Abstract
Heart rate fragmentation (HRF), a marker of abnormal sinoatrial dynamics, was shown to be associated with incident cardiovascular events in the Multi-Ethnic Study of Atherosclerosis (MESA). Here, we test the hypothesis that HRF is also associated with incident atrial fibrillation (AF) in the MESA cohort of participants who underwent in-home polysomnography (PSG) and in two high-risk subgroups: those ≥70 yr taking antihypertensive medication and those with serum concentrations of NH2-terminal prohormone B-type natriuretic peptide (NT-proBNP) >125 pg/ml (top quartile). Heart rate time series (n = 1,858) derived from the ECG channel of the PSG were analyzed using newly developed HRF metrics, traditional heart rate variability (HRV) indices and two widely used nonlinear measures. Eighty-three participants developed AF over a mean follow-up period of 3.83 ± 0.87 yr. A one-standard deviation increase in HRF was associated with a 31% (95% CI: 3–66%) increase in risk of incident AF, in Cox models adjusted for age, height, NT-proBNP, and frequent premature supraventricular complexes. Furthermore, HRF added value to the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE)-AF models. Traditional HRV and nonlinear indices were not significantly associated with incident AF. In the two high-risk subgroups defined above, HRF was also significantly associated with incident AF in unadjusted and adjusted models. These findings support the translational utility of HRF metrics for short-term (∼4-yr) prediction of AF. In addition, they support broadening the concept of atrial remodeling to include electrodynamical remodeling, a term used to refer to pathophysiological alterations in sinus interbeat interval dynamics.
NEW & NOTEWORTHY This study is the first demonstration that heart rate fragmentation (HRF), a marker of anomalous sinoatrial dynamics, is an independent predictor of atrial fibrillation (AF). Traditional measures of heart rate variability and two widely used nonlinear measures were not associated with incident AF in the Multi-Ethnic Study of Atherosclerosis. Fragmentation measures added value to the strongest contemporary predictors of AF, including ECG-derived parameters, coronary calcification score, serum concentrations of NH2-terminal prohormone B-type natriuretic peptide, and supraventricular ectopy. The computational algorithms for quantification of HRF could be readily incorporated into wearable ECG monitoring devices.
Keywords: atrial fibrillation, autonomic nervous system, heart rate fragmentation, heart rate variability, sinus node
INTRODUCTION
Atrial fibrillation (AF), the most common major arrhythmia, is a growing public health problem (14, 54). Substrates of susceptibility include electrophysiological, anatomic, and neuroautonomic perturbations especially prevalent in the aging population and in those with underlying heart disease (44, 54). Not surprisingly, substantial effort is directed at identifying new predictors of incident AF. Widely cited risk factors, in addition to older age, include hypertension, obesity, height, smoking, diabetes (3, 4), increased serum concentration of NH2-terminal prohormone B-type natriuretic peptide (NT-proBNP) (4, 45, 55), coronary artery calcification (CAC) (44), and sleep disordered breathing (34, 35). Additional markers include electrocardiographic (ECG) features such as increased P-wave duration (56) and frequent supraventricular ectopy (20). An active search for genetic markers of AF risk is ongoing (38), but the translational promise of “omic” metrics remains to be realized.
To date, no single or composite index has gained sufficient clinical traction (12) to be incorporated into formal recommendations for the prediction of AF. This knowledge gap motivates the ongoing search for novel, independent AF predictors. Our approach focuses on a new class of biomarkers, “dynamical assays” (15, 24), which are based on the quantification of information encoded in the temporal fluctuations of cardiovascular (CV) signals. The construct derives from the hypothesis that cardioautonomic and electrodynamic perturbations related to the pathogenesis of AF inscribe a dynamical signature on heart rate time series that can be captured with appropriate signal analysis techniques. Because of its repeatability and low-cost profile, this kind of dynamical risk marker is especially attractive in the bourgeoning era of wearable technology. Initially, traditional heart rate variability (HRV) analytics, the major noninvasive probes of cardioautonomic function (32), seemed particularly well suited for assessing CV risk and predicting AF. Unfortunately, HRV measures have not realized their promise (28, 47, 50) with respect to these challenges.
A central problem with HRV analysis derives from its interpretative framework (15–18, 28) that equates increased high-frequency (short-term) variability with increased vagal tone modulation (32). Indeed, higher HRV is a well-established marker of increased cardiac vagal tone modulation in healthy young to middle-aged adults. However, paradoxically, increased HRV can also be seen with advanced aging and atherosclerotic heart disease, which are marked by reduced or impaired parasympathetic modulation (10, 17, 58). Thus, the absence of a monotonic relationship between short-term HRV and degree of vagal tone modulation in adults fundamentally limits the utility of classic HRV analyses in the highest risk populations. However, this limitation does not negate the potential value, both basic and translational, of extracting information encoded in beat-to-beat heart rate dynamics.
Recently, Costa et al. (15, 16) delineated a novel marker of anomalous sinoatrial node (SAN) dynamics, termed heart rate fragmentation (HRF), attributed to pacemaker and/or autonomic impairment, along with a set of metrics for its quantification. Illustrative examples of fragmented versus nonfragmented heart rate dynamics from two participants in the Multi-Ethnic Study of Atherosclerosis (MESA) are showed in Fig. 1. In healthy individuals (Fig. 1, A–C), variations in vagal tone are responsible for the coupling between breathing and heart rate (5, 30). The dynamical signature of this parasympathetic influence is the oscillatory pattern in sinus normal-to-normal (NN) time series (Fig. 1, B) termed respiratory sinus arrhythmia. With aging and CV disease (Fig. 1, D–F) (15–17), fluctuations in heart rate faster than and decoupled from respiration may emerge (Fig. 1, E). Heart rate fragmentation was the term coined to refer to these types of dynamics, which may escape detection from visual inspection of the ECG and graphs obtained from time series analysis such as Poincaré plots and Fourier spectra.
Figure 1.
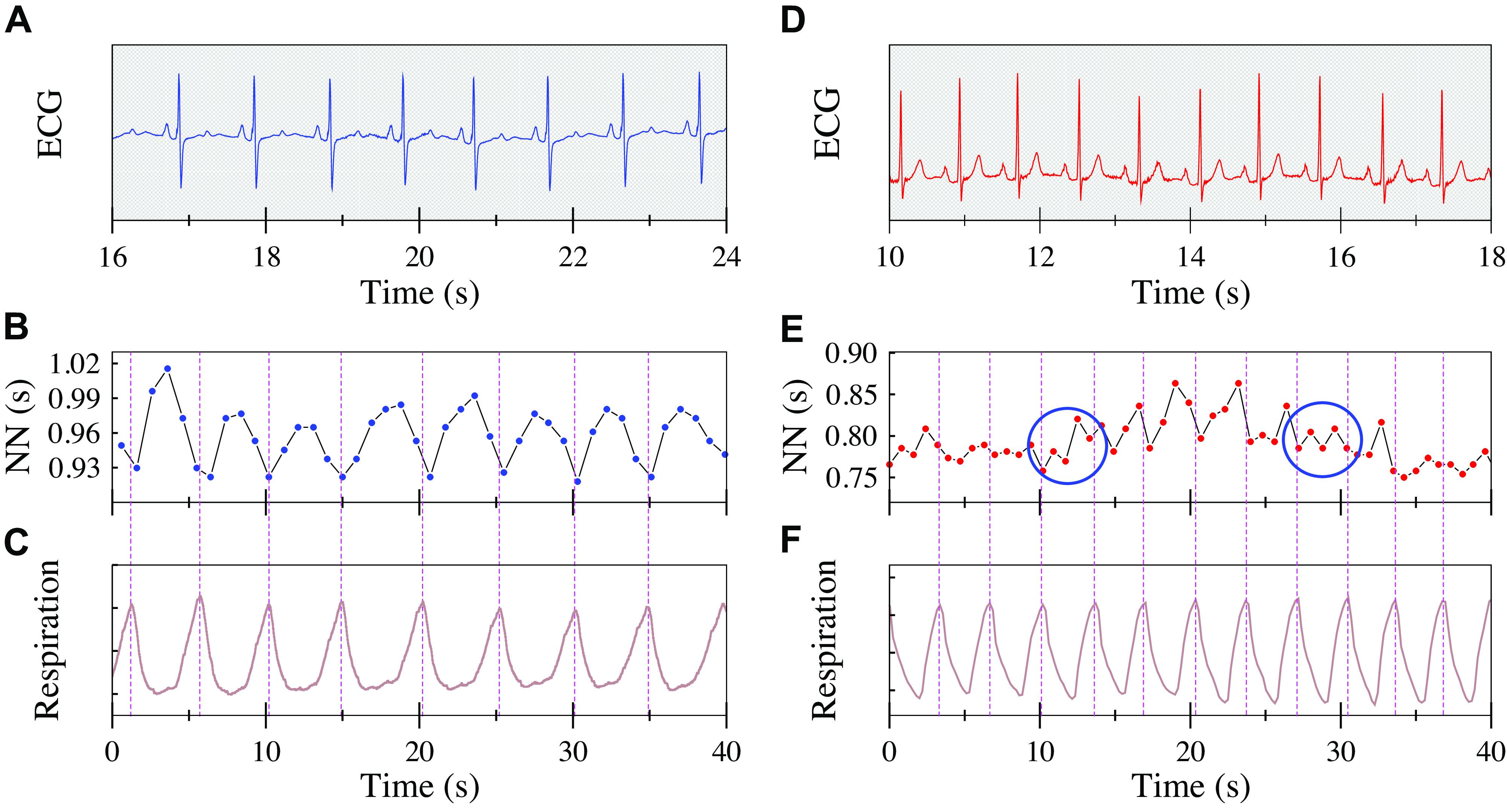
Nonfragmented (A–C) vs. fragmented (D–F) heart rate dynamics. ECG, normal-to-normal (NN) interval time series and respiration (abdominal wall movement) signals during stage N2 sleep from (A–C) a 55 yr-old female with no hypertension, no diabetes, no prevalent or incident atrial fibrillation (AF), and (D–F) from a 75-yr-old female with diabetes, hypertension, and incident AF, who before the sleep study had suffered an ischemic stroke. Dotted vertical lines across B and C, and E and F, mark breathing cycles to facilitate observation of interbeat interval fluctuations within each cycle. Respiratory sinus arrhythmia resulting from the coupling between breathing and interbeat interval can be seen in the younger participant (B and C). Fragmentation, manifested by fluctuations in heart rate faster than the breathing frequency, can be seen in older participant (E and F). The two blue circumferences highlight periods in which the dynamics is maximally fragmented, exhibiting an “up-down-up-down” type of NN interval pattern, despite a surface ECG consistent with “normal sinus rhythm.” (The upward/downward directions of the respiratory signal correspond to inspiration/expiration, respectively.)
The heart rate fragmentation construct resolves the “HRV paradox” (15, 18) by revealing two mechanisms of high-frequency HRV: 1) vagal tone modulation and 2) sinus rhythm fragmentation. The former is a marker of intact heart rate control, the latter of its breakdown.
Costa and colleagues (15–17) previously reported that 1) HRF monotonically increased with cross-sectional age in both healthy subjects and those with coronary artery disease (15, 16); 2) increased HRF, but not reduced HRV, was associated with incident major CV events in MESA (17), and 3) HRF metrics added value to the Framingham and MESA CV risk indices in the prediction of CV events. Given that disrupted cardioautonomic and electrophysiologic control are recognized promoters of atrial arrhythmogenesis in the elderly and in those with advanced coronary artery disease (1, 13, 43), we hypothesized that HRF would be an independent predictor of incident AF, not only in the general MESA population, but also in the two high-risk subgroups defined below.
METHODS
Study Population and Data Collection
The MESA study has been described in detail (6). Briefly, in 2000–2002, 6,814 persons between the ages of 45 and 84 without clinically evident CV disease were recruited at six U.S. field centers. Institutional review boards approved the conduct of the study. Written informed consent was obtained from all participants.
An ancillary sleep study was conducted in 2010–2013 in conjunction with MESA fifth examination. The study enrolled 2,057 participants, who underwent in-home overnight polysomnography (PSG) following a standardized protocol (51). Compared with the participants who did not undergo the sleep examination, those who did were relatively younger (mean, 68 vs. 71 yr), less likely to be hypertensive (58% vs. 62%), and more likely to be Hispanic (23.5% vs. 19.1%) (19). They had lower CAC scores (median [interquartile range]: 30 [0–215] vs. 57 [0–338]) and lower blood concentration of NT-proBNP (71 [35–139] vs. 83 [43–181] pg/ml). There were no differences in sex, body mass index, smoking status, diabetes, and prevalent CV disease.
The PSG data were scored at a centralized sleep reading center, in accordance with published guidelines (51). The ECG channel, sampled at 256 Hz, was processed using Compumedics Somte software (Compumedics, Abbottsville, Australia) for detection and classification of the QRS complexes as normal sinus (N), premature ventricular complexes (PVCs) or premature supraventricular complexes (PSVCs). The automated annotations were reviewed and corrected when necessary by a trained technician. Here, we analyzed NN interbeat interval time series (17). Participants whose records showed one or more of the following were excluded: poor signal quality (n = 33), electronic pacemaker (n = 14), missing annotations for sleep stage or QRS complexes (n = 10), <2 h of combined sleep periods scored as rapid eye movement (REM) or non-REM (n = 14), prevalent AF (n = 118), missing AF adjudication (n = 13) and those for whom (n = 8) the last recorded follow-up was before the PSG study. Overall, heart rate dynamical measures were calculated for 1,858 participants. Our analyses focused on this group and on two high-risk subgroups defined below.
Clinical Follow-Up and Event Classification
In addition to clinical exams, MESA participants were contacted by telephone every 9 to 12 months to obtain information about hospital admissions and medical events. Incident AF (29), including fibrillation and flutter, was identified from 12-lead ECGs, diagnostic codes from hospital admissions and from those enrolled in fee-for-service Medicare, via both inpatient and outpatient Medicare claims. Atrial fibrillation occurring during hospitalizations for cardiac surgery was not counted. Our analyses included cases identified from the date of the PSG study to the end of 2015.
Assessment of Baseline Covariates at MESA Fifth Examination (2010–2012)
Age, sex, race, ethnicity, smoking status, and medication use were self-reported. Race/ethnicity was classified into white, Chinese-American, African-American and Hispanic. In the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE)-AF model, race was reclassified as white or nonwhite. Systolic and diastolic blood pressures (BPs) were measured three times after the participant rested for 5 min; the means of the last two measurements were used here. Diabetes was defined by a fasting blood glucose ≥126 mg/dL or self-reported use of antidiabetic medication. A smoker was a participant who smoked at the time of the PSG or who quit in the previous 12 mo. Antihypertensive medication included β-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors or receptor blockers, vasodilators and diuretics. Two measures of sleep structure included in this study (19) were sleep maintenance efficiency, defined as the percentage of time asleep after sleep onset, and percentage of time in sleep stages N3/N4 [slow wave sleep (SWS)]. Sleep duration was defined as the combined time spent in REM, stages N1, N2, and N3/N4. Respiratory events were quantified by the apnea-hypopnea index (AHI) (19) representing the average number per hour of sleep of all apneas and hypopneas associated with ≥3% oxygen desaturation or an arousal. Nocturnal hypoxemia was measured by the O2 desaturation index (ODI) defined as the average number of episodes with ≥4% decrease in from its baseline value prior to the event, per hour of sleep. Slow-wave sleep, NT-proBNP concentration, AHI, and ODI values were available for all but 19, 37, 21, and 26 participants, respectively. Information for the other baseline covariates was available for the entire cohort (n = 1,858).
Standard 12-lead ECGs were digitally acquired (35). P-wave measures were automatically generated by the GE Marquette 12-SL program 2001 version (GE Marquette, Milwaukee, WI). The P-wave indices included in this study were maximum P-wave duration (among the 12 leads), mean frontal plane P-wave axis, and P-wave terminal force in V1 (duration in milliseconds of the terminal negative part of the P-wave in lead V1 multiplied by its depth in microvolts). The PR interval was calculated as the average value across the 12 leads. Mean and standard deviation (SD) time between MESA exam 5 and the PSG study were 341 and 199 days, respectively (exam 5 occurred first). ECG data were available for 1,837 (99%) out of the 1,858 participants who qualified for heart rate dynamics. The CAC score, derived from computed tomography (CT) imaging, was assessed in 1,433 (77%) participants using the Agatston method (2, 7, 11). Mean and SD time between the PSG and the CT studies were 244 and 231 days, respectively. There were no significant differences in any of the demographic (age, sex, race, and height) and CV risk factors (diabetes, smoking and hypertensive status, body mass index, CV disease prevalence, and NT-proBNP) between those with and without CAC scores.
Criteria for Defining High-Risk Subgroups
We analyzed data from two subgroups known to be at especially high risk of developing AF: 1) those ≥70 yr who were taking antihypertensive medication and 2) those with a serum concentration of NT-proBNP >125 pg/ml (75th percentile), the standard cut-off threshold for elevated NT-proBNP in those below the age of 75.
Heart Rate Dynamical Measures
Heart rate fragmentation metrics.
Heart rate fragmentation was quantified by an ensemble of statistical metrics derived from the analysis of NN interval time series, as described by Costa et al. (15, 16). These metrics are based on counts of heart rate acceleration, deceleration, and no-change intervals. Let ti represent the time of occurrence of a given QRS complex and NNi the time interval (ti – ti−1) between consecutive QRS complexes. Heart rate acceleration, deceleration, and no-change intervals (in seconds) are defined as ΔNNi ≤ −n/SF, ΔNNi ≥ n/SF and −n/SF < ΔNNi < n/SF, respectively, where ΔNNi = NNi – NNi−1, n is a positive integer (in this case, n = 1), and SF is the sampling frequency (in Hz) of the ECG signal. (Note that heart rate and ΔNNi values are inversely related.) Sequences of negative (positive) ΔNN intervals are termed accelerative (decelerative) segments (appendix a, Fig. A1). The length of a segment is the number of ΔNN intervals it contains.
We computed four interrelated indices of overall degree of fragmentation (PIP, ALS, PNNLS, and PNNSS) and the frequency of three specific patterns of heart rate fluctuations, one associated with less fragmented/more “fluent” (healthy) dynamics (W1H) and two other with highly fragmented (pathologic) time series (W3M and W3S). Briefly, PIP, the percentage of “inflection points,” is defined as the combined percentage of transitions from heart rate acceleration to heart rate deceleration and vice-versa (“hard” inflection points), and from heart rate acceleration/deceleration to no-change in heart rate and vice-versa (“soft” inflection points). Mathematically, a given NNi interval is an inflection point if ΔNNi+1 × ΔNNi ≤ 0 and ΔNNi+1 ≠ ΔNNi. The average length of accelerative/decelerative segments, ALS, is defined as the average number of ΔNN intervals in those segments. The overall percentage of ΔNN intervals in long segments, PNNLS, is the number of ΔNN intervals in accelerative/decelerative segments with ≥3 ΔNN intervals over the total number of ΔNN intervals. The percentage of ΔNN intervals in short segments, PNNSS, is the number of ΔNN intervals in accelerative/decelerative segments with <3 ΔNN intervals over the number of ΔNN intervals in accelerative/decelerative segments of any length. More fragmented time series have higher PIP and PNNSS values, and lower ALS and PNNLS values. The three metrics derived from symbolic dynamical analysis, termed W1H, W3S, and W3M, are the percentages of sequences of four consecutive ΔNN intervals, the former with one hard inflection point and the latter two with three inflection points. W3S comprises three soft inflection points. W3M comprises a mixture of soft and hard inflection points: two hard and one soft or two soft and one hard. (Additional details regarding the calculation of these indices are provided in appendix a.) Metrics that decrease in value as fragmentation increases quantify the degree of smoothness of the time series and can be interpreted as “fluency” metrics.
Traditional HRV indices.
Traditional time domain HRV indices (32) were calculated from NN interval time series between sleep onset and sleep termination using a 5-min sliding window (without overlap). Windows with <150 beats and/or < 75% NN intervals were excluded. The following time domain measures were computed: 1) the average of all NN intervals (AVNN), 2) the mean of the SDs of NN intervals in all qualified 5-min window (SDNN), and 3) the root mean square of successive differences between NN intervals (rMSSD). The following traditional frequency domain HRV indices were calculated: 1) the total spectral power of NN intervals between 0.15 and 0.4 Hz (HF), 2) the total spectral power of NN intervals between 0.04 and 0.15 Hz (LF), and 3) the ratio of low- to high-frequency power (LF/HF). Power spectrum estimates were obtained using the Lomb periodogram method. A total of 170,527 windows were analyzed. For each subject, the values from the different windows were averaged. The source codes for HRV computations are available at www.physionet.org (23).
Detrended fluctuation analysis: short-term scaling index.
Detrended fluctuation analysis (46) was developed to quantify the correlation properties of a time series. The method is based on the assessment of the slope of the regression line of the logarithm of F(n) versus the logarithm of n, where F(n) is the root-mean-square fluctuation of the integrated and detrended data, computed using windows of length n. For the analysis of NN interval time series, two indices, α1 and α2, quantifying short- and long-term behavior, respectively, were proposed. We focused on α1, defined as the slope of F(n) versus n, for 4 ≤ n ≤ 11 (48). The source code used for these computations is available at www.physionet.org. Discontinuities in the NN interval time series due to removal of ectopic beats, misdetections, and artifact were dealt with as follows: if the gap was <3 s, an interpolated beat was inserted. If the gap were wider, the segments that preceded and followed the gap were “stitched” together. The α1 exponent was calculated for nonoverlapping segments of 1,000 intervals, and the results were averaged.
Entropy measures: sample entropy.
Sample entropy (SampEn) (53) quantifies the degree of irregularity of a signal. A higher SampEn value implies a more irregular, less predictable signal. Sample entropy is defined as the negative of the natural logarithm of the conditional probability that the (m + 1)th components of two distinct segments match within tolerance r, given that the first m components match within the same tolerance. In this study, we used m = 2 and r = 15% of the time series’ SD.
Statistical Analyses
The summary statistics used to describe continuous variables were the median and the interquartile range, unless otherwise specified. The summary statistics used to describe categorical variables were the number and percentage of participants in each category. In the case of dichotomous variables, the number and percentage of participants were reported for only one of the two categories. To evaluate whether differences in baseline characteristics between groups with and without incident AF were statistically significant, we used the χ2 and Mann-Whitney U tests for categorical and continuous variables, respectively. Variables with skewed distributions were transformed using the function that best normalized their distributions. For ODI and AHI, that function was the square root; for NT-proBNP, rMSSD, SDNN, HF, LF, LF/HF, and CAC score, it was the natural logarithm. (Of note, for the CAC score, we used the natural logarithm of [CAC score +1] due to the occurrence of 0 values.) Two variables, with very skewed distributions, PSVCs and percentage of SWS sleep, were dichotomized as follows: 1 if PSVCs ≥ 0.2% (top quartile), 0 otherwise; 1 if percentage of SWS < 2.3% (bottom quartile), 0 otherwise.
Cox proportional hazards models were used to determine the association of baseline demographic, clinical, and heart rate dynamical characteristics with incident AF. Models with the following adjustments were considered: 1) age and height; 2) age, height, and NT-proBNP; 3) age, height, NT-proBNP, and PSVCs ≥ 0.2%; 4) the components of the CHARGE-AF model, i.e., age, height, weight, white race, diabetes status, smoking (current or quit <1 yr), systolic and diastolic BP, and usage of antihypertensive medication; and 5) NT-proBNP in addition to the components of the CHARGE-AF model. Efron’s method was used to adjudicate ties. The predictive power of these models was assessed using Harrell’s C statistic. Hazard ratios (per one-SD increase in the value of the independent variables) were calculated with 95% confidence intervals (CIs). The Schoenfeld residuals method was used to test the proportional hazards assumption. No violations were noted. Follow-up time was defined as the period from the sleep ancillary study (2010–2013) to incident AF, death, or to the last available follow-up contact, whichever occurred first. The likelihood ratio test was used to compare the fit of two nested models (null vs. null + heart rate dynamical metric). The null hypothesis was that the nested models fitted the data equally well. Rejection of the null hypothesis implied that the larger model fitted the data better, indicating that a given heart rate dynamical metric added value to the null model. Analyses were restricted to participants with complete data. All variables, with the exception of CAC score, had values for more than 98% of the participants.
The terminal force of the P-wave in lead V1 was analyzed both as a continuous and dichotomous (<−4 vs. ≥−4 mV·ms, a standard cut-off threshold (41, 56)) variable. The PR interval was also analyzed both as a continuous and dichotomous variable (≥200 vs. <200 ms, a standard cut-off threshold (41, 56)).
Logistic regression analyses adjusted for age and height were used to quantify the relationship between PSVCs ≥0.2% and the indices of overall degree of HRF (PIP, PNNSS, PNNLS, and ALS).
All analyses were performed using STATA software (version 12.0 for Linux). Statistical significance was set at a P value < 0.05. All P values reported were two-sided.
RESULTS
Baseline Characteristics of the MESA Participants
Over a median [interquartile range] follow-up period of 3.93 [3.45–4.44] years after the PSG study, 83 of the 1,858 participants were identified as having incident AF. Table 1 summarizes demographic, clinical, and heart rate dynamical characteristics of MESA participants with and without incident AF. At the time of the PSG, participants who developed AF during follow-up were ∼6 yr older, 5 cm taller, and had higher systolic BP. A higher proportion of them were male, took antihypertensive medication, and had a density of PSVCs ≥ 0.2% (top quartile). There were no significant differences in weight, diastolic BP, smoking status, diabetes status, and in the percentage of participants with SWS < 2.3%. However, in participants with incident AF the mean concentration of NT-proBNP was twice as high, AHI and ODI values were ∼30% higher, and the median CAC score was more than 10 times higher. Sleep maintenance efficiency and sleep duration were slightly lower in participants with incident AF. Less than 2% of all participants had heart failure or myocardial infarction.
Table 1.
Demographic, clinical, and heart rate dynamic characteristics of MESA participants with no prevalent AF
| All | No-Incident AF | Incident AF | P Value | |
|---|---|---|---|---|
| N | 1,858 | 1,775 | 83 | |
| Demographic and Other Characteristics | ||||
| Age, yr | 67 [60–74] | 66 [60–74] | 72 [68–79] | <0.001 |
| Male sex, n (%) | 857 (46.1) | 807 (45.5) | 50 (60.2) | 0.008 |
| Height, cm | 165 [158–173] | 165 [158–172] | 170 [161–178] | <0.001 |
| Weight, lb | 170 [145–196] | 169 [145–196] | 175 [151–206] | 0.072 |
| Race, n (%) | ||||
| White | 666 (35.8) | 628 (35.4) | 38 (45.8) | 0.257 |
| Chinese-American | 223 (12.1) | c | c | |
| African-American | 518 (27.9) | c | c | |
| Hispanic | 450 (24.2) | 432 (24.3) | 18 (21.7) | |
| Systolic BP, mmHg | 119 [109–135] | 119 [109–134] | 125 [109–146] | 0.037 |
| Diastolic BP, mmHg | 68 [62–75] | 68 [62–75] | 70 [62–77] | 0.482 |
| Antihypertensive Rx, n (%) | 950 (51.1) | 893 (50.3) | 57 (68.7) | 0.001 |
| Diabetes, n (%) | 345 (18.8) | 325 (18.5) | 20 (24.4) | 0.180 |
| Smoking, n (%) | 125 (6.8) | c | c | 0.491 |
| CHF, n (%) | 19 (1.02) | c | c | 0.866 |
| MI, n (%) | 29 (1.56) | c | c | 0.523 |
| NT-proBNP, pg/ml | 66 [33–128] | 64 [33–122] | 134 [65–231] | <0.001 |
| PSVCs ≥ 0.2%, n (%) | 479 (25.8) | 435 (24.5) | 44 (53.0) | <0.001 |
| CAC score | 25.4 [0–195] | 20.1 [0–179] | 247 [73.3–704] | <0.001 |
| Sleep and SDB indices | ||||
| Sleep duration, min | 370 [317–417] | 371 [318–417] | 353 [291–406] | 0.028 |
| Sleep maintenance efficiency, % | 82.8 [74.4–89.5] | 82.8 [74.6–89.6] | 80.9 [72.3–85.5] | 0.018 |
| %SWS < 2.3%, n (%) | 461 (25) | 434 (25) | 27 (33) | 0.079 |
| ODI, events/h | 7.91 [2.84–18.3] | 7.78 [2.80–17.9] | 10.3 [5.43–21.1] | 0.003 |
| AHI , events/h | 17.8 [9.15–32.5] | 17.6 [8.99–31.9] | 24.9 [11.2–39.9] | 0.005 |
| 10-s ECG | ||||
| PR interval, ms | 170 [154–188] | 170 [154–186] | 172 [156–198] | 0.077 |
| PR ≥ 200 ms, n (%) | 260 (14.0) | 242 (13.6) | 18 (21.7) | 0.039 |
| P-wave duration, ms | 112 [104–120] | 112 [104–120] | 116 [110–126] | <0.001 |
| P-axis, ° | 49 [34–61] | 49 [34–61] | 47 [28–60] | 0.569 |
| PWTF in lead V1, mV·ms | −2.20 [−3.39–−0.15] | −2.18 [−3.36–0] | −2.38 [-4.35 – −1.63] | 0.014 |
| PWTF in lead V1 < −4 mV·ms, n (%) | 309 (16.6) | 286 (16.1) | 23 (27.7) | 0.006 |
| HRF indices | ||||
| PIP, % | 58.0 [53.5–63.1] | 57.9 [53.4–62.9] | 62.2 [56.6–66.5] | <0.001 |
| PNNSS, % | 65.9 [55.9–75.7] | 65.7 [55.5–75.4] | 72.2 [63.1–83.4] | <0.001 |
| PNNLS, % | 30.9 [22.2–40.0] | 31.2 [22.5–40.3] | 24.5 [15.5–32.5] | <0.001 |
| ALS | 1.80 [1.64–1.99] | 1.81 [1.65–1.99] | 1.64 [1.54–1.82] | <0.001 |
| W1H, % | 28.5 [20.5–36.4] | 28.8 [20.8–36.5] | 22.2 [16.9–27.8] | <0.001 |
| W3M, % | 8.38 [5.91–11.6] | 8.33 [5.85–11.5] | 9.83 [6.89–14.4] | 0.001 |
| W3S, % | 1.14 [0.59–2.02] | 1.14 [0.59–2.02] | 1.42 [0.65–2.80] | 0.217 |
| HRV indices | ||||
| AVNN, ms | 941 [861–1032] | 941 [861–1032] | 937 [864–1043] | 0.742 |
| ln SDNN, ms | 46.4 [35.2–61.0] | 46.6 [35.3–60.9] | 43.2 [32.3–62.9] | 0.580 |
| ln rMSSD, ms | 28.6 [20.5–42.0] | 28.6 [20.5–41.8] | 29.8 [19.4–44.8] | 0.812 |
| ln HF, ms2 | 374 [193–737] | 373 [194–734] | 417 [181–949] | 0.631 |
| ln LF, ms2 | 565 [307–1115] | 566 [312–1113] | 541 [240–1201] | 0.416 |
| ln LF/HF | 2.01 [1.24–3.50] | 2.01 [1.25–3.52] | 1.89 [1.09–2.85] | 0.041 |
| Nonlinear indices | ||||
| α1 | 1.17 [0.99–1.35] | 1.18 [0.99–1.35] | 1.11 [0.90–1.31] | 0.030 |
| SampEn | 1.16 [0.94–1.38] | 1.16 [0.94–1.38] | 1.17 [0.93–1.36] | 0.610 |
Values of continuous variables are medians and, in brackets, interquartile ranges. Values of categorical variables are numbers of participants and, in parentheses, their percentages. The number of participants included in the study with values for CAC score was 1,433, of which 64 had incident AF. Statistically significant differences (P < 0.05) between groups are highlighted in bold. The number of participants in any subgroup with fewer than 10 individuals is not provided per agreement between Multi-Ethnic Study of Atherosclerosis (MESA) and the Center for Medicare and Medicaid Services. AHI, apnea hypopnea index; ALS, average length of accelerative/decelerative segments; AVNN, the average of all NN intervals; BP, blood pressure; c, cell with <10 participants or cell whose information would allow determination of the number in an adjacent cell with <10 participants; CAC, coronary artery calcification; CHF, chronic heart failure; CI, confidence interval; HF, high-frequency power, the spectral power of NN intervals between 0.15 and 0.4 Hz; HR, hazard ratio; HRF, heart rate fragmentation; HRV, heart rate variability; LF, low-frequency power, the spectral power of NN intervals between 0.04 and 0.15 Hz; LF/HF, the ratio of low- to high-frequency power; MESA, Multi-Ethnic Study of Atherosclerosis; MI, myocardial infarction; NT-proBNP, NH2, terminal prohormone B-type natriuretic peptide; ODI, oxygen desaturation index; PIP, percentage of inflection points; PNNLS, overall percentage of ΔNN intervals in long (≥3) accelerative/decelerative segments; PNNSS, percentage of ΔNN intervals in short (<3) accelerative/decelerative segments; PSVCs, premature supraventricular complexes; PWTFV1, P-wave terminal force in lead V1; Rx, medication; SDB, sleep disorder breathing; rMSSD, the root mean square of successive differences between NN intervals; SampEn, sample entropy; SDNN, the mean of the standard deviations of NN intervals in all 5-min segments; SWS, slow wave sleep; α1, detrended fluctuation analysis short-term index; W1H, percentages of sequences of four consecutive ΔNN intervals with one hard inflection point; W3M, percentages of sequences of four consecutive ΔNN intervals with three inflection points, two hard and one soft or two soft and one hard (“mixed”); W3S, percentages of sequences of four consecutive ΔNN intervals with three soft inflection points.
Among the set of ECG-derived parameters, P-wave duration was significantly longer (∼4%) in participants with incident AF, and P-wave terminal force in lead V1 (PWTFV1) was significantly more negative (∼10%). The percentage of participants with PWTFV1 < −4 mV · ms in the incident AF group was almost double of that in the nonincident AF group. Participants with a PR interval ≥200 ms also had significantly higher rates of incident AF.
The HRF metrics, PIP, PNNSS, W3M, and W3S were ∼7 to 25% higher in those with incident AF (Table 1). Conversely, the (“fluency”) metrics, PNNLS, ALS, and W1H were ∼9% to 23% lower in those with incident AF (Table 1). Among the traditional HRV indices, only LF/HR ratio differed significantly between the groups. Counterintuitively, those who developed AF had a significantly lower LF/HF ratio. Detrended fluctuation analysis short-term exponent, α1, was significantly lower for those who developed AF, while sample entropy did not differ.
Table 2 summarizes demographic and other characteristics of MESA participants in different quartiles of PNNSS and rMSSD. Higher HRF values were associated with older age, more hypertension, more diabetes, elevated NT-proBNP, increased CAC score, a higher percentage of PSVCs, and more cases of incident AF. In contrast, there was no evidence that higher rMSSD was associated with younger age or a more favorable risk profile.
Table 2.
Demographic and other characteristics of MESA participants per quartiles of the PNNSS measure of heart rate fragmentation and of the rMSSD metric of heart rate variability
| PNNSS, % | rMSSD, ms | |||||||
|---|---|---|---|---|---|---|---|---|
| Quartile cut-off values | <55.9 | [55.9–65.9) | [65.9–75.7) | ≥75.7 | <20.5 | [20.5–28.7) | [28.7–42.1) | ≥42.1 |
| Participants, n | 465 | 464 | 465 | 464 | 463 | 462 | 463 | 462 |
| Age, yr | 65 (8) | 66 (8) | 68 (9) | 71 (9) | 68 (9) | 67 (9) | 67 (9) | 68 (10) |
| Male sex, n (%) | 227 (49) | 217 (47) | 210 (45) | 203 (44) | 193 (42) | 196 (42) | 235 (51) | 226 (49) |
| Height, cm | 167 (10) | 166 (10) | 165 (10) | 164 (10) | 165 (10) | 165 (10) | 166 (10) | 166 (10) |
| Weight, lb | 175 (37) | 173 (37) | 171 (42) | 172 (39) | 166 (37) | 175 (40) | 175 (38) | 177 (39) |
| Race, n (%) | ||||||||
| White | 188 (40) | 167 (36) | 172 (37) | 139 (30) | 193 (42) | 159 (34) | 168 (36) | 144 (31) |
| Chinese-American | 55 (12) | 55 (12) | 61 (13) | 53 (11) | 57 (12) | 59 (13) | 58 (13) | 48 (10) |
| African-American | 108 (23) | 130 (28) | 124 (27) | 156 (34) | 101 (22) | 133 (29) | 125 (27) | 156 (34) |
| Hispanic | 114 (25) | 112 (24) | 108 (23) | 116 (25) | 112 (24) | 111 (24) | 112 (24) | 114 (25) |
| Systolic BP, mmHg | 119 (18) | 121 (19) | 124 (22) | 126 (21) | 123 (22) | 122 (19) | 121 (19) | 124 (21) |
| Antihypertensive Rx, n (%) | 185 (40) | 226 (49) | 237 (51) | 302 (65) | 228 (49) | 230 (50) | 239 (52) | 248 (54) |
| Diabetes, n (%) | 69 (15) | 84 (18) | 83 (18) | 109 (24) | 103 (23) | 95 (21) | 73 (16) | 73 (16) |
| Smoking, n (%) | 24 (5) | 34 (7) | 31 (7) | 36 (8) | 42 (9) | 30 (7) | 24 (5) | 29 (6) |
| CHF (yes), n (%) | 2 (0.4) | 3 (0.7) | 4 (0.9) | 10 (2.2) | 3 (0.7) | 3 (0.7) | 9 (1.9) | 4 (0.9) |
| MI (yes), n (%) | 4 (0.9) | 9 (1.9) | 7 (1.5) | 9 (1.9) | 9 (1.9) | 5 (1.1) | 8 (1.7) | 7 (1.5) |
| ln NT-proBNP, pg/ml | 4.0 (1.0) | 4.1 (0.9) | 4.3 (0.9) | 4.5 (1.1) | 4.2 (1.0) | 4.2 (1.0) | 4.1 (1.0) | 4.4 (1.0) |
| PSVCs ≥ 0.2%, n (%) | 58 (12) | 99 (21) | 140 (30) | 182 (39) | 48 (10) | 71 (15) | 121 (26) | 232 (50) |
| ln (CAC score + 1) | 2.6 (2.5) | 2.8 (2.6) | 3.1 (2.6) | 3.5 (2.7) | 3.2 (2.7) | 2.8 (2.6) | 3.1 (2.6) | 3.0 (2.7) |
| Incident AF, n (%) | 7 (1.5) | 20 (4.3) | 23 (5.0) | 33 (7.1) | 23 (5.0) | 16 (3.5) | 20 (4.3) | 22 (4.8) |
Values for continuous variables are their mean and, in parentheses, their standard deviation. The values for the categorical variables are the number of participants in the specified subgroups and, in parentheses, its percentage. AF, atrial fibrillation; BP, blood pressure; CAC, coronary artery calcification; CHF, chronic heart failure; MESA, Multi-Ethnic Study of Atherosclerosis; MI, myocardial infarction; n, number of participants NT-proBNP, NH2-terminal prohormone B-type natriuretic peptide; PSVCs, premature supraventricular complexes. PNNSS, percentage of ΔNN intervals in short (<3) accelerative/decelerative segments; Rx, medication; rMSSD, root mean square of successive differences between NN intervals.
Cox Regression Models of Incident AF Adjusted for Age and Height
Components of the CHARGE-AF model.
Usage of antihypertensive medication was significantly associated with incident AF (Table 3). The other components of the basic CHARGE-AF model (white race, weight, systolic and diastolic BP, smoking, and diabetes status) were not.
Table 3.
Association of demographic, clinical and heart rate dynamical characteristics with incident AF in MESA participants quantified using Cox proportional hazard analysis
| Adj.: Age and Height |
Adj.: Age, Height and NT-proBNP |
Adj.: Age, Height, NT-proBNP and PSVCs ≥ 0.2% |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Demographic and other characteristics | |||||||||
| Age (8.89 yr) | |||||||||
| Sex (Male) | 0.81 | 0.44–1.49 | 0.494 | 1.03 | 0.55–1.93 | 0.916 | 1.01 | 0.54–1.89 | 0.969 |
| Height (9.99 cm) | |||||||||
| Weight (38.7 lb) | 1.08 | 0.82–1.41 | 0.581 | 1.08 | 0.83–1.40 | 0.572 | 1.08 | 0.83–1.40 | 0.583 |
| Race | |||||||||
| White (reference) | |||||||||
| Chinese-American | 0.86 | 0.38–1.97 | 0.714 | 1.08 | 0.44–2.65 | 0.868 | 1.08 | 0.44–2.65 | 0.867 |
| African-American | 0.62 | 0.36–1.07 | 0.089 | 0.80 | 0.46–1.38 | 0.420 | 0.77 | 0.45–1.34 | 0.357 |
| Hispanic | 0.94 | 0.53–1.66 | 0.819 | 1.12 | 0.63–2.00 | 0.699 | 1.13 | 0.63–2.02 | 0.677 |
| Systolic BP (20.2 mmHg) | 1.22 | 0.99–1.50 | 0.061 | 1.08 | 0.88–1.33 | 0.465 | 1.09 | 0.88–1.34 | 0.437 |
| Diastolic BP (9.84 mmHg) | 1.06 | 0.85–1.33 | 0.597 | 1.03 | 0.83–1.28 | 0.780 | 1.02 | 0.82–1.27 | 0.843 |
| Antihypertensive Rx (yes) | 1.65 | 1.03–2.66 | 0.038 | 1.58 | 0.96–2.59 | 0.071 | 1.57 | 0.95–2.57 | 0.077 |
| Diabetes (yes) | 1.28 | 0.77–2.12 | 0.339 | 1.19 | 0.71–1.99 | 0.520 | 1.19 | 0.71–2.00 | 0.512 |
| Smoking (yes) | 1.00 | 0.36–2.76 | 0.999 | 1.06 | 0.38–2.94 | 0.906 | 1.01 | 0.36–2.80 | 0.983 |
| CHF (yes) | 1.14 | 0.16–8.24 | 0.893 | 0.67 | 0.09–4.89 | 0.694 | 0.69 | 0.09–5.03 | 0.714 |
| MI (yes) | 1.17 | 0.29–4.79 | 0.822 | 0.63 | 0.15–2.66 | 0.530 | 0.66 | 0.16–2.81 | 0.578 |
| ln NT-proBNP (1.00 pg/ml) | 1.77 | 1.40–2.23 | <0.001 | ||||||
| PSVCs ≥ 0.2% (yes) | 2.20 | 1.38–3.50 | 0.001 | 1.76 | 1.09–2.85 | 0.022 | |||
| ln (CAC score +1) (2.63) | 1.98 | 1.44–2.70 | <0.001 | 1.77 | 1.28–2.43 | <0.001 | 1.74 | 1.27–2.40 | 0.001 |
| Sleep and SDB indices | |||||||||
| Sleep duration (79 min) | 0.86 | 0.70–1.17 | 0.188 | 0.87 | 0.70–1.07 | 0.187 | 0.87 | 0.70–1.08 | 0.200 |
| Sleep maintenance efficiency (12.0%) | 1.00 | 0.81–1.24 | 0.977 | 1.02 | 0.82–1.27 | 0.844 | 1.03 | 0.83–1.28 | 0.775 |
| %SWS < 2.3% (yes) | 1.06 | 0.66–1.70 | 0.817 | 1.17 | 0.72–1.90 | 0.520 | 1.16 | 0.72–1.87 | 0.545 |
| sqrt (ODI 4%) [1.91 (events/hour)1/2] | 1.18 | 0.96–1.45 | 0.117 | 1.17 | 0.95–1.45 | 0.137 | 1.18 | 0.95–1.46 | 0.131 |
| sqrt (AHI) [1.91 (events/hour)1/2] | 1.24 | 1.00–1.54 | 0.056 | 1.21 | 0.97–1.51 | 0.091 | 1.21 | 0.97–1.51 | 0.093 |
| 12-lead ECG | |||||||||
| PR interval (29.4 ms) | 0.96 | 0.79–1.16 | 0.665 | 0.93 | 0.76–1.12 | 0.435 | 0.93 | 0.77–1.13 | 0.467 |
| PR ≥ 200 ms (yes) | 1.03 | 0.60–1.77 | 0.911 | 0.92 | 0.53–1.61 | 0.778 | 0.92 | 0.53–1.61 | 0.776 |
| P-wave duration (11.8 ms) | 1.24 | 1.01–1.54 | 0.044 | 1.23 | 0.99–1.52 | 0.058 | 1.23 | 1.00–1.52 | 0.053 |
| P-axis (22.1°) | 0.93 | 0.77–1.13 | 0.492 | 0.94 | 0.78–1.13 | 0.500 | 0.95 | 0.79–1.14 | 0.578 |
| PWTF in lead V1 (1.9 mV·ms) | 0.80 | 0.66–0.97 | 0.022 | 0.86 | 0.71–1.05 | 0.137 | 0.86 | 0.70–1.04 | 0.126 |
| PWTF in lead V1 < −4 mV·ms (yes) | 1.79 | 1.11–2.90 | 0.017 | 1.47 | 0.89–2.43 | 0.132 | 1.51 | 0.91–2.50 | 0.109 |
| HRF indices | |||||||||
| PIP (7.2%) | 1.29 | 1.05–1.59 | 0.015 | 1.22 | 0.99–1.50 | 0.067 | 1.18 | 0.96–1.46 | 0.123 |
| PNNSS (13.7%) | 1.43 | 1.13–1.81 | 0.003 | 1.35 | 1.07–1.71 | 0.013 | 1.31 | 1.03–1.66 | 0.025 |
| PNNLS (12.6%) | 0.68 | 0.53–0.86 | 0.001 | 0.72 | 0.56–0.91 | 0.007 | 0.73 | 0.58–0.94 | 0.012 |
| ALS (0.26) | 0.69 | 0.54–0.87 | 0.002 | 0.74 | 0.58–0.94 | 0.014 | 0.77 | 0.60–0.99 | 0.039 |
| W1H (11.3%) | 0.73 | 0.57–0.94 | 0.014 | 0.76 | 0.59–0.99 | 0.038 | 0.77 | 0.60–1.00 | 0.052 |
| W3M (4.56%) | 1.32 | 1.09–1.61 | 0.005 | 1.30 | 1.07–1.57 | 0.007 | 1.36 | 1.12 –1.65 | 0.002 |
| W3S (1.45%) | 1.20 | 1.00–1.44 | 0.050 | 1.21 | 1.01–1.45 | 0.036 | 1.27 | 1.06–1.51 | 0.010 |
| HRV indices | |||||||||
| AVNN (133 ms) | 0.92 | 0.74–1.14 | 0.450 | 0.89 | 0.71–1.10 | 0.283 | 0.87 | 0.70–1.08 | 0.209 |
| ln SDNN (0.44 ms) | 0.92 | 0.75–1.13 | 0.424 | 0.89 | 0.72–1.10 | 0.284 | 0.81 | 0.65–1.01 | 0.066 |
| ln rMSSD (0.60 ms) | 0.97 | 0.80–1.18 | 0.776 | 0.93 | 0.76–1.14 | 0.471 | 0.82 | 0.65–1.03 | 0.083 |
| ln HF (1.11 ms2) | 0.99 | 0.81–1.21 | 0.928 | 0.95 | 0.77–1.17 | 0.614 | 0.84 | 0.67–1.06 | 0.137 |
| ln LF (0.98ms2) | 0.90 | 0.73–1.11 | 0.337 | 0.89 | 0.72–1.10 | 0.274 | 0.82 | 0.65–1.02 | 0.073 |
| ln LF/HF (0.74) | 0.85 | 0.69–1.06 | 0.150 | 0.89 | 0.72–1.12 | 0.321 | 0.96 | 0.76–1.22 | 0.756 |
| Nonlinear indices | |||||||||
| α1 (0.25) | 0.86 | 0.70–1.05 | 0.134 | 0.90 | 0.73–1.11 | 0.335 | 0.97 | 0.78–1.21 | 0.799 |
| SampEn (0.34) | 1.02 | 0.82–1.26 | 0.867 | 1.01 | 0.82–1.25 | 0.944 | 0.99 | 0.80–1.22 | 0.916 |
Each Cox model includes one of the variables listed on the left and one of the following sets of adjustments: 1) age and height (left); 2) age, height, and NT-proBNP (middle); and 3) age, height, NT-proBNP and PSVCs ≥ 0.2% (right). Values shown are standardized hazard ratios (for one-SD increase in the value of the independent variable) and 95% confidence intervals. Statistically significant associations (P < 0.05) are highlighted in bold. The hazard ratio values are for one SD increase in the value of the independent variables. AHI, apnea hypopnea index; ALS, average length of accelerative/decelerative segments; AVNN, the average of all NN intervals; BP, blood pressure; CAC, coronary artery calcification; CHF, chronic heart failure; CI, confidence interval; HF, high-frequency power, the spectral power of NN intervals between 0.15 and 0.4 Hz; HR, hazard ratio; HRF, heart rate fragmentation, HRV, heart rate variability; LF, low-frequency power, the spectral power of NN intervals between 0.04 and 0.15 Hz; LF/HF, the ratio of low- to high-frequency power; MESA, Multi-Ethnic Study of Atherosclerosis; MI, myocardial infarction; NT-proBNP, NH2-terminal prohormone B-type natriuretic peptide; ODI, oxygen desaturation index; PIP, percentage of inflection points; PNNLS, overall percentage of ΔNN intervals in long (≥3) accelerative/decelerative segments; PNNSS, percentage of ΔNN intervals in short (<3) accelerative/decelerative segments; PSVCs, premature supraventricular complexes; PWTFV1, P-wave terminal force in lead V1; Rx, medication; SDB, sleep disorder breathing; rMSSD, the root mean square of successive differences between NN intervals; SampEn, sample entropy; SDNN, the mean of the standard deviations of NN intervals in all 5-min segments; SWS, slow wave sleep; α1, detrended fluctuation analysis short-term index; W1H, percentages of sequences of four consecutive ΔNN intervals with one hard inflection point; W3M, percentages of sequences of four consecutive ΔNN intervals with three inflection points, two hard and one soft or two soft and one hard (“mixed”); W3S, percentages of sequences of four consecutive ΔNN intervals with three soft inflection points.
ECG-derived parameters.
Lower, i.e., more negative, PWTFV1 values were significantly associated with increased AF risk. The association was slightly stronger for the dichotomous variable PWTFV1 < −4 mV · ms (Table 3). A longer P-wave duration was also significantly associated with increased incident AF risk. The PR interval and P axis were not.
Other clinical variables.
A higher CAC score, higher NT-proBNP concentration, and PSVCs ≥ 0.2% were associated with increased AF risk (Table 3). The measures of sleep structure were not associated with incident AF. The selected measures of sleep disorder breathing, AHI and ODI, were higher in those with incident AF, but statistical significance was not reached.
Heart rate dynamical indices.
Heart rate fragmentation metrics, but not traditional HRV and nonlinear dynamical metrics, were significantly associated with incident AF (Table 3). Heart rate fragmentation added significant value to models with age, height, and any of the variables mentioned above that were themselves associated with incident AF.
Among all variables (demographic, clinical, and dynamical), those most strongly associated with incident AF, as quantified by the C-statistic, were NT-proBNP (0.772), CAC score (0.770), ALS (0.752), PNNLS (0.751), W3M (0.750), PIP (0.748), PNNSS (0.748), W3S (0.747), and PSVCs ≥ 0.2% (0.747).
Cox Survival Models Adjusted for Age, Height, and NT-proBNP
In these models (Table 3), only two clinical indices remained significantly associated with incident AF: PSVCs ≥0.2% and the CAC score. Longer P-waves and usage of antihypertensive medication tended to be associated with increased risk of AF, but statistical significance was not reached. Among the dynamical indices, only HRF metrics (with the exception of PIP) remained associated with incident AF.
Cox Survival Models Adjusted for Age, Height, NT-proBNP, and PSVCs ≥ 0.2%
The HRF indices, PNNSS, PNNLS, ALS, W3S, and W3M were significantly associated with incident AF. They added value to the other variables in the model: age, height, NT-proBNP, and PSVCs ≥0.2%, which were also significantly associated with incident AF. Specifically, a one-SD increase in PNNSS was associated with a 31% (3–66%) increase in AF risk. Aside from the HRF indices, CAC score was the only variable significantly associated with incident AF. As in the case of the models adjusted for age, height and NT-proBNP, longer P-waves and usage of antihypertensive medication tended to be associated with increased risk of AF, but statistical significance was not reached (Table 3). Lower HRV also tended to be associated with higher AF risk.
The inclusion of CAC score in the models did not qualitatively change the associations between HRF indices and AF risk. For example, the association between a one-SD increase in PNNSS and AF risk changed from the values shown above to 43% (8–91%) when the CAC score was added to the model. Further adjusting the analyses for the percentage of time in REM, non-REM, %SWS < 2.3%, AHI, or ODI, did not qualitatively change the results. Finally, the results obtained using other threshold values for the definition of frequent PSVCs were qualitatively similar to those presented here.
The CHARGE-AF and the NT-proBNP-Enriched CHARGE-AF Models
Age, height, and usage of antihypertensive medication were the only variables of the CHARGE-AF model significantly associated with incident AF (Table 4). The associations were strongest for the former two components. Consistent with this finding, the hazard ratios for each of the HRF metrics in models adjusted for the components of CHARGE-AF (Table 5) and in those solely adjusted for age and height (Table 3) were almost identical. For example, the hazard ratios for PNNSS in the model adjusted for the components CHARGE-AF and in the one solely adjusted for age and height were 1.42 (1.11–1.81) and 1.43 (1.13–1.81), respectively.
Table 4.
Multivariate Cox survival models
| CHARGE-AF |
CHARGE-AF + HRF |
CHARGE-AF + NT-proBNP |
CHARGE-AF + NT-proBNP + HRF |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Independent Variables | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | ||||
| Age (8.89 yr) | 1.75 | 1.35 | 2.27 | <0.001 | 1.63 | 1.26 | 2.12 | <0.001 | 1.44 | 1.09 | 1.88 | 0.009 | 1.38 | 1.05 | 1.81 | 0.021 |
| White race (yes) | 1.40 | 0.88 | 2.21 | 0.152 | 1.41 | 0.90 | 2.23 | 0.136 | 1.16 | 0.73 | 1.86 | 0.534 | 1.19 | 0.74 | 1.90 | 0.466 |
| Height (9.99 cm) | 1.67 | 1.26 | 2.20 | <0.001 | 1.67 | 1.26 | 2.20 | <0.001 | 1.65 | 1.25 | 2.18 | <0.001 | 1.66 | 1.26 | 2.19 | <0.001 |
| Weight (38.7 lb) | 0.97 | 0.73 | 1.30 | 0.856 | 0.96 | 0.72 | 1.29 | 0.805 | 0.99 | 0.75 | 1.32 | 0.972 | 0.98 | 0.74 | 1.31 | 0.901 |
| Systolic BP (20.2 mmHg) | 1.22 | 0.91 | 1.63 | 0.189 | 1.17 | 0.87 | 1.57 | 0.292 | 1.06 | 0.78 | 1.43 | 0.713 | 1.04 | 0.78 | 1.41 | 0.775 |
| Diastolic BP (9.84 mmHg) | 0.93 | 0.69 | 1.27 | 0.660 | 0.97 | 0.72 | 1.32 | 0.865 | 0.99 | 0.73 | 1.34 | 0.938 | 1.01 | 0.75 | 1.36 | 0.961 |
| Smoking (yes) | 1.04 | 0.38 | 2.89 | 0.934 | 1.01 | 0.36 | 2.80 | 0.988 | 1.08 | 0.39 | 3.00 | 0.885 | 1.02 | 0.37 | 2.85 | 0.966 |
| Antihypertensive Rx (yes) | 1.68 | 1.00 | 2.82 | 0.048 | 1.59 | 0.95 | 2.67 | 0.078 | 1.58 | 0.93 | 2.69 | 0.091 | 1.52 | 0.89 | 2.58 | 0.127 |
| Diabetes (yes) | 1.21 | 0.70 | 2.08 | 0.493 | 1.13 | 0.66 | 1.96 | 0.650 | 1.12 | 0.64 | 1.97 | 0.683 | 1.05 | 0.60 | 1.84 | 0.875 |
| HRF (13.7%) | 1.42 | 1.11 | 1.81 | 0.005 | 1.34 | 1.05 | 1.71 | 0.018 | ||||||||
| ln NT-proBNP (1.00 pg/ml) | 1.69 | 1.32 | 2.15 | <0.001 | 1.61 | 1.25 | 2.06 | <0.001 | ||||||||
Values are standardized hazard ratios (for one-SD increase in the value of the independent variable) and 95% confidence intervals for each of the components included the four models: 1) CHARGE-AF; 2) CHARGE-AF + HRF; 3) NT-proBNP-enriched CHARGE-AF and 4) NT-proBNP-enriched CHARGE-AF + HRF. The HRF metric used was PNNSS. Statistically significant associations (P < 0.05) are highlighted in bold. AF, atrial fibrillation; BP, blood pressure; CHARGE, Cohorts for Heart and Aging Research in Genomic Epidemiology; CI, confidence interval; HR, hazard ratio; HRF, heart rate fragmentation; NT-proBNP, NH2-terminal prohormone B-type natriuretic peptide; Rx, medication.
Table 5.
Cox survival analyses adjusted for all the components of the CHARGE-AF model and for all the components of the NT-proBNP enriched CHARGE-AF model
| Adj.: CHARGE-AF & | ||||||||
|---|---|---|---|---|---|---|---|---|
| Adj.: CHARGE-AF |
NT-proBNP |
|||||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |||
| HRF indices | ||||||||
| PIP (7.2%) | 1.26 | 1.02 | 1.56 | 0.031 | 1.19 | 0.96 | 1.47 | 0.117 |
| PNNSS (13.7%) | 1.42 | 1.11 | 1.81 | 0.005 | 1.34 | 1.05 | 1.71 | 0.018 |
| PNNLS (12.6%) | 0.68 | 0.53 | 0.87 | 0.002 | 0.72 | 0.56 | 0.92 | 0.010 |
| ALS (0.26) | 0.70 | 0.54 | 0.89 | 0.004 | 0.75 | 0.58 | 0.97 | 0.025 |
| W1H (11.3%) | 0.74 | 0.57 | 0.95 | 0.019 | 0.77 | 0.60 | 1.00 | 0.053 |
| W3M (4.56%) | 1.29 | 1.06 | 1.58 | 0.012 | 1.27 | 1.04 | 1.54 | 0.017 |
| W3S (1.45%) | 1.22 | 1.01 | 1.47 | 0.040 | 1.22 | 1.01 | 1.47 | 0.039 |
| HRV indices | ||||||||
| AVNN (133 ms) | 0.90 | 0.72 | 1.14 | 0.390 | 0.90 | 0.71 | 1.13 | 0.350 |
| ln SDNN (0.44 ms) | 0.95 | 0.77 | 1.17 | 0.601 | 0.93 | 0.75 | 1.15 | 0.479 |
| ln rMSSD (0.60 ms) | 0.99 | 0.81 | 1.21 | 0.921 | 0.95 | 0.77 | 1.17 | 0.616 |
| ln HF (1.11 ms2) | 1.01 | 0.82 | 1.24 | 0.938 | 0.97 | 0.79 | 1.20 | 0.774 |
| ln LF (0.98 ms2) | 0.92 | 0.74 | 1.14 | 0.449 | 0.92 | 0.74 | 1.14 | 0.446 |
| ln LF/HF (0.74) | 0.84 | 0.67 | 1.05 | 0.125 | 0.89 | 0.71 | 1.13 | 0.340 |
| Nonlinear indices | ||||||||
| α1 (0.25) | 0.85 | 0.69 | 1.05 | 0.122 | 0.91 | 0.73 | 1.13 | 0.373 |
| SampEn (0.34) | 0.99 | 0.80 | 1.23 | 0.914 | 0.99 | 0.80 | 1.23 | 0.945 |
Values shown are the standardized hazard ratios and 95% confident intervals for each of the heart rate dynamical indices (one dynamical index per model). Statistically significant associations (P < 0.05) are highlighted in bold. Adj., adjustments; ALS, average length of accelerative/decelerative segments; AVNN, the average of all NN intervals; CI, confidence interval; HF, high-frequency power, the spectral power of NN intervals between 0.15 and 0.4 Hz; HR, hazard ratio; HRF, heart rate fragmentation, HRV, heart rate variability; LF, low-frequency power, the spectral power of NN intervals between 0.04 and 0.15 Hz; LF/HF, the ratio of low- to high-frequency power; NT-proBNP, N-terminal prohormone B-type natriuretic peptide; PIP, percentage of inflection points; PNNLS, overall percentage of ΔNN intervals in long (≥3) accelerative/decelerative segments; PNNSS, percentage of ΔNN intervals in short (<3) accelerative/decelerative segments; rMSSD, the root mean square of successive differences between NN intervals; SampEn, sample entropy; SDNN, the mean of the standard deviations of NN intervals in all 5-min segments; α1, detrended fluctuation analysis short-term index; W1H, percentages of sequences of four consecutive ΔNN intervals with one hard inflection point; W3M, percentages of sequences of four consecutive ΔNN intervals with three inflection points, two hard and one soft or two soft and one hard (“mixed”) W3S, percentages of sequences of four consecutive ΔNN intervals with three soft inflection points.
In the NT-proBNP-enriched CHARGE-AF model, age, height and NT-proBNP were the only components significantly associated with incident AF (Table 4). Consistent with this finding, the hazard ratios for each of the HRF metrics in models adjusted for the components of the NT-proBNP-enriched CHARGE-AF (Table 5) and in those solely adjusted for age, height, and NT-proBNP (Table 3) were comparable.
Traditional HRV indices, as well as the two nonlinear metrics included in this study, were unassociated with incident AF, either in models adjusted for the components of CHARGE-AF or in models adjusted for the components of the NT-proBNP-enriched CHARGE-AF model.
The C-statistic for the CHARGE-AF model was 0.736. The inclusion of HRF indices or NT-proBNP in the CHARGE-AF model resulted in significant increases in model performance: 0.748 for PNNSS (P = 0.004) and 0.768 for NT-proBNP (P < 0.001). Notably, the HRF indices PNNSS, PNNLS, ALS, and W3M all added significant value to the NT-proBNP-enriched CHARGE-AF model.
Qualitatively similar results to those presented in this subsection and in the previous ones were obtained when the HRF indices were computed separately from awake, REM, non-REM periods instead of from the entire sleep period. In addition, qualitatively similar results were obtained in analyses restricted to the subgroup of participants (n = 1,433) with CAC scores, i.e., in all models, higher HRF was significantly associated with increased risk of AF.
Association of HRF with Frequent PSVCs (≥0.2%)
In logistic analyses adjusted for age and height, the overall degree of HRF, as quantified by PIP, PNNSS, PNNLS or ALS, was significantly associated with %PSVCs ≥ 0.2%. Specifically, a one-SD increase in PIP and PNNSS (measures whose values are higher in the case of more fragmented dynamics) were associated with a 45% (29–63%) and a 53% (36–73%) increase, in the odds of having an amount of PSVCs above 0.2%, respectively. A one-SD increase in PNNLS and ALS (measures whose values are lower in the case of more fragmented dynamics) were associated with a 32% (23–39%) and a 43% (35–50%) decrease in the odds of having an amount of PSVCs above 0.2%, respectively.
Measures Associated with Incident AF in the High-Risk Subgroup of Participants ≥ 70 yr Taking Antihypertensive Medication
Forty-one of the 490 participants in this group developed AF during follow-up. Height, CAC score, NT-proBNP serum concentration, and PSVCs ≥ 0.2% were the only variables associated with incident AF among the demographic, 12-lead ECG, and other clinical indices included in this study. Heart rate fragmentation indices were the only heart rate dynamical indices associated with incident AF. In models adjusted for height (Table 4), a one-SD increase in PNNSS was associated with a 64% (16–133%) increase in AF risk. In models further adjusted for CAC score, NT-proBNP, or PSVCs ≥0.2%, a one-SD increase in PNNSS was associated with a 66% (10–149%), 47% (4–108%) and 56% (9–123%) increase in AF risk, respectively.
Measures Associated with Incident AF in the High-Risk Subgroup of Participants with a Serum Concentration of NT-proBNP >125 pg/ml
Forty-eight of the 508 participants in this group developed AF during follow-up. In models adjusted for age and height, only three variables were associated with incident AF: CAC score, PSVCs ≥0.2%, and HRF. In these models, a one-SD increase in PNNSS was associated with a 44% (6–97%) increase in AF risk (Table 6).
Table 6.
Cox survival analyses restricted to two high-risk subgroups
|
Subgroup I |
Subgroup II |
|||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |||
| HRF indices | ||||||||
| PIP (7.2%) | 1.32 | 1.01 | 1.72 | 0.043 | 1.25 | 0.97 | 1.60 | 0.083 |
| PNNSS (13.7%) | 1.64 | 1.16 | 2.33 | 0.005 | 1.44 | 1.06 | 1.97 | 0.021 |
| PNNLS (12.6%) | 0.58 | 0.40 | 0.83 | 0.003 | 0.67 | 0.48 | 0.92 | 0.013 |
| ALS (0.26) | 0.61 | 0.43 | 0.86 | 0.005 | 0.64 | 0.46 | 0.88 | 0.006 |
| W1H (11.3%) | 0.65 | 0.45 | 0.94 | 0.020 | 0.71 | 0.51 | 0.98 | 0.039 |
| W3M (4.56%) | 1.36 | 1.05 | 1.76 | 0.019 | 1.26 | 0.99 | 1.60 | 0.058 |
| W3S (1.45%) | 1.31 | 1.02 | 1.68 | 0.033 | 1.22 | 0.98 | 1.52 | 0.074 |
| HRV indices | ||||||||
| AVNN (133 ms) | 0.89 | 0.65 | 1.23 | 0.488 | 0.87 | 0.65 | 1.16 | 0.352 |
| ln SDNN (0.44 ms) | 0.90 | 0.68 | 1.21 | 0.492 | 0.97 | 0.75 | 1.25 | 0.801 |
| ln rMSSD (0.60 ms) | 1.02 | 0.78 | 1.33 | 0.905 | 1.02 | 0.79 | 1.30 | 0.892 |
| ln HF (1.11 ms2) | 1.03 | 0.78 | 1.35 | 0.844 | 1.01 | 0.78 | 1.30 | 0.952 |
| ln LF (0.98 ms2) | 0.88 | 0.65 | 1.18 | 0.382 | 0.92 | 0.71 | 1.20 | 0.562 |
| ln LF/HF (0.74) | 0.73 | 0.53 | 1.01 | 0.056 | 0.85 | 0.64 | 1.13 | 0.259 |
| Nonlinear indices | ||||||||
| α1 (0.25) | 0.73 | 0.55 | 0.98 | 0.037 | 0.86 | 0.66 | 1.12 | 0.257 |
| SampEn (0.34) | 1.17 | 0.87 | 1.56 | 0.302 | 1.01 | 0.78 | 1.31 | 0.947 |
Values shown are standardized hazard ratios and 95% confident intervals for each individual heart rate dynamical index (one dynamical index per model). Participants ≥70 yr and taking antihypertensive medication (subgroup I) and those with a serum concentration of NT-proBNP > 125 (subgroup II). Analyses of subgroup I were adjusted for height, and those of subgroup II were adjusted for age and height. Statistically significant associations (P < 0.05) are highlighted in bold. ALS, average length of accelerative/decelerative segments; AVNN, the average of all NN intervals; CI, confidence interval; HF, high-frequency power, the spectral power of NN intervals between 0.15 and 0.4 Hz; HR, hazard ratio; HRF, heart rate fragmentation, HRV, heart rate variability; LF, low-frequency power, the spectral power of NN intervals between 0.04 and 0.15 Hz; LF/HF, the ratio of low- to high-frequency power; NT-proBNP, NH2-terminal prohormone B-type natriuretic peptide; PIP, percentage of inflection points; PNNLS, percentage of ΔNN intervals in long (≥3) accelerative/decelerative segments; PNNSS, percentage of ΔNN intervals in short (<3) accelerative/decelerative segments; rMSSD, the root mean square of successive differences between NN intervals; SampEn, sample entropy; SDNN, the mean of the standard deviations of NN intervals in all 5-min segments; α1, detrended fluctuation analysis short-term index; W1H, percentages of sequences of four consecutive ΔNN intervals with one hard inflection point; W3M, percentages of sequences of four consecutive ΔNN intervals with three inflection points, two hard and one soft or two soft and one hard (“mixed”); W3S, percentages of sequences of four consecutive ΔNN intervals with three soft inflection points.
DISCUSSION
The present investigation was designed to test the association of measures of HRF, a newly identified property of short-term sinoatrial dynamics, with incident AF in a large multi-ethnic cohort study. Our key findings were that 1) participants with more fragmented dynamics had significantly higher risk of incident AF; 2) HRF added value to components (separately and in combination) of the CHARGE-AF model, the NT-proBNP enriched CHARGE-AF model (3, 4) and to well-established risk factors for AF, namely frequent supraventricular ectopy and CAC score; 3) HRF also added value to the models that included traditional 12-lead ECG signs of left atrial abnormality/enlargement; 4) traditional metrics of HRV and two widely used nonlinear indices of short-term variability (sample entropy and detrended fluctuation analysis α1) were not associated with incident AF; 5) in two high-risk subgroups of participants, those ≥70 yr taking antihypertensive medication and those with a serum concentration of NT-proBNP >125 pg/ml (top quartile), increased HRF was associated with augmented AF risk; 6) twelve-lead ECG parameters were not associated with incident AF either in high-risk subgroups or in the general ancillary sleep study population in models that included NT-proBNP or CAC score. The magnitude and significance of the association between increased HRF and augmented AF risk were neither dependent on the percentages of time in different sleep stages nor on sleep-disordered breathing events quantified by AHI and ODI. Overall, these findings support consideration of HRF metrics as a new, independent biomarker of AF risk.
As shown in Fig. 1, A and B, HRF may escape visual detection during conventional ECG readings. Quantitative analyses of NN interval time series are needed to reveal the key feature of fragmentation, namely, an increased density of changes in heart rate acceleration sign. The most extreme manifestation of HRF is sustained sinus alternans, in which shorter and longer NN intervals alternate while PR intervals remain constant and there are no identifiable changes in surface P-wave morphology. This type of cardiac alternation has been recognized (but not widely reported) for decades (9, 33). Notably, case reports of SAN alternans are primarily from older individuals and those with advanced organic heart disease (9, 15). In addition, Hogue et al. (31) reported that SAN alternans-like patterns were associated with the occurrence of AF in the period just after cardiac surgery. Basic mechanisms of SAN alternans, as well as of other HRF patterns, remain speculative (15–18) and are likely multifactorial and interwoven. Pathogenetic considerations center on degradation of vagal control, and perturbed automaticity and/or conduction in what has been termed the SAN pacemaker complex (22, 25).
Heart Rate Fragmentation: Potential Impact of Undetected Supraventricular Ectopy
Despite the fact that trained technicians reviewed and corrected, when necessary, the ECG beat annotations, it is possible that a certain amount of PSVCs escaped detection. In the presence of undetected PSVCs, the exact percentage of supraventricular ectopy cannot be known. To assess the extent to which undetected PSVCs could bias our results, we created a dichotomous variable that separated participants with and without frequent PSVCs and included this variable in our survival models. In participants with no or just a small amount of labeled PSVCs (e.g., those on the bottom quartile of %PSVCs), a relatively small number of undetected PSVCs would be unlikely to change the participants’ classification from the group of those “without” to the group of those “with” frequent supraventricular ectopy (unless the percentage of labeled PSVCs is very close to the threshold value used to define the dichotomous variable). In participants with a large amount of PSVCs that already belonged to the group of those “with” frequent supraventricular ectopy, consideration of undetected PSVCs would not change these participants’ group affiliation. Thus, the presence of undetected PSVCs would only affect the classification of participants with a percentage of labeled PSVCs close to the threshold used to define frequent supraventricular ectopy. Despite the positive association between HRF and frequent supraventricular ectopy, Cox regression analyses (Table 3) that controlled for PSVCs ≥0.2% (top quartile) showed that HRF indices (PNNSS, PNNLS, ALS, W3S, and W3M) were independently associated with incident AF. Furthermore, HRF metrics remained significantly associated with incident AF independent of the threshold value used in the definition of frequent supraventricular ectopy. This finding indicates that HRF metrics are not surrogate measures of supraventricular ectopy. Of note, the results obtained for the metric W3S could not possibly be affected by undetected PSVCs. The reason is that the dynamical signature of PSVCs, a short-long interbeat interval sequence, i.e., a hard inflection point (↘↗), cannot be part of W3S patterns. By definition, W3S patterns only include soft edges, i.e., transitions from no change in NN interval to increase/decrease (→↗; →↘) in NN and vice-versa (↗→;↘→). (For the same reason, W3S patterns also do not include any portion of bigeminal/trigeminal rhythms, which are characterized by short-long-short-long intervals.)
Possible Links Between Fragmentation and Atrial Fibrillation
The findings of the present study raise speculative consideration of connections between increased HRF and susceptibility to AF, although they do not permit directly addressing basic mechanistic links (1, 40). Fragmented SAN dynamics may be a manifestation of a breakdown in the redundant, diverse intranodal pacemakers and/or the conduction pathways that maintain SAN robustness and help suppress atrial arrhythmogenesis (36). Another factor that inferentially bridges the emergence of HRF and subsequent AF onset is vagal withdrawal. We have previously shown that HRF, a type of nonrespiratory sinus arrhythmia, is most evident with increasing age and advanced atherosclerotic CV disease, settings in which vagal control is most likely to be impaired. Decreased vagal modulation is associated with both increased adrenergic activity (13) and increased inflammatory response (57). The former in turn, may promote supraventricular ectopy, which acts as the initiating trigger for AF, especially with the substrate of atrial fibrosis that is thought to help sustain the micro-reentrant, fibrillatory circuits (27, 40, 54). In this study, we found that increased HRF, while not a surrogate for supraventricular ectopy, is associated with increased percentage of PSVCs. Importantly, from a translational viewpoint, we found that HRF adds value to age, CAC score, NT-proBNP levels and PSVCs in the prediction of incident AF, as well as to other known risk factors.
We note that the impact of vagal cardioautonomics in the pathogenesis of AF is complex and can vary with the physiologic context. Atrial fibrillation has been associated not only with low, but also with high cardiac vagal tone modulation. For example, increased proclivity to AF has been reported in endurance athletes (39), who, typically, are among those with the highest degree of vagal tone. In animal models (42), excessive vagal stimulation consistently promotes AF. However, as noted above, AF is most prevalent in the elderly and in those with advanced heart disease, i.e., in those with diminished cardiac vagal tone modulation (1, 14). Proarrhythmic and proinflammatory effects of decreased vagal tone underlie the emerging area of “neuromodulatory therapeutics,” which is directed at suppressing AF by low-level vagal stimulation (13, 37, 49, 57). Whether HRF, which is a predictor of AF and a strong associate of conditions in which vagal tone is most impaired, will be useful in guiding novel neuromodulatory interventions remains to be demonstrated.
This study supports broadening the conceptual framework of atrial remodeling (21, 40). Currently, atrial remodeling includes the following intertwined categories: structural/anatomic (e.g., chamber enlargement, inflammation and fibrosis; sinus node involution); functional/hemodynamic (e.g., reduced ejection fraction; elevated filling pressures); electrophysiologic (e.g., P-wave abnormalities; intra-atrial conduction delays, sinus node dysfunction, supraventricular ectopy), and neurohormonal (cardioautonomic dysfunction; increased NT-proBNP levels). Heart rate fragmentation can be considered a manifestation of cardiac “electrodynamical” remodeling. This pathophysiologic designation, distinct from the others, relies for its detection and quantification on specific methods of time series analysis such as those we described here. Our findings show that the electrodynamical dimension adds value to the electrophysiological and neurohormonal ones. Future studies are needed to determine whether the new electrodynamical component also adds value to the structural and hemodynamic ones.
Utility of Traditional HRV and Nonlinear Measures for the Prediction of AF
In this study, we did not find any association between traditional HRV measures and incident AF in any of the models. A trend toward lower variability and AF was observed. Previous studies assessing the value of HRV measures in AF prediction have not yielded consistent findings. In a cohort of the Atherosclerosis Risk in Communities Study, Agarwal et al. (1) reported that lower overall HRV, as well as increased “sympathetic/parasympathetic” tone, as inferred from the LF/HF ratio, were independently associated with higher risk of AF. In contrast, in a study of heart rate dynamics in the Osteoporotic Fractures in Men (MrOS), Raman et al. (50) reported that reduced “sympathovagal balance” and increased short-term variability during sleep were associated with future AF. In patients with coronary artery disease, the study by Nortamo et al. (43) also found that a lower LF/HF ratio was significantly associated with increased AF risk. Recently, on the basis of the analysis of three consecutive 10-s ECGs, Habibi et al. (26) reported that “lower values of rMSSD and SDNN and higher values of rMSSD were independently associated with incident AF” in the MESA population. The inconsistency of the results among studies, as well as the finding that both high and low short-term HRV were associated with incident AF, may be attributable to the limitations of traditional HRV measures (8, 15–18, 28). As discussed in further detail in Refs. 15–17, and 28, elevated short-term variability may be due to either increased vagal tone modulation or to increased HRF. In clinical contexts where vagal tone is expected to be low, such as in elderly patients and in those with heart failure, both major risk factors for AF, increased HRV indices of short-term variability, such as HF and rMSSD, are most likely due to fragmentation rather than enhanced vagal tone (15, 28). Results from a previous MESA study that we (17) conducted support this assertion. We found a nonlinear (U-shaped) relationship between HRF and traditional HRV measures of short-term variability in which increased fragmentation was associated with both low and high values of variability. Additionally, we found a U-shaped relationship between HRV and age.
Limitations of the Study
This study has limitations with respect to the possibility of both undetected prevalent and incident paroxysmal AF. Without prolonged monitoring of participants, intermittent AF episodes that are asymptomatic tend to go undetected (52). The adjudication of prevalent paroxysmal AF was based on carefully elaborated MESA protocols, as described in the methods. The possibility that some participants with prevalent paroxysmal AF still eluded detection is an inherent limitation of MESA and other major cohort studies of incident AF. However, this limitation has not precluded others (45, 55) from identifying predictors of incident AF, e.g., of NT-proBNP levels and CAC score, MESA and other populations. From a translational viewpoint, even if HRF metrics were solely surrogate measures of covert, prevalent intermittent AF episode, they would still have clinical “alert” value in identifying patients arguably at the highest risk of developing sustained AF. Similarly, some undetected cases of incident AF events may also have occurred. In appendix b, we present the results of a set of numerical experiments aimed at assessing the potential impact of AF underdetection on the HRF results. We found that undetected incident AF cases (10–20%) would not have been expected to qualitatively change our results. Furthermore, the simulations show that improved detection of AF (≥20%) would likely increase the magnitude of the associations between HRF and incident AF. Finally, we note that underdetection of AF events would be unlikely to change the relative predictive ability of the different variables reported here.
Future Studies
Future large cohort studies are needed to validate the present findings. Additional studies are needed to ascertain whether fragmentation metrics are useful in guiding patient management, specifically, in 1) selecting high-risk patients who would benefit from continuous monitoring (e.g., with cardiac patch monitors or medical wearable devices) for detection of subclinical AF, 2) predicting the success of ablation therapy and assessing its results, and 3) identifying patients in the highest AF risk group who might benefit from prophylactic anticoagulant therapy.
Conclusions
Heart rate fragmentation, a biomarker of altered sinoatrial dynamics, is independently associated with short-term (in this study up to ∼4 yr) risk of incident AF in MESA. The translational potential of this new, noninvasive, and repeatable biomarker is underscored by the findings that 1) HRF adds value to the strongest AF risk factors, including age, NT-proBNP, CAC, and frequent PSVCs and 2) HRF has predictive utility in two subgroups at very high risk of developing AF. More generally, HRF is of interest as it exemplifies a distinct class of biomarkers, termed dynamical assays (18, 24), which extract hidden information from fluctuations in biological variables.
GRANTS
The MESA study was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, 75N92020D00001, 75N92020D00002, 75N92020D00003, 75N92020D00005, 75N92020D00006, and 75N92020D00007 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences. The MESA Sleep ancillary study was supported through R01HL098433. The MESA Atrial Fibrillation ancillary study was supported through R01 HL127659. Coronary calcification data were obtained, in part, under STAR research assistance agreements, no. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the U.S. Environmental Protection Agency. The authors gratefully acknowledge support from the James S. McDonnell Foundation, and National Institutes of Health grants R01HL144510 (to S.H., M.D.C., A.L.G.), R01EB030362 and R01GM104987 (A.L.G., M.D.C.), and R35HL135818 (S.R.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.D.C. and A.L.G. conceived and designed research; M.D.C. and A.L.G. analyzed data; M.D.C., A.L.G., and S.R.H. interpreted results of experiments; M.D.C. prepared figures; M.D.C. and A.L.G. drafted manuscript; M.D.C., S.R., E.S., A.L.G., and S.R.H. edited and revised manuscript; M.D.C., S.R., E.S., A.L.G., and S.R.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the other investigators, the staff, and the participants of the MESA study for valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
APPENDIX A: COMPUTATION OF HEART RATE FRAGMENTATION INDICES
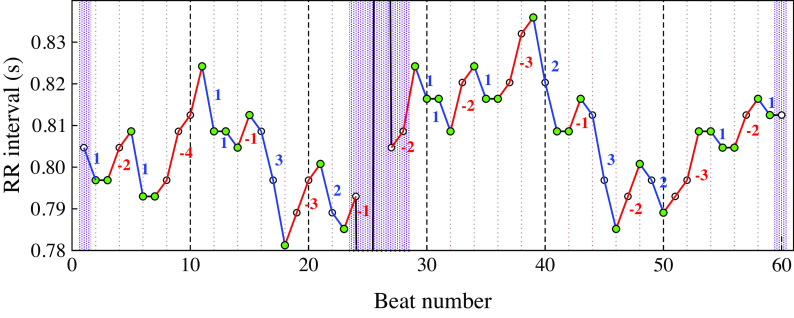
This section is intended to highlight technical details of the computation of HRF indices from cardiac interbeat interval time series. Figure A1 shows a section of a cardiac interbeat (RR) interval time series with a premature ventricular complex.
Green circles mark inflection points. Blue and red lines mark heart rate accelerative and decelerative segments, respectively. Data point no. 25 is a premature ventricular beat (not shown). The purple bars highlight regions excluded from the computation of the indices. These three regions include segments whose real lengths are unknown, two because they are located at the extrema (beginning/end) of the time series and the other (middle) because of the interruption in the time series caused by the premature beat. The time series comprises 60 NN interbeat intervals. After excluding the first, the last and the five data points in the region of the premature beat, the number of NN intervals is 53 and the number of ΔNN intervals is 51. The percentage of inflection points (PIP) is (33/53)·100 = 62.3%. There are 16 hard and 17 soft inflection points, thus PIPH = 16/53 = 30.2% and PIPS = 17/53 = 32.1%. The number of accelerative/decelerative segments of length 1, 2, 3 and 4 is 10, 7, 5 and 1, respectively. The number of ΔNN intervals in these segments is 10·1+7·2+5·3+1·4 = 43. A metric of fragmentation, PNNSS, is defined as the number of ΔNN intervals in short (<3) accelerative/decelerative segments over the total number of ΔNN intervals in accelerative/decelerative segments of any length: (10·1+7·2)/43·100 = 39.5%. A metric of fluency, PNNLS, is defined as the number of ΔNN intervals in long (≥3) accelerative/decelerative segments over the total number of ΔNN intervals in the time series: (5·3+1·4)/51·100% = 37.3%. One may prefer to report the values of the complement of PNNLS, i.e., 100 – PNNLS, instead of PNNLS, since the values of 100 – PNNLS will be higher for more fragmented time series (not lower as in the case of PNNLS). Note that 100 – PNNLS ≠ PNNSS, since the denominators in the definitions of PNNLS and PNNSS are different. The average length of accelerative/decelerative segments (ALS) is the number of ΔNN intervals in accelerative/decelerative segments (43) over the number of different segments (10 + 7 +5 + 1 = 23), i.e., 43/23 = 1.87.
Heart rate fragmentation can be quantified by the frequency of occurrence of specific patterns of NN variability. In HRF analysis, the patterns are clustered by the number and type of inflection points they contained. The higher the number of inflection points, the more fragmented the patterns are.
We considered sequences of five consecutive NN intervals, i.e., of 4 ΔNN intervals. The exact time duration of these segments depends on the heart rate. Typically, their duration varies between 3 s for heart rates of 40 bpm and 6 s for heart rates of 80 bpm. These values fall, as intended, within the traditional limits of the respiratory frequency band, 0.15 to 0.40 Hz, i.e., cycle length of 2.5 to 6.7 s (31).
Let “1” and “−1” represent heart rate accelerative and decelerative intervals, respectively. Let “0” represent an interval in which heart rate does not change. W1H is defined as the combined percentage of all patterns with one hard inflection point. Thus, W1H comprises the following (n = 6) patterns: “111-1,” “11-1-1,” “1-1-1-1,” “-1-1-11,” “-1-111,” and “-1111.” W3M is defined as the combined percentage of all patterns with three (mixed) inflection points, two hard and one soft, or one soft and two hard. W3M comprises the following (n = 14) patterns: “0-110,” “0-11-1,” “01-10,” “01-11,” “-10-11,” “-101-1,” “-110-1,” “-1101,” “1-110,” “10-11,” “101-1,” “1-10-1,” “1-101” and “-11-10.” Given that patterns of 4 consecutive ΔNN intervals can have up to three reversals in heart rate acceleration, W3M patterns are among the most fragmented, while W1H patterns are among the least fragmented (17). W3S is defined as the combined percentage of all patterns with three soft inflection points. W3S comprises the following (n = 8) patterns: “0-10-1,” “0-101,” “010-1,” “0101,” “-10-10,” “-1010,” “10-10,” and “1010.” W3S patterns are also among the most fragmented ones (17).
The first W1H pattern in the sequence presented in Fig. A1 starts at data point no. 8. “-1-1-11.” The first W3M pattern start at data point no. 4: “-110-1.” Overall, there are 15 W1H patterns and 8 W3M. The only W3S pattern (-1010) starts at data point no. 52.
APPENDIX B: QUANTIFICATION OF THE POTENTIAL IMPACT OF UNDETECTED AF CASES ON OUR ANALYSES
As discussed in the text of the article, a potential limitation of this study is the well-known underdetection of paroxysmal or intermittent AF. Accordingly, we conducted a set of numerical experiments aimed at assessing the potential impact of AF underdetection on the HRF results. Specifically, we reran the Cox regression analyses after randomly relabeling a given percentage of incident cases as nonincident for the duration of the follow-up. We chose 10% in one set of experiments and 20% in the other. Each experiment was repeated 10 times to randomly select different subsets of incident cases. The final number of incident cases was 73 and 65, for the experiments with 10% and 20% relabeling, respectively. Results of Cox regression analyses did not qualitatively change. Specifically, for the data sets in which 10% of the incident AF cases were relabeled, the hazard ratios for HRF in models adjusted for age, height, and NT-proBNP varied between 1.29 (P = 0.004) and 1.44 (P = 0.034), in line with the values we found for the original data (1.35). For the data sets in which 20% of incident AF cases were relabeled, the hazard ratios for HRF varied between 1.22 (P = 0.10) and 1.53 (P = 0.002) in models with the same adjustments as those specified above. Although the associations between HRF and incident AF were slightly weaker for the data sets with 20% versus 10% of the incident cases mislabeled, 8 out of the 10 hazard ratios were still statistically significant. These numerical tests support the contention that underdetection of incident AF cases would have tended to reduce the statistical models’ performances with respect to HRF. Thus, the identification of actual undetected cases of incident AF in the original data set (to the extent they existed) would have been expected to improve the results presented in this study.
Fig. A1.
Portion of a cardiac interbeat (R-R) interval time series for illustration of the computation of the fragmentation indices.
REFERENCES
- 1.Agarwal SK, Norby FL, Whitsel EA, Soliman EZ, Chen LY, Loehr LR, Fuster V, Heiss G, Coresh J, Alonso A. Cardiac autonomic dysfunction and incidence of atrial fibrillation: results from 20 years follow-up. J Am Coll Cardiol 69: 291–299, 2017. doi: 10.1016/j.jacc.2016.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15: 827–832, 1990. doi: 10.1016/0735-1097(90)90282-T. [DOI] [PubMed] [Google Scholar]
- 3.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kääb S, Couper D, Harris TB, Soliman EZ, Stricker BH, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc 2: e000102, 2013. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alonso A, Roetker NS, Soliman EZ, Chen LY, Greenland P, Heckbert SR. Prediction of atrial fibrillation in a racially diverse cohort: The Multi-Ethnic Study of Atherosclerosis (MESA). J Am Heart Assoc 5: e003077, 2016. doi: 10.1161/JAHA.115.003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angelone A, Coulter NA Jr. Respiratory sinus arrhythmia: a frequency dependent phenomenon. J Appl Physiol 19: 479–482, 1964. doi: 10.1152/jappl.1964.19.3.479. [DOI] [PubMed] [Google Scholar]
- 6.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 156: 871–881, 2002. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 7.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 111: 1313–1320, 2005. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 8.Billman GE The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol 4: 26, 2013. doi: 10.3389/fphys.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binkley PF, Eaton GM, Nunziata E, Khot U, Cody RJ. Heart rate alternans. Ann Intern Med 122: 115–117, 1995. doi: 10.7326/0003-4819-122-2-199501150-00007. [DOI] [PubMed] [Google Scholar]
- 10.de Bruyne MC, Kors JA, Hoes AW, Klootwijk P, Dekker JM, Hofman A, van Bemmel JH, Grobbee DE. Both decreased and increased heart rate variability on the standard 10-second electrocardiogram predict cardiac mortality in the elderly: the Rotterdam Study. Am J Epidemiol 150: 1282–1288, 1999. doi: 10.1093/oxfordjournals.aje.a009959. [DOI] [PubMed] [Google Scholar]
- 11.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology 234: 35–43, 2005. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 12.Chang KW, Hsu JC, Toomu A, Fox S, Maisel AS. Clinical applications of biomarkers in atrial fibrillation. Am J Med 130: 1351–1357, 2017. doi: 10.1016/j.amjmed.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Chen P-S, Chen LS, Fishbein MC, Lin S-F, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res 114: 1500–1515, 2014. doi: 10.1161/CIRCRESAHA.114.303772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, McCabe P, Noseworthy PA, Perez MV, Turakhia MP; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council . Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity: a scientific statement from the American Heart Association. Circulation 137: e623–e644, 2018. doi: 10.1161/CIR.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa MD, Davis RB, Goldberger AL. Heart rate fragmentation: a new approach to the analysis of cardiac interbeat interval dynamics. Front Physiol 8: 255, 2017. doi: 10.3389/fphys.2017.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa MD, Davis RB, Goldberger AL. Heart rate fragmentation: a symbolic dynamical approach. Front Physiol 8: 827, 2017. doi: 10.3389/fphys.2017.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa MD, Redline S, Davis RB, Heckbert SR, Soliman EZ, Goldberger AL. Heart rate fragmentation as a novel biomarker of adverse cardiovascular events: The Multi-Ethnic Study of Atherosclerosis. Front Physiol 9: 1117, 2018. doi: 10.3389/fphys.2018.01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa MD, Goldberger AL. Heart rate fragmentation: using cardiac pacemaker dynamics to probe the pace of biological aging. Am J Physiol Heart Circ Physiol 316: H1341–H1344, 2019. doi: 10.1152/ajpheart.00110.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dean DA II, Wang R, Jacobs DR Jr, Duprez D, Punjabi NM, Zee PC, Shea S, Watson K, Redline S. A systematic assessment of the association of polysomnographic indices with blood pressure: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep (Basel) 38: 587–596, 2015. doi: 10.5665/sleep.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, Psaty BM, Sotoodehnia N, Gottdiener JS, Marcus GM. Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Ann Intern Med 159: 721–728, 2013. doi: 10.7326/0003-4819-159-11-201312030-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everett TH IV, Wilson EE, Verheule S, Guerra JM, Foreman S, Olgin JE. Structural atrial remodeling alters the substrate and spatiotemporal organization of atrial fibrillation: a comparison in canine models of structural and electrical atrial remodeling. Am J Physiol Heart Circ Physiol 291: H2911–H2923, 2006. doi: 10.1152/ajpheart.01128.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fedorov VV, Glukhov AV, Chang R. Conduction barriers and pathways of the sinoatrial pacemaker complex: their role in normal rhythm and atrial arrhythmias. Am J Physiol Heart Circ Physiol 302: H1773–H1783, 2012. doi: 10.1152/ajpheart.00892.2011. [DOI] [PubMed] [Google Scholar]
- 23.Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, Mietus JE, Moody GB, Peng CK, Stanley HE. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation 101: E215–E220, 2000. doi: 10.1161/01.CIR.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 24.Goldberger AL Giles F. Filley Lecture. Complex systems. Proc Am Thorac Soc 3: 467–471, 2006. doi: 10.1513/pats.200603-028MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glukhov AV, Hage LT, Hansen BJ, Pedraza-Toscano A, Vargas-Pinto P, Hamlin RL, Weiss R, Carnes CA, Billman GE, Fedorov VV. Sinoatrial node reentry in a canine chronic left ventricular infarct model: role of intranodal fibrosis and heterogeneity of refractoriness. Circ Arrhythm Electrophysiol 6: 984–994, 2013. doi: 10.1161/CIRCEP.113.000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Habibi M, Chahal H, Greenland P, Guallar E, Lima JAC, Soliman EZ, Alonso A, Heckbert SR, Nazarian S. Resting heart rate, short-term heart rate variability and incident atrial fibrillation (from the Multi-Ethnic Study of Atherosclerosis (MESA)). Am J Cardiol 124: 1684–1689, 2019. doi: 10.1016/j.amjcard.2019.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LA, Kalyanasundaram A, Lim P, Bratasz A, Powell KA, Simonetti OP, Higgins RSD, Kilic A, Mohler PJ, Janssen PML, Weiss R, Hummel JD, Fedorov VV. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J 36: 2390–2401, 2015. doi: 10.1093/eurheartj/ehv233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayano J, Yuda E. Pitfalls of assessment of autonomic function by heart rate variability. J Physiol Anthropol 38: 3, 2019. doi: 10.1186/s40101-019-0193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heckbert SR, Wiggins KL, Blackshear C, Yang Y, Ding J, Liu J, McKnight B, Alonso A, Austin TR, Benjamin EJ, Curtis LH, Sotoodehnia N, Correa A. Pericardial fat volume and incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis and Jackson Heart Study. Obesity (Silver Spring) 25: 1115–1121, 2017. doi: 10.1002/oby.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirsch JA, Bishop B. Respiratory sinus arrhythmia in humans: how breathing pattern modulates heart rate. Am J Physiol Heart Circ Physiol 241: H620–H629, 1981. doi: 10.1152/ajpheart.1981.241.4.H620. [DOI] [PubMed] [Google Scholar]
- 31.Hogue CW Jr, Domitrovich PP, Stein PK, Despotis GD, Re L, Schuessler RB, Kleiger RE, Rottman JN. RR interval dynamics before atrial fibrillation in patients after coronary artery bypass graft surgery. Circulation 98: 429–434, 1998. doi: 10.1161/01.CIR.98.5.429. [DOI] [PubMed] [Google Scholar]
- 32.Electrophysiology TFES; Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology . Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93: 1043–1065, 1996. doi: 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- 33.Huikuri HV, Seppänen T, Koistinen MJ, Airaksinen J, Ikäheimo MJ, Castellanos A, Myerburg RJ. Abnormalities in beat-to-beat dynamics of heart rate before the spontaneous onset of life-threatening ventricular tachyarrhythmias in patients with prior myocardial infarction. Circulation 93: 1836–1844, 1996. doi: 10.1161/01.CIR.93.10.1836. [DOI] [PubMed] [Google Scholar]
- 34.Kwon Y, Gharib SA, Biggs ML, Jacobs DR Jr, Alonso A, Duprez D, Lima J, Lin GM, Soliman EZ, Mehra R, Redline S, Heckbert SR. Association of sleep characteristics with atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis. Thorax 70: 873–879, 2015. doi: 10.1136/thoraxjnl-2014-206655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwon Y, Misialek JR, Duprez D, Alonso A, Jacobs DR Jr, Heckbert SR, Redline S, Soliman EZ. Association between sleep disordered breathing and electrocardiographic markers of atrial abnormalities: the MESA study. Europace 19: 1759–1766, 2017. doi: 10.1093/europace/euw328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li N, Hansen BJ, Csepe TA, Zhao J, Ignozzi AJ, Sul LV, Zakharkin SO, Kalyanasundaram A, Davis JP, Biesiadecki BJ, Kilic A, Janssen PML, Mohler PJ, Weiss R, Hummel JD, Fedorov VV. Redundant and diverse intranodal pacemakers and conduction pathways protect the human sinoatrial node from failure. Sci Transl Med 9: eaam5607, 2017. doi: 10.1126/scitranslmed.aam5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li S, Scherlag BJ, Yu L, Sheng X, Zhang Y, Ali R, Dong Y, Ghias M, Po SS. Low-level vagosympathetic stimulation: a paradox and potential new modality for the treatment of focal atrial fibrillation. Circ Arrhythm Electrophysiol 2: 645–651, 2009. doi: 10.1161/CIRCEP.109.868331. [DOI] [PubMed] [Google Scholar]
- 38.Lubitz SA, Yin X, Lin HJ, Kolek M, Smith JG, Trompet S, Rienstra M, Rost NS, Teixeira PL, Almgren P, Anderson CD, Chen LY, Engström G, Ford I, Furie KL, Guo X, Larson MG, Lunetta KL, Macfarlane PW, Psaty BM, Soliman EZ, Sotoodehnia N, Stott DJ, Taylor KD, Weng LC, Yao J, Geelhoed B, Verweij N, Siland JE, Kathiresan S, Roselli C, Roden DM, van der Harst P, Darbar D, Jukema JW, Melander O, Rosand J, Rotter JI, Heckbert SR, Ellinor PT, Alonso A, Benjamin EJ; AFGen Consortium . Genetic risk prediction of atrial fibrillation. Circulation 135: 1311–1320, 2017. doi: 10.1161/CIRCULATIONAHA.116.024143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mont L, Elosua R, Brugada J. Endurance sport practice as a risk factor for atrial fibrillation and atrial flutter. Europace 11: 11–17, 2009. doi: 10.1093/europace/eun289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol 1: 62–73, 2008. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen KT, Vittinghoff E, Dewland TA, Mandyam MC, Stein PK, Soliman EZ, Heckbert SR, Marcus GM. Electrocardiographic predictors of incident atrial fibrillation. Am J Cardiol 118: 714–719, 2016. doi: 10.1016/j.amjcard.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishida K, Michael G, Dobrev D, Nattel S. Animal models for atrial fibrillation: clinical insights and scientific opportunities. Europace 12: 160–172, 2010. doi: 10.1093/europace/eup328. [DOI] [PubMed] [Google Scholar]
- 43.Nortamo S, Ukkola O, Kiviniemi A, Tulppo M, Huikuri H, Perkiömäki JS. Impaired cardiac autonomic regulation and long-term risk of atrial fibrillation in patients with coronary artery disease. Heart Rhythm 15: 334–340, 2018. doi: 10.1016/j.hrthm.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 44.O’Neal WT, Efird JT, Dawood FZ, Yeboah J, Alonso A, Heckbert SR, Soliman EZ. Coronary artery calcium and risk of atrial fibrillation (from the multi-ethnic study of atherosclerosis). Am J Cardiol 114: 1707–1712, 2014. doi: 10.1016/j.amjcard.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, Kronmal RA. N-terminal pro-B-type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation 120: 1768–1774, 2009. doi: 10.1161/CIRCULATIONAHA.109.873265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng CK, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 5: 82–87, 1995. doi: 10.1063/1.166141. [DOI] [PubMed] [Google Scholar]
- 47.Perkiömäki J, Ukkola O, Kiviniemi A, Tulppo M, Ylitalo A, Kesäniemi YA, Huikuri H. Heart rate variability findings as a predictor of atrial fibrillation in middle-aged population. J Cardiovasc Electrophysiol 25: 719–724, 2014. doi: 10.1111/jce.12402. [DOI] [PubMed] [Google Scholar]
- 48.Pikkujämsä SM, Mäkikallio TH, Sourander LB, Räihä IJ, Puukka P, Skyttä J, Peng CK, Goldberger AL, Huikuri HV. Cardiac interbeat interval dynamics from childhood to senescence: comparison of conventional and new measures based on fractals and chaos theory. Circulation 100: 393–399, 1999. doi: 10.1161/01.CIR.100.4.393. [DOI] [PubMed] [Google Scholar]
- 49.Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, DiCarlo LA, Ardell JL, Rector TS, Amurthur B, KenKnight BH, Anand IS. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. J Card Fail 20: 808–816, 2014. doi: 10.1016/j.cardfail.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Raman D, Kaffashi F, Lui LY, Sauer WH, Redline S, Stone P, Cawthon PM, Stone KL, Ensrud KE, Ancoli-Israel S, Loparo K, Mehra R. Polysomnographic heart rate variability indices and atrial ectopy associated with incident atrial fibrillation risk in older community-dwelling men. JACC Clin Electrophysiol 3: 451–460, 2017. doi: 10.1016/j.jacep.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Redline S, Budhiraja R, Kapur V, Marcus CL, Mateika JH, Mehra R, Parthasarthy S, Somers VK, Strohl KP, Sulit LG, Gozal D, Wise MS, Quan SF. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med 3: 169–200, 2007. doi: 10.5664/jcsm.26818. [DOI] [PubMed] [Google Scholar]
- 52.Reiffel JA, Verma A, Kowey PR, Halperin JL, Gersh BJ, Wachter R, Pouliot E, Ziegler PD; REVEAL AF Investigators . Incidence of previously undiagnosed atrial fibrillation using insertable cardiac monitors in a high-risk population: The REVEAL AF Study. JAMA Cardiol 2: 1120–1127, 2017. doi: 10.1001/jamacardio.2017.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol 278: H2039–H2049, 2000. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- 54.Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res 120: 1501–1517, 2017. doi: 10.1161/CIRCRESAHA.117.309732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinner MF, Stepas KA, Moser CB, Krijthe BP, Aspelund T, Sotoodehnia N, Fontes JD, Janssens ACJW, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Vasan RS, Wang TJ, Agarwal SK, McManus DD, Franco OH, Yin X, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kääb S, Couper D, Harris TB, Astor BC, Ballantyne CM, Hoogeveen RC, Arai AE, Soliman EZ, Ellinor PT, Stricker BHC, Gudnason V, Heckbert SR, Pencina MJ, Benjamin EJ, Alonso A. B-type natriuretic peptide and C-reactive protein in the prediction of atrial fibrillation risk: the CHARGE-AF Consortium of community-based cohort studies. Europace 16: 1426–1433, 2014. doi: 10.1093/europace/euu175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC Jr. Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke 40: 1204–1211, 2009. [Erratum in Stroke 41: e433, 2010]. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stavrakis S, Humphrey MB, Scherlag BJ, Hu Y, Jackman WM, Nakagawa H, Lockwood D, Lazzara R, Po SS. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol 65: 867–875, 2015. doi: 10.1016/j.jacc.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stein PK, Domitrovich PP, Hui N, Rautaharju P, Gottdiener J. Sometimes higher heart rate variability is not better heart rate variability: results of graphical and nonlinear analyses. J Cardiovasc Electrophysiol 16: 954–959, 2005. doi: 10.1111/j.1540-8167.2005.40788.x. [DOI] [PubMed] [Google Scholar]