Abstract
The usage of flavored electronic nicotine delivery systems (ENDS) is popular, specifically in the teen and young adult age-groups. The possible cardiac toxicity of the flavoring aspect of ENDS is largely unknown. Vaping, a form of electronic nicotine delivery, uses “e-liquid” to generate “e-vapor,” an aerosolized mixture of nicotine and/or flavors. We report our investigation into the cardiotoxic effects of flavored e-liquids. E-vapors containing flavoring aldehydes such as vanillin and cinnamaldehyde, as indicated by mass spectrometry, were more toxic in HL-1 cardiomyocytes than fruit-flavored e-vapor. Exposure of human induced pluripotent stem cell-derived cardiomyocytes to cinnamaldehyde or vanillin-flavored e-vapor affected the beating frequency and prolonged the field potential duration of these cells more than fruit-flavored e-vapor. In addition, vanillin aldehyde-flavored e-vapor reduced the human ether-à-go-go-related gene (hERG)-encoded potassium current in transfected human embryonic kidney cells. In mice, inhalation exposure to vanillin aldehyde-flavored e-vapor for 10 wk caused increased sympathetic predominance in heart rate variability measurements. In vivo inducible ventricular tachycardia was significantly longer, and in optical mapping, the magnitude of ventricular action potential duration alternans was significantly larger in the vanillin aldehyde-flavored e-vapor-exposed mice than in controls. We conclude that the widely popular flavored ENDS are not harm free, and they have a potential for cardiac harm. More studies are needed to further assess their cardiac safety profile and long-term health effects.
NEW & NOTEWORTHY The use of electronic nicotine delivery systems (ENDS) is not harm free. It is not known whether ENDS negatively affect cardiac electrophysiological function. Our study in cell lines and in mice shows that ENDS can compromise cardiac electrophysiology, leading to action potential instability and inducible ventricular arrhythmias. Further investigations are necessary to assess the long-term cardiac safety profile of ENDS products in humans and to better understand how individual components of ENDS affect cardiac toxicity.
Keywords: arrhythmias, cardiac electrophysiology, electronic cigarettes, ENDS, vaping
INTRODUCTION
The use of electronic nicotine delivery systems (ENDS) has been growing. It was recently shown that among high school students, ENDS use increased from 1.5% in 2011 to 20.8% in 2018 (1). From 2017 to 2018 alone, there was a 75% increase in ENDS use by high school students (1).
The popularity of flavored ENDS likely fueled the proliferation of manufacturers and the surge in sales of these products (2). In 2014, it was estimated that there were more than 7,600 different flavored ENDS products from 466 brands (3), and as of this date, these numbers have only increased. The demand for ENDS continues to grow (4), as evident by a dynamic market (3, 5) that is projected to surpass $6 billion in the next couple of years. However, the health effects and particularly the cardiac toxicity of ENDS remain incompletely understood.
Vaping is a form of electronic nicotine delivery. The vaping device heats the “e-liquid” via a coil to generate “e-vapor,” an inhalable smoke-like aerosolized mixture containing nicotine, flavors, and solvent particles. E-liquids are usually a mixture of propylene glycol and vegetable glycerin, flavors, and either nicotine salt or free-base nicotine. E-liquids can be used with different ENDS devices such as the pod-based system, which requires the use of e-liquid with nicotine salt, or the tank-based vaping system, where a “tank” holds the e-liquid with free-base nicotine. Both pod-based and tank-based systems are popular among different age-groups (4).
Several studies investigated the toxicity of e-liquids, and it was shown that flavoring aldehydes could be harmful in cell culture (6–17); however, the possible cardiac electrophysiological toxicity of vaping has not been systematically examined and is not completely understood. Here, we will assess the cardiac electrophysiological toxicity of three e-liquids of different flavors, and we will test the hypothesis that vaping can result in cardiac electrophysiological instability and inducible arrhythmogenesis.
MATERIALS AND METHODS
Vaping Chamber
A rat housing cage GR900 (width = 34.6 cm, length = 39.5 cm, and height = 22.7 cm) (Tecniplast, Buguggiate, Italy) was modified, where the bottom was fitted for the introduction of the mouthpiece of the Smok Species Baby V2 (SMOKTech, Shenzhen, China) vaping device (Fig. 1A). We used the Baby V2 A2 dual subcoils with a total resistance of 0.2 Ω, at 85 W. Inlet and outlet openings were created in the cage’s lid. The inlet opening was used to connect the mouthpiece via a plastic tube with ¼ inner diameter (ID) and 3/8 outer diameter (OD) (Fisher Scientific, Waltham, MA) to a flow meter, 1.4 L/min, which was connected to a silent fish tank air pump. The outlet opening was fitted with a plastic tube that served as the exhaust. A Universal High-powered Door Actuator (Zone Tech, New York, NY)—car door locking mechanism—was fixed alongside the vaping device, aimed at the device’s firing button. The actuator was connected to an AC/DC power adapter. Both the actuator’s power adapter and the air pump were wired into the same cycle timer. Every 2 min, the cycle timer turns on for 5 s. This causes the actuator to push the vaping device’s firing button, and simultaneously, air flows into the mouthpiece, expelling, for 4.7 s, an e-vapor puff at 1.4 L/min inside the cage. The vaping device touch screen displays the duration of the device’s activation every time the firing button is pressed. A total of 60 puffs, 110 mL puff volume, over a 2-h period were delivered. This is consistent with the topography of vaping in ENDS users (18–21). Figure 1B is the tank-based vaping device used and is a diagram of the exposure system.
Figure 1.
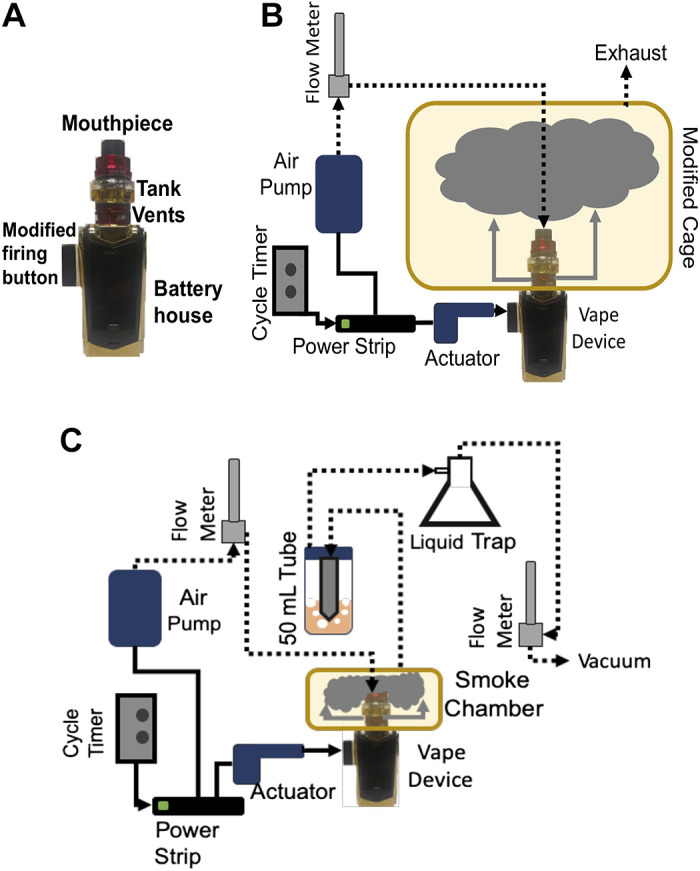
A: picture of the Smok Species Baby V2 vaping device. The black screen displays the duration of the activation every time the firing button is pressed. B: vaping chamber design. C: e-vapor extract generation.
Exposure of Animals to E-vapor
All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of South Florida. Ten, 5-mo-old, C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) of both sexes were used for air control, and 10 mice (5 months old, of both sexes) were used for vaping exposure. Mice were individually housed in ventilated racks, with ad libitum access to food and water. For vaping exposure, the 10 mice were transferred to the vaping cage, and the mice were simultaneously exposed to 4.7-s puffs of vanilla custard-flavored e-vapor at 1.4 L/min, every 2 min for a total of 60 puffs in a 2-h period. Mice were exposed 5 days a week, for a period of 10 wk. Control mice experienced the same handling of the experimental animals. They were placed in a similarly modified chamber, in the same environment, where the only difference was that they were exposed to normal room air. Ten weeks of vaping did not affect the body weight of the animals, which was 29.8 g ± 0.9 in vaped and 29.6 g ± 0.5 in air control mice.
Preparation of E-vapor Extracts
A 10-cm × 10-cm × 7-cm chamber was modified with a bottom opening fitted for the mouthpiece of the Smok Species Baby V2 (SMOKTech) vaping device, and inlet and outlet openings were introduced in the chamber’s sealing top lid. The inlet tube was connected by plastic tubing into the vaping device’s mouthpiece and the flow meter and air pump, as done for the vaping cage described above. The outlet was passed through the cap of a 50-mL conical tube containing cell culture media. Another tube was passed into the 50-mL conical tube cap and connected to a liquid trap flask, then to an air flow meter at 1.4 L/min, and finally to a vacuum. Similar to the vaping machine, every 2 min, the cycle timer turns on for 5 s, causing the actuator to push the vaping device’s firing button; simultaneously, air flows into the mouthpiece, expelling a 4.7-s e-vapor puff at 1.4 L/min inside the chamber. The resulting puff volume is 110 mL, within the limits of reported ENDS user puff size (21). The vacuum pump draws the e-vapor from the chamber at 1.4 L/min, leading to its bubbling into the medium. Ten milliliters of medium was bubbled with 15 puffs of e-vapor, resulting in an e-vapor concentration of 1.5 puffs/mL. For air control, the exact procedure was performed; however, the vaping device was powered off. Dilutions of extracts were performed with fresh, untreated media. We then tested several concentrations of extracts expressed in puffs/mL, as previously done (22). The tested concentrations were 0.075, 0.15, 0.375, and 0.75 puffs/mL. Figure 1C is a diagram of the e-vapor extract system.
E-Liquids
The flavors used in this study were Hawaiian POG (POG), Vanilla Custard (VC) (USA Vape Labs, Huntington Beach, CA), and Apple Jax (APJ) (Epic Juice, Santa Ana, CA). The manufacturer labeled POG flavor as passion fruit, orange, and guava; Vanilla Custard as vanilla custard; and Apple Jax as milky cinnamon apple cereal. These e-liquids are 70% vegetable glycerin/30% propylene glycol (70VG/30PG) and are stated by the manufacturer to contain 6 mg/mL free-base nicotine. We prepared in-house, base only (70% vegetable glycerin/30% propylene glycol) (Sigma-Aldrich) and base plus 6 mg/mL free-base nicotine (Sigma-Aldrich).
Total Particulate Matter Measurements
We measured total particulate matter (TPM) generated from the three different e-liquids using a 25-mm membrane filter (PALL Life Science) placed into a stainless-steel filter holder (Cole-Parmer). E-vapor was generated using the same method and setup used to bubble cell culture medium, but instead of bubbling the vapor in the medium, the 15 puffs were passed through the filter paper. Filter papers were weighed before and after the procedure.
HL1 Cell Culture
HL-1 cells (mouse atrial myocytes) were obtained from the laboratory of Dr. Claycomb (Louisiana State University) and cultured following the recommended protocol (23). Briefly, cells were grown in Claycomb medium (Sigma, St. Louis, MO) and supplemented with 10% FBS (Sigma, St. Louis, MO), 0.1 mM norepinephrine (Sigma, St. Louis, MO), 2 mM l-glutamine (Sigma, St. Louis, MO), and penicillin/streptomycin (100 U/mL/100 µ/mL) on tissue culture plates (Corning, Corning, NY), coated with fibronectin/gelatin (Sigma, St. Louis, MO).
Apoptosis Flow Cytometry
Apoptosis was measured using the FITC annexin V staining assay (BD, Franklin Lakes, NJ). HL-1 cells were plated in 12-well plates, and when confluency reached ∼70%, they were cultured with control medium or with e-vapor extracts. The tested concentrations were 0.075, 0.15, 0.375, and 0.75 puffs/mL, and the duration of exposure was 24 or 48 h. After the incubation period, the cells in each well were lifted with Accutase and resuspended in 5 mL of PBS. Cells were pelleted by centrifugation, washed with PBS, and stained with FITC-labeled annexin V according to the manufacturer’s recommendation. DAPI (3 µM) was added immediately before reading the samples on a BD LSRII Cytometer using the 488-nm and 405-nm lasers for excitation of FITC annexin V and DAPI, respectively. The following controls were included in each experiment: unstained cells, cells stained with FITC annexin V but not with DAPI, and cells stained with DAPI but not with FITC annexin V. Data analysis was carried out using the FlowJo software.
Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Culture and Extracellular Potential Recording
The iCell Cardiomyocytes2 (Cellular Dynamics, Madison, WI) were thawed according to the manufacturer’s protocol, and 50,000 cells/well were plated on the fibronectin (50 µg/mL)-coated 24-well multiple electrode array (MEA) plates (24-well plate-eco) (MED64, Osaka, Japan). Plated cells were incubated at 37°C, 5% CO2 for 1 h to allow attachment of the cells, after which prewarmed maintenance medium (Cellular Dynamics, Madison, WI) was added to the wells. The maintenance medium was changed every 3 days, and after day 7, cells began to beat synchronously, periodically, and spontaneously. The Presto Multielectrode Array (MED64, Osaka, Japan) was used to record extracellular potentials of the spontaneously beating hiPSC-derived cardiomyocytes. The Presto is equipped with an environmental chamber (37°C and 95% O2-5% CO2). After the plate was placed on top of the recording electrodes, 2-min recordings of the extracellular potentials were obtained using the MED64’s MEA Symphony software interface at baseline before addition of the vapor extracts and after addition of the extracts. For each well, the beating rate and the Bazett-corrected field potential durations were calculated in the MED64’s MEA Symphony analysis software.
Patch Clamp
Human embryonic kidney (HEK 293) cells were cotransfected with wild-type hERG and GFP in pcDNA3. Forty-eight hours after transfection, cells were cultured with 0.375 puffs/mL vanilla custard e-vapor or with air control. The hERG current (IKr) was measured by whole cell configuration as usually done (24), using an Axon 700B amplifier and Digidata 1550B A/D converter. The bath solution contained 140 mM NaCl, 2 mM CaCl2, 4 mM KCl, 1 mM MgCl2, 5 mM glucose, and 10 mM HEPES buffer (pH 7.4). The internal solution contained 126 mM KCl, 4 mM Mg-ATP, 2 mM MgSO4, 5 mM EGTA, 0.5 mM CaCl2, and 25 mM HEPES buffer (pH 7.2). Voltage protocol: 10 mV steps from −60 to 60 mV for 3 s and then step to −40 mV for 1 s, and finally hyperpolarized to −120 mV for 0.5 s. Tail current amplitude was quantified and normalized to cell capacitance (nA/pF) and presented in an IV curve.
Mass Spectrometry
E-vapors were generated using the same setup described in Fig. 1. The 10-cm × 10-cm × 7-cm chamber was slightly altered, where the outlet tube was replaced with a septum. Five puffs were generated, each for 4.7 s at an interval of 10 s, at a flow rate of 1.4 L/min. Immediately after the smoke was generated, a 2.5-mL Hamilton glass tight syringe was introduced through the septum to extract 250 µL of e-vapor from the chamber. The smoke was then immediately injected manually into the Aglient 7890B gas chromatograph, mass spectrometer (GC-MS) 5977B. The headspace syringe was cleaned between samples by rinsing repeatedly in pesticide-grade methanol and then thoroughly dried in an incubator for 30 min at 70°C. The GC-MS parameters were optimized based on previous studies (15, 25) and are reported in Table 1. MassHunter Workstation Qualitative Analysis Software (Version B.07.00 SP2) in conjunction with NIST MS Search 2017 Library was used for analysis. Peaks were identified based on a match score factor higher than 700.
Table 1.
GC-MS settings used for qualitative analysis of the e-vapors
| Instrument Parameters | |
|---|---|
| Column | HP-5MS UI (30 m × 0.250 mm × 0.25 μm) |
| Carrier gas flow | He @ 1.00 mL/min (constant flow) |
| Inlet temperature | 250°C |
| Oven program | Ramp 1: 40°C to 170°C @ 10°C/min; hold 2 min; Ramp 2: 8°C/min to 250°C; Ramp 3: 25°C/min to 320°C; hold 5 min. |
| Injection volume | 250 µL, split (20:1) |
| Transfer line temperature | 290°C |
| MS source | Single quadrupole, EI @ 250°C |
| Solvent delay | 1.00 min |
| MS acquisition range | 30–450 amu from 1 min to 32.80 min |
Telemetry and HRV Analysis
Mice were implanted with ECG telemetric devices (ETA-F10, Data Sciences International, St. Paul, MN) using sterile equipment, under general anesthesia with 2% isoflurane, and body temperature maintained at 37°C on a heating pad. The manufacturer’s recommendation for subcutaneous transmitter placement along the lateral flank was followed. The animals were allowed to recover for 14 days after the implantation procedure. ECG recordings in freely moving animals were done using the PhysioTel RPC-3 (Data Sciences International, St. Paul, MN) receivers, after 1, 5, or 10 wk of exposure to vanilla custard e-vapor. The ECGs were recorded using the Ponemah Software (Data Sciences International, St. Paul, MN), at 1 kHz sampling rate, ∼4 h after conclusion of the vaping session, to allow the animals to settle in their cages, after handling and exposure. Thirty-minute ECG strips were prepared and analyzed in MATLAB. Pan–Tompkins algorithm was used to extract the time separation between consecutive R peaks of the ECG. R-R values not contained between mean R-R interval ± 2 standard deviation were inspected for arrhythmias (no arrhythmias were observed) and excluded to obtain normal R-R intervals (NN intervals) (26, 27). Parameterization of HRV was performed in LabView (National Instruments), as previously done (26, 27). Two temporal and two spectral parameters were subsequently further analyzed. The temporal parameters were standard deviation of the normal sinus rhythm beats (SDNN) and the percentage of adjacent NN intervals that differ from each other by more than 6 ms (pNN06). pNN is an indicator of cardiac parasympathetic activity (27, 28), which in humans is usually 50 ms but was adapted to 6 ms in mouse studies due to the high heart rate of the mouse (27). Spectral parameters were calculated from the unmodified periodogram as an estimator of the power spectral density (PSD). Low frequency was defined as 0.15 Hz to 1.5 Hz, and high frequency as 1.5 Hz to 5 Hz, similarly to what is done in mouse HRV studies (26–28).
In Vivo VT Inducibility
Mice were anesthetized (2% isoflurane), and a 1.2-Fr octapolar catheter (Millar, Houston, TX) was placed transvenously into the right atrium and advanced into the right ventricle. Electrograms were recorded using the PowerLab platform (AD Instruments, Colorado Springs, CO). Programmed electrical stimulation for VT induction was performed by pacing the right ventricle at twice diastolic threshold with 1-s bursts from 20 to 50 Hz, in 2-Hz increments (26, 29).
Optical Imaging
Isolated Langendorff perfused mouse hearts were retrogradely perfused with Tyrode’s solution. The preparations were maintained at 37°C and stained with a bolus of voltage-sensitive dye (0.25 mL, 10 μM Di 4 ANEPPS, Sigma, St. Louis, MO). Blebbistatin (7 μM, Sigma, St. Louis, MO) was used as an excitation-contraction uncoupler. Mapping was carried out as we have done extensively. We quantified the action potential duration at 75% repolarization (APD), as we previously did (30, 31). A bipolar, silver-tip stimulation electrode was used to pace the ventricles (5-ms pulses, 2× diastolic threshold) at different frequencies (from 8 to 15 Hz).
Statistics
Data are presented as average ± standard error. Student’s t test and one-way analysis of variance (ANOVA) with Bonferroni correction were used as appropriate, and significance was taken at P < 0.05.
RESULTS
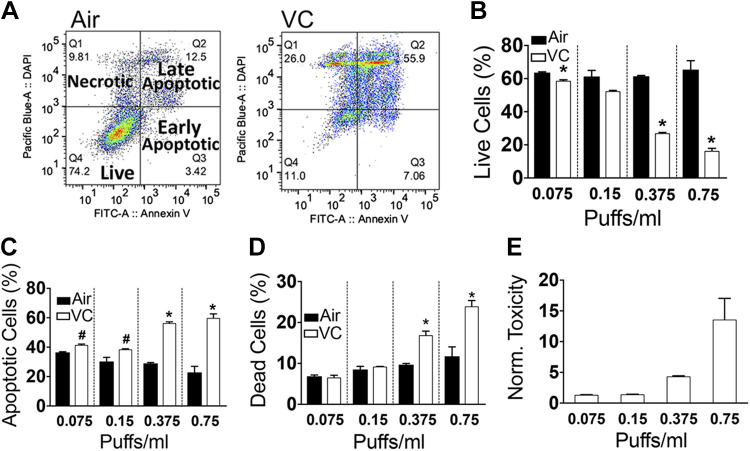
HL-1 mouse atrial cardiomyocytes (23) were cultured for 48 h with air or with 0.075, 0.15, 0.375, and 0.75 puffs/mL vanilla custard (USA Vape Lab, CA) e-vapor extract. Cells were then dissociated with Accutase and stained with FITC-labeled annexin V, a widely used apoptotic marker, and DAPI, a cell viability marker. Figure 2A, left, shows a flow cytometry analysis of air controls. The majority of cells are in the low annexin V and low DAPI live quadrant. Apoptotic cells show high annexin V (right lower and upper quadrants). Necrotic cells show low annexin V and high DAPI labeling. In vanilla custard e-vapor treatment, there was a significant shift of the population from the live quadrant to the necrotic and apoptotic quadrants. Fig. 2B quantifies the percentage of live cells at 0.075, 0.15, 0.375, and 0.75 puffs/mL treatment with air or vanilla custard e-vapor. There was a dose-dependent decrease in the live population as the concentration of vanilla custard e-vapor increased. The percentage of necrotic (Fig. 2D) and apoptotic (Fig. 2C) cells increased with increasing vanilla custard e-vapor extract. Fig. 2E is a plot of the toxicity index (TI) that we calculated as where %DC is the percentage of dead cells, %AC is the percentage of apoptotic cells, and %LC is the percentage of live cells per well. The TI was normalized to that of air at each treatment condition. Figure 2E is the TI normalized to that of air at 0.075, 0.15, 0.375, and 0.75 puffs/mL vanilla custard extract, where TI was 1.3 ± 0.04, 1.4 ± 0.03, 4.3 ± 0.13, and 13.6 ± 3.5, respectively.
Figure 2.
Quantification of annexin V staining in HL-1 cells with flow cytometry. A: flow cytometry analysis of live, apoptotic, and necrotic HL-1 cells cultured for 48 h with 0.75 puffs/mL air or 0.75 puffs/mL vanilla custard (VC) e-vapor extract. B–D: flow cytometry quantification of the percentage of live (B), apoptotic (C), and necrotic (D) cells, in air or vanilla custard e-vapor bubbled medium at 0.075, 0.15, 0.375, and 0.75 puffs/mL. E: toxicity index normalized to that of air. n = 3 in each condition. #P < 0.05 vs. air; *P < 0.01 vs. air, t test.
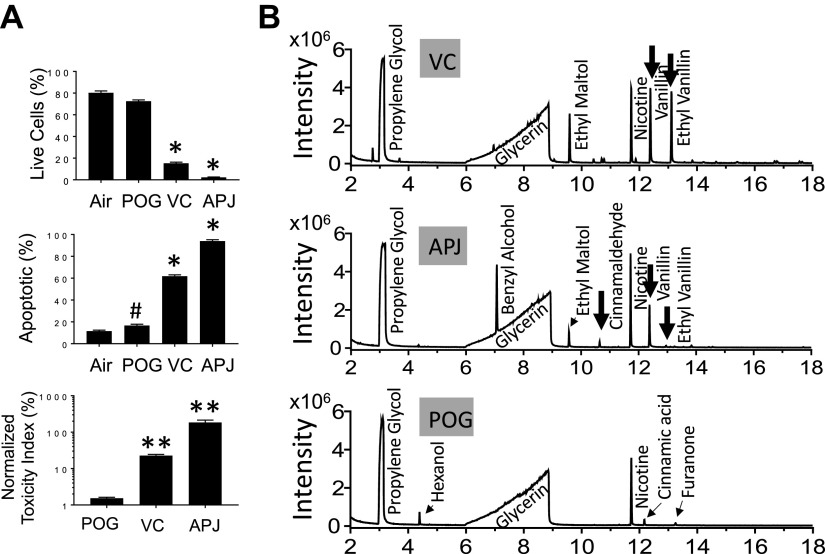
Figure 3A compares the effects of 48-h, 0.75 puffs/mL e-vapor extracts of Hawaiian POG (POG), vanilla custard (VC), and Apple Jax (APJ) or air on HL-1 myocytes. Flow cytometry of annexin V and DAPI staining showed that all three e-liquids decreased viability (top) and increased apoptosis (middle) to different extents (*P < 0.01 vs. air). The bottom shows that VC and APJ were more toxic compared with POG (*P < 0.01), where the respective toxicities were 22.7 ± 1.75, 185 ± 29.9, and 1.53 ± 0.09. The 24-h exposures resulted in lower toxicities compared with 48 h, and the respective toxicities of VC, APJ, and POG at 24 h were 8.9 ± 2.2, 5.3 ± 0.8, and 2.3 ± 0.6.
Figure 3.
A: toxicity of air, POG, vanilla custard (VC), and Apple Jax (APJ) e-liquid vapor extracts at 0.75 puffs/mL, for 48 h in HL1 cells using flow cytometry and annexin V staining. n = 3 in each condition. #P < 0.05 and *P < 0.01 vs. air; **P < 0.01 vs. POG, one-way ANOVA, Bonferroni correction). B: GC-MS chromatograms of vanilla custard (VC), Apple Jax (APJ), and POG. Propylene glycol, glycerin, and nicotine peaks are evident in addition to flavorings peaks corresponding to aldehyde products such as cinnamaldehyde, vanillin, and ethyl vanillin (black arrows). The list of identified constituents is in Table 2.
We measured the total particulate matter (TPM) generated from the three different e-liquids. TPM measurements were not different between the three e-liquids. For vanilla custard, Apple Jax, and POG, the measured respective TPM values were 2.74 mg/puff ± 0.075, 2.67 mg/puff ± 0.060, and 2.66 mg/puff ± 0.067, n = 3 each.
We then used GC-MS to analyze the chemical composition of these e-liquids. Figure 3B shows the total ion chromatograms of vanilla custard (VC), Apple Jax (APJ), and POG. The complete list of identified constituents in these e-liquids is presented in Table 2. The propylene glycol, glycerin, and nicotine peaks are evident (15, 25). In vanilla custard and Apple Jax, but not in POG, flavorings peaks corresponding to aldehyde products such as cinnamaldehyde, vanillin, and ethyl vanillin (black arrows) are present. This is consistent with cell culture studies showing that vanilla- and cinnamon-containing flavors cause higher toxicities (15); however, the studies were done in noncardiomyocyte cell lines (15). Here, we show that in a cardiomyocyte cell line, vanilla- and cinnamon-flavored e-liquids also cause higher toxicities compared with fruit-flavored e-liquids.
Table 2.
GC-MS peaks analysis of the e-vapors
| Retention Time (RT) | Constituentsa | Retention Indexa,b (RI) | Vanilla Custard | Apple Jax | POG |
|---|---|---|---|---|---|
| 2.392 | Acetoin | 713 | ✓ | ||
| 2.961 | Propylene glycol | 740 | ✓ | ✓ | ✓ |
| 3.59 | 3-Hexenol | 857 | ✓ | ||
| 3.633 | Butanoic acid, ethyl ester | 802 | ✓ | ||
| 4.32 | Butanoic acid, 3-methyl-, ethyl ester | 854 | ✓ | ||
| 6.005 | Glycerin | - | ✓ | ✓ | ✓ |
| 6.102 | Piperazine | - | ✓ | ||
| 6.915 | Cyclotene | 1,034 | ✓ | ||
| 6.988 | Benzyl alcohol | 1,036 | ✓ | ||
| 7.183 | Furaneol | 1,070 | ✓ | ||
| 7.762 | d-Limonene | - | ✓ | ||
| 7.908 | Phenol, 2-methoxy | 1,090 | ✓ | ||
| 8.127 | Butanoic acid, 3-methyl-, 3-methylbutyl ester | 1,104 | ✓ | ||
| 8.254 | Maltol | 1,110 | ✓ | ✓ | |
| 9.038 | Benzene, 1,4-dimethoxy | 1,168 | ✓ | ||
| 9.593 | Ethyl maltol | 1,199 | ✓ | ✓ | |
| 10.401 | Benzaldehyde, 4-methoxy | 1,251 | ✓ | ||
| 10.625 | Cinnamaldehyde | 1,270 | ✓ | ||
| 10.703 | Sulfurol | 1,288 | ✓ | ||
| 10.776 | Anisyl alcohol | 1,290 | ✓ | ||
| 11.725 | Pyridine | 1,361 | ✓ | ✓ | ✓ |
| 11.881 | 2(3H)-Furanone, dihydro-5-pentyl- | 1,363 | ✓ | ||
| 12.105 | 2-Propenoic acid | - | ✓ | ||
| 12.154 | Cinnamic acid, methyl ester | 1,379 | ✓ | ||
| 12.397 | Vanillin | 1,404 | ✓ | ✓ | |
| 12.942 | Coumarin | 1,441 | ✓ | ||
| 13.118 | Ethyl vanillin | 1,453 | ✓ | ✓ | |
| 13.244 | 2(3H)-Furanone, 5-hexyldihydro- | 1,470 | ✓ | ✓ | |
| 16.691 | Vanillin propylene glycol acetal | 1,686 | ✓ | ✓ |
NIST 2017 MS database. bsemistandard nonpolar value.
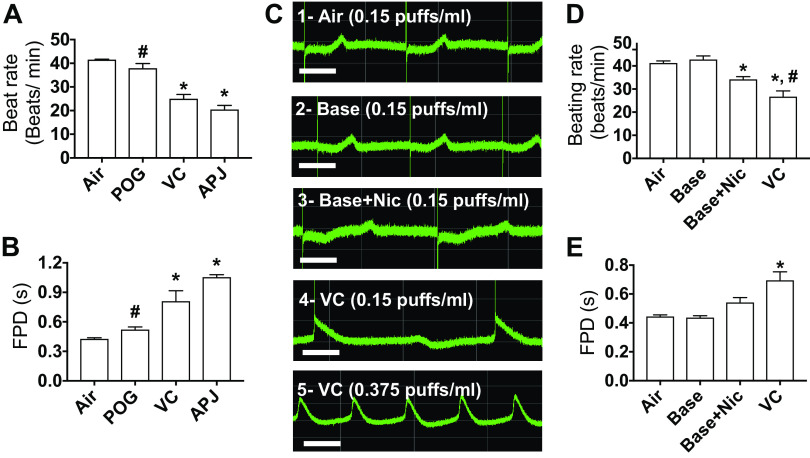
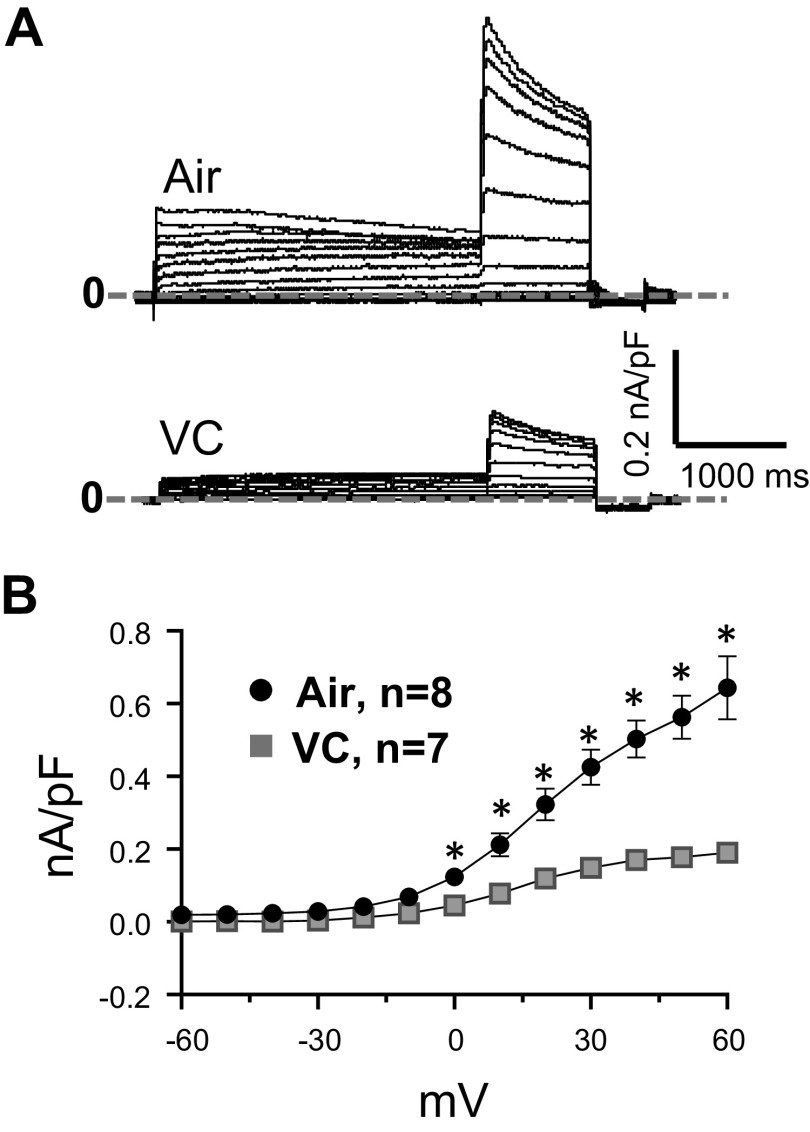
Next, we tested in spontaneously beating hiPSC-derived cardiomyocytes, the effects of the three e-liquids on beating rate and corrected field potential durations. hiPSC-derived cardiomyocytes were seeded in 24-well MEA plates. Each well contained 16 electrodes in a 4 × 4 configuration, 100 µm interelectrode distance. The Presto Multielectrode Array was used to simultaneously record the 16 unipolar extracellular potentials in each well. Only wells that showed spontaneous activity were used. The 48-h exposure to the e-vapors at 0.75 puffs/mL was deadly to the cells. We thus reduced the exposure duration and concentration. At 24 h, and 0.15 puffs/mL, VC and APJ reduced the beating rate (Fig. 4A) and prolonged the corrected field potential duration (FPD) (Fig. 4B) more significantly than POG (*P < 0.05 vs. air, and #P < 0.05 vs. VC and APJ). We then conducted a series of experiments comparing the effects of 0.15 puffs/mL of base only (70VG/30PG), base with nicotine (70VG/30PG plus 6 mg/mL nicotine base), and vanilla custard e-liquid (stated by manufacturer to contain 6 mg/mL free-base nicotine) on beating rate and on the field potential duration in hiPSC-derived cardiomyocytes. Our results showed that base only had no significant effects on the beating rate or the FPD versus air control (Fig. 4C, first two traces, and Fig. 4, D and E). Base with 6 mg/mL nicotine (Base + Nic) significantly decreased the beating rate and tended to increase the FPD versus control (Fig. 4C, third trace, and Fig. 4, D and E), and vanilla custard (base plus 6 mg/mL nicotine plus flavorings) further decreased the beating rate and further increased the FPD (Fig. 4C, fourth trace, and Fig. 4, D and E) (*P < 0.05 vs. air, and #P < 0.05 vs. Base + Nic). This experiment suggested that flavorings in vanilla custard increased the toxicity of base with nicotine. We then tested a higher concentration of vanilla custard (0.375 puffs/mL). Seven of 10 treated wells stopped beating, whereas the three remaining cells showed tachycardia-like activity (74.5 beats/min ± 12.3 vs. 39 beats/min ± 3 at baseline, P < 0.05). The last trace of Fig. 4C shows such tachycardic activity. The prolongation of the FPD suggested that vaping could affect ion channels including the hERG current (IKr). It has been shown in hiPSC-derived cardiomyocytes that IKr block reduces beating rate and increases field potential duration (32). In addition, block of IKr can lead to tachyarrhythmias. Thus, we investigated whether the IKr current is affected by vanilla custard-flavored e-vapor, and we assessed the effects of 0.375 puffs/mL vanilla custard e-vapor on IKr in HEK293 cells transfected with hERG. In Fig. 5A, 24-h treatment with vanilla custard e-vapor caused a reduction in the currents elicited in response to voltage steps from −60 to +60 mV compared with control. In Fig. 5B, the IV relationship of the tail currents indicated that vanilla custard e-vapor significantly inhibited IKr (*P < 0.01, air control vs. VC, t test). As a whole, this set of experiments suggested that flavorings increase the toxicity of base with nicotine and that vaping can affect the human cardiac electrophysiology in part through possible modulation of IKr.
Figure 4.
Effects of 24 h, 0.15 puffs/mL e-vapor exposure on beating rate, and corrected field potential duration in human iPSC-derived cardiomyocytes. Quantification of beating rate (A) and field potential duration (FPD) (B) after 24 h, 0.15 puffs/mL exposure to air control and POG, vanilla custard (VC), and Apple Jax (APJ) e-vapors. *P < 0.05 vs. air; #P < 0.05 vs. VC and APJ, one-way ANOVA, Bonferroni correction, n = 4 each. C: multiple electrodes array (MEA) recordings of extracellular potentials in the spontaneously beating myocytes after 24 h, 0.15 puffs/mL exposure to: air control (trace 1), 70VG/30PG base alone (trace 2), 70VG/30PG base plus 0.6 mg/mL nicotine (trace 3), and VC (trace 4). Trace 5 is 24 h treatment with 0.375 puffs/mL VC. Scale bar = 500 ms. Quantification of beating rate (D) and field potential duration (FPD) (E) after 24 h, 0.15 puffs/mL exposure to air control (n = 10) and base alone (n = 8), base plus 0.6 mg/mL nicotine (n = 5), and vanilla custard (VC, n = 10) e-vapors. *P < 0.05 vs. air; #P < 0.05 vs. base + nicotine, one-way ANOVA, Bonferroni correction. hiPSC, human induced pluripotent stem cells.
Figure 5.
Patch-clamp measurement of hERG in transfected HEK293 cells exposed to vanilla custard. HEK293 cells were cotransfected with hERG and GFP, and exposed for 24 h to 0.375 puffs/mL vanilla custard e-vapor. A: current traces elicited in response to voltage step protocol (10 mV steps from −60 to 60 mV for 3 s, then steps to −40 mV for 1 s, and hyperpolarized to –120 mV for 0.5 s) in an air control (top) and a VC-exposed cell. B: IV curve of the IKr tail current from air control (n = 8) and VC (n = 7)-exposed cells. *P < 0.05, t test. GFP, green fluorescent protein; VC, vanilla custard.
Next, we investigated the effects of 10-wk inhalation exposure to vanilla custard e-vapor, an e-liquid with high toxicity (Figs. 3 and 4), versus air, on heart rate variability parameters in mice instrumented with telemetric ECG. HRV parameters were measured at baseline and 1-, 5-, and 10-wk exposure. Figure 6A shows the histograms of successive differences in NN. The left graph is from an air control mouse. There were no differences between the histograms at baseline (blue trace) and at week 10 (orange trace) of air exposure. The right graph is from a vaped mouse and shows less variation in successive NN differences after 10 wk of vaping (orange histogram) compared with baseline (blue histogram). The dashed lines indicate the marks for 6 ms increase or decrease in successive NN differences.
Figure 6.
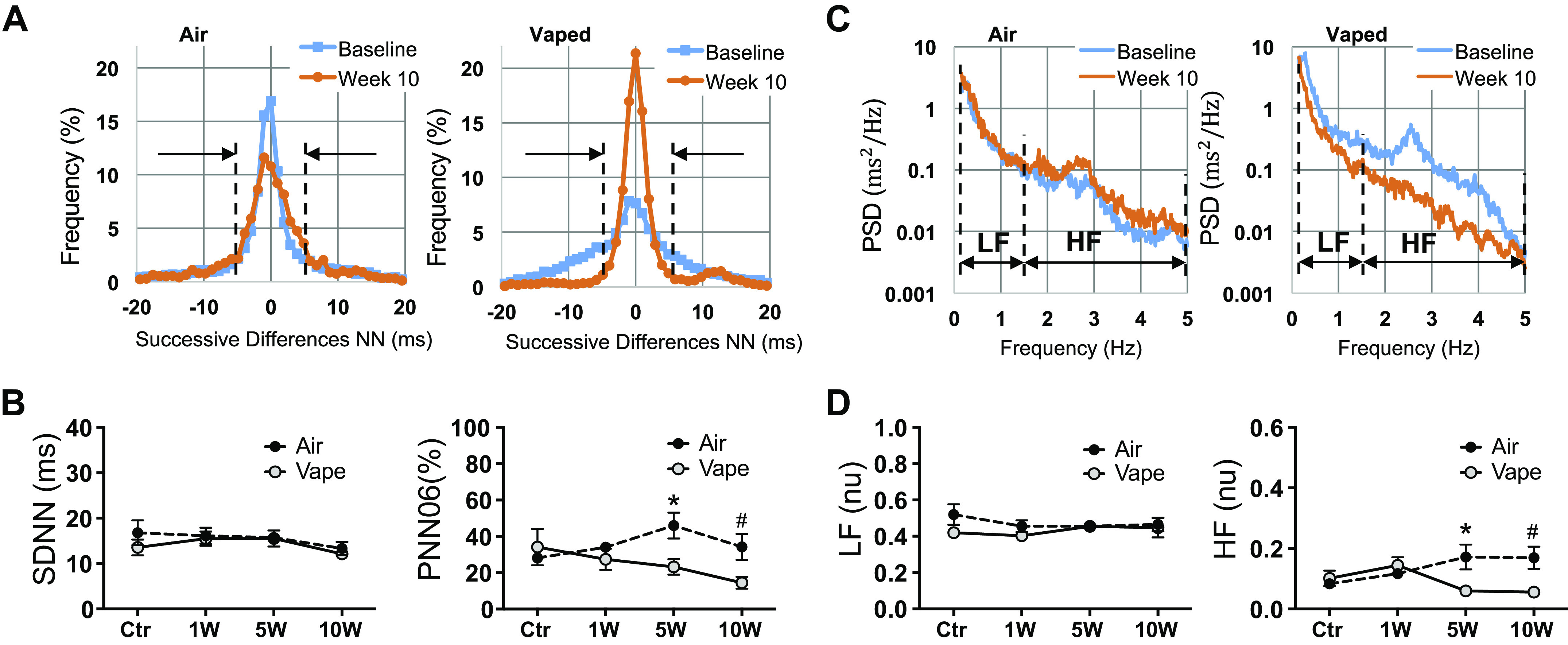
Assessment of heart rate variability in mice exposed to air or vanilla custard e-vapor. A: histograms of the successive differences in NN segments in an air control (left) and a vaped mouse (right) at baseline (blue) and at 10-wk exposure (orange). B: temporal parameters SDNN (ms) and pNN06 (%) at baseline and 1-wk, 5-wk, and 10-wk exposure to air (black circles, n = 5 animals) or vaping (gray circles, n = 5 animals). C: periodograms as estimators of the PSD in an air control (left) and a vaped mouse (right) at baseline (blue) and at 10-wk exposure (orange). D: spectral parameters LF (nu) and HF (nu) at baseline, 1-wk, 5-wk, and 10-wk exposure to air (black circles, n = 5 animals) or vaping (gray circles, n = 5 animals). *P < 0.05, t test, vape vs. air at 5 wk; #P < 0.05, t test, vape vs. air at 10 wk. HF, high frequency; LF, low frequency; SDNN, standard deviation of the normal sinus beats.
The histograms were then used to calculate SDNN, which was not different in mice exposed to air (black circles) versus vaped mice (gray circles) at 0, 1, 5, and 10 wk (Fig. 6B, left). Another temporal HRV parameter that computes the percentage of adjacent NN intervals that differ from each other by more than 6 ms (pNN06) was investigated (Fig. 6B, right). There were no statistically significant differences in pNN06 in mice exposed to air (black circles) versus vaped mice (gray circles) at 0 and 1 wk. However, pNN06 was significantly decreased in vape versus air at 5 and 10 wk (*P < 0.05, vape vs. air, 5 wk. #P < 0.05, vape vs. air, 10 wk). pNN is an indicator of cardiac parasympathetic activity (27,28), which in humans is usually 50 ms, but 6 ms was used in mouse studies due to the high heart rate of the mouse (27). These data suggest that vaping decreased cardiac parasympathetic activity, leading to a sympathetic predominance and, thus, sympathovagal disbalance.
Spectral analysis was also carried out. Figure 6C shows periodograms as estimators of the PSD. PSD from a mouse exposed to air (left graph) is similar at baseline (blue line) and after 10 wk (orange line). The PSD from a vaped mouse (right graph) shows a visible decrease in the high-frequency band after 10 wk of exposure to e-vapor. Quantification of the spectral HRV parameters (low frequency, LF, and high frequency, HF) is shown in Fig. 6D. The LF component did not significantly change; however, HF was significantly lower in vape versus air at 5 and 10 wk (*P < 0.05, vape vs. air, 5 wk; #P < 0.05, vape vs. air, 10 wk). The reduction in HF parallels the finding in the temporal analysis, which showed reduction in pNN06 in vaped mice. This is not surprising since both of these parameters are correlated (27,28). Furthermore, HRV changes in vaped mice are consistent with what has been shown in human subjects who are habitual electronic cigarette users (33) as well as in the setting of acute use (34). Such changes in HRV due to vaping are indicative of possible sympathovagal disbalance in the control of heart rate, which is clinically linked to poor cardiovascular outcomes (28, 35).
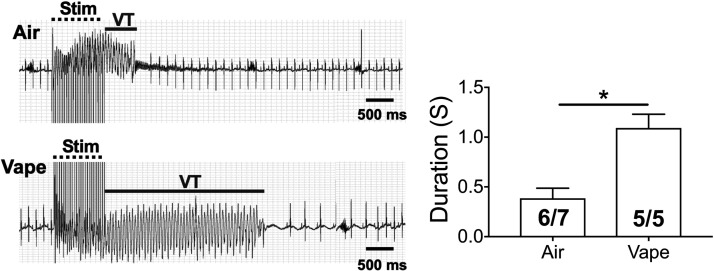
We studied the in vivo inducibility of VT in five wild-type mice exposed to vaping with vanilla custard for 10 wk versus seven mice exposed to air control, using in vivo programmed electrical stimulation. The ECGs of Fig. 7 show that a 1-s, 36-Hz burst stimulus at 1 mA induced a short-lived ventricular tachycardia (VT) episode, after which the heart reverted to sinus rhythm. In the vaped mouse, a similar burst stimulus induced a longer VT episode. Six out of seven air control mice had VT, whereas five out of five vaped animals had VT; however, as shown in the bar graph, the duration of VT episodes was significantly longer in vaped compared with WT mice (t test, P < 0.05).
Figure 7.
In vivo inducibility of VT in mice exposed to air or vanilla custard e-vapor. ECG traces of inducible VT in air control (top) and vaped (bottom) mice are shown. The bar graph compiles the duration of inducible VT episodes in 6 out of 7 control and 5 out of 5 vaped mice. *P < 0.05, t test. Stim: burst pacing stimulation. ECG, electrocardiogram; VT, ventricular tachycardia.
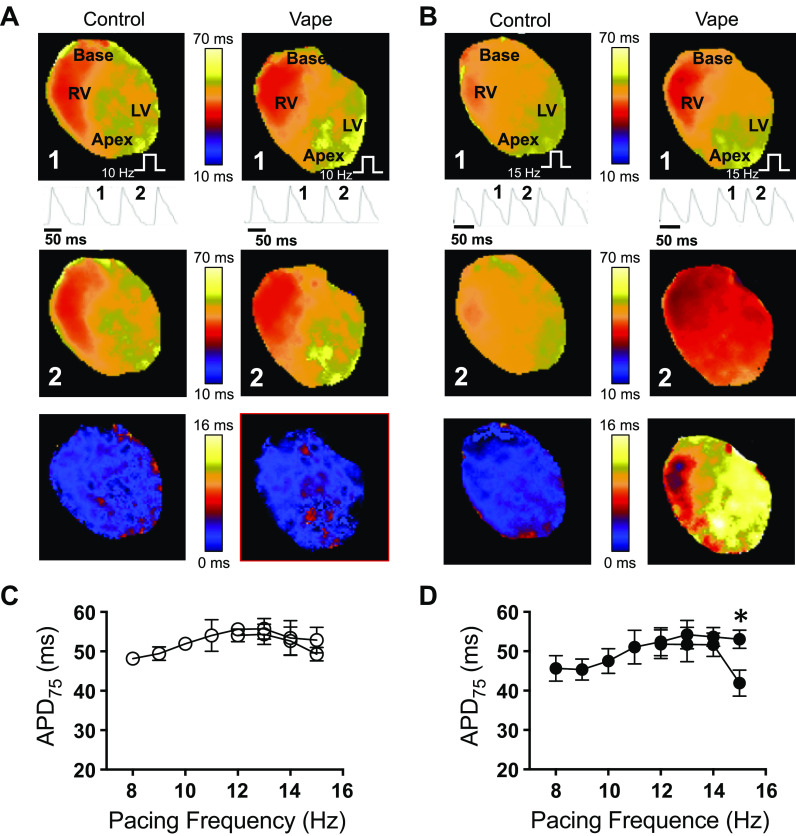
Subsequently, in four air control and three vaped mice, we conducted epicardial optical mapping of voltage. Figure 8A and Figure 8B show maps of action potential duration at 75% repolarization (APD75) of the anterior surface of the ventricles at 10 and 15 Hz pacing, respectively. Pacing was done from the apex, as indicated by the white stair symbol. RV and LV are the right and left ventricles. In Figure 8A, the top maps are representatives of paced beat 1, and the middle maps are representatives of the subsequent paced beat 2 in control and vaped hearts. Single-pixel recordings of the action potentials are underneath the top maps, and they show beat 1 and beat 2. The action potential duration was not different between beat 1 and the subsequent beat 2, as evidenced by the difference map in the bottom (map 1 minus map 2). In Figure 8B, the hearts were paced at 15 Hz. It can be appreciated from the single-pixel recordings of the optical action potential, and from the APD maps, that while in control, the APD did not change considerably between beat 1 and beat 2, action potential duration alternans occurred in the vaped heart, where one beat is long (beat 1), whereas the successive beat is short (beat 2). These APD alternans can be visualized in the difference map (bottom). Figure 8C plots the averaged action potential durations in four control hearts, quantified from the APD maps at different pacing frequencies (from 8 to 15 Hz). No significant alternans occurred. Figure 8D is the averaged action potential durations in three vaped hearts, paced from 8 to 15 Hz. Significant alternans occurred at 15 Hz (*P < 0.01, t test), indicating that vaping induces action potential changes in the heart. It has been shown that alternans are indicators of cardiac electrophysiological instability and could lead to arrhythmogenesis (36).
Figure 8.
Optical mapping in isolated Langendorff-perfused mouse hearts. A and B: first (beat 1) and second (beat 2) rows: APD75 maps of two consecutive beats in pacing at 10 (A) and 15 Hz (B). Single-pixel optical action potential traces are shown. Third row: difference maps of APD75 maps of beat 1 minus beat 2. C and D: average APD75 at different pacing frequencies (8 to 15 Hz) in air control (C, n = 4) and VC-exposed (D, n = 3) mice. *P < 0.05, t test. White step symbol: pacing site. LV, left ventricle; RV, right ventricle; VC, vanilla custard.
DISCUSSION
Flavored ENDS are very popular, and it has been argued that the appeal of flavored ENDS products has fueled the rapid and spectacular growth of this industry (3, 5). Thus, our objective was to investigate the in vitro and in vivo cardiac electrophysiological toxicity of flavorings in ENDS. Our study showed that exposure to ENDS aerosols could result in cellular and whole organ electrophysiological toxicity that includes action potential instability and reduction in heart rate variability parameters. Therefore, it is possible that ENDS are not harm free to the electrophysiological function of the heart.
In HL-1 mouse atrial cardiomyocytes (23), the three e-liquids tested were toxic but to different extents. Aldehydes containing vanilla custard and Apple Jax flavors, as determined by GC-MS, were more detrimental compared with the fruit-flavored e-liquid. This is independent of nicotine content, since these e-liquids are stated to contain 6 mg/mL nicotine. These findings are consistent with similar studies conducted in many other cell lines; however, these cell lines are not relevant to cardiac electrophysiology (8, 11, 12, 14–16). In spontaneously beating hiPSC-derived cardiomyocytes, e-vapor affected the beating rate, prolonged the corrected field potential duration (a surrogate for the QTc interval) (37), and inhibited IKr. This raises the possibility that vaping could be arrhythmogenic since in the heart, prolongation of the QT interval is associated with the development of fast ventricular rhythms that include tachycardia and fibrillation (38).
HRV analysis revealed patterns of change indicative of sympathovagal disbalance in the control of the mouse heart due to exposure to ENDS aerosols where temporal and spectral indices of parasympathetic modulation of heart rate variability were decreased. These changes are consistent with what has been shown in human subjects who are habitual electronic cigarette users (33) as well as in the setting of acute electronic cigarette use (34). It is generally accepted that in HRV measurements, sympathovagal disbalance could correlate with the development of arrhythmias and poor cardiovascular outcome (28, 35). This is in line with the in vivo VT inducibility studies that we conducted, where in vaped mice, inducible VT was more sustained compared with air control mice, and where in optical mapping, vaping resulted in action potential instability that manifested as action potential alternans. It has been shown in human subjects that the use of electronic cigarettes with nicotine affected repolarization indices on the ECG (39), and in mice, it was shown that ENDS constituents including vegetable glycerin caused prolongation of the QT interval (40).
In conclusion, our study suggests that the exposure to ENDS aerosols could result in cardiac electrophysiological toxicity that includes prolongation of repolarization, hERG block, sympathovagal disbalance, inducibility of VT, and action potential alternans. The respiratory system is the major route of ENDS smoke entry into the body, and vaping-related pulmonary illnesses are increasingly documented in the clinic (41). However, similar to combustible tobacco smoking, ENDS use could have potentially harmful effects on the heart. Our work demonstrates that vaping compromises the cardiac electrophysiological integrity, and further studies are needed to further assess the long-term cardiac safety profile of ENDS products.
LIMITATIONS
Our results in the mouse hearts suggested that vaping can lead to inducible ventricular tachyarrhythmias. Other than a case report that attributed the occurrence of atrial fibrillation to vaping in an otherwise healthy teen (42), arrhythmias have not yet been directly linked to the use of ENDS in the young population. Therefore, caution should be exercised when extrapolating the findings in the mouse heart to the human heart due to the presence of many obvious differences including those related to important species differences in the ionic bases of the action potential (43). In the in vitro experiments, we do not know whether the concentrations of ENDS constituents in the bubbled media are similar to those that could actually reach the heart through inhalation exposure to ENDS aerosols. When generating the e-vapor, the full impact of reversing airflow through the mouthpiece is unknown. Plasma or urine nicotine levels were not measured in the vaped mice. HRV measurements were performed 4 h after vaping. The reported nicotine half-life in C57BL/6 mice is ∼9 min (44). Studies have shown that the manufacturer-stated nicotine levels in some ENDS products have been inaccurate (45). The stated nicotine level of 6 mg/mL in the e-liquids we used is not verified and is reported in this manuscript as printed on the products’ labels. In addition, it is not inconceivable that that there may be contaminants (46) or variable grades of nicotine and/or solvents used by different companies. Such possible confounders have not been tested in this work. Further investigations are necessary to assess the long- term cardiac safety profile of ENDS products in humans and to better understand how individual components of ENDS affect cardiac toxicity.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants R21HL138064 and R01HL129136 (to S. F. Noujaim) and American Heart Association postdoctoral fellowship (to B. Chidipi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
O.A., L.C., and S.F.N. conceived and designed research; O.A., M.C., B.C., M.R., M.K., R.S., and S.F.N. performed experiments; O.A., M.C., B.C., J.L.M., M.R., J.S., L.C., and S.F.N., analyzed data; O.A., M.C., J.L.M., M.R., B.H., J.S., L.C., and S.F.N., interpreted results of experiments; O.A., M.C., J.L.M., M.R., J.S., and S.F.N. prepared figures; O.A., M.C., J.L.M., M.R., J.S., and S.F.N. drafted manuscript; T.V.M., B.H., and S.F.N. edited and revised manuscript; J.L.M., T.V.M., B.H., J.S., L.C., and S.F.N. approved final version of manuscript.
REFERENCES
- 1.Kuehn B Youth e-cigarette use. Jama 321: 138, 2019. doi: 10.1001/jama.2018.20655. [DOI] [PubMed] [Google Scholar]
- 2.Prochaska JJ The public health consequences of e-cigarettes: a review by the National Academies of Sciences. A call for more research, a need for regulatory action. Addiction 114: 587–589, 2019. doi: 10.1111/add.14478. [DOI] [PubMed] [Google Scholar]
- 3.Zhu SH, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, Lee M. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control 23: iii3–iii9, 2014. doi: 10.1136/tobaccocontrol-2014-051670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zare S, Nemati M, Zheng Y. A systematic review of consumer preference for e-cigarette attributes: flavor, nicotine strength, and type. PloS One 13: e0194145, 2018. doi: 10.1371/journal.pone.0194145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day HR, Ambrose BK, Schroeder MJ, Corey CG. Point of sale scanner data for rapid surveillance of the e-cigarette market. Tob Regul Sci 3: 325–332, 2017. doi: 10.18001/TRS.3.3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cervellati F, Muresan XM, Sticozzi C, Gambari R, Montagner G, Forman HJ, Torricelli C, Maioli E, Valacchi G. Comparative effects between electronic and cigarette smoke in human keratinocytes and epithelial lung cells. Toxicol In Vitro 28: 999–1005, 2014. doi: 10.1016/j.tiv.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crotty Alexander LE, Drummond CA, Hepokoski M, Mathew D, Moshensky A, Willeford A, Das S, Singh P, Yong Z, Lee JH, Vega K, Du A, Shin J, Javier C, Tian J, Brown JH, Breen EC. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am Journal Physiology Regul, Integr Comp Physiol 314: R834–R847, 2018. doi: 10.1152/ajpregu.00270.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farsalinos KE, Romagna G, Allifranchini E, Ripamonti E, Bocchietto E, Todeschi S, Tsiapras D, Kyrzopoulos S, Voudris V. Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int J Environ Res Public Health 10: 5146–5162, 2013. doi: 10.3390/ijerph10105146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang JH, Lyes M, Sladewski K, Enany S, McEachern E, Mathew DP, Das S, Moshensky A, Bapat S, Pride DT, Ongkeko WM, Crotty Alexander LE. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med 94: 667–679, 2016. doi: 10.1007/s00109-016-1378-3. [DOI] [PubMed] [Google Scholar]
- 10.Khlystov A, Samburova V. Flavoring compounds dominate toxic aldehyde production during E-cigarette vaping. Environ Sci Technol 50: 13080–13085, 2016. doi: 10.1021/acs.est.6b05145. [DOI] [PubMed] [Google Scholar]
- 11.Muthumalage T, Prinz M, Ansah KO, Gerloff J, Sundar IK, Rahman I. Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front Physiol 8: 1130, 2018. doi: 10.3389/fphys.2017.01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palpant NJ, Hofsteen P, Pabon L, Reinecke H, Murry CE. Cardiac development in zebrafish and human embryonic stem cells is inhibited by exposure to tobacco cigarettes and e-cigarettes. PloS One 10: e0126259, 2015. doi: 10.1371/journal.pone.0126259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowell TR, Reeber SL, Lee SL, Harris RA, Nethery RC, Herring AH, Glish GL, Tarran R. Flavored e-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol 313: L52–L66, 2017. doi: 10.1152/ajplung.00392.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sancilio S, Gallorini M, Cataldi A, di Giacomo V. Cytotoxicity and apoptosis induction by e-cigarette fluids in human gingival fibroblasts. Clin Oral Invest 20: 477–483, 2016. doi: 10.1007/s00784-015-1537-x. [DOI] [PubMed] [Google Scholar]
- 15.Sassano MF, Davis ES, Keating JE, Zorn BT, Kochar TK, Wolfgang MC, Glish GL, Tarran R. Evaluation of e-liquid toxicity using an open-source high-throughput screening assay. PLoS Biol 16: e2003904, 2018. doi: 10.1371/journal.pbio.2003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheffler S, Dieken H, Krischenowski O, Forster C, Branscheid D, Aufderheide M. Evaluation of e-cigarette liquid vapor and mainstream cigarette smoke after direct exposure of primary human bronchial epithelial cells. Int J Environ Res Public Health 12: 3915–3925, 2015. doi: 10.3390/ijerph120403915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu V, Rahimy M, Korrapati A, Xuan Y, Zou AE, Krishnan AR, Tsui T, Aguilera JA, Advani S, Crotty Alexander LE, Brumund KT, Wang-Rodriguez J, Ongkeko WM. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol 52: 58–65, 2016. doi: 10.1016/j.oraloncology.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunningham A, Slayford S, Vas C, Gee J, Costigan S, Prasad K. Development, validation and application of a device to measure e-cigarette users' puffing topography. Sci Rep 6: 35071, 2016. doi: 10.1038/srep35071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kośmider L, Jackson A, Leigh N, O'connor R, Goniewicz ML. Circadian puffing behavior and topography among e-cigarette users. Tobacco Reg Sci 4: 41–49, 2018. doi: 10.18001/TRS.4.5.4, 10.18001/TRS.4.4.5. doi: 10.18001/TRS.4.5.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikheev VB, Buehler SS, Brinkman MC, Granville CA, Lane TE, Ivanov A, Cross KM, Clark PI. The application of commercially available mobile cigarette topography devices for e-cigarette vaping behavior measurements. Nicotine Tob Res 22: 681–688, 2020. doi: 10.1093/ntr/nty190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson RJ, Hensel EC, Roundtree KA, Difrancesco AG, Nonnemaker JM, Lee YO. Week long topography study of young adults using electronic cigarettes in their natural environment. PloS One 11: e0164038, 2016. doi: 10.1371/journal.pone.0164038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munakata S, Ishimori K, Kitamura N, Ishikawa S, Takanami Y, Ito S. Oxidative stress responses in human bronchial epithelial cells exposed to cigarette smoke and vapor from tobacco- and nicotine-containing products. Reguly Toxicol Pharmacol 99: 122–128, 2018. doi: 10.1016/j.yrtph.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ. Jr.. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA 95: 2979–2984, 1998.doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertalovitz AC, Badhey MLO, McDonald TV. Synonymous nucleotide modification of the KCNH2 gene affects both mRNA characteristics and translation of the encoded hERG ion channel. J Biol Chem 293: 12120–12136, 2018. doi: 10.1074/jbc.RA118.001805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eddingsaas N, Pagano T, Cummings C, Rahman I, Robinson R, Hensel E. Qualitative analysis of e-liquid emissions as a function of flavor additives using two aerosol capture methods. Int J Environ Res Public Health 15: 323, 2018. doi: 10.3390/ijerph15020323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajab M, Jin H, Welzig CM, Albano A, Aronovitz M, Zhang Y, Park HJ, Link MS, Noujaim SF, Galper JB. Increased inducibility of ventricular tachycardia and decreased heart rate variability in a mouse model for type 1 diabetes: effect of pravastatin. Am J Physiol Heart Circ Physiol 305: H1807–1816, 2013. doi: 10.1152/ajpheart.00979.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thireau J, Zhang BL, Poisson D, Babuty D. Heart rate variability in mice: a theoretical and practical guide. Exp Physiol 93: 83–94, 2008. doi: 10.1113/expphysiol.2007.040733. [DOI] [PubMed] [Google Scholar]
- 28.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health 5: 258, 2017. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin H, Welzig CM, Aronovitz M, Noubary F, Blanton R, Wang B, Rajab M, Albano A, Link MS, Noujaim SF, Park HJ, Galper JB. QRS/T-wave and calcium alternans in a type I diabetic mouse model for spontaneous postmyocardial infarction ventricular tachycardia: a mechanism for the antiarrhythmic effect of statins. Heart Rhythm 14: 1406–1416, 2017. doi: 10.1016/j.hrthm.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noujaim SF, Kaur K, Milstein M, Jones JM, Furspan P, Jiang D, Auerbach DS, Herron T, Meisler MH, Jalife J. A null mutation of the neuronal sodium channel NaV1.6 disrupts action potential propagation and excitation-contraction coupling in the mouse heart. FASEB J 26: 63–72, 2012. doi: 10.1096/fj.10-179770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zarzoso M, Rysevaite K, Milstein ML, Calvo CJ, Kean AC, Atienza F, Pauza DH, Jalife J, Noujaim SF. Nerves projecting from the intrinsic cardiac ganglia of the pulmonary veins modulate sinoatrial node pacemaker function. Cardiovasc Res 99: 566–575, 2013. doi: 10.1093/cvr/cvt081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millard D, Dang Q, Shi H, Zhang X, Strock C, Kraushaar U, Zeng H, Levesque P, Lu HR, Guillon JM, Wu JC, Li Y, Luerman G, Anson B, Guo L, Clements M, Abassi YA, Ross J, Pierson J, Gintant G. Cross-site reliability of human induced pluripotent stem cell-derived cardiomyocyte based safety assays using microelectrode arrays: results from a blinded CiPA pilot study. Toxicol Sci 164: 550–562, 2018. doi: 10.1093/toxsci/kfy110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moheimani RS, Bhetraratana M, Yin F, Peters KM, Gornbein J, Araujo JA, Middlekauff HR. Increased cardiac sympathetic activity and oxidative stress in habitual electronic cigarette users: implications for cardiovascular risk. JAMA Cardiol 2: 278–284, 2017. doi: 10.1001/jamacardio.2016.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moheimani RS, Bhetraratana M, Peters KM, Yang BK, Yin F, Gornbein J, Araujo JA, Middlekauff HR. Sympathomimetic effects of acute e-cigarette use: role of nicotine and non-nicotine constituents. J Am Heart Assoc 6, 2017. doi: 10.1161/JAHA.117.006579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colhoun HM, Francis DP, Rubens MB, Underwood SR, Fuller JH. The association of heart-rate variability with cardiovascular risk factors and coronary artery calcification: a study in type 1 diabetic patients and the general population. Diabetes Care 24: 1108–1114, 2001. doi: 10.2337/diacare.24.6.1108. [DOI] [PubMed] [Google Scholar]
- 36.Tse G, Wong ST, Tse V, Lee YT, Lin HY, Yeo JM. Cardiac dynamics: alternans and arrhythmogenesis. J Arrhythm 32: 411–417, 2016. doi: 10.1016/j.joa.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luerman G, Obejero-Paz C, Brown A, Bruening-Wright A. Optimizing rate correction of field potential duration, a biomarker for QT risk assessment, in human iPSC-cardiomyocytes. Biophysical J 106: 720, 2014. doi: 10.1016/j.bpj.2013.11.3978. [DOI] [Google Scholar]
- 38.Bertino JS, Jr., Owens RC, Jr., Carnes TD, Iannini PB. Gatifloxacin-associated corrected QT interval prolongation, torsades de pointes, and ventricular fibrillation in patients with known risk factors. Clinical Infect Dis 34: 861–863, 2002. doi: 10.1086/339075. [DOI] [PubMed] [Google Scholar]
- 39.Ip M, Diamantakos E, Haptonstall K, Choroomi Y, Moheimani RS, Nguyen KH, Tran E, Gornbein J, Middlekauff HR. Tobacco and electronic cigarettes adversely impact ECG indexes of ventricular repolarization: implication for sudden death risk. Am J Physiol Heart Circ Physiol 318: H1176–H1184, 2020. doi: 10.1152/ajpheart.00738.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carll A, Salatini R, Arab C, Holbrook D, Bhatnagar A, Conklin D. Electronic cigarette aerosols and constituents induce bradycardia and prolonged QT in mice – does e-cigarette use increase cardiovascular disease risk? Circulation 136: A20454, 2017. doi: 10.2337/diacare.24.6.1108. [DOI] [PubMed] [Google Scholar]
- 41.Mukhopadhyay S, Mehrad M, Dammert P, Arrossi AV, Sarda R, Brenner DS, Maldonado F, Choi H, Ghobrial M. Lung biopsy findings in severe pulmonary illness associated with e-cigarette use (Vaping). Am J Clin Pathol 153: 30–39, 2019. doi: 10.1093/ajcp/aqz182. [DOI] [PubMed] [Google Scholar]
- 42.Lowe R, Klingaman C, Golten ADavis , T. Atrial fibrillation with e-cigarette use in an otherwise healthy adolescent male. Pediatrics 146: 312–313, 2020. [Google Scholar]
- 43.Kaese S, Verheule S. Cardiac electrophysiology in mice: a matter of size. Front Physiol 3: 345, 2012. doi: 10.3389/fphys.2012.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siu EC, Tyndale RF. Characterization and comparison of nicotine and cotinine metabolism in vitro and in vivo in DBA/2 and C57BL/6 mice. Mol Pharmacol 71: 826–834, 2007. doi: 10.1124/mol.106.032086. [DOI] [PubMed] [Google Scholar]
- 45.Lisko JG, Tran H, Stanfill SB, Blount BC, Watson CH. Chemical composition and evaluation of nicotine, tobacco alkaloids, pH, and selected flavors in e-cigarette cartridges and refill solutions. Nicotine Tob Res 17: 1270–1278, 2015. doi: 10.1093/ntr/ntu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee MS, Allen JG, Christiani DC. Endotoxin and [formula: see text] contamination in electronic cigarette products sold in the United States. Environ Health Perspect 127: 47008, 2019. doi: 10.1289/EHP3469. [DOI] [PMC free article] [PubMed] [Google Scholar]