: 10.1152/japplphysiol.00788.2001. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum DA, Pretorius M, Gainer JV, Byrne D, Murphey LJ, Painter CA, Vaughan DE, Brown NJ. Ethnicity affects vasodilation, but not endothelial tissue plasminogen activator release, in response to bradykinin. Arterioscler Thromb Vasc Biol 22: 1023–1028, 2002. doi: 10.1161/01.ATV.0000017704.45007.1D. [DOI] [PubMed] [Google Scholar]
- 8.Stein CM, Lang CC, Nelson R, Brown M, Wood AJ. Vasodilation in black Americans: attenuated nitric oxide-mediated responses. Clin Pharmacol Ther 62: 436–443, 1997. doi: 10.1016/S0009-9236(97)90122-3. [DOI] [PubMed] [Google Scholar]
- 9.Stein CM, Lang CC, Singh I, He HB, Wood AJ. Increased vascular adrenergic vasoconstriction and decreased vasodilation in blacks. Additive mechanisms leading to enhanced vascular reactivity. Hypertension 36: 945–951, 2000. doi: 10.1161/01.HYP.36.6.945. [DOI] [PubMed] [Google Scholar]
- 10.Vranish JR, Holwerda SW, Young BE, Credeur DP, Patik JC, Barbosa TC, Keller DM, Fadel PJ. Exaggerated vasoconstriction to spontaneous bursts of muscle sympathetic activity in healthy young black men. Hypertension 71: 192–198, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics. 2019 Update: A Report From the American Heart Association. Circulation 139: e56–e528, 2019. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 12.Selassie A, Wagner CS, Laken ML, Ferguson ML, Ferdinand KC, Egan BM. Progression is accelerated from pre-hypertension to hypertension in blacks. Hypertension 58: 579–587, 2011. doi: 10.1161/HYPERTENSIONAHA.111.177410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner CG, Miller JT, Otis JS, Hayat MJ, Quyyumi AA, Wong BJ. Cutaneous sensory nerve-mediated microvascular vasodilation in normotensive and prehypertensive non-Hispanic Blacks and Whites. Physiol Rep 8: e14437, 2020. doi: 10.14814/phy2.14437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong BJ, Turner CG, Miller JT, Walker DC, Sebeh Y, Hayat MJ, Otis JS, Quyyumi AA. Sensory nerve-mediated and nitric oxide-dependent cutaneous vasodilation in normotensive and prehypertensive non-Hispanic blacks and whites. Am J Physiol Heart Circ Physiol 319: H271–H281, 2020. doi: 10.1152/ajpheart.00177.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feairheller DL, Park JY, Sturgeon KM, Williamson ST, Diaz KM, Veerabhadrappa P, Brown MD. Racial differences in oxidative stress and inflammation: in vitro and in vivo. Clin Transl Sci 4: 32–37, 2011. doi: 10.1111/j.1752-8062.2011.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris KR, Lutz RD, Choi HS, Kamitani T, Chmura K, Chan ED. Role of the NF-kappaB signaling pathway and kappaB cis-regulatory elements on the IRF-1 and iNOS promoter regions in mycobacterial lipoarabinomannan induction of nitric oxide. Infect Immun 71: 1442–1452, 2003. doi: 10.1128/IAI.71.3.1442-1452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim K, Hurr C, Patik JC, Brothers R. Attenuated cutaneous microvascular function in healthy young African Americans: role of intradermal L-arginine supplementation. Microvasc Res 118: 1–6, 2018. doi: 10.1016/j.mvr.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation 109: 2511–2517, 2004. doi: 10.1161/01.CIR.0000129087.81352.7A. [DOI] [PubMed] [Google Scholar]
- 19.Mason RP, Kalinowski L, Jacob RF, Jacoby AM, Malinski T. Nebivolol reduces nitroxidative stress and restores nitric oxide bioavailability in endothelium of black Americans. Circulation 112: 3795–3801, 2005. doi: 10.1161/CIRCULATIONAHA.105.556233. [DOI] [PubMed] [Google Scholar]
- 20.Smith CJ, Santhanam L, Brunin RS, Stanhewicz A, Berkowitz DE, Holowatz LA. Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension 58: 935–942, 2011. doi: 10.1161/HYPERTENSIONAHA.111.178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong HJ, Loh SH, Yen MH. Suppression of the development of hypertension by the inhibition of inducible nitric oxide synthase. Br J Pharmacol 131: 631–637, 2000. doi: 10.1038/sj.bjp.0703603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar U, Chen J, Sapoznikhov V, Canteros G, White BH, Sidhu A. Overexpression of inducible nitric oxide synthase in the kidney of the spontaneously hypertensive rat. Clin Exp Hypertens 27: 17–31, 2005. doi: 10.1081/CEH-200044249. [DOI] [PubMed] [Google Scholar]
- 23.Abularrage CJ, Sidawy AN, Aidinian G, Singh N, Weiswasser JM, Arora S. Evaluation of the microcirculation in vascular disease. J Vasc Surg 42: 574–581, 2005. doi: 10.1016/j.jvs.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Coulon P, Constans J, Gosse P. Impairment of skin blood flow during post-occlusive reactive hyperhemy assessed by laser Doppler flowmetry correlates with renal resistive index. J Hum Hypertens 26: 56–63, 2012. doi: 10.1038/jhh.2010.117. [DOI] [PubMed] [Google Scholar]
- 25.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol (1985) 105: 370–372, 2008. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 26.Kruger A, Stewart J, Sahityani R, O’Riordan E, Thompson C, Adler S, Garrick R, Vallance P, Gologorsky MS. Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: correlation with cardiovascular risk. Kidney Int 70: 157–164, 2006. doi: 10.1038/sj.ki.5001511. [DOI] [PubMed] [Google Scholar]
- 27.Minson C Thermal provocation to evaluate microvascular reactivity in human skin. J Appl Physiol (1985) 109: 1239–1246, 2010. doi: 10.1152/japplphysiol.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison-Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr.. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71: e13–e115, 2018. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 29.Brunt VE, Miner JA, Meendering JR, Kaplan PF, Minson CT. 17β-estradiol and progesterone independently augment cutaneous thermal hyperemia but not reactive hyperemia. Microcirculation 18: 347–355, 2011. doi: 10.1111/j.1549-8719.2011.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charkoudian N, Stephens DP, Pirkle KC, Kosiba WA, Johnson JM. Influence of female reproductive hormones on local thermal control of skin blood flow. J Appl Physiol (1985) 87: 1719–1723, 1999. doi: 10.1152/jappl.1999.87.5.1719. [DOI] [PubMed] [Google Scholar]
- 31.Ketel IJ, Stehouwer CD, Serné EH, Poel DM, Groot L, Kager C, Hompes PG, Homburg R, Twisk JW, Smulders YM, Lambalk CB. Microvascular function has no menstrual-cycle-dependent variation in healthy ovulatory women. Microcirculation 16: 714–724, 2009. doi: 10.3109/10739680903199186. [DOI] [PubMed] [Google Scholar]
- 32.Priest SE, Shenouda N, MacDonald MJ. Effect of sex, menstrual cycle phase, and monophasic oral contraceptive pill use on local and central arterial stiffness in young adults. Am J Physiol Heart Circ Physiol 315: H357–H365, 2018. doi: 10.1152/ajpheart.00039.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossi M, Di Maria C, Erba P, Galetta F, Carpi A, Santoro G. Study of skin vasomotion during phollicular and luteal phase in young healthy women. Clin Hemorheol Microcirc 42: 107–115, 2009. doi: 10.3233/CH-2009-1189. [DOI] [PubMed] [Google Scholar]
- 34.Shenouda N, Priest SE, Rizzuto VI, MacDonald MJ. Brachial artery endothelial function is stable across a menstrual and oral contraceptive pill cycle but lower in premenopausal women than in age-matched men. Am J Physiol Heart Circ Physiol 315: H366–H374, 2018. doi: 10.1152/ajpheart.00102.2018. [DOI] [PubMed] [Google Scholar]
- 35.Stanhewicz AE, Wong BJ. Counterpoint: Investigators should not control for menstrual cycle phase when performing studies of vascular control that include women. J Appl Physiol (1985) 129: 1117–1119, 2020. [DOI] [PubMed] [Google Scholar]
- 36.Hodges G, Chiu C, Kosiba W, Zhao K, Johnson J. The effect of microdialysis needle trauma on cutaneous vascular responses in humans. J Appl Physiol (1985) 106: 1112–1118, 2009. doi: 10.1152/japplphysiol.91508.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunt V, Fujii N, Minson C. Endothelial-derived hyperpolarization contributes to acetylcholine-mediated vasodilation in human skin in a dose-dependent manner. J Appl Physiol (1985) 119: 1015–1022, 2015. doi: 10.1152/japplphysiol.00201.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carter SJ, Hodges GJ. Sensory and sympathetic nerve contributions to the cutaneous vasodilator response from a noxious heat stimulus. Exp Physiol 96: 1208–1217, 2011. doi: 10.1113/expphysiol.2011.059907. [DOI] [PubMed] [Google Scholar]
- 39.Fieger SM, Wong BJ. Adenosine receptor inhibition with theophylline attenuates the skin blood flow response to local heating in humans. Exp Physiol 95: 946–954, 2010. doi: 10.1113/expphysiol.2010.053538. [DOI] [PubMed] [Google Scholar]
- 40.Hodges GJ, Del Pozzi AT, McGarr GW, Mallette MM, Cheung SS. The contribution of sensory nerves to cutaneous vasodilatation of the forearm and leg to local skin heating. Eur J Appl Physiol 115: 2091–2098, 2015. doi: 10.1007/s00421-015-3188-7. [DOI] [PubMed] [Google Scholar]
- 41.Magerl W, Treede R. Heat-evoked vasodilatation in human hairy skin: axon reflexes due to low-level activity of nociceptive afferents. J Physiol 497: 837–848, 1996. doi: 10.1113/jphysiol.1996.sp021814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minson C, Berry L, Joyner M. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol (1985) 91: 1619–1626, 2001. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 43.Wong BJ, Fieger SM. Transient receptor potential vanilloid type-1 (TRPV-1) channels contribute to cutaneous thermal hyperaemia in humans. J Physiol 588: 4317–4326, 2010. doi: 10.1113/jphysiol.2010.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong BJ, Minson CT. Neurokinin-1 receptor desensitization attenuates cutaneous active vasodilatation in humans. J Physiol 577: 1043–1051, 2006. doi: 10.1113/jphysiol.2006.112508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol 560: 941–948, 2004. doi: 10.1113/jphysiol.2004.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi P, Brunt V, Fujii N, Minson C. New approach to measure cutaneous microvascular function: an improved test of NO-mediated vasodilation by thermal hyperemia. J Appl Physiol (1985) 117: 277–283, 2014. doi: 10.1152/japplphysiol.01397.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keen JT, Levitt EL, Hodges GJ, Wong BJ. Short-term dietary nitrate supplementation augments cutaneous vasodilatation and reduces mean arterial pressure in healthy humans. Microvasc Res 98: 48–53, 2015. doi: 10.1016/j.mvr.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Kellogg DL, Liu Y, Kosiba IF, O’Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol (1985) 86: 1185–1190, 1999. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- 49.Kellogg DL, Zhao JL, Wu Y. Endothelial nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. Am J Physiol Heart Circ Physiol 295: H123–H129, 2008. doi: 10.1152/ajpheart.00082.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kellogg D, Zhao J, Wu Y, Johnson J. Antagonism of soluble guanylyl cyclase attenuates cutaneous vasodilation during whole body heat stress and local warming in humans. J Appl Physiol (1985) 110: 1406–1413, 2011. doi: 10.1152/japplphysiol.00702.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paulsen SM, Wurster SH, Nanney LB. Expression of inducible nitric oxide synthase in human burn wounds. Wound Repair Regen 6: 142–148, 1998. doi: 10.1046/j.1524-475X.1998.60208.x. [DOI] [PubMed] [Google Scholar]
- 52.Zamora R, Vodovotz Y, Billiar T. Inducible nitric oxide synthase and inflammatory diseases. Mol Med 6: 347–373, 2000. doi: 10.1007/BF03401781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kröncke KD, Fehsel K, Kolb-Bachofen V. Inducible nitric oxide synthase in human diseases. Clin Exp Immunol 113: 147–156, 1998. doi: 10.1046/j.1365-2249.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin E, Nathan C, Xie Q. Role of interferon regulatory factor 1 in induction of nitric oxide synthase. J Exp Med 180: 977–984, 1994. doi: 10.1084/jem.180.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B_Rel in induction of nitric oxide synthase. J Biol Chem 269: 4705–4708, 1994. [PubMed] [Google Scholar]
- 56.Gunnett CA, Lund DD, McDowell AK, Faraci FM, Heistad DD. Mechanisms of inducible nitric oxide synthase-mediated vascular dysfunction. Arterioscler Thromb Vasc Biol 25: 1617–1622, 2005. doi: 10.1161/01.ATV.0000172626.00296.ba. [DOI] [PubMed] [Google Scholar]
- 57.Jain S, Khera R, Corrales-Medina VF, Townsend RR, Chirinos JA. Inflammation and arterial stiffness in humans. Atherosclerosis 237: 381–390, 2014. doi: 10.1016/j.atherosclerosis.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 58.Brunt VE, Minson CT. KCa channels and epoxyeicosatrienoic acids: major contributors to thermal hyperaemia in human skin. J Physiol 590: 3523–3534, 2012. doi: 10.1113/jphysiol.2012.236398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morris AA, Zhao L, Patel RS, Jones DP, Ahmed Y, Stoyanova N, Gibbons GH, Vaccarino V, Din-Dzietham R, Quyyumi AA. Differences in systemic oxidative stress based on race and the metabolic syndrome: the Morehouse and Emory team up to eliminate health disparities (META-Health) study. Metab Syndr Relat Disord 10: 252–259, 2012. doi: 10.1089/met.2011.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deo SH, Holwerda SW, Keller DM, Fadel PJ. Elevated peripheral blood mononuclear cell-derived superoxide production in healthy young black men. Am J Physiol Heart Circ Physiol 308: H548–H552, 2015. doi: 10.1152/ajpheart.00784.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim K, Brothers RM. Acute consumption of flavanol-rich cocoa beverage improves attenuated cutaneous microvascular function in healthy young African Americans. Microvasc Res 128: 103931, 2020. doi: 10.1016/j.mvr.2019.103931. [DOI] [PubMed] [Google Scholar]