Abstract
Following cardiac injury, increased adrenergic drive plays an important role in compensating for reduced cardiac function. However, chronic excess adrenergic stimulation can be detrimental to cardiac pathophysiology and can also affect other organs including adipose tissue, leading to increased lipolysis. Interestingly, inhibition of adipose triglyceride lipase (ATGL), a rate-limiting enzyme in lipolysis, in adipocytes ameliorates cardiac dysfunction in a heart failure model. Thus, we investigated whether inhibition of adipocyte ATGL can mitigate the adverse cardiac effects of chronic adrenergic stimulation and explored the underlying mechanisms. To do this, isoproterenol (ISO) was continuously administered to C57Bl/6N mice for 2 wk with or without an ATGL inhibitor (Atglistatin). We found that Atglistatin alleviated ISO-induced cardiac remodeling and reduced ISO-induced upregulation of galectin-3, a marker of activated macrophages and a potent inducer of fibrosis, in white adipose tissue (WAT), heart, and the circulation. To test whether the beneficial effects of Atglistatin occur via inhibition of adipocyte ATGL, adipocyte-specific ATGL knockout (atATGL-KO) mice were utilized for similar experiments. Subsequently, the same cardioprotective effects of atATGL-KO following ISO administration were observed. Furthermore, Atglistatin and atATGL-KO abolished ISO-induced galectin-3 secretion from excised WAT. We further demonstrated that activation of cardiac fibroblasts by the conditioned media of ISO-stimulated WAT is galectin-3-dependent. In conclusion, the inhibition of adipocyte ATGL ameliorated ISO-induced cardiac remodeling possibly by reducing galectin-3 secretion from adipose tissue. Thus, inhibition of adipocyte ATGL might be a potential target to prevent some of the adverse effects of chronic excess adrenergic drive.
NEW & NOTEWORTHY The reduction of lipolysis by adipocyte ATGL inhibition ameliorates cardiac remodeling induced by chronic β-adrenergic stimulation likely via reducing galectin-3 secretion from adipose tissue. Our findings highlight that suppressing lipolysis in adipocytes may be a potential therapeutic target for patients with heart failure whose sympathetic nervous system is activated. Furthermore, galectin-3 might be involved in the mechanisms by which excessive lipolysis in adipose tissues influences remote cardiac pathologies and thus warrants further investigation.
Keywords: adipose triglyceride lipase, ATGL, cardiac remodeling, galectin-3, inflammation, isoproterenol
INTRODUCTION
Following cardiac injury that results in cardiac dysfunction, several neurohumoral systems are activated to compensate for the reduced cardiac output (1, 2). Among these, the adrenergic nervous system plays a primary role by increasing cardiac contractility and eliciting a positive chronotropic effect via the release of adrenergic catecholamines into the circulation (2). Although elevated adrenergic catecholamines in patients with heart failure are largely considered to target the heart, other organs/cell types also respond to this adrenergic stimulation. For instance, β-adrenergic stimulation of adipose tissue drives lipolysis via increased activation of lipases including adipose triglyceride lipase (ATGL) (3–5). Unfortunately, enhanced adipose tissue lipolysis can increase proinflammatory cytokines and an increase in systemic inflammation (5, 6). This inflammation creates a feed-forward loop that further impairs cardiac function, thereby further stimulating adrenergic catecholamines and additional lipolysis and inflammation (5). Based on this evidence, it has been postulated that preventing adipose tissue lipolysis by inhibiting adipocyte ATGL may prove to be of benefit in heart failure (5).
Consistent with a previous study (5), we and others (7, 8) have shown that the pharmacological inhibition or genetic deletion of ATGL in adipose tissue decreases adipose tissue lipolysis and ameliorates cardiac dysfunction in a mouse model of pressure overload-induced heart failure. However, the extent to which this improvement in cardiac function in heart failure is directly related to decreases in systemic or cardiac inflammation has yet to be investigated. Nevertheless, a plethora of evidence indicates that the inflammation in adipose tissue contributes to the systemic inflammation associated with innate immune cells (e.g., macrophages), which can contribute to worsening cardiac function and subsequent development of heart failure following cardiac injury (9, 10). In agreement with this, it is now widely accepted that targeting systemic inflammation reduces major adverse cardiovascular events among patients who had a myocardial infarction as shown in the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) (11). Given the importance of ATGL in regulating lipolysis in adipose tissue and its role in increasing systemic inflammation and worsening cardiac function, we hypothesized that inhibition of ATGL in adipose tissue could ameliorate cardiac pathologies induced by excessive adrenergic drive via reduction in systemic inflammation. To test this hypothesis, we examined the cardiac pathophysiological features following isoproterenol (ISO) administration in the presence or absence of Atglistatin, an inhibitor of ATGL (12, 13). Moreover, we conducted similar experiments on adipocyte-specific Atgl-deficient mice (atATGL-KO) (14) to examine whether the effect of Atglistatin originates from inhibition of adipocyte lipolysis.
METHODS
Animals
All protocols involving mice were approved by the University of Alberta Institutional Animal Care and Use Committee and conform to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (eighth edition; revised 2011). The University of Alberta adheres to the principles for biomedical research involving animals developed by the Council for International Organizations of Medical Sciences and complies with the Canadian Council on Animal Care guidelines.
Adipocyte-specific Atgl-deficient mice (atATGL-KO) were generated as previously described (14). Briefly, mice carrying a LoxP-modified ATGL allele (ATGLflox/flox) were crossed to mice expressing Cre-recombinase under the control of the adipocyte-specific Adipoq-Cre mice as previously described (14) to generate experimental ATGLflox/flox +/+ (Control) and ATGLflox/flox Cre/+ (atATGL-KO) mice. Mice were congenic C57Bl/6N at the time of this study. For experiments involving Atglistatin, C57Bl/6N mice were purchased from Charles River Laboratories. All mice were housed individually under standard conditions (25°C, 12:12-h light/dark cycle) with ad libitum access to food and water throughout the study period.
Atglistatin
The ATGL inhibitor, Atglistatin, was synthesized in-house as previously described (12). The purity of Atglistatin synthesized for our study was ≥98%, as confirmed by high-performance liquid chromatography (HPLC). Atglistatin-containing pellets were prepared by adding 2 mmol/kg of Atglistatin powder to ground standard chow diet (Picolab Rodent diet with 5% fat, 21.9% starch, 3.1% sucrose, and 24.1% protein) as described previously (7). Atglistatin-containing pellets and pellets containing no drug were given to each group of mice.
Study Design
To replicate the stimulated sympathetic nervous activity in heart failure, we used ISO, a nonselective β-adrenergic receptor agonist, which induces pathological changes in the heart as well as lipolysis (3, 15). For the pharmacological inhibition study, 7-wk-old male mice were randomly allocated into two groups fed with food containing 2 mmol/kg diet of Atglistatin (Atglistatin group) or the standard chow (Standard group) for 1 wk. After this period, mice were implanted with osmotic pumps (Model 1002, ALZET Tech, Cupertino, CA) containing ISO dissolved in normal saline [30 μg/g body wt (BW)/day] or normal saline only as a vehicle. The pumps were implanted in the subcutaneous region on the backs of mice under continuous inhalational anesthesia using isoflurane (2%–3.5%). Meloxicam (5 mg/kg) was given subcutaneously at the time of surgery and on the following day. The two groups of mice were maintained on the same diet that they were on before implantation of the osmotic pumps for another 2 wk (Fig. 1A). In a separate cohort of mice, 8-wk-old male atATGL-KO mice and their control littermates were treated with ISO or saline (vehicle) delivered via osmotic pumps for 2 wk (Fig. 4A). Following 2 wk of ISO or vehicle administration, all mice were subjected to echocardiography and allowed to recover after anesthesia. Following the recovery period, mice were fasted for 6 h and then euthanized by decapitation for subsequent analysis (Figs. 1A and 4A).
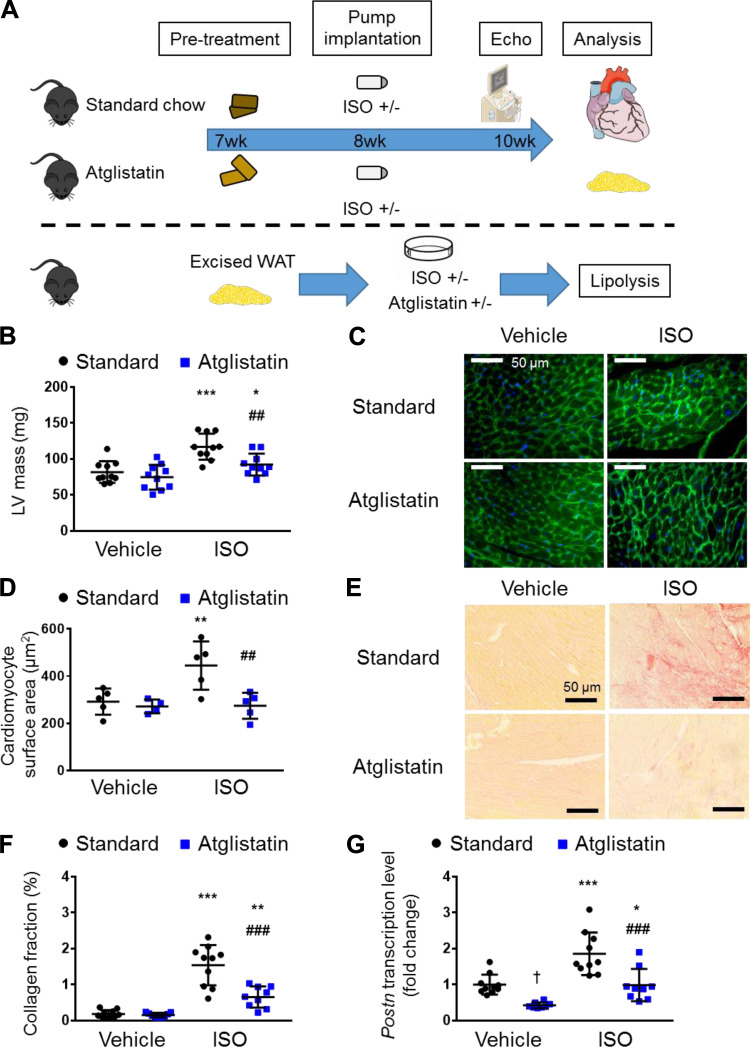
Figure 1.
ATGL inhibition ameliorates cardiac hypertrophy and cardiac fibrosis induced by β-adrenergic stimulation. A: schematic of the Atglistatin model. C57Bl/6N mice were allocated into two groups, fed with standard chow diet or with Atglistatin-containing diet, at 7-wk-old. At 8-wk-old, a mini-osmotic pump containing vehicle or isoproterenol (ISO) was implanted. After 2 wk, the cardiac structure and function were assessed with echocardiography, and then mice were euthanized and tissues collected for further analysis. For assessment of lipolysis, gonadal white adipose tissue (WAT) was excised from C57Bl/6N mice and incubated with or without ISO in the presence or absence of Atglistatin, and then nonesterified fatty acid levels in the medium was measured. B: corrected left ventricular (LV) mass obtained with echocardiography (n = 7–10 mice per group). C: representative images of left ventricle section stained with wheat germ agglutinin conjugated with Alexa Fluor 488 (green) and DAPI (blue). Scale bar represents 50 μm. D: surface area of cardiomyocytes (n = 4–5 mice per group). E: representative images of left ventricle section stained with Picro-Sirius Red staining. Scale bar represents 50 μm. F: proportions of Picro-Sirius Red staining-positive region (n = 9–10 mice per group). G: relative transcript levels of Postn (Periostin) in the heart (n = 9–10 mice per group), normalized to the expression of cyclophilin A. Dots represent individual values. Data are presented as the means ± SD. P values were derived by two-way ANOVA followed by Sidak’s multiple-comparisons test as post hoc. *Comparison with its own vehicle group. †Comparison of standard chow with vehicle group and Atglistatin with vehicle group. #Comparison of standard chow with ISO group to Atglistatin with ISO group. *, †P < 0.05; **, ##P < 0.01; ***, ###P < 0.001. ATGL, adipose triglyceride lipase. Figure 1A was created with art materials provided by Servier Medical Art, licensed under a Creative Common Attribution 3.0 Generic License. http://smart.servier.com/
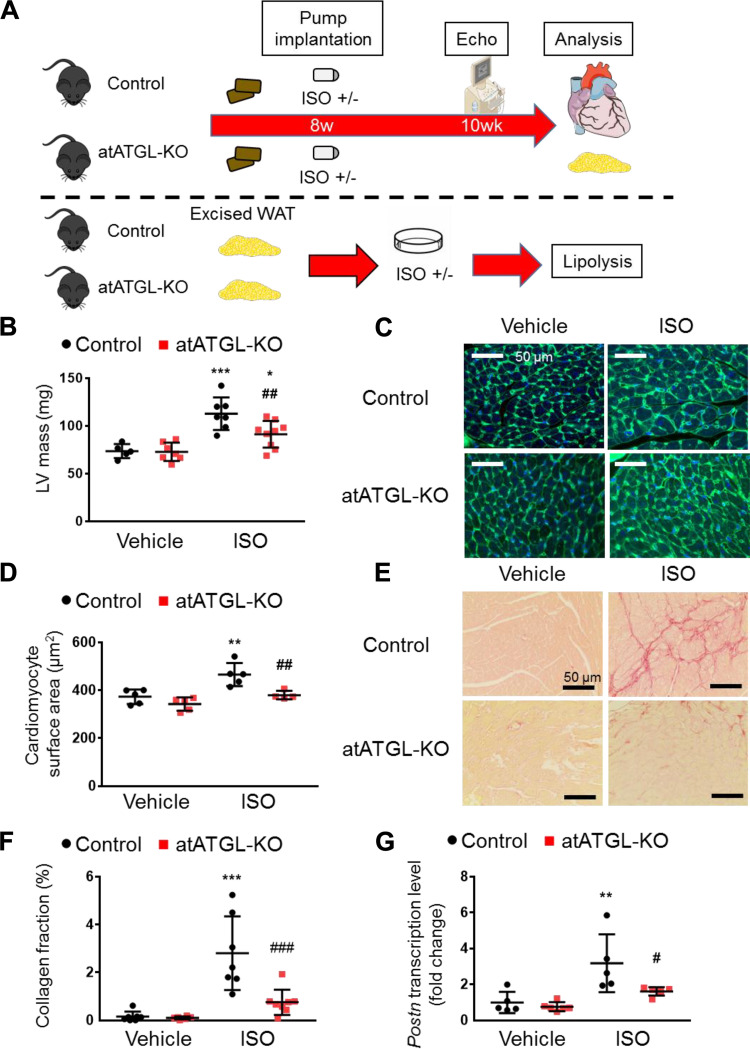
Figure 4.
Genetic Atgl ablation in adipocytes ameliorates isoproterenol-induced cardiac hypertrophy and fibrosis. A: schematic of the genetic model with adipocyte-specific adipose triglyceride lipase knockout (atATGL-KO). At 8-wk-old, a mini-osmotic pump containing vehicle or isoproterenol (ISO) was implanted. After 2 wk, the cardiac structure and function were assessed with echocardiography, and then mice were euthanized and tissues were collected for further analysis. For assessment of lipolysis, gonadal white adipose tissue (WAT) was excised from atATGK-KO mice and control littermates and incubated with or without ISO, and then nonesterified fatty acid (NEFA) level in the medium was measured. B: corrected left ventricular (LV) mass obtained with echocardiography (n = 5–9 mice per group). C: representative images of LV section stained with wheat germ agglutinin conjugated with Alexa Fluor 488 (green) and DAPI (blue). Scale bar represents 50 μm. D: surface area of cardiomyocytes (n = 4–5 mice per group). E: representative images of LV section stained with Picro-Sirius Red staining. Scale bar represents 50 μm. F: proportions of Picro-Sirius Red staining-positive region (n = 6–8 mice per group). G: relative transcript levels of Postn in the heart (n = 5 per group), normalized to cyclophilin A. Dots represent individual values. Data are presented as means ± SD. P values were derived by two-way ANOVA followed by Sidak’s multiple-comparisons test as post hoc. *Comparison with its own vehicle group. #Comparison of control with ISO group to atATGL-KO with ISO group. *, #P < 0.05; **, ##P < 0.01; ***, ###P < 0.001. Figure 4A was created with art materials provided by Servier Medical Art, licensed under a Creative Common Attribution 3.0 Generic License. http://smart.servier.com/
Primary Culture of Adult Murine Cardiac Fibroblasts
The primary adult murine cardiac fibroblasts were obtained as described previously (16–18). Briefly, hearts were excised from C57Bl/6N male mice (8- to 12-wk-old) under isoflurane anesthesia and minced into small pieces. The minced heart tissue was digested with 0.25% trypsin-EDTA at 37°C with gentle stirring. Supernatant that contained cells was collected and centrifuged at 1,200 g for 5 min. The cell suspension was passed through a 100-µm cell strainer (BD Biosciences, San Jose, CA), and the cardiac cells were incubated in Dulbecco’s modified Eagle’s medium (DMEM)-F12 (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum and 100 IU/mL penicillin and 100 µg/mL streptomycin at 37°C in 5% CO2. Because fibroblasts are known to attach to the dishes within 3 h, whereas myocytes require much longer time (19), nonadherent cells were removed and discarded after a 2-h incubation to isolate adherent cells, primarily cardiac fibroblasts. The adherent cells were passaged once when 80% confluence was reached and transferred into a Costar 12-well Clear TC-treated Multiple Well Plates (Corning Inc., Corning, NY). The second generation of fibroblasts was used for all experiments.
Cultured White Adipose Tissue and Conditioned Media Treatment of Primary Cardiac Fibroblasts
Gonadal white adipose tissues (WAT) were excised from 8- to 12-wk-old male mice that were anesthetized by continuous isoflurane inhalation (3%). Following WAT removal, mice were exsanguinated. Excised WAT was placed in Costar 12-well Clear TC-treated Multiple Well Plates (Corning Inc.) with DMEM containing 100 U/mL of penicillin and 100 µg/mL of streptomycin (Life Technologies). Tissue culture dishes were then treated with ISO (100 µM) or the same volume of saline (vehicle) and incubated at 37°C in 5% CO2. Since ISO degrades over time (20), ISO or vehicle were added every 8 h for 24 h. Following the 24 h incubation period, the medium was collected and stored at −80°C. In experiments involving inhibition of ATGL, Atglistatin (50 μM) was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO) to a final concentration of 0.05% in the medium. Since the effect of Atglistatin on lipolysis is known to diminish after 8 h (12, 13), Atglistatin, or the identical amount of DMSO (vehicle), was added to the media every 8 h at the same time of ISO treatment.
The conditioned media of WAT from control or atATGL-KO mice with or without ISO were used to treat the primary cardiac fibroblasts (Fig. 4A). Additionally, to test whether the activation of cardiac fibroblast by the conditioned media is dependent on galectin-3, N-acetyl-d-lactosamine (Sigma-Aldrich) (10 μM), a galectin-3 inhibitor, or recombinant mouse galectin-3 protein (R&D System, Minneapolis, MN) (60 ng/mL) was added to the ISO-treated conditioned media from control and atATGL-KO, respectively. The dose of the galectin-3 inhibitor was chosen from the literature (21), whereas the dose of the recombinant mouse galectin-3 protein was determined based on the differences of the galectin-3 concentration in the ISO-stimulated WAT conditioned media between genotypes. After a 6-h incubation, the culture dish containing primary cardiac fibroblasts was washed twice with Dulbecco’s phosphate-buffered saline (Life Technologies) and total RNA was extracted as described in Quantitative PCR section.
Ex Vivo Lipolysis
The release of nonesterified fatty acid (NEFA) from adipose tissue was examined as described (13, 22). Briefly, for the Atglistatin model, 25 mg of procured WAT was incubated in 250 μL of DMEM containing 2% of fatty acid-free BSA with or without ISO (10 μM) and in the presence or absence of Atglistatin (50 μM) at 37°C in 5% of CO2 (Fig. 1A). For the genetic model, the excised WAT from both genotypes were incubated with or without ISO at the identical conditions as the Atglistatin model (Fig. 4A). Because the rate of ISO-stimulated lipolysis is highest between the first and second hour, after 1-h preincubation, pieces of WAT were transferred to identical fresh media and then incubated for another 1 h, as described previously (22). The media from the last hour of incubation was collected, and the NEFA released was determined by the HR Series NEFA-HR kit (Fujifilm Wako Laboratory Chemicals, Richmond, VA). The pieces of WAT were lysed in lysis buffer (Tris pH 7.4, 20 mM; EDTA 5 mM; Na4P2O7 10 mM; sodium fluoride 100 mM; NP-40 1%), and the protein concentration was determined using Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA). Because there was no further significant increase in NEFA release in the media with a higher concentration than 10 μM ISO (data not shown), 10 μM ISO was chosen to test lipolysis.
Echocardiography
Mice were anesthetized with 1.0%–1.5% isoflurane 1–1.5 L/min 100% oxygen, and in vivo cardiac function was assessed by transthoracic echocardiography using a Vevo 3100 high-resolution imaging system equipped with a 30-MHz transducer (RMV-707B; VisualSonics, Toronto, Canada), as previously described (7).
Quantitative PCR
Total RNA from frozen tissues was isolated using TRIzol reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions and quantified by measuring the absorbance at 260 nm. Thereafter, first-strand cDNA synthesis was performed using 5× All-In-One RT MasterMix, according to the manufacturer’s instructions (Applied Biological Materials, Richmond, Canada). Quantitative PCR was performed using Fast SYBR Green Master Mix (Applied Biosystems) with LightCycler 480 (Roche Life Science). Primer sequences are provided in the Supplemental Table S1 (all Supplemental material is available at https://doi.org/10.6084/m9.figshare.13129745). Primers were synthesized by Integrated DNA Technologies (IDT, Coralville, IA). The real-time PCR data were analyzed using the relative gene expression (ΔΔCt) method, as described previously (23).
Histological Analysis
Paraffin-embedded hearts were sliced into 5-μm-thick sections and used for subsequent histological analyses. Hematoxylin-eosin staining and Picro-Sirius Red staining (Abcam, Cambridge, UK) were performed according to the standard protocol. For wheat germ agglutinin (WGA) staining, sections were treated with WGA conjugated with Alexa Fluor 488 (Thermo Fisher Scientific), and then nuclei were stained with DAPI using ProLong Gold Antifade Mountant with DAPI (Thermo Fisher Scientific).
For immunochemistry, sections were incubated with F4/80 primary antibody (1:50; Cell Signaling Technology, Danvers, MA), followed by the anti-rabbit secondary antibody conjugated with HRP (Vector Laboratories, Burlingame, CA). The signal was visualized with DAB Substrate Kit (Abcam). For immunofluorescence, sections were incubated with primary antibody against Galectin-3/Mac-2 (1:1,000) (Cedarlane, Burlington, Canada), followed by the anti-rat secondary antibody Alexa Fluor 594 conjugated (Thermo Fisher Scientific). Nuclei were stained with DAPI using ProLong Gold Antifade Mountant with DAPI. The images were acquired with Zeiss COLIBRI Fluorescence Microscope (Zeiss, Jena, Germany) for immunofluorescent images or EVOS XL Core (Thermo Fisher Scientific) for bright-field images.
The cross-sectional areas of cardiomyocytes and adipocytes were quantified using ImageJ software (National Institutes of Health, Bethesda, MD) as described previously (24). The proportion of Picro-Sirius Red staining or galectin-3 immunofluorescence positive area was quantified in four to eight randomly selected areas on the left ventricle per mouse using ImageJ software. Subsequently, the quantified values were averaged as the representative data of the individual mouse.
Blood and Medium Chemistry
Blood samples were collected from the decapitated trunks of mice, and then centrifuged at 6,000 g for 20 min. Serum samples were collected and stored at −80°C. Galectin-3 levels in serum and medium were measured by Mouse Galectin-3 DuoSet ELISA (R&D System). The measurement of other serum cytokine and chemokine levels was conducted by Eve Technologies (Calgary, Alberta, Canada). Blood glucose levels were measured with blood from the tail vein using an ACCU-CHEK Advantage Glucometer (Roche Diagnostics, Labal, Quebec, Canada) before euthanasia (6-h fasting) (7). Serum insulin levels were measured with the Mouse Insulin ELISA kit (ALPCO Diagnostic, Salem, NH) as previously described (7). Serum triglyceride and NEFA levels were determined by the LabAssay Triglyceride (Fujifilm Wako) and HR Series NEFA-HR kit (Fujifilm Wako), respectively. As an indicator of systemic insulin resistance, Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was calculated using the following equation:
Statistics
Prior to analysis, outliers were detected by the ROUTE method (Q = 1%). After removing statistically significant outliers, the distributions within a group and variance between groups were examined using Shapiro–Wilk normality test and Brown–Forsythe test, respectively. Results are expressed as the means ± standard deviation (SD) or median ± 95% confidence interval (CI) as indicated in each figure legend. Comparisons between three or more groups were performed using two-way ANOVA followed by Sidak’s post hoc multiple-comparisons test or Kruskal–Wallis test followed by Dunn’s multiple-comparisons test, appropriately. P < 0.05 was considered as the significant difference. GraphPad Prism (v 8.0, GraphPad Software, La Jolla, CA) was used for all statistical analyses.
RESULTS
Pharmacological Inhibition of ATGL Activity Ameliorates ISO-Induced Cardiac Hypertrophy and Fibrosis
To replicate the hyperactivation of the sympathetic nervous system that occurs in the pathogenesis of heart failure (2), we used ISO, a nonselective β-adrenergic agonist, which induces pathological changes in the heart, such as cardiac fibrosis (15, 25), and enhances adipose tissue lipolysis (26). For these experiments, ISO (30 μg/g/day) was administered subcutaneously with the osmotic pump to mice for 2 wk. To test the extent to which the inhibition of adipose ATGL activity alleviates adverse cardiac effects of sympathetic nervous system overstimulation, ISO was given to mice fed with the standard chow (standard group) or Atglistatin-containing food (Atglistatin group) (Fig. 1A). Of importance, Atglistatin is an effective agent that can inhibit adipose tissue and hepatic ATGL but not skeletal or cardiac muscle ATGL (12, 13) and thus avoids any direct deleterious cardiac effects of ATGL inhibition that we have shown previously (27).
Two weeks after continuous ISO administration, cardiac function and structure were determined by echocardiography (Supplemental Table S2). As expected (15), we observed an increase in left ventricular (LV) mass (Fig. 1B) characterized by an increase in cardiac remodeling parameters [such as interventricular septum thickness at end-diastole (IVSTd), left ventricular posterior wall thickness at end-diastole (LVPWTd), left ventricular internal diameter at diastole/systole (LVIDd and LVIDs)] and mild diastolic dysfunctions (decreased E/A and increased E/e′) (Supplemental Table S2) in the ISO-administered mice fed a standard chow diet. These changes were not accompanied by systolic dysfunction, as confirmed by the preservation of percent ejection fraction and stroke volume, demonstrating that this dose and duration of ISO induced only mild changes in cardiac structure and diastolic function (Supplemental Table S2). However, the administration of Atglistatin to these ISO-treated mice significantly suppressed the ISO-induced increase in wall thickness (IVSTd and LVPWTd; Supplemental Table S2) and LV mass (Fig. 1B), indicating that noncardiac ATGL inhibition can prevent cardiac pathological structural remodeling. In agreement with these observations, WGA staining revealed that Atglistatin abolished the ISO-mediated cardiomyocyte hypertrophy (Fig. 1, C and D). Furthermore, Atglistatin significantly reduced ISO-induced collagen deposition in the heart (Fig. 1, E and F) as well as mRNA expression of Postn (Fig. 1G), a hallmark of activated fibroblasts, or myofibroblasts (28). Altogether, these results suggest that Atglistatin treatment ameliorated ISO-induced cardiac hypertrophy and fibrosis independent of a direct cardiac inhibition of ATGL.
Pharmacological Inhibition of ATGL Reduces ISO-Induced Lipolysis and Adipocyte Size and Mitigates the Induction of Galectin-3 Positive Macrophages in ISO-Stimulated WAT
Because the degree of adiposity is correlated with poor prognosis in patients with heart failure (29) and the modulation of ATGL activity in adipose tissue influences adipocyte lipid accumulation (30), we investigated the extent to which the cardiac improvements mediated by Atglistatin were associated with the alteration in WAT histological features. Although ATGL inhibition would be expected to promote adipose tissue lipid droplet accumulation (30), instead Atglistatin dramatically decreased WAT mass and adipocyte size (Fig. 2, A–C), which is consistent with a previous report (13). Because ISO is well known to enhance lipolysis (22, 26), ISO stimulation also reduced WAT mass and adipocyte size, an effect that was further enhanced by Atglistatin (Fig. 2, A–C). Consistent with a previous report showing that the adipocyte size correlates with the circulating triglyceride levels (31), ISO administration also decreased serum triglyceride levels, which was further reduced by Atglistatin treatment (Supplemental Table S3). In addition, we confirmed that Atglistatin treatment suppressed ISO-induced lipolysis in excised WAT (Fig. 2D), although there was no significant change in the serum NEFA levels in fasted status between mice fed with standard chow and Atglistatin-containing food (Supplemental Table S3). Together, these data suggest that Atglistatin treatment reduced triglyceride storage in adipose tissue but did not decrease serum NEFA levels in vivo despite the inhibition of free fatty acid mobilization from WAT. We suspect that this may be due to the pharmacokinetic characteristics of Atglistatin, where the inhibitory effect of adipocyte ATGL diminishes over time (12).
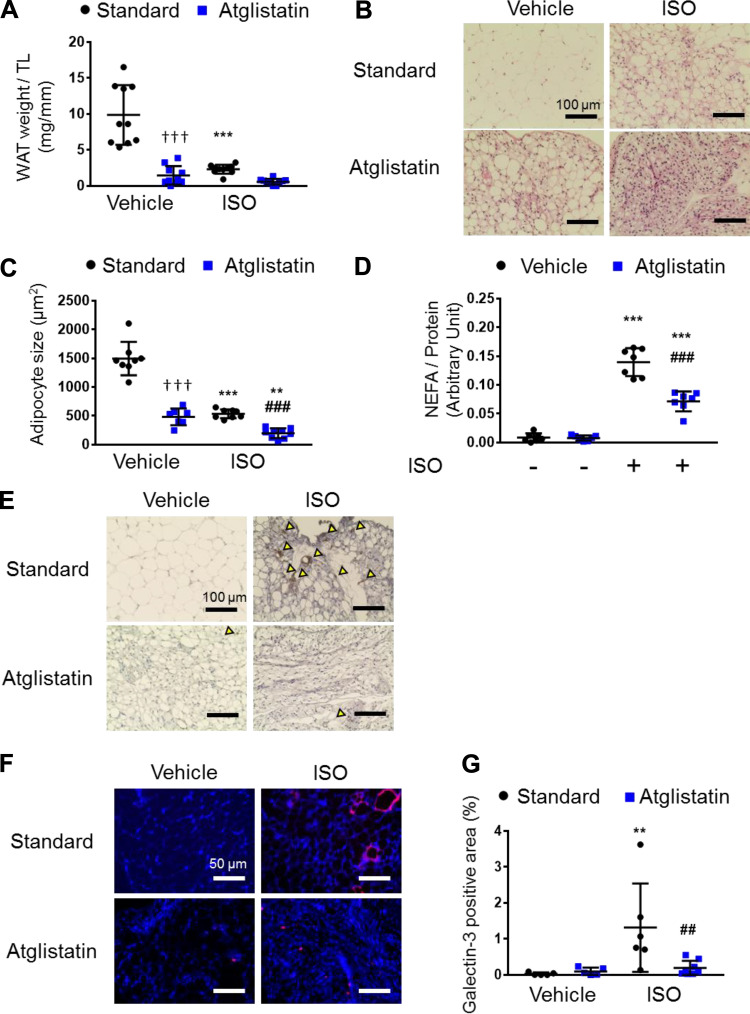
Figure 2.
Atglistatin suppresses isoproterenol (ISO)-induced macrophage infiltration and activation in white adipose tissue (WAT). A: gonadal WAT mass divided by tibial length (TL) (n = 10 per group). B: representative images of hematoxylin and eosin (H&E) staining of gonadal WAT. Scale bar represents 100 μm. C: surface area of adipocyte (n = 7–9 mice per group). D: ex vivo lipolysis by measuring nonesterified fatty acid (NEFA) with or without ISO (10 μM) and with or without Atglistatin (50 μM) (n = 7 per group). E: representative images of immunochemistry with F4/80. Yellow arrow heads indicate positive cells. Scale bar represents 100 μm. F: representative images of immunofluorescence against galectin-3 (red) and DAPI (blue) in the heart tissue. G: quantification of galectin-3-positive area (n = 5–8 mice per group). Scale bar represents 50 μm. Dots represent individual values. Data are presented as the means ± SD. P values were derived by two-way ANOVA followed by Sidak’s multiple-comparisons test as post hoc. *Comparison with its own vehicle group. †Comparison of standard chow with vehicle group and Atglistatin with vehicle group. #Comparison of standard chow with ISO group to Atglistatin with ISO group. *, †, #P < 0.05; **, ##P < 0.01; ***, †††, ###P < 0.001.
Because Atglistatin has been reported to reduce macrophage infiltration in adipose tissue (13), we investigated macrophage markers in WAT to determine whether the similar reduction of macrophage infiltration can be observed during ISO stimulation. Intriguingly, we found that there was less infiltration and activation of macrophages induced by ISO in the presence of Atglistatin, evidenced by reduced WAT expression of F4/80 (a general marker of mouse macrophages), and galectin-3 (a marker of activated macrophages) (32) (Fig. 2, E–G). Overall, we observed that Atglistatin suppressed WAT lipolysis, reduced adipocyte cell size, and prevented ISO-induced recruitment and activation of macrophages in WAT, indicating reduced WAT inflammation in Atglistatin-treated mice.
Pharmacological Inhibition of ATGL in Adipocytes Alleviates ISO-Induced Increase of Galectin-3 Levels in the Heart, Decreases Serum Levels of Galectin-3, and Improves Systemic Insulin Resistance
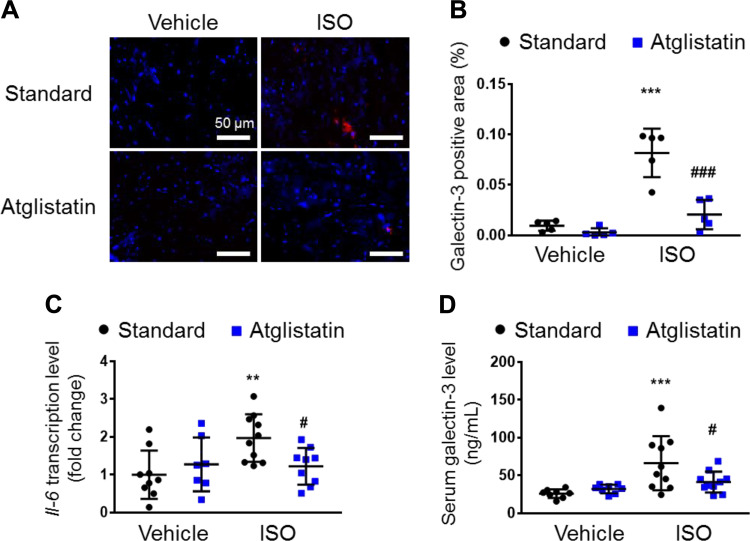
Galectin-3 is secreted from macrophages and plays a pivotal role in macrophage migration as well as activation in a positive feedback fashion (33–35). Moreover, increases in circulating galectin-3 induce cardiac inflammation (36). Therefore, we hypothesized that inflamed adipose tissue secretes galectin-3, which in turn further activates and recruits macrophages to the damaged heart, thereby worsening pathological cardiac remodeling. If this is the case, we would predict that ATGL inhibition might mitigate this pathological interaction between inflamed adipose tissue and the heart. To test this hypothesis, we examined galectin-3 and inflammatory markers in the heart and the circulation of mice fed with standard chow or Atglistatin-containing food. Of interest, immunostaining revealed that Atglistatin treatment significantly reduced the ISO-induced galectin-3 positive areas in the left ventricle (Fig. 3, A and B). Moreover, Atglistatin prevented ISO-induced cardiac mRNA expression of Il-6, a representative cardiac pro-inflammatory cytokine, which largely remained at normal levels in the presence of Atglistatin (Fig. 3C). Furthermore, Atglistatin significantly reduced ISO-induced serum galectin-3 levels (Fig. 3D), but not other cytokines and chemokines (Supplemental Fig. S1).
Figure 3.
Pharmacological inhibition of ATGL reduces isoproterenol-induced inflammation and galectin-3 levels in the heart. A: representative images of immunofluorescence against galectin-3 (red) and DAPI (blue) in the heart tissue. B: quantification of galectin-3-positive areas (n = 5 per group). Scale bar represents 50 μm. C: transcript levels of Il-6 in the heart, normalized to cyclophilin A (n = 7–10 mice per group). D: serum levels of galectin-3 in isoproterenol (ISO)-administered mice with or without Atglistatin treatment (n = 10 mice per group). Dots represent individual values. Data are presented as means ± SD. P values were derived by two-way ANOVA followed by Sidak’s multiple-comparisons test as post. *Comparison with its own vehicle group. #Comparison of standard chow with ISO group to Atglistatin with ISO group. #P < 0.05; **P < 0.01; ***, ###P < 0.001. ATGL, adipose triglyceride lipase.
In addition to the proinflammatory profile, galectin-3 causes cellular and systemic insulin resistance (37). Moreover, because Atglistatin is known to improve insulin sensitivity (13), we measured serum glucose and insulin levels after a 6-h fast and calculated HOMA-IR as an indicator of systemic insulin resistance (Supplemental Table S3). Of note, Atglistatin treatment trended to improve HOMA-IR independent from ISO administration compared with the controls (Supplemental Table S3). Taken together, these data indicate that pharmacological inhibition of ATGL in an noncardiac tissue ameliorates ISO-induced cardiac remodeling, which was associated with lower inflammation and circulating galactin-3 and improved systemic insulin resistance.
Genetic Ablation of Atgl in Adipocytes Phenocopies the Effect of Atglistatin on ISO-Induced Cardiac Remodeling
Because Atglistatin inhibits ATGL activity in the adipose tissue and the liver (12, 13), we wanted to confirm that the beneficial effects of Atglistatin on the heart were due to inhibition of adipocyte ATGL and not hepatic ATGL. To achieve this goal, we conducted similar experiments in mice with targeted genetic deletion of ATGL exclusively in mature adipocytes [adipocyte-specific Atgl knockout (atATGL-KO) mice] (Fig. 4A). After 2 wk of ISO administration, echocardiographic analysis showed that the LV mass that was increased by ISO was significantly lower in atATGL-KO mice compared with their littermates (Fig. 4B). In addition, atATGL-KO mice treated with ISO displayed better diastolic function, indicated by lower E/e′ than their littermates (Supplemental Table S4). Similar to the Atglistatin model, the atATGL-KO and control littermates did not develop systolic dysfunction (Supplemental Table S4). Subsequent histological analysis revealed that the genetic ablation of Atgl in adipocytes also prevented ISO-induced cardiomyocyte hypertrophy (Fig. 4, C and D). Moreover, hearts from atATGL-KO mice showed less collagen accumulation in response to ISO compared with control littermates’ hearts (Fig. 4, E and F). Furthermore, ISO-induced cardiac Postn mRNA expression that increased in control mice was abolished in the atATGL-KO mice (Fig. 4G). Taken together, these data show that the adipocyte-specific targeted deletion of Atgl specifically in adipocyte alleviated the cardiac remodeling induced by ISO in a similar manner as in mice treated with Atglistatin. These findings strongly suggest that the effects of Atglistatin are mediated by ATGL inhibition in adipocytes rather than liver.
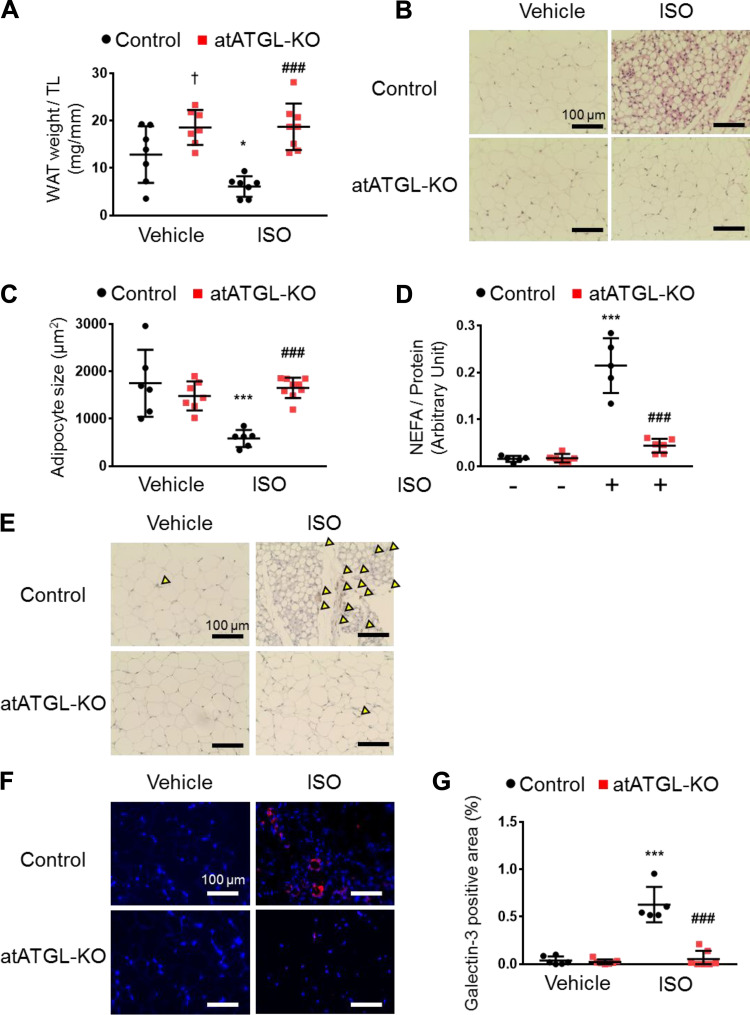
Ablation of Atgl in Adipocytes Reduces ISO-Induced Lipolysis without Altering Adipocyte Size and Mitigates the Induction of Galectin-3-Positive Macrophages in ISO-Stimulated WAT
Because Atglistatin treatment caused a significant histological change in adipocytes as well as mitigation of ISO-induced macrophage recruitment and activation in WAT, we examined those characteristics in the atATGL-KO mice. In contrast to Atglistatin treatment, fat mass and adipocyte size were not affected by the deletion of the Atgl gene in adipocytes in the control condition (Fig. 5, A–C). However, those parameters were reduced significantly after 2 wk of ISO administration in the control littermates but not in atATGL-KO mice (Fig. 5, A–C). Of note, adipocyte-specific Atgl ablation prevented ISO-induced cardiac diastolic dysfunction, yet the atATGL-KO mice had more fat mass than control littermates. This finding is in contrast to previous reports showing that accumulation of fat is a risk factor for the development of heart failure, especially heart failure with preserved ejection fraction (29, 38–40). In agreement with the histological findings, the serum triglyceride levels were reduced by ISO administration in control mice but were preserved in atATGL-KO mice (Supplemental Table S5). Similar to the Atglistatin model, genetic deletion of Atgl in the adipocyte effectively suppressed lipolysis induced by ISO in excised WAT (Fig. 5D), which was more profound than that observed with Atglistatin. Consistent with this, we observed less serum NEFA in atATGL-KO mice than in control mice in vivo (Supplemental Fig. S5). Nevertheless, we found that similar to pharmacological inhibition of ATGL, the deletion of Atgl in adipocytes prevented ISO-induced macrophage infiltration and activation in WAT, evidenced by reduced F4/80 and galectin-3 protein levels (Fig. 5, E–G). This observation suggests that the important factor in adipose inflammation is not the degree of lipid accumulation as previously advocated (10, 41, 42) but that enhanced lipolysis resulting from an ISO-induced increase in ATGL activity may be the main driver of inflammation. More importantly, these data indicate that inhibition of adipocyte ATGL reduces macrophage recruitment and activation and reduces galectin-3 secretion from adipose tissue.
Figure 5.
Genetic Atgl ablation in adipocytes reduces isoproterenol-induced galectin-3 levels in white adipose tissue. A: gonadal white adipose tissue (WAT) mass divided by tibial length (TL) (n = 6–8 mice per group). B: representative images of hematoxylin-eosin (H&E) staining of gonadal WAT. Scale bar represents 100 μm. C: surface area of adipocytes (n = 6–9 mice per group). D: ex vivo lipolysis of WAT by measuring nonesterified fatty acid (NEFA) from control and atATGL-KO mice with or without isoproterenol (ISO) (10 μM) (n = 5–6 per group). E: representative images of immunochemistry with F4/80. Yellow arrow heads indicate positive cells. Scale bar represents 100 μm. F: representative images of immunofluorescent against galectin-3 (red) and DAPI (blue) on gonadal WAT. G: quantification of galectin-3-positive area (n = 5–7 mice per group). Scale bar represents 100 μm. Dots represent individual values. Data are presented as means ± SD. P values were derived by two-way ANOVA followed by Sidak’s multiple-comparisons test as post hoc. *Comparison with its own vehicle group. †Comparison of control with vehicle group and atATGL-KO with vehicle group. #Comparison of control with ISO group to atATGL-KO with ISO group. *, †P < 0.05; ***, ###P < 0.001.
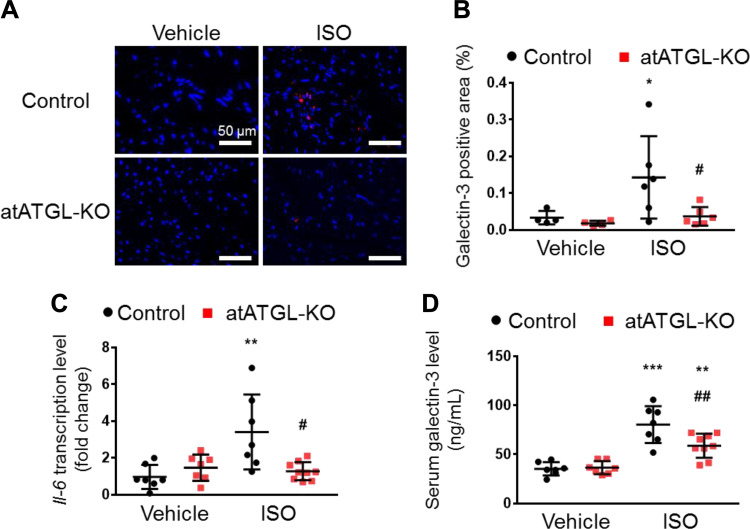
Genetic Deletion of Atgl in Adipocytes Blunts the Induction of Cardiac Inflammation and Galectin-3 Levels in the Heart and Circulation and Improves Insulin Resistance
To determine the extent to which the beneficial cardiac effects mediated by Atglistatin occur via the direct inhibition of ATGL activity in adipocytes, we examined inflammatory and macrophage markers in the heart and circulation of control and atATGL-KO mice treated with ISO. Of interest, immunofluorescence revealed that galectin-3 protein expression levels in hearts from atATGL-KO mice were significantly lower than those in hearts from control littermates following ISO delivery (Fig. 6, A and B). Furthermore, the deletion of adipocyte Atgl prevented the ISO-induced Il-6 mRNA expression in the heart compared to control littermates (Fig. 6C). Moreover, similar to what was observed with Atglistatin, the serum galectin-3 was consistently lower in atATGL-KO mice than in control littermates treated with ISO (Fig. 6D), without any change in other cytokine and chemokine levels (Supplemental Fig. S2).
Figure 6.
Genetic deletion of Atgl in adipocyte reduces isoproterenol-induced inflammation and galectin-3 levels in the heart. A: representative images of immunofluorescent against galectin-3 (red) and DAPI (blue) in the heart tissue. B: quantification of galectin-3-positive area (n = 4–6 per group). Scale bar represents 50 μm. C: transcript levels of Il-6 in the heart normalized to cyclophilin A (n = 7–9 per group). D: serum levels of galectin-3 in isoproterenol (ISO)-administered control and atATGL-KO mice (n = 7–9 mice per group). Dots represent individual values. Data are presented as the means ± SD. P values were derived by two-way ANOVA followed by Sidak’s multiple-comparisons test as post hoc. *Comparison with its own vehicle group. #Comparison of control with ISO group to atATGL-KO with ISO group. *, #P < 0.05; **, ##P < 0.01; ***P < 0.001.
We further examined glucose and insulin levels and calculated the HOMA-IR of control and atATGL-KO mice with or without ISO administration in the same manner as we examined those of Atglistatin-treated mice (Supplemental Table S5). As expected (7, 8, 14), the genetic deletion of adipocyte Atgl significantly reduced circulating insulin levels and improved HOMA-IR compared with the controls regardless of the presence of ISO administration (Supplemental Table S5). Taken together, these observations in the atATGL-KO mice confirm that the amelioration of the ISO-induced adverse cardiac effects with Atglistatin originated from the ATGL inhibition in adipocytes and possibly via reduced production of galectin-3 in adipose tissue and improved systemic insulin resistance.
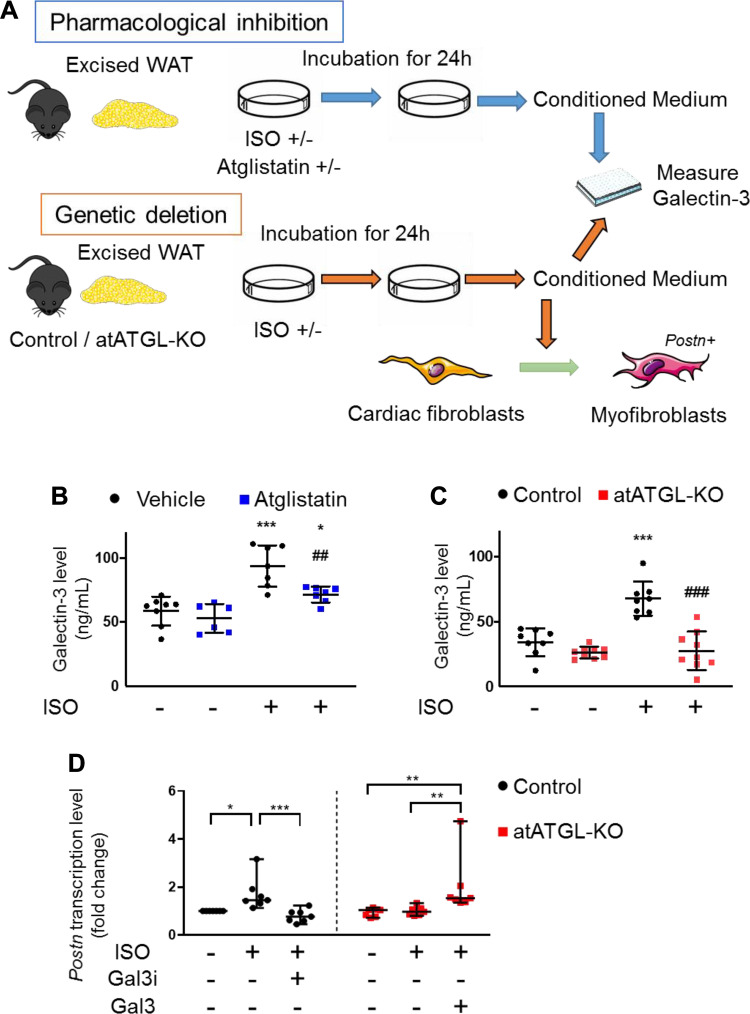
ATGL Inhibition Reduces ISO-Induced Galectin-3 Secretion from Excised WAT
Our data demonstrated a relationship between ATGL inhibition in adipocytes and lower circulating galectin-3 levels. Based on these data, we surmised that the secretion of galectin-3 from ISO-stimulated adipocytes in WAT, could be modulated by inhibition of ATGL activity, resulting in the reduced infiltration of galectin-3 positive macrophages in the heart. To test this hypothesis, excised WAT was incubated with ISO in the presence or absence of Atglistatin (Fig. 7A). Similarly, excised WAT from atATGL-KO or control littermate mice was incubated with ISO (Fig. 7A). In both sets of experiments, galectin-3 protein in the media was measured following ISO stimulation. As expected, the ISO-stimulated galectin-3 secretion from WAT was suppressed by Atglistatin and genetic ablation of Atgl (Fig. 7, B and C). Together, these data indicate that inhibition of ATGL activity in WAT reduces ISO-induced secretion of galectin-3, suggesting that WAT-derived galectin-3 may contribute to structural cardiac remodeling induced by adrenergic drive.
Figure 7.
Adipocyte ATGL inhibition reduces isoproterenol-induced galectin-3 secretion from excised white adipose tissue. A: schematics of ex vivo experiment of ISO stimulation on excised white adipose tissue (WAT). B: galectin-3 levels in the media from the pharmacological inhibition model (n = 6–7 per group). C: galectin-3 levels in the media from the genetic deletion model (n = 8–9 mice per group). D: transcript levels of Postn, normalized to ribosomal protein L32, in cardiac fibroblasts treated with the conditioned media of WAT from control and atATGL-KO mice in the presence or absence of isoproterenol (ISO), N-acetyl-d-lactosamine (Gal3i) (10 μM) or the recombinant murine galectin-3 (Gal3) (60 ng/mL) (n = 7 per group). Dots represent individual values. Data are presented as the means ± SD in B and C the median ± 95% CI in D. P values were derived by two-way ANOVA followed by Sidak’s multiple-comparisons test in (B) and (C) and Kruskal–Wallis test followed by Dunn’s multiple-comparisons test in (D). *Comparison with its own vehicle group. #Comparison of control with ISO group to atATGL-KO with ISO group in (B) and (C). *P < 0.05; **, ##P < 0.01; ***, ###P < 0.001. ATGL, adipose triglyceride lipase. Figure 7A was created with art materials provided by Servier Medical Art, licensed under a Creative Common Attribution 3.0 Generic License. http://smart.servier.com/
Galectin-3 Secreted by ISO-Stimulated White Adipose Tissue Mediates the Activation of Cardiac Fibroblasts
To test whether the increased secretion of galectin-3 from ISO-stimulated WAT causes cardiac remodeling, we treated primary cardiac fibroblasts with the conditioned media from WAT isolated from control or atATGL-KO mice in the presence or absence of ISO (Fig. 7A). Of note, conditioned media taken from ISO-treated WAT originating from control mice increased the transcription levels of Postn in cardiac fibroblasts, whereas conditioned media taken from ISO-treated WAT isolated from atATGL-KO mice did not activate cardiac fibroblasts (Fig. 7D). These findings suggested that ISO was not able to induce WAT isolated from atATGL-KO to secrete a factor that can cause activation of cardiac fibroblasts. To examine whether this activation of cardiac fibroblasts involves a galectin-3-dependent mechanism, we added N-acetyl-d-lactosamine, a galectin-3 inhibitor, to the conditioned media from ISO-treated WAT excised from control mice. Using this approach, we show that the pharmacological inhibition of galectin-3 in the conditioned media resulted in the loss of increased Postn transcription in cardiac fibroblasts (Fig. 7D). Furthermore, the addition of recombinant mouse galectin-3 to the conditioned media of ISO-stimulated WAT excised from atATGL-KO mice increased mRNA levels of Postn (Fig. 7D). Taken together, the data further support the notion that the inhibition or ablation of adipocyte ATGL prevents ISO-induced cardiac remodeling via a mechanism that likely involves decreasing the secretion of galectin-3 from adipose tissue.
DISCUSSION
Following cardiac injury, the sympathetic nervous system plays an important role in compensating for reduced cardiac function. However, the adrenergic hyperstimulation can also contribute to the worsening of cardiac pathologies (1). In addition, adrenergic hyperstimulation induced by cardiac injury promotes WAT lipolysis (3), adding an additional level of putative control mechanisms that contribute to worsening cardiac inflammation and remodeling.
We previously showed that modulation of ATGL, the rate-limiting enzyme in adipocyte lipolysis, ameliorates cardiac dysfunction in a pressure-overload murine model (7). However, the mechanism(s) that may contribute to this effect are largely unknown. In the present study, we show that inhibition of ATGL activity in adipose tissue can ameliorate cardiac damage induced by excessive catecholamine release, at least in part, by reducing galectin-3 secretion from WAT and preventing infiltration of cardiac tissue by activated macrophages. This finding is consistent with that of previous studies showing that inflammation in adipose tissue can alter the subsequent secretion of factors that can contribute to worsening of certain pathologies (13, 41, 43, 44).
There is a well-established positive correlation between visceral fat levels and heart failure (29), which suggests that the degree of adiposity may be responsible for the beneficial cardiac effects of ATGL inhibition. Intriguingly, the pharmacological and genetic modulation of ATGL had different effects on adipose tissue characteristics. Notably, in this study, Atglistatin treatment resulted in greater loss of fat mass than controls, whereas adipocyte-specific knockout of ATGL resulted in less loss of fat mass than controls following ISO stimulation. Consistent with the differences in body composition and histological features, pharmacological and genetic inhibition of adipocyte ATGL exhibited a somewhat different lipid profile. Specifically, Atglistatin reduced triglyceride levels but did not affect NEFA levels in serum compared with the standard chow, whereas atATGL-KO reduced circulating NEFA levels but preserved circulating triglyceride levels following ISO stimulation compared with the control mice. Although the reasons for this are currently unknown, this discrepancy probably originated from the difference in the way adipocyte ATGL was inhibited. For instance, although atATGL-KO constitutively inhibits the adipocyte lipolysis mediated by ATGL, the effect of Atglistatin diminishes over time (12, 13). Thus, this pharmacokinetic characteristic of Atglistatin likely caused the absence of reduction or variability of serum NEFA levels in Atglistatin-treated mice compared with standard chow-treated mice at 6-h after the removal of Atglistatin-containing food. Moreover, Atglistatin has been reported to reduce triglyceride storage in fat likely by suppression of fat absorption via the intestine and increasing energy expenditure (13), which might cause the observed reduction of adipocyte size and triglyceride levels in Atglistatin-treated mice. Thus, we speculate that the combination of these factors likely led to the different lipid profiles observed between the two models.
However, despite the differential response in the model in terms of serum NEFA and triglyceride levels, both pharmacological and genetic models of reduced ATGL action had common protective characteristics in response to adrenergic drive-induced inflammation and cardiac fibrosis. Thus, although we cannot rule out that changes in adiposity and systemic lipid profile play a role in this effect, what is consistent between the two models in this study is the lower recruitment and/or activation of macrophages in WAT in response to ATGL inhibition, evidenced by fewer F4/80 and galectin-3-positive cells. This finding is in agreement with the evidence demonstrating a clear relationship between enhanced ATGL-mediated WAT lipolysis and inflammation as evidenced by increased macrophage infiltration into adipose tissue when lipolysis has been stimulated (5, 14). In fact, macrophages are crucial components that contribute to adipose tissue inflammation and it has been demonstrated that the adipocytes and macrophages synergistically contribute to local adipose inflammation (45). As ATGL is a critical rate-limiting enzyme of lipolysis, it is reasonable that ATGL plays a crucial role in this relationship. Supporting this, it has been reported that the inhibition of ATGL with Atglistatin or genetic deletion of adipocyte Atgl reduced diet- and ISO-induced upregulation of transcript levels of macrophage markers in adipose tissue (13, 14, 46). The data presented herein add to the existing literature and show that inhibition of ATGL or deletion of Atgl in adipocytes significantly reduced lipolysis ex vivo in response to the nonselective β-adrenergic agonist and that this reduced WAT inflammation and galectin-3 secretion can prevent cardiac inflammation and remodeling.
Previous work has shown that systemic insulin resistance is associated with cardiac pathophysiology (47). In addition, the inhibition of adipocyte ATGL, either pharmacologically or genetically, improves glucose homeostasis and insulin resistance (7, 8, 13). In agreement with these findings, we found that both pharmacological and genetic inhibition of adipocyte ATGL improved insulin resistance, which may contribute to amelioration of ISO-induced cardiac remodeling. However, although ISO is known to induce systemic insulin resistance at least in a short-term (48, 49), we observed reduced HOMA-IR by chronic ISO administration in standard chow-fed mice likely due to less insulin levels. Our observation is consistent with a study using chronic ISO infusion in rats, which showed improved insulin sensitivity and glucose clearance likely as a result of β-adrenergic desensitization (50). Given the fact that mice fed with standard chow had improved insulin resistance following ISO administration compared with vehicle-treated mice but developed adverse cardiac remodeling upon ISO administration, the contribution of insulin resistance to the improvement of cardiac remodeling is likely not responsible for the cardiac effects observed in our models.
Galectin-3 belongs to a family of mammalian β-galactoside-binding proteins and has been identified as a macrophage subpopulation marker (32). Although galectin-3 is expressed in small amounts in various cell types, it is most highly expressed in macrophages activated by tissue injury (35, 51, 52). This protein can be found in the cytosol, plasma membrane, and interstitial fluids (32, 53). Recently, it has also been revealed that circulating galectin-3 correlates with the prognosis of the patients with heart failure (54–56). Furthermore, emerging evidence indicates that galectin-3 plays a vital role in the development of inflammation and fibrosis in various organs including the heart (35, 52, 57–61). Thus, there are several ongoing clinical trials designed to test if galectin-3 inhibitors can be used as a treatment for many diseases, including malignancies and fibrotic disorders in various organs (such as ClinicalTrial.gov Identifier: NCT02117362, NCT02575404, and NCT03832946). Of relevance to the current study, Vergaro et al. (52) reported that the inhibition of galectin-3 pathway mitigates ISO-induced cardiac hypertrophy and fibrosis and reduces infiltration of macrophages in the heart in a rodent model. Consistent with this notion, we found reduction of galectin-3 levels in the heart as well as adipose tissue and serum in both Atglistatin-treated mice and atATGL-KO mice following ISO stimulation compared with control mice. Furthermore, we demonstrated that the conditioned media of ISO-stimulated WAT from the control mice activated the primary cardiac fibroblasts and that deletion of adipocyte Atgl abolished the effect in a galectin-3-dependent fashion. To support our finding, a recent study with the acute kidney injury model clearly demonstrated that a remote organ injury can induce cardiac inflammation and fibrosis in galectin-3-dependent fashion (62). These findings suggest that the beneficial cardiac effects with Atglistatin and adipocyte-specific ATGL deletion are influenced by reduced galectin-3 secretion from adipose tissue.
In summary, we observed that pharmacological and genetic inhibition of ATGL activity in adipocytes ameliorated ISO-induced cardiac inflammation and cardiac remodeling. This beneficial effect is likely via suppression of galectin-3 secretion by adipose tissue macrophages due to reduced ISO-stimulated adipocyte lipolysis. These data suggest that inhibition of ATGL-mediated adipocyte lipolysis or the associated macrophage-mediated secretion of galactin-3 may have therapeutic benefit in heart failure and other cardiac pathologies associated with excess sympathetic activity.
GRANTS
This work was supported by grants from Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada (to J.R.B.D). J.R.B.D. is a Canada Research Chair in Molecular Medicine. S.T. was supported by a postdoctoral fellowship from Canadian Institutes of Health Research, from Japan Society for the Promotion of Science Overseas Challenge Program for Young Researchers and Tohoku Kaihatsu Memorial Foundation. Z.H.M. is the recipient of the Alberta Innovates Health Solutions and the Canadian Institutes of Health Research postdoctoral fellowship awards. S.S. was supported by a graduate studentship from Alberta Diabetes Institute. atATGL-KO mouse model was generated with support to E.E.K. by National Institutes of Health Grant R01 DK090166.
DISCLOSURE
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.T. and J.R.B.D. conceived and designed research; S.T., N.S., and A.K.M. performed experiments; S.T., M.F., and N.S. analyzed data; S.T., M.F., Z.H.M., and J.R.B.D. interpreted results of experiments; S.T. and M.F. prepared figures; S.T. and M.F. drafted manuscript; S.T., M.F., Z.H.M., S.S., R.B., E.E.K., and J.R.B.D. edited and revised manuscript; S.T., M.F., N.S., Z.H.M., S.S., A.K.M., R.B., E.E.K., and J.R.B.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the technical assistance of Amy Barr and Joy Paramor from the University of Alberta.
REFERENCES
- 1.Jackson G, Gibbs CR, Davies MK, Lip GY. ABC of heart failure. Pathophysiology. BMJ 320: 167–170, 2000. doi: 10.1136/bmj.320.7228.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res 113: 739–753, 2013. [Erratum in Circ Res 2016 Aug 5; 119(4):e38]. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bezaire V, Langin D. Regulation of adipose tissue lipolysis revisited. Proc Nutr Soc 68: 350–360, 2009. doi: 10.1017/S0029665109990279. [DOI] [PubMed] [Google Scholar]
- 4.Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis—a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res 50: 14–27, 2011. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimizu I, Yoshida Y, Katsuno T, Tateno K, Okada S, Moriya J, Yokoyama M, Nojima A, Ito T, Zechner R, Komuro I, Kobayashi Y, Minamino T. p53-induced adipose tissue inflammation is critically involved in the development of insulin resistance in heart failure. Cell Metab 15: 51–64, 2012. [Erratum in Cell Metab 2012 May 2; 15(5):787]. doi: 10.1016/j.cmet.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Jiang B, Qiao J, Yang Y, Lu Y. Inhibitory effect of paeoniflorin on the inflammatory vicious cycle between adipocytes and macrophages. J Cell Biochem 113: 2560–2566, 2012. doi: 10.1002/jcb.22173. [DOI] [PubMed] [Google Scholar]
- 7.Parajuli N, Takahara S, Matsumura N, Kim TT, Ferdaoussi M, Migglautsch AK, Zechner R, Breinbauer R, Kershaw EE, Dyck JR. Atglistatin ameliorates functional decline in heart failure via adipocyte-specific inhibition of adipose triglyceride lipase. Am J Physiol Heart Circ Physiol 315: H879–H884, 2018. doi: 10.1152/ajpheart.00308.2018. [DOI] [PubMed] [Google Scholar]
- 8.Salatzki J, Foryst-Ludwig A, Bentele K, Blumrich A, Smeir E, Ban Z, Brix S, Grune J, Beyhoff N, Klopfleisch R, Dunst S, Surma MA, Klose C, Rothe M, Heinzel FR, Krannich A, Kershaw EE, Beule D, Schulze PC, Marx N, Kintscher U. Adipose tissue ATGL modifies the cardiac lipidome in pressure-overload-induced left ventricular failure. PLoS Genet 14: e1007171, 2018. doi: 10.1371/journal.pgen.1007171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res 96: 939–949, 2005. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJ, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PR, Troquay RP, Libby P, Glynn RJ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377: 1119–1131, 2017. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 12.Mayer N, Schweiger M, Romauch M, Grabner GF, Eichmann TO, Fuchs E, Ivkovic J, Heier C, Mrak I, Lass A, Hofler G, Fledelius C, Zechner R, Zimmermann R, Breinbauer R. Development of small-molecule inhibitors targeting adipose triglyceride lipase. Nat Chem Biol 9: 785–787, 2013. doi: 10.1038/nchembio.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schweiger M, Romauch M, Schreiber R, Grabner GF, Hutter S, Kotzbeck P, Benedikt P, Eichmann TO, Yamada S, Knittelfelder O, Diwoky C, Doler C, Mayer N, De Cecco W, Breinbauer R, Zimmermann R, Zechner R. Pharmacological inhibition of adipose triglyceride lipase corrects high-fat diet-induced insulin resistance and hepatosteatosis in mice. Nat Commun 8: 14859, 2017. [Erratum in Nat Commun 2017 Apr 25; 8:15490]. doi: 10.1038/ncomms14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoiswohl G, Stefanovic-Racic M, Menke MN, Wills RC, Surlow BA, Basantani MK, Sitnick MT, Cai L, Yazbeck CF, Stolz DB, Pulinilkunnil T, O'Doherty RM, Kershaw EE. Impact of reduced ATGL-mediated adipocyte lipolysis on obesity-associated insulin resistance and inflammation in male mice. Endocrinology 156: 3610–3624, 2015. doi: 10.1210/en.2015-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang SC, Ren S, Rau CD, Wang JJ. Isoproterenol-induced heart failure mouse model using osmotic pump implantation. Methods Mol Biol 1816: 207–220, 2018. doi: 10.1007/978-1-4939-8597-5_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwamiya T, Matsuura K, Masuda S, Shimizu T, Okano T. Cardiac fibroblast-derived VCAM-1 enhances cardiomyocyte proliferation for fabrication of bioengineered cardiac tissue. Regen Ther 4: 92–102, 2016. doi: 10.1016/j.reth.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuura K, Masuda S, Haraguchi Y, Yasuda N, Shimizu T, Hagiwara N, Zandstra PW, Okano T. Creation of mouse embryonic stem cell-derived cardiac cell sheets. Biomaterials 32: 7355–7362, 2011. doi: 10.1016/j.biomaterials.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 18.Simpson P, Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circ Res 50: 101–116, 1982. doi: 10.1161/01.RES.50.1.101. [DOI] [PubMed] [Google Scholar]
- 19.Hyde A, Blondel B, Matter A, Cheneval JP, Filloux B, Girardier L. Homo- and heterocellular junctions in cell cultures: an electrophysiological and morphological study. Pro Brain Res 31: 283–311, 1969. doi: 10.1016/S0079-6123(08)63247-1. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. The international pharmacopoeia. World Health Organization, 2016, 6th ed. https://apps.who.int/phint/2016/index.html#d/b.1. [Google Scholar]
- 21.de Freitas Souza BS, Silva DN, Carvalho RH, de Almeida Sampaio GL, Paredes BD, Franca LA, Azevedo CM, Vasconcelos JF, Meira CS, Neto PC, Macambira SG, da Silva KN, Allahdadi KJ, Tavora F, de Souza Neto JD, Dos Santos RR, Soares MB. Association of cardiac galectin-3 expression, myocarditis, and fibrosis in chronic Chagas disease cardiomyopathy. Am J Pathol 187: 1134–1146, 2017. doi: 10.1016/j.ajpath.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Schweiger M, Eichmann TO, Taschler U, Zimmermann R, Zechner R, Lass A. Measurement of lipolysis. Methods Enzymol 538: 171–193, 2014. doi: 10.1016/B978-0-12-800280-3.00010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byrne NJ, Matsumura N, Maayah ZH, Ferdaoussi M, Takahara S, Darwesh AM, Levasseur JL, Jahng JW, Vos D, Parajuli N, El-Kadi AO, Braam B, Young ME, Verma S, Light PE, Sweeney G, Seubert JM, Dyck JR. Empagliflozin blunts worsening cardiac dysfunction associated with reduced NLRP3 (Nucleotide-Binding Domain-Like Receptor Protein 3) inflammasome activation in heart failure. Circ Heart Fail 13: e006277, 2020. doi: 10.1161/CIRCHEARTFAILURE.119.006277. [DOI] [PubMed] [Google Scholar]
- 24.Takahara S, Inoue SI, Miyagawa-Tomita S, Matsuura K, Nakashima Y, Niihori T, Matsubara Y, Saiki Y, Aoki Y. New Noonan syndrome model mice with RIT1 mutation exhibit cardiac hypertrophy and susceptibility to beta-adrenergic stimulation-induced cardiac fibrosis. EBioMed 42: 43–53, 2019. doi: 10.1016/j.ebiom.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma X, Song Y, Chen C, Fu Y, Shen Q, Li Z, Zhang Y. Distinct actions of intermittent and sustained beta-adrenoceptor stimulation on cardiac remodeling. Sci China Life Sci 54: 493–501, 2011. doi: 10.1007/s11427-011-4183-9. [DOI] [PubMed] [Google Scholar]
- 26.Morimoto C, Tsujita T, Sumida M, Okuda H. Substrate-dependent lipolysis induced by isoproterenol. Biochem Biophys Res Commun 274: 631–634, 2000. doi: 10.1006/bbrc.2000.3190. [DOI] [PubMed] [Google Scholar]
- 27.Kienesberger PC, Pulinilkunnil T, Nagendran J, Young ME, Bogner-Strauss JG, Hackl H, Khadour R, Heydari E, Haemmerle G, Zechner R, Kershaw EE, Dyck JR. Early structural and metabolic cardiac remodelling in response to inducible adipose triglyceride lipase ablation. Cardiovasc Res 99: 442–451, 2013. doi: 10.1093/cvr/cvt124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res 101: 313–321, 2007. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amato MC, Giordano C. Visceral adiposity index: an indicator of adipose tissue dysfunction. Int J Endocrinol 2014: 730827, 2014. doi: 10.1155/2014/730827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science (New York, NY) 312: 734–737, 2006. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 31.Ryden M, Andersson DP, Bergstrom IB, Arner P. Adipose tissue and metabolic alterations: regional differences in fat cell size and number matter, but differently: a cross-sectional study. J Clin Endocrinol Metab 99: E1870–1876, 2014. doi: 10.1210/jc.2014-1526. [DOI] [PubMed] [Google Scholar]
- 32.Ho MK, Springer TA. Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J Immunol 128: 1221–1228, 1982. [PubMed] [Google Scholar]
- 33.Danella Polli C, Alves Toledo K, Franco LH, Sammartino Mariano V, de Oliveira LL, Soares Bernardes E, Roque-Barreira MC, Pereira-da-Silva G. Monocyte migration driven by galectin-3 occurs through distinct mechanisms involving selective interactions with the extracellular matrix. ISRN Inflamm 2013: 1–9, 2013. doi: 10.1155/2013/259256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong R, Zhang M, Hu Q, Zheng S, Soh A, Zheng Y, Yuan H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int J Mol Med 41: 599–614, 2017. doi: 10.3892/ijmm.2017.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, Andre S, Crijns HJ, Gabius HJ, Maessen J, Pinto YM. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 110: 3121–3128, 2004. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 36.Besler C, Lang D, Urban D, Rommel K-P, von Roeder M, Fengler K, Blazek S, Kandolf R, Klingel K, Thiele H, Linke A, Schuler G, Adams V, Lurz P. Plasma and cardiac galectin-3 in patients with heart failure reflects both inflammation and fibrosis: implications for its use as a biomarker. Circ Heart Fail 10: e003804, 2017. doi: 10.1161/CIRCHEARTFAILURE.116.003804. [DOI] [PubMed] [Google Scholar]
- 37.Li P, Liu S, Lu M, Bandyopadhyay G, Oh D, Imamura T, Johnson AM, Sears D, Shen Z, Cui B, Kong L, Hou S, Liang X, Iovino S, Watkins SM, Ying W, Osborn O, Wollam J, Brenner M, Olefsky JM. Hematopoietic-derived galectin-3 causes cellular and systemic insulin resistance. Cell 167: 973–984e912, 2016. doi: 10.1016/j.cell.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eaton CB, Pettinger M, Rossouw J, Martin LW, Foraker R, Quddus A, Liu S, Wampler NS, Hank Wu WC, Manson JE, Margolis K, Johnson KC, Allison M, Corbie-Smith G, Rosamond W, Breathett K, Klein L. Risk factors for incident hospitalized heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women. Circ Heart Fail 9: e002883, 2016. doi: 10.1161/CIRCHEARTFAILURE.115.002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG, Levy D. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail 6: 279–286, 2013. doi: 10.1161/CIRCHEARTFAILURE.112.972828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao VN, Zhao D, Allison MA, Guallar E, Sharma K, Criqui MH, Cushman M, Blumenthal RS, Michos ED. Adiposity and incident heart failure and its subtypes: MESA (Multi-Ethnic Study of Atherosclerosis). JACC Heart Fail 6: 999–1007, 2018. doi: 10.1016/j.jchf.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lau WB, Ohashi K, Wang Y, Ogawa H, Murohara T, Ma X-L, Ouchi N. Role of adipokines in cardiovascular disease. Circ J 81: 920–928, 2017. doi: 10.1253/circj.CJ-17-0458. [DOI] [PubMed] [Google Scholar]
- 42.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr.. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med 7: 22, 2020. doi: 10.3389/fcvm.2020.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimizu I, Yoshida Y, Katsuno T, Minamino T. Adipose tissue inflammation in diabetes and heart failure. Microbes Infect 15: 11–17, 2013. doi: 10.1016/j.micinf.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Berg AH, Lin Y, Lisanti MP, Scherer PE. Adipocyte differentiation induces dynamic changes in NF-kappaB expression and activity. Am J Physiol Endocrinol Metab 287: E1178–1188, 2004. doi: 10.1152/ajpendo.00002.2004. [DOI] [PubMed] [Google Scholar]
- 46.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW, Jr, Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest 120: 3466–3479, 2010. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riehle C, Abel ED. Insulin signaling and heart failure. Circ Res 118: 1151–1169, 2016. doi: 10.1161/CIRCRESAHA.116.306206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoff R, Koh CK. Isoproterenol induced insulin resistance leading to diabetic ketoacidosis in Type 1 diabetes mellitus. Case Rep Endocrinol 2018: 1–3, 2018. doi: 10.1155/2018/4328954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J, Hodel A, Holman GD. Insulin and isoproterenol have opposing roles in the maintenance of cytosol pH and optimal fusion of GLUT4 vesicles with the plasma membrane. J Biol Chem 277: 6559–6566, 2002. doi: 10.1074/jbc.M108610200. [DOI] [PubMed] [Google Scholar]
- 50.Rousseau-Migneron S, Nadeau A, Tancrede G. Effects of isoproterenol on insulin and glucagon secretion in rats treated chronically with isoproterenol. Can J Physiol Pharmacol 58: 275–280, 1980. doi: 10.1139/y80-047. [DOI] [PubMed] [Google Scholar]
- 51.Sciacchitano S, Lavra L, Morgante A, Ulivieri A, Magi FD, Francesco GP, Bellotti C, Salehi LB, Ricci A. . Galectin-3: one molecule for an alphabet of diseases, from A to Z. Int J Mol Sci 19: 379, 2018. doi: 10.3390/ijms19020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vergaro G, Prud’homme M, Fazal L, Merval R, Passino C, Emdin M, Samuel J-L, Cohen Solal A, Delcayre C. Inhibition of galectin-3 pathway prevents isoproterenol-induced left ventricular dysfunction and fibrosis in mice. Hypertension 67: 606–612, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06161. [DOI] [PubMed] [Google Scholar]
- 53.Newlaczyl AU, Yu LG. Galectin-3—a jack-of-all-trades in cancer. Cancer Lett 313: 123–128, 2011. doi: 10.1016/j.canlet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen MN, Su Y, Vizi D, Fang L, Ellims AH, Zhao WB, Kiriazis H, Gao XM, Sadoshima J, Taylor AJ, McMullen JR, Dart AM, Kaye DM, Du XJ. Mechanisms responsible for increased circulating levels of galectin-3 in cardiomyopathy and heart failure. Sci Rep 8: 8213, 2018. doi: 10.1038/s41598-018-26115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128: 1810–1852, 2013. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 56.Wu CK, Su MY, Lee JK, Chiang FT, Hwang JJ, Lin JL, Chen JJ, Liu FT, Tsai CT. Galectin-3 level and the severity of cardiac diastolic dysfunction using cellular and animal models and clinical indices. Sci Rep 5: 17007, 2015. doi: 10.1038/srep17007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Boer RA, Yu L, van Veldhuisen DJ. Galectin-3 in cardiac remodeling and heart failure. Curr Heart Fail Rep 7: 1–8, 2010. [Erratum in Curr Heart Fail Rep. 2012 Sep; 9(3):163]. doi: 10.1007/s11897-010-0004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Desmedt V, Desmedt S, Delanghe JR, Speeckaert R, Speeckaert MM. Galectin-3 in renal pathology: more than just an innocent bystander. Am J Nephrol 43: 305–317, 2016. doi: 10.1159/000446376. [DOI] [PubMed] [Google Scholar]
- 59.Frunza O, Russo I, Saxena A, Shinde AV, Humeres C, Hanif W, Rai V, Su Y, Frangogiannis NG. Myocardial galectin-3 expression is associated with remodeling of the pressure-overloaded heart and may delay the hypertrophic response without affecting survival, dysfunction, and cardiac fibrosis. Am J Pathol 186: 1114–1127, 2016. doi: 10.1016/j.ajpath.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, Sethi T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci USA 103: 5060–5065, 2006. doi: 10.1073/pnas.0511167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu L, Ruifrok WP, Meissner M, Bos EM, van Goor H, Sanjabi B, van der Harst P, Pitt B, Goldstein IJ, Koerts JA, van Veldhuisen DJ, Bank RA, van Gilst WH, Sillje HH, de Boer RA. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail 6: 107–117, 2013. doi: 10.1161/CIRCHEARTFAILURE.112.971168. [DOI] [PubMed] [Google Scholar]
- 62.Prud'homme M, Coutrot M, Michel T, Boutin L, Genest M, Poirier F, Launay JM, Kane B, Kinugasa S, Prakoura N, Vandermeersch S, Cohen-Solal A, Delcayre C, Samuel JL, Mehta R, Gayat E, Mebazaa A, Chadjichristos CE, Legrand M. Acute kidney injury induces remote cardiac damage and dysfunction through the galectin-3 pathway. JACC Basic Transl Sci 4: 717–732, 2019. doi: 10.1016/j.jacbts.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]