Abstract
Aging is associated with heart and vascular dysfunction that contributes to cardiovascular disease (CVD) risk. Clinical data support a sexual dimorphism in the time course of aging-associated CVD. However, the mechanisms driving sex differences in cardiovascular aging and whether they can be modeled in mice have not been explored. Mineralocorticoid receptors (MRs) regulate blood pressure, and we previously demonstrated in male mice that MR expression increases in aging mouse vessels and smooth muscle cell-specific MR deletion (SMC-MR-KO) protects from cardiovascular aging. This study characterizes sex differences in murine cardiovascular aging and the associated sex-specific role of SMC-MR. Aortic stiffness, measured by pulse wave velocity, increased from 3 to 12 mo of age in males but not until 18 mo in females. The timing of the rise in aortic stiffening correlated with the timing of increased aortic MR expression, and aortic stiffness did not increase with age in SMC-MR-KO mice of both sexes. Vascular fibrosis increased at 12 mo in males and later at 18 mo in females; however, fibrosis was attenuated by SMC-MR-KO in males only. In resistance vessels, angiotensin type 1 receptor (AT1R)-mediated vasoconstriction also increased at 12 mo in males and 18 mo in females. ANG II-induced vasoconstriction was decreased in SMC-MR-KO specifically in males in association with decreased AT1R expression. Cardiac systolic function declined in males and females by 18 mo of age, which was prevented by SMC-MR-KO specifically in females. Cardiac perivascular fibrosis increased with age in both sexes accompanied by sex-specific changes in the expression levels of MR-regulated profibrotic genes.
NEW & NOTEWORTHY These data demonstrate that the delayed and steeper decline in cardiovascular function observed in aging females can be modeled in aging mice. Moreover, the mechanisms driving vascular and cardiac aging phenotypes are distinct between males and females. Mineralocorticoid receptors in smooth muscle cells play a significant role in cardiovascular aging in both sexes; however, they do so by distinct mechanisms. Overall, these findings suggest that sex-specific therapies may be necessary to retard the aging process and improve cardiovascular disease outcomes in the aging population.
Keywords: cardiovascular disease, mineralocorticoid receptor, sex differences, vascular aging
INTRODUCTION
Aging is a universal, potent, and currently unmodifiable risk factor for the development of cardiovascular diseases (CVD) in both males and females. Cardiovascular aging is characterized by progressive alterations in vascular and cardiac structure and function that contribute to development of hypertension, coronary artery disease, congestive heart failure, and stroke. Hallmarks of cardiovascular aging include enhanced stiffening and constriction of the arteries and fibrosis and dysfunction of the heart. Although it has been well established in humans that young females are protected from CVD relative to age-matched males, CVD development (i.e., hypertension, heart failure, arterial stiffness) occurs more rapidly in females after menopause such that the risk in older women eventually equals and for some conditions ultimately exceeds that of aging men (1–3). Additional clinical data support that women experience more age-related cardiac remodeling (4, 5) than men, leading to high rates of heart failure with preserved ejection fraction in elderly women (6–8). Furthermore, arterial stiffness (measured by pulse wave velocity) develops later but accelerates faster in women and is tightly associated with risk of heart attack and stroke in both sexes. Specifically, women develop greater aging-associated increases in pulse pressure and wave reflection (measures of vascular stiffness) compared with aging men, which is not explained by intrinsic size differences between men and women (9–11). The accelerated rate of development of arterial stiffness in women may be, in part, due to the removal of estrogen upon menopause, as menopausal status is an independent factor associated with aging-associated arterial stiffness in females (12). Clinical evidence also suggests that enhanced arterial stiffness may be an underlying cause of isolated systolic hypertension (ISH), which is very common in aging women (13, 14). Given that women are also less likely to achieve optimal blood pressure control with current antihypertensive therapeutics (15), these clinical data suggest that sexually dimorphic mechanisms may contribute to age-associated arterial dysfunction and that the determination of these mechanisms may aid in identifying sex-specific therapies to reduce CVD burden in aging women and men. Additional hallmarks of cardiovascular aging are the development of left ventricular (LV) wall thickening and impairments in systolic and diastolic function (16). There are also distinct sex differences observed in cardiac aging; elderly women experience more concentric remodeling of the LV (17) with greater incidence of diastolic dysfunction, ultimately thought to contribute to the higher incidence of heart failure with preserved ejection fraction seen in aging women (18, 19). However, the molecular mechanisms that contribute to the differential manifestations of cardiovascular aging in males and females are currently incompletely understood (11, 16, 20–22).
The majority of preclinical studies exploring mechanisms of cardiovascular aging have been performed in male animals only (23–29), and while clinical data clearly support sex differences in cardiovascular aging, the molecular mechanisms driving these sex differences are poorly understood (16). Indeed, despite the known sex differences in humans, the time course of cardiovascular aging has never been directly compared between male and female mice, and very little is known about the mechanisms of vascular aging in female preclinical models. The identification of mechanisms responsible for the differential CVD development between males and females with aging has the potential to lead to sex-specific therapeutic strategies to prevent or manage CVD, the leading cause of death and disability in the aging population.
We previously established a role for the mineralocorticoid receptor (MR) in vascular smooth muscle cells (SMCs) in cardiovascular aging in male mice (30–32). The MR is a well-known regulator of blood pressure by modulating renal sodium reabsorption in response to the hormone aldosterone. In addition to its role in the kidney, MR is expressed in SMCs that make up the medial layer of blood vessels where it has been shown to contribute to vessel constriction, vascular remodeling, and blood pressure control in male mice (30–33). With regards to aging, we recently demonstrated that SMC-MR contributes to aging-induced cardiovascular stiffness and fibrosis in males (31); however, the role of SMC-MR in female cardiovascular aging is unknown. MR expressed in endothelial cells has been found to contribute to microvascular dysfunction in female, but not male, mice exposed to cardiometabolic risk factors (34). In vitro, the estrogen receptor can inhibit the transcriptional regulatory function of the MR in endothelial cells (35). Based on this literature, we hypothesized that SMC-MR may play a sex-specific role in cardiovascular aging that has never been explored. Therefore, the aims of our study were to 1) determine sex differences in the time course of vascular and cardiac aging in mice; and 2) compare the role of SMC-MR in cardiovascular aging in male and female mice.
MATERIALS AND METHODS
Animal Studies
All mice were handled in accordance with U.S. National Institutes of Health standards, and all procedures were approved by the Tufts Medical Center Institutional Animal Care and Use Committees. Mice with inducible deletion of the mineralocorticoid receptor (MR) gene (Nr3c2) from smooth muscle cells (SMC-MR-KO mice) were generated by crossing floxed MR (MRf/f) mice with SMA-Cre-ERT2 mice (smooth muscle actin promoter driving expression of Cre-ERT2 recombinase that is activated by tamoxifen) as previously described (30–32). For all studies, male and female MRf/f/SMA-Cre-ERT2negative (MR intact) and MRf/f/SMA-Cre-ERT2-positive (SMC-MR-KO) littermates, all on the C57/Bl6J background, were treated with tamoxifen at 6–8 wk of age, resulting in SMC-specific MR deletion in the Cre-positive animals as previously confirmed (32). We compared mice at 3, 12, and 18 mo as these ages represent young, middle aged, and older time points that are analogous to human cardiovascular aging. Specifically, these time points were chosen to model critical times in the progression of sex hormone availability and vascular disease in aging women. By 3 mo of age, male and female mice are considered sexually mature. At 10–14 mo, female mice begin having irregular estrous cycles with decreased fertility. The ability to reproduce in all female mice has been shown to be terminated by 15 mo (36). The average life span of a C57/Bl6J mouse is 18–26 mo; thus the 18-mo time point represents a postreproductive age that may be analogous to the “postmenopausal” phenotype in humans.
Quantitative RT-PCR
Total RNA was extracted from mouse vessels and left ventricles and reverse transcribed, and quantitative RT-PCR was performed with gene-specific primers as previously described (30). For mesenteric vessel mRNA, each n represents vessels from two to three mice pooled together to maximize the total RNA isolated. For all vascular mRNA studies, cycle threshold (Ct) values were normalized to β2-microglobulin (B2m). For the cardiac mRNA studies, Ct values were normalized to GAPDH. Each PCR was performed in triplicate. Specific primers are specified in Table 1.
Table 1.
Gene names, symbols, and primer sequences
| Gene Name | Gene Symbol | Primer Sequence (5′–3′) |
|---|---|---|
| Angiotensin II type 1 b receptor | Agtr1b | |
| Forward | TGGCTTGGCTAGTTTGCCG | |
| Reverse | ACCCAGTCCAATGGGGAGT | |
| Mineralocorticoid receptor | NR3C2 | |
| Forward | GAAGAGCCCCTCTGTTTGCAG | |
| Reverse | TCCTTGAGTGATGGGACTGTG | |
| Beta-2-microglobulin | B2m | |
| Forward | GCTATCCAGAAAACCCCTCAA | |
| Reverse | CATGTCTCGATCCCAGTAGACGGT | |
| Connective tissue growth factor | CTGF | |
| Forward | GGGCCTCTTCTGCGATTTC | |
| Reverse | ATCCAGGCAAGTGCATTGGTA | |
| Collagen type 1-alpha-a | Col1α2 | |
| Forward | AAGGGTCCCTCTGGAGAACC | |
| Reverse | TCTAGAGCCAGGGAGACCCA | |
| Transforming growth factor-β | TGF-β | |
| Forward | TGACGTCACTGGAGTTGTACGG | |
| Reverse | GGTTCATGTCATGGATGGTGC | |
| Glyceraldehyde 3-phosphate dehydrogenase | GAPDH | |
| Forward | GAAGGTCGGTGTGAACGGATTTGG | |
| Reverse | GGTCGTTGATGGCAACAATCTCCAC |
Mesenteric Vessel Wire Myograph Studies
Rings from second- and third-order mesenteric resistance arteries (MRAs) were mounted in a myograph (Danish Myo Technologies) for isometric tension recordings using PowerLab software (AD Instruments). A total of four rings per mouse were used, with n = 3–5 mice for each wire myograph study. Rings were placed under a resting tension of 2 mN in tissue baths containing warmed (37°C), aerated (95% O2-5% CO2) standard physiological saline solution (in mM: 130 NaCl, 4.7 KCl, 1.17 MgSO4, 0.03 EDTA, 1.6 CaCl2, 14.9 NaHCO3, 1.18 KH2PO4, and 5.5 glucose). Administration of 10 μM phenylephrine (PE) was used to test arterial viability, and the presence of intact endothelium was verified by acetylcholine (1 μM)-induced relaxation of a half-maximal PE-induced contraction. The vasoconstriction response to 10 nM angiotensin II was measured.
In Vivo Noninvasive Imaging and Echocardiography
In vivo pulse wave velocity, an index of vascular stiffness, and transthoracic echocardiography were measured in 3-, 12-, and 18-mo-old male and female MR-intact and SMC-MR-KO littermates using Doppler ultrasound (Vevo 2100, VisualSonics, Toronto, ON, Canada). Briefly, mice were anesthetized with isofluorane and placed on a heated platform (37°C). ECG recordings were made via paw contact with pad electrodes. Mice were maintained with ∼2.0% isofluorane during the procedure to maintain a heart rate of 400-450 beats/min. For PWV, the abdominal aorta was imaged and the transit time between the proximal and distal aorta was determined by averaging distances between the foot of the flow waveform and the R-wave of the ECG over five cardiac cycles at each location. PWV was calculated by dividing the distance by the difference in transit times (mm/ms) obtained at each location, as previously described (31). Left ventricular end-diastolic (EDD) and systolic diameter (ESD) were measured by determining the average values from five cardiac cycles obtained via M mode in the short axis view, as previously described (31).
Histology
Mouse carotid vessels were fixed in formalin (10%) and stained with Masson’s trichrome. Collagen content was quantified as percent fibrosis of the total vessel using computerized morphometric analysis software (Image-Pro Premier 9.2, Media Cybernetics, Rockville, MD), as previously described (31, 32). Mouse left ventricle sections were formalin-fixed (10%) and stained with Picrosirius Red. Collagen content was quantified as percent interstitial and perivascular fibrosis, as previously described (31). All histologic analyses were performed by blinded investigators.
Statistics
Within-group differences were assessed with two-factor ANOVA or repeated-measures ANOVA with Tukey post hoc testing. P < 0.05 was considered significant.
RESULTS
Vascular Stiffness Increases Later in Life in Females, Correlates with the Timing of Increased Vascular MR Expression, and Is Prevented in SMC-MR Deficient Males and Females
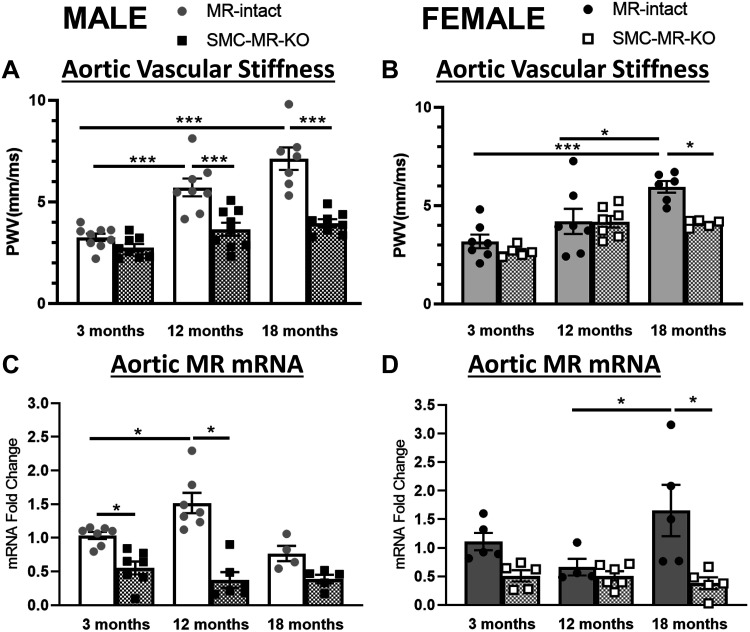
Aortic pulse wave velocity (PWV) was measured in 3-, 12-, and 18-mo-old male and female MR-intact and SMC-MR-KO mice to quantify vascular stiffness. In MR-intact mice, PWV was significantly increased at 12 and 18 mo compared with 3 mo of age in males (Fig. 1A) while in females, PWV was significantly increased only at 18 mo compared with 3 mo of age (Fig. 1B). The rise in aortic stiffness with age did not occur in mice lacking SMC-MR resulting in significantly less vascular stiffness in aged SMC-MR-KO mice compared with MR-intact littermates. Aortic mRNA expression of the MR was also quantified in males and females at 3, 12, and 18 mo of age. MR expression increased from 3 to 12 mo in males but not until 18 mo in females (Fig. 1, C and D). As expected, vascular MR mRNA was significantly decreased in whole aortic tissue from SMC-MR-KO mice in both sexes independent of age. Furthermore, MR expression did not rise with age in aortas from SMC-MR-KO mice supporting that the increase with age likely occurs in SMCs versus endothelial cells. The increase in vascular stiffness in aged mice was prevented in male and female mice lacking SMC-MR, demonstrating a role for MR in aging-associated vascular stiffness in both sexes.
Fig 1.
. Vascular stiffness increases later in life in females, correlates with vascular mineralocorticoid receptor (MR) expression, and is prevented by smooth muscle cell-specific MR deletion (SMC-MR-KO) in males and females. A and B: aortic pulse wave velocity (PWV) was measured in 3-, 12-, and 18-mo-old MR-intact and SMC-MR-KO male (A) and female (B) mice using the transit time method to quantify vascular stiffness (n = 5–9/group). Aortic MR mRNA expression was measured via quantitative RT/PCR in males (C) and females (D) (n = 4–6/group). Differences were assessed via two-way ANOVA with Tukey post hoc testing where appropriate. Data are means ± SE. *P < 0.05 and ***P < 0.001.
Vascular Fibrosis Increases Later in Life in Females and Is Attenuated by Smooth Muscle Cell Mineralocorticoid Receptor Deletion in Males Only
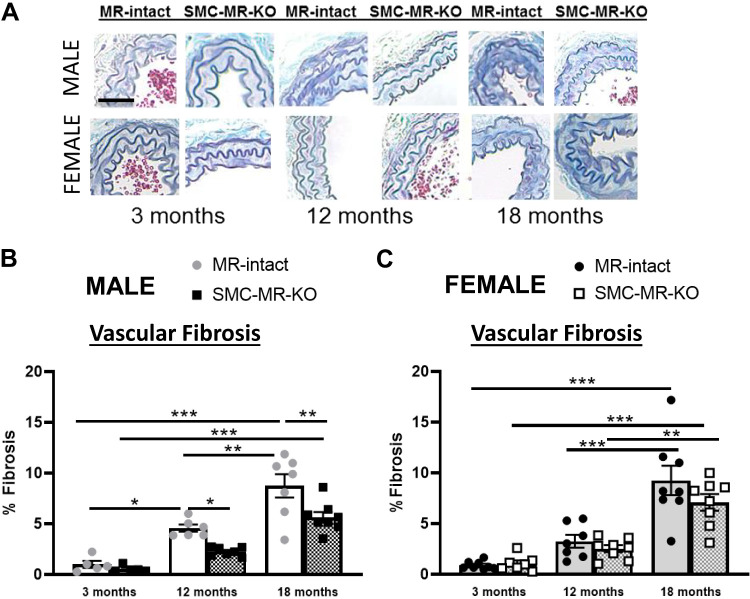
Vascular fibrosis was quantified histologically in carotid artery sections from 3-, 12-, and 18-mo-old male and female MR-intact and SMC-MR-KO mice. As with vascular stiffness (Fig. 1, A and B), carotid fibrosis was significantly increased at 12 and 18 mo of age relative to 3 mo in MR-intact males (Fig. 2, A and B) but was not increased until 18 mo of age in females (Fig. 2, A and C). In males, SMC-MR deletion attenuated aging-associated fibrosis resulting in significantly less fibrosis in 12- and 18-mo-old SMC-MR-KO mice compared with age-matched MR-intact males. In females; however, there was no significant difference in vascular fibrosis between MR-intact and SMC-MR-KO mice at any age.
Fig 2.
Vascular fibrosis increases later in life in females and is attenuated by smooth muscle cell-specific mineralocorticoid receptor deletion (SMC-MR-KO) in males only. Vascular fibrosis was quantified in the medial layer of carotid artery sections stained with Masson’s Trichrome in 3, 12, and 18-mo-old MR-intact and SMC-MR-KO mice (A). Representative images are displayed and then quantified for male (B) and female (C) mice. Scale bar = 10 µm; n = 5–8/group. Differences were assessed via two-way ANOVA with Tukey post hoc testing where appropriate. Data are means ± SE; *P < 0.05, **P < 0.01, ***P < 0.001.
Sex, Age, and SMC-MR Effects on Angiotensin II-Mediated Vasoconstriction
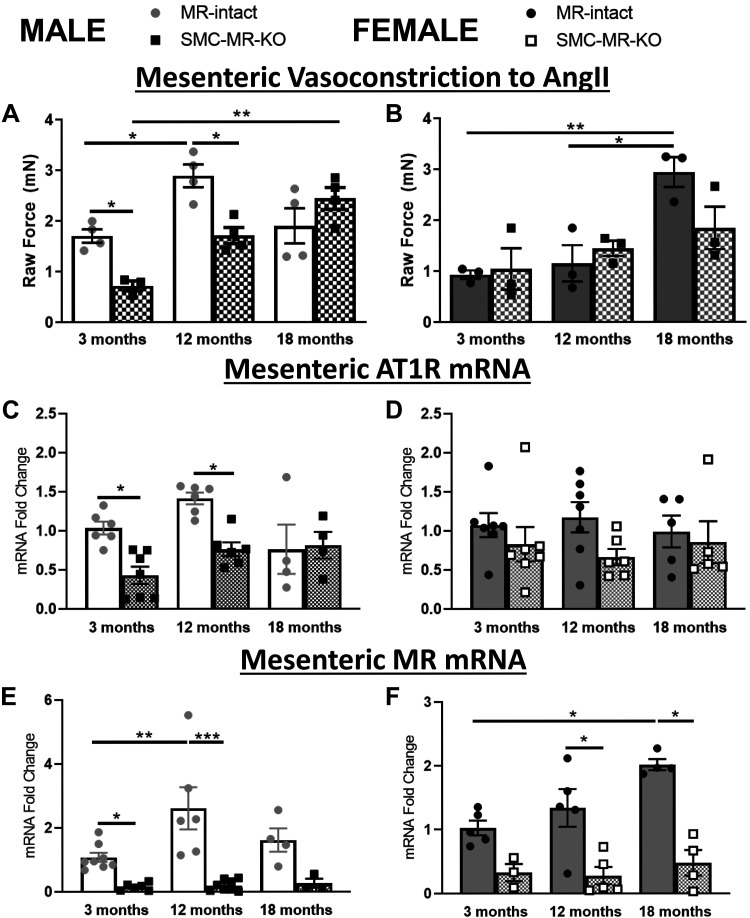
ANG II signaling in the vasculature is known to increase with aging and contributes to vasoconstriction (37). Thus wire myography studies were performed to quantify ANG II-induced vasoconstriction using mesenteric resistance vessels from aging male and female MR-intact and SMC-MR-KO mice. In males, ANG II-mediated vasoconstriction was significantly increased in 12 compared with 3-mo-old mice with no further increase at 18 mo of age (Fig. 3A). ANG II-induced vasoconstriction was significantly decreased in SMC-MR-KO compared with MR-intact males at 3 and 12 mo, supporting a role for SMC-MR in this process in males. ANG II-induced vasoconstriction was also significantly increased in SMC-MR-KO males at 18 compared with 3 mo but vasoconstriction did not differ by genotype at 18 mo supporting that other mechanisms also contribute to enhanced vasoconstriction with aging. In MR-intact females, ANG II-induced vasoconstriction was increased at 18 compared with 3 or 12 mo of age, but vasoconstriction did not significantly change with age in SMC-MR-KO mice despite no significant genotype difference at any age in females (Fig. 3B). Expression of the ANG II type 1b receptor (Agtr1b, the predominant mouse ANG II receptor in mesenteric vessels) was significantly lower in 3- and 12-mo-old SMC-MR-KO males compared with their age-matched MR-intact controls. This difference in Agtr1b expression correlated with differences in ANG II vasoconstriction by genotype in males. In females, Agtr1b expression did not change with age or SMC-MR-KO (Fig. 3, C and D). As in the aorta, mesenteric vessel expression of MR was increased at 12 versus 3 mo of age in males and at 18 versus 3 mo in females and was significantly lower in SMC-MR-KO mice (Fig. 3, E and F).
Fig 3.
Angiotensin II-mediated vasoconstriction increases later in life in females and is attenuated by smooth muscle cell-specific MR deletion (SMC-MR-KO) in males and females. A and B: second and third order mesenteric resistance vessels (MRVs) were isolated from 3-, 12-, and 18-mo-old MR-intact and SMC-MR-KO male (A) and female (B) mice. Vessel tension induced by ANG II (1 × 10−7 M) was determined by wire myography; n = 3–4/group. C–F: total RNA was extracted from MRVs and quantitative RT/PCR was performed to quantify angiotensin II type 1b receptor and mineralocorticoid receptor mRNA expression; n = 3–8/group. Differences were assessed via two-way ANOVA with Tukey post hoc testing where appropriate. MR, mineralocorticoid receptor; ANG II, angiotensin II; AT1R, angiotensin II type 1 receptor. Data are means ± SE; *P < 0.05, **P < 0.01, ***P < 0.001.
Cardiac Function Declines with Advanced Age in Both Sexes and in Females Smooth Muscle Cell Mineralocorticoid Receptor Deletion Is Associated with Improved Cardiac Function
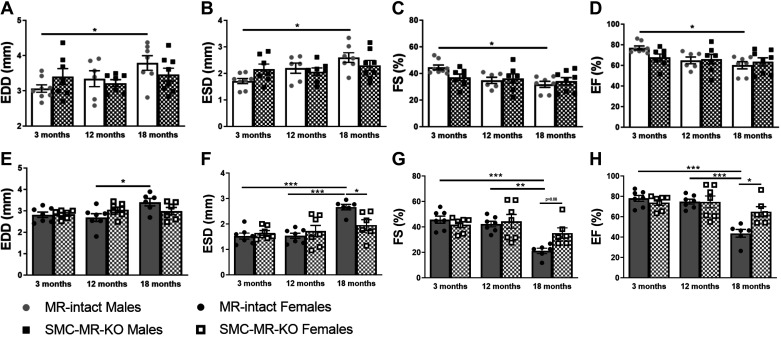
Next, we evaluated the impact of aging on the heart in males compared with females. Cardiac size and systolic function were measured via echocardiography (Fig. 4). The heart dilates with age as demonstrated by increased EDD and end-systolic diameter at 18 compared with 3 mo of age in males (Fig. 4, A and B) and at 18 compared with 12 mo of age in females (Fig. 4, E and F). There is also a decline in systolic function as determined by the fractional shortening (FS) and ejection fraction (EF), both of which declined at 18 versus 3 mo of age in males (Fig. 4, C and D) and compared with 3 mo and 12 mo in females (Fig. 4, G and H). In this case, genotype-specific differences at the 18-mo time point were observed in females only. These data support a potential sex difference in the role of SMC-MR in cardiac aging pathophysiology.
Fig 4.
Cardiac function declines with advanced age in both sexes and specifically females are protected by deletion of smooth muscle cell (SMC)-mineralocorticoid receptor (MR). Echocardiography via ultrasound technique was performed in 3-, 12-, and 18-mo-old male/female MR-intact SMC-specific MR deletion (SMC-MR-KO) mice to determine cardiac function. A–D: males; E–H: females. Differences were assessed via two-way ANOVA with Tukey post hoc testing where appropriate; n = 6–8/group. EDD, end diastolic diameter; ESD; end systolic diameter; FS, fractional shortening; EF, ejection fraction. Data are means ± SE; *P < 0.05, **P < 0.01, ***P < 0.001.
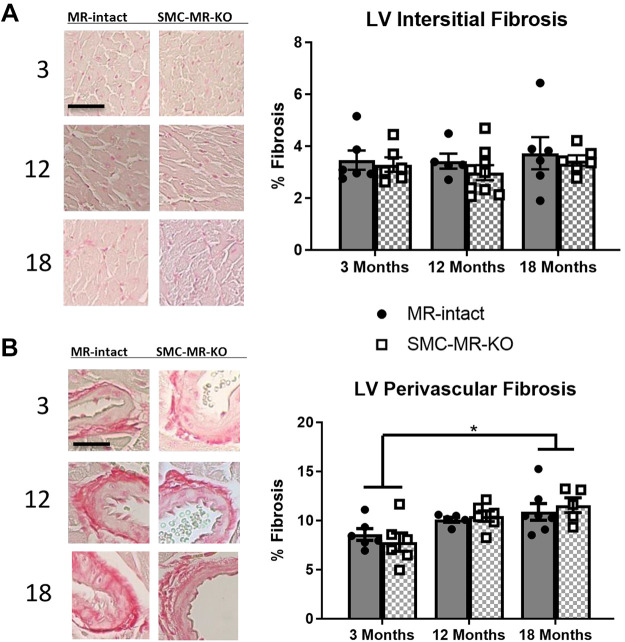
Cardiac Perivascular Fibrosis Increases at 18 Mo of Age in Females Independent of Smooth Muscle Cell Mineralocorticoid Receptor
We previously published that in male mice, cardiac fibrosis increases with age and is significantly attenuated by SMC-MR deletion (31), but this has never been examined in female mice. Thus left ventricular (LV) fibrosis was measured in 3-, 12-, and 18-mo-old MR-intact and SMC-MR-KO female mice. LV interstitial fibrosis was not different between ages or among genotypes in females (Fig. 5A). LV perivascular fibrosis was increased at 18 mo compared with 3 mo of age in MR-intact and SMC-MR-KO females, independent of genotype (Fig. 5B).
Fig 5.
Left ventricular perivascular fibrosis increases later in life in females independent of smooth muscle cell (SMC)-mineralocorticoid receptor (MR). Left ventricular (A) interstitial (scale bars = 50 µm) and (B) perivascular fibrosis (scale bar = 10 µm) were quantified in 3-, 12-, and 18-mo-old MR-intact and SMC-specific MR deletion (SMC-MR-KO) female mice; n = 5–9/group. Differences were assessed via two-way ANOVA. LV, left ventricular. Data are means ± SE; *P < 0.05.
Sex Differences in Fibrotic Gene Expression in the Heart and the Role of Smooth Muscle Cell Mineralocorticoid Receptor
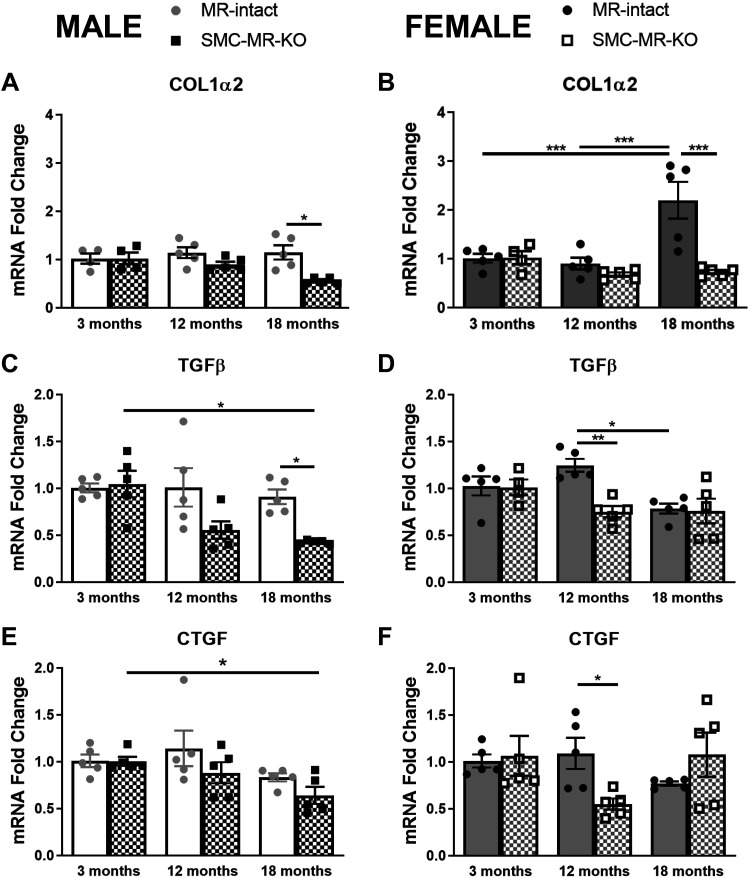
Profibrotic gene expression was measured in left ventricular tissue from male and female mice (Fig. 6). Collagen1α2 (Col1α2) mRNA expression did not change with age in male MR-intact mice; however, 18-mo-old SMC-MR-KO males express less LV Col1α2 than MR-intact littermates (Fig. 6A). In MR-intact females, Col1α2 expression was significantly increased at 18 mo compared with 3 and 12 mo of age. In SMC-MR-KO females, there was no change in Col1α2 expression with aging resulting in significantly less Col1α2 expression in 18-mo-old SMC-MR-KO versus age-matched MR-intact controls (Fig. 6B). Transforming growth factor-β (TGF-β) and connective tissue growth factor (CTGF) are two important regulators of collagen gene expression (38). Thus, LV TGF-β and CTGF mRNA expression were also measured. TGF-β expression did not change with age in MR-intact male mice; however, 18-mo-old SMC-MR-KO males express less TGF-β than MR-intact littermates (Fig. 6C). Similarly, CTGF expression decreased with age in 18-mo-old SMC-MR-KO male mice compared with MR-intact littermates (Fig. 6E). In females, TGF-β and CTGF were lower in 12-mo-old SMC-MR-KO mice compared with age-matched MR-intact littermates (Fig. 6, D and F).
Fig 6.
Sex differences in the impact of aging on cardiac profibrotic gene expression and the role of SMC-MR. Left ventricular mRNA expression of collagen type 1-alpha-2 (Col1a2) (A and B) and the fibrosis regulator transforming growth factor-β (TGF-β) (C and D) and connective tissue growth factor (CTGF) (E and F) was quantified in 3-, 12-, and 18-mo-old MR-intact and SMC-MR-KO male (A, C, and E) and female (B, D, and F) mice; n = 4–5/group. Differences were assessed via two-way ANOVA with Tukey post hoc testing where appropriate. Data are means ± SE; *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
The present study is, to our knowledge, the first to investigate sex differences in vascular and cardiac structure and function with aging in a preclinical mouse model. These measurements in MR-intact male and female littermates provide a frame of reference for sex differences in the time course of cardiovascular aging physiology in C57/Bl6J mice, a commonly used genetic background for transgenic mouse studies. In addition, this study investigated for the first time, the role of smooth muscle cell mineralocorticoid receptor in female cardiovascular aging and directly compared the findings to male littermates. We demonstrated that 1) vascular MR expression, stiffness, and ANG II vasoconstriction all increase with age in both sexes but do so later in life in female mice; 2) SMC-MR contributes to vascular stiffness and ANG II vasoconstriction in both sexes but the mechanisms are sexually dimorphic with MR contributing to vascular fibrosis and angiotensin II receptor expression in males whereas in females the mechanisms are distinct; 3) cardiac systolic function declines with advanced age in both sexes and SMC-MR appears to play a greater role in females; 4) cardiac perivascular fibrosis occurs in aging females and is independent of SMC-MR while in males, cardiac fibrosis is dependent on SMC-MR; and 5) SMC-MR contributes to regulation of cardiac fibrosis regulators CTGF and TGF-β in both sexes, which control collagen production.
Overall, the data support a model in which vascular aging phenotypes occur later in females, depend on SMC-MR in both sexes and are mediated by sexually dimorphic molecular mechanisms. Using 3-mo-old mice to model young adult mice that are able to reproduce, 12-mo-old mice in which females start to lose fertility, and 18-mo-old mice as postreproductive, the present study demonstrates that the hallmarks of cardiovascular aging develop in both aging male and female mice, with most of these changes occurring 6 mo later in females. The delayed cardiovascular aging pattern in female rodents compared with male littermates is analogous to what is observed in human cardiovascular aging (16, 39). These findings support that the aging mouse could be used to investigate the molecular mechanisms driving sexual dimorphisms in aging-associated CVD. Our findings also infer a role for SMC-MR in both male and female cardiovascular aging; however, this role differs among the specific cardiovascular aging parameters we explored for this study, and the molecular mechanisms, while generally not well characterized in females, appear to be distinct from those that have been previously identified in males (30, 31). These data further support that MR antagonist drugs may be effective in the management and treatment of aging-associated cardiovascular disease in males and females, although perhaps via different paradigms (timing, endpoints) that remain to be studied in humans.
Pulse wave velocity studies here show that vascular stiffness rises with age in males and females but that female mice develop arterial stiffness later in life compared with males. The male data are consistent with our previously published data and with the female comparison demonstrating that the aging-associated increase in arterial stiffness is also prevented by SMC-MR-KO in females. These data are the first to explore aging-associated arterial stiffness in female mice and clearly demonstrate that the aging mouse model mimics the sex differences observed in human arterial aging, as it is well-documented that women develop arterial stiffness later in life than males (10, 11, 39). With regards to the mechanism, in addition to aging, SMC-MR has also been shown to contribute to arterial stiffening and vascular fibrosis in young male mice in response to hypertension (40) and vascular injury (41) by a mechanism that may involve the regulation of integrin and VEGF signaling, respectively, but this has never been explored in females. Endothelial cell (EC) MR has been shown to contribute to vascular stiffness in obese female mice (42) by regulating expression of the epithelial sodium channel. EC stiffness is known to increase with age, suggesting that MR in other cells in addition to SMCs could also contribute to aging-associated arterial stiffness but this has never been tested. Our data also show that vascular fibrosis develops later in life in females compared with males but that this does not occur specifically in males when SMC-MR is deleted. In addition to alterations in the extracellular matrix (ECM), recent preclinical studies in males have implicated the vascular smooth muscle cell as a direct source of enhanced arterial stiffness via alterations in the cytoskeleton and in α-smooth muscle actin interactions with the ECM via β1-integrin (28, 43). The potential role of intrinsic vascular SMC stiffness in preclinical models of female aging is unknown; thus the role of SMC-MR in aging-associated SMC stiffness warrants future study in both sexes. Together, these findings suggest that the aging-associated increase in arterial stiffness is driven by SMC-MR in males and females but that the underlying mechanisms are likely sexually dimorphic.
The present study also reveals that vascular MR expression in mesenteric resistance vessels increases later in life in females (12 mo in males, 18 mo in females) as does ANG II vasoconstriction. Previous work by our laboratory has demonstrated that vascular MR expression rises with age in males and is associated with the transcriptional downregulation of microRNA (miR-155) (30). As a result, expression of proconstrictive miR-155 target genes, Agtr1b and L-type calcium channel subunit Cav1.2, rises with age leading to enhanced vasoconstriction and rising blood pressure, all of which were prevented by SMC-MR-KO in males. However, in females, the protective effect of SMC-MR deletion does not correlate with changes in Agtr1b expression as it does in males, supporting a distinct mechanism by which SMC-MR drives ANG II-mediated vasoconstriction in aging females. Interestingly, in male mice lacking SMC-MR, angiotensin II-mediated vasoconstriction ultimately increased from 3 to 18 mo, such that the genotype difference is abolished in 18-mo-old males. These data suggest that additional mechanisms independent of SMC-MR contribute to angiotensin II-mediated vasoconstriction later in life in males. Previous studies have identified aging-associated increases in oxidative stress as contributing to angiotensin II-mediated vasoconstriction in male mice aged 23-31 mo (44). Our own prior work has also demonstrated aging-associated increases in angiotensin II-induced vascular oxidative stress in male mice, which was prevented by SMC-MR-KO (30, 32). Together, the present results suggest that vascular MR expression rises with age in both males and females and contributes to enhanced angiotensin II-mediated vasoconstriction of small resistance arteries via sexually dimorphic mechanisms.
Structural and functional alterations of the heart are also hallmarks of aging. Clinically, left ventricular (LV) wall thickness and fibrotic remodeling increase with age in men and women; however, women exhibit more accelerated increases in LV wall thickness than men (4, 45, 46). We compared cardiac function by echo in aging male and female mice. Our findings show that systolic function, as measured by ejection fraction, declines in aging male and female mice and that this decline in ejection fraction is not statistically significant in SMC-MR-KO males or females. In contrast to females, there is no genotype-specific difference in cardiac function in the males at 18 mo, perhaps suggesting a more exaggerated role for SMC-MR in aging-associated changes in systolic function in females. We recognize that our cardiac function findings in the female mice are in contrast to a previous study (47) that showed only a small decline in EF and FS in female mice at 18 mo of age, more similar to what we observe in males. In that study, Alibhai et. al. (47) demonstrated a significant decline in EF and FS with aging when they induce alterations in circadian rhythm genes or hormonal status, supporting that there might be other differences in the experimental conditions that contribute to differences in cardiac function with aging which remain to be explored. As there are limited studies examining the impact of aging on the heart of female mice, future studies are warranted to fully elucidate aging-associated alterations in cardiac function in females and how that may differ from males.
We have previously shown a role for SMC-MR in aging-associated cardiac fibrosis in males; however, the present findings suggest that females do not exhibit the interstitial cardiac fibrosis phenotype observed in males but rather undergo an increase in perivascular fibrosis with aging that is not impacted by SMC-MR-KO. We also observed sex differences in aging-associated alterations in cardiac fibrosis gene expression. Aged SMC-MR-KO males express less cardiac TGF-β and COL1α2 than MR-intact littermates, and this is associated with decreasing CTGF expression with advancing age in male mice lacking SMC-MR. Importantly, CTGF was previously identified by gene expression profiling as an MR target gene in the vasculature (38) and has been shown to contribute to vascular, cardiac, and renal fibrosis under a variety of pathologies (48). We have previously shown that in male mice SMC-MR-KO was associated with a decrease in fibrotic gene expression in the vasculature (31) with the same pattern of declining CTGF expression in the aging male vasculature when SMC-MR is specifically deleted. This is associated with reduced interstitial and perivascular fibrosis and cardiac stiffness in the male mice (31). This CTGF-related mechanism provides a potential explanation for the protection from cardiac fibrosis in aging SMC-MR-KO males. Interestingly, the pattern of cardiac gene expression and fibrosis is different in the female heart. In female MR-intact mice, COL1α2 expression increases with aging, which is not observed in female SMC-MR-KO mice, providing a potential mechanism for preserved LV function in female KO mice. Although TGF-β and CTGF expression in the heart do not change with aging in females, at 12 mo, the SMC-MR-KO females have lower expression supporting the possibility that the difference in CTGF levels at 12 mo contributes to the subsequent level of collagen expression. Together, these results support that cardiac structure and function decline with age in both male and female mice, which is, in part, prevented by SMC-MR-KO but once again, the mechanisms appear to be sexually dimorphic and support the need for further exploration of the sex-specific mechanisms of aging-associated cardiac dysfunction. In humans, aged women have a greater incidence of diastolic dysfunction than men, as measured by cardiac MRI and the degree of LV fibrosis correlates with the sex differences in aging-associated diastolic dysfunction (49). There are also noted sex differences in cardiac fibrosis in patients with aortic stenosis, hypertension, and arteriosclerosis. Specifically, in AS patients, men have been shown to have a higher level of cardiac fibrosis and enhanced upregulation of type I and type III collagens. Furthermore, in vitro studies have shown that estrogen regulates collagen I and III expression in human cardiac fibroblasts, suggesting that younger females may have less fibrosis due to higher levels of estrogen (50). Our results show that aged MR-intact females have increased cardiac type I collagen expression, which could potentially be due to the decline in circulating estrogen with age, and the lack of this rise in SMC-MR-KO mice suggests that SMC-MR may contribute to this pathology once estrogen is lost but further studies are needed to confirm this possibility.
There are several limitations to our study that warrant acknowledgment. Although the study describes the natural history of cardiovascular aging in male and female mice and demonstrates a role for SMC-MR in the process, the mechanistic studies are correlative without evidence of causation. Thus we can conclude that the mechanisms appear to be different in males and females; however, the details of the molecular mechanisms remain to be determined for each sex. Particularly in females, there are little available data about potential molecular mechanisms; thus extensive and detailed studies are warranted that are beyond the scope of this study. However, these findings confirm that aging male and female mice exhibit cardiovascular aging in a temporal pattern that is similar to that of humans, inferring that this model may be well suited for determining sex-specific mechanisms of aging-associated CVD. Further limitations include the lack of determination of sex hormones in our study. We did not measure estrogen or testosterone in our mice nor did we modulate those hormones; thus we cannot imply that sex hormones have a role in contributing to any of the sex differences that we observed. Additionally, we recognize that we did not include additional male cardiac fibrosis data to be compared with the females in the current study and refer only to published historical data in males (31). Finally, we also did not extend our studies to a later time point in the mouse life span, due to technical challenges involved in aging matching littermates to 24-28 mo when survival begins to decline.
Despite these limitations, there are important clinical implications to this study. Age remains a dominant risk factor for CVD, and there are currently no therapies directly targeting the cardiovascular aging process. As advances in the treatment of cancer and CVD have prolonged median life-expectancy, the aged population continues to grow. By 2030, 20% of the world population will be aged 65 yr or older (51) and hence the prevalence of CVD will increase accordingly. This continued rise of aging-associated CVD poses a significant public health burden and accentuates the need for efficacious therapies to reduce the burden of aging-associated CVD. Given the observed sexually dimorphic timing of the SMC-MR-driven aging-associated alterations in cardiac and vascular function, our findings suggest that MR antagonist therapy may be beneficial in both sexes but might require different timing for improving arterial health in aging males and females. We have previously shown that systemic MR inhibition via low-dose spironolactone reduces vascular stiffness and fibrosis in middle-aged (12–16 mo) male mice (31), but the effects of MR inhibition on cardiovascular aging in female mice has yet to be explored. Indeed, we have observed positive changes in biomarkers related to SMC-MR-driven cardiovascular aging in aged humans treated with MR antagonists, suggesting that MR antagonist therapy may improve cardiovascular outcomes in the aged population via increases in circulating miR-155 and decreases in circulating CTGF. (30, 31). Thus future clinical studies are warranted to address potential sex differences in the timing and efficacy of MR antagonist therapy in cardiovascular aging. Furthermore, the present study is the first to examine sex differences in murine cardiovascular aging. These findings are an important step toward elucidation of the precise mechanisms that contribute to the differential manifestations of aging-associated CVD in men and women. Indeed, future studies are warranted to determine these mechanisms as these data could nominate sex-specific therapies to ease the CVD burden of the aging population.
GRANTS
This work is supported by National Institutes of Health Grants K12-HD-092535 (to J.J.D.) and 5R01-HL-119290 and 3R01-HL-119290-02S1 (to I.Z.J.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.J.D. and I.Z.J. conceived and designed research; J.J.D., S.K.K., and R.M.K. performed experiments; J.J.D., S.K.K., R.M.K., and I.Z.J. analyzed data; J.J.D., S.K.K., R.M.K., and I.Z.J. interpreted results of experiments; J.J.D., S.K.K., R.M.K., and I.Z.J. prepared figures; J.J.D. drafted manuscript; J.J.D., S.K.K., R.M.K., and I.Z.J. edited and revised manuscript; J.J.D., S.K.K., R.M.K., and I.Z.J. approved final version of manuscript.
REFERENCES
- 1.Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Bairey Merz CN, Cheng S. Sex differences in blood pressure trajectories over the life course. JAMA Cardiol 5: 19–26, 2020. doi: 10.1001/jamacardio.2019.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seeland U, Demuth I, Regitz-Zagrosek V, Steinhagen-Thiessen E, Konig M. Sex differences in arterial wave reflection and the role of exogenous and endogenous sex hormones: results of the Berlin Aging Study II. J Hypertens 38: 1040–1046, 2020. doi: 10.1097/HJH.0000000000002386. [DOI] [PubMed] [Google Scholar]
- 3.Stanhewicz AE, Wenner MM, Stachenfeld NS. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am J Physiol Heart Circ Physiol 315: H1569–H1588, 2018. doi: 10.1152/ajpheart.00396.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, Benjamin EJ, Vasan RS. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation 122: 570–578, 2010. doi: 10.1161/CIRCULATIONAHA.110.937821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krumholz HM, Larson M, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol 72: 310–313, 1993. doi: 10.1016/0002-9149(93)90678-6. [DOI] [PubMed] [Google Scholar]
- 6.Barton M, Meyer MR. Heart failure with preserved ejection fraction in women: new clues to causes and treatment. JACC Basic Transl Sci 5: 296–299, 2020. doi: 10.1016/j.jacbts.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuma N, Takimoto E, Ueda K, Liu P, Tajima M, Otsu Y, Kariya T, Harada M, Toko H, Koga K, Blanton RM Jr, Karas RH, Komuro I. Estrogen receptor-alpha non-nuclear signaling confers cardioprotection and is essential to cGMP-PDE5 inhibition efficacy. JACC Basic Transl Sci 5: 282–295, 2020. doi: 10.1016/j.jacbts.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabbatini AR, Kararigas G. Menopause-related estrogen decrease and the pathogenesis of hfpef: jacc review topic of the week. J Am Coll Cardiol 75: 1074–1082, 2020. doi: 10.1016/S0735-1097(20)31701-0, 10.1016/j.jacc.2019.12.049. [DOI] [PubMed] [Google Scholar]
- 9.Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular-arterial interactions. J Am Coll Cardiol 61: 96–103, 2013. doi: 10.1016/j.jacc.2012.08.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dart AM, Kingwell BA, Gatzka CD, Willson K, Liang YL, Berry KL, Wing LM, Reid CM, Ryan P, Beilin LJ, Jennings GL, Johnston CI, McNeil JJ, MacDonald GJ, Morgan TO, West MJ, Cameron JD. Smaller aortic dimensions do not fully account for the greater pulse pressure in elderly female hypertensives. Hypertension 51: 1129–1134, 2008. doi: 10.1161/HYPERTENSIONAHA.107.106310. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 43: 1239–1245, 2004. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 12.Zaydun G, Tomiyama H, Hashimoto H, Arai T, Koji Y, Yambe M, Motobe K, Hori S, Yamashina A. Menopause is an independent factor augmenting the age-related increase in arterial stiffness in the early postmenopausal phase. Atherosclerosis 184: 137–142, 2006. doi: 10.1016/j.atherosclerosis.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 13.Acelajado MC, Oparil S. Hypertension in the elderly. Clin Geriatr Med 25: 391–412, 2009. doi: 10.1016/j.cger.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Martins LC, Figueiredo VN, Quinaglia T, Boer-Martins L, Yugar-Toledo JC, Martin JF, Demacq C, Pimenta E, Calhoun DA, Moreno H, Jr. . Characteristics of resistant hypertension: ageing, body mass index, hyperaldosteronism, cardiac hypertrophy and vascular stiffness. J Hum Hypertens 25: 532–538, 2011. doi: 10.1038/jhh.2010.95. [DOI] [PubMed] [Google Scholar]
- 15.Keyhani S, Scobie JV, Hebert PL, McLaughlin MA. Gender disparities in blood pressure control and cardiovascular care in a national sample of ambulatory care visits. Hypertension 51: 1149–1155, 2008. doi: 10.1161/HYPERTENSIONAHA.107.107342. [DOI] [PubMed] [Google Scholar]
- 16.Merz AA, Cheng S. Sex differences in cardiovascular ageing. Heart 102: 825–831, 2016. doi: 10.1136/heartjnl-2015-308769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gori M, Lam CS, Gupta DK, Santos AB, Cheng S, Shah AM, Claggett B, Zile MR, Kraigher-Krainer E, Pieske B, Voors AA, Packer M, Bransford T, Lefkowitz M, McMurray JJ, Solomon SD, PARAMOUNT Investigators. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur J Heart Fail 16: 535–542, 2014. doi: 10.1002/ejhf.67. [DOI] [PubMed] [Google Scholar]
- 18.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jr., Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 306: 856–863, 2011. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail 13: 18–28, 2011. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DuPont JJ, Kenney RM, Patel AR, Jaffe IZ. Sex differences in mechanisms of arterial stiffness. Br J Pharmacol 176: 4208–4225, 2019. doi: 10.1111/bph.14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller KM, Howlett SE. Sex differences in the biology and pathology of the aging heart. Can J Cardiol 32: 1065–1073, 2016. doi: 10.1016/j.cjca.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell GF, Gudnason V, Launer LJ, Aspelund T, Harris TB. Hemodynamics of increased pulse pressure in older women in the community-based age-Gene/Environment Susceptibility-Reykjavik Study . Hypertension 51: 1123–1128, 2008. doi: 10.1161/HYPERTENSIONAHA.107.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballak DB, Brunt VE, Sapinsley ZJ, Ziemba BP, Richey JJ, Zigler MC, Johnson LC, Gioscia-Ryan RA, Culp-Hill R, Eisenmesser EZ, D'Alessandro A, Dinarello CA, Seals DR. Short-term interleukin-37 treatment improves vascular endothelial function, endurance exercise capacity, and whole-body glucose metabolism in old mice. Aging Cell 19: e13074, 2020. doi: 10.1111/acel.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol 588: 3971–3982, 2010. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleenor BS, Sindler AL, Eng JS, Nair DP, Dodson RB, Seals DR. Sodium nitrite de-stiffening of large elastic arteries with aging: role of normalization of advanced glycation end-products. Exp Gerontol 47: 588–594, 2012. doi: 10.1016/j.exger.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleenor BS, Sindler AL, Marvi NK, Howell KL, Zigler ML, Yoshizawa M, Seals DR. Curcumin ameliorates arterial dysfunction and oxidative stress with aging. Exp Gerontol 48: 269–276, 2013. doi: 10.1016/j.exger.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lesniewski LA, Seals DR, Walker AE, Henson GD, Blimline MW, Trott DW, Bosshardt GC, LaRocca TJ, Lawson BR, Zigler MC, Donato AJ. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, senescence pathways. Aging Cell 16: 17–26, 2017. doi: 10.1111/acel.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sehgel NL, Sun Z, Hong Z, Hunter WC, Hill MA, Vatner DE, Vatner SF, Meininger GA. Augmented vascular smooth muscle cell stiffness and adhesion when hypertension is superimposed on aging. Hypertension 65: 370–377, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Zhao X, Vatner DE, McNulty T, Bishop S, Sun Z, Shen YT, Chen L, Meininger GA, Vatner SF. Extracellular matrix disarray as a mechanism for greater abdominal versus thoracic aortic stiffness with aging in primates. Arterioscler Thromb Vasc Biol 36: 700–706, 2016. doi: 10.1161/ATVBAHA.115.306563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DuPont JJ, McCurley A, Davel AP, McCarthy J, Bender SB, Hong K, Yang Y, Yoo JK, Aronovitz M, Baur WE, Christou DD, Hill MA, Jaffe IZ. Vascular mineralocorticoid receptor regulates microRNA-155 to promote vasoconstriction and rising blood pressure with aging. JCI Insight 1: e88942, 2016. doi: 10.1172/jci.insight.88942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SK, McCurley AT, DuPont JJ, Aronovitz M, Moss ME, Stillman IE, Karumanchi SA, Christou DD, Jaffe IZ. Smooth muscle cell-mineralocorticoid receptor as a mediator of cardiovascular stiffness with aging. Hypertension 71: 609–621, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med 18: 1429–1433, 2012. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarjus A, Belozertseva E, Louis H, El Moghrabi S, Labat C, Lacolley P, Jaisser F, Galmiche G. Role of smooth muscle cell mineralocorticoid receptor in vascular tone. Pflugers Arch 467: 1643–1650, 2015. doi: 10.1007/s00424-014-1616-x. [DOI] [PubMed] [Google Scholar]
- 34.Davel AP, Lu Q, Moss ME, Rao S, Anwar IJ, DuPont JJ, Jaffe IZ. Sex-specific mechanisms of resistance vessel endothelial dysfunction induced by cardiometabolic risk factors. J Am Heart Assoc 7: e007675, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrett Mueller K, Lu Q, Mohammad NN, Luu V, McCurley A, Williams GH, Adler GK, Karas RH, Jaffe IZ. Estrogen receptor inhibits mineralocorticoid receptor transcriptional regulatory function. Endocrinology 155: 4461–4472, 2014. doi: 10.1210/en.2014-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod 27: 327–339, 1982. doi: 10.1095/biolreprod27.2.327. [DOI] [PubMed] [Google Scholar]
- 37.Wang M, Khazan B, Lakatta EG. Central arterial aging and angiotensin II signaling. Curr Hypertens Rev 6: 266–281, 2010. doi: 10.2174/157340210793611668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newfell BG, Iyer LK, Mohammad NN, McGraw AP, Ehsan A, Rosano G, Huang PL, Mendelsohn ME, Jaffe IZ. Aldosterone regulates vascular gene transcription via oxidative stress-dependent and -independent pathways. Arterioscler Thromb Vasc Biol 31: 1871–1880, 2011. doi: 10.1161/ATVBAHA.111.229070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coutinho T Arterial stiffness and its clinical implications in women. Can J Cardiol 30: 756–764, 2014. doi: 10.1016/j.cjca.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 40.Galmiche G, Pizard A, Gueret A, El Moghrabi S, Ouvrard-Pascaud A, Berger S, Challande P, Jaffe IZ, Labat C, Lacolley P, Jaisser F. Smooth muscle cell mineralocorticoid receptors are mandatory for aldosterone-salt to induce vascular stiffness. Hypertension 63: 520–526, 2014. doi: 10.1161/HYPERTENSIONAHA.113.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pruthi D, McCurley A, Aronovitz M, Galayda C, Karumanchi SA, Jaffe IZ. Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors. Arterioscler Thromb Vasc Biol 34: 355–364, 2014. doi: 10.1161/ATVBAHA.113.302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI, Sun Z, Hayden MR, Meininger GA, Mueller KB, Jaffe IZ, Sowers JR. Endothelial mineralocorticoid receptor mediates diet-induced aortic stiffness in females. Circ Res 118: 935–943, 2016. doi: 10.1161/CIRCRESAHA.115.308269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, Resuello RR, Natividad FF, Hunter WC, Genin GM, Elson EL, Vatner DE, Meininger GA, Vatner SF. Short communication: vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res 107: 615–619, 2010. doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dinh QN, Drummond GR, Kemp-Harper BK, Diep H, De Silva TM, Kim HA, Vinh A, Robertson AAB, Cooper MA, Mansell A, Chrissobolis S, Sobey CG. Pressor response to angiotensin II is enhanced in aged mice and associated with inflammation, vasoconstriction and oxidative stress. Aging (Albany NY) 9: 1595–1606, 2017. doi: 10.18632/aging.101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng S, Fernandes VR, Bluemke DA, McClelland RL, Kronmal RA, Lima JA. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: The Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging 2: 191–198, 2009. doi: 10.1161/CIRCIMAGING.108.819938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lieb W, Xanthakis V, Sullivan LM, Aragam J, Pencina MJ, Larson MG, Benjamin EJ, Vasan RS. Longitudinal tracking of left ventricular mass over the adult life course: clinical correlates of short- and long-term change in the framingham offspring study. Circulation 119: 3085–3092, 2009. doi: 10.1161/CIRCULATIONAHA.108.824243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alibhai FJ, Reitz CJ, Peppler WT, Basu P, Sheppard P, Choleris E, Bakovic M, Martino TA. Female ClockΔ19/Δ19 mice are protected from the development of age-dependent cardiomyopathy. Cardiovasc Res 114: 259–271, 2018. doi: 10.1093/cvr/cvx185. [DOI] [PubMed] [Google Scholar]
- 48.Harvey A, Montezano AC, Lopes RA, Rios F, Touyz RM. Vascular fibrosis in aging and hypertension: molecular mechanisms and clinical implications. Can J Cardiol 32: 659–668, 2016. doi: 10.1016/j.cjca.2016.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen YZ, Qiao SB, Hu FH, Yuan JS, Yang WX, Cui JG, Zhang Y, Zhang CL. Left ventricular remodeling and fibrosis: sex differences and relationship with diastolic function in hypertrophic cardiomyopathy. Eur J Radiol 84: 1487–1492, 2015. doi: 10.1016/j.ejrad.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 50.Dworatzek E, Mahmoodzadeh S, Schriever C, Kusumoto K, Kramer L, Santos G, Fliegner D, Leung YK, Ho SM, Zimmermann WH, Lutz S, Regitz-Zagrosek V. . Sex-specific regulation of collagen I and III expression by 17beta-Estradiol in cardiac fibroblasts: role of estrogen receptors. Cardiovasc Res 115: 315–327, 2019. doi: 10.1093/cvr/cvy185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ, American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123: 933–944, 2011. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]